Significance
Plant defenses against herbivorous insects can target the feeding stages and the egg stage. Feeding-induced plant defenses are known to be primed by cues indicating imminent infestation, including sex pheromones. However, priming of egg-induced plant defenses has been unknown so far. Therefore, we studied whether a plant’s response to insect sex pheromones, which might indicate imminent egg depositions, primes defenses against the eggs. Indeed, exposure of pine to the sex pheromones of an herbivorous sawfly primes the tree’s defense against sawfly eggs. The priming effect results in enhanced egg mortality, enhanced accumulation of hydrogen peroxide in egg-laden needles, and differential expression of several defense-related pine genes. These findings open up exciting research perspectives in plant protection from insect infestation.
Keywords: priming, induced plant defense, insect oviposition, Diprion pini, hydrogen peroxide
Abstract
Plants respond to insect infestation with defenses targeting insect eggs on their leaves and the feeding insects. Upon perceiving cues indicating imminent herbivory, such as damage-induced leaf odors emitted by neighboring plants, they are able to prime their defenses against feeding insects. Yet it remains unknown whether plants can amplify their defenses against insect eggs by responding to cues indicating imminent egg deposition. Here, we tested the hypothesis that a plant strengthens its defenses against insect eggs by responding to insect sex pheromones. Our study shows that preexposure of Pinus sylvestris to pine sawfly sex pheromones reduces the survival rate of subsequently laid sawfly eggs. Exposure to pheromones does not significantly affect the pine needle water content, but results in increased needle hydrogen peroxide concentrations and increased expression of defense-related pine genes such as SOD (superoxide dismutase), LOX (lipoxygenase), PAL (phenylalanine ammonia lyase), and PR-1 (pathogenesis related protein 1) after egg deposition. These results support our hypothesis that plant responses to sex pheromones emitted by an herbivorous insect can boost plant defensive responses to insect egg deposition, thus highlighting the ability of a plant to mobilize its defenses very early against an initial phase of insect attack, the egg deposition.
Plants can respond to a wide array of volatile compounds released from microbes, plants, and insects (1–4). Plant responses to odors indicative of biotic stress (pathogens, herbivores) enable them to improve their stress management (5).
Volatile compounds released from damaged plants provide cues indicating herbivory. The perception of herbivory-induced leaf volatiles primes the defensive responses of undamaged plants to imminent herbivory, thus rendering their antiherbivore defense more potent (6–9). Priming of plant defense is an effective way to improve infestation-inducible defense against herbivores (10, 11).
Priming of inducible plant defenses against herbivory is not only mediated by plant volatiles. Plants can also take insect-released volatile compounds as an indicator of impending herbivory, as demonstrated in an exciting study of goldenrod plants exposed to a putative male gall fly sex pheromone, (E,S)-conophthorin, a spiroacetal (3, 4). Exposure of goldenrod to conophthorin primes the plant’s defenses against herbivory by insects specialized on goldenrod plants, thus suggesting a coevolved signal–response pattern.
Priming of inducible plant defenses against insect eggs has thus far not been studied, although insect egg depositions can induce changes in the plant´s primary metabolism (12) as well as defensive plant responses capable of killing those same eggs (13). For example, several plant species form necrotic tissue at the site of egg deposition; this response may result in desiccation of the eggs and/or their detachment from leaves (13, 14). Egg-induced growth of novel plant tissue can squeeze and thus kill the eggs (15). Plants can also produce ovicidal compounds in response to egg deposition (16). In addition, many plant species have been shown to change their leaf odor in response to insect egg deposition; the egg-induced leaf odor attracts parasitic wasps that kill the eggs (17). Since insect mating precedes egg deposition, cues like insect sex pheromones might serve as reliable stimuli indicative of imminent egg deposition, thus eliciting plant responses harming the eggs.
Here we present a study testing the hypothesis that exposure of a plant to insect sex pheromones primes the plant’s defensive response to insect eggs. We used young Pinus sylvestris trees and the pine sawfly Diprion pini to test this hypothesis in the laboratory. These plant and insect species are well suited as a model for several reasons. Conifer forests in the Northern Hemisphere are frequently heavily damaged by sawfly larvae feeding gregariously upon pine needles. Scots pine defends itself against D. pini eggs by accumulating reactive oxygen species (ROS) (18) and by releasing egg-induced needle volatiles that attract egg parasitoids (13). The male-attracting sex pheromone components of D. pini females, (2S,3R,7R)-3,7-dimethyl-2-tridecanyl acetate and propionate, have been intensively studied and are synthetically available (19).
Our results show that exposure of pine to the sex pheromones of a female sawfly primes the tree’s defenses against sawfly eggs and results in enhanced egg mortality, enhanced accumulation of hydrogen peroxide in pine needles, and differential regulation of defense-related pine genes. These results provide evidence that plants are capable of strengthening their defense against a very early step of insect infestation, the egg deposition, by responding to cues preceding egg depositions.
Results and Discussion
Survival Rates of Sawfly Eggs Are Lower on Pine Previously Exposed to Sawfly Sex Pheromones.
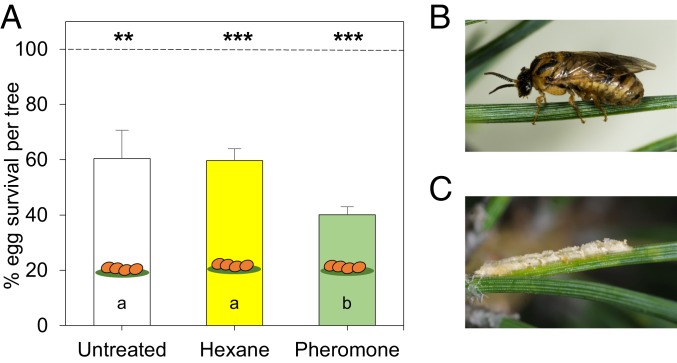
We compared survival rates of D. pini eggs that have been deposited on small, 3-y-old P. sylvestris trees previously exposed for 1 d to D. pini sex pheromones or, as a control, to the pheromone solvent hexane. After 24 h of pheromone (or hexane) exposure, D. pini females were allowed to oviposit for 1 d on the needles of these trees. A D. pini female inserts her eggs in a row (about 15 eggs per row) into a pine needle. After 12 to 14 d (egg incubation time), the larvae hatch from surviving eggs. We exposed the trees to a pheromone dose comparable to that which pine trees are exposed to during a mass D. pini outbreak (SI Appendix, Table S1). Exposure of pine trees to the pheromones significantly affected the pines’ resistance against sawfly eggs. The mean (±SE) survival rate of eggs on trees previously exposed to the pheromone (40.07 ± 2.89%) was significantly lower than the survival rate of eggs on untreated controls (60.37 ± 10.25%) and on trees exposed to the solvent hexane (59.65 ± 4.35%) (Fig. 1 and SI Appendix, Tables S2 and S3). The hexane treatment had no impact on the egg survival rate. This may be due to the high volatility of this solvent. Prior to treatment, the dispensers with hexane and the dispensers with pheromone dissolved in hexane were kept for 30 min in a fume cupboard, where the solvent evaporated; thereafter, pine trees were exposed to the dispensers for 24 h. The low survival rate of D. pini eggs on untreated trees in the absence of natural enemies and at favorable abiotic conditions indicates that P. sylvestris can directly defend itself against the eggs, as also suggested by an earlier study (18). The results here show that preexposure of pine to D. pini sex pheromones results in further reduction of the sawfly´s egg survival rate.
