Abstract
Background
This evidence review was conducted to inform the accompanying clinical practice guideline on the management of cyclic vomiting syndrome (CVS) in adults.
Methods
We followed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework and focused on interventions aimed at prophylactic management and abortive treatment of adults with CVS. Specifically, this evidence review addresses the following clinical questions: (a) Should the following pharmacologic agents be used for prophylaxis of CVS: amitriptyline, topiramate, aprepitant, zonisamide/levetiracetam, or mitochondrial supplements? (b) Should the following pharmacologic agents be used for abortive treatment: triptans or aprepitant?
Results
We found very low‐quality evidence to support the use of the following agents for prophylactic and abortive treatment of CVS: amitriptyline, topiramate, aprepitant, zonisamide/levetiracetam, and mitochondrial supplements. We have moderate certainty of evidence for the use of triptans as abortive therapy. We found limited evidence to support the use of ondansetron and the treatment of co‐morbid conditions and complementary therapies.
Conclusions
This evidence review helps inform the accompanying guideline for the management of adults with CVS which is aimed at helping clinicians, patients, and policymakers, and should improve patient outcomes.
Keywords: cyclic vomiting, technical review, treatment
Abbreviations
- AE
adverse event
- CI
confidence interval
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- LR
likelihood ratio
- OR
odds ratio
- PICO
population, intervention, comparator, and outcome
- RCT
randomized control trial
- RR
relative risk
1. INTRODUCTION
Cyclic vomiting syndrome (CVS) is a chronic, debilitating illness that is characterized by recurrent episodes of intense nausea and vomiting. Although the true prevalence of CVS in adults in the general population remains uncertain, it is not a rare disorder. A recent population‐based study noted that the US prevalence was 2% among adults, mirroring prevalence estimates in children.1 Another estimated that ~10% of outpatients presenting to a tertiary gastroenterology clinic met the Rome III criteria for the illness;2 however, even in this clinical setting, CVS was considered as a potential diagnosis in only a small minority of these patients. This finding highlights the poor recognition of CVS in adults by clinicians, with many patients continuing to suffer for several years before receiving a diagnosis of CVS. Concerted messaging and increased awareness campaigns should minimize this clinical recognition gap. Recognizing CVS in adults is critical, as there are several fairly effective prophylactic and abortive therapies to treat the disorder.
This evidence review represents a foundational effort by the American Neurogastroenterology and Motility Society (ANMS) and the Cyclic Vomiting Syndrome Association (CVSA) to develop recommendations based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework to provide a robust guideline for best practices in the management of CVS. This review addresses focused clinical questions on the use of pharmacologic agents for prophylactic and abortive therapies for the management of patients with CVS and was used to inform the development of the accompanying clinical practice guidelines. Panel members were selected by the CVS guideline committee task chair (T.V.), co‐chair (B.L.), and former ANMS council member (B.M.) and the CVSA based on their clinical and methodological expertise. All members of the panel underwent a thorough vetting process for potential conflicts of interest.
2. METHODS
2.1. Overview
This evidence review was developed using the GRADE framework to develop clinically focused questions, and identify, synthesize, and evaluate the quality of the supporting evidence to inform a recommendation.3
2.2. Formulation of clinical questions
Through an iterative process, the panel developed focused clinical questions on the role of specific therapeutics in the management of CVS. The PICO format was used which frames a clinical question by defining a specific population (P), intervention (I), comparator (C), and outcomes (O) (Table 1). The population was adult patients with CVS. The intervention was one of numerous therapies used in CVS. The preferred comparator was placebo. Relevant patient‐centered outcomes were considered and rated in terms of importance. All PICO questions formed the basis for a literature search which is detailed below.
Table 1.
PICO questions
| PICO questions | Method | |||
|---|---|---|---|---|
| Population | Intervention(s) | Comparator | Outcomes | |
| Prophylactic therapy | ||||
| Adults with CVS | 1. TCAs 2. Topiramate 3. Zonisamide Levetiracetam 4. Aprepitant 5. Mitochondrial supplements CoQ10, L‐Carnitine Riboflavin | Placebo or usual care | 1. Complete response or partial response or subjective improvement (reduction in frequency or duration or severity of CVS symptoms) 2. Decrease in frequency or duration or severity of CVS attacks (if reported separately) 3. Reduction in numbers of hospitalizations of ED visits per year 4. Adverse effects—% of patients discontinuing treatment | GRADE |
| Abortive therapy | ||||
| Adults with CVS | 6. Triptans 7. 5HT3 antagonists Ondansetron 8. Aprepitant | Placebo or usual care | 1. Complete response or partial response or subjective improvement (reduction in frequency or duration or severity of CVS symptoms) 2. Decrease in frequency or duration or severity of CVS attacks (if reported separately) 3. Reduction in numbers of hospitalizations of ED visits per year 4. Adverse effects—% of patients discontinuing treatment | GRADE and narrative review |
2.3. Outcomes
Outcomes were grouped into two broad categories for prophylactic and abortive therapies. We arrived at a consensus as to what measurements would be acceptable for each outcome. Outcomes were rated by the group on a scale of 1 (not important) to 9 (critically important) for medical decision making. It was understood that data on all outcomes would not be available in the published literature.
2.4. Systematic review process
2.4.1. Search strategy
The literature search was performed initially in June 2016 and updated in February 2018, with the aid of a research librarian (C.S.). Details of the search strategy are reported in the Online Supplement. Individual studies were identified via searches of three bibliographic databases: PubMed (includes MEDLINE), SCOPUS (a large, multidisciplinary database), and CINAHL (the Cumulative Index to Nursing and Allied Health Literature). Given the acknowledged possibility of diagnostic misclassification, individual search strategies included the following terms: cyclic vomiting; cyclical vomiting; cannabinoid hyperemesis; functional vomiting; abdominal migraine; and periodic syndrome. The searches excluded animal‐only studies and non–English language studies. The search strategy was iteratively developed through refinement with author input to maximize sensitivity. Given the limited total literature, a single search was conducted for all PICO Questions.
For all PICOs, the a priori intent was to rely upon high‐quality systematic reviews for evidence synthesis, particularly those that synthesized data from randomized control trials (RCTs). If systematic reviews of RCTs were not available, we would then look to individual RCTs to generate summary estimates if possible. In the absence of systematic reviews of RCTs or individual RCTs, systematic reviews of observational studies and observational studies were then considered to inform the evidence. Case series of fewer than 10 individuals were excluded, as were narrative reviews.
2.4.2. Study selection criteria
The reviewers utilized the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines to develop the review. A PRISMA flow diagram is included in Figure 1. The titles/abstracts from the database searches were uploaded to Covidence (http://covidence.org), a Web‐based application that facilitates screening and reviewing studies for systematic reviews. All titles and abstracts were screened by two researchers (R.S. and S.S.) with disagreements regarding inclusion and exclusion resolved by discussion. Inclusion criteria included any articles that might be relevant to the included PICO questions. Exclusion criteria were principally around study design as mentioned above. A total of 1469 non‐duplicate articles were found, and 572 full‐text articles were then reviewed. One author (R.S.) extracted data from full‐text articles into a standardized data collection form with accuracy of data extraction confirmed by several members of the systematic review committee. Study characteristics and data extraction are reported in Table 2a,b.
Figure 1.
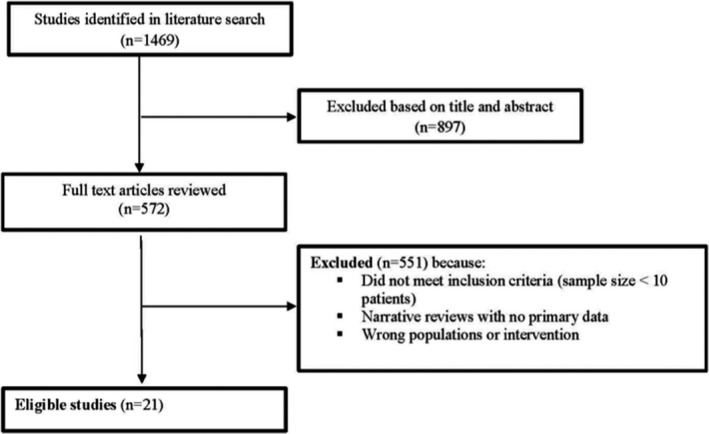
PRISMA flow diagram
Table 2.
