Abstract
Aim:
Physical therapy and exercise are considered essential components in the management of Parkinson's disease (PD). Using our retrospective data and years of experience in assigning persons with PD to multilevel group classes we propose a two-part physical therapy decision-making tool consisting of participant and exercise program considerations.
Methods:
Retrospective medical record review and therapist consensus identified evaluation considerations determined to aide clinical decision making. The ability of these variables (i.e., demographics, clinical characteristics, clinical measures cut-offs) to predict the class assignment decision of PD-specialized physical therapists was evaluated using discriminant function analysis.
Results:
Therapist-assigned groups differed significantly on all clinical measures (p < 0.001) which provided the categorical data required for discriminant analysis. Using all variables, the discriminant function analysis predicted class assignment of the therapists with 79% agreement.
Conclusion:
This proposed tool provides a framework that may guide the process for increasing access to multilevel group classes.
Keywords: : cognitive–motor challenge, community, exercise prescription, functional mobility, group exercise, motor learning, physical therapy, rehabilitation paradigms, sustained practice
Background
Physical therapy and exercise are now considered an essential component in the management of Parkinson's disease (PD), complementary to pharmaceutical and surgical approaches [1,2]. Studies support that habitual exercisers may have better function and quality of life and slower disease progression [3,4]. This is supported by multiple reviews and meta-analyses [5–9], physical therapy guidelines [10,11] and consensus statements by the scientific community [12–16]. Unfortunately, only 34% of persons with PD (PwP) are prescribed medication or physical therapy at diagnosis or within the first year [17]. This lost opportunity occurs at the time exercise in early onset PD has been shown to reduce symptoms [18], improve postural control and dopamine signaling [4,19], upregulate neurotrophic factors [20] and enhance learning capacity [21]. In addition, optimal dopaminergic medication may further enhance functional gains from exercise [16,22,23] and increase the ability and motivation to exercise [24]. Combined with low activity levels [25], apathy [26] and stigma as early features of PD [27], this opportunity quickly dissipates into a negative cycle of isolation [27,28], reduced QoL [29] and faster disease progression [30]. To optimize the PwP's social and emotional well-being and ability to fully participate in exercise and life [31], early and ongoing person-centered care is necessary [32].
The challenge to healthcare professionals is how to deliver rehabilitation and community exercise resources if we expect PwP to exercise and remain as physically active as possible for the long-term, starting at diagnosis [14,33,34]. Physical therapy and exercise are often used interchangeably, and while both contribute to daily physical activity, they each offer unique and potentially additive benefits [23,35–38]. While the benefits of either physical therapy or exercise quickly disappear (3–6 months), recent reviews suggest that benefits may be extended with longer training periods, intermittent therapy bouts, tapered supervision or sustained practice [16,39–41]. Since lifelong ongoing physical therapy is not an option, we propose that the integration of rehabilitation goals (e.g., functional skill training) with community exercise resources offers one solution for providing sustained practice that may optimize retention of functional benefits gained in therapy [16,22,23,36,37,39–46]. In addition, community exercise may be complementary to individual therapy programs, enhancing fitness, social-emotional well-being and improving self-efficacy, motivation and nonmotor symptoms [35,45–48].
Recently, there has been a proliferation of community exercise programs with varied evidence, expertise and focus [49,50]. Evidence is emerging to support many different nonconventional programs which may include boxing [51], aquatics [52], Nordic walking [53,54], dance [55], Tai Chi [56–58] and yoga [59,60]. However, the level of instructor expertise related to safe and effective exercise instruction for people with a progressive neurodegenerative disease, and the specificity of training directed at PD symptoms or impairments underlying functional mobility is either not known or limited [49]. It is also not known how community group exercise instructors can adequately challenge PwP physically or cognitively in large classes with varied age, fitness and comorbidities, complex and often worsening sensorimotor, autonomic, attention and executive function symptoms, and high fall risk (even when newly diagnosed) [49,61–63]. We propose that physical therapists who network with community exercise instructors to integrate personalized rehabilitation goals and concerns, coordinate referrals and stratify PwP into more homogenous group exercise classes may extend and enhance benefits from therapy, reduce the rate of functional decline in PwP, improve long-term compliance and limit injury.
We are unaware of any comprehensive screening guidelines to best stratify PwP into multilevel group classes with similar functional level within each class. Some community programs may use Hoehn and Yahr (H&Y) stages [64], ask participants to self-select their group by categories (easy, moderate, vigorous) or allow instructors to screen and identify community walkers or individuals with fall risk choosing from a variety of fitness tools (e.g., physical activity readiness questionnaire [65]) and norms (e.g., gait speed). While these criteria may be adequate for exercise programming for the older adult, they may not necessarily consider factors specific to PwP that could contribute to falls, poor outcomes or attrition. Such factors can include cognitive function (e.g., problem-solving, dual-tasking), motor symptoms (e.g., bradyphrenia, hypophonia, swallowing difficulties), nonmotor symptoms (e.g., stress, anxiety, cardiac autonomic dysfunction, fatigue), medication side effects (e.g., dyskinesias, dystonias, impulsivity) and personal factors (e.g., stigma, apathy, need for assistance or cueing, loss of self-efficacy) [66].
The purpose of this retrospective research study is to summarize the components of a proof of concept clinical decision-making tool for physical therapists for the purposes of stratification of PwP into multilevel group classes. The decision-making tool is the result of PD-specialized physical therapists evaluating and assigning PwP to multilevel group classes in a clinic devoted to lifelong access to integrated physical therapy and group exercise. PD-specialized refers to the level of practical experience and overall knowledge of PD of each physical therapist involved in this study. Each physical therapist had 3–4 years of experience evaluating and providing individualized therapy for an average of 4–5 PwP daily. In addition, they teach multilevel group exercise classes 1–2 times per week, participate 1–2 times per year in continuing education and conferences related to PD, and teach physical therapy students, group instructors and volunteers about PD, exercise and safety essentials and the research underlying the rationale for PD-specific neuroplasticity-principled exercise programming.
While previous research has suggested a clinical decision-making framework for physical therapists evaluating a client with neurological deficits [67], no framework exists to guide physical therapists in their clinical decision-making when evaluating the client with PD for physical therapy and community exercise needs. The American Physical Therapy Association's Guide to Physical Therapist Practice outlines principles of physical therapist patient and client management and mentions various factors that contribute to the decision-making process including the client's decline in functional independence, cognitive status, social support and accessibility and availability of resources [68]. We propose that our PD-specific clinical decision-making tool can guide the physical therapy plan of care, including prescribing and updating exercise recommendations and networking with community resources to integrate PD-specific therapy and fitness goals. Helping PwP with varied functional mobility and fitness find group exercise classes that more closely match their functional needs allows class format and goals to be as similar as possible to one-on-one therapy. This may promote lifelong adherence to a more PD-specific, personalized, effective, safe and challenging exercise program [39,61].
Methods
Study design
To develop the proposed clinical decision-making tool, this retrospective cohort study investigated the process by which PD-specialized physical therapists stratified PwP into multilevel group exercise classes that shared similar functional goals and neuroplasticity-principled aspects of training. The data from the initial evaluations presented in this study were part of a larger medical record review examining long-term adherence and benefits for participants who consistently attended our rehabilitation and exercise programs. Study procedures were reviewed by the university institutional review board (IRB) and exempted from IRB reporting requirements (procuring informed consent from participants), as a retrospective chart review from which personal identifiers cannot be linked to the extracted data.