Fig. 1.
Impact of exposure of P. sylvestris to sex pheromones of pine sawflies (D. pini) on sawfly egg survival rates. (A) Percentage (mean + SE) survival of D. pini eggs on untreated pine trees (n = 6), pine trees exposed to hexane (n = 8), and pine trees exposed to the pheromones (dissolved in hexane) (n = 8) for 24 h prior to egg deposition by 2 females per tree. Total number of eggs on untreated trees is 100% = 915 (mean number of eggs per tree ± SE: 152.5 ± 20.81), on hexane-treated trees is 100% = 1170 (mean ± SE: 146.3 ± 11.48), and on pheromone-treated trees is 100% = 858 (mean ± SE: 107.3 ± 11.76). Difference between numbers of eggs laid on the differently treated trees is not significant (n.s.) (ANOVA). Difference between numbers of laid eggs and hatched eggs within a treatment: **P < 0.01; ***P < 0.001 (paired t tests). Different letters in bars indicate significant differences (P < 0.05) in survival rates among treatments (ANOVA followed by multiple pairwise t tests and a Benjamini−Hochberg P value correction) (compare SI Appendix, Tables S2 and S3). (B) D. pini female on P. sylvestris. (C) Egg row of D. pini on a pine needle.
Pheromone Exposure Promotes Hydrogen Peroxide Accumulation in Egg-Laden Pine Needles.
That preexposure of pines to sawfly sex pheromones significantly reduced survival of D. pini eggs raised the question of what causes this ecological effect. At the immediate interface between insect egg and plant, environmental humidity and leaf hydrogen peroxide concentrations are known to affect development of insect eggs and their survival (18, 20, 21). The humidity to which an insect egg is exposed is not only determined by air humidity but also by leaf water content. An increase in leaf hydrogen peroxide concentration and accumulation of other ROS in response to insect eggs may result in formation of necrotic plant tissue (22). This plant response provides an environment in which eggs of several insect species have been shown to suffer increased mortality (20, 23). Formation of necrotic tissue has been described for pines in response to D. pini egg deposition (18), but whether ROS accumulation in response to D. pini eggs is amplified by prior exposure of pines to pheromones is unknown.
Therefore, we investigated whether exposure of pine trees to pheromones 1) reduces the pine needle water content, thus possibly resulting in desiccation of the eggs, or 2) enhances the concentration of pine needle hydrogen peroxide concentrations, thus directly harming the eggs or resulting in amplified plant defense signaling (24). The needle water and hydrogen peroxide contents were analyzed 2 and 12 d after pheromone exposure, that is, 1 and 11 d, respectively, after egg deposition (Fig. 2 and SI Appendix, Fig. S1).
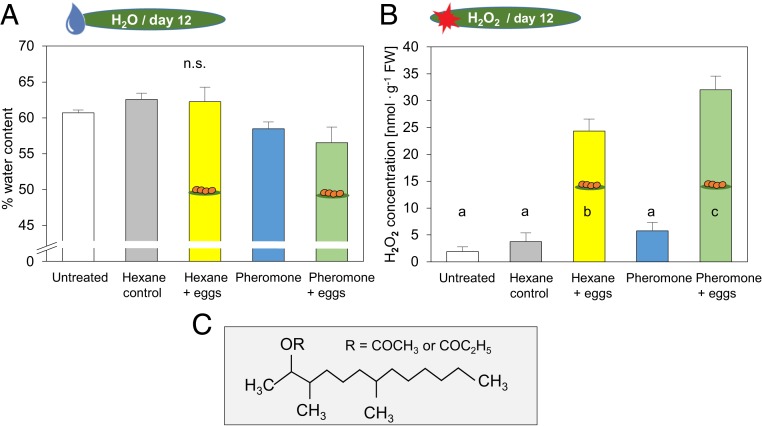
Fig. 2.
(A) Water contents and (B) hydrogen peroxide concentrations of P. sylvestris after exposure to sawfly sex pheromones and subsequent egg deposition. Measurements were conducted 12 d after pheromone exposure, that is, 11 d after egg deposition, at the end of the egg incubation period. Water concentrations and hydrogen peroxide concentrations were determined in pine needles from untreated trees, from trees exposed to the solvent hexane (without eggs: hexane control; with eggs: hexane + eggs), and from trees exposed to the pheromones (dissolved in hexane) (without eggs: pheromone; with eggs: pheromone + eggs). Means + SE of water contents and hydrogen peroxide concentrations are given (n = 5 for water content untreated; n = 8 for all other treatments). All data were evaluated by ANOVA and, for the hydrogen peroxide concentrations, by multiple pairwise t tests and a Benjamini−Hochberg P value correction (different lowercase letters in bars indicate significant differences at P < 0.01) (compare SI Appendix, Table S2). (C) Chemical structure of D. pini sex pheromone components.
The water content of pine needles exposed to the different treatments was similar, and no significant differences between treatments were detected at any of the 2 measurement time points after pheromone exposure (Fig. 2A and SI Appendix, Fig. S1A and Table S2).
Hydrogen peroxide accumulated in egg-laden needles at the end of egg incubation time (i.e., 11 d after egg deposition) (Fig. 2B). This egg-induced accumulation of hydrogen peroxide was significantly enhanced by the pheromone treatment 12 d earlier. In contrast, the pheromone treatment had no effect on the needle hydrogen peroxide concentration of the egg-free pines. Nor did exposure of the pines to hexane affect the needle hydrogen peroxide concentration (Fig. 2B and SI Appendix, Table S2). No induction of hydrogen peroxide accumulation was detectable shortly (1 d) after egg deposition. Nor did a preceding pheromone exposure affect the hydrogen peroxide concentration of pine needles shortly after egg deposition (SI Appendix, Fig. S1B and Table S2).
Thus, the pheromone-mediated strengthening of pine resistance against sawfly eggs is associated with enhanced accumulation of hydrogen peroxide in the pine needles, which becomes evident at the end of the egg incubation time. The enhanced hydrogen peroxide concentration might directly exert a detrimental effect on the eggs (20) and/or serve as an intensified early defense signal (24, 25). Several studies have shown an increase in plant hydrogen peroxide concentrations in response to wounding or herbivory (26–30) and to insect egg deposition (18, 20, 22). While a wound-induced increase in hydrogen peroxide concentration is known to be detectable almost immediately in response to herbivory (e.g., refs. 27 and 28), egg-induced increases have been observed only several days (22) after the egg treatment or at the end of the egg incubation time (18, 20). Here we show that exposure of a plant to a female insect sex pheromone (Fig. 2C), that is, an environmental cue indicating impending insect egg deposition, can even further promote the (egg) infestation-induced hydrogen peroxide accumulation.
Pheromone Exposure Results in Changes of Expression of Defense-Related Pine Genes.
