Abstract
Purpose of review
Long-acting HIV treatment and prevention (LAHTP) can address some of the achievement gaps of daily oral therapy to bring us closer to achieving Joint United Nations Programme on HIV/AIDS Fast-track goals. Implementing these new technologies presents individual-level, population-level, and health systems-level opportunities and challenges.
Recent findings
To optimize LAHTP implementation and impact, decision-makers should define and gather relevant data to inform their investment case within the existing health systems context. Programmatic observations from scale-up of antiretroviral therapy, oral preexposure prophylaxis, voluntary medical male circumcision, and family planning offer lessons as planning begins for implementation of LAHTP. Additional data intelligence should be derived from formative studies, pragmatic clinical trials, epidemiologic and economic modeling of LAHTP. Key implementation issues that need to be addressed include optimal communication strategies for demand creation; target setting; logistics and supply chain of commodities needed for LAHTP delivery; human resource planning; defining and operationalizing monitoring and evaluating metrics; integration into health systems.
Summary
Successful LAHTP implementation can bolster treatment and prevention coverage levels if implementation issues outlined above are proactively addressed in parallel with research and development so that health systems can more rapidly integrate new technologies as they gain regulatory approval.
Keywords: HIV, implementation, long-acting, prevention, treatment
INTRODUCTION
Significant progress has been made towards the Joint United Nations Programme on HIV/AIDS (UNAIDS) 2020 fast-track goal to end the HIV epidemic by identifying 90% of people living with HIV, sustaining 90% of those diagnosed on antiretroviral therapy (ART), and achieving viral suppression in 90% of those on ART [1–3]. However, few countries have achieved the fast-track treatment targets. Even fewer have contributed to the global target of initiating 3.1 million persons-per-year on oral preexposure prophylaxis (PrEP) by 2020 [4–6]. Long-acting HIV treatment and prevention (LAHTP) have the potential to address some of the achievement gaps that persist with daily oral therapy by expanding the method choice, improving adherence and quality of life, and potentially reducing the burdens on the health system [7▪▪]. Long-acting injectable cabotegravir (LA-Cab) alone for prevention and in combination with Rilpivirine for treatment are two of the more mature LAHTP products in the research and development (R&D) pathway. They will be used illustratively throughout this review to discuss LAHTP implementation.
Planning for implementation of LAHTP should build on knowledge gained from recent implementation and scale-up of relevant biomedical interventions (e.g., ART, voluntary medical male circumcision (VMMC), family planning and more recently, oral PrEP programs), as well as from data generated within ongoing trials of these technologies. Applying an implementation science lens, we propose and use a conceptual model of implementation that takes a health-systems and population perspective to examine recent data for LAHTP implementation (Fig. 1). Decision-makers and funders may consider these when planning for the introduction, scale-up and institutionalization of LAHTP within health systems. The conceptual model prioritizes decision-making based on data intelligence for implementation of LAHTP within a country's investment case. Health systems will need to specify the intervention components needed to deliver LAHTP to priority populations within a comprehensive health package and outline an implementation pathway. Below we elaborate on each component of the conceptual model, referencing relevant lessons from R&D of LAHTP and implementation of other biomedical interventions mentioned above.
FIGURE 1.
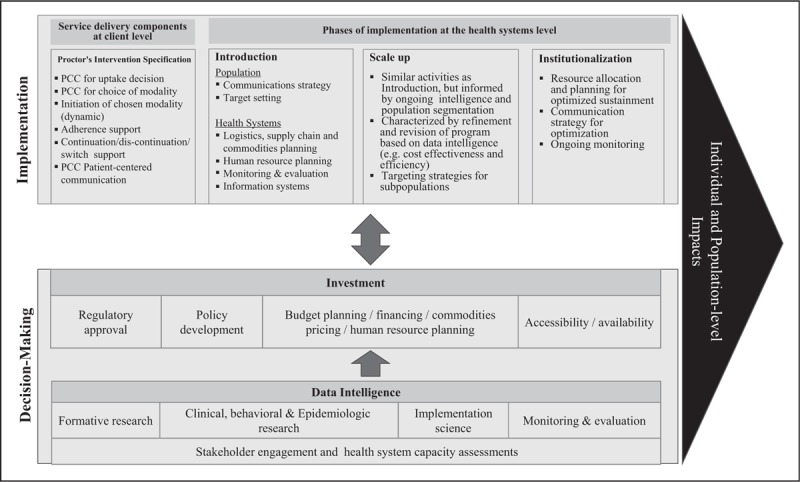
Conceptual model of long-acting treatment and prevention implementation.
