SUMMARY
BACKGROUND
Dietary avoidance is recommended for peanut allergies. We evaluated sustained effects of peanut allergy oral immunotherapy (OIT) in the first randomized long-term study in adults and children.
METHODS
Peanut allergic participants (7–55 years-old) underwent a double-blind, placebo-controlled, phase 2 study. Participants randomized 2·4:1·4:1 in a 2×2 block design received placebo (oat flour) or peanut protein. Active therapy participants were built up to and maintained on 4 g peanut protein through week 104 then either discontinued peanut (peanut-0) or ingested 300 mg daily (peanut-300) for 52 weeks. Double-blind, placebo-controlled, food challenges (DBPCFCs) to 4 g peanut protein were conducted at baseline and weeks 104, 117, 130, 143 and 156. The primary endpoint was passing DBPCFCs at 104 and 117 weeks. This trial is registered at ClinicalTrials.gov ().
FINDINGS
Participants recruited between April 15, 2014 and March 2, 2016 were enrolled into peanut-0 (60), peanut-300 (35) and placebo (25) arms. At week 117, 35% peanut-0 and 4% placebo participants achieved success (OR [95%CI] 12·7 [1·8, 554·8]). While time to treatment failure (i.e., failing DBPCFCs to 4 g peanut over time) was significantly longer in peanut-300 vs. peanut-0, the proportion of participants passing DBPCFCs in peanut-300 declined significantly from week 104 to 156 (weeks 104 vs 156; 83% vs. 37%, OR [95%CI] 7·9 [2·4, 29·9]). The most common adverse events (AEs) were mild gastrointestinal symptoms; AEs decreased over time in all arms. In peanut-0, in which 13% passed DBPCFCs at week 156, higher baseline peanut-specific IgG4/IgE and lower Ara h 2 IgE and basophil activation responses were associated with sustained unresponsiveness.
INTERPRETATION
Peanut OIT can desensitize most peanut-allergic individuals to 4 g peanut protein but discontinuation, or even reduction to 300 mg daily, increases the likelihood of regaining clinical reactivity to peanut. Baseline blood tests correlate with week 117 treatment outcomes.
INTRODUCTION
Among food-allergic individuals, estimated in the United States to be approximately 5–8% of children and 4% of adults, those with peanut allergies have the highest likelihood of anaphylaxis.1,2 Rates of accidental peanut ingestion as high as 12·4% annually have been reported.3 Oral immunotherapy (OIT) is being investigated for desensitizing patients to their food allergens, and the majority of trials have been conducted with peanut OIT.4–7 Recent meta-analyses of peanut OIT studies conclude that desensitization is successful, however, side effects in the first year are prevalent during build-up and maintenance (depending on the dose).8,9 Varying maintenance doses (300–5,000 mg)10 have been utilized with the idea that higher maintenance doses may lead to lack of clinical reactivity after discontinuation (sustained unresponsiveness10). However, when peanut OIT is not discontinued, lower amounts in long-term dosing may minimize adverse events (AEs) and improve compliance with daily dosing, a key feature of continued desensitization and safety.11–13 Studies investigating sustained unresponsiveness for milk, egg, or peanut, or for multiple allergens have had varying success (28–78%), depending on the specific allergen, age at therapy initiation, duration of OIT maintenance, and the period of discontinuation.14–18 For example, our peanut OIT pilot study with 24 months of 4 g maintenance reported 35% of participants remained unresponsive after 3 months of discontinuation.15
We therefore designed a single-site, double-blind, randomized, long-term phase 2 study to determine whether such sustained unresponsiveness was higher in participants treated with peanut OIT followed by discontinuation for 3 months vs. those on placebo. We also were interested in the sustained effect of discontinuation for up to 1 year and tested this using standardized and validated double-blind, placebo-controlled food challenges (DBPCFCs) every 3 months. Additionally, recent studies indicate that AEs related to peanut OIT are prevalent in the first year of therapy8 while continued consumption of peanut after OIT reduces recurrence of clinical reactivity.19 However, compliance is sub-optimal5,20 as peanut-allergic participants find it hard to consume large amounts of peanuts long-term as a maintenance dose.12 To improve compliance, we lowered the maintenance dose to 300 mg peanut protein and compared sustained responses upon serial DBPCFCs between the groups continuing long-term 300 mg vs. 0 mg peanut protein (peanut-300 and peanut-0, respectively). This is the first large long-term study performing sequential withdrawal of peanut OIT, testing 300 mg of peanut or withdrawal long term, and evaluating biomarkers associated with clinical outcome. Our goal was to use our results to inform clinicians and patients regarding the long-term outcomes of discontinuation of peanut OIT.
METHODS
Study Design
We conducted a double-blind, randomized, placebo-controlled, long-term phase 2 study of peanut immunotherapy at a single site at the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. The clinical research protocol was approved by the Division of Allergy, Immunology, and Transplantation (DAIT)/National Institute of Allergy and Infectious Diseases (NIAID) Allergy and Asthma Data Safety Management Board, the DAIT/NIAID Clinical Review Committee, the Stanford Institutional Review Board, and the Food and Drug Administration (FDA). The study was conducted in conformity with the current revision of the Declaration of Helsinki, and with the International Conference for Harmonization Good Clinical Practice (ICH-GCP) regulations and guidelines.21
Participants
Adult and pediatric peanut-allergic participants ages 7–55 years were screened (figure 1). Inclusion criteria included a positive DBPCFC (≤500 mg of peanut protein), a positive skin prick test (SPT) result (≥5 mm wheal diameter above the negative control), and peanut-specific IgE of >4 kU/L. Exclusion criteria included severe or uncontrolled asthma, history of eosinophilic gastrointestinal disease, and history of sensitivity to oats. See the Protocol for a more detailed list of inclusion and exclusion criteria. Participants were recruited for screening from our registry and referrals to our Center. All participants gave written informed consent and assent as appropriate, and the clinical and laboratory-based features associated with severity during screening food challenges in this cohort have previously been reported.22 During screening and every 3 months during study participation, all participants were trained on how and when to use epinephrine auto-injector devices to treat allergic symptoms. Participants were encouraged to use epinephrine for any respiratory symptoms (cough, shortness of breath, change in voice, etc.), widespread hives or erythema, repetitive vomiting, persistent abdominal pain, angioedema of the face, or feeling faint. They were also educated on when and how to use anti-histamines (Food Allergy Research & Education (FARE) Emergency Care Plan).23
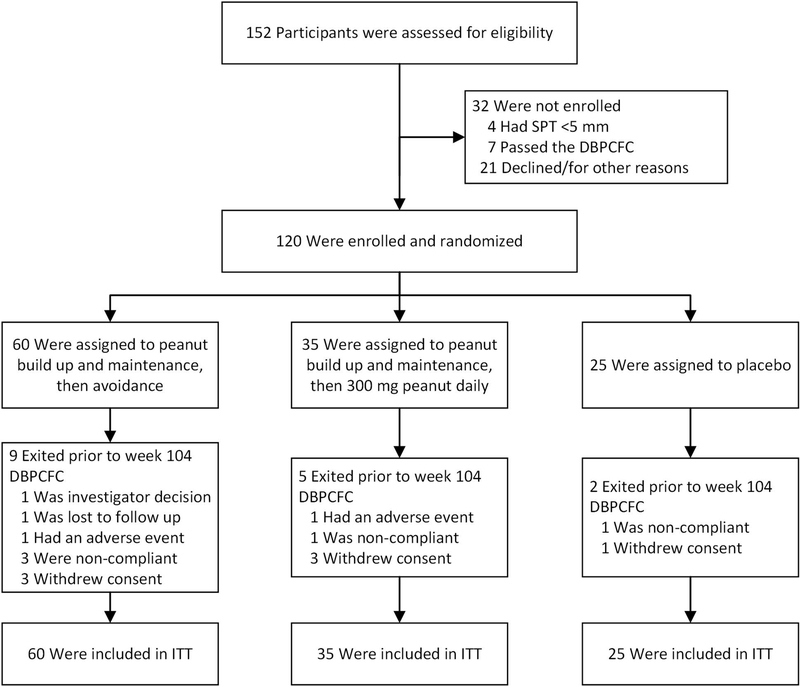
Figure 1.