To figure out whether, and if so how, exposure of pines to sawfly sex pheromones affects expression of defense-related pine genes, we ran qPCR analyses of needles from trees treated in different ways (Table 1 and SI Appendix, Table S4). Samples were harvested 2 and 12 d after pheromone or hexane exposure to differentiate between early and late treatment effects. We selected the following genes (for information on sequences, see SI Appendix, Tables S5 and S6): PsRboh (sequence homolog to a respiratory burst oxidase—plant NADPH oxidase), involved in ROS production; PsSOD (superoxide dismutase) encoding an enzyme catalyzing hydrogen peroxide formation; and PsCAT (sequence homolog to catalase) and PsAPX (sequence homolog to ascorbate peroxidase), both of which are involved in ROS degradation (25). We tested expression levels of a putative lipoxygenase encoding gene (PsLOX) initiating the jasmonic acid (JA) pathway (31) and of PsPDF putatively encoding a plant defensin, which is inducible by early JA- and ethylene-mediated defense signaling (32). Additionally, we determined transcript levels of PsPR-1 (sequence homolog to pathogenesis-related protein 1), which is inducible by insect egg depositions on Arabidopsis thaliana (22, 33). Because accumulation of phenylpropanoid derivatives is involved in egg-mediated strengthening of antiherbivore defenses in several plant species (33–35), we also determined transcript levels of PsPAL encoding a putative phenylalanine ammonia lyase, an enzyme at the entrance of the phenylpropanoid pathway (36). As the hexane treatment did not affect expression of the genes tested (SI Appendix, Table S7), we normalized the gene expression levels of all other treatments to those determined for hexane-treated pines.
Table 1.
Expression of selected genes of P. sylvestris after exposure to sawfly sex pheromones and egg deposition
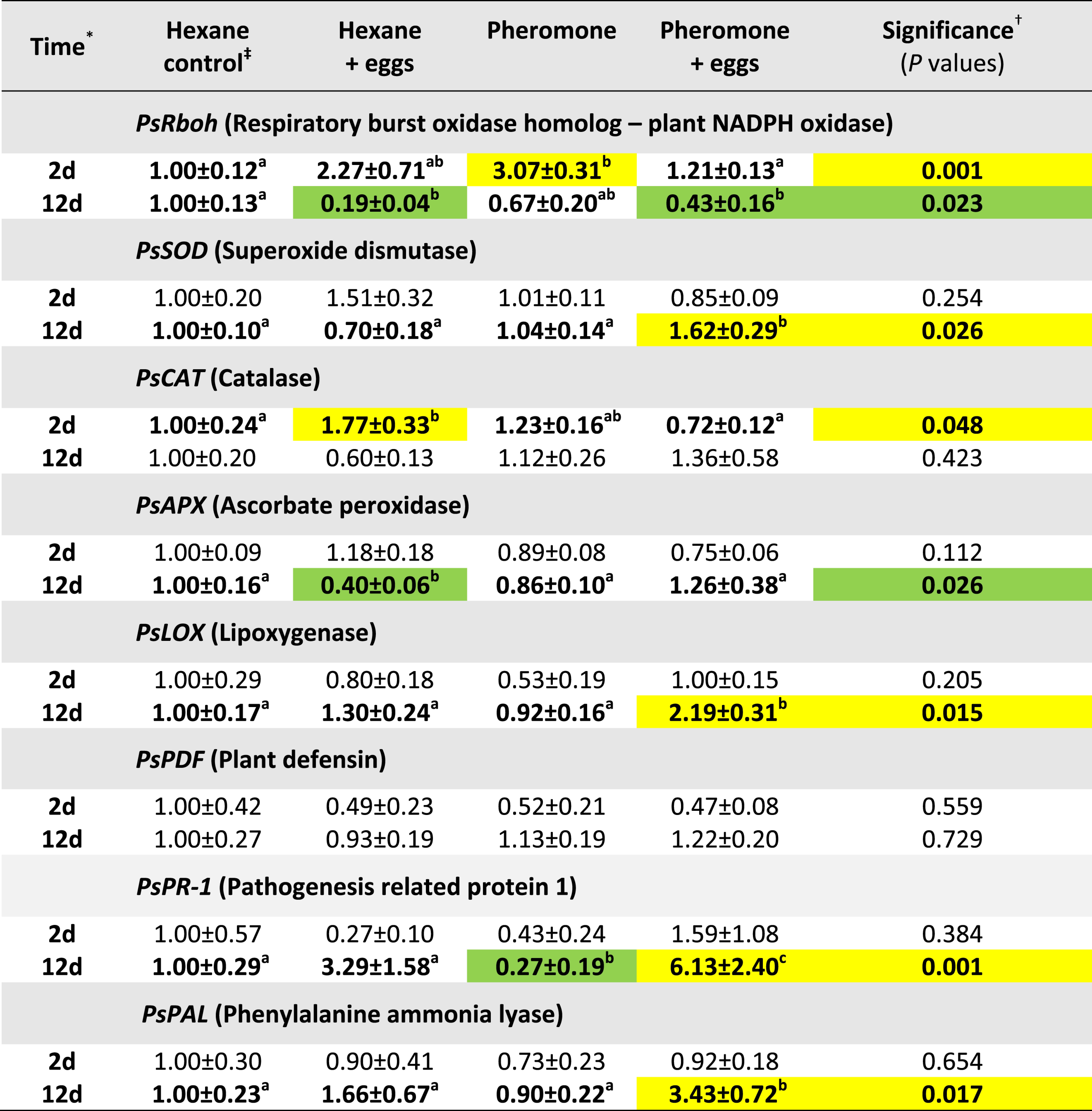 |
Relative transcript abundance (mean ± SE) after treatment with pure hexane (the pheromone solvent; hexane control), with hexane and subsequent egg depositions (hexane + eggs), with pheromones dissolved in hexane only (pheromone), or with pheromone and subsequent egg deposition (pheromone + eggs); n = 5 to 8 trees for each treatment. Green highlights: transcript abundance significantly decreased as compared to hexane control. Yellow highlights: transcript abundance significantly increased as compared to hexane control. Within a line: numbers in bold with different lowercase letters denote statistical differences (P ≤ 0.05).
Days after start of pheromone exposure for 24 h; 2d = directly after 1 d of egg deposition.
Expression values determined in untreated control trees did not differ from those in the “hexane control” (SI Appendix, Table S7).
Significance values (P) were calculated by Kruskal−Wallis H tests (compare SI Appendix, Table S4). Significant differences between 2 treatments were evaluated by a post hoc Conover−Iman test with a Benjamini−Hochberg correction for multiple comparisons.
The pheromone exposure per se affected expression of only 2 of the 8 genes investigated. Shortly (2 d) after sawfly pheromone exposure, expression levels of PsRboh were significantly higher. Priming plants for improved resistance against phytopathogens by preexposure to pathogens or to priming chemicals such as β-aminobutyric acid also results in enhanced expression of RbohD in A. thaliana (37). In contrast to PsRboh, none of the other genes showed significantly altered transcript levels at this early time point after treatment. Twelve days after pheromone exposure, expression levels of PsPR-1 were significantly reduced in pheromone-treated trees.
Sawfly egg deposition without prior exposure of the pines to pheromones affected expression of pine catalase PsCAT, whose transcript levels were significantly higher only shortly after egg deposition, but not later. Expression levels of PsRboh coding for a putative ROS-generating enzyme and of PsAPX coding for a putative hydrogen peroxide detoxifying enzyme were lower at the later sampling time. When trying to relate these data to the hydrogen peroxide concentrations shown in Fig. 2B, these findings suggest that the high hydrogen peroxide levels in needles of egg-deposited pine trees are not due to Rboh-mediated production of ROS. This interpretation is in line with a previous study (22), which found no indication that Rboh is involved in hydrogen peroxide accumulation induced by application of butterfly egg extracts on A. thaliana. However, reduced degradation of ROS because of reduced availability of the ROS-degrading enzyme PsAPX at the end of the egg incubation period (Table 1) might at least contribute to the high hydrogen peroxide concentrations in egg-laden pine needles.
