Abstract
Objective:
N-acetylcysteine (NAC) is beneficial in psychiatric conditions, including schizophrenia. Patients with schizophrenia exhibit mesolimbic dopamine hyperfunction consequent to an endogenous sensitization process. This sensitization can be modeled in rodents by repeated exposure to psychostimulants, provoking an enduring amplified response at subsequent exposure. The aim of this study was to investigate the effects of NAC on amphetamine sensitization in mice.
Methods:
D-amphetamine was administered to C57BL/6 mice three times a week for 3 weeks; the dose was increased weekly from 1 to 3 mg/kg. NAC (60 mg/kg) or saline was administered intraperitoneally before saline or amphetamine during the second and third weeks. After a 4-week washout period, latent inhibition (LI) and the locomotor response to amphetamine 2 mg/kg were assessed.
Results:
Sensitization disrupted LI and amplified the locomotor response; NAC disrupted LI in control mice. In sensitized animals, NAC attenuated the enhanced locomotion but failed to prevent LI disruption.
Conclusion:
NAC warrants consideration as a candidate for early intervention in ultra-high risk subjects due to its safety profile and the relevance of its mechanism of action. Supplementing this proposition, we report that NAC attenuates sensitization-induced locomotor enhancement in mice. The finding that NAC disrupted LI incites a cautionary note and requires clarification.
Keywords: Schizophrenia, acetylcysteine, amphetamine
Introduction
The long-standing dopamine hypothesis of schizophrenia postulates that mesolimbic dopamine hyperfunction underlies the psychotic symptoms of the disorder. This dopamine hyperfunction is considered the result of an “endogenous sensitization” process,1,2 matching neuroimaging studies that show enhanced striatal dopamine release in response to acute amphetamine challenge in patients with schizophrenia.3,4 Moreover, repeated consumption of high doses of amphetamine (or other stimulants) often results in the progressive development of a psychotic state that closely resembles paranoid schizophrenia.5,6
The amplified sensitivity to amphetamine seen in patients with schizophrenia can be recapitulated in rodents by repeated exposure to psychostimulants, a sensitization process leading to increased response to a later exposure to the same drug. Animals sensitized to amphetamine display enhanced response to the drug even months or years after its withdrawal, illustrating this is an enduring phenomenon.7 Because amphetamine sensitization induces behavioral and neurochemical abnormalities similar to those observed in schizophrenia, it has become a widely accepted animal model of the disorder.8,9
N-acetylcysteine (NAC) is a safe and well-tolerated drug, clinically used as a mucolytic and antidote against paracetamol toxicity.10,11 NAC is a glutathione precursor that exerts antioxidant, anti-inflammatory, anti-apoptotic and neurotrophic actions.12-14 NAC also modulates glutamate release through activation of the cystine-glutamate antiporter expressed in astrocytes at extra-synaptic sites.15,16 The effects of this promising drug in psychiatry17 were assessed in several clinical trials,18 which demonstrated the beneficial effect of NAC as an adjunctive treatment for neuropsychiatric disorders.
The purpose of this study was to investigate the effects of NAC on amphetamine sensitization. We used an escalating dose protocol that induces hyperlocomotion, latent inhibition (LI) disruption, and prepulse inhibition deficits,19-21 which are behavioral abnormalities related to schizophrenia.
Methods
Animals
A total of 72 C57BL/6 male mice (2 months old) were obtained from Universidade Federal de Pelotas and maintained under controlled environmental conditions (reversed 12-h light/dark cycle with lights on at 7:00 a.m. and constant temperature of 22±1°C) with free access to food and water. All procedures were approved by the ethics committee of Hospital de Clínicas de Porto Alegre (approval no. 13-0457), and were in agreement with the principles applicable to the care and use of laboratory animals.22 All efforts were made to minimize the number of animals used and their suffering.
Drugs
NAC and D-amphetamine were purchased from Sigma-Aldrich (St. Louis, MO, USA). All drugs were dissolved in saline (0.9% NaCl). Solutions were freshly prepared and injected intraperitoneally (i.p.) at final volumes of 5 mL/kg.
