Summary
Forward genetic screens play a key role in the identification of genes contributing to plant stress tolerance. Using a screen for freezing sensitivity, we have identified a novel freezing tolerance gene, SENSITIVE‐TO‐FREEZING8, in Arabidopsis thaliana.
We identified SFR8 using recombination‐based mapping and whole‐genome sequencing. As SFR8 was predicted to have an effect on cell wall composition, we used GC‐MS and polyacrylamide gel electrophoresis to measure cell‐wall fucose and boron (B)‐dependent dimerization of the cell‐wall pectic domain rhamnogalacturonan II (RGII) in planta. After treatments to promote borate‐bridging of RGII, we assessed freeze‐induced damage in wild‐type and sfr8 plants by measuring electrolyte leakage from freeze‐thawed leaf discs.
We mapped the sfr8 mutation to MUR1, a gene encoding the fucose biosynthetic enzyme GDP‐d‐mannose‐4,6‐dehydratase. sfr8 cell walls exhibited low cell‐wall fucose levels and reduced RGII bridging. Freezing sensitivity of sfr8 mutants was ameliorated by B supplementation, which can restore RGII dimerization. B transport mutants with reduced RGII dimerization were also freezing‐sensitive.
Our research identifies a role for the structure and composition of the plant primary cell wall in determining basal plant freezing tolerance and highlights the specific importance of fucosylation, most likely through its effect on the ability of RGII pectin to dimerize.
Keywords: Arabidopsis thaliana, boron, cell wall, freezing tolerance, fucose, pectin, Rhamnogalacturonan II, sfr8
Introduction
Plants vary enormously in their capacity to tolerate low temperature. Whilst some tropical species are susceptible to mild chilling, others from cooler parts of the world can tolerate severe sub‐zero temperatures (Burke et al., 1976; Levitt, 1980). Freezing damage manifests itself as dehydration of the cytoplasm and damage to cellular membranes. At very low temperatures, protein denaturation is an additional problem (Thomashow, 1999). During freezing, ice crystals form in the extracellular space because the water in the apoplast has a lower osmotic potential than in the cytoplasm. This leads to very low water potential in the apoplast; therefore, water is drawn out of the protoplast causing dehydration (Thomashow, 1999; Pearce, 2001). Dehydration stress is thus a major component of freeze‐induced damage in plants. Indeed, freezing and drought share many consequences and tolerance of both conditions is effected by some of the same mechanisms, including the upregulation of genes encoding dehydrins (Thomashow, 2010; Nakashima et al., 2014). Upon thawing, if water returns to the cytosol in overabundance or too quickly, the plasma membrane bursts, a phenomenon termed expansion‐induced lysis (EIL) (Uemura et al., 2006), resulting in cell death. Further damage is caused at lower freezing temperatures by the aggregation of membrane lipids into non‐bilayer structures (Gordon‐Kamm & Steponkus, 1984).
The cell wall (CW) provides structural integrity whilst allowing flexibility, extensibility and growth (Cosgrove, 2005). The CW is typically composed of three biochemically distinct components; the primary CW and middle lamella are secreted from the cell first whilst the secondary CW is normally secreted after growth ceases (Popper, 2008). The secondary CW is usually only present in tissues that require structural reinforcement, such as the xylem (Meents et al., 2018). The middle lamella is a pectin‐rich layer shared by adjacent cells and has a number of functions in addition to its role of separating neighbouring cells (Zamil & Geitmann, 2017) whilst the primary CW consists of cellulose microfibrils embedded in a matrix of hemicelluloses and pectins as well as a large number of proteins (Keegstra, 2010). Although much attention has been focussed on the plasma membrane as the major site of damage during and after freezing, the CW can also be significantly affected and may undergo collapse (cytorrhysis) in response to dehydration when extracellular ice forms (Levitt, 1980). Not all plant species undergo CW collapse after freezing and not all CWs exhibit the same growth of ice crystals under similar freezing conditions; it has been suggested that the nature of the CW may be an important determinant of plant freezing tolerance (Rajashekar & Lafta, 1996; Gusta & Wisniewski, 2013). Apoplastic ice‐binding proteins (IBPs) are also important in reducing the growth of ice crystals in the extracellular compartment (Bredow & Walker, 2017).
Some species from temperate parts of the world are capable of cold acclimation (CA), a phenomenon whereby they gain in freezing tolerance (FT) after exposure to low non‐freezing temperatures for a period of days or weeks before the onset of freezing (Thomashow, 1999; Browse & Xin, 2001; Smallwood & Bowles, 2002; Baxter, 2014). During CA, major transcriptional reprogramming occurs (Fowler & Thomashow, 2002; Hannah et al., 2005; Kaplan et al., 2007). This is accompanied by large‐scale changes in the metabolome that bring about increased production of compatible solutes (Stitt & Hurry, 2002; Cook et al., 2004; Kaplan et al., 2007), changes in membrane structure and composition (Steponkus & Lynch, 1989; Uemura & Steponkus, 1994; Uemura et al., 1995), and changes in growth and morphology (Thomashow, 1999). Changes in CW composition (Takahashi et al., 2019), and increased CW thickness and strength also occur (Rajashekar & Lafta, 1996; Stefanowska et al., 1999).
The CBF (C‐Repeat binding factor) transcription factors (CBF1, CBF2 and CBF3 genes; also known as DREB1B, DREB1C and DREB1A (Shinwari et al., 1998) control the expression of a large number of cold‐regulated (COR) genes encoding proteins with functions associated with CA (Gilmour et al., 2004). The CBFs have been well‐documented elsewhere (for a recent review see (Ding et al., 2019)). Overexpression of the CBFs causes constitutive FT without the requirement for CA (Jaglo‐Ottosen et al., 1998) although a major obstacle preventing CBF exploitation in crop protection has been the negative effect on growth due to their promotion of DELLA activity (Achard et al., 2008). For this reason, it is pertinent to search for novel routes to FT that could be used to engineer tolerant plants without such severe growth penalties.
A forward genetic screen for Arabidopsis mutants that were susceptible to freezing even after CA identified a number of sensitive‐to‐freezing (sfr) mutants (Warren et al., 1996) and several SFR genes have been cloned and their contribution to FT elucidated. SFR2 acts via chloroplast membrane lipid remodelling (Moellering et al., 2010), SFR3 affects cuticle wax deposition (Amid et al., 2012) and SFR6 encodes the MED16 subunit of the Mediator transcriptional coactivator complex (Knight et al., 2009; Hemsley et al., 2014) and controls the expression of CBF‐regulated genes.
In this study, we present the mapping and cloning of a novel FT gene, SENSITIVE‐TO‐FREEZING8 (SFR8). We demonstrate that reduced pectin fucosylation and crosslinking in the CW of sfr8 mutants is associated with a compromised ability to tolerate freezing temperatures, identifying the CW as a target for the genetic control of FT.
Materials and Methods
Plant materials
bor1‐3 (SALK_37312), bor2‐1 (SALK_56473) and a bor1‐3bor2‐1 double mutant were a kind gift from Kyoko Miwa (Hokkaido University). mur2, mur1‐1 and mur1‐2 (N6243, N6244 and N8565) were obtained from the Nottingham Arabidopsis Stock Centre (NASC).
Sequencing of the sfr8 mutant genome and analysis of polymorphisms
Illumina‐based whole‐genome sequencing of sfr8 genomic DNA was carried out at The Genome Analysis Centre (now the Earlham Institute, Norwich, UK). The raw data were analysed using the open source platform Galaxy (https://main.g2.bx.psu.edu/) and mapped to the TAIR10 reference genome (Lamesch et al., 2012) with the mapping tool Bowtie (https://main.g2.bx.psu.edu/) using the default settings. The output was read using the Integrative Genomics Viewer (IGV) software (http://www.broadinstitute.org/software/igv/). The threshold was set manually to 0.7 in order to detect mutations that appear in 70% of reads or more. The previously defined mapping interval was scanned manually for bases that differed from the reference genome sequence. After this analysis, four SNPs remained candidates for the sfr8 mutation (Supporting Information Table S1).
Production of the complementation lines
The MUR1 coding sequence was amplified from Col‐0 wild‐type cDNA using the primers 5′‐CACCATGGCGTCAGAGAACAACG‐3′ and 5′‐TCAAGGTTGCTGCTTAGCATC‐3′ and cloned into the Gateway entry vector pENTR‐D‐TOPO before transfer to the destination vector pK7WG2 (Karimi et al., 2002) to allow expression in plants under the control of the 35S CaMV promoter. sfr8 mutant plants were transformed with the construct using the floral dip method (Clough & Bent, 1998) and kanamycin‐resistant transformants selected.
Plant growth and visual freezing assay
Seeds were sown on MS agar medium as described previously (Hemsley et al., 2014). After 8–10 d seedlings were transferred to 44‐mm peat plugs (LBS Horticulture, Colne, UK) and grown in short days (20°C; 12 h : 12 h, light : dark; 150‐200 µE m−2 s−1 light) for a further 4 wk before transfer to acclimating conditions (5°C; 10 h : 14 h, light : dark; 150 µE m−2 s−1 light), if used, for 2 wk. Plants were then transferred to a freezing chamber set to −8.5°C for 24 h in darkness. Plants were removed and allowed to thaw at 4°C for 8 h. For boron (B) supplementation experiments, plants were grown on half‐strength MS medium to reduce levels of available B then transferred to peat plugs at 8–10 d old and grown in short days as before. Plants were watered once a wk with deionised water with or without 20 mg l−1 boric acid (BA) supplementation. Further deionised water was supplied as necessary. Plants were transferred to cold acclimating conditions at 5 wk with the same supplementation regime for 2 wk before freeze testing.
Electrolyte leakage assays
Plants grown and cold‐acclimated (where applicable) as described above were subjected to an electrolyte leakage (EL) assay as we have described previously (Hemsley et al., 2014) but modified to use leaf discs, except in the experiments using bor mutants, which had smaller leaves. Six replicate samples per genotype per temperature tested were prepared, each consisting of three 8‐mm‐diameter leaf discs taken from the same plant using a cork borer.
Inhibition of fucosylation with 2f‐fucose
Peracetylated 2‐fluoro‐2‐deoxy‐l‐fucose (2f‐fucose, Merck Millipore, Nottingham, UK) was dissolved in DMSO to give a stock solution of 10 mM. Wild‐type Arabidopsis (Col‐0) seedlings were grown on half‐strength MS medium supplemented with 2f‐fucose at 2.5 µM, 10 µM or 25 µM or 0.25% (v/v) DMSO (control, corresponding to the highest concentration of DMSO present in the 2f‐fucose treatments). Seedlings were grown for 14 d before being assessed for freezing damage using the EL assay. Approximately 10 mg of seedlings was used for each of the replicate samples.
