Abstract
Neisseria meningitidis is a Gram‐negative bacterium that asymptomatically colonises the nasopharynx of humans. For an unknown reason, N. meningitidis can cross the nasopharyngeal barrier and invade the bloodstream where it becomes one of the most harmful extracellular bacterial pathogen. This infectious cycle involves the colonisation of two different environments. (a) In the nasopharynx, N. meningitidis grow on the top of mucus‐producing epithelial cells surrounded by a complex microbiota. To survive and grow in this challenging environment, the meningococcus expresses specific virulence factors such as polymorphic toxins and MDAΦ. (b) Meningococci have the ability to survive in the extra cellular fluids including blood and cerebrospinal fluid. The interaction of N. meningitidis with human endothelial cells leads to the formation of typical microcolonies that extend overtime and promote vascular injury, disseminated intravascular coagulation, and acute inflammation. In this review, we will focus on the interplay between N. meningitidis and these two different niches at the cellular and molecular level and discuss the use of inhibitors of piliation as a potent therapeutic approach.
1. INTRODUCTION
Neisseria meningitidis (the meningococcus) is a Gram‐negative bacterium that asymptomatically colonises the nasopharynx of 4% to 20% of humans (Christensen, May, Bowen, Hickman, & Trotter, 2010). For unknown reasons, N. meningitidis may invade the bloodstream where it becomes one of the most harmful extracellular bacterial pathogen. In some cases, meningococcemia will rapidly progress toward a septic shock leading in the worst cases to a purpura fulminans, an acute systemic inflammatory response associated with an intravascular coagulation and tissue necrosis (Bonazzi et al., 2018; Brandtzaeg & van Deuren, 2012; Capel et al., 2017; Lecuyer et al., 2018; Lecuyer, Borgel, Nassif, & Coureuil, 2017). Alternatively, N. meningitidis can be responsible for cerebrospinal meningitis after crossing the blood brain barrier (Coureuil, Lecuyer, Bourdoulous, & Nassif, 2017; Simonis & Schubert‐Unkmeir, 2016). The fatality ratio of meningococcal disease is 10–15% and may be up to 40% in case of purpura fulminans, with 20% of the survivors having permanent sequelae (Centers for Disease Control and Prevention (CDC), 2017).
The infectious cycle of N. meningitidis involves the colonisation of two different environments. (a) The natural habitat of N. meningitidis is the human nasopharynx from where bacteria are transmitted from person to person by aerosol droplets or direct contact with contaminated fluids. In the nasopharynx, N. meningitidis grows on the top of mucus‐producing epithelial cells surrounded by a complex microbiota. (b) During pathogenesis, meningococci have the ability to survive in the extra cellular fluids including blood and cerebrospinal fluid. The interaction of N. meningitidis with human endothelial cells is very unusual because it leads to the formation of typical microcolonies that extend overtime to ultimately fill in the microvessels. In this review, we will focus on the interplay between N. meningitidis and these two different niches at the cellular and molecular level.
2. COLONISATION OF THE NASOPHARYNX
The nasopharynx is lined by two types of epithelia: a stratified squamous epithelium that covers 60% of the nasopharynx and a columnar respiratory epithelium (Ali, 1965; Freeman & Kahwaji, 2018). Airway epithelial cells are covered by a 10–12‐μm thick airway surface liquid, itself composed of a low‐viscosity periciliary liquid layer and a high‐viscosity mucus facing the lumen and containing mucins polymers and antimicrobial peptides (Brandtzaeg, 2009; Cole, Dewan, & Ganz, 1999; Ganz, 2002). The airway mucus plays the role of a physical barrier (Fahy & Dickey, 2010; Lillehoj, Kato, Lu, & Kim, 2013). It also facilitates the elimination of particles or bacteria by the mucociliary clearance. Indeed, airway epithelial cells expressed cilia that beat synchronously to allow the clearance of the airway surface liquid (at a speed of 6.9 ± 0.7 mm/min [Hoegger et al., 2014]) from the lung to the pharynx and from the nose to the pharynx from where the mucus is swallowed (Paul et al., 2013). This mechanism is considered as the main defence against microorganisms and particles. Continuously drained from other compartments of the airways and unable to escape the mucus clearance, N. meningitidis is restrained to the nasopharyngeal mucosa at the crossroads of the two mucociliary escalators.
The airway mucus also possesses bacteriostatic and bacteriolytic properties that limit the growth of bacteria. (a) The mucus is a poor nutritive medium with low concentration of glucose (Garnett et al., 2016) and iron (Smith, Lamont, Anderson, & Reid, 2013), and (b) it is enriched in antimicrobial peptides/proteins (such as β‐defensins and cathelicidin LL‐37; Bals & Hiemstra, 2004), components of the complement system, and specific secretory immunoglobulin A that function by preventing attachment of bacteria to the components of the mucus itself (De Rose, Molloy, Gohy, Pilette, & Greene, 2018). The natural niche of meningococci is the human nasopharynx, and these bacteria are perfectly adapted to this niche. N. meningitidis is able to use several carbon sources including glucose, pyruvate, and lactate, the latter being found in the mucus in the millimolar range during inflammation of the airways (Bensel et al., 2011). It is also particularly well equipped to capture iron (see below #3). N. meningitidis expresses IgA protease and is extremely well equipped to survive against the innate immune system through expression of a polysaccharide capsule, the lipooligosaccharide (LOS) (Lo, Tang, & Exley, 2009), and the factor H binding protein (Seib et al., 2009; for an extended review, see Laver, Hughes, & Read, 2015). Meningococci also express the MtrCDE efflux pump that efflux antimicrobial peptides (Handing, Ragland, Bharathan, & Criss, 2018; Rouquette, Harmon, & Shafer, 1999). On the other hand, N. meningitidis is lack classical toxins, proteases, or type VI secretion system.
The mucus is highly colonised with a complex microbiota (Biesbroek et al., 2014; Cremers et al., 2014; Esposito & Principi, 2018; Santee et al., 2016; Wang et al., 2018) that does not seem to be favourable for colonisation by other bacterial species. Nonetheless, recent studies have revealed that families of polymorphic toxins and the presence of a prophage designated MDAΦ could play an essential role in the colonisation of the nasopharynx by meningococci, the former by allowing N. meningitidis to fight the local microbiota and the latter by increasing the colonisation rate.
2.1. Polymorphic toxins
Polymorphic toxins (PT) are multidomain secreted proteins primarily involved in competition between bacteria (Jamet & Nassif, 2015a; Jamet & Nassif, 2015b; Zhang, de Souza, Anantharaman, Iyer, & Aravind, 2012). Each family is defined by a conserved N‐terminal region and diverse C‐terminal toxic domains (e.g., nucleases, pore forming, or protein‐modifying activities). In PT systems, a gene encoding a protective immunity protein is always located immediately downstream of the toxin gene (Figure 1a). This immunity protein protects the toxin‐producing cell both from autointoxication and from toxin produced by other strains (Jamet & Nassif, 2015a; Jamet & Nassif, 2015b; Zhang et al., 2012). N. meningitidis possesses two distinct families of PTs encoded by maf and tps loci, which are not related to one another but share several common features of PT. The most obvious similarity lies in their typical gene organisation. Indeed, downstream of the full‐length toxin gene and its cognate immunity gene, there is a variable number of 5′ truncated toxin genes called CT(C‐terminal)‐cassettes, also associated with their specific immunity gene (Arenas, Schipper, van Ulsen, van der Ende, & Tommassen, 2013; Jamet et al., 2015). These alternative toxic domains could potentially allow antigenic variation by recombination with the full‐length toxin gene (i.e., mafB or tpsA; Arenas et al., 2013; Arenas et al., 2015; Jamet et al., 2015). Hence, CT‐cassettes constitute a potential reservoir of toxic domains. Strikingly, the number of CT‐cassettes and the nature of the toxic activities they encode are highly variable from one strain to another. Because CT‐cassettes are always found associated with their cognate specific immunity genes, it suggests that their repertoire of toxin‐immunity modules could determine the ability of strains to compete or cooperate with each other (Figure 1a).
Figure 1.
