Abstract
Saccharomyces cerevisiae Tel1 is the ortholog of human ATM kinase and initiates a cell cycle checkpoint in response to dsDNA breaks (DSBs). Tel1ATM kinase is activated synergistically by naked dsDNA and the Mre11-Rad50-Xrs2NBS1 complex (MRX). A multisubunit protein complex, which is related to human shelterin, protects telomeres from being recognized as DSBs, thereby preventing a Tel1ATM checkpoint response. However, at very short telomeres, Tel1ATM can be recruited and activated by the MRX complex, resulting in telomere elongation. Conversely, at long telomeres, Rap1-interacting-factor 2 (Rif2) is instrumental in suppressing Tel1 activity. Here, using an in vitro reconstituted Tel1 kinase activation assay, we show that Rif2 inhibits MRX-dependent Tel1 kinase activity. Rif2 discharges the ATP-bound form of Rad50, which is essential for all MRX-dependent activities. This conclusion is further strengthened by experiments with a Rad50 allosteric ATPase mutant that maps outside the conserved ATP binding pocket. We propose a model in which Rif2 attenuates Tel1 activity at telomeres by acting directly on Rad50 and discharging its activated ATP-bound state, thereby rendering the MRX complex incompetent for Tel1 activation. These findings expand our understanding of the mechanism by which Rif2 controls telomere length.
Keywords: ATPase, ataxia telangiectasia, DNA damage response, telomere, checkpoint control, double-stranded break, MRX, Rad50 ATPase, Rif2, telomere control, Tel1 kinase, ATM kinase, cell cycle checkpoint
Introduction
Telomeres are DNA-protein complexes that define the physical ends of linear eukaryotic chromosomes. Telomeric DNA is composed of short, tandem G-rich repeats that extend for thousands of bases at the 3′-ends of chromosomes. Proper establishment and maintenance of telomeres is essential for genome stability and cell survival (1, 2). Telomeres progressively shorten as cells divide. Telomere shortening is counteracted by telomerase, a reverse transcriptase that synthesizes the G-rich telomeric repeats de novo (3). Yeast cells and primary mammalian cells that are unable to maintain telomeres progress into senescence, which is mediated by a DNA damage–signaling pathway (4). Telomere-induced senescence underlies a plethora of human genetic disorders that share short telomere as a common molecular defect (5, 6). On the other hand, in humans, the inappropriate elongation of telomere leads to cancer predisposition and aids in the survival of cancer cells (7).
In addition to telomerase, telomere DNA repeats are bound by a set of proteins that assemble into a protective capping structure called shelterin (8). This DNA-protein structure serves two important functions. First, it protects chromosome ends from nucleolytic degradation and from being recognized as dsDNA breaks (DSBs).3 Improper recognition of chromosome ends as DSBs would elicit a DNA-damage checkpoint and promote repair, which at telomeres could result in telomeric fusions. Second, shelterin regulates telomere length by controlling access of telomerase and its activity (9, 10). The shelterin-analogous complex in Saccharomyces cerevisiae is composed of many proteins which include the Rap1-Rif1-Rif2 complex and the Cdc13-Stn1-Ten1 complex. The Rap1-Rif1-Rif2 protein complex associates with the double-stranded TG(1–3) repeat and is a pivotal player in telomere homeostasis (11–13). The Cdc13-Stn1-Ten1 complex forms RPA-like (replication protein A) trimeric complex that binds to the single-stranded 3′ overhang and prevents its nucleolytic degradation(14, 15). In addition to these major telomere-associated proteins, there are other enzymes that mediate both end protection and telomere length control. Among these are Tel1 checkpoint kinase, the ortholog of human ATM, and the MRX(N) complex composed of Mre11, Rad50, and Xrs2 (human NBS1) (16). The Rad50 subunit binds ATP and has weak ATPase activity that is stimulated a few -fold in the presence of dsDNA. Disabling ATP binding to Rad50 results in a rad50Δ phenotype (17).
The double-stranded telomeric regions of S. cerevisiae contain extended repetitive arrays of high-affinity Rap1-binding sites (11, 18, 19). Rap1 also recruits Rif1 and Rif2 to telomeres. Both of these proteins bind to the C-terminal domain of Rap1, and this interaction is required for their recruitment(12, 13, 20, 21). Deletion of Rif1 and Rif2 leads to telomere elongation, and deletion of both has an additive effect. Strains that express a Rap1 variant lacking the C-terminal domain confer a telomere phenotype similar to rif1Δ rif2Δ double deletion (13). Based on these genetic observations, current models propose that long telomeres, by virtue of their size, are able to support the binding of an increased number of Rap1-Rif1-Rif2 complexes (Fig. 1A) (20), leading to an increased repressive effect on telomere elongation. Conversely, short telomeres bind fewer of these repressive complexes and are more frequently elongated. Tethering Rif1 and Rif2 to telomeres by other means than binding to Rap1 also limits telomere elongation (22, 23). This supports the model that Rif1 and Rif2, and not Rap1, are responsible for the repressive effect. The preferential extension of short telomeres coupled with the restrained elongation of long telomeres maintains telomere length homeostasis.
