Supplemental Digital Content is available in the text
Keywords: glucosamine, gut microbiome, osteoarthritis, undenatured type 2 collagen
Purpose of review
Osteoarthritis is a debilitating disease leading to joint degeneration, inflammation, pain, and disability. Despite efforts to develop a disease modifying treatment, the only accepted and available clinical approaches involve palliation. Although many factors contribute to the development of osteoarthritis, the gut microbiome has recently emerged as an important pathogenic factor in osteoarthritis initiation and progression. This review examines the literature to date regarding the link between the gut microbiome and osteoarthritis.
Recent findings
Studies showing correlations between serum levels of bacterial metabolites and joint degeneration were the first links connecting a dysbiosis of the gut microbiome with osteoarthritis. Further investigations have demonstrated that microbial community shifts induced by antibiotics, a germ-free environment or high-fat are important underlying factors in joint homeostasis and osteoarthritis. It follows that strategies to manipulate the microbiome have demonstrated efficacy in mitigating joint degeneration in osteoarthritis. Moreover, we have observed that dietary supplementation with nutraceuticals that are joint protective may exert their influence via shifts in the gut microbiome.
Summary
Although role of the microbiome in osteoarthritis is an area of intense study, no clear mechanism of action has been determined. Increased understanding of how the two factors interact may provide mechanistic insight into osteoarthritis and lead to disease modifying treatments.
INTRODUCTION
Osteoarthritis is a multifaceted whole joint disease involving degeneration of articular cartilage, subchondral bone sclerosis and synovial inflammation with these combine symptoms culminating in joint pain and disability [1]. Impacting more than 10% of the US population, osteoarthritis poses an enormous economic burden comprised of medical care costs, lost wages, and reduced economic productivity, with significant impact on the quality-of-life of its sufferers [2,3]. Despite its clinical and financial ramifications, there are currently no approved disease modifying osteoarthritis drugs available, with symptom palliation the only alternative [4]. Osteoarthritis is now recognized as a disease of complex cause, including age, injury, genetics, sex, and obesity as central contributing factors [5]. A commonality shared by many of these contributors is chronic systemic inflammation [6–11], and emerging research has firmly linking the inflammation of obesity and osteoarthritis initiation and progression [11,12,13▪▪].
The microbiome is the totality of the microbial ecosystems that exist within and on the human body, including both organisms and their secreted products [14–16]. The microbiome is a crucial component of the holobiont, and has significant ramifications for human health [17]. A growing understanding of the gut microbiome ascribes to it both endocrine and immunological function [18,19]. The number of bacterial cells in the human microbiome likely exceeds the number of host cells, with this ratio ranging anywhere from 1 : 1 up to estimates that bacterial cells outnumber human cells by a factor of 100 [20,21]. Wet mass of the microbiome has been calculated to equal that of the human kidney, supporting the notion that it could fundamentally serve an endocrine-like regulatory role.
Defining a healthy microbiome is a difficult endeavor owing to the large variations in what could be considered ‘normal’ caused by diurnal rhythms, immune status, diet, genetics, and many other variables [22–26]. However, shifts in the microbiome have an established role in numerous diseases, including amyotrophic lateral sclerosis, Parkinson's disease, Alzheimer's disease, rheumatoid arthritis (RA), Crohn's disease, type 2 diabetes, metabolic syndrome, and osteoporosis [27–32,33▪▪]. A common factor in many of these diseases is chronic local and systemic inflammation, and it is now understood that the gut microbiome contributes to inflammation by inducing the production of proinflammatory cytokines by host immune cells and by production of inflammatory bacterial metabolites [34]. Given that osteoarthritis is now understood to involve an inflammatory component, particular in the context of obesity [35,36], and as it is established that the gut microbiome is a regulator of inflammation [37–39], studies investigating the connections between the gut microbiome and the degenerative processes of osteoarthritis are crucial. In this review we will provide a state of the field assessment on work connecting the gut, joint, and osteoarthritis.
Most of our knowledge about the role of the gut microbiome in the development of inflammation-driven diseases has been generated outside the context of the skeletal system. Recently, however, a number of studies have provided data suggesting a role for the gut microbiome in bone homeostasis, RA, and most recently in osteoarthritis initiation and progression. In the context of osteoarthritis, these studies have used various rodent models of joint degenerative disease such as high-fat diet (HFD)-induced obesity, mechanical over-loading, surgical induction, and genetically prone rodent models; in all cases potential contributions of the gut microbiota to progression of osteoarthritis have been suggested. Table 1 provides a summary of the key published works that contribute to the current state of the field. Hinting at human relevance, a couple studies have suggested that patients diagnosed with osteoarthritis possess a quantifiable dysbiosis in the gut microbiome, supporting the concept that there is an osteoarthritis-associated microbial shift that may be pathogenic. As obesity and metabolic syndrome are known to be caused by gut microbiome dysbiosis and are important risk factors in the development of osteoarthritis [6,40], it follows that among all causes of osteoarthritis, potential causation in this context has been the most extensively investigated. Studies have shown that human obesity is associated with increased osteoarthritis risk in both weight bearing and nonweight bearing joints [41], suggesting the possibility of systemic players in the development of the osteoarthritis of obesity. In addition, studies on obesity in conjunction with other causes of osteoarthritis, such as injury [destabilization of the medial meniscus (DMM) or intra-articular fracture surgery] have reported an enhanced osteoarthritis severity, thus suggested a synergic effect of obesity [41,42].
