Key Points
Question
Are changes in circulating progenitor cells during exercise associated with future cardiovascular events in individuals with coronary artery disease?
Findings
In this cohort study of 454 patients with coronary artery disease, for every 50% reduction in circulating progenitor cell counts during exercise stress, the adverse event risk more than doubled. Stress-induced ischemia was no longer associated with outcomes after stress-induced changes in circulating progenitor cell counts were included in the analysis.
Meaning
Among patients with coronary artery disease, a decrease in circulating progenitor cell counts during exercise is associated with a worse prognosis and is a stronger factor in outcomes than the presence of stress-induced myocardial ischemia.
Abstract
Importance
Stem and progenitor cells mobilize from the bone marrow in response to myocardial ischemia. However, the association between the change in circulating progenitor cell (CPC) counts and disease prognosis among patients with ischemia is unknown.
Objective
To investigate the association between the change in CPC counts during stress testing and the risk of adverse cardiovascular events in patients with stable coronary artery disease (CAD).
Design, Setting, and Participants
This prospective cohort study included a population-based sample of 454 patients with stable CAD who were recruited between June 1, 2011, and August 15, 2014, at Emory University-affiliated hospitals and followed up for 3 years. Data were analyzed from September 15, 2018, to October 15, 2018.
Exposures
Myocardial perfusion imaging with technetium Tc 99m sestamibi at rest and 30 to 60 minutes after conventional stress testing.
Main Outcomes and Measures
Circulating progenitor cells were enumerated with flow cytometry as CD34-expressing mononuclear cells (CD45med/CD34+), with additional quantification of subsets coexpressing the chemokine (C-X-C motif) receptor 4 (CD34+/CXCR4+). Changes in CPC counts were calculated as poststress minus resting CPC counts. Cox proportional hazards regression models were used to identify factors associated with the combined end point of cardiovascular death and myocardial infarction after adjusting for clinical covariates, including age, sex, race, smoking history, body mass index, and history of heart failure, hypertension, dyslipidemia, and diabetes.
Results
Of the 454 patients (mean [SD] age, 63 [9] years; 76% men) with stable CAD enrolled in the study, 142 (31.3%) had stress-induced ischemia and 312 (68.7%) did not, as measured by single-photon emission computed tomography. During stress testing, patients with stress-induced ischemia had a mean decrease of 20.2% (interquartile range [IQR], −45.3 to 5.5; P < .001) in their CD34+/CXCR4+ counts, and patients without stress-induced ischemia had a mean increase of 3.2% (IQR, −20.6 to 35.1; P < .001) in their CD34+/CXCR4+ counts. Twenty-four patients (5.2%) experienced adverse events. After adjustment, baseline CPC counts were associated with worse adverse outcomes, but this association was not present after stress-induced ischemia was included in the model. However, the change in CPC counts during exercise remained significantly associated with adverse events (hazard ratio, 2.59; 95% CI, 1.15-5.32, per 50% CD34+/CXCR4+ count decrease), even after adjustment for clinical variables and the presence of ischemia. The discrimination of risk factors associated with incident adverse events improved (increase in C statistic from 0.72 to 0.77; P = .003) with the addition of the change in CD34+/CXCR4+ counts to a model that included clinical characteristics, baseline CPC count, and ischemia.
Conclusions and Relevance
In this study of patients with CAD, a decrease in CPC counts during exercise is associated with a worse disease prognosis compared with the presence of stress-induced myocardial ischemia. Further studies are needed to evaluate whether strategies to improve CPC responses during exercise stress will be associated with improvements in the prognosis of patients with CAD.
This cohort study of 454 patients with stable coronary artery disease investigates the association between the change in circulating progenitor cell counts during exercise and the risk of adverse cardiovascular events among patients with and without stress-induced myocardial ischemia.
Introduction
Coronary artery disease (CAD) remains the predominant cause of mortality worldwide, and its prevalence continues to increase.1 There is a need for comprehensive risk stratification in individuals with CAD to identify those at risk of adverse outcomes. Documentation of stress-induced myocardial ischemia, often by single-photon emission computed tomography (SPECT) myocardial perfusion imaging or echocardiography, has become the primary method used to stratify risk among patients with CAD.2,3,4 However, the imaging associated with stress testing is expensive, and SPECT has been reported to expose participants to substantial levels of radiation.5,6 It remains unknown whether alterations in biomarkers associated with ischemia can be used as surrogates for the traditional assessment of ischemia.
There is now extensive evidence to suggest that circulating progenitor cells (CPCs) and resident stem cells are associated with repair and regeneration after vascular and myocardial injury. Circulating progenitor cells can be characterized as CD34-expressing mononuclear cells that originate primarily from the bone marrow and can be quantified using flow cytometry.7,8,9,10 Mononuclear cells expressing both CD34+ and chemokine (C-X-C motif) receptor 4 (CXCR4+) permit homing of progenitor cells to stromal cell–derived factor-1 α (SDF-1α)-enriched hypoxic environments and further characterize CPCs with the capacity for homing and tissue repair11; CD34+ cells have greater myocardial reparative potential than unselected populations, and reduced numbers of CPCs are associated with an increase in incident adverse cardiovascular outcomes.12,13,14,15,16
A previous study has reported that patients with CAD who develop myocardial ischemia during physical exercise experience a decrease in CD34+/CXCR4+ counts and an increase in circulating SDF-1α levels, changes not observed in patients without ischemia.17 Moreover, the magnitude of ischemia was associated with a proportionately greater decrease in CPC counts.17 Whether changes in CPC counts during exercise are associated with adverse cardiovascular events is unknown and was the subject of our investigation. We measured changes in CPC counts among patients with CAD undergoing exercise stress testing with SPECT perfusion imaging and explored the association between these changes and incident cardiovascular events. Our hypothesis was that the change in CPC counts during exercise would be associated with adverse outcomes in patients with CAD.