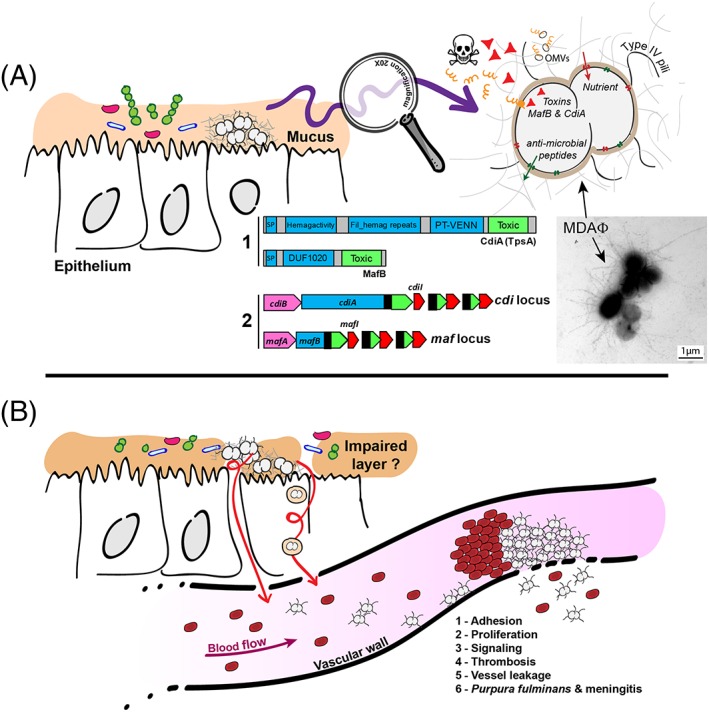
Neisseria meningitidis, a commensal and pathogenic bacterium. A. N. meningitidis grow in the mucus in nasopharynx where it encounters a poor nutritive medium and a rich microbiota. Meningococci survive by expressing capsule, LOS, the MtrCDE efflux pump, and factors that capture nutrients. N. meningitidis also express two families of polymorphic toxins: MafB and CdiA. A1 depicts the domain organisation found in CdiA and MafB polymorphic toxins constituted by a conserved N‐terminal domain (blue boxes) apposed to a toxic domain in the variable C‐terminal region (green boxes). Many toxic activities have been reported for toxic domains (Zhang et al., 2012). SP, signal peptide; Hemag activity, hemagglutination activity domain, also called a “TPS domain” (PF05860); fil hemag repeats, filamentous hemagglutinin repeats (PF13332); PT‐VENN, pre‐toxin domain with a VENN motif; DUF1020, domain of unknown function 1020. A2: Simplified genomic organisations of maf and cdi loci. Full‐length toxin genes cdiA and mafB are depicted in blue with their extremity encoding the toxic activity depicted in green. Genes encoding immunity proteins are depicted in red (cdiI and mafI). Open Reading Frame (ORF) encoding alternative C‐terminal toxic domains of CdiA and MafB are depicted in green surrounded with dotted lines. cdiB encodes the dedicated transporter of CdiA toxin, whereas the role of mafA in MafB secretion is unknown. Black boxes indicate regions potentially involved in recombination. Left panel: aggregates harvested from the biomass covering a monolayer of FaDu cells infected by the meningococcal Z5463 strain (Bille et al., 2017) and labelled by the anti‐MDA polyclonal antibody coupled to 8 nm‐diameter gold particles. Representative picture of meningococcal MDAΦ phage‐dependant aggregates. Bar: 1 μm. B. For an unknown reason, meningococci cross the epithelial layer and enter the bloodstream where bacteria adhere to the vascular wall. Adhesive bacteria proliferate and induce an active signalling that leads to better adhesion and opening of the vascular barrier, vessel leakage, and massive thrombosis
The tps loci encode two‐partner secretion systems (TPS). An outer membrane transporter generically named TpsB allows the translocation of a very large filamentous protein (>2,000 amino acid residues) generically named TpsA (Hodak et al., 2006; ur Rahman, Arenas, Ozturk, Dekker, & van Ulsen, 2014). TpsA proteins are well‐known to carry adhesive properties as described for the filamentous hemagglutinin adhesin from Bordetella pertussis (Hodak et al., 2006). Several roles have been attributed to meningococcal TpsA proteins including adhesion to epithelial cells (Schmitt et al., 2007) and promotion of biofilm formation (Neil & Apicella, 2009). A TpsA protein carrying a toxic domain at its C‐terminus is usually renamed CdiA toxins (Aoki et al., 2005). CdiA toxins have been initially described in Escherichia coli, where they have been shown to mediate a contact‐dependent inhibition of the growth of neighbouring bacteria ((Contact‐dependent inhibitor (CDI); Aoki et al., 2005). A role in interbacterial competition has been experimentally confirmed for a tps locus in the meningococcal strain B16B6 belonging to the hypervirulent cc11 clone (Arenas et al., 2013). This suggests that other meningococcal TpsA proteins harbouring various CT toxic domains could constitute functional CDI systems able to mediate growth inhibition of bacteria that lack protective immunity protein.
The MafB family is restricted to the Neisseria genus, in contrast with CdiA and most other PT families that are found in several genera (Zhang et al., 2012). Strikingly, maf genes represent 2% of the genome of pathogenic Neisseria and are likely to play important roles for pathogenesis of this genus (Jamet et al., 2015). Maf proteins are encoded by genes belonging to the multiple adhesin family (maf). MafA is a putative adhesin because it has been shown to interact with a specific glycolipid found on mammalian cells (Paruchuri, Seifert, Ajioka, Karlsson, & So, 1990). There are three maf genomic islands with conserved chromosomic location in meningococcal genomes that harboured mafB and mafI genes (Jamet et al., 2015). Anne Jamet et al. recently demonstrated that mafB genes encode secreted polymorphic toxins specifically neutralised by immunity proteins encoded by cognate mafI genes (Jamet et al., 2015). Of note, apart from the toxic domain that can be the same in a CdiA or a MafB toxin, MafB proteins do not show any similarity with TpsA proteins. Nor there is any similarity between MafA and CdiB proteins. While MafB proteins are secreted by meningococcus, their mode of secretion remains unknown, and there is no clue of a role of MafA in MafB secretion. The presence of MafA adhesin and MafB toxins in outer‐membrane vesicles (OMVs; Zielke, Wierzbicki, Weber, Gafken, & Sikora, 2014) suggests that OMVs could be a vehicle for delivery of MafB toxins to neighbouring bacteria or even to eukaryotic cells because MafA is able to bind cellular glycolipids (Paruchuri et al., 1990). Hence, MafB toxins could have multiple roles in vivo during pathogenesis or commensalism, which remain to be deciphered.
2.2. The MDA filamentous phage
Filamentous bacteriophages are part of the horizontally mobile elements (Mai‐Prochnow et al., 2015). As example, CTXΦ of Vibrio cholerae, which encodes the cholera toxin, can transduce nontoxigenic strains into toxigenic strains. The Pf bacteriophages of Pseudomonas aeruginosa are involved in the formation of biofilm by inducing cell death and the subsequent release of bacterial DNA (Rice et al., 2009). Filamentous bacteriophages are also involved in horizontal gene transfer (VPIΦ of V. cholerae), increase of motility (RSS1Φ of Ralstonia solanacearum, SW1Φ of Shewanella piezotolerans) and formation of host morphotypic variants (Cf1tΦ of Xanthomonas campestris, Pf4Φ, and Pf6Φ of P. aeruginosa; Mai‐Prochnow et al., 2015).
Whole genomes comparison using a collection of meningococci of defined pathogenic potential allowed the identification of an 8‐kb island that was associated with invasive infections. This island, designated MDA for Meningococcal Disease Associated island, encodes a functional filamentous prophage able to produce infectious filamentous phage particles (MDAΦ; Bille et al., 2005; Bille et al., 2008). The MDAΦ particles, each about 1,200 nm long, are secreted through the type IV pilus secretin PilQ and form a mesh of long bundles of filaments anchored to meningococci (Meyer et al., 2016). To infect naive recipient strains, MDAΦ particles use type IV pili as receptor and benefit from pilus retraction to access the cytoplasm of new hosts. Interestingly, production of MDAΦ particles and type IV pili seems to be mutually exclusive. Bille et al. demonstrated that MDAΦ particles form large bundles surrounding and connecting bacteria. These bacteria‐bacteria interactions increase the biomass of encapsulated meningococci interacting with monolayer of epithelial cells (Bille et al., 2017). Altogether, these data suggest that MDAΦ increases the bacterial load at the site of entry that in turn enhances the probability of bacterial translocation into the bloodstream and/or the dissemination of the bacteria in the general population.