Table 1.
Literature review summary
| Study | Animal model | OA inducing method | Intervention | OA assessment method | Microbiome Assessment | Conclusion |
| Collins et al., 2015 [43] | Rats (male) | High fat/high sucrose diet for 28 weeks. (40% energy from fat and 45% from sucrose) | N/A | Cartilage histological evaluation (Modified Mankin scores), synovial and sub-chondral bone assessment (OARSI), serum and synovial fluid biomarkers analysis | Fecal sample 16s sequencing | Higher Mankin scores, and higher level of serum LPS in obese animals. -->Lactobacillus species and Methanobrevibacter spp. abundance correlated with Mankin scores. |
| Huang et al., 2016 [44] | Humans (male and female) | N/A | N/A | Knee Radiography, Etarfolatide SPECT/CT scans, serum and synovial fluid biomarker analysis, WOMAC questionnaire, NHANES-I pain scores | N/A | Serum LPS and LBP levels were associated with an increased abundance of activated macrophages in the knee joint capsule and synovium; they were also associated with osteophyte formation, knee joint space narrowing, and WOMAC scores. |
| Ulici et al., 2018 [45▪] | Germ Free Mice (male) | DMM surgery | N/A | Histologic cartilage evaluation (ACS scores), osteophyte and synovial membrane evaluation | Fecal samples 16s sequencing | Reduced ACS scores in germ free mice compared to the control group. Differences in abundance of microbes detected in high and low ACS mice. |
| Schott et al., 2018 [13▪▪] | Mice (male) | High fat diet induced obesity (60% kcal from fat for 12 weeks) and DMM surgery | Oligofructose (prebiotic) | Cartilage histological evaluation (OARSI scores), IHC and IF of cartilage and synovium, PAST cells flow cytometry, Micro CT scan of mineralized meniscus, serum biomarkers analysis | Fecal samples 16s sequencing | Prebiotic supplementation reduced OA severity observed in HFD mice. Bifidobacterium pseudolongum abundance increased with prebiotic treatment; its levels were inversely related to the OA severity, systemic, and colon inflammation. |
| Guss et al., 2019 [47] | Mice (male) | Metabolic disorder (TLR5KO), High fat diet induced obesity (60% energy from fat) | Tibial mechanical loading (2 weeks or 6 weeks) | Cartilage histological evaluation (OARSI scores and Modified Mankin scores), Micro CT evaluation of the subchondral bone trabecular architecture, serum biomarker analysis | Fecal samples 16s sequencing | Long term (6 weeks) mechanical loading is necessary to induce OA. Mild metabolic disorder is not sufficient to develop severe OA. Disturbing metabolic disorder with antibiotics reduces OA severity. |
| Rios et al., 2019 [49▪▪] | Rats (male) | High fat/high sucrose diet for 28 weeks (20% energy from fat, 50% from sucrose) | Prebiotic fibers supplementation and exercise (12 weeks) | Cartilage histological evaluation (Modified Mankin scores), synovial and sub-chondral bone assessment (OARSI), serum and synovial fluid biomarker analysis | Cecal matter 16s sequencing | Prebiotic fibers, exercise, and the combination of both prevented OA development. Prebiotic treatment increased Bifidobacterium and Roseburia; they decreased Closteridium leptum and Akkemansia muciniphila. |
| Henrotin et al., 2019 [50▪] | Dunkin Hartley Guinea pigs (male) | Spontaneous development | Lyophilized inactivated culture from Bifidobacteri-um longum CBi0703 (12 weeks) | Cartilage histological evaluation (OARSI scores), serum biomarkers analysis | N/A | B. longum oral administration decreased cartilage damage and decreased type II collagen degradation. |
Studies investigating the connection between osteoarthritis development and the gut microbiome in rodent models of osteoarthritis are enumerated. Relevant studies were identified through a PubMed search for articles in English published in peer-reviewed journals. We used multiple search terms capturing osteoarthritis, joint, cartilage, gut microbiome and microbiota. Articles selected were reviewed by all coauthors for final inclusion. ACS, articular cartilage structure; CT, computed tomography; HFD, high-fat diet; IF, immunofluorescence; IHC, immunohistochemistry; LBP, LPS binding protein; LPS, lipopolysaccharides; OA, osteoarthritis; SPECT, single-photon emission computerized tomography.
Box 1.