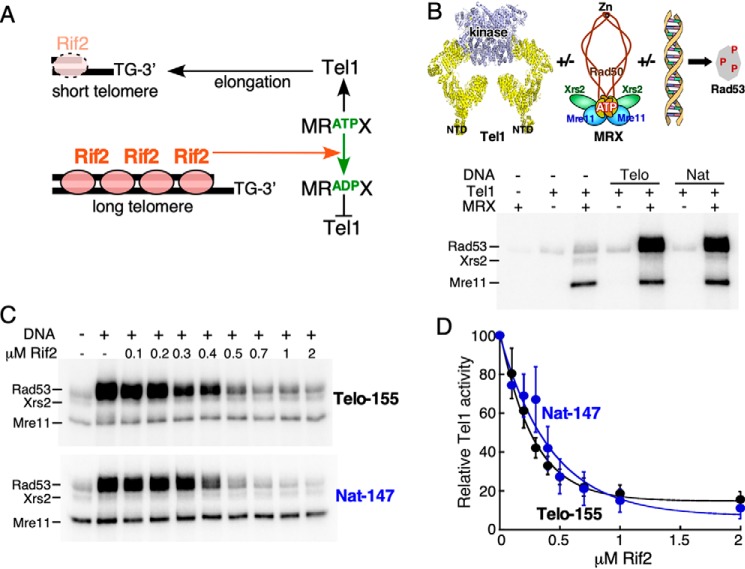
Figure 1.
Rif2 inhibits MRX-dependent activation of Tel1 kinase. A, simplified model for the regulatory activity of Rif2. Note that localization of Rif2 to telomers requires interaction with Rap1 (not shown). B, top, schematic representation of in vitro kinase assay using purified proteins. The mutations in Rad50(ΔCC48) are near the Zn-binding site. Bottom, standard kinase reactions (see “Experimental procedures”) contained the indicated factors, and either 10 nm Telo-155 or Nat-147 DNA where indicated. C, standard kinase reaction with 30 nm MRX, and either 10 nm Telo-155 or Nat-147 DNA, contained the indicated concentrations of Rif2. D, quantification of four independent replicates of the assay shown in C. Relative Tel1 activity of the reaction without Rif2 was set to 100 (lane 2). Averages and S.E. are shown. Half-maximal inhibition was observed at 200–300 nm Rif2.
The protein kinase Tel1 coordinates checkpoint activation in response to DSBs (24). Tel1's recruitment to DSBs and the subsequent activation of its protein kinase activity depends on MRX (25). This central three-subunit complex consists of Mre11 nuclease, Rad50 ATPase, and the auxiliary subunit Xrs2 (NBS1) (26, 27). In addition to its role in DNA break repair, Tel1 is also involved in telomere length regulation (16). Tel1 specifically associates with short telomeres, and tel1Δ mutants show a defect in telomerase-mediated telomere elongation (28–31). Although the exact mechanism of Tel1-mediated telomere length regulation is unknown, its kinase activity is essential because cells expressing kinase-dead Tel1 show similar telomere length defects as tel1Δ cells (24, 32). Tel1 binding to telomeres has been shown to depend on the C-terminal domain of the Xrs2 subunit of MRX (25). Deletion of any of the subunits of MRX, or destructive mutation of the ATP-binding site in Rad50 shows the same telomere-shortening phenotype as a tel1Δ mutant, or a tel1Δ MRX double mutant (16, 28, 33). This indicates that Tel1 and MRX require its Rad50 ATPase to positively regulate telomere length in the same pathway.
The mechanism by which Rif2 controls telomere length remains elusive. Recent studies have suggested that Rif2 attenuates the functions of Tel1 and MRX at telomeres. Rif2 interacts with Xrs2 in the same region as Tel1 does, prompting the model that Rif2 counteracts MRX-dependent recruitment of Tel1 to telomeres by simple competition (22). Here, we have studied the role of Rif2 in the MRX-dependent activation of Tel1 in a reconstituted biochemical system. Our data show that Rif2 inhibits MRX-dependent stimulation of Tel1, independent of interaction with Xrs2. A recent study has shown that Rif2 stimulates the ATPase activity of Rad50 (34). We show here that Rif2 acts directly on Rad50 by discharging its activated ATP-bound state, thereby making the MRX complex incompetent for Tel1 activation.
Results and discussion
Rif2 inhibits MRX-dependent activation of Tel1 kinase
We have employed an in vitro assay using purified proteins to examine the potential role that Rif2 plays in regulating the activities of Tel1 and MRX. Tel1's physiological substrate is Rad53, the effector kinase in the cell cycle checkpoint pathway (35). Phosphorylation of Rad53 was used as a direct readout of Tel1 kinase activity. We showed previously that individually MRX or DNA caused a 2- to 3-fold stimulation of Tel1 kinase activity; however, when both are present, a greatly synergistic stimulation of Tel1 kinase was observed (36) (Fig. 1B). This strong stimulation is independent of DNA sequence; both random DNA (Nat) and telomeric repeat DNA (Telo) showed a similar response.
To test whether Rif2 has a function in the MRX-dependent activation of Tel1, we titrated Rif2 into the complete assay, containing both MRX and DNA (Fig. 1C). Rif2 inhibited Tel1 kinase in this assay, as shown by a reduction in Rad53 phosphorylation, independent of the nature of the DNA sequence (telomeric versus nontelomeric (Nat)) (Fig. 1, C and D). We note that full inhibition of MRX-dependent kinase activity requires high concentrations of Rif2, suggesting weak interactions with the complex. Moreover, in our assay, the Rif2-mediated inhibition is independent of DNA-bound Rap1 (Fig. S1A). In the cell, inhibition at telomeres occurs via the tethering of Rif2 to telomeres through Rap1. However, Rap1-Rif2 binding is weak (37), suggesting that extended arrays of Rap1-Rif2 may be required for full inhibition in vivo. This result is consistent with the genetic observation that Rap1's requirement for telomere length control can be bypassed by artificially tethering Rif2 to telomeres (21). Taken together, these observations support a model that is based on genetic data, i.e. that Rif2 attenuates the functions of Tel1 and MRX at telomeres. In a control experiment, we determined that the Rif2-mediated inhibition is not specific to Tel1's physiological phosphorylation target Rad53. A heterologous substrate, human PHAS-I, also showed severely reduced phosphorylation by Tel1 in this assay when Rif2 was added (Fig. S1B).