Cell‐wall sugar analysis
Plants were grown on full‐strength MS agar medium for 14 d as described above and cell wall sugars extracted following standard procedures (York et al., 1986), outlined in detail below. Approximately 100 mg of tissue was harvested and ground in 70% (v/v) ethanol at 80°C to remove the alcohol‐soluble fraction, and then dried overnight in a vacuum dryer. Samples were then rehydrated in 500 µl water in a sonicating water bath before the addition of 100 µg inositol as internal standard and samples incubated in 2 M trifluoroacetic acid for 2 h at 110°C. After this, 800 µl of supernatant was transferred to glass vials and dried under N2 at 40°C. Once dry, 400 µl of 1 M hydrochloric acid in methanol was added and samples incubated at 80°C overnight. Samples were then dried under N2 at 40°C until completely dry then 400 µl of 1‐(trimethylsilyl)imidazole/pyridine 1 : 4 (v/v) (Sigma, Poole, Dorset, UK) mixture added and incubated at 80°C. After 30 min, samples were dried under N2 at 40°C; the residue was suspended in 1 ml hexane and vortexed vigorously. Samples were centrifuged and transferred to clean glass vials to remove the salt, before partitioning with an equal volume of water to hexane. After vortexing vigorously, an upper and lower phase was generated, with the upper hexane phase being transferred to GC‐MS vials for analysis. The GC‐MS analyses were performed using a single‐quadrupole Shimadzu QP‐2010‐Plus system fitted with a Restek Rxi‐5Sil column (30 m, 0.25 mm ID). Samples were introduced by split injection and the carrier gas was helium. The injector temperature was 250°C and the initial oven temperature was 140°C, increasing at 2°C min−1 to 180°C and held at this temperature for 5 min before increasing to 275°C at 10°C min−1, held for 10 min. Seven monosaccharides (arabinose, fucose, galactose, glucose, mannose, xylose and inositol obtained from Supelco and Sigma‐Aldrich) were used as reference standards as described previously (Lobine et al., 2018). Quantification was carried out using inositol as the internal standard.
RGII analysis by gel electrophoresis
Leaves were harvested from 5‐wk‐old plants, ground in liquid nitrogen and c. 50 mg of the ground tissue used to prepare alcohol‐insoluble residue (AIR). Tissue was washed twice in 96% ethanol (v/v) at room temperature, followed by a second incubation in fresh ethanol for 16 h at 37°C. Samples were then incubated in 40 ml of 1 M Na2CO3 (pH 11.5) for 16 h at 4°C after which samples were acidified with a slight excess of acetic acid, washed in 500 µL of acetone and dried overnight. The AIR (c. 5 mg) was digested in 1 ml of 2 U ml−1 endopolygalacturonase (EPG; Megazyme, Irishtown, County Wicklow, Ireland) suspended in pyridine/acetic acid/water (1 : 1 : 98) with 0.5% chlorobutanol for 16 h at room temperature and the pectin digest separated using polyacrylamide gel electrophoresis as described in Chormova et al. (2014). Briefly, 12 µl of sample was mixed with 3 µl of buffer (0.63 M Tris‐HCl, 0.25% (w/v) bromophenol blue, 50% (v/v) glycerol, pH 8.8) and electrophoresed through a 26.4% polyacrylamide gel (buffer 50 mM Tris‐HCl, 38 mM glycine, pH 9) for 75 min at 200 V. The gel was then fixed in ethanol/acetic acid/water (4 : 1 : 5) for 30 min then washed with water three times for 1 min followed by treatment with 400 µM sodium thiosulphate (1 min), water (1 min, three times), 6 mM silver nitrate in 10 mM formaldehyde (20 min), water (20 s, twice) and 0.28 M Na2CO3 containing 8 µM sodium thiosulphate and 64 mM formaldehyde for 2–10 min. Colour development was stopped by addition of 0.33 M Tris base in 2% (v/v) acetic acid. RGII monomer and dimer standards were included for comparison.
Statistical analyses
For EL experiments, percentage electrolyte leakage values from two or three biological replicate experiments were arcsine‐transformed. A linear mixed effects model (Kuznetsova et al., 2016) was computed using R software (R Core Team, 2016), with genotype and any treatment (i.e. BA supplementation) specified as fixed terms, and experiment specified as a random effect. For the BA supplementation experiments, results were analysed by a two‐way ANOVA at each temperature point with an interaction term specified between genotype and BA. For other EL experiments, a one‐way ANOVA was carried out to determine the effect of genotype on the level of EL. Significant differences in leakage between genotypes and/or treatments was assessed using a least‐squares means comparison (Lenth, 2016) at each temperature datapoint. A one‐way ANOVA and least‐squares means comparison was also carried out to assess the significance of cell‐wall fucose levels in different genotypes, with experiment specified as a random effect.
Results
Identification of sensitive‐to‐freezing8 as an allele of mur1
A screen for ethyl methanesulphonate (EMS)‐induced Arabidopsis mutants that were freezing‐sensitive even after cold acclimation (CA) was carried out previously, identifying sensitive‐to‐freezing mutations 1‐7 (sfr1‐7) (Warren et al., 1996). sfr8 and sfr9 were subsequently isolated (Thorlby et al., 1999). 1 wk after a 24‐h freezing treatment at −8.5°C, cold‐acclimated wild‐type (WT) plants showed signs of recovery and the majority of leaf tissue was green whilst sfr8 mutant plants exhibited almost complete chlorosis and failed to regrow subsequently (Fig. 1a). The sfr8 mutation had no obvious effect on the expression of the CBF genes or the two CBF‐controlled COR genes KIN2 and GOLS3 (Fig. S1), indicating that SFR8 was unlikely to control freezing tolerance (FT) by acting upstream of the CBFs. SFR8 was previously mapped to chromosome 3 (Thorlby et al., 1999) between markers CDC2a (69 cM) and BGL1 (75.4 cM); equating to the interval between the genes At3g48750 and At3g57270 (Fig. 1b). We took an Illumina whole‐genome sequencing approach to identifying SNPs within this interval and we mapped these to the TAIR10 Arabidopsis reference genome (Lamesch et al., 2012). SNPs appearing in <70% of reads were disregarded. After quality control, four SNPs were identified in the interval as having the potential to be the sfr8 mutation (Table S1). Sanger sequencing of the regions containing these putative SNPS confirmed all four of them to be present in sfr8 genomic DNA and homozygous. One of these (Chr3:18684521) did not fall within an annotated gene and so was not pursued further; a second SNP, at Chr3:20966136, fell within an intron and was considered unlikely to be the cause of the sfr8 phenotype (Table S1). The remaining two SNPs were in two genes: At3g50910 and At3g51160. We obtained homozygous insertional mutants for these genes from NASC. Two homozygous insertions into At3g50910 were confirmed as exhibiting reduced transcript levels: SALK_074693C and SALK_132810C (Fig. S2). These were tested for FT but showed no difference to WT plants, indicating that the sfr8 mutation was not in At3g50910 (Fig. S2).
Figure 1.
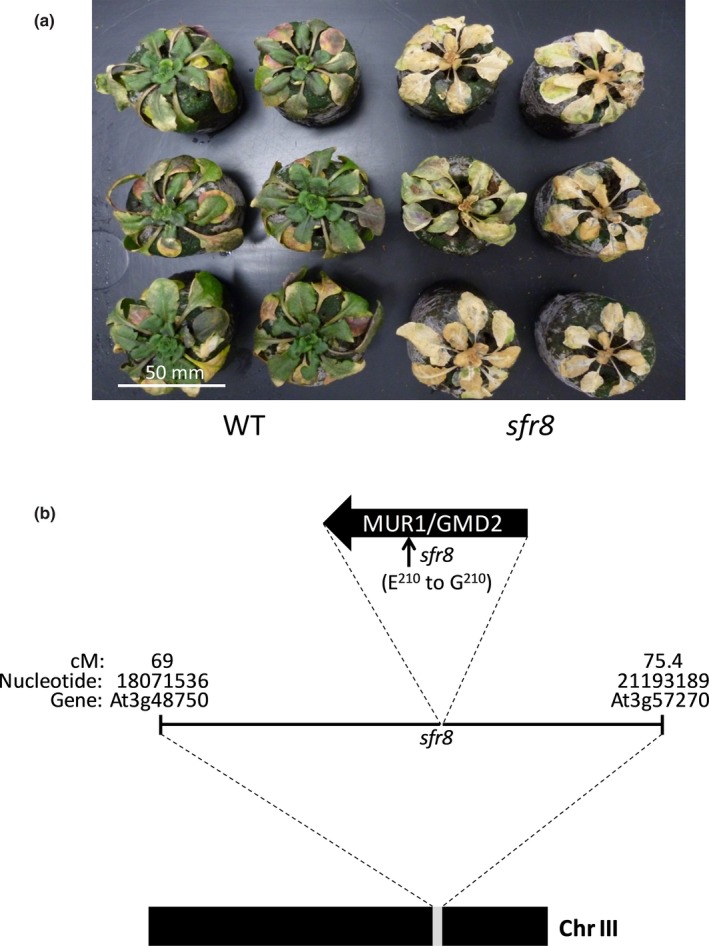
Fine mapping of the sensitive‐to‐freezing8 (sfr8) mutation. (a) Cold‐acclimated Col‐0 wild‐type (WT) Arabidopsis thaliana and sfr8 mutant plants 1 wk after a 24‐h freezing treatment at −8.5°C. Plants were grown for 5 wk before acclimating at 5°C for 2 wk. (b) Diagrammatic representation of the interval determined on chromosome III as containing the sfr8 mutation.
For At3g51160 (MUR1), we were unable to isolate viable homozygous mutants from any of three insertion lines (SALK_027379, SALK_027387 and SALK_027279); therefore, we obtained two EMS lines, mur1‐1 and mur1‐2 (Reiter et al., 1993) (Fig. S3). mur1‐1 and mur1‐2, like sfr8, were more freezing‐sensitive than WT after CA (Fig. 2). We also observed that all three mutants displayed a similar rounded leaf shape when compared to Col‐0 WT (Fig. 2). The sfr8, mur1‐1 and mur1‐2 mutations all result in amino acid substitutions to the MUR1 protein; sfr8 is identical to the previously described mur1‐4 (Fig. S3; Bonin et al., 1997). These observations strongly indicated that sfr8 is a mutant in the MUR1 gene and, therefore, MUR1 confers FT.
Figure 2.

sfr8 and mur1 mutants are both freezing‐sensitive. Cold‐acclimated Col‐0 wild‐type (WT) Arabidopsis thaliana and sfr8 mutant plants alongside mur1‐1 and mur1‐2 mutants 1 wk after a 24‐h freezing treatment at −8.5°C (upper two rows) or without freezing (lower two rows). Plants were grown for 5 wk before acclimating at 5°C for 2 wk.
