Abstract
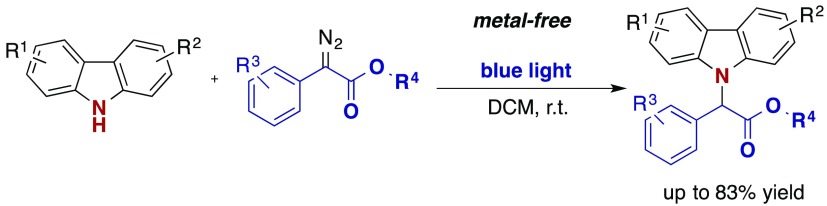
Metal-free N–H functionalization reactions represent an important strategy for sustainable C–N coupling reactions. In this report, we describe the visible light photolysis of aryl diazoacetates in the presence of some N-heterocycles that enables mild, metal-free N–H functionalization reactions of carbazole and azepine heterocycles (15 examples, up to 83% yield).
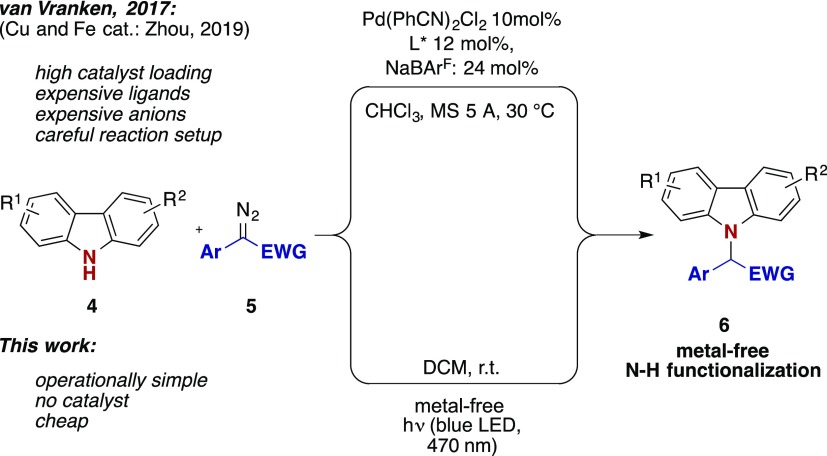
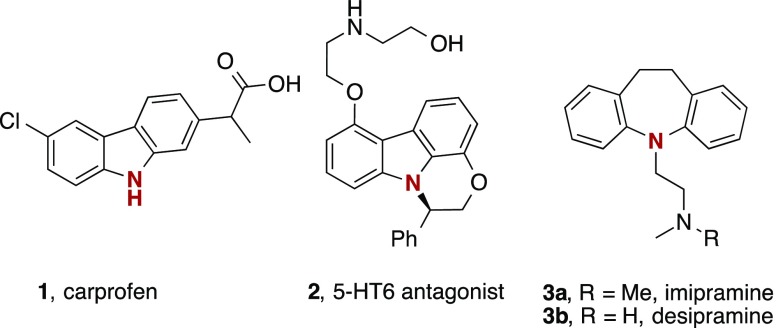
Palladium, copper, or even iron catalysts have been utilized to enable the (enantioselective) insertion of carbenes into the N–H bond of carbazoles (Scheme 1) to yield N-functionalized carbazoles.1,2 Although highly efficient, the limitations of these methods lie in the necessity of high catalyst loadings, the application of expensive ligands, and weakly coordinating anions (i.e., BArF), which impacts the atom efficiency of the overall process.3N-Functionalized carbazoles are particularly important as biologically active compounds and pharmaceuticals,4 and are of prime interest in the fields of organic materials and polymers (Figure 1).5 A broad array of synthetic methods exist to either de novo synthesize functionalized carbazole scaffolds,6 or to selectively introduce new functional groups onto an appropriately prefunctionalized carbazole ring. The direct functionalizations of C–H or N–H bonds of the parent heterocycle via cross-dehydrogenative coupling, C–H activation, or the direct insertion of highly reactive carbenes or radicals are examples that allow the most atom-economic strategy to decorate the carbazole framework and is thus of major interest in organic synthesis.7
Scheme 1. Carbene N-Carbazolation.
Figure 1.
Bioactive compounds based on carbazole and dibenzoazepine.
Metal-free carbene transfer reactions8 represent a long-standing challenge in synthetic methodology, and typical approaches involve e.g. the UV photolysis9 or the thermolysis of diazoalkanes,10 yet with limitations to substrate scope and/or applicability. Only recently, the visible light photolysis of diazoalkanes8,11−14 attracted the interest of synthetic chemists and is currently emerging as an attractive pathway toward sustainable carbene transfer reactions. This methodology now enables the selective photolysis of diazoalkanes in the presence of noncolored substrates under mild reaction conditions while other reaction partners and/or products remain untouched. In the past year, different groups reported on their efforts in metal-free cycloaddition,11,12 rearrangement,12,13 esterification,11 N–H functionalization of basic amines,11 or olefination reactions.14 Indole was reported to undergo an efficient C–H functioanlization reaction with aryldiazoacetates under photochemical conditions.11 In this context, the development of a (metal-free) insertion reaction of a carbene fragment into the N–H bond of carbazoles would open up new pathways to valuable compounds or late stage functionalization of well-known drugs (Scheme 1). In view of our interest in modern synthetic methods revolving around the strategic carbazole structure7 and carbene transfer reactions,12,13,15 we envisioned a metal-free synthetic scenario for the N–H functionalization of heterocycles via the visible light photolysis of diazoalkanes, which would avoid the usual transition metal complexes, ligands, and additives.
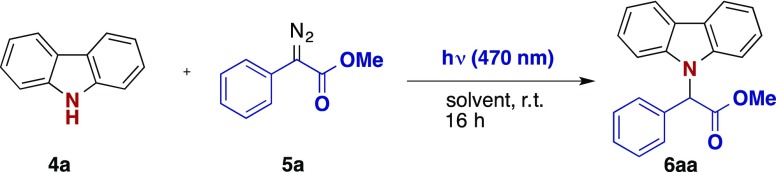
In a first step the model reaction of carbazole 4a with methyl phenyldiazoacetate (5a) was investigated. Different solvents, concentrations, and equivalents were tested, to optimize the reaction conditions (Table 1). We identified DCM as a suitable solvent and verified that neither increasing nor decreasing the reaction concentration improved the yield (entries 13–14). Moreover, an excess of the diazo coupling partner was beneficial to the yield. The change to a slow addition protocol increased the yield slightly (entry 12). Importantly, no reaction was observed when the reaction mixture is kept in the dark (entry 15). Notably, in all reactions, exclusive N–H functionalization occurred and the product of a formal C–H functionalization was not observed.
