Abstract
NL003 is a plasmid engineered to simultaneously express two isoforms of hepatocyte growth factor. This phase II study was performed to assess the clinical safety and efficacy of intramuscular injection of NL003 in critical limb ischemia (CLI) patients for 6 months. Two hundred patients (Rutherford scale 4–5) were randomly assigned: placebo (n = 50), low-dose NL003 (n = 50), middle-dose NL003 (n = 50), or high-dose NL003 (n = 50). The drug was administered in the affected limb of 197 patients on days 0, 14, and 28. No significant differences in the incidence of adverse events (AEs) or serious AEs were found among the groups. At 6 months, pain severity was significantly reduced in all NL003 groups, but not in the placebo group (p < 0.05). The proportion of patients with complete ulcer healing in the high-dose group was significantly higher than that of the placebo group (p = 0.0095). There were no statistically significant differences in transcutaneous oxygen pressure (TcPO2), ankle-brachial index (ABI), or toe-brachial index (TBI) value among the four groups throughout the study period. These results provide the first effective evidence of significant improvements in total healing of ulcers in treated legs, complete pain relief without analgesics, and safety for NL003 in patients with Rutherford stage 4–5.
Keywords: critical limb ischemia, peripheral arterial disease, gene therapy, angiogenesis, hepatocyte growth factor, HGF, naked plasmid, NL003
Critical limb ischemia is a disease causing rest pain and ulcer of limbs. In this issue of Molecular Therapy, Gu et al. present evidence demonstrating that NL003 was benefiting in phase II. It is suggested that NL003 has the potential to treat critical limb ischemia.
Introduction
Critical limb ischemia (CLI) is one of the most severe conditions associated with peripheral arterial occlusive diseases (PAODs), manifesting as ischemic rest pain, ulceration, and gangrene in lower extremities, and belonging to category 4–6 of the Rutherford classification or Fontaine stage III–IV.1 In CLI patients, macrovascular lesions induce decreased perfusion in the distal part of the lower limbs. Thus, the nutrient supply from the blood to the target tissue and microcirculation exchange is severely compromised. A number of CLI patients also undergo amputations in their affected lower limbs because of long-term chronic ischemia.2 As in Western countries, CLI is a serious clinical phenomenon that is also widespread in China, particularly among the senior citizen population. It is estimated that approximately 5 million Chinese individuals suffer from CLI.3,4
According to guidelines on the clinical investigation of medicinal products for the treatment of PAOD from the European Medicines Agency, the aims of primary treatment for CLI patients are to improve their quality of life via complete relief of pain while off analgesics (Rutherford 4 or Fontaine III) and to completely heal all necroses and ulcerations in the leg (Rutherford 5–6 or Fontaine IV).5 Current treatment methods for CLI patients include endovascular intervention or surgical bypass; however, no alternative effective pharmacological therapies exist that can modify the disease conditions.1,2,6 Therefore, a novel medical approach to treat CLI at fundamental levels is urgently needed.
Hepatocyte growth factor (HGF) is a well-known angiogenic, antifibrotic, and neurotropic factor that works through the c-Met/HGF signaling pathway. HGF expression is necessary for the proliferation, migration, and survival of epithelial and endothelial cells related to tissue repair of a variety of organs.7 In humans, there are two isoforms of HGF: the proteins consist of 723 and 728 amino acids (HGF723 and HGF728), which are produced via alternative splicing. Shima et al.8 reported that HGF723 and HGF728 are distinguishable in their biological activities in various cell types and in their physicochemical properties relating their solubility.8 However, it was later discovered that coexpression of two HGF isoforms is better suited for optimum biological functionality, as evidenced by enhanced migration activities of human umbilical vein smooth muscle cells and mouse myoblast cell lines compared with those of a single isoform of HGF and vascular endothelial growth factor (VEGF). Consistent with in vitro data, ischemic hindlimb rabbit and ischemic heart disease rat models have demonstrated that expression of both HGF723 and HGF728 can more efficiently induce angiogenic and/or anti-fibrotic activities than using single forms only: VEGF or HGF728.9,10
NL003 (pCK-HGF-X7) is a plasmid DNA encoding a genomic cDNA hybrid human HGF sequence designed to simultaneously express two wild-type isoforms of HGF: HGF723 and HGF728.9 Results from two phase I studies and one phase II study for CLI in China and the United States suggest that plasmid DNA injected via the intramuscular route is safe and well tolerated, and might have clinical benefits through reduced rest pain and improved complete wound closure.11,12 This large-scale, double-blind, placebo-controlled phase II clinical trial was performed to evaluate the safety and efficacy of NL003 in CLI patients, and to provide rationales for clinical endpoints and an appropriate dose for phase III clinical trials.