The protein kinase activity of Tel1 is required for all Tel1-mediated transactions, including telomere regulation in vivo (24, 32). However, the inhibitory activity of Rif2 does not proceed through Tel1 directly. Rif2 inhibited neither the basal Tel1 kinase activity (not shown) nor the DNA-stimulated Tel1 kinase activity (Fig. S1C). These data suggest that Rif2 inhibition may proceed through MRX.
The Xrs2 subunit of MRX is dispensable for Rif2's inhibitory activity in vitro
Tel1 recruitment to telomeres and dsDNA breaks requires its association with the C terminus of Xrs2 (25). A C-terminal truncation of Xrs2 (human NBS1) attenuates MRX function without affecting the stability of the MRX complex in budding yeast, fission yeast, and human cells (25, 38–40). Moreover, Rif2 interacts with Xrs2 within the same C-terminal region as Tel1. This has led to the proposal of a model by which Rif2 may exert its inhibitory role by abrogating the binding of Tel1 to Xrs2, and thereby to MRX (22). One prediction of this model is that inhibition depends on Xrs2.
In a recent study, we showed that Rad50 is the critical subunit of MRX for full activation of Tel1 (36). In addition to DNA and Rad50, either Mre11 or Xrs2, but not both, is also required. Thus, both the Mre11-Rad50 and Rad50-Xrs2 pairs, but not the Mre11-Xrs2 pair, function efficiently in vitro (36). We tested whether Rif2 inhibited the activation of Tel1 by the different dimeric pairs of MRX. Rif2 inhibited Tel1 activation by MRX and by RX, and also by MR lacking Xrs2 (Fig. 2, A and B). These data suggest that Rif2 enforces its inhibition through the Rad50 subunit, which is the common subunit in all complexes. The observation of a functional relationship between Rad50 and Rif2 prompted us to test for their physical interaction. GST-tagged Rif2 was able to pull down Rad50 with an efficiency comparable to that of the positive control Mre11, a known interactor of Rad50 (41) (Fig. 2C, compare lane 1 with lane 3).
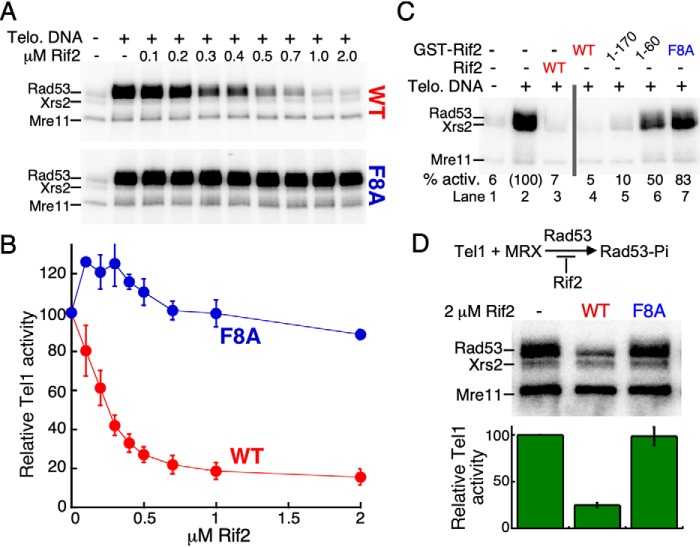
Figure 2.
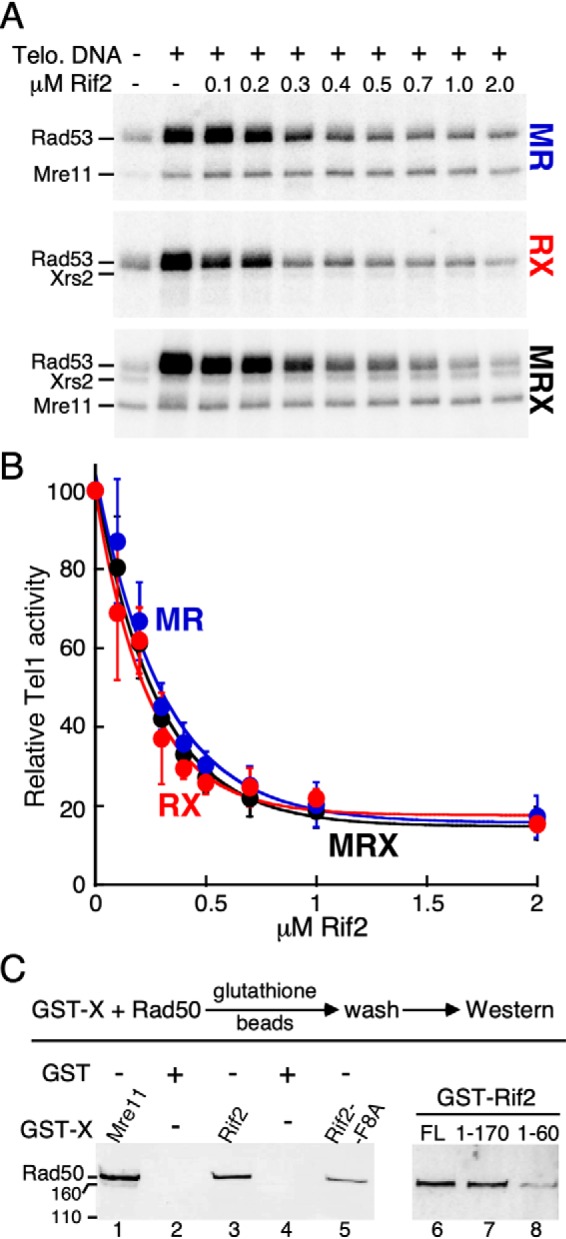
The Xrs2 subunit of MRX is dispensable for Rif2's inhibitory activity in vitro. A, standard kinase reaction with increasing concentrations of Rif2, and 30 nm MR, RX, or MRX complex. A representative experiment is shown. B, quantification of three independent experiments with S.E. The reaction without Rif2 (lane 2) was set to 100. C, purified Rad50 subunit was incubated with either GST-tagged Mre11, Rif2-WT, Rif2-F8A, Rif2 (1–170), or Rif2 (1–60). The beads were washed and bound Rad50 was analyzed by 7% SDS-PAGE, followed by Western blotting with anti-Rad50 antibody, exactly as described (36). GST (lanes 2 and 4) was used as a negative control.