Table 1. Reaction Optimization.
| no.a | solvent | 4a:5a ratio | other | yield (%) |
|---|---|---|---|---|
| 1 | toluene | 1:2 | 50 | |
| 2 | n-hexane | 1:2 | 24 | |
| 3 | CHCl3 | 1:2 | 68 | |
| 4 | DCM | 1:2 | 80 | |
| 5 | 1,2-DCE | 1:2 | 73 | |
| 6 | EtOAc | 1:2 | 67 | |
| 7 | CH3CN | 1:2 | 69 | |
| 8 | THF | 1:2 | 67 | |
| 9 | DCM | 1:4 | 60 | |
| 10 | DCM | 1:1 | 76 | |
| 11 | DCM | 2:1 | 55 | |
| 12b | DCM | 1:2 | slow add. | 83 |
| 13 | DCM | 1:2 | 0.5 mL | 76 |
| 14 | DCM | 1:2 | 2 mL | 71 |
| 15c | DCM | 1:2 | dark | no reaction |
Reaction conditions: 4a (0.4 mmol) and 5a (0.8 mmol) were dissolved in 1 mL of solvent and were irradiated at room temperature with blue LEDs (1 W, 470 nm) overnight (16 h).
4a and 5a were each dissolved in 0.5 mL of DCM, 5a being slowly added over the course of 6 h by a syringe pump, under otherwise identical conditions.
The reaction mixture was stirred in the dark.
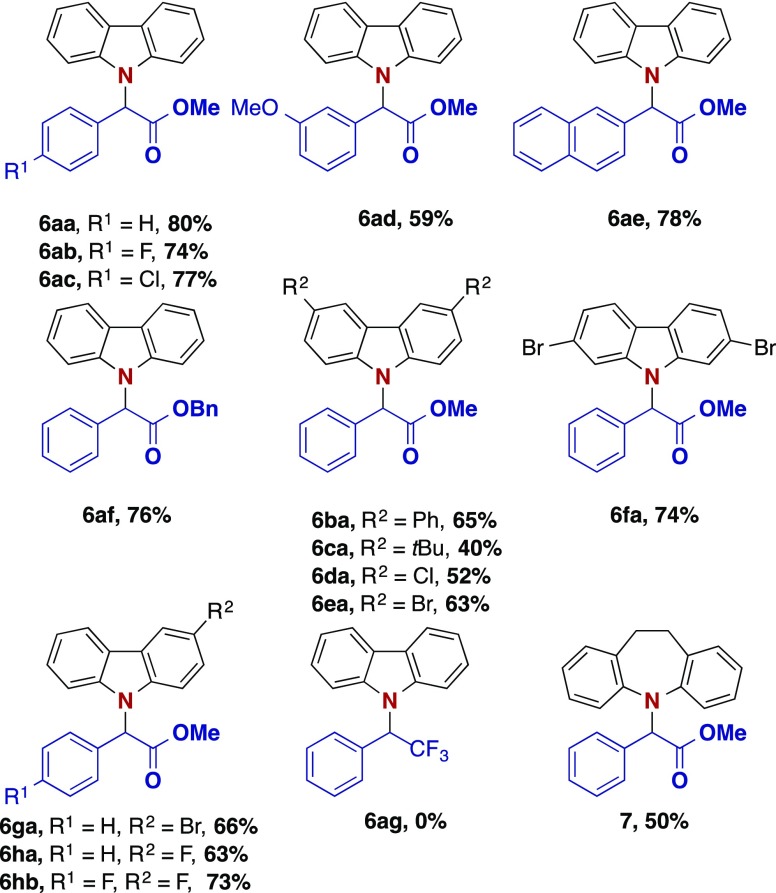
With these very simple optimized conditions in hand (Table 1, entry 4), we next explored the functional group tolerance on both the carbazole 4 and the diazoalkane 5 (Scheme 2). Different phenyl diazoacetates were compatible with the optimized reaction conditions, and the N–H functionalization products were obtained in moderate to good yields (Scheme 2). Furthermore, diverse functional groups were tolerated, notably a number of halides (X = F, Cl, Br), leaving the possibility for further functionalization reactions. Notably, no product formation was observed when changing the diazo compound to (1-diazo-2,2,2-trifluoroethyl)benzene. In a last step mono- and disubstituted carbazole derivatives were investigated, and the corresponding insertion products were isolated in moderate to good yield. To our delight 10,11-dihydro-5H-dibenz[b,f]azepine, an important bioactive antidepressant drug precursor16 showed promising reactivity (product 7, 50%).
Scheme 2. Reaction Scope.
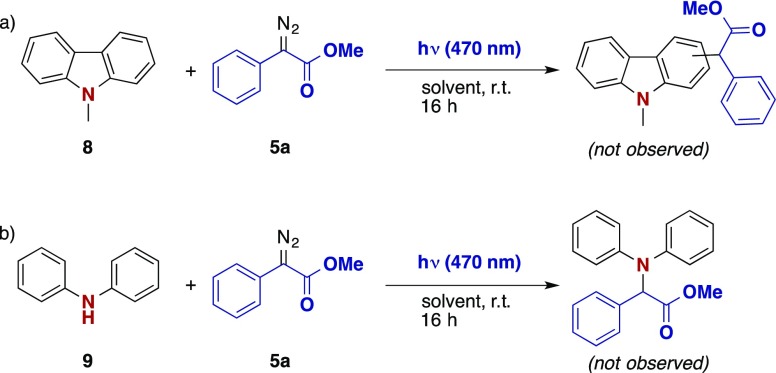
When using the N-methyl carbazole 8, only the decomposition reaction of the diazoalkane, e.g. to the corresponding diazine, was observed and not even trace amounts of the C–H insertion reaction did occur under the optimized reaction conditions (Scheme 3a), which underlines the different reactivities of light-mediated and metal-catalyzed carbene transfer reactions.1,12b When changing the substrate from carbazole to diphenylamine 9, no N–H insertion reaction was observed, which might be attributed to steric hindrance caused by the two phenyl rings.
Scheme 3. Reaction with (a) N-Protected Carbazole and (b) Diphenylamine.
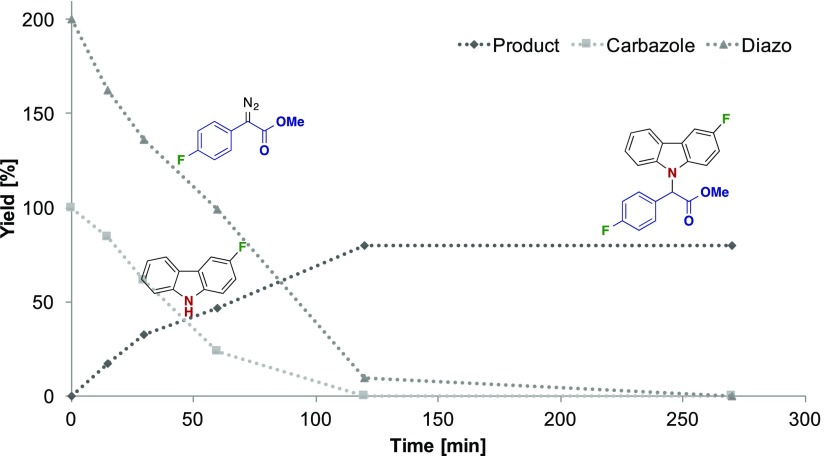
Finally, we monitored the conversion of the starting materials 4h and 5b, and the formation of the N–H insertion product (6hb) over time, by means of 19F NMR. The insertion reaction is almost complete within the first 2 h for those two substrates. Moreover, the excess of the diazo coupling partner disappears in about the same time, highlighting the competing decomposition processes (e.g., diazine formation) at play of the carbene intermediate and/or diazoalkane (Figure 2).