Methods
Study Population
A total of 454 patients with stable CAD who had previously participated in the Mental Stress Ischemia Prognosis Study were enrolled in this prospective cohort study; participants were recruited between June 1, 2011, and August 215, 2014, at Emory University-affiliated hospitals and followed up for 3 years.18 Data were analyzed from September 15, 2018, to October 15, 2018. The research protocol was approved by the institutional review board of the Emory University School of Medicine institutional review board, and all participants provided informed consent.
The presence of CAD was identified based on either the presence of angiographic atherosclerosis, a history of myocardial infarction (MI) or revascularization, or a positive nuclear stress test result. Patients were excluded if they had a recent (less than 2 months) history of acute coronary syndrome or decompensated heart failure, end-stage renal disease, systolic blood pressure greater than 180 mm Hg or diastolic blood pressure greater than 110 mm Hg on the day of the test, or unstable psychiatric conditions. Clinical information, including previous CAD-related events, risk factors for CAD, coronary angiography results, and current medications, was documented using standardized questionnaires and medical record reviews.
Stress Testing and SPECT Imaging
Antianginal medications, including beta blockers, calcium channel blockers, and nitrates, were not administered to patients for at least 12 hours before the stress testing. Patients were tested in the morning after completing a 12-hour fast. All patients underwent treadmill exercise stress testing according to the Bruce protocol. The SPECT myocardial perfusion imaging was performed with technetium Tc 99m sestamibi, both at rest and 30 to 60 minutes after conventional stress testing. Stress-rest SPECT results were independently evaluated and interpreted by 2 experienced readers who were blinded to the patients’ medical histories.
The number and severity of perfusion defects were visually compared between the rest and stress images using a 17-segment model. Each segment was scored from 0 to 4, with 0 indicating no defect and 4 indicating severe defect. Ischemia was defined as a new impairment with a score of 2 or greater in any segment or as the worsening of a preexisting impairment by at least 2 points if in a single segment or by at least 1 point if in 2 or more contiguous segments.19 The magnitude of the ischemic myocardium was calculated as the summed difference score divided by 68, then multiplied by 100.20
CPC Measurements
We measured CPC counts using flow cytometry at rest and 45 minutes after stress testing.12,21 After patients completed an overnight fast, venous blood samples were collected in tubes containing EDTA and processed within 4 hours.14 Mononuclear cells enriched for CPCs were enumerated using flow cytometry as CD45med cells coexpressing CD34, CD133, and/or CXCR4 epitopes. A total of 300 μL of peripheral blood was incubated with 7 μL of fluorochrome-labeled monoclonal PE mouse antihuman CD181 antibody (BD Biosciences), 15 μL of PerCP antihuman CD45 antibody (BD Biosciences), 3 μL of PE/Cy7–conjugated antihuman CD184 (CXCR4) antibody (EBioscience), and 10 μL of human antibody CD133-APC (Miltenyi Biotec) in the dark for 15 minutes. To lyse red blood cells, 1.2 mL of ammonium chloride lysing buffer was added. Subsequently, 1.2 mL of staining medium (phosphate-buffered saline with 3% heat-inactivated serum and 0.1% sodium azide) was added to stop the lysing reaction.
After mixing, samples were centrifuged at 1500 rpm for 5 minutes, then washed with phosphate-buffered saline. Cells were then suspended in 500 μL of staining medium and mixed and run on a BD FACSCanto II flow cytometer (BD Biosciences) within 4 hours. Before flow cytometry was performed, 100 μL of Invitrogen AccuCheck counting beads (ThermoFisher Scientific) was added to act as an internal standard for direct estimation of the concentration of target cell subsets. At least 2.5 million events were acquired from the cytometer. Flow data were analyzed using Flowjo software, version 8 (BD).
The absolute mononuclear cell count was estimated as the sum of lymphocytes and monocytes using a Coulter AcT diff cell counter (Beckman Coulter). The selection of CD45med cells excluded CD45bright and CD45− cells. Exclusion of the rare CD45bright cells helped to eliminate lymphoblasts, and exclusion of CD45− cells helped to eliminate nonhematopoietic progenitors, such as mesenchymal or osteoprogenitor cells, as these cells are typically CD45−. In 20 samples that were repeatedly analyzed on 2 occasions by the same technician, the coefficients of variation of the cell types were 2.9% for CD34+ and 6.5% for CD34+/CXCR4+.
Follow-up and Outcomes
Follow-up was conducted to evaluate the primary end point of cardiovascular death and MI. Personnel who were blinded to the study data ascertained adverse events for all participants by performing follow-up clinic visits at 1 and 2 years, phone calls at 3 years, medical record reviews, and queries of the Social Security Death Index. Cause of death was ascertained by 2 cardiologists (A.S. and A.A.Q.), with a third arbitrator (V.V.) in case of disagreement. Cardiovascular death was defined as death associated with cardiovascular ischemia, including fatal MI, stroke, peripheral arterial disease, or sudden death associated with an unknown but presumed cardiovascular event in CAD patients. Medical records were accessed or requested to validate all self-reported MI events, which were defined using standard criteria.22 Follow-up data were not available for 3 patients (0.5%).