The N-terminal region of Rif2 is essential for its regulatory role
To gain a better understanding of how Rif2 attenuates the MRX-dependent activation of Tel1, we sought to identify the region of Rif2 responsible for its inhibitory role. The extreme N-terminal region of Rif2 has been implicated in its telomere length regulatory function in vivo (23, 37). This region is predicted to be largely unstructured and lies outside of the Orc4-homology domain (37). Specifically, the Rif2-F8A mutation completely abrogated Rif2's functionality in yeast (23). This point mutant confers a similar telomere phenotype to that of a rif2D mutant. We tested the F8A variant of Rif2 in our in vitro system. Rif2-F8A showed no inhibition of the MRX-dependent activation of Tel1 (Fig. 3, A and B). We carried out several control experiments to test the integrity of the F8A mutant. First, Rif2 shows a weak DNA-binding activity; this activity is unaltered in the mutant (Fig. S3C). Second, the interaction between Rad50 and Rif2 is not affected by the F8A mutation (Fig. 2C, lane 5). Furthermore, both the Rad50-Rif2 and Rad50-Rif2F8A interactions are unaffected by the presence of Mg-ATP in the assay (Fig. S2A). Therefore, the interaction between Rif2 and Rad50 alone is not sufficient to mediate inhibition.
Figure 3.
The N-terminal region of Rif2 is important for its inhibitory role. A, standard kinase reaction with increasing concentrations of either Rif2-WT or Rif2-F8A. A representative experiment is shown. B, quantification of three independent experiments with S.E. Relative Tel1 activity of the reaction without Rif2 was set to 100 (lane 2). C, standard kinase assays contained 2 μm indicated Rif2 or GST-Rif2 variants. D, standard kinase assays were carried out without DNA and Rif2 as indicated. Reactions were incubated for 30 min. Bottom, averages and S.E. are derived from three independent experiments. The reaction without Rif2 (lane 1) was set to 100.
We next queried whether the entire Rif2 protein is required for inhibition. In yeast, the artificial tethering of Rif2 (1–170) to telomeres via Tet-operator sites restored the regulatory function to a rif2Δ mutant (22). In an independent study, fusion of Rif2 (1–60) to Rap1, which ensures telomere localization by artificial means, similarly restored telomere length control to the rif2Δ mutant (23). In our in vitro assay, the GST-Rif2 (1–170) truncation showed comparable inhibition to full-length GST-Rif2 (Fig. 3C, lanes 4 and 5), whereas the GST-Rif2 (1–60) truncation showed low but detectable inhibition (50%, lane 6). The weaker inhibitory activity of Rif2 (1–60) compared with Rif2 (1–170) and full-length Rif2 is consistent with the decreased interaction between Rif2 (1–60) with Rad50 (Fig. 2C, compare lane 8 with lanes 6 and 7). Parenthetically, the extreme N-terminal location of the essential Phe (at position 8 for most Saccharomyces species, Table S1) might suggest that its mode of action involves insertion of the entire N-terminal peptide into a hydrophobic cavity of Rad50. However, in control experiments, we show that the presence of the N-terminal GST-domain did not interfere with the function of Rif2 (Fig. 3C and Fig. S3, A and B).
Finally, we tested whether the inhibitory function of Rif2 requires both MRX and DNA, even though the DNA-stimulated Tel1 kinase activity is not inhibited by Rif2 (Fig. S1C). One proposed model for the regulatory role of Rif2 is that Rif2 changes the DNA end-tethering properties of MRX (34). This model can be tested because MRX stimulates the kinase activity of Tel1 2- to 3-fold in the absence of DNA (Fig. 1B) (36). Rif2 or Rif2-F8A was added to standard Tel1 kinase reactions containing MRX but no DNA. Rif2 inhibited the MRX-dependent stimulation of Tel1 whereas the mutant was ineffective (Fig. 3D). Interestingly, the Tel1-catalyzed phosphorylation of Mre11 and Xrs2 is not affected by Rif2. This result is consistent with our previous observation that the phosphorylation of these two subunits by Tel1 is largely constitutive (36).
Rif2, but not Rif2-F8A, stimulates the ATPase activity of MRX
It is important to make a distinction between ATP binding by Rad50 and hydrolysis of the bound ATP (ATPase activity). Several studies have shown that mutants that are deficient for ATP binding are phenotypically similar to rad50Δ in checkpoint activation and DNA repair (17, 36). However, ATP binding and not its hydrolysis drives the activation of Tel1ATM kinase in the complex with DNA and MRX(N) (42, 43). This raises the possibility that Rif2 acts by modulating the ATPase activity of MRX. In fact, in one study, Rif2 was shown to stimulate the ATPase activity of MRX by about 2-fold (34). Our own assessment of the effect of Rif2 on the MRX ATPase activity recapitulates this observation and extends it. Rif2 stimulated the ATPase activity of MRX either in the absence (Fig. S4B) or the presence of DNA (Fig. 4A). In contrast, Rif2-F8A showed no detectable stimulation of the MRX ATPase (Fig. 4A and Fig. S4B). Considering that none of the other activities of Rif2 is altered in the F8A mutant (DNA binding, interaction with Rad50), the most straightforward conclusion from these data is that the increase in the ATPase of MRX is associated with loss of Tel1-specific MRX activity.