Figure 2.
19F NMR conversion of 4h and 5b as well as formation of 6hb over time, with hexafluorobenzene as the internal standard.
In summary, we reported on a metal-free insertion reaction of carbenes into the N–H bond of carbazoles, induced by low-energy blue light. The simple reaction protocol allows the direct functionalization of the carbazole backbone without the exclusion of moisture or air. This blue light induced reactivity certainly represents an important step in the field of metal-free intermolecular C–N bond forming reactions,17 for possible applications in drug synthesis. Indeed, we expect it will inspire the development of future photochemical C–N bond forming methods.
Experimental Section
All commercially available compounds were used without further purification; chemicals were purchased from Fluorochem, TCI, Sigma-Aldrich, and Alfa Aesar. Solvents used for reactions were p.A. grade, and solvents for column chromatography were technical grade and distilled before use; solvent mixtures are understood as volume/volume.
1H, 19F, and 13C NMR were recorded on a Varian AV600/AV400 or an Agilent DD2 400 NMR spectrometer in CDCl3. HRMS data were recorded on a ThermoFisher Scientific LTQ Orbitrap XL using ESI ionization or on a Finnigan MAT 95 using EI ionization at 70 eV. IR spectra were recorded on a PerkinElmer-100 spectrometer and are reported in terms of frequency of absorption (cm–1). Syringe pump: Chemyx Inc. Model Fusion 710. LEDs used in this manuscript were purchased from Conrad Electronics: High Power LED-Module, 1 W, 23 lm, 10°, 470 nm, art.nr. 180711-62. Reactions were irradiated from 1.5 cm, the temperature was room temperature, and cooling was realized with a fan.
Important Safety Note
Handling of diazo compounds should only be done in a well-ventilated fume cupboard using an additional blast shield. No incidents occurred during the preparation of this manuscript, yet the reader should be aware of the carcinogenicity and explosiveness of the herein described diazo compounds. General safety precautions when working with diazo compounds should be followed. Any reactions described in this manuscript should not be performed without strict risk assessment and proper safety precautions.
Synthesis of Diazoalkanes
The aryldiazoacetates 5a,185b,185c,185d,195e,19 and 5f(19) were prepared according to literature procedures. (1-Diazo-2,2,2-trifluoroethyl)benzene (5g) was prepared according to literature procedures.20
General Reaction Procedure
In a reaction tube carbazole 4 (0.4 mmol, 1.0 equiv) and diazo compound 5 (0.8 mmol, 2.0 equiv) were dissolved in 1.0 mL of DCM. The reaction mixture was irradiated with blue LEDs (470 nm; 1 W) overnight (16 h) at room temperature. The product was purified by column chromatography; eluent: n-hexane/EtOAc = 40:1 → 20:1. Solid products were recrystallized using n-pentane as solvent.
Procedure for Kinetic Measurements
For the kinetic measurements, carbazole 4b (0.1 mmol, 18.5 mg, 1.0 equiv) and diazo compound 5g (0.2 mmol, 38.8 mg, 2.0 equiv) were dissolved in 1.0 mL of DCM. Hexafluorobenzene (0.0167 mmol, 1.93 μL, 0.0167 equiv) was added as an internal standard. The reaction mixture was irradiated with blue LEDs (470 nm; 1 W) for different reaction times. The amounts of product 6gb, the carbazole 4b, and the diazo compound 5g were then determined by 19F NMR spectroscopy of the crude reaction mixture and quantified against the internal standard.
Methyl 2-(9H-Carbazol-9-yl)-2-phenylacetate (6aa)
Compound 6aa was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless solid in 80% yield (101.3 mg). 1H NMR (600 MHz, Chloroform-d): δ = 8.16–8.07 (m, 2H), 7.39–7.35 (m, 2H), 7.35–7.31 (m, 3H), 7.29–7.21 (m, 6H), 6.63 (s, 1H), 3.78 (s, 3H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.8, 140.2, 134.0, 128.7, 128.4, 127.4, 125.8, 123.6, 120.3, 119.8, 110.2, 60.29*, 60.26*, 52.8 ppm. *Peaks belong to one Carbon; indication of two different conformers. Data according to literature.1
Methyl 2-(9H-Carbazol-9-yl)-2-(4-fluorophenyl)acetate (6ab)
Compound 6ab was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless solid in 74% yield (98.2 mg). 1H NMR (600 MHz, Chloroform-d): δ = 8.12 (dq, J = 7.8, 1.0 Hz, 2H), 7.38 (ddt, J = 8.3, 7.2, 1.2 Hz, 2H), 7.28–7.16 (m, 6H), 7.09–6.92 (m, 2H), 6.56 (s, 1H), 3.77 (d, J = 1.0 Hz, 3H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.6, 162.5 (d, J = 247.3 Hz), 139.9, 129.7 (d, J = 2.5 Hz), 129.21 (d, J = 8.2 Hz), 125.8, 123.5, 120.3, 119.8, 115.6 (d, J = 21.8 Hz), 109.9, 59.5, 52.8 ppm. 19F NMR (564 MHz, Chloroform-d): δ = −113.5 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C21H16NFO2Na+ 356.1057; Found 356.1057. IR (KBr): 3424, 3079, 2955, 2328, 2209, 2160, 2114, 2014, 1897, 1717, 1600, 1509, 1452, 1380, 1331, 1270, 1208, 1160, 1106, 1071, 1038, 1006, 932, 901, 838, 806, 747, 676 cm–1.
Methyl 2-(9H-Carbazol-9-yl)-2-(4-chlorophenyl)acetate (6ac)
Compound 6ac was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless solid in 77% yield (108 mg). 1H NMR (600 MHz, Chloroform-d): δ = 8.12 (dd, J = 7.7, 1.0 Hz, 2H), 7.38 (ddt, J = 8.1, 7.1, 0.9 Hz, 2H), 7.30–7.27 (m, 4H), 7.25–7.22 (m, 2H), 7.19–7.15 (m, 2H), 6.55 (s, 1H), 3.76 (s, 3H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.4, 139.9, 134.2, 132.4, 128.8, 128.7, 125.9, 123.5, 120.3, 119.95, 109.92, 59.5, 52.9 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C21H16NClO2Na+ 372.0761; Found 372.0766. IR (KBr): 3820, 3478, 3057, 2950, 2839, 2658, 2476, 2316, 2223, 2176, 2075, 2022, 1899, 1744, 1595, 1485, 1447, 1334, 1195, 1085, 999, 931, 888, 825, 748 cm–1.
