Abstract
Immune checkpoint inhibitors are novel biologic agents to treat cancer by inhibiting the regulatory interactions that limit T cell cytotoxicity to tumours. Current agents target either CTLA-4 or the PD-1/PD-L1 axis. Because checkpoints may also regulate autoreactivity, immune checkpoint inhibitor therapy is complicated by side effects known as immune-related adverse events (irAEs). The aim of this article is to review the mechanisms of these events. irAEs can involve different tissues and include arthritis and other rheumatic manifestations. The frequency of irAEs is related to the checkpoint inhibited, with the combination of agents more toxic. Because of their severity, irAEs can limit therapy and require immunosuppressive treatment. The mechanisms leading to irAEs are likely similar to those promoting anti-tumour responses and involve expansion of the T cell repertoire; furthermore, immune checkpoint inhibitors can affect B cell responses and induce autoantibody production. Better understanding of the mechanisms of irAEs will be important to improve patient outcome as well as quality of life during treatment.
Keywords: checkpoint, co-stimulation, CTLA-4, PD-1, PD-L1, arthritis, autoreactivity, T cell, B cell, autoantibodies
Rheumatology key messages
Checkpoint inhibition can promote anti-cancer responses by blocking regulatory interactions limiting T cell cytotoxicity.
Checkpoint inhibition can lead to immune-related adverse events that limit cancer treatment.
Immune-related adverse events result from changes in patterns of T and B cell expression.
Introduction
Immune checkpoint inhibitors (ICIs) are biologic agents that represent a revolutionary approach to treating cancer [1–3]. Rather than killing cancer cells directly, these agents reset the checks and balances that regulate T cell cytotoxicity against tumours. This approach exploits the capacity of the immune system to defend against malignancy by T cells targeting tumour neoantigens [4]. These cells may fail to prevent or eradicate cancer, however, because of regulatory interactions known as checkpoints. With checkpoints blocked by ICIs, a cytotoxic T cell response can emerge and provide powerful anti-tumour activity.
While ICIs have improved the survival of patients with previously untreatable cancers, the benefits have come at the cost of serious side effects known as immune-related adverse events (irAEs) [5–8]; these side effects can limit cancer therapy and necessitate treatment. Clinically diverse, irAEs affect the skin, gastrointestinal tract, lung, heart and endocrine system. irAEs can also cause arthritis and related rheumatic disease (Table 1) [9–16].
Table 1.
Immune-related adverse events according to specialty
| Gastroenterology | Dermatology | Endocrinology | Rheumatology |
|---|---|---|---|
| Colitis Hepatitis Pancreatitis | Vitiligo Skin rasha Alopecia | Thyroiditis Adrenal insufficiency Hypophysitis Type I diabetes Pituitary disorders | Inflammatory arthritis Sicca syndrome Polymyalgia rheumatica Myositis/myocarditis Uveitis |
Skin rash includes psoriasis and psoriaform rashes.
In many respects, irAEs are not unexpected since the checkpoints that inhibit the response to tumour antigens would likely also inhibit autoreactivity. As such, irAEs would appear an almost inevitable consequence of ICIs [17, 18]. In view of the frequency and severity of irAEs, delineating their mechanisms is important in developing strategies for prevention and treatment. While a damaging consequence of immunotherapy, irAEs nevertheless provide a setting for elucidating the mechanisms that govern autoreactivity.
Checkpoint inhibitors
Currently, seven immune checkpoint blocking agents have received approval by the Food and Drug Administration to treat cancer. These agents target cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), also known as CD152; programmed cell death protein-1 (PD-1); and programmed cell death ligand-1 (PD-L1) (Table 2). As shown in many clinical trials, irAEs commonly complicate ICI therapy and clinically present with autoimmune or autoinflammatory manifestations. In general, anti-CTLA-4 agents lead to more frequent irAEs than do anti-PD-1/anti-PD-L1 agents. Combined checkpoint blockade (CCB) is associated with an increased frequency of irAEs along with higher levels of C-reactive protein [19].
Table 2.
FDA approved immune checkpoint inhibitors
| Immune checkpoint inhibitor | Year of FDA approval | Mechanism of action | Indications |
|---|---|---|---|
| Ipilimumab | 2011 | Anti-CTLA-4 | Melanoma, CRC, MCC, RCC |
| Pembrolizumab | 2014 | Anti-PD-1 | Melanoma, HCC, NSCLC, PMBCL, cervical cancer, gastric/gastroesophageal carcinoma, solid tumour, urothelial carcinoma, Hodgkin lymphoma, HNSCC |
| Nivolumab | 2014 | Anti-PD-1 | Melanoma, NSCLC, SCLC, CRC, RCC, HCC, urothelial carcinoma, HNSCC, Hodgkin lymphoma |
| Atezolizumab | 2016 | Anti-PD-L1 | NSCLC, urothelial carcinoma |
| Durvalumab | 2017 | Anti-PD-L1 | NSCLC, urothelial carcinoma |
| Avelumab | 2017 | Anti-PD-L1 | Urothelial carcinoma, MCC |
| Cemiplimab | 2018 | Anti-PD-1 | CSCC |
CSCC: cutaneous squamous cell carcinoma; CTLA-4: cytotoxic T-lymphocyte antigen 4; CRC: colorectal cancer; FDA: Food and Drug Administration; HCC: hepatocellular carcinoma; HNSCC: head and neck squamous cell carcinoma; MCC: Merkel cell carcinoma; NSCLC: non-small cell lung cancer; PD-1: programmed cell death protein-1; PD-L1: programmed cell death ligand-1; PMBCL: primary mediastinal large B cell lymphoma; RCC: renal cell carcinoma; SCLC: small cell lung cancer.
