Abstract
Syncytin (Syn)-2 is an important fusogenic protein that contributes to the formation of the placental syncytiotrophoblast. Galectin (Gal)-1, a soluble lectin, is also involved in trophoblast cell fusion and modulates the interaction of certain retroviral envelopes with their cellular receptor. This study aimed to investigate the association between Syn-2 and Gal-1 during human trophoblast cell fusion. This association was evaluated in vitro on primary villous cytotrophoblasts (vCTBs) and cell lines using recombinant Gal-1 and Syn-2–pseudotyped viruses. Using lactose, a Gal antagonist, and Gal-1–specific small interfering RNA (siRNA) transfections, we confirmed the implication of Gal-1 in vCTBs and BeWo cell fusion, although RT-PCR and ELISA analyses suggested that Gal-1 alone did not induce syncytialization. Infection assays showed a specific and significant effect of Gal-1 on the infectivity of Syn-2–pseudotyped viruses that depended on the expression of major facilitator superfamily domain-containing 2A (MFSD2a). Moreover, Gal-3, another placental Gal, did not modulate the infectivity of Syn-2–positive viruses, strengthening the specific association between Gal-1 and Syn-2. Interestingly, Gal-1 significantly reduced the infectivity of Syn-1–pseudotyped viruses, suggesting the opposite effects of Gal-1 on Syn-1 and -2. Finally, coimmunoprecipitation experiments showed a glycan-dependent interaction between Syn-2–bearing virions and Gal-1. We conclude that Gal-1 specifically interacts with Syn-2 and possibly regulates Syn-2/MFSD2a interaction during syncytialization of trophoblastic cells.—Toudic, C., Vargas, A., Xiao, Y., St-Pierre, G., Bannert, N., Lafond, J., Rassart, É., Sato, S., Barbeau, B. Galectin-1 interacts with the human endogenous retroviral envelope protein syncytin-2 and potentiates trophoblast fusion in humans.
Keywords: ERVFRD-1, β-galactoside-binding lectin, villous cytotrophoblasts, syncytialization
The syncytiotrophoblast (STB) layer of the human placenta is a syncytial structure with endocrine, immunologic, and nutritive functions, ensuring a successful pregnancy through nutrient and gas exchanges between maternal and fetal blood circulation (1, 2). The STB originates from the differentiation of trophoblast cells into villous cytotrophoblast (vCTB), which fuse to form a syncytium (3). Constant fusion of vCTB is in fact needed throughout pregnancy to stably maintain the STB structure and compensate for its continuous shedding. Trophoblast syncytialization is a complex and highly regulated process, requiring cell cycle exit, bridging of adjacent cells membranes, and final fusion of cell membranes and also involves transcription and growth factors, cytokines, hormones, tight- and gap-junction proteins, cytoskeleton-remodeling proteins, galectins (Gals), and human endogenous retroviral (ERV) proteins (3–7).
Two human ERV envelope proteins, syncytin (Syn)-1 and Syn-2 are determinant for vCTB fusion (8–12). These ancient retroviral genes were acquired several million years ago after the infection of germ cells by 2 different retroviruses, leading to the presence of ERV group W member 1, envelope (ERVW-1) and ERV group FRD member 1, envelope (ERVFRD-1) proviral DNA in the human genome (8, 10, 13). These envelope glycoproteins display typical features of retroviral envelopes, being expressed as precursors (gp73 and gp75, respectively) cleaved into surface and transmembrane (TM) subunits (14, 15). The fusogenic properties of Syn-1 and -2 depend on their interaction with specific receptors, namely solute carrier family 1 [(SLC1)-A4; ASCT1] and SLC1A5 (ASCT2) for Syn-1 (16) and major facilitator superfamily domain-containing 2A (MFSD2a) for Syn-2 (17, 18). Despite the fact that both syncytins are involved in vCTB fusion, these 2 proteins seem to have nonredundant functions. First, unlike Syn-2 (11, 19), Syn-1 expression is not restricted to vCTB, being expressed in STB and in nonfusogenic extravillous trophoblast (EVT) cells (8, 20, 21). In addition, Syn-1 is expressed in proliferative cells and promotes the G1/S transition, whereas Syn-2 expression is restricted to resting vCTB (19, 22). Interestingly, Syn-1 has also been associated with the regulation of apoptosis (23–25). Our previous results suggest that Syn-2 is a major fusogenic protein in STB formation because inhibition of Syn-2 expression in trophoblastic-like BeWo cells and primary vCTB had a strong impact on syncytialization (12). Furthermore, in preeclampsia (PE), a hypertensive obstetrical syndrome with abnormal placentation (26), Syn-2 expression is significantly decreased in PE placentas and strongly correlates with severity of symptoms (15, 27). In addition to their fusogenic function in the placenta, Syn-1 and -2 carry functional immunosuppressive domains in their TM subunit (28, 29). Moreover, Syn-1 and -2 are incorporated in circulating placental extracellular vesicles and participate in their cellular uptake (28, 30).
Other factors are known to be implicated in placental development and trophoblast syncytialization, such as various Gals. This family of proteins contains 19 members sharing a conserved 130 aa carbohydrate-recognition domain (CRD) that confers a binding affinity toward β-galactoside residues (31). Gals play important functions in development, immune regulation, cell proliferation, migration, and adhesion and are key players in pathologies, such as cancer and obstetrical disorders (5, 31, 32). Indeed, secreted Gal-1 and -3 family members are known to be important in several aspects of embryo implantation and placental function (5, 32–42). Gal-1 forms functional homodimers containing 2 CRDs, whereas Gal-3 oligomerizes upon binding to its glycan ligands, thereby becoming multivalent in glycan binding. Because of this bi- or multivalent conformation, Gal-1 and -3 can cross-link glycoproteins expressed on the same cell membrane or on 2 neighboring cells (43–46). In the first-trimester placenta, Gal-1 and -3 are expressed in vCTB, EVT, and in the decidua (34, 47–49). Interestingly, previous studies have shown that Gal-1 binds to STB and to BeWo membranes and is implicated in the differentiation and fusion of trophoblastic cells (34, 35, 37, 50–53). In addition, Gal-1 expression is often dysregulated in placenta from women suffering from obstetrical disorders or spontaneous abortion (32, 47, 49, 54).
Gal-1 has also been shown to play a role in cell-pathogen interactions (31) and to enhance the infectivity of HIV-1 and human T-cell leukemia virus type 1 retroviruses (55, 56). Specifically, Gal-1 increased the interaction of HIV-1 gp120 to its cellular receptor CD4, which led to increased HIV-1 infectivity and replication (57). As both Gal-1 and Syn-2 are implicated in the physiopathology of placentation and Gal-1 enhances the interaction of retroviral envelopes with their cellular receptor, we herein investigated the role of Gal-1 in the interaction of the endogenous retroviral protein Syn-2 with its cellular receptor. Using pseudotyped viruses, our results demonstrate that, unlike Gal-3, Gal-1 increases Syn-2–dependent infection at an early step and that this increase correlates with binding affinity of Gal-1 toward Syn-2.
MATERIALS AND METHODS
Ethical approval
Placental tissues used in this study were obtained from uncomplicated term pregnancies (gestational weeks 37–41) after informed consent was signed by recruited pregnant women and were collected after spontaneous vaginal or C-section delivery, in accordance with the guidelines of the ethical committee of St. Luc Hospital of Centre Hospitalier Universitaire de Montréal (CHUM) (Montréal, QC, Canada). Exclusion criteria were as follows: chronic hypertension, diabetes mellitus and gestational diabetes, fetal chromosomal abnormalities, HIV and Hepatitis (B, C) infections, multiple pregnancy, metabolic diseases, renal and liver diseases, cancer, and smoking.
Primary cells and cell lines
Primary vCTBs were isolated from normal term placentas (n = 3) according to a previously published protocol and cultured for 4 d during which they differentiate and fuse to form large syncytia (27, 58, 59). The purity of each cytotrophoblast preparation was assessed by flow cytometry using FITC-conjugated monoclonal antibody against cytokeratin-7, a specific trophoblast marker, (CBL194F; MilliporeSigma, Burlington, MA, USA) and only cultures of more than 96% purity were used in this study. Briefly, vCTB (106 cells) were fixed in 2% paraformaldehyde for 15 min at room temperature and washed 3 times in PBS. Cells were incubated with a blocking solution [5% bovine serum albumin (BSA; A7906; MilliporeSigma) in PBS 1×] in the presence of human Fc receptor blocking reagent (130-059-901, MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) for 1 h at room temperature. Cells were washed 3 times in PBS and incubated with FITC-conjugated anti-cytokeratin-7 (dilution 1/500) or FITC-conjugated isotypic control antibodies for 1 h at room temperature. Following 3 washes in PBS, stained vCTB were resuspended in PBS, and fluorescent signals were detected and analyzed with the BD Accuri C6 Flow Cytometer (BD Bioscience, San Jose, CA, USA). All experiments with primary vCTB were done in triplicate under normoxia conditions. Human embryonic kidney (HEK) 293T, adenocarcinoma HeLa, and choriocarcinoma BeWo cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). BeWo is a trophoblast-derived choriocarcinoma cell line frequently used as a fusion model for trophoblast cells that forms syncytia upon activation of the cAMP pathway (12, 60). HEK293T and HeLa cells were grown in DMEM containing 2 mM glutamine, and BeWo cells were maintained in Ham’s F12 medium (Wisent, St-Jean-Baptiste, QC, Canada). All media were supplemented with 10% fetal bovine serum (FBS) (Wisent), and cells were maintained at 37°C in a 5% CO2 atmosphere without antibiotics and antimycotics.
Recombinant Gal-1 (rGal-1) and Gal-3 production
Recombinant (r) Gals were purified as previously described with minor modifications (61–65). Briefly, Terrific Broth containing ampicillin was inoculated with Escherichia coli BL21 (DE3), which carries the expression plasmid of either human Gal-1 or human Gal-3 [kindly provided by Dr. Jun Hirabayashi and Dr. Kenichi Kasai (Teikyo University, Tokyo, Japan)], and incubated overnight at 37°C. Recombinant protein expression was induced by addition of 1 mM isopropyl-β-d-thiogalactoside for 3 h. Bacteria pellets were resuspended in 10 ml ice cold buffer [22 mM Tris-HCl pH 7.5, 5 mM EDTA, 1 mM DTT, and a protease inhibitor cocktail (MilliporeSigma)] and sonicated for 30 s at 120 W (8 times,1-min interval) on ice. Lysates were subjected to ultracentrifugation at 112,500 g for 30 min at 4°C (T70.1 rotor) in a L8-80M centrifuge (Beckman Coulter, Brea, CA, USA). Supernatants were then passed on α-lactose agarose column (MilliporeSigma). After washing with PBS, Gal-1 or Gal-3 were eluted with 10 ml of 150 mM lactose (MilliporeSigma) in PBS and collected in 1 ml fractions. For Gal-1, fractions that contained the Gal were pooled and incubated overnight at 4°C with 100 mM iodoacetamide for carboxymethylation of cysteine residues, which are otherwise susceptible for oxidation (57). Free iodoacetamide and lactose were then removed by a series of dialysis against PBS. Fractions that contained Gal-3 were pooled, and lactose was removed using a HiPrep 26/10 Desalting Column (GE Healthcare, Chicago, IL, USA). Proteins were further applied to Acticlean Etox (Sterogene Bioseparations, Carlsbad, CA, USA) to remove endotoxins and then filter-sterilized using syringe filters (0.22-µm pore size) (MilliporeSigma). Protein concentration was determined by the Bradford assay. Finally, endotoxin activity was assessed by the LAL assay (QCL-1000 Assay; Lonza, Basel Switzerland). The hemagglutination assay was used to evaluate Gal-1 and -3 activities before use. Recombinant Gal-1 was biotinylated with the EZ-link Maleimide-PEG2-Biotin (Thermo Fisher Scientific, Waltham, MA, USA) in the presence of 50 mM lactose (MilliporeSigma), an antagonist of Gals and purified again to remove the biotinylated reagent and lactose.
