Abstract
Apoptosis and autophagy are dynamic processes that determine the fate of cells. Vitamin D receptor (VDR) deficiency in the intestine leads to abnormal Paneth cells and impaired autophagy function. Here, we will elucidate the mechanisms of the intestinal epithelial VDR regulation of autophagy and apoptosis. We used in vivo VDRlox and VDR∆IEC mice and ex vivo organoids generated from small intestine and colon tissues. We found that VDR deficiency induced more apoptotic cells and significantly increased cell death in the small intestine and colon of VDR∆IEC mice. The proapoptotic protein B-cell lymphoma 2 (BCL-2) associated X protein (Bax) was enhanced, whereas autophagy related 16 like 1 (ATG16L1) and Beclin-1 were decreased in the intestines of VDRΔIEC mice. Apoptosis induced by Bax reduced autophagy by decreasing Beclin-1. Physical interactions between Beclin-1 and Bcl-2 were increased in the VDR-deficient epithelia from mice. The growth of VDR∆IEC organoids was significantly slower with fewer Paneth cells than that of VDR+/+ organoids. The expression levels of Beclin-1 and lysozyme were decreased in VDR∆IEC organoids. Bacterial endotoxin levels were high in the serum from VDR∆IEC mice and made mice susceptible to colitis. In the organoids and colitis IL-10−/− mice, vitamin D3 treatment increased VDR and ATG16L1 protein expression levels, which activated autophagic responses. In summary, intestinal epithelial VDR regulates autophagy and apoptosis through ATG16L1 and Beclin-1. Our studies provide fundamental insights into the tissue-specific function of VDR in modulating the balance between autophagy and apoptosis.—Lu, R., Zhang, Y.-G., Xia, Y., Sun, J. Imbalance of autophagy and apoptosis in intestinal epithelium lacking the vitamin D receptor.
Keywords: Beclin-1, colonoids, enteroids, inflammation, VDR
Intestinal epithelial cells (IECs) play a critical role in intestinal barrier function, host defense, and inflammation (1–8). IECs participate in inflammatory responses and host defense directly through dynamic processes such as autophagy and apoptosis and indirectly through the production of cytokines that recruit innate and adaptive immune cells to mucosal damage sites (3). Autophagy and apoptosis share common stimuli and signaling pathways and exhibit some degree of mutual inhibition; these factors determine the fate of IECs. Apoptosis and autophagy balance is critical for maintaining the normal functions of IECs. Therefore, it is important to understand the key regulators in controlling the balance between apoptosis and autophagy.
The vitamin D receptor (VDR) regulates the biologic actions of the active vitamin D metabolite 1α,25-dihydroxy vitamin D3. The VDR is highly expressed in the small intestine and colon. The VDR is a key player in proliferation and differentiation, intestinal barrier function, innate immunity, host defense, and intestinal and microbial homeostasis (9). VDR levels are altered in various human diseases, including infection, colitis, and cancer (10). Our previous study demonstrated the intestinal epithelial VDR regulation of Paneth cells is dependent on the autophagy pathway through autophagy related 16 like 1 (ATG16L1) (11). Both ATG16L1 and Beclin-1 were regulated by activated caspase-3, which necessitates the activation of the apoptotic process (12–14). Apoptosis and autophagy may be coregulated in the same directions because the antiapoptotic B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma–extra large (Bcl-xL) proteins negatively regulate autophagy by binding to Beclin-1 (15–18). However, the fundamental role of the VDR in regulating autophagy and apoptosis in IECs is not explored.
In the current study, we used our established animal models and organoids (including enteroids and colonoids) to investigate the physiologic changes in IECs without VDR expression. Enteroids were derived from small intestine tissue, and colonoids were derived from colon tissue. These systems allow us to investigate the molecular mechanisms of intestinal VDR and cell death in a physiologic related context. The traditional model of treating cell lines with vitamin D3 that guided early vitamin studies is now giving way to a model with a more complex mechanism of action. We aimed to provide deeper understanding by investigating mechanisms of intestinal VDR in healthy and inflamed states using organoids, conditional-knockout mice, and transgenic mouse models.
MATERIALS AND METHODS
Animal models
VDRloxP/loxP mice were originally developed by Dr. Geert Carmeliet (Department of Clinical and Experimental Medicine, KU Leuven, Leuven, Belgium). VDRΔIEC mice were obtained by crossing VDRloxP/loxP mice with villin-cre mice (004586; The Jackson Laboratory, Bar Harbor, ME, USA). The VDRΔIEC mutant mice were then backcrossed to the C57BL/6J genetic background for several generations to generate the current strain. IL-10−/− mice were purchased from The Jackson Laboratory (C57BL/6J; 002251) (11). Experiments were performed on 2–3-mo-old mice (both male and female). The mice were provided with water ad libitum and maintained in a room with a 12-h light/dark cycle. All animal work was approved by the University of Illinois at Chicago Committee on Animal Resources.
Induction of dextran sulfate sodium–colitis
Mice (male and female, 2–3 mo old) were administered 5% dextran sulfate sodium (DSS) (MW = 40–50 kDa; Thermo Fisher Scientific, Waltham, MA, USA) dissolved in filter-purified and sterilized water ad libitum for the experimental period. Animals were weighed daily. At d 7 after DSS administration, mice were euthanized under anesthesia. Severity of colitis was quantified by a disease activity index determined by weight loss, fecal blood, and diarrhea. Intestines were harvested, fixed in 10% formalin (pH 7.4), processed, and paraffin embedded. Sections (5 μm) were stained with hematoxylin and eosin. Blinded histologic inflammatory scores were performed by a validated scoring system by a trained pathologist (11).
Vitamin D treatment in the IL-10−/− mice
IL-10−/− mice (male and female, 6–8 wk old) were gavaged by 0.2 μg 1,25 D3 in 0.1 ml corn oil 3 times per week for 4 wk. Mice were euthanized under anesthesia. Intestinal tissues were collected for analysis.
Mouse intestinal organoid culture
Mouse small intestines (mostly the jejunum and ileum) or colons were removed immediately after cervical dislocation. The stools were flushed out with ice-cold PBS (100 IU/ml penicillin and 100 µg/ml streptomycin), and the small intestines and colon were dissected, opened longitudinally, and cut into small (∼1 cm) pieces. The tissues were incubated in PBS with 2 mM EDTA for 30 min at 4°C on a rocker and then moved to PBS with 54.9 mM d-sorbitol and 43.4 mM sucrose. The tissues were vortexed for 1–2 min and filtered through a 70-µm sterile cell strainer. The crypts were collected by centrifugation at 150 g for 10 min at 4°C. A total of ∼500 crypts were suspended in 50 µl growth factor–reduced phenol-free Matrigel (BD Biosciences, San Jose, CA, USA). Next, a 50-µl droplet of the Matrigel-crypt mix was placed in the center well of a 12-well plate. After 30 min of polymerization, 650 µl mouse IntestiCult Organoid Growth Medium (Stemcell Technologies, Vancouver, BC, Canada) was overlain. For colonoids, 30 ng/ml Wnt family member 3a was added into the medium. The medium was changed every 2–3 d. For passaging, the organoids were removed from the Matrigel and broken up mechanically by passing through a syringe and needle (27G; BD Biosciences); then, the samples were transferred to fresh Matrigel. Passaging was performed every 7–10 d with a 1:4 split ratio. Each condition was examined in triplicate with multiple (>10) organoids in each sample (19, 20). The shape of the organoids (including budding) and the total area of the organoid cultures were examined.