Characteristics of included studies
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author/year | Medications | Abortive vs. Prophylactic treatment | Adult or pediatric | Study design | Country | N (# patients for which medication data reported) | Population characteristics | Duration of follow‐up | |
| 1 | Badihian et al. (2018) | Amitriptyline: 0.5 mg/kg, increased to 1 mg/kg after 1 week OR Cyproheptadine: 0.1 mg/kg, increased to 0.2 mg/kg after 1 week Medications were delivered to patient at 2‐week intervals. | Prophylactic | Pediatric | Randomized trial of amitriptyline vs cyproheptadine. MD visits every 2 weeks over 6 months | Iran | 70 eligible, 64 randomized (32/arm), 0 lost to follow‐up | Diagnosis of CVS (based on Rome III criteria). 3‐15 years old. 2 treatment groups not statistically different | 6 months |
| 2 | Shearer et al. (2016) | Amitriptyline (median 45 mg, range 10‐140 mg) | Prophylactic | Adults | Retrospective cohort | United Kingdom | 14 patients on amitriptyline. | Diagnosis of CVS based on “symptoms compatible with CVS.” Mean age ~ 30 years | 1‐5 years |
| 3 | Hikita et al. (2016) | Valproic acid Valproic acid with phenobarbital Phenobarbital Amitriptyline Phenytoin Carbamazepine Cyproheptadine Primidone Propranolol Clonidine hydrochloride (Dosages not specified for all medications) | Prophylactic | Pediatric | Retrospective cohort | Japan | 18 received prophylactic medications | CVS diagnosis based on International Classification of Headache Disorders criteria | 5 years |
| 4 | Sezer et al. (2016) | Topiramate (started at 25 mg at night. Increased to a maximum of 75 mg/ day if needed. OR Propranolol (started at 1 mg/kg/day, increased after 1 month if needed, goal 1.4 mg/kg/day) | Prophylactic | Pediatric | Retrospective chart review and patient questionnaire. | Turkey | 38 (16 patients on topiramate and 22 patients on propranolol) | CVS diagnosed by Rome III criteria and International Headache Society Classification. Topiramate vs. Propranolol group had significantly: Less episodes of vomiting/cycle before treatment Fewer attack numbers/yr. after treatment Fewer median duration of cycles in hour Fewer peak number of emeses per hour | At least 12 months |
| 5 | Jensen et al. (2015) | Tricyclic antidepressants Phenergan Ondansetron L‐Carnitine Co‐Q10 Showers Marijuana (Dosages not described) | Abortive and prophylactic | Adults | Cross‐sectional. Phenomenological approach. | USA | 16 | Diagnostic criteria for CVS not mentioned. Median age at diagnosis 25 years. Patients with CVS recruited from Cyclic Vomiting Syndrome Association Web site, Facebook page, and newsletter. Survey and qualitative interviews of patients with CVS | Cross‐sectional so single time point |
| 6 | Moses et al. (2014) | Amitriptyline was started at a dose of 0.2 mg/kg per day, once daily at bedtime and increased as clinically indicated and tolerated by the patient. Cyproheptadine was started at 0.25‐0.5 mg/kg once daily at bedtime and “infrequently” divided twice a day. Ondansetron: The dose used was 0.3 mg/kg per dose every 6 hours as needed for continued vomiting or nausea. | Abortive and prophylactic | Pediatric | Retrospective cohort | USA | 106 | CVS diagnosis based on Rome III criteria: Personal history of migraine was reported in 25% of the children and a family history of migraine in 72% | Not specified |
| 7 | Treepongkaruna et al. (2014) | Amitriptyline Propranolol Cyproheptadine Sodium valproate (Medication dosages not explicitly stated but prior clinical practice guidelines referenced for dosages) | Prophylactic | Pediatric | Retrospective cohort | Thailand | 29 patients received either propranolol (n = 11), cyproheptadine (n = 4), and amitriptyline (n = 14). Long‐term outcomes were available in 19 patients. | Age (mean ± std.) 11.3 ± 4.9 years | 3 months to 5 years |
| 8 | Cristofori et al. (2014) | Aprepitant Abortive: 125 mg in early prodrome, then if needed: a) If < 15 kg, 80 mg (Day 1), 40 mg (Days 2/3); b) if 15‐20 kg, 80 mg (Days 1/2/3), c) if > 20 kg, 80 mg (Days 2/3). Prophylactic (twice/week): 40 mg if < 40 kg, 80 mg if 41‐59 kg, 125 mg if > 60 kg Abortive regimen of aprepitant given if prodromal phase* suggested imminent CVS attack. Otherwise, prophylactic aprepitant dose given. All oral administration. *Prodromal phase= symptoms such as nausea, anorexia, change in mood, anxiety, dizziness and autonomic symptoms *Some children > 60 kg | Abortive and prophylactic | Pediatric | Retrospective cohort | United Kingdom | 41 total 16 prophylactic (though outcome data only collected on 15). 25 abortive | Met NASPGHAN criteria for CVS. All either failed or could not tolerate prior treatment. No statistically significant differences between abortive/prophylaxis groups except that abortive group had a higher rate of prodromal events (21/25 vs 3/15). Current treatment for abortive group Propranolol (0.5‐1 mg/kg/day): 13/25 (52%) Amitriptyline (1‐1.5 mg/kg/day): 10/25 (40%) Co‐Enzyme Q10 (10 mg/kg/day): 6/25 (24%) L‐Carnitine (100 mg/kg/day): 4/25 (16%) Current treatment for prophylaxis group: Propranolol (0.5‐1 mg/kg/day): 9/15 (60%) Amitriptyline (1‐1.5 mg/kg/day): 7/15 (46%) Co‐Enzyme Q10 (10 mg/kg/day): 5/15 (33%) L‐Carnitine (100 mg/kg/day): 3/15 (20%) | Abortive: median 30 months, range 16‐60 months Prophylactic: median 24 months, range 18‐60 months |
| 9 | Kumar et al. (2012) | Abortive Triptans (dose not mentioned) Hot showers Prophylactic Tricyclic antidepressants (TCAs, goal 1 mg/kg/day Topiramate (dose not mentioned) Mitochondrial therapy: (Carnitine 1 gram twice daily, Co‐enzyme Q‐10 200 mg twice daily, riboflavin 100 mg once daily) | Abortive and prophylactic | Adult | Retrospective chart review plus “prospective” standardized questionnaire. | USA | 101. Follow‐up available in 76/101(75%) on medical therapy. | Met Rome III Criteria for CVS. Mean age 27 years Anxiety (47%) Depression (49%) Dysautonomia (64%) 30/70 (43%) personal history of migraine 41/64 (64%) had a family history of migraine | Mean duration of follow‐up was 11.2 ± 6.2 months. |
| 10 | Lee et al. (2012) | TCA Propranolol L‐carnitine and amitriptyline Erythromycin Coenzyme Q Phenobarbital Valproate Pizotifen Cyproheptadine L‐Carnitine Sumatriptan Zonisamide/Levetiracetam | Abortive and prophylactic | Adult and pediatric | Systematic review. | Not applicable | 1 prospective cohort study 24 retrospective cohort studies. 1093 patients, of whom 377 were adults | Literature search MEDLINE via Ovid (January 1948 to October 2011) and EMBASE (January 1980 to October 2011). References searched as well. Case reports excluded. CVS diagnosis based on Rome III criteria. Adult CVS patients (vs pediatric) had a significantly higher family history of headache/migraine (56% vs 28% (P = 0.006)) | Not reported |
| 11 | Boles et al. (2011) | *Dietary: “3 + 3 diet” (3 meals and 3 snacks a day including between meals and at bed‐ time), and the avoidance of fasting. • Co‐enzyme Q10: Maximum blood level > 3.0 mg/L • L‐carnitine: Maximum blood level > 40 micromolar • Amitriptyline/Nortriptyline: Maximum blood level > 150 ng/ml • Cyproheptadine: Maximum dosage of 0.5 mg/kg/day • Topiramate: Maximum dosage of 200 mg twice a day (in adolescents and adults). Used as a last resort medication Dosages were increased until one of the following occurred: • Resolution of vomiting episodes • Intolerable side effects that failed a reduction in dosage followed by a slow dosage increase | Abortive and prophylactic | Adult and pediatric | Retrospective cohort | USA | 42 met inclusion criteria, data available on 30 patients | CVS diagnoses based on ICD‐9 and NASPGHAN and Rome III criteria. Age: 3 ‐26 years (median 12). The age of onset of vomiting episodes was 1 week to 15 years, with a median of 4 years. Of 42 patients, 74% with chronic pain, 74% with GI dysmotility, 57% with “functional or autonomic‐related conditions,” 31% with mental health disorders, 36% with “cognitive disorders” | Minimum 2 years |
| 12 | Hikita et al. (2011) | Sumatriptan subcutaneous injection (age x4 + 20)/(100x3 mg or nasal spray versus nasal spray (20 mg) | Abortive | Adult and pediatric | Prospective non‐randomized trial | Japan | 12 patients enrolled; 11 children and 1 adult | Diagnosed with CVS by International Classification of Headache Disorders. Family history migraine in a first‐degree relative in 33% (4 of 12) patients. | Not applicable |
| 13 | Boles et al. (2010) | Amitriptyline (ranged from < 0.5 mg/kg/ day), medium, and high (≥1.0 mg/kg/day) Coenzyme Q‐10: (ranged from ≥ 10 mg/kg/day or ≥ 300 mg in a patient ≥ 30 kg) | Prophylactic | Adult and pediatric Child and adolescent data combined to create a “pediatric‐onset” group. | Retrospective cohort | Not applicable | Amitriptyline: 249 subjects Co‐enzyme Q 10: 32 subjects | Patients met Rome III and NASPGHAN for CVS. Recruitment via newsletters, the Cyclic Vomiting Syndrome Association Web site, emails to members and associated physicians. | Cross‐sectional study (no follow‐up available) |
| 14 | Hejazi et al. (2010) | Tricyclic antidepressants (amitriptyline, nortriptyline, or doxepin, started at 10 to 25 mg, goal 1 mg/kg. Actual doses achieved were 0.25 to 3 mg/kg (range: 15 to 200 mg/daily; average dose 100 mg at bedtime). | Prophylactic | Adult | Open‐Label Prospective cohort Office visits, telephone interviews, and questionnaires at time zero and every 6‐month intervals. | USA | 41 | All met the Rome III criteria for CVS 12 pts had migraine headaches. 3 pts had a family history of migraine 22/46 (53%) current/past marijuana use. In addition to TCAs, 7 patients on L‐carnitine/CoQ 10, 3 patients on topiramate, but treatment effect from these medications was not reported. | Up to 2 years |
| 15 | Haghihat et al. (2007) | Amitriptyline (1 mg/kg per day) OR Propranolol (1 mg/kg per day) | Prophylactic | Pediatric | Randomized trial of amitriptyline vs propranolol | Iran | 164 patients (81 on propranolol and 83 on amitriptyline) | CVS diagnosis: >/= 3 episodes of intractable, self‐limited, non‐bilious vomiting, separated by symptom‐free intervals. 8/164 (5%) history of seizures (on anticonvulsants) 90/164 (55%) older children with recurrent headaches; in 20%, symptoms were typical of migraine. 39/164 (24%): family history migraine | 6 months to 12 years |
| 16 | Namin et al. (2007) | Amitriptyline (target dose of 1 mg/kg/day over 1‐2 months. Mean daily dose 75 mg (range 50‐150 mg) | Prophylactic | Adults | Single‐arm cohort study, prospective. This cohort of patients was studied in a 2‐year follow‐up program. | USA | 31 eligible. 27 received amitriptyline, 24 on it for at least 3 months. 15 patients on it for at least 12 months. | Rome III criteria for CVS The Hamilton Rating Scale for Anxiety: 84% had an anxiety disorder Zung Depression Inventory: 78% suffered from mild‐to‐severe depression. 4/31 (13%) patients reported migraine, 14/31(45%) had a family history of migraine. | 2 years |
| 17 | Clouse et al. (2007) | Zonisamide (median dose, 400 mg/d) Levetiracetam (median dose, 1000 mg/d) | Prophylactic | Adults | Retrospective chart review and patient interview | USA | 20 (16 on Zonisamide, 4 on Levetiracetam) | CVS diagnosis: >/= 3 discrete stereotypical episodes of severe vomiting separated by symptom‐free intervals of at least 2 weeks with no structural or metabolic explanation for the subjects. All patients who had failed or could not tolerate tricyclic antidepressant monotherapy Mean age 38.5 ± 3.2 years, 18 White, 10 males | Mean of 9.5 ± 1.8 months |