Gal-1 ELISA
BeWo cells (105 cells in triplicate wells) were cultured in 6-well plates (Bio Basic, Markham, ON, Canada) in regular media for 24 and 48 h before conditioned media was collected and cleared from cell debris by centrifugation (300 g) and filtration through a 0.22-µm filter. The release of endogenous Gal-1 was assessed by an in-house ELISA, as previously published (56). Briefly, an anti–Gal-1 antibody (rabbit polyclonal 0.5 µg/ml, generated in-house) was used to coat an ELISA plate overnight at 4°C. Standards and samples were loaded on the plate, incubated for 2 h at room temperature before addition of a biotinylated rabbit polyclonal anti–Gal-1 antibody (0.25 µg/ml) for 1 h at room temperature. After 3 washing steps, 100 µl of streptavidin–horseradish peroxidase (HRP) (1/4000) was added to each well and incubated for 45 min at room temperature. After removal of unbound streptavidin-HRP, the plate was incubated with 100 µl of 3,3′,5,5′-tetramethylbenzidine, and the reaction was stopped with 100 µl of 0.18 M H2SO4. The 450-nm absorbance was measured and Gal-1 concentration was determined using Gal-1 standards.
Plasmids
The HIV-1 NL4.3 envelope–deficient luciferase expressing pNL4.3env-Luc vector was obtained from the National Institutes of Health (NIH) AIDS Reagent Program (Germantown, MD, USA) and the equivalent green fluorescence protein (GFP)-expressing pNL4.3env-eGFP construct was a kind gift from Dr. Eric Cohen (Institut de Recherches Cliniques de Montréal, Montreal, QC, Canada). The expression vector for the pLP/vesicular stomatitis virus G-protein (VSV-G) was obtained from the ViraPower Lentiviral Packaging Mix Kit (K497500; Thermo Fisher Scientific). The phCMV1-Syn-2 expression vector has been previously described (12). The phCMV-Syn-2-Flag construct was obtained by substituting the Syn-2 cDNA sequence corresponding to 75–82 aa with the Flag-tag (DYKDDDDK) by inverse PCR (forward 5′-GACTACAAAGACGATGACGACAAGTCCTATCGATAGG-3′ and reverse 5′-CAGATTAGGGTCCCATCGATAGG-3′). phCMV1-eGFP was obtained by insertion of the eGFP cDNA amplified from the pEGFP-C1-Rab7a expression vector (a kind gift from Dr. Robert Lodge, Institut de Recherches Cliniques de Montréal, Montreal, QC, Canada) with forward (5′-CTCGAGATGGTGAGCAAGGGCGAG-3′) and reverse primers (5′-GGATCCTTACTTGTACAGCTCGTCCATGC-3′) using the XhoI and BamHI sites of the phCMV1 vector (Genlantis, San Diego, CA, USA). The Syn-1 expression vector was obtained by cloning the codon-optimized Syn-1 cDNA sequence (GenScript, Hong Kong, China) in the pTH vector (66) through HindIII and NotI sites. The pLVX-MFSD2a-myc construct was generated by cloning the MFSD2a cDNA from the BamHI/NotI-digested pEF6-MFSD2a vector (67) (a kind gift from Dr. Tommaso Dragani and Dr. Francesca Colombo, Instituto Nazionale Tumori, Milan, Italy) into the pLVX-Puro plasmid (Takara Bio, Kusatsu, Japan), in which a NotI site was added next to the BamHI site. The myc tag was then inserted at the C-terminal end of MFSD2a by inverse PCR using forward 5′-GAGCAGAAGCTGATCAGCGAGGAGGACCTGTAGCGGCCGAGATCCCGCGACTC-3′ and reverse 5′-GAGGATGCTAGCCAGC-3′ primers.
Generation of stable MFSD2a-overexpressing HEK293T cell clones
HEK293T cells were cotransfected with pLP1, pLP2, and pVSV-G (ViraPower Lentiviral packaging Mix kit K497500; Thermo Fisher Scientific) in the presence of polyethylenimine at a 1 µg DNA: 7 µl polyethylenimine (PEI) ratio (PEI, 1 µg/µl solution) (23966; Polysciences, Warrington, PA, USA). HEK293T cells (1.5 × 105 cells) were next infected with VSV-G–pseudotyped MFSD2a-myc-expressing LVX virions. At 48 h postinfection, complete medium containing 1 µg/ml puromycin (MilliporeSigma) was added to the cells for selection. Colonies were then individually trypsinized and separately plated in 6-well plates. MFSD2a expression was analyzed for each clone by Western blot, and positive clones were further expanded.
Production of pseudotyped NL4.3 viruses and titration
Envelope-defective HIV-1 proviral NL4.3 expressing either GFP or luciferase were used to produce CMVeGFP (negative control), Syn-2, or VSV-G–pseudotyped virions. HEK293T cells (2 × 106) were cotransfected using PEI (1 µg DNA: 7 µl PEI) with 5 µg of pNL4.3env-Luc or pNL4.3env-eGFP and 1.5 µg of either phCMV1-Syn-2, phCMV1-eGFP or pLP/VSV-G. Cells were then replenished with fresh medium, which was next harvested between 36 and 40 h after transfection. Supernatants were centrifuged at 300 g for 5 min, passed through a 0.22-µm pore size sterile syringe filter, measured in aliquots, and stored at −80°C before use. Virus-containing supernatants (10 µl) were next quantified by the addition of 40 µl of a reaction mix [60 mM Tris-HCl pH 7.9, 6 mM MgCl2, 0.2 M KCl, 0.6 mM EGTA, 0.06% Triton X-100, 2.5% ethylene glycol, 6 mM DTT, 0.4 mM glutathione (GSH), 0.025 U poly(rA) oligo-dT, 2.5 µCi 3H-dTTP] and incubation for 2 h at 37°C. The reaction was stopped with 150 µl of cold 10% trichloroacetic acid at 4°C for 30 min. Samples were then loaded on a Millipore Multiscreen Glass Fiber FC Plate (MilliporeSigma) and aspirated with a Millipore Multiscreen Manifold (MilliporeSigma). The plate was washed once with 200 µl 10% cold trichloroacetic acid, and twice with 200 µl cold 95% ethanol. Filters were transferred in scintillation vial containing 4 ml of scintillation solution. Reverse transcriptase (RT) activity was estimated by detection of the radioisotope with the Tri-Carb 2800TR liquid scintillation analyzer and the Quanta Smart software (PerkinElmer, Waltham, MA, USA).
Infection assay with pseudotyped virions
Cells (5 × 104) were plated in 24-well plates and left for 16 h at 37°C. When HeLa cells were used, cells were transfected with 0.5 µg of the pLVX-MFSD2amyc expression vector using the Lipofectamine 2000 Transfection Reagent (11668030; Thermo Fisher Scientific) 24 h before infection. A volume of 200 µl of Syn-2–pseudotyped NL4.3-based viruses was used for infection, whereas added volume of Syn-1, control Env-, and VSV-G–pseudotyped virions were adjusted according to measured RT activities of virus stocks. Cells were infected in a final volume of 200 µl, in FBS-free DMEM medium in the presence of 8 µg/ml of polybrene (MilliporeSigma). For infection experiments in the presence of Gals, unless otherwise stated, 4 µM of rGal-1 or rGal-3 were added to viral preparations before infection. As controls, an equal volume of PBS was added to viral preparations (virus only), whereas 50 mM lactose (L3750; MilliporeSigma) was combined with 4 µM of recombinant galectins for other control samples (Gal + lactose). Cell-virus mixtures were finally spinoculated at 1000 g for 2 h at 25 or 4°C to increase the interaction between virus and cells (16). Cells were then washed with PBS, and fresh complete medium was added to the cells, which were incubated for 24 h at 37°C. Infected cells were either detected by fluorescent microscopy for pseudotyped NL4.3env-GFP viruses or by luciferase assay for pseudotyped NL4.3env-Luc viruses. For GFP detection, infected cells were washed with warm PBS and then fixed in a 2% paraformaldehyde solution for 15 min at room temperature. After 3 washes, fluorescent cells were observed under an Eclipse Ti microscope (Nikon Canada, Mississauga, ON, Canada) with the Plan Fluor ELWD ×20/0.45 objective. Three to 5 representative fields were captured for each condition, and representative images of 2 independent experiments were selected. For luciferase assays, infected cells were washed once with warm PBS before lysis in 150 µl of cold 1× luciferase lysis buffer (25 mM Tris pH 7.8, 2 mM DTT, 1% Triton X-100, 10% glycerol). Cells were lysed for 20 min at 4°C, and the lysate was cleared by centrifugation at 16,000 g for 10 min at 4°C. Each sample (25 µl) was added to a 96-well luminometer plate in triplicate, and the luciferase activity was measured using a luciferase buffer [20 mM tricine, 1.07 mM (MgCO3)4·Mg(OH)2·5 H2O, 2.67 mM MgSO4, 0.1 mM EDTA, 220 μM coenzyme A, 4.7 μM d-luciferin potassium salt, 530 μM ATP, and 33.3 mM DTT] with the Dynex MLX microplate luminometer (68). Luciferase activities were calculated as the mean relative light unit (RLU) value ± sem of triplicate samples and normalized for the protein concentration of each sample. Infection assays were repeated 3 times.
Cell fusion assay
vCTBs (1.5 × 106) and BeWo (8 × 104) cells were cultured in 24-well plates on poly-D- or poly-l-lysine–coated glass coverslips (microcover glass 12 mm circle; VWR International, Radanor, PA, USA), respectively. vCTBs were cultured for 4 d in regular medium containing PBS or with medium containing 50 mM lactose. At d3–d4 of differentiation, cells were fixed with cold methanol for 30 min at −20°C. BeWo cells were cultured in the presence of 0.5% DMSO (D2650; MilliporeSigma) or 50 µM forskolin (F6886; MilliporeSigma) for 24 h before changing the medium with medium containing either PBS or 50 mM lactose. Cells were treated for another 24 h before fixation for 30 min in cold methanol at −20°C. For experiments with small interfering RNA (siRNA) transfections, 15 nM of Flexitube siRNA (SI00035924 5′-CGCCAACACCATCGTGTGCAA-3′ and SI02628269 5′-CGCCAGCAACCTGAATCTCAA-3′) or control siRNA (SI03650325) were transfected in BeWo cells using the Hiperfect reagent (Qiagen, Germantown, MD, USA) following the manufacturer’s instructions. BeWo cells were treated with 50 µM forskolin or 0.5% DMSO 16 h after transfection and fixed after 48 h. Both cell types were washed 3 times with PBS and then blocked with a solution of PBS containing 2% FBS, 2% BSA, and 2% human serum for 1 h at room temperature. For both vCTBs and BeWo cells, the plasma membrane was stained with a mouse monoclonal anti-desmoplakin antibody (1/300 dilution, ab16434 anti-desmoplakin I+II; Abcam, Cambridge, MA, USA) for 1 h at room temperature followed by 3 washes in PBS. Cells were then incubated with the Alexa Fluor 488-conjugated goat anti-mouse antibody (1/1000, A11001; Thermo Fisher Scientific) for 1 h at room temperature. For nuclear staining, vCTBs were incubated for 30 min with 50 µg/ml propidium iodide (P4170; MilliporeSigma) at room temperature, whereras BeWo cells were treated with the DAPI NucBlue Cell Stain ReadyProbes reagent (R37605; Thermo Fisher Scientific) for 45 min at room temperature. Cells were washed 3 times with PBS before coverslips were mounted on a drop of ProLong Gold antifade reagent (P36930; Thermo Fisher Scientific). Cells were visualized with an A1R Nikon confocal unit attached to the inverted Eclipse Ti microscope suited for a Plan Fluor ×20/0.8 numerical aperture oil objective (Nikon Canada). A syncytium was defined as an agglomeration of 3 nuclei or more in the same cytoplasm (27). After analysis of microscopic fields (5–10/condition), a cellular fusion index was calculated by counting the number of nuclei in syncytia over the total number of nuclei per field and multiplying the results by 100 to obtain a percentage. An average percentage was calculated for each condition, and the experiment was repeated 3 times. For each experiment, around 1000 nuclei were counted per condition. A final cellular fusion index (means ± sem) is presented and represents the final average of 3 values obtained from 3 independent experiments.
