Abstract
Amyloid-β (Aβ) is derived from the proteolytic processing of amyloid precursor protein (APP), and the deposition of extracellular Aβ to form amyloid plaques is a pathologic hallmark of Alzheimer’s disease (AD). Although reducing Aβ generation and accumulation has been proposed as a means of treating the disease, adverse side effects and unsatisfactory efficacy have been reported in several clinical trials that sought to lower Aβ levels. Engulfment adaptor phosphotyrosine-binding (PTB) domain containing 1 (GULP1) is a molecular adaptor that has been shown to interact with APP to alter Aβ production. Therefore, the modulation of the GULP1-APP interaction may be an alternative approach to reducing Aβ. However, the mechanisms that regulate GULP1-APP binding remain elusive. As GULP1 is a phosphoprotein, and because phosphorylation is a common mechanism that regulates protein interaction, we anticipated that GULP1 phosphorylation would influence GULP1-APP interaction and thereby Aβ production. We show here that the phosphorylation of GULP1 threonine 35 (T35) reduces GULP1-APP interaction and suppresses the stimulatory effect of GULP1 on APP processing. The residue is phosphorylated by an isoform of atypical PKC (PKCζ). Overexpression of PKCζ reduces both GULP1-APP interaction and GULP1-mediated Aβ generation. Moreover, the activation of PKCζ via insulin suppresses APP processing. In contrast, GULP1-mediated APP processing is enhanced in PKCζ knockout cells. Similarly, PKC ι, another member of atypical PKC, also decreases GULP1-mediated APP processing. Intriguingly, our X-ray crystal structure of GULP1 PTB-APP intracellular domain (AICD) peptide reveals that GULP1 T35 is not located at the GULP1-AICD binding interface; rather, it immediately precedes the β1-α2 loop that forms a portion of the binding groove for the APP helix αC. Phosphorylating the residue may induce an allosteric effect on the conformation of the binding groove. Our results indicate that GULP1 T35 phosphorylation is a mechanism for the regulation of GULP1-APP interaction and thereby APP processing. Moreover, the activation of atypical PKC, such as by insulin, may confer a beneficial effect on AD by lowering GULP1-mediated Aβ production.—Chau, D. D.-L., Yung, K. W.-Y., Chan, W. W.-L., An, Y., Hao, Y., Chan, H.-Y. E., Ngo, J. C.-K., Lau, K.-F. Attenuation of amyloid-β generation by atypical protein kinase C–mediated phosphorylation of engulfment adaptor PTB domain containing 1 threonine 35.
Keywords: Alzheimer’s disease, GULP1, amyloid precursor protein
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder that currently has no effective treatment or cure. A pathologic hallmark of AD is the presence of amyloid-β (Aβ) peptide–containing senile plaques. Aβ is derived from amyloid precursor protein (APP) processing via the catalytic cleavage of β- and γ-secretases. The aberrant generation and accumulation of Aβ are believed to be central to the pathogenesis of AD. Hence, reducing Aβ production and enhancing Aβ clearance are straightforward strategies for treating AD. Although several APP secretase inhibitors have been developed to decrease Aβ generation, unsatisfactory results have been obtained because these inhibitors have significant undesirable effects via their influence on the processing of substrates other than APP. Indeed, a phase III clinical trial of a γ-secretase inhibitor was recently halted because of the reported CNS side effects (1). On the other hand, Eli Lilly recently announced that the Aβ antibody drug solanezumab, which was supposed to enhance the clearance of Aβ, failed to demonstrate efficacy in a phase III clinical trial (2). Alternative therapeutic approaches for the reduction of Aβ levels are clearly desirable.
An increasing body of evidence suggests that APP intracellular domain (AICD)-interacting proteins (AIPs) can influence APP metabolism and Aβ generation [see reviews (3, 4)]. Recently, our group and others have identified engulfment adaptor phosphotyrosine-binding domain (PTB) domain containing 1 (GULP1) as a novel AIP that alters APP processing and Aβ generation (5, 6). Such interaction is mediated by GULP1 PTB domain, and APP NPTY motif (5). Because GULP1-APP interaction plays a role in APP processing, targeting this interaction may be an alternative therapeutic strategy to modulate Aβ production. However, the mechanism by which GULP1-APP interaction is regulated remains unknown. An important mechanism that regulates protein-protein interactions is phosphorylation. Phosphoproteomic and mass spectrometric analyses from various laboratories have shown that GULP1 is a phosphoprotein (7–10); however, the biologic significance of these phosphorylated (phospho) residues is largely unknown. Among these residues, T35 within GULP1 PTB is conserved in various species; therefore, GULP1 T35 phosphorylation may play a role in the modulation of GULP1-APP interaction and in turn APP processing. We show here that GULP1 T35 phosphomimetic mutation reduces GULP1-APP interaction and, most importantly, Aβ generation. Moreover, we identify atypical PKCs, including PKCζ and PKCι, as candidate kinases for GULP1 T35. The activation of PKCζ by insulin enhances GULP1 phosphorylation and decreases APP processing. To gain further insight into how GULP1 T35 phosphorylation modulates GULP1-APP interaction, the crystal structure of GULP1 PTB in complex with APP NPTY is also elucidated in our study [Protein Data Bank (PDB; http://www.rcsb.org/) crystal structure of the GULP1 PTB domain-APP peptide complex ID: 6ITU].
MATERIALS AND METHODS
Plasmids
Mammalian expression construct for APP695, hemagglutinin (HA)-tagged full-length GULP1 and untagged GULP1 were as described (5, 11, 12). Bacterial glutathione-S-transferase (GST)-APPc and 6X-H-GULP1 PTB (residues 1 to 168) were as previously described (5, 11). GULP1 T35 phosphomimetic and dephosphomimetic mutants (i.e., T35A and T35D, respectively) were generated by QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) using the following primers: for T35A, 5′-CAAAGTTTCTTGGCAGTGCAGAAGTGGAACAGCC-3′; 5′-GGCTGTTCCACTTCTGCACTGCCAAGAAACTTTG-3′; for T35D, 5′-GCAAAGTTTCTTGGCAGTGACGAAGTGGAACAGCC-3′; 5′-CTTTTGGCTGTTCCACTTCGTCACTGCCAAGAAAC-3′. Other GULP1 phosphomimetic mutants were generated by the same approach. FLAG-tagged PKCζ was generated by subcloning rat PKCζ cDNA (generated by RT-PCR from rat neurons) into pCMV-Tag2B. Mammalian expression vector of PKCι was obtained from Addgene (Watertown, MA, USA). APP-GAL4 construct consisting of human APP695 followed by the entire GAL4 transcription factor (pRc-CMV-APP695) was as previously described (13). Mammalian expression construct of myc-GULP1 is prepared by subcloning human GULP1 cDNA into pCMV-Myc (Takara Bio, Mountain View, CA, USA). Upstream activation sequence–dependent firefly luciferase reporter pFR-Luc and transfection efficiency vector Renilla luciferase phRL-TK plasmids were obtained from Stratagene (San Diego, CA, USA) and Promega (Madison, WI, USA), respectively.
Antibodies
Rat anti-GULP1 and rabbit anti-APP were as described (5). Mouse anti-APP (22C11) was purchased from Merck (Darmstadt, Germany). Anti-HA (12CA5) was purchased from Roche (Basel, Switzerland). Anti-FLAG (M2) was purchased from MilliporeSigma (Burlington, MA, USA). Goat anti-GULP1 (P19), rabbit anti-PKCζ (C20), and mouse anti–α-tubulin (DM1A) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Rabbit anti-PKCζ (C24E6) and anti-PKCι/λ (C83H11) were purchased from Cell Signaling Technology (Danvers, MA, USA). Rabbit anti-PKCζ phospho-T410 (EP1491Y) was purchased from Abcam (Cambridge, MA, USA). Mouse IgG control was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Cell culture and transfection
Chinese hamster ovary (CHO), human embryonic kidney 293 (HEK293), and SHSY5Y cells were cultured as previously described (5, 14) and transfected either by X-tremeGene 9 (Roche), X-tremeGene HP (Roche), or polyethylenimine (Polysciences, Warrington, PA, USA).
Generation of PKCζ and PKCι knockout cells
PKCζ and PKCι knockout HEK293 cells were generated by clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 system. Two pairs of single guide RNA sequences were designed by an online CRISPR design server (http://crispr.mit.edu/) to target the exon 1 of human PRKCZ gene (guide 1: sense 5′-CGCCTCAAGGCGCATTACGG-3′, anti-sense 5′-CCGTAATGCGCCTTGAGGCG-3′; guide 2: sense 5′-AGGACCGGCCCCAAGATGGA-3′, anti-sense 5′-TCCATCTTGGGGCCGGTCCT-3′) and the exon 1 of PRKCI (guide 3: sense 5′-CGCGGTAGTAGGCTTTCACC-3′, anti-sense 5′-GGTGAAAGCCTACTACCGCG-3′; guide 4: sense 5′-ACTACCGCGGGTGAGTGTCC-3′, anti-sense 5′-GGACACTCACCCGCGGTAGT-3′). The guide oligonucleotides were cloned into pSpCas9(BB)-2A-Puro (PX459) as described (15). The PX459-sgRNA plasmids were cotransfected into HEK293 cells. Twenty-four hours post-transfection, the cells were selected with puromycin (6 μg/ml). The puromycin resistance cells were selected and grown individually. The cell lysates were then analyzed by immunoblotting using a rabbit anti-PKCζ and anti-PKCι antibodies (Cell Signaling Technology).
Dephosphorylation of GULP1
Untransfected and GULP1-transfected CHO cell lysates were dephosphorylated using λ-protein phosphatase (New England Biolabs, Ipswich, MA, USA) as previously described (16). The samples were then resolved by high-resolution NuPAGE Bis-Tris Precast Gels (Thermo Fisher Scientific). GULP1 was detected by a rat anti-GULP1 antibody (5).
Protein expression and purification
Recombinant GULP1 PTB protein was expressed with an N-terminal hexa-histidine (i.e., 6X-H-GULP1 PTB) in Escherichia coli Rosetta 2 (DE3) strain. The 6X-H-GULP1 PTB fusion protein was purified by Ni-NTA agarose (Qiagen, Germantown, MD, USA). Then it was loaded onto Superdex 75 column (Pharmacia, Piscataway, NJ, USA) and eluted in 20 mM glycine (pH 9), 250 mM NaCl, 2 mM DTT, and 1 mM benzamidine using an AKTA system (Pharmarcia). The purified protein was concentrated to ∼16 mg/ml, frozen in liquid nitrogen, and stored at −80°C.
Cocrystallization GULP1 PTB:APP peptide complex and data collection
Seven hundred micromolars (final concentration) of GULP1-PTB were mixed with 1.4 mM (final concentration) APP peptide (NGYENPTYKFFE). Small crystals were obtained by hanging drop vapor diffusion at 16°C using the Hampton Screen (Hampton Research, Aliso Viejo, CA, USA). The drops contained 1 μl protein-peptide mixture mixed with 1 μl of reservoir solution. Optimal condition consisted of 100 mM glycine (pH 3.3), 2000 mM (NH4)2SO4, 50 mM NaCl, and 50 mM KCl in the reservoir. All crystals were cryoprotected with the addition of 20% ethylene glycol (v/v) and flash frozen in liquid nitrogen prior to data collection. X-ray diffraction data were collected at 110 K by an in-house FR-E + X-ray generator and R- AXIS IV++ imaging plate detectors (Rigaku, Tokyo, Japan) in the Centre for Protein Science and Crystallography in CUHK. All X-ray data were processed using the iMosflm programS (MRC Laboratory of Molecular Biology, Cambridge, United Kingdom). The crystals belong to P3221 space group (a = b = 63.91, c = 108.77) with 1 complex molecule per asymmetric unit.
Structure determination and refinement
To determine the structure by the molecular replacement method, a homology structure model of GULP1 PTB was constructed from the known primary sequence of GULP1 with the structure of Disabled-1 (Dab1) PTB domain as the template (PDB ID: 1NTV). The GULP1 PTB:APP complex structure was solved by molecular replacement using the program Phaser (http://www.phaser.com/) with the homology model as the search model, yielding clear rotation function and translation peaks. After rigid-body refinement using the program REFMAC (https://www2.mrc-lmb.cam.ac.uk/groups/murshudov/content/refmac/refmac.html), Fo-Fc electron density map calculated with the model unveiled large positive peaks and readily interpretable density for the APP peptide. The complex model was then built manually with the program Coot (https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/) and refined with REFMAC. The Rcryst and Rfree were 20.7 and 22.5%, respectively, for data from 32 to 2.17 Å. The final model of the binary complex includes residues 1–168 of GULP1 and residues 1–28 of the peptide.
