Abstract
Suppressor interacting 3a (Sin3a) is a scaffold component of the chromatin repressive complex Sin3/histone deacetylase (Hdac). Sin3a has been shown as a hub gene driving preimplantation development in both mice and humans. However, its precise functions during preimplantation development remain unclear. Here, we show that the embryos arrested at morula stage upon specific depletion of Sin3a in mouse early embryos. Given the reduced cell number in Sin3a-depleted embryos, blocked cell proliferation is observed, likely because of the increased level of Trp53 acetylation at lysine 379. Moreover, we found that Sin3a depletion reduces Cdx2 and Tir Na Nog (Nanog), suggesting a failure of the first cell fate decision. In addition, we noted a striking increase of genome-wide DNA methylation, likely attributed to the increased nuclear DNA methyltransferase 1 observed in Sin3a-depleted embryos. Notably, RNA sequencing analyses showed 717 genes are differentially expressed, and Gene Ontology analysis of down-regulated genes (e.g., Hdac1) revealed top enriched terms involving protein deacetylation. Consistently, we confirmed a significant decrease of Hdac1 mRNA and protein abundance. Importantly, the development and Trp53 acetylation in Sin3a-depleted embryos could be rescued by expression of Hdac1 but not Hdac2. In summary, our results indicate a vital role of Sin3a in safeguarding the developmental progression through the morula-to-blastocyst transition via Hdac1.—Zhao, P., Li, S., Wang, H., Dang, Y., Wang, L., Liu, T., Wang, S., Li, X., Zhang, K. Sin3a regulates the developmental progression through morula-to-blastocyst transition via Hdac1.
Keywords: preimplantation, Hdac2, trophectoderm, pluripotency, embryo
Early embryo mortality is one major limiting factor causing infertility or subfertility in domestic animals as well as humans (1, 2). For example, 37% of total embryonic loss occurs during the first week after insemination in cattle (1, 2). Previous studies have also shown that oocytes and early embryos are sensitive to various environmental cues (3–5). For example, the use of assisted reproductive technologies (including in vitro fertilization) has been associated with poor developmental outcome of the offspring (6–10). To address these challenges, a fundamental understanding of epigenetic regulation during early embryo development is required.
Contrasting with somatic cells, preimplantation embryos undergo a remarkable reprograming in epigenetics, including DNA methylations, histone modifications, histone variants, and chromatin remodeling (11). The preimplantation embryo is therefore a valuable research model for epigenetic scientists to dissect physiologic relevance of epigenetic modifications. Increasing evidence has demonstrated that incomplete epigenetic reprogramming is a major contributing factor to developmental failure or poor developmental outcome associated with the use of assisted reproductive technologies (12–14). Indeed, reproductive cloning of macaque monkeys via somatic-cell nuclear transfer is feasible when H3K9me3 demethylase [lysine demethylase 4D (KDM4D)] is employed to correct H3K9me3 level (12).
The Sin3/histone deacetylase (Hdac) complex is known as a chromatin repressive complex and is conserved between yeast and man (15, 16). Given that Sin3a cannot directly bind to DNA, it acts as a scaffold protein for other transcription factors with DNA-binding activities, and therefore tethers Hdac1/2 to target genes to induce transcription silencing. Many studies have demonstrated that Sin3 complex not only represses gene expression but promotes transcription in a cell context–dependent manner (16, 17).
Knockout (KO) of genes encoding core components in Sin3/Hdac [Hdac1 (18), Sin3a (19), and Suds3 (20)] individually leads to early embryo lethality, indicating a requirement for the complex during early embryonic development. Sin3a, Suds3, and Hdac1/2 are detectable throughout preimplantation development (21–23). Single-cell RNA sequencing (RNA-seq) analyses reveal that Sin3a is a conserved hub gene of mouse and human transcriptome networks in preimplantation embryos, suggesting a key role in driving preimplantation development (24). Compatible with this thought, depletion of maternal Sin3a suppresses embryonic development beyond the 2-cell stage and disrupts gene expression during the oocyte-to-embryo transition (21). However, it remains unclear for the precise role of Sin3a on preimplantation development, in particular the morula-to-blastocyst transition.
In this study, we showed that silencing Sin3a via microinjection of small interfering RNAs (siRNAs) into zygotes results in developmental arrest during the morula-to-blastocyst transition in mice. Concurrently, cell proliferation was blocked, likely owing to the increased Trp53 acetylation observed in knockdown (KD) embryos. We observed a failure of the cell fate decision to generate trophectoderm (TE) and inner cell mass (ICM) with reduced expression of key lineage-specific genes Cdx2 and Nanog. Genome-wide DNA methylation was up-regulated, which likely arose from the increase of nuclear DNA methyltransferase (Dnmt)1. Importantly, Sin3a ablation triggered a significant reduction of Hdac1, which may contribute to the increased Trp53 acetylation. Injection of exogenous Hdac1 but not Hdac2 successfully rescued the development of Sin3a-depleted embryos as well as Trp53 acetylation. Overall, we propose that Sin3a complex plays an essential role during the morula-to-blastocyst transition via regulation of Hdac1.
MATERIALS AND METHODS
Mouse embryo collection and culture
All mice were bred under a 12-h light/dark cycle. Eight-to-ten-week-old female mice bred from C57BL/6 × DBA2 (B6D2 F1; Beijing Vital River Laboratory Animal Technology, Beijing, China) were superovulated by injecting pregnant mare serum gonadotropin (10 IU; Sansheng Pharmaceutical, Xinxiang City, China) followed by human chorionic gonadotropin (hCG; 10 IU; Sansheng Pharmaceutical) 46–48 h later. After hCG injection, B6D2 F1 female mice were mated to B6D2 F1 males. Then, 20–22 h after hCG injection, zygotes were collected by flushing the oviduct with M2 medium (MilliporeSigma, Burlington, MA, USA) and hyaluronidase (MilliporeSigma) used to discard enclosing cumulus cells. Mouse embryos were cultured in KSOM medium that balanced overnight prior to culture. Embryos were incubated at 37°C under 5% CO2 in air. All experiments using lab animals were carried out based on the guidelines for the care and use of lab animals and approved by Zhejiang University.
siRNA synthesis
RNA interference in mouse early embryos was performed as previously described in refs. 22 and 25. siRNA oligo sequences were synthesized by GenePharma (Shanghai, China). We performed Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi) analysis for all siRNAs to avoid nonspecific targeting. The sense and antisense oligo sequences of the siRNAs used in the present study were as follows: Sin3a-mus-584 (sense: 5′-GGUUUCAGCUGUGCCACAATT-3′; antisense: 5′-UUGUGGCACAGCUGAAACCTT-3′), Sin3a-mus-816 (sense: 5′-CCUCAGGUCUACAAUGAUUTT-3′; antisense: 5′-AAUCAUUGUAGACCUGAGGTT-3′), and Sin3a-mus-893 (sense: 5′-CCGAGUGUCCCAGCUAUUUTT-3′; antisense: 5′-AAAUAGCUGGGACACUCGGTT-3′).
In vitro transcription
Wild-type Sin3a, Hdac1, and Hdac2 (coding sequences) were amplified from cDNA libraries built from mouse tissues. Amplicons were subcloned into T7-driven vectors. Mutants for Hdac1 (26) were constructed as previously described. All sequences were validated by Sanger sequencing before use. To synthesize cRNAs in vitro, expression plasmids were linearized and were transcribed in vitro, capped, and poly(A)-tailed according to the manual (T7 mMessage mMachine Ultra Kit; Thermo Fisher Scientific, Waltham, MA, USA). cRNAs were extracted and purified by MegaClear Kit (Thermo Fisher Scientific).
Microinjection
Microinjection of siRNA and cRNAs into mouse zygotes was performed as previously described in refs. 22 and 25. siRNAs (20 µM) and cRNAs (700 ng/µl) were injected into the cytoplasm of zygotes with a Piezo machine (Eppendorf, Hamburg, Germany) and micromanipulator (TransferMan; Eppendorf). siRNAs and cRNAs were filled into the microinjector, and constant outward flow was adjusted and maintained to allow successful microinjection and avoid the incidence of embryo lysis after injection. Ten picoliters of RNA solution was microinjected per zygote.