In addition to currently recognized irAEs, the spectrum of these side effects may change as ICIs are used to treat different malignancies and patient populations. Possible influences include age, sex, comorbidities like pre-existent autoimmune disease, prior anti-cancer treatment and the composition of the microbiome [20–22]. Interestingly, older patients may be at particular risk for side effects because of age-related changes in the immune system [23]. While irAEs can be considered ‘off-target’ since they are distinct from the anti-tumour effect, some studies indicate that the occurrence of irAEs may be associated with improved tumour response [24]. Table 3 indicates factors that may influence the development of irAEs.
Table 3.
Potential influences on irAE development
| Malignancy-related factors | Underlying host factors |
|---|---|
| Age Genetic predisposition to autoimmunity Pre-existing autoimmune disease Microbiome |
Anti-CTLA-4 agents vs anti-PD-1/anti-PD-L1 agents.
Use of anti-CTLA-4 therapy followed by anti-PD-1/anti-PD-L1 therapy or vice versa. CCB: combined checkpoint blockade; ICI: immune checkpoint inhibitor; irAE: immune-related adverse event.
The mechanisms of ICIs
ICIs act on the basic mechanisms regulating the T cell response to antigen. As is now well recognized, T cell activation requires two signals: TCR recognition of antigen and co-stimulation. For the first signal, antigen recognition occurs in the context of MHC molecules on antigen presenting cells (APCs). Co-stimulation occurs between membrane-bound molecules on T cells and APCs, with the interaction of CD28 molecules on T cells with CD80/86 molecules on APCs a key event in co-stimulation (Fig. 1) [25, 26].
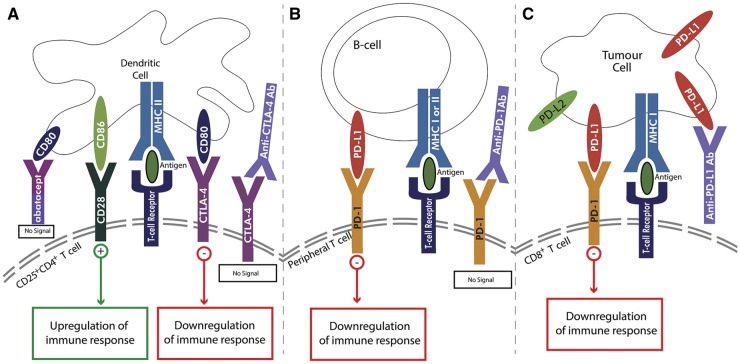
Fig. 1.
Two-step signalling process for activation of naïve T cells
Antigen presenting cells (APCs) such as dendritic cells (DCs) or B cells present antigen to T cells via MHC class I or II molecules (signal 1). The co-stimulatory signal occurs with binding of CD80/86 on an APC (A) to the CD28 receptor on the CD25+CD4+ T cell resulting in upregulation of immune responses (signal 2). Alternatively, a co-inhibitory signal can occur with binding of the cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) receptor on the CD25+CD4+ T cell to CD80/86 (B) or binding of PD-1 on the peripheral T cell to PD-L1 or PD-L2 on an APC (B); both pathways result in downregulation of immune responses. Tumour cells can evade immune system recognition via upregulation of PD-L1 or PD-L2 on the tumour cell surface (C) to bind with CD8+ T cells resulting in downregulation of immune response. DC: dendritic cell; MHC: major histocompatibility complex.
Following activation of T cells, the expression of CTLA-4 is induced. CTLA-4 is expressed on both activated T cells and on a subset of CD25+CD4+ T cells called T-regulatory (T-reg) cells [26]. A member of the immunoglobulin supergene family, CTLA-4 is ∼30% homologous with CD28; CTLA-4 binds CD80/86 with higher affinity and avidity than CD28. The binding of CTLA-4 by CD80/86 decreases T cell-mediated immune responses by reducing IL-2 and IL-2 receptor expression [27]. Another mechanism by which CTLA-4 can regulate immunity is via its effects on T regulatory (T-reg) cells [28].
While anti-CTLA-4 antibodies are termed checkpoint inhibitors, these agents may have other actions that may manifest in certain locales (i.e. tumour microenviroment) and involve other immune cell types [29–31]. Thus, treatment with anti-CTLA-4 can eliminate T-reg cells in a tumour microenvironment via Fc-receptor-mediated interactions. The relationship between a local reduction of T-reg cells and the emergence of irAEs is not clear since this mechanism seems most relevant for an established site of inflammation.