Isothermal titration calorimetry
Isothermal titration calorimetry (ITC) measurements were performed using an iTC200 microcalorimeter (Malvern Panalytical, Malvern, United Kingdom) at 25°C with a stirring speed of 750 rpm. All protein samples were dissolved in a buffer containing 50 mM NaH2PO4, pH 7.5, and 300 mM NaCl. The titrations were carried out by injecting 40 μl of 0.5 mM AICD peptide (NGYENPTYKFFE) into solutions of 0.4 mM GULP11–168, GULP11–168 T35A, or GULP11–168 T35D at time intervals of 90 s. To calculate the dissociation constant, the titration data were analyzed using the program Origin 7.0 (https://www.originlab.com/index.aspx?go) and fitted by one-site binding model.
Protein binding assays
GST-fusion protein pull-down and coimmunoprecipitation assays were performed as described (17). In brief, GST-AICD fusion protein was expressed in E. coli BL21 and captured by glutathione sepharose 4B according to the manufacturer’s instructions (GE Healthcare, Chicago, IL, USA). GST-AICD baits were used in pull-down assays from GULP1 transfected cells, which were harvested in ice-cold lysis buffer that consisted of 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and Complete protease inhibitor (Roche). The cell lysates were incubated with the baits at 4°C for an hour. The captured proteins were then isolated by boiling in SDS-PAGE sample buffer and analyzed by SDS-PAGE and immunoblotting. GULP1 was immunoblotted with 12CA5 anti-HA antibody (Roche) against the N-terminal HA-tag.
For immunoprecipitation, CHO cells transfected with APP + HA-GULP1 and APP + HA-GULP1 T35D were harvested in ice-cold lysis buffer. HA-GULP1 was immunoprecipitated from cell lysates using mouse anti-HA antibody 12CA5 (Roche) for 16 h at 4°C. The antibody was captured by Protein G Sepharose (MilliporeSigma) for 2 h at 4°C, and the immunoprecipitates were washed 3 times with ice-cold lysis buffer. Proteins in the immunoprecipitates were analyzed by SDS-PAGE and Western blotting. APP and GULP1 in the immunoprecipitates were detected by a rabbit anti-APP antibody (18) and a mouse anti-HA antibody (5), respectively.
For in vitro binding assay, recombinant GULP1 PTB and GULP1 PTB T35A were incubated with FLAG-tagged PKCζ isolated from transfected cells by immunoprecipitation using an anti-FLAG antibody (M2) (MilliporeSigma) for 10 min at 30°C. The GULP1 PTB proteins were then incubated with recombinant GST-APPc at 4°C for an hour. GULP1 PTB and GST-APPc in the pull-downs were detected by a rabbit anti-APP antibody and a rat anti-GULP1 antibody, respectively.
Proximity ligation assay
Proximity ligation assay (PLA) was performed by using a Duolink In Situ–Fluorescence kit (MilliporeSigma). In brief, HEK293 cells were seeded on coverslips 24 h before transfection. Cells were transfected with either APP + GULP1, APP + GULP1 T35D, or APP + GULP1 + PKCζ. The cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 24 h post-transfection. After blocking with 5% fetal bovine serum in PBS at 37°C for 1 h, the cells were then incubated with goat anti-GULP1 P19 (Santa Cruz Biotechnology) and mouse anti-APP 22C11 (Merck) for 1 h at room temperature to probe for GULP1 and APP, respectively. The cells were then washed 3 times with 1× Wash Buffer A (Thermo Fisher Scientific), and followed by incubation with Duolink In Situ PLA probe anti-mouse PLUS and anti-goat MINUS at 37°C for 1 h in a humid incubator. After incubation, the cells were washed 3 times with 1× Wash Buffer A. Ligation was performed by adding 1× ligation stock and diluted ligase at 37°C for 30 min that was then followed by 2 washes with 1× Wash Buffer A. Amplification was carried out in a darkened humid incubator by incubating the cells with 1× amplification stock and diluted polymerase at 37°C for 100 min. Then the cells were washed 2 times with 1× Wash Buffer B and then 1 time with 0.01× Wash Buffer B. The coverslips were mounted with Duolink In Situ mounting medium with DAPI. Images were captured using an Olympus IX71 fluorescence microscope with an UPlanSAPo ×60 water immersion objective (Tokyo Japan). Fluorescence images were captured by a Nikon DS-Qi2 camera (Tokyo, Japan), and the fluorescence signals were quantified by the Object Count tool in Nikon NIS Elements. Cells were also stained with anti–β-tubulin as a morphology marker.
APP-GAL4 cleavage reporter assay
APP-GAL4 cleavage reporter assay was performed as previously described (5, 19). Cells were transfected with the relevant constructs together with pRc-CMV-APP695, pFR-Luc, and phRL-TK. phRL-TK, which expresses the Renilla luciferase, was used as a control to quantify transfection efficiency. Cells were harvested in Dual-Glo luciferase substrate (Promega) at 24 h post-transfection. The firefly luciferase activities produced by pFR-Luc were measured by a luminometer (Perkin Elmer, Waltham, MA, USA). Then, the Renilla luciferase activities produced by the phRL-TK were assayed by adding equal volume of Dual-Glo Stop&Glo substrate and analyzed by the luminometer. The firefly luciferase activity was normalized to the corresponding Renilla luciferase activity.
Tricine–SDS-PAGE analysis for APP carboxyl-terminal fragments
APP C-terminal fragments (CTFs) were analyzed by 16% tricine–SDS-PAGE essentially as described (5). The cells were harvested in 1× tricine sample buffer containing 2 mM phenanthroline, 10% 2-ME, and Complete protease inhibitor (Roche). Immunoblot analyses were performed using a rabbit anti-APP antibody that recognizes the last 21 aa residues of APP (18, 20, 21). The relative amounts of APP CTFs on the immunoblots were analyzed by Image Studio (Li-Cor Biosciences, Lincoln, NE, USA).
Aβ ELISA assay
Human Aβ40 level in the cell culture medium was analyzed using the high sensitivity human amyloid β40 ELISA kit (MilliporeSigma), which recognizes the C-terminal end of human Aβ40 peptide, as previously described (5, 14). In brief, HEK293 cells were transfected with human APP, APP + PKCζ, APP + GULP1, APP + GULP1 T35A, APP + GULP1 + PKCζ, and APP + GULP1 T35A + PKCζ. The cells were replenished with fresh medium 48 h post-transfection. After 7 h, the medium was collected, diluted as appropriate in sample diluent and added to the ELISA plate. After overnight incubation at 4°C with primary antibody, the ELISA plate was washed 5 times with wash buffer and steptavidin-peroxidase-conjugate was added. Following a 1-h room temperature incubation and washing, colorimetric substrate solution was added to the ELISA plate. The colorimetric signal development was then stopped by adding stop solution. Signals from ELISA were measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
Kinase finder radiometric protein kinase assays
Kinase Finder radiometric protein kinase assay was performed by ProQinase (Freiburg, Germany). In brief, a biotinylated peptide of GULP1 (AKFLGSTEVEQPK; residues 29–41) was incubated with various kinases from a panel of 190 serine/threonine kinases and reaction cocktails (60 mM HEPES-NaOH, pH 7.5, 3 mM MgCl2, 3 mM MnCl2, 3 μM Na-orthovanadate, 1.2 mM DTT, 50 μg/ml PEG20000, 1 μM [γ-33P]ATP) at 30°C for 60 min. The reactions were terminated by adding an appropriate amount of stop solution (4.7 M NaCl/35 mM EDTA), and then transferred to streptavidin-coated 96-well FlashPlate PLUS plates (Perkin Elmer). The plates were incubated at room temperature for 30 min and then washed 3 times with 0.9% NaCl. Radioactive 33P signals were measured by a microplate scintillation counter.
In vitro kinase assay
In vitro kinase assays were performed as previously described (22). GST-GULP126–43 and GST-GULP126–43 T35A were expressed in E. coli and purified with glutathione sepharose 4B according to the manufacturer’s instructions (GE Healthcare). FLAG-tagged PKCζ was isolated by immunoprecipitation using an anti-FLAG antibody (M2) (MilliporeSigma). One microgram of each substrate was incubated with immunoprecipitated PKCζ in 1× kinase buffer (60 mM HEPES, pH 7.5, 3 mM MgCl2, 3 mM MnCl2, 1.2 mM DTT, 3 μM sodium orthovanadate), 0.185 MBq [γ-[32P]]ATP, and 20 μM ATP for 5 or 30 min at 30°C. Reactions were stopped by addition of SDS-PAGE sample buffer. After boiling, samples were separated on 12% (w/v) acrylamide SDS-PAGE gels, and the gels were then subjected to autoradiography.
Statistical analyses
Statistical analyses were performed using 1-way ANOVA tests with LSD post hoc test. Significance is indicated between different treatments when P < 0.05. Error bars shown are either sd or sem.
Mass spectrometric analysis
Single phosphoprotein mass spectrometric analysis was performed by the Beijing Genomics Institute (BGI; Shenzhen, China). In brief, recombinant GULP11–168 was phosphorylated in vitro by PKCζ as previously described. The reaction was quenched by the addition of SDS sample buffer and then separated with SDS-PAGE. The protein band corresponding to the size of recombinant GULP11–168 was excised from Coomassie blue-stained gels and destained, and then dehydrated with acetonitrile (ACN). Proteins in gel band were reduced with 10 mM DTT (1 h at 56°C), alkylated with 55 mM iodoacetamide (45 min in dark), and then subjected to in-gel trypsin digestion [10 ng/μl in 25 mM (NH4)HCO3 at 37°C]. Peptides in gel slices were extracted with 50% ACN) in water once and followed by 100% ACN. Phosphopeptides were enriched by using TiO2. The enriched sample was separated using UltiMate 3000 UHPLC (Thermo Fisher Scientific) at 300 nl/min using self-packed C18 column (25-cm length × 75-μm inside diameter, 3 μm particle). Solvent gradient conditions were as follows: 0–5 min, 5% B buffer (98% ACN, 0.1% formic acid); 5–45 min, 5–25% B buffer; 45–50 min, 25–35% B buffer; 50–52 min, 35–80% B buffer; 52–54 min, 80% B buffer; 54–60 min, 5% B buffer. Separated peptide samples were ionized via nanoelectrospray ionization source and analyzed with Q-Extractive HF Mass Spectrometer (Thermo Fisher Scientific) via higher-energy collisional dissociation with data-dependent analysis. Peptides were analyzed for MS scans [350–1500 m/z (mass/charge ratio), 120,000 resolution], followed by tandem mass spectrometry scan of the 10 most intense precursors with charge states from +2 to +6 with a dynamic exclusion for 30 s. Raw data were processed with Mascot v.2.3.02 (Matrix Science) (23). The tandem mass spectrometry spectra were searched against the UniProt Human database (https://www.uniprot.org/; uniport_homo_sapiens_filter.fa, 20355 sequences). The search was performed with the following parameters: Electrospray ionization Fourier transform ion cyclotron resonance as the instrument type; 20 ppm for peptide mass tolerance and 0.05 Da for fragment mass tolerance. Trypsin as enzyme allowing maximal 2 missed cleavages. Cysteine carbamidomethyl was set as a fixed modification; methionine oxidation, N-terminal glutamine to pyroglutamate and serine; and threonine and tyrosine phosphorylation were set as variable modifications. Mascot results were filtered with Percolator (http://percolator.ms) (24) using a false discovery rate ≤1% (peptide-spectrum match–level false discovery rate ≤0.01). Phosphorylation site localization was performed with phosphoRS (25) to identify confident phospho-residues with P ≥ 0.75.
