Abstract
BACKGROUND
Deaths by exsanguination in trauma are preventable with hemorrhage control and resuscitation with allogeneic blood products (ABPs). The ideal transfusion ratio is unknown. We compared efficacy and safety of high transfusion ratios of FFP:RBC and PLT:RBC with low ratios in trauma.
STUDY DESIGN AND METHODS
Medline, Embase, Cochrane, and Controlled Clinical Trials Register were searched. Observational and randomized data were included. Risk of bias was assessed using validated tools. Primary outcome was 24‐h and 30‐day mortality. Secondary outcomes were exposure to ABPs and improvement of coagulopathy. Meta‐analysis was conducted using a random‐effects model. Strength and evidence quality were graded using GRADE profile
RESULTS
55 studies were included (2 randomized and 53 observational), with low and moderate risk of bias, respectively, and overall low evidence quality. The two RCTs showed no mortality difference (odds ratio [OR], 1.35; 95% confidence interval [CI], 0.40‐4.59). Observational studies reported lower mortality in high FFP:RBCs ratio (OR, 0.38 [95% CI, 0.22‐0.68] for 1:1 vs. <1:1; OR, 0.42 [95% CI, 0.22‐0.81] for 1:1.5 vs. <1:1.5; and OR, 0.47 [95% CI, 0.31‐0.71] for 1:2 vs. <1:2, respectively). Meta‐analyses in observational studies showed no difference in exposure to ABPs. No data on coagulopathy for meta‐analysis was identified.
CONCLUSIONS
Meta‐analyses in observational studies suggest survival benefit and no difference in exposure to ABPs. No survival benefit in RCTs was identified. These conflicting results should be interpreted with caution. Studies are mostly observational, with relatively small sample sizes, nonrandom treatment allocation, and high potential for confounding. Further research is warranted.
ABBREVIATIONS
- ATC
acute trauma coagulopathy
- ABPs
allogeneic blood products
- IQR
interquartile range
- INR
international normalized ratio
- MTP(s)
massive transfusion protocol(s)
- NOS
Newcastle–Ottawa Scale
- PCC
prothrombin complex concentrate
- PT
prothrombin time
- RCT(s)
randomized controlled trial(s)
- ROTEM
rotational thrombelastometry
- TEG
thromboelastography
Trauma is responsible for one in 10 deaths worldwide and is the leading cause of mortality in individuals younger than 35 years of age.1, 2 Hemorrhage accounts for 40% of all trauma deaths.3 Bleeding is exacerbated by acute trauma coagulopathy (ATC),3, 4 which is present before resuscitation in 25% of patients.4, 5 Deaths by exsanguination and coagulopathy are potentially preventable with rapid hemorrhage control and proper resuscitation strategies.5
Resuscitation strategies targeted at ATC have demonstrated a lower risk of death from exsanguination, possibly mediated by early reversal of coagulopathy with subsequent decreased bleeding.5, 6 However, the intrinsic mechanisms of ATC are still controversial, such as the balance between inhibition of procoagulant pathways and fibrinolysis.7 Due to this uncertainty, the amount of fresh‐frozen plasma (FFP) and platelets (PLTs) that should be used in relation to the amount of red blood cells (RBCs) transfused or the importance of other clotting factor concentrates are unknown. The principle of damage control resuscitation, which is characterized by early administration of blood products using a FFP:PLTs:RBCs ratio of 1:1:1, aims to prevent and correct the ATC while minimizing the use of crystalloids. These principles have demonstrated improved outcomes compared to traditional resuscitation practices.7, 8, 9
However, several studies have questioned the principle of 1:1:1 ratio, reporting improved outcomes with other fixed transfusion ratios of FFP:PLTs:RBCs, FFP:RBCs, or PLTs:RBCs9 or using viscoelastic methods10, 11, 12, 13 such as thromboelastography (TEG [Haemonetics Corporation]) and rotational thrombelastometry (ROTEM [Tem Innovations GmbH]) to direct resuscitation. These tests provide global information on the dynamics of clot development, stabilization, and dissolution, reflecting in vivo hemostasis and provide a more goal‐directed approach to transfusion.12 Currently, there are no data supporting the optimal approach, with centers using massive transfusion protocols (MTPs) with different fixed transfusion ratios on admission, followed by transfusion guided by laboratory tests or TEG/ROTEM.14, 15, 16, 17 Other centers use a more goal‐directed approach with TEG/ROTEM assessment at admission, using other concentrate of clotting factors, such as prothrombin complex concentrate (PCC; Octaplex), fibrinogen concentrate, and antifibrinolytic drugs.13, 14, 15, 16, 17 We sought to review the evidence supporting different transfusion ratios of FFP and PLTs to RBCs or FFP or PLTs to RBCs. Specifically, we aimed to compare the effects of high transfusion ratio of FFP and/or PLTs to RBCs to low ratios on in‐hospital mortality, exposure to allogeneic blood products, and coagulopathy.
MATERIALS AND METHODS
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.18 Meta‐analyses were performed according to the guidelines from the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) group.19
Studies
This review included prospective and retrospective cohort studies with a control group (e.g., observational studies comparing high vs. low transfusion ratios) and randomized controlled trials (RCTs). To be included, studies should have reported at least one outcome of interest. We excluded observational studies that addressed other approaches such as transfusion guided by viscoelastic methods or studies that addressed the use of other concentrate of clotting factors. Case reports, case series, and conference proceedings were also excluded.
Participants
Studies were included if they were conducted in adult trauma patients (≥15 years old) with an important risk of bleeding as per each study definition. Studies should have included patients who received at least 1 unit of RBCs within the first 24 hours postadmission.
Interventions
Intervention was the resuscitation of trauma patients using a high fixed transfusion ratio of FFP and PLTs to RBCs or FFP or PLTs to RBCs, as defined in each study. The control was a low fixed transfusion ratio of FFP and PLTs to RBCs or FFP or RBCs to RBCs, also defined and compared in each study.
Outcome measures
The primary outcome was in‐hospital mortality assessed at two time points: at 24 hours of admission and at 30 days of admission. Secondary outcomes were: 1) cumulative number of allogeneic RBCs, FFP, and PLT units transfused in 24 hours postadmission and 2) effect on the ATC, represented by values of international normalized ratio (INR), fibrinogen, and TEG or ROTEM variables, all measured within 24 hours postadmission.
Search methods
We searched Medline (from 1946 to July 31, 2018), Embase (1947 to July 31, 2018), Cochrane Controlled Trials Register (from inception to July 31, 2018), http://clinicaltrials.gov (http://www.clinicaltrials.gov), and Google Scholar (first 200 hits). The search was not restricted by date, language, or publication status. Search terms were defined a priori and by reviewing the MeSH terms of articles identified in preliminary literature searches. The search strategy was based on the Medline search strategy and was modified as necessary for the other databases. A sensitive search strategy combining MeSH headings and the keywords “transfusion ratios/component transfusion/balanced resuscitation/hemostatic resuscitation/bleeding/trauma coagulopathy/trauma induced coagulopathy/acute coagulopathy of trauma and shock/acute trauma coagulopathy” AND “injury/trauma” was used.
