Summary
Antimicrobial peptides secreted by intestinal immune and epithelial cells are important effectors of innate immunity. They play an essential role in the maintenance of intestinal homeostasis by limiting microbial epithelium interactions and preventing unnecessary microbe‐driven inflammation. Pancreatitis‐associated protein (PAP) belongs to Regenerating islet‐derived III proteins family and is a C‐type (Ca+2 dependent) lectin. PAP protein plays a protective effect presenting anti‐inflammatory properties able to reduce the severity of colitis, preserving gut barrier and epithelial inflammation. Here, we sought to determine whether PAP delivered at intestinal lumen by recombinant Lactococcus lactis strain (LL‐PAP) before and after chemically induced colitis is able to reduce the severity in two models of colitis. After construction and characterization of our recombinant strains, we tested their effects in dinitro‐benzenesulfonic‐acid (DNBS) and Dextran sulfate sodium (DSS) colitis model. After the DNBS challenge, mice treated with LL‐PAP presented less severe colitis compared with PBS and LL‐empty‐treated mice groups. After the DSS challenge, no protective effects of LL‐PAP could be detected. We determined that after 5 days administration, LL‐PAP increase butyrate producer's bacteria, especially Eubacterium plexicaudatum. Based on our findings, we hypothesize that a treatment with LL‐PAP shifts the microbiota preventing the severity of colon inflammation in DNBS colitis model. These protective roles of LL‐PAP in DNBS colitis model might be through intestinal microbiota modulation.
Introduction
Inflammatory bowel diseases (IBD) are chronic inflammatory disorders located in the large and/or small intestine, including ulcerative colitis and Crohn's disease. These diseases are multifactorial driven mainly by an inappropriate immune response to gut microbes in a genetically predisposed host (M'Koma, 2013; Nielsen, 2014). This group of diseases has a substantial socio‐economic impact worldwide, being a significant health problem in Western societies, affecting 1 in 1000 individuals, and characterized by chronic, nonspecific inflammation in the large and/or small intestine. Indeed, these patients have relapse and remit into long‐term morbidity. At the present day, there is no permanent drug cure; therefore, their treatment represents a medical challenge (Steidler et al., 2000; M'Koma, 2013; Nielsen, 2014; Eisenstein, 2016). Some of the existing treatments for IBD include anti‐inflammatory and immunosuppressive drugs presenting severe side‐effects. In later years, there has been a landmark of discoveries and advancements for the therapeutic intervention of IBD but new tools are still required (Steidler et al., 2000; Vandenbroucke et al., 2010; Nielsen, 2014; Santos Rocha et al., 2014; Pigneur and Sokol, 2016; de Souza et al., 2017; Hasenoehrl et al., 2017; Weingarden and Vaughn, 2017).
Therapeutic proteins are gaining increased popularity, due to their low toxicity and minimal nonspecific effect, which allow them increased applicability to multiple disease (Choonara et al., 2014; Liu et al., 2018). Antimicrobial peptides (AMPs) secreted by intestinal immune, epithelial cells and lymphocytes belong to the important effectors of innate immunity compartment, serving as a first line of the defence against pathogens. AMPs are key regulators in the host–microbiota relationships by restricting contact between commensal bacteria and epithelial surface (Gironella et al., 2005; Cash et al., 2006; Ismail et al., 2009; Natividad et al., 2013).
Pancreatitis‐associated protein (PAP) belongs to the Reg gene family. PAP was first found in regenerating pancreatitis islets in rat encoding a small group of proteins involved in the control of epithelial cell proliferation and wound healing in various organs, including pancreas and intestine (Keim et al., 1984; Gironella et al., 2005; Granlund et al., 2011; Yang et al., 2011; Marafini et al., 2014). PAP is mainly synthesized by goblet cells and enterocytes in the colon and in the small intestine by Paneth cells located in the crypt (Ogawa et al., 2003; Cash et al., 2006; Natividad et al., 2013; Nunes et al., 2014). Its presence in the intestinal lumen will limit the contact between resident microbes composed largely of commensal microorganisms and mucosal surface (Mukherjee and Hooper, 2015). Several studies demonstrated the expression of RegIIIγ/PAP in the intestine correlated with the richness of microbiota composition. They observed low expression of RegIIIγ in germ‐free mice but markedly increases after bacterial colonization (Cash et al., 2006; Ismail et al., 2009; Natividad et al., 2013; Mukherjee and Hooper, 2015). PAP expressed by intraepithelial lymphocytes (IEL) and epithelial cells (IEC) also requires cytokine signals from innate lymphocyte cells (ILCs) subsets. One of them, the ILC3, produces IL22, which binds to IL22R (receptor) on epithelial cells and modulates epithelial function and AMP production, such as RegIIIγ, warranting the intestinal epithelial homeostasis (Natividad et al., 2013; Lamas et al., 2016). Regarding the intestinal homeostasis and PAP, recent work has shown the transgenic mice expressing PAP in the pancreas were more resistant to colitis development. Those mice presented microbiota diversity able to drive an anti‐inflammatory environment ensuring the epithelial integrity and function (Darnaud et al., 2018).
Several works showed the use of living genetically engineered strains, for example, food‐grade bacterium Lactococcus lactis delivering therapeutic molecules in situ as being promising to treat different human diseases as allergy (Adel‐Patient et al., 2005; Daniel et al., 2006), cancer (Bermudez‐Humaran et al., 2005), obesity (Bermudez‐Humaran et al., 2007) or IBD (Steidler et al., 2000; Foligne et al., 2007; Motta et al., 2012; Hanson et al., 2014). In this study, we investigate mucosal release of PAP using a well‐described system that enables oral delivery of biopharmaceuticals to the gastrointestinal tract by genetically engineered L. lactis. We show exogenous PAP delivered by recombinant L. lactis might shape the intestinal microbiota and thus act against the inflammatory process taking place in IBD. It may be useful as an intervention approach to maintain the intestinal homeostasis or prevent the intestinal dysbiosis caused by genetic predisposition to IBD.
Results
Characterization of human PAP production by L. lactis
PAP cDNA was inserted in pSEC or pCYT vectors, obtaining thus pSEC‐PAP and pCYT‐PAP, in order to produce PAP secreted or cytoplasmic. Then pSEC‐PAP and pCYT‐PAP were introduced in L. lactis strain NZ9000 where PAP expression was induced by nisin. We used then enzyme‐linked immunosorbent assay (ELISA) to test the ability of our recombinant strains to produce and secrete human PAP. Highest PAP production was obtained with strains transformed with pSEC:PAP (Fig. 1). Recombinant strain NZ9000 containing pSEC:PAP (LL‐PAP) was used in further experiments. A band of ~19 kDa in the cytoplasm was detected in nisin‐induced cultures of the LL‐PAP by western‐blot (data not shown).
Figure 1.