RNA isolation, reverse transcription, and quantitative PCR
Total RNA extraction in early embryos (n = 10–15/group/replicate) was performed based on the manual using the Arcturus PicoPure RNA Isolation Kit (Thermo Fisher Scientific). Reverse transcription was conducted using SuperScript II Reverse Transcriptase (Thermo Fisher Scientific). To quantify gene expression differences between KD and control groups, real-time PCR was performed on a StepOne System using FastStart Universal SYBR Green Master Mix (Roche, Basel, Switzerland). Quantification was normalized to H2A histone family member Z (H2afz). One embryo equivalent of cDNA was used for each real-time PCR reaction with a minimum of 3 replicates for all data shown. Primers used in the present study were as follows: H2afz (27): forward-5′-TCCAGTGGACTGTATCTCTGTGA-3′, reverse-5′-GACTCGAATGCAGAAATTTGG-3′; Sin3a: forward-5′-GCTCCTGGACACAGAAGAGG-3′, reverse-5′-TGTGCCAGATGTTCTCGAAG-3′; Sin3b: forward-5′-GTGGAGGACGCTCTCACCTA-3′, reverse-5′-CGATGCTCTGGCTTTTGAACT-3′; Cdx2: forward-5′-CAAGGACGTGAGCATGTATCC-3′, reverse-5′-GTAACCACCGTAGTCCGGGTA-3′; Hdac1: forward-5′-TGAAGCCTCACCGAATCCG-3′, reverse-5′-GGGCGAATAGAACGCAGGA-3′; Hdac2: forward-5′-GGAGGAGGCTACACAATCCG-3′, reverse-5′-TCTGGAGTGTTCTGGTTTGTCA-3′; Nanog (28): forward-5′-CAAGGGTCTGCTACTGAGATGCTCTG-3′, reverse-5′-TTTTGTTTGGGACTGGTAGAAGAATCAG-3′; Oct4 (28): forward-5′-CTCCCGAGGAGTCCCAGGACAT-3′, reverse-5′-GATGGTGGTCTGGCTGAACACCT-3′; TEA domain transcription factor 4 (Tead4) forward-5′-TGATGCAGAGGGTGTATGGA-3′, reverse-5′-GATCAGCTCATTCCGACCAT-3′; yes-associated protein 1 (Yap1): forward-5′-GTCCTCCTTTGAGATCCCTGA-3′, reverse-5′-TGTTGTTGTCTGATCGTTGTGAT-3′; ribosomal protein (Rp)l3: forward-5′-GGAAAGTGAAGAGCTTCCCTAAG-3′, reverse-5′-CTGTCAACTTCCCGGACGA-3′; Rpl7: forward-5′-ACCGCACTGAGATTCGGATG-3′, reverse-5′-GAACCTTACGAACCTTTGGGC-3′; Rpl10a: forward-5′-ATGAGCAGCAAAGTCTCACG-3′, reverse-5′-GGTCGTAGTTCTTCAGGCTGAT-3′; angiomotin (Amot): forward-5′-CCGCCAGAATACCCTTTCAAG-3′, reverse-5′-CTCATCAGTTGCCCCTCTGT-3′; large tumor suppressor kinase 2 (Lats2) (29): forward-5′-TGCGAGTCATCAAGCAGACC-3′, reverse-5′-ACTTGGCTCTACTGCTGTGC-3′; lysine demethylase 6B (Kdm6b): forward-5′-TGAAGAACGTCAAGTCCATTGTG-3′, reverse-5′-TCCCGCTGTACCTGACAGT-3′; Scm-like with four Mbt domains 2 (Sfmbt2): forward-5′-AAGATAACCGGCTCAGCAAATG-3′, reverse-5′-TCTCTTCCAAATAGTCTCCCCAG-3′; orthodenticle homeobox 2 (Otx2): forward-5′-TATCTAAAGCAACCGCCTTACG-3′, reverse-5′-AAGTCCATACCCGAAGTGGTC-3′; lysine demethylase 3A (Kdm3a): forward-5′-GTGACACAACCATTTTCAACCTG-3′, reverse-5′-CACCCTGTTGGCAGTTCTTC-3′; mesoderm-specific transcript (Mest): forward-5′-GTGGTGGGTCCAAGTAGGG-3′, reverse-5′-AAGCACAACTATCTCAGGGCT-3′; paternally-expressed gene 3 (Peg3): forward-5′-CCAAGAGAACTGCCTACCCA-3′; reverse-5′-TCCCTTGCTCTTCCGGATTT-3′; Meg3: forward-5′-CGAGGACTTCACGCACAAC-3′; reverse-5′-TTACAGTTGGAGGGTCCTGG-3′; small nuclear ribonucleoprotein-associated protein N (Snrpn): forward-5′-ATGCAAAACAGCCAGAACGT-3′; reverse-5′-CACACGAGCAATGCCAGTAT-3′; and Igf2: forward-5′-GTGTGTGTCAGCCAAGCATG-3′, reverse-5′-CAAATGTGGGGACACAGAGG-3′.
RNA-seq library construction and bioinformatics
Mouse morulae (E2.75) were harvested from nonspecific siRNA-injected control (NC) and KD groups (n = 3; 60 embryos/group/replicate). Total RNA extraction was performed using a PicoPure RNA Isolation Kit based on the manufacturer’s manual. Before RNA isolation, equal amounts of green fluorescent protein and red fluorescent protein mRNAs (2 × 106 copies) were added to each group as a spike-in control. mRNA separation was achieved using oligo(dT)25 beads. Sequencing libraries were constructed with NEBNext Ultra RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s instructions. In brief, mRNAs were fragmented followed by reverse transcription. The cDNA library underwent end repair, poly(A)-tailing, adaptor ligation, and PCR amplification for 12–15 cycles to prepare sequencing libraries. Libraries were sequenced using Illumina HiSeq X Ten (Illumina, San Diego, CA, USA). The sequencing reads were assigned directly to transcripts and analyzed using Salmon (https://combine-lab.github.io/salmon/) (30, 31). Analysis of differential gene expression was carried out using the DESeq2 package [adjusted P < 0.05 and Log2 (fold change) >0.8 or <−0.8] (32). Bioinformatics analysis [Gene Ontology (GO; http://geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG; https://www.genome.jp/kegg/kegg1.html)] of differentially expressed genes (DEGs) was performed by the Database for Annotation, Visualization, and Integrated Discovery (DAVID; https://david.ncifcrf.gov/). The data set can be accessed at the Gene Expression Omnibus (GSE131058; https://www.ncbi.nlm.nih.gov/geo/).
Bisulfite sequencing
Groups of 5–6 morulae were lysed and bisulfite converted according to the manufacturer’s instruction using an EZ DNA Methylation-Direct Kit (Zymo, Irvine, CA, USA). One-fifth embryo equivalent of the mutagenized DNA was used for each first-round PCR reaction. Five microliters of first-round PCR product was used as a template for each second-round PCR reaction. The PCR products were subcloned into pMD18-T vectors, and at least 10 clones per group were sequenced for each PCR reaction. Sequence data were analyzed using CodonCode Aligner software (https://www.codoncode.com/aligner/download.htm). DNA strands that contained identical patterns were counted only once. The experiment was performed 3 times.
Immunofluorescence
Mouse embryos were fixed using 4% paraformaldehyde (MilliporeSigma) in PBS for 10 min and permeabilized with 0.5% Triton X-100 and PBS for 30 min at room temperature. Fixed embryos were blocked in 10% fetal bovine serum, 0.1% Triton X-100, and PBS for 1 h. Then, embryos were incubated with primary antibodies for 1 h at room temperature or overnight at 4°C. Primary antibodies used are listed in Supplemental Table S1. Incubation with secondary antibodies was performed at 37°C for 1 h. DNA was counterstained with DAPI, and samples were mounted into and imaged using a Zeiss LSM780 confocal microscope system (Carl Zeiss, Oberkochen, Germany) with ×40 oil UPlanFLN objective (numerical aperture = 1.4) and ×1.8 digital zoom. Z stacks were obtained with 6-μm intervals between optical sections.