MUR1 was originally identified in a forward genetic screen for cell wall (CW) mutants, in which mutants were selected on the basis of altered CW polysaccharide composition, with mur1 exhibiting vastly reduced levels of shoot CW fucose (Reiter et al., 1993). This is because MUR1 encodes an isoform of GDP‐d‐mannose‐4,6‐dehydratase (GMD2), the enzyme catalysing the first step in the de novo synthesis of activated fucose, GDP‐l‐fucose (Bonin et al., 1997). Using a thin‐layer chromatography assay developed by Bonin et al. (1997), we showed that, as expected, extracts from sfr8 mutant plants were unable to convert GDP‐d‐mannose substrate into GDP‐l‐fucose, unlike WT plants, indicating that the mutation in MUR1 found in sfr8 results in the production of non‐functional GDP‐d‐mannose‐4,6‐dehydratase (Fig. S4). Two other sfr mutants, including sfr4, which is deficient in sucrose accumulation (Uemura et al., 2003), showed WT levels of conversion (Fig. S4). As a result of reduced fucose synthesis mur1 mutants show reduced incorporation of fucose into CW polysaccharides (Reiter et al., 1993) and glycoproteins (Rayon et al., 1999). We found CW fucose incorporation in sfr8 mutants was reduced to levels similar to those seen in mur1‐1 (Fig. 3). A one‐way ANOVA followed by a least‐squares means comparison showed that fucose levels were significantly decreased compared to WT plants (P < 0.01).
Figure 3.
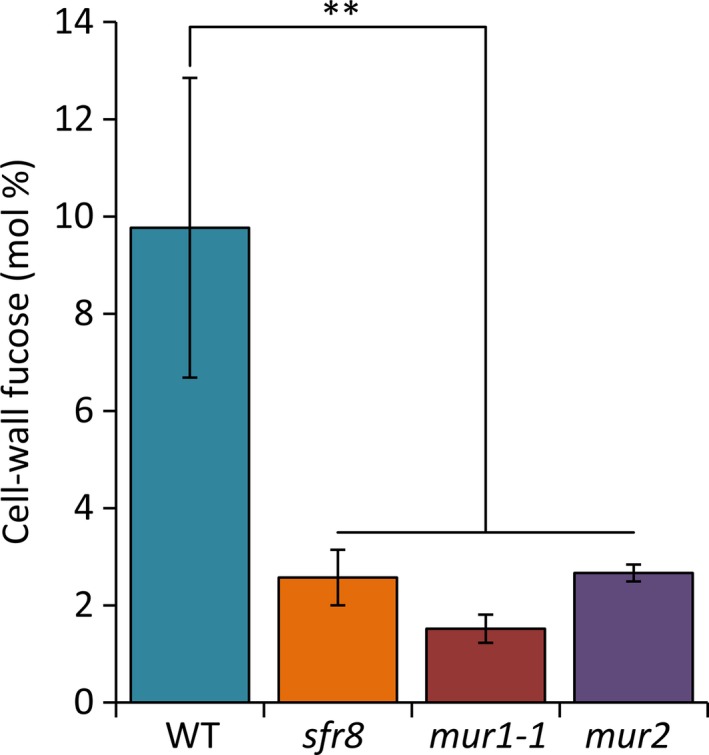
Cell‐wall fucose levels are reduced in sfr8, mur1‐1 and mur2 mutants. Col‐0 wild‐type (WT) Arabidopsis thaliana, sfr8, mur1‐1 and mur2 plants were grown on MS agar for 14 d after which samples of alcohol insoluble residue (AIR) were prepared and cell‐wall fucose content (mol%) analysed by GC‐MS. Data presented are the mean values from two independent biological replicates and were analysed using a least‐squares means comparison (**, P < 0.01). Error bars represent ± 1 SE.
To confirm finally that SFR8 and MUR1 were allelic, we created complemented lines by transforming sfr8 mutant plants with a vector containing the MUR1 coding sequence under the control of the 35S CaMV promoter. We identified 3 independent complemented lines (lines 1, 8 and 14) with FT restored to WT levels (Fig. 4, Fig. S5a). Line 14 was selected for further study (referred to hereafter as sfr8‐C). We confirmed that the low levels of CW fucose observed in sfr8 mutants were restored to WT levels in sfr8‐C plants (Fig. S6). RGII dimerization, which is dependent on fucosylation of RGII domains, was also restored in sfr8‐C (Fig. 4b). Levels of FT after CA were assessed in the mutant and line sfr8‐C using a quantitative electrolyte leakage (EL) assay to measure the degree of cellular damage after freezing. A one‐way ANOVA showed a significant effect of genotype on EL for all three temperatures tested over three biological replicate experiments (***, P < 0.001; *, P < 0.05; Fig. 4a). sfr8 mutants showed greater leakage than WT plants at the three freezing temperatures tested, consistent with greater sensitivity to freezing. However, the complemented line, sfr8‐C, showed significantly lower levels of leakage than sfr8 mutants, similar to those of WT plants, indicating that the MUR1 gene could complement the freezing‐sensitive phenotype of sfr8. Consistent with these quantitative measures of damage, the WT and complemented line showed less visible damage after freezing than did the sfr8 mutant (Fig. S5b). These data allowed us to confirm SFR8 as MUR1 and to show that MUR1 plays a role in FT.
Figure 4.
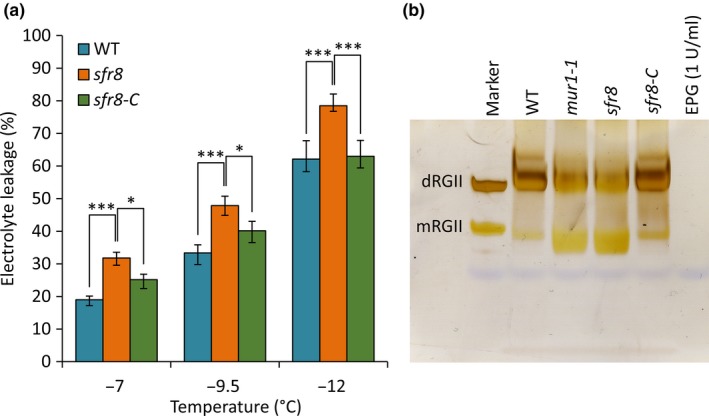
sfr8 can be complemented by MUR1. (a) Electrolyte leakage values from Col‐0 wild‐type (WT) Arabidopsis thaliana, sfr8 and sfr8‐C (complemented line). Plants were grown for 5 wk before acclimating at 5°C for 2 wk. Values represent percentage loss of electrolytes from leaf discs when exposed to temperatures of −7, −9.5 and −12°C. Each data point represents the average of three separate biological replicate experiments. Each experiment used six replicate tubes per genotype per temperature, with three leaf discs per tube. Arcsine‐transformed percentage leakage data were analysed by a least‐squares means comparison at each temperature point (*, P < 0.05; ***, P < 0.001). Error bars represent ± 1 SE of arcsine‐transformed data. (b) The proportion of RGII in the dimerized form is greater in WT Arabidopsis thaliana and sfr8‐C plants than it is in mur1‐1 or sfr8 mutants, which show higher proportions of the monomer form. Col‐0 wild‐type (WT), mur1‐1, sfr8 and sfr8‐C plants were grown at 20°C for 5 wk. Samples of alcohol insoluble residue (AIR) were produced from leaves of plants and digested with endopolygalacturonase (EPG), and products analysed by polyacrylamide gel electrophoresis. The first lane on the gel shows RGII monomer (mRGII) and dimer (dRGII) standards (0.8 µg of each were loaded). mRGII and dRGII products were stained with silver nitrate.
A fucosylation event is necessary for full freezing tolerance
Having demonstrated genetic linkage between the MUR1/SFR8 gene, CW fucose content, RGII dimerization and FT we used an EL assay to test for the ability of supplementary fucose to restore FT in mur1 or sfr8 mutants. FT was restored in mur1‐1 and sfr8 mutants that had been sprayed with supplementary fucose (Fig. S7), confirming that low levels of cellular fucose are linked to freezing sensitivity. Application of fucose to WT plants did not alter EL, indicating that at the levels applied, fucose supplementation had no significant effect per se on WT tolerance (for instance, as a compatible solute).
Our data indicated that either the level of free fucose levels or fucosylation of other molecules was important for maintaining WT levels of FT. To test whether fucosylation contributes to FT we assessed the effect of an inhibitor of fucosylation, 2‐fluoro‐2‐l‐fucose (2f‐fucose) (Dumont et al., 2015; Villalobos et al., 2015) on WT plants. Non‐acclimated seedlings grown on agar supplemented with the inhibitor were subjected to EL analysis to assess their FT. EL analysis of non‐acclimated plants is, from necessity performed at higher temperatures than those used for acclimated plants, as they are less freezing tolerant (Gilmour et al., 2000). Similarly, testing seedlings rather than mature rosette plants necessitates the use of less severe temperatures (Xin & Browse, 1998). A one‐way ANOVA showed a significant effect of 2f‐fucose treatment at all temperatures tested (***, P < 0.001, *, P < 0.05, Fig. 5). The two higher concentrations of 10 and 25 µM were effective in increasing the damage to WT Arabidopsis frozen at −3 and −5°C, whilst all three concentrations were effective at −7°C (Fig. 5). These data indicate that fucosylation contributes to FT but they do not allow identification of the specific fucosylation events that are required.
Figure 5.
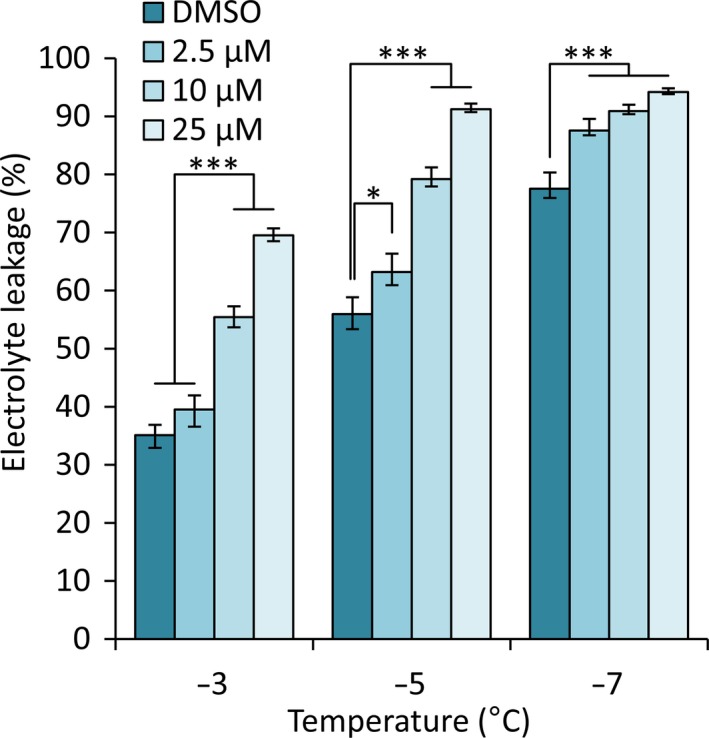
Freezing tolerance is reduced by 2f‐fucose, a fucose synthesis inhibitor, in a concentration‐dependent manner. Electrolyte leakage values from Col‐0 wild‐type (WT) Arabidopsis thaliana plants treated with DMSO and three different concentrations of 2f‐fucose. Plants were grown on MS agar containing DMSO, 2.5 µM, 10 µM or 25 µM of 2f‐fucose for 2 wk. Values represent percentage loss of electrolytes from seedlings when exposed to temperatures of −3, −5 and −7°C. Each data point represents the average of three separate biological replicate experiments. Each experiment used six replicate tubes per treatment per temperature, with c. 10 mg seedlings per tube. Arcsine‐transformed percentage leakage data were analysed by a least‐squares means comparison at each temperature point (*, P < 0.05; ***, P < 0.001). Error bars represent ± 1 SE of arcsine‐transformed data.