Trial profile: ITT: intention-to-treat. DBPCFC: double-blind, placebo-controlled food challenge.
Randomization and masking
Participants were randomized using a computerized system in a 2 by 2 block design into 3 arms 2·4:1·4:1 to: (1) build up to and maintain 4 g peanut OIT through week 104 followed by avoidance (peanut-0), (2) build up to and maintain 4 g peanut OIT through week 104 and then continue dosing at 300 mg daily (peanut-300), or (3) placebo throughout (figures 1 and s1). This upfront randomization was performed because our statistical analysis plan was focused on intention-to-treat (ITT). A Master Randomization Assignment List was kept by the investigational pharmacist in a locked cabinet. The pharmacist verified the randomization number and notified the Good Manufacturing Practice (GMP) facility (at Stanford) of the treatment assignment. Strict compliance with documentation of randomization procedures was essential to ensure that there was a reliable, verifiable link between the study participant’s study ID and treatment assignment. Therefore, the randomization number was recorded on a study Case Report Form (CRF). At the end of the study, the Master Randomization List with all randomization numbers and corresponding treatment assignments was provided as an Excel file to the biostatistician as a further check on the randomization process. In order to reduce all biases, during DBPCFCs, the order of peanut and placebo flour were randomly permuted and both the patient and study staff were blinded to the randomized order and the peanut/placebo involved in the specific challenge. Only objective allergic reactions were used for eligibility and for endpoint testing. In the discontinuation phase, blinding was ensured through protocol-regulated procedures in which each participant received the same volume of dry weight flour (peanut vs. oat) and the study team and participants remained blinded (sections 3.1 and 3.4, protocol, appendix).
Following written standard operating procedures, a study monitor verified that the clinical trial was conducted and data were generated, documented (recorded), and reported in compliance with the protocol, GCP, and applicable regulatory requirements. Stanford implemented quality control procedures beginning with the data entry system and generated data quality control checks on the database.
Procedures
All participants were screened using published standardized procedures for SPTs, peanut-specific and total IgE tests, and DBPCFCs, as detailed in the protocol (section 8.4 and synopsis, protocol, appendix). Eligible participants were randomized into 1 of 3 arms (peanut-0, peanut-300, or placebo). Participants in all 3 groups underwent build up to and maintenance of 4 g OIT (peanut or placebo) through week 104. This was followed by avoidance of peanut protein in the peanut-0 group (who received placebo flour), continued dosing at 300 mg daily in the peanut-300 group, and continued daily dosing of placebo in the placebo group for an additional 52 weeks. Discontinuation of peanut OIT after week 104 (peanut-0) was a functional definition, as these participants did undergo DBPCFCs every 3 months if they tolerated a cumulative dose of 4 g at the previous challenge without dose-limiting symptoms.
The food flours that we used were peanut flour (Byrd Mill) and oat flour (Tate and Lyle), dispensed through our Food Flour/Powder GMP facility at Stanford (as per FDA guidelines; see supplemental methods for more details). Any mg or g amount in the text of this manuscript denotes peanut protein.
DBPCFCs to 4 g peanut protein were conducted at baseline, weeks 104, 117, 130, 143 and 156. All food challenges were performed using standardized, staged doses and were deemed positive if objective symptoms were diagnosed by trained personnel. Participants who failed a 4 g DBPCFC were not re-challenged. Peanut and placebo food challenges at screening were dosed in the following increments of peanut protein (mg): 5, 20, 50, 100, 100, 100 and 125; or placebo (oat): 5, 20, 50, 100, 100, 100 and 125. Subsequent food challenge dosing starting at week 104 and every 3 months thereafter were dosed in the following increments of peanut protein (mg): 5, 50, 220, 625, 1000, 1050 and 1050 or placebo (oat): 5, 50, 220, 625, 1000, 1050 and 1050. Doses were given every 15 to 60 minutes at the discretion of the investigator.
Allergy skin tests, basophil activation tests, and assays of peanut-specific serum IgE (sIgE) and IgG4 were performed using published and validated techniques.24,25 SPTs were performed at baseline, weeks 104 and 117. Total IgE was evaluated at baseline. Peanut-specific IgE and IgG4 levels and component IgE (Ara h 1, 2, 3, 8 and 9) were measured at baseline, weeks 104 and 117 using standardized methods in CLIA-approved laboratories (Johns Hopkins University for peanut-specific IgE, peanut IgG4, and component IgE testing, and Stanford Clinical laboratories for total IgE testing). Basophil activation tests in responses to peanut protein at 0·1, 1, 10, 100 and 1000 ng/ml were performed using whole blood as described in Mukai et al.24 Basophils were gated as CD123+HLA- cells and percentage of CD63high cells was quantified by flow cytometry. Basophil responses are represented by AUC (area under the curve) of %CD63high basophils as a function of the peanut dose. Safety parameters and compliance were monitored throughout the study with standardized grading systems26 for allergic and non-allergic reactions using daily diaries and in-clinic research unit responses. Frequent instruction was given by trained personnel on use of reaction medications and dosing.
Outcomes
The primary endpoint was defined as the proportion of participants who reached and passed the DBPCFC (i.e., no objective reaction of grade 1 or more according to Bock’s criteria26) to a cumulative dose of 4 g at both 104 and 117 weeks.
Secondary endpoints included: (1) the proportion of peanut-0 participants who passed a DBPCFC after 6, 9 and 12 months of discontinuation, (2) proportion of peanut-allergic participants who successfully completed the build-up phase of peanut OIT to the highest dose (4,000 mg of peanut protein) with only mild objective symptoms, (3) proportion of peanut-allergic participants who successfully underwent the build-up and maintenance phases of peanut OIT with only mild objective symptoms, and (4) proportion of participants in all 3 arms who were able to undergo DBPCFCs with no clinical reactivity after initiating OIT.
Safety outcomes were determined by Common Terminology Criteria for adverse events (CTCAE) version 4·03 criteria and documented per regulatory guidelines.27 We determined skin prick wheal sizes and basophil activation responses for placebo, peanut-0, and peanut-300 participants who underwent DBPCFCs at baseline, and weeks 104 and 117 and peanut sIgE and Total IgE at additional weeks of 130, 143, and 156.
Statistical analysis
Using a binomial test of proportions with 2-sided alpha level 0·05, the subject sample sizes of 60 in the peanut-0 and 25 in the placebo arms yielded 90% power to detect a difference in the success rates, assuming rates of success in the peanut-0 arm vs placebo of 0·35 and 0·05, respectively.
We designed our study and analysis plan before participant enrollment. Analyses not specified in the protocol are considered post-hoc. The primary efficacy analysis focused on the ITT population (all randomized participants) and compared the peanut-0 vs. placebo arms using Fisher’s exact test. Using our endpoint definition (passing DBPCFC at 104 and 117 weeks), randomized participants who dropped out of the study or had allergic reactions to the week 104 DBPCFC were included in the analysis as failures. Additional analyses on biomarkers used the per-protocol population, defined as only those who passed a previous challenge and returned for the next challenge.