3. INVASION OF THE BLOODSTREAM FROM THE PORT OF ENTRY
The mechanisms by which meningococci leave the nasopharynx and invade the bloodstream remain unknown. Active translocation of N. meningitidis following bacterial internalisation and trafficking within intracellular vacuoles is one of the major hypothesis, especially considering that the outer membrane proteins Opa and Opc were involved in an active process of internalisation that could be followed by translocation of bacteria through cellular monolayers (Billker, Popp, Gray‐Owen, & Meyer, 2000; de Vries, van Der Ende, van Putten, & Dankert, 1996; Schmitter et al., 2007; Virji, Makepeace, Ferguson, Achtman, & Moxon, 1993). Internalisation of meningococci may also be enhanced by other bacterial factors such as NadA (Bozza et al., 2014; Montanari et al., 2012), GltT‐GltM (Takahashi, Kim, & Watanabe, 2011), AutB (Arenas et al., 2016), or interaction of porin with TLR2 host receptor (Toussi, Wetzler, Liu, & Massari, 2016). Interestingly, two studies observed that infection of fully differentiated epithelial cells (Calu‐3 cell line) restrained meningococci at the apical domain (Barrile et al., 2015; Sutherland, Quattroni, Exley, & Tang, 2010), suggesting that differentiated epithelial cells prevent the dissemination of meningococci. Further experiments using Calu‐3 cells in air‐liquid interface culture, a model in which cells are grown with the apical domain facing the air, which generates a model more morphologically representative of the airway epithelium with a more rugged apical topography and greater glycoprotein secretion (Grainger, Greenwell, Lockley, Martin, & Forbes, 2006), will be needed to address the question of physiologically relevant colonisation of epithelial cells. It should be pointed out that circumstances, such as viral infections (Hubert, Watier, Garnerin, & Richardson, 1992) or climatic conditions (Sultan, Labadi, Guegan, & Janicot, 2005) possibly responsible for damaging the airway epithelium can favour meningococcal infections.
4. BLOOD‐BORNE COLONISATION OF THE VASCULAR COMPARTMENT
Once in the blood, the meningococcus benefits from the same virulence factors that ensure its survival in mucus to survive and proliferate in the blood: the polysaccharidic capsule and the LOS, the factor H binding protein (fHBP), the MtrCDE efflux pump, up to four different transporters to capture iron including transferrin binding proteins, lactoferrin binding proteins, and two independent heme transport systems that recognise haemoglobin and haptoglobin‐haemoglobin (HmbR and HpuAB, respectively; Perkins‐Balding, Ratliff‐Griffin, & Stojiljkovic, 2004). The virulence factor appearing to be specific to blood infection is the type IV pilus, which is the only mean by which capsulated meningococci adhere to endothelial cells in vivo (Join‐Lambert et al., 2013)
4.1. The importance of endothelial cell colonisation, the Achilles' heel of meningococci
Examination of post‐mortem samples of meningococcemia has shown that N. meningitidis forms large colonies at the apical surface of capillary endothelial cells throughout the body including spleen, skin, liver, kidney, heart, and brain. Retraction of endothelial cells, capillary disruption, haemorrhages, and luminal thrombi is observed. These observations are uncommon compared with most invasive bacterial pathogens. Thus, the specific ability of N. meningitidis to colonise and specifically interact with peripheral and brain microvessels is likely responsible for its capacity to both cross the blood–brain barrier and induce a thrombotic/leakage syndrome that in severe forms lead to purpura fulminans. Meningococcal interaction with endothelial cells is specific of human cells. Meningococci are unable to interact with any non‐human cells, thus hampering the ability to study the mechanisms and the consequences of this interaction in vivo. Recently, a humanised model using severe combined immunodeficiency mice grafted with human skin was developed (Join‐Lambert et al., 2013). In this model, where vessels of human origin from the graft anastomose to the mice vessels, blood‐borne N. meningitidis readily adhere to human endothelial cells. Bacterial‐induced vascular damages are very similar to those described in patients, including perivascular infiltrates, thrombosis, and vascular leakage associated with massively infected vessels (Harrison et al., 2002) that ultimately lead to the death of infected grafted animals (Capel et al., 2017). In addition, vascular damages are localised only in vessels colonised by meningococci. Capel et al. showed that adhesion to human skin vessels is a prerequisite for virulence of N. meningitidis and the maintenance of a sustained bacteremia because bacteria unable to adhere to microvessels are rapidly cleared from the bloodstream (Capel et al., 2017). Altogether, this points out the essential role of microvascular colonisation in meningococcal pathogenesis (Figure 1b).
Due to the predominant role of meningococcal adhesion in pathogenesis, targeting bacterial colonisation to microvessels represents a particularly promising strategy in the treatment of invasive meningococcal infections. Recently, Denis et al. have identified a family of compounds, which promotes within minutes the loss of type IV pili (see next paragraph) and, subsequently, alter all the functions carried by these structures, including twitching motility and adherence to human endothelial cells (Denis et al., 2019). Using the humanised mouse model of skin infection, they showed that these compounds exert a strong protective effect against the pathophysiological events occurring during meningococcemia. They reduce colonisation of the human vessels by circulating meningococci and prevent subsequent vascular dysfunctions, intravascular coagulation, and overwhelming inflammation, the hallmarks of invasive meningococcal infections. Finally, in consistence with the role of the vascular niche in promoting sustained bacteraemia leading to mice lethality (Capel et al., 2017), these compounds reduce bacteraemia and increase mice survival. In association with antibiotics, they reduce vascular inflammatory and thrombotic responses that were shown to be highly detrimental and correlated with the severity of the disease in patients (Girardin, Grau, Dayer, Roux‐Lombard, & Lambert, 1988; van Deuren et al., 1995; Waage, Brandtzaeg, Halstensen, Kierulf, & Espevik, 1989).
The identified molecules (trifluoperazine and thioridazine) belong to phenothiazines, a family of compounds previously used in human medicine to treat psychotic disorders. Due to the well‐conserved set of proteins involved in type IV pilus biosynthesis in Gram‐negative bacteria, interfering with Tfp‐mediated host cell interaction by these phenothiazines represents an attractive strategy to modify the course of diseases induced by piliated bacterial pathogens. Data strongly suggest that these phenothiazines target the sodium pumping NADH:ubiquinone oxidoreductase complex (Na+‐NQR). This protein complex, highly conserved among numerous nonpathogenic and pathogenic bacteria, creates a sodium motive force through the translocation of Na+ across the inner cell membrane. In many pathogenic bacteria, Na+ gradient is an entry site for electrons into the respiratory chain toward ATP synthesis or to sustain ionic homeostasis, nutrient transports, flagellum rotation, and other essential processes (Juarez & Barquera, 2012; Reyes‐Prieto, Barquera, & Juarez, 2014). Interestingly, meningococcal isogenic mutants, devoid of individual nqr genes were poorly piliated and aggregative, thus linking piliation and NQR‐mediated Na+ pumping activity. How this sodium gradient affects type IV pilus dynamics remains to be explored.
4.2. Type IV pili and their interaction with human cell receptors
Tfp are long and dynamic polymeric fibres made of pilin monomers (Egelman, 2017; Kolappan et al., 2016). This fibre is assembled from a platform in the inner membrane (PilM,N,O,P,G). Two ATPases permit the elongation (PilF) and the retraction (PilT) of the fibre that protrudes through the secretin PilQ (Hospenthal, Costa, & Waksman, 2017). The fibre is composed of the monomeric core pilin PilE and of three minor pilins that are responsible for Tfp‐associated phenotypes. ComP is responsible for natural competence for DNA transformation (Brown, Helaine, Carbonnelle, & Pelicic, 2010; Cehovin et al., 2013); PilV is involved in adhesion and signalling to human cells (Bernard et al., 2014; Mikaty et al., 2009), and the minor pilin PilX is essential to promote interbacterial interactions, a process named aggregation (Helaine et al., 2005; Helaine, Dyer, Nassif, Pelicic, & Forest, 2007). Aggregation of bacteria is allowed by the interaction between antiparallel pili and is a key aspect of colonisation. Indeed, a PilX‐defective mutant is piliated and unable to form aggregates or colonies on the top of endothelial cells. This interaction between antiparallel pili also exerts forces on both fibres that are responsible for a transition between two conformations: a packed and an elongated conformation. This transition reveals new epitopes, previously buried into the fibre and involved in the interaction with cellular receptors (see below; Biais, Higashi, Brujic, So, & Sheetz, 2010; Brissac, Mikaty, Dumenil, Coureuil, & Nassif, 2012; Kolappan et al., 2016). Furthermore, the dynamic of bacterial aggregates is key during colonisation of the blood capillary network. Bonazzi et al. recently revealed that the ATPase PilT drives adaptation of aggregates to tubular geometry found in capillaries, a property necessary for colonisation of blood vessels (Bonazzi et al., 2018).