Box 1.
no caption available
DECISION-MAKING: DATA INTELLIGENCE
Data intelligence refers to information derived from monitoring and evaluation (M&E) of service delivery, as well as data from service-supporting systems such as laboratories, supply chain and logistics. Other critical sources of data intelligence to inform implementation include, but are not limited to, formative studies, mathematical modeling studies of impact, cost and cost-effectiveness, preclinical, clinical, behavioral, and epidemiological studies conducted throughout R&D (Fig. 1).
Formative studies typically assess acceptability and interest of novel technologies among different communities and populations. Formative studies of user preferences for prevention modalities found long-acting options to be preferred, but reasons varied by age, sex, geography, and sample. Some young women preferred long-acting prevention if synchronized with contraception, for their potential to be used discretely, or for facilitating adherence [8,9▪]. MSM and providers preferred LA-Cab for assurance of adherence, and convenience (despite consistent reports of pain at the administration site) [10,11,12▪,13]. In the treatment context, long-acting products were perceived to simplify and demedicalize care [14▪,15]. However, such preference studies are still quite limited in scope and number and do not adequately capture the various populations in which LAHTP would be implemented [16]. User preferences also should be taken with caution as studies of VMMC acceptance and desire did not directly translate to uptake [17▪▪,18].
Clinical, behavioral, and epidemiological data generated throughout R&D provide implementation insights as well. HIV Prevention Trials Network 083 and 084, ongoing phase 2b/3 studies of LA-Cab for HIV prevention in men and women respectively, have reported on the challenges with the extensive preparation time of the injectable [19]. This challenge may be exacerbated in lesser-resourced program settings. Pragmatic trials could study how to address these challenges in real-world settings with constraints in time, space, human, and financial resources, to inform targets, service delivery models, and follow-up frequency [20▪,21,22▪,23]. Moreover, these data would improve epidemiologic and economic modeling that increasingly inform ministries’ implementation plans. Mathematical models used to estimate epidemiological impact (e.g., number of new infections, infections averted, deaths, and disability-adjusted life-years), program targets, costs, and cost-effectiveness have been used extensively to guide decision-making on scale-up of ART, VMMC, as well as the Dapivirine Ring that is currently under regulatory review [24–26]. Modeling studies in various epidemic settings advocated for judicious use of PrEP at the population level as greatest PrEP impact would be seen in highest risk groups; yet tacit program knowledge and experience suggest there may be benefits for expanded access [27▪]. Monitoring facility-level, program-level, and subpopulation factors that produce variation in delivery, even within a trial context, can accelerate translation.
DECISION-MAKING: INVESTMENT
Rigorous and relevant data can inform the speed and coverage levels at which decision-makers (e.g., governments, donors, funders, community) invest in translating LAHTP into routine public health services (Fig. 1). Reed et al.[17▪▪] highlighted the value of data intelligence optimized through stakeholder consultation for investing in VMMC scale-up beyond 15 million circumcisions in a decade (Table 1). Meyer-Rath et al. recently reported on South Africa's use of the UNAIDS investment case model with stakeholder consultation to optimize their investment in an effective mix of HIV and tuberculosis interventions for the next 20 years [28▪,29]. The exercise illuminated the dearth of impact, cost, and cost-effectiveness data, critical for ministries to plan programs. Broader use of the UNAIDS investment case and implementation science frameworks can help codify common implementation issues and emergent strategies for investment. In addition, cost data relevant to implementation could be collected during the R&D process so that decision makers are armed with such data when determining investment levels [28▪].
Table 1.