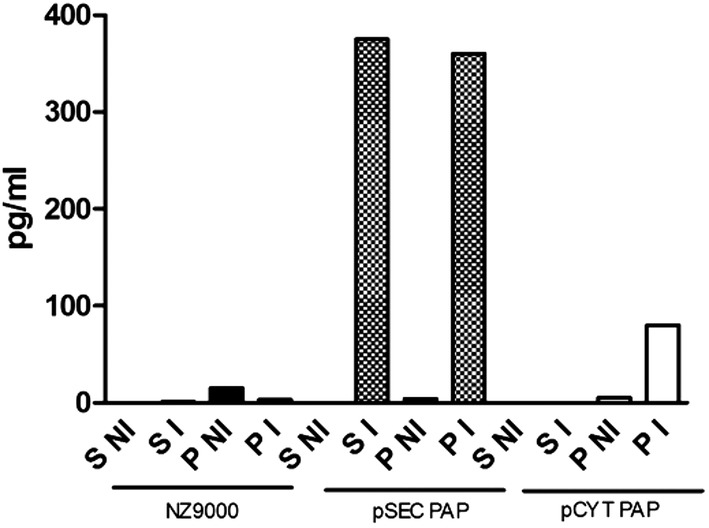
Characterization of human PAP production by Lactococcus lactis. PAP was identified in the pellet and supernatant of nisin‐induced recombinant L. lactis PAP culture by ELISA. S NI = supernatant from non‐induced culture; S I = supernatant from induced culture; P NI = pellet from non‐induced culture; and P I = pellet from induced culture. NZ9000: L. lactis control strain, containing the plasmid pNIS empty; pSECPAP: L. lactis strain secreting PAP; and pCYTPAP: L. lactis strain expressing PAP into the cytoplasm.
LL‐PAP treatment reduces the severity of DNBS‐induced acute colitis but does not prevent damages in DSS‐induced colitis
In order to evaluate the effects of LL‐PAP in vivo, we used two well‐established chemically induced colitis models, DNBS and DSS (Rochat et al., 2007; Chassaing et al., 2014; Martin et al., 2014a; Martin et al., 2014b; Eissa et al., 2017).
Briefly, mice received a pre‐treatment (LL‐empty or LL‐PAP or PBS) for 7 days. The last day, faecal samples were recovered. At day 7, the intra‐rectal injection of DNBS (150 mg kg–1) diluted in 30% ethanol was performed. After the DNBS challenge, mice were continuously treated with the same recombinant bacteria or PBS for 4 days more. Finally, mice were sacrificed on day 10 when MLN and colon were sampled.
Animals treated with LL‐PAP lost less weight than PBS‐ or LL‐empty administered mice (Fig. 2A). LL‐empty‐treated mice did not start to regain weight at D4 after DNBS even if the difference with the PBS group is not statistically significant (Fig. 2A). Other parameters such as macroscopic (Fig. 2B) and microscopic scores (Fig. 2C) were reduced by ~75% and ~50% respectively in LL‐PAP group compared with PBS or LL‐empty group. All parameters analysed here showed LL‐PAP mice developed less severe colitis compared with LL‐empty and PBS‐treated mice.
Figure 2.
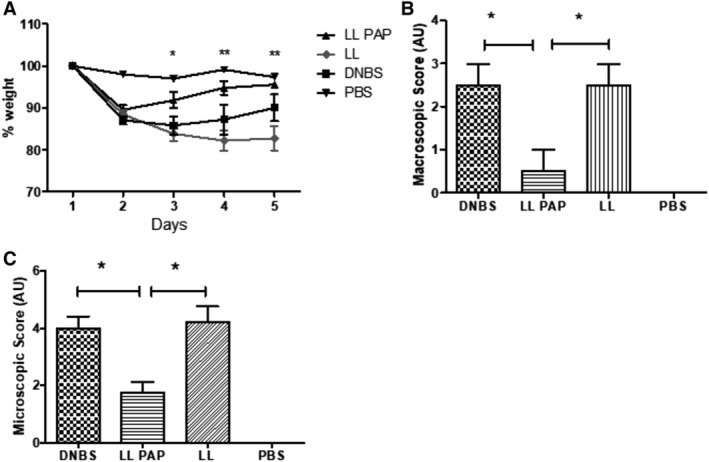
Effect of LL‐PAP on DNBS‐induced colitis. Mice were orally administered with LL‐empty or LL‐PAP during 5 days before and 4 days after intra‐rectal injection of DNBS. Mice were sacrificed 4 days after DNBS injection.A. Percentage of weight loss among the groups. B. Macroscopic score. C. Microscopic score.
DSS experiment were performed as follow, mice were orally administered with LL‐empty or LL‐PAP during all experiment long. After 7 days, they received 2.5% DSS solution diluted in drink water ad libitum. The solution was changed every 3 days. After 7 days of DSS, mice returned to normal drinking water to allow recovery for 5 days and then were sacrificed. There is no difference in the weight loss (Fig. 3A) neither in the other parameters measured, such as consistency and presence of blood in the faeces (Disease Activity Index) (Fig. 3B). All parameters analysed here showed LL‐PAP did not affect the severity of DSS colitis. Regarding the absence of protective effect on these two major readouts, no further analysis was performed on DSS experiment.
Figure 3.
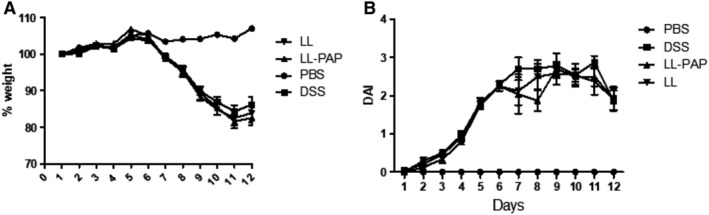
Effect of LL‐PAP on DSS‐induced colitis. Mice were orally administered with LL‐empty or LL‐PAP during 5 days before and 12 days after DSS administration. Mice were sacrificed 12 days after DSS administration.A. Percentage of weight loss among the groups. B. Disease Activity Index.
LL‐PAP treatment is able to modulate the immune response in DNBS‐induced colitis model
In order to know the effects of PAP delivered by L. lactis on the immune response in DNBS‐induced colitis in mice, amount of cytokines IFNγ, IL12p70, IL4, TSLP, IL‐17 and TGF‐β were assayed in the supernatant of activated lymphocytes isolated from MLN and protein extracts from colon tissue of those mice.
In MLN supernatant, secretion of pro inflammatory cytokines IL‐12, IFN‐γ, and IL17 was decreased in LL‐PAP‐treated mice compared with LL‐empty‐treated mice (Fig. 4). While the anti‐inflammatory cytokines, such as TGF‐β and TSLP were expressed at higher level in LL‐PAP treated mice. IL‐4 concentration was not different among the groups (Fig. 4).
Figure 4.