Interestingly, pheromone treatment followed by egg deposition resulted in enhanced expression of PsSOD-encoding superoxide dismutase, which catalyzes the formation of hydrogen peroxide. This result is in line with the higher hydrogen peroxide concentrations in pheromone-treated, egg-deposited needles at the end of the egg incubation period (i.e., 12 d after pheromone treatment). In contrast, expression levels of PsRboh, producing superoxide radicals as substrate for SOD, were low at this time point in pheromone-treated, egg-deposited pine needles. Regulation of hydrogen peroxide concentrations may not only be mediated by the expression of genes encoding ROS generating and degrading enzymes. Also, the activation of these enzymes and other factors like a change in the abundance of ROS scavenging secondary compounds might have contributed to hydrogen peroxide accumulation in pheromone-exposed, egg-deposited needles. In A. thaliana, ROS accumulation is important for egg-induced up-regulation of PR-1 (22). In pines, expression of PsPR-1 was significantly up-regulated in the pheromone-exposed, egg-deposited needles with the highest hydrogen peroxide concentrations. These results suggest that PsPR-1 needs a high ROS level to be significantly up-regulated in response to sawfly egg deposition. In addition to expression of PsSOD and PsPR-1, expression levels of PsLOX were also enhanced in trees preexposed to pheromones and subsequently to egg deposition. Hence, the pheromone preexposure resulted in significant up-regulation of both a salicylic acid (SA)-responsive gene (PR-1) (32) and PsLOX, a gene involved in JA signaling (31), suggesting that both JA and SA signaling are involved in pheromone-mediated priming of plant defense against insect eggs. Despite numerous studies showing antagonistic interactions between JA- and SA-mediated plant defenses (38), our finding supports the growing evidence that these hormones can also act synergistically in a dose- and kinetics-dependent manner (33, 39). Expression of PsPDF was not affected by either treatment. However, PsPAL was significantly up-regulated in pheromone-exposed, egg-laden needles when sampled 12 d after pheromone treatment. Phenylalanine ammonia lyase catalyzes the biosynthesis of cinnamic acid, which is a precursor of numerous compounds formed along the phenylpropanoid pathway, among them compounds that contribute to plant cell wall lignification (36), which might impair larval hatching from D. pini eggs inserted into needle tissue.
Altogether, exposure of pine trees to sawfly sex pheromones affected the expression of several defense-related genes in a time-dependent manner after egg deposition (Table 1 and SI Appendix, Table S4). The combinatory effects of pheromone exposure and subsequent egg deposition on the expression of PsSOD, PsLOX, PsPR-1, and PsPAL are striking. Hence, the pheromone exposure primes the enhanced expression of these genes in response to the sawfly’s egg deposition.
Sawfly Females Show No Electrophysiological Response to Their Pheromones.
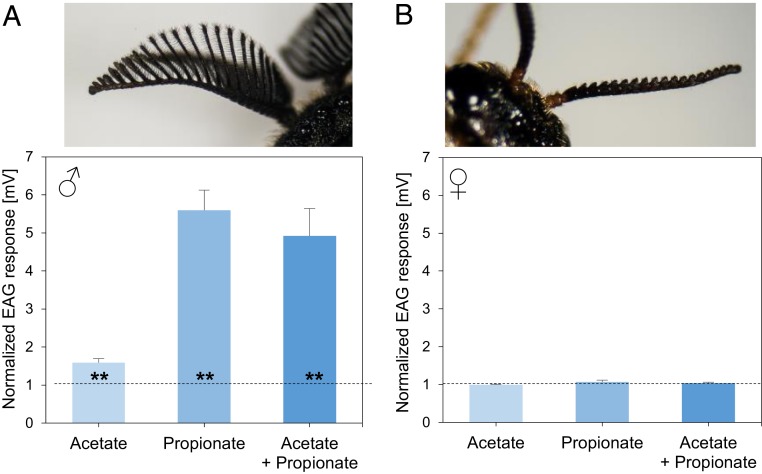
Because pines and pine sawflies share an evolutionary history of about 50 million years (40), we asked whether the sawflies have developed a counteractive strategy to cope with the pheromone-mediated defenses of pines against their eggs. If D. pini females are able to detect their own pheromones, they might disperse away from sites with high pheromone concentrations, thus avoiding competition for resources, as has been observed in females of some lepidopteran species, which are capable of autodetecting their own male-attracting sex pheromones (41). However, our electroantennogram (EAG) studies did not support this hypothesis. While D. pini male antennae clearly responded to both pheromone components, D. pini female antennae did not show these responses (Fig. 3 and SI Appendix, Table S8). We checked by gas chromatography−mass spectrometry (GC-MS) analyses whether pheromone traces were still left on pheromone-exposed pine needles when the trees were exposed to D. pini females for oviposition and, thus, might be perceivable by contact. However, no pheromone traces were detectable on pine needles at the time when females were exposed to the trees. The ability of a D. pini female to lay numerous eggs—a hundred eggs or more—might be a means to maintain a critical population density despite the pine’s effective defense against them.
Fig. 3.
Electrophysiological response of (A) male and (B) female antennae of D. pini to sex pheromone components. Acetate: (2S,3R,7R)-3,7-dimethyl-2-tridecanyl acetate. Propionate: (2S,3R,7R)-3,7-dimethyl-2-tridecanyl propionate. Data show the responses to 500 ng of each pheromone component tested separately (acetate, propionate), or to a blend of both components (acetate + propionate) with 500 ng of each component, that is, 1,000 ng of pheromone in total. Each test odor (acetate, propionate, or the blend) was offered to n = 8 antennae of each sex. Data show means + SE of responses normalized to responses to ambient air and hexane, which were set to value 1 (dashed line). The antennal response to air was almost the same as the one to hexane. Statistical difference of the response to the pheromone from the response to air/hexane was evaluated by the Wilcoxon matched pairs test (**P < 0.01) (compare SI Appendix, Table S8).
Another possibility of counteradaptation to the pheromone-mediated defenses of pine against D. pini eggs could be avoidance of oviposition on pheromone-exposed pine because of pheromone-induced oviposition-deterring changes in the needles. Further studies are necessary to investigate this possible counteradaptation. Such a counteradaptation of an herbivorous insect to pheromone-primed defense against herbivory is suggested by results of the study of goldenrod plants exposed to male gall fly emissions; fewer oviposition punctures were detected in male-exposed plants than in control plants (3); however, in this study, the survival of gall fly eggs and gall fly larval feeding upon the previously male-exposed plants could not be recorded. Nevertheless, these gall fly performance parameters are expected to be reduced because exposure of goldenrod plants to male gall fly emissions and their major component, conophthorin, primed the plants for improved defense against feeding damage by other goldenrod-specialized insects than the gall fly (3, 4).
Conclusion.
Our study highlights that plant defense against eggs can be primed by an insect’s sex pheromone, which reliably indicates an impending very first step of plant infestation, the egg deposition. Hence, these findings show that a plant cannot only be primed for improved defense against impending feeding damage (3–11) but can even prepare its defense against insect eggs, which indicate impending larval feeding damage. Thus, the ability to respond to insect pheromones allows a plant to resist even the very beginnings of insect infestation, the eggs, in a more efficient way. These results suggest that such an early and enhanced defensive response to the eggs might save costs of investment in later feeding-induced defense against hatching larvae, because the greater egg mortality results in reduced abundance of hungry larvae that will hatch from surviving eggs. While constitutive defenses of pine have been shown to trade off with inducible ones and growth rates, possible costs of priming have not been studied yet in pine (42). The costs of priming of plant antiherbivore defenses—measurable by, for example, reduced seed set, aboveground or belowground growth rate, and resistance against other biotic threats like phytopathogens—are considered to depend on various factors, among them the reliability of the priming cue, the presence of priming-sensitive targets, and resource availability and competition (7, 10, 43–45).