Statistical Analysis
Continuous variables are presented as mean (SD) or median (interquartile range [IQR]), and categorical variables are presented as proportions. Differences between groups were assessed using a t test for continuous variables and χ2 or Fisher exact test for categorical variables where appropriate. The Wilcoxon signed rank test was used to compare CPC counts and biomarker levels before and after stress testing. Spearman rank correlation coefficients were used to examine associations between CPC counts and continuous variables. For variables that are not normally distributed (eg, CPCs and biomarkers), the base-2 logarithm transformation was used to calculate percentage change during stress testing.
The association between CPC counts and adverse events (cardiovascular death and MI) was examined using Kaplan-Meier curves and Cox proportional hazards regression models. For Kaplan-Meier curves, patients were divided into 2 groups based on whether they had a decrease or an increase in their CPC counts after stress testing. Multivariable competing-risks Cox proportional hazard regression models were constructed to assess the association between stress-induced ischemia and change in CPC counts after stress testing and adverse events after adjusting for baseline demographics (age, sex, race, smoking history, and body mass index [calculated as weight in kilograms divided by height in meters squared]) and cardiovascular risk factors (history of heart failure, hypertension, dyslipidemia, and diabetes). Noncardiac death before the diagnosis of MI was considered as a competing event using the Fine and Gray method.23
We tested the incremental value of the change in CPC counts after stress testing to the identification of factors associated with adverse events by adding it to a model that included stress-induced ischemia, traditional risk factors, and baseline CPC counts. The Harrell C statistic (ie, area under the receiver operating characteristic curve), category-free net reclassification improvement, and integrated discrimination improvement were calculated as indices of risk discrimination.24 Significance testing was 2-sided with a significance threshold of P < .05, and all statistical analyses were performed using Stata software, version 14.0 (StataCorp).
Results
Of the 454 patients (mean [SD] age, 62 [9] years; 76% men) enrolled in the study, 142 (31.3%) had stress-induced ischemia and 312 (68.7%) did not, as measured by SPECT (Table 1). Patients with ischemia had higher rates of previous coronary bypass surgery, but no other significant differences in baseline characteristics, comorbidities, or medication use were noted between patients with and without myocardial ischemia (Table 1). As expected, participants with ischemia were more likely to report angina during exercise; however, no significant differences between the groups were observed with respect to heart rate, systolic blood pressure, or rate-pressure product at rest or during exercise (Table 1).
Table 1. Baseline Characteristics of Patients With and Without Stress-Induced Ischemia.
| Characteristic | No. (%) | P Valuea | ||
|---|---|---|---|---|
| Total Patients (N = 454) | Patients Without Ischemia (n = 312) | Patients With Ischemia (n = 142) | ||
| Demographics | ||||
| Age, mean (SD), y | 63 (9) | 63 (9) | 63 (9) | .48 |
| Men | 352 (77.5) | 238 (76.3) | 114 (80.3) | .34 |
| Black race | 114 (25.1) | 75 (24.0) | 39 (27.5) | .43 |
| Smoking history | 199 (43.8) | 141 (45.6) | 58 (40.8) | .60 |
| BMI, mean (SD) | 29.1 (5.1) | 29.2 (5.3) | 29.0 (5.1) | .81 |
| GFR, mean (SD) | 94.8 (31.4) | 94.4 (31.2) | 95.2 (32.4) | .71 |
| EF, mean (SD) | 53 (10) | 54 (10) | 52 (11) | .11 |
| Comorbidities | ||||
| History of hypertension | 332 (73.1) | 229 (73.4) | 103 (72.5) | .84 |
| History of diabetes | 124 (27.3) | 82 (26.3) | 42 (29.6) | .45 |
| CABG | 138 (30.4) | 84 (26.9) | 54 (38.0) | .02 |
| PCI | 251 (55.3) | 170 (54.5) | 81 (57.0) | .61 |
| MI | 169 (37.2) | 114 (36.5) | 55 (38.7) | .65 |
| History of heart failure | 66 (14.5) | 39 (12.5) | 27 (19.3) | .08 |
| Cerebrovascular disease | 28 (6.2) | 22 (7.1) | 6 (4.2) | .24 |
| Medications | ||||
| Aspirin | 406 (89.4) | 277 (88.8) | 129 (90.8) | .50 |
| ACEI/ARB | 208 (45.8) | 137 (43.9) | 71 (50.0) | .22 |
| β-Blocker | 317 (69.8) | 218 (69.9) | 99 (69.7) | .97 |
| Statin | 397 (87.4) | 267 (85.6) | 130 (91.5) | .10 |
| Stress test parameters | ||||
| Angina during stress test | 34 (8.2) | 12 (4.1) | 22 (17.6) | <.001 |
| Heart rate, mean (SD) | ||||
| At rest | 66.4 (12.2) | 65.7 (13.1) | 65.2 (10.7) | .71 |
| During exercise | 115 (14) | 116 (14) | 114 (12) | .09 |
| SBP, mean (SD) | ||||
| At rest | 135 (19) | 134 (18) | 133 (19) | .60 |
| During exercise | 161 (20) | 163 (19) | 160 (22) | .10 |
| RPP, mean (SD) | ||||
| At rest | 9051 (1921) | 9092 (1981) | 8901 (1911) | .77 |
| During exercise | 18 251 (4481) | 18 631 (4100) | 17 881 (4871) | .09 |