Summary of the 10 key lessons learned during voluntary medical male circumcision scale-up with application to oral HIV preexposure prophylaxis implementation
| Lesson | Topic | Lesson learned during VMMC scale-up and its application to oral PrEP implementation |
| 1 | Establish safety surveillance | Systems normative agencies should institute mandatory immediate reporting at the global level of all instances of the most consequential adverse events related to new HIV interventions and standardize the recognition, grading, and clinical management of such adverse events |
| 2 | Engage communities and encourage government ownership | Before rolling out new HIV prevention services, community opinion leaders must be engaged to ensure buyin of the intervention. Government ownership of the overall intervention is also critical |
| 3 | Innovate demand creation activities | Demand creation for consumers of HIV prevention services or products should include plans to target early and late adopters, focus on subpopulations most at risk and involve novel strategies, which use multidisciplinary efforts, such as market research, behavioral economics and human-centred design-based demand creation |
| 4 | Create service delivery models | A variety of service delivery models, such as school-based, community-based and mobile clinic-based services, are needed to effectively reach different populations for initial and follow-up services |
| 5 | Coordinate complex supply chains | There should be regional and national coordination of stakeholders to develop supply chain management systems to ensure an adequate supply of all the essential commodities, including medications and laboratory supplies |
| 6 | Utilize mathematical models to forecast impact and identify bestplaced investments | Mathematical modeling to forecast the epidemiologic and economic impact provides compelling estimates for policymakers around reduced HIV incidence and cost-effectiveness and can help hone programs to ensure the greatest impact possible |
| 7 | Plan for sustainable programs | It is important to develop sustainable HIV prevention programs with the transition of implementation by external donors to national governments and this effort entails long-term financial and technical planning |
| 8 | Anticipate technological advances | Newer prevention technologies should be embraced as they become available and current implementation efforts (i.e. both demand-related and supply-related efforts) should lay the groundwork to promote more rapid rollout of mechanisms for overcoming the different challenges that accompany newer technology |
| 9 | Leverage programs as gateways to other services | HIV prevention interventions can be the gateway for offering other on-site or off-site comprehensive health services (e.g. harm reduction for intravenous drug users and mental healthcare services) to both HIV infected and uninfected individuals |
| 10 | Coordinate global advocacy | There will need to be coordinated efforts by stakeholder advocates to build consensus around global and regional advocacy and key policy guidance to advance implementation worldwide. |
PrEP, preexposure prophylaxis; VMMC, voluntary medical male circumcision. Reproduced with permission [20▪].
Additional activities requiring significant public health program planning and financing for successful implementation include coordination with regulatory agencies to expedite product approval, coordination with regulatory agencies to expedite product approval (Fig. 1). Nearly 4 years after WHO recommended PrEP use for persons at substantial HIV risk, many countries have not developed a clear national PrEP policy, registered generic PrEP for use, or defined access points for PrEP [30]. Kenya, one of the sub-Saharan African countries with the largest HIV burden, has been the first in the region to roll-out PrEP nationally, highlighting the role of leadership from the government in expanding resources to coordinate stakeholders in the development and implementation of a national strategy [31].
IMPLEMENTATION: SERVICE DELIVERY
Planning for implementation begins with specification of the intervention package, that is, what does it takes for a particular health service setting to deliver LAHTP to potential beneficiaries. From a health systems perspective, introduction of the intervention requires strategic prioritization of where, by whom, to whom, and how services will be introduced and scaled-up. Formative studies of use and preferences like those mentioned above, can better characterize sub-population preferences for delivery modalities, describe reasons for preference that can be addressed through communication strategies, and can be used for target setting and planning for impact.