Initial attachment of N. meningitidis to endothelial cells requires the interaction of tfp with human cell receptors. Several receptors were proposed as being able to interact with Tfp: the complement regulatory protein CD46, the Laminin receptor, or the platelet activating factor receptor (Jen et al., 2013; Kallstrom, Liszewski, Atkinson, & Jonsson, 1997; Kirchner, Heuer, & Meyer, 2005; Orihuela et al., 2009). During the last decade, CD147 (also known as Basigin/Emmprin) has been identified as an important adhesion receptor on both brain and peripheral endothelial cells. CD147 is a member of the immunoglobulin (Ig) superfamily comprising two Ig‐like domains (Iacono, Brown, Greene, & Saouaf, 2007). CD147 is massively recruited at sites of N. meningitidis adhesion. This recruitment precedes cytoskeletal rearrangement specific of meningococcal signalling onto endothelial cells (Carbonnelle et al., 2009). Bernard et al. showed that CD147 directly interacts with recombinant PilE and PilV, in contrast to other minor pilins: PilX and ComP (Bernard et al., 2014). Interestingly, CD147 is associated in a preformed complex with the α‐actinin‐4 scaffolding protein and the β2‐adrenergic receptor (β2AR; family of G protein coupled receptor [GPCR]). This pre‐existing association facilitates the activation of the β2AR by the two pilins PilE and PilV that activate and amplify a βarrestin‐dependent signalling pathway leading to the enrichment of ERM proteins. These proteins anchor the actin network at the site of bacterial adhesion in a structure named cortical plaque (Coureuil et al., 2010; Maissa et al., 2017; Merz, Enns, & So, 1999; Slanina, Hebling, Hauck, & Schubert‐Unkmeir, 2012). Actin polymerisation is the consequence of the local recruitment of ErbB2, small Rho GTPases, and that of the Src tyrosine kinase activation. This cell signalling is responsible for the elongation of host‐cell membrane protrusions that stabilise bacterial colonies at the surface of blood vessels (Coureuil et al., 2010; Lambotin et al., 2005; Mikaty et al., 2009). Besides, it has been recently proposed that the interaction of Tfp with host‐cell plasma membrane triggers its remodelling as discrete and dynamic protrusions in a process reminiscent of membrane wetting (referred to as 1D‐wetting; Charles‐Orszag et al., 2018). The authors suggest that membrane 1D‐wetting drives subsequent actin polymerisation and recruitment of cortical plaque associated proteins. In this context, the exact role of 1D‐wetting in β2AR‐induced remodelling of the apical surface is not clear; all the more since the involvement of this former phenomenon in meningococcal‐induced signalling has not been formally proven.
The nature of the epitope targeted by meningococcal Tfp on their cellular receptors is still unknown. To date, studies aiming at demonstrating receptor‐pilin interaction never revealed how both partners interact with each other. Some evidences suggested that Tfp from other pathogens interact with host cell carbohydrates. For instance, Tfp of Pseudomonas aeruginosa bind complex N‐glycans (Bucior, Pielage, & Engel, 2012) and that of Vibrio parahaemolyticus interact with chitin, a long‐chain polymer of N‐acetylglucosamine (Frischkorn, Stojanovski, & Paranjpye, 2013). A recent study by Mubaiwai et al. revealed that Tfp of the meningococcal strain MC58 can interact with complex N‐glycan such as GD2 ganglioside, a glycan composed of a core GalNAcβ1‐4Galβ1‐4Glc, and a terminal sialic acid (Neu5Ac; Mubaiwa et al., 2017). However, this glycan is only expressed in the cerebellum and peripheral nerves in humans and cannot account for the ability of meningococci to colonise human vessels (Ahmed & Cheung, 2014). Another open question is the role of the mechanical forces applied by Tfp. Indeed, using optical tweezers, Biais et al. showed that retraction of a single pilus generates forces up to 110 pN. Bundles of Tfp, which result from the association of eight to 10 pili, act as coordinated retractable units. Thus, bundles can generate retraction forces in the nanonewton range (Biais, Ladoux, Higashi, So, & Sheetz, 2008). Interestingly, some GPCRs were described as mechanosensors and, as such, may sense membrane tension. For instance, angiotensin II type 1 receptor is capable of sensing membrane stretching (Wang, Hanada, Gareri, & Rockman, 2018) and parathyroid hormone type 1 receptor senses mechanochemical signals in preosteoblatic cells (Zhang, Frangos, & Chachisvilis, 2009). The possibility that CD147 and/or the β2AR can be involved in mechanosensing of Tfp pulling forces needs to be further studied.
4.3. Consequences of blood vessels colonisation
A major consequence of bacterial adhesion to endothelial cells is the loss of vessels integrity. N. meningitidis causes vascular leakage through several parallel pathways that ultimately lead to the disassembly of both adherens and tight junctions, which is likely to be responsible in vivo for the peripheral leakage syndrome and in the brain for the crossing of the blood–brain barrier. Following bacterial adhesion onto endothelial cells, the adherens‐junction protein VE‐cadherin is relocated at sites of bacterial adhesion via the mislocalization of the Par3/6 polarity complex (Coureuil et al., 2009). Besides, N. meningitidis also promotes the cleavage of occludin (a component of the tight junctions) by the metalloproteinase MMP‐8, further altering the sealing of intercellular junctions (Schubert‐Unkmeir et al., 2010). In addition, N. meningitidis activates other signalling events in endothelial cells, but their exact consequences on endothelium integrity have not been assessed yet. Meningococci induce host‐cell calcium release from intracellular stores (Asmat, Tenenbaum, Jonsson, Schwerk, & Schroten, 2014); p21 and cyclin G2‐dependent cell cycle arrest at S phase (Oosthuysen, Mueller, Dittrich, & Schubert‐Unkmeir, 2016); Acid sphingomyelinase (ASM) and ceramide exposure in ErbB2 containing domain at plasma membrane (Simonis, Hebling, Gulbins, Schneider‐Schaulies, & Schubert‐Unkmeir, 2014).
Another major consequence of meningococcal interaction with endothelial cells is an increase of the procoagulant activity of the endothelium. Indeed, meningococcemia is very often complicated by thrombosis events. Up to 70% of patients develop a cutaneous purpura that reflects skin microvascular thrombosis and red blood cell extravasation (Thompson et al., 2006). Moreover, about 25% of the patients develop a purpura fulminans syndrome that associates extensive cutaneous thrombosis, ischemic necrosis of skin and organs (such as the adrenal glands), and a severe septic shock (Powars et al., 1993). All these thrombotic events are far more common in meningococcemia than in any other bacterial infections, suggesting that meningococci induce a specific dysregulation of coagulation. The humanised mouse model of meningococcal vascular colonisation described above has unveiled an important step in thrombosis development during meningococcemia: In this model, thrombosis was specifically associated with meningococcal adhesion to human endothelial cells. These results suggest that meningococcal interaction with endothelial cells is a key contributor to thrombosis. While meningococci express common prothrombotic factors (such as the LOS that mediates tissue factor expression [Mirlashari, Hoiby, Holst, & Lyberg, 2001; Ovstebo et al., 2012; Schlichting, Lyberg, Solberg, & Andersen, 1993]), a recent work has demonstrated that meningococcal Tfp‐mediated adhesion on endothelial cells specifically induces the activation of a membranous protease, a disintegrin metalloprotease 10, which subsequently cleaves the endothelial protein C receptor (EPCR) in a process known as shedding. This shedding is detrimental in the context of meningococcemia. Indeed, EPCR normally binds Protein C (PC), and this binding accelerates the rate of PC activation. Activated Protein C (aPC) is a potent anticoagulant that cleaves several coagulation factors and by such it prevents any overwhelming coagulation activation. An acquired or congenital severe deficit in PC is per se a cause of non‐infectious purpura fulminans, demonstrating the key role of this protein in preventing this syndrome. Hence, meningococcal Tfp‐mediated adhesion on endothelial cells, by inducing EPCR shedding, impairs the aPC negative feedback on coagulation (Lecuyer et al., 2018). So far, such dysregulation of coagulation system seems to be specific to the meningococcus. Besides, fibrinolysis that ends with fibrin clot destruction is also impaired during meningococcal infection. Indeed, meningococcal LOS induces the release of the fibrinolysis inhibitor plasminogen activator inhibitor by monocytes, and this certainly contributes to the extensive microvascular thrombosis (Mirlashari et al., 2001; Schlichting et al., 1993).
5. CONCLUSION
Many studies aiming at understanding how N. meningitidis colonise the pharyngeal niche, disseminate, and colonise blood vessels have been carried out and gave a clear overview of N. meningitidis interaction with epithelial and endothelial cells, providing a promising route to the treatment of invasive meningococcal diseases. However, so far, the lack of suitable models has limited our understanding of meningococcal colonisation of the upper airway or that of the opening of the blood–brain barrier. The understanding of the “colonisation phase” of N. meningitidis is a prerequisite to addressing the dissemination route responsible for bloodstream dissemination.
ACKNOWLEDGEMENTS
The laboratory of X. N. is supported by the “Fondation pour la Recherche Médical,” The “Agence Nationale de la Recherche,” INSERM, CNRS, and the University Paris Descartes. X. N. and S. B. were supported by collaborative research grants from the Agence Nationale de la Recherche of France (ANR‐14‐CE14‐0010 and ANR‐14‐IFEC14‐0006). M. C. was supported by a research grants from the Agence Nationale de la Recherche of France (ANR‐15‐CE15‐0002‐01).