Results
Baseline Demographic and Medical History
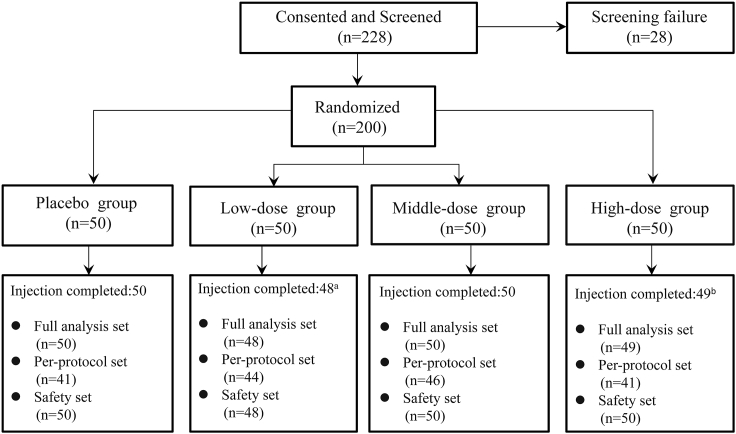
As shown in Figure 1, 228 CLI patients provided written informed consent and were subjected to screening in accordance with the inclusion and exclusion criteria. Twenty-eight patients were excluded after failing the screening process. The eligible 200 patients were selected and then randomly assigned to the placebo (n = 50), low-dose (n = 50), middle-dose (n = 50), or high-dose group (n = 50). A total of 197 out of 200 patients (98.5%) received all three scheduled doses, but two patients in the low-dose group prior to the dosing and one patient in the high-dose group after the first dosing voluntarily withdrew. Finally, 172 patients (86%) completed the study. Table 1 summarizes the demographics, medical histories, and standard medical treatments of the participating patients. Of note, baseline characteristics were well balanced, and no significant differences were observed among the four groups.
Figure 1.
Patient Enrollment
aTwo patients voluntarily withdrew from the study without dosing. bOne patient withdrew from the study after the first dosing because of an unavailable age.
Table 1.
Demographics
| Placebo (n = 50) | Low Dose (n = 48) | Medium Dose (n = 50) | High Dose (n = 49) | p Value | |
|---|---|---|---|---|---|
| Age (year) | 60.28 ± 13.04 | 56.38 ± 13.85 | 55.44 ± 12.82 | 54.94 ± 12.04 | 0.1703 |
| Sex | 0.3379 | ||||
| Male | 40 (80.00%) | 39 (81.25%) | 45 (90.00%) | 44 (89.80%) | |
| Female | 10 (20.00%) | 9 (18.75%) | 5 (10.00%) | 5 (10.20%) | |
| Race | 0.5652 | ||||
| Chinese Han | 50 (100.00%) | 47 (97.92%) | 49 (98.00%) | 47 (95.92%) | |
| Other | 0 (0.00%) | 1 (2.08%) | 1 (2.00%) | 2 (4.08%) | |
| Prior limb procedures | |||||
| PTA and bypass | 19 (38.00%) | 28 (58.33%) | 20 (40.00%) | 17 (34.69%) | 0.0882 |
| Drug therapy for PAD | 49 (98.00%) | 46 (95.83%) | 46 (92.00%) | 45 (91.84%) | 0.4826 |
| Risk factors | |||||
| Hyperlipidemia | 15 (30.00%) | 14 (29.17%) | 9 (18.00%) | 14 (28.57%) | 0.4716 |
| Hypertension | 21 (42.00%) | 17 (35.42%) | 16 (32.00%) | 17 (34.69%) | 0.7643 |
| Diabetes | 19 (38.00%) | 18 (37.50%) | 17 (34.00%) | 17 (34.69%) | 0.9698 |
| Variety of disease | 0.7908 | ||||
| ASO | 33 (66.00%) | 33 (68.75%) | 30 (60.00%) | 30 (61.22%) | |
| TAO | 17 (34.00%) | 15 (31.25%) | 20 (40.00%) | 19 (38.78%) | |
| Rutherford class | 0.9452 | ||||
| Rutherford 4 | 24 | 22 | 21 | 23 | |
| Rutherford 5 | 26 | 26 | 29 | 26 | |
Safety Endpoints
No unexpected severe adverse events (AEs) were observed during the 6-month study period after the study drug or placebo injection. A total of 434 AEs were reported in 164 patients (164/197, 83%). Of these, 337 cases (337/434, 77.6%) were resolved before the study ended; most AEs (268/434, 61.8%) were classified as mild. Seventeen injection-site reactions occurred in 13 patients (4 cases in 3 placebo patients, 5 cases in 3 low-dose patients, 4 cases in 4 middle-dose patients, and 4 cases in 3 high-dose patients) over the course of the trial. All 17 of these reactions were classified as mild, manifesting as pain, itching, erythema, rash, swelling, and/or bruising in the injection sites. All of these reactions were completely resolved during the follow-up period. At 6 months postinjection, 32 serious AEs (SAEs) were experienced in 30 out of 197 patients (15.2%) but were not related to the study drug. There were no statistically significant differences in the occurrence of AEs, SAEs, or injection-site reactions among the three NL003 and placebo groups.