Immunoblotting
Mouse intestine tissues were lysed in lysis buffer [1% Triton X-100, 150 mM NaCl, 10 mM Tris (pH 7.4), 1 mM EDTA, 1 mM EGTA (pH 8.0), 0.2 mM sodium orthovanadate, and protease inhibitor cocktail], and the protein concentrations were measured. Equal amounts of protein were separated by SDS-PAGE, transferred onto nitrocellulose membranes, and immunoblotted with anti-ATG16L1 (Abcam, Cambridge, MA, USA), anti-p53 upregulated modulator or apoptosis (Puma), anti-lysozyme (Cell Signaling Technology, Danvers, MA, USA), anti–Beclin-1, anti–sequestosome 1/p62 (BD Biosciences), anti–Bcl-2, anti–BCL-2 associated X protein (Bax), anti–Bcl-xL, anti–cleaved caspase-3, and anti-VDR antibodies (Santa Cruz Biotechnology, Dallas, TX, USA) as previously described in refs. 21 and 22.
Real-time quantitative PCR analysis
Total mRNA was extracted from scraped mouse colonic epithelial cells using Trizol reagent (Thermo Fisher Scientific) and reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). cDNA was then subjected to real-time PCR (SYBR Green PCR Kit; Bio-Rad) with primers (Table 1). Percent expression was calculated as the ratio of the normalized value of each sample relative to that of the corresponding untreated control samples. All real-time PCR reactions were performed in triplicate.
TABLE 1.
Primers for real-time PCR
| Primer sequence, 5′–3′ |
||
|---|---|---|
| Name | Forward | Reverse |
| Bax | AGACAGGGGCCTTTTTGCTAC | AATTCGCCGGAGACACTCG |
| Bcl-2 | ATGCCTTTGTGGAACTATATGGC | GGTATGCACCCAGAGTGATGC |
| Bcl-xL | ACATCCCAGCTTCACATAACCC | CCATCCCGAAAGAGTTCATTCAC |
| VDR | ATGCGGCAATCTCCATTGAAG | GAATGTGCCTCGGATCTGTGG |
| Atg16L1 | CAGAGCAGCTACTAAGCGACT | AAAAGGGGAGATTCGGACAGA |
| Beclin-1 | ATGGAGGGGTCTAAGGCGTC | TGGGCTGTGGTAAGTAATGGA |
| LYZ | GAGACCGAAGCACCGACTATG | CGGTTTTGACATTGTGTTCGC |
| β-actin | TGTTACCAACTGGGACGACA | CTGGGTCATCTTTTCACGGT |
Immunohistochemistry
Intestinal tissues were fixed in 10% buffered formalin and processed the next day with standard techniques as previously described in refs. 22–25. The slides were stained with anti–caspase-3 (Santa Cruz Biotechnology) and anti-Beclin-1 antibodies (BD Biosciences).
Immunofluorescent staining of organoids
A total of 40 μl of the Matrigel-containing organoids was plated into 1 well of an 8-well chamber slide. To analyze proliferation by bromodeoxyuridine (BrdU) incorporation assays, 10 μM BrdU was added to the wells 6 h prior to fixation. The medium was removed, and 300 μl freshly prepared 4% paraformaldehyde in PBS buffer was added for 20 min. The samples were permeabilizated in 0.5% Triton X-100 for 20 min, followed by incubation for 1 h in blocking solution (1% bovine serum albumin, 0.05% Tween, and 0.2% Triton X-100 in PBS) to reduce nonspecific background signals. The samples were incubated overnight with anti-BrdU antibody (Abcam), anti-lysozyme, and anti-Mucin2 antibodies (Cell Signaling Technology) at 4°C; then, the samples were processed for immunofluorescence as previously described by Liao et al. (21).
TUNEL staining
Apoptotic cells were detected by TUNEL staining in 10% buffered formalin–fixed paraffin-embedded tissue sections using an in situ cell death detection kit from Roche (Basel, Switzerland) and fluorescence detection. The paraffin was removed by incubating the slides for 3 min each in the following: xylene, 100% ethanol (EtOH), 96% EtOH, and 80% EtOH. The slides were rinsed with distilled water and washed with Tris-buffered saline (TBS); the liquid was then carefully removed. Next, 200 μl proteinase K (10 μg/ml) was added to completely cover the sections, and the slides were incubated for 15 min at room temperature. The slides were washed 2 × 3 min with TBS. A total of 200 μl 0.1% Triton X-100 was added to completely cover the sections, which were incubated for 2 min at room temperature. Then, the slides were washed 2 × 3 min with TBS, and the liquid was carefully removed. Next, 10–20 μl TUNEL mix was added, and the sections were coverslipped and incubated for 60 min at 37°C in the dark. The slides were washed 3 × 3 min with TBS, and the liquid was carefully removed. Next, 50–100 μl propidium iodid (PI) (1 μg/ml) was added to completely cover the sections, which were then incubated for 5 min at room temperature. PI was removed, and the slides were washed with TBS for 5 min. Finally, the coverslips were mounted using an aqueous-based fluorescence mounting medium, and the slides were stored in the dark.
LPS detection
LPS in serum samples was measured with limulus amebocyte lysate (LAL) chromogenic endpoint assays (HIT302; Hycult Biotech, Plymouth Meeting, PA, USA) according to the manufacturer’s indications. The samples were diluted 1:4 with endotoxin-free water and then heated at 75°C for 5 min in a warm plate to denature the protein before the reaction. A standard curve was generated and used to calculate the concentrations, which were expressed as EU/ml, in the serum samples.
Quantification of fecal lipocalin 2 by ELISA
Freshly collected fecal samples were reconstituted in PBS containing 0.1% Tween 20 (100 mg/ml) and vortexed for 20 min to get a homogenous fecal suspension. These samples were then centrifuged for 10 min at 12,000 rpm and 4°C. Clear supernatants were collected. Fecal lipocalin 2 (Lcn-2) level was estimated using Duoset murine Lcn2 ELISA Kit (R&D Systems, Minneapolis, MN, USA).