| 18 | Boles et al. (2006) | Abortive IV dextrose Ondansetron Lorazepam Promethazine Sumatriptan Prophylactic Amitriptyline Cyproheptadine Propranolol (Dosages not listed) | Abortive and prophylactic | Adult and pediatric | Cross‐sectional study via clinical questionnaire. Random recruitment from Cyclic Vomiting Syndrome Association, USA/Canada. | USA and Canada | 23 CVS plus (CVS + neuromuscular disease) 44 CVS minus (no neuromuscular disease) 13 subjects with CVS that were not subgrouped | CVS plus group had earlier age of onset of symptoms. Definition of CVS not explicitly specified. | Cross‐sectional (no follow‐up available) |
| 19 | Aanpreung et al. (2002) | Amitriptyline Pizotifen Propranolol Dosage not specified | Prophylactic | Pediatric | Retrospective cohort | Siriraj Hospital, Thailand | 25 | CVS diagnosis: >/= 3 discrete, stereotypic episodes of nausea and vomiting; each > 12 hours; more than 7 days between episodes; and no structural or metabolic explanation for the symptoms. 6/25 with headaches (of these 3 had family history of migraine) | Patients were on medications for at least 3 months |
| 20 | Prakash et al. (1999) | Amitriptyline Doxepin Nortriptyline Desipramine Imipramine | Prophylactic | Adults | Retrospective chart review. Had comparison group (functional nausea/abdominal pain) | USA | 17 | CVS definition: a) > /=3 discrete, stereotypic episodes of nausea and vomiting, each lasting 12 hours; b) 7 days between episodes; c) complete resolution of symptoms between episodes; and d) no structural or metabolic explanation for the symptoms. 4 patients with migraine headache. | 23 ± 7 months |
| 21 | Li et al. (1999) | Supportive therapyIV hydration Antiemetic/Prokinetic therapy Phenothiazines Prokinetic Agents Ondansetron Antimigraine therapies Propranolol Cyproheptadine Amitriptyline Sumatriptan (Dosages not described) | Abortive and prophylactic | Pediatric | Retrospective chart review and structured interviews | USA | 214 | CVS diagnosis made based on clinical judgment. 176/214 (82%) Migraine‐related CVS (family history of migraines, subsequent development of migraines) 38/214 (18%) Non–migraine‐related CVS | Median follow‐up 17.5 months |
| 22 | Andersen et al. (1997) | Amitriptyline (10‐200 mg) Cyproheptadine (2‐4 mg) | Prophylactic | Pediatric | Retrospective chart review | USA | 27 | Patients fulfilling referenced “diagnostic criteria for CVS” and treated prophylactically. 50% with parent/sibling with migraine. 8/27 children with concomitant medical/psychiatric conditions, including depression, hyperactivity, and developmental delay. | 5 months to 10 years |
| (b) | ||||
|---|---|---|---|---|
| Author/year | Outcomes measured | Results | Adverse events | |
| 1 | Badihian et al. (2018) | Primary outcomes measured in last 2 months of study: a) Severity of attacks b) Medication response: Complete remission: Complete resolution of disease 50‐99% remission: >50% decrease in # or duration of attacks <50% remission: <50% decrease in # or duration of attacks | Complete remission: (P‐value = 0.206) Amitriptyline: 21/32 (65.6%) Cyproheptadine: 16/32 (50%) 50‐99% remission: (P value = 1) Amitriptyline: 8/32 (25%) Cyproheptadine 8/32 (25%) <50% Remission: (P value = 0.1) Amitriptyline 3/32, 9.4% Cyproheptadine 8/32, 25% | Amitriptyline: 3/32 (9%) with sedative effects, weight gain or constipation. None discontinued medication Cyproheptadine: 2/32 (6%) with increased appetite or temporary restlessness. None discontinued medication |
| 1 | Shearer et al. (2016) | Full remission: No further attacks during follow‐up Substantial improvement in symptoms: physician's global assessment, with a reduction in the frequency of attacks of CVS No response | Full remission: 6/14 (43%) Substantial improvement: 2/14 (14%) No response: 3/14 (21%) Lost to follow‐up: 3/14 (21%) | Not reported |
| 3 | Hikita et al. (2016) | “Response”: less than two attacks per year “Non‐responders”: more than three attacks per year | Responder/Non‐responder/Total patients on listed medication Valproic acid (9/4/15) Valproic acid with phenobarbital (4/0/4) Phenobarbital (3/6/9) Amitriptyline (1/4/5) Phenytoin (0/4/4) Carbamazepine (0/2/2) Cyproheptadine (0/2/2) Primidone (0/1/1) Propranolol (0/1/1) Clonidine hydrochloride (0/1/1) | Not reported |
| 4 | Sezer et al. (2016) | Responders: ≥ 50% reduction in attacks Non‐responders: <50% reduction in attacks | No attacks for 1 year (P = 0.05) Propranolol group (13/22,59%) Topiramate group (13/16, 81%) ≥ 50% decrease in attacks/year (P = 0.11) Propranolol group (5/22, 23%) Topiramate group (2/16,13%) Responder rates (P = 0.001) (Combination of zero attacks and ≥ 50% decrease) (A composite endpoint): Propranolol 18/22 (81%) Topiramate group 15/16 (94%) Non‐responder rates (P = 0.01) Propranolol 4/22 (18%) Topiramate 1/16 (6%) | No dropouts from adverse events. No stat sig difference in adverse events. Topiramate group: 2 patients with drowsiness and dizziness Propranolol group: 3 patients with drowsiness, nervousness, and dizziness. |
| 5 | Jensen et al. (2015) | Thematic saturation | Two themes emerged from the data: 1) Perceived lack of knowledge among healthcare providers (difficulty receiving a diagnosis, inappropriate treatment in the acute care facility, avoidance of care) 2) Response to CVS‐related treatments (abortive treatment, self‐management) a) Prophylactic treatment ‐4/16 (25%) currently on TCAs. ‐1/16 (6%) stated that “ Phenergan, ondansetron, amitriptyline, L‐carnitine, and Co‐Q10 have all been “minimal in their success with my treatments.” b) Abortive treatment ‐10/16 (63%) said ondansetron was most effective abortive medication ‐7/16 (44%) said benzodiazepines effective ‐ 6/16 (38%) said antimigraine medications least effective abortive medication c) Self‐Management ‐Marijuana use: 6/16 (38%) using MJ (daily use as prophylactic or abortive) 2/16 (13%) tried MJ but had no symptom relief ‐Hot‐water bathing (11/16) 69% (used for helpful in nausea and pain) 5/8 that used MJ also reported hot‐water bathing. 6/16 (38%) described hot‐water bathing and did not discuss marijuana consumption | TCAs: 1/4 reported somnolence 6/16 (38%) no longer taking TCAs because “side effects, ineffectiveness, or minimal effectiveness.” One patient reported “severe constipation, dry mouth, mental side effects” |
| 6 | Moses et al. (2014) | Response to acute medications was defined as any of the following: (1) decreased severity or duration of acute episodes, (2) complete resolution of acute episodes, or (3) avoidance of emergency department visit. Response to prophylactic medications was defined as any of the following: (1) resolution of abnormalities since initiating treatment, (2) decreased frequency or duration of acute episodes (within the last 6 months), or (3) decreased number of emergency department visits (within the last 1 year) | Prophylaxis: Amitriptyline: 23/40 (58%) with “clinical benefit” Cyproheptadine: 30/61 (49%) with “clinical benefit” Abortive: Ondansetron: 56/85 (66%) reported “improvement” | Not reported |
| 7 | Treepongkaruna et al. (2014) | Short‐term outcomes (3‐6 months after treatment initiation) Good response: Remission (no attacks after treatment) OR marked improvement No response: <50% decrease in attack frequency Long‐term outcomes (at least 2‐year follow‐up Frequency of vomiting: 1) Excellent: no episodes 2) Good: 1‐2 episodes 3) Poor: 3 or more episodes/year | Short‐term outcomes Good response: Amitriptyline 11/15 (73%) (significantly better than propranolol P = 0.04) Propranolol 5/14 (36%) Cyproheptadine 0/4 (0%) Sodium valproate 1/3 (33%) Long‐term outcomes (diagnosis ≥ 2 years) Data available on 19 patients Amitriptyline: 7/9 (78%) patients who had it as a first‐line drug had good or excellent response. Propranolol: 4/7 (57%) had good response when used as first‐line medication | “Adverse reaction” reported in 1 patient, but medication involved was not specified |
| 8 | Cristofori et al. (2014) | Primary outcome: (CVS episodes) Complete response: No episodes Partial response: ≥50% decrease in both frequency (# episodes/year) and intensity (episode duration in days) No response: <50% decrease in episode frequency and intensity. Secondary outcomes: Number of CVS episodes/ year Number of hospital admissions/year CVS episode duration Number of vomits/hour Symptom‐free interval length (days) School attendance percentage | Prophylactic group had higher aprepitant doses than the abortive group. All outcomes below measured at 12 months Abortive: 1) Primary outcomes Complete response: 3/25 (12%) Partial response 16/25 (64%) No response 6/25 (24%) 2) Secondary outcomes (All statistically significant) CVS episodes/year: Baseline 12 (9.5‐16.5) to 6 (2‐8.5) Hospital admissions/year: Baseline 9 (6‐12) to 2.5 (1‐5.5) Duration of episodes: Baseline 5 (3.5‐7) to 1 (0.75‐2) Number vomits/episode: Baseline 9 (7‐10) to 4 (2‐4.5) Duration of interepisodic period (days): Baseline 30 (21‐35) to 60 (40‐180) % school attendance: Baseline 65 (57.5‐74) to 80 (72‐87.5) Prophylaxis: 1) Primary outcomes Complete response 3/16 (19%) Partial response 10/16 (62%) No response 2/16 (19%) 2) Secondary outcomes (All statistically significant) CVS episodes/year: Baseline 12 (9‐14) to 3 (2‐6) Hospital admissions/year: Baseline 8 (6‐12) to 2 (1‐4) Duration of episodes: Baseline 5 (4‐7) to 3 (1‐3) Number vomits/episode: Baseline 9 (7‐10) to 6 (5‐8) Duration of interspersed period (days): Baseline 30 (21‐40) to 120 (60‐180) % School attendance: Baseline 67 (58‐72) to 81 (78‐85) | Side effects only in prophylactic group affecting 5/16 (31%) Hiccup 3/16 (19%) Asthenia/fatigue 2/16 (12.5%) Increased appetite 2/16 (12.5%) Mild headache 1/16 (6%) Severe migraine 1/16 (6%) Only child with migraine stopped medication. |
| 9 | Kumar et al. (2012) | Complete response (≥ 80% amelioration in symptom duration, frequency, and severity) Partial response (50‐80% reduction in symptom duration, frequency, and severity) No response (< 50% reduction in symptom duration, frequency, and severity) | Complete response in 44/76 (58%). Of these, 36/44 ~90% taking TCAs, 6/44 ~15% taking topiramate, 13/44 ~30% taking L‐carnitine, 13/44 ~ 30% taking Co‐Q10. Partial response achieved in 21/76 (28%). Of these, 20/21 ~95% taking TCAs, 8/21 ~40% taking topiramate, 11/21 ~50% taking L‐carnitine, 7/21 35% taking CoQ‐10. No response in 11/76 (14%). Of these, 8/11 ~70% taking TCAs, 3/11 30% taking topiramate, 3/11 ~25% taking L‐carnitine, and 1/11 ~10% taking CoQ‐10. Medication‐specific response data that were provided Triptans: 64/76 (83%) could abort episodes Hot showers: 38/73 (52%) improved (reported in 25/35 (71%) marijuana users vs 19/56 (34%) non‐users) (P = 0.01). Cold showers: 1/73 (1%) improved | Tricyclic antidepressants: 18/70 patients (26%) had to stop (bad dreams, behavioral changes, and increased somnolence). |
| 10 | Lee et al. (2012) | Response to treatment (“either decreased frequency/duration/intensity of attacks”) | Response of pediatric CVS patients to medication TCA 168/244 (68%) Propranolol 79/91 (87%) L‐carnitine and amitriptyline 23/30 (77%) Erythromycin 13/20 (65%) Coenzyme Q 12/18 (67%) Phenobarbital 11/14 (78.6%) Valproate 13/13 (100%) Pizotifen 4/8 (50%) Cyproheptadine 5/6 (83%) L‐Carnitine 6/6 (100%) Response of adult CVS patients to medication TCA 180/237 (76%) Sumatriptan 21/37 (57%) Zonisamide/Levetiracetam 15/20 (75%) Co‐Q10 5/7 (71%) No statistically significant difference between children and adults | Not reported |