Coureuil M, Jamet A, Bille E, Lécuyer H, Bourdoulous S, Nassif X. Molecular interactions between Neisseria meningitidis and its human host. Cellular Microbiology. 2019;21:e13063 10.1111/cmi.13063
Contributor Information
Mathieu Coureuil, Email: mathieu.coureuil@inserm.fr.
Xavier Nassif, Email: xavier.nassif@inserm.fr.
REFERENCES
- Ahmed, M. , & Cheung, N. K. (2014). Engineering anti‐GD2 monoclonal antibodies for cancer immunotherapy. FEBS Letters, 588, 288–297. 10.1016/j.febslet.2013.11.030 [DOI] [PubMed] [Google Scholar]
- Ali, M. Y. (1965). Histology of the human nasopharyngeal mucosa. Journal of Anatomy, 99, 657–672. [PMC free article] [PubMed] [Google Scholar]
- Aoki, S. K. , Pamma, R. , Hernday, A. D. , Bickham, J. E. , Braaten, B. A. , & Low, D. A. (2005). Contact‐dependent inhibition of growth in Escherichia coli. Science (New York, N.Y.), 309, 1245–1248. 10.1126/science.1115109 [DOI] [PubMed] [Google Scholar]
- Arenas, J. , de Maat, V. , Caton, L. , Krekorian, M. , Herrero, J. C. , Ferrara, F. , & Tommassen, J. (2015). Fratricide activity of MafB protein of N. meningitidis strain B16B6. BMC Microbiology, 15, 156 10.1186/s12866-015-0493-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas, J. , Paganelli, F. L. , Rodriguez‐Castano, P. , Cano‐Crespo, S. , van der Ende, A. , van Putten, J. P. , & Tommassen, J. (2016). Expression of the Gene for Autotransporter AutB of Neisseria meningitidis Affects Biofilm Formation and Epithelial Transmigration. Frontiers in Cellular and Infection Microbiology, 6, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas, J. , Schipper, K. , van Ulsen, P. , van der Ende, A. , & Tommassen, J. (2013). Domain exchange at the 3′ end of the gene encoding the fratricide meningococcal two‐partner secretion protein A. BMC Genomics, 14, 622 10.1186/1471-2164-14-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmat, T. M. , Tenenbaum, T. , Jonsson, A. B. , Schwerk, C. , & Schroten, H. (2014). Impact of calcium signaling during infection of Neisseria meningitidis to human brain microvascular endothelial cells. PLoS ONE, 9, e114474 10.1371/journal.pone.0114474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals, R. , & Hiemstra, P. S. (2004). Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. The European Respiratory Journal, 23, 327–333. 10.1183/09031936.03.00098803 [DOI] [PubMed] [Google Scholar]
- Barrile, R. , Kasendra, M. , Paccani, S. R. , Merola, M. , Pizza, M. , Baldari, C. , … Aricò, B. (2015). Neisseria meningitidis subverts the polarized organization and intracellular trafficking of host cells to cross the epithelial barrier. Cellular Microbiology, 17(9), 1365–1375. [DOI] [PubMed] [Google Scholar]
- Bensel, T. , Stotz, M. , Borneff‐Lipp, M. , Wollschlager, B. , Wienke, A. , Taccetti, G. , … Worlitzsch, D. (2011). Lactate in cystic fibrosis sputum. Journal of Cystic Fibrosis: Official Journal of the European Cystic Fibrosis Society, 10, 37–44. 10.1016/j.jcf.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Bernard, S. C. , Simpson, N. , Join‐Lambert, O. , Federici, C. , Laran‐Chich, M. P. , Maissa, N. , … Bourdoulous, S. (2014). Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nature Medicine, 20, 725–731. 10.1038/nm.3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biais, N. , Higashi, D. L. , Brujic, J. , So, M. , & Sheetz, M. P. (2010). Force‐dependent polymorphism in type IV pili reveals hidden epitopes. Proceedings of the National Academy of Sciences of the United States of America, 107, 11358–11363. 10.1073/pnas.0911328107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biais, N. , Ladoux, B. , Higashi, D. , So, M. , & Sheetz, M. (2008). Cooperative retraction of bundled type IV pili enables nanonewton force generation. PLoS Biology, 6, e87 10.1371/journal.pbio.0060087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesbroek, G. , Tsivtsivadze, E. , Sanders, E. A. , Montijn, R. , Veenhoven, R. H. , Keijser, B. J. , & Bogaert, D. (2014). Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. American Journal of Respiratory and Critical Care Medicine, 190, 1283–1292. 10.1164/rccm.201407-1240OC [DOI] [PubMed] [Google Scholar]
- Bille, E. , Meyer, J. , Jamet, A. , Euphrasie, D. , Barnier, J. P. , Brissac, T. , … Nassif, X. (2017). A virulence‐associated filamentous bacteriophage of Neisseria meningitidis increases host‐cell colonisation. PLoS Pathogens, 13, e1006495 10.1371/journal.ppat.1006495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bille, E. , Ure, R. , Gray, S. J. , Kaczmarski, E. B. , McCarthy, N. D. , Nassif, X. , … Tinsley, C. R. (2008). Association of a bacteriophage with meningococcal disease in young adults. PLoS ONE, 3, e3885 10.1371/journal.pone.0003885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bille, E. , Zahar, J. R. , Perrin, A. , Morelle, S. , Kriz, P. , Jolley, K. A. , … Tinsley, C. R. (2005). A chromosomally integrated bacteriophage in invasive meningococci. The Journal of Experimental Medicine, 201, 1905–1913. 10.1084/jem.20050112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billker, O. , Popp, A. , Gray‐Owen, S. D. , & Meyer, T. F. (2000). The structural basis of CEACAM‐receptor targeting by neisserial Opa proteins. Trends in Microbiology, 8, 258–260; discussion 260‐251. 10.1016/S0966-842X(00)01771-6 [DOI] [PubMed] [Google Scholar]
- Bonazzi, D. , Lo Schiavo, V. , Machata, S. , Djafer‐Cherif, I. , Nivoit, P. , Manriquez, V. , … Duménil, G. (2018). Intermittent Pili‐Mediated Forces Fluidize Neisseria meningitidis Aggregates Promoting Vascular Colonization. Cell, 174, 143–155 e116. 10.1016/j.cell.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Bozza, G. , Capitani, M. , Montanari, P. , Benucci, B. , Biancucci, M. , Nardi‐Dei, V. , … Merola, M. (2014). Role of ARF6, Rab11 and external Hsp90 in the trafficking and recycling of recombinant‐soluble Neisseria meningitidis adhesin A (rNadA) in human epithelial cells. PLoS ONE, 9, e110047 10.1371/journal.pone.0110047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg, P. (2009). Mucosal immunity: induction, dissemination, and effector functions. Scandinavian Journal of Immunology, 70, 505–515. 10.1111/j.1365-3083.2009.02319.x [DOI] [PubMed] [Google Scholar]
- Brandtzaeg, P. , & van Deuren, M. (2012). Classification and pathogenesis of meningococcal infections. Methods in Molecular Biology, 799, 21–35. 10.1007/978-1-61779-346-2_2 [DOI] [PubMed] [Google Scholar]
- Brissac, T. , Mikaty, G. , Dumenil, G. , Coureuil, M. , & Nassif, X. (2012). The meningococcal minor pilin PilX is responsible for type IV pilus conformational changes associated with signaling to endothelial cells. Infection and Immunity, 80, 3297–3306. 10.1128/IAI.00369-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. R. , Helaine, S. , Carbonnelle, E. , & Pelicic, V. (2010). Systematic Functional Analysis Reveals That a Set of Seven Genes Is Involved in Fine‐Tuning of the Multiple Functions Mediated by Type IV Pili in Neisseria meningitidis. Infection and Immunity, 78, 3053–3063. 10.1128/IAI.00099-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucior, I. , Pielage, J. F. , & Engel, J. N. (2012). Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathogens, 8, e1002616 10.1371/journal.ppat.1002616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel, E. , Barnier, J. P. , Zomer, A. L. , Bole‐Feysot, C. , Nussbaumer, T. , Jamet, A. , … Coureuil, M. (2017). Peripheral blood vessels are a niche for blood‐borne meningococci. Virulence, 8, 1808–1819. 10.1080/21505594.2017.1391446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonnelle, E. , Hill, D. J. , Morand, P. , Griffiths, N. J. , Bourdoulous, S. , Murillo, I. , … Virji, M. (2009). Meningococcal interactions with the host. Vaccine, 27(Suppl 2), B78–B89. 10.1016/j.vaccine.2009.04.069 [DOI] [PubMed] [Google Scholar]
- Centersfor Disease Control and Prevention (CDC) . 2017. http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/mening.pdf.