The percentages of participants with any AEs were compared descriptively across treatment arms.
The Supplementary Appendix and Study Protocol contain more detailed information about the statistical analysis plan. Tests for primary and secondary analyses were 2-sided and conducted at the 0·05 level of significance. All analyses were conducted using R software v3·5·0.
Data sharing
The trial dataset will be available to appropriate academic parties on request from the corresponding author, in accordance with the data sharing policies of Stanford University, with input from the investigator group where applicable, subject to submission of a suitable study protocol and analysis plan, on publication of all initial trial results.
Role of the funding source
The NIAID but no other funders was involved in the study design, collection and interpretation of the data, analysis, writing of the report, and in the decision to submit the manuscript for publication. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Participant overview
Participants were recruited between April 15, 2014 and March 2, 2016. Trial eligibility was evaluated in 152 participants (figure 1); 32 were ineligible or declined to participate in the trial and 120 were ultimately randomized and underwent peanut or placebo OIT as specified in the Methods Section. The overall dropout rate was 13%. Dropout rates across arms were not significantly different (Fisher’s exact test, P=0·73) nor were there any significant differences in baseline clinical parameters or lab-based features between dropouts and those who continued in the study.
Baseline characteristics for the ITT population by randomization arm were similar, with the exception of asthma history (table 1). The median age at enrollment was 11 years, and 22 participants were 18 years or older. The median cumulative tolerated dose on screening DBPCFC was 25 mg for each arm. More than two thirds of participants had a history of comorbid conditions including asthma (67%), atopic dermatitis (73%), and allergic rhinitis (75%). The median age at peanut allergy diagnosis was 1·5 years with a median duration of peanut allergy of 9 years.
Table 1.
Demographics and immunological characteristics of the intention-to-treat (ITT) population at baseline
| Characteristics | Total (n = 120) |
Treatment | ||
|---|---|---|---|---|
| Peanut-0 (n = 60) |
Peanut-300 (n = 35) |
Placebo (n = 25) |
||
| Age (years) | 11 (8, 15) | 10 (9, 13) | 11 (8, 17) | 11 (9, 16) |
| Male | 81 (68%) | 39 (65%) | 23 (66%) | 19 (76%) |
| Hispanic | 3 (3%) | 0 (0%) | 1 (3%) | 2 (8%) |
| BMI | 17·4 (15·7, 21·8) | 17·2 (15·6, 20·8) | 17·4 (15·7, 22·4) | 17·7 (16·2, 24·3) |
| Race | ||||
| Caucasian | 74 (62%) | 35 (58%) | 22 (63%) | 17 (68%) |
| Black or African American | 2 (2%) | 0 | 1 (3%) | 1 (4%) |
| Asian | 32 (27%) | 15 (25%) | 11 (31%) | 6 (24%) |
| Native American / Pacific Islander | 1 (<1%) | 1 (2%) | 0 | 0 |
| Multiple | 11 (9%) | 9 (15%) | 1 (3%) | 1 (4%) |
| History of comorbid conditions | ||||
| Asthma | 80 (67%) | 39 (65%) | 19 (54%) | 22 (88%)* |
| Atopic dermatitis | 87 (73%) | 47 (78%) | 22 (63%) | 18 (72%) |
| Allergic rhinitis | 90 (75%) | 42 (70%) | 27 (77%) | 21 (84%) |
| Number of doctor diagnosed food allergies | 4 (1, 7) | 4 (1, 7) | 3 (2, 7) | 5 (2, 7) |
| Age at diagnosis of peanut allergy (years) | 1·5 (1, 2·5) | 1·1 (1, 2) | 1·5 (1, 2·8) | 2 (1·5, 3) |
| Duration of peanut allergy (years) | 9 (7, 13·2) | 9·2 (7, 12) | 8·2 (6·4, 15·3) |
9 (7, 13) |
| CTD in baseline peanut challenge (mg) | 25 (5, 75) | 25 (5, 75) | 25 (5, 75) | 25 (5, 75) |
| Total serum IgE (kU/L) | 526·5 (272·5, 1240·0) | 512 (234·3, 1342·8) | 544 (325·5, 798) | 458 (271, 1040) |
| Peanut-specific IgE (kU/L) | 79·1 (12·9, 200·3) | 75·7 (13·1, 282·5) | 99·3 (15·3, 132·5) | 88·1 (7·6, 183) |
| Peanut component IgE (kU/L) | ||||
| Ara h 1 IgE | 14·3 (0·9, 73·7) | 14·8 (0·8, 55·5) | 10·1 (1·2, 69·7) | 33·2 (1·2, 84·6) |
| Ara h 2 IgE | 34·9 (4·1, 88·6) | 37·1 (4·6, 131) | 35·7 (5·0, 82·6) | 20·7 (4·1, 66·0) |
| Ara h 3 IgE | 2·3 (0·1, 15·7) | 1·9 (0·1, 16·7) | 2·9 (0·1, 12·0) | 2·7 (0·2, 17·3) |
| Ara h 8 IgE | 0 (0, 0·7) | 0 (0, 0·7) | 0 (0, 2·6) | 0 (0, 0·4) |
| Ara h 9 IgE | 0 (0, 0) | 0 (0, 0·1) | 0 (0, 0·1) | 0 (0, 0) |
| Peanut-specific IgG4 (ng/L) | 0·8 (0·3, 1·9) | 0·5 (0·3, 2·5) | 1·2 (0·5, 1·6) | 0·8 (0·3, 1·4) |
| Peanut skin prick test wheal diameter (mm) | 12 (8·5, 16·8) | 13·3 (8·5, 18·1) | 11·5 (8·5, 15·5) | 11·5 (9·5, 14·5) |
Data are n (%) or median (1st and 3rd quartile). CTD = cumulative tolerated dose. Peanut-0 = oral immunotherapy with peanut discontinuation arm; Peanut-300 = oral immunotherapy with peanut continuation arm (300 mg). None of the differences among the 3 treatment groups were statistically significant except for asthma status of the placebo vs other 3 treatment groups (*P < 0·05).
Most ITT participants reached a 4 g maintenance dose between weeks 42 and 60 (figure S2). The time to reach 4 g of maintenance was significantly different by treatment arm, with placebo having a median time to maintenance (to oat flour) of 52 weeks compared to 53 and 57 weeks (to peanut) in peanut-300 and peanut-0, respectively (P=0·015).
Assessment of clinical outcomes
At week 104, 51/60 (85%) in the peanut-0 arm passed the food challenge versus 1/25 (4%) assigned to placebo (table 2). Three months later, 21/60 (35%) assigned to peanut-0 passed the 4 g challenge and reached the primary endpoint versus 1/25 (4%) on placebo (odds ratio [OR] = 12·7, 95% confidence interval [CI] = 1·8–554·8, P=0·0024). Due to a significant difference in history of asthma between the peanut-0 and placebo arms (P=0·013), the primary analysis was also conducted adjusting for asthma history. The association between the primary endpoint and treatment remained significant after adjusting for asthma history (OR = 13·4, 95% CI = 2·5–249·4, P=0·015).
Table 2.