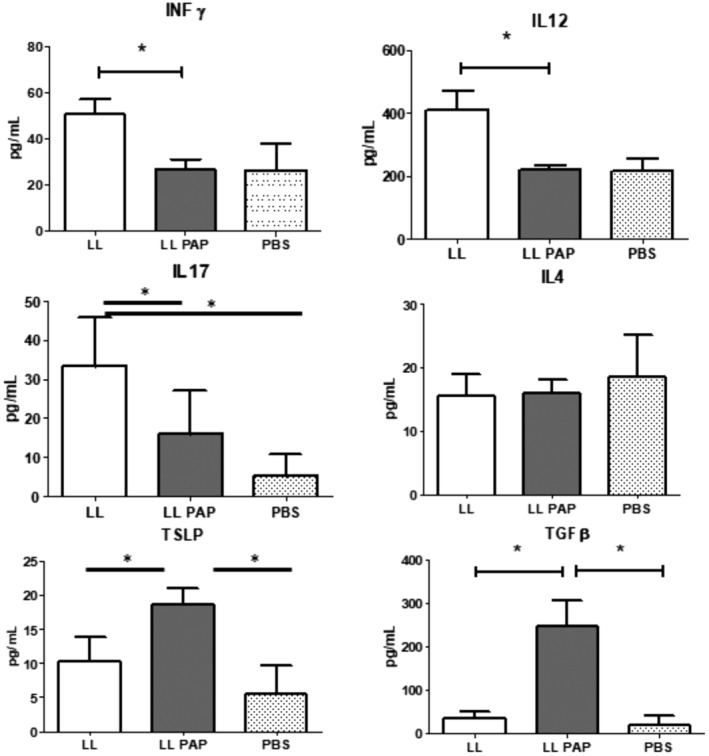
Cytokine production in mesenteric lymph nodes. Mice were orally administered with LL or LL‐PAP during 5 days before and 4 days after intra‐rectal injection of DNBS. Mice were sacrificed 4 days after DNBS injection. Cells were isolated from MLN and re‐stimulated in vitro by anti‐CD3 and anti‐CD28 during 48 h. Supernatants were recovered and cytokine measured using ELISA kits.
In colon extracts, we observed a decrease of IL‐17 production between LL‐empty and LL‐PAP‐treated mice, while TSLP level was increased (Fig. 5). No other differences could be detected.
Figure 5.
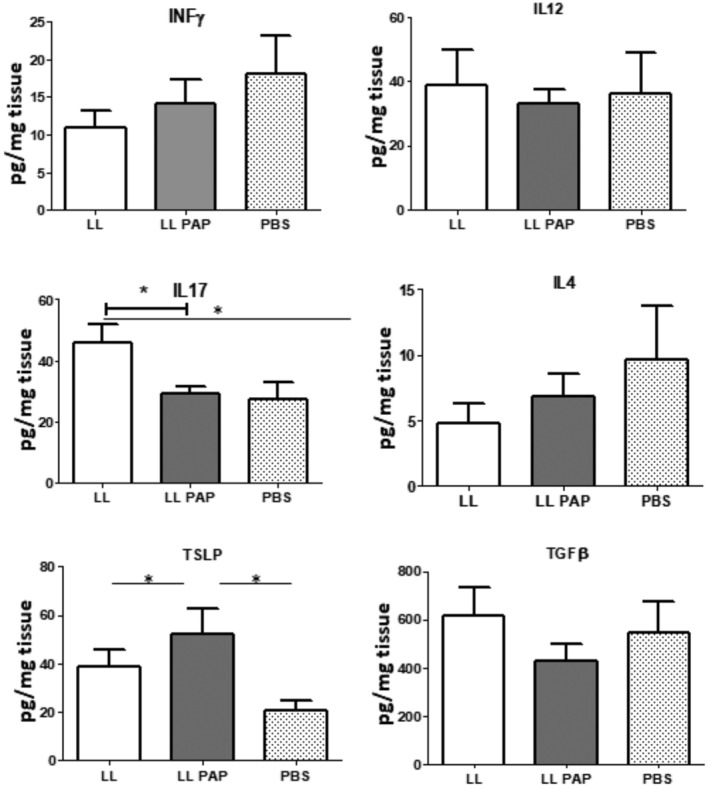
Cytokine production in colon.Mice were orally administered with LL‐empty or LL‐PAP during 5 days before and 4 days after intra‐rectal injection of DNBS. Mice were sacrificed 4 days after DNBS injection. Colon from each mouse was mashed in 1 ml of PBS using Gentle Max and cytokines were measured using ELISA kits.
Treg population in the intestinal lamina propria is restored
Considering the increase of TGF‐β in MLN induced by LL‐PAP, we hypothesized that the protective effect of PAP could be mediated by Treg cells. Thus, we isolated cells from intestinal lamina propria from LL‐empty, LL‐PAP, non‐inflamed and inflamed controls mice to measure the Treg cells population using flow cytometry. LL‐PAP, LL‐empty and non‐inflamed mice (PBS) presented the same percentage of CD4+FoxP3+ cell (Fig. 6).
Figure 6.
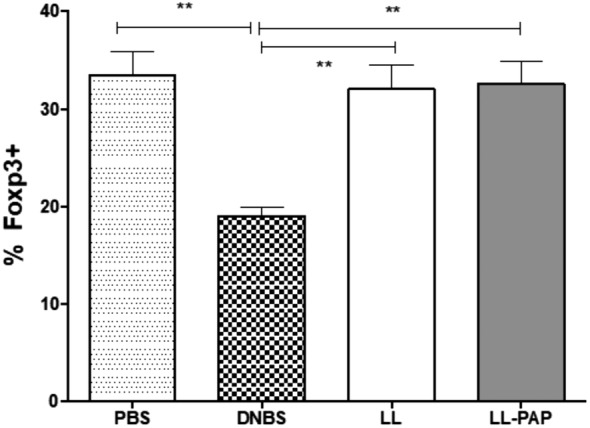
Percentage of FoxP3+ cells population from intestinal lamina propria. Mice were orally administered with LL‐empty or LL‐PAP during 5 days before and 4 days after intra‐rectal injection of DNBS. Mice were sacrificed 4 days after DNBS injection. Cells isolated from intestinal lamina propria from PBS, DNBS, LL‐empty and LL‐PAP treated mice were stained with anti‐CD4+ and anti‐FoxP3+ and analysed by Flow cytometer. The percentage of cells obtained is represented in the graph.
PAP shaped the intestinal microbiota after oral gavage
To assess the impact of LL‐PAP on gut microbiota, we repeated the protocol previously described in Fig. 2. C57BL/6 mice were treated with LL‐PAP or LL‐empty during 5 days by oral gavage then DNBS was administered intra‐rectally and mice were sacrificed at D9. Fresh faecal samples were collected from each mouse at two different time point: D5 (before DNBS administration) and D9 (before the sacrifice). These samples were sent for 16S rRNA sequencing. The bar‐coded sequencing provided 237,945 usable reads (6012 operational taxonomic units [OTUs] with 99% identity threshold) from 10 faecal samples. Oral administration of LL‐PAP for 5 days remarkably shifted the overall structure of gut microbiota in vivo. Principal coordinates analysis (PCoA) separate clearly the two microbiota (Fig. 7A). The boxplots showed an increasing of alpha‐diversity into the LL‐PAP‐treated mice microbiota population compared with LL‐treated mice (Fig. 7B). The differential abundance test shows that eight OTUs are different between LL and LL‐PAP‐treated (Fig. 7C). The relative abundance of OTUs belonging to the families Lachnospiraceae and Ruminococcaceae, to the Ruminoclostridium genus, and the species Eubacterium plexicaudatum is highly increased in mice LL‐PAP‐treated compared with LL‐empty. In the other hand, bacteria from the genus Clostridium strict sense 1 and one OTU from the Lachnospiraceae NK4A136 group are moderately decreased in those mice (Fig. 7C).
Figure 7.