- Cehovin, A. , Simpson, P. J. , McDowell, M. A. , Brown, D. R. , Noschese, R. , Pallett, M. , … Pelicic, V. (2013). Specific DNA recognition mediated by a type IV pilin. Proceedings of the National Academy of Sciences of the United States of America, 110, 3065–3070. 10.1073/pnas.1218832110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles‐Orszag, A. , Tsai, F. C. , Bonazzi, D. , Manriquez, V. , Sachse, M. , Mallet, A. , … Duménil, G. (2018). Adhesion to nanofibers drives cell membrane remodeling through one‐dimensional wetting. Nature Communications, 9, 4450 10.1038/s41467-018-06948-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, H. , May, M. , Bowen, L. , Hickman, M. , & Trotter, C. L. (2010). Meningococcal carriage by age: a systematic review and meta‐analysis. The Lancet Infectious Diseases, 10, 853–861. 10.1016/S1473-3099(10)70251-6 [DOI] [PubMed] [Google Scholar]
- Cole, A. M. , Dewan, P. , & Ganz, T. (1999). Innate antimicrobial activity of nasal secretions. Infection and Immunity, 67, 3267–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureuil, M. , Lecuyer, H. , Bourdoulous, S. , & Nassif, X. (2017). A journey into the brain: insight into how bacterial pathogens cross blood‐brain barriers. Nature Reviews Microbiology, 15, 149–159. 10.1038/nrmicro.2016.178 [DOI] [PubMed] [Google Scholar]
- Coureuil, M. , Lecuyer, H. , Scott, M. G. , Boularan, C. , Enslen, H. , Soyer, M. , … Marullo, S. (2010). Meningococcus Hijacks a beta2‐adrenoceptor/beta‐Arrestin pathway to cross brain microvasculature endothelium. Cell, 143, 1149–1160. 10.1016/j.cell.2010.11.035 [DOI] [PubMed] [Google Scholar]
- Coureuil, M. , Mikaty, G. , Miller, F. , Lecuyer, H. , Bernard, C. , Bourdoulous, S. , … Nassif, X. (2009). Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science (New York, N.Y.), 325, 83–87. 10.1126/science.1173196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers, A. J. , Zomer, A. L. , Gritzfeld, J. F. , Ferwerda, G. , van Hijum, S. A. , Ferreira, D. M. , … Hermans, P. W. (2014). The adult nasopharyngeal microbiome as a determinant of pneumococcal acquisition. Microbiome, 2, 44 10.1186/2049-2618-2-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rose, V. , Molloy, K. , Gohy, S. , Pilette, C. , & Greene, C. M. (2018). Airway Epithelium Dysfunction in Cystic Fibrosis and COPD. Mediators of Inflammation, 2018, 1309746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries, F. P. , van Der Ende, A. , van Putten, J. P. , & Dankert, J. (1996). Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infection and Immunity, 64, 2998–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis, K. , Le Bris, M. , Le Guennec, L. , Barnier, J. P. , Faure, C. , Gouge, A. , … Bourdoulous, S. (2019). Targeting Type IV pili as an antivirulence strategy against invasive meningococcal disease. Nature Microbiology, 4, 972–984. 10.1038/s41564-019-0395-8 [DOI] [PubMed] [Google Scholar]
- Egelman, E. H. (2017). Cryo‐EM of bacterial pili and archaeal flagellar filaments. Current Opinion in Structural Biology, 46, 31–37. 10.1016/j.sbi.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito, S. , & Principi, N. (2018). Impact of nasopharyngeal microbiota on the development of respiratory tract diseases. European Journal of Clinical Microbiology & Infectious Diseases, 37, 1–7. 10.1007/s10096-017-3076-7 [DOI] [PubMed] [Google Scholar]
- Fahy, J. V. , & Dickey, B. F. (2010). Airway mucus function and dysfunction. The New England Journal of Medicine, 363, 2233–2247. 10.1056/NEJMra0910061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, S. C. , & Kahwaji, C. I. (2018). Physiology, Nasal. Treasure Island (FL): StatPearls. [PubMed] [Google Scholar]
- Frischkorn, K. R. , Stojanovski, A. , & Paranjpye, R. (2013). Vibrio parahaemolyticus type IV pili mediate interactions with diatom‐derived chitin and point to an unexplored mechanism of environmental persistence. Environmental Microbiology, 15, 1416–1427. 10.1111/1462-2920.12093 [DOI] [PubMed] [Google Scholar]
- Ganz, T. (2002). Antimicrobial polypeptides in host defense of the respiratory tract. The Journal of Clinical Investigation, 109, 693–697. 10.1172/JCI0215218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett, J. P. , Kalsi, K. K. , Sobotta, M. , Bearham, J. , Carr, G. , Powell, J. , … Baines, D. L. (2016). Hyperglycaemia and Pseudomonas aeruginosa acidify cystic fibrosis airway surface liquid by elevating epithelial monocarboxylate transporter 2 dependent lactate‐H(+) secretion. Scientific Reports, 6, 37955 10.1038/srep37955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin, E. , Grau, G. E. , Dayer, J. M. , Roux‐Lombard, P. , & Lambert, P. H. (1988). Tumor necrosis factor and interleukin‐1 in the serum of children with severe infectious purpura. The New England Journal of Medicine, 319, 397–400. 10.1056/NEJM198808183190703 [DOI] [PubMed] [Google Scholar]
- Grainger, C. I. , Greenwell, L. L. , Lockley, D. J. , Martin, G. P. , & Forbes, B. (2006). Culture of Calu‐3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharmaceutical Research, 23, 1482–1490. 10.1007/s11095-006-0255-0 [DOI] [PubMed] [Google Scholar]
- Handing, J. W. , Ragland, S. A. , Bharathan, U. V. , & Criss, A. K. (2018). The MtrCDE Efflux Pump Contributes to Survival of Neisseria gonorrhoeae From Human Neutrophils and Their Antimicrobial Components. Frontiers in Microbiology, 9, 2688 10.3389/fmicb.2018.02688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, O. B. , Robertson, B. D. , Faust, S. N. , Jepson, M. A. , Goldin, R. D. , Levin, M. , & Heyderman, R. S. (2002). Analysis of pathogen‐host cell interactions in purpura fulminans: expression of capsule, type IV pili, and PorA by Neisseria meningitidis in vivo. Infection and Immunity, 70, 5193–5201. 10.1128/IAI.70.9.5193-5201.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helaine, S. , Carbonnelle, E. , Prouvensier, L. , Beretti, J. L. , Nassif, X. , & Pelicic, V. (2005). PilX, a pilus‐associated protein essential for bacterial aggregation, is a key to pilus‐facilitated attachment of Neisseria meningitidis to human cells. Molecular Microbiology, 55, 65–77. 10.1111/j.1365-2958.2004.04372.x [DOI] [PubMed] [Google Scholar]
- Helaine, S. , Dyer, D. H. , Nassif, X. , Pelicic, V. , & Forest, K. T. (2007). 3D structure/function analysis of PilX reveals how minor pilins can modulate the virulence properties of type IV pili. Proceedings of the National Academy of Sciences of the United States of America, 104, 15888–15893. 10.1073/pnas.0707581104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodak, H. , Clantin, B. , Willery, E. , Villeret, V. , Locht, C. , & Jacob‐Dubuisson, F. (2006). Secretion signal of the filamentous haemagglutinin, a model two‐partner secretion substrate. Molecular Microbiology, 61, 368–382. 10.1111/j.1365-2958.2006.05242.x [DOI] [PubMed] [Google Scholar]
- Hoegger, M. J. , Awadalla, M. , Namati, E. , Itani, O. A. , Fischer, A. J. , Tucker, A. J. , … Welsh, M. J. (2014). Assessing mucociliary transport of single particles in vivo shows variable speed and preference for the ventral trachea in newborn pigs. Proceedings of the National Academy of Sciences of the United States of America, 111, 2355–2360. 10.1073/pnas.1323633111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hospenthal, M. K. , Costa, T. R. D. , & Waksman, G. (2017). A comprehensive guide to pilus biogenesis in Gram‐negative bacteria. Nature Reviews Microbiology, 15, 365–379. 10.1038/nrmicro.2017.40 [DOI] [PubMed] [Google Scholar]
- Hubert, B. , Watier, L. , Garnerin, P. , & Richardson, S. (1992). Meningococcal disease and influenza‐like syndrome: a new approach to an old question. The Journal of Infectious Diseases, 166, 542–545. 10.1093/infdis/166.3.542 [DOI] [PubMed] [Google Scholar]