Western blotting
Embryo lysis was performed on ice using RIPA lysis buffer (Beyotime Biotechnology, Haimen, China) supplemented with 1 mM PMSF (Beyotime Biotechnology). Equal numbers of embryos were used in each group for each replicate. Proteins were extracted using 8% SDS-PAGE and transferred to a PVDF membrane (MilliporeSigma). Then, the membrane was blocked with 5% nonfat milk and incubated with primary antibodies overnight at 4°C and secondary antibodies for 1.5 h at room temperature. Signals were detected with Westar Nova v.2.0 (Cyanagen, Bologna, Italy).
Statistical analysis
All experiments were performed ≥3 times unless stated. Differences between 2 groups were determined by 2-tailed, unpaired Student’s t tests. Immunofluorescence (IF) was analyzed by ImageJ (National Institutes of Health, Bethesda, MD, USA). Graphs were made using Prism (GraphPad, La Jolla, CA, USA). A value of P < 0.05 was shown to be statistically significant. RNA-seq results were analyzed with R (http://www.rproject.org). Results were described as means ± sem.
RESULTS
Developmental arrest at morula stage in Sin3a-depleted embryos
Previous studies from others and our laboratory demonstrated extensive expression of genes encoding Sin3a complex components, including Hdac1/2, Suds3, and Sin3a, during preimplantation development (21–23). To evaluate the physiologic relevance of the Sin3a complex, we first investigated colocalization of 2 central components of Sin3a complex, Sin3a, and Hdac1. IF analyses confirmed that Sin3a and Hdac1 are colocalized in the nucleoplasm throughout preimplantation development (Supplemental Fig. S1). Combined with the evidence that Hdac1 and 2 are also colocalized in preimplantaion embryos (23), it suggests that the Sin3a complex is functional during preimplantation development.
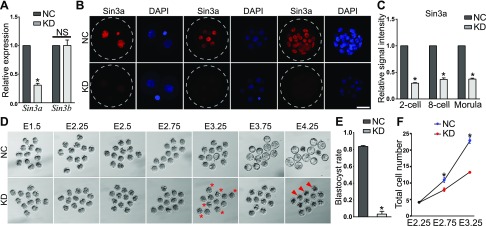
To determine the functional role of Sin3a, we injected a cocktail of 3 siRNAs targeting Sin3a into in vivo–derived mouse zygotes. Compared with zygotes injected with NCs, Sin3a siRNA injection (KD) led to a 70% decrease in the amount of Sin3a mRNAs and no change in expression of Sin3b, a highly homologous gene to Sin3a, suggesting a specific targeting of injected siRNAs (Fig. 1A; P < 0.05). IF analyses confirmed Sin3a was dramatically depleted from 2-cell to morula stage (Fig. 1B, C; P < 0.05).
Figure 1.
Specific depletion of Sin3a leads to developmental failure during the morula-to-blastocyst transition. A) Validation of KD efficiency of Sin3a siRNAs by qPCR. In vivo–derived mouse zygotes were microinjected with Sin3a siRNAs (20 µM; cKD) or NC. Embryos were collected at morula stage (5–10 embryos per pool; n = 3) for qPCR against Sin3a and Sin3b, a homolog of Sin3a. B) Immunostaining validation of Sin3a KD efficiency (n = 3; 5–10 embryos were analyzed/group/replicate). Scale bar, 25 µm. C) Quantification of Sin3a signal intensity for the experiment described in B. D) Representative photos of NC and KD embryos from E1.5 to E4.25. Asterisk indicates abnormal embryos; arrowhead denotes degenerating embryos. E) Blastocyst rate in NC and KD groups at E4 (n = 3). Data are presented as means ± sem. *P < 0.05. F) Total cell number per embryo was reduced from E2.75 in KD groups (n = 3; P < 0.05).
Next, we monitored the developmental potential of these embryos after siRNA injection. KD embryos did not show detectable morphologic differences until embryonic d (E)2.75, when nearly all embryos underwent compaction in NC embryos (Fig. 1D). However, examination of KD embryos at E3.25 revealed that the surface of a subset of KD morulae became unflattened, whereas NC embryos still appeared compact, suggesting ongoing degeneration in KD embryos. We found that Sin3a depletion strikingly reduced the capacity of blastocyst formation with only 3.0% KD embryos developing to blastocyst stage vs. 82.3% in NC groups at E3.75 (Fig. 1E). Moreover, we ruled out the possibility of developmental delay because KD embryos appeared even worse and began to collapse when cultured further until E4.25. In conformity with the observed phenotypic differences, reduced cell numbers were found in KD groups at E2.75 and the reduction was greater at E3.25 (Fig. 1F). Overall, these results suggest that Sin3a is essential for the developmental progression through the morula-to-blastocyst transition.
RNA-seq analysis of embryos deficient of Sin3a
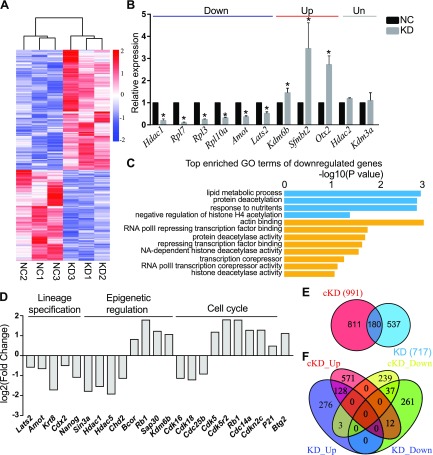
The Sin3a complex has been thought to induce transcriptional repression by binding to promoters of target genes (16). To explore the underlying consequences of the developmental failure associated with Sin3a-depleted embryos, we compared global gene expression between NC and KD morulae by RNA-seq analysis. The embryos were collected at E2.75, when they are morphologically indistinguishable. The differential expression of 717 genes accounts for the transcriptome changes between the 2 groups, of which 407 were up-regulated and 310 were down-regulated in KD morulae (Fig. 3A). RNA-seq results were validated with real-time PCR analysis against a group of representative transcripts (down: Hdac1, Rpl7, Rpl3, Rpl10a, Amot, and Lats2; Up: Kdm6b, Sfmbt2, and Otx2; unchanged: Hdac2 and Kdm3a; Fig. 3B). To shed insight into the role of Sin3a in modulating preimplantation development, GO analysis was performed. Consistent with the reduced cell numbers observed in KD embryos, analysis of up-regulated genes indicated that the enriched GO terms involved negative regulation of cell proliferation and cell growth (Supplemental Fig. S2A). However, the most strikingly enriched GO terms of down-regulated genes included histone H4 acetylation, protein deacetylase activity, transcriptional corepressor, and Hdac activity (Fig. 2C). These results indicated that KD morulae had already deviated from NC morulae at the molecular level, although they were morphologically indistinguishable. What is more, it suggests that Sin3a acts not only as a transcriptional repressor but transcriptional activator during preimplantation development.
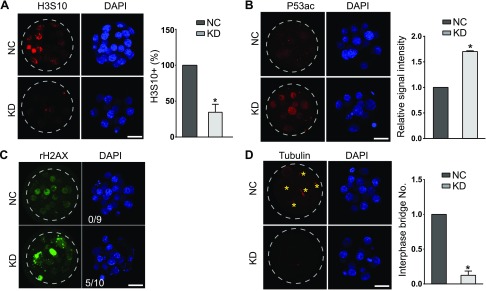
Figure 3.
Sin3a depletion results in cell proliferation failure and increased Trp53 acetylation. A) Immunostaining analyses of phosphorylation of H3S10, a hallmark for late G2 and mitosis, in mouse morulae (5–10 embryos/group; n = 3). Scale bar, 25 μm. B) Immunostaining analyses of Trp53K379 in morulae. The signal intensity of Trp53K79 was increased significantly (5–10 embryos/ group; n = 3. Scale bar, 25 μm). Nuclei were labeled with DAPI. C) Immunostaining analyses of γ-H2AX, an established marker for DNA damage in morulae. The experiment was replicated 3 times with 9 NC and 10 KD morulae analyzed. Scale bar, 25 μm. D) Immunocytochemical examination of α-tubulin, a marker for microtubule bridges, in morulae. Asterisk indicates microtubule bridge; n = 3; 5–10 embryos were analyzed/group each time. Scale bar, 25 μm.
Figure 2.