While the PD-1–PD-L1 axis also regulates T cells, the outcome is distinct from that of CTLA-4. PD-1 is a member of the immunoglobulin supergene family, with activation of peripheral T cells and B cells inducing its expression. The main action of PD-1 appears to be the maintenance of peripheral tolerance [32]. PD-1 interacts with two ligands in the peripheral tissues: PD-L1 and PD-L2. PD-L1 is expressed on resting B cells, T cells, macrophages and dendritic cells [33]. PD-L2 is uncommonly expressed on resting immune cells, but its production can be induced by pro-inflammatory cytokines [33].
Signalling via both CTLA-4 and PD-1 converges on Akt, although the pathways and consequences of antibody inhibition are distinct [34]. Akt is a serine threonine kinase that plays a key role in the regulation of processes such as metabolism, apoptosis and proliferation. For T cells, ligation of CD28 leads to activation of phosphatidylinositol 3-kinase (PI3K) whose products bind to Akt, promoting its phosphorylation. Whereas PD-1 signalling can antagonize PI3K directly, the effects of CTLA-4 occur via the phosphatase called PP2A. As such, anti-CTLA-4 and anti-PD-1 act differently suggesting that combination therapy may lead to more global effects that are not observed with either therapy alone; this situation could lead to increased effectiveness against cancer as well as increased incidence of irAEs.
Together, these findings indicate that the actions of anti-CTLA-4 and anti-PD-1/PD-L1 differ in terms of the stage of T cell activation, downstream pathway affected and localization of action. These differences have been reflected in terminology [35]. Anti-CTLA-4 and anti-PD-1/PD-L1 antibodies have recently been termed ‘immune enhancers’ and ‘immune normalizers’, respectively. The latter terminology is consistent with the idea that anti-PD-1 ICIs ‘normalize’ T cell immunity in the tumour microenvironment [35]. Consistent with differences in their mechanism, PD-1 ICIs have greater activity and less toxicity compared with anti-CTLA-4 ICIs in melanoma patients [36–38].
CTLA-4 and PD-1 in autoimmune disease
Immune homeostasis can prevent autoimmune disease and the CTLA-4 and PD-1 pathways are vital for this balance. Evidence for this role derives from studies on knockout mice that lack these checkpoint molecules [39, 40]. Thus, mice lacking CTLA-4 develop an aggressive immune-mediated condition characterized by the extensive infiltration of activated lymphocytes in lymph nodes, spleen and thymus. Relevant to irAEs, infiltration of lymphocytes occurs in the heart, lung, liver and pancreas but, interestingly, not in the kidney. Antibody levels in the knockout mice are also strikingly elevated.
The lack of kidney involvement in the knockout mice is interesting because of the high levels of immunoglobulin in these mice. Often, increases in immunoglobulin production in genetically manipulated mice are commonly associated with anti-DNA and other antinuclear antibodies that characterize SLE, a prototype autoimmune disease with glomerulonephritis. In studies thus far, development of either antinuclear antibodies or lupus-like illness has not been a prominent feature of irAEs, perhaps suggesting a distinct pattern of B cell expression that occurs after CTLA-4 inhibition in humans and mice.
While germline knockout of CTLA-4 can lead to autoimmunity, the role of CTLA-4 is complex and may include cell autonomous and non-autonomous effects operating at different steps in tolerance. Studies by Klocke et al. on mice undergoing conditional deletion of CTLA-4 shed light on these mechanisms [41]. With conditional deletion in adult mice, CTLA-4 deficiency leads to lymphoproliferation, pneumonitis, gastritis and insulitis among other manifestations. In contrast to congenital deficiency, acquired deficiency in adult mice is not fatal.
With adult deficiency, disease can be transferred by T cells, although abnormalities among B cells also develop including hyperglobulinaemia as well as antibodies to insulin, gastric antigen and the Ro52 protein. Despite these serological abnormalities, the levels of anti-DNA, an important lupus marker, are less prominent. Autoantibody findings, therefore, appear more selective than might be anticipated with a global breakdown in tolerance.
Unlike the situation with germline-deficient mice that develop rapid fatality, with CTLA-4 deletion during adulthood, there is adequate time to test experimental induction of autoimmunity. As studies by Klocke et al. showed, collagen-induced arthritis is more severe in mice with adult CTLA-4 deficiency than in wild-type mice [41]. Levels of induced antibodies to collagen are also increased. In contrast, mice with adult CTLA-4 deficiency are protected from the experimental allergic encephalomyelitis induced by myelin oligodendrocyte glycoprotein (MOG) peptide immunization; with a MOG protein-induced model, however, experimental allergic encephalomyelitis was delayed but not prevented.