A number of CW components undergo fucosylation in WT plants, including arabinogalactan proteins (AGPs), xyloglucans (XyG) and pectins (Nakamura et al., 2001; Perrin et al., 2003; Wu et al., 2010). In order to ascertain whether the reduced FT we observed in sfr8 was linked to a general lack of fucosylation in the CW or to the fucosylation of one particular component, we investigated the effect of the mur2 mutation on FT. mur2 mutants have a mutation in the FUT1 gene, which encodes a XyG‐specific fucosyltransferase that fucosylates the hemicellulosic CW component XyG (Vanzin et al., 2002). Whilst mur2 mutants showed reduced fucose incorporation into the CW fraction (Fig. 3) they exhibited minimal differences in freezing sensitivity (Fig. S5b, S8). This indicates that it is unlikely that fucosylation of XyGs plays a role in plant FT, but does not eliminate the possibility that either or both AGP and pectin fucosylation might do.
Borate bridging of the pectic domain rhamnogalacturonan II is associated with freezing tolerance
Plant CW pectins comprise three main domains: homogalacturonans (HG) and rhamnogalacturonans I and II (RGI and RGII; Harholt et al., 2010). Arabidopsis RGII domains have six side chains (side chains A to F; (Ndeh et al., 2017)), two of which are usually fucosylated in WT plants (O'Neill et al., 2001). mur1 mutants lack fucosylation of side chain A and exhibit reduced growth and CW mechanical strength as a result (Reiter et al., 1993; O’Neill et al., 2001). Fucosylation of side chain A is necessary if RGII domains are to dimerize efficiently and stably via a borate‐diester crosslink formed between the apiose residues of two RGII A chains (Kobayashi et al., 1996). Borate bridging of RGII domains mainly occurs before or during secretion of the pectic polysaccharides into the wall (Chormova et al., 2014), probably in association with plasma‐membrane glycolipids (Voxeur & Fry, 2014). In mur1 mutants l‐galactose substitutes for l‐fucose in side chain A (Reuhs et al., 2004) but the side chain is truncated (Pabst et al., 2013; Sechet et al., 2018) and dimerization is consequently reduced (O'Neill et al., 2001; Voxeur et al., 2017; Sechet et al., 2018).
Restoration of mur1 phenotypes by boron (B) supplementation has been recognised as confirmation that a phenotype is associated specifically with reduced borate bridging of RGII due to the lack of fucose in side chain A, rather than attributable to a reduction in other fucosylation events (O'Neill et al., 2001; Ryden et al., 2003; Voxeur et al., 2017; Feng et al., 2018; Sechet et al., 2018). RGII crosslinking, growth and the tensile strength breaking force of mur1 mutant inflorescences were all restored to levels approaching those of WT plants in mur1 mutants by supplementation with additional boric acid (BA) (O'Neill et al., 2001; Ryden et al., 2003; Sechet et al., 2018). To test whether FT could be restored by BA, we grew WT, mur1‐1 and sfr8 plants with or without BA supplementation and cold‐acclimated them. The visible phenotype associated with mutation of the MUR1 gene was restored in mutant plants by BA supplementation (Fig. S9); in the absence of supplementation mutants exhibited the reduced petiole length and rounded, non‐serrated leaves typical of mur1 (O'Neill et al., 2001; Goncalves et al., 2017). Whilst non‐supplemented sfr8 and mur1‐1 mutants showed the expected freezing‐sensitive phenotype and greater levels of EL than WT, sfr8 and mur1‐1 mutants supplemented with BA both showed reduced levels of damage similar to WT plants. A two‐way ANOVA showed a significant effect of genotype on EL (P < 0.001) and a significant interaction of genotype with BA (P < 0.05) for all temperatures tested, highlighting the fact that BA reduced the EL of both mutants, but had no effect on WT plants. A least‐squares means comparison showed that sfr8 and mur1‐1 leakage was significantly greater than leakage from WT and BA‐supplemented WT plants or BA‐supplemented mutant plants (P < 0.001) (Fig. 6a,b). These data suggested that RGII dimerization is required for full WT levels of FT.
Figure 6.
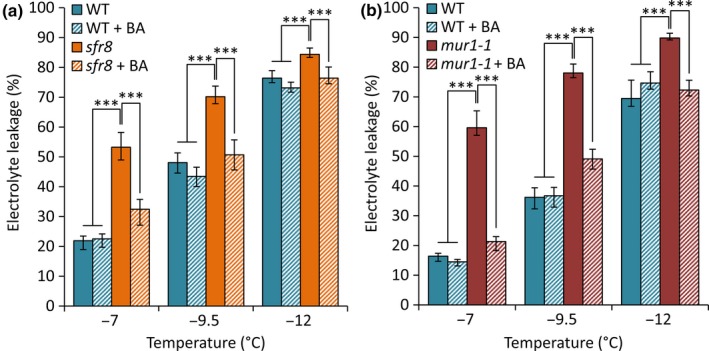
Addition of boric acid restores freezing tolerance in sfr8 and mur1‐1 mutant plants. Electrolyte leakage values from Col‐0 wild‐type (WT) Arabidopsis thaliana, (a) sfr8 and (b) mur1‐1 plants with and without boric acid (BA) supplementation. Plants were grown for 5 wk before acclimating at 5°C for 2 wk. Values represent percentage loss of electrolytes from leaf discs when exposed to temperatures of −7, −9.5 and −12°C. Each data point represents the average of three separate biological replicate experiments. Each experiment used six replicate tubes per genotype/treatment per temperature, with three leaf discs per tube. Arcsine‐transformed percentage leakage data were analysed by a least‐squares means comparison at each temperature point (***, P < 0.001). Error bars represent ± 1 SE of arcsine‐transformed data.
Cell‐wall fucosylation, and most likely RGII crosslinking, contribute to basal freezing tolerance
Our data raised the possibility that MUR1‐mediated RGII dimerization might be part of the WT CA process that leads to improved FT. Using the Genevestigator tool (genevestigator.com/gv/) (Zimmermann et al., 2004) we found that MUR1 was not upregulated by overexpression of the CA‐specific transcription factors CBF2 (Vogel et al., 2005) or CBF3 (Chan et al., 2012) (Table S2). Neither was MUR1 misregulated in the cbfs triple mutant (Jia et al., 2016). Consistent with this, a search for transcription factor binding sites within the MUR1 promoter using the AGRIS promoter database (agris‐knowledgebase.org/cite.html) (Yilmaz et al., 2011) revealed no CBF binding motifs. This indicated that MUR1 is not a target of the CBF transcription factors but did not eliminate the possibility that it could be upregulated in response to CA independently of the CBFs. However, we found that MUR1 was not induced by exposure to 5°C for 1, 3, 6 or 12 h or 1, 4 or 7 d (Fig. S10a), unlike the cold‐inducible gene KIN2. This was consistent with published transcriptomic data that shows no significant increase in MUR1 transcript levels in plants transferred to CA conditions (Fig. S10b; Table S3; (Calixto et al., 2018)) and with reports that fucose levels do not increase during CA in Arabidopsis (Cook et al., 2004; Takahashi et al., 2019) or Pisum sativum (Baldwin et al., 2014). Therefore, we hypothesised that MUR1 is unlikely to contribute to FT by increasing fucose levels during CA and is more likely to influence basal FT. We confirmed that sfr8 was more sensitive‐to‐freezing at −5 or −7°C than WT even when not acclimated (P < 0.001) and that its tolerance of these temperatures did improve significantly after CA (P < 0.001) but failed to reach WT levels (Fig. 7). Consistent with our findings, non‐acclimated sfr8 were also more susceptible to freezing than WT at less severe freezing temperatures (−2 and −4°C) (Fig. S11).
Figure 7.
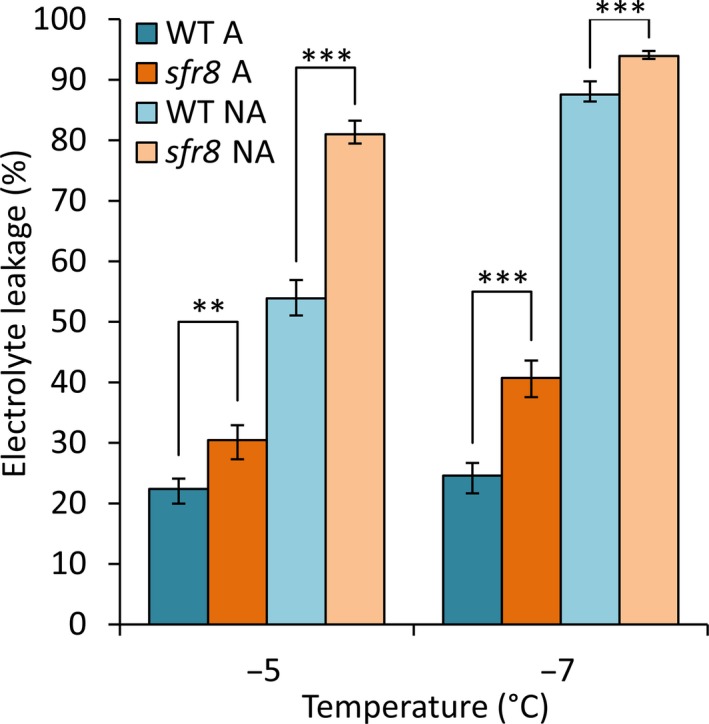
sfr8 mutants are more susceptible to freezing than WT with and without cold acclimation. Electrolyte leakage values from Col‐0 wild‐type (WT) Arabidopsis thaliana and sfr8. Non‐acclimated (NA) plants were grown for 5 wk, cold acclimated (A) plants were grown for 5 wk and then acclimated at 5°C for 2 wk. Values represent percentage loss of electrolytes from leaf discs when exposed to temperatures of −5 and −7°C. Each data point represents the average of two separate biological replicate experiments. Each experiment used six replicate tubes per genotype per temperature, with three leaf discs per tube. Arcsine‐transformed percentage leakage data were analysed by a least‐squares means comparison at each temperature point (**, P < 0.01; ***, P < 0.001). Error bars represent ± 1 SE of arcsine‐transformed data.