Cell death detection
Intestinal tissues were harvested from VDRlox and VDR∆IEC mice, and then the Cell Death Detection ELISAPLUS Photometric Enzyme Immunoassay Kit (MilliporeSigma, Burlington, MA, USA) was used for the quantitative determination of cytoplasmic histone-associated DNA fragments.
Statistical analysis
All statistical analyses were performed with Prism 5 (GraphPad Software, La Jolla, CA, USA). The results are shown as mean values ± sem. All data were analyzed using 2-tailed tests, and the differences were considered to be statistically significant at a value of P < 0.05. The differences between 2 samples were analyzed by Student’s t test. The differences among ≥3 groups were analyzed using 1-way ANOVA or Kruskal–Wallis 1-way ANOVA, depending on whether the variances differed or not.
RESULTS
Different apoptosis responses in the small and large bowels
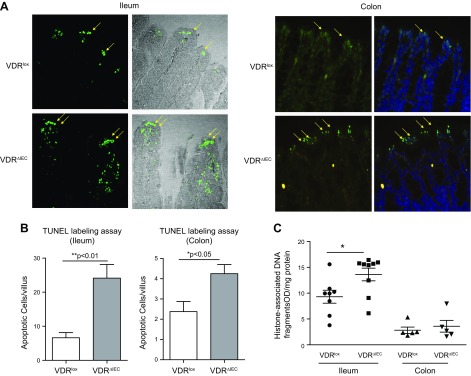
We collected tissue samples from the colon and small intestine of VDRlox and VDRΔIEC mice. We performed TUNEL staining to investigate the apoptotic cells in the intestine. We found fewer apoptotic epithelial cells in VDRlox mice than in VDRΔIEC mice in both the small intestine and colon (Fig. 1A, B). We then quantified cell death by ELISA (Sigma-Aldrich). We found greater cell death in VDRΔIEC mice in the small intestine and in the colon than those in the in VDRlox mice (Fig. 1C).
Figure 1.
VDR deficiency increased the number of apoptotic cells in VDR∆IEC mice. A) Apoptotic cells were determined with TUNEL assays. Small intestines from VDRlox and VDR∆IEC mice: TUNEL signals (green) are indicated by the yellow arrows. Colon tissues from the VDRlox and VDR∆IEC mice: TUNEL signaling (green) was counterstained with DAPI nuclear stain (blue). B) A significant increase in the number of apoptotic cells in the villi was noted in VDR∆IEC mice compared with that in the VDRlox mice. The data are expressed as means ± sem (n = 6; Student’s t test). *P < 0.05, **P < 0.01. C) ELISA was used to quantitate cell death, and we found significantly increased cell death in the intestines of VDR∆IEC mice. Intestinal tissues were harvested from VDRlox and VDR∆IEC mice; then, cell death was determined with ELISA kits according to the manufacturer’s instructions. The data are expressed as means ± sem; Student’s t test (small intestine, n = 9; colon, n = 5). *P < 0.05.
Cleaved caspase-3, an apoptotic marker, was enhanced in VDR∆IEC mice
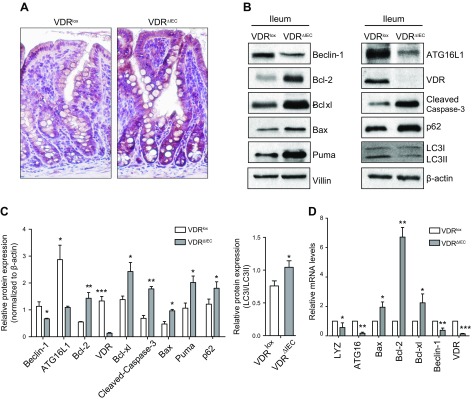
Caspases are both the initiators and effectors of apoptotic cascades (26). Enhanced cleaved caspase-3 in the intestines of VDR∆IEC mice was found by immunohistochemistry (Fig. 2A). We also used Western blotting to quantify the procaspase-3 and cleaved caspase-3 protein levels. We found that VDR deletion significantly increased cleaved caspase-3 in VDR∆IEC mice (Fig. 2B, C).
Figure 2.
Apoptosis- and autophagy-related genes were regulated by intestinal epithelial VDR. A) Enhanced cleaved caspase-3 in VDR∆IEC mice according to immunohistochemistry staining. Representative images of cleaved caspase-3 immunohistochemistry in small intestinal crypts from VDRlox and VDR∆IEC mice. B) Western blots for Beclin-1, Bcl-2, Bcl-xL, Bax, P62, LC3I/II, PUMA, and cleaved caspase-3 in the small intestines of VDR∆IEC and VDRlox mice. We found reduced levels of Beclin-1 and enhanced levels of Bcl-2, Bcl-xL, P62, and cleaved caspase-3. C) The intensity of gene expression in the intestinal tissues was analyzed by Western blotting, and the ratio of their expression to that of β-actin was calculated. D) Real-time PCR for Beclin-1, Bcl-2, Bcl-xL, Bax, LYZ, and ATG16L1 in the intestines of VDR∆IEC and VDRlox mice. LC3, autophagy-related proteins, light chain 3; LYZ, lysozyme. The data represent means ± sem; Student’s t test (n = 5 mice/group). *P < 0.05, **P < 0.01, ***P < 0.001.
Apoptosis induced by Bax reduced autophagy by enhancing Beclin-1 in VDRΔIEC mice
Apoptosis induced by the proapoptotic protein Bax reduces autophagy by enhancing the caspase-mediated cleavage of Beclin-1 (15). We thus examined the expression levels of apoptosis genes (Bcl-2, Bcl-xL, and Bax). Our data show that Bax was enhanced in the intestines of VDRΔIEC mice, whereas Beclin-1 was decreased in the intestines of VDRΔIEC mice (Fig. 2B, D). In VDRΔIEC mice, we also found increased levels of PUMA, a regulator of apoptosis (Fig. 2B). Taken together, Beclin-1 was reduced, and Bcl-2, Bcl-xL, and cleaved caspase-3 were enhanced in the small intestine of VDRΔIEC mice. P62 is known to accumulate in autophagy-deficient mice (11, 27). We further found that p62 protein in the small intestine is significantly increased in the VDRΔIEC mice compared with that in the VDRlox mice (Fig. 2B, C).