In another approach to elucidate irAEs, Lute et al. investigated the relationship between autoimmunity and the anti-tumour effect in human CTLA-4 knock-in mice by comparing the activity of three monoclonal antibodies differing in CTLA-4 binding [42]. These studies showed that the antibodies varied in their anti-tumour activity as well as the development of autoimmunity although these activities were not linked. Interestingly, in this model system, animals with tumours treated with anti-CTLA-4 showed robust anti-DNA responses although the magnitude of these responses varied among the antibodies. These findings suggest that the presence of a tumour may influence the development of autoimmunity because of tumour-associated immune disturbances or release of self-antigen by a tumour undergoing cytotoxicity.
The development of abatacept, a CTLA-4–Fc fusion protein that competes with CD28 for CD80/86 binding, also attests to the role of CTLA-4 in the pathogenesis of RA [43]. While this therapy is effective in the treatment of rheumatoid arthritis, it has not been used to treat irAE arthritis, likely because of concerns on its effect on anti-tumour responses [44].
The role of PD-1/PD-L1
The link between autoimmunity and PD-1 was first demonstrated in studies of PD-1-deficient murine models [45]. Depending on the strain, mice lacking PD-1 develop a lupus-like disease marked by glomerulonephritis and renal deposition of IgG3 and C3. In addition, in these studies, the majority of PD-1-deficient mice also developed inflammatory arthritis as shown by histology [45]. In an extension of this model, Nishimura et al. crossed the PD-1 knockout mice with mice of the B6-lpr/lpr strain, which develop lymphoproliferation because of genetic deficiency of Fas [45]. In these studies, autoimmunity in the cross of B6-lpr/lpr by PD-1−/− mice was accelerated in comparison with that observed in B6-lpr/lpr mice, displaying greater lymphadenopathy as well as immune complex glomerulonephritis.
The serological findings in these mice are interesting in terms of the mechanisms for irAEs. Thus, the B6-PD-1−/− mice do not display antibodies to either anti-DNA or RF. Furthermore, the B6-PD-1−/− by lpr/lpr mice showed an increase in IgG2a anti-DNA but not IgG3 anti-DNA. As in the case of the CTLA-4 knockout, the PD-1 knockout mice did not lead to serological findings of SLE. In other studies, Nishimura et al. demonstrated autoimmune dilated cardiomyopathy with severe heart failure in PD-1 knockout mice [46].
While both CTLA-4 and PD-1 knockout mice develop immune-mediated conditions, the disease is quite different. Whereas CTLA-4 knockout mice die rapidly, disease in mice lacking PD-1 is gradual, with disease developing after a year. With inhibition of either checkpoint, it appears that background genes can influence the development of autoimmunity.
Evidence for the role of PD-1 in arthritis also comes from studies of patients with RA demonstrating high levels of PD-1 in synovial fluid. Liu et al. showed positive correlation of serum levels of soluble PD-1 with DAS28 scores, a measure of RA disease activity [47]. In other studies, Guo et al. showed increased PD-1 expression in synovial tissue of RA patients compared with osteoarthritis patients or normal controls. There was also statistically significant elevation in soluble PD-1 in the serum of ACPA positive RA patients compared with seronegative RA patients [48].
Mechanisms of T cell reactivity in irAEs
Since the antigen specificity of T cells mediating irAEs is unknown, studies of the mechanisms of these side effects have assessed general features of the T cell repertoire as a clue to the aetiology. Studies by Robert et al. demonstrated that treatment with tremelimumab (an anti-CTLA-4 monoclonal antibody) can increase the number of unique productively rearranged TCR V-β sequences in the blood of patients with metastatic melanoma [49]. Using measures called richness and the Shannon diversity index, importantly, this study showed that patients with and without irAEs differed in terms of the total number of productive TCR V β sequences in the complementarity determining region (CDR3). While these differences were noted in terms of the frequency of irAEs, responders and non-responders in terms of the anti-tumour response showed similar patterns of sequence expression.
While this study did not show the expansion of particular clones in the peripheral blood, Robert et al. posited that TCR expansion may be considered a pharmacodynamic effect of ICIs that reflects the overall immune activation. Since the emergence of irAEs and the increase in clonal diversity may be associated, these findings suggest that irAEs may result from a mobilization of large numbers of T cells, some of which are autoreactive. The lack of correlation of TCR diversity with treatment effect may further suggest that autoreactive and anti-tumour cells represent distinct populations.
A study by Oh and colleagues reached a similar conclusion [50]. This study involved patients with metastatic castrate-resistant prostate cancer treated with ipilimumab in association with GM-CSF. In this study, treatment with ipilimumab led to a greater diversification of the T cell repertoire in those patients who developed irAEs in comparison to those without this complication. This diversification was notable in the number of clonotypes that were increased along with the presence of newly detected clones.