Our data indicated that fucose‐dependent borate‐dimerization of RGII plays a role in FT irrespective of CA. To add further support to this conclusion we tested other mutants defective in borate dimerization of RGII for damage following freezing without prior CA. BOR1 and BOR2 are plasma membrane B transporters and mutants in either BOR1 or BOR2 require supplementary BA to maintain normal wild‐type growth and CW structure (Miwa et al., 2013). After growth under B‐limiting conditions bor1‐3 and a double mutant bor2‐1bor1‐3 both showed reduced RGII dimerization, as evidenced by a higher monomer : dimer ratio (Fig. 8b). Levels of EL were significantly higher in freeze‐thawed leaves of these mutants (P < 0.001) than in leaves of WT plants subjected to the same freezing temperatures (Fig. 8a). Together, these data strongly indicate that the lack of fucose in sfr8 plants results in freezing sensitivity specifically as a consequence of reduced RGII dimerization.
Figure 8.
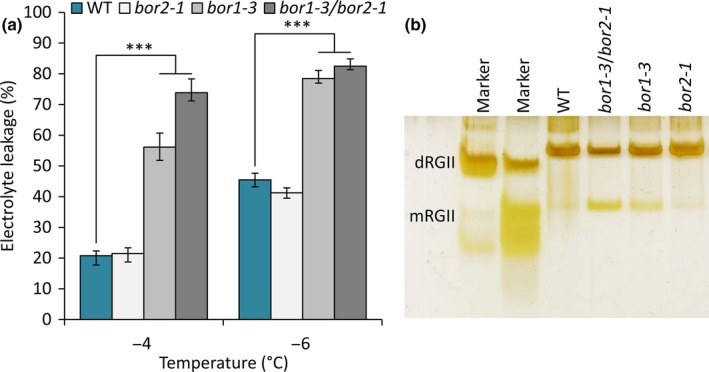
Boron transporter mutants are susceptible to freezing. (a) Electrolyte leakage values from Col‐0 wild‐type (WT) Arabidopsis thaliana, bor2‐1, bor1‐3 and bor1‐3bor2‐1 plants. Plants were grown for 5 wk under short day conditions and boron‐limiting conditions and not acclimated. Values represent percentage loss of electrolytes from leaves when exposed to temperatures of −4 and −6°C. Because bor mutants are smaller than wild‐type plants, experiments were conducted using size‐matched leaves and repeated using age‐matched leaves and the same results were observed. Each data point represents the average of three separate biological replicate experiments. Each experiment used six replicate tubes per genotype per temperature, with three leaves per tube. Arcsine‐transformed percentage leakage data were analysed by a least‐squares means comparison at each temperature point (***, P < 0.001). Error bars represent ± 1 SE calculated from arcsine‐transformed data. (b) The proportion of RGII in the dimerized form is greater in WT and bor2‐1 plants than it is in the bor1‐3 or bor1‐3bor2‐1 mutants, which show higher proportions of the monomerised form. Samples of alcohol insoluble residue (AIR) were produced from leaves of plants and digested with endopolygalacturonase (EPG), and products analysed by polyacrylamide gel electrophoresis. The first lanes on the gel show RGII monomer (mRGII) and dimer (dRGII). mRGII and dRGII products were stained with silver nitrate.
Discussion
Research into the genetic control of plant freezing tolerance (FT) has, to date, focussed largely on the damage to cellular membranes and the cytoplasmic dehydration that occur during freezing stress. Here we describe the identification of a novel FT gene, SFR8, through fine mapping and genome sequencing of a sensitive‐to‐freezing EMS mutant and we demonstrate that SFR8 acts through modifying the composition and function of the plant primary cell wall (CW).
Other studies have implicated the CW in regulating FT. For example, reduced biosynthesis of lignin, a component of the secondary CW, results in greater FT (Ji et al., 2015). SFR8 (At3g51160/MUR1) was originally identified in a screen for mutants with altered CW sugar composition (Reiter et al., 1993). Levels of fucose in the shoot of mur1 mutants were barely detectable and mur1‐1 mutants exhibited reduced mechanical strength and growth (Reiter et al., 1993; Zablackis et al., 1996). MUR1 was subsequently identified as GMD2, an isoform of the first enzyme in the biosynthetic pathway for GDP‐l‐fucose (Bonin et al., 1997). We show that CW‐fucose incorporation is reduced in sfr8 mutants similarly to mur1‐1 (Fig. 3). We show that SFR8 is allelic with MUR1; mur1‐1, mur1‐2 and sfr8 representing three different SNPs in At3g51160 that each cause a different amino acid substitution in the MUR1 protein (Fig. 2, Figs S3, S5b). mur1 and sfr8 plants exhibited a similar physical appearance to one another, sfr8 exhibiting the cup‐shaped cotyledons and rounded leaves that have been previously reported for mur1 mutants and associated with the effects of reduced fucosylation (O'Neill et al., 2001; Goncalves et al., 2017) (Fig. 2); however, they were not severely dwarfed as reported previously (O'Neill et al., 2001). We consider this likely to be due to the fact we grew our plants under short day conditions (typical for CA experiments), and our growth regime may have been less B‐limiting than those used previously. FT was impaired in fucose‐deficient mur1/sfr8 mutants but this could be restored by spraying with fucose (Fig. S7). By using 2f‐fucose, a competitive inhibitor of a broad spectrum of cellular fucosylation events (Dumont et al., 2015; Villalobos et al., 2015) we were able to mimic the effect of the sfr8 mutation, observing increased tissue damage after freezing in WT seedlings (Fig. 5). This suggests that FT is dependent on a fucosylation event rather than on fucose itself.
Both the protein and polysaccharide components of the CW are subject to fucosylation in WT plants, with N‐linked glycoproteins, arabinogalactan proteins (AGPs), the hemicellulose xyloglucan (XyG) and the pectic domains RGI and RGII all being targets. Previous work has shown that in mur1 mutants l‐fucose (l‐fuc) and 2‐O‐methyl l‐fucose are replaced by l‐galactose (l‐gal) and 2‐O‐methyl l‐galactose respectively in CW polysaccharides but that this substitution has little or no effect on the structure and function of XyGs (Zablackis et al., 1996). This suggested that lack of XyG fucosylation is unlikely to be the cause of freezing sensitivity in sfr8. This view is supported by our observation that the mur2 mutant, which lacks functional XyG fucosyl transferase, FUT1 (Vanzin et al., 2002), showed little sensitivity to freezing despite the significant reduction in its CW fucose level (Figs S5b, S8).
In contrast with XyG, the structure of RGII is substantially altered by substitution of l‐fuc with l‐gal (Reuhs et al., 2004). Pectic RGII in wild‐type plants is predominantly crosslinked (dimerized) via borate‐diester linkages between apiose sugars in side chain A of RGII monomers (Kobayashi et al., 1996); dimerized RGII is associated with elasticity and mechanical strength in the CW (Ryden et al., 2003). RGII domains are highly negatively charged (thus mutually repulsive) and the formation of crosslinks between them via borate bridges is strongly enhanced by cationic ‘chaperones’ e.g. extensins (Chormova & Fry, 2016). Substitution of l‐fuc with l‐gal in RGII results in a lower proportion of full‐length side chains, which reduces the opportunities for dimerization (O'Neill et al., 2001; Pabst et al., 2013). Whilst WT Arabidopsis RGII is c. 95% dimerized, only c. 50% of RGII was reported to be dimerized in mur1 mutants (O'Neill et al., 2001). Consistent with this, B deficiency causes similar CW abnormalities to those seen in mur1 plants (Fleischer et al., 1999). Our RGII analysis shows reduced RGII dimerization in sfr8 and mur1 plants (Fig. 4b) similar to the qualitative differences in RGII dimer : monomer ratio observed in a recent study (Voxeur et al., 2017).
We, therefore, hypothesised that impaired FT in sfr8 could be attributed to lack of RGII dimerization. Previous work has shown that RGII crosslinking can be restored in mur1 mutants by the application of supplementary boric acid (BA) (O'Neill et al., 2001; Sechet et al., 2018), a treatment that also results in restoration of normal CW strength, cell integrity and growth (O'Neill et al., 2001; Ryden et al., 2003; Feng et al., 2018). Fucose generated by MUR1 is necessary for proper establishment of leaf boundary domains by the CUP‐shaped cotyledon transcription factor CUC2 (Goncalves et al., 2017). CUC2‐overexpressing lines show long leaves with serrated edges whereas the mur1 mutation suppresses this and renders leaves more cup‐shaped. The effect of mur1 on leaf shape is known not to be a consequence of reduced XG fucosylation and it has been suggested it may be the result of impaired RGII crosslinking (Goncalves et al., 2018). The reversion of leaf shape we observe after BA supplementation would support this view (Fig. S9). Having established that our BA‐watering regime restored the fucose‐dependent growth defect in mur1 mutants we compared FT in sfr8 and mur1‐1 with and without BA supplementation (Fig. 6). BA supplementation reduced the sensitivity of sfr8 and mur1‐1 to freeze damage, indicating that fucosylation‐dependent borate‐dimerization of RGII is required for full FT.
RGII crosslinking is the main cellular function that has been described for B in plants (O'Neill et al., 2004; Funakawa & Miwa, 2015) with RGII described as possibly the only site of B binding in the CW. However, potential roles for B in CW‐membrane attachment and membrane structure have been proposed (Funakawa & Miwa, 2015) and membrane‐associated B‐interacting proteins isolated from plants (Wimmer et al., 2009). It is conceivable that our BA treatment could have affected alternative B‐dependent aspects of cell structure and integrity and we cannot exclude the possibility that B may play other roles in FT that involve such interactions. However, whereas BA supplementation improved FT in sfr8 plants, we observed no effect of BA supplementation on the FT of WT plants (Fig. 6). This indicates that the role of B in FT we observe intersects with a fucosylation event. N‐glycosylated proteins and AGPs lack fucosylation in mur1 mutants (Rayon et al., 1999; Tryfona et al., 2012; Zhang et al., 2018) so it remains a possibility that lack of CW protein fucosylation is responsible for the reduced FT of mur1/sfr8. However, no published evidence suggests that any phenotype caused by a defect in protein fucosylation can be restored by B supplementation and it is difficult to envisage a mechanism whereby this could occur. In fact the short‐root phenotype of mur1, attributed to reduced fucosylation of AGPs but not RGII, was not restored by B supplementation (van Hengel & Roberts, 2002). These published data and the effect of both fucosylation and B on FT together support our conclusion that the restoration of FT in sfr8 by supplementary B is most logically explained by FT being dependent upon dimerization of fucosylated RGII.