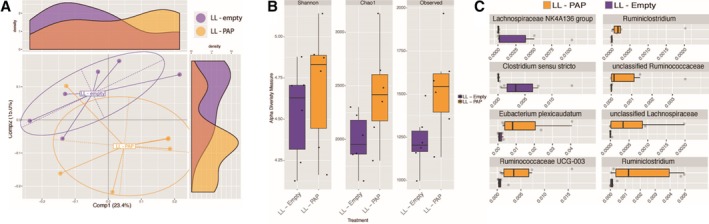
Intestinal microbiota analysis after oral gavage with LL‐PAP. A. Principal coordinate analysis. B. The α‐diversity into the LL‐PAP treated mice microbiota population compared with LL‐empty‐treated mice. C. Comparison of the relative abundance of OTUs in LL‐empty and LL‐PAP groups. [Color figure can be viewed at http://wileyonlinelibrary.com]
At D9, after DNBS challenge, PCoA showed us that microbiota from mice administered with DNBS, treated are not with our recLAB, are clustered together and thus are not different (Fig. 8). DNBS treatment, therefore, seems to be the main changing factor.
Figure 8.
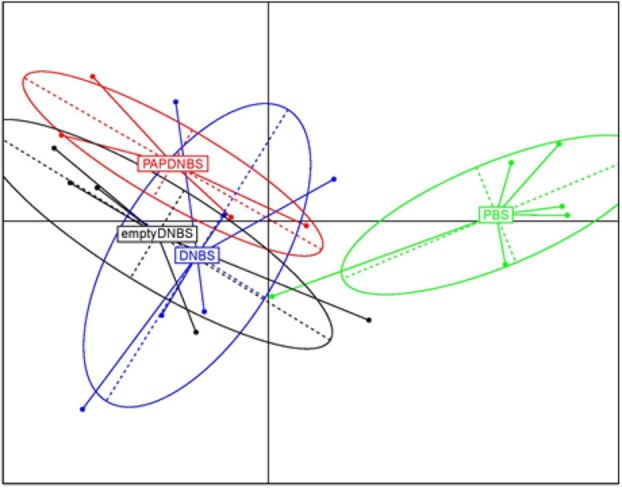
Intestinal microbiota analysis after DNBS administration.PCoA of microbiota from mice orally administered with LL‐empty or LL‐PAP during 5 days before and 4 days after intra‐rectal injection of DNBS. [Color figure can be viewed at http://wileyonlinelibrary.com]
Discussion
Our goal was to describe the anti‐inflammatory properties of PAP and its impact in the intestinal homeostasis using recombinant lactococci. Previous work had shown that an overexpression, or oral and rectal administration, of antimicrobials led to significant changes in gut microbiota composition; however, the underlying mechanisms and health benefits provided by these changes remain to be demonstrated (Darnaud et al., 2018).
In order to understand how PAP improves the health status after DNBS challenge, we figured out the severity of colitis through cytokine profile, macroscopic and microscopic parameters from these mice. In our study, we treated mice with LL‐empty and LL‐PAP before and after DNBS challenge. Therefore, we hypothesized that once delivered into the intestinal lumen by L. lactis, PAP will play an important anti‐inflammatory role. Our results confirm our hypothesis as LL‐PAP mice recovered weight faster than the other groups. Moreover, microscopic score presented low severity lesion markers, showing a preserved intestinal architecture (low mucosal and/or submucosal inflammatory infiltrate, no edema and neither punctate erosion, and the muscularis mucosae intact). Furthermore, the macroscopic score shown a lower mucus level production and no adhesion, as well as no haemorrhage neither ulceration compared with the other groups (Wallace et al., 1995;Dothel et al., 2013 ; Aubry et al., 2015 ; Eissa et al., 2017). These lower pathological scores in LL‐PAP treated mice were correlated with a decrease of IFNγ and IL12 (Th1) as well IL17 (Th17) cells producing in MLNs. However, no difference was found in IFNγ and IL12 (Th1) production in colonic tissue, but IL17 was less produced in LL‐PAP mice compared with LL mice. Taken altogether, as those cytokines are known as pro‐inflammatory and they are involved in the progression of IBD, these results suggest lower local inflammation able to reduce the severity of the disease as well as the histological scores (Dothel et al., 2013; Aubry et al., 2015; Eissa et al., 2017). In the same time, those mice showed an increase of TGF‐β and TSLP in MLN or in colonic tissue. TSLP was first discovered in a thymic stromal cell line and is mainly produced by non‐haematopoietic cells, such as epithelial cells in response to stress stimuli (Aubry et al., 2016). Previous study showed recombinant L. lactis secreting TSLP was able to reduce the severity as well as delay in the inflammation caused by DSS in mice (Aubry et al., 2016). TGF‐β is involved in the Treg cell differentiation and anti‐inflammatory status (Nakamura et al., 2001; Wan and Flavell, 2007). The relation between IL‐17 and TGF‐β is very important. TGF‐β is a pleiotropic cytokine required for the differentiation of Treg and Th17 cells. However, TGF‐β is non‐redundantly required to the development of Treg cells, but dispensable for the differentiation of Th17. On the other hand, high concentration of TGF‐β favours Foxp3+Treg cells (Chen et al., 2003; Zhou et al., 2007; Omenetti and Pizarro, 2015). Treg cells could be the key element in the maintenance of the intestinal integrity, preventing all inflammation markers, such as macroscopic, microscopic scores, and cytokine profile. In order to know whether LL‐PAP increase the Treg cells population, improving the health status of those mice, we isolated T cells from lamina propria from all groups (PBS, DNBS, LL‐empty and LL‐PAP) to access the Treg cells population. The percentage of Treg cells present in lamina propria of LL‐empty and LL‐PAP treated mice were the same, moreover both restore the Treg cells population after DNBS challenge at the same level to the non‐inflamed group (PBS). So, the protective effect of LL‐PAP administration versus LL‐empty cannot be explained by a difference in Treg population in the lamina propria at D9. However, we cannot exclude that a difference in Treg population could have been observed earlier.
In order to explain the protective effect of PAP, we hypothesized the microbiota shaping as a potential mechanism by which PAP prevents the mucosal barrier damage. The composition of a host's intestinal microbiota drives the type of mucosal and systemic immune response by affecting the proportion and number of functionally distinct T cells subsets. In particular, the microbiota affects the differentiation of intestinal T cells, which play crucial role in maintaining mucosal barrier of functions, besides controlling immunological homeostasis (Asano et al., 2013; Atarashi et al., 2013). Our results showed that the microbiota composition was different in both groups (LL‐empty and LL‐PAP treated mice) before DNBS challenge. Treatment by LL‐PAP increased the α‐diversity. Diversity is known now to be very important to resist against various pathologies. Moreover, β‐diversity analysis through PCoA confirmed that the two groups (LL‐empty and LL‐PAP) have different microbiota. At the genus level, we could see that LL‐PAP treatment increased Ruminoclostridium, Ruminococcaceae and Lachnospiraceae. These genuses belong to Firmicutes phylum, which are mainly butyrate producers. Butyrate has a protective role against colitis by improving gut barrier function, increasing AMPs production, interacting with the immune system to drive to an anti‐inflammatory profile, and reducing oxidative stress (Riviere et al., 2016). Remarkably, we noticed an increase of the specie E. plexicaudatum. Compared with the other bacteria modified by LL‐PAP treatment E. plexicaudatum is highly abundant, 1%–5% of total. E. plexicaudatum is a member of the altered Schaedler flora (Dewhirst et al., 1999) and described as a butyrate producer (Wilkins et al., 1974). Butyrate‐producing bacteria are reported to be decreased in HFD‐fed animals and in some human's diseases such as IBD and obesity (Zhang et al., 2016). We can suppose the abundance of this bacterium in the microbiota of LL‐PAP mice was able to prevent inflammatory signals, and consequently able to inhibit Th17 and Th1 differentiation. This explanation fits with our findings and confirms that somehow this bacterium was able to improve the intestinal health in LL‐PAP treated mice after DNBS challenge. Nevertheless, at D9, after DNBS administration, no difference of microbiota composition was observed when we compared mice treated or not with our recombinant LAB. DNBS was the main changing factor. Hence, mice can prevent tissue damage and regain weight, albeit they do not completely recover their previous microbiota.