Efficacy outcomes for primary endpoint and major secondary endpoints
| Primary Endpoint | ||||
|---|---|---|---|---|
| Peanut-0 (n = 60) |
Placebo (n = 25) |
OR (95% CI) |
P-value1 | |
| Passing the week 117 DBPCFC to peanut | 21/60 (35%) | 1/25 (4%) | 12·7 (1·8, 554·8) | 0·0024 |
| Secondary Endpoints | ||||
| Peanut-0 (n = 60) |
Placebo (n = 25) |
OR (95% CI) |
P-value1 | |
| Passing the DBPCFC to peanut at… | ||||
| Week 104 (Desensitization) | 51/60 (85%) | 1/25 (4%) | 124·2 (16·6, 5473·4) | <0·001 |
| Week 130 | 12/60 (20%) | 1/25 (4%) | 5·9 (0·8, 266·7) | 0·10 |
| Week 143 | 9/60 (15%) | 1/25 (4%) | 4·2 (0·5, 193·1) | 0·27 |
| Week 156 | 8/60 (13%) | 1/25 (4%) | 3·6 (0·4, 170·4) | 0·27 |
| Complete build-up phase to 4000 mg (peanut or oat flour) with only mild symptoms2 | 15/60 (25%) |
13/25 (52%)3 | 0·3 (0·1, 0·8) |
0·023 |
| Complete build-up and maintenance phases (peanut or oat flour) with only mild symptoms2 | 13/60 (22%) |
13/25 (52%)3 | 0·3 (0·1, 0·7) |
0·0092 |
| Inability to tolerate at least 1000 mg (peanut or oat flour) | 9/60 (15%) | 2/25 (8%)3 | 0·5 (0·05, 2·7) | 0·50 |
| Peanut-0 (n = 60) |
Peanut-300 (n = 35) |
OR (95% CI) |
P-value1 | |
| Passing the DBPCFC to peanut at… | ||||
| Week 104 (Desensitization) | 51/60 (85%) | 29/35 (83%) | 1·2 (0·3, 4·1) | 0·78 |
| Week 117 | 21/60 (35%) | 19/35 (54%) | 0·5 (0·2, 1·2) | 0·086 |
| Week 130 | 12/60 (20%) | 15/35 (43%) | 0·3 (0·1, 0·9) | 0·021 |
| Week 143 | 9/60 (15%) | 13/35 (37%) | 0·3 (0·1, 0·9) | 0·022 |
| Week 156 | 8/60 (13%) | 13/35 (37%) | 0·4 (0·1, 0·8) | 0·010 |
| Complete build-up phase to 4000 mg peanut with only mild symptoms2 | 15/60 (25%) | 12/35 (34%) | 0·6 (0·3, 1·6) | 0·35 |
| Complete build-up and maintenance phases (to peanut) with only mild symptoms2 | 13/60 (22%) | 11/35 (31%) | 0·6 (0·2, 1·5) | 0·33 |
| Inability to tolerate at least 1000 mg peanut | 9/60 (15%) | 6/35 (17%) | 1·2 (0·3, 4·1) | 0·78 |
Fisher’s exact test. Peanut-0 = oral immunotherapy with peanut discontinuation arm; Peanut-300 = oral immunotherapy with peanut continuation arm (300 mg). DBPCFC = double-blind placebo-controlled food challenge.
Mild symptoms are AEs with CTCAE grade = 1.
Since placebo received oat flour, these counts correspond to rates to be expected in the untreated peanut population.
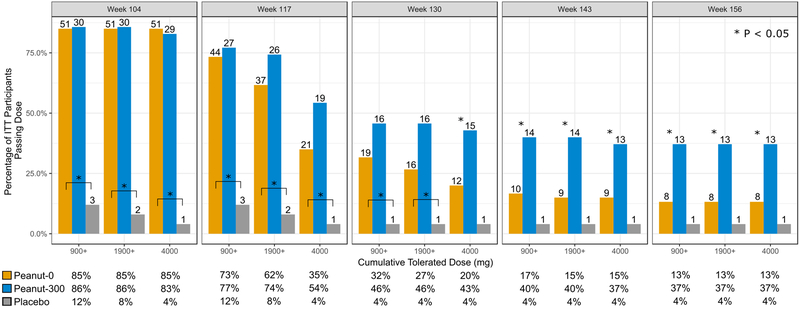
Success in passing a DBPCFC with no clinical reaction was significantly different between placebo vs. the combined peanut treatment arms (peanut-0 and peanut-300) at all challenge time points (table S1a). Reaction thresholds at all challenge time points in both peanut arms improved from the baseline food challenge. At week 117, 73% and 77% of the peanut-0 and peanut-300 participants, respectively, tolerated at least 900 mg cumulative peanut and 85% and 83% passed the 275 mg cumulative peanut dose (approximately 1 peanut kernel; data not shown). At week 130 DBPCFCs, 32% of the peanut-0 arm and 46% of the peanut-300 arm tolerated at least 900 mg. However, starting at week 143, there were significant differences between peanut arms in the ability to tolerate even the 900 mg cumulative peanut doses (figure 2).
Figure 2.
Percentage of participants who tolerated cumulative peanut challenge dose of 900 mg, 1900 mg and 4 g by DBPCFC week and treatment arm for the ITT population. Number on the top of each bar is the number of participants. P-values based on Fisher’s exact test between peanut-0 (orange bar) and peanut-300 (blue bar), or, highlighted by brackets, comparisons between placebo and peanut-0. Although not noted in the figure, all comparisons between placebo and peanut-300 had P < 0.05. Further detailed percentages are provided below each panel. Peanut-0 = oral immunotherapy with peanut discontinuation arm; Peanut-300 = oral immunotherapy with peanut continuation arm (300 mg).
Challenge success over time
Thirteen percent of participants were successful in the peanut-0 arm at week 156, having avoided daily peanut for 1 year, excepting the exposure to peanut at food challenges every 3 months (figure 2). Notably, compared to peanut-0, maintaining with 300 mg peanut daily allowed for greater (37%, peanut-300) success at week 156.
There was no statistically significant difference in success between peanut-0 vs. peanut-300 at the week 117 DBPCFC (table 2, week 117: 35% vs. 54%, P=0·09). However, at all subsequent DBPCFCs, peanut-0 participants were less likely to reach 4 g vs. those in the peanut-300 arm and the delta between the two arms increased (week 130: 20% vs. 43%, P=0·021; week 143: 15% vs 37%, P=0·022; week 156: 13% vs 37%, P=0·010). Importantly, ingesting 300 mg peanut daily until week 156 did not sustain the rate of desensitization to 4 g observed at week 104 (37% vs. 83%, P<0·001).
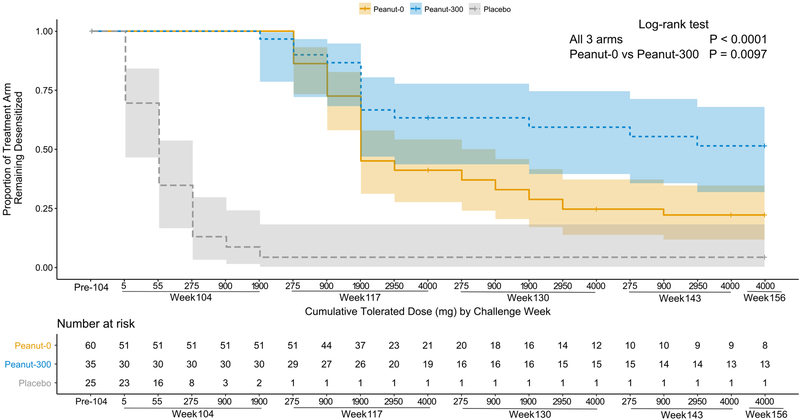
There was a significant difference in time-to-failure at DBPCFC (where week and cumulative tolerated dose are jointly considered) among the 3 treatment arms overall (P<0·001) and between peanut-300 and peanut-0 (figure 3, hazard ratio [HR] = 0·5, 95% CI = 0·2–0·8; P=0·0097). The time-to-failure curves show that the rate of failure plateaus over time in both peanut arms, albeit with greater success in peanut-300.