In further support of this conclusion, we observed that the B transporter mutants bor1‐3 and bor2‐1bor1‐3, which show reduced RGII‐borate dimerization whilst suffering no deficit in fucose synthesis, were freezing‐sensitive (Fig. 8). This strongly suggests that crosslinking of RGII monomers via borate‐diester linkages is a specific requirement for FT and that the freezing‐sensitivity of mur1/sfr8 is not a consequence of any other impaired fucosylation event. BOR1 and BOR2 encode plasma membrane‐localised efflux‐type B transporters expressed in root cells (Takano et al., 2002; Miwa et al., 2013) and under B‐limiting conditions mutants show reduced B uptake (Miwa et al., 2013). Whilst previous work showed that bor2‐1 mutants had reduced RGII crosslinking in root cells whereas bor1‐3 did not (Miwa et al., 2013), we saw the opposite effect in rosette leaf tissue, bor1‐3 having a noticeable effect on RGII monomer : dimer ratio in plants grown on low levels of B (Fig. S9b). A recent study showed that regenerative xylem formation occurred in response to reduced RGII dimerization in stems of bor1‐3, similar to mur1 mutants (Voxeur et al., 2017), therefore, it appears likely that BOR1 makes the greater contribution to RGII dimerization in aerial tissues. In addition to self‐dimerization, RGII has been reported as forming B‐containing complexes with plasma membrane glycosylinositol phosphorylceramides (GIPCs) in Rosa cell cultures and binding to AGP‐extensin in symbiotic infection threads via B‐dependent linkages (Reguera et al., 2010; Voxeur & Fry, 2014). It is possible that fucosylated RGII contributes to FT via interactions like these as well as through RGII dimerization.
It is interesting to note recent work demonstrating a role for RGII dimerization in maintaining CW integrity in plants challenged with salinity stress (Feng et al., 2018; Sechet et al., 2018), suggesting a wider role for RGII crosslinking in the response to abiotic stress. RGII dimerization via borate‐diester linkages has a number of consequences in plant CWs: increasing wall thickness (Ishii et al., 2001), elasticity and mechanical strength (Ryden et al., 2003) and decreasing CW pore size (Fleischer et al., 1999). Accordingly, restricting B supply rapidly reduces the elasticity of CWs (Findeklee & Goldbach, 1996) and renders tissues brittle (Blevins & Lukaszewski, 1998). Our data suggest that one or more of these properties may be important in determining plant FT. During CA, CW strength and thickness increase whilst pore size decreases (Rajashekar & Lafta, 1996; Kubacka‐Zebalska & Kacperska, 1999; Stefanowska et al., 1999; Arias et al., 2015), further supporting the suggestion that these CW properties are important for FT.
As the cell’s barrier against the external environment, the CW is the site of extracellular ice nucleation (Wisniewski et al., 1997; Pearce, 2001). It has in the past been suggested that the qualitative nature of the CW may determine how easily, and where, ice can nucleate, affecting the level of freezing damage (Burke et al., 1976; McCully et al., 2004). Smaller pore sizes have been shown to restrict the propagation of ice within plants (Ashworth & Abeles, 1984) and CW rigidity has been associated with reduced levels of freeze‐induced dehydration (Rajashekar & Burke, 1996). Increased CW rigidity may also help the cell deal with the mechanical strain imposed by extracellular ice formation (Smallwood & Bowles, 2002). Pectin crosslinking (albeit via calcium bridges) was proposed to play a role in preventing ice nucleation back in 1991 when it was suggested it might exert this effect through modifying the CW’s permeability to water and through altering pore size (Wisniewski et al., 1991). It is possible, therefore, that crosslinking of pectic RGII promotes FT by reducing the opportunities for extracellular ice nucleation and propagation.
A growing number of observations show that the CW undergoes extensive remodelling in response to abiotic stresses (Tenhaken, 2014; Le Gall et al., 2015), suggesting that it may play a role in tolerance to these conditions and recent work indicates the extent of CW modification that occurs during CA (Willick et al., 2018; Takahashi et al., 2019). Pectin methylesterification, a major determinant of calcium crosslinking of pectic homogalacturonan (HG) domains, alters during CA (Solecka et al., 2008; Baldwin et al., 2014) and one recent study indicates that inhibition of pectin methylesterase activity compromises FT (Chen et al., 2018). This is consistent with a positive role for pectin crosslinking in FT. Our results indicate that RGII crosslinking is also important for plant FT, although our data suggest that it plays a role in basal FT rather than the CA response (Fig. 7, Figs S10, S11).
In conclusion, we have demonstrated that fucosylated RGII pectin in the primary CW plays a role in determining plant FT, most likely through B‐dependent dimerization. Future work will determine whether CW pectin crosslinking brings about FT by altering wall elasticity, strength and/or ice nucleation and propagation and whether these effects are specific to RGII or common to all CW components that influence these parameters. Understanding of the mechanistic basis of this phenomenon will allow the identification of further targets for crop improvement.
Author contributions
HK, MRK, SCF and PEP designed the experiments, analysed the data and wrote the paper; PEP, OK, MS, SJS, GT, IC, RAB, MD, NR and DS conducted the experiments.
Supporting information
Fig. S1 Expression of CBF1‐3 and the CBF target genes KIN2 and GOLS3 are all expressed to normal wild‐type levels in sfr8.
Fig. S2 Two insertional mutants for candidate gene At3g50910 fail to show reduced freezing tolerance after cold acclimation.
Fig. S3 Nucleotide and amino acid sequence of MUR1 showing the SNPs and amino acid substitutions in the mutants mur1‐1, mur1‐2, mur1‐3 and sfr8.
Fig. S4 sfr8 fails to convert GDP‐mannose to GDP‐fucose.
Fig. S5 sfr8 can be complemented by the MUR1 coding sequence.
Fig. S6 Cell‐wall fucose content is restored to wild‐type levels in sfr8 mutants complemented with MUR1.
Fig. S7 Fucose supplementation restores the freezing‐sensitive phenotype of sfr8 and mur1‐1 but does not further improve freezing tolerance in wild‐type plants.
Fig. S8 mur2 mutants are not impaired in freezing tolerance.
Fig. S9 The boric acid watering regime restores the WT visible phenotype in mur1.
Fig. S10 MUR1 is not inducible by low temperature.
Fig. S11 sfr8 is more sensitive‐to‐freezing than WT even without cold acclimation.
Table S1 Candidate SNPs identified using Galaxy.
Table S2 MUR1 is not upregulated by CBF overexpression.
Table S3 MUR1 is not differentially expressed in response to cold acclimation.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
This work was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) through research grant (BB/J007331/1) and BBSRC Doctoral Training Partnership studentships awarded to PEP (award ref 1518540) and NR (BB/M011186/1). The RGII electrophoresis work in the Fry laboratory was supported by the Comisión Nacional de Investigación Científica y Tecnológia (Conicyt), Chile, through a research grant to DS and a BBSRC grant (BB/H000690/1) to SCF; RAB is grateful for a Commonwealth Scholarship (BDCS‐2016‐64) in support of her work. We would like to thank the Genome Analysis Centre (TGAC, Norwich, now The Earlham Centre) for providing the NGS data and the Nottingham Arabidopsis Seeds stock Centre (NASC) for supplying seeds of the mutants used. We are most grateful to Kyoko Miwa (Hokkaido University) for the gift of bor mutant seeds and advice on their growth. We thank Rebecca Manning (Durham) for technical assistance and Sarah Gratton (undergraduate student, Durham) for work optimising the EL method. We are grateful to Dr Wayne Dawson (Durham) for advice on the statistical analysis and we thank Prof. Chris Somerville (University of California, Berkeley) for many useful discussions. We also thank Prof John Brown and Dr Nikoleta Tzioutziou (James Hutton Institute, Scotland) for permission to use their transcriptomic data.