RNA-seq analysis of embryos deficient of Sin3a. A) Heatmap showing DEGs between KD and NC embryos. B) Validation of RNA-seq results on expression levels of representative genes. Three biologic replicates were performed with 5–10 morula collected for each group. *P < 0.05. C) GO analysis of down-regulated genes in cKD morula. The data indicate enriched GO terms related to epigenetic regulation, cell proliferation, and apoptosis. D) Overrepresentation of genes related to lineage specification, epigenetic regulation, and cell cycle among DEGs. E) Venn diagram showing the relationship of transcriptome changes identified by RNA-seq in Hdac1 cKD and Sin3a KD morulae. Number of DEGs is indicated in parentheses. F) Venn diagram illustrating the relationship of up- and down-regulated transcripts identified by RNA-seq in Hdac1 cKD and Sin3a KD morulae.
Among DEGs, we observed key genes associated (Fig. 2D) with lineage specification (Krt8, Cdx2, Nanog, Amot, and Lats2), genes associated with epigenetic regulation (Hdac1, Sap30, Hdac5, Chd2, Kdm6b, Bcor, and Rb1), and cell cycle [cyclin-dependent kinase inhibitor (Cdkn2c), P21, Btg2, Cdc14a, Rb1, Cdk5r2, Cdc25b, Cdk18, Cdk16, and Cdk5], suggesting abnormalities in these biologic processes may contribute to the phenotype of KD embryos as described above.
Because both Sin3a and Hdac1/2 are core components of the Sin3a complex, we asked whether there is a correlation between the molecular consequences of Sin3a KD and Hdac1/2 co-KD (cKD) embryos. Surprisingly, there is a remarkable difference between the transcriptomes of Sin3a KD and Hdac1/2 cKD embryos. Compared with 717 in Sin3a KD embryos, there were slightly more profound changes of transcriptome detected in Hdac1/2 cKD embryos, with differential expression in 991 genes (Fig. 2E). However, among DEGs, there were only 180 genes found in common, 71.1% of which were up-regulated (Fig. 2F), consolidating the primary role of the Sin3a complex in transcriptional repression.
Depletion of Sin3a impairs cell cycle progression and genome stability in preimplantation embryos
Because the majority of KD embryos manifested growth retardation and abnormal expression of genes was associated with cell cycle progression in RNA-seq analyses, we thus investigated the cell proliferation status by labeling with phosphorylated histone H3 serine 10 (H3S10), a marker for late G2/M phase. A previous study reported mouse embryonic stem cells generated from Sin3a-null blastocysts are subject to cell cycle arrest at the G2/M checkpoint (33). Similarly, we observed that the incidence of H3S10-positive cells was also markedly reduced in KD morulae relative to NC controls (P < 0.05; Fig. 3A), implying a proliferation defect. However, no difference in apoptosis was detected in KD embryos.
Sin3a has been shown to bind to critical cell cycle regulatory proteins including Trp53 (34). Trp53 plays a critical role in triggering cell cycle arrest. Trp53 is activated via multiple mechanisms, including post-translational modifications. Among them, Trp53 could be acetylated in an Hdac1-dependent manner (26). We observed that the amount of Trp53 acetylation at Lys-369 (Trp53K379ac) was increased by more than 1.5-fold in KD compared with the NC group (Fig. 3B). In concert with the increase in Trp53K379ac, we noted up-regulated expression of Trp53 target genes (P21, Btg2, and Rb1; Fig. 2D), which are well-characterized as cell cycle inhibitors (35–37). Thus, these results suggest cell cycle arrest may arise from increased Trp53K379ac in KD embryos.
Trp53 activation is induced by stress signals, including DNA damage (38). Sin3a has been shown to regulate DNA double-strand breaks repair in somatic cells and embryonic stem cells (33). Therefore, we examined whether observed cell cycle arrest as well as increased Trp53K379ac might be caused by a failure to resolve DNA damage by staining for phosphorylated H2AX histone (γ-H2AX), an established marker for DNA damage. We found that γ-H2AX–positive cells were increased in KD embryos (50%) compared with NC controls (Fig. 3C).
A recent report identified microtubule bridges between blastomeres in preimplantation embryos with critical roles in protein transport, such as E-cadherin (39). The number of interphase bridges was reduced in KD embryos, suggesting defective cell-cell communications (Fig. 3D). Overall, these results suggest that Sin3a is required for cell proliferation and genome stability in mouse preimplantation embryos.
Lineage specification is compromised in Sin3a KD embryos
The first lineage specification takes place during the morula-to-blastocyst transition and gives rise to ICM and TE (40). Moreover, we also noted down-regulated expression of key lineage-specific genes among DEGs from RNA-seq analyses (Fig. 2D). To better understand the early differentiation program in KD embryos, we examined the expression pattern of the marker genes (TE specific: Cdx2; pluripotency marker: Oct4 and Nanog) at mRNA and protein levels. We found the amounts of Cdx2 and Nanog mRNA and protein were both reduced in KD morulae (Fig. 4A, B, D), whereas Oct4 mRNA and protein levels were unchanged (Fig. 4C, D), suggesting a failure of the first lineage specification.
Figure 4.
Sin3a depletion leads to a failure of the first lineage specification event. A) qPCR analysis of key lineage-specific genes (TE: Cdx2, Tead4, and Yap1; ICM: Oct4 and Nanog) in morulae (n = 3 pools of 5–10 embryos/group). B–D) Immunocytochemical analysis of Cdx2 and Nanog in morulae and blastocysts after RNA interference. The intensity of Cdx2 and Nanog was diminished in KD (n = 3 pools of 5–10 embryos/group). *P < 0.05. E) Normal distribution pattern of E-cadherin and Ctnnb1, 2 adhesion molecules, in KD morulae. F) No difference was detected for Yap1 and phosphorylated Yap (pYap) between NC and KD morulae.
Polarization of embryos and Hippo-signaling pathways are 2 upstream biologic processes to induce TE-specific programs (40). Two cell adhesion molecules E-cadherin and β-catenin (Ctnnb1) were analyzed. E-cadherin plays a crucial role in the process of mouse embryo compaction and the maintenance of epithelial cells (41). Although Ctnnb1-null embryos could develop to blastocyst stage, abnormal shape and size were noted during periimplantation development. Ctnnb1-null embryos are also prone to fission, indicating a requirement for blastocyst integrity (42). IF analyses revealed a normal expression level and localization of E-cadherin and Ctnnb1 in KD embryos (Fig. 4E). Tead4 and Yap1 are 2 critical components of the Hippo-signaling pathway (40). Gene expression assay showed that no difference in the expression of Tead4 and Yap1 was found between NC and KD embryos (Fig. 4A). IF results confirmed that no difference of Yap1 signal was detected with its main distribution in the nucleus in outer cells of each KD morula (Fig. 4F). Phosphorylated Yap was primarily localized in the cytoplasm in inner cells of both NC and KD morulae (Fig. 4F). Taken together, these data suggest that Sin3a regulates the first lineage specification event independent of embryo polarization and Hippo pathway.
Reduced dimethylation of histone H3 lysine 9 was detected in Sin3a KD embryos
The Sin3a complex plays an important role in maintaining chromatin configuration (20, 43). Consistently, our previous study showed Suds3 KD led to a global decrease in histone H3 lysine 9 dimethylation (H3K9me2) during preimplantation development (22). To shed insight on how Sin3a plays its role in regulating gene expression, we performed IF analyses of several established histone marks. The signal intensity of H3K9me2, a hallmark of transcription repression, was diminished significantly in KD embryos (Fig. 5A, B). However, no change was found for a closely related histone modification, H3K9me3 (Fig. 5C). Moreover, the signal intensity of H3K4me3 (Fig. 5D), a marker for transcriptional activation, and H3K27me3 (Fig. 5E), a marker for transcriptional repression, were not affected in KD embryos. Collectively, these data suggest that reduced H3K9me2 may be responsible for the up-regulated gene expression observed in Sin3a KD embryos.
Figure 5.
Sin3a depletion results in a decrease of dimethylation of histone H3 lysine 9. Immunocytochemical detection of histone marks for transcription repression (H3K9me2, H3K9me3, and H3K27me3) and transcription activation (H3K4me3) in Sin3a-depleted morulae (n = 3 pools of 5–10 embryos/group. *P < 0.05. The signal intensity of H3K9me2 (A, B) was reduced significantly in KD embryos, whereas no change was found for H3K9me3 (C), H3K4me3 (D), and H3K27me3 (E). Scale bar, 25 μm.