Coimmunoprecipitation
Virus particle–containing supernatants were collected, cleared from dead cells by centrifugation at 300 g, and filtered through a 0.22-µm syringe filter. Viral supernatants were then concentrated using Amicon Ultra (4 ml), 10 kDa cutoff centrifugal filter units (UFC801024; MilliporeSigma) following the manufacturer’s instructions. Protein G Sepharose Xtra Magnetic Beads (GE Healthcare) were washed 3 times with cold PBS–0.1% Tween-20 (PBST) and incubated with 2 µg/ml anti-Flag antibody (F1804, mouse monoclonal Flag M2; MilliporeSigma) vs. 2 µg/ml isotypic anti-GFP antibody [GFP (B2) HRP, sc-9996; Santa Cruz Biotechnology, Dallas, TX, USA] for 2 h at room temperature on a rotation wheel. Beads were washed twice with cold PBST and 200 µl of concentrated viral supernatant were incubated with 5 µl of Flag- or isotypic control-coupled magnetic beads overnight at 4°C on a rotation wheel. Beads were then washed twice with cold PBST and then incubated with PBS or with Gal-1 (4 µM) supplemented with 50 mM lactose for 90 min. Beads were washed 3 times with cold PBST, and virus/Gal-1 complexes were eluted in 25 µl of elution buffer [1× NuPage sample reducing agent (Novex; Thermo Fisher Scientific), 1× Laemmli buffer in PBS] at 85°C for 10 min. Resulting samples were next analyzed by Western blot. For reverse coimmunoprecipitation, streptavidin sepharose magnetic beads (11205D, Dynabeads M-280 Streptavidin; Thermo Fisher Scientific) were washed 3 times in cold PBST before use. Streptavidin beads (25 µl/sample) were incubated with 4 µM recombinant biotinylated Gal-1 for 1 h at room temperature. Following 3 washes in cold PBST, Gal-1–coupled beads were incubated with 200 µl of Syn-2-Flag or 20 µl of env- control viral supernatants overnight at 4°C. After incubation, the beads were washed 3 times with cold PBST. Protein complexes were eluted using 25 µl of elution buffer (1× NuPage, 1× Laemmli buffer in PBS) and boiled at 85°C for 10 min before Western blot analysis.
Western blot analysis
Cell extracts (30 µg) were loaded on 12% acrylamide-bis acrylamide gels. For coimmunoprecipitation experiments, 25 µl of immunoprecipitated samples and input were loaded on 14% acrylamide-bis acrylamide gels. Migrated proteins were then transferred on 0.45 µm Amersham Hybond (GE Healthcare) or 0.2 µm Immun-Blot (Bio-Rad, Hercules, CA, USA) PVDF membranes. Membranes were blocked in PBST containing 5% BSA (MilliporeSigma) or 5% nonfat dry milk for 90 min. at room temperature and incubated overnight at 4°C with primary antibodies: mouse monoclonal anti–HIV-1 p24 antibody (1/200 dilution in PBST, from hybridoma 183-H12-5C, NIH AIDS Reagent Program), mouse monoclonal anti-Myc antibody (1/2000 from hybridoma MYC 9E10, purchased from ATCC), mouse monoclonal anti-Flag M2 antibody, (1/2000, F1804; MilliporeSigma), rabbit polyclonal anti-galectin-1 antibody (1/3000, ab25138; Abcam), or mouse monoclonal anti–glutaraldehyde-3-phosphate dehydrogenase antibody (1/2000, sc32233; Santa Cruz Biotechnology). Membranes were washed 3 times in PBST for 10 min before incubation with secondary antibodies: ECL HRP-conjugated sheep anti-mouse or donkey anti-rabbit IgG antibodies (1/5000, NA931; GE Healthcare). Proteins were detected following three 10 min washes in PBST in 10 ml of revealing solution (Sol. A: 0.1 M Tris-HCl pH 10.5, 18% H2O2 mixed with Sol B: 0.1 M Tris-HCl pH 10.5, 450 µM p-coumaric acid, 7.5 mM luminol) using the Fusion Fx7 apparatus (Montreal Biotech, Dorval, QC, Canada).
RT-PCR
BeWo (106 cells) cells were treated with either 0.5% DMSO or 50 µM forskolin for 6–24 h and total RNA was extracted with Trizol (Thermo Fisher Scientific) following manufacturer’s instructions. cDNA synthesis was performed on 3 µg of RNA with the High RT (RNAse H-) Kit following the manufacturer’s protocol (Bio Basic). Gal-1 and actin cDNA were specifically PCR amplified using the following sets of forward and reverse primers: Gal-1 forward 5′-GACTCAATCATGGCTTGTGTCTG-3′, Gal-1 reverse 5′-GCTGATTTCAGTCAAAGGCCACAC-3′, actin forward 5′-CGTGACATTAAGGAGAAGCTGT-3′, actin reverse 5′-CTCAGGAGGAGCAATGATCTTGAT-3′.
Statistical analysis
Unless otherwise mentioned, the results were obtained from 3 independent experiments and Prism 6 (GraphPad Software, La Jolla, CA, USA) was used for all statistical analyses. For comparison of 2 means, the unpaired Student t test was used. When multiple means were analyzed, 1- and 2-way ANOVA followed by a Bonferroni multiple comparison test was chosen.
RESULTS
Gal-1 is involved in the syncytialization of trophoblastic cells
A previous study had demonstrated that soluble Gal-1 was capable of stimulating cytotrophoblast fusion (35), although the mechanism behind this induction has not been clearly explained. We first sought to confirm the fusion-inducing capacity of Gal-1 in both primary vCTB and BeWo cells.
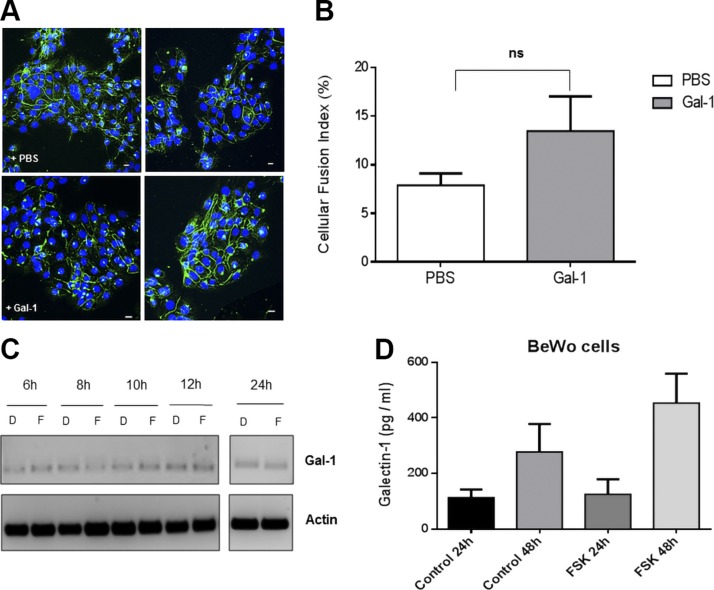
Freshly isolated vCTBs were initially cultured in the presence or absence of lactose, an antagonist of Gals, for 4 d, and cell fusion was observed by confocal microscopy after staining of cell membranes and nuclei. We observed that, in the presence of lactose, the size and number of syncytia was reduced compared with the control condition without lactose (Fig. 1A). We repeated this experiment with the choriocarcinoma BeWo cell line, which can fuse when treated with forskolin, in a Syn-1– and Syn-2–dependent manner (12, 69). BeWo cells were treated with either DMSO or 50 μM forskolin in the presence of lactose or not, and cell fusion was analyzed by measurement of a cellular fusion index. Lactose treatment had no significant effect on background levels of BeWo cell fusion, as shown by confocal images and by calculation of the cell fusion index (6.2 ± 0.65% for the PBS treatment vs. 7.3 ± 2.0% for the lactose treatment), whereas it significantly reduced fusion of forskolin-treated cells (21.3 ± 2.4% with PBS vs. 11.7 ± 1.2% with lactose, P = 0.037) (Fig. 1B). Because lactose is an antagonist of several Gals, we used commercially available siRNA directed against human Gal-1 to assess its role during trophoblastic cell fusion. BeWo cells were thus transfected with Gal-1–specific siRNA vs. control siRNA (scramble siRNAs) (Fig. 1C). Stained cells were analyzed for syncytium formation by confocal microscopy, and the results showed that scrambled siRNAs did not alter the levels of syncytialization upon forskolin treatment, whereas transfection of Gal-1 siRNA decreased syncytium formation in forskolin-induced BeWo cells (Fig. 1C). Our results thus confirmed the involvement of Gal-1 in syncytialization of trophoblastic cells.
Figure 1.
Gal-1 is necessary for optimal fusion of trophoblastic cells. A) Primary vCTBs were cultivated for 4 d in absence (left panels) or presence (right panels) of lactose. Plasma membrane (green) and nuclei (red). B) BeWo cells were treated with DMSO or forskolin (FSK; 50 µM) for 24 h before addition of PBS or lactose. Plasma membrane (green), nuclei (blue). Cellular fusion index, expressed as percentage of fusion, was calculated for each condition using the following formula: (NS/NT) × 100, in which NS is the number of nuclei in syncytia and NT is the total number of nuclei. *P < 0.05 (2-way ANOVA). C) BeWo cells were transfected with scrambled or Gal-1 siRNA and were then treated with either DMSO or FSK (50 µM). Cells were fixed and plasma membrane (green) and nuclei (red) stained 24 h after treatment to visualize cell fusion events. Lac, lactose; ns, not significant. Scale bar, 10 µm.
Gal-1 does not induce BeWo cell fusion and is not modulated by forskolin
Previous studies showed that the treatment of BeWo cells with extracellular Gal-1 was sufficient to induce syncytialization (35, 53). We wished to test these results in BeWo cells using a purification protocol for recombinant Gal-1, which has been demonstrated to lead to purified protein of high quality, retaining functional properties (57, 61). BeWo cells were thus directly treated with Gal-1 or PBS (control cells) and assessed for cell fusion, as depicted in Fig. 2A, B. Gal-1–treated BeWo cells had a slight increase in syncytia number compared with control cells, but the difference was not significant (7.89 ± 1.24% for PBS-treated cells and 13.5 ± 3.56% for the Gal-1–treated cells, P = 0.2114). These results were also confirmed by simple visual analysis of images taken from confocal microscopy, showing no marked induction of cell fusion upon addition of Gal-1. Furthermore, when BeWo cells were treated with Gal-1, the expression and promoter activity of Syn-2 was not altered, unlike in cells incubated with forskolin (unpublished results).
Figure 2.
Gal-1 alone does not induce cell fusion and is not modulated by cell activation. A) Confocal microscopy images of BeWo cells. Twenty-four hours after treatment with PBS (upper panel) or 4 µM recombinant Gal-1 (lower panel), BeWo cells were fixed and membranes (green) and nuclei (blue) were stained. Scale bar, 10 µm. B) The cellular fusion index was calculated from 3 independent experiments. C) RT-PCR analysis of Gal-1 and actin mRNA expression over time in DMSO- (D) or forskolin (F)-treated BeWo cells. D) ELISA analysis of the secretion of Gal-1 from BeWo cells. Conditioned media of DMSO- (control) or FSK-treated cells were collected 24 and 48 h after treatment and Gal-1 expression level was measured. Ns, not significant.
Because a determinant role of Gal-1 in cell fusion would be expected to lead to an induction of its expression during cell fusion, we also tested if Gal-1 levels changed during forskolin-induced cellular fusion by analyzing its expression level through RT-PCR (Fig. 2C). Interestingly, levels of transcripts did not vary between the DMSO- and forskolin-treated cells. Similarly, when the levels of released Gal-1 were measured by ELISA in the medium of BeWo cells at 24 and 48 h after forskolin treatment, no significant increase was observed when compared with control cells (treated with DMSO). The mean concentration of Gal-1 ± sem was 113 ± 29 pg/ml in DMSO-treated and 124.5 ± 54.5 pg/ml in forskolin-treated BeWo cells at 24 h, and 277 ± 101 vs. 453 ± 106 pg/ml at 48 h (Fig. 2D). Our data hence suggested that Gal-1 acts in combination with other factors during syncytialization of trophoblastic cells but cannot induce fusion on its own.