no caption available
STUDIES CONNECTING GUT MICROBIOME AND OSTEOARTHRITIS
Study of a potential gut microbiome-joint axis in homeostasis and disease is a nascent area of study. Early work involved rodent models of osteoarthritis and in humans diagnosed with osteoarthritis, revealing a correlation between increased levels of circulatory inflammatory markers including bacterially produced lipopolysaccharides (LPS) that correlate with osteoarthritis severity, suggesting that microbiome-derived proinflammatory metabolites are players in osteoarthritis [43,44]. In a study of rats fed high-fat/high-sugar diet for 28 weeks, Collins et al. showed increased cartilage damage in obese animals and established a direct correlation between serum LPS levels and Mankin histological scores. When the gut microbiome composition was examined by 16S sequencing, they detected an increase in Lactobacillus spp. and Methanobrevibacter spp. abundance with a strong predictive relationship with histological score [43]. Significantly, in gnotobiotic mice, Ulici et al.[45▪] showed a decline in the severity of posttraumatic osteoarthritis in the germ-free situation; this provided evidence for a role of the gut microbiome in osteoarthritis pathogenesis in this model. Suggesting relevance in humans, in 25 patients with knee osteoarthritis, Huang et al.[44] established a link between serum and synovial fluid LPS levels with activated macrophages in the knee joint capsule and synovium, joint space narrowing, osteophyte formation, and increased WOMAC scores (i.e., worse symptoms). Connecting these LPS-osteoarthritis associations with a possible dysbiosis in the gut microbiome occurred in a study of the Rotterdam cohort. In 1444 participants enrolled in the Rotterdam study-III with hip and/or knee osteoarthritis, an association between increased WOMAC score and abundance of microbes in the proinflammatory Streptococcus taxa was identified [46▪]. This study further established human relevance, prompting the field to more carefully examine the role of a dysbiotic community or individual taxa as pathogenic in osteoarthritis.
To investigate the role of metabolic dysfunction in the absence of overt obesity on gut-joint associations and the involvement of individual taxa in this context, Guss et al. used a murine genetic model of metabolic syndrome (Toll-like receptor-5 deficiency) in combination with osteoarthritis-inducing mechanical overloading (2 or 6 weeks). The authors compared the impact of the metabolic disorder in this context to previously studied HFD-induced osteoarthritis models. Evaluation of histological changes in the cartilage indicated more severe osteoarthritis in the HFD-fed group; in this group they detected metabolic irregularities, increased body fat, systemic inflammation and the expected gut microbiome dysbiosis which included an increased abundance of Firmicutes. They concluded that while metabolic irregularities were observed in Toll-like receptor-5 deficient mice, alone they were not sufficient to induce osteoarthritis. Rather, they showed that increased levels of LPS in HFD-fed mice was associated with higher OARSI scores and a dysbiosis involving expansion of Firmicutes, suggesting an association between microbial components and development of osteoarthritis [47].
In an article providing evidence that gut microbiome dysbiosis in obesity is more than just associated with osteoarthritis, Schott et al.[13▪▪] examined microbial community shifts in HFD-fed obese mice with an overlay of DMM injury to synchronize initiation of disease. Histological evaluation showed increased cartilage degradation in HFD-fed obese mice and 16S sequencing confirmed a gut dysbiosis. Importantly, when HFD-fed obese mice were supplemented with the indigestible fiber oligofructose, the obese gut dysbiosis was mitigated and osteoarthritis progression was essentially halted. Bifidobacterium pseudolongum, a species with known anti-inflammatory properties [48] that was lost in HFD-fed mice, was restored following oligofructose supplementation [13▪▪]. Conversely, proinflammatory Peptococcaceae and Peptostreptococcaceae family members that were present in the obese cohort were completed ablated in oligofructose-supplemented mice. These findings provided the first published evidence that shaping of the microbial community with an indigestible prebiotic fiber, lacking direct effects on host biology, could be disease modifying in osteoarthritis; implication from the work indicate a causal link between gut microbiome dysbiosis and osteoarthritis.
Consistent with the Schott et al. findings, HFD-fed rats supplemented with oligofructose also showed delayed development of obesity-associated osteoarthritis [49▪▪]. In this report, Rios et al. demonstrated that a maximum protection could be achieved by combining the supplement with exercise. Paralleling the Schott et al. study, 16S sequencing demonstrated an increase in Bifidobacterium and Roseburia and a decrease in Clostridium leptum and Akkermansia muciniphila levels as a result of oligofructose supplementation [49▪▪]. Another recent study in a guinea pig model of spontaneous osteoarthritis showed that the oral administration of a lyophilized inactivated culture of Bifidobacterium longum CBi0703 reduces cartilage structural lesions and cartilage degradation markers, providing an overall joint protective effect [50▪]. Future studies on microbes from the Bifidobacterium taxa and their metabolites may be important in establishing a gut-joint axis and may represent a subset of new approaches to treat osteoarthritis that involve targeting the gut microbiome.
NUTRACEUTICALS MAY IMPROVE OSTEOARTHRITIS OUTCOMES VIA EFFECTS ON THE GUT MICROBIOME
Glucosamine, chondroitin sulfate, and undenatured type 2 collagen (UT2C) are nutraceuticals marketed as dietary supplements supportive of joint health. In the US alone, these compounds make up a multibillion-dollar industry that spans both human and animal use [51]. In the first major article detailing the protective effects of nutraceuticals on joint disease, Trentham et al.[52] demonstrated that oral consumption of rooster comb type 2 collagen had beneficial effects on biological, structural, and pain outcomes in patients with RA flare. Although this provocative finding was met with broad skepticism because of uncertainty regarding mechanism of action, it was an important early piece of evidence for use of cartilage component-based nutraceuticals to treat joint arthropathies. Various preclinical experiments have also supported the notion that oral supplementation with ‘joint protective’ nutraceuticals is effective at mitigating osteoarthritis. For example, Dar et al.[53] demonstrated that daily oral consumption of hydrolyzed type I collagen is chondroprotective in murine post traumatic osteoarthritis. Similarly, Bagi et al.[54] demonstrated that oral supplementation with undenatured native chicken type 2 collagen reduced joint degeneration in a rat model of posttraumatic osteoarthritis. As oral supplements composed of cartilage and soft tissue matrix components are the only agents with clinical data supporting positive patient-reported functional improvement in osteoarthritis [55–57], we and others have speculated that positive results may be due to an unappreciated action of these agents as prebiotics that can affect the gut microbiome.