Three cases of death were reported during the study period. All deaths were deemed unrelated to the drug injections. Two placebo patients died of stroke and heart failure at 95 and 32 days after the first treatment, respectively. One mortality case from the high-dose group was caused by acute myocardial infarction, which occurred 95 days postinjection.
Serum levels of the human HGF protein were measured using ELISA before injection and on days 0, 14, 28, 60, 90, and 180 after injection. Its level remained relatively stable throughout the follow-up period, without a noticeable change, and no HGF antibodies were detected (data not shown).
Efficacy Endpoints
Rest Pain
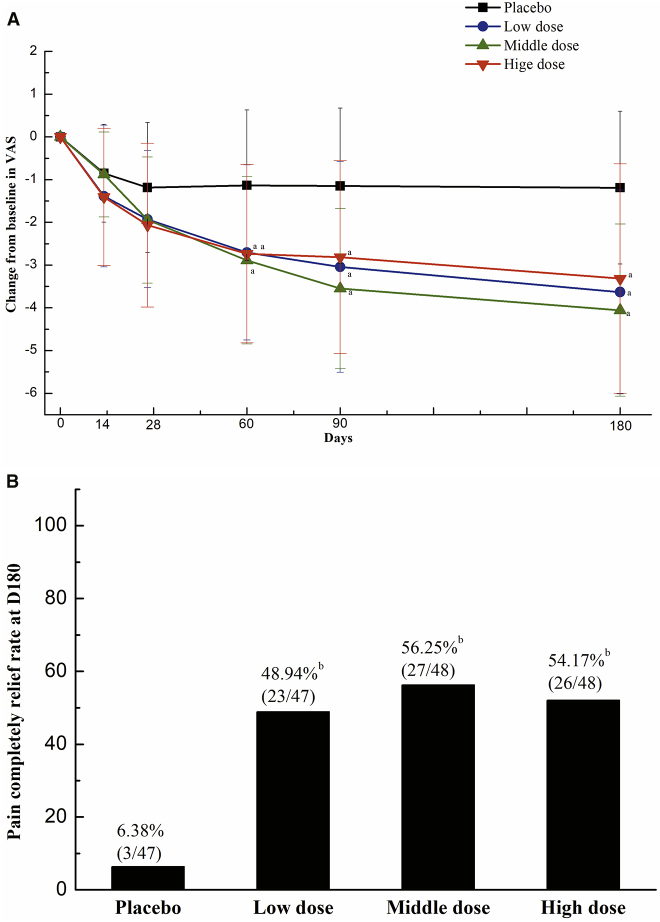
Rest pain is an important clinical symptom that seriously compromises the quality of life of CLI patients. The level of the rest pain was measured using the visual analog scale (VAS) (cm) on days 0, 14, 28, 60, 90, and 180. There were no significant differences in the baseline pain severity among the four groups (placebo, 4.63 ± 1.85; low dose, 4.95 ± 1.92; middle dose, 5.17 ± 1.75; high dose, 4.95 ± 2.30; p = 0.6133). As shown in Figure 2A, the pain severity in all NL003 groups significantly improved at 60, 90, and 180 days after the first treatment compared with that of the placebo group. Furthermore, the proportion of patients with complete pain reduction while off analgesics was evaluated on day 180 postinjection in patients (Rutherford 4–5). As shown in Figure 2B, the proportion of patients with complete pain relief in all NL003 groups was significantly higher than that of the placebo group (p < 0.05; 6.38% in placebo versus 54.17%, 56.25%, or 48.94% in high-dose, middle-dose, or low-dose group, respectively). There were no significant differences in the proportion of patients with complete pain relief among the groups on day 0 (data not shown).
Figure 2.
Summary of the Change in Pain Score during the Study and the Rate of Complete Pain Relief without Analgesics at the Last Visit
(A) Changes in pain severity over the 6-month follow-up. ap < 0.05 among all four groups by one-way ANOVA. (B) Percentage of patients achieving complete pain relief while off analgesics on day 180. bp < 0.05 by Fisher’s exact test, placebo versus low-, middle-, or high-dose, respectively.
Ulcer Healing
Ulcer is one of the most visible clinical manifestations in patients with CLI. If ulcers were present prior to the first injection on day 0, photographs of each ulceration area were captured on days 14, 28, 60, 90, and 180 for comparison. As shown in Table 2, the percentages of patients achieving complete ulcer closure at 6 months posttreatment were 42.11%, 52.17%, and 66.67% for the low-dose, middle-dose, and high-dose groups, respectively, whereas the placebo group reported 27.27%. The rate of patients with complete ulcer healing in the high-dose group was significantly higher than that of the placebo group (p = 0.0095, placebo versus high-dose group), although there was no significant difference in the middle- or low-dose group when compared with the placebo group. Additionally, >50% ulcer healing, a clinically meaningful reduction,13,14 was significantly observed in the high-, middle-, and low-dose groups. The mean change in ulcer size (cm2) from day 0 to the last visit of each patient was similar among all groups.