Recently, Darnaud et al. described that enteric delivery of PAP modifies the intestinal microbiota composition and controls inflammation. Similarly to our results, they showed that expression of PAP shift the composition of intestinal microbiota toward enrichment in clostridiales (Rumincoccaceae, Lachnospiraceae) (Darnaud et al., 2018). It has to be noted that a more deep comparison between are results on microbiota composition is difficult because the data were not treated and compared using the same analysis pipeline. Nevertheless, in contrary to our results they have a strong protective effect in DSS‐induced colitis model of PAP expression. One of the most important differences between our two studies is that they used transgenic mice (TG) overexpressing PAP in liver, delivering thus the AMP in the lower part of the intestinal tract. The delivery of PAP using recombinant LAB strategy has more chance to occur in the upper part of the intestine than in the lower part. Indeed, lactococci are highly sensitive to low pH and generally to the biochemical and physical–chemical conditions of the intestinal tract. They do not colonize and after entering the intestine, they do not survive more than few hours (Drouault et al., 1999). Thus, they deliver their load rapidly in the small intestine. It has to be noted too, that they describe a mild protective effect of a 100 μg intra‐rectal injection of recombinant PAP. In our case, we administer daily a quantity of PAP estimated around few hundreds of picograms which is far from what Darnaud et al. have injected.
Taken altogether, our results allow us to conclude LL‐PAP was able to shift the microbiota through an enriched butyrate‐producers microbiota that could be able to prevent intestinal epithelial damage, weight loss, and inflammatory status after DNBS challenge. More studies should be performed to demonstrate the role of the microbiota but we can propose E. plexicaudatum as a potential probiotic used to prevent intestinal inflammation damages.
Experimental procedures
Cloning of the human PAP gene in L. lactis
A 478‐bp DNA fragment encoding for mature human PAP (i.e., without the signal peptide) was PCR amplified from the pSPORT1:PAP vector (Itoh and Teraoka, 1993) using primers NsiI‐PAP (5′‐CC AATGCATCAGAAGAACCCCAGAGGGAACTG‐3′) and EcoRI‐PAP (5’‐GGGAATTCA CTCAGTCCCTAGTCAGTGAACTTGCAGACA‐3′). The resulting fragment was directly digested with NsiI and EcoRI enzymes (restriction sites on the primers are indicated in bold and italics) and cloned into purified backbone isolated from the NsiI‐EcoRI‐cut pSEC‐E7 vector (Bermudez‐Humaran et al., 2002) resulting in pSEC:PAP or NsiI‐EcoRI‐cut pCYT‐E7 vector resulting in pCYT:PAP. Both plasmids were introduced into L. lactis strain NZ9000 carrying the regulatory genes nisR and nisK (Mierau and Kleerebezem, 2005) to obtain the strain LL‐PAP. pSEC:PAP was also introduced into NZ9000htrA‐ (Cortes‐Perez et al., 2006). As a negative control, NZ9000 was transformed with a pSEC empty vector to generate strain LL. Recombinant L. lactis clones were selected by the addition of 10 μg ml–1 chloramphenicol.
Inducible expression of PAP
For the induction of PAP expression from the nisin promoter, strains were grown in M17 medium (Difco) supplemented with 1% glucose (GM17) at 30°C without agitation until an optical density at 600 nm of 0.6. Recombinants L. lactis were selected by the addition of 10 μg ml–1 chloramphenicol. Afterwards, the strains were induced with 10 ng of nisin (Sigma) per ml for 2 h. L. lactis culture extraction and immunoblotting assays were performed as follows, using a polyclonal serum specific from Human Reg3A (R&D Systems). Protein samples were prepared from 2 ml of induced culture at a DO600 = 1. After centrifugation (5 min, 10,000 rpm), the cell pellet and supernatant were treated separately. The supernatants were treated with 100 μl of 100% trichloroacetic acid to precipitate proteins. Samples were incubated for 1 h on ice, and proteins were recovered from the pellets after centrifugation at 4°C for 30 min at 13,000 rpm. The cell fractions were resuspended in PBS supplemented with anti‐protease and sonicated (6 cycles of 10 s sonicating and 10 s rest) on ice. Sodium dodecyl sulfate‐polyacrylamide gel electrophoresis, Western blotting, and immunodetection were performed as previously described (Le Loir et al., 1998; Bermudez‐Humaran et al., 2002).
The concentrations of PAP secreted in the medium and retained in cell fractions were assessed by an ELISA kit (Dynabio) too. Human commercial PAP (BioVendor) was used as a control in Western blotting and ELISA.
Animals
Specific pathogen‐free C57BL/6 mice (6–8 weeks old; Janvier, France) were maintained under normal husbandry conditions in the animal facilities of the National Institute of Agricultural Research (UEIERP, INRA, Jouy‐en‐Josas, France). All animal experiments began after 1 week of acclimation and were performed according to European Community rules of animal care and with authorization 78‐149 of the French Veterinary Services.
Induction of acute colitis and bacteria administration
The protocol of DNBS‐induced acute colitis is detailed in Fig. 2. Briefly, mice of approximately 20 g were fully anaesthetised by intraperitoneal (i.p.) injection of 150 μl of 0.1% ketamine (Imalgene 1000, Merial, France) and 0.06% xylazine (Rompun) and a 3.5 catheter (French catheter, Solomon Scientific) attached to a tuberculin syringe was inserted into the colon. A dose of 150 mg kg–1 of DNBS solution (ICN, Biomedical Inc.) in 30% ethanol (EtOH) was then injected intra‐rectally to induce colitis. Control mice (without colitis) received only 30% EtOH. Mice were fed with 5 × 109 CFU in 200 μl of either LL or LL‐PAP in PBS, or PBS alone daily for 9 days. Weight loss was monitored daily to assess the severity of colitis. Inflammation was monitored 4 days after DNBS administration by cytokine productions, macroscopic and microscopic analysis.
The protocol of DSS‐induced acute realized was based on previous protocols. Briefly, at D0 colitis was induced by adding 2.5% (w/v) of Dextran sulfate sodium salt (DSS) of a molecular weight of 36,000–50,000 (MPBio) to the drinking water for 7 days. The mice were sacrificed at D12 (DSS recovery) after the DSS induction. For the recovery phase, DSS colitis induction was followed by 5 days of recovery with normal drinking water. Mice were monitored daily for weight loss and Disease Activity Index (DAI) has been calculated according to the protocol established by Cooper and colleagues (1993). Mice have been sacrificed by cervical dislocation. Mesenteric lymph node (MLN) cells were isolated and colon have been harvested for protein extraction and histological assessment.