RESULTS
GULP1 is a phosphoprotein, and the phosphomimetic mutation of GULP1 T35 reduces APP processing
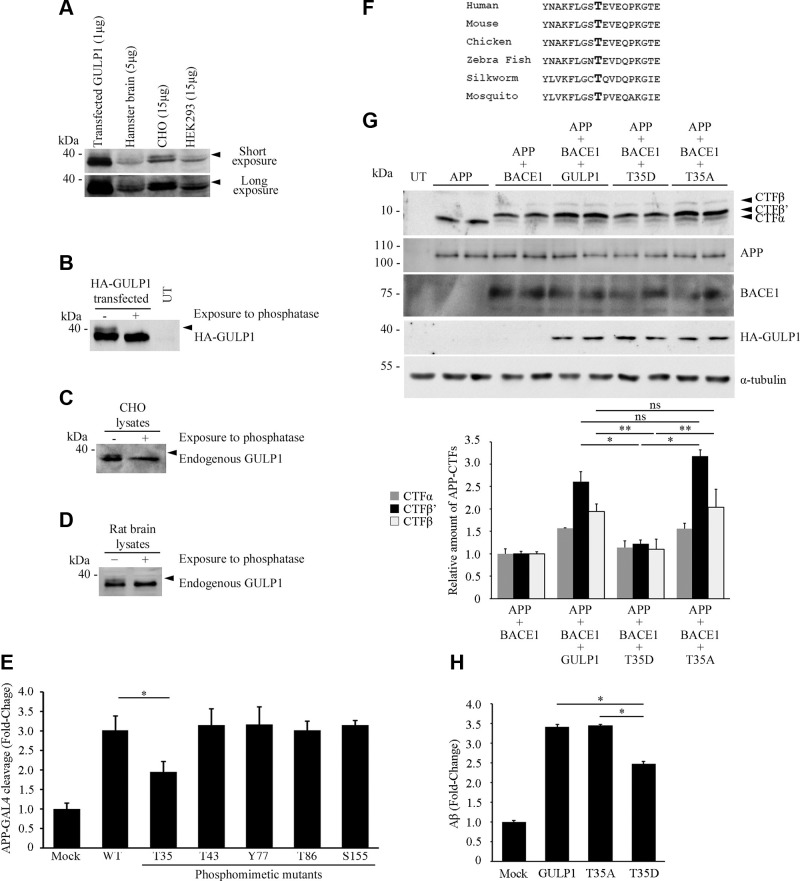
Upon examination of CHO cells transfected with GULP1, we noticed that a band with a reduction in electrophoretic mobility was detected by anti-GULP1 antibody (Fig. 1A). Similarly, upshifted bands were observed in the immunoblotting analysis of endogenous GULP1 from hamster brain, untransfected CHO, and HEK293 cell lysates (Fig. 1A). A reduction in electrophoretic mobility is a characteristic of many phosphoproteins [see Ref. (16)]. To formally test whether the upshifted bands were phospho-GULP1, untransfected and GULP1 transfected CHO cell lysates were treated with λ protein phosphatase, which can remove phosphate from proteins. This treatment led to the disappearance of the slower migrating GULP1 species in GULP1-transfected, untransfected CHO, and rat brain lysates (Fig. 1B–D). Our observations are in agreement with the finding of various mass spectrometric studies, namely, that GULP1 is a phosphoprotein (26–28).
Figure 1.
GULP1 is a phosphoprotein, and GULP1 T35 phosphomimetic mutation reduces GULP1-mediated APP processing. A) HEK293, CHO, hamster brain, and GULP1-transfected CHO cell lysates were fractionated by a high-resolution NuPAGE Bis-Tris Precast Gel and then subjected to Western blot analysis using a rat anti-GULP1 antibody. Two closely migrating GULP1 bands were detected by the anti-GULP1 antibody in all the samples (arrowed). Transfected GULP1 was used as a size control. Two exposures of the same blot are shown. The amounts of protein lysates in each lane are indicated. B–D) Untransfected (UT) HA-GULP1–transfected CHO (B), UT CHO cells (C), and rat brain (D) were harvested in 1× dephosphorylation buffer. The protein lysates were incubated at 30°C either with (+) or without (−) λ-protein phosphatase for 60 min. The samples were then analyzed by NuPAGE Bis-Tris Precast Gels and Western blotting. Transfected HA-GULP1 (B) and endogenous GULP1 (C, D) were detected by a mouse anti-HA 12CA5 (Roche) and a rat anti-GULP1, respectively. The slow-migrating GULP1 bands (arrows) (B–D) disappeared after λ-protein phosphatase treatment. E) CHO cells were cotransfected with APP-GAL4, pFR-Luc, and phRL-TK together with either empty vector control plasmid (mock) or different phosphomimetic mutants of GULP1. GULP1 T35 mutant shows reduced ability to stimulate GAL4-APP cleavage as compared with GULP1 WT; n = 5. Results are means ± sd. *P < 0.001 compared with GULP1 transfected cells. F) Alignment of GULP1 sequence around T35 (bolded; reference to human) from various species. G) Immunoblot analysis of APP CTFs from CHO cells transfected with APP, APP + BACE1, APP + BACE1 + GULP1, APP + BACE1 + GULP1 T35D, or APP + BACE1 + GULP1 T35A (top panel). The expression of transfected APP, BACE1, and GULP1 were also determined. The amounts of APP CTF-α, -β′ and -β′ were quantified. Full-length APP and APP CTFs were detected by a rabbit anti-APP. HA-GULP1 and BACE1-myc were probed by a mouse anti-HA (12CA5) and a mouse anti-myc (9B11), respectively. Data for graphs were obtained from 3 independent experiments. Ns, not significant (n = 3). Results are means ± sd. *P < 0.001, **P < 0.01. H) CHO cells were cotransfected with APP either mock, GULP1, GULP1 T35A, or GULP1 T35D. Forty-eight hours post-transfection, cell culture medium was aspirated and changed to fresh medium. The level of secreted Aβx-40 was assayed using an ELISA kit 7 h after the change of medium (n = 5). *P < 0.001 compared with GULP1 transfected cells. The effect of GULP1 on Aβx-40 liberation is reduced by GULP1 T35 phosphomimetic mutation.
It has been reported that phosphorylation of AIPs alters APP processing, including residues within their PTB domains (14, 19, 29, 30). Five phospho-residues within the GULP1 PTB domain have been reported in several mass spectrometric studies (26–28). To test whether these GULP1 phospho-residues influence APP processing, phosphomimetic mutants of the residues were generated, and their effects on APP processing were determined using APP-GAL4 cleaving reporter assays, a system for monitoring the release of AICD (5, 31–33). In the APP-GAL4 reporter assay system, APP is fused with the yeast transcription factor GAL4 (including both DNA binding and activation domains). AICD-GAL4 is released after proteolytic cleavage and translocates to the nucleus, in which GAL4 stimulates the transcription of the GAL4-dependent firefly luciferase reporter gene. In this assay, APP-GAL4 was transfected with either empty vector, wild type (WT), or various phosphomimetic mutants of GULP1 into cells. As reported (5), GULP1 stimulated APP-GAL4 cleavage. Remarkably, the phosphomimetic mutant for GULP1 T35 reduced the cleavage of APP by ∼40% as compared with the WT counterpart. No other phosphomimetic mutants showed any significant effect in the assay (Fig. 1E). Our findings suggest a functional role for GULP1 T35, a highly conserved residue among various animal species (Fig. 1F).
In addition to the release of AICD, GULP1 has been shown to potentiate both α- and β-secretase processing of APP with the most notable effect at the β-cleavage site (5). We, therefore, inquired if GULP1 T35 phosphorylation alters such APP cleavage by analyzing APP CTF generation. In line with a previous report (5), overexpression of GULP1 promoted the generation of APP CTF-α (1.5-fold), -β’ (2.5-fold), and -β (2-fold), in particular CTF-β’. A similar effect was observed in GULP1 T35A–transfected cells. However, GULP1 T35D phosphomimetic mutant did not show any significant effect on the generation of APP CTFs (Fig. 1G).
To further confirm the effects of GULP1 T35 phosphorylation on APP processing, Aβ40 secretion was determined from cells transfected with human APP, APP + GULP1, APP + GULP1 T35A, and APP + GULP1 T35D. Consistent with our previous findings (5), overexpression of GULP1 increased the production of Aβ40 in mammalian cells; however, the effect of GULP1 T35D on Aβ40 secretion was significantly reduced compared with WT GULP1 and GULP1 T35A nonphosphomimetic mutant (Fig. 1H). Taken together, our findings indicate that GULP1 T35 phosphorylation reduces GULP1-mediated APP processing and Aβ secretion by suppressing both β-secretase and γ-secretase cleavage of APP.
GULP1 T35D phosphomimetic mutation reduces GULP1-APP interaction
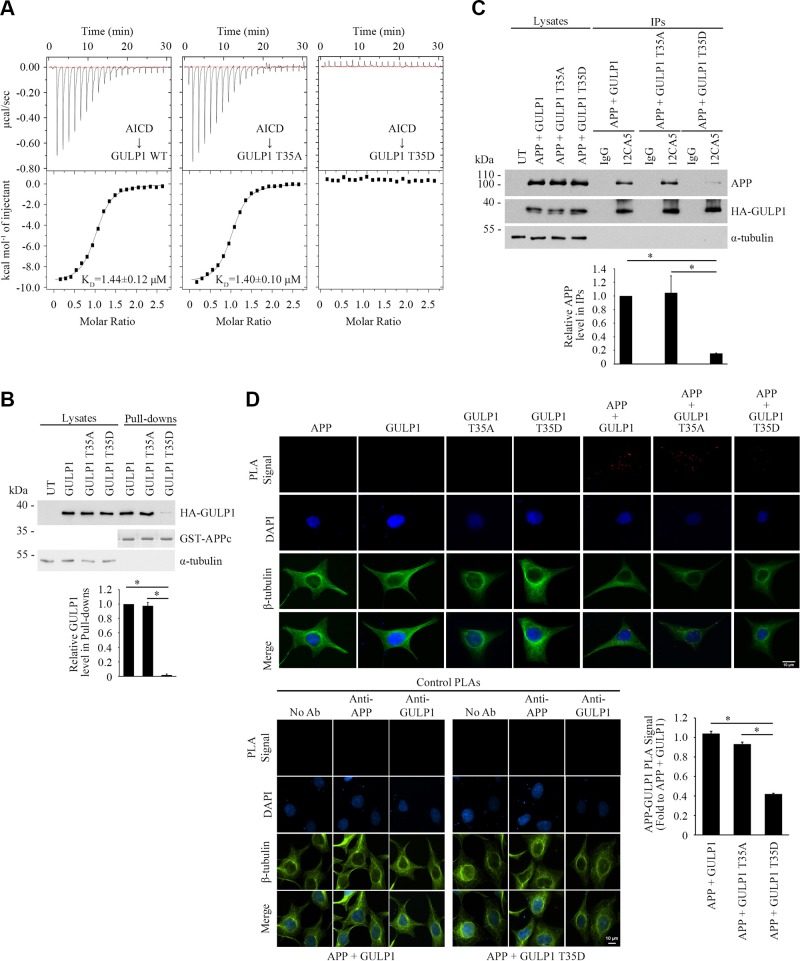
We showed that GULP1 PTB interacts with APP NPTY motif (5); therefore, we anticipated that GULP1 T35 phosphorylation regulates APP processing via the modulation of GULP1-APP interaction. To test this notion, we perform ITC experiments to characterize the interaction between a 12-mer AICD peptide and GULP1 PTB with or without T35 mutation. The heat generated by the dilution of AICD peptide was determined by titrating the peptide into buffer only and then subtracting it from each binding titration curve. The measured equilibrium dissociation constants (KD) of both GULP1 WT and GULP1 T35A were ∼1.4 μM, indicating that the mutation of T35 to alanine did not affect GULP1-APP interaction (Fig. 2A). In contrast, ITC revealed no detectable binding of AICD to the GULP1 T35D mutant, which suggests that the phosphorylation of T35 disrupts the interaction between GULP1 and APP. In line with the results of ITC, the amount of GULP1 T35D mutant pulled down by GST-AICD was markedly reduced in the GST pull-down assay (Fig. 2B). A similar effect was also observed in coimmunoprecipitation assays, in which GULP1-APP interaction was decreased by GULP1 T35D phosphomimetic mutation but not the WT and T35A mutation (Fig. 2C).
Figure 2.
GULP1 T35 phosphomimetic mutation reduces GULP1-APP interaction. A) ITC-based measurements of the binding between AICD peptide and PTB domains from WT GULP1 (left), GULP1 T35A (middle), or GULP1 T35D (right). B) Bacterially expressed GST-APPc was used as bait for GST pull-down assay from GULP1, GULP1 T35A, or GULP1 T35D–transfected cell lysate. GULP1 was detected by a rat anti-GULP1. Middle panel: Coomassie Blue staining of GST-APPc bait used. C) Coimmunoprecipitation was performed from CHO cells transfected with APP + GULP1, APP + GULP1 T35A, or APP + GULP1 T35D using a mouse anti-HA antibody 12CA5 to the N-terminal HA-tag of GULP1. APP and GULP1 in the immunoprecipitates (IPs) were detected by a rabbit anti-APP and a rat anti-GULP1, respectively. Data for graphs (B, C) were obtained from 3 independent experiments (n = 3). Results are means ± sd. *P < 0.001. D) GULP1 T35D reduces GULP1-APP interaction in PLA. HEK293 cells were transfected with APP + GULP1, APP + GULP1 T35A, or APP + GULP1 T35D. Fewer PLA signals were observed in APP + GULP1 T35D cotransfected cells. No signal was detected in APP, GULP1, GULP1 T35A, or GULP1 T35D singly transfected cells. β-Tubulin and DAPI were used as morphology and nucleus markers, respectively. Representative images are shown (upper left panel). Bar chart shows relative PLA signal (fold to APP + GULP1). Data were obtained from at least 60 cells per transfection and the experiments were repeated 3 times. Error bars are sem. *P < 0.001. No-antibody (No Ab), anti-APP, and anti-GULP1 control PLAs were performed for APP + GULP1 and APP + GULP1 T35D transfected cells (lower panel).