Analysis of infection of Syn-2–pseudotyped viruses
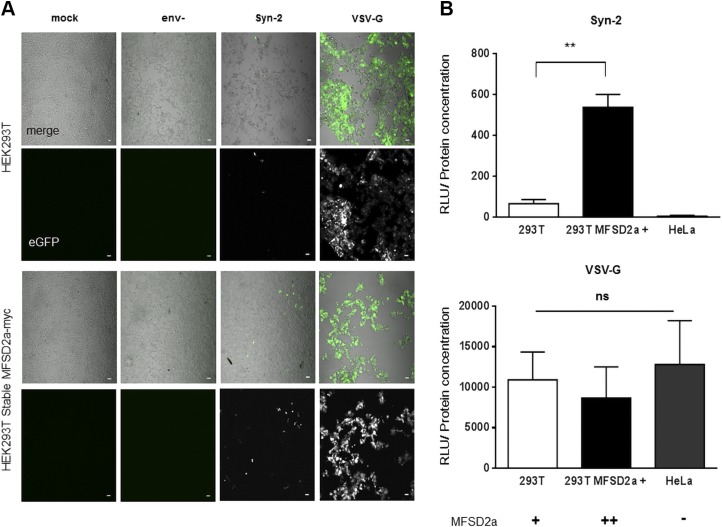
As previous studies had demonstrated that Gal-1 increases infection of retroviruses, such as HIV-1 and human T-cell leukemia virus type 1, and that this was mediated by its binding to the viral envelope protein (55–57), we speculated that extracellular Gal-1 could equally act on the interaction of Syn-2 with MFSD2a to increase cell fusion. To assess this, we generated Syn-2–pseudotyped viruses, expressing either GFP or luciferase. The infectivity of Syn-2–pseudotyped viruses was compared with 2 controls, 1 pseudotyped with the envelope VSV-G and the other lacking a viral envelope protein (env-). Because BeWo cells are resistant to infection with Syn-2–pseudotyped viruses, a feature that was already reported with cell-free HIV-1 infection (70, 71), we used permissive HEK293T cells for our infection assays (72). We also generated MFSD2a-overexpressing HEK293T cells by stably transfecting an MFSD2amyc expression vector. MFSD2amyc expression was analyzed in each generated clone, which were subsequently tested for infection with env- and Syn-2–pseudotyped viruses (Supplemental Fig. S1A, B). We first used the GFP-expressing pseudotyped viruses to infect parental or MFSD2a-overexpressing HEK293T cells by spinoculation of viral preparations. As presented in Fig. 3A and as expected, env- viral particles were noninfectious, whereas Syn-2–pseudotyped viruses were significantly less infectious than the VSV-G control (upper panel, Syn-2 vs. VSV-G). However, Syn-2–expressing viruses were notably more infectious in MFSD2a-overexpressing HEK293T cells, whereas no such difference was noted for VSV-G–pseudotyped viruses (Fig. 3A). These results were confirmed when luciferase-expressing pseudotyped viruses were tested. Results showed that the infectivity of Syn-2–pseudotyped viruses correlated with MFSD2a expression levels, with a significant increase of infection in the stably MFSD2a-overexpressing cells compared with parental 293T cells (Fig. 3B; 66.37 ± 19.23 for parental HEK293T cells vs. 537.7 ± 61.88 for the HEK293T MFSD2a cells, P = 0.0019) and no infection of HeLa cells, which are known to be deficient for MFSD2a expression (17). As expected, VSV-G–pseudotyped viruses presented no difference in the extent of infection between the different tested cells.
Figure 3.
Syn-2–pseudotyped viruses are infectious in MFSD2a-expressing cells. A) Confocal microscopy images of infected cells. Parental and stably MFSD2a-overexpressing HEK293T cells were mock-infected or infected with env-, Syn-2–, and VSV-G–pseudotyped viruses and GFP expression was next detected by confocal microscopy. Scale bar, 10 µm. B) Luciferase activities were measured from HEK293T and HeLa cells infected with luciferase-expressing Syn-2–pseudotyped viruses (top panel) or VSV-G–pseudotyped viruses (bottom panel). For each sample, luciferase activity was quantified 24 h postinfection (expressed as RLU) and normalized against protein concentration. Signs (+ and –) under the graphs indicate MFSD2a cellular expression level. These results are representative of 3 independent experiments. Ns, not significant **P < 0.01 (unpaired Student t test).
Gal-1 but not Gal-3 increases Syn-2–dependent infection
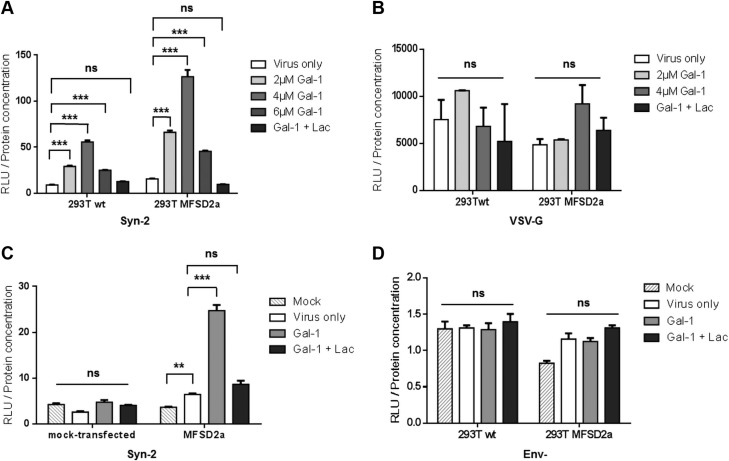
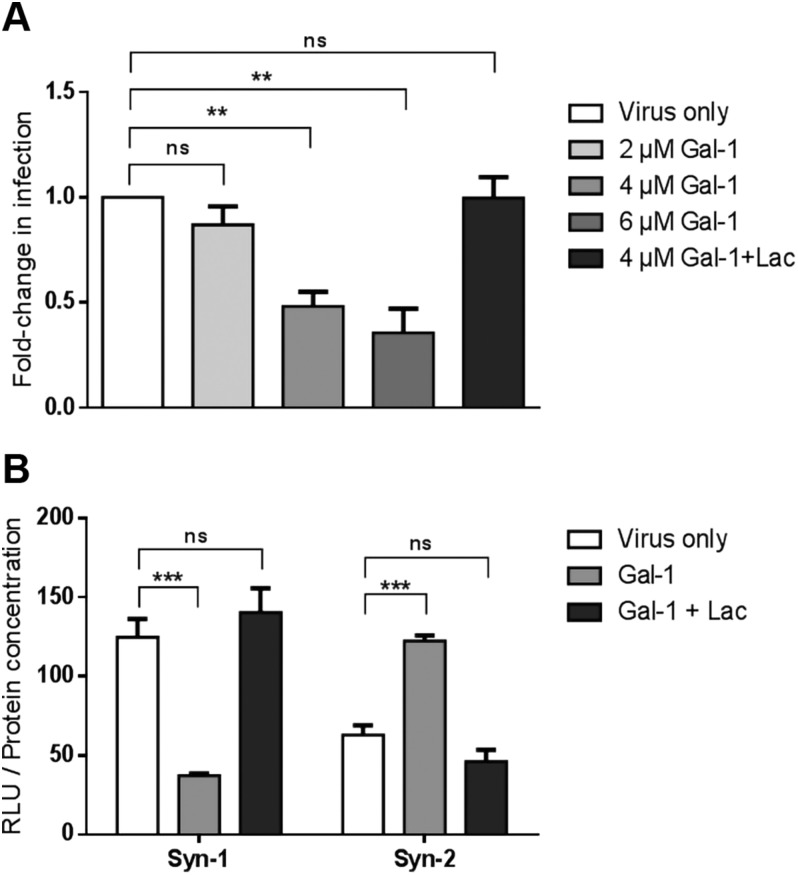
We next addressed the role of Gal-1 on the infectivity of Syn-2–pseudotyped viruses. Parental and MFSD2a stably expressing HEK293T cells were infected in presence or absence of increasing concentrations of Gal-1 (Fig. 4A, B). We found a significant dose-dependent increase in the infectivity of Syn-2-expressing viruses with Gal-1 (compared with the virus-only condition) on both parental and MFSD2a-overexpressing HEK293T cells, with optimal response being at a concentration of 4 µM Gal-1 (Fig. 4A). Interestingly, Gal-1 did not affect the infectivity of VSV-G–pseudotyped viruses (Fig. 4B, P = 0.304 by 2-way ANOVA). The specificity of Gal-1–induced increase of the infectivity of Syn-2–pseudotyped viruses was further revealed by its inhibition when lactose was added. As concentration above 4 µM resulted in lower induction (Fig. 4A), this concentration was chosen for subsequent experiments.
Figure 4.
Gal-1 specifically increases the infectivity of Syn-2–pseudotyped viruses in a MFSD2a-dependent manner. A, B) Parental- and MFSD2a-expressing HEK293T cells were infected (triplicate samples) with luciferase-expressing Syn-2– (A) or VSV-G– (B) pseudotyped viruses in the presence of PBS (virus only, white bars), increasing doses of Gal-1 (gray bars), or 4 µM of Gal-1 and lactose (dark gray bars). C) HeLa cells were mock-transfected or transfected with a MFSD2a expression vector and infected with mock (striped bars) or Syn-2–pseudotyped viruses combined with PBS (white bars), 4 µM Gal-1 (light gray bars), or 4 µM Gal-1 and lactose (dark gray bars). D) Parental and MFSD2a-expressing HEK293T cells were mock-infected (striped bars) or infected with Env- control viruses in the presence of PBS (white bars), 4 µM Gal-1 (light gray bars), or 4 μM Gal-1 + lactose (dark gray bars). Luciferase activities (expressed as RLU) were measured 24 h postinfection and normalized against protein concentration of each sample. The mean luciferase activity ± sem of triplicate samples is presented. Results are representative of 2 (C) or 3 (A, B, D) independent experiments. Lac, lactose; ns, not significant. **P < 0.01, ***P < 0.001 (2-way ANOVA).
As many glycosylated proteins are present in retroviral envelopes (73), we next sought to determine if Gal-1 could enhance the binding of viral particles to cell membranes in an env-independent manner. HeLa cells, which do not express MFSD2a protein and are resistant to infection by Syn-2–pseudotyped Simian immunodeficiency virus virions (17), were thus transfected with the MFSD2a expression vector and infected with Syn-2–pseudotyped viruses with or without Gal-1. The data presented in Fig. 4C indeed confirmed that the effect of Gal-1 depended on the expression of MFSD2a, as Gal-1 did not increase the infection of mock-transfected HeLa cells (Fig. 4C) but significantly increased the infection of MFSD2a-transfected cells. Moreover, env- viruses remained noninfectious in the presence of Gal-1 (Fig. 4D), showing that the observed effect of Gal-1 on Syn-2–pseudotyped viruses depended on the interaction between an envelope protein and its receptor. We also compared the level of infection of Syn-2–pseudotyped viruses between addition of Gal-1 at the start of the infection vs. pretreatment of cells prior to infection and found no differences in the Gal-1–mediated enhanced infectivity of Syn-2 viruses between the 2 conditions (unpublished results).