Amphetamine sensitization protocol
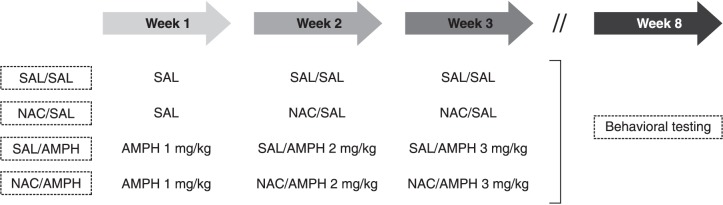
The sensitization protocol was based on the work of Tenn et al.20 To increase the translational value of the approach, this model is designed to mimic the prodromal state of schizophrenia, and treatment start is delayed until the second week to mimic early intervention. Animals were randomly assigned across four groups, as depicted in Figure 1. Injections were administered three times per week over 3 weeks. Amphetamine doses were progressively increased from 1 to 3 mg/kg from the first to the third week. NAC (60 mg/kg) was given immediately before saline or amphetamine during the second and third weeks. Animals were returned to their home cages immediately after the injections. Behavioral tests were performed in the same animals after a 4-week washout starting after the last amphetamine administration. NAC dose was based on a previous study of ours showing that 60 mg/kg NAC prevents increased sensitivity to amphetamine in social isolation-reared mice.23
Figure 1. Experimental design. Drugs were administered intraperitoneally three times a week for 3 weeks. Amphetamine (AMPH) doses were increased weekly (1 to 3 mg/kg). N-acetylcysteine (NAC, 60 mg/kg) or saline (SAL) was given immediately before saline or amphetamine during the second and third weeks. Animals were returned to their home cages after injections. Behavioral tests started after a 4-week washout. Animals were assigned to the following experimental groups: SAL/SAL, NAC/SAL, SAL/AMPH, or NAC/AMPH.
Latent inhibition (LI)
LI was assessed in a conditioned active avoidance procedure using a two-way shuttle box (Insight Equipamentos Científicos, Ribeirão Preto, Brazil). In the first phase of the test, animals from each of the four treatment groups were allocated to a preexposure (PE) condition: conditioned stimulus (CS)-PE or non-preexposure (NPE). The PE subjects were placed in the apparatus and presented with 100 discrete exposures to a 5-s burst of white noise (85 dBA), with a random interstimulus interval of 40±15 s. The NPE subjects were placed in the apparatus for an equivalent period without any stimulus presentation. On conditioning day (24 h later), all subjects received a total of 100 avoidance trials presented with an intertrial interval of 40±15 s; each trial began with the onset of the noise (CS). If the animal shuttled within 5 s of CS onset, the CS was terminated and the animal avoided the electric shock (unconditioned stimulus [US]) on that trial. Avoidance failure led to an electric foot shock (0.3 mA) presented in conjunction with the CS that could last for a maximum of 2 s but could be terminated by a shuttle response during this period (i.e., an escape response). Conditioned avoidance learning was indexed as the mean number of avoidance responses recorded on successive blocks of 10 trials.
Locomotion
A week after LI, animals were subjected to an amphetamine challenge (2 mg/kg, i.p.) in an open-field arena to assess locomotor activity. The apparatus consisted of four identical square arenas (40×40×40 cm) located in a testing room under dim diffused light. A webcam connected to a computer was mounted directly above the four arenas, and the videos were analyzed with ANY-Maze tracking software (Stoelting Co., Wood Dale, IL, USA). The animals were injected i.p. with vehicle solution and immediately placed in the apparatus to measure basal locomotor activity for 30 min; subsequently, they were treated with amphetamine and immediately returned to the same arena, where the locomotor response was monitored for 90 min.
Statistics
Data were analyzed using ANOVA. Fisher’s least significant difference (LSD) post-hoc tests were used whenever significant interactions were obtained by the initial ANOVAs. Statistical significance was set at p < 0.05.