Genome-wide DNA methylation was perturbed in KD morulae with increased nuclear Dnmt1
A global DNA demethylation occurs following fertilization during preimplantation development (11). Loss of core components of Sin3a complex, including Hdac1/2 and Sin3a, results in reduced global DNA methylation and disturbs genome imprinting during mouse oogenesis (21). We sought to determine whether global DNA methylation was affected as well in KD embryos. Surprisingly, we detected that the amount of global 5′-methylcytosine (5mc) signal was increased by 50% in KD morulae (Fig. 6A). This increase in 5mc could be ascribable to its abnormal conversion to 5-hydroxymethylcytosine (5hmc) by ten-eleven translocation methylcytosine dioxygenase family enzymes (11). However, no change in 5hmc signal intensity was found (Fig. 6A), ruling out such possibility.
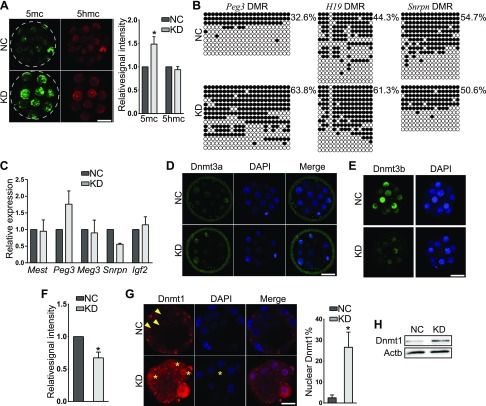
Figure 6.
Sin3a KD increases genome-wide DNA methylation and nuclear Dnmt1 content. A) Immunocytochemical analysis of 5mc and 5hmc in morulae. The intensity of 5mc, but not 5hmc, was increased in KD embryos (n = 3; 5–10 embryos were analyzed/group each time. *P < 0.05. B) Bisulfite sequencing analysis of DMRs of Peg3, H19, and Snrpn in KD embryos at the morula stage. C) qPCR analysis of well-characterized imprinted genes, including Mest, Peg3, maternally expressed 3 (Meg3), Snrpn, and Igf2 in morulae (n = 3 pools of 5–10 embryos each group. *P < 0.05. D–F) Immunocytochemical detection of Dnmt3a (D) and Dnmt3b (E, F). Sin3a deficiency results in no change in Dnmt3a but an increase of Dnmt3b signal intensity (F). G) Dnmt1 is distributed mainly in the cytoplasm (arrowheads) of control embryos but more nuclear Dnmt1 (asterisks) detected in KD embryos. The experiment was conducted 3 times and 5–10 embryos were analyzed per group each time.*P < 0.05. Scale bar, 25 µm. H) Immunoblot analysis shows Dnmt1 protein abundance was increased in response to Sin3a KD. Two replicates were performed, and similar results obtained.
DNA methylation plays a critical role in regulating expression of imprinted genes, including H19, Peg3, and Snrpn (44). Bisulfite sequencing analyses revealed the methylation level of differentially methylated regions (DMRs) of H19 and Peg3, but not Snrpn, was strikingly increased in KD embryos (Fig. 6B). However, quantitative PCR (qPCR) analyses showed Peg3 was up-regulated and Snrpn was down-regulated (Fig. 6C). Thus, these results confirmed that Sin3a deficiency increases DNA methylation level at specific loci. Moreover, it suggests that transcription of Peg3 and Snrpn is also subject to DNA methylation-independent mechanism.
There are 3 major Dnmts (Dnmt1, Dnmt3a, and 3b) involved in establishing DNA methylation during preimplantation development (45). We next explored if the 5mc increase was caused by misregulation of these Dnmts. We detected no difference of the mRNA level of Dnmt1, 3a, and 3b from RNA-seq results (unpublished results). IF analyses showed that the distribution and signal intensity of Dnmt3a were not changed, whereas Dnmt3b signal intensity was diminished in KD morulae, which is not likely responsible for the increased 5mc (Fig. 6D–F). Dnmt1 was mainly distributed in the cytoplasm in wild-type morulae as previously reported in ref. 46. However, Dnmt1 was detected in the nucleus in a greater number of blastomeres of KD morulae than the NC group (Fig. 6G). Immunoblot assay confirmed that the amount of Dnmt1 protein was increased (Fig. 6H). Overall, these results suggest that Sin3a regulates global 5mc via regulation of the Dnmt1 protein abundance and localization.
Sin3a is required for proper expression of Hdac1 during preimplantation development
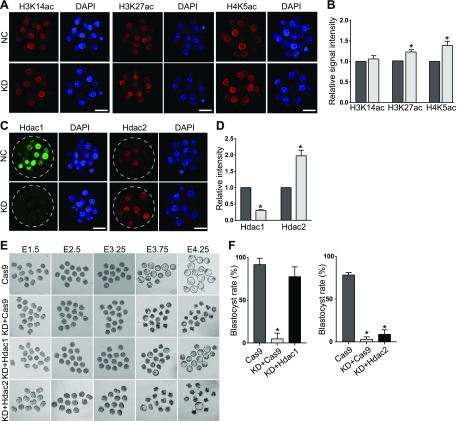
Because GO analysis of down-regulated genes enriched terms associated with histone deacetylation and Hdac1 was significantly down-regulated in KD morulae, we first set out to perform IF against representative histone acetylations, including histone H3 lysine 14 acetylation (H3K14ac), histone H3 lysine 27 acetylation (H3K27ac), and histone H4 lysine 5 acetylation (H4K5ac). No change was found for H3K14ac; however, the amount of H3K27ac and H4K5ac was obviously improved, suggesting a compromised activity of Hdac1/2 (Fig. 7A, B). qPCR results confirmed that Hdac1 mRNA abundance decreased dramatically in KD embryos, whereas the Hdac2 mRNA level was not changed (Fig. 2B). Correspondingly, Hdac1 signal intensity was decreased, but surprisingly, Hdac2 signal intensity was increased in Sin3a-deficient embryos (Fig. 7C, D).
Figure 7.
Sin3a depletion results in decreased Hdac1 and injection of Hdac1 cRNA can rescue the developmental failure of Sin3a-depleted embryos. A) Immunocytochemical detection of representative histone acetylations (H3K14ac, H3K27ac, and H4K5ac) in Sin3a-depleted morulae (n = 3 pools of 5–10 embryos per group. *P < 0.05. B) Quantification of the signal intensity for the experiment in A. The signal intensity of H3K27ac and H4K5ac was increased significantly in KD embryos, whereaa no change was found for H3K14ac. Scale bar, 25 μm. C, D) Hdac1 signal was diminished; however, Hdac2 signal intensity was increased in response to Sin3a depletion (n = 3 pools of 5–10 embryos/group). *P < 0.05. E, F) Expression of Hdac1 in Sin3a KD embryos (KD+Hdac1) rescued the development of Sin3a KD embryos (KD+Cas9). As a control, Hdac2 expression did not rescue the development. *P < 0.05.