Autophagy regulators were changed in the small intestines of VDRΔIEC mice
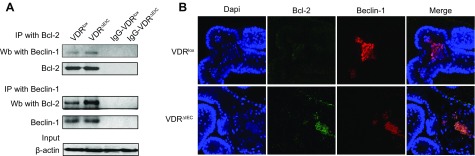
Beclin-1 has roles in both cell survival and cell death (28). Cleavage by cell proteases to remove the N-terminal BCL-2–interacting domain converts Beclin-1 to a proapoptotic protein (29). However, under conditions that favor autophagy, Beclin-1 forms a complex with activating molecule in Beclin-1-regulated autophagy, phosphatidylinositol 3-kinase (VPS)34, and VPS15 to initiate autophagy (28). Our previous study demonstrated that VDR deletion reduced ATG16L1 (11). The identification of an autophagy defect and apoptosis in VDR-deficient cells led us to further examine Beclin-1 in IECs. Bcl-2 inhibits the proautophagy function of Beclin-1 (16). Thus, we determined whether VDR-deficient cells have defective autophagy and apoptosis functions. We then investigated whether the VDR regulates Beclin-1 directly to alter apoptosis and autophagy balance. We found that the physical interaction between Beclin-1 and Bcl-2 was increased in VDR-deficient epithelia in mice (Fig. 3A). Using costaining, we identified increased levels of colocalized Bcl-2 and Beclin-1 in the enteroids of VDRΔIEC mice (Fig. 3B).
Figure 3.
Intestinal VDR affected the Bcl-2 and Beclin-1 complex interaction. A) Bcl-2 and Beclin-1 interaction in VDRlox and VDR∆IEC mice was detected by immunoprecipitation. Physical interactions between Beclin-1 and Bcl-2 were increased in the VDR-deficient epithelia from mice. B) The gene expression levels of Bcl-2 and Beclin-1 in the enteroids from VDRlox and VDR∆IEC enteroids were detected by costaining. We identified increased Bcl-2 and Beclin-1 colocalization in the VDRΔIEC mice according to immunofluorescence staining. IP, immunoprecipitation; Wb, Western blot.
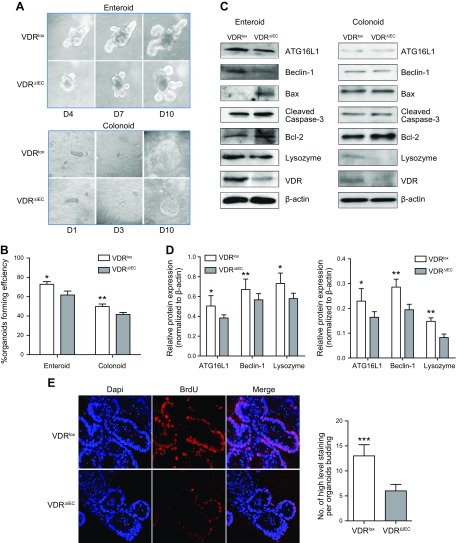
VDRΔIEC organoids had enhanced apoptosis
To rule out confounding factors that may contribute to intestinal epithelial VDR regulation, we have established a crypt cell culture organoid model derived from mice to study cellular and molecular changes of epithelial cells (20, 27, 30). The enteroids were derived from small intestine tissue, and the colonoids were derived from colon tissue (Fig. 4A). This system allows us to investigate the molecular mechanisms of intestinal VDR and cell death in vitro. The organoid-forming efficiency after 10 d was compared between the VDR∆IEC group and the VDRlox control group (Fig. 4B). Organoids formed significantly slower in the VDRΔIEC group than in the VDRlox group. Then, the protein expression in the VDR∆IEC and VDRlox organoids was detected by Western blotting. The regulators of autophagy and apoptosis were examined using the enteroids and colonoids. Lysozyme, Beclin-1, and ATG16L1 protein levels were lower in the VDR∆IEC group than those in the VDRlox group (Fig. 4C, D). Furthermore, we found that cell proliferation was insignificantly lower in the VDR∆IEC enteroids according to the BrdU staining (Fig. 4E).
Figure 4.
Growth was significantly slower in VDR∆IEC organoids than in VDRlox organoids. A) The growth of VDR∆IEC and VDRlox organoids from the small intestine and colon. B) The number of organoids formed from 1 × 105 single crypt cells was used to calculate the efficiency. The organoid-forming efficiency of the VDR∆IEC group relative to the VDRlox control group after 10 d is shown. The data represent the mean ± sem of 3 independent experiments. Statistical analysis was performed by 1-way ANOVA (n = 6/group). *P < 0.05, **P < 0.01. C) Protein expression in VDR∆IEC and VDRlox organoids according to Western blotting. Decreased lysozyme, Beclin-1, and ATG16L1 protein levels were found in the VDR∆IEC mice compared with the VDRlox group. D) The intensity of gene expression was analyzed by Western blotting, and the ratio of their expression to that of β-actin was calculated. The data represent means ± sem; Student’s t test (n = 5/group). *P < 0.05, **P < 0.01. E) BrdU staining in the enteroids. Proliferation was insignificantly lower in the VDR∆IEC enteroids. D, day. The data represent means ± sem; Student’s t test (n = 10/group). ***P < 0.001.
Changes of specialized epithelial cells in VDR∆IEC organoids
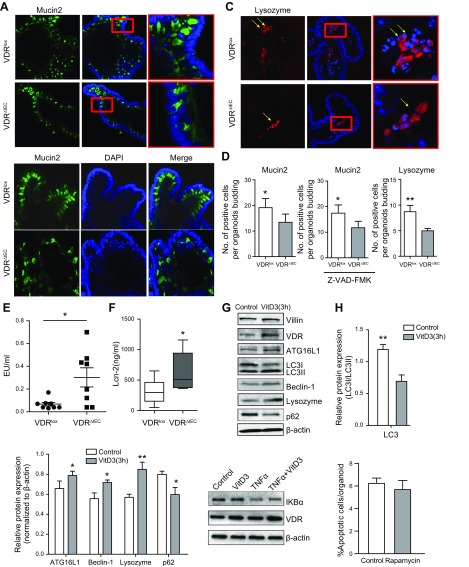
Using the stem cell–derived organoid systems, we investigated the influence of intestinal VDR on the specialized epithelial cells. Goblet cells secrete the main component of mucus in intestine. We detected the goblet cell marker Mucin2 and found that the Mucin2 expression level was lower in VDR∆IEC enteroids than in VDRlox enteroids (Fig. 5A, D). To examine whether the change in Mucin2 was apoptosis dependent, we then treated the enteroids with an apoptosis inhibitor carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]- fluoromethylketone (Z-VAD-FMK; 50 μm for 72 h). We found that Mucin2 expression was still lower in VDR∆IEC enteroids (Fig. 5B, D). Therefore, the reduced Mucin2 expression might be a survival-independent function regulated by VDR. Lysozyme is a downstream autophagy marker in Paneth cells (specialized IECs). We then measured lysozyme distribution with immunofluorescence staining in organoids. Lysozyme expression level was lower in VDR∆IEC organoids than in VDRlox organoids (Fig. 5C, D).
Figure 5.