Figure 3.
Time until challenge failure. Drop in risk at the pre-104 time point refers to participants who did not reach the week 104 challenge (i.e., drop outs). Participants who passed the last performed challenge but did not complete the next challenge, or dropped out prior to the week 104 challenge, were censored at their last cumulative tolerated dose (denoted by the vertical tick in the Kaplan-Meier curve). Colored bands represent the 95% confidence intervals. Peanut-0 = peanut oral immunotherapy with peanut discontinuation arm; Peanut-300 = oral immunotherapy with peanut continuation arm (300 mg).
Safety summary
Across all arms, the percentage of participants with AEs decreased by study year (table 3). The most common AE was gastrointestinal, followed by skin. Events requiring injectable epinephrine resolved within a few minutes with no sequelae. There were no episodes of hypoxia or neurological compromise (table S2). Two serious adverse events (SAEs) led to individual participant discontinuation. One peanut-0 participant developed eosinophilic esophagitis (EoE) and his symptoms resolved after termination from the study. The other SAE, of anaphylaxis, occurred in a peanut-300 participant due to vigorous exercise; this was reversed after 1 dose of injectable epinephrine.
Table 3.
Safety summary – Number and percent of participants experiencing AEs
| Study arm and period | Number of participants* | Any adverse event | Organ system | Treated | Grade AE1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrointestinal2 | General3 | Respiratory | Skin | Other4 | 1 | 2 | 3 | ||||
| Year 1 | |||||||||||
| Peanut-0 | 60 | 57 (95%) | 52 (87%) |
11 (18%) |
27 (45%) | 34 (57%) |
5 (8%) |
50 (83%) | 53 (88%) | 42 (70%) | 1 (2%) |
| Peanut-300 | 35 | 32 (91%) | 29 (83%) |
9 (26%) |
18 (51%) | 17 (49%) |
2 (6%) |
28 (80%) | 30 (86%) | 23 (66%) | 0 |
| Placebo | 25 | 16 (64%) | 12 (48%) |
1 (4%) |
6 (24%) |
10 (40%) |
1 (4%) |
11 (44%) | 13 (52%) | 11 (44%) | 1 (4%) |
| Year 2 | |||||||||||
| Peanut-0 | 55 | 40 (73%) | 35 (64%) |
15 (27%) |
21 (38%) | 25 (45%) |
2 (4%) |
35 (64%) | 34 (62%) | 29 (53%) | 3 (5%) |
| Peanut-300 | 31 | 20 (65%) | 15 (48%) |
5 (16%) |
9 (29%) |
11 (35%) |
2 (6%) |
18 (58%) | 16 (52%) | 13 (42%) | 1 (3%) |
| Placebo | 23 | 6 (26%) |
5 (22%) |
1 (3%) |
3 (13%) |
2 (9%) |
0 | 3 (13%) |
6 (26%) | 2 (9%) |
0 |
| Year 3 | |||||||||||
| Peanut-0 | 51 | 1 (2%) |
1 (2%) |
0 | 0 | 0 | 0 | 0 | 1 (2%) |
0 | 0 |
| Peanut-300 | 30 | 6 (20%) |
4 (13%) |
0 | 3 (10%) |
2 (7%) |
0 | 6 (20%) |
4 (13%) | 6 (20%) | 0 |
| Placebo | 20 | 1 (5%) |
1 (5%) |
0 | 1 (5%) |
0 | 0 | 0 | 1 (5%) |
0 | 0 |
Number of subjects with at least 1 AE during the year out of the number of subjects with doses during that year. Peanut-0 = oral immunotherapy with discontinuation arm; Peanut-300 = oral immunotherapy with peanut continuation arm (300 mg).
CTCAE v.4·03 grade where 1, 2, 3 is grade 1, 2, and 3, respectively.
Gastrointestinal includes itchy oropharynx.
General indicates skin reactions at injection site.
Other indicates anxiety or eye reactions.
Up to week 104, there was no significant difference in peanut-0 vs. peanut-300 in the percent who completed build-up and maintenance with only mild symptoms (22% vs. 31%, P=0·33, table 2). However, there was a significant difference in the percentage of peanut-0 vs. peanut-300 participants experiencing any AEs in year 3 (2% vs 20%, P=0·0093, table 3).
A higher ratio of baseline peanut-specific IgE to total IgE was significantly associated with higher percentage of doses related to AEs throughout the entire study, adjusting for treatment arm (Estimate = 0·9, 95% CI = 0·3–1.4, Q = 0·021; figure S3).
Biomarker associations with treatment success
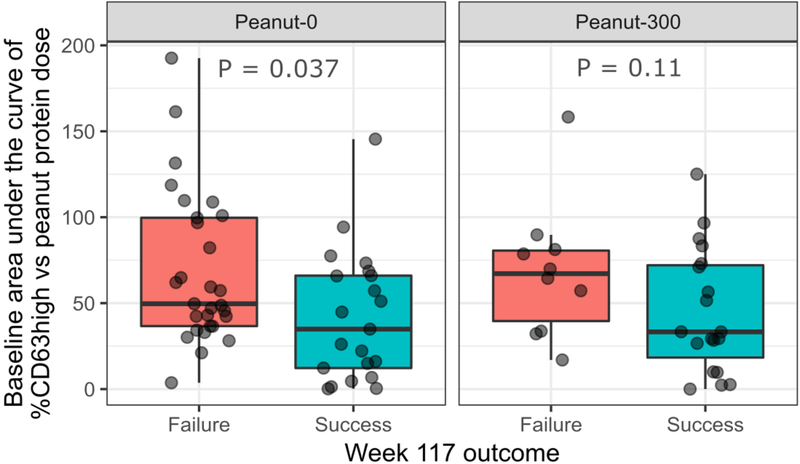
Lower basophil levels of baseline CD63 AUC were observed in peanut-0 per-protocol participants who passed week 117 challenge compared to those who failed (P=0·037, figure 4) (AUC [95% CI]: 0·68 [0·52–0·83]). Lower Ara h 2 IgE and peanut sIgE were significantly associated with week 117 success in both the peanut-0 and peanut-300 arms (P<0·001 and P=0·0048, P<0·001 and P=0·033 respectively, figure S4) and had excellent ability to distinguish between success and failure at week 117 (AUC [95% CI]: 0·83 [0·74–0·92] and 0·81 [0·71–0·90], respectively).
Figure 4.
Baseline basophil %CD63high area under the curve by treatment arm and week 117 outcome (red = failure, green = success). Baseline CD63high area under the curve by treatment arm and week 117 outcome for per-protocol participants. P-values were calculated using the Mann-Whitney U test. Peanut-0 = oral immunotherapy with discontinuation arm; Peanut-300 = oral immunotherapy with peanut continuation arm (300 mg).
A higher ratio of peanut sIgG4 to peanut sIgE was significantly associated with week 117 success only in the peanut-0 arm (P<0·001, AUC [95% CI]: 0·79 [0·66–0·92]) and not in the peanut-300 arm (P=0·66).