Data abstraction
Two review authors (RS, PPD) not blinded to journal, institutions, or authors independently examined all abstracts of the studies identified by the search and determined the eligibility of each study. Any disagreements were resolved by consensus or with another review author (LTDL or AAM). Titles and abstracts of every record retrieved were screened to determine which of the studies should be undergo a full‐text review. Full texts of the studies with questionable eligibility or considered eligible were retrieved in this phase for evaluation. The reference lists of the retrieved articles were also searched for additional citations. Only published data were included. Investigators were not contacted for obtaining further data. Data were also collected independently by two review authors (RS and AAM).
Risk of bias assessment and GRADE profile
Risk of bias was assessed by two review authors (LTDL and RS) for each included study. Any disagreement was resolved through discussion and consensus with a third author (BN). Each included study was classified as RCT or observational cohort study, and the risk of bias was assessed differently according to each type of study design. For RCTs, the Cochrane Collaboration's tool20 was used, which assesses bias in the domains of sequence generation, allocation concealment, blinding of outcomes, incomplete outcome data, selective outcome reporting, and baseline imbalances. For cohort studies, risk of bias was assessed using the Newcastle–Ottawa Scale (NOS),21 which defines patient groups as comparable in either the design or analysis when the effect of the exposure is adjusted for confounders. This tool assesses risk of bias in the domains of selection of exposed and nonexposed cohorts, comparability of cohorts, assessment of outcomes, and adequacy of follow‐up. For observational studies score of 3 or less was considered high risk of bias, 4 to 6 was considered moderate risk of bias, and 7 or more was low risk of bias. Quality of evidence for mortality and exposure to allogeneic blood products was evaluated using GRADE criteria, which included evaluation of each outcome for five criteria: risk of bias, inconsistency, imprecision, indirectness, and publication bias. It was classified as high, moderate, low, or very low (http://www.gradepro.org, Version 3.6.1, McMaster University 2014).
Statistical analyses
Studies were combined in meta‐analyses if there was enough clinical and methodologic homogeneity. Studies were analyzed separately according to their design (observational or randomized). Clinical and methodologic heterogeneity across the studies was assessed by examining the details of the subjects, the baseline data, the interventions, and the outcomes to determine whether the studies were sufficiently similar. Statistical heterogeneity was determined using the I2 statistic and the chi‐square test. High values of both tests (I2 > 40%, a nonsignificant chi‐square [p <0.05], respectively) demonstrate high levels of inconsistency and heterogeneity. Heterogeneity was further investigated observing the variations in the effect sizes across studies and overlapping of confidence intervals (CIs), which were used while performing the GRADE profile. Pooling of overall estimates was performed using generic inverse variance weighting methods. With these methods, each study estimate of the relative treatment is given a weight that is equal to the inverse of the variance of the effect estimate (i.e., one divided by the standard error squared). Studies were grouped according to the transfusion ratios of FFP:PLTs:RBCs, FFP:RBCs, or PLTs:RBCs reported for conducting meta‐analyses. Before‐and‐after studies were removed from the pooled analyses to avoid secular trends in practice patterns.
Review Manager 5.3 software (RevMan 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, 2015) was used to carry out quantitative analyses. A random‐effects model was used as this approach accommodates clinical and statistical variations. Heterogeneity was explored. Odds ratio (OR) and 95% CI were used as statistical measures for mortality as a dichotomous outcome. Mean and standard deviation (SD) was the statistical measure used to describe exposure to allogeneic blood products and coagulopathy. Studies that reported transfusion data in medians and interquartile ranges (IQRs) and mean and SD were estimated using the sample size, median, and the IQR as demonstrated in the method published by Wan.22
RESULTS
Included studies (Fig. 1)
Figure 1.
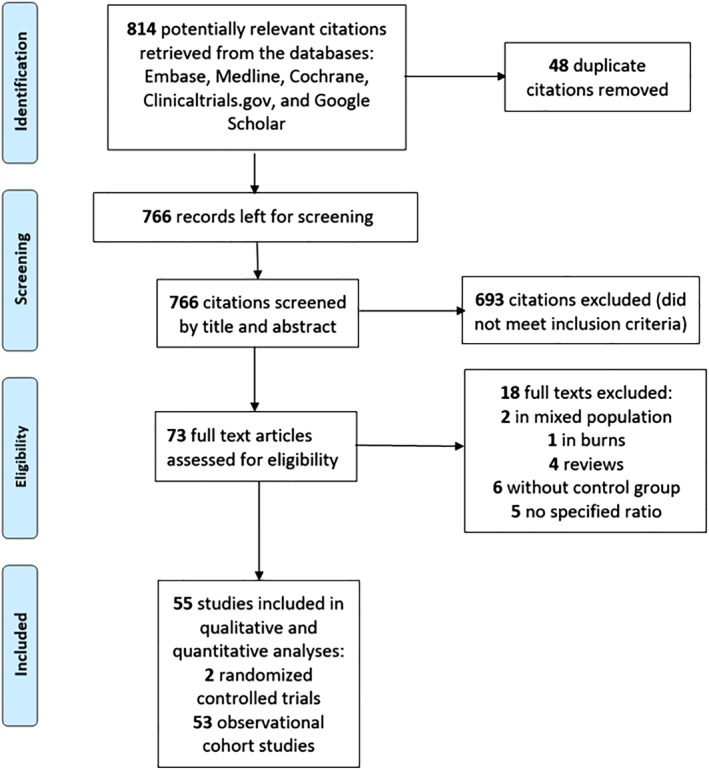
PRISMA flow chart of study selection. [Color figure can be viewed at http://wileyonlinelibrary.com]
The electronic search identified 814 potentially relevant studies of which 73 were selected for full‐text review and from these 55 studies met the inclusion criteria.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 There was excellent agreement between the reviewers for study inclusion (Cohen's Kappa, 0.88).78
Clinical characteristics
RCTs (Table S1 , available as supporting information in the online version of this paper.)
Two RCTs23, 24 were included (n = 749 patients). The study conducted by Holcomb and colleagues23 was a multicenter trial and the study conducted by Nascimento and colleagues24 was a single‐center feasibility trial. The Injury Severity Score of participants across both studies ranged from 26 to 41, and the age ranged from 34 to 41 years. Most participants were male.
Observational cohort studies (Table S1 )
Fifty‐three observational cohort studies25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 were included (n = 27,228 patients). Thirty‐one were retrospective,26, 29, 34, 36, 38, 42, 44, 45, 46, 47, 49, 50, 51, 52, 53, 54, 56, 57, 58, 59, 60, 61, 62, 65, 66, 68, 69, 70, 72, 73, 74, 76, 77 13 were prospective,25, 27, 28, 30, 31, 35, 37, 40, 41, 55, 67, 70, 71 and nine had a before‐and‐after design.31, 32, 39, 43, 48, 63, 64, 68, 75 Nineteen studies were multicenter,27, 28, 29, 32, 37, 41, 46, 49, 50, 51, 54, 55, 56, 57, 60, 69, 70, 72, 74 and 3425, 26, 30, 31, 33, 34, 35, 36, 38, 39, 40, 42, 43, 44, 45, 47, 48, 52, 53, 58, 59, 61, 62, 63, 64, 65, 66, 67, 68, 71, 73, 75, 76, 77 were conducted in single trauma centers. The mean ± SD age of patients across all studies was 38.3 ± 6.8 years and the mean ± SD Injury Severity Score was 31.6 ± 6.2. Most patients were male (mean ± SD 76.5% ± 9.2%).