As demonstrated in this study, changes in T cell populations occurred early after treatment, suggesting a relationship with the subsequent development of irAEs. Nevertheless, toxicity in general occurs later in the course of therapy. Given the time lag, it is possible that pathogenic T cells gradually emerge from the larger number of clonotypes that occur after checkpoint blockade. Interestingly, the time of onset of inflammatory arthritis (IA) ranges from 7 weeks to 24 months, which is later than that of other non-rheumatic irAEs. In patients with melanoma who develop IA, the average time of onset is 6–24 months, suggesting that the onset of IA may be affected by the nature of the T cell population in the periphery as well as tumour microenvironment in different types of cancers [44, 51, 52].
Mechanisms of B cell reactivity in irAEs
While the goal of ICI therapy is to increase cytotoxic T cells, these agents can also affect B cells either directly or indirectly. The data on B cell changes after ICI therapy are limited, although a study by Das et al. demonstrated that patients with advanced melanoma receiving CCB had a significant decrease in the number of circulating B cells after treatment [53]. This effect was not observed in patients treated with anti-CTLA-4 or anti-PD-1 monotherapy. These investigators also demonstrated an increase in the number of plasmablasts and plasma levels of CXCL13, a marker of germinal centre activation in humans, with CCB in comparison to monotherapy. Furthermore, B cells following CCB showed increased clonality in terms of the expression of immunoglobulin genes [53].
In the Das et al. study, the early changes in B cells correlated with the higher rates of grade 3 or higher irAEs 6 months after CCB. Together, these findings suggest that B cells may be important contributors of autoimmunity following CCB. In this regard, a subset of CD21lo memory B cells appeared to be particularly affected by CCB as shown by restricted expression of PD-1 on the CD21lo subset, increased B cells with a CD21lo genomic profile and increased IFN-γ signalling in CD21lo B cells after CCB [53]. This B cell subset was previously described as a functionally and phenotypically distinct population on the path to long-lived plasma cells [54]. Patients who are haploinsufficient for CTLA-4 exhibit similar B cell findings to those observed following CCB [55].
In contrast to studies on T cells, evidence of B cell autoreactivity is clearer for certain conditions. Thus, patients with thyroid abnormalities (i.e., thyroiditis and hypothyroidism) display autoantibodies to thyroidal antigen [56, 57]. Similarly, patients who develop diabetes on ICIs show antibodies to islet cell antigens as well as glutamic acid decardoxylase-65 [58, 59]. Although autoantibodies may be present in patients who develop rheumatic irAEs, most patients are seronegative [10, 14, 16, 51, 60, 61].
To delineate further autoantibody production following ICI, de Moel and colleagues screened pre-treatment and post-treatment samples of patients with advanced melanoma with a large panel of autoantigens [62]. The data indicated that, of 127 patients in the study, 20% were positive for any autoantibody prior to treatment while 29% were positive after treatment. Among patients who were antibody negative before treatment, 19.2% developed new autoantibodies, with anti-TPO (thyroperoxidase) and anti-TG (thyroglobulin) being the most common. The relationship between emergence of autoantibodies and irAEs was not significant. Data showed that 78.9% of patients who expressed any autoantibody had an irAE while only 57.5% who were autoantibody negative had an irAE.
Since ICIs can be given together or sequentially, de Moel et al. investigated the responses of patients who had developed an autoantibody during ipilimumab treatment and then received PD-1 blockade. These studies showed that 44.4% of patients who expressed anti-thyroid antibodies during therapy with anti-CTLA-4 and then received anti-PD-1 therapy had evidence of thyroid dysfunction. While these findings could suggest that anti-PD-1 can promote B cell autoimmunity initiated by anti-CTLA-4, irAEs can take time to develop.
Pre-existing autoimmune disease and immune checkpoint therapy
Another setting to elucidate the mechanisms of irAEs concerns the response to ICIs in patients with pre-existing autoimmune disease. In these patients, ICIs can result in a flare in the underlying disease up to 50% of the time [63–65]. In a retrospective review of patients with pre-existing autoimmune disease, Abdel-Wahab et al. noted differences in the occurrence of irAEs in patients with active vs inactive autoimmune disease at the time of ICI initiation [65]. This same review noted that patients on immunosuppressive therapy at the start of ICI therapy had a lower incidence of irAEs. In this analysis, patients with irAEs were more likely to achieve partial or complete tumoral response compared with those without irAEs. In patients with pre-existing autoimmune disease, more disease flares were observed with anti-PD-1/PD-L1 agents compared with de novo irAEs reported with anti-CTLA-4 agents [65].
Menzies et al. showed an increase in the frequency of flares in patients with active pre-existing autoimmune disease compared with those with clinically inactive disease. Furthermore, this study showed that, while the risk of flare of pre-existing autoimmune disease was ∼50%, the rate of de novo irAEs appeared similar to rates observed in clinical trials that excluded such patients [64]. For patients who experienced irAEs with anti-CTLA-4 ICI, then changed to anti-PD-1 ICI, recurrence of the same irAE was uncommon despite frequent irAEs [64]. Johnson et al. described a 50% combined risk of either flare of pre-existing autoimmune disease or development of a new irAE [63]. Together, these findings suggest a predisposition to the development of irAEs, although the risk may depend on the ICI used.