Higher peanut specific IgE, and Ara h 1 and Ara h 2 IgE, at baseline were associated with lower odds of success at all subsequent challenges (week 130, 143 and 156) (table S4 and figures S5=S6). Among participants who reached the week 117 challenge (i.e. per-protocol), participants who failed the week 117 challenge in both peanut-0 and peanut-300 had significantly different trajectories of peanut sIgE compared to those that passed the week 117 challenge (P=0·020 for peanut-0 and P=0·0093 for peanut-300, figure S6a). Differences also existed between peanut-300 week 117 success and failures for total IgE (P<0·001). Among those that reached the week 130 challenge, there were significant differences in trajectories of peanut sIgE and the ratio of peanut sIgE to total IgE within the peanut-300 week 130 successes and failures (P<0·001 for both, figure S6b).
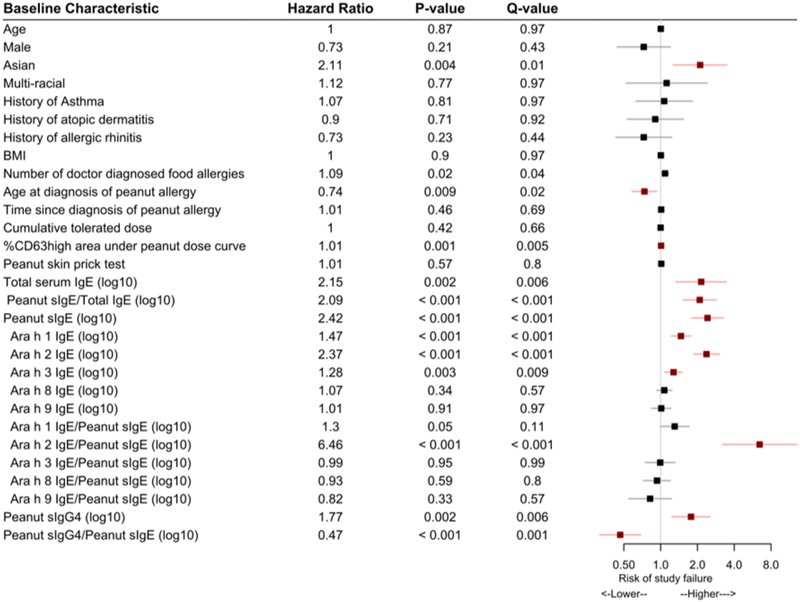
Extending the above analyses to incorporate all of a participant’s challenge outcomes during year 3 and cumulative tolerated doses, similar associations were found between peanut-specific biomarkers and overall study outcome (figure 5). Specifically, a higher ratio of Ara h 2 IgE to peanut sIgE was significantly associated with a higher risk of treatment failure (HR = 6·46, Q<0·001). By contrast, neither the cumulative tolerated peanut dose at baseline, age, gender, years with peanut allergy disease, atopic co-morbidity, nor peanut skin prick wheal size, were associated with higher risk of treatment failure (figure 5).
Figure 5.
Hazard of study failure among baseline characteristics using dose and time to failure. Each characteristic was fit to a Cox proportional hazards model adjusting for treatment arm. An estimate above 1 (gray line) denotes a higher risk of treatment failure, while an estimate below 1 denotes a lower risk of study failure. Q-value is the FDR-adjusted P-value. Characteristics with significant Q-values are highlighted in red. BMI: body mass index.
DISCUSSION
With peanut allergy therapies in varying stages of clinical development, and some nearing FDA approval, critical questions remain regarding the durability of treatment effects and the appropriate maintenance doses. A recent meta-analysis8 (PACE study) concluded that while desensitization to peanut is achievable, allergic reactions commonly occur during the first year of build-up and maintenance to peanut. In commenting on the PACE study, Roberts et al. discussed the importance of identifying ‘information about which participants benefited most from the intervention’.28 Two things are clear from the evaluation of these 12 trials that included more than 1,000 participants. First, data are lacking regarding the safety profile of peanut OIT after one year of therapy. Second, biomarkers have not been identified for determining whether patients could stop or decrease therapy long term to minimize adverse reactions while still staying desensitized.
We think that our study has begun to answer these questions. Our controlled, randomized, double-blind, long-term study was designed to evaluate 2 years of peanut therapy followed by either 1 year of discontinuation vs. 1 year of lower maintenance OIT dosing after the 2 years of peanut OIT. We found that participants who underwent peanut OIT for 2 years were 120·8 fold more likely than those in the placebo arm to pass a peanut food challenge at 2 years and, even after a subsequent 3-month peanut avoidance period, they were 12·7 fold more likely to pass than those in the placebo group. We captured safety data for 3 years of OIT to understand long term side effects of OIT and found reductions of side effects, including AEs due to accidental ingestions (9% in year 1 to 2% in year 2 for the combined peanut arms compared to 12% in year 1 to 16% in year 2 for the placebo arm) (table S5).
Importantly, even though we did exclude participants with a history of severe asthma or a history of hypotension or severe anaphylaxis, our cohort was highly sensitive to baseline peanut challenges conducted at screening.22 Nonetheless, most (85%) were able to be successfully desensitized at week 104. Despite a decline in clinical success at subsequent challenges, participants attained higher cumulative tolerated doses than those achieved at screening. After 2 years of peanut OIT, only 35% of participants demonstrated durability of sustained unresponsiveness to 4 g (about 13–16 peanut kernels) at week 117. Notably, some participants (13%) withdrawn from OIT maintained sustained unresponsiveness to 4 g of peanut up to week 156 -- a year after cessation of peanut treatment. This is important, given that many patients are likely to self-test withdrawal from peanut OIT by deviating away from daily doses in ‘real life’.12
Additionally, we also tested, in a blinded fashion, long-term maintenance therapy with 300 mg peanut (an identical dose as in a prior phase 3 study of peanut OIT5), the equivalent of taking 1 peanut kernel a day to try to maintain a state of desensitization, and assessed AEs at this lower dose. In our cohort, 83% of participants on peanut maintenance of 4 g daily passed the desensitization challenge at week 104, and this group then received a reduced maintenance dose of 300 mg of peanut. In this peanut-300 arm, only 54% passed a 4 g challenge to peanut at week 117, and the rate subsequently declined further (figures 3 and 4). Ultimately, continuing daily OIT with 300 mg peanut to week 156 gave similar results as full discontinuation of peanut at week 117 (37% vs. 35%). Moreover, we found differences in an individual’s ability to ingest approximately 1 vs. 4 g over time, which could be important for the safe ingestion of various quantities and types of peanut-containing foods and an important message to convey in discussing patients’ ultimate wishes with peanut therapy. Accordingly, the optimal dose for maintaining desensitization to 13–16 peanuts requires further testing, but is likely to be greater than 300 mg per day. However, it is important to balance maintenance considerations with patient goals, which may include limiting the daily intake of peanut protein and safety considerations such as preparedness for reactions to home doses vs experiencing reactions to inadvertent accidental exposures.28
Although success continued to diminish over time with continued avoidance in peanut-0 and continued maintenance in peanut-300, there appeared to be a plateauing of the rate of decline in both groups, suggesting there may be a subset of individuals in both groups who are ‘resistant’ to the loss of desensitization. The size of this subset was larger in the peanut-300 cohort compared to peanut-0, however, there are clearly participants in each group that remain unresponsive over time. Biomarkers we identified in an individual at baseline, such as lower IgE to Ara h 1–3, peanut sIgE, or peanut IgE/IgG4 ratio, or a lower basophil activation test response to peanut, are associated with improved success rates. These results warrant further investigation, particularly regarding the mechanisms which may contribute to such long-term sustained unresponsiveness and the criteria which may be useful for identifying the subgroups most responsive to therapy.