| 11 | Boles et al. (2011) | • Resolution (episodes resolved, allowing for one episode a year with an obvious trigger). • > 75% improvement (between 75 and 100% response in at least one episode parameter*). • > 50‐75% improvement (between 50 and 75% response in at least one episode parameter*) • Treatment failure (< 50% improvement in both episode parameters*) *Episode parameter = episode frequency and episode duration | Co‐Q 10/L‐carnitine: Resolution: 3 patients >75% improvement: 3 patients Amitriptyline Resolution: 1 patient Treatment failure: 1 patient Amitriptyline + Co‐Q10: Resolution: 3 patients (in one of these cases, amitriptyline was not tolerated, yet episodes resolved on topiramate + co‐enzyme Q10) Amitriptyline + L‐carnitine: Resolution: 2 patients Amitriptyline + Co‐Q10 + L‐carnitine: Resolution: 10 patients >50% improvement: 1 patient Treatment failure: 2 patients Cyproheptadine Resolution: 1 patient Cyproheptadine + Co‐Q10: Resolution: 1 patient Cyproheptadine + L‐Carnitine: Resolution 1 patient Cyproheptadine + Co‐Q10 + L‐carnitine: Resolution: 1 patient Topiramate ± Co‐Q10 or L‐carnitine Resolution: 2 patients | Amitriptyline: 2 treatment discontinuations (prolonged QTc, narcolepsy) 1 treatment reduction (“behavioral and emotional effects”) 3 non‐treatment limiting side effect (increased frustration in two, one also with insomnia, one with dizziness) Co‐enzyme Q10: 1 discontinued because of a pseudo‐porphyria rash. Cyproheptadine: 1 with lethargy 2 with “vague non‐specific sensations” |
| 12 | Hikita et al. (2011) | Complete response: (no vomiting after treatment) Effective response: (vomiting frequency reduced by >/= 50%) Non‐effective response: (the treatment was not effective in preventing vomiting) | 11 patients received sumatriptan subcutaneous injection: Complete resolution: 4/11(36%) Effective response: 5/11 (46%) Non‐effective response: 2/11 (18%) Patients with a family history migraine more likely to respond (defined as complete and effective response) (P = 0.04) 5 patients received nasal spray: Complete resolution: 1/5 Effective response: 1/5 Non‐effective response: 3/5 | Not observed |
| 13 | Boles et al. (2010) | Episode frequency Episode duration Number of emeses Nausea severity Episode improvement: “compound measurement scored positive if at least one of the above four parameters was scored as positive.” A reduction of at least 50% in each parameter was co‐scored as positive and any lesser response as negative. | Amitriptyline* Episode frequency: 88/162 (54%) Episode duration: 78/155 (50%) Number of Emesis: 70/154 (45%) Nausea severity: 68/157 (43%) Episode improvement: 127/177 (72%) Coenzyme Q10* Episode frequency: 11/22 (50%) Episode duration: 8/22 (36%) Number of emesis: 8/20 (40%) Nausea severity: 11/25 (44%) Episode improvement: 17/25 (68%) *All reported as 50% reduction in a symptom Statistically significantly higher patient satisfaction with CoQ10 | Amitriptyline side effects (statistically higher than with CoQ‐10) Side effects reported: 102/202 (50%) Discontinued due to side effects: 42/198 (21%) Coenzyme Q10: Side effects: 0/28 (0%) Discontinued due to side effects: 0/28 (0%) |
| 14 | Hejazi et al. (2010) | Frequency of CVS episodes per year Duration of CVS episodes (# days) Number of ED visits and/or hospitalizations Outcomes measured after first and second year of follow‐up visits. | Frequency of CVS episodes/year (Statistically significant change at Year 1 and Year 2) Baseline 17.8 ± 8.3 (4.5‐180) Year 1 5.4 ± 3.8 (1‐54) Year 2 3.3 ± 2.8 (1‐42) Duration of CVS episode (days): (Stat significant change at Year 1 and Year 2) Baseline 6.7 ± 6.1 (0.2‐30) Year 1 2.5 ± 2.7 (0‐14) Year 2 2.2 ± 2.4 (0‐10) #ED visits/hospitalizations/year (Stat significant change at Year 1 and Year 2) Baseline 15 ± 13.4 (1‐27) Year 1 4.2 ± 5 (0‐20) Year 2 3.3 ± 3.6 (0‐14) 36/4 (88%) patients 88% reported less ED visits, decreased frequency, and duration of CVS episodes and improved clinical status based on subjective global assessment. Over a half of those who agreed to stop MJ during follow‐up indicated a positive impact in decreasing CVS episodes. | Side effects in 14/41 (34%) (1/3 of this group needed to reduce dosage to alleviate side effects) Dry mouth 5 (12%) Somnolence 4 (9%) Chronic fatigue 3 (7%) Constipation 2 (4%) Blurred vision 1 (2%) Mild hallucinations 1 (2%) 1 patient stopped medication due to severe hallucinations |
| 15 | Haghihat et al. (2007) | Severity of CVS attacks Frequency of attacks | Amitriptyline (does not seem to be statistically significant) Good response (decrease in the frequency and severity of attacks): 46/83 (56%) Propranolol (statistically significantly) Good response (decrease in the frequency and severity of attacks): 74/81 (92%) | Amitriptyline: “Significant” number of had side effects of including irritability, agitation, insomnia, or lethargy* (number of patients not specified) Propranolol: No side effects reported |
| 16 | Namin et al. (2007) | Completed CVS questionnaire Hamilton Rating Scale for Anxiety (HAM‐A), Zung Depression Inventory Visual Analog Scale (Pain) at 3 months Visual Analog Scale (Pain) after 12 months Subjective improvement (SI) in daily nausea (After 12 months) SI in pain SI in vomiting | Amitriptyline: 5 patients had improvement in VAS at 3 months: 22/27(81%) 24 patients on amitriptyline for at least 3 months: 93% had a “favorable response with decreased frequency and severity of their symptoms.” 15/27 took at least 12 months of amitriptyline: VAS of 6.1 points (significant mean improvement in symptoms) Showers: 72% reported hot showers; heating pads and lying down in a quiet setting could ameliorate or completely relieve their symptoms. Marijuana (MJ) use: 13/31 daily use (7/13 thought MJ therapeutic, 2/13 said CVS resolved after stopping MJ, 4/13 said no relationship between MJ and symptoms). | Amitriptyline: 1/27 discontinued: hypersomnia and palpitations. |
| 17 | Clouse et al. (2007) | Reduction of vomiting episodes Frequency of vomiting Episodes Likert scale: (Score >/=2 for favorable clinical response) 0: no significant improvement or worse 1: slight improvement, requiring treatment changes 2: moderate improvement, regimen stable but symptoms not completely resolved 3: clinical remission and complete patient satisfaction Interview data obtained at “point of last clinical contact” | Chart review: Favorable outcome—15/20 (75%) Zonisamide: 12/16 and Levetiracetam: 3/4 (no statistical difference) Interviewing: Improved overall (18/20) (2/16 no change) Less severe vomiting (12/16) (4/16 no change) Shorter episodes (7/16) (9/16 no change) Frequency of vomiting episodes decreased significantly after initiation of antiepileptic drug therapy (1.3 to 0.5 episodes/month) | Medication side effects classified as: Severe: fatigue, confusion, headache, and dizziness Moderate: depression, muscle weakness, dizziness, difficulty sleeping, poor concentration/memory, confusion, or tiredness/fatigue Mild: agitation/irritability, poor appetite, runny nose/cough, difficulty sleeping, poor concentration/memory, headache, confusion, and tiredness/fatigue Severe side effects: reported in 4 patients, which were eliminated in 75% (3/4) of subjects once switched to the other antiepileptic (including 1 patient with angioedema on levetiracetam). 1/20 reported antiepileptic drugs intolerable despite switching drugs or dosage. ` Moderate side effects: 5/20 Mild side effects: 4/20 |
| 18 | Boles et al. (2006) | “Treatment benefit” (specifics not defined) | Treatment (includes CVS+ and CVS ‐ groups) Prophylactic (reported benefit)* Amitriptyline 16/31 (52%) Cyproheptadine 11/18 (61%) Propranolol: 5/15 (33%) Abortive (reported benefit)* IV Dextrose: 21/24 (88%) Ondansetron 42/52 (81%) Lorazepam: 23/30 (77%) Promethazine: 13/23 (57%) Sumatriptan: 8/8 (100%) | Prophylactic Amitriptyline: 5/31 with lethargy (2 subjects), mood swings, abdominal cramping, and syncope Cyproheptadine: 3/18 with weight gain (2), behavioral change, and hallucinations Propranolol: 0/15 Abortive IV Dextrose: 0/21 Ondansetron: 2/42 with palpitations and allergy Lorazepam: 2/30 with “untoward” reaction* Promethazine: 0/23 Sumatriptan succinate: 1/8 with hallucinations |
| 19 | Aanpreung et al. (2002) | Good response: absence of vomiting or few episodes of vomiting Fair response: persistence of vomiting but improvement with less frequency and less intense episodes of vomiting Poor response: no response. Severity of disease Severe (>20 emeses/day) Moderate (10‐19 emeses/day) Mild (<10 emeses/day) Medication “effective” if good/fair response. Medication “non‐effective” if poor response. | Pizotifen: 8/25 received pizotifen Good response: 3 (2 with mild disease, 1 with moderate) Fair response: 1 (1 with severe disease) Poor response: 4 (3 had severe disease) In 3 patients, pizotifen changed to amitriptyline Amitriptyline (18/25 patents received) Good response: 11 (4 with mild disease, 5 with moderate, 2 with severe) Fair response: 4 (2 with mild disease, 2 with moderate disease) No response: 3 (3 with severe disease) Propranolol (2/25 received) Good response: 1 (1 with moderate disease) No response: 1 (1 with severe disease) No statistical difference in response between pizotifen and amitriptyline | No adverse events observed |
| 20 | Prakash et al. (1999) | Complete remission of symptoms (rating of 3) Partial response (rating of 2) Likert‐type response scale: 0: no significant improvement or worse; 1: slight improvement 2: moderate improvement 3: clinical remission | CVS group: Complete remission: 3/17 (17.6%) Partial response: 10/17 (58.8%) No response: 4/17 (23.5%) Response was manifest as decreased cycle frequency (3, 17.6%), decreased intensity of symptoms (6, 35.3%), and shortening of the vomiting cycles (1, 5.9%). | 4/17(23.5%) had side effects that included sedation, emotional lability, and sleep disturbances. No discontinuation due to side effects. |
| 21 | Li et al. (1999) | Demographic characteristics Vomiting pattern Associated symptoms Triggering events Medication response (>50% reduction in vomiting or episodes) | No statistically significant difference between migraine‐associated and non–migraine‐associated CVS Medication response (abortive and prophylactic) Propranolol (migraine‐associated CVS (52/73 (71%) vs non‐migraine CVS (8/21 38%) Cyproheptadine (migraine‐associated CVS (15/32 (47%)) vs non‐migraine CVS (0/5 pts) Amitriptyline (migraine‐associated CVS (12/16 (75%) vs non‐migraine CVS (1/1(100%)) Sumatriptan (migraine‐associated CVS (24/35 (69%)) vs non‐migraine CVS (1/3 (33%). No statistically significant difference in sumatriptan usage between groups | None reported |
| 22 | Andersen et al. (1997) | Complete response: no attacks Partial response: ≥ 50% reduction in frequency of attacks No response: < 50% decrease in frequency of attacks | Complete remission Amitriptyline 16/22 (73%) Cyproheptadine: 4/6 (66%) Partial remission Amitriptyline: 4/22 (18%) Cyproheptadine: 1/6(17%) No response Amitriptyline: 2/22 (10%) Cyproheptadine: 1/6 (16.7%) | “Side effects for both medications included sedation and weight gain due to increased appetite.” Specific numbers not provided. |
2.5. Statistical analysis
Given the size and heterogeneity of included studies, the majority of results were suitable to narrative summary. Quantitative outcomes were calculated using Open Meta (http://www.cebm.brown.edu/openmeta/).