Table 2.
Summary of Ulcer Healing during the 6-Month Follow-Up Period
| Treatment Group | No. of Patients Having Ulcers at Baseline | No. of Completely Healed Patients at Last Visit (%) | No. of ≥50% Healed Patients at Last Visit (%) | Ulcers Changed in Sizea (cm2) (95% CI) |
|---|---|---|---|---|
| Placebo | 22 | 6 (27.27) | 9 (40.91) | −1.36 ± 3.32 (−2.91 to 0.20) |
| Low dose | 19 | 8 (42.11) | 15 (78.95)b | −1.75 ± 2.52 (−3.01 to 0.50) |
| Middle dose | 23 | 12 (52.17) | 15 (65.22) | 0.92 ± 8.33 (−2.87 to 4.72) |
| High dose | 18 | 12 (66.67)c | 14 (77.78)d | 0.71 ± 5.76 (−2.16 to 3.58) |
Ulcer changed in size = last ulcer measurement − baseline ulcer measurement.
p = 0.0251 versus placebo.
p = 0.0243 versus placebo.
p = 0.0266 versus placebo.
Skin Perfusion
The skin perfusion was measured at 28, 60, 90, and 180 days postinjection with transcutaneous oxygen pressure (TcPO2). The ratio of the TcPO2 value in the foot to the chest was calculated as TcPO2 dorsum foot/TcPO2 chest. There were no statistically significant differences in TcPO2 values among the four groups throughout the study period (Table 3).
Table 3.
Parameters TcPO2, ABI, and TBI
| Treatment Group | Baseline | Day 28 | Day 60 | Day 90 | Day 180 |
|---|---|---|---|---|---|
| TcPO2Measurements (mm Hg) | |||||
| Placebo | 22.62 ± 15.69 | 23.53 ± 15.26 | 26.93 ± 16.20 | 31.79 ± 17.89 | 35.46 ± 16.50 |
| Low dose | 19.48 ± 14.02 | 22.49 ± 16.76 | 22.84 ± 16.46 | 27.26 ± 19.81 | 28.12 ± 15.22 |
| Middle dose | 20.24 ± 15.19 | 26.23 ± 19.69 | 29.40 ± 16.90 | 30.45 ± 15.75 | 28.71 ± 16.12 |
| High dose | 24.61 ± 16.67 | 26.18 ± 19.95 | 27.14 ± 17.12 | 29.59 ± 14.71 | 29.78 ± 17.52 |
| ABI Measurements (Index) | |||||
| Placebo | 0.46 ± 0.32 | 0.46 ± 0.26 | 0.48 ± 0.27 | 0.57 ± 0.22 | 0.57 ± 0.27 |
| Low dose | 0.46 ± 0.37 | 0.37 ± 0.28 | 0.43 ± 0.27 | 0.51 ± 0.31 | 0.48 ± 0.33 |
| Middle dose | 0.41 ± 0.29 | 0.46 ± 0.23 | 0.55 ± 0.23 | 0.56 ± 0.24 | 0.60 ± 0.23 |
| High dose | 0.45 ± 0.28 | 0.48 ± 0.25 | 0.52 ± 0.24 | 0.57 ± 0.23 | 0.61 ± 0.24 |
| TBI Measurements (Index) | |||||
| Placebo | 0.12 ± 0.18 | 0.12 ± 0.19 | 0.16 ± 0.20 | 0.17 ± 0.19 | 0.18 ± 0.22 |
| Low dose | 0.08 ± 0.12 | 0.09 ± 0.15 | 0.10 ± 0.14 | 0.15 ± 0.23 | 0.15 ± 0.19 |
| Middle dose | 0.09 ± 0.19 | 0.09 ± 0.13 | 0.10 ± 0.14 | 0.14 ± 0.21 | 0.14 ± 0.20 |
| High dose | 0.08 ± 0.14 | 0.08 ± 0.15 | 0.11 ± 0.19 | 0.15 ± 0.23 | 0.18 ± 0.22 |
Hemodynamic Assessments
Ankle-brachial index (ABI) and toe-brachial index (TBI) were measured on days 0, 28, 60, 90, and 180. Neither ABI nor TBI was significantly different across treatment groups at any given time (Table 3).
Amputations
A total of 16 patients (8.0%, 16/200) had amputations in their study limbs after the study drug injection (placebo or NL003). There were 10 major amputations in the study limbs of 10 patients (placebo, n = 2; low dose, n = 2; middle dose, n = 2; and high dose, n = 4) and 6 minor amputations in 6 patients (placebo, n = 1; low dose, n = 2; middle dose, n = 0; and high dose, n = 3). There were no significant differences in the amputation rate among the groups.