Suggesting a potential mode of biological action that involves shaping of the gut microbiome, several studies have documented microbial shifts is response to chondroitin sulfate [58–60]. In the Liu et al.[58] study particularly, supplementation was associated with reductions in inflammatory Proteobacteria and increases in a Bacteroidetes taxa that blocks stress-induced intestinal inflammation. These shifts suggest a potential mode of action in osteoarthritis. To address this possibility, we supplemented mice on a chow-based diet with two popular joint-protective nutraceuticals (see Supplementary data, http://links.lww.com/COR/A48). Mice were supplemented daily with either 0.31 mg/g of body weight of glucosamine or 2 μg/g of body weight of UT2C to examine their joint protective capabilities and to examine changes in the gut microbiome. In chow fed mice, osteoarthritis was initiated by trauma (DMM surgery), and as expected, we observed degenerative change to the cartilage in injured joints compared with sham-operated controls. In mice given the same injury but fed a diet supplemented with either glucosamine or UT2C, we observed a deceleration of degenerative change (Fig. 1a–c), evidenced by a general improvement in OARSI scores of mice supplemented with glucosamine (2.16 ± 0.98) or UT2C (1.88 ± 0.84) compared with the vehicle control (2.54 ± 0.52). Histomorphometric analysis of joint cartilage revealed increased tibia total cartilage area, tibia uncalcified cartilage area, and number of Safranin O+ chondrocytes all in both glucosamine and UT2C supplemented mice compared with the vehicle control (Fig. 1d and e).
FIGURE 1.
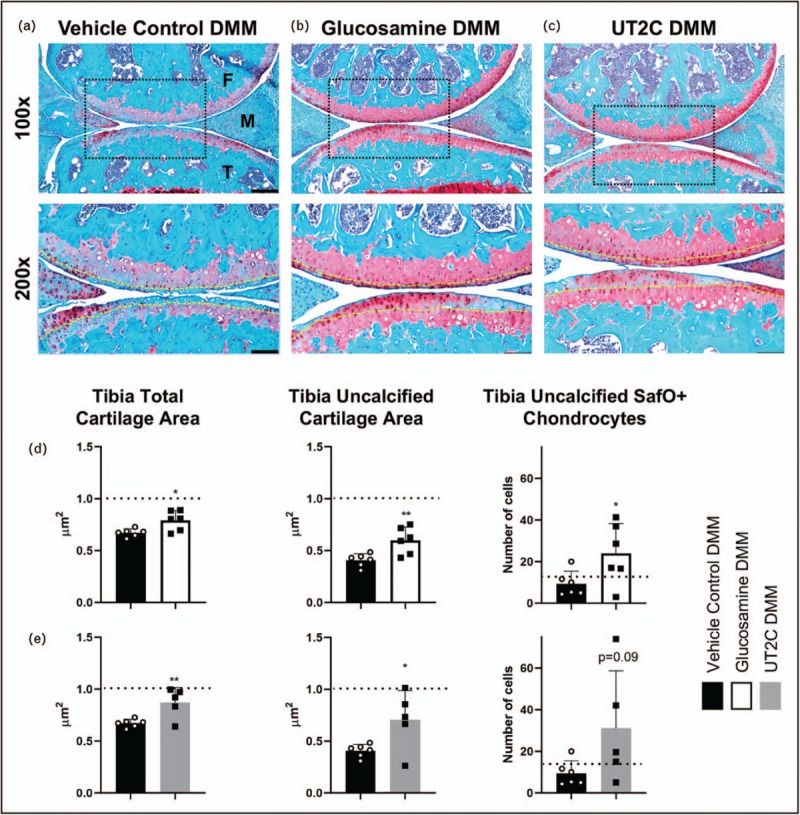
Glucosamine and undenatured type II collagen ameliorate posttraumatic osteoarthritis compared with vehicle control. Sham or DMM surgery was initiated on the knee joint 2 weeks after either glucosamine or undenatured type II collagen were introduced in the diet. Representative Safranin O/Fast Green stains collected from mice fed vehicle control (a) glucosamine (b) or undenatured type II collagen (c) are presented (Yellow dotted line = tidemark, Black dotted line box = region of interested viewed at higher magnification, F = femur, M = meniscus, T = tibia, Scale bar at 100× = 100 μm, Scale bar at 200× = 50 μm). Sections like those in (a–c) were used for histomorphometry. Tibia cartilage area, tibia uncalcified cartilage area, and SafO+ chondrocytes were quantified for mice fed glucosamine (d) and undenatured type II collagen (e) compared with vehicle control. Dashed black lines indicate measurement on sham knee joints. Data shown represent mean (n ≥ 5) ± SD; statistical significance was determined using Student's t test ∗P < 0.5, ∗∗P < 0.01. DMM, destabilization of the medial meniscus.