Case studies illustrating mechanisms
In a cohort at Duke University Medical Center, we have had two patients with autoantibodies consistent with rheumatoid arthritis and Sjogren’s syndrome but who did not develop symptoms of disease until after starting ICI. The RA patient was a 53-year-old man with advanced melanoma who had a high titre ACPA but negative rheumatoid factor at the time of evaluation. While asymptomatic prior to therapy, his joint symptoms started after 2 months on pembrolizumab.
The patient with Sjogren’s syndrome was a 70-year-old woman with non-small cell lung cancer who, at the time of evaluation, had a positive ANA of 1 : 2560 titre by immunofluorescence (homogeneous and speckled patterns), antibodies to SS-A (Ro60), inflammatory arthritis, and sicca symptoms (bilateral corneal erosions). She also did not develop symptoms until after starting CCB with ipilimumab and pembrolizumab. Her sicca symptoms started first, with inflammatory arthritis developing later. Both patients required long-term immunosuppression to control their symptoms, despite discontinuation of ICI (Table 4).
Table 4.
Patients with autoantibody positive rheumatic disease after immune checkpoint therapy
| Patient age/sex | Cancer | Rheumatic diagnosis | Serologies | ICI (MOA) | ICI initiation, month/year | Onset of clinical symptoms (after first dose ICI) | DMARDs (current) | Tumour response | Next steps |
|---|---|---|---|---|---|---|---|---|---|
| 53 yo M | Melanoma | RA | Neg RF, +ACPA | Pembrolizumab (anti-PD-1) | 04/2017 | 2 months | Leflunomide + PDN | Progression | Change to ipilimumab (anti-CTLA-4) |
| 70 yo F | NSCLC | Sjogren’s syndrome | +ANA 1: 2560, +SSA | Pembrolizumab (anti-PD-1) + ipilumumab (anti-CTLA-4) | 06/2016 | 6 months | Leflunomide + PDN | Stable disease | Surveillance CCB dc’d (03/2018) |
ANA: anti-nuclear antibody; CCB: combined checkpoint blockade; CTLA-4: cytotoxic T-lymphocyte antigen-4; dc’d: discontinued; F: female; ICI: immune checkpoint inhibitor; M: male; MOA: mechanism of action; PD-1: programmed cell death protein-1; PDN: prednisone; SSA: SS antibody A; yo: year old.
Consideration of the serological and clinical findings of these two patients raises questions about the mechanism for irAEs and the most informative terminology to describe their disease. In general, autoantibody production in RA, SLE and related autoimmune diseases pre-dates symptomatology by many years in a state known as pre-autoimmunity. For our patients, the initiation of ICI may have provided a trigger for the transition from pre-autoimmunity to autoimmunity. As such, the development of disease in pre-autoimmune individuals who are predisposed to disease may be mechanistically distinct from the development of an irAE in a patient without risk factors or pre-existent serological findings.
Immunosuppression and tumour response
Since patients with pre-existent autoimmune or inflammatory disease may be receiving immunosuppressive therapy at the time of ICI therapy, their ability to respond to ICIs is an important question and relates to the mechanistic relationship of checkpoint inhibition and auto-reactivity. In a study by Menzies et al. patients with advanced melanoma and pre-existing autoimmune disease demonstrated a lower response rate to anti-PD-1 therapy if they were on immunosuppressive therapy at treatment onset than those not on immunosuppression [64]. It is not clear, however, whether the patients who were not on immunosuppression had previously been in remission or whether immunosuppressive therapies had been discontinued to facilitate ICI treatment.
In other studies by Kobayashi et al. and Raptopoulou et al. patients with rheumatological conditions like RA and Sjogren’s were more likely to flare on anti-PD-1 therapy compared with other autoimmune diseases; this effect may be related to the role of PD-1 positive T cells in rheumatological disease discussed above [66, 67].
Lee et al. reported that seven of eight patients with pre-existing RA on immunosuppressive therapy showed partial response or stable disease [68]. One explanation for a more robust treatment response concerns changes in immune function in seropositive RA; because of pre-existing defective T-reg suppressive function, activation of cancer-specific cytotoxic T cells may occur more readily [69].
Treatment of rheumatic irAEs
Treatment of irAEs is often necessary to reduce symptoms and to allow ICI therapy to proceed. For ICI-associated IA, Naidoo et al. proposed an algorithm for evaluation and management [70]. This algorithm includes recommendations for NSAIDs, prednisone, and both non-biologic and biologic DMARDs. One case series by Kim et al. demonstrated clear and sustained response of ICI-associated IA to IL-6 inhibition [71]. Evidence thus far indicates that these recommended treatments do not impair the anti-tumour response suggesting differences in the pathways leading to irAEs and the anti-tumour response.
Conclusion
The use of ICIs has led to a revolution in the treatment of cancer although improved outcomes have been associated with unique side effects known as immune-related adverse events. To advance this important new treatment modality, future studies will need to define the relationship between anti-tumour and anti-self reactivity, develop biomarkers for prediction, and assess new approaches for prevention and treatment, especially in patients with pre-existent autoimmune disease. In view of the number of checkpoints that operate in the immune system, the coming years will see very exciting mechanistic research to maximize anti-cancer responses while minimizing anti-self responses.