Evaluations and group class sessions occurred at a community-centered clinic that provides ongoing access to research-based PD-specific physical therapy and integrated onsite multilevel group exercise programming. Participants were required to complete an initial physical therapy evaluation prior to group class attendance, and they were offered physical therapy bouts or tapered supervision as needed to proactively address personal goals or problem areas. In addition, participants who met minimum criteria were offered group exercise programming designed to enhance and extend the benefits of physical therapy. Exercise classes were taught by group instructors (i.e., therapists and exercise professionals) who were trained in the application of neuroplasticity-principled PD-specific functional skill training. If participants met minimum criteria and chose to participate in onsite group exercise, they were assigned to a specific class by the evaluating physical therapist and required to complete additional physical therapy evaluations at 6 months, 12 months and annually thereafter. Multiple levels of group classes were available, ranging from lowest to highest levels of fitness and function (Moves 1, Moves 2, Moves 3 and Moves 4, respectively).
Description of moves classes
Table 1 outlines the differences between Moves 1, 2, 3 and 4 classes that impacted the evaluating therapists' class recommendation with the intention of optimizing the participants' safety and challenge. All Moves classes included progressive aerobic training (20–30 min per class of interval training on treadmills, stationary bikes and elliptical machines utilizing the modified Borg Scale [69] with moderate to vigorous intensities targeted during high intensity intervals) and amplitude-focused functional skill training in multiple positions including standing, seated, quadruped, prone and supine (30–40 min per class) for an hour-long class offered three-times per week. Instruction mode, skilled task dosing, balance exercises, frequency of transitions, cognitive–motor complexity and environmental complexity varied by class. Moves 1 and Moves 2 instructors used equipment for visual, auditory and tactile cues while they concurrently modeled the exercises. Moves 3 and Moves 4 instructors demonstrated all circuit stations before the participants began the circuit on their own, requiring substantial participant recall. Circuit stations were designed to challenge strength, agility and balance using equipment such as bosu balls, bungees, cones and weights.
Table 1. . Description of moves classes.
| Class level | Moves 1 | Moves 2 | Moves 3 | Moves 4 |
|---|---|---|---|---|
| Key principles (modes of practice) | Progressive aerobics on equipment (treadmills, stationary bikes, ellipticals) Amplitude-focused functional skill training in multiple positions including standing, seated, quadruped, prone and supine |
|||
| Instruction mode | Concurrent modeling of exercises | Demonstration of multiple circuit stations | ||
| Skilled task dosing | Lower repetitions, slower paced | More repetitions, faster paced | Fewer stations | More stations |
| Balance exercises | Static | Static | Dynamic | Dynamic |
| Frequency of transitions (e.g., sit to stand, supine to quadruped, stand to prone) | Low | Moderate to low | Moderate to high | High |
| cognitive–motor complexity | Low | Moderate to low | Moderate to high | High |
| Environmental complexity | Low | Moderate to low | Moderate to high | High |
Skilled task dosing was altered with more repetitions and faster pacing of exercises in Moves 2 compared with Moves 1 and more stations for Moves 4 compared with Moves 3. Moves 1 and 2 spent most of the skill training portion of class performing exercises in standing, sitting, quadruped, prone, or supine requiring static balance while Moves 3 and 4 spent most of the skill training portion of class performing exercises in similar positions but requiring more dynamic balance (e.g., forward/backward walking, sidestepping, navigating obstacles and uneven surfaces, crawling, scooting). Frequency of transitions (e.g., sit to stand, supine to quadruped, stand to prone) was manipulated ranging from low to high from Moves 1 to Moves 4, respectively.
cognitive–motor complexity was altered with the use of sequencing (e.g., functional combinations of movements such as sit to stand plus forward step in standing), dual tasking (e.g., naming countries while walking over obstacles), timing (e.g., use of metronome and music) and problem solving (e.g., partner work, interacting with equipment) with increasing complexity from low to high from Moves 1 to Moves 4, respectively. Environmental complexity varied by group including participant numbers (e.g., more participants in higher level classes) and visuospatial demands (e.g., more obstacles to navigate in higher level classes) with increasing complexity from low to high from Moves 1 to Moves 4, respectively.
Medical records selection
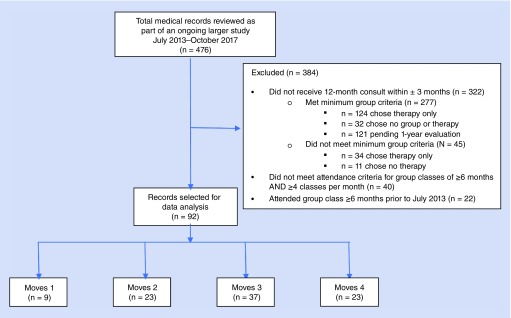
Medical records of all participants who completed an initial evaluation from July 2013 through October 2017 (N = 476) were systematically reviewed and the data were entered into a database by a co-author not involved in direct patient care (Figure 1). To create the cohort for use in this project, participants' records were excluded from this larger dataset if they did not meet the selection criteria of having a 12-month evaluation ( ± 3 months) (N = 322); attending group class ≥6 months between the initial and the 12-month evaluation; and averaging ≥4 classes per month in the months attended (N = 40). In addition, participants' data were excluded if they attended group classes more than 6 months prior to the start date for implementing formal group class criteria and evaluation processes (July 2013) (N = 22). The remaining participant records (N = 92) were included in the data analysis (see Physical Therapy Evaluation – Group Assignment Data below for more detail on reasons for exclusion).
Figure 1. . Flow diagram of medical records selection criteria for data analysis.
This remaining cohort joined group classes on a rolling basis within one month after initial evaluation. Classes were offered 3 days per week and attendance was reflective of vacations, illnesses, injuries and each individual's varying level of resources and commitment. Group class participation was retrospectively examined through paper and electronic (MINDBODY®, CA, USA) attendance records. We chose these minimal attendance requirements over the course of 1 year because we are interested in investigating the benefits of attending multilevel group classes in the future.
Physical therapy evaluation: demographic & clinical data
Data considered most important to aide clinical decision making were extracted from selected participant records. This included demographics, clinical characteristics, clinical measures and group assignments at initial evaluation. Demographic information included gender and age. Clinical characteristics included years with PD diagnosis, amount of levodopa medication (levodopa equivalent daily dose, the Montreal Cognitive Assessment (MoCA) [70], self-reported history of falls or freezing of gait in the past 6 months (yes/no) and disease severity as determined by H&Y stage [64]. Time of diagnosis was established by participant recall and used to determine years with PD diagnosis. Hoehn and Yahr stages at baseline were determined retroactively by one physical therapist reviewing clinical descriptions and tests for bilateral motor involvement, postural instability (pull test or push/release test), use of walking aids or wheelchair, reported need for assistance with activities of daily living and confinement to bed (Table 2).
Table 2. . Participant demographics and clinical characteristics.
| Moves 1 (n = 9) | Moves 2 (n = 23) | Moves 3 (n = 37) | Moves 4 (n = 23) | Group difference (p-value) | |
|---|---|---|---|---|---|
| Male:female | 6:3 | 15:8 | 29:8 | 17:6 | 0.700 |
| Age (years [SD]) | 76.5 (6.3) | 76.2 (6.3) | 73.5 (6.7) | 63.1 (6.5) | <0.001 |
| Age range (years) | 66–83 | 67–87 | 56–84 | 53–75 | – |
| Years with diagnosis (years [SD]) | 4.6 (7.7) | 3.6 (5.1) | 3.7 (4.5) | 2.6 (4.4) | 0.717 |
| Levodopa equivalent daily dose (mg [SD]) (# of participants [%])† | 407 (194) 7/9 (78%) | 292 (275) 18/23 (78%) | 494 (403) 29/37 (78%) | 511 (336) 18/23 (78%) | 0.192 |
| Hoehn and Yahr (out of 5) | 3.0 (1.2) | 2.1 (.7) | 1.9 (.7) | 1.3 (.6) | <0.001 |
| MoCA (out of 30) (# of participants [%])† | 22.8 (5.3) 6/9 (67%) | 25.6 (2.5) 19/23 (82%) | 26.5 (2.0) 28/37 (76%) | 27.4 (1.7) 16/23 (70%) | 0.002 |
| Falls reported (yes/no) (# of participants [%])† | 5/9 (56%) | 9/23 (39%) | 14/35 (40%) | 5/21 (24%) | 0.403 |
| Freezing reported (yes/no) (# of participants [%])† | 6/8 (75%) | 5/19 (26%) | 8/34 (24%) | 2/20 (10%) | 0.025 |
Data not available on all participants.