Scots pine is shown here to improve its defense against insect eggs by responding to the insect’s sex pheromones with changes in the expression of its own defense-related genes and increased accumulation of egg-induced hydrogen peroxide. Our results provide the basis for further research addressing the questions arising here, such as about the specificity of the pine’s response to sawfly pheromones, the specificity of the response effects, and the perception of these pheromones. Components similar to the D. pini pheromonal components are released by closely related sawfly species. Females of other diprionid genera than Diprion emit esters similar to the D. pini pheromonal esters, for example, esters with an alcohol component having a longer or shorter chain length than tridecanol or with other methylation patterns of tridecanol than in the D. pini pheromonal compounds (46). The sawfly Diprion jingyuanensis, a pest of Chinese pine (Pinus tabulaeformis), has been shown to be attracted by the D. pini propionate pheromonal compound, suggesting that this is also a pheromone of D. jingyuanensis (47). Whether the Chinese pine species responds similarly to the pheromone and whether the eggs of D. jingyuanensis react similarly to the tree´s defense remains to be addressed in future studies. The lipophilic character of D. pini sex pheromones might facilitate direct interactions with the plant’s plasma membrane, and thus change transmembrane ion fluxes and initiate early defense signaling (2). In addition to these proximate questions on the mechanisms involved, it will be interesting to address evolutionary ecology aspects of this pheromone-mediated plant defense strategy. If the ability to respond to insect sex pheromones by priming defenses against insect eggs is widespread among plants, this might place some selective pressure on pheromone communication among insects and on their oviposition behaviors. Furthermore, if the priming effect shown by our study is not limited to the species studied here, but extends to other ones relevant in, for example, agriculture and viticulture, application of the pheromone-mediated mating disruption technology in integrated insect pest management not only will cause negative effects on the fertilization of females due to olfactory insect disorientation (48) but will also reduce survival of insect eggs due to pheromone-primed plant defense.
Materials and Methods
Experimental Organisms.
Three-year-old pine trees (P. sylvestris) were obtained from a tree nursery (Schlegel & Co.) and used for the experiments. The small trees (45 to 55 cm high) were kept in a greenhouse under long-day conditions (18:6 h light:dark, average temperature 20 °C) until the experiment started.
The pine sawfly D. pini was reared in the laboratory on P. sylvestris. The sawfly rearing was based on specimens collected in the surroundings of Goettingen, Germany, and in the Berlin−Brandenburg area, Germany. Branches of P. sylvestris were obtained from a forest northwest of Berlin and offered to D. pini females for oviposition and to larvae for feeding. The sawflies were reared according to established protocols (49, 50). The development of D. pini from egg to adult takes from 50 to 55 d under the given laboratory conditions (18:6 h light:dark, 20 °C, 70% relative humidity). The adults mate and start egg depositions within several days after emergence from cocoons. No distinct mate calling behavior has been described for D. pini females, nor has it been observed by us. When we observed mating couples, they were sitting on the pine needles.
Plant Treatments.
Prior to each experiment, trees from the greenhouse were acclimatized for 3 d in a climate chamber at 20 °C, 18:6 h light:dark, 70% relative humidity, 155 µmol photons per square meter per second. To avoid cross-contamination with volatiles from plants that had been treated differently, the small trees were placed in Plexiglas cylinders (60 cm height, 9.5 litre), which were ventilated by charcoal-filtered air (inflowing and outflowing air: ∼200 mL⋅min−1). As described above, the D. pini sex pheromones were dissolved in hexane; therefore, we also treated the trees with hexane only. Specifically, we used the following types of pine treatments (n = 5 to 8 trees each) for later analysis of needle water content, hydrogen peroxide concentrations and gene expression levels: treatment a, untreated pines; treatment b, exposure of pines to hexane for 24 h; treatment c, exposure of pines to hexane for 24 h and subsequent egg deposition for 24 h; treatment d, exposure of pines to D. pini sex pheromones (dissolved in hexane) for 24 h; and treatment e, exposure of pines to D. pini sex pheromones for 24 h and subsequent egg deposition for 24 h.
For treatment b, we applied 100 µL of hexane to a cotton wool pad (diameter: 5.6 cm, thickness: 0.4 cm) as the dispenser. To allow evaporation of the hexane, pads were kept for 30 min under the fume hood prior to exposure to the trees. Following hexane evaporation, a pad was placed into the aforementioned Plexiglas cylinder, along with a pine tree, for 24 h.
For treatment c, the plants were treated as in b, and, thereafter, 2 D. pini females were allowed to oviposit on the tree for 24 h. Only trees with at least 4 egg rows were used for the experiments.
Treatments d and e were conducted as described for b and c except that 100 µL of a pheromone solution was applied to the cotton wool pad instead of only hexane. The trees were exposed to the pheromone components for 24 h, because we expect a high pheromone concentration to be present for at least a day in a pine forest, where a mass outbreak of D. pini takes place (SI Appendix, SI Material and Methods).
Pheromones.
Previous field and electrophysiological studies showed that the acetate and propionate esters of (2S,3R,7R)-3,7-dimethyl-2-tridecanol are the active, male-attracting components of the sex pheromone released by female D. pini (51, 52). Synthesized esters dissolved in hexane were obtained from the laboratory of Olle Anderbrant (Lund University, Sweden). The pheromones were synthesized by Helen Edlund and Erik Hedenström at Mid Sweden University (52, 53). We controlled the pheromone purity and concentration using GC-MS (Agilent 7890 A GC model coupled to an Agilent 5975 C MS unit).
To determine the concentration of the pheromone components, 10 ng⋅µL−1 methyl undecanoate (Sigma Aldrich) was used as an internal standard. We injected 1 µL of a 1/100 and of a 1/1,000 dilution of the obtained pheromone solution in hexane (including the internal standard) in splitless mode (injector temperature 300 °C; J&W DB-5-ms capillary column: length: 30 m; inner diameter: 0.25 mm; film thickness: 0.25 µm). Helium was used as carrier gas, with an inlet pressure of 0.1 bar and an outlet pressure of 50 kPa. The following program was used for analysis: 4-min hold at 40 °C followed by a temperature increase of 10 °C⋅min−1 until 180 °C, followed by a temperature increase of 20 °C⋅min−1 until 280 °C, and a 5-min hold at the end of the program. The column effluent was ionized by electron impact ionization at 70 eV (mass range from 35 to 600 m/z).
The pheromone solution, which the plants were exposed to, contained both pheromone components, each at a concentration of 50 ng⋅µL−1 hexane. We determined the release rate of the pheromones from the cotton pads by GC-MS analyses as described in SI Appendix, SI Material and Methods. The results confirmed that the release rate was equivalent to the release rate of a high abundance of D. pini females, that is, 270 to 450 females. The upper end of this range is similar to the number (around 400 females per tree) counted during a mass outbreak in the surroundings of Berlin (SI Appendix, SI Material and Methods).
To determine whether pheromone residues were left on pine needles when the trees were exposed to D. pini egg deposition, we extracted pine needles 6 h after the end of a 24-h pheromone exposure time and analyzed the extract by GC-MS as described in SI Appendix, SI Material and Methods.