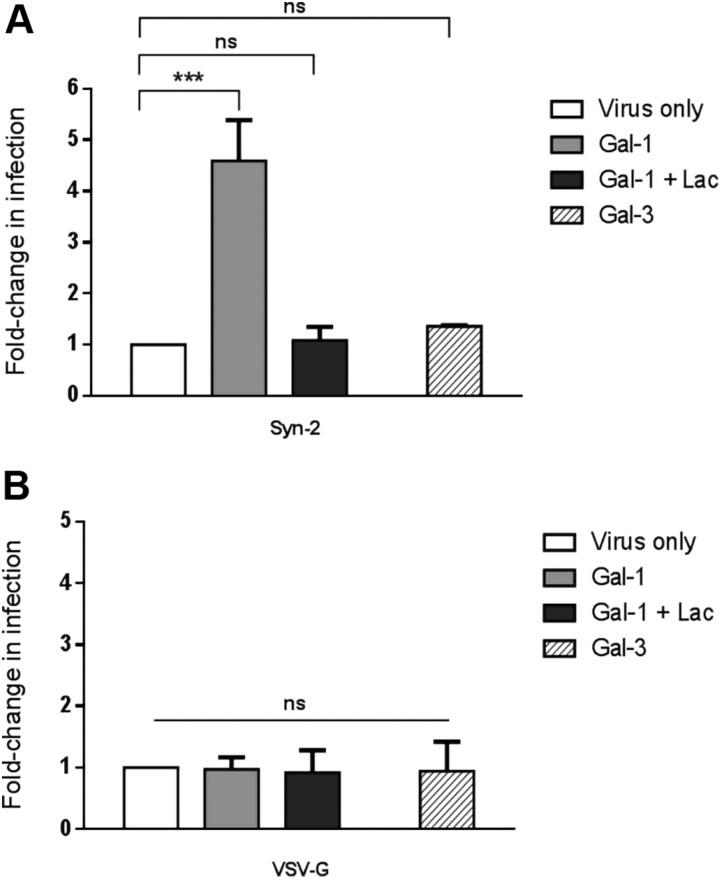
Because Gal-3 has important functions in the placenta (36), we wished to see if it could also modulate the infectivity of Syn-2–pseudotyped viruses. For this purpose, HEK293T cells were infected with luciferase-expressing pseudotyped viruses in the presence of either Gal-1 or Gal-3 (Fig. 5A). As expected, the addition of Gal-1 during infection significantly increased the infectivity of Syn-2–pseudotyped viruses, which was blocked by the addition of lactose (Fig. 5A). In contrast, Gal-3 did not significantly change the infectivity of Syn-2–expressing viruses. Gal-1 and Gal-3 showed no effect on the infectivity of VSV-G-pseudotyped viruses (Fig. 5B). These results hence demonstrated that, unlike Gal-3, Gal-1 had a specific enhancing effect over the infectivity of Syn-2–pseudotyped viruses.
Figure 5.
Gal-1 but not Gal-3 increases the infection rate of Syn-2–expressing viruses. HEK293T cells were infected (triplicate samples) with luciferase-expressing Syn-2– (A) or VSV-G– (B) pseudotyped viruses in the presence of PBS (virus only, white bars), 4 µM Gal-1 (light gray bars), 4 µM Gal-1 and lactose (dark gray bars), or 4 µM Gal-3 (crossed bars). The mean normalized luciferase activity ± sem was calculated for triplicates, and the results of 3 independent experiments are expressed as fold-change in infection over the virus only condition. Ns, not significant. ***P < 0.001 (2-way ANOVA).
Syn-1 and -2 behave differently in the presence of Gal-1
The fusogenic function of Syn-1 and -2 in the placenta is well established, and redundant functions have been suggested for both endogenous retroviral proteins, despite the fact that previous results from our group strongly support a more important role by Syn-2 (27). The demonstration that Gal-1 could potentiate the interaction of Syn-2 with its cellular receptor prompted us to investigate the response of Syn-1 to the addition of Gal-1. Infection of HEK293T cells with Syn-1–pseudotyped viruses in presence of increasing concentrations of Gal-1 surprisingly showed a dose-dependent decrease in infectivity, which was restored by the addition of lactose (Fig. 6A). Comparison between Syn-1– and Syn-2–pseudotyped viruses added to HEK293T cells in the presence of 4 µM Gal-1 confirmed the increase of infection of Syn-2–expressing-viruses, whereas a significant decrease in the infectivity of Syn-1–pseudotyped viruses was again measured (Fig. 6B). For each virus, the addition of lactose counteracted the Gal-1 modulation on their infectivity. In these experiments, cell viability was tested for all conditions and revealed no significant variation in between samples (unpublished results).
Figure 6.
Gal-1 differently affects Syn-1– and Syn-2–pseudotyped viruses. A) HEK293T cells were infected by Syn-1–pseudotyped viruses (triplicate samples) in the presence of PBS (virus only, white bars), increasing doses of recombinant Gal-1 (gray bars), or 4 μM Gal-1 + lactose (dark gray bars). Mean normalized luciferase activities ± sem was calculated, and the results of 3 independent experiments are expressed as fold-change in infectivity. **P < 0.01 (1-way ANOVA). B) HEK293T cells were infected with Syn-1– or Syn-2–pseudotyped viruses in the presence of PBS (white bars), 4 μM Gal-1 (gray bars), or 4 μM Gal-1 + lactose (dark gray bars). The mean normalized luciferase activity ± sem was calculated. Lac, lactose. Results are representative of 3 independent experiments. Ns, not significant ***P < 0.001 (2-way ANOVA).
Gal-1 potentiates the attachment of Syn-2–pseudotyped viruses to the cell surface
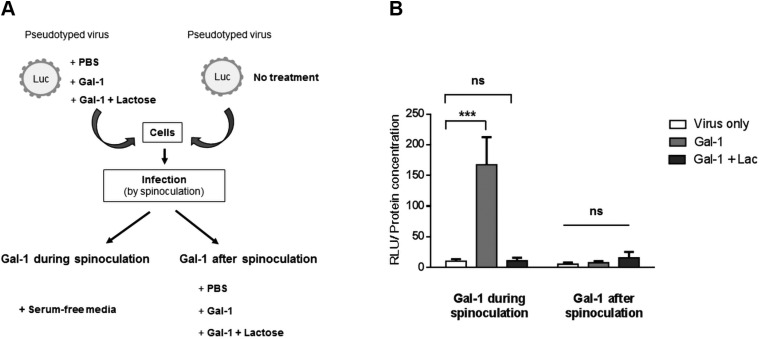
The infection cycle of enveloped viruses starts with the attachment of the viral envelope to its specific receptor and/or coreceptor expressed on the surface of the targeted cell. Following receptor binding, the viral particle can fuse directly with the cell plasma membrane or is first endocytosed and then fused with the endosomal membrane after internalization. Our results showed that Gal-1 increased the infectivity of Syn-2–pseudotyped viruses in a MFSD2a-dependent manner. We then wanted to determine if Gal-1 acted on the binding of Syn-2 with MFSD2a or on the fusion activity of Syn-2 after its interaction with MFSD2a. Thus, infection experiments with Syn-2–pseudotyped viruses were first performed at 4°C to block endocytosis followed by a shift to 37°C. Gal-1 was either added during the early phase of viral attachment or postattachment, i.e., before or after spinoculation, respectively (Fig. 7A). Results presented in Fig. 7B showed that Gal-1 increased the infection of HEK293T cells by Syn-2–pseudotyped viruses only when present during the attachment phase. Indeed, Gal-1 had a significant impact on viral infectivity during the binding of pseudotyped viruses but not after its binding at the fusion step. Syn-2–pseudotyped viruses showed similar infectivity than the virus-only condition when lactose was combined to Gal-1 during infection. These results hence provided evidence that Gal-1 acts at the early binding step of infection of Syn-2–pseudotyped viruses.
Figure 7.
Gal-1 enhances the attachment of Syn-2–pseudotyped viruses to cells. A) Schematic representation of the experiment. B) HEK293T cells (triplicate samples) were infected by Syn-2–pseudotyped viruses at 4°C in the presence or absence of PBS (virus only), 4 µM Gal-1 (light gray bars), or 4 µM Gal-1 + lactose (Lact; dark gray bars) (Gal-1 during spinoculation). After spinoculation, cells infected without Gal-1 were incubated for 1 h at 37°C with either PBS, 4 µM Gal-1, or 4 µM Gal-1 + lactose (Gal-1 after spinoculation). At 24 h postinfection, normalized luciferase activities were calculated and are represented as the mean normalized luciferase activity ± sem of 3 independent experiments. Ns, not significant ***P < 0.001 (2-way ANOVA).
Higher association between Gal-1 and viral particles in the presence of Syn-2
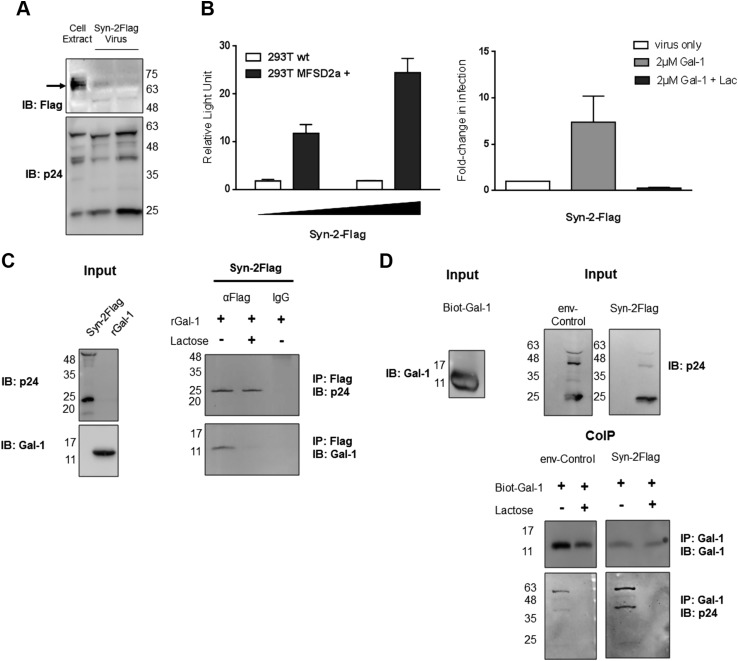
We were next interested in testing if Gal-1 showed higher affinity toward Syn-2–pseudotyped viruses. In order to address this, coimmunoprecipitation experiments using viral particles and recombinant Gal-1 were conducted. We first constructed a Flag-tagged Syn-2 expression vector and produced viral particles pseudotyped with this tagged version of Syn-2 (Fig. 8). Importantly, the addition of a tag at position 75–82 did not alter the fusogenic capacity of Syn-2 (Supplemental Fig. S1C). The expression of the protein could be further detected by Western blot analysis of lysed viral particles despite the weakness of the signal (Fig. 8A). In addition, compared with untagged Syn-2–pseudotyped viruses, Syn-2-Flag–pseudotyped viruses were similarly infectious toward parental and MFSD2a-overexpressing HEK293T cells and responded to the addition of Gal-1 during infection (Fig. 8B). Flag-tagged viral particles incubated with Gal-1 were then immunoprecipitated with anti-Flag or isotypic control antibody-coupled beads and analyzed by Western blot. As shown in Fig. 8C, Gal-1 was coimmunoprecipitated with Syn-2-Flag–pseudotyped viruses (detected by the presence of the HIV-1 p24 capsid) and the addition of lactose to the combination of Gal-1 and pseudotyped viruses competed out the binding of Gal-1. A reverse coimmunoprecipitation approach was next carried out with Syn-2-Flag and env- control pseudotyped viruses. A biotinylated version of recombinant Gal-1 was bound to streptavidin beads and used to coimmunoprecipitate pseudotyped viruses (Fig. 8D). Upon Western blot analyses of extracts before and after coimmunoprecipitation, the selective presence of p24 (and other more abundant noncleaved versions) was seen only on viruses incubated with Gal-1. The association of the viruses to Gal-1 was lost upon addition of lactose prior to immunoprecipitation (Fig. 8D). Importantly, the p24 signal was markedly more prominent for Syn-2–pseudotyped viruses in comparison with control env- viruses, whereas no such differences were observed in terms of immunoprecipitated Gal-1 or viral-associated p24 prior to immunoprecipitation.
Figure 8.