MoCA: Montreal Cognitive Assessment; SD: Standard deviation.
Clinical measures at initial evaluation were chosen for their relevance to participation in this clinic's group exercise class and documented psychometric properties supporting their use in PD. These measures are summarized in Table 3 and included: assessment of functional strength (five times sit to stand [FTSS] [71] with excellent reliability [test/retest interclass correlation coefficient (ICC) = 0.91 [72]]); floor transfer (transfer stand to prone to stand (floor transfer), reliability not established); backward walking (3-m backward walk [3MBW] [73,74], reliability not established); walking endurance (6-min walk test [6MWT] [75], excellent test/retest reliability [ICC = 0.95] [76]); gait speed (10-m walk test at preferred speed [10MWT- PS] and 10-m walk test fast and safe [10MWT-FS] [77,78], excellent test/retest reliability [ICC = 0.96 and 0.97, respectively] [76]); and single and dual task functional mobility (timed up and go test fast and safe [TUG-FS] and with concurrent serial-three subtraction, cognitive [TUG-FScog] [79], good test/retest reliability [ICC = 0.85] [76]).
Table 3. . Clinical measures.
| Clinical measure | Moves 1 | Moves 2 | Moves 3 | Moves 4 | p-value |
|---|---|---|---|---|---|
| FTSS (s) | 37.25 (6.67) | 19.80 (2.48) | 12.65 (0.65) | 9.70 (0.54) | <0.001 |
| Floor transfer (s) | 60.94 (23.89) | 19.80 (3.74) | 10.55 (0.95) | 5.40 (0.36) | <0.001 |
| 3MBW (s) | 9.14 (2.36) | 5.30 (0.51) | 3.80 (0.25) | 2.71 (0.18) | <0.001 |
| 6MWT (m) | 307.13 (41.36) | 351.15 (20.47) | 435.55 (11.33) | 518.76 (15.08) | <0.001 |
| Gait speed 10MWT-PS (m/s) | 0.81 (0.09) | 0.92 (0.04) | 1.09 (0.03) | 1.25 (0.04) | <0.001 |
| Gait speed 10MWT-FS (m/s) | 1.08 (0.14) | 1.27 (0.06) | 1.53 (0.04) | 1.74 (0.06) | <0.001 |
| TUG-FS (s) | 16.25 (2.46) | 10.89 (1.27) | 7.76 (0.28) | 5.82 (0.24) | <0.001 |
| TUG-FScog (s) | 22.49 (3.80) | 13.47 (1.00) | 9.89 (0.56) | 7.35 (0.52) | <0.001 |
Mean (standard error) for all baseline outcome measures of the four subject groups.
3MBW: 3-m backward walk; 6MWT: 6-min walk test; Gait speed; 10MWT-FS: 10-m walk test – fast and safe; 10MWT-PS: 10-m walk test – preferred speed – gait speed; Floor transfer: Transfer stand to prone to stand; FTSS: Five times sit to stand; TUG-FS: Timed up and go test – fast and safe; TUG-FScog: Timed up and go test – fast and safe with a concurrent cognitive task.
Physical therapy evaluation: group assignment data
To be eligible for assignment to onsite group classes, the evaluating physical therapist first determined if minimum group class criteria were met. Therapists established these criteria for group class eligibility concurrent with development of the physical therapy evaluation for the purpose of establishing a baseline level of safety that was required for participation in the existing group exercise classes offered at our facility. Considerations unique to our setting included participants' required assistance with activities to be performed in class (e.g., transferring to and from the floor, performing progressive aerobic training on cardio equipment) and class format factors specific to this clinic's setting (e.g., walking into building and around classroom safely). Therefore, minimum criteria for participation in group classes included: ambulation with or without an assistive device ≥500 feet with supervision, floor transfer (standing to prone to standing) with a chair and/or minimal assistance, use of aerobic exercise equipment (e.g., stationary bicycles, treadmills, elliptical machines) with minimal assistance and ability to follow simple, single step instructions.
If minimum group class criteria were met and the participant chose to join class, therapists assigned PwP to one of four group class levels (Moves 1, Moves 2, Moves 3 or Moves 4) using clinical judgment from information gathered at the initial evaluation including demographics and clinical characteristics (Table 2), and performance on clinical measures (Table 3). Participants who had high-risk cardiovascular conditions requiring close monitoring with exercise or other health conditions contraindicated to participation in an exercise program were deemed ineligible for group class at the therapist's discretion. If participants did not meet the suggested minimum group class criteria, therapists recommended one-on-one therapy only with goals set to eventually qualify for a class if participant desired. Participants who did not meet minimum criteria upon evaluation along with participants who met minimum criteria but preferred to receive one-on-one physical therapy did not require 12-month follow up and were excluded from the analysis (see Figure 1).
Statistical analysis
The ability of the demographics, clinical characteristics and clinical measures of the proposed clinical decision-making tool to predict the classification decisions of the PD-specialized therapists was evaluated using discriminant function analysis. To identify four levels of descriptors of PwP participants, we computed and report descriptive statistics (means, standard deviations, counts) for baseline demographic, clinical characteristics and clinical measures across assigned class levels for all participants (N = 92). Differences among groups were evaluated using chi-square tests and generalized linear models, as appropriate for the metric of the variable. To establish the categorical data required for discriminant analysis, cut-off scores for each clinical measure for each group were calculated using two standard deviations from the mean. These cut-off scores categories were converted into an ordinal scale reflecting proposed group level assignment, 1 through 4, for each measure.
Of the nine participants in Moves 1, only three had all demographics, clinical characteristics and outcome measures, therefore, the discriminant function analysis was performed using only three groups (Moves 2 [n = 1 excluded], Moves 3 [n = 4 excluded] and Moves 4 [n = 2 excluded]; n = 83). Missing data occurred for levodopa equivalent daily dose, MoCA and FTSS in this cohort, as these measurements were not all in place at the start date for the retrospective chart review. Therefore, to represent participants who had completed all measures on evaluation, a subset of the cohort (n = 76) were included in the discriminant function analysis.
Emphasis for the discriminant function analysis was placed on the percentage of participants in the subset falling within the a priori cut-off scores for clinical measures, along with selected demographic and clinical characteristics. Therapist-assigned group was entered as the outcome variable into the discriminant function analysis. Finally, to predict the therapists classification decisions, the ordinal classification scores for seven clinical measures (floor transfer, 3MBW, 6MWT, 10MWT-PS, 10MWT-FS, TUG-FS, TUG-FScog) for each individual were entered as predictors, along with participant age, duration of PD, H&Y score and a binary variable indicating whether or not they had experienced a fall in the prior 6 months. An alpha of 0.05, two-tailed, was used as the criterion for statistical significance, and no corrections were made for multiplicity. SPSS ver 25 (IBM Corp., NY, USA) was used to conduct the analyses.