Methyl 2-(9H-Carbazol-9-yl)-2-(3-methoxyphenyl)acetate (6ad)
Compound 6ad was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless solid in 59% yield (81.1 mg). 1H NMR (400 MHz, Chloroform-d): δ = 8.16–8.00 (m, 2H), 7.36 (ddd, J = 8.3, 7.0, 1.3 Hz, 2H), 7.30–7.15 (m, 5H), 6.91–6.75 (m, 3H), 6.57 (s, 1H), 3.76 (s, 3H), 3.68 (s, 3H) ppm. 13C{1H} NMR (101 MHz, Chloroform-d): δ = 169.6, 159.8, 140.2, 135.5, 129.7, 125.7, 123.6, 120.2, 119.7, 119.6, 113.6, 113.4, 110.2, 60.2, 55.2, 52.7 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C22H19NO3Na+ 368.1257; Found 368.1256. IR (KBr): 3852, 3504, 3048, 2981, 2942, 2837, 2660, 2316, 2170, 2072, 1984, 1873, 1756, 1596, 1484, 1448, 1386, 1336, 1291, 1257, 1159, 1051, 994, 933, 875, 803, 749 cm–1.
Methyl 2-(9H-Carbazol-9-yl)-2-(naphthalen-2-yl)acetate (6ae)
Compound 6ae was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless solid in 78% yield (112 mg). 1H NMR (600 MHz, Chloroform-d): δ = 8.14 (dt, J = 7.8, 0.9 Hz, 2H), 7.84–7.79 (m, 1H), 7.79–7.73 (m, 3H), 7.52–7.46 (m, 2H), 7.35 (ddd, J = 8.3, 7.0, 1.3 Hz, 5H), 7.32–7.23 (m, 2H), 6.77 (s, 1H), 3.82 (s, 3H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.8, 140.2, 132.98, 132.97, 131.4, 128.6, 128.2, 127.6, 126.5, 126.4, 126.3, 125.8, 125.1, 123.6, 120.2, 119.8, 110.1, 60.4, 52.8 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C25H20NO2+ 366.1488; Found 366.1475. IR (KBr): 3443, 3058, 2930, 2670, 2339, 2159, 2021, 1922, 1724, 1597, 1437, 1380, 1328, 1241, 1168, 1122, 1012, 953, 900, 860, 815, 744 cm–1.
Benzyl 2-(9H-Carbazol-9-yl)-2-phenylacetate (6af)
Compound 6af was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a red oil in 76% yield (119 mg). 1H NMR (400 MHz, Chloroform-d): δ = 8.13–8.05 (m, 2H), 7.36–7.16 (m, 14H), 7.17–7.05 (m, 2H), 6.63 (s, 1H), 5.20 (s, 2H) ppm. 13C{1H} NMR (101 MHz, Chloroform-d): δ = 169.1, 140.2, 134.9, 133.9, 128.6, 128.4, 128.32, 128.30, 128.2, 127.4, 125.7, 123.6, 120.2, 119.7, 110.3, 67.5, 60.5 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C27H21NO2Na+ 414.1464; Found 414.1467. IR (KBr): 3467, 3050, 2950, 2667, 2327, 2162, 1890, 1741, 1596, 1485, 1450, 1383, 1330, 1162, 1068, 970, 845, 809, 736 cm–1.
Methyl 2-(3,6-Diphenyl-9H-carbazol-9-yl)-2-phenylacetate (6ba)
Compound 6ba was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless solid in 65% yield (122 mg). 1H NMR (600 MHz, Chloroform-d): δ = 8.37 (d, J = 1.6 Hz, 2H), 7.73–7.69 (m, 4H), 7.63 (dd, J = 8.5, 1.9 Hz, 2H), 7.50–7.44 (m, 4H), 7.38–7.28 (m, 9H), 6.66 (s, 1H), 3.83 (s, 3H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.7, 141.7, 140.1, 133.8, 133.4, 128.8, 128.7, 128.5, 127.4, 127.2, 126.6, 125.5, 124.2, 118.8, 110.5, 60.5, 52.8 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C33H25NO2Na+ 490.1777; Found 490.1770. IR (KBr): 3477, 3031, 2924, 2853, 2250, 2154, 1957, 1878, 1744, 1601, 1474, 1384, 1340 1270, 1201, 1163, 1073, 1004, 906, 882, 840, 811, 759, 730, 696, 658 cm–1.
Methyl 2-(3,6-Di-tert-butyl-9H-carbazol-9-yl)-2-phenylacetate (6ca)
Compound 6ca was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 80:1 → 40:1) as a colorless solid in 40% yield (67.6 mg). 1H NMR (600 MHz, Chloroform-d): δ = 8.13–8.04 (m, 2H), 7.40 (dd, J = 8.7, 2.0 Hz, 2H), 7.35–7.30 (m, 3H), 7.26 (s, 2H), 7.14 (d, J = 8.7 Hz, 2H), 6.56 (s, 1H), 3.77 (s, 3H), 1.44 (s, 18H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.9, 142.4, 138.6, 134.3, 128.6, 128.2, 127.5, 123.4, 123.3, 116.1, 109.4, 60.3, 52.6, 34.6, 31.9 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C29H33NO2Na+ 450.2403; Found 450.2402. IR (KBr): 3418, 3057, 2956, 2905, 2868, 2323, 2168, 2124, 2034, 1952, 1874, 1743, 1691, 1604, 1473, 1388, 1360, 1327, 1296, 1260, 1291, 1166, 1106, 1057, 1031, 1003, 903, 878, 840, 806, 735, 696 cm–1.
Methyl 2-(3,6-Dichloro-9H-carbazol-9-yl)-2-phenylacetate (6da)
Compound 6da was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless solid in 58% yield (88.8 mg). 1H NMR (600 MHz, Chloroform-d): δ = 8.00 (d, J = 2.1 Hz, 2H), 7.37–7.30 (m, 5H), 7.21–7.16 (m, 2H), 7.15 (d, J = 8.8 Hz, 2H), 6.54 (s, 1H), 3.80 (s, 3H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.2, 139.0, 133.2, 128.8, 128.7, 127.2, 126.5, 125.7, 123.8, 120.1, 111.5, 60.5, 52.8 ppm. Data according to literature.1
Methyl 2-(3,6-Dibromo-9H-carbazol-9-yl)-2-phenylacetate (6ea)
Compound 6ea was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless solid in 63% yield (120 mg). 1H NMR (600 MHz, Chloroform-d): δ = 8.16 (d, J = 2.0 Hz, 2H), 7.45 (dd, J = 8.8, 2.0 Hz, 2H), 7.39–7.30 (m, 3H), 7.21–7.14 (m, 2H), 7.11 (d, J = 8.8 Hz, 2H), 6.54 (s, 1H), 3.80 (s, 3H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.2, 139.1, 133.1, 129.2, 128.9, 128.7, 127.2, 124.2, 123.2, 113.0, 111.9, 60.5, 52.9 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C21H15Br2NO2Na+ 493.9361; Found 493.9361. IR (KBr): 3470, 3201, 3063, 2951, 2847, 2654, 2520, 2335, 2193, 2110, 1972, 1903, 1861, 1823, 1742, 1592, 1469, 1434, 1380, 1332, 1280, 1204, 1172, 1112, 1054, 1007, 981, 944, 903, 863, 826, 790, 732, 696 cm–1.