Lactose (Lact)-sensitive interaction between Syn-2–pseudotyped viruses and Gal-1. A) Tagged Syn-2–pseudotyped viruses and HEK293T-producing cells were analyzed by Western blot for p24 and Syn-2. B) Parental or stably MFSD2a-overexpressing HEK293T cells were infected with an increasing dose of Syn-2-Flag–pseudotyped viruses (left graph) or treated with PBS (white bar), Gal-1 (light gray bar), or Gal-1 + lactose (dark gray bar) (right graph). Mean normalized luciferase activity of triplicates ± sem expressed as RLU was calculated and presented on the left graph. For the right graph, fold-change in infection compared with the virus-only condition (calculated from 2 independent experiments) are presented. C, D) Gal-1 (including the biotinylated version in D) was incubated in the presence of env- and Syn-2-Flag–pseudotyped viruses and coimmunoprecipitated (CoIP) with anti-Flag antibodies (C) or streptavidin beads (D). Immunoprecipitated and input samples were analyzed by Western blot with anti–Gal-1 and anti-p24 antibodies. IB, immunoblot; IP, immunoprecipitate.
These results hence strongly suggest that Gal-1 interacts more importantly with Syn-2–pseudotyped viruses and that, consequently, Gal-1 binds to Syn-2.
DISCUSSION
The formation of the STB layer in the human placenta is a highly regulated process that involves many actors including transcription factors [glial cells missing transcription factor 1 (GCM1), cAMP response element-binding protein (CREB)] (69, 74, 75), hormones (human chorionic gonadotropin, estradiol) (2), fusogenic proteins (Syn-1 and -2) (8, 9, 12, 15), gap- and tight-junction proteins, adhesion proteins (connexin 43, ZO-1, cadherin-11) (76–78), and galectins (Gal-1) (35). Over the years, molecular mechanisms leading to cytotrophoblast cell fusion have been unraveled (3). However, many questions remain regarding the connection between all these syncytialization factors.
In this study, using pseudotyped viruses, we show for the first time a functional association between 2 proteins involved in vCTB fusion, namely, the endogenous retroviral protein Syn-2 and a β-galactoside–binding lectin, Gal-1. We found that Gal-1 specifically and significantly increased infectivity of Syn-2–pseudotyped viruses in MFSD2a-expressing cells and, inversely, reduced the infectivity of Syn-1–pseudotyped viruses. We present evidence that Gal-1 acts on the binding of Syn-2 to the cell surface, by cross-linking Syn-2–expressing viral particles and MFSD2a-expressing cells.
The role of endogenous retroviral proteins in STB formation is well established (8, 27), whereas the role of galectins in this specific placental process has been less extensively studied, except for the first identified member of this family, Gal-1 (35, 37). In this study, we first confirmed the role of Gal-1 in the fusion of vCTB and BeWo cells by the use of lactose, an antagonist of Gals, and Gal-1–specific siRNA. Although the involvement of Gal-1 in trophoblast fusion was already known, the mechanism behind its mode of action remained ill-defined (35, 37, 53). Our present results demonstrated that Gal-1 on its own could not induce cell fusion and that its expression was not modulated upon induced syncytialization. This thereby suggested that levels of Gal-1 in the media of unstimulated BeWo cells are sufficient for the protein to exert its function during syncytialization but further required additional factors involved in fusion. This is in contrast to a previous study by Fischer et al., which showed that Gal-1 on its own could induce trophoblast cell fusion. Differences in the purification protocol of the tested Gal-1 could explain the discrepancy between these results. It should also be noted that no lactose treatment was performed in the former study, which would have helped to assess the specificity of the observed Gal-1–induced cell fusion and Syn-1 expression (35).
Based on previous studies showing an association between Gal-1 and retroviral infection, we thereby tested if Syn-2 might be directly mediating the effect of Gal-1 through the use of Syn-2–pseudotyped viruses and HEK293T cells, which has been previously shown to be infected by such pseudotyped viruses (17, 72). Indeed, addition of Gal-1 during infection significantly increased the infectivity of Syn-2–pseudotyped viruses in a dose-dependent manner but had no effect on VSV-G–pseudotyped or env- viruses. Interestingly, high levels of Gal-1 resulted in reduced infectivity of Syn-2–pseudotyped viruses and could suggest that Gal-1 binds to other sites on the protein at higher doses and sterically inhibits the binding of Syn-2 with MFSD2a at high concentrations. Coimmunoprecipitation experiments between purified Gal-1 and pseudotyped viruses further suggested that Syn-2 contributed importantly to virus–Gal-1 interaction and that this effect was CRD-dependent (sensitive to the addition of lactose). The specific effect of Gal-1 on Syn-2–pseudotyped viruses was also confirmed to depend on the interaction with MFSD2a, as HeLa cells, which do not express this protein, were not infected by these viruses, even in the presence of Gal-1. Altogether, these results showed that, even though Gal-1 is expected to bind to viral particles when no envelope proteins are present, its activity still depends on the expression of Syn-2 and MFSD2a.
Our results also support that Gal-1 acts at the early step (before fusion) of infection of the pseudotyped viruses. The same results have been observed for both HIV-1 and the Nipah virus, in which Gal-1 increased the binding of viral particles to target cells (55, 57, 79). For these viruses, the N-linked glycan responsible for the binding of Gal-1 was shown to localize in close proximity to the fusion peptide or their envelope protein. Interestingly, previous work by Cui et al. has identified 8 N-linked glycans attached to asparagine residues on the surface subunit of Syn-2 and 1 in the TM (80), whereas no O-glycosylations have been reported to date on Syn-2. Thus, N-glycans are likely implicated in the binding of Gal-1 with Syn-2, and further analyses will be needed to identify which glycans are necessary for this interaction.
The effect of Gal-3, another important placental galectin (32, 81), was also tested. No difference in infection by Syn-2–pseudotyped viruses was measured. This result is surprising as Gal-3 can bind with high affinity to a wider array of glycans than Gal-1 (43). However, a similar selective impact of Gal-1 vs. -3 on the viral infectivity of HIV-1 has been previously reported (55). A subsequent study argues that selective binding of Gal-1 to native gp120 is likely dependent on peripheral N-acetyllactosamine sugars, whereas Gal-3 binding requires internal N-acetyllactosamine groups that are inaccessible because of the envelope folding (57). It is possible that the tridimensional conformation of Syn-2 similarly allows selective binding of Gal-1.
Very interestingly, when the infectivity of Syn-1–pseudotyped viruses was tested in the presence of Gal-1 and compared with Syn-2–pseudotyped viruses, we observed an inverse effect. Indeed, addition of increasing doses of Gal-1 during infection resulted in a lactose-sensitive decrease in infection by pseudotyped viruses. Syn-1 and -2 possess different numbers of N-glycosylation sites (14, 80). However, the arrangement and composition of the carbohydrates are unknown. Their receptors, SLC1A4, SLC1A5, and MFSD2a, all have 2 N-glycosylation sites (82, 83). Thus, the difference in the number, composition, and arrangement of carbohydrates of Syn-1, Syn-2, and their receptors likely determines the differential effect of Gal-1 toward Syn-1 and -2. Based on these premises, we are proposing that Gal-1 upon interaction with either Syn-1 and/or its receptors is positioned in an orientation that leads to the blockade of key regions required for the interaction between Syn-1 and its receptors. Certain N-glycosylation sites in Syn-1, SLC1A4, or SLC1A5 with affinity toward Gal-1 would thereby be responsible for this competitive binding of Gal-1 toward one of these interacting partners, leading to weakening of their association. It is, however, important to emphasize that, because glycosylation varies between cells, we cannot be certain that glycan composition of Syn proteins or their receptors in HEK293T reflects the in vivo glycosylation state of these proteins in the placenta. This is particularly relevant, as Syn-1 and -2 are differentially expressed in the placenta, with Syn-1 and the SLC1A4 receptor being expressed in fusogenic vCTB and nonfusogenic EVT (20, 21) and Syn-2 being restricted to vCTB (11, 19). Interestingly, the analysis of the N-glycan composition of STB, vCTB, and EVT cells showed differences in the structural organization of carbohydrates, with vCTB and STB bearing mainly biantennary N-glycans and EVT multiantennary N-glycans (84). Importantly, binding affinity of Gal proteins toward bi- and multiantennary N-glycans differs (43, 57) and could consequently impact the susceptibility of glycosylated Syn-1 and Syn-2 and/or their receptors toward a modulatory effect by Gal-1 on their fusogenic properties. Further analysis of the composition of Syn-1 and -2 glycans as well as biomolecular analysis are needed to identify the glycosylation residues responsible for the binding of Gal-1 and understand potential different binding of Gal-1 toward Syn proteins.
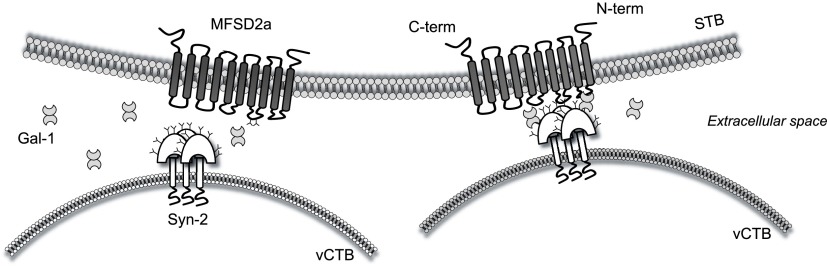
Altogether, our results show a specific and functional association between the endogenous retroviral protein Syn-2 and the β-galactoside–binding protein Gal-1, indicating an opposite response of Syn-1 and -2 to Gal-1. We propose that Gal-1 could favor or stabilize the interaction between Syn-2 and its cellular receptor MFSD2a during syncytialization of trophoblast cells. This could be achieved by bridge formation of Gal-1 between Syn-2 and MFSD2a (Fig. 9). Furthermore, as we and others have shown that Syn-1 and -2 are present at the surface of placental extracellular vesicles (28, 30), our results further argue that Gal-1 could also play a role in the cellular internalization or fusion of these vesicles to target cells expressing their receptor. The functional association between Gal-1 and Syn-2 is of specific interest in the context of PE, in which both proteins are dysregulated (15, 27, 32, 85, 86). More studies are needed to further address this new regulatory mechanism in the context of this placental disorder.
Figure 9.
Proposed model of the association between Gal-1, Syn-2, and MFSD2a during vCTB fusion. Gal-1 binds to N-glycans (Y) on Syn-2 and MFSD2a and stabilizes their interaction thereby optimizing vCTB fusion. The 2 N-glycosylation sites positioned on the second extracellular loop of MFSD2a are shown.
ACKNOWLEDGMENTS
The authors thank Denis Flipo, Xavier Elisseeff, and Antoine Beaulieu (all from Université du Québec à Montréal) for their technical assistance. The authors are also grateful to the researchers who supplied expression vectors. This study was supported by the National Sciences and Engineering Research Council of Canada (298527-2013), and by an operating grant from the Canadian Institutes of Health Research (MOP130561). The authors declare no conflicts of interest.