Discussion
In the present phase II clinical trial, our findings suggest that the intramuscular injection of NL003 (pCK-HGF-X7) into the affected limb of CLI patients not only is safe but also improves two important clinical parameters. The differences in pain severity and ulcer healing were significantly improved in all treated groups compared with the placebo group 6 months after the study drug initiation. Additionally, the administration of NL003 into muscles was safe and well tolerated while producing data consistent with a previous phase I study.7
Strategies have been explored, particularly for CLI as a proof-of-concept (POC) setting using genes, proteins, and cells over the last 25 years, with an aim to fundamentally cure the clinical symptoms through the induction of neovascularization. Despite intense efforts, most clinical studies conducted so far have produced unsatisfactory results. The phase II study reported by Nikol et al.15 showed that the use of NV1FGF significantly reduced the risk for major amputation and death assessed at secondary endpoints compared with placebo, although there was no statistically significant improvement in ulcer healing, evaluated as the primary endpoint, over that of placebo. These data provided the basis for the initiation of a large phase III study in 525 patients, namely, TAMARIS. Unfortunately, the primary combined endpoint (time to major amputation or death) of the TAMARIS study was not achieved, nor were the secondary endpoints (all amputations, skin lesion status, pain intensity, and so on).16 In two HGF trials published by Powell et al.,12,17 HGF treatment significantly increased the mean change in TcPO2 from baseline at 6 months in the high-dose group compared with placebo. Additionally, the change in TBI and VAS score from baseline in the HGF-treated patients was significantly improved at 6 months compared with that of placebo. However, there were no statistically significant differences in the incidence of complete wound healing, major amputation, or death at 6 months among the groups.12,17 In another HGF study reported by Shigematsu et al.,13 there was a significant improvement in ischemic ulcer from baseline in the HGF-treated group compared with that of the placebo group at 12 weeks, although there was no significant pain relief. Recently, a report by Kibbe et al.14 showed the potential bioactivity of plasmids expressing two HGF isoforms, as documented by a significant improvement in tissue oxygenation and ulcer healing, although no effectiveness was observed for rest pain.17
In patients administered NL003, our results showed that two important clinical parameters, namely, pain relief and ulcer healing, were improved in the high-dose NL003-treated group compared with the placebo group. The pain level was decreased in a dose-dependent manner, and the difference between the placebo and all NL003 treatment groups was statistically significant at 2, 3, and 6 months following the first injection. The effect on the reduction in pain severity of the three injections at 2-week intervals lasted for the entire 6-month period. However, there were no statistically significant differences in TcPO2, ABI, or TBI value among the four groups throughout the study period.
Interestingly, complete improvements in pain relief without analgesics and healing of all ulcers were observed in the high-dose group compared with the placebo group. These clinically important symptoms have especially been mentioned as primary endpoints in guidelines on the clinical investigation of PAOD published by the European Medicines Agency. These results have not previously been achieved in placebo-controlled CLI studies of other gene therapies using growth factors (HGF, FGF, or VEGF).11, 12, 13, 14,17, 18, 19 The dosing scheme and treatment regimen of this study are superior to those of other HGF studies. Regarding the dosing schemes, in the current study, the administration followed the dose-escalation scheme: 3 injections of 4 mg (total 12 mg in the low-dose group), 6 mg (total 18 mg in the middle-dose group), or 8 mg (total 24 mg in the high-dose group) per visit at 2-week intervals. In the HGF-STAT trial, the dosing scheme was employed with two or three injections of 0.4 mg or 4 mg per visit at the same interval (total 1.2 mg in the low-dose, 8 mg in the middle-dose, or 12 mg in the high-dose group).19 In the VM202 trial, the prolonged dosing scheme for maintaining HGF level was employed with two or four injections of 4 mg per visit within the same interval (total 8 mg in the low-dose or 16 mg in the high-dose group). Second, the treatment regimen, including injection sites, differs among the three trials. In the current study, NL003 was injected into the target muscles surrounding occluded vessels using angiography-guided dosing. Unlike this regimen, the plasmid DNA was delivered into the planned area regardless of occlusion sites in the VM202 and HGF-STAT trials. For clinical efficacy in CLI patients, the dose-escalation scheme and angiography-guided dosing in this trial were far better options than the prolonged scheme and planned dosing. Our superior results over the other trials suggest that three injections of 8 mg of NL003 per visit with angiography-guided dosing could be a more suitable option to improve CLI conditions.
In conclusion, this phase II, double-blind, randomized, placebo-controlled clinical trial provides the first effective evidence of significant improvements in total healing of ulcers in treated legs, complete pain relief, and safety for NL003 in patients with Rutherford category 4–5. Phase III clinical trials were approved by the Chinese Food and Drug Administration (FDA) in September 2017. These data support the performance of a phase III clinical trial in patients with CLI for the first new NL003 drug approval from the Chinese FDA.
Materials and Methods
Design
This phase II clinical trial was a double-blind, randomized, placebo-controlled study to evaluate the safety, tolerability, and efficacy of NL003 injected via the intramuscular route in patients with CLI (clinicaltrials.gov: NCT01548378). This study was conducted from March 2012 (first patient in) to June 2014 (last patient out). This study was approved by the China Food and Drug Administration (CFDA approval number: 2011L01681) and the institutional review boards of nine clinical sites in China. It adhered to Good Clinical Practice and the Declaration of Helsinki.