| Magnitude of ischemia | NA | NA | 7.1 (4.6) | |
| Hematocrit at rest, mean (SD) | 40.5 (4.4) | 40.3 (4.1) | 40.5 (4.2) | .60 |
| Hematocrit after exercise, mean (SD) | 41.3 (4.5) | 41.2 (4.2) | 41.7 (4.0) | .45 |
| CPC counts, median (IQR), cells/mL | ||||
| CD34+ | ||||
| At rest | 1642 (1034 to 2446) | 1647 (1054 to 2460) | 1590 (983 to 2430) | .81 |
| Absolute change from rest to stress | 10 (−378 to 362) | 53 (−274 to 523) | −172 (−619 to 266) | .003 |
| Percentage change from rest to stress | 0.6 (−21.0 to 27.3) | 6.1 (−12.3 to 40.6) | −10.2 (−28.1 to 5.5) | .006 |
| CD34+/CXCR4+ | ||||
| At rest | 654 (355 to 1051) | 650 (359 to 1049) | 659 (331 to 1032) | .76 |
| Change from rest to stress | −16 (−322 to 391) | 29 (−222 to 545) | −110 (−421 to 83) | .001 |
| Percentage change from rest to stress | −2.3 (−35.3 to 79.4) | 3.2 (−20.2 to 35.2) | −20.2 (−45.6 to 5.3) | <.001 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft; CPC, circulating progenitor cell; EF, ejection fraction; GFR, glomerular filtration rate; IQR, interquartile range; MI, myocardial infarction; NA, not applicable; PCI, percutaneous coronary intervention; RPP, rate pressure product; SBP, systolic blood pressure.
P value comparing patients with and without ischemia.
Change in CPC Counts During Stress Testing
The resting CPC counts were not significantly different between patients with and without stress-induced ischemia (Table 1). Patients with stress-induced ischemia had a median decrease of 20.2% (IQR, −45.3to 5.5; P < .001) in their circulating CD34+/CXCR4+ cell counts, whereas those without ischemia had a median increase of 3.2% (IQR, −20.6 to 35.1; P < .001) after stress testing.
In bivariate analyses, black patients had a greater overall increase in the number of CD34+ and CD34+/CXCR4+ cells during exercise compared with white patients (Table 2). As reported in a previous study,17 a significant negative association was noted between the magnitude of ischemia during stress and the decrease in circulating CD34+ and CD34+/CXCR4+ cell counts (Table 2). These factors remained significant in a multivariable regression analysis that included demographic variables, such as age, sex, race, CAD risk factors, and comorbidities (smoking history, body mass index, and history of heart failure, hypertension, dyslipidemia, and diabetes). Thus, after adjustment, for every unit increase in the ischemic defect, the CD34+ cell counts were 13% lower after exercise stress.
Table 2. Bivariate Associations Between Changes in CPCs With Stress Testing and Clinical Characteristics.
| Variable | Change in CD34+ | Change in CD34+/CXCR4+ | ||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| Demographics | ||||
| Age, y | 0.08 | .18 | 0.12 | .05 |
| Men | −0.05 | .38 | −0.05 | .39 |
| Black race | 0.18 | .004 | 0.09 | .03 |
| Smoking history | 0.07 | .24 | −0.05 | .37 |
| BMI | −0.01 | .79 | 0.04 | .48 |
| Comorbidity | ||||
| Hypertension | 0.09 | .13 | 0.08 | .16 |
| Diabetes | 0.04 | .45 | 0.04 | .47 |
| History of CABG | −0.01 | .77 | −0.04 | .52 |
| History of PCI | 0.02 | .71 | −0.04 | .50 |
| Heart failure | −0.01 | .81 | −0.001 | .98 |
| Magnitude of ischemia | −0.14 | .006 | −0.18 | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft; CPC, circulating progenitor cell; PCI, percutaneous coronary intervention.
Change in CPC Counts During Exercise Stress and Adverse Outcomes
During a median follow-up of 3 years (IQR, 2.4–3.7 years), 24 patients (5.2%) experienced adverse events, including 12 cardiovascular deaths and 12 MIs. After adjustment for clinical and demographic variables (age, sex, race, smoking history, body mass index, and history of heart failure, hypertension, dyslipidemia, and diabetes), the presence of stress-induced ischemia was associated with adverse events (hazard ratio [HR], 2.79; 95% CI, 1.55-5.03).
In a Cox proportional hazards regression analysis adjusting for the above variables, the baseline CD34+/CXCR4+ count was associated with adverse events; for each 50% lower CPC count, the adverse event rate increased by 48% (HR, 1.48; 95% CI, 1.23-2.95; Table 3). However, after further adjustment for stress-induced ischemia, the baseline CPC count was no longer a significant factor in identifying the risk of adverse outcomes.
Table 3. Association Between CPC Counts and Risk of Cardiovascular Death and Myocardial Infarctiona.
| Variable | Hazard Ratio (95% CI) | ||
|---|---|---|---|
| Model 1b | Model 2 (CD34+)c | Model 3 (CD34+/CXCR4+)d | |
| Baseline CPC Counte | |||
| CD34+ | 1.26 (1.10-2.10) | 1.18 (0.91-2.14) | NA |
| CD34+/CXCR4+ | 1.48 (1.23-2.95) | NA | 1.17 (0.80-2.02) |
| Poststress CPC Counte | |||
| CD34+ | 1.64 (1.11-2.43) | 1.58 (1.09-2.34) | NA |
| CD34+/CXCR4+ | 1.84 (1.34-3.12) | NA | 1.77 (1.22-2.98) |
| Change in CPC Counte | |||
| CD34+ | 2.12 (1.25-3.52) | 1.98 (1.16-3.24) | NA |
| CD34+/CXCR4+ | 2.70 (1.06-7.12) | NA | 2.65 (1.06-6.54) |
Abbreviations: CPC, circulating progenitor cell; NA, not applicable.