Glossary
- BSA
bovine serum albumin
- CRD
carbohydrate-recognition domain
- env
envelope
- EVT
extravillous trophoblast
- FBS
fetal bovine serum
- Gal
galectin
- GFP
green fluorescence protein
- HEK
human embryonic kidney
- HRP
horseradish peroxidase
- MFSD2a
major facilitator superfamily domain-containing 2A
- PBST
PBS–0.1% Tween-20
- PE
preeclampsia
- PEI
polyethylenimine
- RLU
relative light unit
- siRNA
small interfering RNA
- SLC1
solute carrier family 1
- STB
syncytiotrophoblast
- Syn
syncytin
- TM
transmembrane
- vCTB
villous cytotrophoblast
- VSV-G
vesicular stomatitis virus G-protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
C. Toudic designed and executed the experiments, analyzed and critically discussed the results, and drafted the manuscript; A. Vargas, Y. Xiao, and G. St-Pierre executed experimental work; N. Bannert contributed new reagents; N. Bannert, J. Lafond, and É. Rassart contributed to the critical discussion of the results; S. Sato participated in the study design, provided expert analysis of the results, and finalized the manuscript; and B. Barbeau designed and coordinated the study and helped in drafting and finalizing the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Huppertz B., Gauster M. (2011) Trophoblast fusion. Adv. Exp. Med. Biol. 713, 81–95 [DOI] [PubMed] [Google Scholar]
- 2.Costa M. A. (2016) Scrutinising the regulators of syncytialization and their expression in pregnancy-related conditions. Mol. Cell. Endocrinol. 420, 180–193 [DOI] [PubMed] [Google Scholar]
- 3.Gerbaud P., Pidoux G. (2015) Review: an overview of molecular events occurring in human trophoblast fusion. Placenta 36 (Suppl 1), S35–S42 [DOI] [PubMed] [Google Scholar]
- 4.Gauster M., Siwetz M., Huppertz B. (2009) Fusion of villous trophoblast can be visualized by localizing active caspase 8. Placenta 30, 547–550 [DOI] [PubMed] [Google Scholar]
- 5.Barrientos G., Freitag N., Tirado-González I., Unverdorben L., Jeschke U., Thijssen V. L., Blois S. M. (2014) Involvement of galectin-1 in reproduction: past, present and future. Hum. Reprod. Update 20, 175–193 [DOI] [PubMed] [Google Scholar]
- 6.Lokossou A. G., Toudic C., Barbeau B. (2014) Implication of human endogenous retrovirus envelope proteins in placental functions. Viruses 6, 4609–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toudic C., Elisseeff X., Lokossou A. G., Barbeau B. (2017) Roles of endogenous retrovirus-encoded Syncytins in human placentation. In Human Retrotransposons in Health and Disease (Cristofari G., ed.), pp. 215–238, Springer International Publishing, Cham, Switzerland: [Google Scholar]
- 8.Mi S., Lee X., Li X., Veldman G. M., Finnerty H., Racie L., LaVallie E., Tang X. Y., Edouard P., Howes S., Keith J. C., Jr., McCoy J. M. (2000) Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403, 785–789 [DOI] [PubMed] [Google Scholar]
- 9.Frendo J. L., Olivier D., Cheynet V., Blond J. L., Bouton O., Vidaud M., Rabreau M., Evain-Brion D., Mallet F. (2003) Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol. Cell. Biol. 23, 3566–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaise S., de Parseval N., Bénit L., Heidmann T. (2003) Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. USA 100, 13013–13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malassiné A., Blaise S., Handschuh K., Lalucque H., Dupressoir A., Evain-Brion D., Heidmann T. (2007) Expression of the fusogenic HERV-FRD Env glycoprotein (syncytin 2) in human placenta is restricted to villous cytotrophoblastic cells. Placenta 28, 185–191 [DOI] [PubMed] [Google Scholar]
- 12.Vargas A., Moreau J., Landry S., LeBellego F., Toufaily C., Rassart E., Lafond J., Barbeau B. (2009) Syncytin-2 plays an important role in the fusion of human trophoblast cells. J. Mol. Biol. 392, 301–318 [DOI] [PubMed] [Google Scholar]
- 13.Esnault C., Cornelis G., Heidmann O., Heidmann T. (2013) Differential evolutionary fate of an ancestral primate endogenous retrovirus envelope gene, the EnvV syncytin, captured for a function in placentation. PLoS Genet. 9, e1003400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheynet V., Ruggieri A., Oriol G., Blond J. L., Boson B., Vachot L., Verrier B., Cosset F. L., Mallet F. (2005) Synthesis, assembly, and processing of the Env ERVWE1/syncytin human endogenous retroviral envelope. J. Virol. 79, 5585–5593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C. P., Chen L. F., Yang S. R., Chen C. Y., Ko C. C., Chang G. D., Chen H. (2008) Functional characterization of the human placental fusogenic membrane protein syncytin 2. Biol. Reprod. 79, 815–823 [DOI] [PubMed] [Google Scholar]
- 16.Lavillette D., Marin M., Ruggieri A., Mallet F., Cosset F. L., Kabat D. (2002) The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J. Virol. 76, 6442–6452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esnault C., Priet S., Ribet D., Vernochet C., Bruls T., Lavialle C., Weissenbach J., Heidmann T. (2008) A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc. Natl. Acad. Sci. USA 105, 17532–17537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toufaily C., Vargas A., Lemire M., Lafond J., Rassart E., Barbeau B. (2013) MFSD2a, the Syncytin-2 receptor, is important for trophoblast fusion. Placenta 34, 85–88 [DOI] [PubMed] [Google Scholar]
- 19.Lu X., Wang R., Zhu C., Wang H., Lin H. Y., Gu Y., Cross J. C., Wang H. (2017) Fine-tuned and cell-cycle-restricted expression of fusogenic protein syncytin-2 maintains functional placental syncytia. Cell Rep. 21, 1150–1159; erratum: 23, 3979 [DOI] [PubMed] [Google Scholar]
- 20.Malassiné A., Handschuh K., Tsatsaris V., Gerbaud P., Cheynet V., Oriol G., Mallet F., Evain-Brion D. (2005) Expression of HERV-W Env glycoprotein (syncytin) in the extravillous trophoblast of first trimester human placenta. Placenta 26, 556–562 [DOI] [PubMed] [Google Scholar]
- 21.Muir A., Lever A. M., Moffett A. (2006) Human endogenous retrovirus-W envelope (syncytin) is expressed in both villous and extravillous trophoblast populations. J. Gen. Virol. 87, 2067–2071 [DOI] [PubMed] [Google Scholar]
- 22.Huang Q., Li J., Wang F., Oliver M. T., Tipton T., Gao Y., Jiang S. W. (2013) Syncytin-1 modulates placental trophoblast cell proliferation by promoting G1/S transition. Cell. Signal. 25, 1027–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knerr I., Schnare M., Hermann K., Kausler S., Lehner M., Vogler T., Rascher W., Meissner U. (2007) Fusiogenic endogenous-retroviral syncytin-1 exerts anti-apoptotic functions in staurosporine-challenged CHO cells. Apoptosis 12, 37–43 [DOI] [PubMed] [Google Scholar]
- 24.Knerr I., Söder S., Licha E., Aigner T., Rascher W. (2008) Response of HEK293 and CHO cells overexpressing fusiogenic syncytin-1 to mitochondrion-mediated apoptosis induced by antimycin A. J. Cell. Biochem. 105, 766–775 [DOI] [PubMed] [Google Scholar]
- 25.Huang Q., Chen H., Wang F., Brost B. C., Li J., Gao Y., Li Z., Gao Y., Jiang S. W. (2014) Reduced syncytin-1 expression in choriocarcinoma BeWo cells activates the calpain1-AIF-mediated apoptosis, implication for preeclampsia. Cell. Mol. Life Sci. 71, 3151–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher S. J. (2015) Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 213(Suppl):S115–S122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vargas A., Toufaily C., LeBellego F., Rassart É., Lafond J., Barbeau B. (2011) Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod. Sci. 18, 1085–1091 [DOI] [PubMed] [Google Scholar]
- 28.Tolosa J. M., Schjenken J. E., Clifton V. L., Vargas A., Barbeau B., Lowry P., Maiti K., Smith R. (2012) The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta 33, 933–941 [DOI] [PubMed] [Google Scholar]
- 29.Lokossou A. G., Toudic C., Nguyen P. T., Elisseeff X., Vargas A., Rassart É., Lafond J., Leduc L., Bourgault S., Gilbert C., Scorza T., Tolosa J., Barbeau B. (2019) Endogenous retrovirus-encoded Syncytin-2 contributes to exosome-mediated immunosuppression of T cells. Biol. Reprod., ioz124 [DOI] [PubMed] [Google Scholar]
- 30.Vargas A., Zhou S., Éthier-Chiasson M., Flipo D., Lafond J., Gilbert C., Barbeau B. (2014) Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J. 28, 3703–3719 [DOI] [PubMed] [Google Scholar]
- 31.Vasta G. R., Feng C., González-Montalbán N., Mancini J., Yang L., Abernathy K., Frost G., Palm C. (2017) Functions of galectins as ‘self/non-self’-recognition and effector factors. Pathog. Dis. 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeschke U., Mayr D., Schiessl B., Mylonas I., Schulze S., Kuhn C., Friese K., Walzel H. (2007) Expression of galectin-1, -3 (gal-1, gal-3) and the Thomsen-Friedenreich (TF) antigen in normal, IUGR, preeclamptic and HELLP placentas. Placenta 28, 1165–1173 [DOI] [PubMed] [Google Scholar]
- 33.Maquoi E., van den Brûle F. A., Castronovo V., Foidart J. M. (1997) Changes in the distribution pattern of galectin-1 and galectin-3 in human placenta correlates with the differentiation pathways of trophoblasts. Placenta 18, 433–439 [DOI] [PubMed] [Google Scholar]
- 34.Vićovac L., Janković M., Cuperlović M. (1998) Galectin-1 and -3 in cells of the first trimester placental bed. Hum. Reprod. 13, 730–735 [DOI] [PubMed] [Google Scholar]
- 35.Fischer I., Redel S., Hofmann S., Kuhn C., Friese K., Walzel H., Jeschke U. (2010) Stimulation of syncytium formation in vitro in human trophoblast cells by galectin-1. Placenta 31, 825–832 [DOI] [PubMed] [Google Scholar]
- 36.Jeschke U., Hutter S., Heublein S., Vrekoussis T., Andergassen U., Unverdorben L., Papadakis G., Makrigiannakis A. (2013) Expression and function of galectins in the endometrium and at the human feto-maternal interface. Placenta 34, 863–872 [DOI] [PubMed] [Google Scholar]
- 37.Hutter S., Morales-Prieto D. M., Andergassen U., Tschakert L., Kuhn C., Hofmann S., Markert U. R., Jeschke U. (2016) Gal-1 silenced trophoblast tumor cells (BeWo) show decreased syncytium formation and different miRNA production compared to non-target silenced BeWo cells. Cell. Adh. Migr. 10, 28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poirier F., Timmons P. M., Chan C. T., Guénet J. L., Rigby P. W. (1992) Expression of the L14 lectin during mouse embryogenesis suggests multiple roles during pre- and post-implantation development. Development 115, 143–155 [DOI] [PubMed] [Google Scholar]
- 39.Fowlis D., Colnot C., Ripoche M. A., Poirier F. (1995) Galectin-3 is expressed in the notochord, developing bones, and skin of the postimplantation mouse embryo. Dev. Dyn. 203, 241–251 [DOI] [PubMed] [Google Scholar]
- 40.Ramhorst R. E., Giribaldi L., Fraccaroli L., Toscano M. A., Stupirski J. C., Romero M. D., Durand E. S., Rubinstein N., Blaschitz A., Sedlmayr P., Genti-Raimondi S., Fainboim L., Rabinovich G. A. (2012) Galectin-1 confers immune privilege to human trophoblast: implications in recurrent fetal loss. Glycobiology 22, 1374–1386 [DOI] [PubMed] [Google Scholar]
- 41.Blois S. M., Ilarregui J. M., Tometten M., Garcia M., Orsal A. S., Cordo-Russo R., Toscano M. A., Bianco G. A., Kobelt P., Handjiski B., Tirado I., Markert U. R., Klapp B. F., Poirier F., Szekeres-Bartho J., Rabinovich G. A., Arck P. C. (2007) A pivotal role for galectin-1 in fetomaternal tolerance. Nat. Med. 13, 1450–1457; erratum: 15, 584 [DOI] [PubMed] [Google Scholar]
- 42.Blidner A. G., Rabinovich G. A. (2013) ‘Sweetening’ pregnancy: galectins at the fetomaternal interface. Am. J. Reprod. Immunol. 69, 369–382 [DOI] [PubMed] [Google Scholar]
- 43.Hirabayashi J., Hashidate T., Arata Y., Nishi N., Nakamura T., Hirashima M., Urashima T., Oka T., Futai M., Muller W. E., Yagi F., Kasai K. (2002) Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572, 232–254 [DOI] [PubMed] [Google Scholar]