Interventions
RCTs (Table S1 )
The PROPPR (Pragmatic, Randomized Optimal Platelet and Plasma Ratios) trial23 assessed the ratio of 1:1:1 versus 1:1:2 (FFP:PLTs:RBCs) and the feasibility trial24 assessed the ratio of 1:1:1 versus laboratory‐guided transfusion.
Observational cohort studies (Table S1 )
Different fixed transfusion ratios were evaluated across the studies. Thirty‐three studies28, 29, 30, 33, 34, 35, 36, 37, 38, 39, 40, 42, 46, 48, 51, 52, 53, 55, 56, 57, 58, 59, 61, 62, 64, 66, 67, 70, 71, 72, 73, 76, 77 addressed FFP:RBCs, two54, 65 addressed PLT:RBCs, and 1426, 27, 41, 43, 44, 45, 47, 49, 50, 60, 63, 68, 74, 75 addressed both FFP:RBCs and PLTs:RBCs. Four studies32, 43, 63, 69 examined fibrinogen:RBCs or cryoprecipitate:RBCs and three25, 31, 32 examined FFP:PLTs:RBCs. Ratios of FFP:RBCs and PLTs:RBCs ranged both from 1:1 to 1:8 and from 1:1 to 1:9, respectively.
Risk of bias
RCTs (Table S2 , available as supporting information in the online version of this paper)
Both studies had low risk of bias in all domains assessed. We did not penalize the studies due to lack of blinding because blinding intervention is not feasible in this setting.
Observational cohort studies (Table S2 )
The NOS21 scores were more than 6 for all studies.
OUTCOMES
Mortality
RCTs (Table S3 , available as supporting information in the online version of this paper)
The PROPPR trial23 assessed two different ratios of FFP:PLTs:RBCs (high, 1:1:1 vs. low, 1:1:2) and reported no difference in 24‐hour and 30‐day mortality. However, there was a lower rate of death from exsanguination at 24 hours (9.2% vs. 14.6% in 1:1:2 group; difference, −5.4% [95% CI, −10.4% to −0.5%]; p = 0.03). The other study, a feasibility trial24 not powered for mortality, reported no difference in 28‐day mortality between both groups (1:1:1 vs. laboratory‐guided transfusion), with a relative risk of 2.27 (95% CI = 0.98‐9.63).
Observational cohort studies (Table S3 )
Fifty studies25, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 reported 24‐hour and/or 30‐day mortality. Only six studies30, 40, 54, 57, 66, 76 scored nine in the NOS and demonstrated a low risk of bias. Overall, these 50 studies had an adequate balance of risk factors across the two groups and accounted for confounders in their analyses. They reported that higher transfusion ratios were associated with higher rates of survival. Only one retrospective study40 using regression analyses with Cox proportional hazard models reported no difference in mortality within the first 6 hours after admission across three ratios of FFP:RBCs (1:1.5, 1:1.5‐1:2, and <1:2; p = 0.535).
Exposure to allogeneic blood products
RCTs (Table S3 )
The PROPPR trial23 reported that the 1:1:1 ratio group received more FFP (median of 7 units vs. 5 units, p < 0.001) and PLTs (12 units vs. 6 units, p < 0.001). However, patients received similar amount of RBCs (9 units) within the first 24 hours in both arms. Nascimento and coworkers24 demonstrated no difference in the amount of transfused RBCs, FFP, and PLTs across groups.
Observational cohort studies (Table S3 )
Twenty‐nine studies25, 28, 31, 32, 34, 35, 37, 39, 41, 42, 43, 44, 45, 46, 48, 49, 51, 52, 55, 58, 61, 62, 63, 65, 67, 68, 70, 73, 74 reported comparisons between the number of units transfused in the high‐ and low‐transfusion‐ratio groups. Of these, 11 studies24, 28, 34, 42, 43, 52, 55, 58, 62, 67, 68 reported a statistical difference in RBC transfusion, 2024, 28, 31, 32, 35, 37, 44, 45, 46, 49, 51, 52, 55, 62, 66, 67, 68, 70, 73, 74 reported a statistical difference in FFP transfusion, and 1424, 31, 32, 35, 41, 44, 45, 51, 52, 55, 62, 70, 73, 74 reported a statistical difference in PLT transfusion. A composite outcome of exposure to blood products (RBC + FFP + PLT + fibrinogen products) was reported in only one study.27
Coagulopathy
RCTs (Table S3 )
The PROPPR trial23 reported significant difference in achievement of clinical hemostasis in the 1:1:1 ratio group compared to 1:1:2 (86% vs. 78%, p = 0.006). However, laboratory coagulopathy was not assessed in this trial. The feasibility trial24 assessed values of INR, PLT count, and fibrinogen and reported no significant differences.
Observational cohort studies (Table S3 )
Nine studies24, 28, 34, 36, 44, 53, 64, 67, 69 assessed laboratory coagulation variables. Prothrombin time (PT) and INR were assessed in seven studies28, 34, 36, 44, 53, 64, 67 and showed conflicting results. Bui and coworkers30 reported a nonsignificant difference in values of INR before and after resuscitation (0.09 ± 0.5 to −0.07 ± 0.13, p = 0.37). Kutcher and colleagues35 reported that values of PT normalized in 60 and 20% of patients receiving high and low transfusion ratios, respectively. Sperry and coworkers70 reported no difference between values of INR on admission and after resuscitation (1.76 ± 2 vs. 1.89 ± 1 p = 0.45). Conversely, Kashuk and colleagues73 reported significant increased values of INR 6 hours postresuscitation with higher FFP:RBCs ratio (1.4 ± 0.5 vs. 2.4 ± 9.1, p not reported). ROTEM variables were assessed in a single study37 that showed a nonsignificant trend in the proportion of patients developing coagulopathy according to the number of RBC units transfused (40% on admission, 58% after 4 units of RBCs, and 81% after 8 units of RBCs), as measured by clotting amplitude at 5 minutes. Furthermore, the authors reported that FFP and PLTs did not improve coagulation variables; however, therapy combining FFP, PLTs, and cryoprecipitate improved ROTEM values.
Meta‐analyses (Figs. 2, 3, 4 and Table 1)
Figure 2.