Data were derived using Cox regression analysis.
Model 1 was adjusted for baseline demographics (age, sex, race, smoking history, body mass index [calculated as weight in kilograms divided by height in meters squared]) and cardiovascular risk factors (history of heart failure, hypertension, dyslipidemia, and diabetes).
Model 2 was adjusted for all variables in model 1 plus the presence of myocardial ischemia and the baseline CD34+ count.
Model 3 was adjusted for all variables in model 1 plus the presence of myocardial ischemia and the baseline CD34+/CXCR4+ count.
Per 50% decrease.
Poststress CPC counts were more strongly associated with adverse events compared with baseline CPC counts (eFigure in the Supplement). Even after adjustment for exercise stress results and resting CPC count, the poststress CPC counts remained independently associated with adverse events. Thus, after all the aforementioned adjustments, the HR for the poststress CD34+/CXCR4+ counts was 1.84 (95% CI, 1.34-3) for each 50% decrease in CPC count (Table 3). Similar results were observed for the CD34+ subset.
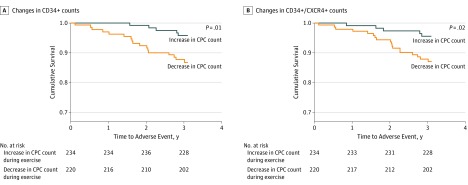
In addition, the change in CPC counts during exercise stress was the strongest factor in identifying the risk of adverse events, even after adjustment for the presence of ischemia during stress and the baseline CPC count, with an adjusted HR for CD34+/CXCR4+ cells of 2.59 (95% CI, 1.15-5.32) for each 50% further decrease in CPC count after stress testing (Table 3; Figure). Results were similar for the CD34+ subset. The Kaplan-Meier survival analysis for participants with a decrease compared with an increase in CPC counts during exercise stress is shown in the Figure. Moreover, stress-induced ischemia was no longer significantly associated with outcomes (HR, 1.28; 95% CI, 0.68-2.21) after stress-induced changes in CPC counts were included in the model.
Figure. Association Between Changes in Circulating Progenitor Cells (CPCs) and Exercise and Incident Cardiovascular Death and Nonfatal Myocardial Infarction.
A, Changes in CD34+ counts. B, Changes in CD34+/CXCR4+ counts. All CPC counts were measured after exercise stress testing.
Because CD34+/CXCR4+ cells are a subset of CD34+ cells, and both CPC subsets were associated with adverse outcomes, we further investigated whether the CD34+/CXCR4+ cells were the primary factors associated with outcomes among the CD34+ population by examining the association between the subgroup of CD34+ cells that did not express a CXCR4 epitope, CD34+/CXCR4− cell counts, and adverse outcomes. There was no association between either poststress CD34+/CXCR4− cells (HR, 1.10; 95% CI, 0.85-1.64) or changes in CD34+/CXCR4− cells during exercise stress (HR, 1.12; 95% CI, 0.88-1.76) and the risk of cardiovascular death and MI.
Risk Discrimination Testing
We tested the incremental value of adding the change in CPC counts during stress testing to a model with traditional risk factors, baseline CPC counts, and the presence or absence of ischemia during stress testing to the identification of factors associated with incident cardiovascular death and MI. The addition of the change in CD34+/CXCR4+ counts during exercise, the C statistic, the category-free net reclassification index, and the integrative discrimination improvement significantly improved the discrimination of risk factors associated with incident cardiovascular death and MI compared with the clinical model that included stress ischemia alone (Table 4).
Table 4. Risk Analysis for Changes in CPC Counts During Exercise for Composite Event Rate of Cardiovascular Death and Myocardial Infarction.
| Variable | C Statistic (95% CI) | Change in C Statistic (95% CI) | Continuous NRI (95% CI) | IDI (95% CI) |
|---|---|---|---|---|
| Modela | 0.72 (0.62-0.80) | NA | NA | NA |
| Model plus CD34+ change | 0.76 (0.69-0.86) | 0.04 (0.01-0.11) | 0.50 (0.21-0.71) | 0.03 (0.01-0.08) |
| Model plus CD34+/CXCR4+ change | 0.77 (0.71-0.91) | 0.06 (0.02-0.13) | 0.54 (0.28-0.79) | 0.04 (0.01-0.11) |
Abbreviations: CPC, circulating progenitor cell; IDI, integrative discrimination improvement; NA, not applicable; NRI, net reclassification index.
The model includes age; sex; race; smoking history; body mass index (calculated as weight in kilograms divided by height in meters squared); history of heart failure, hypertension, dyslipidemia, and diabetes; baseline CD34+ levels; baseline CXCR4+ levels; and positive stress test results.