Specialized epithelial cells in VDR∆IEC enteroids. A) Detection of the goblet cell marker Mucin2 (green) in enteroids. B) Enteroids were treated with 50 μm Z-VAD-FMK (apoptosis inhibitor) for 72 h, and then the expression of Mucin2 (green) were detected. C) Detection of the autophagy downstream marker lysozyme (red) in enteroids. D) Lysozyme and Mucin2 expression were lower in VDR∆IEC organoids than in VDRlox organoids. The data represent means ± sem; Student’s t test (n = 10/group). *P < 0.05, **P < 0.01. E) More bacterial endotoxin was found in serum samples from VDR∆IEC mice. Serum samples from VDRlox and VDR∆IEC mice were measured for bacterial endotoxin levels with LAL chromogenic endpoint assays. The data are expressed as means ± sem. (n = 8; Student’s t test). *P < 0.05. F) The expression level of Lcn-2 was higher in VDR∆IEC mice than in VDRlox mice. Student’s t test (n = 10/group). *P < 0.05. G) Vitamin D enhanced autophagy and reduced apoptosis in VDR+/+ organoids. Enteroids from VDR+/+ mice were treated with 20 nM vitamin D3 for 3 and 24 h. Increased VDR and ATG16L1 protein levels were found in VDR+/+ organoids treated with vitamin D. H, I) The intensity of gene expression in the intestinal tissues was analyzed by Western blotting, and the ratio of their expression to that of LC3I/II (H) or β-actin (I) was calculated. The data represent means ± sem; Student’s t test (n = 3/group). *P < 0.05, **P < 0.01. J) Enteroids from VDR+/+ mice were treated with 20 nM vitamin D3 for 3 h and then added with 0.5 ng/ml TNFα for 30 min. Vitamin D3 (VitD3) can increase the expression of VDR and affect the expression of IκBα. K) TUNEL assay of VDR∆IEC enteroids treated with 100 nM rapamycin for 24 h. Apoptotic cells per enteroids were accounted. LC3, autophagy-related proteins, light chain 3. Data are expressed as means ± sem (n = 30 enteroids).
In the gastrointestinal tract, tissue barrier integrity is particularly important. The loss of the mucosal barrier leads to the increased exposure of underlying tissues to inflammatory ligands in the gut lumen (1, 31). Serum samples from VDRlox and VDR∆IEC mice were used to measure bacterial endotoxin with LAL chromogenic endpoint assays. We found more bacterial endotoxin LPS in VDR∆IEC mice than in VDRlox mice (Fig. 5E). Level of Lcn-2 has been used as a marker of intestinal inflammation (32). We found that the expression level of fecal Lcn-2 was significantly higher in VDR∆IEC mice than in VDRlox mice (Fig. 5F).
Vitamin D enhanced autophagy and reduced apoptosis in enteroid
Vitamin D3 is known to activate autophagy (33, 34), and it binds with VDR and increased VDR expression. We treated enteroids with vitamin D3 for 3 h. We showed that the protein levels of VDR, ATG16L1, and lysozyme in enteroids were increased by vitamin D3 treatment, suggesting activated autophagic responses through ATG16L1 as a target (Fig. 5G–I). However, in the small intestine–derived enteroid from VDR∆IEC mice, there was no change because of lack of receptor response to the vitamin D3 treatment. Furthermore, we found that vitamin D3 treatment reduced the degradation of IκBα, an inhibitor of the proinflammatory signaling in enteroids treated with TNF-α. There data indicate that activation of VDR could protect the enteroids against inflammation induced by TNF-α (Fig. 5J).
Rapamycin is known to up-regulate autophagy. Here, we treated the VDR-deficient enteroids with rapamycin and found that the autophagy activator could not inhibit apoptosis in VDR∆IEC enteroids (Fig. 5K).
VDR was required to enhance autophagy and reduced apoptosis in vivo
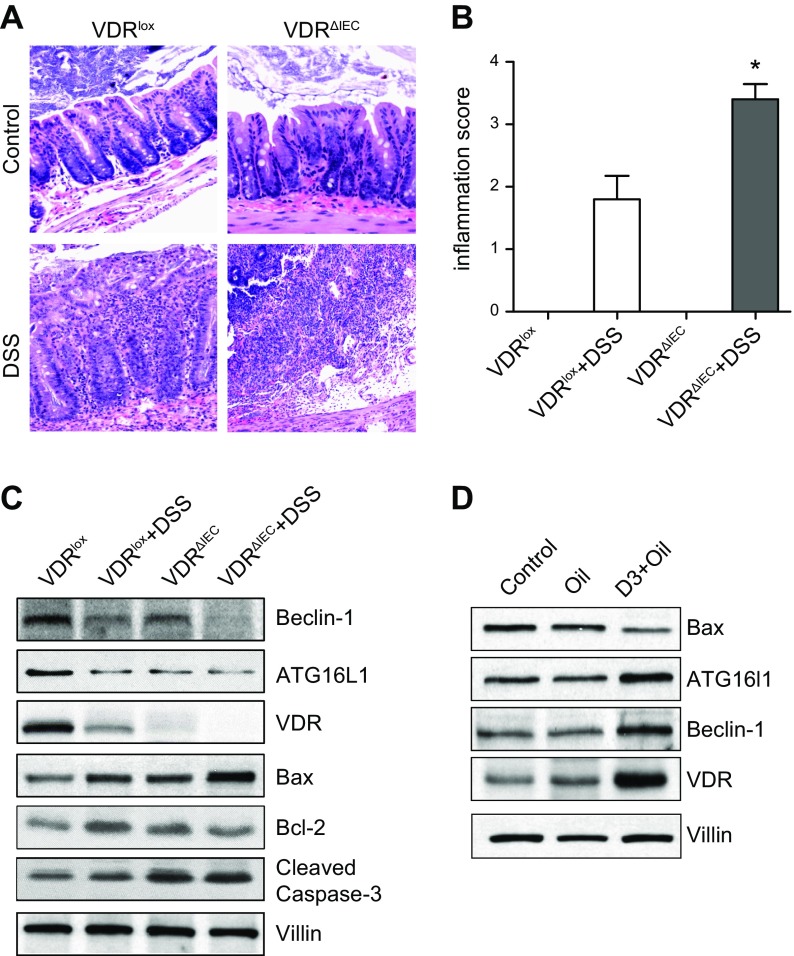
We then investigated the VDRlox and VDRΔIEC mice in a chemically induced acute inflammatory model in vivo. Mice were administrated with 5% DSS for 5 d. Inflammatory scores of the mouse intestine showed that VDRΔIEC mice were susceptible to chemical injury (Fig. 6A, B). We showed that the protein levels of Bax and cleaved caspase-3 in the VDRΔIEC mice group were increased by DSS treatment compared with that of VDRlox mice group (Fig. 6C).
Figure 6.