Macroscopic and microscopic damage scores
Mice were sacrificed by cervical dislocation and the abdominal cavity was opened, the colon was removed and opened longitudinally and damage was immediately assessed macroscopically. Macroscopic scores were recorded using a previously described system (Martin et al., 2014a,b). Briefly, the macroscopic criteria (assessed on a scale from 0 to 5) include macroscopic mucosal damages such as ulcers, thickening of the colon wall, the presence of adhesions between the colon and other intra‐abdominal organs, the consistency of faecal material (as an indicator of diarrhoea) and the presence of hyperemia.
Large intestines pieces were removed, visually inspected for macroscopic evaluation, and prepared for histological evaluation using standard methods. Serial paraffin sections of 4 μm were made and stained with haematoxylin–eosin for light microscopy examination. Each parameter (erythema, haemorrhage, edema, stricture formation, ulceration, faecal blood, presence of mucus, diarrhoea and adhesions) was awarded 1 point if observed in tissue examination. The extent of colonic damage and inflammation was assessed using a standard histopathological grading system: histological findings identical to normal mice (grade 0); mild mucosal and/or submucosal inflammatory infiltrate (admixture of neutrophils) and edema, punctate mucosal erosions often associated with capillary proliferation, Muscularis mucosae intact (grade 1); grade 1 changes involving 50% of the specimen (grade 2); prominent inflammatory infiltrate and edema (neutrophils usually predominating) frequently with deeper areas of ulceration extending through the muscularis mucosae into the submucosa; rare inflammatory cells invading the muscularis propriae but without muscle necrosis (grade 3); grade 3 changes involving 50% of the specimen (grade 4); extensive ulceration with coagulative necrosis bordered inferiorly by numerous neutrophils and lesser numbers of mononuclear cells; necrosis extends deeply into the muscularis propria (grade 5); grade 5 changes involving 50% of the specimen (grade 6).
Cytokine assays
Mesenteric lymph nodes (MLN) cells were isolated from mice and cultured in RPMI culture medium (Lonza) with 100 unit of streptomycin, penicillin (PAA Laboratories) and 10% SVF (Lonza) at 2 × 106 cells/well. Cells were re‐activated with 4 μg ml–1 pre‐coated anti‐mouse antibody CD3e and CD28 (eBioscience). Concentrations of cytokines IL‐12, IL‐17, IL‐4, TSLP, and INF‐γ (Mabtech) and TGF‐β (R&D), in medium were assessed by ELISA after 48 h of incubation (37°C/5%CO2).
One centimetre of colonic tissue was weighing and mashed by Gentle MaxTM (Miltenyl Biotec) in 1 ml of PBS plus anti‐protease (Roche). The lysate was centrifuged and the supernatant used to measure cytokine level by ELISA. The cytokines tested were IFNγ, IL12, IL4, IL17, TSLP (Mabtech) and TGF‐β (R&D systems), and the concentration was normalized by microgram of tissue.
Lamina propria isolation
Black C57BL/6 mice were administered with 109 CFU of LL, LL‐PAP or PBS for 7 days before DNBS challenge. After 4 days since the challenge, animals were euthanized, colon were recovered to perform lamina propria extraction. After cleaning the tissue, digestion using DNAse and Liberase (Roche) was performed during 30 min at 37°C with constant shaking. The digested tissue was mashed in a cell stainer (100 μm) and collected in complete medium (RPMI sigma). After centrifugation, cells resuspended in Percoll 40% were underlaid on 3 ml of Percoll 80%, tubes were centrifuged during 20 min, 600 g (without break), and the ring formed in the middle of the two phases was collected into another tube. Cells were centrifuged, washed with complete RPMI medium and counted using a flow cytometer.
Treg cells population
Staining was performed according to manufacturer recommendations: around 106 cells/well were inserted in an opaque, white 96‐Well Plate V format (Grener), centrifuged at 37°C for 2 min/2000 rpm. Supernatant was discarded, cells were washed with PBS before being incubated with anti‐mouse CD3e APC‐efluor 780, CD4 PE‐Cy5 conjugated L3 T4 and CD16/CD32 antibodies (diluted in PBS1× containing 2% FBS—PBS1XFluo). All antibodies are used at final concentration of 1 μg ml–1. Plate was incubated 20 min at 4°C protected from light and next centrifuged at 37°C for 2 min/2000 rpm. Supernatant was then discarded and incubated with PBS1XFluo, washed and resuspended with fixation/permeabilization solution. Cells were kept for 30 min at 4°C protected light, centrifuged, and incubated with anti‐mouse/Rat Foxp3‐FITC antibody for 30 min (prepared in permeabilization buffer). Cells were washed and resuspended with PBS for reading in the flow cytometer. Leukocytes were gated using forward scatter and side scatter, and within the leukocyte gates, leucocytes were identified as Th cells (CD3+, CD4+) and the population of Treg was identified as CD3+CD4+FoxP3+. CD16/CD32 are expressed in B cells, monocytes/macrophages, NK cells, granulocytes, mast cells and dendritic cells, and used as control.
Statistical analysis
The GraphPad software (GraphPad Sofware, La Jolla) was used for statistical analysis. Results are presented as bar graphs or dot plots with means±SEM. Most comparisons involved one‐way analysis of variance followed by the Bonferroni multiple comparison post hoc analysis. For data sets that were non‐Gaussian or based on a score or on a percentage, the non‐parametric Mann–Whitney test was used. A p value of less than 0.05 was considered significant.
Bioinformatics analysis
The assembled sequences were dereplicated and singletons were removed using the Vsearch tool using the ‘derep_fulllength’ command. The dereplicated sequences were clustered into 99% identity groups to constitute the OTUs through the ‘cluster_fast’ command. The initial reads were mapped to the constructed OTUs to quantify each Taxonomic Unit using the ‘usearch_global’ Vsearch tool (Rognes et al., 2016). The taxonomic assignment was performed by the TAG.ME (Pires et al., 2018) R package using 515F‐806R model.
Statistical analysis—The differential abundant OTUs were identified using the Deseq2 (Love et al., 2014) with an adjusted p value threshold of 0.05. The Beta‐Diversity visualization was performed through the PCoA using the Jensen‐Shannon distance matrix.
Acknowledgement
We thank CAPES‐Cofecub, CNPq (NMB, PBVB, RDC) and Carnot Qualiment (MA) for funding. All authors have no conflict of interest to declare. We thank the members of Animal Facility (INRA, Jouy en Josas, France): Jérôme Pottier, Charline Pontlevoy, Mathilde Bauducel, Marlène Héry and Andre Tiffoche for all help and the hosting and caring of the mice. We also thank C. Aubry, R. Martin, B. Lamas, G. da Costa, M.L. Richard, E. Jacouton, C. Michon, C. Severiano, D. Rama, T. Alain, J. Natividad, and S. Le Guin for fruitful discussion or technical help.