PLAs were performed to further confirm the above observations and to investigate whether GULP1 T35 phosphorylation reduces GULP1-APP interaction in cells. As shown in Fig. 2D (upper panel), fluorescence PLA signals were observed in APP + GULP1 cotransfected cells, which suggests that the 2 proteins interact in cells. Notably, the amount of PLA signal was reduced significantly in APP + GULP1 T35D cotransfected cells but not in APP + GULP1 T35A. We did not detect any signal from APP, GULP1, GULP1 T35A, and GULP1 T35D singly transfected cells. Similarly, no signal was observed in the no-antibody, anti-GULP1, and anti-APP control PLAs (Fig. 2D, lower panel). Together, our data indicate that the phosphorylation of GULP1 T35 decreases GULP1-mediated APP processing by reducing GULP1-APP interaction.
PKCζ phosphorylates GULP1 T35 and reduces GULP1-mediated APP processing by decreasing GULP1-APP interaction
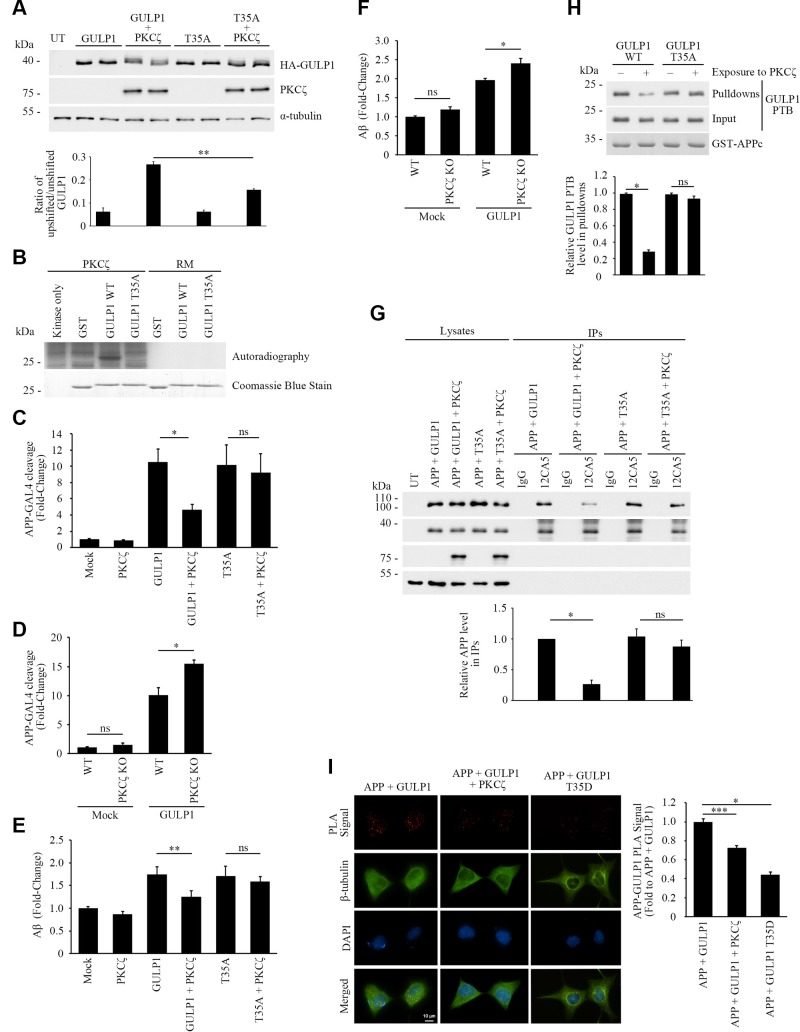
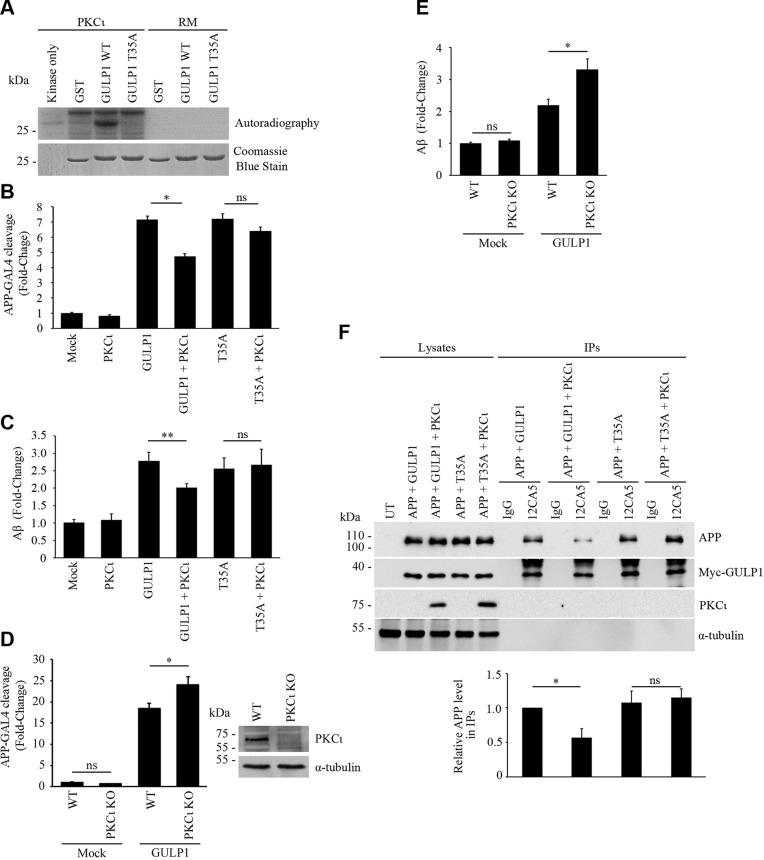
We used a commercial Kinase Finder approach (ProQinase) to screen for GULP1 T35 kinase(s). In this assay, a peptide composed of residues 29–41 of GULP1 (AKFLGSTEVEQPK) was subjected to in vitro radiometric protein kinase assay on a panel of 190 serine/threonine kinases. The results show that PKCζ was the highest-scoring kinase in the assay that could phosphorylate the GULP1 peptide (unpublished data). To test whether PKCζ phosphorylates GULP1 in vivo, we analyzed the electrophoretic mobility of GULP1 in SDS-PAGE from GULP1 and GULP1+PKCζ transfected cells. In Fig. 3A, a weak upshifted form of GULP1 was observed in GULP1 singly transfected lysate. The ratio of upshifted/unshifted GULP1 was markedly increased in GULP1 + PKCζ cotransfected cells. A slight increase of the ratio was also observed in GULP1 T35A + PKCζ cotransfected cells, which might due to the phosphorylation of GULP1 S155 (please see below). Nevertheless, this finding suggests that PKCζ enhances the phosphorylation of GULP1.
Figure 3.
PKCζ phosphorylates GULP1 T35 and reduces the effect of GULP1 on APP processing. A) HA-GULP1 and HA-GULP1 T35A were transfected to CHO cells together with or without PKCζ. Cell lysates were resolved on a high-resolution NuPAGE Bis-Tris Precast gel for determination of GULP1 migration pattern. Expression of FLAG-tagged PKCζ was confirmed by immunoblotting using anti-FLAG M2 antibody. α-Tubulin was detected by the anti–α-tubulin DM1A antibody as loading control. The amounts of unshifted and unshifted GULP1 were quantified by densitometry using Image Studio and their ratios in different transfected cells were illustrated. UT, untransfected (n = 5). **P < 0.01. B) Bacterially expressed GST-GULP126–43 (WT) or GST-GULP126–43 T35A (T35A) were incubated with PKCζ immunoprecipitated from transfected cell lysate together with (γ-[32P])-ATP for 30 min at 30°C. RM is the reaction mix only without kinase. Upper panel: autoradiograph; lower panel: Coomassie Blue staining PKCζ phosphorylates GULP1 at T35. C) HEK293 were cotransfected with APP-GAL4, pFR-Luc, and phRL-TK together with the indicated constructs. PKCζ reduces the effect of GULP1-mediated APP-GAL4 cleavage via phosphorylation of T35. Ns, not significant (n = 5). Results are means ± sd. *P < 0.001 compared with GULP1 transfected cells. D) WT or PKCζ knockout (KO) HEK293 cells were cotransfected with APP-GAL4, pFR-Luc, and phRL-TK together with the indicated constructs. GULP1-mediated APP-GAL4 cleavage was enhanced in PKCζ KO cells. Ns, not significant (n = 5). *P < 0.001. E) Cells were cotransfected with APP and the indicated constructs, and the level of secreted Aβ40 was assayed. PKCζ suppresses GULP1-enhanced Aβ40 liberation through phosphorylation of T35 (n = 5). Results are means ± sd. **P < 0.01 compared with GULP1 transfected cells. F) WT or PKCζ KO HEK293 cells were cotransfected with APP and the indicated constructs, and the level of secreted Aβ40 was assayed. GULP1-mediated Aβ40 secretion was increased in PKCζ knockout cells (n = 5). Results are means ± sd. *P < 0.001. G) PKCζ reduces GULP1-APP interaction in coimmunoprecipitation assay. Coimmunoprecipitation was performed from cells transfected with APP + HA-GULP1, APP + HA-GULP1 + PKCζ, APP + HA-GULP1 T35A, or APP + HA-GULP1 T35A + PKCζ using a mouse anti-HA antibody 12CA5. APP and HA-GULP1 in the immunoprecipitates (IPs) were detected by a rabbit anti-APP and 12CA5; n = 3. *P < 0.001. H) PKCζ reduces GULP1-APP interaction in vitro. Recombinant GULP1 PTB and GULP1 PTB T35A were phosphorylated in vitro by PKCζ. GST-APPc was used as bait to pull down the recombinant proteins. The amount of GULP1 PTB and GST-APPc was revealed by a rat anti-GULP1 and a rabbit anti-APP, respectively; n = 3. Results are means ± sd. *P < 0.001. I) PKCζ reduces GULP1-APP interaction in PLA. Cells were transfected with APP + GULP1, APP + GULP1 + PKCζ, or APP + GULP1 T35D. Fewer PLA signals were observed in APP + GULP1+ PKCζ and APP + GULP1 T35D cotransfected cells. β-Tubulin and DAPI were used as morphology and nucleus markers, respectively. Representative images are shown. Bar chart shows relative PLA signal (fold to APP + GULP1). Data were obtained from at least 60 cells per transfection and the experiments were repeated 3 times. Error bars = sem. *P < 0.001, ***P < 0.05.
In addition to T35, another phosphorylable residue, S34, is present in the GULP1 peptide used in the Kinase Finder assay. To further confirm this observation and to preclude the possibility of PKCζ phosphorylating the nearby S34, in vitro kinase assays were performed with WT and T35A GST-fusion protein containing GULP1 fragment (residues 26–43) as substrates. PKCζ was transfected into the cells and then isolated from the cell lysate by immunoprecipitation for in vitro kinase assays. We found that PKCζ could phosphorylate recombinant WT GULP1 fragment but not the GULP1 T35A mutant (Fig. 3B, upper panel). To further confirm the result, recombinant PKCζ phospho-GULP1 PTB was analyzed by single phosphoprotein mass spectrometry. We obtained over 66% sequence coverage and identified a peptide (FLGSTEVEQPK) with phospho-T35 (Supplemental Fig. S1). No peptide with phospho-S34 was detected. Interestingly, a peptide with phospho-S155 (FLESGGKDVETRK) was also found (Supplemental Fig. S2). However, GULP1 S155 phosphomimetic mutant did not affect APP processing (Fig. 1E) and GULP1-APP interaction (Supplemental Fig. S2). Although its roles remain to be determined, our findings suggest that GULP1 S155 phosphorylation does not participate in APP metabolism. Nevertheless, our findings suggest that GULP1 T35 is a PKCζ target residue and such phosphorylation reduces GULP1-mediated APP processing.
We next tested whether PKCζ affects GULP1-mediated APP processing. GULP1-mediated APP-GAL4 cleavage in transfected cells was measured in the presence or absence of PKCζ. Overexpression of PKCζ resulted in a significant reduction in the effect of GULP1 on APP-GAL4 cleavage. Conversely, PKCζ showed no effect on the GULP1 T35A mutant, which can preclude phosphorylation at T35 (Fig. 3C). In contrast, GULP1-mediated APP-GAL4 cleavage was significantly enhanced in PKCζ knockout cells (Fig. 3D). We also determined the effect of PKCζ on GULP1-mediated Aβ production. As in our previous report, GULP1 enhanced Aβ secretion in mammalian cells (5). In agreement with the APP-GAL4 cleavage assay, cotransfection of PKCζ resulted in a significant reduction in the effect of GULP1, but not the GULP1 T35A mutant, on Aβ secretion (Fig. 3E). Moreover, GULP1-mediated Aβ generation was increased in the PKCζ knockout cells (Fig. 3F).