Epinephrine use was encouraged in this study to pro-actively treat mild, moderate, and severe symptoms (i.e. wheezing, persistent cough, angioedema, etc., table S2), to prevent more allergic reactions or for relief of prolonged symptoms (i.e., abdominal pain). This frequent use of pro-active epinephrine was expected in peanut OIT and safety results were reviewed by the National Institutes of Health (NIH) Data and Safety Monitoring Board (DSMB), Institutional Review Board (IRB), and FDA. Epinephrine use declined within the peanut arms with longer duration on study drug (19%, 13%, 2%, from years 1, 2, and 3, respectively; table S3). Further studies using other immunotherapy regimens (i.e., vaccines, biologics, adjuvants, epitopes) to decrease safety risks and to permit inclusion of more severe phenotypes of food allergy will be helpful in the future, but our study was focused on long-term therapy outcomes with continuation or discontinuation of peanut OIT.
In general, we did not find differences in safety or efficacy outcomes between adults and children at week 117, although the study was not designed to formally test the differences in these age groups. There were 22 participants over 18 years old in the study; we found no differences compared to younger participants at week 117 in AE rates, DBPCFC outcomes,29,30 or peanut-0 or peanut-300 success. The dropout rate was increased in adults vs. children (32% vs. 9%). A recent phase 3 peanut OIT study also had a higher dropout rate in the adult cohort.5 Further research is indicated in adults with food allergies.
Our study revealed AE rates similar to those of other single food allergen OIT studies.5,6,8,31 Our SAE rate of 2% within the combined peanut arms over 3 years was less than what has been described in other studies (6% rate of SAEs reported in the PACE study of 1 year of peanut OIT).8 When evaluating AEs at the participant level by time on study drug, we observed a decline in AEs with increased duration on a 4 g maintenance dose during the second year (from the first to second study year: peanut-0 95% to 73%; peanut-300 91% to 65%). Despite experiencing AEs, the majority of participants in the peanut arms who were tested at week 104 for desensitization success to 4 g of peanut (approximately 13–16 peanuts) were successful in reaching the efficacy endpoint. AEs diminished in the peanut-0 arm in the third year and were significantly fewer than in the peanut-300 arm. Reactions can still occur during maintenance dosing with 300 mg (as opposed to minimizing the potential for AEs with avoidance) and, therefore, continued vigilance against adverse reactions is essential. It is unclear whether the reduction in the percent of participants experiencing AEs in year 3 is due to the reduction of peanut maintenance dose or longer duration on therapy. We hypothesize that that patients in both the peanut and placebo arms decided to comply to achieve peanut desensitization, despite encountering AEs, due to the possibility of decreasing chances of accidental allergic reactions after the study was over. Additionally, we speculate that continued avoidance with periodic challenges as “immune boosts to desensitization” may prove to be a safer and still effective maintenance therapy approach in a subset of individuals.
Chu et al.8 and Roberts et al.28 suggested identifying specific groups that could benefit from adjunctive or other lines of therapy compared to OIT alone if side effects were too frequent or too severe. To that end, we are investigating eosinophilic inflammation during the course of OIT to determine if biologics should be given as adjunctive therapies to OIT.32 One of our participants developed biopsy-proven EoE. But the incidence of EoE in this study (1/120 or 0·8%) is lower than what has been reported in the literature.33,34 In the present cohort, we also defined a more severe subset of participants at baseline through basophil activation markers and clinical phenotype.22 Higher levels of peanut sIgE/Total IgE, peanut sIgE, Ara h 1, Ara h 2, and Ara h 1 IgE/peanut sIgE at baseline in participants were associated with increased frequencies of AEs during active peanut OIT. All of these markers except for Ara h 1 IgE/peanut sIgE were also significantly associated with clinical outcome (Figure 5). Patients with these higher biomarker values would have a lower likelihood of benefitting overall due to AEs; however, despite experiencing AEs, participants were motivated to continue in the study. These findings could improve clinical management during OIT by helping to identify proper treatment algorithms and monitoring safety according to clinical endotypes (clinical features and immunological biomarkers).
There are limitations to our study. We tested the ability to tolerate 4 g of peanut at week 104 after achieving a maintenance dose of 4 g of peanut. Serial testing thereafter was conducted only in those individuals who passed the 4 g challenge, and this may have selected for those with a boosted immune responses in both arms. The optimal treatment dose remains to be determined. Furthermore, our findings could be specific for peanut. Safety in OIT studies, including ours, should continue to be monitored carefully during the study and in the long term.
OIT is a promising approach for those with peanut allergy. At high doses, OIT can desensitize most peanut-allergic patients to 4 g of peanut, but discontinuation of peanut, or even ingesting peanut at 300 mg daily after desensitization, decreases the likelihood of tolerating peanut at previously attained thresholds. Our data demonstrate that a lower basophil activation test (CD63), peanut-specific IgE, and Ara h 1 and 2-specific IgE, and a higher peanut-specific IgG4/IgE, were associated with sustained unresponsiveness and had acceptable to excellent discrimination ability (AUC ranging from 0·68 for CD63 to 0·86 for Ara h 2 sIgE). Excluding CD63, all the above biomarkers were also significantly associated with success in the continued desensitization arm (peanut-300) and some were additionally associated with AEs (figure S3). This is the first time to our knowledge that all of these markers were found associated with clinical outcomes in a single clinical trial.35–38 These markers may help the practitioner in identifying good candidates for OIT and/or those individuals who warrant increased vigilance against allergic reactions during OIT. Optimal maintenance dosing and regimens still need to be further elucidated for improved safety, and must involve informed conversations with patients to identify attainable goals.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed with the terms “long-term,” “food allergy,” and “oral immunotherapy” for articles published on or before May 1, 2019, with no start date or language restrictions. Oral immunotherapy (OIT) has been shown to be safe and effective in the majority of participants in desensitizing patients to peanuts. However, questions remain about the durability of desensitization. Current data suggest that continued ingestion may be necessary to maintain the desensitized state after successful OIT in the majority of individuals; however, there is currently considerable variability in the age at therapy initiation, duration of OIT maintenance, maintenance dose, and the period of discontinuation of treatment for testing sustained unresponsiveness.
Added value of this study
This study provides data on long-term outcomes after successful peanut desensitization, informing clinicians about the care of peanut allergic patients after OIT. This is the first large, randomized study to perform sequential clinical and immunological testing of withdrawal of peanut OIT or continued low dose peanut maintenance (300 mg) while also evaluating biomarkers of clinical outcomes for 3 years.
Implications of all the available evidence
Peanut OIT can desensitize most peanut-allergic individuals to 4 g peanut protein but discontinuation, or even a reduction to 300 mg daily, increases the likelihood of regaining clinical reactivity to peanut. The data also suggest that those with lower basophil activation levels at baseline are more likely to have sustained unresponsiveness after 2 years of therapy.
ACKNOWLEDGEMENTS
This work was supported by the NIAID AADCRC U19AI104209 (Galli, Nadeau, Boyd and Chinthrajah), Sean N. Parker Center for Allergy and Asthma Research at Stanford University, Myra Reinhard Foundation, Food Allergy Research & Education (FARE) Center of Excellence, EAT (End Allergies Together), Trip Advisor Foundation, and the Crown Family Foundation (endowment for Boyd). All study drug was manufactured through the Stanford GMP facility as per GCP and GMP guidelines. All QA/QC was performed as per FDA GMP guidelines. We thank Dana Tupa, Sayantani Sindher, Vanitha Sampath, the staff, nurses, dieticians, study coordinators, and, especially, the participants and their families, who participated in the study.