References
- Adel‐Patient, K. , Ah‐Leung, S. , Creminon, C. , Nouaille, S. , Chatel, J.M. , Langella, P. , and Wal, J.M. (2005) Oral administration of recombinant Lactococcus lactis expressing bovine beta‐lactoglobulin partially prevents mice from sensitization. Clin Exp Allergy 35: 539–546. [DOI] [PubMed] [Google Scholar]
- Asano, K. , Yoshimura, S. , and Nakane, A. (2013) Alteration of intestinal microbiota in mice orally administered with salmon cartilage proteoglycan, a prophylactic agent. PLoS One 8: e75008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi, K. , Tanoue, T. , Oshima, K. , Suda, W. , Nagano, Y. , Nishikawa, H. , et al (2013) Treg induction by a rationally selected mixture of clostridia strains from the human microbiota. Nature 500: 232–236. [DOI] [PubMed] [Google Scholar]
- Aubry, C. , Michon, C. , Chain, F. , Chvatchenko, Y. , Goffin, L. , Zimmerli, S.C. , et al (2015) Protective effect of TSLP delivered at the gut mucosa level by recombinant lactic acid bacteria in DSS‐induced colitis mouse model. Microb Cell Fact 14: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry, C. , Michon, C. , Chain, F. , Chvatchko, Y. , Goffin, L. , Zimmerli, S.C. , et al (2016) Protective effect of TSLP delivered at the gut mucosa level by recombinant lactic acid bacteria in DSS‐induced colitis mouse model (vol 14, 176, 2015). Microb Cell Fact 15: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez‐Humaran, L.G. , Cortes‐Perez, N.G. , Lefevre, F. , Guimaraes, V. , Rabot, S. , Alcocer‐Gonzalez, J.M. , et al (2005) A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL‐12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16‐induced tumors. J Immunol 175: 7297–7302. [DOI] [PubMed] [Google Scholar]
- Bermudez‐Humaran, L.G. , Langella, P. , Miyoshi, A. , Gruss, A. , Guerra, R.T. , Montes de Oca‐Luna, R. , and Le Loir, Y. (2002) Production of human papillomavirus type 16 E7 protein in Lactococcus lactis . Appl Environ Microbiol 68: 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez‐Humaran, L.G. , Nouaille, S. , Zilberfarb, V. , Corthier, G. , Gruss, A. , Langella, P. , and Issad, T. (2007) Effects of intranasal administration of a leptin‐secreting Lactococcus lactis recombinant on food intake, body weight, and immune response of mice. Appl Environ Microbiol 73: 5300–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash, H.L. , Whitham, C.V. , Behrendt, C.L. , and Hooper, L.V. (2006) Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313: 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing, B. , Aitken, J.D. , Malleshappa, M. , and Vijay‐Kumar, M. (2014) Dextran sulfate sodium (DSS)‐induced colitis in mice. Curr Protoc Immunol 104 Unit 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Jin, W. , Hardegen, N. , Lei, K.J. , Li, L. , Marinos, N. , et al (2003) Conversion of peripheral CD4+CD25‐ naive T cells to CD4+CD25+ regulatory T cells by TGF‐beta induction of transcription factor Foxp3. J Exp Med 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choonara, B.F. , Choonara, Y.E. , Kumar, P. , Bijukumar, D. , du Toit, L.C. , and Pillay, V. (2014) A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol Adv 32: 1269–1282. [DOI] [PubMed] [Google Scholar]
- Cooper, H.S. , Murthy, S.N. , Shah, R.S. , and Sedergran, D.J. (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249. [PubMed] [Google Scholar]
- Cortes‐Perez, N.G. , Poquet, I. , Oliveira, M. , Gratadoux, J.J. , Madsen, S.M. , Miyoshi, A. , et al (2006) Construction and characterization of a Lactococcus lactis strain deficient in intracellular ClpP and extracellular HtrA proteases. Microbiology 152: 2611–2618. [DOI] [PubMed] [Google Scholar]
- Daniel, C. , Repa, A. , Wild, C. , Pollak, A. , Pot, B. , Breiteneder, H. , et al (2006) Modulation of allergic immune responses by mucosal application of recombinant lactic acid bacteria producing the major birch pollen allergen Bet v 1. Allergy 61: 812–819. [DOI] [PubMed] [Google Scholar]
- Darnaud, M. , Dos Santos, A. , Gonzalez, P. , Augui, S. , Lacoste, C. , Desterke, C. , et al (2018) Enteric delivery of regenerating family member 3 alpha alters the intestinal microbiota and controls inflammation in mice with colitis. Gastroenterology 154: 1009–1023 e1014. [DOI] [PubMed] [Google Scholar]
- de Souza, H.S.P. , Fiocchi, C. , and Iliopoulos, D. (2017) The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol 14: 739–749. [DOI] [PubMed] [Google Scholar]
- Dewhirst, F.E. , Chien, C.C. , Paster, B.J. , Ericson, R.L. , Orcutt, R.P. , Schauer, D.B. , and Fox, J.G. (1999) Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl Environ Microbiol 65: 3287–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dothel, G. , Vasina, V. , Barbara, G. , and De Ponti, F. (2013) Animal models of chemically induced intestinal inflammation: predictivity and ethical issues. Pharmacol Ther 139: 71–86. [DOI] [PubMed] [Google Scholar]
- Drouault, S. , Corthier, G. , Ehrlich, S.D. , and Renault, P. (1999) Survival, physiology, and lysis of Lactococcus lactis in the digestive tract. ApplEnvironMicrobiol 65: 4881–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein, M. (2016) Biology: a slow‐motion epidemic. Nature 540: S98–S99. [DOI] [PubMed] [Google Scholar]
- Eissa, N. , Kermarrec, L. , Hussein, H. , Bernstein, C.N. , and Ghia, J.E. (2017) Appropriateness of reference genes for normalizing messenger RNA in mouse 2,4‐dinitrobenzene sulfonic acid (DNBS)‐induced colitis using quantitative real time PCR. Sci Rep 7: 42427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foligne, B. , Dessein, R. , Marceau, M. , Poiret, S. , Chamaillard, M. , Pot, B. , et al (2007) Prevention and treatment of colitis with Lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein. Gastroenterology 133: 862–874. [DOI] [PubMed] [Google Scholar]
- Gironella, M. , Iovanna, J.L. , Sans, M. , Gil, F. , Penalva, M. , Closa, D. , et al (2005) Anti‐inflammatory effects of pancreatitis associated protein in inflammatory bowel disease. Gut 54: 1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granlund, A. , Beisvag, V. , Torp, S.H. , Flatberg, A. , Kleveland, P.M. , Ostvik, A.E. , et al (2011) Activation of REG family proteins in colitis. Scand J Gastroenterol 46: 1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, M.L. , Hixon, J.A. , Li, W. , Felber, B.K. , Anver, M.R. , Stewart, C.A. , et al (2014) Oral delivery of IL‐27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology 146: 210–221.e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenoehrl, C. , Storr, M. , and Schicho, R. (2017) Cannabinoids for treating inflammatory bowel diseases: where are we and where do we go? Expert Rev Gastroenterol Hepatol 11: 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail, A.S. , Behrendt, C.L. , and Hooper, L.V. (2009) Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol 182: 3047–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, T. , and Teraoka, H. (1993) Cloning and tissue‐specific expression of cDNAs for the human and mouse homologs of rat pancreatitis‐associated protein (PAP). Biochim Biophys Acta 1172: 184–186. [DOI] [PubMed] [Google Scholar]