VDR in DSS-induced colitis models. A) VDRlox and VDRΔIEC mice were administrated with 5% DSS. Representative hematoxylin and eosin histology of colon from VDRlox and VDRΔIEC mice with or without DSS treatment. B) Inflammatory scores of the mouse intestine showed that VDRΔIEC mice were susceptible to chemical injury (n = 5/group). *P < 0.05 compared with VDRlox mice. C) Western blots for Beclin-1, Bcl-2, ATG16L1, and cleaved caspase-3 in the intestines of VDR∆IEC and VDRlox mice. D) IL-10−/− mice were gavaged by 0.2 μg 1,25D3 in 0.1 ml corn oil 3 times per week for 4 wk. Colon tissues were collected for Western blotting. Vitamin D treatment could increase the expression levels of VDR, ATG16L1, and Beclin-1 in the IL-10−/− mice.
Colitis in IL-10–deficient mice is a genetically engineered model that has been extensively used to dissect inflammatory bowel disease etiology (35). IL-10−/− mice were gavaged by 0.2 μg 1,25 D3 in 0.1 ml corn oil 3 times per week for 4 wk. We can find decreased intestinal VDR and ATG16L1 in the IL-10−/− mice. Vitamin D treatment could enhance the expression of ATG16L1 and Beclin-1 and reduce Bax in colon (Fig. 6D).
DISCUSSION
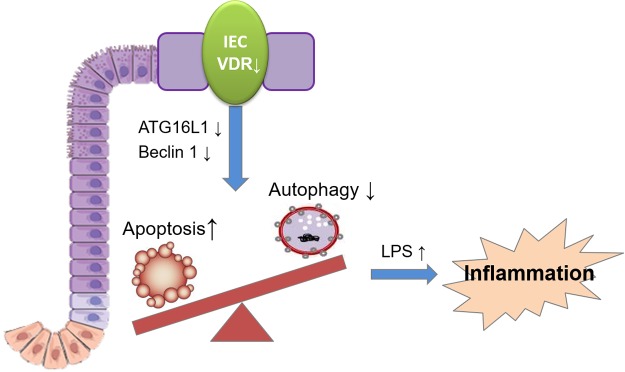
We investigated the tissue-specific role of intestinal epithelial VDR in cell death and survival using enteroinds, colonoids, and mouse models. We report that the VDR controls the autophagy and apoptosis checkpoint in IECs by maintaining Beclin-1 and ATG16L1 expression (summarized in Fig. 7). VDRΔIEC mice have reduced Beclin-1 levels and enhanced proapoptotic Bcl-2 and cleavage of caspase-3 levels. Thus, VDR loss in the intestinal epithelium impairs autophagy and enhances cell death through apoptosis. Our studies indicate that intestinal VDR may be a crucial factor in all of these processes because it determines the fate of cells: whether they undergo apoptosis or autophagy. Our data also suggest that enteroid and colonoid models could reflect the physiologic responses in vivo and be suitably applied to molecular and cellular mechanistic studies.
Figure 7.
A working model of intestinal epithelial VDR in apoptosis and autophagy balance. The VDR controls the autophagy and apoptosis checkpoint in IECs by maintaining the expressions of Beclin-1 and ATG16L1. VDR deficiency leads to impaired autophagy and enhanced cell death, thus increasing serum LPS and promoting inflammation.
Our previous study demonstrated the intestinal epithelial VDR regulation of Paneth cells is dependent on the autophagy pathway (11). ATG16L1 is a direct target of VDR. P62 is an autophagy substrate that is used as a reporter of autophagy activity, and increased p62 protein can be found in VDRΔIEC mice (11). Beclin-1 is a key determining factor for whether cells undergo autophagy or apoptosis (36). Here, we further demonstrate the reduced Paneth cells in enteroids and decreased ATG16L1 in VDR deficiency cells. In fact, Beclin-1 connects autophagy and apoptosis (15–18). Both ATG16L1 and Beclin-1 are regulated by activated caspase-3, which causes the activation of the apoptotic process (12–14). Apoptosis and autophagy may be coregulated in the same directions because the antiapoptotic Bcl-2 and Bcl-xL proteins negatively regulate autophagy by binding to Beclin-1 (15–18). During sustained exposure to apoptotic stimuli, the caspase-mediated cleavage of Beclin-1 generates fragments (N and C) that lose their ability to induce autophagy. The C-terminal fragment translocates to the mitochondria and sensitizes cells to apoptotic signals. Although the apoptosis-associated cleavage of Beclin-1 and autophagy related 5 inactivates autophagy, the cleavage of autophagy related 4D cysteine peptidase by caspase-3 generates a fragment with increased autophagy activity. Moreover, autophagy inhibits apoptosis partly by degrading active caspase-8 or by preventing the activation of Bid by Beclin-1 (36). The absence of the VDR in IECs through regulating Beclin-1 and ATG16L1 leads to enhanced apoptosis seen in our current models.
Apoptosis induced by the proapoptotic protein Bax reduced autophagy by enhancing the caspase-mediated cleavage of Beclin-1 (15). Recently, it has been shown that caspases participate in regulating the crosstalk between autophagy and apoptosis. Caspase-3 is a predominant effector caspase that participates in apoptosis. However, accumulating evidence has shown that caspase-3 is essential for autophagic activity. A study reported that during staurosporine-induced apoptosis, caspase-3 could cleave Beclin-1 at positions 124 and 149. This cleavage inhibited autophagy and activated apoptosis in HeLa cells (37). Another study found that caspase-3 together with other caspases cleaved Beclin-1 in the process of apoptosis. This effect is regulated by IL-3 deprivation in the culture medium, which blocks autophagic activity and promotes proapoptotic stimuli. The Beclin-1 C-terminal fragment is localized to the mitochondria. There, it sensitizes cells to apoptosis (14). Our data indicate that lack of VDR changed the level of caspase-3 and also the interaction of Beclin-1 and Bcl-2, which contribute to the observed imbalance of apoptosis and autophagy.
We have reported on apoptosis and autophagy in various diseases models, including amyotrophic lateral sclerosis, necrotizing enterocolitis, infectious diseases, and virus-infected intestines treated with Lactobacillus rhamnosus (11, 38–40) as well as apoptosis (22, 41) and protein interactions (22, 42, 43). Interestingly, we found that apoptosis and autophagy in host-microbial interactions significantly contribute to the dynamic process that determines the fate of IECs and pathogens. A recent study using whole-body VDR-knockout mice showed that VDR signaling attenuated PUMA induction in IECs, leading to a reduction in IEC apoptosis (44). The VDR acts to minimize tissue inflammation by mitigating cell death, which prevents the release of intracellular inflammatory mediators and maintains tissue barrier integrity. In the gastrointestinal tract, tissue barrier integrity is particularly important because mucosal barrier loss leads to the increased exposure of underlying tissues to inflammatory ligands in the gut lumen. Our current data show the increased bacterial LPS in the serum of VDR∆IEC mice. The VDR is an important mediator that regulates intestinal barrier function (31, 45, 46). Thus, the VDR role in epithelial cells need be further investigated in the context of inflammation and infection in vivo.