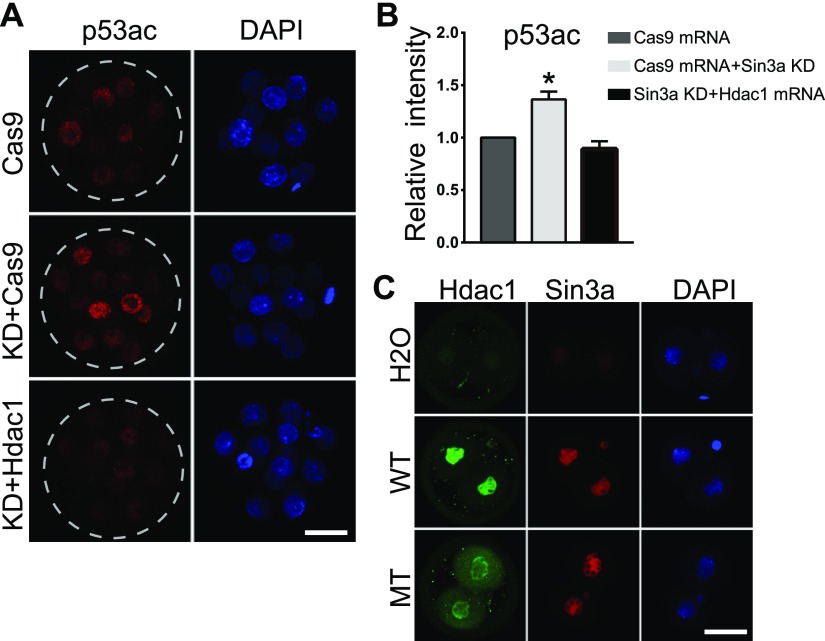
Hdac1 plays a key role in early embryogenesis (18, 23). To test if Hdac1 could rescue the developmental capability of KD embryos, we expressed Hdac1 in Sin3a KD embryos by microinjecting Hdac1 cRNA into KD embryos (KD+Hdac1). Expression of Hdac1 was confirmed successful as evidenced by increased Hdac1 signal intensity in KD+Hdac1 embryos (Supplemental Fig. S3A). Surprisingly, we also detected a significant increase of Sin3a signal intensity in KD+Hdac1 embryos relative to embryos injected with either CRISPR-associated protein 9 (Cas9) mRNA alone (Cas9 mRNA, control) or Sin3a siRNA and Cas9 mRNA (KD+Cas9) (Supplemental Fig. S3B). Following culture, we observed that the developmental capability was almost completely rescued to pass the blastocyst stage (Fig. 7E, F). As a control, exogenous Hdac2 cRNA injection did not rescue the developmental potential (Fig. 7E, F). Moreover, Trp53K379ac was also restored in response to exogenous Hdac1 cRNA injection (Fig. 8A, B), suggesting an Hdac1-depedent pattern of Trp53K379ac during preimplantation development. Nonetheless, there is no rescue of 5mc in the KD+Hdac1 group relative to the KD group (Supplemental Fig. S3C).
Figure 8.
Injection of Hdac1 cRNA successfully restored the level of Trp53 acetylation in Sin3a-depleted embryos. A) Immunostaining analyses of Trp53K379 in morulae. The signal intensity of Trp53K79 in Sin3a-deficient embryos was restored in response to Hdac1 cRNA injection (KD+Hdac1 vs. KD+Cas9; 5–10 embryos per group; n = 3. Scale bar, 25 μm). B) Quantification of the signal intensity for the experiment in A. C) Hdac1 was mutated at the deacetylase site and mRNA was produced in vitro. Wild-type Hdac1 (WT) and mutant Hdac1 (MT) was introduced into zygotes, and 2-cell embryos were collected for immunocytochemical analysis. Hdac1 expression induced up-regulation of Sin3a regardless of the deacetylase activity of Hdac1.
Given the increase of Sin3a in KD+Hdac1 embryos (Supplemental Fig. S3B), we sought to ask if Hdac1 overexpression is directly responsible for the increase. We performed overexpression experiment by injecting Hdac1 cRNA alone into mouse zygotes. As expected, Sin3a signal intensity was also obviously increased (Fig. 8C). Moreover, no change in Sin3a mRNA abundance was detected in Hdac1 overexpressed embryos (unpublished results), suggesting that Hdac1 posttranslationally regulates Sin3a. Because of the direct interaction between Sin3a and Hdac1 and the central function of Hdac1 in lysine acetylation, we tested the requirement of Hdac1’s deacetylation activity on its role in Sin3a protein abundance. Results showed Sin3a was also improved in Hdac1 mutant-expressed embryos (Fig. 8C), suggesting that Hdac1 regulates Sin3a independent of its deacetylase activity.
DISCUSSION
Although the Sin3a complex was defined 20 yr ago (15), its precise role during preimplantation development has not been addressed. In the present study, we have disclosed the developmental failure of Sin3a-deficient embryos during preimplantation development. Meanwhile, the transcriptome profile is disturbed with enriched genes associated with cell cycle progression, lineage specification, and epigenetic regulation. Indeed, cell proliferation is blocked, which is likely a result of the increased Trp53K379ac in response to Sin3a depletion. The first lineage specification program is disrupted with reduced expression of key lineage-specific genes, including Cdx2 and Nanog. Genome-wide DNA methylation is increased with altered nuclear Dnmt1 content. Importantly, Hdac1 mRNA and protein are severely compromised, and exogenous Hdac1 rescues the developmental potential of KD embryos. Overall, these developmental abnormalities contribute together to the developmental arrest of Sin3a-deficient embryos during the morula-to-blastocyst transition. We propose that Sin3a regulates the developmental progression through the morula-to-blastocyst transition via Hdac1.
The Sin3a/Hdac complex is conventionally thought of as a genome-wide transcriptional repressor via histone deacetylation (15). Nonetheless, previous studies from others and our lab suggest the complex is also involved in transcriptional activation (17, 22, 43). Here, we observed that 771 transcripts were differentially expressed, either up-regulated or down-regulated. Thus, Sin3a is not only involved in transcription repression but transcriptional activation. It is likely that Sin3a does not simply play a role via Hdac activity. First, we observed a more severe defect in Sin3a KD embryos (nearly 100% blastocyst formation failure) compared with 50% in Hdac1/2 cKD embryos (unpublished results). Second, the amount of histone acetylation was increased relatively mildly in Sin3a KD compared with those in Hdac1/2 cKD embryos (unpublished results). Third, the increased global 5mc level was observed in KD embryos, suggesting that Sin3a is involved in transcriptional activation via inhibiting DNA methylation. Fourth, changes in the transcriptome profile differ significantly between Sin3a KD and Hdac1/2 cKD embryos (Fig. 2E, F). In addition, conditional KO of Sin3a in mouse oocytes results in a moderate phenotype compared with the one in conditional KO of Hdac1/2 (47). Taken together, Sin3a likely regulates gene expression through both Hdac1/2-dependent and -independent mechanisms. In agreement with our findings, Sin3a deletion leads to a more severe defect in lungs relative to deletion of Hdac1/2, and there is only partial overlap of transcriptome changes between them (48, 49).
Inhibited cell proliferation, increased Trp53K379ac, and reduced microtubule bridges were associated with Sin3a deficiency in preimplantation embryos (Fig. 3). Previous reports show that Sin3a is required for the viability and cell cycle progression in pluripotent cells (33) and could regulate cell cycle inhibitor Cdkn1a and Cdkn1c in mouse embryonic fibroblast cells (50). Here, we show that deficiency of Sin3a leads to a cell proliferation block at G2/M. Correspondingly, we observed up-regulation of the cell cycle checkpoint inhibitor Cdkn2c. Indeed, Sin3a has been shown to directly bind to Cdkn2c promoter, suggesting that Sin3a acts as a transcriptional repressor of Cdkn2c. Increased expression of several Trp53 target genes, p21, Btg2, and Rb1 (Fig. 2D), which encode negative cell cycle regulators (35–37), further consolidates the critical role of Sin3a in regulating cell cycle progression during preimplantation development.
It is likely the cell cycle arrest observed in Sin3a KD embryos could be attributed to the increased acetylation of Trp53. Sin3a has been reported to regulate Trp53 acetylation (34). We also recently determined Trp53 deacetylation is directly regulated by the deacetylase activity of Hdac1 during preimplantation development (unpublished results). Given Hdac1 mRNA and protein were reduced significantly here, we propose that reduced Hdac1 activity accounts for increased Trp53K379ac. Indeed, exogenous Hdac1 injection can successfully restore the Trp53K379ac level (Fig. 8A). Previously, Sin3a has been shown to be involved in apoptosis in cells and tissues (19, 33). However, we have not detected changes in apoptosis in Sin3a-deficient preimplantation embryos, suggesting a stage-dependent or context-dependent role of Sin3a in regulating cellular apoptosis.