- Keim, V. , Rohr, G. , Stockert, H.G. , and Haberich, F.J. (1984) An additional secretory protein in the rat pancreas. Digestion 29: 242–249. [DOI] [PubMed] [Google Scholar]
- Lamas, B. , Richard, M.L. , Leducq, V. , Pham, H.P. , Michel, M.L. , Da Costa, G. , et al (2016) CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 22: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Loir, Y. , Gruss, A. , Ehrlich, S.D. , and Langella, P. (1998) A nine‐residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis . J Bacteriol 180: 1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. , Li, S. , Zhang, Q. , Xu, Z. , Wang, J. , and Sun, H. (2018) Oral engineered Bifidobacterium longum expressing rhMnSOD to suppress experimental colitis. Int Immunopharmacol 57: 25–32. [DOI] [PubMed] [Google Scholar]
- Love, M.I. , Huber, W. , and Anders, S. (2014) Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marafini, I. , Di Sabatino, A. , Zorzi, F. , Monteleone, I. , Sedda, S. , Cupi, M.L. , et al (2014) Serum regenerating islet‐derived 3‐alpha is a biomarker of mucosal enteropathies. Aliment Pharmacol Ther 40: 974–981. [DOI] [PubMed] [Google Scholar]
- Martin, R. , Chain, F. , Miquel, S. , Lu, J. , Gratadoux, J.J. , Sokol, H. , et al (2014a) The commensal bacterium Faecalibacterium prausnitzii is protective in DNBS‐induced chronic moderate and severe colitis models. Inflamm Bowel Dis 20: 417–430. [DOI] [PubMed] [Google Scholar]
- Martin, R. , Martin, R. , Chain, F. , Chain, F. , Miquel, S. , Miquel, S. , et al (2014b) Effects in the use of a genetically engineered strain of Lactococcus lactis delivering in situ IL‐10 as a therapy to treat low‐grade colon inflammation. Hum Vaccin Immunother 10: 1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierau, I. , and Kleerebezem, M. (2005) 10 years of the nisin‐controlled gene expression system (NICE) in Lactococcus lactis . Appl Microbiol Biotechnol 68: 705–717. [DOI] [PubMed] [Google Scholar]
- M'Koma, A.E. (2013) Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol 6: 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta, J.P. , Bermudez‐Humaran, L.G. , Deraison, C. , Martin, L. , Rolland, C. , Rousset, P. , et al (2012) Food‐grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci Transl Med 4: 158ra144. [DOI] [PubMed] [Google Scholar]
- Mukherjee, S. , and Hooper, L.V. (2015) Antimicrobial defense of the intestine. Immunity 42: 28–39. [DOI] [PubMed] [Google Scholar]
- Nakamura, K. , Kitani, A. , and Strober, W. (2001) Cell contact‐dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface‐bound transforming growth factor beta. J Exp Med 194: 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad, J.M. , Hayes, C.L. , Motta, J.P. , Jury, J. , Galipeau, H.J. , Philip, V. , et al (2013) Differential induction of antimicrobial REGIII by the intestinal microbiota and Bifidobacterium breve NCC2950. Appl Environ Microbiol 79: 7745–7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, O.H. (2014) New strategies for treatment of inflammatory bowel disease. Front Med (Lausanne) 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes, T. , Bernardazzi, C. , and de Souza, H.S. (2014) Cell death and inflammatory bowel diseases: apoptosis, necrosis, and autophagy in the intestinal epithelium. Biomed Res Int 2014: 218493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, H. , Fukushima, K. , Naito, H. , Funayama, Y. , Unno, M. , Takahashi, K. , et al (2003) Increased expression of HIP/PAP and regenerating gene III in human inflammatory bowel disease and a murine bacterial reconstitution model. Inflamm Bowel Dis 9: 162–170. [DOI] [PubMed] [Google Scholar]
- Omenetti, S. , and Pizarro, T.T. (2015) The Treg/Th17 Axis: a dynamic balance regulated by the gut microbiome. Front Immunol 6: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigneur, B. , and Sokol, H. (2016) Fecal microbiota transplantation in inflammatory bowel disease: the quest for the holy grail. Mucosal Immunol 9: 1360–1365. [DOI] [PubMed] [Google Scholar]
- Pires, D.E.V. , Oliveira, F.S. , Correa, F.B. , Morais, D.K. , and Fernandes, G.R. (2018) TAG.ME: Taxonomic Assignment of Genetic Markers for Ecology. bioRxiv. [Google Scholar]
- Riviere, A. , Selak, M. , Lantin, D. , Leroy, F. , and De Vuyst, L. (2016) Bifidobacteria and butyrate‐producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 7: 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat, T. , Bermudez‐Humaran, L. , Gratadoux, J.J. , Fourage, C. , Hoebler, C. , Corthier, G. , and Langella, P. (2007) Anti‐inflammatory effects of Lactobacillus casei BL23 producing or not a manganese‐dependant catalase on DSS‐induced colitis in mice. Microb Cell Fact 6: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognes, T. , Flouri, T. , Nichols, B. , Quince, C. , and Mahe, F. (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4: e2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos Rocha, C. , Gomes‐Santos, A.C. , Garcias Moreira, T. , de Azevedo, M. , Diniz Luerce, T. , Mariadassou, M. , et al (2014) Local and systemic immune mechanisms underlying the anti‐colitis effects of the dairy bacterium Lactobacillus delbrueckii . PLoS One 9: e85923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidler, L. , Hans, W. , Schotte, L. , Neirynck, S. , Obermeier, F. , Falk, W. , et al (2000) Treatment of murine colitis by Lactococcus lactis secreting interleukin‐10. Science 289: 1352–1355. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke, K. , de Haard, H. , Beirnaert, E. , Dreier, T. , Lauwereys, M. , Huyck, L. , et al (2010) Orally administered L. lactis secreting an anti‐TNF nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol 3: 49–56. [DOI] [PubMed] [Google Scholar]
- Wallace, J.L. , Le, T. , Carter, L. , Appleyard, C.B. , and Beck, P.L. (1995) Hapten‐induced chronic colitis in the rat: alternatives to trinitrobenzene sulfonic acid. J Pharmacol Toxicol Methods 33: 237–239. [DOI] [PubMed] [Google Scholar]
- Wan, Y.Y. , and Flavell, R.A. (2007) Yin‐Yang' functions of transforming growth factor‐beta and T regulatory cells in immune regulation. Immunol Rev 220: 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarden, A.R. , and Vaughn, B.P. (2017) Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 8: 238–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins, T.D. , Fulghum, R.S. , and Wilkins, J.H. (1974) Eubacterium plexicaudatum sp. nov., an Anaerobic Bacterium with a Subpolar Tuft of Flagella, Isolated from a Mouse Cecum. Int J Syst Evol Microbiol 24: 408–411. [Google Scholar]
- Yang, X. , Jin, H. , Liu, K. , Gu, Q. , and Xu, X. (2011) A novel peptide derived from human pancreatitis‐associated protein inhibits inflammation in vivo and in vitro and blocks NF‐kappa B signaling pathway. PLoS One 6: e29155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Ning, Z. , Mayne, J. , Moore, J.I. , Li, J. , Butcher, J. , et al (2016) MetaPro‐IQ: a universal metaproteomic approach to studying human and mouse gut microbiota. Microbiome 4: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L. , Ivanov, I.I. , Spolski, R. , Min, R. , Shenderov, K. , Egawa, T. , et al (2007) IL‐6 programs T(H)‐17 cell differentiation by promoting sequential engagement of the IL‐21 and IL‐23 pathways. Nat Immunol 8: 967–974. [DOI] [PubMed] [Google Scholar]