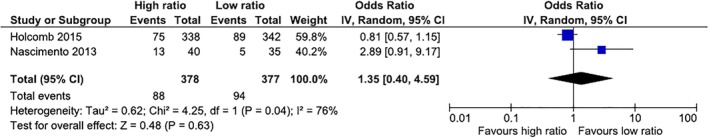
Thirty‐day mortality in RCTs. High ratios in both studies were 1:1:1 (FFP:PLTs:RBCs in Holcomb's and RBCs:FFP:PLTs in Nascimento's trial). Low ratio in Holcomb's trial was 1:1:2 (FFP:PLTs:RBCs) and in Nascimento's trial it was 1.8:1:0.7 (RBCs:FFP:PLTs). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
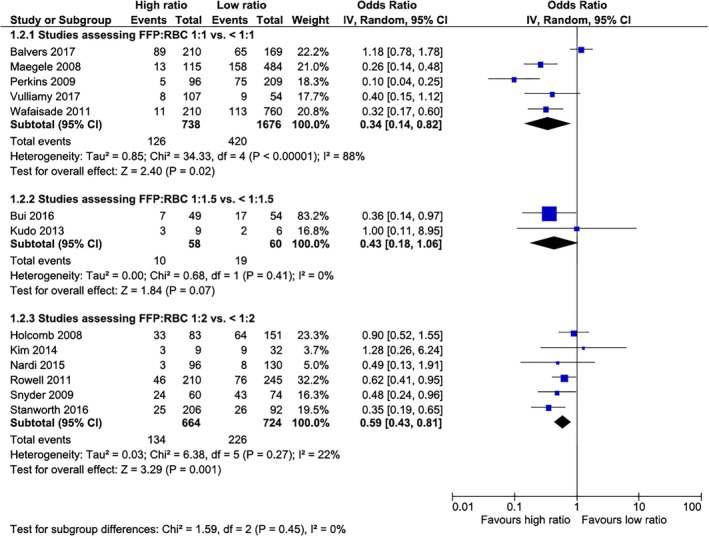
Twenty‐four‐hour mortality in non‐RCTs according to the FFP:RBC ratio assessed. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
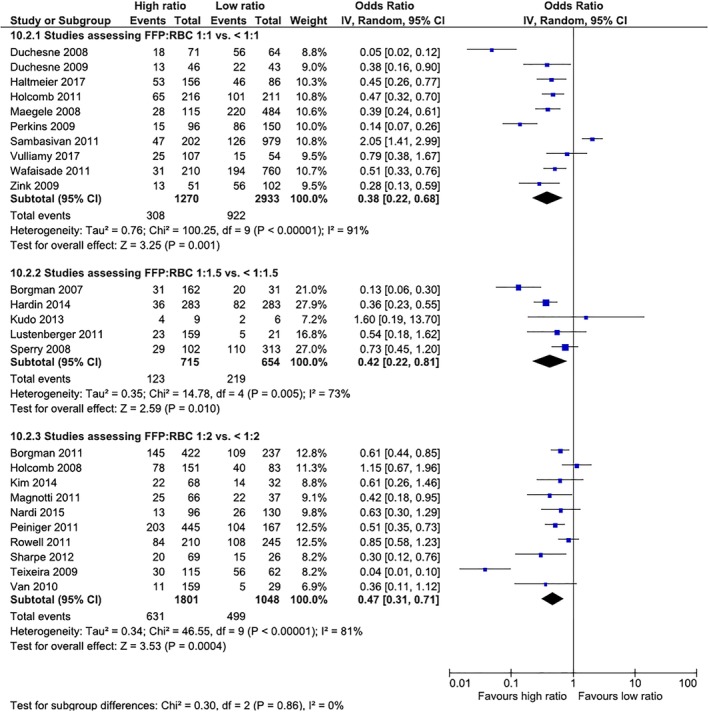
Thirty‐day mortality in non‐RCTs according to the FFP:RBC ratio assessed. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Meta‐analyses on the exposure to allogeneic blood products in the included studies
| Transfusion ratio | Blood product exposure (units) | Pooled mean difference | 95% CI | p (overall effect) | I2 |
|---|---|---|---|---|---|
| RCTs | |||||
| FFP:PLTs:RBCs 1:1:1 vs. <1:1:1 | RBCs | –0.65 | –1.75 to 0.45 | 0.25 | 0% |
| FFP:PLTs:RBCs 1:1:1 vs. <1:1:1 | FFP | 1.91 | 0.92 to 2.90 | 0.0002 | 0% |
| FFP:PLTs:RBCs 1:1:1 vs. <1:1:1 | PLTs | 3.28 | –2.66 to 9.23 | 0.28 | 87% |
| Observational cohort studies | |||||
| FFP:RBCs 1:1 vs. <1:1 | RBCs | –0.68 | –2.68 to 1.33 | 0.51 | 59% |
| FFP:RBCs 1:1 vs. <1:1 | FFP | 8.88 | 3.76 to 14.0 | 0.0007 | 95% |
| FFP:RBCs 1:1 vs. <1:1 | PLTs | 0.80 | –0.75 to 2.36 | 0.31 | 84% |
| FFP:RBCs 1:1.5 vs. <1:1.5 | RBCs | –0.26 | –6.28 to 5.76 | 0.93 | 98% |
| FFP:RBCs 1:1.5 vs. <1:1.5 | FFP | 9.65 | 5.35 to 13.95 | < 0.0001 | 98% |
| FFP:RBCs 1:1.5 vs. <1:1.5 | PLTs | 1.04 | –0.48 to 2.55 | 0.18 | 88% |
| FFP:RBCs 1:2 vs. <1:2 | RBCs | –1.12 | –4.27 to 2.03 | 0.48 | 91% |
| FFP:RBCs 1:2 vs. <1:2 | FFP | 9.24 | 3.95 to 14.53 | 0.0006 | 98% |
| FFP:RBCs 1:2 vs. <1:2 | PLTs | 7.82 | –2.83 to 18.47 | 0.15 | 98% |
| PLTs:RBCs 1:1 vs. <1:1 | RBCs | –0.39 | –5.121 to 4.32 | 0.87 | 86% |
Studies addressing mortality and exposure to allogeneic blood products were combined for the purpose of meta‐analyses (Figs. 2, 3, 4). Studies addressing coagulopathy and other transfusion ratios with fibrinogen or cryoprecipitate, for example, were not included in meta‐analyses as insufficient data were available to be pooled. Furthermore, before‐and‐after studies were not included in the quantitative analyses. Publication bias was not assessed with funnel plots, as the number of studies on each analysis was less than 10. However, the included studies had a mix of small and large sample sizes, with both positive and negative results, which denotes low probability of publication bias. For exposure to allogeneic blood products, studies were combined according to the blood product assessed (FFP:RBCs or PLTs:RBCs) and according to the ratio assessed (Table 1). Four groups of studies were identified according to the fixed transfusion ratios reported, as follows: 1) studies that reported FFP:RBC ratio of 1:1 versus less than 1:1; 2) studies that reported FFP:RBC ratios of 1:1.5 versus less than 1:1.5; 3) studies that reported FFP:RBC ratios of 1:2 versus less than 1:2; and studies that reported PLT:RBC ratio of 1:2 versus less than 1:2. The latter group was represented by only two studies.55, 60 All mortality and transfusion analyses were conducted separately for each study group.
Mortality in the two RCTs was not significantly different (Fig. 2). For observational cohort studies, mortality was lower in the intervention groups in all three different transfusion ratios of FFP:RBCs assessed (1:1 vs. <1:1, 1:1.5 vs. < 1:1.5, and 1:2 vs. <1:2; Fig. 3). For both 24‐hour and 30‐day mortality, results were similar, with a survival benefit in the intervention group for the three ratios assessed, demonstrating a dose effect, with increasing ORs from 1:1, 1:1.5, and 1:2 FFP:RBC transfusion ratios.
We did not find a difference in the number of units of RBCs and PLTs transfused across groups in the RCTs (Table 1). However, greater rates of FFP transfusion were evident in the high‐ratio groups, as expected. In the observational cohort studies, exposure to FFP in all three fixed transfusion ratios assessed was also higher in the intervention groups. Conversely, no difference was found for RBCs and PLTs across all three ratios.
GRADE evidence profile (Tables S4 and S5, available as supporting information in the online version of this paper)
Overall, the evidence was of low quality for both mortality and exposure to allogeneic blood products (see details for each item addressed in the tables).