Results
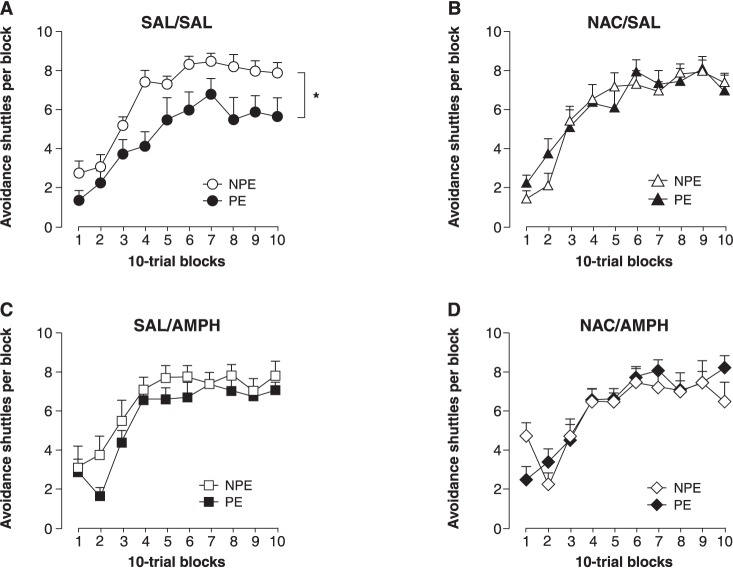
Figure 2 shows the effects of amphetamine sensitization and NAC on LI. A LI effect was observed in control (SAL/SAL) animals (Figure 2A); consistent with previous studies, the sensitization protocol led to a disruption in LI (Figure 2C). NAC abolished the LI effect in the control group (Figure 2B) and failed to counteract the sensitization-induced LI disruption (Figure 2D); 2×10 (PE condition × 10-trial blocks) ANOVAs restricted to each of the four experimental groups revealed a significant PE main effect only for the SAL/SAL group (F1,11 = 13.9, p < 0.01).
Figure 2. Effects of amphetamine (AMPH) sensitization and N-acetylcysteine (NAC) on latent inhibition (LI). LI was observed in the control group (saline – SAL/SAL) (A), and disrupted by amphetamine (SAL/AMPH) (C). NAC abolished LI in control animals (NAC/SAL) (B) and failed to prevent amphetamine sensitization-induced disruption of LI (NAC/AMPH) (D). Mean + standard error, n=7-9. NPE = non-preexposed; PE = preexposed. * p < 0.01, concerning main effect of preexposure condition.
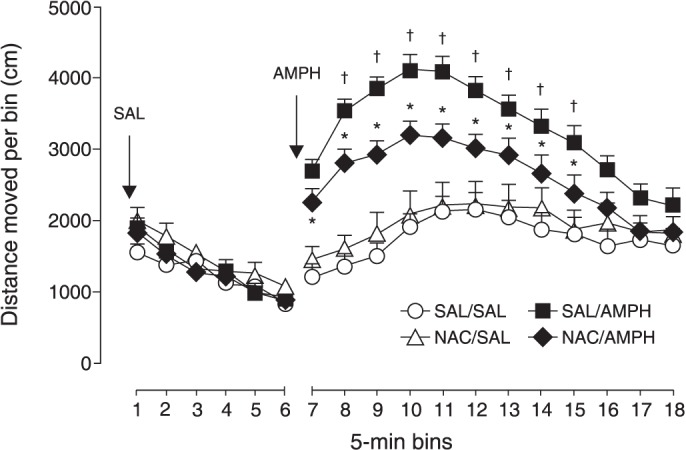
Figure 3 shows that N-acetylcysteine (NAC) attenuated the hyperlocomotion induced by the sensitization protocol (pretreatment × bins interaction: F11,429 = 16.5, p < 0.0001), without affecting basal locomotor activity.
Figure 3. Effects of amphetamine sensitization and N-acetylcysteine (NAC) on locomotion. NAC partially prevented the increased locomotion induced by amphetamine (AMPH) challenge in sensitized animals. Mean + standard error, n=10-11. * p < 0.05 compared to saline (SAL/SAL); † p < 0.05 compared to NAC/AMPH.