In summary, we have demonstrated an essential function for intestinal epithelial VDR in cell survival. The VDR is effectively anti-inflammatory in the intestine. We have described a fundamental mechanism through which the intestinal epithelial VDR promotes cell survival through ATG16L1 and Beclin-1. VDR deficiency leads to impaired autophagy and enhanced cell death, particularly in cardiomyopathy, type 2 diabetes, ischemia-reperfusion injury, microbial infections, and cancers (47, 48). The VDR is also important for normal cell proliferation, tissue repair, and remodeling programs (10, 38, 49–52). We provide new insights into the tissue-specific function of the VDR as a molecular rheostat that controls the balance between cell autophagy and apoptosis. This rheostat could be malfunctioned during intestinal inflammation, resulting in more severe mucosal damage. As such, intestinal VDR may represent a useful therapeutic target in a diverse range of human diseases.
ACKNOWLEDGMENTS
The authors acknowledge U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK105118 and R01DK114126; U.S. Department of Defense Grant BC160450P1, Funding Opportunity W81XWH-17-1-0039; and the University of Illinois Cancer Center (to J.S.). R.L. is a recipient of the American Gastroenterological Association (AGA) Young Investigator Award, and orally presented some of these data at the Digestive Disease Week 2017. The authors declare no conflicts of interest.
Glossary
- ATG16L1
autophagy related 16 like 1
- Bax
Bcl-2 associated X protein
- BCL-2
B-cell lymphoma 2
- Bcl-xL
B-cell lymphoma–extra large
- BrdU
bromodeoxyuridine
- DSS
dextran sulfate sodium
- EtOH
ethanol
- IEC
intestinal epithelial cell
- LAL
limulus amebocyte lysate
- Lcn-2
lipocalin 2
- PUMA, anti-p53 upregulated modulator or apoptosis:
TBS, Tris-buffered saline
- VDR
vitamin D receptor
AUTHOR CONTRIBUTIONS
J. Sun designed research and obtained funds; R. Lu and Y.-G. Zhang performed research; R. Lu, Y.-G. Zhang, and Y. Xia analyzed data; and all authors wrote the manuscript.
REFERENCES
- 1.Shen L., Turner J. R. (2006) Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G577–G582 [DOI] [PubMed] [Google Scholar]
- 2.Laukoetter M. G., Nava P., Lee W. Y., Severson E. A., Capaldo C. T., Babbin B. A., Williams I. R., Koval M., Peatman E., Campbell J. A., Dermody T. S., Nusrat A., Parkos C. A. (2007) JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 204, 3067–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blikslager A. T., Moeser A. J., Gookin J. L., Jones S. L., Odle J. (2007) Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 87, 545–564 [DOI] [PubMed] [Google Scholar]
- 4.Farhadi A., Banan A., Fields J., Keshavarzian A. (2003) Intestinal barrier: an interface between health and disease. J. Gastroenterol. Hepatol. 18, 479–497 [DOI] [PubMed] [Google Scholar]
- 5.Rajapaksa T. E., Stover-Hamer M., Fernandez X., Eckelhoefer H. A., Lo D. D. (2010) Claudin 4-targeted protein incorporated into PLGA nanoparticles can mediate M cell targeted delivery. J. Control. Release 142, 196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling J., Liao H., Clark R., Wong M. S., Lo D. D. (2008) Structural constraints for the binding of short peptides to claudin-4 revealed by surface plasmon resonance. J. Biol. Chem. 283, 30585–30595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naydenov N. G., Harris G., Morales V., Ivanov A. I. (2012) Loss of a membrane trafficking protein αSNAP induces non-canonical autophagy in human epithelia. Cell Cycle 11, 4613–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumagin R., Brazil J. C., Nava P., Nishio H., Alam A., Luissint A. C., Weber D. A., Neish A. S., Nusrat A., Parkos C. A. (2016) Neutrophil interactions with epithelial-expressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunol. 9, 1151–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J. (2010) Vitamin D and mucosal immune function. Curr. Opin. Gastroenterol. 26, 591–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J. (2017) The role of vitamin D and vitamin D receptors in colon cancer. Clin. Transl. Gastroenterol. 8, e103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S., Zhang Y. G., Lu R., Xia Y., Zhou D., Petrof E. O., Claud E. C., Chen D., Chang E. B., Carmeliet G., Sun J. (2015) Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 64, 1082–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy A., Li Y., Peng I., Reichelt M., Katakam A. K., Noubade R., Roose-Girma M., DeVoss J., Diehl L., Graham R. R., van Lookeren Campagne M. (2014) A Crohn’s disease variant in Atg16l1 enhances its degradation by caspase 3. Nature 506, 456–462 [DOI] [PubMed] [Google Scholar]
- 13.Rubinstein A. D., Kimchi A. (2012) Life in the balance - a mechanistic view of the crosstalk between autophagy and apoptosis. J. Cell Sci. 125, 5259–5268 [DOI] [PubMed] [Google Scholar]
- 14.Wirawan E., Vande Walle L., Kersse K., Cornelis S., Claerhout S., Vanoverberghe I., Roelandt R., De Rycke R., Verspurten J., Declercq W., Agostinis P., Vanden Berghe T., Lippens S., Vandenabeele P. (2010) Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 1, e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo S., Rubinsztein D. C. (2010) Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 17, 268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiuri M. C., Criollo A., Kroemer G. (2010) Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. EMBO J. 29, 515–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariño G., Niso-Santano M., Baehrecke E. H., Kroemer G. (2014) Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 15, 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salminen A., Kaarniranta K., Kauppinen A. (2013) Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: impact on the aging process. Ageing Res. Rev. 12, 520–534 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y. G., Sun J. (2016) Study bacteria-host interactions using intestinal organoids. [E-pub ahead of print] Methods Mol. Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y. G., Wu S., Xia Y., Sun J. (2014) Salmonella-infected crypt-derived intestinal organoid culture system for host-bacterial interactions. Physiol. Rep. 2, e12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao A. P., Petrof E. O., Kuppireddi S., Zhao Y., Xia Y., Claud E. C., Sun J. (2008) Salmonella type III effector AvrA stabilizes cell tight junctions to inhibit inflammation in intestinal epithelial cells. PLoS One 3, e2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan Y., Liao A. P., Kuppireddi S., Ye Z., Ciancio M. J., Sun J. (2007) beta-Catenin activity negatively regulates bacteria-induced inflammation. Lab. Invest. 87, 613–624 [DOI] [PubMed] [Google Scholar]