16S sequencing of DNA extracted from fecal pellets harvested from these mice revealed changes in the abundance of Bacteroidetes, Actinobacteria, and Firmicutes, among others (Fig. 2a). Firmicutes and Bacteroidetes are typically the dominant phyla of the vertebrate microbiome [61], and an increase in Firmicutes and decrease in Bacteroidetes has been associated with proinflammatory states in both humans and mice [62,63]. The change in the ratio may be linked with inflammation as an effect of both different responses to caloric intake and increased ability to extract calories from food [64,65]. Related to this, we found an increased Bacteroidetes/Firmicutes ratio in the microbiome of mice fed either glucosamine or UT2C compared with the vehicle control (Fig. 2a), consistent with a potential anti-inflammatory shift.
FIGURE 2.
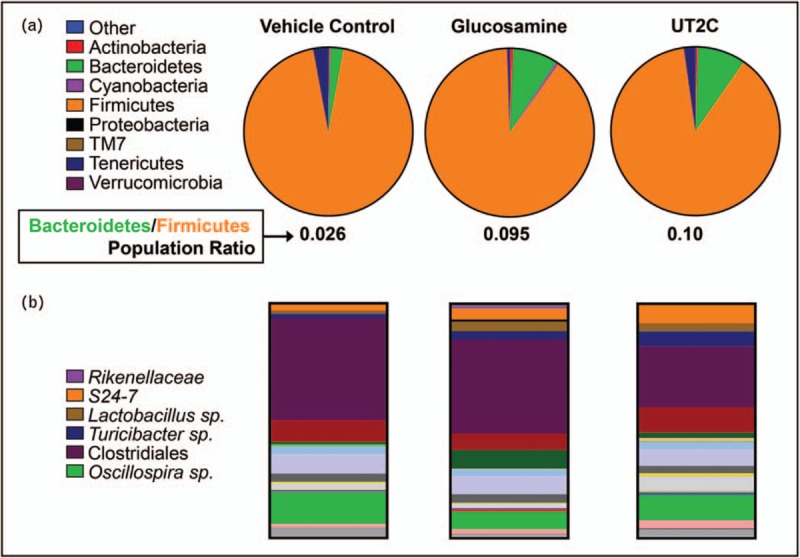
Supplementation with glucosamine or undenatured type II collagen impacts the gut microbiome. (a) Phylum level relative abundances change when either glucosamine or undenatured type II collagen are included in the diet for 12 weeks following injury compared with a vehicle control. Data shown are average relative abundances of phyla in each experimental group (n = 3). (b) Relative abundance of operational taxonomic unit in the gut microbiome determined by fecal sampling is changed by supplementation with either glucosamine or undenatured type II collagen. Data shown are average relative abundances of operational taxonomic unit in each experimental group (n = 3).
Numerous differences at the level of individual operational taxonomic units (OTUs) were found when either glucosamine or UT2C supplemented mice were compared with the vehicle control (Fig. 2b). The family Rikenellaceae and the Bacteroidales family S24–7 were significantly increased in mice fed glucosamine, while the supplement caused the opposite trend in an Oscillospira species (Fig. 3a). Dietary supplementation with UT2C produced even more changes in the abundance of individual OTUs than glucosamine when compared with the vehicle control. As with glucosamine, UT2C increased the abundance of S24-7 and decreased the amount of Oscillospira (Fig. 3b). Both Lactobacillus and Turicibacter levels were increased in mice given UT2C compared with vehicle control, and certain Clostridiales taxa decreased as well (Fig. 3b). Significantly, Rikenellaceae family members are reduced in proinflammatory models of induced colitis [66]. Their increased abundance in mice fed glucosamine may suggest that this family can contribute to a lower inflammatory state. The absence of a change in Rikenellaceae in the UT2C-fed mice may indicate a glucosamine-specific effect. We also found Turicibacter to be significantly increased in mice fed UT2C compared with vehicle control or glucosamine. This genus has been found to be expanded in RA [67] although it has also been shown to be reduced in HFDs [68].
FIGURE 3.