Intervention specification
Proctor et al.[32] propose that an important, and often overlooked step in the science of health service delivery is defining the parameters of a given health intervention including: the actor (who will deliver each component of the intervention), action (what each actor does to deliver the intervention), action target (what is the relevant target for each intervention component), temporality (when does each component of the intervention occur), dose (how often is the intervention component provided), implementation outcome affected (what is the specific outcome each component will affect), justification (what is the evidence for the intervention component). For instance, confirmation of HIV-negative status could lead to provider-patient collaboratively deciding on a method choice (i.e., oral vs. long-acting), as has been done in the family planning field where multimodal contraception is expanding [33]. Patient-centered communication can influence subsequent counseling on adherence and persistence, potential side effects, adverse events, regimen-switching and health visit frequency [34]. The intervention then is not solely the injection for the long-acting agent, but the decision-making counseling that goes into a patient deciding whether to initiate PrEP and if so; which method to choose; and subsequent actions to support adherence, persistence to a given prevention method; as well as how and when to switch and stop. In the process of intervention specification, constructs that are key to daily oral pill-taking will need to be reconceptualized in the era of LAHTP. For instance, it is likely that adherence support may become less critical for LAHTP compared with persistence support (i.e., strategies to help users remain appropriately engaged in care). Follow-up visit schedules may differ for individuals on daily oral pills versus those on LAHTP, and an individual's prior use pattern for oral PrEP could influence choice of prevention product and/or discontinuation. Counseling will need to address these factors. Finally, counseling on how to safely stop LAHTP will need to be uniquely considered, particularly with a product like LA-Cabotegravir for prevention where the tail produces subtherapeutic levels for months after discontinuation [35▪,36].
Implementation: from introduction to institutionalization
Key implementation issues that will need to be considered in planning for the introduction, scale-up and institutionalization of LAHTP include: communications, target setting, logistics, and supply chain, health force training, M&E for safety, effectiveness and impact, and information systems. Though the operationalization of each of these issues will differ by stage of implementation (i.e., introduction, scale-up, institutionalization), these focal issues have common underlying themes across the stages of implementation and will therefore be discussed jointly below.
Communication strategies
The introduction and scale-up of a new product should include a communications strategy that addresses various levels of the health system including policy makers, all cadres of health providers, opinion leaders in communities and potential end-users. The strategy should include media surveillance to proactively address negative and erroneous communication. As detailed by Reed et al.[17▪▪] to be effective throughout scale-up, a communication strategy should recognize population segmentation and how messaging to accompany introduction (i.e., attracting early adopters) might differ from messaging as health systems move towards scale-up and institutionalization. One leading example is a customizable, digital PrEP communications tool which can be utilized to develop targeted communications strategies and demand generation materials for various subpopulations, service delivery settings, and at national and subnational levels [37▪]. It may be possible to integrate and test preliminary long-acting prevention messaging into such a platform during R&D.
Early demand generation for PrEP prioritized rational and functional messaging approaches that conveyed clinical information (e.g., side effect profiles, efficacy estimates) without attending to affective drivers of PrEP-related decision-making and behavior (see Meyers et al. in this issue). Demand creation strategies for LAHTP should build on lessons learned from the proactive, empowering, sex-positive campaigns developed in Chicago (PrEP4Love) [38], Kenya [39], Lesotho (OPTIONS), South Africa, and Zimbabwe.
Target setting
Setting targets for LAHTP as part of a comprehensive package of services should ideally be done through a consultative stakeholder engagement process [17▪▪] to ensure that such targets are set with input from affected communities. Target setting will involve understanding the fraction of the population that remains in need, an operational definition of eligible individuals, and clearly defined metrics of uptake and use. In contexts in which incidence data to determine risk are lacking, operationalizing WHO's definition of ‘individuals at substantial risk’ [2,40] will need to be resolved irrespective of PrEP formulation. Sex differences in PrEP recommendations further complicate target setting, as WHO released updated recommendations (July 2019) for event-driven PrEP in men [3]. Nonetheless, setting PrEP targets helps decision-makers, service providers and data managers to strategize on the direction of the PrEP program and determine financing for adequate human resource and commodity planning. These complexities in background risk, sex, indications for use of the intervention and adherence has prompted novel methods for setting targets including estimating ‘population in need’ and ‘infections averted’ [7▪▪,41]. On the treatment side, the leading regimen is currently intended as maintenance for virally suppressed individual and this indication has implications for setting program targets as well [14▪,20▪].
Logistics, supply chain, and commodities planning
Once the intervention package has been specified and targets and service delivery approach have been determined through a consultative stakeholder engagement process, programs will need to procure the relevant commodities. Careful planning exercises to ensure that supply and demand are well matched should reduce the risk of commodity stock-outs and expiries. For example, limited manufacturing capacity for Raltegravir and its high unit cost delayed its roll-out as first line ART for neonates [22▪].