Discussion
In the present study, we showed that mice can be sensitized by treatment with amphetamine in an escalating dose schedule, as measured by the magnified locomotor response to amphetamine challenge after 4 weeks of withdrawal. NAC partially counteracted the neuroadaptive changes that determine long-term sensitization to the effect of amphetamine on locomotion. However, NAC failed to prevent amphetamine-induced LI disruption. Of note, NAC disrupted LI in non-sensitized (control) animals.
Glutamate pathways may contribute to the neurochemical mechanisms that underlie sensitization. Psychostimulants enhance glutamate release in the nucleus accumbens,24 activating N-methyl-D-aspartate (NMDA) receptors; the resulting increase in intracellular calcium concentration is a prerequisite for sensitization.25,26 NAC, by activating the cystine-glutamate antiporter, increases extrasynaptic glutamate, resulting in stimulation of metabotropic glutamate receptors 2/3 (mGluR2/3), with a consequent decrease in synaptic glutamate release.15,16,27 This mechanism may underlie the counteracting effects of NAC on amphetamine sensitization, especially since microdialysis studies have shown that NAC prevents the increase in glutamate levels induced by cocaine and phencyclidine, as well as the increased locomotion induced by these drugs.16,28
Several studies have reported protective effects of NAC against the neurotoxic effects of psychostimulants.29-32 These neurotoxic effects are attained by continuous delivery or multiple injections of amphetamine at high doses; the maintenance of elevated amphetamine levels in the brain for several days damages dopamine terminals in the striatum, a process accompanied by increased oxidative stress.33,34 The antioxidant properties of NAC are likely to play an important protective role in the neurotoxic paradigm.33-35 The intermittent administration (three times a week) and escalating low-dose amphetamine regimen used herein do not lead to the pronounced neurotoxic effects observed with successive high amphetamine doses or continuous delivery.7,36
Repeated amphetamine administration protocols are also used to model bipolar disorder and drug abuse. Modeling mania, Valvassori et al.37 reported that NAC (20 mg/kg three times a day) did not prevent the hyperactivity induced by 1 week of daily amphetamine in rats. Investigating the effects of NAC on methamphetamine abuse, Fukami et al.29 showed that NAC (100 mg/kg) prevented the behavioral sensitization produced by five daily injections of methamphetamine in rats. The amphetamine regimen used in this study, which adds dose progression to the mere repetition of amphetamine used in other protocols, leads to behavioral and neurochemical alterations observed in patients with schizophrenia and at-risk subjects.19-21 In the present study, NAC treatment was initiated a week after amphetamine exposure, and different protective results might have been obtained if NAC treatment had been initiated along with amphetamine.
LI, which is disrupted in patients with schizophrenia, is a measure of selective associative learning in which repeated exposure to a non-reinforced stimulus delays subsequent conditioning with that stimulus.38,39 NAC did not prevent the amphetamine sensitization-induced disruption of LI, and in fact disrupted it in non-sensitized mice. Several drugs disrupt LI, including antipsychotics,40-43 and this NAC-induced LI disruption remains to be examined. The use of different amphetamine schedules suggests that deficits in information processing are mediated by different neuronal mechanisms than those underlying locomotor sensitization.8 Different neurochemical bases for these two phenomena would be consistent with the differential effects of NAC on locomotor sensitization and LI.
Limitations of this study include the use of only one dose of NAC and the absence of associated neurochemical measures. Although modulation of glutamate release might be implicated in the effects of NAC on the behavioral parameters here assessed, the precise mechanism remains to be elucidated. Nevertheless, this study advances the hypothesis that NAC may be advantageous for preventing the neurochemical changes that are thought to characterize the prodromal state in patients at risk of developing full-blown schizophrenia.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
We are grateful to the UEA team, especially to Marta Cioato and Fabíola Schons Meyer, for animal husbandry and care; and to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the fellowships granted to APH and EE, respectively. CSG received research funding from Fundo de Incentivo à Pesquisa – Hospital de Clínicas de Porto Alegre (FIPE-HCPA, grant 13-0457).