Determination of Egg Survival.
To determine the effect of pheromone exposure on the survival rate of D. pini eggs, 2 sawfly females were offered an untreated pine tree, a hexane-exposed tree, or a pheromone-exposed tree for a period of 24 h. We counted the number of eggs and larvae hatching on each tree. The egg survival rate was calculated by relating the number of eggs laid to the number of larvae hatching from the eggs per tree. Egg survival rates were determined on n = 6 untreated trees, n = 8 hexane-exposed trees, and n = 8 pheromone-exposed trees.
Determination of Pine Needle Water Content and Hydrogen Peroxide Concentration.
To determine the water content of needles from the differently treated trees, we harvested 3 to 4 needles that were adjacent to the oviposition site. The needles were sampled 1) 2 d after pheromone or hexane exposure (i.e., 1 d after egg deposition) and 2) at the end of the egg incubation period, shortly before larvae would hatch, that is, 12 d after pheromone or hexane exposure and 11 d after egg deposition (egg incubation is around 12 to 14 d in the abiotic conditions used). Needles from equivalent positions and in comparable quantities were harvested from egg-free trees. Immediately after harvesting, the needles were weighed. The needles were then dried for 72 h in an oven (60 °C) and weighed once again. Based on these weights, the relative water content (percent) was calculated. Drying for more than 72 h showed no further weight loss. We determined the water content of needles taken from n = 5 untreated trees and n = 8 trees subjected to the aforementioned treatments.
To determine the hydrogen peroxide concentrations of needles from the differently treated trees, we used the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Molecular Probes by Invitrogen), which provides a fluorescing product with hydrogen peroxide. Our protocol followed the manufacturer’s recommendations modified after Chakraborty et al. (54). Needles were harvested from similar tree positions and at the same time points as described above for determining the water content. The needles were immediately transferred to liquid nitrogen after being detached from the experimental trees and were ground to a powder. A sample of 30 mg of powdered needle tissue per tree was mixed with 250 µL (0.05 M; pH 7.4) of sodium phosphate buffer and placed on a shaker with 50 rpm at 25 °C for 30 min. Thereafter, the needle sample was centrifuged at 15,000 × g for 15 min, and the supernatant was centrifuged again at 15,000 × g for 2 min. A sample (50 μL) was taken from the final supernatant and incubated with 50 μL of a solution consisting of 100 μM Amplex Red reagent and 0.2 U⋅mL−1 horseradish peroxidase. The incubation took 30 min at 30 °C in dark conditions. To prepare samples with distinct hydrogen peroxide concentrations for recording a reference standard curve, samples with hydrogen peroxide concentrations ranging from 0 to 30 µM H2O2 were prepared according to the protocol provided with the kit. These samples were incubated with the Amplex Red reagent and horseradish peroxidase as described for the needle samples. After incubation and centrifugation, the fluorescence of each sample (50 µL; 3 technical replicates) was determined by using an Infinite 200 PRO plate reader (Tecan Life Science) (excitation: 560 nm; emission: 590 nm). The hydrogen peroxide concentrations were calculated based on the standard curve value and then divided by 30 mg (needle sample weight). The hydrogen peroxide concentration was determined in needles taken from n = 8 trees of each treatment, as well as from n = 8 untreated trees.
Gene Expression Analysis.
Needles were collected from sites adjacent to the oviposition site (about 1 g per tree) and from equivalent positions and in comparable quantities from egg-free trees. We harvested the needles at the same time points after pheromone exposure and egg deposition as described above for determining the water content. Needles that had been immediately frozen in liquid nitrogen after sampling were powdered. A powdered needle sample (50 mg) was used for RNA extraction with the InviTrap Spin Plant RNA Mini Kit (Stratec). RNA was eluted in 50 µL of nuclease-free H2O, and contaminating DNA remains were digested with the TURBO DNA free kit (ThermoFisher Scientific). RNA integrity and purity were checked by analysis on a 1.1% agarose gel in 1× TAE buffer with 0.006% EtBr. A volume of 10 µL of the sample was diluted 1:1 with 2× RNA loading dye (ThermoFisher Scientific), heated for 10 min to 70 °C, and placed on ice immediately afterward. A volume of 4 µL of the RiboRuler High Range RNA Ladder (ThermoFisher Scientific) was treated likewise. After loading samples, the gel was run for 90 min at 120 V. Spectrophotometric determination of the RNA concentration was performed on a Multiscan GO microplate spectrophotometer (ThermoFisher Scientific) by measuring absorbance at 260 nm.
For synthesis of cDNA, 500 ng of extracted RNA was used as a template for reverse transcription utilizing the AMV-RT (avian myeloblastosis virus reverse transkriptase) native enzyme (Roboklon). The RNA was mixed with 1 µL of Oligo dT20 (50 µM) and 2 µL of dNTPs (10 mM) and filled up to a reaction volume of 14 µL with nuclease-free H2O. The mixture was incubated for 5 min at 65 °C, followed by 5 min incubation at 4 °C. To start the reaction, 4 µL of 5× RT buffer (Roboklon), 0.5 µL of RNase inhibitor (Roboklon; 30 U⋅µL−1), 1 µL of 100 mM DTT (dithiothreitol), and 1 µL of AMV-RT native (Roboklon; 10 U⋅µL−1) were added and heated to 42 °C for 15 min and to 50 °C for 45 min. To inactivate the AMV-RT enzyme, the mixture was finally heated to 80 °C for 10 min and thereafter cooled on ice.
Primers (SI Appendix, Table S5) for the selected genes and for the housekeeping genes ubiquitin (PsUBI), cytochrome subunit 6 (PsPETB), and chloroplast ATPase beta subunit (PscATP) were designed and evaluated according to the MIQE guidelines (minimum information for publication of quantitative real-time PCR experiments) (55, 56) with the online tool named PRIMER-BLAST (57). For genes for which no annotated template sequences have been published for P. sylvestris (LOX; PR-1; PETB; cATP; UBI), we searched in BLAST (basic local alignment search tool), EST (expressed sequence tags), and nr databases for Pinus sequences, which showed high homology with annotated sequences from other plant species. Primers were designed based on sequences with the lowest E value, and the identity of the PCR products was evaluated by Sanger sequencing at Seqlab and BLAST analysis (SI Appendix, Table S6) (58).
We performed qPCR analyses using the qPCRBIO SyGreen Mix Lo-Rox kit (Nippon Genetics Europe) on an MX3005P (Stratagene) cycler. For the qPCR reactions, 12.5 ng of cDNA was mixed with 5 µL of qPCRBIO SyGreen Mix Lo-Rox Master Mix (Nippon Genetics Europe) and 0.17 µL of each primer (10 pmol⋅µL−1) and filled up to a 10-µL reaction volume with nuclease-free H2O. To control for primer dimerization, H2O controls were run, and, to control for genomic DNA contamination, DNase-treated RNA from each sample was used. Each reaction was performed with 3 technical replicates under the following running conditions: after an initial heating step of 2 min at 95 °C, 40 cycles of 5 s at 95 °C, followed by 30 s at 60 °C, were performed. At the end of each cycle, the fluorescence was measured twice. Following the 40 cycles of PCR amplification, a dissociation curve ranging from 55 °C to 95 °C in 1 °C steps was measured to check for primer dimer reaction products. Cq (cycle quantification value) values and PCR efficiency of all reactions were calculated with LinRegPCR version 2015.2 (59). Normalization of response genes to the reference genes PsUBI, PsPETB, and PsCATP was performed as described by Vandesompele et al. (60). Gene expression analyses were conducted with samples taken from n = 5 to 8 trees of each treatment.