DISCUSSION
Main findings
This systematic review summarizes the evidence pertaining to the use of high transfusion ratios compared to low transfusion ratios in bleeding adult trauma patients. The evidence is represented by two RCTs and 53 observational studies. The randomized data reported no difference in mortality (with one of the RCTs not powered to detect mortality difference). Additionally, low‐quality observational evidence suggests a survival benefit in all high transfusion ratios assessed (FFP:RBCs of 1:1 vs. <1:1, 1:1.5 vs. <1:1.5, and 1:2 vs. <1:2 and PLT: RBCs 1:2 vs. <1:2) in pooled analyses of observational studies for 24‐hour and 30‐day mortality (primary outcome). Low‐quality evidence demonstrated no difference in exposure to allogeneic blood products within the first 24 hours. As expected, there was increased number of FFP and PLT units transfused in all three fixed ratios groups. Laboratory measures of coagulopathy were reported in a few studies and reported inconsistent results, however, demonstrating an overall trend to improvement of coagulation tests.
Treatment of severely bleeding and coagulopathic trauma patients has evolved considerably over the past two decades. MTPs have been adopted for the treatment of this population.79 Additionally, in limited military observational data, the concept of component therapy with higher fixed transfusion ratios of FFP and/or PLTs to RBCs has shown a survival benefit leading to an extension of its use to civilian trauma population and other settings.80 Currently, most trauma centers use MTPs designed to achieve administration of high amounts of FFP and PLTs early in the resuscitation process.79 The evolving knowledge on fixed component transfusion ratios provided the rationale for three important studies23, 24, 41 that explored the feasibility of 1:1:1 ratio, time to achieve a balanced amount of blood products, and clinical outcomes. The PROMMTT study,41 a multicenter, prospective observational cohort study, was designed to assess the timing of transfusion of blood products and its association with in‐hospital mortality. In this study, most patients achieved a balanced transfusion between 3 and 6 hours postadmission. Authors reported a decreased 24‐hour mortality, which corroborates with previous data.77 The second study, a RCT,24 showed that 1:1:1 ratio of FFP:PLTs:RBCs was feasible; however, the authors reported a high rate of wastage of FFP. The trial was not powered to detect mortality difference. The PROPPR trial23 was the first RCT to assess 1:1:1 ratio of FFP and PLTs to RBCs, compared to 1:1:2 ratio, and assess mortality as primary outcome. The study did not show mortality difference at 24 hours or 30 days postadmission. However, PROPPR addressed several limitations from previous RCTs, such as lack of blinding treatment assignment, survival and selection biases, and small sample sizes. An important limitation was that the trial was not powered to detect a smaller, but still clinically meaningful, difference. As a consequence, the trial did not exclude a potential benefit of the 1:1:1 ratio of less than 10% for 24‐hour mortality. A difference in mortality due to exsanguination was identified. The authors recommended a 1:1:1 ratio to be used while patients are still actively bleeding and then transitioning to a lab‐guided strategy once bleeding is controlled. These well‐designed studies corroborated with previous evidence which emphasized that clinicians should use a higher rate of FFP:RBCs and PLTs:RBCs while activating their MTPs.
More recently, clinicians are evaluating a targeted goal‐direct approach.81 Conventional laboratory tests such as PT and INR, initially used to guide transfusion, have now been replaced by viscoelastic tests in some American and European centers.81 TEG and ROTEM can identify the specific coagulation defects and guide the clinicians to transfuse the most appropriate blood products.14, 81 Several studies in trauma and nontrauma settings have demonstrated that these tests are able to identify the nuances of ATC and predict need for massive transfusion.82 An RCT83 that assessed trauma patients randomized to either TEG‐directed therapy, or conventional coagulation assays, demonstrated that the former resulted in similar numbers of RBCs but less FFP and PLTs transfused within the first 2 hours of resuscitation, and 28‐day survival improved. In cardiac surgery, a meta‐analysis84 of 8332 patients (nine RCTs and seven observational studies) reported a significant decrease in the odds of receiving allogeneic blood products, decreased reoperations due to postoperative hemorrhage, decreased postoperative acute kidney injury, and fewer thromboembolic events. It is expected that the role of TEG and ROTEM to target specific coagulation defects in different clinical and surgical settings will be defined within the near future.
In parallel, the use of FFP in trauma resuscitation has been done in the prehospital setting. Recently, two RCTs85, 86 using FFP during transport of bleeding trauma patients demonstrated a survival benefit, calling attention for a possible advantage in using FFP or other clotting factors very early after injury. Additionally, plasma has been replaced or supplemented with other concentrate of clotting factors in other clinical/surgical settings in some European countries. Some centers have been adopting different plasma‐derived and recombinant coagulation factor preparations, such as PCC (Octaplex), fibrinogen concentrate (RiasTAP), and other coagulation factors.14, 16, 87 The use is variable in different clinical settings, including trauma. For example, in a retrospective study,87 bleeding trauma patients receiving PCC and/or fibrinogen concentrate without FFP were matched to similar patients receiving FFP without coagulation factor concentrates. Patients receiving PCC were transfused with less allogeneic blood products and had less multiple organ failure but had no survival benefit. Further evidence of non‐FFP/PLT use has been growing and experimental research is under way, such as in transfusion of cold‐stored and frozen PLTs,88, 89 fibrinogen concentrate administration,14, 15, 16, 17, 90, 91 and cryoprecipitate14, 15, 16, 17, 92 and the use of antifibrinolytic drugs such as tranexamic acid.14, 15, 16, 17, 93, 94, 95
Strengths and weaknesses of this review and future research
This is the first meta‐analyses to report exposure to allogeneic blood products in resuscitation using fixed component transfusion ratios. This review also updates the evidence previously published in other systematic reviews.96, 97, 98, 99 In addition, this is the first review assessing transfusion ratios of FFP and PLT in trauma patients exclusively and to use the GRADE profile to assess the evidence in trauma patients. The main limitation of the review is that most data are observational and thus survival bias, confounding, and publication bias are unavoidable. Furthermore, retrospective data included different definitions of high and low fixed transfusion ratios, which limited the analysis and pooling of the data between which constitutes high and low ratios uniformly across the studies. Therefore, due to the variability of the definition and center‐specific transfusion protocols with differences in blood and blood products' availability and patient's characteristics, we followed what the authors defined as high and low ratios based on their settings, adopting a very pragmatic methodologic approach to our current analyses. Future research is strongly warranted, including well‐designed prospective or RCTs to generate a more robust knowledge on which population will best benefit from fixed component transfusion, including which component and at which ratio will it be beneficial for important outcomes. Future studies are needed to address substitutes of component therapy, such as whole blood, cryoprecipitate, fibrinogen concentrate, Factor XIII, PCC, and antifibrinolytic drugs or a combination of these. Moreover, the goal‐directed approach offered by TEG and ROTEM still needs its place established in trauma and other perioperative and clinical settings, and studies should include meaningful clinical outcomes as primary endpoints.