Results
Summary of data analysis
Participant demographics and clinical characteristics with p-values for the differences among groups are provided in Table 2. There were statistically significant differences between groups on age, disease severity (H&Y), executive function (MoCA) and self-reported freezing of gait. Summary statistics for clinical measures by group are provided in Table 3. There were statistically significant differences between groups on all clinical measures.
Using selected demographic and clinical measures (Tables 2 & 3) excluding the Moves 1 group and those with missing data (n = 76 with all measures), the discriminant function analysis predicted 79% of the therapists' decisions, indicating these measures can contribute to the decision-making process. The prediction was reduced to 74% when H&Y scores were removed from the analysis, implying that using only H&Y to stratify individuals to group exercise classes may have limitations when for assigning PwP to homogeneous exercise groups. Indeed, H&Y scores alone predicted only 41% of the therapist's decision. We suggest that even when including H&Y scores, the unpredictability in group class assignment (∼21%) may be explained by other personal factors and exercise program considerations that our PD-specialized therapists deemed important as described in the next sections.
While we recommend using all the demographic and clinical measures in the model just described, many clinical settings may not have the resources to obtain all seven clinical measures on evaluation. Therefore, we further analyzed the prediction of stratification to three levels of exercise classes (Moves 2, 3 and 4) and found that using only three clinical measures (3MBW, floor transfer and TUG-FS), age and years with PD predicted the therapists assignments at 74% (n = 81 had all measures).
Proposed two-part clinical decision-making tool
We propose a two-part clinical decision-making tool consisting of participant considerations (Table 4) and exercise program considerations (Table 5).
Table 4. . Proposed clinical decision-making tool part 1: participant considerations.
| Class level | Moves 1 | Moves 2 | Moves 3 | Moves 4 | |
|---|---|---|---|---|---|
| Personal factors | PD diagnosis duration | Long | Moderate | Moderate | Short |
| Age | Older | Older | Slightly younger | Younger | |
| Exercise experience | Low | Low | Moderate | High | |
| Activities of daily living assistance | Minimal | Supervision | Independent | Independent | |
| Self-reported frequency of falls in past 6 months | High | Moderate | Moderate | Low | |
| Balance confidence | Low | Moderate | Moderate | High | |
| Freezing of gait severity | High | Moderate | Low | Low | |
| Use of assistive device | Home | Community | None | None | |
| Impact of comorbidities on exercise | High | Moderate | Minimal | None | |
| Community participation | Low | Moderate | Moderate | High | |
| Executive functioning | Poor | Fair | Good | Good | |
| Self-monitoring | Poor | Fair | Good | Good | |
| Self-efficacy | Low | Moderate | Moderate | High | |
| Clinical measures cut-offs | Five times sit to stand | >30 s | <30 s | <17 s | <12 s |
| Floor transfer | >40 s | <40 s | <18 s | <8 s | |
| 3-m backward walk | >12 s | <12 s | <8s | <5 s | |
| 6-min walk test | <250 m | >250 m | >325 m | >400 m | |
| Gait speed; (preferred speed) | <0.65 m/s | >0.65 m/s | >0.85 m/s | >1.00 m/s | |
| Gait speed; (fast and safe) | <0.90 m/s | >0.90 m/s | >1.20 m/s | >1.40 m/s | |
| Timed up and go (fast and safe) | >17 s | <17 s | <10 s | <7 s | |
| Timed up and go – cognitive (fast and safe) | >20 s | <20 s | <15 s | <10 s | |
| Montreal Cognitive Assessment (out of 30) | <22 | >22 | >24 | >26 |
Table 5. . Proposed clinical decision-making tool part 2: exercise program considerations.
| Class level | Moves 1 | Moves 2 | Moves 3 | Moves 4 | |
|---|---|---|---|---|---|
| Level of assistance with class activities | Aerobic equipment operation | Minimal | Supervision | Independent | Independent |
| Rating of perceived exertion | Moderate | Minimal | Independent | Independent | |
| Over ground balance activities | Contact guard assist | Contact guard assist | Supervision | Independent | |
| Complex sequencing and dual tasking | Moderate | Minimal | Supervision | Independent | |
| Floor transfer/floor exercise | Minimal | Supervision | Supervision | Independent | |
| Class format factors | Key principles (modes of practice) | Progressive aerobics on equipment (treadmills, stationary bikes, ellipticals); | |||
| Amplitude-focused functional skill training in multiple positions including standing, seated, quadruped, prone and supine | |||||
| Instruction mode | Concurrent modeling of exercises | Demonstration of multiple circuit stations | |||
| Skilled task dosing | Lower repetitions, slower paced | More repetitions, faster paced | Fewer stations | More stations | |
| Balance exercises | Static | Static | Dynamic | Dynamic | |
| Frequency of transitions (e.g., sit to stand, supine to quadruped, stand to prone) | Low | Moderate to low | Moderate to high | High | |
| cognitive–motor complexity | Low | Moderate to low | Moderate to high | High | |
| Environmental complexity | Low | Moderate to low | Moderate to high | High | |
Part 1: participant considerations
Participant considerations include personal factors and clinical measure cut-off scores (Table 4). In addition to the personal factors discussed in Table 2 and used in our analysis, our PD-specialized physical therapists gathered other information via clinical observation to aid in decision making and did not necessarily measure each factor. These are all combined in Table 4 and include: PD diagnosis duration, age, exercise experience, activities of daily living assistance, self-reported falls, balance confidence, freezing of gait, use of assistive device, comorbidities, community participation, cognition, self-monitoring and self-efficacy. Self-monitoring refers to a participant's ability to regulate his or her own safety in a group setting when performing activities specific to that class. Community participation refers to activities performed in public such as going out to lunch with friends, volunteering, or going to church. Clinical measures include cut-off scores for the FTSS, floor transfer, 3MBW, 6MWT, 10MWT- PS, 10MWT-FS, TUG-FS and TUG-FScog.
Part 2: exercise program considerations
Exercise program considerations for our clinic included participants' level of assistance with class activities and class format factors specific to this clinic's setting (Table 5). Level of assistance with class activities includes operation of aerobic equipment, comprehension of rating of perceived exertion, over ground balance activities, complex sequencing and dual tasking, floor transfer and floor exercise. Class format factors include the key principles of practice: progressive aerobic training and amplitude-focused functional skill training. Other class format factors include instruction mode, skilled task dosing, balance exercises, frequency of transitions, cognitive–motor complexity and environmental complexity. These exercise program considerations may be customized for different exercise programs and settings (see Discussion: application to other exercise programs).
Discussion
This process of assigning PwP to multilevel group classes in our clinic inspired the retroactively designed clinical decision-making tool that we propose here. Our initial results showing statistical difference among group classes on all clinical measures revealed that stratification was largely achieved by our PD-specialized therapists using much of the information from the initial evaluation (Tables 2 & 3). These results confirmed the heterogeneity in functional mobility across disease severity that typically would occur in most community exercise classes [49] and further motivated our efforts to design a proof of concept clinical decision-making tool. The discriminant function analysis supports that other PD-specialized therapists using demographics and cut-offs (defined in this study for common clinical measures) should be able to achieve the maximum predictive ability. While for greater generalizability to clinical situations in which capturing all the measures is not possible or too onerous, or for therapists with fewer levels of classes, they may want to use the fewest factors to retain a reasonably high predictive value. Assignments can be further personalized and improved by referring to the other personal factors and exercise program considerations defined by our therapists that affected their group class assignment (Tables 4 & 5). This could allow for fine-tuning of the decision-making process and may explain the 21% discrepancy in assignment decisions we quantified in our analysis. The examples discussed below illustrate how therapists can use this tool to initiate and adapt the personalized exercise prescription.