References
- Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P. 2008. The cold‐inducible CBF1 factor‐dependent signaling pathway modulates the accumulation of the growth‐repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20: 2117–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amid A, Lytovchenko A, Fernie AR, Warren G, Thorlby GJ. 2012. The sensitive to freezing3 mutation of Arabidopsis thaliana is a cold‐sensitive allele of homomeric acetyl‐CoA carboxylase that results in cold‐induced cuticle deficiencies. Journal of Experimental Botany 63: 5289–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias NS, Bucci SJ, Scholz FG, Goldstein G. 2015. Freezing avoidance by supercooling in Olea europaea cultivars: the role of apoplastic water, solute content and cell wall rigidity. Plant, Cell & Environment 38: 2061–2070. [DOI] [PubMed] [Google Scholar]
- Ashworth EN, Abeles FB. 1984. Freezing behavior of water in small pores and the possible role in the freezing of plant tissues. Plant Physiology 76: 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin L, Domon JM, Klimek JF, Fournet F, Sellier H, Gillet F, Pelloux J, Lejeune‐Henaut I, Carpita NC, Rayon C. 2014. Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 104: 37–47. [DOI] [PubMed] [Google Scholar]
- Baxter R. 2014. Plant acclimation and adaptation to cold environments In: Franklin KA, Wigge PA, eds. Temperature and plant development. Oxford, UK: Wiley Blackwell, 19–48. [Google Scholar]
- Blevins DG, Lukaszewski KM. 1998. Boron in plant structure and function. Annual Review of Plant Physiology and Plant Molecular Biology 49: 481–500. [DOI] [PubMed] [Google Scholar]
- Bonin CP, Potter I, Vanzin GF, Reiter WD. 1997. The MUR1 gene of Arabidopsis thaliana encodes an isoform of GDP‐d‐mannose‐4,6‐dehydratase, catalyzing the first step in the de novo synthesis of GDP‐l‐fucose. Proceedings of the National Academy of Sciences, USA 94: 2085–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredow M, Walker VK. 2017. Ice‐binding proteins in plants. Frontiers in Plant Science 8: 2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, Xin Z. 2001. Temperature sensing and cold acclimation. Current Opinion in Plant Biology 4: 241–246. [DOI] [PubMed] [Google Scholar]
- Burke MJ, Gusta LV, Quamme HA, Weiser CJ, Li PH. 1976. Freezing and injury in plants. Annual Review of Plant Physiology and Plant Molecular Biology 27: 507–528. [Google Scholar]
- Calixto CPG, Guo W, James AB, Tzioutziou NA, Entizne JC, Panter PE, Knight H, Nimmo H, Zhang R, Brown JWS. 2018. Rapid and dynamic alternative splicing impacts the Arabidopsis cold response transcriptome. Plant Cell 30: 1424–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Z, Bigelow PJ, Loescher W, Grumet R. 2012. Comparison of salt stress resistance genes in transgenic Arabidopsis thaliana indicates that extent of transcriptomic change may not predict secondary phenotypic or fitness effects. Plant Biotechnology Journal 10: 284–300. [DOI] [PubMed] [Google Scholar]
- Chen J, Chen X, Zhang Q, Zhang Y, Ou X, An L, Feng H, Zhao Z. 2018. A cold‐induced pectin methyl‐esterase inhibitor gene contributes negatively to freezing tolerance but positively to salt tolerance in Arabidopsis. Journal of Plant Physiology 222: 67–78. [DOI] [PubMed] [Google Scholar]
- Chormova D, Fry SC. 2016. Boron bridging of rhamnogalacturonan‐II is promoted in vitro by cationic chaperones, including polyhistidine and wall glycoproteins. New Phytologist 209: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chormova D, Messenger DJ, Fry SC. 2014. Rhamnogalacturonan‐II cross‐linking of plant pectins via boron bridges occurs during polysaccharide synthesis and/or secretion. Plant Signaling & Behaviour 9: e28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana . The Plant Journal 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Cook D, Fowler S, Fiehn O, Thomashow MF. 2004. A prominent role for the CBF cold response pathway in configuring the low‐temperature metabolome of Arabidopsis . Proceedings of the National Academy of Sciences, USA 101: 15243–15248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. 2005. Growth of the plant cell wall. Nature Reviews Molecular Cell Biology 6: 850–861. [DOI] [PubMed] [Google Scholar]
- Ding Y, Shi Y, Yang S. 2019. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytologist 222: 1690–1704. [DOI] [PubMed] [Google Scholar]
- Dumont M, Lehner A, Bardor M, Burel C, Vauzeilles B, Lerouxel O, Anderson CT, Mollet JC, Lerouge P. 2015. Inhibition of fucosylation of cell wall components by 2‐fluoro 2‐deoxy‐l‐fucose induces defects in root cell elongation. The Plant Journal 84: 1137–1151. [DOI] [PubMed] [Google Scholar]
- Feng W, Kita D, Peaucelle A, Cartwright HN, Doan V, Duan Q, Liu MC, Maman J, Steinhorst L, Schmitz‐Thom I et al 2018. The FERONIA receptor kinase maintains cell‐wall integrity during salt stress through Ca2+ signaling. Current Biology 28: 666–675 e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeklee P, Goldbach HE. 1996. Rapid effects of boron deficiency on cell wall elasticity modulus in Cucurbita pepo roots. Botanica Acta 109: 463–465. [Google Scholar]
- Fleischer A, O'Neill MA, Ehwald R. 1999. The pore size of non‐graminaceous plant cell walls is rapidly decreased by borate ester cross‐linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiology 121: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S, Thomashow MF. 2002. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14: 1675–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakawa H, Miwa K. 2015. Synthesis of borate cross‐linked rhamnogalacturonan II. Frontiers in Plant Science 6: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Fowler SG, Thomashow MF. 2004. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Molecular Biology 54: 767–781. [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF. 2000. Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiology 124: 1854–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves B, Maugarny‐Cales A, Adroher B, Cortizo M, Borrega N, Blein T, Hasson A, Gineau E, Mouille G, Laufs P et al 2017. GDP‐l‐fucose is required for boundary definition in plants. Journal of Experimental Botany 68: 5801–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves B, Sechet J, Arnaud N. 2018. Xyloglucans fucosylation defects do not alter plant boundary domain definition. Plant Signaling & Behavior 13: e1430545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon‐Kamm WJ, Steponkus PL. 1984. Lamellar‐to‐hexagonalII phase transitions in the plasma membrane of isolated protoplasts after freeze‐induced dehydration. Proceedings of the National Academy of Sciences, USA 81: 6373–6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusta LV, Wisniewski M. 2013. Understanding plant cold hardiness: an opinion. Physiologia Plantarum 147: 4–14. [DOI] [PubMed] [Google Scholar]
- Hannah MA, Heyer AG, Hincha DK. 2005. A global survey of gene regulation during cold acclimation in Arabidopsis thaliana . PLoS Genetics 1: e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harholt J, Suttangkakul A, Vibe Scheller H. 2010. Biosynthesis of pectin. Plant Physiology 153: 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley PA, Hurst CH, Kaliyadasa E, Lamb R, Knight MR, De Cothi EA, Steele JF, Knight H. 2014. The Arabidopsis mediator complex subunits MED16, MED14, and MED2 regulate mediator and RNA polymerase II recruitment to CBF‐responsive cold‐regulated genes. Plant Cell 26: 465–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Matsunaga T, Hayashi N. 2001. Formation of rhamnogalacturonan II‐borate dimer in pectin determines cell wall thickness of pumpkin tissue. Plant Physiology 126: 1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo‐Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF. 1998. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280: 104–106. [DOI] [PubMed] [Google Scholar]
- Ji H, Wang Y, Cloix C, Li K, Jenkins GI, Wang S, Shang Z, Shi Y, Yang S, Li X. 2015. The Arabidopsis RCC1 Family protein TCF1 regulates freezing tolerance and cold acclimation through modulating lignin biosynthesis. PLoS Genetics 11: e1005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Ding Y, Shi Y, Zhang X, Gong Z, Yang S. 2016. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis . New Phytologist 212: 345–353. [DOI] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Sung DY, Zhao W, Popp M, Porat R, Guy CL. 2007. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold‐regulated gene expression with modifications in metabolite content. The Plant Journal 50: 967–981. [DOI] [PubMed] [Google Scholar]
- Karimi M, Inze D, Depicker A. 2002. GATEWAY vectors for Agrobacterium‐mediated plant transformation. Trends in Plant Science 7: 193–195. [DOI] [PubMed] [Google Scholar]
- Keegstra K. 2010. Plant cell walls. Plant Physiology 154: 483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Mugford SG, Ulker B, Gao D, Thorlby G, Knight MR. 2009. Identification of SFR6, a key component in cold acclimation acting post‐translationally on CBF function. The Plant Journal 58: 97–108. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Matoh T, Azuma J. 1996. Two chains of rhamnogalacturonan II are cross‐linked by borate‐diol ester bonds in higher plant cell walls. Plant Physiology 110: 1017–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubacka‐Zebalska M, Kacperska A. 1999. Low temperature‐induced modifications of cell wall content and polysaccharide composition in leaves of winter oilseed rape (Brassica napus L‐var. oleifera L.). Plant Science 148: 59–67. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. 2016. lmerTest: tests in linear mixed effects models. R package, v.2.0‐32. [WWW document] URL https://CRAN.R-project.org/package=lmerTest.
- Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, Muller R, Dreher K, Alexander DL, Garcia‐Hernandez M et al 2012. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Research 40: D1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall H, Philippe F, Domon JM, Gillet F, Pelloux J, Rayon C. 2015. Cell wall metabolism in response to abiotic stress. Plants 4: 112–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth RV. 2016. Least‐squares means: the R package lsmeans. Journal of Statistical Software 69: 1–33. [Google Scholar]
- Levitt J. 1980. Responses of plants to environmental stresses: Volume I: Chilling, freezing and high temperature stresses. New York, NY, USA: Academic Press. [Google Scholar]
- Lobine D, Cummins I, Govinden‐Soulange J, Ranghoo‐Sanmukhiya M, Lindsey K, Chazot PL, Ambler CA, Grellscheid S, Sharples G, Lall N et al 2018. Medicinal mascarene aloes: an audit of their phytotherapeutic potential. Fitoterapia 124: 120–126. [DOI] [PubMed] [Google Scholar]
- McCully ME, Canny MJ, Huang CX. 2004. The management of extracellular ice by petioles of frost‐resistant herbaceous plants. Annals of Botany 94: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meents MJ, Watanabe Y, Samuels AL. 2018. The cell biology of secondary cell wall biosynthesis. Annals of Botany 121: 1107–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Wakuta S, Takada S, Ide K, Takano J, Naito S, Omori H, Matsunaga T, Fujiwara T. 2013. Roles of BOR2, a boron exporter, in cross linking of rhamnogalacturonan II and root elongation under boron limitation in Arabidopsis. Plant Physiology 163: 1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering ER, Muthan B, Benning C. 2010. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330: 226–228. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Furuta H, Maeda H, Nagamatsu Y, Yoshimoto A. 2001. Analysis of structural components and molecular construction of soybean soluble polysaccharides by stepwise enzymatic degradation. Bioscience Biotechnology Biochemistry 65: 2249–2258. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Yamaguchi‐Shinozaki K, Shinozaki K. 2014. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Frontiers in Plant Science 5: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndeh D, Rogowski A, Cartmell A, Luis AS, Basle A, Gray J, Venditto I, Briggs J, Zhang X, Labourel A et al 2017. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 544: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MA, Eberhard S, Albersheim P, Darvill AG. 2001. Requirement of borate cross‐linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294: 846–849. [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Ishii T, Albersheim P, Darvill AG. 2004. Rhamnogalacturonan II: structure and function of a borate cross‐linked cell wall pectic polysaccharide. Annual Review of Plant Biology 55: 109–139. [DOI] [PubMed] [Google Scholar]