CONCLUSIONS
Randomized data have not shown a mortality benefit from a higher transfusion ratio of 1:1:1 compared with 1:1:2 or standard care. Additionally, low quality observational evidence demonstrates a survival benefit, and conflicting results on exposure to allogeneic blood products and improvement of coagulopathy in patients who received high‐transfusion ratios. However, these results should be interpreted with extreme caution, as the research in this area is limited by relatively small sample sizes, lack of clinical trials, and the observational nature of most studies with nonrandom treatment allocation and the high probability for confounding. Additionally, fixed transfusion ratios may be potentially replaced in the future with a more targeted approach with different plasma‐derived and recombinant coagulation factor preparations using viscoelastic tests and decreasing the use of allogeneic blood products. Ultimately, larger prospective RCTs with several thousands of patients would be required to determine the best transfusion ratio or determine if the goal‐directed approach is the answer, which might not be ever feasible due to challenges in timely recruitment of severely bleeding trauma patients and costs involved. Currently, a balanced approach to transfusion while patients are actively bleeding is accepted by American and European guidelines.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Table S1. Characteristics of the studies included in the systematic review
Table S2. Main findings of the included studies in the systematic review
Table S3. Risk of bias assessment of the studies included in the systematic review
Table S4. GRADE quality assessment profile for mortality and summary of findings
Table S5. GRADE quality assessment profile for exposure to allogeneic blood products and summary of findings
ACKNOWLEDGMENTS
We thank Mr. Henry Lam for assistance with the search strategy. This manuscript was performed under contract with DRDC (Defense research and Development Canada) and CIMVHR (Canadian Institute for Military and Veteran Health research).
Correction added on 6 November, 2019, after first online publication: The originally published abstract was incomplete. The full abstract has now been included.
REFERENCES
- 1.US Centers for Disease Control and Prevention. Injury prevention and control: data and statistics (WISQARS), 2014. Accessed September 2019.
- 2. Gruen RL, Jurkovich GJ, McIntyre LK, et al. Patterns of errors contributing to trauma mortality: lessons learned from 2,594 deaths. Ann Surg 2006;244:371‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma 2008;65:748‐54. [DOI] [PubMed] [Google Scholar]
- 4. Tisherman SA, Schmicker RH, Brasel KJ, et al. Detailed description of all deaths in both the Shock and Traumatic Brain Injury Hypertonic Saline Trials of the Resuscitation Outcomes Consortium. Ann Surg 2014;252:149‐57. 10.1097/SLA.0b013e3181df0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stahel P, Moore E, Schreier L, et al. Transfusion strategies in postinjury coagulopathy. Curr Opin Anaesthesiol 2009;22:289‐98. [DOI] [PubMed] [Google Scholar]
- 6. Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011;254:598‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johansson PI, Srensen AM, Larsen CF, et al. Low hemorrhage‐related mortality in trauma patients in a level I trauma center employing transfusion packages and early thromboelastography‐directed hemostatic resuscitation with plasma and platelets. Transfusion 2013;53:3088‐99. [DOI] [PubMed] [Google Scholar]
- 8. Langan NR, Eckert M, Martin MJ. Changing patterns of in‐hospital deaths following implementation of damage control resuscitation practices in US forward military treatment facilities. JAMA Surg 2014;149:904‐12. [DOI] [PubMed] [Google Scholar]
- 9. Johnson JL, Moore EE, Kashuk JL, et al. Effect of blood products transfusion on the development of post injury multiple organ failure. Arch Surg 2010;145:973‐7. [DOI] [PubMed] [Google Scholar]
- 10. Da Luz LT, Nascimento B, Rizoli S. Thromboelastography (TEG): practical considerations on its clinical use in trauma resuscitation. Scand J Trauma Resusc Emerg Med 2013;21:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Da Luz LT, Nascimento B, Shankarakutty AK, et al. Effect of thromboelastography (TEG) and rotational thromboelastometry (ROTEM) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Crit Care 2014;18:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veigas PV, Callum J, Rizoli S, et al. A systematic review on the rotational thromboelastometry (ROTEM) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med 2016;24:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brohi K, Eaglestone S. Traumatic coagulopathy and massive transfusion: improving outcomes and saving blood. South Hampton, UK: NIHR Journals Library; 2017. Programme Grants for Applied Research. [PubMed]
- 14. Johansson PI, Stensballe J, Oliveri R, et al. How I treat patients with massive hemorrhage. Blood 2014;124:3052‐8. [DOI] [PubMed] [Google Scholar]
- 15. Cannon, JW , Khan M, Raja AS, et al. Damage control resuscitation in patients with severe traumatic hemorrhage [Internet]. Chicago: Eastern Association for the Surgey of Trauma; 2017. [cited 2019 Apr 17]. Available from: https://www.east.org/education/practice-management-guidelines/damage-control-resuscitation-in-patients-with-severe-traumatic-hemorrhage. [DOI] [PubMed]
- 16. Glen J, Constanti M, Brohi K, et al. Assessment and initial management of major trauma: summary of NICE guideline. BMJ 2016;353:i3051. [DOI] [PubMed] [Google Scholar]
- 17.ACS TQIP best practice guidelines [Internet]. Chicago: American College of Surgeons; 2018 [cited 2019 Apr 17]. Available from: https://www.facs.org/quality-programs/trauma/tqp/center-programs/tqip/best-practice.
- 18. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1‐e34. [DOI] [PubMed] [Google Scholar]
- 19. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008‐12. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses [Internet]. Ottawa: Ottawa Hospital Research Institute; c2019 [cited 2013 Mar]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 22. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nascimento B, Callum J, Tien H, et al. Effect of a fixed‐ratio (1:1:1) transfusion protocol versus laboratory‐results‐guided transfusion in patients with severe trauma: a randomized feasibility trial. CMAJ 2013;185:E583‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vulliamy P, Gillespie S, Gall LS, et al. Platelet transfusions reduce fibrinolysis but do not restore platelet function during trauma hemorrhage. J Trauma Acute Care Surg 2017;83:388‐97. [DOI] [PubMed] [Google Scholar]
- 26. Haltmeier T, Benjamin E, Gruen JP, et al. Decreased mortality in patients with isolated severe blunt traumatic brain injury receiving higher plasma to packed red blood cells transfusion ratio. Injury 2017;49:62‐6. [DOI] [PubMed] [Google Scholar]
- 27. Balvers K, van Dieren S, Baksaas‐Aasen K, et al. Combined effect of therapeutic strategies for bleeding injury on early survival, transfusion needs and correction of coagulopathy. Br J Surg 2017;104:222‐9. [DOI] [PubMed] [Google Scholar]
- 28. Stanworth SJ, Davenport R, Curry N, et al. Mortality from trauma haemorrhage and opportunities for improvement in transfusion practice. Br J Surg 2016;103:357‐65. [DOI] [PubMed] [Google Scholar]