We observed that Sin3a KD down-regulated Hdac1 expression, and exogenous Hdac1 could rescue developmental capability of Sin3a KD embryos. The rescue effect is specifically induced by Hdac1, because injecting cRNA of Hdac2, a homolog of Hdac1, did not rescue the development of Sin3a KD embryos. Given that Hdac1 is dispensable for blastocyst formation (22, 23), the morula arrest of Sin3a KD embryos cannot be explained solely by the reduced Hdac1. In accordance with this thought, we demonstrated that Hdac1 injection promoted a significant increase of Sin3a, which may partly account for the significant rescue effect of Hdac1. We further determined that Hdac1 overexpression does not affect Sin3a mRNA levels (unpublished results), suggesting that Hdac1 may regulate the post-translational stability of Sin3a. Regulation of Sin3a via acetylation seems unlikely because mutating the deacetylase site of Hdac1 still induces Sin3a increase. Maternal Sin3a undergoes degradation in a proteasome-dependent manner during maternal-to-zygotic transition (21). It warrants further investigation as to how Hdac1 affects the stability of Sin3a and if Hdac1 is directly involved in the proteasome degradation of Sin3a.
DNA methylation is the most well-characterized epigenetic modification that plays a crucial role in transcriptional repression (11, 45). It takes place predominantly at CpG islands, with 70% of gene promoters in mammalian genomes containing CpG islands (45). Generally, transcriptionally active promoters are hypomethylated, and hypermethylation of CpG in a promoter is linked with transcriptional repression (45). Although double KO of Hdac1/2 in oocytes leads to a significant reduction of global 5mc and genomic imprinting affected, Sin3a KO results in slight and moderate changes in DNA methylation and genomic imprinting (47). Here, we show that Sin3a KD causes a significant increase of 5mc globally, suggesting a developmental context-dependent role of Sin3a in regulation of DNA methylation. Given the increased methylation levels at DMRs of several imprinted genes, their expression was not down-regulated, implicating that other regulatory factors than DNA methylation were involved in maintaining their proper expression.
In summary, the research described here clearly demonstrates Sin3a as an essential epigenetic player governing preimplantation development. Notably, ablation of Sin3a causes a developmental failure beyond morula stage. Moreover, Sin3a allows for the proper transcriptome profile, cell cycle progression, and DNA damage repair, as well as the establishment of microtubule bridges. We demonstrate that Hdac1 activity is severely disrupted after Sin3a ablation, likely through regulation of Hdac1 transcription. Supplementing exogenous Hdac1 mRNA could restore the Trp53 acetylation and the development progression in KD embryos. Moreover, Hdac1 is also critical for maintaining Sin3a stability during preimplantation development. The present study suggests that the Sin3a complex is involved in creating competent conditions required for developmental progression during the morula-to-blastocyst transition via regulating Hdac1 expression.
ACKNOWLEDGMENTS
The authors thank all members of the K.Z. laboratory for their helpful discussions and comments. This study was supported by National Natural Science Foundation of China (31672416 and 31872348 to K.Z.), the Zhejiang Provincial Natural Science Foundation of China (LY19C180002 to H.W.), and the Foundation of Shanghai Key Laboratory of Veterinary Biotechnology (klab201708). The authors declare no conflicts of interest.
Glossary
- γ-H2AX
phosphorylated H2AX histone
- 5hmc
5′-hydroxmethylcystosine
- 5mc
5′-methylcytosine
- Amot
angiomotin
- Cas9
CRISPR-associated protein 9
- Cdkn
cyclin-dependent kinase inhibitor
- cKD
co-knockdown
- Ctnnb1
β-catenin
- DEG
differentially expressed gene
- DMR
differentially methylated region
- Dnmt
DNA methyltransferase
- GO
Gene Ontology
- Hdac
histone deacetylase
- H3K9ac
histone H3 lysine 9 acetylation
- H3K9me2
histone H3 lysine 9 dimethylation
- H3K9me3
histone H3 lysine 9 trimethylation
- H3K27ac
histone H3 lysine 27 acetylation
- H3S10
histone H3 serine 10
- H4K5ac
histone H4 lysine 5 acetylation
- hCG
human chorionic gonadotropin
- ICM
inner cell mass
- IF
immunofluorescence
- KD
knockdown
- KDM6B
lysine demethylase 6B
- KO
knockout
- Lats2
large tumor suppressor kinase 2
- Nanog
Tir Na Nog
- NC
nonspecific siRNA-injected control
- qPCR
quantitative PCR
- RNA-seq
RNA sequencing
- Sin3a
suppressor interacting 3a
- siRNA
small interfering RNA
- Snrpn
small nuclear ribonucleoprotein-associated protein N
- TE
trophectoderm
- Tead4
TEA domain transcription factor 4
- Trp53K379ac
Trp53 acetylation at lysine 379
- Yap1
yes-associated protein 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. Zhao, S. Wang, X. Li, and K. Zhang conceived the project and designed research; P. Zhao, S. Li, Y. Dang, and K. Zhang analyzed data; P. Zhao, S. Li, H. Wang, Y. Dang, L. Wang, and T. Liu performed research; and P. Zhao and K. Zhang wrote the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Wiltbank M. C., Baez G. M., Garcia-Guerra A., Toledo M. Z., Monteiro P. L., Melo L. F., Ochoa J. C., Santos J. E., Sartori R. (2016) Pivotal periods for pregnancy loss during the first trimester of gestation in lactating dairy cows. Theriogenology 86, 239–253 [DOI] [PubMed] [Google Scholar]
- 2.Spencer T. E. (2013) Early pregnancy: concepts, challenges, and potential solutions. Anim. Front. 3, 48–55 [Google Scholar]
- 3.Ealy A. D., Wooldridge L. K., McCoski S. R. (2019) BOARD INVITED REVIEW: post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 97, 2555–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krisher R. L. (2013) In vivo and in vitro environmental effects on mammalian oocyte quality. Annu. Rev. Anim. Biosci. 1, 393–417 [DOI] [PubMed] [Google Scholar]
- 5.Fleming T. P., Watkins A. J., Velazquez M. A., Mathers J. C., Prentice A. M., Stephenson J., Barker M., Saffery R., Yajnik C. S., Eckert J. J., Hanson M. A., Forrester T., Gluckman P. D., Godfrey K. M. (2018) Origins of lifetime health around the time of conception: causes and consequences. Lancet 391, 1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z., Hagen D. E., Elsik C. G., Ji T., Morris C. J., Moon L. E., Rivera R. M. (2015) Characterization of global loss of imprinting in fetal overgrowth syndrome induced by assisted reproduction. Proc. Natl. Acad. Sci. USA 112, 4618–4623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mann M. R. W., Lee S. S., Doherty A. S., Verona R. I., Nolen L. D., Schultz R. M., Bartolomei M. S. (2004) Selective loss of imprinting in the placenta following preimplantation development in culture. Development 131, 3727–3735 [DOI] [PubMed] [Google Scholar]
- 8.Wen J., Jiang J., Ding C., Dai J., Liu Y., Xia Y., Liu J., Hu Z. (2012) Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil. Steril. 97, 1331–1337.e1–e4 [DOI] [PubMed] [Google Scholar]
- 9.Rivera R. M., Stein P., Weaver J. R., Mager J., Schultz R. M., Bartolomei M. S. (2008) Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum. Mol. Genet. 17, 1–14 [DOI] [PubMed] [Google Scholar]
- 10.Market-Velker B. A., Zhang L., Magri L. S., Bonvissuto A. C., Mann M. R. W. (2010) Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum. Mol. Genet. 19, 36–51 [DOI] [PubMed] [Google Scholar]
- 11.Eckersley-Maslin M. A., Alda-Catalinas C., Reik W. (2018) Dynamics of the epigenetic landscape during the maternal-to-zygotic transition. Nat. Rev. Mol. Cell Biol. 19, 436–450 [DOI] [PubMed] [Google Scholar]
- 12.Liu Z., Cai Y., Wang Y., Nie Y., Zhang C., Xu Y., Zhang X., Lu Y., Wang Z., Poo M., Sun Q. (2018) Cloning of macaque monkeys by somatic cell nuclear transfer. Cell 174, 245 [DOI] [PubMed] [Google Scholar]
- 13.Matoba S., Liu Y., Lu F., Iwabuchi K. A., Shen L., Inoue A., Zhang Y. (2014) Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell 159, 884–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W., Liu X., Wang C., Gao Y., Gao R., Kou X., Zhao Y., Li J., Wu Y., Xiu W., Wang S., Yin J., Liu W., Cai T., Wang H., Zhang Y., Gao S. (2016) Identification of key factors conquering developmental arrest of somatic cell cloned embryos by combining embryo biopsy and single-cell sequencing. Cell Discov. 2, 16010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassig C. A., Fleischer T. C., Billin A. N., Schreiber S. L., Ayer D. E. (1997) Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell 89, 341–347 [DOI] [PubMed] [Google Scholar]