- Pabst M, Fischl RM, Brecker L, Morelle W, Fauland A, Kofeler H, Altmann F, Leonard R. 2013. Rhamnogalacturonan II structure shows variation in the side chains monosaccharide composition and methylation status within and across different plant species. The Plant Journal 76: 61–72. [DOI] [PubMed] [Google Scholar]
- Pearce RS. 2001. Plant freezing and damage. Annals of Botany 87: 417–424. [Google Scholar]
- Perrin RM, Jia Z, Wagner TA, O'Neill MA, Sarria R, York WS, Raikhel NV, Keegstra K. 2003. Analysis of xyloglucan fucosylation in Arabidopsis. Plant Physiology 132: 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA. 2008. Evolution and diversity of green plant cell walls. Current Opinion in Plant Biology 11: 286–292. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2016. R: A language and environment for statistical computing, v.3.2.5. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rajashekar CB, Burke MJ. 1996. Freezing characteristics of rigid plant tissues (development of cell tension during extracellular freezing). Plant Physiology 111: 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekar CB, Lafta A. 1996. Cell‐wall changes and cell tension in response to cold acclimation and exogenous abscisic acid in leaves and cell cultures. Plant Physiology 111: 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayon C, Cabanes‐Macheteau M, Loutelier‐Bourhis C, Salliot‐Maire I, Lemoine J, Reiter WD, Lerouge P, Faye L. 1999. Characterization of N‐glycans from Arabidopsis. Application to a fucose‐deficient mutant. Plant Physiology 119: 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera M, Abreu I, Brewin NJ, Bonilla I, Bolanos L. 2010. Borate promotes the formation of a complex between legume AGP‐extensin and hamnogalacturonan II and enhances production of Rhizobium capsular polysaccharide during infection thread development in Pisum sativum symbiotic root nodules. Plant, Cell & Environment 33: 2112–2120. [DOI] [PubMed] [Google Scholar]
- Reiter WD, Chapple CC, Somerville CR. 1993. Altered growth and cell walls in a fucose‐deficient mutant of Arabidopsis. Science 261: 1032–1035. [DOI] [PubMed] [Google Scholar]
- Reuhs BL, Glenn J, Stephens SB, Kim JS, Christie DB, Glushka JG, Zablackis E, Albersheim P, Darvill AG, O'Neill MA. 2004. l‐Galactose replaces l‐fucose in the pectic polysaccharide rhamnogalacturonan II synthesized by the l‐fucose‐deficient mur1 Arabidopsis mutant. Planta 219: 147–157. [DOI] [PubMed] [Google Scholar]
- Ryden P, Sugimoto‐Shirasu K, Smith AC, Findlay K, Reiter WD, McCann MC. 2003. Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross‐linked microfibrillar network and rhamnogalacturonan II‐borate complexes. Plant Physiology 132: 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechet J, Htwe S, Urbanowicz B, Agyeman A, Feng W, Ishikawa T, Colomes M, Kumar KS, Kawai‐Yamada M, Dinneny JR et al 2018. Suppression of Arabidopsis GGLT1 affects growth by reducing the L‐galactose content and borate cross‐linking of rhamnogalacturonan‐II. The Plant Journal 96: 1036–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinwari ZK, Nakashima K, Miura S, Kasuga M, Seki M, Yamaguchi‐Shinozaki K, Shinozaki K. 1998. An Arabidopsis gene family encoding DRE/CRT binding proteins involved in low‐temperature‐responsive gene expression. Biochemical and Biophysical Research Communications 250: 161–170. [DOI] [PubMed] [Google Scholar]
- Smallwood M, Bowles DJ. 2002. Plants in a cold climate. Philosophical Transactions of the Royal Society of London B: Biological Sciences 357: 831–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecka D, Zebrowski J, Kacperska A. 2008. Are pectins involved in cold acclimation and de‐acclimation of winter oil‐seed rape plants? Annals of Botany 101: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanowska M, Kuras M, Kubacka‐Zebalska M, Kacperska A. 1999. Low temperature affects pattern of leaf growth and structure of cell walls in winter oilseed rape (Brassica napus L., var. oliefera L.). Annals of Botany 84: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus PL, Lynch DV. 1989. Freeze/thaw‐induced destabilization of the plasma membrane and the effects of cold acclimation. Journal of Bioenergy & Biomembranes 21: 21–41. [DOI] [PubMed] [Google Scholar]
- Stitt M, Hurry V. 2002. A plant for all seasons: alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis . Current Opinion in Plant Biology 5: 199–206. [DOI] [PubMed] [Google Scholar]
- Takahashi D, Gorka M, Erban A, Graf A, Kopka J, Zuther E, Hincha DK. 2019. Both cold and sub‐zero acclimation induce cell wall modification and changes in the extracellular proteome in Arabidopsis thaliana . Scientific Reports 9: 2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T. 2002. Arabidopsis boron transporter for xylem loading. Nature 420: 337–340. [DOI] [PubMed] [Google Scholar]
- Tenhaken R. 2014. Cell wall remodeling under abiotic stress. Frontiers in Plant Science 5: 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. 2010. Plant cold acclimation identifying gene regulons involved in freezing tolerance. Vitro Cellular & Developmental Biology–Animal 46: S78–S79. [Google Scholar]
- Thomashow MF. 1999. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annual Review of Plant Physiology and Plant Molecular Biology 50: 571–599. [DOI] [PubMed] [Google Scholar]
- Thorlby G, Veale E, Butcher K, Warren G. 1999. Map positions of SFR genes in relation to other freezing‐related genes of Arabidopsis thaliana . The Plant Journal 17: 445–452. [DOI] [PubMed] [Google Scholar]
- Tryfona T, Liang HC, Kotake T, Tsumuraya Y, Stephens E, Dupree P. 2012. Structural characterization of Arabidopsis leaf arabinogalactan polysaccharides. Plant Physiology 160: 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Joseph RA, Steponkus PL. 1995. Cold acclimation of Arabidopsis thaliana (Effect on plasma membrane lipid composition and freeze‐induced lesions). Plant Physiology 109: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Steponkus PL. 1994. A Contrast of the plasma membrane lipid composition of oat and rye leaves in relation to freezing tolerance. Plant Physiology 104: 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Tominaga Y, Nakagawara C, Shigematsu S, Minami A, Kawamura Y. 2006. Responses of the plasma membrane to low temperatures. Physiologia Plantarum 126: 81–89. [Google Scholar]
- Uemura M, Warren G, Steponkus PL. 2003. Freezing sensitivity in the sfr4 mutant of Arabidopsis is due to low sugar content and is manifested by loss of osmotic responsiveness. Plant Physiology 131: 1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hengel AJ, Roberts K. 2002. Fucosylated arabinogalactan‐proteins are required for full root cell elongation in arabidopsis. The Plant Journal 32: 105–113. [DOI] [PubMed] [Google Scholar]
- Vanzin GF, Madson M, Carpita NC, Raikhel NV, Keegstra K, Reiter WD. 2002. The mur2 mutant of Arabidopsis thaliana lacks fucosylated xyloglucan because of a lesion in fucosyltransferase AtFUT1. Proceedings of the National Academy of Sciences, USA 99: 3340–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos JA, Yi BR, Wallace IS. 2015. 2‐Fluoro‐L‐fucose is a metabolically incorporated inhibitor of plant cell wall polysaccharide fucosylation. PLoS ONE 10: e0139091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF. 2005. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. The Plant Journal 41: 195–211. [DOI] [PubMed] [Google Scholar]
- Voxeur A, Fry SC. 2014. Glycosylinositol phosphorylceramides from Rosa cell cultures are boron‐bridged in the plasma membrane and form complexes with rhamnogalacturonan II. The Plant Journal 79: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voxeur A, Soubigou‐Taconnat L, Legee F, Sakai K, Antelme S, Durand‐Tardif M, Lapierre C, Sibout R. 2017. Altered lignification in mur1–1 a mutant deficient in GDP‐L‐fucose synthesis with reduced RG‐II cross linking. PLoS ONE 12: e0184820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G, McKown R, Marin AL, Teutonico R. 1996. Isolation of mutations affecting the development of freezing tolerance in Arabidopsis thaliana (L.) Heynh. Plant Physiology 111: 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willick IR, Takahashi D, Fowler DB, Uemura M, Tanino KK. 2018. Tissue‐specific changes in apoplastic proteins and cell wall structure during cold acclimation of winter wheat crowns. Journal of Experimental Botany 69: 1221–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer MA, Lochnit G, Bassil E, Muhling KH, Goldbach HE. 2009. Membrane‐associated, boron‐interacting proteins isolated by boronate affinity chromatography. Plant & Cell Physiology 50: 1292–1304. [DOI] [PubMed] [Google Scholar]
- Wisniewski M, Davis G, Arora R. 1991. Effect of macerase, oxalic acid, and EGTA on deep supercooling and pit membrane structure of xylem parenchyma of peach. Plant Physiology 96: 1354–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski M, Lindow SE, Ashworth EN. 1997. Observations of ice nucleation and propagation in plants using infrared video thermography. Plant Physiology 113: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Williams M, Bernard S, Driouich A, Showalter AM, Faik A. 2010. Functional identification of two nonredundant Arabidopsis alpha(1,2)fucosyltransferases specific to arabinogalactan proteins. Journal of Biological Chemistry 285: 13638–13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Browse J. 1998. eskimo1 mutants of Arabidopsis are constitutively freezing‐tolerant. Proceedings of the National Academy of Sciences, USA 95: 7799–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A, Mejia‐Guerra MK, Kurz K, Liang X, Welch L, Grotewold E. 2011. AGRIS: the Arabidopsis Gene Regulatory Information Server, an update. Nucleic Acids Research 39: D1118–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York WS, Darvill AG, Mcneil M, Stevenson TT, Albersheim P. 1986. Isolation and characterization of plant‐cell walls and cell‐wall components. Methods in Enzymology 118: 3–40. [Google Scholar]
- Zablackis E, York WS, Pauly M, Hantus S, Reiter WD, Chapple CC, Albersheim P, Darvill A. 1996. Substitution of L‐fucose by L‐galactose in cell walls of Arabidopsis mur1 . Science 272: 1808–1810. [DOI] [PubMed] [Google Scholar]
- Zamil MS, Geitmann A. 2017. The middle lamella‐more than a glue. Physical Biology 14: 015004. [DOI] [PubMed] [Google Scholar]
- Zhang L, Paasch BC, Chen J, Day B, He SY. 2018. An important role of l‐fucose biosynthesis and protein fucosylation genes in Arabidopsis immunity. New Phytologist 222: 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch‐Hoffmann M, Hennig L, Gruissem W. 2004. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiology 136: 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Expression of CBF1‐3 and the CBF target genes KIN2 and GOLS3 are all expressed to normal wild‐type levels in sfr8.
Fig. S2 Two insertional mutants for candidate gene At3g50910 fail to show reduced freezing tolerance after cold acclimation.
Fig. S3 Nucleotide and amino acid sequence of MUR1 showing the SNPs and amino acid substitutions in the mutants mur1‐1, mur1‐2, mur1‐3 and sfr8.
Fig. S4 sfr8 fails to convert GDP‐mannose to GDP‐fucose.
Fig. S5 sfr8 can be complemented by the MUR1 coding sequence.
Fig. S6 Cell‐wall fucose content is restored to wild‐type levels in sfr8 mutants complemented with MUR1.
Fig. S7 Fucose supplementation restores the freezing‐sensitive phenotype of sfr8 and mur1‐1 but does not further improve freezing tolerance in wild‐type plants.
Fig. S8 mur2 mutants are not impaired in freezing tolerance.
Fig. S9 The boric acid watering regime restores the WT visible phenotype in mur1.
Fig. S10 MUR1 is not inducible by low temperature.
Fig. S11 sfr8 is more sensitive‐to‐freezing than WT even without cold acclimation.
Table S1 Candidate SNPs identified using Galaxy.
Table S2 MUR1 is not upregulated by CBF overexpression.
Table S3 MUR1 is not differentially expressed in response to cold acclimation.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.