Additional complexities with delivering LAHTP include: continued inclusion of oral formulations to ensure safety in the initial phase of LA-Cab use; increased resistance monitoring due to missed acute infection or the LA-Cab tail described above; such resistance monitoring is not routinely done in current programs; increased use of syringes and risk of greater biological waste and appropriate disposal; and manufacturing limitations of the antiretroviral, biologic, and/or delivery device [23].
Human resource planning
Provider attitudes and familiarity with interventions have been shown to influence delivery and quality of health services. In South Africa, providers’ comfort level with managing PrEP attributed to nearly 50% of unwarranted PrEP discontinuation during the earliest phase of a PrEP demonstration study (personal communication, Quarraisha Abdool Karim, January 2019). Refresher training for providers reduced discontinuation levels in patients. In the context of long-acting reversible contraceptives, provider's self-reported confidence in their administration, and in conducting patient-centered counseling were identified as drivers of uptake in the patient population [42]. In both family planning and VMMC programs, supportive supervision and cross training has been shown to be a critical part of training to improve competencies [42,43]. Successful implementation of LAHTP will require healthcare providers to be knowledgeable, have behavioral and communication skills to engage in patient-centered communication, and understand linkage and referral procedures to connect patients to providers who can administer the intervention [44–46,47▪]. Task-shifting and task-sharing, as has been done in ART, VMMC, and family planning programs, is an effective strategy to explore to efficiently and effectively deliver LAHTP [17▪▪,48,49].
Monitoring and evaluation
While indicators to measure overall treatment coverage and coverage disaggregated by age, sex, and subgroups have been used globally, measures for treatment adherence and quality of services remain ill-defined and inconsistently measured. WHO's implementation module for M&E of PrEP recommended several core indicators for monitoring: uptake, early continuation, toxicity, and seroconversion [2]. Countries are still in the early stages of implementing PrEP and, to our knowledge, the WHO-recommended core indicators have not been universally adopted or validated in program settings. The definitions, frequency of monitoring and suggested levels of disaggregation of these core indicators (and others) will need to be revisited to include LAHTP. Reed et al.[17▪▪] highlighted the need for active surveillance beyond reported adverse events observed in clinical trials. Methods to adequately surveil and report adverse events will be needed, particularly in vulnerable populations, to ensure that PrEP, including long-acting formulation, is delivered safely and effectively. At all levels of the health system, routine monitoring will help to forecast demand, ensure a sufficient supply of required commodities, identify areas for improvement in services, and evaluate programs and impact.
Information systems
The evolution of health information systems from manual to digital data collection, management, and reporting presents a unique opportunity to include metrics and visit schedules for new modalities of long-acting agents. Maturing interoperability of these information systems through comparable platforms and effective data exchange will facilitate more seamless data triangulation between HIV prevention and treatment programs as well as other relevant sectors, that should ideally improve effective implementation of LAHTP. Lastly, optimal client-centered care will require that information systems be nimble and can reflect the rapidly-evolving data landscape.
CONCLUSION
Once introduced to the market, LAHTP regimens have great potential to improve HIV program coverage levels and help reach ambitious goals to end AIDS globally. Successful implementation at scale can draw from historic lessons of biomedical innovations, and application of implementation science frameworks to systematically scale-up services and institutionalize programs. This work can begin now, while LAHTP products are still in R&D phase to accelerate implementation and impact.
Acknowledgements
The information provided does not necessarily reflect the views of USAID or the United States Government.