The patients who were eligible for the study were randomized 1:1:1:1 to placebo (0.9% saline), low-dose (12 mg), middle-dose (18 mg), and high-dose NL003 (24 mg) groups. These patients were followed for 6 months after the NL003 injection to assess safety, tolerability, and efficacy.
Inclusion and Exclusion Criteria
The enrolled patients in this study were CLI patients identified by the investigator as not suitable for vascular intervention or surgical procedures. Patients were enrolled if they were male or a nonpregnant, nonlactating female between 30 and 80 years of age and were diagnosed with CLI (Rutherford category 4–5). CLI was diagnosed if there was a resting ankle systolic pressure of ≤70 mm Hg, a resting toe systolic pressure of ≤50 mm Hg in the affected limb, or transcutaneous oxygen pressure of ≤30 mm Hg if ankle systolic pressure measurements were not feasible. The patients were also diagnosed with significant stenosis (≥75%) of one or more superficial femoral or infra-popliteal arteries as verified by angiography. In addition, the patients were willing to maintain current standard medications and ulcer management for CLI, and were diagnosed with no meaningful results in cancer evaluation.
Patients were excluded if they had undergone a successful revascularization procedure or sympathectomy within 3 months prior to study entry, required affected limb amputation within 4 weeks, were diagnosed with acute advanced CLI, had significant stenosis (≥75%) of the aorto-iliac artery, had active infection or deep ulceration exposing a bone or tendon in the target limb, or had an estimated life expectancy of less than 1 year. We also excluded patients with heart failure (New York Heart Association [NYHA] class 3–4), stroke, myocardial infarction, or unstable angina within 3 months from the screening; uncontrolled hypertension (>180 mm Hg of sustained systolic blood pressure or >110 mm Hg of diastolic blood pressure); advanced liver disease, including cirrhosis, jaundice, ascites, or bleeding varices; HIV positivity; active hepatitis B or C infection; clinically significant abnormal laboratory findings; proliferative retinopathy, malignancy history within 5 years or new findings of malignancy; current immunosuppressive medications, chemotherapy or radiation therapy; or a history of drug or alcohol abuse/dependency within 12 months. Furthermore, patients requiring >100 mg/day of acetylsalicylic acid, a Cox-2 inhibitor, or high-dose steroid, or who had participated in other clinical trials within the last 3 months were excluded.
Study Drug and Injections
NL003 was manufactured by Beijing Northland Biotech (Beijing, China) in accordance with Good Manufacturing Practice. The drug product of NL003 was supplied in a glass vial containing 2 mg in 1 mL of 0.9% normal saline and was stored between −10°C and −25°C. The placebo was used with 0.9% normal saline. The liquid form of NL003 was indistinguishable from the saline for placebo. For injections, the drug product was diluted to a concentration of 0.5 mg/mL using saline.
At each injection visit (days 0, 14, and 28), the patients received 32 injections (0.5 mL/site) intramuscularly in the calf of the target limb, unilaterally (Table 4). The injections were performed from the proximal to the distal part of the calf in accordance with the verification of muscle compartments with occluded arteries by angiography. The injection sites were no closer than 2 cm apart.
Table 4.
Dosing Scheme
| Treatment Group | Dose (mg) | Dose Level (per Injection) | Daily Dose (No. of Injections) |
||
|---|---|---|---|---|---|
| Day 0 | Day 14 | Day 28 | |||
| Placebo (n = 50) | 0 | 0.5 mL of saline | Saline (32) | Saline (32) | Saline (32) |
| Low dose (n = 50) | 12 | 0.25 mg per 0.5 mL of NL003 or 0.5 mL of saline | 4 mg (16) Saline (16) |
4 mg (16) Saline (16) |
4 mg (16) Saline (16) |
| Medium dose (n = 50) | 18 | 0.25 mg per 0.5 mL of NL003 or 0.5 mL of saline | 6 mg (24) Saline (8) |
6 mg (24) Saline (8) |
6 mg (24) Saline (8) |
| High dose (n = 50) | 24 | 0.25 mg per 0.5 mL of NL003 | 8 mg (32) | 8 mg (32) | 8 mg (32) |
Efficacy Endpoints
Coprimary endpoints of efficacy evaluation were the differences in pain severity (Rutherford 4–5) and ulcer sizes (Rutherford 5 only) between baseline and 6 months after the study drug initiation.
Secondary endpoints included the change in skin perfusion measured by transcutaneous oxygen pressure (TcPO2), ankle-brachial index (ABI), toe-brachial index (TBI), amputation rate, and mortality from baseline to 6-month follow-up. ABI, TBI, and TcPO2 were performed at the screening period and 28, 60, 90, and 180 days postinjection. In addition, according to EMA guidance, the clinical endpoints of the medicinal product for CLI treatment were the proportions of patients with complete relief of rest pain without analgesics (Rutherford 4–5) and with complete healing of all ischemic ulcers in the treated leg (Rutherford 5 only). Pain severity measured by a VAS was conducted at screening, day 0 before the treatments, and at 14, 28, 60, 90, and 180 days after study drug or placebo administration. Ulcer size was measured on days 0, 14, 28, 60, 90, and 180.