Figure 4.
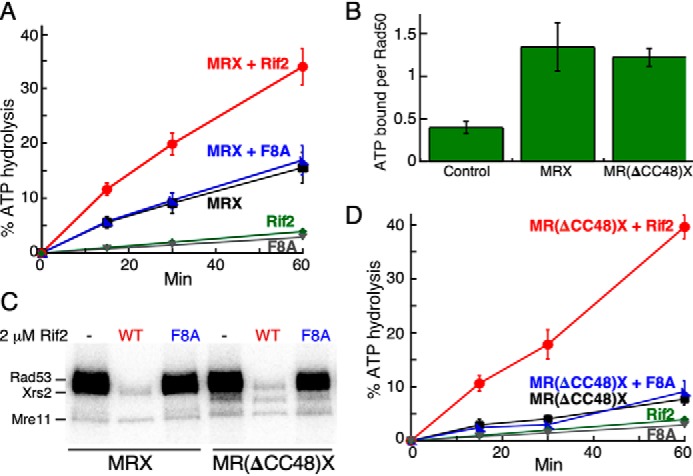
Rif2 stimulates the ATPase activity of MRX. A, standard ATPase assays with DNA and WT MRX contained either no Rif2 (black), 2 μm Rif2 (red), or Rif2-F8A (blue). Negative controls contained 2 μm Rif2 (green) or Rif2-F8A (gray), but no MRX. B, nitrocellulose filter binding assays of [γ-32P]ATP to WT or mutant MRX, as described (36). Control, no MRX. Quantification of three independent experiments with S.E. is shown. C, Tel1 kinase assays with 30 nm MRX or M(Rad50ΔCC-48)X and 2 mm Rif2 or Rif2-F8A as indicated. D, ATPase reactions as in A, but with M(Rad50ΔCC-48)X. The green Rif2 and gray Rif2-F8A curves, minus MRX, are from the same data set as shown in A.
To understand how Rif2 interferes with the MRX-mediated activation of Tel1 through the Rad50 ATPase, we used an allosteric ATPase mutant of Rad50. Rad50(ΔCC48) has two point mutations (S685R, Y688R) and lacks an additional 104 amino acids of the coiled-coiled domain near the zinc hook motif (Fig. 1B) (33, 44–46). The rad50-ΔCC48 mutant is defective for Tel1-mediated checkpoint signaling in response to DNA double-stranded breaks (33). In addition, the mutant has shortened telomeres. In biochemical studies, the mutant MR(ΔCC48)X complex lacks ATPase activity (33). We surmise that the effects of these mutations are allosteric in nature because they are distal from the conserved ATP-binding domains (Fig. 1B), and indeed, the mutant MRX complex still binds ATP like WT (Fig. 4B). Consequently, the observation that this mutant complex also activates Tel1 like WT was as expected (Fig. 4C, lanes 1 and 4). However, because the mutant showed little or no basal ATPase activity, the observed inhibition by Rif2 was surprising (Fig. 4C, lanes 2 and 5; Fig. S4A). Remarkably, we found that Rif2, but not Rif2-F8A, caused a potent stimulation of the MR(ΔCC48)X ATPase activity (Fig. 4D). As observed with WT MRX, the Rif2-stimulated ATPase activity of MR(ΔCC48)X does not require the presence of effector DNA (Fig. S4, B and C).
A new model for Rif2-mediated regulation of Tel1 kinase
The Rad50(ΔCC48) mutant has given us a unique window into observing the regulatory function of Rif2. The ATPase activity of WT MRX is stimulated about 2-fold by either DNA or Rif2, and when both are present, the activity is additive (Fig. 4A and S4D). In contrast, the mutant MRX complex with or without DNA shows limited ATPase activity (Fig. S4E). Yet, with or without DNA, Rif2 causes a robust stimulation of its ATPase (Fig. 4D and Fig. S4C). Under any of these conditions, mutant Rif2-F8A does not affect the MRX ATPase. These data suggest that the conformational/structural changes that occur in MRX when the bound ATP hydrolyzes are principally different whether DNA or Rif2 is the effector molecule.
One straightforward explanation for these observations that we considered is that the physical association of MRX with Tel1 is disrupted by Rif2, but not by Rif2-F8A. However, no disruption of binding was observed (Fig. S2B), and perhaps that result was not surprising, because Tel1 has been shown to interact with each of the three MRX subunits (36). Given the difference between Rif2 and Rif2-F8A, we propose that inhibition is a two-step process. The first step involves binding of Rif2 to Rad50, which does not require the critical Phe (Fig. 2C). In the second step, the Phe-8 region of Rif2 likely alters the ATP-binding pocket of Rad50, stimulating its hydrolysis, and possibly inducing a conformational state of Rad50 that is competent for binding Tel1, but not productive for kinase stimulation. This proposal is supported by the observation that several different structures of MRX can exist, depending on DNA- and/or ATP-bound states (47–49).
Experimental procedures
DNA substrates
The DNA substrates used were a linearized 2 kb DNA (by BamHI), derived from plasmid pUC19, a 147-mer natural DNA sequence, derived from the Widom 601 sequence (50). The 155-mer yeast telomeric repeat sequence was derived from the left arm of chromosome 4. The TG(1–3) repeats account for 155 bp. Terminal AflII restriction sites were used for cloning in pUC19 and subsequent cleavage.
Protein expression and purification
Tel1, MRX, and MRX subcomplexes and Rad53-kd were purified as described (36). The MR(ΔCC48)X mutant complex was expressed in Sf9 cells, and purified on Xrs2-FLAG-affinity beads.4 Rap full-length and Rap1 (1–622) were purified as described (51).