Our phosphomimetic study indicates that GULP1 T35 phosphorylation reduces APP processing by reducing GULP1-APP interaction. Therefore, we performed a coimmunoprecipitation assay to investigate whether PKCζ alters GULP1-APP interaction. To do this, GULP1 was immunoprecipitated from APP + GULP1, APP + GULP1 + PKCζ, APP + GULP1 T35A, and APP + GULP1 T35A + PKCζ transfected cell lysates. The immunoprecipitates were analyzed for APP. As shown in Fig. 3G, a reduced amount of APP was coimmunoprecipitated with GULP1 in the presence of PKCζ. However, such effect was not observed in the cells with GULP1 T35A mutant, which precludes phosphorylation of the residue. Likewise, the amount of WT GULP1 PTB domain, but not the T35A mutant, pulled down by GST-APPc bait was markedly decreased if the domain was preincubated with PKCζ (Fig. 3H). PLAs were performed to further confirm the effects of PKCζ upon GULP1-APP interaction in cells. As with coimmunoprecipitation assay, GULP1-APP PLA signals were significantly reduced when PKCζ was cotransfected (Fig. 3I). Similarly, PLA signals were markedly decreased in APP + GULP1 T35D cotransfected cells (Fig. 3I). Taken together, our data suggest that PKCζ phosphorylates GULP1 T35 to reduce GULP1-APP interaction and in turn APP processing.
Insulin stimulates PKCζ and suppresses GULP1-mediated APP processing
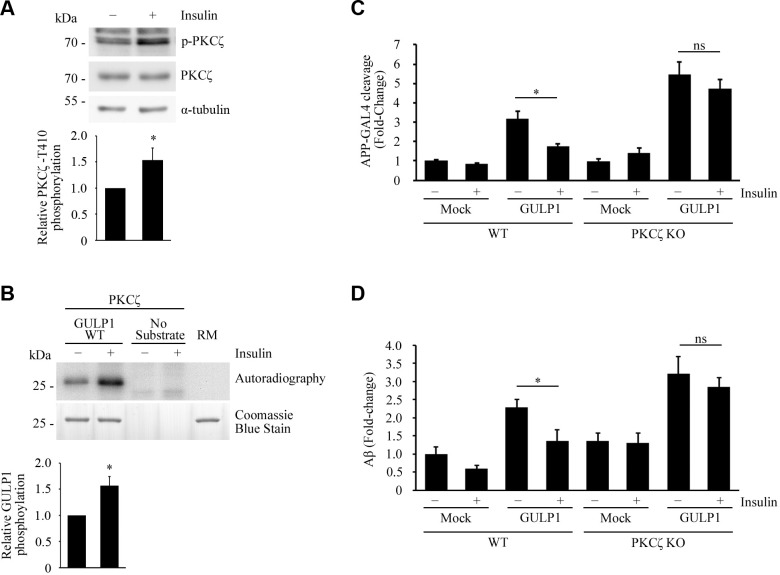
Aberrant insulin signaling is associated with AD. In fact, insulin has been shown to inhibit APP processing (34, 35). Interestingly, PKCζ is reported to be activated by insulin (36–38). These findings prompted us to investigate the relationship between insulin, PKCζ, and GULP1-mediated APP processing. As in previous reports, insulin was found to activate PKCζ, which is revealed by the level of PKCζ T410 phosphorylation (Fig. 4A), a marker of the kinase activation (39–41). In addition, PKCζ isolated from insulin-treated cells was more potent in the phosphorylation of GULP1 in in vitro kinase assay (Fig. 4B).
Figure 4.
Insulin stimulates PKCζ and suppresses GULP1-mediated APP processing. A) Serum-starved HEK293 cells were incubated in culture medium containing 0 (−) or 100 nM (+) insulin. T410-phospho-PKCζ, total PKCζ, and α-tubulin in cell lysates were detected by a rabbit anti-PKCζ phospho-T410, rabbit anti-PKCζ, and anti–α-tubulin DM1A antibody, respectively. Insulin stimulates phosphorylation of PKCζ T410. Bar chart shows relative PKCζ T410 phosphorylation (n = 3). *P < 0.001. B) Bacterially expressed GST-GULP126–43 was incubated with PKCζ immunoprecipitated from untreated (−) or 100 nM insulin-treated (+) transfected cell lysate together with (γ-[32P])-ATP for 5 min at 30°C. RM is the reaction mix only without kinase. Upper panel: autoradiograph; lower panel: Coomassie Blue staining. Insulin potentiates GULP1 T35 phosphorylation by PKCζ. Bar chart shows relative GULP1 phosphorylation (n = 3). *P < 0.001. C) WT or PKCζ knockout (KO) HEK293 cells were cotransfected with APP-GAL4, pFR-Luc, and phRL-TK together with the indicated constructs. Twenty-four hours post-transfection, cells were serum starved for 4 h and then treated with either 0 (−) or 100 nM (+) insulin for 12 h. Insulin reduces the effect of GULP1-mediated APP-GAL4 cleavage in WT cells but not in PKCζ KO cells. Ns, not significant (n = 5). *P < 0.001. D) WT or PKCζ KO HEK293 cells were cotransfected with APP and the indicated constructs. Twenty-four hours post-transfection, cells were serum starved for 4 h and then treated with either 0 (−) or 100 nM (+) of insulin for 17 h. The cells were replenished with fresh medium containing 0 (−) or 100 nM (+) of insulin for 7 h and the level of secreted Aβ40 was assayed. Insulin reduces the effect of GULP1-mediated Aβ40 production in WT cells but not in PKCζ KO cells. Ns, not significant (n = 5). *P < 0.001.
Next, APP-GAL4 cleavage was used to test whether insulin influences GULP1-mediated APP processing. As shown in Fig. 4C, GULP1-mediated APP-GAL4 cleavage was significantly reduced upon insulin treatment in WT cells. Similar to the result in Fig. 3D, GULP1-mediated APP-GAL4 cleavage was markedly enhanced in PKCζ knockout cells than the WT counterpart. However, unlike in WT cells, insulin did not show any significant effect on GULP1-mediated APP-GAL4 cleavage in PKCζ knockout cells (Fig. 4C). In a similar manner, insulin suppressed GULP1-mediated generation of Aβ in the WT cells but not in PKCζ knockout cells (Fig. 4D). Collectively, our findings suggest that insulin reduces APP processing, at least in part, via the activation of PKCζ-mediated GULP1 phosphorylation.
PKCι phosphorylates GULP1 T35 and reduces GULP1-mediated APP processing
In addition to PKCζ, PKCι (also known as PKCλ in rodents) is another member of atypical PKC (42). They have been shown to phosphorylate the same substrates [see Refs. (43, 44)]. Therefore, it is possible that PKCι also targets GULP1 T35. To test this, in vitro kinase assay was conducted. Intriguingly, PKCι could also phosphorylate recombinant WT GULP1 fragment but not the GULP1 T35A mutant (Fig. 5A).
Figure 5.
PKCι phosphorylates GULP1 T35 and reduces the effect of GULP1 on APP processing. A) Bacterially expressed GST-GULP126–43 (WT) or GST-GULP126–43 T35A (T35A) were incubated with PKCι immunoprecipitated from transfected cell lysate together with (γ-[32P])-ATP for 30 min at 30°C. RM is the reaction mix only without kinase. Upper panel: autoradiograph; lower panel: Coomassie Blue staining. PKCι phosphorylates GULP1 at T35. B) HEK293 were cotransfected with APP-GAL4, pFR-Luc, and phRL-TK together with the indicated constructs. PKCι reduces the effect of GULP1-mediated APP-GAL4 cleavage via phosphorylation of T35. ns, not significant (n = 5). Results are means ± sd. **P < 0.01 compared with GULP1 transfected cells. C) Cells were cotransfected with APP and the indicated constructs, and the level of secreted Aβ40 was assayed. PKCι suppresses GULP1-enhanced Aβ liberation through phosphorylation of T35 (n = 5). Results are means ± sd. **P < 0.01 compared with GULP1 transfected cells. D) WT or PKCι knockout (KO) HEK293 cells were cotransfected with APP-GAL4, pFR-Luc, and phRL-TK together with the indicated constructs. GULP1-mediated APP-GAL4 cleavage was enhanced in PKCι KO cells. Left panel: Immunoblot demonstrates KO of PKCι; n = 5. Results are means ± sd. *P < 0.001. E) WT or PKCι KO HEK293 cells were cotransfected with APP and the indicated constructs, and the level of secreted Aβ40 was assayed. GULP1-mediated Aβ40 secretion was increased in PKCι KO cells (n = 5). Results are means ± sd. *P < 0.001. F) PKCι reduces GULP1-APP interaction in coimmunoprecipitation assay. Coimmunoprecipitation was performed from cells transfected with APP + myc-GULP1, APP + myc-GULP1 + PKCι, APP + myc-GULP1 T35A, or APP + myc-GULP1 T35A + PKCι using a mouse anti-myc antibody 9B11. APP and myc-GULP1 in the immunoprecipitates (IPs) were detected by a rabbit anti-APP and a rabbit anti-myc, respectively. Ns, not significant (n = 3). *P < 0.001.
Next, we tested if PKCι alters APP processing. Like PKCζ, overexpression of PKCι could suppress APP-GAL4 cleavage and Aβ generation in cells cotransfected with WT GULP1 but not the T35A mutant (Fig. 5B, C). Conversely, GULP1-mediated APP-GAL4 cleavage and Aβ production were significantly enhanced in PKCι knockout cells (Fig. 5D, E). Moreover, a reduced amount of APP was coimmunoprecipitated with WT GULP1, but not the T35A mutant, in the presence of PKCι (Fig. 5F). Hence, our findings suggest that both PKCι and PKCζ phosphorylate GULP1 T35 and reduce GULP1-mediated APP processing.
Structure of GULP1 PTB in complex with the NPTY motif of APP
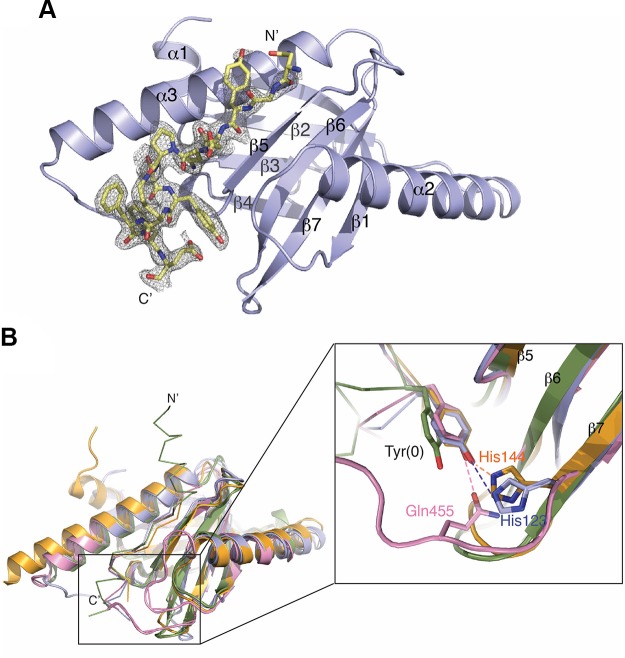
To gain molecular insights into the mechanism by which GULP1 PTB interacts with APP, we determined the X-ray crystal structure of GULP1 PTB in complex with a 12-mer AICD peptide (NGYENPTYKFFE) to a resolution of 2.17 Å (Rfree = 22.5% and Rwork = 20.7%) (Table 1). The peptide covers the classic PTB binding site, and the entire sequence was observed in the crystal structure (Fig. 6A). The complex structure reveals that GULP1 PTB adopts the canonical Pleckstrin homology fold, which consists of a 7-stranded β-sandwich capped by a C-terminal helix. GULP1 PTB, like the mammalian disabled-2 (Dab2) PTB domain, contains a short α-helix (α1) at the N terminus, and like the Dab2, X11, and FE65 PTB domains, it contains a large α-helix (α2) inserted between the β1 and β2 strands. In the complex, the N-terminal region of APP peptide (aa NGYE) forms an anti-parallel β-interaction with the β5 strand of the PTB, where the C-terminal region (aa TYKFFE) forms a short α-helix (αC) docked in a groove formed by the β1-α2 loop, the β4-β5 loop, and helix α3. Noteworthy, similar to the PTB domains of other APP binding adaptor proteins like FE65, Dab, and X11, GULP1 PTB does not require the tyrosine within the NPTY motif of APP to be phosphorylated for binding (5, 45, 46). An overlay of the model of the GULP1 PTB:APP complex with those of Dab2:APP, X11 PTB:APP, and FE65 PTB2:APP complexes reveals that although the anti-parallel β-interaction between the bound peptide and the β5 strand is similar in all structures, the unphosphorylated NPTY motif binds to the PTBs of GULP1, Dab2, and X11, but not to that of FE65, in the same mode (Fig. 6B) (47–49). In particular, although the lengths and the C-terminal folds of the APP peptides are different, the unphosphorylated tyrosines in GULP1, Dab2, and X11 structures adopt similar rotamers and are stabilized by hydrogen bonding to a side-chain from the respective PTB (H123 in GULP1, H144 in Dab2, and Q455 in X11, respectively) (Fig. 6B, inset). Such stabilizing interaction is missing in the FE65 structure, where the unphosphorylated tyrosine is positioned solely via van der Waals interaction with surrounding residues. Our structure thus provides the molecule basis on how GULP1 and APP interact in a phosphorylation-independent manner and suggests that there is no conserved mechanism for the recognition of the unphosphorylated NPTY motif.