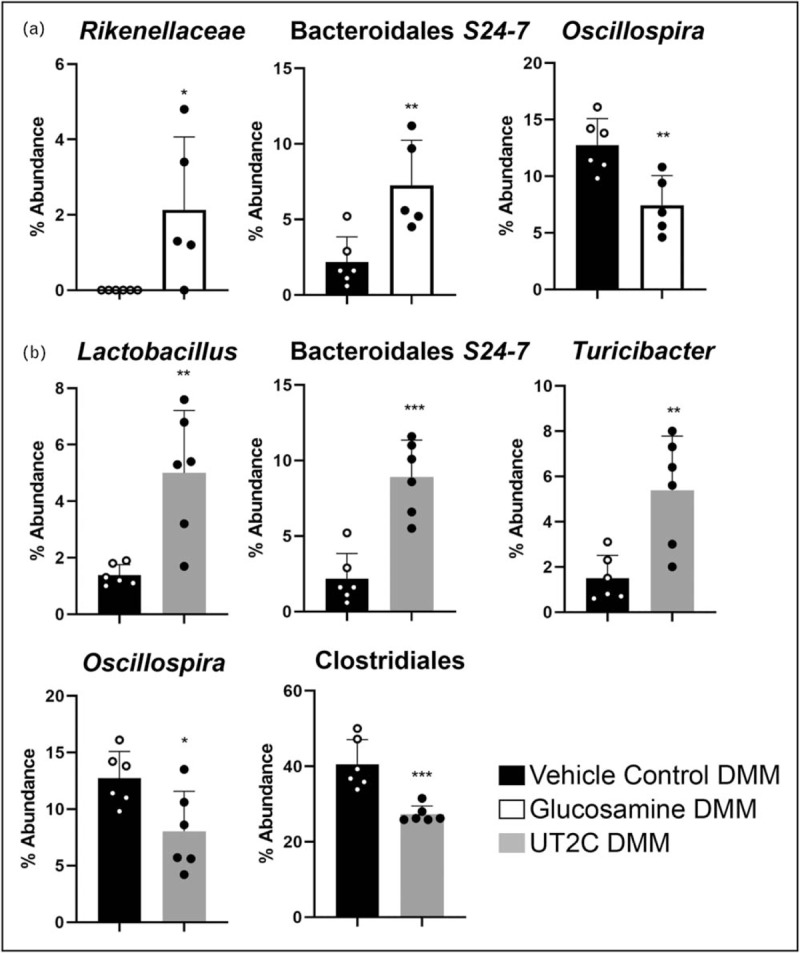
Individual operational taxonomic unit are significantly changed by supplementation with glucosamine or undenatured type II collage. (a) Supplementation with glucosamine for 14 weeks caused significant changes in abundance of three operational taxonomic unit compared with vehicle control. (b) Supplementation with undenatured type II collagen for 14 weeks caused significant changes in 5 operational taxonomic unit. Data shown represent mean % abundance (n ≥ 5) ± SD. Significance was determined using Student's t test. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
The family S24-7, also known as Muribaculaceae and Candidatus Homeothermaceae, is a dominant member of the murine gut microbiome [61,69]. Like Rikenellaceae, they have also been shown to decrease in colitis [66], and here we demonstrate their increase with both glucosamine and UT2C supplementation. Recent work has shown that HFD, a cause of systemic inflammation, is associated with a decrease in both Rikenellaceae and S24-7[70]. In addition, S24-7 are most often found in herbivores or omnivores with a high percentage of plant material in the diet; they ferment glucans to yield short-chain fatty acids that can have anti-inflammatory effects [61,71]. The biological impact of changes in S24-7 may be more complex than a simple antiinflammation as they are increased in diabetes-sensitive mice fed a HFD as well as after remission of induced colitis [61].
Nutraceuticals are long standing supplements implicated in joint health whose efficacy has been difficult to define with biological measures. Defining the mechanism of action has been problematic due to their relative inability to reach the joint. New findings presented here reveal that mice fed either glucosamine or UT2C supplements demonstrate changes in both joint health and microbiome. Tibia cartilage, uncalcified cartilage, and Safranin O+ chondrocytes are all increased in supplemented mice compared with vehicle controls. Global changes in phyla, Bacteroidetes/Firmicutes ratio, and individual OTUs in mice given supplements mirror improvements in the joint. Together, these parallel phenotypes suggest that nutraceuticals may exert beneficial action on joint health through modulation of the gut microbiome; further proof of causal links will require deeper investigation.
CONCLUSION
Emerging research provides compelling evidence of a link between the gut microbiome and development of osteoarthritis. Most of the studies to date have identified interesting associations, with candidate interventions involving indigestible prebiotics providing the best evidence of a causal linkage between the gut and joints. Moving forward, the field must perform deep analysis of causation using fecal microbiota transplant methods and metabolomic study of molecular mediators produced by microbiota that can impact host biology and induce – or protect from – joint degenerative disease. In addition to defining the pathogenic role the gut microbiome plays in osteoarthritis, establishing this connection provides the opportunity for development of new and effective disease modifying osteoarthritis therapeutics. In fact, it is possible that the only commonly used intervention with evidence of improvement in osteoarthritis symptoms, namely nutraceuticals, act indirectly via effects on the gut niche.
Acknowledgements
The authors wish to acknowledge the outstanding technical contributions of Sarah Mack and Kathy Maltby of the Histology, Biochemistry and Molecular Imaging Core in the Center for Musculoskeletal Research at the University of Rochester Medical Center. We also would like to thank Alex Grier of the Genomics Research Center at the University of Rochester Medical Center for performing the 16S rRNA bacterial sequencing to and providing bioinformatics support. This work was supported by NIH NCATS TR000042 (M.J.Z.), NIH NIAMS P50 AR054041-5471 (M.J.Z.), Core services supported by NIH NIAMS P30 AR061307 and P30 AR069665, and DOD W81XWH1910807 (M.J.Z.).
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Supplementary Material
Lacey J. Favazzo, Honey Hendesi and David A. Villani contributed equally to the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.co-rheumatology.com).