Funding: This paper was published as part of a supplement funded by an educational grant from BMS.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ribas A, Wolchok JD.. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei SC, Duffy CR, Allison JP.. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–86. [DOI] [PubMed] [Google Scholar]
- 4. Galon J, Angell HK, Bedognetti D, Marincola FM.. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013;39:11–26. [DOI] [PubMed] [Google Scholar]
- 5. Stucci S, Palmirotta R, Passarelli A. et al. Immune-related adverse events during anticancer immunotherapy: pathogenesis and management. Oncol Lett 2017;14:5671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suarez-Almazor ME, Kim ST, Abdel-Wahab N, Diab A.. Review: immune-related adverse events with use of checkpoint inhibitors for immunotherapy of cancer. Arthritis Rheumatol 2017;69:687–99. [DOI] [PubMed] [Google Scholar]
- 7. Palmieri DJ, Carlino MS.. Immune checkpoint inhibitor toxicity. Curr Oncol Rep 2018;20:72. [DOI] [PubMed] [Google Scholar]
- 8. Sandigursky S, Mor A.. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Curr Rheumatol Rep 2018;20:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ban BH, Crowe JL, Graham RM.. Rheumatology case report: immune-related arthritis associated with ipilimumab. Rheumatologist 2017;11:1–7. [Google Scholar]
- 10. Cappelli LC, Gutierrez AK, Bingham CO 3rd, Shah AA.. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res 2017;69:1751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cappelli LC, Shah AA, Bingham CO 3rd. Immune-related adverse effects of cancer immunotherapy – implications for rheumatology. Rheum Dis Clin North Am 2017;43:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garel B, Kramkimel N, Trouvin AP, Frantz C, Dupin N.. Pembrolizumab-induced polymyalgia rheumatica in two patients with metastatic melanoma. Joint Bone Spine 2017;84:233–4. [DOI] [PubMed] [Google Scholar]
- 13. Goldstein BL, Gedmintas L, Todd DJ.. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of CTLA-4. Arthritis Rheumatol 2014;66:768–9. [DOI] [PubMed] [Google Scholar]
- 14. Manusow JS, Khoja L, Pesin N, Joshua AM, Mandelcorn ED.. Retinal vasculitis and ocular vitreous metastasis following complete response to PD-1 inhibition in a patient with metastatic cutaneous melanoma. J Immunol Cancer 2014;2:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Minor DR, Bunker SR, Doyle J.. Lymphocytic vasculitis of the uterus in a patient with melanoma receiving ipilimumab. J Clin Oncol 2013;31:e356. [DOI] [PubMed] [Google Scholar]
- 16. Sheik Ali S, Goddard AL, Luke JJ. et al. Drug-associated dermatomyositis following ipilimumab therapy: a novel immune-mediated adverse event associated with cytotoxic T-lymphocyte antigen 4 blockade. JAMA Dermatol 2015;151:195. [DOI] [PubMed] [Google Scholar]
- 17. June CH, Warshauer JT, Bluestone JA.. Is autoimmunity the Achilles' heel of cancer immunotherapy? Nat Med 2017;23:540–7. [DOI] [PubMed] [Google Scholar]
- 18. Bucktrout SL, Bluestone JA, Ramsdell F.. Recent advances in immunotherapies: from infection and autoimmunity, to cancer, and back again. Genome Med 2018;10:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cappelli LC, Brahmer JR, Forde PM. et al. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum 2018;48:553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sivan A, Corrales L, Hubert N. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015;350:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vetizou M, Pitt JM, Daillere R. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dubin K, Callahan MK, Ren B. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Comm 2016;7:10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bouchlaka MN, Sckisel GD, Chen M. et al. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med 2013;210:2223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rogado J, Sanchez-Torres JM, Romero-Laorden N. et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer 2019;109:21–7. [DOI] [PubMed] [Google Scholar]
- 25. Ravetch JV, Lanier LL.. Immune inhibitory receptors. Science 2000;290:84–9. [DOI] [PubMed] [Google Scholar]
- 26. Alegre ML, Frauwirth KA, Thompson CB.. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol 2001;1:220–8. [DOI] [PubMed] [Google Scholar]
- 27. Phan GQ, Yang JC, Sherry RM. et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA 2003;100:8372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takahashi T, Tagami T, Yamazaki S. et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med 2000;192:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simpson TR, Li F, Montalvo-Ortiz W. et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013;210:1695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arce Vargas F, Furness AJS, Litchfield K. et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell 2018;33:649–63.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Du X, Tang F, Liu M. et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res 2018;28:416–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okazaki T, Honjo T.. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 2007;19:813–24. [DOI] [PubMed] [Google Scholar]
- 33. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med 2016;375:1767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parry RV, Chemnitz JM, Frauwirth KA. et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanmamed MF, Chen L.. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell 2018;175:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Larkin J, Chiarion-Sileni V, Gonzalez R. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robert C, Schachter J, Long GV. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- 38. Weber J, Mandala M, Del Vecchio M. et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–35. [DOI] [PubMed] [Google Scholar]