TABLE 1.
X-ray data collection and model refinement statistics
| Variable |
GULP1 PTB-APP peptide |
|---|---|
| Data collection | |
| Space group | P3221 |
| Cell dimensions | |
| a, b, c (Å) | 63.91, 63.91, 108.77 |
| α, β, γ (deg) | 90, 90, 120 |
| Resolution (Å) | 32–2.17 |
| Rmerge (%) | 5.6 (36.9)a |
| I/σI | 26.8 (5.46) |
| Completeness (%) | 97.6 |
| Redundancy | 5.3 (10.9) |
| Refinement | |
| Resolution (Å) | 32–2.17 |
| Rwork/Rfree (%) | 20.7/22.5 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.014 |
| Bond angles (deg) | 1.68 |
| Ramachandran favored (%) | 96 |
| Ramachandran outliers (%) | 0 |
Number in parentheses refer to the highest resolution shells.
Figure 6.
Crystal structure of GULP1 PTB in complex with APP peptide. A) Overall structure of APP peptide bound to GULP1 PTB. Fo-Fc electron density map (contoured at 3σ) revealed large positive peaks and clearly indicates the presence of the APP peptide. B) Comparison of the overall structure of GULP1 PTB-APP structure and other PTB-APP complexes. GULP1 PTB-APP peptide complex (blue) is overlaid with Dab2 PTB-APP peptide complex [orange, PDB code: 1M7E], FE65 PTB-APP peptide complex (green, PDB code 3DXD), and X11 PTB-APP peptide complex (pink, PDB code: 1X11). The inset shows the side-chains of Tyr(0) in different complexes, except that of the FE65-APP peptide complex, interact with the PTBs in similar conformation. The side-chain of Tyr(0) of the APP peptide in the X11 PTB-APP complex structure is stabilized by Q455 from the β6-β7 loop (pink). On the other hand, Tyr(0) in the GULP1 PTB-APP and Dab2 PTB-APP complex structures interacts with H144 and H123 of the β7 strand of GULP1 (blue) and Dab2 (orange) respectively.
DISCUSSION
Aberrant kinase activity has been implicated in the pathogenesis of AD by influencing various pathways, including those that modulate the generation of Aβ. For instance, cyclin-dependent kinase 5 and JNK are reported to promote Aβ production by phosphorylation of APP T668 (50, 51). Although the exact phosphorylation target is not known, constitutively active Akt reduces Aβ generation via the suppression of APP trafficking (52). Apparently, proper kinase activity is critical to maintain normal brain physiology toward APP. In this study, we demonstrate that atypical PKC isoforms, PKCζ and PKCι, inhibit APP processing via the phosphorylation of GULP1 T35. Atypical PKCs, a subfamily of PKC that is insensitive to Ca2+ and diacylglycerol, have been shown to participate in various neuronal processes. For instance, both PKCζ and PKCι are implicated in synaptic plasticity (53–57). The inhibition of PKCζ results in the strong suppression of Rac1-mediated neurite outgrowth in subventricular zone–derived neurons (58). Likewise, PKCι is essential for Wnt3a-mediated neurite outgrowth (59). Primary dendrite specification of Purkinje cells is reported to be regulated by the kinase (60). In addition to physiologic processes, atypical PKCs are also implicated in AD. The mRNA expression levels of both PKCζ and PKCι are down-regulated in the affected regions of AD brain (61). The brain PKCζ level has been reported to decrease with age (62), and a postmortem examination has revealed a reduction in PKCζ activity in the brains of patients with AD (63). In addition, PKMζ and PKCι are found sequestered into neurofibrillary tangles in the brains of patients with AD, which is suggested to inhibit the normal activities of the kinases (64). It is worthy of note that the activation of PKCζ has been shown to improve cognitive function in adult mice, and the PKCζ activator metformin enhances spatial memory function (65) and confers a neuroprotective effect against Aβ-mediated long-term potentiation suppression (66). PKCι has also been shown to ameliorate Aβ toxicity in neural cells (67). However, the precise mechanism(s) by which atypical PKCs confer these beneficial effects remains unclear. Nevertheless, our findings provide new insight into the mechanism by which atypical PKCs participate in the APP metabolism via alteration of the GULP1-APP interaction.
Mounting evidence suggests that AIPs can influence the functions of APP, such as Aβ production (3, 68). Several AIPs, including GULP1, contain a PTB domain through which they interact with the NPTY motif of APP. Classic PTB domains interact with phospho-NPXpY motifs (pY = phosphotyrosine; X = any amino acid) in which the phosphorylation of the tyrosine can serve as a mechanism to modulate the interaction. However, GULP1 PTB belongs to another class of PTB in which PTB-NPXY interaction is independent of such tyrosine phosphorylation (5, 69, 70). Hence, an alternative mechanisms may operate to regulate GULP1-APP interaction. We show here that GULP1 T35 phosphorylation attenuates GULP1-APP interaction and GULP1-mediated APP processing. Of note, such threonine residue in GULP1 is conserved in several other APP-interacting PTB domains including X11s and ARH. Interestingly, our phosphomimetic study suggests that X11γ-APP interaction is also reduced by corresponding threonine phosphorylation (our unpublished observation). It is, therefore, possible that the upstream kinases that phosphorylate such threonine residues regulate the functions of APP in a spatial and temporal manner by determining which AIP, at least for some, binds to APP.
Notably, the effects of some AIPs, including GULP1, FE65, and X11, on APP processing remain controversial (5, 6, 20, 68, 71–78). The reasons for such conflicting observations are largely unknown. Interestingly, these AIPs are phosphoproteins, and their phosphorylation has been shown to alter APP processing. For instance, phosphorylation of FE65 PTB2 S610 reduces FE65-APP interaction and APP processing, whereas FE65 S579 phosphorylation promotes Aβ generation (14, 19). The phosphorylation of the X11β N-terminal region has been shown to promote the sorting of APP to autophagosome and to reduce Aβ secretion (29). As this study provides further evidence that the effects of AIPs on APP processing can be altered by phosphorylation, the phosphorylation status of AIPs in various cell types and model systems may contribute, at least in part, to the contradictory observations described above.
As mentioned above, phosphorylation of S610 in the PTB2 domain of FE65 was also shown to reduce its interaction with APP (14). Although both residues are located in the PTB domains, the phosphorylation of FE65 S610 and GULP1 T35 may alter their corresponding interaction with APP via different mechanisms. FE65 S610 is situated in the binding interface of FE65/APP. Once S610 is phosphorylated, the side-chain points out into solvent to disrupt APP binding because of a steric clash with APP Y687 (14). However, GULP1 T35 is located at the N-terminal region of the β1 strand and does not make direct contact with the APP peptide. However, because T35 immediately precedes the β1-α2 loop that forms part of the binding groove for the APP helix αC, we speculate that T35 phosphorylation may induce an allosteric effect on the conformation of the loop and therefore affect APP binding. Specifically, conformational change of the β1-α2 loop may alter the rotamer of H123 and disrupt the binding of the NPTY motif of APP. In addition, the ionic interaction between Lys41 and the Glu N-terminal to the NPTY motif, which is unique in the GULP1-APP structure, may likely be broken. Such phosphorylation-induced conformational change via an allosteric effect is not uncommon in phosphoproteins. For instance, phosphorylation at the C terminus of Rap1b, a member of the Ras family of small G proteins, communicates allosterically with the distal N-terminal effector binding domain and affects effector protein interaction (79). Further structural, biophysical, and computational studies are required to elucidate the molecular mechanism by which T35 phosphorylation inhibits APP binding.
The exact mechanism(s) by which GULP1 alters APP processing remains elusive. Endosome is a major subcellular compartment for β- and γ-secretase cleavages of APP (80). Interestingly, GULP1 has been shown to influence endocytic trafficking of various lipoprotein receptor-related protein ligands (81). Moreover, GULP1 has been suggested to participate in endocytic trafficking as it interacts with clathrin (82). Noteworthy, several PKC isoforms are implicated in regulating APP trafficking (83–85). Although it remains to be determined, GULP1 T35 phosphorylation by atypical PKCs could function in controlling the trafficking of APP via altering GULP1-APP interaction.
A meta-analysis indicated that type 2 diabetes is a risk factor for AD (86). Moreover, there is evidence to suggest that insulin influences APP metabolism (34, 35, 87). For example, insulin is reported to inhibit Aβ generation, and insulin injection has been shown to lower brain Aβ and improve memory in 3xTg-AD mice (88). Moreover, intranasal administration of insulin improves cognitive performance in animal model and clinical trials (89–91). Hence, aberrant insulin signaling is proposed to be associated with the pathogenesis of AD (92). However, the mechanism by which insulin exerts the above beneficial effects remains unclear. Notably, insulin resistance has been shown to cause the down-regulation of PKCζ activation (93), and a reduction in PKCζ activity has been observed in patients with AD (63). Additionally, insulin-stimulated atypical PKC activities are significantly lowered in patients with diabetes (94). As both PKCζ and PKCι were found to be activated by insulin (36, 37, 94, 95), the increased risk of AD in patients with type 2 diabetes may be due to a reduction in insulin-mediated atypical PKC activation. Together with our current findings, the activation of atypical PKC by insulin may decrease APP processing, at least in part, by the phosphorylation of GULP1.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Tommaso Russo (University of Naples Federico II, Naples, Italy) for pRc-CMV-APP695, and Kodi Ravichandran (University of Virginia, Charlottesville, Virginia, USA) for pEBB GULP1. This work was supported by funds from the Health and Medical Research Fund of Hong Kong, the Research Grants Council Hong Kong, The Chinese University of Hong Kong (CUHK) Direct Grant Scheme, United College Endowment Fund and the TUYF Charitable Trust. The authors declare no conflicts of interest.
Glossary
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- AICD
APP intracellular domain
- AIP
AICD–interacting proteins
- APP
amyloid precursor protein
- CHO
Chinese hamster ovary
- Dab2
disabled-2
- GST
glutathione-S-transferase
- GULP1
engulfment adaptor PTB domain containing 1
- HA
hemagglutinin
- HEK293
human embryonic kidney 293
- ITC
isothermal titration calorimetry
- phospho
phosphorylated
- PLA
proximity ligation assay
- PTB
phosphotyrosine-binding domain
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. D.-L. Chau, H.-Y. E. Chan, J. C.-K. Ngo, and K.-F. Lau conceived the study and designed the experiments; D. D.-L. Chau, K. W.-Y. Yung, W. W.-L. Chan, Y. An, Y. Hao, and J. C.-K. Ngo performed the experiments; D. D.-L. Chau, H.-Y. E. Chan, J. C.-K. Ngo, and K.-F. Lau analyzed the data and wrote the paper; and all authors read and approved the final manuscript.