Discussion
In patients with stable CAD, the magnitude of change in the CPC counts during exercise stress testing was associated with a higher risk of death and MI during follow-up, independent of other clinical risk factors, the presence or absence of stress-induced myocardial ischemia, and the resting CPC count. An increase in CD34+/CXCR4+ counts during exercise stress was associated with increases in survival compared with a decrease in CPC counts during exercise stress. Importantly, the change in CPC counts during exercise stress was associated with significant improvements in the discrimination of future risk of death and MI compared with a standard clinical model that included risk factors, reversible ischemia, and baseline CPC counts, as indicated by the significant improvement in the C statistic and other reclassification indices. Therefore, a decrease in CPC counts during exercise appears to be an independent factor associated with a high risk of adverse events in patients with stable CAD, even after adjusting for known clinical risk factors and the presence of ischemia.
Studies have previously reported that lower baseline CPC counts are associated with cardiovascular risk factors, vascular dysfunction, and all-cause mortality in patients with CAD, acute coronary syndromes, peripheral arterial disease, and congestive heart failure.12,13,14,15,16,25,26,27 In the present study, baseline CPC counts were also associated with a higher risk of death and MI during follow-up. However, when both baseline CPC counts and changes in CPC counts during stress testing were included in the Cox proportional hazards regression models, only the change in CPC counts was independently associated with adverse events.
Myocellular ischemia mobilizes progenitor cells from the bone marrow into the peripheral circulation.28,29 Following myocardial injury, the transient hypoxic microenvironment recruits CPCs to the site of injury and promotes local repair and regeneration.28 Stromal cell–derived factor-1 α is known to mediate the mobilization and migration of CPCs in vivo,29,30 and the expression of SDF-1α is increased during tissue hypoxia, in which it constitutes a homing signal for recruitment of CPCs to the ischemic myocardium.31,32 The binding of SDF-1α to its receptor, CXCR4, promotes the homing of CPCs by activating phosphoinositide-3 kinase–dependent chemotaxis and cell migration.33,34,35 A previous study has reported an inverse association between the change in SDF-1α levels and the change in CPC counts during exercise stress testing, especially among the CD34+/CXCR4+ subgroup.17 In the present study, we suggest that the magnitude of this decrease in CD34+/CXCR4+ counts during stress testing is independently associated with a higher risk of incident adverse events.
We found that measurement of the changes in CPC counts during exercise stress was more accurate in identifying factors associated with incident adverse events than detection of the magnitude of ischemia, even with SPECT imaging. These results suggest that measuring CPC counts during stress testing may be an inexpensive and more sensitive method of evaluating the association between exercise stress and disease prognosis in patients with stable CAD compared with measurement of reversible ischemia using conventional imaging technologies, which are expensive and may be associated with radiation exposure.
Several potential mechanisms may explain the association between decreased CPC counts after exercise and worse outcomes. The lower CPC count in the peripheral blood after stress-induced ischemia may reflect the homing of cells expressing CXCR4 into the ischemic myocardium during stress testing. Previous studies have indicated that intense bouts of acute exercise are associated with transient increases in CPC counts in the peripheral blood by mobilizing these cells from the bone marrow.36,37 Although the exact mechanisms responsible for CPC mobilization during exercise have not been fully elucidated, increases in angiogenic factors, such as vascular endothelial growth factor and granulocyte colony-stimulating factor37,38 as well as norepinephrine levels, have been reported to be associated with the magnitude of increase in CPC counts.39
Our findings support previous studies, in which exercise stress testing was associated with an increase in CPC counts among patients without exercise-induced ischemia. However, in patients with exercise-induced ischemia, we observed a decrease in both CPC subsets 45 minutes after exercise stress testing. We also found an inverse linear association between the magnitude of the decrease in CD34+/CXCR4+ counts and the magnitude of ischemia during exercise; this inverse linear association was not observed among subsets that did not express CXCR4. These findings could be explained by the possible homing of CPC subsets expressing the CXCR marker to sites of ischemic myocardium during exercise. In support of this hypothesis, previous studies have reported avid homing of intracoronary-injected CPC cells into the viable but ischemic peri-infarct tissue in patients with a recent MI40,41 as well as increased myocardial engraftment of CPCs in patients with microvascular ischemia detected through coronary flow reserve.42
Another possible mechanism that may explain the association between reduced CPC counts and worse outcomes could be that the lack of an increase in CPC counts in response to ischemia was owing to abnormalities in the mobilization of the progenitor cells from the bone marrow rather than the homing of the cells to the ischemic myocardium. Although we did not assess the regenerative capacity of the CPCs in the present study, previous studies have reported that, in patients with acute MI, lower CPC counts were associated with lower bone marrow CD34+ counts and worse long-term outcomes.16 We excluded the possibility that the observed CPC changes were owing to differences in the intensity of exercise and the associated hemoconcentration between patients with and without ischemia by noting similar changes in both groups.
In addition to exercise, aging and the presence of cardiovascular risk factors modulate the number of CPCs.43,44,45,46 Both experimental and clinical studies have reported that mobilization of progenitor cells from the bone marrow to the peripheral circulation is a homeostatic response to tissue ischemia, which is preserved in young and healthy individuals and diminishes with aging.43,44,45,46 A previous study has also reported that the presence of cardiovascular risk factors is associated with modifications to this age-related decrease in CPC counts, such that those with a higher risk factor burden experience a progressively steeper age-related decrease in their CPC counts.46 These data suggest that therapeutic interventions, such as optimization of risk factor control, may be associated with improvements in cardiovascular outcomes by preserving the endogenous regenerative capacity.
We also found that black patients had a greater degree of mobilization during exercise compared with white patients, despite the fact that black patients had lower resting CPC counts.47 However, race was not associated with adverse events, and the change in CPC counts remained significantly associated with worse outcomes after adjusting for race.