2.6. Quality or certainty of evidence
The GRADE approach was used to rate the certainty in the evidence. In this approach, direct evidence from RCTs starts at high quality and can be rated down to levels of moderate, low, and very low quality, based on risk of bias in the body of evidence (or study quality), indirectness (addressing a different but related population, intervention, or outcome, from the one of interest), imprecision (of the summary estimate and boundaries of 95% CI), inconsistency (or heterogeneity in the results of the included studies), and/or publication bias. Due to inherent limitations in observational studies (selection bias, unmeasured confounding, etc.), evidence derived from observational studies starts at low quality and then is potentially downgraded based on the aforementioned factors or upgraded in case of dose‐response relationship and large magnitude of effect. High‐quality evidence suggests that we are confident of the quality of the evidence and/or the direction and magnitude of the effect estimate, and any new data are unlikely to alter this. Moderate certainty suggests that we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty suggests that our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Finally, very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. Judgments about the certainty in the evidence were made via discussion among the panel, and any disagreements were resolved by group consensus.
2.7. Evidence‐to‐decision framework
Information from this review was used in combination with factors such as patients’ values and preferences, cost‐effectiveness data (if available), and resource utilization to inform the development of the clinical guideline.
3. RESULTS
3.1. Overview
Study details are presented in Table 2a,b and summarized for each PICO question in the accompanying evidence profiles. The team acknowledges the limited evidence for CVS with few randomized control trials or high‐quality observational studies leaving us with low‐ or very low‐quality certainty in the evidence across outcomes. Given the paucity of literature on the topic, studies of all populations (adult and pediatric) were included with the assumption that the pathophysiology of CVS was similar in adults and adolescents, and that the effects of the various interventions may be generalizable across some populations. Finally, there was variability in criteria used to diagnose CVS, medication exposures (eg, dosage and length of treatment) that were not consistently reported, and variable definitions for “response to treatment” used by authors across studies.
3.2. Prophylactic therapy
3.2.1. Should tricyclic antidepressants (TCAs) be used as prophylactic therapy in adults with CVS?
Key message
There is very low certainty in the evidence that TCAs should be used as prophylactic therapy in CVS. See Table 3 for full evidence profile.
Table 3.
Should TCAs be used as prophylactic therapy in adults with CVS?
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | TCAs | Placebo | Relative (95% CI) | Absolute (95% CI) | ||
| Complete/Partial response or symptom improvement (variably defined in each study; follow‐up range 5 months to 5 years) | ||||||||||||
| 14 | Observational studies [Link] | Serious [Link] | Serious [Link] | Serious[Link] | Not serious | None | 413/600 (70%; range 61‐77%) of patients had complete or partial response to treatment or symptom improvement across 14 studies | ⨁◯◯◯ VERY LOW | CRITICAL | |||
| Reduction in duration or severity of CVS symptoms; follow‐up 2 years | ||||||||||||
| 1 Hejazi 2010 | Observational study, n = 41 | Not serious | Not serious | Not serious | Serious[Link] | None | Reduction in duration of CVS episodes from baseline 6.7 ± 6.1 (days) to 2.2 ± 2.4 (days). | ⨁◯◯◯ VERY LOW | IMPORTANT | |||
| Reduction in number of episodes; follow‐up 2 years | ||||||||||||
| 1 Hejazi 2010 | Observational study, n = 41 | Not serious | Not serious | Not serious | Serious[Link] | None | Reduction in number of episodes from baseline (mean) 17.8 ± 8.3 to 3.3 ± 2.8. | ⨁◯◯◯ VERY LOW | IMPORTANT | |||
| Reduction in hospitalizations/ED visits | ||||||||||||
| 1 Hejazi 2010 | Observational study, n = 41 | Not serious | Not serious | Not serious | Serious[Link] | None | Reduction in number of hospitalizations reported: baseline 15 ± 13.4 down to 3.3 ± 3.6. | ⨁◯◯◯ VERY LOW | IMPORTANT | |||
| Adverse effects leading to treatment discontinuation[Link] | ||||||||||||
| See narrative. | ⨁◯◯◯ VERY LOW | IMPORTANT | ||||||||||
Overall, 14 studies (including the intervention arm from 2 RCTs) were included in this analysis.
There were issues around selection bias, no intention to treat analysis, confounding, co‐interventions with mitochondrial supplements, and variable follow‐up. The outcomes were variably reported across the different studies: from complete response (no attacks), partial response (50% reduction in frequency and duration) to “good response, fair response, poor response,” to the use of a visual analog scale to “subjective improvement.”
We rated down for inconsistency (high I‐squared).
We rated down for indirectness as 6 studies were conducted in the pediatric population.
There were few events, and the sample size was small.
Variably reported across studies. See narrative.
Potential benefits/harms
Fourteen studies met inclusion criteria and were used to inform this question: These included 2 randomized trials and 12 observational studies.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 Data from the randomized trials were converted to a single‐arm cohort of amitriptyline for inclusion into a summary estimate for amitriptyline's symptomatic effect. A summary estimate from all included data revealed that approximately 70% of patients with CVS exhibited a symptom response (variably defined for variable durations). Six studies were from pediatric populations, four studies from adult populations, and four studies from mixed adult/pediatric populations (see Table 2a,b). Across these studies, 413/600 (70%) of patients reported complete or partial improvement with a decrease in frequency, duration, or severity of CVS symptoms when treated with a TCA, most commonly amitriptyline. Hejazi et al. in an open‐label study of 46 adult patients demonstrated not only a marked reduction in the number of CVS episodes from 17 to 3, and in the duration of a CVS episode from 6 to 2 days, but also a reduction in the number of ED visits/hospitalizations from 15 to 3.3 with AT. Nine studies reported on adverse events, the most common being sedation and weight gain. Boles et al. 2010 had one of the largest patient cohorts and noted that 72/139 pediatric patients and 39/54 (72%) adults experienced TCA‐related side effects and 29/137 pediatric patients and 13/61 (21%) adult patients discontinued amitriptyline because of side effects.7 However, adverse events leading to treatment discontinuation were not systematically reported across the studies.
Certainty of evidence
The overall certainty of the evidence was judged to be very low. Risk of bias was a concern (lack of control group and possible selection bias in the observational studies, and lack of obvious blinding and an intention to treat analysis in the randomized trials). There was also concern regarding inconsistency, indirectness (many of the studies included only pediatric patients), and imprecision (for a few of the outcomes).
3.2.2. Should topiramate be used as prophylactic therapy in adults with CVS?
Key message
There is very low certainty in the evidence that topiramate should be used as prophylactic therapy in CVS. See Table 4 for full evidence profile
Table 4.
Should topiramate be used as prophylactic therapy in adults with CVS?
| Quality assessment | № of patients | Effect | Quality | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Valproic acid | Relative (95% CI) | Absolute (95% CI) | ||
| Complete response (free from attack for at least 1 year) | |||||||||||
| 1 Sezer 2016 | Observational study, N = 16 | Not serious[Link] | Not serious | Serious[Link] | Serious[Link] | None | 81% were free from attacks at 12 months | ⨁◯◯◯ VERY LOW | CRITICAL | ||
| Partial response (50% reduction in both frequency and intensity of CVS symptoms); follow‐up 12 months | |||||||||||
| 1 | Observational study, N = 16 | Not serious[Link] | Not serious | Serious[Link] | Serious[Link] | None | 13% achieved a partial response (50% reduction in symptoms) | ⨁◯◯◯ VERY LOW | CRITICAL | ||
| Reduction in duration or severity of CVS symptoms; follow‐up 12 months | |||||||||||
| 1 | Observational study, N = 16 | Not serious[Link] | Not serious | Serious[Link] | Serious[Link] | None | Reduction in median duration of cycles from baseline 17.0 ± 5.1 to 11.0 ± 2.2 hours. Reduction in episodes of vomiting per cycle from baseline 14.0 ± 2.3 to 12.0 ± 1.4 | ⨁◯◯◯ VERY LOW | IMPORTANT | ||
| Reduction in number of episodes; follow‐up 12 months | |||||||||||
| 1 | Observational study, N = 16 | Not serious[Link] | Not serious | Serious[Link] | Serious[Link] | None | Decrease in number of attacks per year from 5.0 ± 0.1 to 1.0 ± 0.4 | ⨁◯◯◯ VERY LOW | IMPORTANT | ||
| Reduction in hospitalizations/ED visits—NOT REPORTED | |||||||||||
| Adverse effects leading to treatment discontinuation; follow‐up 12 months | |||||||||||
| 1 | Observational study, N = 13 | Not serious[Link] | Not serious | Serious[Link] | Serious[Link] | None | None observed | ⨁◯◯◯ VERY LOW | IMPORTANT | ||
This was a retrospective study based on chart review.
The study (Sezer 2016) included 16 pediatric patients. Overall responders (≥ 50% reduction) = 94% (partial or complete response). In one additional study (Kumar 2012), 17/76 adult patients received topiramate but there was not enough detail provided for the analysis (as patients may also have been treated with amitriptyline and mitochondrial supplements. In this study, overall response was 86% (≥ 50% reduction in frequency of CVS episodes).
There were few events and small numbers of patients.
Potential benefits/harms
One study met inclusion criteria that investigated the role of topiramate in CVS.15 Sezer et al. investigated the use of topiramate (n = 16) and propranolol (n = 22) in 38 pediatric patients with CVS in a retrospective cohort study in Turkey. At baseline, the topiramate group (compared to the propranolol group) had significantly fewer episodes of vomiting/cycle before treatment, fewer attacks/year after treatment, decreased median duration of cycles, and fewer peak number of emeses/hour during an attack. As such, patients in the topiramate group might have been less severe prior to treatment than the propranolol group. Patients were followed for 1 year. At follow‐up, responder rates (patients who had zero attacks in the year following treatment or patients that a ≥ 50% reduction in attacks) were significantly higher in the topiramate group 15/16 (94%) compared to the propranolol group 18/22 (81%). In the topiramate arm, 81% became episode free and 13% showed at least ≥ 50% reduction in number of episodes. Per the study, the four patients who were non‐responsive to propranolol were treated with topiramate, and all of them had a “satisfactory response,” though this was not clearly defined by the authors. The one patient who was non‐responsive to topiramate was also non‐responsive to other medications, including propranolol, amitriptyline, and cyproheptadine. One additional study reported on topiramate use in adults (Kumar et al.); in this study, 18/92 adults were treated with topiramate, but not enough detail was provided to discern the efficacy of topiramate alone, as patients in this cohort also received treatment with amitriptyline and mitochondrial supplements. 12
In the study by Sezer et al., there were no dropouts from adverse events, and no statistically significant difference in adverse events between the propranolol and topiramate groups.15 Two patients experienced drowsiness and dizziness with topiramate, and mean weight loss after the end of 12 months was 1.1 ± 0.5 kg (2.9%).
Certainty in effects
The overall certainty in the effects was very low due to concerns about study quality, imprecision (few events and small sample size), and indirectness (the study population was pediatric patients)
3.2.3. Should aprepitant be used as prophylactic therapy in adults with CVS?
Key message
In patients with CVS, there is very low certainty in the evidence for the use of aprepitant as prophylactic therapy in CVS. See Table 5 for full evidence profile
Table 5.