Financial support and sponsorship
K.M. receives funding support for work related to implementation of PrEP (R01MH19884, PI: K.M.) and planning for implementation for long-acting prevention technologies (R01MH106380, PI: Golub) from the NIH.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Ghys P. AIDS by the numbers: where do we stand with 90–90–90? 2017; Miami, FL: IAPAC, Available at: https://www.iapac.org/909090-workshop/presentations/909090tw17-Sa1200-Ghys.pdf. [Accessed online on 10 September 2019]. [Google Scholar]
- 2.World Health Organization, WHO. WHO implementation tool for preexposure prophylaxis of HIV infection. Module on monitoring and evaluation. 2017; Available at: https://www.who.int/hiv/pub/prep/prep-implementation-tool/en/. [Accessed online on 10 September 2019]. [Google Scholar]
- 3.World Health Organization, WHO. What's the 2+1+1? Event-driven oral preexposure prophylaxis to prevent HIV for men who have sex with men: update to WHO's recommendation on oral PrEP. 2019; Available at: https://www.who.int/hiv/pub/prep/211/en/. [Accessed online on 10 September 2019]. [Google Scholar]
- 4.Stover J, Bollinger L, Izazola JA, et al. What is required to end the AIDS epidemic as a public health threat by 2030? The cost and impact of the fast-track approach. PLoS One 2016; 11:e0154893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS, UNAIDS. Factsheet: global AIDS update. 2019; Available at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. [Accessed online on 10 September 2019]. [Google Scholar]
- 6.UNAIDS, UNAIDS. Data 2018. 2019; Available at: https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf. [Accessed online on 9 September 2019]. [Google Scholar]
- 7▪▪.Pyra MN, Haberer JE, Hasen N, et al. Global implementation of PrEP for HIV prevention: setting expectations for impact. J Int AIDS Soc 2019; 22:e25370. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article delineated the implementation challenges related to global scale-up of preexposure prophylaxis (PrEP), specifically uptake, adherence, and persistent use. Also, the article attempted to define novel metrics for measuring achievement given the complexity in operationalizing ‘persons at substantial HIV risk’ for prevention.
- 8.Flexner C, Thomas DL, Swindells S. Creating demand for long-acting formulations for the treatment and prevention of HIV, tuberculosis, and viral hepatitis. Curr Opin HIV AIDS 2019; 14:13–20. [DOI] [PubMed] [Google Scholar]
- 9▪.van der Straten A, Agot K, Ahmed K, et al. The tablets, ring, injections as options (TRIO) study: what young African women chose and used for future HIV and pregnancy prevention. J Int AIDS Soc 2018; 21:e25094. [DOI] [PMC free article] [PubMed] [Google Scholar]; Strong experimental design exploring user preferences with inclusion of pragmatic, real-world considerations for implementation.
- 10.Kerrigan D, Mantsios A, Grant R, et al. Expanding the menu of HIV prevention options: a qualitative study of experiences with long-acting injectable cabotegravir as PrEP in the context of a phase II trial in the United States. AIDS Behav 2018; 22:3540–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyers K, Rodriguez K, Brill AL, et al. Lessons for patient education around long-acting injectable PrEP: findings from a mixed-method study of phase II trial participants. AIDS Behav 2018; 22:1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Calder BJ, Schieffer RJ, Bryndza Tfaily E, et al. Qualitative consumer research on acceptance of long-acting pre-exposure prophylaxis products among men having sex with men and medical practitioners in the United States. AIDS Res Hum Retroviruses 2018; 34:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]; A qualitative research study to probe the value that long-acting modalities have for potential end users, including clients and providers, to inform product promotion strategies.
- 13.Meyers K, Wu Y, Brill A, et al. To switch or not to switch: Intentions to switch to injectable PrEP among gay and bisexual men with at least twelve months oral PrEP experience. PLoS One 2018; 13:e0200296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Fernandez C, van Halsema CL. Evaluating cabotegravir/rilpivirine long-acting, injectable in the treatment of HIV infection: emerging data and therapeutic potential. HIV AIDS 2019; 11:179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article goes beyond a summary of the evidence around cabotegravir and rilpivirine combination injectable therapy to discuss implications of such a regimen for clinical practices and challenges that are likely to require the attention of clinicians and health systems.
- 15.Kerrigan D, Mantsios A, Gorgolas M, et al. Experiences with long acting injectable ART: a qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One 2018; 13:e0190487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nachman S, Townsend CL, Abrams EJ, et al. Long-acting or extended-release antiretroviral products for HIV treatment and prevention in infants, children, adolescents, and pregnant and breastfeeding women: knowledge gaps and research priorities. Lancet HIV 2019; 6:e552–e558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪▪.Reed JB, Patel RR, Baggaley R. Lessons from a decade of voluntary medical male circumcision implementation and their application to HIV preexposure prophylaxis scale up. Int J STD AIDS 2018; 29:1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article synthesized 10 lessons from the scale-up of voluntary medical male circumcision with application to the roll out of oral PrEP. The 10 lessons can be usefully extrapolated to long-acting technologies.