- 23.Lu R., Wu S., Zhang Y. G., Xia Y., Liu X., Zheng Y., Chen H., Schaefer K. L., Zhou Z., Bissonnette M., Li L., Sun J. (2014) Enteric bacterial protein AvrA promotes colonic tumorigenesis and activates colonic beta-catenin signaling pathway. Oncogenesis 3, e105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu R., Liu X., Wu S., Xia Y., Zhang Y. G., Petrof E. O., Claud E. C., Sun J. (2012) Consistent activation of the β-catenin pathway by Salmonella type-three secretion effector protein AvrA in chronically infected intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1113–G1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X., Lu R., Wu S., Sun J. (2010) Salmonella regulation of intestinal stem cells through the Wnt/beta-catenin pathway. FEBS Lett. 584, 911–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parrish A. B., Freel C. D., Kornbluth S. (2013) Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 5, a008672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y. G., Zhu X., Lu R., Messer J. S., Xia Y., Chang E. B., Sun J. (2019) Intestinal epithelial HMGB1 inhibits bacterial infection via STAT3 regulation of autophagy. Autophagy 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu D., Zhang T., Xiao J., Zhu K., Wei R., Wu Z., Meng H., Li Y., Yuan J. (2015) Modification of BECN1 by ISG15 plays a crucial role in autophagy regulation by type I IFN/interferon. Autophagy 11, 617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu X., Messer J. S., Wang Y., Lin F., Cham C. M., Chang J., Billiar T. R., Lotze M. T., Boone D. L., Chang E. B. (2015) Cytosolic HMGB1 controls the cellular autophagy/apoptosis checkpoint during inflammation. J. Clin. Invest. 125, 1098–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu R., Voigt R. M., Zhang Y., Kato I., Xia Y., Forsyth C. B., Keshavarzian A., Sun J. (2017) Alcohol injury damages intestinal stem cells. Alcohol. Clin. Exp. Res. 41, 727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y. G., Lu R., Xia Y., Zhou D., Petrof E., Claud E. C., Sun J. (2019) Lack of vitamin D receptor leads to hyperfunction of claudin-2 in intestinal inflammatory responses. Inflamm. Bowel Dis. 25, 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chassaing B., Srinivasan G., Delgado M. A., Young A. N., Gewirtz A. T., Vijay-Kumar M. (2012) Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One 7, e44328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Høyer-Hansen M., Bastholm L., Mathiasen I. S., Elling F., Jäättelä M. (2005) Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 12, 1297–1309 [DOI] [PubMed] [Google Scholar]
- 34.Demasters G., Di X., Newsham I., Shiu R., Gewirtz D. A. (2006) Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3 analogue, EB 1089, through promotion of autophagy and interference with proliferative recovery. Mol. Cancer Ther. 5, 2786–2797 [DOI] [PubMed] [Google Scholar]
- 35.Eun C. S., Mishima Y., Wohlgemuth S., Liu B., Bower M., Carroll I. M., Sartor R. B. (2014) Induction of bacterial antigen-specific colitis by a simplified human microbiota consortium in gnotobiotic interleukin-10-/- mice. Infect. Immun. 82, 2239–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang R., Zeh H. J., Lotze M. T., Tang D. (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 18, 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y., Zhao L., Liu L., Gao P., Tian W., Wang X., Jin H., Xu H., Chen Q. (2010) Beclin 1 cleavage by caspase-3 inactivates autophagy and promotes apoptosis. Protein Cell 1, 468–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu S., Yuan L., Zhang Y., Liu F., Li G., Wen K., Kocher J., Yang X., Sun J. (2013) Probiotic Lactobacillus rhamnosus GG mono-association suppresses human rotavirus-induced autophagy in the gnotobiotic piglet intestine. Gut Pathog. 5, 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao Y., Ma C., Yi J., Wu S., Luo G., Xu X., Lin P. H., Sun J., Zhou J. (2015) Suppressed autophagy flux in skeletal muscle of an amyotrophic lateral sclerosis mouse model during disease progression. Physiol. Rep. 3, e12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y., Shiou S. R., Guo Y., Lu L., Westerhoff M., Sun J., Petrof E. O., Claud E. C. (2013) Erythropoietin protects epithelial cells from excessive autophagy and apoptosis in experimental neonatal necrotizing enterocolitis. PLoS One 8, e69620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collier-Hyams L. S., Zeng H., Sun J., Tomlinson A. D., Bao Z. Q., Chen H., Madara J. L., Orth K., Neish A. S. (2002) Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J. Immunol. 169, 2846–2850 [DOI] [PubMed] [Google Scholar]
- 42.Wu S., Ye Z., Liu X., Zhao Y., Xia Y., Steiner A., Petrof E. O., Claud E. C., Sun J. (2010) Salmonella typhimurium infection increases p53 acetylation in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G784–G794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu S., Liao A. P., Xia Y., Li Y. C., Li J. D., Sartor R. B., Sun J. (2010) Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am. J. Pathol. 177, 686–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W., Chen Y., Golan M. A., Annunziata M. L., Du J., Dougherty U., Kong J., Musch M., Huang Y., Pekow J., Zheng C., Bissonnette M., Hanauer S. B., Li Y. C. (2013) Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J. Clin. Invest. 123, 3983–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y. G., Wu S., Lu R., Zhou D., Zhou J., Carmeliet G., Petrof E., Claud E. C., Sun J. (2015) Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci. Rep. 5, 10642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y. G., Wu S., Sun J. (2013) Vitamin D, vitamin D receptor, and tissue barriers. Tissue Barriers 1, e23118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bakke D., Chatterjee I., Agrawal A., Dai Y., Sun J. (2018) Regulation of microbiota by vitamin D receptor: a nuclear weapon in metabolic diseases. Nucl. Receptor Res. 5, 101377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bakke D., Sun J. (2018) Ancient nuclear receptor VDR with new functions: microbiome and inflammation. Inflamm. Bowel Dis. 24, 1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glenn D. J., Cardema M. C., Gardner D. G. (2016) Amplification of lipotoxic cardiomyopathy in the VDR gene knockout mouse. J. Steroid Biochem. Mol. Biol. 164, 292–298 [DOI] [PubMed] [Google Scholar]
- 50.Angel B., Lera L., Márquez C., Albala C. (2018) The association of VDR polymorphisms and type 2 diabetes in older people living in community in Santiago de Chile. Nutr. Diabetes 8, 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao T., Ying X., Zhao Y., Yuan A., He Q., Tong H., Ding S., Liu J., Peng X., Gao E., Pu J., He B. (2015) Vitamin D receptor activation protects against myocardial reperfusion injury through inhibition of apoptosis and modulation of autophagy. Antioxid. Redox Signal. 22, 633–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luderer H. F., Nazarian R. M., Zhu E. D., Demay M. B. (2013) Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-β signaling during the inflammatory response to cutaneous injury. Endocrinology 154, 16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]