Methyl 2-(2,7-Dibromo-9H-carbazol-9-yl)-2-phenylacetate (6fa)
Compound 6fa was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless solid in 74% yield (141 mg). 1H NMR (600 MHz, Chloroform-d): δ = 7.93–7.87 (m, 2H), 7.41–7.32 (m, 7H), 7.22–7.18 (m, 2H), 6.49 (s, 1H), 3.84 (s, 3H). 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.1, 141.1, 132.9, 128.9, 128.8, 127.3, 123.5, 122.0, 121.3, 119.8, 113.5, 60.5, 53.0 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C21H15NBr2O2Na+ 493.9361; Found 493.9360. IR (KBr): 3872, 3399, 2951, 2325, 2093, 1990, 1887, 1810, 1739, 1688, 1589, 1498, 1477, 1446, 1419, 1368, 1325, 1237, 1205, 1173, 1135, 1054, 994, 961, 889, 849, 796, 731, 698, 665 cm–1.
Methyl 2-(3-Bromo-9H-carbazol-9-yl)-2-phenylacetate (6ga)
Compound 6ga was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless solid in 66% yield (104 mg). 1H NMR (600 MHz, Chloroform-d): δ = 8.21 (d, J = 2.0 Hz, 1H), 8.07–8.04 (m, 1H), 7.43–7.38 (m, 2H), 7.35–7.32 (m, 3H), 7.31–7.26 (m, 2H), 7.22–7.19 (m, 2H), 7.07 (d, J = 8.7 Hz, 1H), 6.58 (s, 1H), 3.79 (s, 3H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.4, 140.6, 138.7, 133.5, 128.7, 128.5, 128.3, 127.3, 126.5, 125.4, 122.9, 122.5, 120.4, 120.2, 112.6, 112.0, 110.0, 60.3, 52.8 ppm. Data according to literature.1
Methyl 2-(3-Fluoro-9H-carbazol-9-yl)-2-phenylacetate (6ha)
Compound 6ha was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless oil in 63% yield (83.6 mg). 1H NMR (600 MHz, Chloroform-d): δ = 8.06 (dt, J = 7.7, 0.9 Hz, 1H), 7.75 (dd, J = 8.7, 2.6 Hz, 1H), 7.40 (ddd, J = 8.3, 7.1, 1.3 Hz, 1H), 7.36–7.33 (m, 3H), 7.31–7.24 (m, 2H), 7.24–7.21 (m, 2H), 7.14–7.11 (m, 1H), 7.07 (td, J = 9.0, 2.6 Hz, 1H), 5.30 (s, 1H), 3.79 (s, 3H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.6, 157.6 (d, J = 236.9 Hz), 141.1, 136.3, 133.8, 128.7, 128.4, 127.3, 126.3, 124.2 (d, J = 9.3 Hz), 123.1 (d, J = 4.1 Hz), 120.5, 119.7, 113.4 (d, J = 25.3 Hz), 111.1 (d, J = 8.9 Hz), 110.1, 105.9 (d, J = 23.8 Hz), 60.4, 52.7 ppm. 19F NMR (564 MHz, Chloroform-d): δ = −124.2 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C21H16NFO2Na+ 356.1058; Found 356.1057. IR (KBr): 3418, 3059, 2951, 2849, 2661, 2329, 2086, 1739, 1592, 1484, 1452, 1388, 1330, 1275, 1206, 1165, 1066, 1000, 868, 800, 736, 694 cm–1.
Methyl 2-(3-Fluoro-9H-carbazol-9-yl)-2-(4-fluorophenyl)acetate (6hb)
Compound 6hb was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a yellow oil in 73% yield (103 mg). 1H NMR (600 MHz, Chloroform-d): δ = 8.12–8.02 (m, 1H), 7.75 (dd, J = 8.7, 2.5 Hz, 1H), 7.43–7.39 (m, 1H), 7.29–7.22 (m, 2H), 7.21–7.16 (m, 2H), 7.14–7.07 (m, 2H), 7.04–6.99 (m, 2H), 6.54 (s, 1H), 3.78 (s, 3H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 169.4, 162.5 (d, J = 247.9 Hz), 157.7 (d, J = 236.9 Hz), 141.0, 136.2, 129.5 (d, J = 3.4 Hz), 129.1 (d, J = 8.3 Hz), 126.5, 124.2 (d, J = 9.4 Hz), 123.1, 120.6, 119.9, 115.7 (d, J = 21.8 Hz), 113.5 (d, J = 25.3 Hz), 110.9 (d, J = 9.0 Hz), 109.9, 106.0 (d, J = 23.7 Hz), 59.7, 52.8 ppm. 19F NMR (564 MHz, Chloroform-d): δ = −113.24, −124.00 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C21H16NO2F2+ 352.1143; Found 352.1143. IR (KBr): 3787, 3465, 3055, 2954, 2670, 2323, 2110, 2001, 1892, 1744, 1604, 1509, 1486, 1455, 1386, 1328, 1276, 1227, 1163, 1067, 1003, 905, 860, 834, 800, 732 cm–1.
Methyl 2-(10,11-Dihydro-5H-dibenzo[b,f]azepin-5-yl)-2-phenylacetate (7)
Compound 7 was prepared according to the general procedure and was obtained after column chromatography (n-hexane/EtOAc 40:1 → 20:1) as a colorless solid in 50% yield (68.5 mg). 1H NMR (600 MHz, Chloroform-d): δ = 7.58–7.51 (m, 2H), 7.24–7.20 (m, 2H), 7.19–7.14 (m, 1H), 7.12–7.06 (m, 2H), 6.99–6.94 (m, 4H), 6.90–6.82 (m, 2H), 5.93 (s, 1H), 3.49 (s, 3H), 3.32 (br, 4H) ppm. 13C{1H} NMR (151 MHz, Chloroform-d): δ = 171.8, 147.4, 136.2, 135.1, 129.9, 128.7, 128.4, 128.2, 126.0, 123.5, 120.6, 68.1, 52.39*, 52.36*, 31.6 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C23H22NO2+ 344.1645; Found 344.1650. IR (KBr): 3059, 2928, 2671, 2330, 2242, 2115, 1913, 1820, 1738, 1587, 1486, 1444, 1345, 1297, 1235, 1195, 1164, 1030, 991, 905, 815, 722 cm–1. *Peaks belong to one carbon; indication of two different conformers.
Acknowledgments
This work was supported by ERC project 716136: “2O2ACTIVATION.” (F.W.P.). R.M.K. gratefully acknowledges the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project No. 408033718.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.joc.9b01753.