Proposed clinical decision-making tool: illustrative examples
The clinical decision-making process is holistic and person-centered and allows for blending the science and art of physical therapy for optimal outcomes. The categories in Tables 4 & 5 are meant to guide the decision-making process with the goal of maximizing the participants' level of physical and cognitive challenge while also ensuring safety in a group setting. For example, initially the therapist considered placing participant A in Moves 4 based on low fall risk, high balance confidence and minimal comorbidities. However, older age, low exercise experience and low community participation led the therapist to ultimately decide Moves 3 for group class placement. In another case, participant B's fair self-monitoring, need for contact guard assistance with over ground balance activities and class format considerations (moderate to high cognitive–motor and environmental complexity) led the therapist to recommend Moves 2. In addition to guiding the decision-making process, the tool can be used to identify therapy goals. For example, if participant B's goal was to improve his level of assistance with over ground balance activities from contact guard assistance to supervision, he could qualify for a higher level class (Moves 3). Another participant C's goal may be to improve his level of assistance with floor transfers from minimal assistance to supervision, allowing him to also move to a higher level class, from Moves 1 to Moves 2, respectively.
Proposed clinical decision-making tool: framework for communication to coordinate care between rehabilitation & community
The clinical decision-making tool may also be used as a framework for communication between physical therapists and group exercise instructors to coordinate care between the rehabilitative and community settings. For example, upon referral to a group class, the evaluating physical therapist should communicate factors that might impact exercise to the instructors including comorbidities (e.g., cardiac history, joint replacements) or mobility concerns (e.g., assistance needed with floor transfer, difficulties with dual tasking). The tool can also be used for professionals to communicate participant changes over time including a hospitalization or sickness that warrants a bout of therapy and perhaps a change in class level until baseline function can be restored [40,80]. Participants may also improve to a functional level where a more challenging group class is appropriate and ideal for continued adherence and positive outcomes. On the other hand, an instructor could report concerns to the referring physical therapist of a participant having more anxiety, absences, dyskinesias or increasing difficulty with performing exercises in class.
Through ongoing monitoring, therapists and exercise instructors can recognize when a change in motor or cognitive performance is more than day-to-day variability, and then be able to intervene immediately. Day-to-day, even hourly variability in performance was particularly the case for people with advanced disease with freezing of gait, medication fluctuations and cognitive impairments. Although our Moves 1 participants were fewer in number and more likely to have missing measures it is remarkable that they continue to attend, even if more sporadically. The need to coordinate between rehabilitation and community and other medical, financial, or wellness resources is even more essential in moderate to advanced disease. This tool can promote coordination of care that should begin upon diagnosis and continue throughout the participants' lifespan.
Over the years, we have developed a process facilitating timely and efficient communication between our physical therapists and instructors regarding a participants' change in health status and observed concerns in order to reduce attrition, functional decline and adverse events such as falls or injuries. We also have recognized the importance of physical therapy re-evaluations and therapy bouts to address these health status changes or observed concerns early; with the intention of keeping participants in their class as long as possible or returning them to prior level of function as soon as possible. The decision-making tool also provides a way for therapists to work with participants on setting rehabilitation goals that may impact their eligibility for class, and it allows for transparency and clear communication with participants on the need for class level changes for optimal safety, participation and challenge. The clinical decision-making tool can generalize to other rehabilitation and community centers by offering a framework for communication with a common language between rehabilitation and exercise professionals when discussing participant care.
Proposed clinical decision-making tool: application to other exercise programs
Studies suggest aerobic exercise and the intensity, complexity and specificity of exercise is important for learning [16,21,23,36–40,42–44]. We designed the clinical decision-making tool based upon the activities specific to our setting, which included progressive aerobic training and functional learning-principled skill training (e.g., floor exercise and dynamic-balance activities). We altered the instruction mode, skilled task dosing, balance exercises, frequency of transitions between exercise positions, cognitive–motor complexity and environmental complexity factors to optimize safety and challenge relative to our class levels and assistance required for class activities (Table 5). Many of these exercise program considerations in Part 2 of the proposed clinical decision-making tool will be affected by maximum class size, space limitations and availability of volunteers. We suggest therapists adapt Part 2 of the clinical decision-making tool for the unique goals and activities of their recommended exercise class. They may also use this tool to help others grow community exercise programming that takes into account the unique needs of PwP to be challenged yet safe. For example, the factors described in the clinical decision-making tool can be customized for exercise programs that include other modalities of intervention including boxing, dancing, Tai Chi and cycling. Therapists can then determine what other assessments may be needed to evaluate the participants' ability to safely perform the activities specific to their setting. For example, assessment of dynamic balance would be relevant for a Tango class and assessment of floor transfer would be relevant for a high-level yoga class. However, the requirement for dance partners or the availability of volunteers to cue or guard may impact the therapists' choice of assessments or assignment.
Future directions
In this study, we propose a two-part clinical decision-making tool that can be refined and tested over time for the purpose of extending the benefits of physical therapy through the sustained practice of rehabilitation goals in community multilevel group exercise classes. We predict that participating in an integrated rehab and group exercise model where PwP continue to sustain practice of therapy goals in a group exercise program that allows for the appropriate level of physical and cognitive challenge will provide additive and complementary benefits in the long-term. Future studies could examine these questions and compare benefits of integrated therapy and group to therapy alone or group alone conditions. Studies are also needed to investigate the validity and reliability of the decision-making tool for group exercise class stratification when compared with more typical mixed class design. In addition, studies are needed that utilize validated tools to measure self-efficacy, comorbidities, postural control and self-reported balance confidence to further investigate how these factors impact group exercise class assignment and to investigate their sensitivity to detect change over time. It is also important to look at versions of the tool that can be used by community instructors.
Limitations
The findings from this study are limited by the design of a retrospective study of real-world longitudinal data collection in an operational PD-specialized clinic. Due to time constraints in a clinic setting, many factors in Table 4 were gathered by self or family report or clinical observation rather than directly measured. Recall bias and presence of mild cognitive impairment could limit accuracy of results for self-reported fall and freezing history. Measurement error associated with the retrospective determination of H&Y staging by a physical therapist may have influenced the low predictability of this variable. To be able to capture the core clinical measures we needed for group assignment purposes, we did not have the time to add additional measures to the evaluation. Future studies could include other measurements to capture more specific mobility and postural control deficits for establishing cut-offs for group assignments. The timing of evaluations was influenced by the day-to-day scheduling conflicts of a busy physical therapy clinic. While we did not attempt to control for timing of medication dosing for evaluations or class participation, medication side effects were considered as they related to functional mobility, safety and ability to exert physical effort in a group setting and contributed to the development of Tables 4 & 5.
Conclusion
We propose a two-part physical therapy clinical decision-making tool that was designed from a combination of data from a retrospective chart review and clinical experience with assigning PwP to multilevel group classes. We suggest that physical therapists are uniquely qualified to manage the lifelong exercise prescription for PwP and serve as liaisons with community exercise professionals to optimize therapy outcomes. Physical therapists' scope of practice [81,82] allows them to comprehensively assess PwP and make appropriate plan of care recommendations for physical therapy and community group exercise participation. This tool may assist physical therapists manage the lifelong exercise prescription for PwP as it includes assessments of domains within and specific to a therapist's scope of practice. Physical therapists can use this tool to establish plans of care, assign participants to multilevel group classes, re-evaluate participants' needs over time and make referrals or consult with other healthcare professionals.