The Rif2 WT gene was cloned in pGEX-6p-1 vector at EcoRI/XhoI restriction sites and overexpressed in Rosetta2(DE3)-pLysS as described for Rap1 (51). GST-Rif2 overexpression cells were lysed by sonication in lysis buffer (50 mm sodium phosphate buffer, pH 7.3, 400 mm sodium chloride, 10% glycerol, 1 mm EDTA, 1 mm DTT, and 0.1 mm PMSF). Cells debris was spun down, and 0.2% polyethyleneimine was added to the supernatant. After centrifugation the supernatant was incubated overnight with GSH-Sepharose 4 Fast Flow resin (GE Healthcare). The resin was then extensively washed with lysis buffer and lysis buffer containing 1 m sodium chloride. GST-Rif2 was finally eluted with 25 mm reduced GSH in lysis buffer and dialyzed overnight against heparin buffer (25 mm Tris buffer, pH 7.4, 150 mm NaCl, 10% glycerol, 1 mm EDTA, and 1 mm DTT). The dialyzed protein was loaded on a Poros 50 HE Heparin column (Life Technologies, Inc.) and eluted with heparin buffer containing 300 mm NaCl. Rif2 WT was purified using the same protocol, except that PreScission Protease was added during dialysis against heparin buffer. Rif2F8A and Rif2F8S were generated by standard site-specific mutagenesis using the Rif2 gene cloned in the pGEX6p-1 vector as template. Rif2F8A and GST-Rif2F8A, Rif2F8S and GST-Rif2F8S were expressed and purified using the procedure reported above for the Rif2 WT constructs. The Rif 2 truncation construct (1–170 and 1–60) were expressed and purified similarly, except that the GST tag was kept on the purified truncated proteins. All Rif2 proteins were dialyzed against storage buffer (20 mm HEPES, pH 7.4, 400 mm NaCl, 40% (v/v) glycerol, 0.5 mm EDTA, and 1 mm DTT) and stored at −80 °C.
Tel1 kinase assay
The standard 10 μl assay contained 25 mm HEPES-NaOH pH 7.6, 80 mm NaCl, 7 mm Mg-acetate, 100 μg/ml BSA, 1 mm DTT, 50 μm ATP, 0.05 μCi [γ-32P]ATP, 200 nm GST-Rad53-kd, 30 nm MRX, and 10 nm Telo DNA. Reactions were initiated with 5 nm Tel1 at 30 °C for 15 min, stopped with 4 μl of 2.5× SDS-PAGE loading dye, boiled, and separated on 7% SDS-PAGE gels. Gels were dried and exposed to a phosphor screen (GE Healthcare). Deviations from the standard assay are indicated in the legends to figures. Data were analyzed with ImageQuant software and plotted using KaleidaGraph software.
ATPase assay
Standard 20 μl ATPase reactions contained 50 μm [α-32P]ATP, 100 nm MRX, and 10 nm linearized pUC19 DNA in buffer containing 25 mm HEPES-NaOH, pH 7.6, 80 mm NaCl, 7 mm Mg-acetate, and 1 mm DTT. At indicated times at 30 °C, 5 ml aliquots were quenched in 1.5 μl of 150 mm EDTA/1% SDS. Radioactive ATP and ADP were separated by PEI-Cellulose TLC and quantified as described in Ref. 36.
GST-pulldown and ATP-binding experiments
Pulldown experiments in Fig. 2C and Fig. S2, and ATP binding in Fig. 4B were performed exactly as described in Ref. 36.
Author contributions
S. H., P. D. B., R. G., J. H. P., and P. M. B. conceptualization; S. H., P. D. B., R. G., M. H., J. H. P., and P. M. B. resources; S. H., P. D. B., R. G., J. H. P., and P. M. B. data curation; S. H., R. G., J. H. P., and P. M. B. formal analysis; S. H., R. G., J. H. P., and P. M. B. validation; S. H., P. D. B., R. G., M. H., J. H. P., and P. M. B. investigation; S. H., R. G., J. H. P., and P. M. B. visualization; S. H., P. D. B., R. G., J. H. P., and P. M. B. methodology; S. H., R. G., J. H. P., and P. M. B. writing-original draft; S. H., P. D. B., R. G., M. H., J. H. P., and P. M. B. writing-review and editing; R. G., M. H., J. H. P., and P. M. B. supervision; R. G., M. H., J. H. P., and P. M. B. funding acquisition; R. G., J. H. P., and P. M. B. project administration.
Supplementary Material
This work was supported in part by the National Institutes of Health Grants GM118129 (to P. M. B.); GM098509 (to R. G.); and GM59413, P30-CA008748, and U54-OD020355 (to J. H. P.) and by an NSF Graduate Research Fellowship 2014157291 (to S. H.). J. H. P. is a consultant for Novus Biologicals, and a scientific advisor for Atropos Therapeutics. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S4 and Table S1.
M. Hohl and J.H. Petrini, submitted for publication.
- DSBs
- dsDNA breaks
- ATM
- ataxia telangiectasia-mutated
- MRX
- Mre11-Rad50-Xrs2NBS1 complex
- Nat
- random DNA
- Telo
- telomeric repeat DNA.