Sawfly Antennal Responses to Pheromones.
Electrophysiological antennal responses of D. pini adult males and females to their sex pheromones [(2S,3R,7R)-3,7-dimethyl-2-tridecanyl acetate and propionate] were recorded by EAG. We chilled the sawflies by each sawfly at 4 °C for several minutes and then cut off the antenna at its base, where we inserted the reference electrode, that is, a glass electrode filled with Ringer solution (NaCl 128.3 mmol/L, KCl 4.7 mmol/L, CaCl2 2.6 mmol/L) and linked with a grounded Ag wire. The tip of the antenna was connected to the recording glass electrode filled with Ringer as well and linked via an interface (IDAC 2; Syntech) to a PC for signal recording. To record the electrophysiological response of an antenna to the pheromones, we applied 500 ng of the acetate pheromone component, or 500 ng of the propionate pheromone component, or 500 ng of each of the components as a blend on a filter paper (28 mm2) (5 µL of pheromone solution in hexane; these quantities are equivalent to that released by about 27 to 45 D. pini females). For control measurements, 5 µL of hexane was applied to a filter paper. Prior to exposure to the antenna, the solvent was allowed to evaporate for 15 min. Thereafter, the filter paper with the test odor was inserted into a Pasteur pipette, which was connected to a stimulus controller (CS-05; Syntech), which allows puffing the test odor in a standardized manner to the antenna (flow: 20 mL/s; stimulus time: 0.5 s). Each antenna was first exposed to ambient air and then to one of the test odors or to the control solvent. The EAG signals (millivolts) were amplified 100-fold by a microelectrode amplifier and recorded by EAG software (Syntech). The EAG signals were evaluated by normalizing the responses to test odors (R-t) to the responses to ambient air (R-a) by dividing the signals (R-t/R-a). Likewise, the responses to the solvent hexane (R-h) were normalized to those to air (R-h/R-a). Thereafter, the air-normalized response to the solvent hexane was set to value 1.0 (R-h/R-a divided by R-h/R-a = 1), and the air-normalized responses to the test odors were adjusted accordingly (R-t/R-a divided by R-h/R-a). The signals recorded in response to the solvent were almost the same as those in response to ambient air. We determined the responses of n = 8 antennae (taken from 8 individuals) of each sex.
Data Analysis.
The gene expression data were evaluated with the statistical software R version 3.4.1 (61) using the packages car, lawstat, and PMCMR. All other data were evaluated with the statistical software SigmaPlot version 11.0 (Systat Software GmbH, 2008). All datasets were tested for normal distribution by the Shapiro−Wilk test. Variance homogeneity was measured with Levene’s test. Normally distributed data (with variance homogeneity) were subjected to parametric tests, and nonnormally distributed data were subjected to nonparametric tests. All tests (and respective P values) were run 2-sided with confidence intervals of 95%. To analyze the difference between the recorded egg survival rates per pine treatment and the theoretically possible survival rate (100% survival of all deposited eggs), we used the paired t test. To analyze whether the survival rates, the water content, and hydrogen peroxide concentrations differed among treatments, we used an ANOVA, and, in the case of statistical significance, we further analyzed the data by multiple pairwise t tests and a Benjamini−Hochberg P value correction. Statistical details are given in SI Appendix, Tables S2 and S3. The qPCR data were normalized to the expression values recorded in the treatment “hexane control.” The expression values in the hexane control treatment did not differ from those in the untreated samples, as analyzed by the Mann−Whitney U test in the case of nonnormally distributed data and by the paired t test in the case of normally distributed data (SI Appendix, Table S7). Differences in expression values between the hexane control and the other treatments were evaluated by Kruskal−Wallis H test followed by a pairwise comparison with the Conover−Iman test, with a Benjamini−Hochberg correction for multiple comparisons (Table 1 and SI Appendix, Table S4). To analyze the difference in electrophysiological antennal responses to the hexane solvent and the pheromone components, we used the Wilcoxon matched pairs test (Fig. 3 and SI Appendix, Table S8).
Supplementary Material
Acknowledgments
We are grateful to Ute Braun, Beate Eisermann, and Gabriele Haberberger, Freie Universität Berlin (FUB), for rearing the sawflies. Many thanks are owed to Jona Höfflin, FUB, for his technical assistance and his support with the GC-MS analyses. We thank Gunnar Bröhan, FUB, for his support in designing the primers. We are further grateful to the Collaborative Research Centre 973 (Project B1, FUB) for its support. We also thank Helen Edlund and Erik Hedenström from Mid Sweden University, Sundsvall, for the synthesis of pine sawfly pheromones. A.A.-C. has been financially supported by the European Research Council Synergy Grant ERC-SyG-2013-610028 IMBALANCE-P and a grant from the German Academic Exchange Service (Grant 572142227).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Sequences of Pinus sylvestris PCR products as well as the template accession numbers in Genbank for the primer design and the annotation information referred to in this paper have been deposited at the repository of the Max-Planck-Institute for Molecular Plant Physiology with open access at https://primedb.mpimp-golm.mpg.de/index.html?sid=reviewer&pid=4afbcf5cea03476a56b57e44eb58e261.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910991116/-/DCSupplemental.