Should aprepitant be used as prophylactic therapy in adults with CVS?
| Certainty assessment | Impact | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |||
| Complete response (no episodes) (follow‐up: 12 months) | |||||||||
| 1 Cristofori 2014 | Observational study, n = 16 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | 3/16 (19%) of patients had no further episodes at 12 months | ⨁◯◯◯ VERY LOW | CRITICAL |
| Partial response: ≥50% decrease in both frequency (# episodes/year) and intensity (episode duration in days); follow‐up: 12 months | |||||||||
| 1 | Observational study, n = 16 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | 10/16 (62%) had a partial response | ⨁◯◯◯ VERY LOW | IMPORTANT |
| CVS episode duration (follow‐up: 12 months) | |||||||||
| 1 | Observational study, n = 16 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | Reduction in the duration of episodes (days): Baseline 5 (4‐7) to 3 (1‐3). Reduction in number vomits/episode: Baseline 9 (7‐10) to 6 (5‐8). | ⨁◯◯◯ VERY LOW | IMPORTANT |
| Reduction in number of CVS episodes/year (follow‐up: 12 months) | |||||||||
| 1 | Observational study, n = 16 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | CVS episodes/year: Baseline 12 (9‐14) to 3 (2‐6) at 12 months | ⨁◯◯◯ VERY LOW | IMPORTANT |
| Reduction in hospitalizations/year (follow‐up: 12 months) | |||||||||
| 1 | Observational study, n = 16 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | Reduction in number of hospital admissions/year from baseline 8 (6‐12) to 2 (1‐4) at 12 months | ⨁◯◯◯ VERY LOW | IMPORTANT |
| Symptom‐free interval length (days) (follow‐up: 12 months) | |||||||||
| 1 | Observational study, n = 16 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | Duration of interspersed period (days): Baseline 30 (21‐40) to 120 (60‐180) at 12 months | ⨁◯◯◯ VERY LOW | IMPORTANT |
| School attendance (follow‐up: 12 months) | |||||||||
| 1 | Observational study, n = 16 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | Increase in school attendance: 67% (58‐72) to 81% (78‐85) at 12 months | ⨁◯◯◯ VERY LOW | IMPORTANT |
| Adverse effects (follow‐up: 12 months)[Link] | |||||||||
| 1 | Observational study, n = 16 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | Only one child with migraine stopped the medication (1/16) | ⨁◯◯◯ VERY LOW | IMPORTANT |
This was a retrospective cohort study with no control population and concerns about possible selection bias. The study included cohorts who received prophylaxis and abortive treatment. Only the patients who received prophylaxis are presented here.
The patient population included pediatric patients that failed prior CVS treatments and were on several concomitant medications.
Side effects were reported only in the prophylactic group affecting 5/16, 31%: hiccup (3/16, 19%), asthenia/fatigue (2/16, 12.5%), increased appetite (2/16, 12.5%), mild headache (1/16, 6%), and severe migraine (1/16, 6%).
Potential benefits/harms
One observational study investigated the use of aprepitant both as abortive therapy and as prophylactic therapy in CVS.16 This study by Cristofori et al., published in 2014, included pediatric patients and was retrospective in design, collecting data from administrative, pharmacy, and clinical databases as well as telephone interviews with parents of patients. The 41 included patients met NASPGHAN criteria for diagnosis of CVS and had failed or could not tolerate past treatments (Table 2a,b). Forty‐one children and adolescents were included with 25 being administered aprepitant as an abortive medication and 16 as prophylaxis. Some adolescents in this group weighed > 60 kg. There was no control group. Patients were given an “abortive” regimen of aprepitant if they had a prodromal phase that suggested an imminent CVS attack. With respect to co‐interventions, individuals were also being treated with propranolol 9/15 (60%), amitriptyline 7/15 (46%), coenzyme Q10 5/15 (33%), and L‐carnitine 3/15 (20%).16
The outcomes were complete response (no CVS episodes), partial response (≥50% reduction in both frequency and intensity of CVS symptoms), no response (<50% reduction in CVS frequency and intensity), CVS episodes/year, hospital admissions/year, duration of episodes, number of vomits/episode, duration of interspersed period (days), and percentage of school attendance. All outcomes (for abortive and prophylactic groups) were measured at a 12‐month follow‐up time point.
In the prophylactic group, at 12‐month follow‐up, 19% of individuals achieved a complete response (3/16) and 62% (10/16) achieved a partial response. Overall, 82% (13/16) achieved either complete or partial response. Two children failed to respond (2/16, 19%).
With respect to adverse events, in the prophylaxis group, one patient discontinued therapy due to severe migraine (1/16, 6%). Other side effects noted included hiccups (3/16, 19%), asthenia/fatigue (2/16, 12.5%), increased appetite (2/16, 12.5%), and mild headache (1/16, 6%).
Certainty of evidence
The certainty in the evidence was very low due to concern for risk of bias (lack of a control population, possible selection bias and confounding). There was also concern regarding indirectness, given that the study included a population that failed prior CVS treatments, and was on several concomitant medications. Some adolescents were at an adult weight (>60 kg) in the prophylactic group and were dosed accordingly, making this less of a concern.
3.2.4. Should zonisamide or levetiracetam be used as prophylactic therapy in adults with CVS?
Key message
In patients with CVS, there is very low certainty in the evidence for the use of zonisamide or levetiracetam as prophylactic therapy. See Table 6 for full evidence profile
Table 6.
Should (antiepileptics) zonisamide or levetiracetam be used as prophylactic therapy in adults with CVS?
| Certainty assessment | Impact | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |||
| Symptomatic Improvement assessed by Likert scale: 0 (no significant improvement/worse) to 3 (clinical remission and complete satisfaction); follow‐up ~9 months[Link] | |||||||||
| 1 Clouse (2007) | Observational study, n = 20 | Serious [Link] | Not serious | Serious [Link] | Serious [Link] | None | “Favorable outcome” 15/20 (chart review); “Better” 18/20 patients (patient interviews); 12/16 had less severe vomiting (4: no change); 7/16 had shorter episodes (9: no change) | ⨁◯◯◯ VERY LOW | CRITICAL |
| Reduction in number of episodes/episode frequency (per month); median follow‐up ~9 months | |||||||||
| 1 | Observational study, n = 20 | Serious [Link] | Not serious | Serious [Link] | Serious [Link] | None | Reduction in the number of episodes per month: Baseline: 1.3 ± 0.3 to 0.5 ± 0.2 episodes/month | ⨁◯◯◯ VERY LOW | IMPORTANT |
| Reduction in hospitalizations/ED visits—NOT REPORTED | |||||||||
| Adverse effects (AEs); follow‐up ~9 months[Link] | |||||||||
| 1 | Observational study, n = 20 | Serious [Link] | Not serious | Serious [Link] | Serious [Link] | None | Severe AEs: 4/20 (20%). One subject on levetiracetam developed angioedema, which resolved when switched to zonisamide. One subject discontinued therapy in spite of switching drugs and dosages | ⨁◯◯◯ VERY LOW | IMPORTANT |
A score ≥ 2 was required for a “favorable” clinical response. “Better” as a clinical response was not defined. Likert scale: 0 = no significant improvement or worse; 1 = slight improvement, requiring treatment changes; 2 = moderate improvement, regimen stable but symptoms not completely resolved; and 3 = clinical remission and complete patient satisfaction with therapy. Of the 20 patients with a “favorable” clinical response, 12/16 received zonisamide and 3/4 received levetiracetam.
This retrospective study was based on chart review and patient interviews with no control group and concerns for possible selection bias, baseline confounding, and awareness of treatment when measuring outcome (no blinding).
This patient population was adults who were unresponsive to TCAs.
We rated down for imprecision due to the small sample size and few events.
Severe side effects: fatigue, confusion, headache, and dizziness (4/20) which were eliminated in 3 of 4 patients once antiepileptic was switched to the other. Moderate side effects: depression, muscle weakness, dizziness, difficulty sleeping, poor concentration/memory, confusion, or tiredness/fatigue (5/20).
Potential benefits/harms
One retrospective study met inclusion criteria.17 Clouse et al. reviewed outpatient records and conducted interviews of 20 adult patients with CVS who had received prophylactic zonisamide (median dose, 400 mg/day) or levetiracetam (median dose, 1000 mg/day) when tricyclic antidepressants (TCAs) alone had failed, were intolerable, or unsuitable. Sixteen patients were treated with zonisamide and four with levetiracetam for CVS prophylactic therapy. Median follow‐up after initiation of the intervention was 10 months.
Outcomes measured included episode frequency and change in symptoms. A score ≥ 2 was required for a “favorable” clinical response. “Better” as a clinical response was not defined. The study used the following Likert scale: 0 = no significant improvement or worse; 1 = slight improvement, requiring treatment changes; 2 = moderate improvement, regimen stable but symptoms not completely resolved; and 3 = clinical remission and complete patient satisfaction with therapy. Twelve out of 16 patients in the zonisamide group and 3 out of 4 in the levetiracetam group reported a favorable clinical response. Frequency of vomiting episodes decreased significantly after initiation of either zonisamide or levetiracetam from 1.3 to 0.5 episodes/month. In total, 18/20 (90%) stated that they were better on drug therapy (2 unchanged, 0 worse). There were no data on number of hospitalizations or ED visits.
Four subjects out of 20 reported “severe” side effects consisting of fatigue, confusion, headache, and dizziness, which were eliminated in 3/4 of these patients once they switched to the other antiepileptic. Two of these 4 patients were noted to have concomitant use of TCAs, and 1 of the 4 patients was on a high dose of levetiracetam (3000 mg/day). Five subjects out of 20 reported depression, muscle weakness, difficulty sleeping, dizziness, poor concentration/memory, confusion, or tiredness/fatigue. One subject on levetiracetam developed angioedema, which resolved when switched to zonisamide. Only one subject out of 20 reported antiepileptic drugs intolerable in spite of switching drugs and dosages.
Certainty in the evidence
The certainty in the evidence was very low. We rated down for risk of bias and imprecision (small sample size, raising concern about optimal information size).
3.2.5. Should mitochondrial supplements be used as prophylactic therapy in adults with CVS?
Key message
In patients with CVS, there is very low certainty in the evidence for the use of mitochondrial supplements, such as Co‐enzyme Q10, and riboflavin as prophylactic therapy. See Table 7 for full evidence profile.
Table 7.
Should mitochondrial supplements be used as prophylactic therapy in adults with CVS?
| Quality assessment | № of patients | Effect | Quality | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Mitochondrial supplements* | Relative (95% CI) | Absolute (95% CI) | ||
| Complete/Partial response—NOT REPORTED | |||||||||||
| Reduction in duration or severity of CVS symptoms | |||||||||||
| 1 Boles (2010) | Observational study, N = 32 | Not serious[Link] | Not serious | Serious[Link] | Serious[Link] | None | Using varying doses of CoQ10, 68% of subjects had improvement in symptoms. | ⨁◯◯◯ VERY LOW | IMPORTANT | ||
| Reduction in number of episodes—NOT REPORTED | |||||||||||
| IMPORTANT | |||||||||||
| Adverse effects leading to treatment discontinuation | |||||||||||
| 1 Boles (2010) | Observational study, N = 28 | Not serious[Link] | Not serious | Serious[Link] | Serious[Link] | None | Out of 28 participants on CoQ10, 0 side effects were reported. | ⨁◯◯◯ VERY LOW | IMPORTANT | ||
This was a retrospective study based on chart review.