References
- 1. Wellinger R. J., and Zakian V. A. (2012) Everything you ever wanted to know about Saccharomyces cerevisiae telomeres: Beginning to end. Genetics 191, 1073–1105 10.1534/genetics.111.137851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gomes N. M., Shay J. W., and Wright W. E. (2010) Telomere biology in Metazoa. FEBS Lett. 584, 3741–3751 10.1016/j.febslet.2010.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greider C. W., and Blackburn E. H. (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43, 405–413 10.1016/0092-8674(85)90170-9 [DOI] [PubMed] [Google Scholar]
- 4. d'Adda di Fagagna F., Reaper P. M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N. P., and Jackson S. P. (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 10.1038/nature02118 [DOI] [PubMed] [Google Scholar]
- 5. Armanios M., and Blackburn E. H. (2012) The telomere syndromes. Nat. Rev. Genet. 13, 693–704 10.1038/nrg3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khincha P. P., and Savage S. A. (2013) Genomic characterization of the inherited bone marrow failure syndromes. Semin. Hematol. 50, 333–347 10.1053/j.seminhematol.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., and Shay J. W. (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 10.1126/science.7605428 [DOI] [PubMed] [Google Scholar]
- 8. Palm W., and de Lange T. (2008) How shelterin protects mammalian telomeres. Annu. Rev. Genet. 42, 301–334 10.1146/annurev.genet.41.110306.130350 [DOI] [PubMed] [Google Scholar]
- 9. Bianchi A., and Shore D. (2008) How telomerase reaches its end: Mechanism of telomerase regulation by the telomeric complex. Mol. Cell 31, 153–165 10.1016/j.molcel.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 10. Smogorzewska A., and de Lange T. (2004) Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 73, 177–208 10.1146/annurev.biochem.73.071403.160049 [DOI] [PubMed] [Google Scholar]
- 11. Longtine M. S., Wilson N. M., Petracek M. E., and Berman J. (1989) A yeast telomere binding activity binds to two related telomere sequence motifs and is indistinguishable from RAP1. Curr. Genet. 16, 225–239 10.1007/BF00422108 [DOI] [PubMed] [Google Scholar]
- 12. Hardy C. F., Sussel L., and Shore D. (1992) A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 6, 801–814 10.1101/gad.6.5.801 [DOI] [PubMed] [Google Scholar]
- 13. Wotton D., and Shore D. (1997) A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 11, 748–760 10.1101/gad.11.6.748 [DOI] [PubMed] [Google Scholar]
- 14. Grandin N., Reed S. I., and Charbonneau M. (1997) Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes Dev. 11, 512–527 10.1101/gad.11.4.512 [DOI] [PubMed] [Google Scholar]
- 15. Gao H., Cervantes R. B., Mandell E. K., Otero J. H., and Lundblad V. (2007) RPA-like proteins mediate yeast telomere function. Nat. Struct. Mol. Biol. 14, 208–214 10.1038/nsmb1205 [DOI] [PubMed] [Google Scholar]
- 16. Ritchie K. B., and Petes T. D. (2000) The Mre11p/Rad50p/Xrs2p complex and the Tel1p function in a single pathway for telomere maintenance in yeast. Genetics 155, 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen L., Trujillo K. M., Van Komen S., Roh D. H., Krejci L., Lewis L. K., Resnick M. A., Sung P., and Tomkinson A. E. (2005) Effect of amino acid substitutions in the rad50 ATP binding domain on DNA double strand break repair in yeast. J. Biol. Chem. 280, 2620–2627 10.1074/jbc.M410192200 [DOI] [PubMed] [Google Scholar]
- 18. Gilson E., Roberge M., Giraldo R., Rhodes D., and Gasser S. M. (1993) Distortion of the DNA double helix by RAP1 at silencers and multiple telomeric binding sites. J. Mol. Biol. 231, 293–310 10.1006/jmbi.1993.1283 [DOI] [PubMed] [Google Scholar]
- 19. Williams T. L., Levy D. L., Maki-Yonekura S., Yonekura K., and Blackburn E. H. (2010) Characterization of the yeast telomere nucleoprotein core: Rap1 binds independently to each recognition site. J. Biol. Chem. 285, 35814–35824 10.1074/jbc.M110.170167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marcand S., Gilson E., and Shore D. (1997) A protein-counting mechanism for telomere length regulation in yeast. Science 275, 986–990 10.1126/science.275.5302.986 [DOI] [PubMed] [Google Scholar]
- 21. Levy D. L., and Blackburn E. H. (2004) Counting of Rif1p and Rif2p on Saccharomyces cerevisiae telomeres regulates telomere length. Mol. Cell. Biol. 24, 10857–10867 10.1128/MCB.24.24.10857-10867.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirano Y., Fukunaga K., and Sugimoto K. (2009) Rif1 and rif2 inhibit localization of tel1 to DNA ends. Mol. Cell 33, 312–322 10.1016/j.molcel.2008.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaizer H., Connelly C. J., Bettridge K., Viggiani C., and Greider C. W. (2015) Regulation of telomere length requires a conserved N-terminal domain of Rif2 in Saccharomyces cerevisiae. Genetics 201, 573–586 10.1534/genetics.115.177899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mallory J. C., and Petes T. D. (2000) Protein kinase activity of Tel1p and Mec1p, two Saccharomyces cerevisiae proteins related to the human ATM protein kinase. Proc. Natl. Acad. Sci. U.S.A. 97, 13749–13754 10.1073/pnas.250475697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakada D., Matsumoto K., and Sugimoto K. (2003) ATM-related Tel1 associates with double-strand breaks through an Xrs2-dependent mechanism. Genes Dev. 17, 1957–1962 10.1101/gad.1099003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paull T. T. (2018) 20 years of Mre11 biology: No end in sight. Mol. Cell 71, 419–427 10.1016/j.molcel.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 27. Oh J., and Symington L. S. (2018) Role of the Mre11 complex in preserving genome integrity. Genes (Basel) 9, E589 10.3390/genes9120589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hector R. E., Shtofman R. L., Ray A., Chen B. R., Nyun T., Berkner K. L., and Runge K. W. (2007) Tel1p preferentially associates with short telomeres to stimulate their elongation. Mol. Cell 27, 851–858 10.1016/j.molcel.2007.08.007 [DOI] [PubMed] [Google Scholar]