Safety Endpoints
The safety and tolerability of NL003 injected via the intramuscular route in CLI patients were evaluated during the 6-month follow-up. Over the course of the study, the assessment of adverse events, ophthalmologic examination using retinal fundoscopy, cancer screening, physical examination, and various laboratory tests were conducted. The evaluation of adverse events was performed according to the frequency of adverse events and their relationship with the study drug, injection procedure, and underlying disease. To assess the changes in the levels of NL003 plasmid DNA, whole blood was collected from each patient on days 0, 14, and 28 preinjection and days 14, 28, 60, 90, and 180 postinjection. Changes in the level of plasma HGF were evaluated at days 0, 14, and 28 before the injection and at days 60, 90, and 180 after the injection using an ELISA kit (R&D Systems, Minneapolis, MN, USA).
Sample Size Calculation
The sample size was calculated based on the VAS change from baseline using PASS Version 15.0. The effect sizes of the primary endpoints referred to the mean change in the VAS value in the treatment group from CLI. The statistical power was 90%, and the two-sided significance level was 0.05. The allocation ratio was 1:1:1:1 for the four groups (low-, middle-, and high-dose, and placebo groups). In the case of VAS, the mean changes were −1.9 and 0.06 in the treatment and placebo groups, respectively. The SD was assumed to be 3 in each group. Assuming the common SD was 3 and the high-dose/middle-dose/low-dose groups had the same mean of −1.9 and the placebo group mean was 0.06, the total sample size needed was 152 patients. Considering the likely dropout rate and other conditions, 200 patients were determined for the reasonable sample size in this study.
Statistical Analysis
All statistical analyses were done with SAS 9.4 (SAS Institute, Cary, NC, USA) in the Statistical Analysis Department, Beijing Panacea Technology. All efficacy and safety analyses were conducted based on the full analysis and safety population, that is, including all patients randomized and treated with the investigational product at least once. The last observation carried forward method imputed missing values of the VAS information. The statistical significance level was set to 0.05. Bivariate associations were assessed using the chi-square or Fisher’s exact test for categorical variables. Continuous variables were analyzed using one-way ANOVA followed by Dunnett’s adjusted multiple comparison. For VAS analysis, patients who scored 0 points at screening could not provide valid data. Furthermore, for the last visit for pain relief analysis, patients who had been using analgesic at the day 180 VAS assessment were excluded.
Author Contributions
Y.G. designed and conducted the trial and wrote the manuscript. S.C., Q.W., Changjian Liu, B.J., W.G., Changwei Liu, T.C., C.S., and F.Z. conducted the trial. C.H. and Y.L. participated in the post-trial data processing.
Conflicts of Interest
The authors declare no competing interests. C.H. and Y.L. are employees of the sponsor.
Acknowledgments
The authors retain sole responsibility for the data and manuscript content. This trial was funded by Beijing Northland Biotech, Co. (Beijing, China).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.10.017.
Supplemental Information
References
- 1.Norgren L., Hiatt W.R., Dormandy J.A., Nehler M.R., Harris K.A., Fowkes F.G., TASC II Working Group Inter-society consensus for the management of peripheral arterial disease (TASC II) J. Vasc. Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Mohler E., 3rd, Giri J., ACC. AHA Management of peripheral arterial disease patients: comparing the ACC/AHA and TASC-II guidelines. Curr. Med. Res. Opin. 2008;24:2509–2522. doi: 10.1185/03007990802274379. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes F.G.R., Rudan D., Rudan I., Aboyans V., Denenberg J.O., McDermott M.M., Norman P.E., Sampson U.K.A., Williams L.J., Mensah G.A., Criqui M.H. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 4.Farber A., Eberhardt R.T. The current state of critical limb ischemia: a systematic review. JAMA Surg. 2016;151:1070–1077. doi: 10.1001/jamasurg.2016.2018. [DOI] [PubMed] [Google Scholar]
- 5.Note for guidance on clinical investigation of medicinal products for the treatment of peripheral arterial occlusive disease. European Agency for the Evaluation of Medicinal Products, Committee for Proprietary Medicinal Products (EMEA CPMP), April 25, 2002, CPMP/EWP/714/98. https://www.ema.europa.eu/en/documents/scientific-guideline/note-guidance-clinical-investigation-medicinal-products-treatment-peripheral-arterial-occlusive_en.pdf.