The studies included adult and pediatric patients.
There were few events and small numbers of patients.
Potential benefits/harms
The only comparative study to evaluate the efficacy of Coenzyme Q10 was conducted by Boles et al. (2010).7 In this study, the authors compared the efficacy of Coenzyme Q10 to amitriptyline in patients with CVS via an Internet‐based survey that asked subjects about their response to treatment. Eleven out of 22 subjects, using varying doses of Coenzyme Q10, reported a 50% reduction in episode frequency, 8/22 reported a 50% reduction in episode duration, and 8/20 reported a 50% reduction in nausea severity. Out of 28 participants on Coenzyme Q10, no side effects were reported. The survey did not allow a physician to confirm if the patient truly had CVS and was subject to recall and self‐selection bias. No published studies reported on the efficacy of riboflavin in CVS patients. The Boles 2011 study included riboflavin but did not report on response for these patients.
The majority of studies that reported on the use of mitochondrial supplements was not amenable to providing estimates on the efficacy of mitochondrial supplements because these were used as co‐therapy in conjunction with other agents or because lack of reporting of outcomes specific to mitochondrial therapy.2, 7, 8, 10, 16, 18
Data on the reported prevalence of mitochondrial supplement therapy as co‐interventions are reviewed below. The Lee et al. (2012) systematic review was not used to inform this outcome because it either included studies that did not meet our inclusion criteria or included studies that as discussed below, used supplements as co‐therapy.19 Kumar 2012 conducted a retrospective analysis of 101 patients who met Rome III criteria for CVS. Of the 44/76 patients who achieved a “complete response” with medical therapy, approximately ~30% were taking Co‐enzyme Q10. Of those with a “partial response” (21/76) to medical therapy, 35% were taking Coenzyme Q10. Of the 11/76 patients with “no response” to medical therapy, 10% were taking Coenzyme Q10.
Boles 2011 conducted a retrospective study in adult and pediatric populations with CVS and reported on outcomes of a 2‐year case series in which 30 patients were treated with multiple agents, which often included mitochondrial supplements. Individual effect from the mitochondrial supplements could not be determined from the result, though the combination of amitriptyline, Coenzyme Q10, and L‐carnitine was used most frequently. Two articles by Hejazi et al. described outcomes of an open‐labeled study for adults with CVS treated with TCA.10, 20 Seventeen percent of the 46 patients took L‐carnitine and/or Coenzyme Q10. The second study by Hejazi reported outcomes on 132 patients and focused on comparing non‐responders and responders to TCA therapy. This study also had 17% of patients on L‐carnitine/Co‐enzyme Q10. There seemed to be an overlap in the patient population between both of these studies. With respect to adverse effects, in the Boles 2010 study, there were no reported side effects (0/20).
Certainty of evidence
The certainty in the evidence was deemed to be very low due to concerns about study quality, indirectness, and imprecision (retrospective design, lack of a control population, probable selection bias, pediatric population, small sample size, and confounding). No pooled effect estimate or range of effects could be calculated.
3.3. Abortive medications
3.3.1. Should triptans be used as abortive therapy in adults with CVS?
Key message
There is moderate certainty in the evidence for the use of triptans as abortive therapy in CVS, primarily based on indirect data. See Table 8 for full evidence profile.
Table 8.
Should triptans be used as abortive therapy in adults with CVS?
| Quality assessment | № of patients | Effect | Quality | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | triptans | Relative (95% CI) | Absolute (95% CI) | ||
| Treatment response (variably defined in each study) | |||||||||||
| 3 Kumar (2012) Hikita (2011) Li (1999_ | Observational studies | Serious [Link] , [Link] | Not serious | Serious [Link] | Serious [Link] | None | The range of effects was 36‐82% response (across the 3 studies) in aborting an episode or preventing an attack | ⨁◯◯◯ VERY LOW | CRITICAL | ||
| INDIRECT EVIDENCE‐Relief of (or improvement in) nausea within 2 hours in migraine headache patients[Link] | |||||||||||
| 8 | Randomized trials (overview of srs) | Not serious | Not serious | Serious [Link] | Not serious | None | The range of effects for reduction in nausea symptoms within 2 hours was 45% to 76% (across 8 RCTs); higher rates of symptom improvement were seen with intranasal (50‐60% range) and subcutaneous medication (76%). | ⨁⨁⨁◯ MODERATE | CRITICAL | ||
| Reduction in number of CVS episodes | |||||||||||
| 1 Hikita (2011) | Observational study, n = 12 | Serious [Link] | Not serious | Serious [Link] | Serious [Link] | None | In 11 patients with 35 attacks, response was seen in 19 attacks (subcutaneous). In 5 patients with 6 attacks, response was seen in 2 attacks (nasal spray). | ⨁◯◯◯ VERY LOW | IMPORTANT | ||
| Reduction in hospitalizations/ED visits—NOT REPORTED | |||||||||||
| Adverse effects leading to treatment discontinuation[Link] | |||||||||||
| 3 | Observational studies | Serious[Link] | Not serious | Serious [Link] | Serious[Link] | None | No adverse events observed across the three studies in CVS patients. | ⨁◯◯◯ VERY LOW | IMPORTANT | ||
The observational studies were at risk for selection bias.
The outcome was variably defined across studies: “medication response” or “benefit” which may represent complete/partial response or symptom improvement. Li et al. found that 69% of kids (24/35) had improvement in nausea symptoms (defined as a > 50% reduction in vomiting episodes with subcutaneous sumatriptan). Hikita et al. found 54% of attacks in 11 kids/1 adult were responsive to sumatriptan therapy (defined as complete improvement or at least a 50% reduction in vomiting frequency).
Some studies were conducted in pediatric populations, and some data come from a CVS–migraine‐associated phenotype.
We rated down for imprecision due to the small sample size and few events.
An overview of SRs was used to provide indirect evidence to support the use of triptans for nausea and vomiting. These 8 studies were conducted in individuals with migraine headaches, and nausea relief was a secondary outcome. This estimate was derived from the Cochrane overview of SRs by Derry et al. Sumatriptan (all routes of administration) for acute migraine attacks in adults‐overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2014, Issue5, Art. No. CD009108.
The Hikita 2011 study included 1 adult and 11 pediatric patients.
No data on adverse events leading to treatment discontinuation were provided in the Derry et al. SR. AEs were generally described as mild or moderate and self‐limited. No cardiovascular problems were noted.
Potential benefits/harms
We identified four studies that met inclusion criteria and that reported on the use of triptans as abortive therapy in CVS. One systematic review of treatments for CVS was not included below because it only reviewed the Hikita 2011 study.21 We additionally looked for indirect evidence in the migraine literature to help inform outcomes, such as nausea and vomiting.22
Kumar 2012 conducted a retrospective review of adult and pediatric patients seen at the Medical College of Wisconsin who met Rome III criteria for CVS.12 Data were collected on 101 patients through chart review and patient questionnaires. Response data were not available on all patients, though it was noted that triptan medications “aborted” CVS episodes in 64/77 (83%) of patients.
Hikita 2011 studied one adult and eleven pediatric patients in a prospective cohort study that took place at Teikyo University Hospital in Japan.21 Patients had been diagnosed with severe CVS by a pediatric neurologist per the International Classification of Headache Disorders. Patients were given sumatriptan, as either a subcutaneous injection or a nasal spray; the average dose administered was not specified. Measured outcomes included “complete response” (no vomiting after treatment), “effective response” (vomiting frequency reduced by ≥ 50%), or “non‐effective response” (the treatment was not effective in preventing vomiting). For the 11 patients receiving subcutaneous sumatriptan injection, 4/11 had complete resolution, 5/11 had effective response, and 2/11 had a non‐effective response. Patients with a family history migraine were more likely to respond (“complete” and “effective”). Among the five patients who received nasal spray, 1/5 had complete resolution, 1/5 had effective response, and 3/5 had non‐effective response.
Li 1999 published a retrospective cohort study in of 214 children from Columbus Children's Hospital with a clinical diagnosis of CVS. 23The purpose of the study was to descriptively compare the characteristics of those with migraine‐associated CVS versus those with non–migraine‐associated CVS. The diagnosis of CVS was made as a clinical diagnosis by treating clinicians. Median follow‐up was 17.5 months. Measured outcomes included demographic characteristics, vomiting pattern, associated symptoms, triggering events, and medication response. The migraine‐associated CVS group (with either self or family history of migraines) compared to non–migraine‐associated CVS had fewer emeses/episode, more abdominal pain, and more triggering events for their CVS episodes. Li et al. found that 24/35 (69%) of children had improvement in symptoms (defined as a ≥ 50% reduction in vomiting episodes) with subcutaneous sumatriptan.
Indirect estimates for the effect of sumatriptan on symptom reduction (nausea and vomiting) were derived from the migraine headache literature. 22 In a systematic review of patients with migraine headaches, but not necessarily CVS, of 8 randomized control trials, 45% to 76% of individuals with migraine headaches experienced a reduction in nausea symptoms within 2 hours with triptans and higher rates of symptom improvement were seen in individuals receiving sumatriptan by either intranasal (50%‐60% range) and subcutaneous routes (76%).22
Among the 3 studies that included data on the use of triptans as abortive therapy in CVS, no adverse events were reported. 12, 23 Furthermore, no data on adverse events leading to treatment discontinuation were provided in the Derry et al. Cochrane systematic review. Adverse effects were generally described as mild or moderate and self‐limited. No cardiovascular problems were noted.
Certainty in the evidence
Indirect estimates influenced the certainty of the evidence supporting the utility of triptans as abortive therapy in CVS. With regard to the outcome of relief of nausea at 2 hours, we had moderate certainty in the beneficial effect of triptans, as presented by the summary estimate yielded from a meta‐analysis of eight RCTs. We downgraded for indirectness as the population studied was patients with migraine headaches (CVS is in the subgroup of periodic syndromes that include migraine and its equivalents).
With regard to the outcome of treatment response and adverse events (across the three studies in CVS patients), the certainty in the evidence was deemed to be very low. We downgraded due to risk of selection bias, imprecision (concern for fragility in the estimate due to suboptimal information size), and indirectness, because some studies were conducted in pediatric populations and some data come from a CVS–migraine‐associated phenotype.
3.3.2. Should 5‐HT3 antagonists be used as abortive therapy in adults with CVS?
No published studies examining the use of ondansetron as abortive therapy for CVS were identified despite its widespread use in CVS. No GRADE evidence profile was created.
3.3.3. Should aprepitant be used as abortive therapy in adults with CVS?
Key message
In patients with CVS, there is very low certainty in the evidence for the use of aprepitant as abortive therapy. See Table 9 for full evidence profile
Table 9.