- Iacono, K. T. , Brown, A. L. , Greene, M. I. , & Saouaf, S. J. (2007). CD147 immunoglobulin superfamily receptor function and role in pathology. Experimental and Molecular Pathology, 83, 283–295. 10.1016/j.yexmp.2007.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet, A. , Jousset, A. B. , Euphrasie, D. , Mukorako, P. , Boucharlat, A. , Ducousso, A. , … Nassif, X. (2015). A new family of secreted toxins in pathogenic Neisseria species. PLoS Pathogens, 11, e1004592 10.1371/journal.ppat.1004592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet, A. , & Nassif, X. (2015a). Characterization of the Maf family of polymorphic toxins in pathogenic Neisseria species. Microbial Cell, 2, 88–90. 10.15698/mic2015.03.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet, A. , & Nassif, X. (2015b). New Players in the Toxin Field: Polymorphic Toxin Systems in Bacteria. MBio, 6, e00285–e00215. 10.1128/mBio.00285-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen, F. E. , Warren, M. J. , Schulz, B. L. , Power, P. M. , Swords, W. E. , Weiser, J. N. , … Jennings, M. P. (2013). Dual Pili Post‐translational Modifications Synergize to Mediate Meningococcal Adherence to Platelet Activating Factor Receptor on Human Airway Cells. PLoS Pathogens, 9, e1003377 10.1371/journal.ppat.1003377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Join‐Lambert, O. , Lecuyer, H. , Miller, F. , Lelievre, L. , Jamet, A. , Furio, L. , … Nassif, X. (2013). Meningococcal interaction to microvasculature triggers the tissular lesions of purpura fulminans. The Journal of Infectious Diseases, 208, 1590–1597. 10.1093/infdis/jit301 [DOI] [PubMed] [Google Scholar]
- Juarez, O. , & Barquera, B. (2012). Insights into the mechanism of electron transfer and sodium translocation of the Na(+)‐pumping NADH:quinone oxidoreductase. Biochimica et Biophysica Acta, 1817, 1823–1832. 10.1016/j.bbabio.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallstrom, H. , Liszewski, M. K. , Atkinson, J. P. , & Jonsson, A. B. (1997). Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Molecular Microbiology, 25, 639–647. 10.1046/j.1365-2958.1997.4841857.x [DOI] [PubMed] [Google Scholar]
- Kirchner, M. , Heuer, D. , & Meyer, T. F. (2005). CD46‐independent binding of neisserial type IV pili and the major pilus adhesin, PilC, to human epithelial cells. Infection and Immunity, 73, 3072–3082. 10.1128/IAI.73.5.3072-3082.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolappan, S. , Coureuil, M. , Yu, X. , Nassif, X. , Egelman, E. H. , & Craig, L. (2016). Structure of the Neisseria meningitidis Type IV pilus. Nature Communications, 7, 13015 10.1038/ncomms13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambotin, M. , Hoffmann, I. , Laran‐chich, M. , Nassif, X. , Couraud, P. O. , & Bourdoulous, S. (2005). Invasion of endothelial cells by Neisseria meningitidis requires cortactin recruitment by a PI3‐Kinase/Rac1 signalling pathway triggered by the lipo‐oligosaccharide. Journal of Cell Science, 118, 3805–3816. 10.1242/jcs.02514 [DOI] [PubMed] [Google Scholar]
- Laver, J. R. , Hughes, S. E. , & Read, R. C. (2015). Neisserial Molecular Adaptations to the Nasopharyngeal Niche. Advances in Microbial Physiology, 66, 323–355. 10.1016/bs.ampbs.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Lecuyer H, Borgel D, Nassif X, Coureuil M. 2017. Pathogenesis of meningococcal purpura fulminans. Pathogens and Disease, 75 10.1093/femspd/ftx027 [DOI] [PubMed] [Google Scholar]
- Lecuyer, H. , Virion, Z. , Barnier, J. P. , Matczak, S. , Bourdoulous, S. , Bianchini, E. , … Coureuil, M. (2018). An ADAM‐10 dependent EPCR shedding links meningococcal interaction with endothelial cells to purpura fulminans. PLoS Pathogens, 14, e1006981 10.1371/journal.ppat.1006981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj, E. P. , Kato, K. , Lu, W. , & Kim, K. C. (2013). Cellular and molecular biology of airway mucins. International Review of Cell and Molecular Biology, 303, 139–202. 10.1016/B978-0-12-407697-6.00004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, H. , Tang, C. M. , & Exley, R. M. (2009). Mechanisms of avoidance of host immunity by Neisseria meningitidis and its effect on vaccine development. The Lancet Infectious Diseases, 9, 418–427. 10.1016/S1473-3099(09)70132-X [DOI] [PubMed] [Google Scholar]
- Mai‐Prochnow, A. , Hui, J. G. , Kjelleberg, S. , Rakonjac, J. , McDougald, D. , & Rice, S. A. (2015). Big things in small packages: the genetics of filamentous phage and effects on fitness of their host. FEMS Microbiology Reviews, 39, 465–487. 10.1093/femsre/fuu007 [DOI] [PubMed] [Google Scholar]
- Maissa, N. , Covarelli, V. , Janel, S. , Durel, B. , Simpson, N. , Bernard, S. C. , … Bourdoulous, S. (2017). Strength of Neisseria meningitidis binding to endothelial cells requires highly‐ordered CD147/beta2‐adrenoceptor clusters assembled by alpha‐actinin‐4. Nature Communications, 8, 15764 10.1038/ncomms15764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz, A. J. , Enns, C. A. , & So, M. (1999). Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Molecular Microbiology, 32, 1316–1332. 10.1046/j.1365-2958.1999.01459.x [DOI] [PubMed] [Google Scholar]
- Meyer, J. , Brissac, T. , Frapy, E. , Omer, H. , Euphrasie, D. , Bonavita, A. , … Bille, E. (2016). Characterization of MDAPhi, a temperate filamentous bacteriophage of Neisseria meningitidis. Microbiology (Reading, England), 162, 268–282. 10.1099/mic.0.000215 [DOI] [PubMed] [Google Scholar]
- Mikaty, G. , Soyer, M. , Mairey, E. , Henry, N. , Dyer, D. , Forest, K. T. , … Duménil, G. (2009). Extracellular bacterial pathogen induces host cell surface reorganization to resist shear stress. PLoS Pathogens, 5, e1000314 10.1371/journal.ppat.1000314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirlashari, M. R. , Hoiby, E. A. , Holst, J. , & Lyberg, T. (2001). Outer membrane vesicles from Neisseria meningitidis: effects on tissue factor and plasminogen activator inhibitor‐2 production in human monocytes. Thrombosis Research, 102, 375–380. 10.1016/S0049-3848(01)00256-0 [DOI] [PubMed] [Google Scholar]
- Montanari, P. , Bozza, G. , Capecchi, B. , Caproni, E. , Barrile, R. , Norais, N. , … Merola, M. (2012). Human heat shock protein (Hsp) 90 interferes with Neisseria meningitidis adhesin A (NadA)‐mediated adhesion and invasion. Cellular Microbiology, 14, 368–385. 10.1111/j.1462-5822.2011.01722.x [DOI] [PubMed] [Google Scholar]
- Mubaiwa, T. D. , Hartley‐Tassell, L. E. , Semchenko, E. A. , Jen, F. E. , Srikhanta, Y. N. , Day, C. J. , … Seib, K. L. (2017). The glycointeractome of serogroup B Neisseria meningitidis strain MC58. Scientific Reports, 7, 5693 10.1038/s41598-017-05894-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil, R. B. , & Apicella, M. A. (2009). Role of HrpA in biofilm formation of Neisseria meningitidis and regulation of the hrpBAS transcripts. Infection and Immunity, 77, 2285–2293. 10.1128/IAI.01502-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuysen, W. F. , Mueller, T. , Dittrich, M. T. , & Schubert‐Unkmeir, A. (2016). Neisseria meningitidis causes cell cycle arrest of human brain microvascular endothelial cells at S phase via p21 and cyclin G2. Cellular Microbiology, 18, 46–65. 10.1111/cmi.12482 [DOI] [PubMed] [Google Scholar]
- Orihuela, C. J. , Mahdavi, J. , Thornton, J. , Mann, B. , Wooldridge, K. G. , Abouseada, N. , … Tuomanen, E. I. (2009). Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. The Journal of Clinical Investigation, 119, 1638–1646. 10.1172/JCI36759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovstebo, R. , Aass, H. C. , Haug, K. B. , Troseid, A. M. , Gopinathan, U. , Kierulf, P. , … Henriksson, C. E. (2012). LPS from Neisseria meningitidis is crucial for inducing monocyte‐ and microparticle‐associated tissue factor activity but not for tissue factor expression. Innate Immunity, 18, 580–591. 10.1177/1753425911428230 [DOI] [PubMed] [Google Scholar]
- Paruchuri, D. K. , Seifert, H. S. , Ajioka, R. S. , Karlsson, K. A. , & So, M. (1990). Identification and characterization of a Neisseria gonorrhoeae gene encoding a glycolipid‐binding adhesin. Proceedings of the National Academy of Sciences of the United States of America, 87, 333–337. 10.1073/pnas.87.1.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P, Johnson P, Ramaswamy P, Ramadoss S, Geetha B, Subhashini AS. 2013. The effect of ageing on nasal Mucociliary clearance in women: A pilot study.