- 18.Wambura M, Mahler H, Grund JM, et al. Increasing voluntary medical male circumcision uptake among adult men in Tanzania. AIDS 2017; 31:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HIV Prevention Trials Network, HPTN. Tips and lessons learned from HPTN 084 Sites. 2019; Available at: https://www.hptn.org/sites/default/files/inline-files/Tips%20and%20Lessons%20Learned%20from%20HPTN%20084%20Sites%20V3.0%2031May2019.pdf. [Accessed 5 September 2019]. [Google Scholar]
- 20▪.Barnhart M. Long-acting HIV treatment and prevention: closer to the threshold. Glob Health Sci Pract 2017; 5:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]; The summary and characterization of long-acting technology was written in a manner that will be accessible to many stakeholders.
- 21.Malati C, Rosenfeld J, Mowafy S, et al. Dealing with large-scale supply lines when introducing new regimens. Curr Opin HIV AIDS 2017; 12:408–413. [DOI] [PubMed] [Google Scholar]
- 22▪.Malati CY, Golin R, O’Brien L, et al. Pursuing use of optimal formulations for paediatric HIV epidemic control – a look at the use of LPV/r oral pellets and oral granules. J Int AIDS Soc 2019; 22:e25267. [DOI] [PMC free article] [PubMed] [Google Scholar]; Highlighted key issues in supply chain of antiretroviral therapy (ART) that is also relevant for long-acting technologies.
- 23.Mobula L, Barnhart M, Malati C, et al. Long-acting, injectable antiretroviral therapy for the management of HIV infection: an update on a potential game-changer. J AIDS Clin Res 2015; 6:466.doi: 10.4172/2155-6113.1000466. [Google Scholar]
- 24.Kripke K, Chimbwandira F, Mwandi Z, et al. Voluntary medical male circumcision for HIV prevention in malawi: modeling the impact and cost of focusing the program by client age and geography. PloS one 2016; 11:e0156521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reidy M, Gardiner E, Pretorius C, et al. Evaluating the potential impact and cost-effectiveness of dapivirine vaginal ring preexposure prophylaxis for HIV prevention. PLoS One 2019; 14:e0218710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Peng P, Wu Y, et al. Modelling the epidemiological impact and cost-effectiveness of PrEP for HIV transmission in MSM in China. AIDS Behav 2019; 23:523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪.Sarkar S, Corso P, Ebrahim-Zadeh S, et al. Cost-effectiveness of HIV prevention interventions in sub-Saharan Africa: a systematic review. EClinicalMedicine 2019; 10:10–31. [DOI] [PMC free article] [PubMed] [Google Scholar]; The systematic review usefully summarized evidence from economic evaluations of the full range of HIV prevention interventions in sub-Saharan Africa. Conducting these interventions across intervention types should be particularly useful to policymakers and other stakeholders involved in setting priorities in resource-constrained settings.
- 28▪.Meyer-Rath G, van Rensburg C, Chiu C, et al. The per-patient costs of HIV services in South Africa: systematic review and application in the South African HIV investment case. PloS one 2019; 14:e0210497. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article highlighted the persistent absence of cost data in the literature and highlighted its tremendous utility in decision-making on how to prioritize certain investments within a comprehensive program. The analytic approach and proactive stakeholder engagement in such a quantitative exercise was noteworthy.