- 44.Cooper D. N., Barondes S. H. (1999) God must love galectins; he made so many of them. Glycobiology 9, 979–984 [DOI] [PubMed] [Google Scholar]
- 45.Sato S., Hughes R. C. (1994) Control of Mac-2 surface expression on murine macrophage cell lines. Eur. J. Immunol. 24, 216–221 [DOI] [PubMed] [Google Scholar]
- 46.Hirabayashi J., Kasai K. (1993) The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology 3, 297–304 [DOI] [PubMed] [Google Scholar]
- 47.Tirado-González I., Freitag N., Barrientos G., Shaikly V., Nagaeva O., Strand M., Kjellberg L., Klapp B. F., Mincheva-Nilsson L., Cohen M., Blois S. M. (2013) Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Hum. Reprod. 19, 43–53 [DOI] [PubMed] [Google Scholar]
- 48.Unverdorben L., Jeschke U., Santoso L., Hofmann S., Kuhn C., Arck P., Hutter S. (2016) Comparative analyses on expression of galectins1-4, 7-10 and 12 in first trimester placenta, decidua and isolated trophoblast cells in vitro. Histol. Histopathol. 31, 1095–1111 [DOI] [PubMed] [Google Scholar]
- 49.Unverdorben L., Haufe T., Santoso L., Hofmann S., Jeschke U., Hutter S. (2016) Prototype and chimera-type galectins in placentas with spontaneous and recurrent miscarriages. Int. J. Mol. Sci. 17, E644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeschke U., Reimer T., Bergemann C., Wiest I., Schulze S., Friese K., Walzel H. (2004) Binding of galectin-1 (gal-1) on trophoblast cells and inhibition of hormone production of trophoblast tumor cells in vitro by gal-1. Histochem. Cell Biol. 121, 501–508 [DOI] [PubMed] [Google Scholar]
- 51.Jeschke U., Karsten U., Wiest I., Schulze S., Kuhn C., Friese K., Walzel H. (2006) Binding of galectin-1 (gal-1) to the Thomsen-Friedenreich (TF) antigen on trophoblast cells and inhibition of proliferation of trophoblast tumor cells in vitro by gal-1 or an anti-TF antibody. Histochem. Cell Biol. 126, 437–444 [DOI] [PubMed] [Google Scholar]
- 52.Fischer I., Schulze S., Kuhn C., Friese K., Walzel H., Markert U. R., Jeschke U. (2009) Inhibiton of RET and JAK2 signals and upregulation of VEGFR3 phosphorylation in vitro by galectin-1 in trophoblast tumor cells BeWo. Placenta 30, 1078–1082 [DOI] [PubMed] [Google Scholar]
- 53.Fischer I., Weber M., Kuhn C., Fitzgerald J. S., Schulze S., Friese K., Walzel H., Markert U. R., Jeschke U. (2011) Is galectin-1 a trigger for trophoblast cell fusion?: the MAP-kinase pathway and syncytium formation in trophoblast tumour cells BeWo. Mol. Hum. Reprod. 17, 747–757 [DOI] [PubMed] [Google Scholar]
- 54.Than N. G., Erez O., Wildman D. E., Tarca A. L., Edwin S. S., Abbas A., Hotra J., Kusanovic J. P., Gotsch F., Hassan S. S., Espinoza J., Papp Z., Romero R. (2008) Severe preeclampsia is characterized by increased placental expression of galectin-1. J. Matern. Fetal Neonatal Med. 21, 429–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ouellet M., Mercier S., Pelletier I., Bounou S., Roy J., Hirabayashi J., Sato S., Tremblay M. J. (2005) Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J. Immunol. 174, 4120–4126 [DOI] [PubMed] [Google Scholar]
- 56.Gauthier S., Pelletier I., Ouellet M., Vargas A., Tremblay M. J., Sato S., Barbeau B. (2008) Induction of galectin-1 expression by HTLV-I Tax and its impact on HTLV-I infectivity. Retrovirology 5, 105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.St-Pierre C., Manya H., Ouellet M., Clark G. F., Endo T., Tremblay M. J., Sato S. (2011) Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J. Virol. 85, 11742–11751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kliman H. J., Nestler J. E., Sermasi E., Sanger J. M., Strauss J. F., III (1986) Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118, 1567–1582 [DOI] [PubMed] [Google Scholar]
- 59.Le Bellego F., Vaillancourt C., Lafond J. (2009) Isolation and culture of term human cytotrophoblast cells and in vitro methods for studying human cytotrophoblast cells’ calcium uptake. Methods Mol. Biol. 550, 73–87 [DOI] [PubMed] [Google Scholar]
- 60.Wice B., Menton D., Geuze H., Schwartz A. L. (1990) Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp. Cell Res. 186, 306–316 [DOI] [PubMed] [Google Scholar]
- 61.Mercier S., St-Pierre C., Pelletier I., Ouellet M., Tremblay M. J., Sato S. (2008) Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology 371, 121–129 [DOI] [PubMed] [Google Scholar]
- 62.Bhaumik P., St-Pierre G., Milot V., St-Pierre C., Sato S. (2013) Galectin-3 facilitates neutrophil recruitment as an innate immune response to a parasitic protozoa cutaneous infection. J. Immunol. 190, 630–640 [DOI] [PubMed] [Google Scholar]
- 63.Sato S., Ouellet N., Pelletier I., Simard M., Rancourt A., Bergeron M. G. (2002) Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J. Immunol. 168, 1813–1822 [DOI] [PubMed] [Google Scholar]
- 64.St-Pierre C., Ouellet M., Tremblay M. J., Sato S. (2010) Galectin-1 and HIV-1 infection. Methods Enzymol. 480, 267–294 [DOI] [PubMed] [Google Scholar]
- 65.Ouellet M., St-Pierre C., Tremblay M. J., Sato S. (2015) Effect of galectins on viral transmission. Methods Mol. Biol. 1207, 397–420 [DOI] [PubMed] [Google Scholar]
- 66.Hong S., Klein E. A., Das Gupta J., Hanke K., Weight C. J., Nguyen C., Gaughan C., Kim K. A., Bannert N., Kirchhoff F., Munch J., Silverman R. H. (2009) Fibrils of prostatic acid phosphatase fragments boost infections with XMRV (xenotropic murine leukemia virus-related virus), a human retrovirus associated with prostate cancer. J. Virol. 83, 6995–7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spinola M., Falvella F. S., Colombo F., Sullivan J. P., Shames D. S., Girard L., Spessotto P., Minna J. D., Dragani T. A. (2010) MFSD2A is a novel lung tumor suppressor gene modulating cell cycle and matrix attachment. Mol. Cancer 9, 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemieux A. M., Paré M. E., Audet B., Legault E., Lefort S., Boucher N., Landry S., van Opijnen T., Berkhout B., Naghavi M. H., Tremblay M. J., Barbeau B. (2004) T-cell activation leads to poor activation of the HIV-1 clade E long terminal repeat and weak association of nuclear factor-kappaB and NFAT with its enhancer region. J. Biol. Chem. 279, 52949–52960 [DOI] [PubMed] [Google Scholar]
- 69.Toufaily C., Lokossou A. G., Vargas A., Rassart É., Barbeau B. (2015) A CRE/AP-1-like motif is essential for induced syncytin-2 expression and fusion in human trophoblast-like model. PLoS One 10, e0121468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dolcini G., Derrien M., Chaouat G., Barré-Sinoussi F., Menu E. (2003) Cell-free HIV type 1 infection is restricted in the human trophoblast choriocarcinoma BeWo cell line, even with expression of CD4, CXCR4 and CCR5. AIDS Res. Hum. Retroviruses 19, 857–864 [DOI] [PubMed] [Google Scholar]
- 71.Vidricaire G., Tardif M. R., Tremblay M. J. (2003) The low viral production in trophoblastic cells is due to a high endocytic internalization of the human immunodeficiency virus type 1 and can be overcome by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-1. J. Biol. Chem. 278, 15832–15841 [DOI] [PubMed] [Google Scholar]
- 72.Blaise S., Ruggieri A., Dewannieux M., Cosset F. L., Heidmann T. (2004) Identification of an envelope protein from the FRD family of human endogenous retroviruses (HERV-FRD) conferring infectivity and functional conservation among simians. J. Virol. 78, 1050–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Segura M. M., Garnier A., Di Falco M. R., Whissell G., Meneses-Acosta A., Arcand N., Kamen A. (2008) Identification of host proteins associated with retroviral vector particles by proteomic analysis of highly purified vector preparations. J. Virol. 82, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schubert S. W., Abendroth A., Kilian K., Vogler T., Mayr B., Knerr I., Hashemolhosseini S. (2008) bZIP-Type transcription factors CREB and OASIS bind and stimulate the promoter of the mammalian transcription factor GCMa/Gcm1 in trophoblast cells. Nucleic Acids Res. 36, 3834–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu C., Shen K., Lin M., Chen P., Lin C., Chang G. D., Chen H. (2002) GCMa regulates the syncytin-mediated trophoblastic fusion. J. Biol. Chem. 277, 50062–50068 [DOI] [PubMed] [Google Scholar]
- 76.Pidoux G., Gerbaud P., Gnidehou S., Grynberg M., Geneau G., Guibourdenche J., Carette D., Cronier L., Evain-Brion D., Malassiné A., Frendo J. L. (2010) ZO-1 is involved in trophoblastic cell differentiation in human placenta. Am. J. Physiol. Cell Physiol. 298, C1517–C1526 [DOI] [PubMed] [Google Scholar]
- 77.Getsios S., MacCalman C. D. (2003) Cadherin-11 modulates the terminal differentiation and fusion of human trophoblastic cells in vitro. Dev. Biol. 257, 41–54 [DOI] [PubMed] [Google Scholar]
- 78.Frendo J. L., Cronier L., Bertin G., Guibourdenche J., Vidaud M., Evain-Brion D., Malassine A. (2003) Involvement of connexin 43 in human trophoblast cell fusion and differentiation. J. Cell Sci. 116, 3413–3421 [DOI] [PubMed] [Google Scholar]
- 79.Garner O. B., Yun T., Pernet O., Aguilar H. C., Park A., Bowden T. A., Freiberg A. N., Lee B., Baum L. G. (2015) Timing of galectin-1 exposure differentially modulates Nipah virus entry and syncytium formation in endothelial cells. J. Virol. 89, 2520–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cui L., Wang H., Lu X., Wang R., Zheng R., Li Y., Yang X., Jia W. T., Zhao Y., Wang Y., Wang H., Wang Y. L., Zhu C., Lin H. Y., Wang H. (2016) Effects of individually silenced N-glycosylation sites and non-synonymous single-nucleotide polymorphisms on the fusogenic function of human syncytin-2. Cell Adhes. Migr. 10, 39–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dalton P., Christian H. C., Redman C. W., Sargent I. L., Boyd C. A. (2007) Membrane trafficking of CD98 and its ligand galectin 3 in BeWo cells--implication for placental cell fusion. FEBS J. 274, 2715–2727 [DOI] [PubMed] [Google Scholar]
- 82.Marin M., Lavillette D., Kelly S. M., Kabat D. (2003) N-linked glycosylation and sequence changes in a critical negative control region of the ASCT1 and ASCT2 neutral amino acid transporters determine their retroviral receptor functions. J. Virol. 77, 2936–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reiling J. H., Clish C. B., Carette J. E., Varadarajan M., Brummelkamp T. R., Sabatini D. M. (2011) A haploid genetic screen identifies the major facilitator domain containing 2A (MFSD2A) transporter as a key mediator in the response to tunicamycin. Proc. Natl. Acad. Sci. USA 108, 11756–11765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Q., Pang P. C., Cohen M. E., Longtine M. S., Schust D. J., Haslam S. M., Blois S. M., Dell A., Clark G. F. (2016) Evidence for differential glycosylation of trophoblast cell types. Mol. Cell. Proteomics 15, 1857–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freitag N., Tirado-González I., Barrientos G., Herse F., Thijssen V. L., Weedon-Fekjær S. M., Schulz H., Wallukat G., Klapp B. F., Nevers T., Sharma S., Staff A. C., Dechend R., Blois S. M. (2013) Interfering with Gal-1-mediated angiogenesis contributes to the pathogenesis of preeclampsia. Proc. Natl. Acad. Sci. USA 110, 11451–11456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hirashima C., Ohkuchi A., Nagayama S., Suzuki H., Takahashi K., Ogoyama M., Takahashi H., Shirasuna K., Matsubara S. (2018) Galectin-1 as a novel risk factor for both gestational hypertension and preeclampsia, specifially its expression at a low level in the second trimester and a high level after onset. Hypertens. Res. 41, 45–52 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.