- 29. Hagiwara A, Kushimoto S, Kato H, et al. Can early aggressive administration of fresh frozen plasma improve outcomes in patients with severe blunt trauma? A report by the Japanese Association for the Surgery of Trauma. Shock 2016;45:495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bui E, Inaba K, Ebadat A, et al. The impact of increased plasma ratios in massively transfused trauma patients: a prospective analysis. Eur J Trauma Emerg Surg 2016;42:519‐25. [DOI] [PubMed] [Google Scholar]
- 31. Baysinger K, Barnett ME, Ott M, et al. What's in the Box? The effectiveness of a low‐volume massive transfusion protocol. Am Surg 2016;82:602‐27. [PubMed] [Google Scholar]
- 32. Nardi G, Agostini V, Rondinelli B, et al. Trauma‐induced coagulopathy: impact of the early coagulation support protocol on blood product consumption, mortality and costs. Crit Care 2015;19:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glaser J, Vasquez M, Cardarelli C, et al. Ratio‐driven resuscitation predicts early fascial closure in the combat wounded. J Trauma Acute Care Surg 2015;79(Suppl 2):S188‐92. [DOI] [PubMed] [Google Scholar]
- 34. Mitra B, Gabbe BJ, Kaukonen KM, et al. Long‐term outcomes of patients receiving a massive transfusion after trauma. Shock 2014;42:307‐12. [DOI] [PubMed] [Google Scholar]
- 35. Kutcher ME, Kornblith LZ, Vilardi RF, et al. The natural history and effect of resuscitation ratio on coagulation after trauma: a prospective cohort study. Ann Surg 2014;260:1103‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim Y, Lee K, Kim J, et al. Application of damage control resuscitation strategies to patients with severe traumatic hemorrhage: review of plasma to packed red blood cell ratios at a single institution. J Korean Med Sci 2014;29:1007‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khan S, Davenport R, Raza I, et al. Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma hemorrhage. Intensive Care Med 2015;41:239‐47. [DOI] [PubMed] [Google Scholar]
- 38. Hardin MO, Ritchie JD, Aden JK, et al. Plasma‐to‐red cell ratio and mechanism of injury in massively transfused combat casualties. Mil Med 2014;179:92‐8. [DOI] [PubMed] [Google Scholar]
- 39. Kutcher ME, Komblith LZ, Narayan R, et al. A paradigm shift in trauma resuscitation: evaluation of evolving massive transfusion practices. JAMA Surg 2013;148:834‐40. [DOI] [PubMed] [Google Scholar]
- 40. Kudo D, Sasaki J, Akaishi S, et al. Efficacy of a high FFP: PRBC transfusion ratio on the survival of severely injured patients: a retrospective study in a single tertiary emergency center in Japan. Surg Today 2014;44:653‐61. [DOI] [PubMed] [Google Scholar]
- 41. Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time‐varying treatment with competing risks. JAMA Surg 2013;148:127‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Halmin M, Boström F, Brattström O, et al. Effect of plasma‐to‐RBC ratios in trauma patients: a cohort study with time‐dependent data. Crit Care Med 2013;41:1905‐14. [DOI] [PubMed] [Google Scholar]
- 43. Sisak K, Soeyland K, McLeod M, et al. Massive transfusion in trauma: blood product ratios should be measured at 6 hours. ANZ J Surg 2012;82:161‐7. [DOI] [PubMed] [Google Scholar]
- 44. Sharpe JP, Weinberg JA, Magnotti LJ, et al. Accounting for differences in transfusion volume: are all massive transfusions created equal? J Trauma Acute Care Surg 2012;72:1536‐40. [DOI] [PubMed] [Google Scholar]
- 45. Brown JB, Cohen MJ, Minei JP, et al. Debunking the survival bias myth: characterization of mortality during the initial 24 hours for patients requiring massive transfusion. J Trauma Acute Care Surg 2012;73:358‐64. [DOI] [PubMed] [Google Scholar]
- 46. Wafaisade A, Maegele M, Lefering R, et al. High plasma to red blood cell ratios are associated with lower mortality rates in patients receiving multiple transfusion (4 ≤ red blood cell units <10) during acute trauma resuscitation. J Trauma 2011;70:81‐8. [DOI] [PubMed] [Google Scholar]
- 47. Spinella PC, Wade CE, Blackbourne LH, et al. The association of blood component use ratios with the survival of massively transfused trauma patients with and without severe brain injury. J Trauma 2011;71(Suppl 3):S343‐52. [DOI] [PubMed] [Google Scholar]
- 48. Simmons JW, White CE, Eastridge BJ, et al. Impact of improved combat casualty care on combat wounded undergoing exploratory laparotomy and massive transfusion. J Trauma 2011;71(Suppl):S82‐6. [DOI] [PubMed] [Google Scholar]
- 49. Sambasivan CN, Kunio NR, Nair PV, et al. High ratios of plasma and platelets to packed red blood cells do not affect mortality in nonmassively transfused patients. J Trauma 2011;71(Suppl 3):S329‐36. [DOI] [PubMed] [Google Scholar]
- 50. Rowell SE, Barbosa RR, Diggs BS, et al. Effect of high product ratio massive transfusion on mortality in blunt and penetrating trauma patients. J Trauma 2011;71(Suppl 3):S353‐7. [DOI] [PubMed] [Google Scholar]
- 51. Peiniger S, Nienaber U, Lefering R, et al. Balanced massive transfusion ratios in multiple injury patients with traumatic brain injury. Crit Care 2011;15:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Magnotti LJ, Zarzaur BL, Fischer PE, et al. Improved survival after hemostatic resuscitation: does the emperor have no clothes? J Trauma 2011;70:97‐102. [DOI] [PubMed] [Google Scholar]
- 53. Lustenberger T, Frischknecht A, Brüesch M, et al. Blood component ratios in massively transfused, blunt trauma patients – a time dependent covariate analysis. J Trauma 2011;71:1144‐50. [DOI] [PubMed] [Google Scholar]
- 54. Holcomb JB, Zarzabal LA, Michalek JE, et al. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma 2011;71(Suppl 3):S318‐28. [DOI] [PubMed] [Google Scholar]
- 55. Davenport R, Curry N, Manson J, et al. Hemostatic effects of fresh frozen plasma may be maximal at red cell ratios of 1:2. J Trauma 2011;70:90‐5. [DOI] [PubMed] [Google Scholar]
- 56. Brown LM, Aro SO, Cohen MJ, et al. A high fresh frozen plasma: packed red blood cell transfusion ratio decreases mortality in all massively transfused trauma patients regardless of admission international normalized ratio. J Trauma 2011;71(Suppl 3):S358‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Borgman MA, Spinella PC, Holcomb JB, et al. The effect of FFP: RBC ratio on morbidity and mortality in trauma patients based on transfusion prediction score. Vox Sang 2011;101:44‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Van PY, Sambasivan CN, Wade CE, et al. High transfusion ratios are not associated with increased complication rates in patients with severe extremity injuries. J Trauma 2010;69(Suppl 1):S64‐8. [DOI] [PubMed] [Google Scholar]
- 59. Mitra B, Mori A, Cameron PA, et al. Fresh frozen plasma (FFP) use during massive blood transfusion in trauma resuscitation. Injury 2010;41:35‐9. [DOI] [PubMed] [Google Scholar]
- 60. Zink KA, Sambasivan CN, Holcomb JB, et al. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg 2009;197:565‐70. [DOI] [PubMed] [Google Scholar]
- 61. Teixeira PG, Inaba K, Shulman I, et al. Impact of plasma transfusion in massively transfused trauma patients. J Trauma 2009;66:693‐7. [DOI] [PubMed] [Google Scholar]
- 62. Snyder CW, Weinberg JA, McGwin G Jr, et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma 2009;66:358‐62. [DOI] [PubMed] [Google Scholar]