- 6.Hirsch A.T., Haskal Z.J., Hertzer N.R., Bakal C.W., Creager M.A., Halperin J.L., Hiratzka L.F., Murphy W.R., Olin J.W., Puschett J.B., American Association for Vascular Surgery/Society for Vascular Surgery. Society for Cardiovascular Angiography and Interventions. Society for Vascular Medicine and Biology. Society of Interventional Radiology. ACC/AHA Task Force on Practice Guidelines ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J. Vasc. Interv. Radiol. 2006;17:1383–1397. doi: 10.1097/01.RVI.0000240426.53079.46. quiz 1398. [DOI] [PubMed] [Google Scholar]
- 7.Gu Y., Zhang J., Guo L., Cui S., Li X., Ding D., Kim J.M., Ho S.H., Hahn W., Kim S. A phase I clinical study of naked DNA expressing two isoforms of hepatocyte growth factor to treat patients with critical limb ischemia. J. Gene Med. 2011;13:602–610. doi: 10.1002/jgm.1614. [DOI] [PubMed] [Google Scholar]
- 8.Shima N., Tsuda E., Goto M., Yano K., Hayasaka H., Ueda M., Higashio K. Hepatocyte growth factor and its variant with a deletion of five amino acids are distinguishable in their biological activity and tertiary structure. Biochem. Biophys. Res. Commun. 1994;200:808–815. doi: 10.1006/bbrc.1994.1523. [DOI] [PubMed] [Google Scholar]
- 9.Pyun W.B., Hahn W., Kim D.S., Yoo W.S., Lee S.D., Won J.H., Rho B.S., Park Z.Y., Kim J.M., Kim S. Naked DNA expressing two isoforms of hepatocyte growth factor induces collateral artery augmentation in a rabbit model of limb ischemia. Gene Ther. 2010;17:1442–1452. doi: 10.1038/gt.2010.101. [DOI] [PubMed] [Google Scholar]
- 10.Hahn W., Pyun W.B., Kim D.S., Yoo W.S., Lee S.D., Won J.H., Shin G.J., Kim J.M., Kim S. Enhanced cardioprotective effects by coexpression of two isoforms of hepatocyte growth factor from naked plasmid DNA in a rat ischemic heart disease model. J. Gene Med. 2011;13:549–555. doi: 10.1002/jgm.1603. [DOI] [PubMed] [Google Scholar]
- 11.Henry T.D., Hirsch A.T., Goldman J., Wang Y.L., Lips D.L., McMillan W.D., Duval S., Biggs T.A., Keo H.H. Safety of a non-viral plasmid-encoding dual isoforms of hepatocyte growth factor in critical limb ischemia patients: a phase I study. Gene Ther. 2011;18:788–794. doi: 10.1038/gt.2011.21. [DOI] [PubMed] [Google Scholar]
- 12.Powell R.J., Goodney P., Mendelsohn F.O., Moen E.K., Annex B.H., HGF-0205 Trial Investigators Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF-0205 trial. J. Vasc. Surg. 2010;52:1525–1530. doi: 10.1016/j.jvs.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shigematsu H., Yasuda K., Iwai T., Sasajima T., Ishimaru S., Ohashi Y., Yamaguchi T., Ogihara T., Morishita R. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17:1152–1161. doi: 10.1038/gt.2010.51. [DOI] [PubMed] [Google Scholar]
- 14.Kibbe M.R., Hirsch A.T., Mendelsohn F.O., Davies M.G., Pham H., Saucedo J., Marston W., Pyun W.B., Min S.K., Peterson B.G. Safety and efficacy of plasmid DNA expressing two isoforms of hepatocyte growth factor in patients with critical limb ischemia. Gene Ther. 2016;23:306–312. doi: 10.1038/gt.2015.110. [DOI] [PubMed] [Google Scholar]
- 15.Nikol S., Baumgartner I., van Belle E., Diehm C., Visona A., Capogrossi M.C., Ferreira-Maldent N., Gallino A., Wyatt M.G., Wijesinghe L.D. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol. Ther. 2008;16:972–978. doi: 10.1038/mt.2008.33. [DOI] [PubMed] [Google Scholar]
- 16.Belch J., Hiatt W.R., Baumgartner I., Driver I.V., Nikol S., Norgren L., Van Belle E., TAMARIS Committees and Investigators Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–1937. doi: 10.1016/S0140-6736(11)60394-2. [DOI] [PubMed] [Google Scholar]
- 17.Powell R.J., Simons M., Mendelsohn F.O., Daniel G., Henry T.D., Koga M., Morishita R., Annex B.H. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. [DOI] [PubMed] [Google Scholar]
- 18.Morishita R., Aoki M., Hashiya N., Makino H., Yamasaki K., Azuma J., Sawa Y., Matsuda H., Kaneda Y., Ogihara T. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension. 2004;44:203–209. doi: 10.1161/01.HYP.0000136394.08900.ed. [DOI] [PubMed] [Google Scholar]
- 19.Suda H., Murakami A., Kaga T., Tomioka H., Morishita R. Beperminogene perplasmid for the treatment of critical limb ischemia. Expert Rev. Cardiovasc. Ther. 2014;12:1145–1156. doi: 10.1586/14779072.2014.955850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.