PwP are particularly vulnerable to adopting a less physically active lifestyle after diagnosis and may experience stress, injury, sleep disorders and illnesses that have been shown to negate the benefits of exercise [28,83]. Physical therapists can identify and address these issues and other barriers to exercise during re-evaluations including comorbidities, nonmotor symptoms and psychosocial factors that may interfere with PwP's willingness and capacity to fully participate in exercise and life [28,66,83–86]. This tool can serve as a guide for communication and coordination of care between therapists and exercise professionals to safely challenge PwP in the rehabilitation and community setting.
The decision-making tool also allows for transparency and clear communication with instructors and participants on the need for class level changes for optimal safety, participation and challenge. Many PD-specific community classes include PwP with varying levels of mobility making it challenging for the instructor to optimally challenge each participant and potentially leading to participant dropout. The results of this study indicate that PwP present differently and that stratification into the appropriate level of class could contribute to long-term adherence to exercise. All domains assessed in the initial evaluations are within physical therapists' scope of practice indicating the importance of their involvement in PD exercise prescription. Our proposed clinical decision-making tool offers a management perspective for the PD exercise prescription where physical therapists are the practitioners of choice to bridge the gap between the medical system and the community promoting long-term adherence to exercise. Results of this study could guide other clinics, therapists and exercise professionals in establishing criteria for multilevel community exercise classes for PwP. In addition, our results highlight the need for these multilevel classes to promote long-term adherence to exercise and appropriate challenge point within the specific therapeutic intervention or exercise program.
Summary points.
Physical therapy and exercise are now considered an essential component in the management of Parkinson's disease (PD) and should begin at the time of diagnosis of PD, when there is the greatest potential for putting off motor deterioration and slowing disease progression.
The challenge to healthcare professionals is how to deliver rehabilitation and community exercise resources if we expect persons with PD (PwP) to exercise and remain as physically active as possible for the long-term, starting at diagnosis.
We suggest that the integration of personalized rehabilitation goals into group exercise programs allows for the sustained practice of functional mobility while harnessing the additive and complimentary benefits of exercise.
To maximize each participants' level of physical and cognitive challenge for achieving functional goals, we propose a process for stratifying individuals into more homogeneous group exercise classes.
The two-part physical therapy clinical decision-making tool for group class stratification summarized here was designed from a combination of clinical judgment and participant characteristics (personal factors and clinical measures) acquired from a retrospective chart review at a clinic experienced with assigning PwP to multilevel group classes.
Discriminant function analysis revealed that seven clinical measures and four demographic characteristics predicted 79% of the PD-specialized therapists' decisions to three levels of exercise classes.
This tool may help guide the physical therapy plan of care, including prescribing and updating the exercise prescription and networking with community resources to integrate therapy and fitness goals for optimal motor and cognitive challenge.
The clinical decision-making tool may be used as a framework for communication between physical therapists and fitness professionals to convey precautions, need for class reassignment and need for changes to specific class considerations.
Physical therapists are uniquely qualified to manage the lifelong exercise prescription for PwP, incorporating the myriad of factors impacting ongoing access to rehabilitation and exercise and serving as liaisons between the medical and community professionals.
The integration of physical therapy and group exercise goals offers one solution for extending the benefits of therapy, reducing the rate of functional decline in PwP and contributing to a possible disease-modifying effect.
Acknowledgments
AJ Elkins is currently attending the University of Colorado Physical Therapy Program, Anschutz Medical Campus, Aurora, CO 80045, USA.
Footnotes
Financial & competing interests disclosure
BG Farley is Founder and Chief Scientific Officer, EE Borchers and JK Bazan-Wigle are physical therapists, and AJ Elkins was a research assistant and all were employees of NeuroFit Networks, Inc. DBA Parkinson Wellness Recovery, a 501(c)(3) nonprofit organization that manages both the physical therapy clinic and neurofitness and wellness center where these data were collected. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This retrospective study involved the use of de-identified data only. The authors obtained a waiver from the A.T. Still University Institutional Review Board for conduct of this study. A data sharing agreement was established b/w TL McIsaac and BG Farley for the purposes of statistical analysis and interpretation.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Cheng EM, Tonn S, Swain-Eng R. et al. Quality improvement in neurology: AAN Parkinson disease quality measures: report of the Quality Measurement and Reporting Subcommittee of the American Academy of Neurology. Neurology 75(22), 2021–2027 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox SH, Katzenschlager R, Lim SY. et al. The Movement Disorder Society Evidence-Based Medicine Review update: treatments for the motor symptoms of Parkinson's disease. Mov. Disord. 26(Suppl. 3), S2–S41 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Rafferty MR, Schmidt PN, Luo ST. et al. Regular exercise, quality of life, and mobility in Parkinson's disease: a longitudinal analysis of National Parkinson Foundation Quality Improvement Initiative Data. J. Parkinsons Dis. 7(1), 193–202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • The first longitudinal study on self-reported exercise habits in persons with Parkinson's disease to show that consistent exercisers, or those that began exercise after their baseline, had smaller declines in quality of life and functional mobility over 2 years compared with people who did not exercise regularly.
- 4.Sacheli MA, Murray DK, Vafai N. et al. Habitual exercisers versus sedentary subjects with Parkinson's disease: multimodal PET and fMRI study. Mov. Disord. 33(12), 1945–1950 (2018). [DOI] [PubMed] [Google Scholar]; • First study to use imaging of dorsal and ventral striatal pathways to compare exercisers with sedentary persons with mild to moderate Parkinson's disease. Exercisers showed more robust dopamine connectivity in both pathways and decreased motor and non-motor symptoms.
- 5.Perry SIB, Nelissen PM, Siemonsma P, Lucas C. The effect of functional-task training on activities of daily living for people with Parkinson's disease, a systematic review with meta-analysis. Complement. Ther. Med. 42, 312–321 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Shen X, Wong-Yu IS, Mak MK. Effects of exercise on falls, balance, and gait ability in Parkinson's disease: a meta-analysis. Neurorehabil. Neural Repair 30(6), 512–527 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Tomlinson CL, Patel S, Meek C. et al. Physiotherapy intervention in Parkinson's disease: systematic review and meta-analysis (structured abstract). BMJ 345(3), e5004 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uhrbrand A, Stenager E, Pedersen MS, Dalgas U. Parkinson's disease and intensive exercise therapy – a systematic review and meta-analysis of randomized controlled trials. J. Neurol. Sci. 353(1), 9–19 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Ni Meng, Hazzard JB, Signorile J LC. Exercise guidelines for gait function in Parkinson's disease: a systematic review and meta-analysis. Neurorehabil. Neural Repair 32(10), 872–886 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Keus SH, Munneke M, Nijkrake MJ, Kwakkel G, Bloem BR. Physical therapy in Parkinson's disease: evolution and future challenges. Mov. Disord. 24(1), 1–14 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Keus SHJ, Munneke M, Graziano M. et al. European Physiotherapy Guideline for Parkinson's disease. KNGF/ParkinsonNet, The Netherlands: (2014). http://www.parkinsonnet.info/guidelines [Google Scholar]
- 12.Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson's disease: current outlook and future challenges. Parkinsonism Relat. Disord. 22(Suppl. 1), S60–S64; 61p (2016). [DOI] [PubMed] [Google Scholar]
- 13.Archibald N, Miller N, Rochester L. Neurorehabilitation in Parkinson disease. Handbook Clin. Neurol. 110, 435–442 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Canning CG. Rehabilitation in Parkinson's disease – the challenge to provide early and ongoing, evidence-based, patient-centred care. Arquivos de neuro-psiquiatria 71(12), 917–919 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Kim SD, Allen NE, Canning CG, Fung VSC. Chapter 11 – Parkinson disease In: Handbook of Clinical Neurology, Day BL, Lord SR (). Elsevier, 159, 173–193 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Ferrazzoli D, Ortelli P, Madeo G, Giladi N, Petzinger GM, Frazzitta G. Basal ganglia and beyond: the interplay between motor and cognitive aspects in Parkinson's disease rehabilitation. Neurosci. Biobehav. Rev. 90, 294–308 (2018). [DOI] [PubMed] [Google Scholar]; •• An expert consensus suggesting that rehabilitation approaches should engage cognition in the relearning of habitual motor behaviors and integrate progressive aerobics exercise to be most effective.