- 39. Tivol EA, Borriello F, Schweitzer AN. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3:541–7. [DOI] [PubMed] [Google Scholar]
- 40. Waterhouse P, Penninger JM, Timms E. et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science 1995;270:985–8. [DOI] [PubMed] [Google Scholar]
- 41. Klocke K, Sakaguchi S, Holmdahl R, Wing K.. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc Natl Acad Sci USA 2016;113:E2383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lute KD, May KF Jr, Lu P. et al. Human CTLA4 knock-in mice unravel the quantitative link between tumor immunity and autoimmunity induced by anti-CTLA-4 antibodies. Blood 2005;106:3127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van der Vlist M, Kuball J, Radstake TR, Meyaard L.. Immune checkpoints and rheumatic diseases: what can cancer immunotherapy teach us? Nat Rev Rheumatol 2016;12:593–604. [DOI] [PubMed] [Google Scholar]
- 44. Cappelli LC, Gutierrez AK, Baer AN. et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nishimura H, Nose M, Hiai H, Minato N, Honjo T.. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999;11:141–51. [DOI] [PubMed] [Google Scholar]
- 46. Nishimura H, Okazaki T, Tanaka Y. et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001;291:319–22. [DOI] [PubMed] [Google Scholar]
- 47. Liu C, Jiang J, Gao L. et al. Soluble PD-1 aggravates progression of collagen-induced arthritis through Th1 and Th17 pathways. Arthritis Res Ther 2015;17:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo Y, Walsh AM, Canavan M. et al. Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PLoS One 2018;13:e0192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Robert L, Tsoi J, Wang X. et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res 2014;20:2424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oh DY, Cham J, Zhang L. et al. Immune toxicities elicted by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Res 2017;77:1322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Calabrese C, Kirchner E, Kontzias K, Velcheti V, Calabrese LH.. Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD open 2017;3:e000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lidar M, Giat E, Garelick D. et al. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev 2018;17:284–9. [DOI] [PubMed] [Google Scholar]
- 53. Das R, Bar N, Ferreira M. et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 2018;128:715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lau D, Lan LY, Andrews SF. et al. Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci Immunol 2017;2:eaai8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kuehn HS, Ouyang W, Lo B. et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 2014;345:1623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Orlov S, Salari F, Kashat L, Walfish PG.. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab 2015;100:1738–41. [DOI] [PubMed] [Google Scholar]
- 57. Osorio JC, Ni A, Chaft JE. et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hughes J, Vudattu N, Sznol M. et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 2015;38:e55–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stamatouli AM, Quandt Z, Perdigoto AL. et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 2018;67:1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kostine M, Rouxel L, Barnetche T. et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer—clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis 2018;77:393–8. [DOI] [PubMed] [Google Scholar]
- 61. Smith MH, Bass AR.. Arthritis after cancer immunotherapy: symptom duration and treatment response. Arthritis Care Res 2019;71:362–6. [DOI] [PubMed] [Google Scholar]
- 62. de Moel EC, Rozeman EA, Kapiteijn EH. et al. Autoantibody development under treatment with immune-checkpoint inhibitors. Cancer Immunol Res 2019;7:6–11. [DOI] [PubMed] [Google Scholar]
- 63. Johnson DB, Sullivan RJ, Ott PA. et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2016;2:234–40. [DOI] [PubMed] [Google Scholar]
- 64. Menzies AM, Johnson DB, Ramanujam S. et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–76. [DOI] [PubMed] [Google Scholar]
- 65. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME.. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med 2018;168:121–30. [DOI] [PubMed] [Google Scholar]
- 66. Kobayashi M, Kawano S, Hatachi S. et al. Enhanced expression of programmed death-1 (PD-1)/PD-L1 in salivary glands of patients with Sjogren's syndrome. J Rheumatol 2005;32:2156–63. [PubMed] [Google Scholar]
- 67. Raptopoulou AP, Bertsias G, Makrygiannakis D. et al. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum 2010;62:1870–80. [DOI] [PubMed] [Google Scholar]
- 68. Lee B, Wong A, Kee D. et al. The use of ipilimumab in patients with rheumatoid arthritis and metastatic melanoma. Ann Oncol 2016;27:1174–7. [DOI] [PubMed] [Google Scholar]
- 69. Pieper J, Herrath J, Raghavan S. et al. CTLA4-Ig (abatacept) therapy modulates T cell effector functions in autoantibody-positive rheumatoid arthritis patients. BMC Immunol 2013;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Naidoo J, Cappelli LC, Forde PM. et al. Inflammatory arthritis: a newly recognized adverse event of immune checkpoint blockade. Oncologist 2017;22:627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim ST, Tayar J, Trinh VA. et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis 2017;76:2061–4. [DOI] [PubMed] [Google Scholar]