- Perkins‐Balding, D. , Ratliff‐Griffin, M. , & Stojiljkovic, I. (2004). Iron transport systems in Neisseria meningitidis. Microbiology and Molecular Biology Reviews: MMBR, 68, 154–171. 10.1128/MMBR.68.1.154-171.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powars, D. , Larsen, R. , Johnson, J. , Hulbert, T. , Sun, T. , Patch, M. J. , … Chan, L. (1993). Epidemic meningococcemia and purpura fulminans with induced protein C deficiency. Clinical Infectious Diseases, 17, 254–261. 10.1093/clinids/17.2.254 [DOI] [PubMed] [Google Scholar]
- Reyes‐Prieto, A. , Barquera, B. , & Juarez, O. (2014). Origin and evolution of the sodium ‐pumping NADH: ubiquinone oxidoreductase. PLoS ONE, 9, e96696 10.1371/journal.pone.0096696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, S. A. , Tan, C. H. , Mikkelsen, P. J. , Kung, V. , Woo, J. , Tay, M. , … Kjelleberg, S. (2009). The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. The ISME Journal, 3, 271–282. 10.1038/ismej.2008.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouquette, C. , Harmon, J. B. , & Shafer, W. M. (1999). Induction of the mtrCDE‐encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC‐like protein. Molecular Microbiology, 33, 651–658. 10.1046/j.1365-2958.1999.01517.x [DOI] [PubMed] [Google Scholar]
- Santee, C. A. , Nagalingam, N. A. , Faruqi, A. A. , DeMuri, G. P. , Gern, J. E. , Wald, E. R. , & Lynch, S. V. (2016). Nasopharyngeal microbiota composition of children is related to the frequency of upper respiratory infection and acute sinusitis. Microbiome, 4, 34 10.1186/s40168-016-0179-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting, E. , Lyberg, T. , Solberg, O. , & Andersen, B. M. (1993). Endotoxin liberation from Neisseria meningitidis correlates to their ability to induce procoagulant and fibrinolytic factors in human monocytes. Scandinavian Journal of Infectious Diseases, 25, 585–594. 10.3109/00365549309008547 [DOI] [PubMed] [Google Scholar]
- Schmitt, C. , Turner, D. , Boesl, M. , Abele, M. , Frosch, M. , & Kurzai, O. (2007). A functional two‐partner secretion system contributes to adhesion of Neisseria meningitidis to epithelial cells. Journal of Bacteriology, 189, 7968–7976. 10.1128/JB.00851-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter, T. , Pils, S. , Weibel, S. , Agerer, F. , Peterson, L. , Buntru, A. , … Hauck, C. R. (2007). Opa proteins of pathogenic neisseriae initiate Src kinase‐dependent or lipid raft‐mediated uptake via distinct human carcinoembryonic antigen‐related cell adhesion molecule isoforms. Infection and Immunity, 75, 4116–4126. 10.1128/IAI.01835-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert‐Unkmeir, A. , Konrad, C. , Slanina, H. , Czapek, F. , Hebling, S. , & Frosch, M. (2010). Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: a role for MMP‐8. PLoS Pathogens, 6, e1000874 10.1371/journal.ppat.1000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib, K. L. , Serruto, D. , Oriente, F. , Delany, I. , Adu‐Bobie, J. , Veggi, D. , … Pizza, M. (2009). Factor H‐binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL‐37. Infection and Immunity, 77, 292–299. 10.1128/IAI.01071-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonis, A. , Hebling, S. , Gulbins, E. , Schneider‐Schaulies, S. , & Schubert‐Unkmeir, A. (2014). Differential activation of acid sphingomyelinase and ceramide release determines invasiveness of Neisseria meningitidis into brain endothelial cells. PLoS Pathogens, 10, e1004160 10.1371/journal.ppat.1004160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonis, A. , & Schubert‐Unkmeir, A. (2016). Interactions of meningococcal virulence factors with endothelial cells at the human blood‐cerebrospinal fluid barrier and their role in pathogenicity. FEBS Letters, 590, 3854–3867. 10.1002/1873-3468.12344 [DOI] [PubMed] [Google Scholar]
- Slanina, H. , Hebling, S. , Hauck, C. R. , & Schubert‐Unkmeir, A. (2012). Cell invasion by Neisseria meningitidis requires a functional interplay between the focal adhesion kinase, Src and cortactin. PLoS ONE, 7, e39613 10.1371/journal.pone.0039613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D. J. , Lamont, I. L. , Anderson, G. J. , & Reid, D. W. (2013). Targeting iron uptake to control Pseudomonas aeruginosa infections in cystic fibrosis. The European Respiratory Journal, 42, 1723–1736. 10.1183/09031936.00124012 [DOI] [PubMed] [Google Scholar]
- Sultan, B. , Labadi, K. , Guegan, J. F. , & Janicot, S. (2005). Climate drives the meningitis epidemics onset in west Africa. PLoS Medicine, 2, e6 10.1371/journal.pmed.0020006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland, T. C. , Quattroni, P. , Exley, R. M. , & Tang, C. M. (2010). Transcellular passage of Neisseria meningitidis across a polarized respiratory epithelium. Infection and Immunity, 78, 3832–3847. 10.1128/IAI.01377-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H. , Kim, K. S. , & Watanabe, H. (2011). Meningococcal internalization into human endothelial and epithelial cells is triggered by the influx of extracellular L‐glutamate via GltT L‐glutamate ABC transporter in Neisseria meningitidis. Infection and Immunity, 79, 380–392. 10.1128/IAI.00497-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, M. J. , Ninis, N. , Perera, R. , Mayon‐White, R. , Phillips, C. , Bailey, L. , … Levin, M. (2006). Clinical recognition of meningococcal disease in children and adolescents. Lancet, 367, 397–403. 10.1016/S0140-6736(06)67932-4 [DOI] [PubMed] [Google Scholar]
- Toussi, D. N. , Wetzler, L. M. , Liu, X. , & Massari, P. (2016). Neisseriae internalization by epithelial cells is enhanced by TLR2 stimulation. Microbes and Infection, 18, 627–638. 10.1016/j.micinf.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ur Rahman, S. , Arenas, J. , Ozturk, H. , Dekker, N. , & van Ulsen, P. (2014). The polypeptide transport‐associated (POTRA) domains of TpsB transporters determine the system specificity of two‐partner secretion systems. The Journal of Biological Chemistry, 289, 19799–19809. 10.1074/jbc.M113.544627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deuren, M. , van der Ven‐Jongekrijg, J. , Bartelink, A. K. , van Dalen, R. , Sauerwein, R. W. , & van der Meer, J. W. (1995). Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. The Journal of Infectious Diseases, 172, 433–439. 10.1093/infdis/172.2.433 [DOI] [PubMed] [Google Scholar]
- Virji, M. , Makepeace, K. , Ferguson, D. J. , Achtman, M. , & Moxon, E. R. (1993). Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Molecular Microbiology, 10, 499–510. 10.1111/j.1365-2958.1993.tb00922.x [DOI] [PubMed] [Google Scholar]
- Waage, A. , Brandtzaeg, P. , Halstensen, A. , Kierulf, P. , & Espevik, T. (1989). The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. The Journal of Experimental Medicine, 169, 333–338. 10.1084/jem.169.1.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Dai, W. , Feng, X. , Zhou, Q. , Yang, Y. , Li, S. , & Zheng, Y. (2018). Microbiota Composition in Upper Respiratory Tracts of Healthy Children in Shenzhen, China, Differed with Respiratory Sites and Ages. BioMed Research International, 2018, 6515670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Hanada, K. , Gareri, C. , & Rockman, H. A. (2018). Mechanoactivation of the angiotensin II type 1 receptor induces beta‐arrestin‐biased signaling through Galphai coupling. Journal of Cellular Biochemistry, 119, 3586–3597. 10.1002/jcb.26552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , de Souza, R. F. , Anantharaman, V. , Iyer, L. M. , & Aravind, L. (2012). Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biology Direct, 7, 18 10.1186/1745-6150-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. L. , Frangos, J. A. , & Chachisvilis, M. (2009). Mechanical stimulus alters conformation of type 1 parathyroid hormone receptor in bone cells. American Journal of Physiology, 296, C1391–C1399. 10.1152/ajpcell.00549.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke, R. A. , Wierzbicki, I. H. , Weber, J. V. , Gafken, P. R. , & Sikora, A. E. (2014). Quantitative proteomics of the Neisseria gonorrhoeae cell envelope and membrane vesicles for the discovery of potential therapeutic targets. Molecular & Cellular Proteomics, 13, 1299–1317. 10.1074/mcp.M113.029538 [DOI] [PMC free article] [PubMed] [Google Scholar]