- 17.Dahodwala N, Xie M, Noll E, Siderowf A, Mandell DS. Treatment disparities in Parkinson's disease. Ann. Neurol. 66(2), 142–145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schenkman M, Moore CG, Kohrt WM. et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a Phase 2 randomized clinical trial. JAMA Neurol. 75(2), 219–226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher BE, Li Q, Nacca A. et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson's disease. Neuroreport 24(10), 509–514 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Hirsch MA, Van Wegen EEH, Newman MA, Heyn PC. Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson's disease: a systematic review and meta-analysis. Transl. Neurodegener. 7, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duchesne C, Gheysen F, Bore A. et al. Influence of aerobic exercise training on the neural correlates of motor learning in Parkinson's disease individuals. Neuroimage Clin. 12, 559–569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Evidence that aerobics exercise training primes dopamine circuits involved in sequential movements and may enhance learning capacity when combined with the practice of sequential tasks.
- 22.Wu J, Kung J, Dong J. et al. Distinct connectivity and functionality of aldehyde dehydrogenase 1a1-positive nigrostriatal dopaminergic neurons in motor learning. Cell Rep. 28, 1167–1181.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlskog JE. Aerobic exercise: evidence for a direct brain effect to slow Parkinson disease progression. Mayo Clin. Proc. 93(3), 360–372 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Chong TT, Bonnelle V, Manohar S. et al. Dopamine enhances willingness to exert effort for reward in Parkinson's disease. Cortex 69, 40–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lord S, Godfrey A, Galna B, Mhiripiri D, Burn D, Rochester L. Ambulatory activity in incident Parkinson's: more than meets the eye? J. Neurol. 260(12), 2964–2972 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Benito-Leon J, Cubo E, Coronell C, Group AS. Impact of apathy on health-related quality of life in recently diagnosed Parkinson's disease: the ANIMO study. Mov. Disord. 27(2), 211–218 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Maffoni M, Giardini A, Pierobon A, Ferrazzoli D, Frazzitta G. Stigma experienced by Parkinson's disease patients: a descriptive review of qualitative studies. Parkinsons Dis. 2017, 7203259 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauzé M, Daneault J-F, Duval C. The effects of physical activity in Parkinson's disease: a review. J. Parkinsons Dis. 6(4), 685–698 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma HI, Saint-Hilaire M, Thomas CA, Tickle-Degnen L. Stigma as a key determinant of health-related quality of life in Parkinson's disease. Qual. Life Res. 25(12), 3037–3045 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mischley LK, Lau RC, Weiss NS. Use of a self-rating scale of the nature and severity of symptoms in Parkinson's Disease (PRO-PD): correlation with quality of life and existing scales of disease severity. NPJ Parkinsons Dis. 3(1), 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekker MS, Janssen S, Nonnekes J, Bloem BR, De Vries NM. Neurorehabilitation for Parkinson's disease: future perspectives for behavioural adaptation. Parkinsonism Relat. Disord. 22(Suppl. 1), S73–S77 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Buetow SA, Martinez-Martin P, Hirsch MA, Okun MS. Beyond patient-centered care: person-centered care for Parkinson's disease. NPJ Parkinsons Dis. 2, 16019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsch MA, Farley BG. Exercise and neuroplasticity in persons living with Parkinson's disease. Eur. J. Phys. Rehabil. Med. 45(2), 215–229 (2009). [PubMed] [Google Scholar]
- 34.Hirsch MA, Iyer SS, Englert D, Sanjak M. Promoting exercise in Parkinson's disease through community-based participatory research. Neurodegener. Dis. Manag. 1(5), 365–377 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King LA, Wilhelm J, Chen Y. et al. Effects of group, individual, and home exercise in persons with Parkinson disease: a randomized clinical trial. J. Neurol. Phys. Ther. 39(4), 204–212 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The first study that compared differing benefits of administering the same motor-cognitive exercise approach for PwP in different settings supporting a combination of both group and one-on-one physical therapy as the most effective way to benefit functional mobility, while calling into question the usefulness of a home exercise.
- 36.Duchesne C, Lungu O, Nadeau A. et al. Enhancing both motor and cognitive functioning in Parkinson's disease: aerobic exercise as a rehabilitative intervention. Brain Cogn. 99, 68–77 (2015). [DOI] [PubMed] [Google Scholar]; • This study showed aerobic exercise (small group, supervised, 3×/week, 1 hour) in early PD and healthy controls not only improved fitness, but cognition and motor sequence learning functions important for everyday movements.
- 37.Petzinger GM, Fisher BE, Mcewen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol. 12(7), 716–726 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marusiak J, Fisher BE, Jaskólska A. et al. Eight weeks of aerobic interval training improves psychomotor function in patients with Parkinson's disease-randomized controlled trial. Int. J. Environ. Res. Public Health 16(5), E880 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mak MK, Wong-Yu IS, Shen X, Chung CL. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat. Rev. Neurol. 13, 689–703 (2017). [DOI] [PubMed] [Google Scholar]; •• First review of the long-term effects of physical therapy and exercise on Parkinson's disease; at least 12 weeks of training and at least 12 weeks of follow-up following end of treatment.
- 40.Frazzitta G, Maestri R, Bertotti G. et al. Intensive rehabilitation treatment in early Parkinson's disease: a randomized pilot study with a 2-year follow-up. Neurorehabil. Neural Repair 29(2), 123–131 (2015). [DOI] [PubMed] [Google Scholar]; •• First study of 2-year results showing intensive rehabilitation early in PD might slow progression of motor decay and delay the need for medication increases.
- 41.Levitt J, Chitnis S, Walker-Batson D. The effects of the “SPEAK OUT! ®” and “LOUD Crowd®” voice programs for Parkinson disease. Int. J. Health Sci. 3(2), 13–19 (2015). [Google Scholar]; •• The first study showing that benefits of intensive one-on-one speech therapy were maintained (vocal intensity) or even additive (voice related quality of life) through sustained practice in an 8-week group maintenance program.
- 42.Jakowec MW, Wang Z, Holschneider D, Beeler J, Petzinger GM. Engaging cognitive circuits to promote motor recovery in degenerative disorders. exercise as a learning modality. J. Hum. Kinet. 52, 35–51 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steib S, Wanner P, Adler W, Winkler J, Klucken J, Pfeifer K. A single bout of aerobic exercise improves motor skill consolidation in Parkinson's disease. Front. Aging Neurosci. 10, 328 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong-Yu IS, Mak MK. Multi-dimensional balance training programme improves balance and gait performance in people with Parkinson's disease: a pragmatic randomized controlled trial with 12-month follow-up. Parkinsonism Relat. Disord. 21(6), 615–621 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Corcos DM, Robichaud JA, David FJ. et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson's disease. Mov. Disord. 28(9), 1230–1240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenkman M. et al. Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys. Ther. 92(11), 1395–410 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uc EY, Doerschug KC, Magnotta V. et al. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology 83(5), 413–425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.States RA, Sweeny TL, Rossi A, Spierer DK, Salem Y. Physical functioning after 1, 3, and 5 years of exercise among people with Parkinson's disease: a longitudinal observational study. J. Geriatr. Phys. Ther. 40(3), 127–134 (2017). [DOI] [PubMed] [Google Scholar]; •• This observational study showed that PwP will participate in a community group exercise program one to two visits per week over multiple years. While many participants showed no worsening on a number of measures, attrition ranged from 10 to 40% per year highlighting the need for physical therapist to work with community resources to optimize the exercise prescription.