REFERENCES
- 1.Liu Y, Ding W, Wang HL, et al. Gut microbiota and obesity-associated osteoarthritis. Osteoarthritis Cartilage 2019; 27:1257–1265. [DOI] [PubMed] [Google Scholar]
- 2.Zhao X, Shah D, Gandhi K, et al. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthritis Cartilage 2019; 27:1618–1626. [DOI] [PubMed] [Google Scholar]
- 3.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum 2009; 60:3546–3553. [DOI] [PubMed] [Google Scholar]
- 4.Ghouri A, Conaghan PG. Update on novel pharmacological therapies for osteoarthritis. Ther Adv Musculoskelet Dis 2019; 11:1759720x19864492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol 2014; 28:5–15. [DOI] [PubMed] [Google Scholar]
- 6.Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol 2013; 25:114–118. [DOI] [PubMed] [Google Scholar]
- 7.Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage 2015; 23:1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage 2015; 23:1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perruccio AV, Chandran V, Power JD, et al. Systemic inflammation and painful joint burden in osteoarthritis: a matter of sex? Osteoarthritis Cartilage 2017; 25:53–59. [DOI] [PubMed] [Google Scholar]
- 10.Punzi L, Galozzi P, Luisetto R, et al. Posttraumatic arthritis: overview on pathogenic mechanisms and role of inflammation. RMD Open 2016; 2:e000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers EL, Reynard LN, Loughlin J. The role of inflammation-related genes in osteoarthritis. Osteoarthritis Cartilage 2015; 23:1933–1938. [DOI] [PubMed] [Google Scholar]
- 12.Portune KJ, Benitez-Paez A, Del Pulgar EM, et al. Gut microbiota, diet, and obesity-related disorders – the good, the bad, and the future challenges. Mol Nutr Food Res 2017; 61: doi: 10.1002/mnfr.201600252. [DOI] [PubMed] [Google Scholar]
- 13▪▪.Schott EM, Farnsworth CW, Grier A, et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight 2018; 3: pii: 95997. doi: 10.1172/jci.insight.95997. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study is the first to suggest that gut dysbiosis manipulation through prebiotics can improve osteoarthritis outcomes. It used both 16S sequencing and RNA-Seq as outcome measures.
- 14.Kundu P, Blacher E, Elinav E, et al. Our gut microbiome: the evolving inner. Cell 2017; 171:1481–1493. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med 2016; 8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez CJ, Guss JD, Luna M, Goldring SR. Links between the microbiome and bone. J Bone Miner Res 2016; 31:1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van de Guchte M, Blottiere HM, Dore J. Humans as holobionts: implications for prevention and therapy. Microbiome 2018; 6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke G, Stilling RM, Kennedy PJ, et al. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol 2014; 28:1221–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazar V, Ditu LM, Pircalabioru GG, et al. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol 2018; 9:1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016; 14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016; 164:337–340. [DOI] [PubMed] [Google Scholar]
- 22.D’Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta 2015; 451:97–102. [DOI] [PubMed] [Google Scholar]
- 23.McBurney MI, Davis C, Fraser CM, et al. Establishing what constitutes a healthy human gut microbiome: state of the science, regulatory considerations, and future directions. J Nutr 2019; doi: 10.1093/jn/nxz154. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurilshikov A, Wijmenga C, Fu J, Zhernakova A. Host genetics and gut microbiome: challenges and perspectives. Trends Immunol 2017; 38:633–647. [DOI] [PubMed] [Google Scholar]
- 25.Deschasaux M, Bouter KE, Prodan A, et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med 2018; 24:1526–1531. [DOI] [PubMed] [Google Scholar]
- 26.Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res 2017; 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell 2016; 167:1469–1480. e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh A, Molinaro A, Stahlman M, et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 2018; 175:947–961. e917. [DOI] [PubMed] [Google Scholar]
- 29.Wu HJ, Ivanov II, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010; 32:815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blacher E, Bashiardes S, Shapiro H, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 2019; 572:474–480. [DOI] [PubMed] [Google Scholar]
- 31.Imhann F, Vich Vila A, Bonder MJ, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2018; 67:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minter MR, Zhang C, Leone V, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep 2016; 6:30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪▪.Tyagi AM, Yu M, Darby TM, et al. The microbial metabolite butyrate stimulates bone formation via T regulatory cell-mediated regulation of WNT10B expression. Immunity 2018; 49:1116–1131.e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]; In an ovariectomized mouse model, probiotics impact gut microbiome butyrate production. Butyrate increased expansion of regulatory T cells in the bone marrow and intestine. This microbiome/T-cell axis plays a role in bone anabolism.
- 34.Levy M, Thaiss CA, Zeevi D, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 2015; 163:1428–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamada D, Maynard R, Schott E, et al. Suppressive effects of insulin on tumor necrosis factor-dependent early osteoarthritic changes associated with obesity and type 2 diabetes mellitus. Arthritis Rheumatol 2016; 68:1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 2013; 5:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hand TW, Vujkovic-Cvijin I, Ridaura VK, Belkaid Y. Linking the microbiota, chronic disease, and the immune system. Trends Endocrinol Metab 2016; 27:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017; 542:177–185. [DOI] [PubMed] [Google Scholar]
- 39.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013; 38:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffin TM, Huebner JL, Kraus VB, et al. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum 2012; 64:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yusuf E, Nelissen RG, Ioan-Facsinay A, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis 2010; 69:761–765. [DOI] [PubMed] [Google Scholar]
- 42.Louer CR, Furman BD, Huebner JL, et al. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheum 2012; 64:3220–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins KH, Paul HA, Reimer RA, et al. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Osteoarthritis Cartilage 2015; 23:1989–1998. [DOI] [PubMed] [Google Scholar]
- 44.Huang ZY, Stabler T, Pei FX, Kraus VB. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthritis Cartilage 2016; 24:1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪.Ulici V, Kelley KL, Azcarate-Peril MA, et al. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthritis Cartilage 2018; 26:1098–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]; DMM-induced osteoarthritis in germ-free mice and specific pathogen-free mice was compared histologically. This study demonstrated that the absence of certain microbial communities significantly reduces osteoarthritis development.