REFERENCES
- 1.Schor N. F. (2011) What the halted phase III γ-secretase inhibitor trial may (or may not) be telling us. Ann. Neurol. 69, 237–239 [DOI] [PubMed] [Google Scholar]
- 2.Doody R. S., Thomas R. G., Farlow M., Iwatsubo T., Vellas B., Joffe S., Kieburtz K., Raman R., Sun X., Aisen P. S., Siemers E., Liu-Seifert H., Mohs R.; Alzheimer’s Disease Cooperative Study Steering Committee ; Solanezumab Study Group (2014) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 370, 311–321 [DOI] [PubMed] [Google Scholar]
- 3.Buoso E., Lanni C., Schettini G., Govoni S., Racchi M. (2010) beta-Amyloid precursor protein metabolism: focus on the functions and degradation of its intracellular domain. Pharmacol. Res. 62, 308–317 [DOI] [PubMed] [Google Scholar]
- 4.Bukhari H., Glotzbach A., Kolbe K., Leonhardt G., Loosse C., Müller T. (2017) Small things matter: implications of APP intracellular domain AICD nuclear signaling in the progression and pathogenesis of Alzheimer’s disease. Prog. Neurobiol. 156, 189–213 [DOI] [PubMed] [Google Scholar]
- 5.Hao Y., Perkinton M. S., Chan W. W., Chan H. Y., Miller C. C., Lau K. F. (2011) GULP1 is a novel APP-interacting protein that alters APP processing. Biochem. J. 436, 631–639; erratum: 437, 576 [DOI] [PubMed] [Google Scholar]
- 6.Beyer A. S., von Einem B., Schwanzar D., Keller I. E., Hellrung A., Thal D. R., Ingelsson M., Makarova A., Deng M., Chhabra E. S., Pröpper C., Böckers T. M., Hyman B. T., von Arnim C. A. (2012) Engulfment adapter PTB domain containing 1 interacts with and affects processing of the amyloid-β precursor protein. Neurobiol. Aging 33, 732–743 [DOI] [PubMed] [Google Scholar]
- 7.Cole S. L., Vassar R. (2008) The role of amyloid precursor protein processing by BACE1, the β-secretase, in Alzheimer disease pathophysiology. J. Biol. Chem. 283, 29621–29625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nekhoroshkova E., Albert S., Becker M., Rapp U. R. (2009) A-RAF kinase functions in ARF6 regulated endocytic membrane traffic. PLoS One 4, e4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R. Q., Yang Q. K., Lu B. W., Yi W., Cantin G., Chen Y. L., Fearns C., Yates J. R., III, Lee J. D. (2009) CDC25B mediates rapamycin-induced oncogenic responses in cancer cells. Cancer Res. 69, 2663–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantin G. T., Yi W., Lu B., Park S. K., Xu T., Lee J. D., Yates J. R., III (2008) Combining protein-based IMAC, peptide-based IMAC, and MudPIT for efficient phosphoproteomic analysis. J. Proteome Res. 7, 1346–1351 [DOI] [PubMed] [Google Scholar]
- 11.Besançon R., Lorenzi A., Cruts M., Radawiec S., Sturtz F., Broussolle E., Chazot G., van Broeckhoven C., Chamba G., Vandenberghe A. (1998) Missense mutation in exon 11 (Codon 378) of the presenilin-1 gene in a French family with early-onset Alzheimer’s disease and transmission study by mismatch enhanced allele specific amplification. Mutations in brief no. 141. Online. besancon@rockefeller1.univ.lyon1.fr. Hum. Mutat. 11, 481 [DOI] [PubMed] [Google Scholar]
- 12.Aboitiz F., Montiel J., López J. (2002) An hypothesis on the early evolution of the development of the isocortex. Brain Res. Bull. 57, 481–483 [DOI] [PubMed] [Google Scholar]
- 13.Zambrano N., Gianni D., Bruni P., Passaro F., Telese F., Russo T. (2004) Fe65 is not involved in the platelet-derived growth factor-induced processing of Alzheimer’s amyloid precursor protein, which activates its caspase-directed cleavage. J. Biol. Chem. 279, 16161–16169 [DOI] [PubMed] [Google Scholar]
- 14.Chow W. N., Ngo J. C., Li W., Chen Y. W., Tam K. M., Chan H. Y., Miller C. C., Lau K. F. (2015) Phosphorylation of FE65 Ser610 by serum- and glucocorticoid-induced kinase 1 modulates Alzheimer’s disease amyloid precursor protein processing. Biochem. J. 470, 303–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., Zhang F. (2013) Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau K. F., Howlett D. R., Kesavapany S., Standen C. L., Dingwall C., McLoughlin D. M., Miller C. C. (2002) Cyclin-dependent kinase-5/p35 phosphorylates Presenilin 1 to regulate carboxy-terminal fragment stability. Mol. Cell. Neurosci. 20, 13–20 [DOI] [PubMed] [Google Scholar]
- 17.Li W., Tam K. M. V., Chan W. W. R., Koon A. C., Ngo J. C. K., Chan H. Y. E., Lau K. F. (2018) Neuronal adaptor FE65 stimulates Rac1-mediated neurite outgrowth by recruiting and activating ELMO1. J. Biol. Chem. 293, 7674–7688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau K. F., McLoughlin D. M., Standen C., Miller C. C. (2000) X11 α and x11 β interact with presenilin-1 via their PDZ domains. Mol. Cell. Neurosci. 16, 557–565 [DOI] [PubMed] [Google Scholar]
- 19.Lee Y. S., Chow W. N. V., Lau K. F. (2017) Phosphorylation of FE65 at threonine 579 by GSK3β stimulates amyloid precursor protein processing. Sci. Rep. 7, 12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J. H., Lau K. F., Perkinton M. S., Standen C. L., Rogelj B., Falinska A., McLoughlin D. M., Miller C. C. (2004) The neuronal adaptor protein X11β reduces amyloid β-protein levels and amyloid plaque formation in the brains of transgenic mice. J. Biol. Chem. 279, 49099–49104 [DOI] [PubMed] [Google Scholar]
- 21.Lau K. F., Chan W. M., Perkinton M. S., Tudor E. L., Chang R. C., Chan H. Y., McLoughlin D. M., Miller C. C. (2008) Dexras1 interacts with FE65 to regulate FE65-amyloid precursor protein-dependent transcription. J. Biol. Chem. 283, 34728–34737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bredeston L. M., Rega A. F. (2002) Pre-steady-state phosphorylation and dephosphorylation of detergent-purified plasma-membrane Ca2+-ATPase. Biochem. J. 361, 355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 24.Käll L., Canterbury J. D., Weston J., Noble W. S., MacCoss M. J. (2007) Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 4, 923–925 [DOI] [PubMed] [Google Scholar]
- 25.Taus T., Köcher T., Pichler P., Paschke C., Schmidt A., Henrich C., Mechtler K. (2011) Universal and confident phosphorylation site localization using phosphoRS. J. Proteome Res. 10, 5354–5362 [DOI] [PubMed] [Google Scholar]
- 26.Sui S., Wang J., Yang B., Song L., Zhang J., Chen M., Liu J., Lu Z., Cai Y., Chen S., Bi W., Zhu Y., He F., Qian X. (2008) Phosphoproteome analysis of the human Chang liver cells using SCX and a complementary mass spectrometric strategy. Proteomics 8, 2024–2034 [DOI] [PubMed] [Google Scholar]
- 27.Mertins P., Yang F., Liu T., Mani D. R., Petyuk V. A., Gillette M. A., Clauser K. R., Qiao J. W., Gritsenko M. A., Moore R. J., Levine D. A., Townsend R., Erdmann-Gilmore P., Snider J. E., Davies S. R., Ruggles K. V., Fenyo D., Kitchens R. T., Li S., Olvera N., Dao F., Rodriguez H., Chan D. W., Liebler D., White F., Rodland K. D., Mills G. B., Smith R. D., Paulovich A. G., Ellis M., Carr S. A. (2014) Ischemia in tumors induces early and sustained phosphorylation changes in stress kinase pathways but does not affect global protein levels. Mol. Cell. Proteomics 13, 1690–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H. Y., Tseng V. S., Chen L. C., Chang Y. C., Ping P., Liao C. C., Tsay Y. G., Yu J. S., Liao P. C. (2009) Combining alkaline phosphatase treatment and hybrid linear ion trap/Orbitrap high mass accuracy liquid chromatography-mass spectrometry data for the efficient and confident identification of protein phosphorylation. Anal. Chem. 81, 7778–7787 [DOI] [PubMed] [Google Scholar]
- 29.Chaufty J., Sullivan S. E., Ho A. (2012) Intracellular amyloid precursor protein sorting and amyloid-β secretion are regulated by Src-mediated phosphorylation of Mint2. J. Neurosci. 32, 9613–9625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunning C. J., Black H. L., Andrews K. L., Davenport E. C., Conboy M., Chawla S., Dowle A. A., Ashford D., Thomas J. R., Evans G. J. (2016) Multisite tyrosine phosphorylation of the N-terminus of Mint1/X11α by Src kinase regulates the trafficking of amyloid precursor protein. J. Neurochem. 137, 518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoey S. E., Williams R. J., Perkinton M. S. (2009) Synaptic NMDA receptor activation stimulates α-secretase amyloid precursor protein processing and inhibits amyloid-β production. J. Neurosci. 29, 4442–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gianni D., Zambrano N., Bimonte M., Minopoli G., Mercken L., Talamo F., Scaloni A., Russo T. (2003) Platelet-derived growth factor induces the beta-gamma-secretase-mediated cleavage of Alzheimer’s amyloid precursor protein through a Src-Rac-dependent pathway. J. Biol. Chem. 278, 9290–9297 [DOI] [PubMed] [Google Scholar]
- 33.Law B. M., Guest A. L., Pullen M. W. J., Perkinton M. S., Williams R. J. (2018) Increased Foxo3a nuclear translocation and activity is an early neuronal response to βγ-secretase-mediated processing of the amyloid-β protein precursor: utility of an AβPP-GAL4 reporter assay. J. Alzheimers Dis. 61, 673–688 [DOI] [PubMed] [Google Scholar]
- 34.Devi L., Alldred M. J., Ginsberg S. D., Ohno M. (2012) Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer’s disease. PLoS One 7, e32792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Yu S., Gao S. J., Hu J. P., Wang Y., Liu H. X. (2014) Insulin inhibits Abeta production through modulation of APP processing in a cellular model of Alzheimer’s disease. Neuroendocrinol. Lett. 35, 224–229 [PubMed] [Google Scholar]
- 36.Standaert M. L., Bandyopadhyay G., Perez L., Price D., Galloway L., Poklepovic A., Sajan M. P., Cenni V., Sirri A., Moscat J., Toker A., Farese R. V. (1999) Insulin activates protein kinases C-zeta and C-lambda by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J. Biol. Chem. 274, 25308–25316 [DOI] [PubMed] [Google Scholar]
- 37.Standaert M. L., Galloway L., Karnam P., Bandyopadhyay G., Moscat J., Farese R. V. (1997) Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J. Biol. Chem. 272, 30075–30082 [DOI] [PubMed] [Google Scholar]
- 38.Bandyopadhyay G., Standaert M. L., Zhao L., Yu B., Avignon A., Galloway L., Karnam P., Moscat J., Farese R. V. (1997) Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J. Biol. Chem. 272, 2551–2558 [DOI] [PubMed] [Google Scholar]
- 39.Le Good J. A., Brindley D. N. (2004) Molecular mechanisms regulating protein kinase Czeta turnover and cellular transformation. Biochem. J. 378, 83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Standaert M. L., Bandyopadhyay G., Kanoh Y., Sajan M. P., Farese R. V. (2001) Insulin and PIP3 activate PKC-zeta by mechanisms that are both dependent and independent of phosphorylation of activation loop (T410) and autophosphorylation (T560) sites. Biochemistry 40, 249–255 [DOI] [PubMed] [Google Scholar]
- 41.Mayanglambam A., Bhavanasi D., Vijayan K. V., Kunapuli S. P. (2011) Differential dephosphorylation of the protein kinase C-zeta (PKCζ) in an integrin αIIbβ3-dependent manner in platelets. Biochem. Pharmacol. 82, 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drummond M. L., Prehoda K. E. (2016) Molecular control of atypical protein kinase C: tipping the balance between self-renewal and differentiation. J. Mol. Biol. 428, 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunaratne A., Thai B. L., Di Guglielmo G. M. (2013) Atypical protein kinase C phosphorylates Par6 and facilitates transforming growth factor β-induced epithelial-to-mesenchymal transition. Mol. Cell. Biol. 33, 874–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taniguchi T., Kawamata T., Mukai H., Hasegawa H., Isagawa T., Yasuda M., Hashimoto T., Terashima A., Nakai M., Mori H., Ono Y., Tanaka C. (2001) Phosphorylation of tau is regulated by PKN. J. Biol. Chem. 276, 10025–10031 [DOI] [PubMed] [Google Scholar]