FUNDING
National Institutes of Allergy and Infectious Disease.
Footnotes
DECLARATION OF INTERESTS
Dr. Kari Nadeau: NIAID CoFAR, NIAID Immune Tolerance Network, NHLBI Data and Safety Monitoring Board, NIAID grant awardee, NHLBI grant awardee, NIEHS grant awardee, EPA grant awardee, FARE Center of Excellence Director, WAO Center of Excellence Director, sponsored research for clinical studies from Aimmune, DBV, AnaptysBio, Astellas, Novartis, Regeneron, Adare, Sanofi, Stallergenes-Greer. Dr. Sharon Chinthrajah receives grant support from CoFAR NIAID, Aimmune, DBV Technologies, Astellas, AnaptysBio, Novartis, Regeneron, Stallergenes-Greer, and Boehringer Ingelheim, and is a scientific advisory board member for Alladapt Immunotherapeutics. Dr. Stephen J. Galli receives grant support from NIAID, NIAMS, and the Tobacco-Related Disease Research Program (U. California). Dr. Scott Boyd receives grant support from NIAID, has been an expert witness in a patent lawsuit involving Amgen, Regeneron and Sanofi, and is a scientific advisory board member for the Food Allergy Fund. Dr. Holden Maecker receives grant support from NIAID and Amgen.
REFERENCES
- 1.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011;128:e9–17. [DOI] [PubMed] [Google Scholar]
- 2.Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010;126:S1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherkaoui S, Ben-Shoshan M, Alizadehfar R, et al. Accidental exposures to peanut in a large cohort of Canadian children with peanut allergy. Clin Transl Allergy 2015;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feuille E, Nowak-Wegrzyn A. Oral Immunotherapy for Food Allergies. Ann Nutr Metab 2016;68 Suppl 1:19–31. [DOI] [PubMed] [Google Scholar]
- 5.Palisade Group of Clinical Investigators. AR101 Oral Immunotherapy for Peanut Allergy. N Engl J Med 2018;379:1991–2001. [DOI] [PubMed] [Google Scholar]
- 6.Anagnostou K, Islam S, King Y, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet 2014;383:1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bird JA, Spergel JM, Jones SM, et al. Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. J Allergy Clin Immunol Pract 2018;6:476–85 e3. [DOI] [PubMed] [Google Scholar]
- 8.Chu DK, Wood RA, French S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet 2019;393:2222–32. [DOI] [PubMed] [Google Scholar]
- 9.Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy 2017;72:1133–47. [DOI] [PubMed] [Google Scholar]
- 10.Bluemchen K, Eiwegger T. Oral peanut immunotherapy - How much is too much? How much is enough? Allergy 2018;74:220–2. [DOI] [PubMed] [Google Scholar]
- 11.Reier-Nilsen T, Michelsen MM, Lodrup Carlsen KC, et al. Feasibility of desensitizing children highly allergic to peanut by high-dose oral immunotherapy. Allergy 2019;74:337–48. [DOI] [PubMed] [Google Scholar]
- 12.Nachshon L, Goldberg MR, Katz Y, Levy MB, Elizur A. Long-term outcome of peanut oral immunotherapy-Real-life experience. Pediatr Allergy Immunol 2018;29:519–26. [DOI] [PubMed] [Google Scholar]
- 13.Blumchen K, Trendelenburg V, Ahrens F, et al. Efficacy, Safety, and Quality of Life in a Multicenter, Randomized, Placebo-Controlled Trial of Low-Dose Peanut Oral Immunotherapy in Children with Peanut Allergy. J Allergy Clin Immunol Pract 2019;7:479–91 e10. [DOI] [PubMed] [Google Scholar]
- 14.Jones SM, Burks AW, Keet C, et al. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J Allergy Clin Immunol 2016;137:1117–27 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syed A, Garcia MA, Lyu SC, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol 2014;133:500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood RA, Kim JS, Lindblad R, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol 2016;137:1103–10 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vickery BP, Scurlock AM, Kulis M, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol 2014;133:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andorf S, Purington N, Kumar D, et al. A Phase 2 Randomized Controlled Multisite Study Using Omalizumab-facilitated Rapid Desensitization to Test Continued vs Discontinued Dosing in Multifood Allergic Individuals. EClinicalMedicine 2019;7:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jhamnani RD, Frischmeyer-Guerrerio P. Desensitization for Peanut Allergies in Children. Curr Treat Options Allergy 2016;3:282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andorf S, Manohar M, Dominguez T, et al. Feasibility of sustained response through long-term dosing in food allergy immunotherapy. Allergy Asthma Clin Immunol 2017;13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Group IEW. ICH harmonized tripartite guideline: guideline for good clinical practice. 1996. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. (accessed June 6 2019). [PubMed]
- 22.Chinthrajah RS, Purington N, Andorf S, et al. Development of a tool predicting severity of allergic reaction during peanut challenge. Ann Allergy Asthma Immunol 2018;121:69–76 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food Allergy Research & Education. Food Allergy & Anaphylaxis Emergency Care Plan. 2018. https://www.foodallergy.org/sites/default/files/2018-06/emergency-care-plan.pdf (accessed June 6 2019).
- 24.Mukai K, Gaudenzio N, Gupta S, et al. Assessing basophil activation by using flow cytometry and mass cytometry in blood stored 24 hours before analysis. J Allergy Clin Immunol 2017;139:889–99.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos AF, James LK, Bahnson HT, et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol 2015;135:1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bock SA, Sampson HA, Atkins FM, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol 1988;82:986–97. [DOI] [PubMed] [Google Scholar]
- 27.Common Terminology Criteria for Adverse Events (CTCAE) v. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf. (accessed June 6 2019).
- 28.Roberts G, Angier E. Peanut oral immunotherapy: balancing benefits and risks for individuals. Lancet 2019;393:2180–81. [DOI] [PubMed] [Google Scholar]
- 29.Purington N, Chinthrajah RS, Long A, et al. Eliciting Dose and Safety Outcomes From a Large Dataset of Standardized Multiple Food Challenges. Front Immunol 2018;9:2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sindher S, Long AJ, Purington N, et al. Analysis of a large standardized food challenge data set to determine predictors of positive outcome across multiple allergens. Front Immunol 2018;9:2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virkud YV, Burks AW, Steele PH, et al. Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy. J Allergy Clin Immunol 2017;139:882–8 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright BL, Fernandez-Becker NQ, Kambham N, et al. Baseline gastrointestinal eosinophilia is common in oral immunotherapy subjects with IgE-mediated peanut allergy. Front Immunol 2018;22:2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petroni D, Spergel JM. Eosinophilic esophagitis and symptoms possibly related to eosinophilic esophagitis in oral immunotherapy. Ann Allergy Asthma Immunol 2018;120:237–40 e4. [DOI] [PubMed] [Google Scholar]
- 34.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 2014;113:624–9. [DOI] [PubMed] [Google Scholar]
- 35.Kulis M, Yue X, Guo R, et al. High- and low-dose oral immunotherapy similarly suppress pro-allergic cytokines and basophil activation in young children. Clin Exp Allergy 2018;49:180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorelik M, Narisety SD, Guerrerio AL, et al. Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol 2015;135:1283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright BL, Kulis M, Orgel KA, et al. Component-resolved analysis of IgA, IgE, and IgG4 during egg OIT identifies markers associated with sustained unresponsiveness. Allergy 2016;71:1552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frischmeyer-Guerrerio PA, Masilamani M, Gu W, et al. Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy. J Allergy Clin Immunol 2017;140:1043–53 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.