- 46▪.Boer CG, Radjabzadeh D, Uitterlinden AG, et al. The role of the gut microbiome in osteoarthritis and joint pain. Osteoarthritis and Cartilage 2017; 25:S10. [Google Scholar]; The study provided the first evidence in humans that a gut microbiome dysbiosis accompanies osteoarthritis pain. It used 16S sequencing to study fecal material from participants in the Rotterdam cohort.
- 47.Guss JD, Ziemian SN, Luna M, et al. The effects of metabolic syndrome, obesity, and the gut microbiome on load-induced osteoarthritis. Osteoarthritis Cartilage 2019; 27:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khokhlova EV, Smeianov VV, Efimov BA, et al. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol Immunol 2012; 56:27–39. [DOI] [PubMed] [Google Scholar]
- 49▪▪.Rios JL, Bomhof MR, Reimer RA, et al. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci Rep 2019; 9:3893. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study confirmed findings in Schott et al. regarding the protective efficacy of oligofructose in osteoarthritis. An overlay of exercise appears to further protect against progressive degeneration. The study used 16S sequencing to examine the gut microbial community.
- 50▪.Henrotin Y, Patrier S, Pralus A, et al. Protective actions of oral administration of bifidobacterium longum CBi0703 in spontaneous osteoarthritis in Dunkin Hartley guinea pig model. Cartilage 2019; 1947603519841674.doi: 10.1177/1947603519841674. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study used a genetic model of osteoarthritis and confirmed the impact of known anti-inflammatory bacteria in osteoarthritis inhibition.
- 51.Silbert JE. Dietary glucosamine under question. Glycobiology 2009; 19:564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trentham DE, Dynesius-Trentham RA, Orav EJ, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science 1993; 261:1727–1730. [DOI] [PubMed] [Google Scholar]
- 53.Dar QA, Schott EM, Catheline SE, et al. Daily oral consumption of hydrolyzed type 1 collagen is chondroprotective and anti-inflammatory in murine posttraumatic osteoarthritis. PLoS One 2017; 12:e0174705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bagi CM, Berryman ER, Teo S, Lane NE. Oral administration of undenatured native chicken type II collagen (UC-II) diminished deterioration of articular cartilage in a rat model of osteoarthritis (OA). Osteoarthritis Cartilage 2017; 25:2080–2090. [DOI] [PubMed] [Google Scholar]
- 55.Bottegoni C, Muzzarelli RA, Giovannini F, et al. Oral chondroprotection with nutraceuticals made of chondroitin sulphate plus glucosamine sulphate in osteoarthritis. Carbohydr Polym 2014; 109:126–138. [DOI] [PubMed] [Google Scholar]
- 56.Henrotin Y, Lambert C, Richette P. Importance of synovitis in osteoarthritis: evidence for the use of glycosaminoglycans against synovial inflammation. Semin Arthritis Rheum 2014; 43:579–587. [DOI] [PubMed] [Google Scholar]
- 57.Bruyere O, Reginster JY. Glucosamine and chondroitin sulfate as therapeutic agents for knee and hip osteoarthritis. Drugs Aging 2007; 24:573–580. [DOI] [PubMed] [Google Scholar]
- 58.Liu F, Zhang N, Li Z, et al. Chondroitin sulfate disaccharides modified the structure and function of the murine gut microbiome under healthy and stressed conditions. Sci Rep 2017; 7:6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shang Q, Yin Y, Zhu L, et al. Degradation of chondroitin sulfate by the gut microbiota of Chinese individuals. Int J Biol Macromol 2016; 86:112–118. [DOI] [PubMed] [Google Scholar]
- 60.Shang Q, Shi J, Song G, et al. Structural modulation of gut microbiota by chondroitin sulfate and its oligosaccharide. Int J Biol Macromol 2016; 89:489–498. [DOI] [PubMed] [Google Scholar]
- 61.Ormerod KL, Wood DL, Lachner N, et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016; 4:1600252.doi: 10.1002/mnfr.201600252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ley RE, Backhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005; 102:11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444:1022–1023. [DOI] [PubMed] [Google Scholar]
- 64.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004; 101:15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013; 500:541–546. [DOI] [PubMed] [Google Scholar]
- 66.Osaka T, Moriyama E, Arai S, et al. Meta-analysis of fecal microbiota and metabolites in experimental colitic mice during the inflammatory and healing phases. Nutrients 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen J, Wright K, Davis JM, et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med 2016; 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo X, Li J, Tang R, et al. High fat diet alters gut microbiota and the expression of paneth cell-antimicrobial peptides preceding changes of circulating inflammatory cytokines. Mediators Inflamm 2017; 2017:9474896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lagkouvardos I, Pukall R, Abt B, et al. The mouse intestinal bacterial collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat Microbiol 2016; 1:16131. [DOI] [PubMed] [Google Scholar]
- 70.Liu S, Qin P, Wang J. High-fat diet alters the intestinal microbiota in streptozotocin-induced type 2 diabetic mice. Microorganisms 2019; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tian X, Hellman J, Horswill AR, et al. Elevated gut microbiome-derived propionate levels are associated with reduced sterile lung inflammation and bacterial immunity in mice. Front Microbiol 2019; 10:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