Copies of NMR spectra, picture of the reaction setup (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Arredondo V.; Hiew S. C.; Gutman E. S.; Premachandra I. D. U. A.; Van Vranken D. L. Enantioselective Palladium-Catalyzed Carbene Insertion into the N–H Bonds of Aromatic Heterocycles. Angew. Chem., Int. Ed. 2017, 56, 4156. 10.1002/anie.201611845. [DOI] [PubMed] [Google Scholar]
- Shen H.-Q.; Wu B.; Xie H.-P.; Zhou Y.-G. Preparation of Axially Chiral 2,2′-Biimidazole Ligands through Remote Chirality Delivery and Their Application in Asymmetric Carbene Insertion into N–H of Carbazoles. Org. Lett. 2019, 21, 2712. 10.1021/acs.orglett.9b00687. [DOI] [PubMed] [Google Scholar]
- Sheldon R. A. Fundamentals of Green Chemistry: Efficiency in Reaction Design. Chem. Soc. Rev. 2012, 41, 1437. 10.1039/C1CS15219J. [DOI] [PubMed] [Google Scholar]
- Selected examples:; a Molette J.; Routier J.; Abla N.; Besson D.; Bombrun A.; Brun R.; Burt H.; Georgi K.; Kaiser M.; Nwaka S.; Muzerelle M.; Scheer A. Identification and Optimization of an Aminoalcohol-Carbazole Series with Antimalarial Properties. ACS Med. Chem. Lett. 2013, 4, 1037. 10.1021/ml400015f. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yamaguchi A. D.; Yamaguchi J.; Itami K. C-H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem., Int. Ed. 2012, 51, 8960. 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]; c Schmidt A. W.; Reddy K. R.; Knoelker H.-J. Occurrence, Biogenesis, and Synthesis of Biologically Active Carbazole Alkaloids. Chem. Rev. 2012, 112, 3193. 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]
- Selected references:; a Sotzing G. A.; Reddinger J. L.; Katritzky A. R.; Soloducho J.; Musgrave R.; Reynolds J. R. Multiply Colored Electrochromic Carbazole-Based Polymers. Chem. Mater. 1997, 9, 1578. 10.1021/cm960630t. [DOI] [Google Scholar]; b Morin J.-F.; Ades D.; Leclerc M.; Siove A. Polycarbazoles: 25 Years of Progress. Macromol. Rapid Commun. 2005, 26, 761. 10.1002/marc.200500096. [DOI] [Google Scholar]; c Chen Y.-M.; Lin Y.-T.; Su H.-C.; Wu C.; Wong K.-T. Nonconjugated Hybrid of Carbazole and Fluorene: A Novel Host Material for Highly Efficient Green and Red Phosphorescent OLEDs. Org. Lett. 2005, 7, 5361. 10.1021/ol051977h. [DOI] [PubMed] [Google Scholar]; d Boudreault P.- L. T.; Wakim S.; Blouin N.; Simard M.; Tessier C.; Tao Y.; Leclerc M. Synthesis, Characterization, and Application of Indolo[3,2-b]carbazole Semiconductors. J. Am. Chem. Soc. 2007, 129, 9125. 10.1021/ja071923y. [DOI] [PubMed] [Google Scholar]; e Kim J.; Kwon Y. S.; Shin W. S.; Moon S.-J.; Park T. Carbazole-Based Copolymers: Effects of Conjugation Breaks and Steric Hindrance. Macromolecules 2011, 44, 1909. 10.1021/ma102467w. [DOI] [Google Scholar]; f Albrecht K.; Yamamoto K. Dendritic Structure Having a Potential Gradient: New Synthesis and Properties of Carbazole Dendrimers. J. Am. Chem. Soc. 2009, 131, 2244. 10.1021/ja807312e. [DOI] [PubMed] [Google Scholar]; g Kato S.; Shimizu S.; Kobayashi A.; Yoshihara T.; Tobita S.; Nakamura Y. Systematic Structure–Property Investigations on a Series of Alternating Carbazole–Thiophene Oligomers. J. Org. Chem. 2014, 79, 618. 10.1021/jo402416f. [DOI] [PubMed] [Google Scholar]; h Li J.; Grimsdale A. C. Carbazole-Based Polymers for Organic Photovoltaic Devices. Chem. Soc. Rev. 2010, 39, 2399. 10.1039/b915995a. [DOI] [PubMed] [Google Scholar]; i Li M. C3–C3′ and C6–C6′ Oxidative Couplings of Carbazoles. Chem. - Eur. J. 2019, 25, 1142. 10.1002/chem.201803246. [DOI] [PubMed] [Google Scholar]
- Selected references on the de novo synthesis of carbazoles:; a Shimizu M.; Hirano K.; Satoh T.; Miura M. Waste-Free Synthesis of Condensed Heterocyclic Compounds by Rhodium-Catalyzed Oxidative Coupling of Substituted Arene or Heteroarene Carboxylic Acids with Alkynes. J. Org. Chem. 2009, 74, 3478. 10.1021/jo900396z. [DOI] [PubMed] [Google Scholar]; b Buden M. E.; Vaillard V. A.; Martin S. E.; Rossi R. A. Synthesis of Carbazoles by Intramolecular Arylation of Diarylamide Anions. J. Org. Chem. 2009, 74, 4490. 10.1021/jo9006249. [DOI] [PubMed] [Google Scholar]; c Yamashita M.; Horiguchi H.; Hirano K.; Satoh T.; Miura M. Fused Ring Construction around Pyrrole, Indole, and Related Compounds via Palladium-Catalyzed Oxidative Coupling with Alkynes. J. Org. Chem. 2009, 74, 7481. 10.1021/jo9016698. [DOI] [PubMed] [Google Scholar]; d Watanabe T.; Oishi S.; Fujii N.; Ohno H. Palladium-Catalyzed Direct Synthesis of Carbazoles via One-Pot N-Arylation and Oxidative Biaryl Coupling: Synthesis and Mechanistic Study. J. Org. Chem. 2009, 74, 4720. 10.1021/jo9003376. [DOI] [PubMed] [Google Scholar]; e Yamashita M.; Hirano K.; Satoh T.; Miura M. Synthesis of Condensed Heteroaromatic Compounds by Palladium-Catalyzed Oxidative Coupling of Heteroarene Carboxylic Acids with Alkynes. Org. Lett. 2009, 11, 2337. 10.1021/ol900736s. [DOI] [PubMed] [Google Scholar]; f Chen C.-C.; Chin L.-Y.; Yang S.-C.; Wu M.-J. Synthetic Development and Mechanistic Study on Pd(II)-Catalyzed Cyclization of Enediynes to Benzo[a]carbazoles. Org. Lett. 2010, 12, 5652. 10.1021/ol1024458. [DOI] [PubMed] [Google Scholar]; g Namjoshi O. A.; Gryboski A.; Fonseca G. O.; Van Linn M. L.; Wang Z.; Deschamps J. R.; Cook J. M. Development of a Two-Step Route to 3-PBC and βCCt, Two Agents Active against Alcohol Self-Administration in Rodent and Primate Models. J. Org. Chem. 2011, 76, 4721. 10.1021/jo200425m. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Chen C.-C.; Yang S.-C.; Wu M.-J. Iodine-Mediated Cascade Cyclization of Enediynes to Iodinated Benzo[a]carbazoles. J. Org. Chem. 2011, 76, 10269. 10.1021/jo201795t. [DOI] [PubMed] [Google Scholar]; i Wang L.; Li G.; Liu Y. Gold-Catalyzed Deacylative Cycloisomerization Reactions of 3-Acylindole/ynes: A New Approach for Carbazole Synthesis. Org. Lett. 2011, 13, 3786. 10.1021/ol2012154. [DOI] [PubMed] [Google Scholar]; j Gao H.; Xu Q.-L.; Yousufuddin M.; Ess D. H.; Kürti L. Rapid Synthesis of Fused N-Heterocycles by Transition-Metal-Free Electrophilic Amination of Arene C-H Bonds. Angew. Chem., Int. Ed. 2014, 53, 2701. 10.1002/anie.201309973. [DOI] [PubMed] [Google Scholar]; For a review on bioactive carbazole alkaloids, see:; k Knölker H.-J.; Reddy K. R. Isolation and Synthesis of Biologically Active Carbazole Alkaloids. Chem. Rev. 2002, 102, 4303. 10.1021/cr020059j. [DOI] [PubMed] [Google Scholar]
- Selected references on carbazole functionalization:; a Louillat M.-L.; Biafora A.; Legros F.; Patureau F. W. Ruthenium-Catalyzed Cross-Dehydrogenative ortho-N-Carbazolation of Diarylamines: Versatile Access to Unsymmetrical Diamines. Angew. Chem., Int. Ed. 2014, 53, 3505. 10.1002/anie.201308601. [DOI] [PubMed] [Google Scholar]; b Biafora A.; Patureau F. W. Perspective on Ruthenium–Copper-Mediated Dehydrogenative C–N Bond Formation. Synlett 2014, 25, 2525. 10.1055/s-0034-1378999. [DOI] [Google Scholar]; c Jones A. W.; Rank C. K.; Becker Y.; Malchau C.; Funes-Ardoiz I.; Maseras F.; Patureau F. W. Accelerated Ru–Cu Trinuclear Cooperative C–H Bond Functionalization of Carbazoles: A Kinetic and Computational Investigation. Chem. - Eur. J. 2018, 24, 15178. 10.1002/chem.201802886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ciszewski L. W.; Rybicka-Jasinska K.; Gryko D. Recent Developments in Photochemical Reactions of Diazo Compounds. Org. Biomol. Chem. 2019, 17, 432. 10.1039/C8OB02703J. [DOI] [PubMed] [Google Scholar]; b Candeias N. R.; Afonso C. A. M. Developments in the Photochemistry of Diazo Compounds. Curr. Org. Chem. 2009, 13, 763. 10.2174/138527209788167231. [DOI] [Google Scholar]; c Galkina O. S.; Rodina L. L. Photochemical Transformations of Diazocarbonyl Compounds: Expected and Novel Reactions. Russ. Chem. Rev. 2016, 85, 537. 10.1070/RCR4519. [DOI] [Google Scholar]; d Empel C.; Koenigs R. M. Sustainable Carbene Transfer Reactions with Iron and Light. Synlett 2019, 10.1055/s-0037-1611874. [DOI] [Google Scholar]; e Meerwein H.; Rathjen H.; Werner H. Die Methylierung von RH-Verbindungen mittels Diazomethans unter Mitwirkung des Lichtes. Ber. Dtsch. Chem. Ges. B 1942, 75, 1610. 10.1002/cber.19420751229. [DOI] [Google Scholar]; f von E. Doering W.; Knox L.; Jones M. Notes. Reaction of Methylene with Diethyl Ether and Tetrahydrofuran. J. Org. Chem. 1959, 24, 136. 10.1021/jo01083a627. [DOI] [Google Scholar]; g Corey E. J.; Felix A. M. A New Synthetic Approach to the Penicillins. J. Am. Chem. Soc. 1965, 87, 2518. 10.1021/ja01089a055. [DOI] [PubMed] [Google Scholar]; h Minh T. D.; Strausz O. P.; Gunning H. E. Photochemistry of diazo esters. II. Reaction path. J. Am. Chem. Soc. 1969, 91, 1261. 10.1021/ja01033a064. [DOI] [Google Scholar]; i Lowe G.; Parker J. Photochemical conversion of α-diazo-amides and -esters into β-lactams and β- and γ-lactones. J. Chem. Soc. D 1971, 0, 577. 10.1039/C29710000577. [DOI] [Google Scholar]; j Wydila J.; Thornton E. R. Photolysis studies of α-diazoamides the effect of carboxamide substituents on selectivity ratios. Tetrahedron Lett. 1983, 24, 233. 10.1016/S0040-4039(00)81373-8. [DOI] [Google Scholar]; k Rando R. R. J. J. Am. Chem. Soc. 1972, 94, 1629. 10.1021/ja00760a033. [DOI] [Google Scholar]; l Wang J.; Burdzinski G.; Gustafson T. L.; Platz M. S. Ultrafast Study of p-Biphenylyldiazomethane and p-Biphenylylcarbene. J. Org. Chem. 2006, 71, 6221. 10.1021/jo061029+. [DOI] [PubMed] [Google Scholar]
- a Liang Y.; Jiao L.; Zhang S.; Xu J. Microwave- and Photoirradiation-Induced Staudinger Reactions of Cyclic Imines and Ketenes Generated from α-Diazoketones. A Further Investigation into the Stereochemical Process. J. Org. Chem. 2005, 70, 334. 10.1021/jo048328o. [DOI] [PubMed] [Google Scholar]; b Wang J.; Burdzinski G.; Kubicki J.; Platz M. S. Ultrafast UV–Vis and IR Studies of p-Biphenylyl Acetyl and Carbomethoxy Carbenes. J. Am. Chem. Soc. 2008, 130, 11195. 10.1021/ja803096p. [DOI] [PubMed] [Google Scholar]; c Nakatani K.; Maekawa S.; Tanabe; Saito K. I. alpha-Diazo Ketones as Photochemical DNA Cleavers: A Mimic for the Radical Generating System of Neocarzinostatin Chromophore. J. Am. Chem. Soc. 1995, 117, 10635. 10.1021/ja00148a005. [DOI] [Google Scholar]
- Selected references:; a Hansen S. R.; Spangler J. E.; Hansen J. H.; Davies H. M. L. Metal-Free N–H Insertions of Donor/Acceptor Carbenes. Org. Lett. 2012, 14, 4626. 10.1021/ol3020754. [DOI] [PubMed] [Google Scholar]; b Luo X.; Chen G.; He L.; Huang X. Amination of Diazocarbonyl Compounds: N–H Insertion under Metal-Free Conditions. J. Org. Chem. 2016, 81, 2943. 10.1021/acs.joc.6b00233. [DOI] [PubMed] [Google Scholar]; c Barroso R.; Jimenez A.; Perez-Aguilar M. C.; Cabal M.-P.; Valdes C. Synthesis of 1,3-Diaryl-3-Trifluoromethylcyclopropenes by Transition-Metal-Free Reaction of 2,2,2-Trifluoroacetophenone Tosylhydrazones with Alkynes: the Effect of the Trifluoromethyl Group. Chem. Commun. 2016, 52, 3677. 10.1039/C5CC10472F. [DOI] [PubMed] [Google Scholar]