- 45.Borg J. P., Ooi J., Levy E., Margolis B. (1996) The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol. Cell. Biol. 16, 6229–6241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner M. J., Stacey M. M., Liu B. A., Pawson T. (2013) Molecular mechanisms of SH2- and PTB-domain-containing proteins in receptor tyrosine kinase signaling. Cold Spring Harb. Perspect. Biol. 5, a008987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radzimanowski J., Simon B., Sattler M., Beyreuther K., Sinning I., Wild K. (2008) Structure of the intracellular domain of the amyloid precursor protein in complex with Fe65-PTB2. EMBO Rep. 9, 1134–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yun M., Keshvara L., Park C. G., Zhang Y. M., Dickerson J. B., Zheng J., Rock C. O., Curran T., Park H. W. (2003) Crystal structures of the Dab homology domains of mouse disabled 1 and 2. J. Biol. Chem. 278, 36572–36581 [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z., Lee C. H., Mandiyan V., Borg J. P., Margolis B., Schlessinger J., Kuriyan J. (1997) Sequence-specific recognition of the internalization motif of the Alzheimer’s amyloid precursor protein by the X11 PTB domain. EMBO J. 16, 6141–6150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee M. S., Kao S. C., Lemere C. A., Xia W., Tseng H. C., Zhou Y., Neve R., Ahlijanian M. K., Tsai L. H. (2003) APP processing is regulated by cytoplasmic phosphorylation. J. Cell Biol. 163, 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colombo A., Bastone A., Ploia C., Sclip A., Salmona M., Forloni G., Borsello T. (2009) JNK regulates APP cleavage and degradation in a model of Alzheimer’s disease. Neurobiol. Dis. 33, 518–525 [DOI] [PubMed] [Google Scholar]
- 52.Shineman D. W., Dain A. S., Kim M. L., Lee V. M. (2009) Constitutively active Akt inhibits trafficking of amyloid precursor protein and amyloid precursor protein metabolites through feedback inhibition of phosphoinositide 3-kinase. Biochemistry 48, 3787–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly M. T., Crary J. F., Sacktor T. C. (2007) Regulation of protein kinase Mzeta synthesis by multiple kinases in long-term potentiation. J. Neurosci. 27, 3439–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frankland P. W., Josselyn S. A. (2013) Neuroscience: memory and the single molecule. Nature 493, 312–313 [DOI] [PubMed] [Google Scholar]
- 55.Glanzman D. L. (2013) PKM and the maintenance of memory. F1000 Biol. Rep. 5, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matt L., Hell J. W. (2013) PKCλ: a new player in LTP coming to the rescue of PKCζ’s faltering role in LTP? EMBO J. 32, 1348–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren S. Q., Yan J. Z., Zhang X. Y., Bu Y. F., Pan W. W., Yao W., Tian T., Lu W. (2013) PKCλ is critical in AMPA receptor phosphorylation and synaptic incorporation during LTP. EMBO J. 32, 1365–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khodosevich K., Monyer H. (2010) Signaling involved in neurite outgrowth of postnatally born subventricular zone neurons in vitro. BMC Neurosci. 11, 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greer Y. E., Fields A. P., Brown A. M., Rubin J. S. (2013) Atypical protein kinase Cι is required for Wnt3a-dependent neurite outgrowth and binds to phosphorylated dishevelled 2. J. Biol. Chem. 288, 9438–9446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanabe K., Kani S., Shimizu T., Bae Y. K., Abe T., Hibi M. (2010) Atypical protein kinase C regulates primary dendrite specification of cerebellar Purkinje cells by localizing Golgi apparatus. J. Neurosci. 30, 16983–16992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang W. S., Dunckley T., Beach T. G., Grover A., Mastroeni D., Ramsey K., Caselli R. J., Kukull W. A., McKeel D., Morris J. C., Hulette C. M., Schmechel D., Reiman E. M., Rogers J., Stephan D. A. (2008) Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiol. Genomics 33, 240–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldberg M., Steinberg S. F. (1996) Tissue-specific developmental regulation of protein kinase C isoforms. Biochem. Pharmacol. 51, 1089–1093 [DOI] [PubMed] [Google Scholar]
- 63.Moore P., White J., Christiansen V., Grammas P. (1998) Protein kinase C-zeta activity but not level is decreased in Alzheimer’s disease microvessels. Neurosci. Lett. 254, 29–32 [DOI] [PubMed] [Google Scholar]
- 64.Crary J. F., Shao C. Y., Mirra S. S., Hernandez A. I., Sacktor T. C. (2006) Atypical protein kinase C in neurodegenerative disease I: PKMzeta aggregates with limbic neurofibrillary tangles and AMPA receptors in Alzheimer disease. J. Neuropathol. Exp. Neurol. 65, 319–326 [DOI] [PubMed] [Google Scholar]
- 65.Wang J., Gallagher D., DeVito L. M., Cancino G. I., Tsui D., He L., Keller G. M., Frankland P. W., Kaplan D. R., Miller F. D. (2012) Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 11, 23–35 [DOI] [PubMed] [Google Scholar]
- 66.Asadbegi M., Yaghmaei P., Salehi I., Ebrahim-Habibi A., Komaki A. (2016) Neuroprotective effects of metformin against Aβ-mediated inhibition of long-term potentiation in rats fed a high-fat diet. Brain Res. Bull. 121, 178–185 [DOI] [PubMed] [Google Scholar]
- 67.Xie J., Guo Q., Zhu H., Wooten M. W., Mattson M. P. (2000) Protein kinase C iota protects neural cells against apoptosis induced by amyloid beta-peptide. Brain Res. Mol. Brain Res. 82, 107–113 [DOI] [PubMed] [Google Scholar]
- 68.Taru H., Suzuki T. (2009) Regulation of the physiological function and metabolism of AbetaPP by AbetaPP binding proteins. J. Alzheimers Dis. 18, 253–265 [DOI] [PubMed] [Google Scholar]
- 69.Uhlik M. T., Temple B., Bencharit S., Kimple A. J., Siderovski D. P., Johnson G. L. (2005) Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J. Mol. Biol. 345, 1–20 [DOI] [PubMed] [Google Scholar]
- 70.Yan K. S., Kuti M., Zhou M. M. (2002) PTB or not PTB -- that is the question. FEBS Lett. 513, 67–70 [DOI] [PubMed] [Google Scholar]
- 71.Santiard-Baron D., Langui D., Delehedde M., Delatour B., Schombert B., Touchet N., Tremp G., Paul M. F., Blanchard V., Sergeant N., Delacourte A., Duyckaerts C., Pradier L., Mercken L. (2005) Expression of human FE65 in amyloid precursor protein transgenic mice is associated with a reduction in β-amyloid load. J. Neurochem. 93, 330–338 [DOI] [PubMed] [Google Scholar]
- 72.Guénette S., Chang Y., Hiesberger T., Richardson J. A., Eckman C. B., Eckman E. A., Hammer R. E., Herz J. (2006) Essential roles for the FE65 amyloid precursor protein-interacting proteins in brain development. EMBO J. 25, 420–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang B., Hu Q., Hearn M. G., Shimizu K., Ware C. B., Liggitt D. H., Jin L. W., Cool B. H., Storm D. R., Martin G. M. (2004) Isoform-specific knockout of FE65 leads to impaired learning and memory. J. Neurosci. Res. 75, 12–24 [DOI] [PubMed] [Google Scholar]
- 74.Lee J. H., Lau K. F., Perkinton M. S., Standen C. L., Shemilt S. J., Mercken L., Cooper J. D., McLoughlin D. M., Miller C. C. (2003) The neuronal adaptor protein X11α reduces Abeta levels in the brains of Alzheimer’s APPswe Tg2576 transgenic mice. J. Biol. Chem. 278, 47025–47029 [DOI] [PubMed] [Google Scholar]
- 75.Ho A., Liu X., Südhof T. C. (2008) Deletion of Mint proteins decreases amyloid production in transgenic mouse models of Alzheimer’s disease. J. Neurosci. 28, 14392–14400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saluja I., Paulson H., Gupta A., Turner R. S. (2009) X11alpha haploinsufficiency enhances Abeta amyloid deposition in Alzheimer’s disease transgenic mice. Neurobiol. Dis. 36, 162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saito Y., Sano Y., Vassar R., Gandy S., Nakaya T., Yamamoto T., Suzuki T. (2008) X11 proteins regulate the translocation of amyloid beta-protein precursor (APP) into detergent-resistant membrane and suppress the amyloidogenic cleavage of APP by beta-site-cleaving enzyme in brain. J. Biol. Chem. 283, 35763–35771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sano Y., Syuzo-Takabatake A., Nakaya T., Saito Y., Tomita S., Itohara S., Suzuki T. (2006) Enhanced amyloidogenic metabolism of the amyloid beta-protein precursor in the X11L-deficient mouse brain. J. Biol. Chem. 281, 37853–37860 [DOI] [PubMed] [Google Scholar]
- 79.Edreira M. M., Li S., Hochbaum D., Wong S., Gorfe A. A., Ribeiro-Neto F., Woods V. L., Jr., Altschuler D. L. (2009) Phosphorylation-induced conformational changes in Rap1b: allosteric effects on switch domains and effector loop. J. Biol. Chem. 284, 27480–27486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haass C., Kaether C., Thinakaran G., Sisodia S. (2012) Trafficking and proteolytic processing of APP. Cold Spring Harb. Perspect. Med. 2, a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kiss R. S., Ma Z., Nakada-Tsukui K., Brugnera E., Vassiliou G., McBride H. M., Ravichandran K. S., Marcel Y. L. (2006) The lipoprotein receptor-related protein-1 (LRP) adapter protein GULP mediates trafficking of the LRP ligand prosaposin, leading to sphingolipid and free cholesterol accumulation in late endosomes and impaired efflux. J. Biol. Chem. 281, 12081–12092 [DOI] [PubMed] [Google Scholar]
- 82.Martins-Silva C., Ferreira L. T., Cyr M., Koenen J., Fernandes D. R., Carvalho N. R., Ribeiro C. B., Marion S., Chavez-Olortegui C., Prado M. A., Prado V. F. (2006) A rat homologue of CED-6 is expressed in neurons and interacts with clathrin. Brain Res. 1119, 1–12 [DOI] [PubMed] [Google Scholar]
- 83.Koo E. H. (1997) Phorbol esters affect multiple steps in beta-amyloid precursor protein trafficking and amyloid beta-protein production. Mol. Med. 3, 204–211 [PMC free article] [PubMed] [Google Scholar]
- 84.Xu H., Greengard P., Gandy S. (1995) Regulated formation of Golgi secretory vesicles containing Alzheimer beta-amyloid precursor protein. J. Biol. Chem. 270, 23243–23245 [DOI] [PubMed] [Google Scholar]
- 85.Wang X., Zhou X., Li G., Zhang Y., Wu Y., Song W. (2017) Modifications and trafficking of APP in the pathogenesis of Alzheimer’s disease. Front. Mol. Neurosci. 10, 294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Profenno L. A., Porsteinsson A. P., Faraone S. V. (2010) Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry 67, 505–512 [DOI] [PubMed] [Google Scholar]
- 87.Watson G. S., Peskind E. R., Asthana S., Purganan K., Wait C., Chapman D., Schwartz M. W., Plymate S., Craft S. (2003) Insulin increases CSF Abeta42 levels in normal older adults. Neurology 60, 1899–1903 [DOI] [PubMed] [Google Scholar]
- 88.Vandal M., White P. J., Tremblay C., St-Amour I., Chevrier G., Emond V., Lefrançois D., Virgili J., Planel E., Giguere Y., Marette A., Calon F. (2014) Insulin reverses the high-fat diet-induced increase in brain Aβ and improves memory in an animal model of Alzheimer disease. Diabetes 63, 4291–4301 [DOI] [PubMed] [Google Scholar]
- 89.Maimaiti S., Anderson K. L., DeMoll C., Brewer L. D., Rauh B. A., Gant J. C., Blalock E. M., Porter N. M., Thibault O. (2016) Intranasal insulin improves age-related cognitive deficits and reverses electrophysiological correlates of brain aging. J. Gerontol. A. Biol. Sci. Med. Sci. 71, 30–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reger M. A., Watson G. S., Green P. S., Wilkinson C. W., Baker L. D., Cholerton B., Fishel M. A., Plymate S. R., Breitner J. C., DeGroodt W., Mehta P., Craft S. (2008) Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70, 440–448; erratum: 71, 866 [DOI] [PubMed] [Google Scholar]
- 91.Reger M. A., Watson G. S., Green P. S., Baker L. D., Cholerton B., Fishel M. A., Plymate S. R., Cherrier M. M., Schellenberg G. D., Frey W. H., II, Craft S. (2008) Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J. Alzheimers Dis. 13, 323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arnold S. E., Arvanitakis Z., Macauley-Rambach S. L., Koenig A. M., Wang H. Y., Ahima R. S., Craft S., Gandy S., Buettner C., Stoeckel L. E., Holtzman D. M., Nathan D. M. (2018) Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat. Rev. Neurol. 14, 168–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beeson M., Sajan M. P., Dizon M., Grebenev D., Gomez-Daspet J., Miura A., Kanoh Y., Powe J., Bandyopadhyay G., Standaert M. L., Farese R. V. (2003) Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes 52, 1926–1934 [DOI] [PubMed] [Google Scholar]
- 94.Kim Y. B., Kotani K., Ciaraldi T. P., Henry R. R., Kahn B. B. (2003) Insulin-stimulated protein kinase C lambda/zeta activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes: reversal with weight reduction. Diabetes 52, 1935–1942 [DOI] [PubMed] [Google Scholar]
- 95.Sajan M. P., Nimal S., Mastorides S., Acevedo-Duncan M., Kahn C. R., Fields A. P., Braun U., Leitges M., Farese R. V. (2012) Correction of metabolic abnormalities in a rodent model of obesity, metabolic syndrome, and type 2 diabetes mellitus by inhibitors of hepatic protein kinase C-ι. Metabolism 61, 459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.