References
- 1.Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Brain Res Rev. 2000;31:371–84. doi: 10.1016/s0165-0173(99)00054-5. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman JA, Sheitman BB, Kinon BJ. Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology. 1997;17:205–29. doi: 10.1016/S0893-133X(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 3.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–40. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 5.Bell DS. The experimental reproduction of amphetamine psychosis. Arch Gen Psychiatry. 1973;29:35–40. doi: 10.1001/archpsyc.1973.04200010020003. [DOI] [PubMed] [Google Scholar]
- 6.Snyder SH. Amphetamine psychosis: a ‘model’ schizophrenia mediated by catecholamines. Am J Psychiatry. 1973;130:61–7. doi: 10.1176/ajp.130.1.61. [DOI] [PubMed] [Google Scholar]
- 7.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–98. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 8.Peleg-Raibstein D, Knuesel I, Feldon J. Amphetamine sensitization in rats as an animal model of schizophrenia. Behav Brain Res. 2008;191:190–201. doi: 10.1016/j.bbr.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Peleg-Raibstein D, Yee BK, Feldon J, Hauser J. The amphetamine sensitization model of schizophrenia: relevance beyond psychotic symptoms? Psychopharmacology (Berl). 2009;206:603–21. doi: 10.1007/s00213-009-1514-7. [DOI] [PubMed] [Google Scholar]
- 10.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285–92. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadowska AM. N-Acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2012;6:127–35. doi: 10.1177/1753465812437563. [DOI] [PubMed] [Google Scholar]
- 12.Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. N-Acetylcysteine–a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol. 2007;7:355–9. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karalija A, Novikova LN, Kingham PJ, Wiberg M, Novikov LN. The effects of N-acetyl-cysteine and acetyl-L-carnitine on neural survival, neuroinflammation and regeneration following spinal cord injury. Neuroscience. 2014;269:143–51. doi: 10.1016/j.neuroscience.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 14.Palacio JR, Markert UR, Martínez P. Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflamm Res. 2011;60:695–704. doi: 10.1007/s00011-011-0323-8. [DOI] [PubMed] [Google Scholar]
- 15.Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci. 2003;1003:349–51. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- 16.Baker DA, Madayag A, Kristiansen LV, Meador-Woodruff JH, Haroutunian V, Raju I. Contribution of cystine-glutamate antiporters to the psychotomimetic effects of phencyclidine. Neuropsychopharmacology. 2008;33:1760–72. doi: 10.1038/sj.npp.1301532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34:167–77. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Deepmala, Slattery J, Kumar N, Delhey L, Berk M, Dean O, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Tenn CC, Fletcher PJ, Kapur S. Amphetamine-sensitized animals show a sensorimotor gating and neurochemical abnormality similar to that of schizophrenia. Schizophr Res. 2003;64:103–14. doi: 10.1016/s0920-9964(03)00009-4. [DOI] [PubMed] [Google Scholar]
- 20.Tenn CC, Fletcher PJ, Kapur S. A putative animal model of the ‘prodromal’ state of schizophrenia. Biol Psychiatry. 2005;57:586–93. doi: 10.1016/j.biopsych.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Tenn CC, Kapur S, Fletcher PJ. Sensitization to amphetamine, but not phencyclidine, disrupts prepulse inhibition and latent inhibition. Psychopharmacology (Berl). 2005;180:366–76. doi: 10.1007/s00213-005-2253-z. [DOI] [PubMed] [Google Scholar]
- 22.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the care and use of laboratory animals. Washington: National Academies; 2011. [Google Scholar]
- 23.Herrmann AP, Benvenutti R, Pilz LK, Elisabetsky E. N-acetylcysteine prevents increased amphetamine sensitivity in social isolation-reared mice. Schizophr Res. 2014;155:109–11. doi: 10.1016/j.schres.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Wolf ME, Xue CJ, Li Y, Wavak D. Amphetamine increases glutamate efflux in the rat ventral tegmental area by a mechanism involving glutamate transporters and reactive oxygen species. J Neurochem. 2000;75:1634–44. doi: 10.1046/j.1471-4159.2000.0751634.x. [DOI] [PubMed] [Google Scholar]