- 16.Laugesen A., Helin K. (2014) Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell 14, 735–751 [DOI] [PubMed] [Google Scholar]
- 17.Icardi L., Mori R., Gesellchen V., Eyckerman S., De Cauwer L., Verhelst J., Vercauteren K., Saelens X., Meuleman P., Leroux-Roels G., De Bosscher K., Boutros M., Tavernier J. (2012) The Sin3a repressor complex is a master regulator of STAT transcriptional activity. Proc. Natl. Acad. Sci. USA 109, 12058–12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagger G., O’Carroll D., Rembold M., Khier H., Tischler J., Weitzer G., Schuettengruber B., Hauser C., Brunmeir R., Jenuwein T., Seiser C. (2002) Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 21, 2672–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowley S. M., Iritani B. M., Mendrysa S. M., Xu T., Cheng P. F., Yada J., Liggitt H. D., Eisenman R. N. (2005) The mSin3A chromatin-modifying complex is essential for embryogenesis and T-cell development. Mol. Cell. Biol. 25, 6990–7004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David G., Turner G. M., Yao Y., Protopopov A., DePinho R. A. (2003) mSin3-associated protein, mSds3, is essential for pericentric heterochromatin formation and chromosome segregation in mammalian cells. Genes Dev. 17, 2396–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jimenez R., Melo E. O., Davydenko O., Ma J., Mainigi M., Franke V., Schultz R. M. (2015) Maternal SIN3A regulates reprogramming of gene expression during mouse preimplantation development. Biol. Reprod. 93, 89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang K., Dai X., Wallingford M. C., Mager J. (2013) Depletion of Suds3 reveals an essential role in early lineage specification. Dev. Biol. 373, 359–372 [DOI] [PubMed] [Google Scholar]
- 23.Ma P., Schultz R. M. (2008) Histone deacetylase 1 (HDAC1) regulates histone acetylation, development, and gene expression in preimplantation mouse embryos. Dev. Biol. 319, 110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue Z., Huang K., Cai C., Cai L., Jiang C. Y., Feng Y., Liu Z., Zeng Q., Cheng L., Sun Y. E., Liu J. Y., Horvath S., Fan G. (2013) Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 500, 593–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang K., Haversat J. M., Mager J. (2013) CTR9/PAF1c regulates molecular lineage identity, histone H3K36 trimethylation and genomic imprinting during preimplantation development. Dev. Biol. 383, 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito A., Kawaguchi Y., Lai C. H., Kovacs J. J., Higashimoto Y., Appella E., Yao T. P. (2002) MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 21, 6236–6245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki S., Nozawa Y., Tsukamoto S., Kaneko T., Manabe I., Imai H., Minami N. (2015) CHD1 acts via the Hmgpi pathway to regulate mouse early embryogenesis. Development 142, 2375–2384 [DOI] [PubMed] [Google Scholar]
- 28.Wang J., Rao S., Chu J., Shen X., Levasseur D. N., Theunissen T. W., Orkin S. H. (2006) A protein interaction network for pluripotency of embryonic stem cells. Nature 444, 364–368 [DOI] [PubMed] [Google Scholar]
- 29.Cao Z., Carey T. S., Ganguly A., Wilson C. A., Paul S., Knott J. G. (2015) Transcription factor AP-2γ induces early Cdx2 expression and represses HIPPO signaling to specify the trophectoderm lineage. Development 142, 1606–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patro R., Duggal G., Love M. I., Irizarry R. A., Kingsford C. (2017) Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahraeian S. M. E., Mohiyuddin M., Sebra R., Tilgner H., Afshar P. T., Au K. F., Bani Asadi N., Gerstein M. B., Wong W. H., Snyder M. P., Schadt E., Lam H. Y. K. (2017) Gaining comprehensive biological insight into the transcriptome by performing a broad-spectrum RNA-seq analysis. Nat. Commun. 8, 59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Love M. I., Huber W., Anders S. (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonel P., Demmers J., Tan D. W. M., Watt F., Hendrich B. D. (2012) Sin3a is essential for the genome integrity and viability of pluripotent cells. Dev. Biol. 363, 62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zilfou J. T., Hoffman W. H., Sank M., George D. L., Murphy M. (2001) The corepressor mSin3a interacts with the proline-rich domain of p53 and protects p53 from proteasome-mediated degradation. Mol. Cell. Biol. 21, 3974–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bunz F., Dutriaux A., Lengauer C., Waldman T., Zhou S., Brown J. P., Sedivy J. M., Kinzler K. W., Vogelstein B. (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282, 1497–1501 [DOI] [PubMed] [Google Scholar]
- 36.Harrington E. A., Bruce J. L., Harlow E., Dyson N. (1998) pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc. Natl. Acad. Sci. USA 95, 11945–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouault J. P., Falette N., Guéhenneux F., Guillot C., Rimokh R., Wang Q., Berthet C., Moyret-Lalle C., Savatier P., Pain B., Shaw P., Berger R., Samarut J., Magaud J. P., Ozturk M., Samarut C., Puisieux A. (1996) Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nat. Genet. 14, 482–486 [DOI] [PubMed] [Google Scholar]
- 38.Kruiswijk F., Labuschagne C. F., Vousden K. H. (2015) p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 16, 393–405 [DOI] [PubMed] [Google Scholar]
- 39.Zenker J., White M. D., Templin R. M., Parton R. G., Thorn-Seshold O., Bissiere S., Plachta N. (2017) A microtubule-organizing center directing intracellular transport in the early mouse embryo. Science 357, 925–928 [DOI] [PubMed] [Google Scholar]
- 40.Rossant J. (2018) Genetic control of early cell lineages in the mammalian embryo. Annu. Rev. Genet. 52, 185–201 [DOI] [PubMed] [Google Scholar]
- 41.Riethmacher D., Brinkmann V., Birchmeier C. (1995) A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc. Natl. Acad. Sci. USA 92, 855–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messerschmidt D., de Vries W. N., Lorthongpanich C., Balu S., Solter D., Knowles B. B. (2016) β-catenin-mediated adhesion is required for successful preimplantation mouse embryo development. Development 143, 1993–1999 [DOI] [PubMed] [Google Scholar]
- 43.Ma P., Pan H., Montgomery R. L., Olson E. N., Schultz R. M. (2012) Compensatory functions of histone deacetylase 1 (HDAC1) and HDAC2 regulate transcription and apoptosis during mouse oocyte development. Proc. Natl. Acad. Sci. USA 109, E481–E489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tucci V., Isles A. R., Kelsey G., Ferguson-Smith A. C.; Erice Imprinting Group (2019) Genomic imprinting and physiological processes in mammals. Cell 176, 952–965 [DOI] [PubMed] [Google Scholar]
- 45.Li E., Zhang Y. (2014) DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 6, a019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimura H., Tanaka S., Shiota K. (1999) A novel domain of Dnmt1 is necessary for nuclear localization. Mol. Biol. Cell 10, 285a [Google Scholar]
- 47.Ma P., de Waal E., Weaver J. R., Bartolomei M. S., Schultz R. M. (2015) A DNMT3A2-HDAC2 complex is essential for genomic imprinting and genome integrity in mouse oocytes. Cell Rep. 13, 1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao C., Carraro G., Konda B., Guan X., Mizuno T., Chiba N., Kostelny M., Kurkciyan A., David G., McQualter J. L., Stripp B. R. (2017) Sin3a regulates epithelial progenitor cell fate during lung development. Development 144, 2618–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y., Tian Y., Morley M. P., Lu M. M., Demayo F. J., Olson E. N., Morrisey E. E. (2013) Development and regeneration of Sox2+ endoderm progenitors are regulated by a Hdac1/2-Bmp4/Rb1 regulatory pathway. Dev. Cell 24, 345–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji Q., Hu H., Yang F., Yuan J., Yang Y., Jiang L., Qian Y., Jiang B., Zou Y., Wang Y., Shao C., Gong Y. (2014) CRL4B interacts with and coordinates the SIN3A-HDAC complex to repress CDKN1A and drive cell cycle progression. J. Cell Sci. 127, 4679–4691 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.