- 63. Shaz BH, Dente CJ, Nicholas J, et al. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion 2010;50:493‐500. [DOI] [PubMed] [Google Scholar]
- 64. Riskin DJ, Tasi TC, Riskin L, et al. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg 2009;209:198‐205. [DOI] [PubMed] [Google Scholar]
- 65. Perkins JG, Cap AP, Spinella PC, et al. An evaluation of the impact of apheresis platelets used in te setting of massively transfused trauma patients. J Trauma 2009;66(Suppl):S77‐84. [DOI] [PubMed] [Google Scholar]
- 66. Duchesne JC, Islam TM, Stuke L, et al. Hemostatic resuscitation during surgery improves survival in patients with traumatic‐induced coagulopathy. J Trauma 2009;67:33‐7. [DOI] [PubMed] [Google Scholar]
- 67. Dente CJ, Shaz BH, Nicholas JM, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma 2009;66:1616‐24. [DOI] [PubMed] [Google Scholar]
- 68. Cotton BA, Au BK, Nunez TC, et al. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma 2009;66:41‐8. [DOI] [PubMed] [Google Scholar]
- 69. Stinger HK, Spinella PC, Perkins JG, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma 2008;64(Suppl):S79‐85. [DOI] [PubMed] [Google Scholar]
- 70. Sperry JL, Ochoa JB, Gunn SR, et al. An FFP:PRBC transfusion >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma 2008;65:986‐93. [DOI] [PubMed] [Google Scholar]
- 71. Scalea TM, Bochicchio KM, Lumpkins K, et al. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg 2008;248:578‐84. [DOI] [PubMed] [Google Scholar]
- 72. Maegele M, Lefering R, Paffrath T, et al. Red‐blood‐cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Geselleschaft für Unfallchirurgie. Vox Sang 2008;95:112‐9. [DOI] [PubMed] [Google Scholar]
- 73. Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma: packed red blood cells the answer? J Trauma 2008;65:261‐70. [DOI] [PubMed] [Google Scholar]
- 74. Holcomb JB, Wade CE, MIchalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg 2008;248:447‐58. [DOI] [PubMed] [Google Scholar]
- 75. Gunter OL Jr, Au BK, Isbell JM, et al. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma 2008;65:527‐34. [DOI] [PubMed] [Google Scholar]
- 76. Duchesne JC, Hunt JP, Wahl G, et al. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma 2008;65:272‐6. [DOI] [PubMed] [Google Scholar]
- 77. Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma 2007;63:805‐13. [DOI] [PubMed] [Google Scholar]
- 78. Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull 1968;70:213‐20. [DOI] [PubMed] [Google Scholar]
- 79. Flint AWJ, McQuilten ZK, Wood EM. Massive transfusions for critical bleeding: is everything old new again? Transfus Med 2018;28:140‐9. [DOI] [PubMed] [Google Scholar]
- 80. Callum J, Nascimento B, Alam A. Massive haemorrhage protocol: what's the best protocol? ISBT Sci Ser 2016;11:297‐306. [Google Scholar]
- 81. Curry NS, Davenport R, Pavord S, et al. The use of viscoelastic haemostatic assays in the management of major bleeding. A British Society for Haematology Guideline. Br J Haematol 2018;182:789‐806. 10.1111/bjh.15524. [DOI] [PubMed] [Google Scholar]
- 82. Hagemo JS, Christiaans SC, Stanworth SJ, et al. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Crit Care 2015;19:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gonzalez DE, Moore EE, Moore HB, et al. Goal‐directed hemostatic resuscitation of trauma‐induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg 2016;263:1051‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Deppe AC, Weber C, Zimmermann J, et al. Point‐of‐care thromboelastography/thromboelastometry‐based coagulation management in cardiac surgery: a meta‐analysis of 8332 patients. J Surg Res 2016;203:424‐33. [DOI] [PubMed] [Google Scholar]
- 85. Sperry JL, Guyette FX, Brown JB, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med 2018;379:315‐26. [DOI] [PubMed] [Google Scholar]
- 86. Moore HB, Moore EE, Chapman MP, et al. Plasma‐first resuscitation to treat hemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet 2018;392:283‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nienaber U, Innerhofer P, Westermann I, et al. The impact of fresh frozen plasma vs coagulation factor concentrates on morbidity and mortality in trauma‐associated haemorrhage and massive transfusion. Injury 2011;42:697‐701. [DOI] [PubMed] [Google Scholar]
- 88. Noorman F, van Dongen TT, Plat MJ, et al. Transfusion: −80°C frozen blood products are safe and effective in military casualty care. PLoS One 2016:12, e0168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Aubron C, Flint AW, Ozier Y, et al. Transfusion of stored platelets: balancing risks and product availability. Int J Clin Trans Med 2016;4:133‐8. [Google Scholar]
- 90. McQuilten ZK, Bailey M, Cameron PA, et al. Fibrinogen concentration and use of fibrinogen supplementation with cryoprecipitate in patients with critical bleeding receiving massive transfusion: a bi‐national cohort study. Br J Haematol 2016;179:131‐41. [DOI] [PubMed] [Google Scholar]
- 91. Collins PW, Solomon C, Sutor K. Theoretical modelling of fibrinogen supplementation with therapeutic plasma, cryoprecipitate, or fibrinogen concentrate. Br J Anaesth 2014;113:585‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Curry N, Rourke C, Davenport R, et al. Early cryoprecipitate for major haemorrhage in trauma: a randomized controlled feasibility trial. Br J Anaesth 2015;115:76‐83. [DOI] [PubMed] [Google Scholar]
- 93. Ramirez RJ, Spinella PC, Bochicchio GV. Tranexamic acid update in trauma. Crit Care Clin 2017;33:85‐99. [DOI] [PubMed] [Google Scholar]
- 94. Meizoso JP, Dudaryk R, Mulder MB, et al. Increased risk of fibrinolysis shutdown among severely injured trauma patients receiving tranexamic acid. J Trauma Acute Care Surg 2018;84:426‐32. [DOI] [PubMed] [Google Scholar]
- 95. Moore EE, Moore HB, Gonzalez E, et al. Rationale for the selective administration of tranexamic acid to inhibit fibrinolysis in the severely injured patient. Transfusion 2016;56(Suppl 2):S110‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bhangu A, Nepogodiev D, Doughty H, et al. Meta‐analysis of plasma to red blood cell ratios and mortality in massive blood transfusions for trauma. Inj Int J Care Inj 2013;44:1693‐9. [DOI] [PubMed] [Google Scholar]
- 97. Hallet J, Lauzier F, Mailloux O, et al. The use of higher platelet: RBC transfusion ratio in the acute phase of trauma resuscitation: A systematic review. Crit Care Med 2013;41:2800‐11. [DOI] [PubMed] [Google Scholar]
- 98. Rahouma M, Kamel M, Jodeh D, et al. Does a balanced transfusion ratio of plasma to packed red blood cells improve outcomes in both trauma and surgical patients? A meta‐analysis of randomized controlled trials and observational studies. Am J Surg 2018;216:342‐50. [DOI] [PubMed] [Google Scholar]
- 99. McQuilten ZK, Crighton G, Brunskill S, et al. Optimal dose, timing and ratio of blood products in massive transfusion: results from a systematic review. Transfus Med Rev 2018;32:6‐15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of the studies included in the systematic review
Table S2. Main findings of the included studies in the systematic review
Table S3. Risk of bias assessment of the studies included in the systematic review
Table S4. GRADE quality assessment profile for mortality and summary of findings
Table S5. GRADE quality assessment profile for exposure to allogeneic blood products and summary of findings