References
- 1.Sharifi R., Ryu C. M., Revisiting bacterial volatile-mediated plant growth promotion: Lessons from the past and objectives for the future. Ann. Bot. 122, 349–358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erb M., Volatiles as inducers and suppressors of plant defense and immunity-origins, specificity, perception and signaling. Curr. Opin. Plant Biol. 44, 117–121 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Helms A. M., De Moraes C. M., Tooker J. F., Mescher M. C., Exposure of Solidago altissima plants to volatile emissions of an insect antagonist (Eurosta solidaginis) deters subsequent herbivory. Proc. Natl. Acad. Sci. U.S.A. 110, 199–204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms A. M., et al. , Identification of an insect-produced olfactory cue that primes plant defenses. Nat. Commun. 8, 337 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilker M., Schmülling T., Stress priming, memory, and signalling in plants. Plant Cell Environ. 42, 753–761 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Dicke M., Baldwin I. T., The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help.’ Trends Plant Sci. 15, 167–175 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Heil M., Karban R., Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 25, 137–144 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Ton J., et al. , Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 49, 16–26 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Frost C. J., Mescher M. C., Carlson J. E., De Moraes C. M., Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiol. 146, 818–824 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilker M., et al. , Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. Camb. Philos. Soc. 91, 1118–1133 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Medina A., et al. , Recognizing plant defense priming. Trends Plant Sci. 21, 818–822 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Schröder R., Forstreuter M., Hilker M., A plant notices insect egg deposition and changes its rate of photosynthesis. Plant Physiol. 138, 470–477 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilker M., Fatouros N. E., Resisting the onset of herbivore attack: Plants perceive and respond to insect eggs. Curr. Opin. Plant Biol. 32, 9–16 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Reymond P., Perception, signaling and molecular basis of oviposition-mediated plant responses. Planta 238, 247–258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desurmont G. A., Hérard F., Agrawal A. A., Oviposition strategy as a means of local adaptation to plant defence in native and invasive populations of the viburnum leaf beetle. Proc. Biol. Sci. 279, 952–958 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J. O., Nakayama N., Toda K., Tebayashi S., Kim C. S., Structural determination of elicitors in Sogatella furcifera (Horváth) that induce Japonica rice plant varieties (Oryza sativa L.) to produce an ovicidal substance against S. furcifera eggs. Biosci. Biotechnol. Biochem. 78, 937–942 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Hilker M., Fatouros N. E., Plant responses to insect egg deposition. Annu. Rev. Entomol. 60, 493–515 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Bittner N., Trauer-Kizilelma U., Hilker M., Early plant defence against insect attack: Involvement of reactive oxygen species in plant responses to insect egg deposition. Planta 245, 993–1007 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Anderbrant O., et al. , Release of sex pheromone and its precursors in the pine sawfly Diprion pini (Hym., Diprionidae). Chemoecology 15, 147–151 (2005). [Google Scholar]
- 20.Geuss D., Stelzer S., Lortzing T., Steppuhn A., Solanum dulcamara’s response to eggs of an insect herbivore comprises ovicidal hydrogen peroxide production. Plant Cell Environ. 40, 2663–2677 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Griese E., Dicke M., Hilker M., Fatouros N. E., Plant response to butterfly eggs: Inducibility, severity and success of egg-killing leaf necrosis depends on plant genotype and egg clustering. Sci. Rep. 7, 7316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gouhier-Darimont C., Schmiesing A., Bonnet C., Lassueur S., Reymond P., Signalling of Arabidopsis thaliana response to Pieris brassicae eggs shares similarities with PAMP-triggered immunity. J. Exp. Bot. 64, 665–674 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fatouros N. E., et al. , Synergistic effects of direct and indirect defences on herbivore egg survival in a wild crucifer. Proc. Biol. Sci. 281, 20141254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baxter A., Mittler R., Suzukí N., ROS as key players in plant stress signalling. J. Exp. Bot. 65, 1229–1240 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Apel K., Hirt H., Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Bi J. L., Felton G. W., Foliar oxidative stress and insect herbivory: Primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J. Chem. Ecol. 21, 1511–1530 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Orozco-Cardenas M., Ryan C. A., Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc. Natl. Acad. Sci. U.S.A. 96, 6553–6557 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maffei M. E., et al. , Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 140, 1022–1035 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maffei M. E., Mithöfer A., Boland W., Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release. Phytochemistry 68, 2946–2959 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Wu J., Baldwin I. T., Herbivory-induced signalling in plants: Perception and action. Plant Cell Environ. 32, 1161–1174 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Wasternack C., Song S., Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 68, 1303–1321 (2017). [DOI] [PubMed] [Google Scholar]
- 32.van Loon L. C., Rep M., Pieterse C. M., Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 44, 135–162 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Lortzing V., et al. , Insect egg deposition renders plant defence against hatching larvae more effective in a salicylic acid-dependent manner. Plant Cell Environ. 42, 1019–1032 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Bandoly M., Grichnik R., Hilker M., Steppuhn A., Priming of anti-herbivore defence in Nicotiana attenuata by insect oviposition: Herbivore-specific effects. Plant Cell Environ. 39, 848–859 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Austel N., Eilers E. J., Meiners T., Hilker M., Elm leaves ‘warned’ by insect egg deposition reduce survival of hatching larvae by a shift in their quantitative leaf metabolite pattern. Plant Cell Environ. 39, 366–376 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Vogt T., Phenylpropanoid biosynthesis. Mol. Plant 3, 2–20 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Pastor V., et al. , Fine tuning of reactive oxygen species homeostasis regulates primed immune responses in Arabidopsis. Mol. Plant Microbe Interact. 26, 1334–1344 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Thaler J. S., Humphrey P. T., Whiteman N. K., Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 17, 260–270 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Liu L., et al. , Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 7, 13099 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Reilly J. E., Dos Reis M., Donoghue P. C. J., Dating tips for divergence-time estimation. Trends Genet. 31, 637–650 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Holdcraft R., Rodriguez-Saona C., Stelinski L. L., Pheromone autodetection: Evidence and implications. Insects 7, 1–29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreira X., et al. , Trade-offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecol. Lett. 17, 537–546 (2014). [DOI] [PubMed] [Google Scholar]
- 43.van Hulten M., Pelser M., van Loon L. C., Pieterse C. M. J., Ton J., Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 103, 5602–5607 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Douma J. C., Vermeulen P. J., Poelman E. H., Dicke M., Anten N. P. R., When does it pay off to prime for defense? A modeling analysis. New Phytol. 216, 782–797 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yip E. C., Tooker J. F., Mescher M. C., De Moraes C. M., Costs of plant defense priming: Exposure to volatile cues from a specialist herbivore increases short-term growth but reduces rhizome production in tall goldenrod (Solidago altissima). BMC Plant Biol. 19, 209 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keeling C. I., Plettner E., Slessor K. N., “Hymenopteran semiochemicals” in The Chemistry of Pheromones and Other Semiochemicals I, Schulz S., Ed. (Topics in Current Chemistry, Springer, 2004), vol. 239, pp. 133–177. [DOI] [PubMed] [Google Scholar]
- 47.Chen G. F., et al. , A preliminary study on the sex pheromone of Diprion jingyuanensis Xiao et Zhang. Chin. J. Biol. Control 13, 61–64 (1997). [Google Scholar]
- 48.Witzgall P., Kirsch P., Cork A., Sex pheromones and their impact on pest management. J. Chem. Ecol. 36, 80–100 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Bombosch S., Ramakers P. M. J., Zur Dauerzucht von Gilpinia hercyniae Htg. Z. Pflanzenkr. Pflanzenschutz 83, 40–44 (1976). [Google Scholar]
- 50.Eichhorn O., Dauerzucht von Diprion pini L. (Hym.: Diprionidae) im Laboratorium unter Berücksichtigung der Fotoperiode. Anz. Schädlingskunde Pflanzenschutz Umweltschutz 49, 38–41 (1976). [Google Scholar]
- 51.Anderbrant O., et al. , Electrophysiological and morphological characteristics of pheromone receptors in male pine sawflies, Diprion pini (Hymenoptera: Diprionidae), and behavioural response to some compounds. J. Insect Physiol. 41, 395–401 (1995). [Google Scholar]
- 52.Hedenström E., et al. , Sex pheromone of the pine sawfly, Gilpinia pallida: Chemical identification, synthesis, and biological activity. J. Chem. Ecol. 32, 2525–2541 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Bergström G., et al. , Sex pheromone of the pine sawfly Diprion pini (Hymenoptera: Diprionidae): Chemical identification, synthesis and biological activity. Experientia 51, 370–380 (1995). [Google Scholar]
- 54.Chakraborty S., et al. , Quantification of hydrogen peroxide in plant tissues using Amplex Red. Methods 109, 105–113 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Bustin S. A., et al. , The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Taylor S., Wakem M., Dijkman G., Alsarraj M., Nguyen M., A practical approach to RT-qPCR—Publishing data that conform to the MIQE guidelines. Methods 50, S1–S5 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Ye J., et al. , Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13, 134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bittner N., Hundacker J., Achotegui-Castells A., Anderbrant O., Hilker M. (2019) Defense of Scots pine against sawfly eggs (Diprion pini) is primed by exposure to sawfly sex pheromones. PrimeDB. https://primedb.mpimp-golm.mpg.de/index.html?sid=reviewer&pid=4afbcf5cea03476a56b57e44eb58e261. Deposited 25 October 2019. [DOI] [PMC free article] [PubMed]
- 59.Ruijter J. M., et al. , Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 37, e45 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vandesompele J., et al. , Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.R Development Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.