- Jurberg I.; Davies H. M. L. Blue Light-Promoted Photolysis of Aryldiazoacetates. Chem. Sci. 2018, 9, 5112. 10.1039/C8SC01165F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hommelsheim R.; Guo Y.; Yang Z.; Empel C.; Koenigs R. M. Blue-Light-Induced Carbene-Transfer Reactions of Diazoalkanes. Angew. Chem., Int. Ed. 2019, 58, 1203. 10.1002/anie.201811991. [DOI] [PubMed] [Google Scholar]; b He F.; Koenigs R. M. Visible Light Mediated, Metal-Free Carbene Transfer Reactions of Diazoalkanes with Propargylic Alcohols. Chem. Commun. 2019, 55, 4881. 10.1039/C9CC00927B. [DOI] [PubMed] [Google Scholar]
- a Jana S.; Koenigs R. M. Doyle-Kirmse Rearrangement Reactions of Difluoroacetates. Asian J. Org. Chem. 2019, 8, 683. 10.1002/ajoc.201900099. [DOI] [Google Scholar]; b Yang Z.; Guo Y.; Koenigs R. M. Photochemical, Metal-Free Sigmatropic Rearrangement Reactions of Sulfur Ylides. Chem. - Eur. J. 2019, 25, 6703. 10.1002/chem.201900597. [DOI] [PubMed] [Google Scholar]; c Yang J.; Wang J.; Huang H.; Qin G.; Jiang Y.; Xiao T. gem-Difluoroallylation of Aryl Diazoesters via Catalyst-Free, Blue-Light-Mediated Formal Doyle–Kirmse Reaction. Org. Lett. 2019, 21, 2654. 10.1021/acs.orglett.9b00647. [DOI] [PubMed] [Google Scholar]; d Yang Z.; Guo Y.; Koenigs R. M. Solvent-Dependent, Rhodium Catalysed Rearrangement Reactions of Sulfur Ylides. Chem. Commun. 2019, 55, 8410. 10.1039/C9CC03809D. [DOI] [PubMed] [Google Scholar]
- Xiao T.; Mei M.; He Y.; Zhou L. Blue Light-Promoted Cross-Coupling of Aryldiazoacetates and Diazocarbonyl Compounds. Chem. Commun. 2018, 54, 8865. 10.1039/C8CC04609C. [DOI] [PubMed] [Google Scholar]
- a Hock K. J.; Knorrscheidt A.; Hommelsheim R.; Ho J.; Weissenborn M. J.; Koenigs R. M. Tryptamine Synthesis by Iron Porphyrin Catalyzed C–H Functionalization of Indoles with Diazoacetonitrile. Angew. Chem., Int. Ed. 2019, 58, 3630. 10.1002/anie.201813631. [DOI] [PubMed] [Google Scholar]; b Empel C.; Hock K. J.; Koenigs R. M. Dealkylative Intercepted Rearrangement Reactions of Sulfur Ylides. Chem. Commun. 2019, 55, 338. 10.1039/C8CC08821G. [DOI] [PubMed] [Google Scholar]; c Hock K. J.; Spitzner R.; Koenigs R. M. Towards Nitrile-Substituted Cyclopropanes – a Slow-Release Protocol for Safe and Scalable Applications of Diazo Acetonitrile. Green Chem. 2017, 19, 2118. 10.1039/C7GC00602K. [DOI] [Google Scholar]; d Hock K. J.; Mertens L.; Hommelsheim R.; Spitzner R.; Koenigs R. M. Enabling Iron Catalyzed Doyle–Kirmse Rearrangement Reactions with in situ Generated Diazo Compounds. Chem. Commun. 2017, 53, 6577. 10.1039/C7CC02801F. [DOI] [PubMed] [Google Scholar]
- Klebe G.Wirkstoffdesign; Spektrum Akad. Verlag: Heidelberg, 2009. [Google Scholar]
- a Louillat-Habermeyer M.-L.; Jin R.; Patureau F. W. O2-Mediated Dehydrogenative Amination of Phenols. Angew. Chem., Int. Ed. 2015, 54, 4102. 10.1002/anie.201500089. [DOI] [PubMed] [Google Scholar]; See also:; b Jin R.; Patureau F. W. Mild, Periodate-Mediated, Dehydrogenative C–N Bond Formation with Phenothiazines and Phenols. Org. Lett. 2016, 18, 4491. 10.1021/acs.orglett.6b02223. [DOI] [PubMed] [Google Scholar]; c Zhao Y.; Huang B.; Yang C.; Xia W. Visible-Light-Promoted Direct Amination of Phenols via Oxidative Cross-Dehydrogenative Coupling Reaction. Org. Lett. 2016, 18, 3326. 10.1021/acs.orglett.6b01371. [DOI] [PubMed] [Google Scholar]; d Zhao Y.; Huang B.; Yang C.; Li B.; Gou B.; Xia W. Photocatalytic Cross-Dehydrogenative Amination Reactions between Phenols and Diarylamines. ACS Catal. 2017, 7, 2446. 10.1021/acscatal.7b00192. [DOI] [Google Scholar]; e Tang S.; Wang S.; Liu Y.; Cong H.; Lei A. Electrochemical Oxidative C–H Amination of Phenols: Access to Triarylamine Derivatives. Angew. Chem., Int. Ed. 2018, 57, 4737. 10.1002/anie.201800240. [DOI] [PubMed] [Google Scholar]; f Tang S.; Zeng L.; Lei A. Oxidative R1–H/R2–H Cross-Coupling with Hydrogen Evolution. J. Am. Chem. Soc. 2018, 140, 13128. 10.1021/jacs.8b07327. [DOI] [PubMed] [Google Scholar]; g Bering L.; D'Ottavio L.; Sirvinskaite G.; Antonchick A. P. Nitrosonium Ion Catalysis: Aerobic, Metal-Free Cross-Dehydrogenative Carbon–Heteroatom Bond Formation. Chem. Commun. 2018, 54, 13022. 10.1039/C8CC08328B. [DOI] [PubMed] [Google Scholar]; h Goswami M.; Konkel A.; Rahimi M.; Louillat-Habermeyer M.-L.; Kelm H.; Jin R.; de Bruin B.; Patureau F. W. Mechanism of the Dehydrogenative Phenothiazination of Phenols. Chem. - Eur. J. 2018, 24, 11936. 10.1002/chem.201800730. [DOI] [PMC free article] [PubMed] [Google Scholar]; I Patureau F. W.ChemCatChem, The Phenol-Phenothiazine Coupling: an Oxidative Click Concept. 2019, 10.1002/cctc.201900152. [DOI] [Google Scholar]
- Keipour H.; Ollevier T. Iron-Catalyzed Carbene Insertion Reactions of α-Diazoesters into Si–H Bonds. Org. Lett. 2017, 19, 5736. 10.1021/acs.orglett.7b02488. [DOI] [PubMed] [Google Scholar]
- Chan W.-W.; Yeung S.-H.; Zhou Z.; Chan A. S. C.; Yu W.-Y. Ruthenium Catalyzed Directing Group-Free C2-Selective Carbenoid Functionalization of Indoles by α-Aryldiazoesters. Org. Lett. 2010, 12, 604. 10.1021/ol9028226. [DOI] [PubMed] [Google Scholar]
- Emer E.; Twilton J.; Tredwell M.; Calderwood S.; Collier T. L.; Liégault B.; Taillefer M. Diversity-Oriented Approach to CF3CHF-, CF3CFBr-, CF3CF2-, (CF3)2CH-, and CF3(SCF3)CH-Substituted Arenes from 1-(Diazo-2,2,2-trifluoroethyl)arenes. Org. Lett. 2014, 16, 6004. 10.1021/ol5030184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.