Strengths and Limitations
The strengths of our study include the large number of patients investigated, the detailed information gathered on CPC phenotypes at rest and after exercise stress testing, and the long-term follow-up conducted. Measurements of myocardial ischemia and CPC levels were performed using state-of-the-art reproducible techniques. The study’s limitations include the lack of functional assessments of CPCs, which would have required long-term cell cultures; thus, these assessments were not feasible to perform in this study. In addition, the single-study nature, together with the relatively small number of events, limits the generalizability of our findings. Furthermore, we only assessed regenerative capacity using circulating CD34+ cell subsets; however, other progenitor cell pools may be more informative. Our study included patients with known CAD; thus, our findings may not apply to the general population.
Conclusions
Our study highlights the potential significance of measuring CPC counts during stress testing as a novel method of stratifying risk in patients with known CAD. Although stress SPECT imaging for the detection of ischemic defects has been considered a valuable tool, our results suggest that measuring the change in CPC counts may improve the discrimination of future risk of cardiovascular events compared with a standard clinical model that includes stress testing alone. Further investigation is needed to evaluate whether therapeutic interventions aimed at increasing CPC mobilization will be associated with improvements in outcomes.
eFigure. Kaplan–Meier Curves for Association Between Resting CPC Counts (A and B), Poststress CPC Subsets (C and D), and Incident Cardiovascular Death and Nonfatal Myocardial Infarction
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. ; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 2.Ladenheim ML, Pollock BH, Rozanski A, et al. Extent and severity of myocardial hypoperfusion as predictors of prognosis in patients with suspected coronary artery disease. J Am Coll Cardiol. 1986;7(3):464-471. doi: 10.1016/S0735-1097(86)80454-5 [DOI] [PubMed] [Google Scholar]
- 3.Hachamovitch R, Berman DS, Kiat H, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93(5):905-914. doi: 10.1161/01.CIR.93.5.905 [DOI] [PubMed] [Google Scholar]
- 4.Farzaneh-Far A, Borges-Neto S. Ischemic burden, treatment allocation, and outcomes in stable coronary artery disease. Circ Cardiovasc Imaging. 2011;4(6):746-753. doi: 10.1161/CIRCIMAGING.111.970111 [DOI] [PubMed] [Google Scholar]
- 5.Einstein AJ. Multiple opportunities to reduce radiation dose from myocardial perfusion imaging. Eur J Nucl Med Mol Imaging. 2013;40(5):649-651. doi: 10.1007/s00259-013-2355-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thom H, West NE, Hughes V, et al. ; CECaT Study Group . Cost-effectiveness of initial stress cardiovascular MR, stress SPECT or stress echocardiography as a gate-keeper test, compared with upfront invasive coronary angiography in the investigation and management of patients with stable chest pain: mid-term outcomes from the CECaT randomised controlled trial. BMJ Open. 2014;4(2):e003419. doi: 10.1136/bmjopen-2013-003419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221-228. doi: 10.1161/01.RES.85.3.221 [DOI] [PubMed] [Google Scholar]
- 8.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105(1):71-77. doi: 10.1172/JCI8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964-967. doi: 10.1126/science.275.5302.964 [DOI] [PubMed] [Google Scholar]
- 10.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343-353. doi: 10.1161/01.RES.0000137877.89448.78 [DOI] [PubMed] [Google Scholar]
- 11.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. CXCR4 expression determines functional activity of bone marrow–derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29(11):1802-1809. doi: 10.1161/ATVBAHA.109.194688 [DOI] [PubMed] [Google Scholar]
- 12.Patel RS, Li Q, Ghasemzadeh N, et al. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res. 2015;116(2):289-297. doi: 10.1161/CIRCRESAHA.116.304187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999-1007. doi: 10.1056/NEJMoa043814 [DOI] [PubMed] [Google Scholar]
- 14.Roura S, Galvez-Monton C, Fernandez MA, Lupon J, Bayes-Genis A. Circulating endothelial progenitor cells: potential biomarkers for idiopathic dilated cardiomyopathy. J Cardiovasc Transl Res. 2016;9(1):80-84. doi: 10.1007/s12265-015-9671-z [DOI] [PubMed] [Google Scholar]
- 15.Hammadah M, Al Mheid I, Wilmot K, et al. Telomere shortening, regenerative capacity, and cardiovascular outcomes. Circ Res. 2017;120(7):1130-1138. doi: 10.1161/CIRCRESAHA.116.309421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samman Tahhan A, Hammadah M, Raad M, et al. Progenitor cells and clinical outcomes in patients with acute coronary syndromes. Circ Res. 2018;122(11):1565-1575. doi: 10.1161/CIRCRESAHA.118.312821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammadah M, Samman Tahhan AS, Al Mheid I, et al. Myocardial ischemia and mobilization of circulating progenitor cells. J Am Heart Assoc. 2018;7(4):e007504. doi: 10.1161/JAHA.117.007504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammadah M, Al Mheid I, Wilmot K, et al. The Mental Stress Ischemia Prognosis Study: objectives, study design, and prevalence of inducible ischemia. Psychosom Med. 2017;79(3):311-317. doi: 10.1097/PSY.0000000000000442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holly TA, Abbott BG, Al-Mallah M, et al. ; American Society of Nuclear Cardiology . Single photon-emission computed tomography. J Nucl Cardiol. 2010;17(5):941-973. doi: 10.1007/s12350-010-9246-y [DOI] [PubMed] [Google Scholar]