- 25.Licata SC, Freeman AY, Pierce-Bancroft AF, Pierce RC. Repeated stimulation of L-type calcium channels in the rat ventral tegmental area mimics the initiation of behavioral sensitization to cocaine. Psychopharmacology (Berl). 2000;152:110–8. doi: 10.1007/s002130000518. [DOI] [PubMed] [Google Scholar]
- 26.Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1998;286:1171–6. [PubMed] [Google Scholar]
- 27.Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 2011;36:78–86. doi: 10.1503/jpn.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–76. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, Iyo M. Effect of antioxidant N-acetyl-L-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Res. 2004;1016:90–5. doi: 10.1016/j.brainres.2004.04.072. [DOI] [PubMed] [Google Scholar]
- 30.Achat-Mendes C, Anderson KL, Itzhak Y. Impairment in consolidation of learned place preference following dopaminergic neurotoxicity in mice is ameliorated by N-acetylcysteine but not D1 and D2 dopamine receptor agonists. Neuropsychopharmacology. 2007;32:531–41. doi: 10.1038/sj.npp.1301119. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Shimizu E, et al. Effects of N-acetyl-L-cysteine on the reduction of brain dopamine transporters in monkey treated with methamphetamine. Ann N Y Acad Sci. 2004;1025:231–5. doi: 10.1196/annals.1316.028. [DOI] [PubMed] [Google Scholar]
- 32.Wan FJ, Tung CS, Shiah IS, Lin HC. Effects of alpha-phenyl-N-tert-butyl nitrone and N-acetylcysteine on hydroxyl radical formation and dopamine depletion in the rat striatum produced by d-amphetamine. Eur Neuropsychopharmacol. 2006;16:147–53. doi: 10.1016/j.euroneuro.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remião F, et al. Toxicity of amphetamines: an update. Arch Toxicol. 2012;86:1167–231. doi: 10.1007/s00204-012-0815-5. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci. 2010;1187:101–21. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellison GD, Eison MS. Continuous amphetamine intoxication: an animal model of the acute psychotic episode. Psychol Med. 1983;13:751–61. doi: 10.1017/s003329170005145x. [DOI] [PubMed] [Google Scholar]
- 36.Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26:821–7. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- 37.Valvassori SS, Petronilho FC, Réus GZ, Steckert AV, Oliveira VBM, Boeck CR, et al. Effect of N-acetylcysteine and/or deferoxamine on oxidative stress and hyperactivity in an animal model of mania. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1064–8. doi: 10.1016/j.pnpbp.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Weiner I. The ‘two-headed’ latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology (Berl). 2003;169:257–97. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- 39.Weiner I, Arad M. Using the pharmacology of latent inhibition to model domains of pathology in schizophrenia and their treatment. Behav Brain Res. 2009;204:369–86. doi: 10.1016/j.bbr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Moser PC, Hitchcock JM, Lister S, Moran PM. The pharmacology of latent inhibition as an animal model of schizophrenia. Brain Res Brain Res Rev. 2000;33:275–307. doi: 10.1016/s0165-0173(00)00026-6. [DOI] [PubMed] [Google Scholar]
- 41.Cassaday HJ, Hodges H, Gray JA. The effects of ritanserin, RU 24969 and 8-OH-DPAT on latent inhibition in the rat. J Psychopharmacol. 1993;7:63–71. doi: 10.1177/026988119300700110. [DOI] [PubMed] [Google Scholar]
- 42.Shadach E, Gaisler I, Schiller D, Weiner I. The latent inhibition model dissociates between clozapine, haloperidol, and ritanserin. Neuropsychopharmacology. 2000;23:151–61. doi: 10.1016/S0893-133X(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 43.Weiner I, Schiller D, Gaisler-Salomon I. Disruption and potentiation of latent inhibition by risperidone: the latent inhibition model of atypical antipsychotic action. Neuropsychopharmacology. 2003;28:499–509. doi: 10.1038/sj.npp.1300069. [DOI] [PubMed] [Google Scholar]