- 49.Domingos J, Dean J, Godinho C, Melo F. Proliferation of community exercise programs with limited evidence and expertise: Safety implications. Mov. Disord. 33(8), 1365–1366 (2018). [DOI] [PubMed] [Google Scholar]; • Recent comment on the importance of evaluating community exercise programs for people with Parkinson's disease.
- 50.Ramaswamy B, Jones J, Carroll C. Exercise for people with Parkinson's: a practical approach. Pract. Neurol. 18(5), 399–406 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Combs SA, Diehl MD, Staples WH. et al. Boxing training for patients with Parkinson disease: a case series. Phys. Ther. 91(1), 132–142 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Carroll LM, Volpe D, Morris ME, Saunders J, Clifford AM. Aquatic exercise therapy for people with Parkinson disease: a randomized controlled trial. Arch. Phys. Med. Rehabil. 98(4), 631–638 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Franzoni LT, Monteiro EP, Oliveira HB. et al. A 9-week nordic and free walking improve postural balance in Parkinson's disease. Sports Med. Int. Open 2(2), E28–E34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reuter I, Mehnert S, Leone P, Kaps M, Oechsner M, Engelhardt M. Effects of a flexibility and relaxation programme, walking, and nordic walking on Parkinson's disease. J. Aging Res. 2011, 232473 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foster ER, Golden L, Duncan RP, Earhart GM. Community-based Argentine tango dance program is associated with increased activity participation among individuals with Parkinson's disease. Arch. Phys. Med. Rehabil. 94(2), 240–249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao Q, Leung A, Yang Y. et al. Effects of Tai Chi on balance and fall prevention in Parkinson's disease: a randomized controlled trial. Clin. Rehabil. 28(8), 748–753 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Li F, Harmer P, Fitzgerald K. et al. Tai chi and postural stability in patients with Parkinson's disease. N. Engl. J. Med. 366(6), 511–519 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li F, Harmer P, Liu Y. et al. A randomized controlled trial of patient-reported outcomes with tai chi exercise in Parkinson's disease. Mov. Disord. 29(4), 539–545 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hawkins BL, Van Puymbroeck M, Walter A. et al. Perceived activities and participation outcomes of a yoga intervention for individuals with Parkinson's disease: a mixed methods study. Int. J. Yoga Ther. 28(1), 51–61 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Ni M, Signorile JF, Mooney K. et al. Comparative effect of power training and high-speed yoga on motor function in older patients with Parkinson disease. Arch. Phys. Med. Rehabil. 97(3), 345–354.e315 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Guadagnoli MA, Lee TD. Challenge point: a framework for conceptualizing the effects of various practice conditions in motor learning. J. Mot. Behav. 36(2), 212–224 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Van Der Kolk NM, King LA. Effects of exercise on mobility in people with Parkinson's disease. Mov. Disord. 28(11), 1587–1596 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Voss TS, Elm JJ, Wielinski CL. et al. Fall frequency and risk assessment in early Parkinson's disease. Parkinsonism Relat. Disord. 18(7), 837–841 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 17(5), 427–442 (1967). [DOI] [PubMed] [Google Scholar]
- 65.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q). Can. J. Sport Sci. 17(4), 338–345 (1992). [PubMed] [Google Scholar]
- 66.King LA, Priest KC, Nutt J. et al. Comorbidity and functional mobility in persons with Parkinson disease. Arch. Phys. Med. Rehabil. 95(11), 2152–2157 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schenkman M, Deutsch JE, Gill-Body KM. An integrated framework for decision making in neurologic physical therapist practice. Phys. Ther. 86(12), 1681–1702 (2006). [DOI] [PubMed] [Google Scholar]
- 68.APTA. Guide to Physical Therapist Practice 3.0. (2014). http://guidetoptpractice.apta.org/
- 69.Borg G, Borg E. A new generation of scaling methods: level-anchored ratio scaling. Psychologica 28(3), 15–45 (2001). [Google Scholar]
- 70.Nazem S, Siderowf AD, Duda JE. et al. Montreal cognitive assessment performance in patients with Parkinson's disease with “normal” global cognition according to Mini-Mental State Examination score. J. Am. Geriatr. Soc. 57(2), 304–308 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bohannon RW. Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept. Mot. Skills 103(1), 215–222 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Paul SS, Canning CG, Sherrington C, Fung VS. Reproducibility of measures of leg muscle power, leg muscle strength, postural sway and mobility in people with Parkinson's disease. Gait Posture 36(3), 639–642 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Carter V, Jain T, James J, Cornwall M, Aldrich A, De Heer HD. The 3-m backwards walk and retrospective falls: diagnostic accuracy of a novel clinical measure. J. Geriatr. Phys. Ther. 42(4), 249–255 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Hackney ME, Earhart GM. Backward walking in Parkinson's disease. Mov. Disord. 24(2), 218–223 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 166(1), 111–117 (2002). [DOI] [PubMed] [Google Scholar]
- 76.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-Item Short-Form Health Survey, and the Unified Parkinson Disease Rating Scale in people with parkinsonism [corrected] [published erratum appears in Phys. Ther. 90(3), 462 (2010)]. Phys. Ther. 88(6), 733–746 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing 26(1), 15–19 (1997). [DOI] [PubMed] [Google Scholar]
- 78.Duncan RP, Earhart GM. Should one measure balance or gait to best predict falls among people with Parkinson disease?. Parkinsons Dis. 2012, 6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go test. Phys. Ther. 80(9), 896–903 (2000). [PubMed] [Google Scholar]
- 80.Fullard ME, Thibault DP, Hill A. et al. Utilization of rehabilitation therapy services in Parkinson disease in the United States. Neurology 89(11), 1162–1169 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.APTA. The Physical Therapist Scope of Practice. 2019(1/23/2019) (2018). https://www.apta.org/ScopeOfPractice/ [Google Scholar]
- 82.Dean E, Al-Obaidi S, De Andrade AD. et al. The First Physical Therapy Summit on Global Health: implications and recommendations for the 21st Century. Physiother. Theory Pract. 27(8), 531–547 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Ellis T, Cavanaugh JT, Earhart GM. et al. Factors associated with exercise behavior in people with Parkinson disease. Phys. Ther. 91(12), 1838–1848 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ellis T, Boudreau JK, Deangelis TR. et al. Barriers to exercise in people with Parkinson disease. Phys. Ther. 93(5), 628–636 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gray WK, Hildreth A, Bilclough JA, Wood BH, Baker K, Walker RW. Physical assessment as a predictor of mortality in people with Parkinson's disease: a study over 7 years. Mov. Disord. 24(13), 1934–1940 (2009). [DOI] [PubMed] [Google Scholar]
- 86.Speelman AD, Van Nimwegen M, Bloem BR, Munneke M. Evaluation of implementation of the ParkFit program: a multifaceted intervention aimed to promote physical activity in patients with Parkinson's disease. Physiotherapy 100(2), 134–141 (2014). [DOI] [PubMed] [Google Scholar]