- 29. Chang M., Arneric M., and Lingner J. (2007) Telomerase repeat addition processivity is increased at critically short telomeres in a Tel1-dependent manner in Saccharomyces cerevisiae. Genes Dev. 21, 2485–2494 10.1101/gad.1588807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sabourin M., Tuzon C. T., and Zakian V. A. (2007) Telomerase and Tel1p preferentially associate with short telomeres in S. cerevisiae. Mol. Cell 27, 550–561 10.1016/j.molcel.2007.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viscardi V., Bonetti D., Cartagena-Lirola H., Lucchini G., and Longhese M. P. (2007) MRX-dependent DNA damage response to short telomeres. Mol. Biol. Cell 18, 3047–3058 10.1091/mbc.e07-03-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greenwell P. W., Kronmal S. L., Porter S. E., Gassenhuber J., Obermaier B., and Petes T. D. (1995) TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell 82, 823–829 10.1016/0092-8674(95)90479-4 [DOI] [PubMed] [Google Scholar]
- 33. Park Y. B., Hohl M., Padjasek M., Jeong E., Jin K. S., Krezel A., Petrini J. H., and Cho Y. (2017) Eukaryotic Rad50 functions as a rod-shaped dimer. Nat. Struct. Mol. Biol. 24, 248–257 10.1038/nsmb.3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cassani C., Gobbini E., Wang W., Niu H., Clerici M., Sung P., and Longhese M. P. (2016) Tel1 and Rif2 regulate MRX functions in end-tethering and repair of DNA double-strand breaks. PLoS Biol. 14, e1002387 10.1371/journal.pbio.1002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Usui T., Ogawa H., and Petrini J. H. (2001) A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol. Cell 7, 1255–1266 10.1016/S1097-2765(01)00270-2 [DOI] [PubMed] [Google Scholar]
- 36. Hailemariam S., Kumar S., and Burgers P. M. (2019) Activation of Tel1(ATM) kinase requires Rad50 ATPase and long nucleosome-free DNA but no DNA ends. J. Biol. Chem. 294, 10120–10130 10.1074/jbc.RA119.008410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi T., Bunker R. D., Mattarocci S., Ribeyre C., Faty M., Gut H., Scrima A., Rass U., Rubin S. M., Shore D., and Thoma N. H. (2013) Rif1 and Rif2 shape telomere function and architecture through multivalent Rap1 interactions. Cell 153, 1340–1353 10.1016/j.cell.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 38. Difilippantonio S., Celeste A., Kruhlak M. J., Lee Y., Difilippantonio M. J., Feigenbaum L., Jackson S. P., McKinnon P. J., and Nussenzweig A. (2007) Distinct domains in Nbs1 regulate irradiation-induced checkpoints and apoptosis. J. Exp. Med. 204, 1003–1011 10.1084/jem.20070319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. You Z., Chahwan C., Bailis J., Hunter T., and Russell P. (2005) ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol. Cell. Biol. 25, 5363–5379 10.1128/MCB.25.13.5363-5379.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stracker T. H., Morales M., Couto S. S., Hussein H., and Petrini J. H. (2007) The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex. Nature 447, 218–221 10.1038/nature05740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Usui T., Ohta T., Oshiumi H., Tomizawa J., Ogawa H., and Ogawa T. (1998) Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell 95, 705–716 10.1016/S0092-8674(00)81640-2 [DOI] [PubMed] [Google Scholar]
- 42. Cassani C., Vertemara J., Bassani M., Marsella A., Tisi R., Zampella G., and Longhese M. P. (2019) The ATP-bound conformation of the Mre11-Rad50 complex is essential for Tel1/ATM activation. Nucleic Acids Res. 47, 3550–3567 10.1093/nar/gkz038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deshpande R. A., Lee J. H., and Paull T. T. (2017) Rad50 ATPase activity is regulated by DNA ends and requires coordination of both active sites. Nucleic Acids Res. 45, 5255–5268 10.1093/nar/gkx173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hohl M., Kochańczyk T., Tous C., Aguilera A., Krel A., and Petrini J. H. (2015) Interdependence of the rad50 hook and globular domain functions. Mol. Cell 57, 479–491 10.1016/j.molcel.2014.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hohl M., Kwon Y., Galvan S. M., Xue X., Tous C., Aguilera A., Sung P., and Petrini J. H. (2011) The Rad50 coiled-coil domain is indispensable for Mre11 complex functions. Nat. Struct. Mol. Biol. 18, 1124–1131 10.1038/nsmb.2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiltzius J. J., Hohl M., Fleming J. C., and Petrini J. H. (2005) The Rad50 hook domain is a critical determinant of Mre11 complex functions. Nat. Struct. Mol. Biol. 12, 403–407 10.1038/nsmb928 [DOI] [PubMed] [Google Scholar]
- 47. Lim H. S., Kim J. S., Park Y. B., Gwon G. H., and Cho Y. (2011) Crystal structure of the Mre11-Rad50-ATPγS complex: Understanding the interplay between Mre11 and Rad50. Genes Dev. 25, 1091–1104 10.1101/gad.2037811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu Y., Sung S., Kim Y., Li F., Gwon G., Jo A., Kim A. K., Kim T., Song O. K., Lee S. E., and Cho Y. (2016) ATP-dependent DNA binding, unwinding, and resection by the Mre11/Rad50 complex. EMBO J. 35, 743–758 10.15252/embj.201592462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Möckel C., Lammens K., Schele A., and Hopfner K. P. (2012) ATP driven structural changes of the bacterial Mre11:Rad50 catalytic head complex. Nucleic Acids Res. 40, 914–927 10.1093/nar/gkr749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lowary P. T., and Widom J. (1998) New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 276, 19–42 10.1006/jmbi.1997.1494 [DOI] [PubMed] [Google Scholar]
- 51. Feldmann E. A., and Galletto R. (2014) The DNA-binding domain of yeast Rap1 interacts with double-stranded DNA in multiple binding modes. Biochemistry 53, 7471–7483 10.1021/bi501049b [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.