- 20.Vaccarino V, Wilmot K, Al Mheid I, et al. Sex differences in mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Heart Assoc. 2016;5(9):e003630. doi: 10.1161/JAHA.116.003630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahar EA, Mou L, Hayek SS, Quyyumi AA, Waller EK. Flow cytometric data analysis of circulating progenitor cell stability. Data Brief. 2016;10:346-348. doi: 10.1016/j.dib.2016.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020-2035. doi: 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 23.Zhou B, Latouche A, Rocha V, Fine J. Competing risks regression for stratified data. Biometrics. 2011;67(2):661-670. doi: 10.1111/j.1541-0420.2010.01493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 25.Samman Tahhan A, Hammadah M, Sandesara PB, et al. Progenitor cells and clinical outcomes in patients with heart failure. Circ Heart Fail. 2017;10(8):e004106. doi: 10.1161/CIRCHEARTFAILURE.117.004106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45(9):1441-1448. doi: 10.1016/j.jacc.2004.12.074 [DOI] [PubMed] [Google Scholar]
- 27.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593-600. doi: 10.1056/NEJMoa022287 [DOI] [PubMed] [Google Scholar]
- 28.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell–derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385):697-703. doi: 10.1016/S0140-6736(03)14232-8 [DOI] [PubMed] [Google Scholar]
- 29.Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell–derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97(11):3354-3360. doi: 10.1182/blood.V97.11.3354 [DOI] [PubMed] [Google Scholar]
- 30.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635-638. doi: 10.1038/382635a0 [DOI] [PubMed] [Google Scholar]
- 31.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858-864. doi: 10.1038/nm1075 [DOI] [PubMed] [Google Scholar]
- 32.Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med. 2005;15(2):57-63. doi: 10.1016/j.tcm.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 33.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768(4):952-963. doi: 10.1016/j.bbamem.2006.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganju RK, Brubaker SA, Meyer J, et al. The alpha-chemokine, stromal cell–derived factor-1 alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273(36):23169-23175. doi: 10.1074/jbc.273.36.23169 [DOI] [PubMed] [Google Scholar]
- 35.Vicente-Manzanares M, Rey M, Jones DR, et al. Involvement of phosphatidylinositol 3-kinase in stromal cell–derived factor-1 alpha-induced lymphocyte polarization and chemotaxis. J Immunol. 1999;163(7):4001-4012. [PubMed] [Google Scholar]
- 36.Kroepfl JM, Pekovits K, Stelzer I, et al. Exercise increases the frequency of circulating hematopoietic progenitor cells, but reduces hematopoietic colony-forming capacity. Stem Cells Dev. 2012;21(16):2915-2925. doi: 10.1089/scd.2012.0017 [DOI] [PubMed] [Google Scholar]
- 37.Mobius-Winkler S, Hilberg T, Menzel K, et al. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J Appl Physiol (1985). 2009;107(6):1943-1950. doi: 10.1152/japplphysiol.00532.2009 [DOI] [PubMed] [Google Scholar]
- 38.Domanchuk K, Ferrucci L, Guralnik JM, et al. Progenitor cell release plus exercise to improve functional performance in peripheral artery disease: the PROPEL Study. Contemp Clin Trials. 2013;36(2):502-509. doi: 10.1016/j.cct.2013.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010;1192:139-144. doi: 10.1111/j.1749-6632.2010.05390.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musialek P, Tekieli L, Kostkiewicz M, et al. Randomized transcoronary delivery of CD34(+) cells with perfusion versus stop-flow method in patients with recent myocardial infarction: early cardiac retention of 99(m)Tc-labeled cells activity. J Nucl Cardiol. 2011;18(1):104-116. doi: 10.1007/s12350-010-9326-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111(17):2198-2202. doi: 10.1161/01.CIR.0000163546.27639.AA [DOI] [PubMed] [Google Scholar]
- 42.Schachinger V, Aicher A, Dobert N, et al. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation. 2008;118(14):1425-1432. doi: 10.1161/CIRCULATIONAHA.108.777102 [DOI] [PubMed] [Google Scholar]
- 43.Zhu S, Liu X, Li Y, Goldschmidt-Clermont PJ, Dong C. Aging in the atherosclerosis milieu may accelerate the consumption of bone marrow endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27(1):113-119. doi: 10.1161/01.ATV.0000252035.12881.d0 [DOI] [PubMed] [Google Scholar]
- 44.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108(4):457-463. doi: 10.1161/01.CIR.0000082924.75945.48 [DOI] [PubMed] [Google Scholar]
- 45.Cohen KS, Cheng S, Larson MG, et al. Circulating CD34(+) progenitor cell frequency is associated with clinical and genetic factors. Blood. 2013;121(8):e50-e56. doi: 10.1182/blood-2012-05-424846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al Mheid I, Hayek SS, Ko YA, et al. Age and human regenerative capacity impact of cardiovascular risk factors. Circ Res. 2016;119(7):801-809. doi: 10.1161/CIRCRESAHA.116.308461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samman Tahhan A, Hammadah M, Kelli HM, et al. Circulating progenitor cells and racial differences. Circ Res. 2018;123(4):467-476. doi: 10.1161/CIRCRESAHA.118.313282 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Kaplan–Meier Curves for Association Between Resting CPC Counts (A and B), Poststress CPC Subsets (C and D), and Incident Cardiovascular Death and Nonfatal Myocardial Infarction