Should aprepitant be used as abortive therapy in adults with CVS?
| Certainty assessment | Impact | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |||
| Complete response (no episodes) (follow‐up: 12 months) | |||||||||
| 1 Cristofori (2014) | Observational studies, n = 25 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | 3/25 (12%) of patients had no further episodes | ⨁◯◯◯ VERY LOW | CRITICAL |
| Partial response: (≥50% decrease in both frequency (# episodes/year) and intensity (episode duration in days)) (follow‐up: 12 months) | |||||||||
| 1 | Observational studies, n = 25 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | 16/25 (64%) of patients had partial response | ⨁◯◯◯ VERY LOW | CRITICAL |
| CVS episode duration (follow‐up: 12 months) | |||||||||
| 1 | Observational studies, n = 25 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | Reduction in duration of episodes: Baseline 5 (3.5‐7) to 1 (0.75‐2). Reduction in number vomits/episode: Baseline 9 (7‐10) to 4 (2‐4.5). | ⨁◯◯◯ VERY LOW | IMPORTANT |
| Reduction in number of CVS episodes/year (follow‐up: 12 months) | |||||||||
| 1 | Observational studies, n = 25 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | CVS episodes/year: Baseline 12 (9.5‐16.5) to 6 (2‐8.5) at 12 months | ⨁◯◯◯ VERY LOW | IMPORTANT |
| Reduction in hospitalizations/year (follow‐up: 12 months) | |||||||||
| 1 | Observational studies, n = 25 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | Reduction in number of hospital admissions/year: Baseline 9 (6‐12) to 2.5 (1‐5.5) | ⨁◯◯◯ VERY LOW | IMPORTANT |
| Symptom‐free interval length (days) (follow‐up: 12 months) | |||||||||
| 1 | Observational studies, n = 25 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | Duration of interspersed period (days): Baseline 30 (21‐35) to 60 (40‐180) at 12 months | ⨁◯◯◯ VERY LOW | IMPORTANT |
| School attendance (follow‐up: 12 months) | |||||||||
| 1 | Observational studies, n = 25 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | Increase in school attendance: 65% (57.5‐74) to 80% (72‐87.5) at 12 months | ⨁◯◯◯ VERY LOW | IMPORTANT |
| Adverse Events leading to treatment discontinuation (follow‐up: 12 months) | |||||||||
| 1 | Observational studies, n = 25 | Serious [Link] | Not serious | Serious [Link] | Not serious | None | None reported in abortive group | ⨁◯◯◯ VERY LOW | IMPORTANT |
This was a retrospective cohort study with no control population and concerns about possible selection bias. The study included cohorts who received prophylaxis and abortive treatment. Only the patients who received abortive therapy are presented here.
The patient population included pediatric patients that failed prior CVS treatments and were on several concomitant medications.
Potential benefits/harms
One observational study investigated the use of aprepitant as abortive therapy and as prophylactic therapy in CVS.16 The study included pediatric patients and was retrospective in design, collecting data from administrative, pharmacy, and clinical databases as well as telephone interviews with patients’ parents (see section on aprepitant as prophylactic therapy in CVS for more details). In the abortive group, at a 12‐month follow‐up time point, 12% (3/25) achieved a complete response and 64% (16/25) achieved a partial response. Overall, 76% (19/25) achieved either a complete or partial response. Six children had no response (6/25, 24%). It was difficult to discern how often patients received the medication in the abortive group. There were no noted adverse events from aprepitant administration in the abortive group.
Certainty in the evidence
The certainty in the evidence was deemed to be very low, for the same reasons discussed in the prophylactic group. Certainty was reduced by risk of bias (lack of a control population, possible selection bias, and confounding). There was also concern regarding indirectness, given that the study included a population that failed prior CVS treatments, and was on several concomitant medications.
3.3.4. Should we screen for and treat co‐morbid conditions, such as anxiety, depression, migraine headache, autonomic dysfunction, sleep disorders, and substance use in adults with CVS?
No published studies were found that explicitly addressed this question. No GRADE evidence profile was created.
3.3.5. Should meditation, relaxation, and biofeedback be used as complementary therapy in adults with CVS?
No published studies were found that explicitly addressed this question. No GRADE evidence profile was created.
3.3.6. Areas of limited/insufficient evidence
Three recommendations (recommendations 7, 9, and 10) that are presented in the accompanying manuscript were deemed consensus recommendations and no GRADE evidence profile was created. Recommendation 7 addresses the role of 5‐HT3 antagonists, such as ondansetron, as abortive therapy for CVS. Acknowledging the lack of direct evidence to inform this clinical question, the committee relied on indirect evidence on the efficacy of ondansetron in patients with chemotherapy‐induced nausea and vomiting (CINV) and postoperative nausea and vomiting (PONV) in treating acute, delayed, and anticipatory nausea and vomiting to inform the recommendation. For recommendations 9 and 10, there was insufficient evidence in the published literature examining the role of screening and treatment of co‐morbid conditions on CVS symptoms and the effects of complementary therapies on CVS symptoms. For these two recommendations, the committee made consensus‐based recommendations based on their large collective experience of managing adult and pediatric CVS patients and their observations in clinical practice as well as the recognition that the treatment of CVS, a functional disorder, should be based on a biopsychosocial care model, integrating lifestyle modification, prophylactic, and/or abortive medications, and evidenced‐based psychotherapy to address psychiatric co‐morbidity. Finally, the guideline also includes consensus statements that address the diagnosis and workup of CVS patients as well as a narrative review and sample protocol for treatment of CVS patients in the ED.
4. CONCLUSIONS
This evidence review is based on the GRADE framework and was developed to inform the clinical practice guideline for the management of CVS, which should ultimately improve patient outcomes and reduce morbidity associated with this chronic and often, debilitating illness.
DISCLOSURES
None of the authors had any competing interests. Full disclosure statement included. Dr. Sharaf was supported by Academy Health DSSF, NY State ECRIP Award, NCI K07CA216326 and NCI R01CA211723. Dr. Sharaf is a paid consultant for the non‐profit Institute for Clinical and Economic Review, Boston, Massachusetts. Dr. Sultan has no financial disclosures.
ACKNOWLEDGMENTS
We are grateful to Dr. Benson Massey whose counsel and guidance have been invaluable in completing this work.
Sharaf RN, Venkatesan T, Shah R, et al. Management of cyclic vomiting syndrome in adults: Evidence review. Neurogastroenterol Motil. 2019;31(Suppl. 2):e13605 10.1111/nmo.13605
Funding information
This work was funded by the Cyclic Vomiting Syndrome Association (CVSA), a non‐profit organization for patients with CVS and their caregivers.
REFERENCES
- 1. Abu‐Arafeh I, Russell G. Cyclical vomiting syndrome in children: a population‐based study. J Pediatr Gastroenterol Nutr. 1995;21:454‐458. [DOI] [PubMed] [Google Scholar]
- 2. Sagar RC, Sood R, Gracie DJ, et al. Cyclic vomiting syndrome is a prevalent and under‐recognized condition in the gastroenterology outpatient clinic. Neurogastroenterol Motil. 2018;30:. [DOI] [PubMed] [Google Scholar]
- 3. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aanpreung P, Vajaradul C. Cyclic vomiting syndrome in Thai children. J Med Assoc Thai. 2002;85(Suppl 2):S743‐S748. [PubMed] [Google Scholar]
- 5. Andersen JM, Sugerman KS, Lockhart JR, Weinberg WA. Effective prophylactic therapy for cyclic vomiting syndrome in children using amitriptyline or cyproheptadine. Pediatrics. 1997;100:977‐981. [DOI] [PubMed] [Google Scholar]
- 6. Badihian N, Saneian H, Badihian S, Yaghini O. Prophylactic Therapy of Cyclic Vomiting Syndrome in Children: Comparison of Amitriptyline and Cyproheptadine: A Randomized Clinical Trial. Am J Gastroenterol. 2018;113:135‐140. [DOI] [PubMed] [Google Scholar]
- 7. Boles RG, Lovett‐Barr MR, Preston A, Li BU, Adams K. Treatment of cyclic vomiting syndrome with co‐enzyme Q10 and amitriptyline, a retrospective study. BMC Neurol. 2010;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boles RG. High degree of efficacy in the treatment of cyclic vomiting syndrome with combined co‐enzyme Q10, L‐carnitine and amitriptyline, a case series. BMC Neurol. 2011;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haghighat M, Rafie SM, Dehghani SM, Fallahi GH, Nejabat M. Cyclic vomiting syndrome in children: experience with 181 cases from southern Iran. World J Gastroenterol. 2007;13:1833‐1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hejazi RA, Reddymasu SC, Namin F, Lavenbarg T, Foran P, McCallum RW. Efficacy of tricyclic antidepressant therapy in adults with cyclic vomiting syndrome: a two‐year follow‐up study. J Clin Gastroenterol. 2010;44:18‐21. [DOI] [PubMed] [Google Scholar]
- 11. Hikita T, Kodama H, Ogita K, Kaneko S, Nakamoto N, Mimaki M. Cyclic Vomiting Syndrome in Infants and Children: A Clinical Follow‐Up Study. Pediatr Neurol. 2016;57:29‐33. [DOI] [PubMed] [Google Scholar]
- 12. Kumar N, Bashar Q, Reddy N, et al. Cyclic Vomiting Syndrome (CVS): is there a difference based on onset of symptoms–pediatric versus adult? BMC Gastroenterol. 2012;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moses J, Keilman A, Worley S, Radhakrishnan K, Rothner AD, Parikh S. Approach to the diagnosis and treatment of cyclic vomiting syndrome: a large single‐center experience with 106 patients. Pediatr Neurol. 2014;50:569‐573. [DOI] [PubMed] [Google Scholar]
- 14. Namin F, Patel J, Lin Z, et al. Clinical, psychiatric and manometric profile of cyclic vomiting syndrome in adults and response to tricyclic therapy. Neurogastroenterol Motil. 2007;19:196‐202. [DOI] [PubMed] [Google Scholar]
- 15. Sezer OB, Sezer T. A New Approach to the Prophylaxis of Cyclic Vomiting: Topiramate. J Neurogastroenterol Motil. 2016;22:656‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cristofori F, Thapar N, Saliakellis E, et al. Efficacy of the neurokinin‐1 receptor antagonist aprepitant in children with cyclical vomiting syndrome. Aliment Pharmacol Ther. 2014;40:309‐317. [DOI] [PubMed] [Google Scholar]
- 17. Clouse RE, Sayuk GS, Lustman PJ, Prakash C. Zonisamide or levetiracetam for adults with cyclic vomiting syndrome: a case series. Clin Gastroenterol Hepatol. 2007;5:44‐48. [DOI] [PubMed] [Google Scholar]
- 18. Jensen AD. Challenges With Acute Care and Response to Treatment Among Adult Patients With Cyclic Vomiting Syndrome. Gastroenterol Nurs. 2015;38:469‐476. [DOI] [PubMed] [Google Scholar]
- 19. Lee LY, Abbott L, Mahlangu B, Moodie SJ, Anderson S. The management of cyclic vomiting syndrome: a systematic review. Eur J Gastroenterol Hepatol. 2012;24:1001‐1006. [DOI] [PubMed] [Google Scholar]
- 20. Hejazi RA, Lavenbarg TH, Foran P, McCallum RW. Who are the nonresponders to standard treatment with tricyclic antidepressant agents for cyclic vomiting syndrome in adults? Aliment Pharmacol Ther. 2010;31:295‐301. [DOI] [PubMed] [Google Scholar]
- 21. Hikita T, Kodama H, Kaneko S, et al. Sumatriptan as a treatment for cyclic vomiting syndrome: a clinical trial. Cephalalgia. 2011;31:504‐507. [DOI] [PubMed] [Google Scholar]
- 22. Derry CJ, Derry S, Moore RA. Sumatriptan (all routes of administration) for acute migraine attacks in adults ‐ overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;:CD009108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li BU, Murray RD, Heitlinger LA, Robbins JL, Hayes JR. Is cyclic vomiting syndrome related to migraine? J Pediatr. 1999;134:567‐572. [DOI] [PubMed] [Google Scholar]