- 29.Schwartlander B, Stover J, Hallett T, et al. Towards an improved investment approach for an effective response to HIV/AIDS. Lancet 2011; 377:2031–2041. [DOI] [PubMed] [Google Scholar]
- 30. [[Accessed October 23, 2019]]. HIV Prevention Market Manager, Global PrEP Tracker, Country Updates. https://www.prepwatch.org/in-practice/country-updates/ [Google Scholar]
- 31.Masyuko S, Mukui I, Njathi O, et al. Preexposure prophylaxis rollout in a national public sector program: the Kenyan case study. Sex Health 2018; 15:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci 2013; 8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesnewski R, Prine L, Ginzburg R. Preventing gaps when switching contraceptives. Am Fam Phys 2011; 83:567–570. [PubMed] [Google Scholar]
- 34.King A, Hoppe RB. Best practice’ for patient-centered communication: a narrative review. J Grad Med Educ 2013; 5:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪.Markowitz M, Frank I, Grant RM, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV 2017; 4:e331–e340. [DOI] [PubMed] [Google Scholar]; Seminal study of long-acting cabotegravir.
- 36.Landowitz R. [[Accessed October 3, 2019]]. Tail-phase safety, tolerability and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected individuals: HPTN 077 final results. Oral late-breaker abstract OA15.06LB. R4P; Madrid, Spain 2018. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002690. [Google Scholar]
- 37▪.Schwartz K, Ferrigno B, Vining S, et al. PrEP communications accelerator: a digital demand creation tool for sub-Saharan Africa. Sex Health 2018; 15:570–577. [DOI] [PubMed] [Google Scholar]; Innovative and comprehensive approach to operationalizing communications strategy and demand creation.
- 38.Dehlin JM, Stillwagon R, Pickett J, et al. #PrEP4Love: an evaluation of a sex-positive HIV prevention campaign. JMIR Public Health Surveill 2019; 5:e12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The National AIDS and STI's Control Programme (NASCOP), Ministry of Health, Kenya, National AIDS & STI Control Programme (NASCOP); Ministry of Health. Framework for the implementation of preexposure prophylaxis of HIV in Kenya, Nairobi, Kenya: NASCOP. 2017; Available at: https://www.prepwatch.org/wp-content/uploads/2017/05/Kenya_PrEP_Implementation_Framework.pdf. [Accessed online on 1 September 2019]. [Google Scholar]
- 40.Coleman R. Setting the scene, setting the targets. The Joint United Nations Programme on HIV/AIDS prevention targets of 2016 and estimating global preexposure prophylaxis targets. Sex Health 2018; 15:485–488. [DOI] [PubMed] [Google Scholar]
- 41.Dunn DT, Glidden DV. The connection between the averted infections ratio and the rate ratio in active-control trials of preexposure prophylaxis agents. Stat Commun Infect Dis 2019; 11:20190006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy MK, Stoffel C, Nolan M, Haider S. Interdependent barriers to providing adolescents with long-acting reversible contraception: qualitative insights from providers. J Pediatr Adolesc Gynecol 2016; 29:436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.PEPFAR. Best practices for VMMC site operations: a service guide for site operations chapter five – VMMC skills training and training of supervisors. 2016; Available at: https://aidsfree.usaid.gov/resources/pepfars-best-practices-vmmc-site-operations-0. [Accessed online on 25 August 2019]. [Google Scholar]
- 44.Krakower D, Ware N, Mitty JA, et al. HIV providers’ perceived barriers and facilitators to implementing preexposure prophylaxis in care settings: a qualitative study. AIDS Behav 2014; 18:1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krakower DS, Mayer KH. The role of healthcare providers in the roll out of preexposure prophylaxis. Curr Opin HIV AIDS 2016; 11:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu PM, Shearer LS, Edelman EJ. Educating the primary care clinician on preexposure prophylaxis for human immunodeficiency virus: a teachable moment. JAMA Intern Med 2016; 176:890–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪.O’Malley G, Barnabee G, Mugwanya K. Scaling-up PrEP delivery in sub-Saharan Africa: what can we learn from the scale-up of ART? Curr HIV AIDS Rep 2019; 16:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]; Characterized many parallel issues between ART and PrEP that are also applicable to long-acting HIV technologies.
- 48.Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health 2019; 4:e001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mugwanya KK, Irungu E, Bukusi E, et al. Scale up of PrEP integrated in public health HIV care clinics: a protocol for a stepped-wedge cluster-randomized rollout in Kenya. Implement Sci 2018; 13:118. [DOI] [PMC free article] [PubMed] [Google Scholar]


