Abstract
Root-tissue colonizing bacteria demonstrated with multiple PGP traits from sorghum plants were identified as Ochrobactrum sp. EB-165, Microbacterium sp. EB-65, Enterobacter sp. EB-14 and Enterobacter cloacae strain EB-48 on the basis of 16S rRNA gene sequencing. Here, the in vivo experiments using ½-MS media and ½-MS media + 15% PEG 8000 (for inducing drought stress) indicated stress tolerance imparting ability of these rhizobacterial endophytes in a non-stay green and senescent genotype (R-16) of sorghum. In the experiment with sterile soilrite mix base, seed bacterization with these isolates showed improved plant growth specifically the roots, in terms of root length (~ 44.2 to 50.8% over controls), root dry weight (~ 91.3 to 99.8% over controls) and root surface area (~ 1 to 1.5 fold over controls) under drought stress. Rhizobacterial endophytes were successful, not only in providing better cellular osmotic adjustment in leaves (≥ 1-fold increase in proline accumulation over controls), but favorable physiological responses like Relative Water Content (RWC) and cell Membrane Stability Index (MSI) in the inoculated plants during the drought stress induction. Up-regulation of drought responsive genes like sbP5CS2 and sbP5CS1 was observed in these endophytes-treated plants as compared to untreated control and Escherichia coli DH5α (negative control)-treated plants. Interestingly, the stress imparting traits of rhizobacterial endophytes, including up-regulation of specific genes, were observed during sorghum seedling growth only under drought stresses. The results of this study lead to the conclusion that the potential endophytic rhizobacterial interactions can contribute to plant growth promotion as well as induced stress tolerance in sorghum.
Keywords: Rhizobacterial endophytes, Multi-trait PGPR, Proline, RWC, MSI, Sorghum, Drought tolerance
Introduction
Plants are naturally associated with microorganisms both externally and internally in various ways. Rhizosphere and phyllosphere-derived microorganisms, which are either bacteria or fungi, penetrate the interior of the plant and colonize the intercellular spaces and vascular tissues. This colonization causes beneficial/symbiotic, neutral or pathogenic interactions with the plants (Rosenblueth and Martínez-Romero 2006; Compant et al. 2010). In an endosymbiotic beneficial interaction, such as the symbiosis of legumes with rhizobia or the formation of arbuscular mycorrhiza with fungi, the development of an organized symbiotic structure is a common phenomenon, where the microsymbionts are intracellularly accommodated within a host membrane. Mycorrhizal fungi and rhizobial symbiosis provide plants with mineral nutrients and fixed nitrogen, respectively, in exchange for plant-derived carbon. On the contrary, “endophytic interactions” are characterized by the colonization of plant inter- and intracellular spaces by the diverse group of bacteria and fungi (Reinhold-Hurek and Hurek 2011; Govindasamy et al. 2018). Many of these microorganisms that reside within plant tissues (endophytes) do not cause any plant diseases but often contribute significantly to the nutrient supply of their host plant and can help the plant to overcome a variety of biotic or abiotic stresses. Though, few bacterial and fungal species have been well characterized, the identities and the functions of large majority of microbial species in the plant root–shoot microbiome (rhizosphere, root inter- and intracellular spaces) are yet unknown.
Plants are endophytically colonized by bacteria belonging to different phylogenetic groups such as Proteobacteria, Firmicutes, Actinobacteria, Bacteroides, etc. Under these phylogenetic groups, Pseudomonas, Enterobacter, Klebsiella, Serratia, Rhizobium, Bacillus, and Streptomyces are amongst those commonly found genera in different agricultural crops (Compant et al. 2010; Reinhold-Hurek and Hurek 2011; George et al. 2013; Govindasamy et al. 2014). The structural composition of endophytic bacterial communities depends on the genotype of the host, plant tissue and its vegetation stage. Also, the species composition is significantly influenced by the plant stress and soil types (Hirsch and Mauchline 2012; Govindasamy et al. 2017). Numerous reports have shown that endophytic bacteria can have the capacity to improve plant growth through various mechanisms. One such mechanism of systemic resistance against biotic stresses (insects, pathogens and nematodes), as induced by rhizobacterial/bacterial endophytic interactions, has been well documented (Kloepper et al. 2004; Senthilkumar et al. 2009; Govindasamy et al. 2011; Pieterse et al. 2014). Similarly, the concept of induced systemic tolerance (IST) has been proposed for rhizobacterial/bacterial endophyte-induced physical and chemical changes in plants leading to the enhanced tolerance against various abiotic stresses (Dimkpa et al. 2009; Yang et al. 2009). The possible mechanisms of IST include increasing host plant nutrition, promotion of plant growth by secretion of phytohormones, improved plant osmotic regulation, balancing the plant hormones and reactive oxygen species (ROS) production; and inducing expression of host plant stress-responsive genes (Sziderics et al. 2007; Govindasamy et al. 2011). Most of the above mechanisms that induce abiotic stress tolerance were documented in the model plants with cultured rhizobacteria/bacterial or fungal endophytes.
Scarcity of soil water is a severe environmental constraint to plant productivity and drought-induced loss in crop yield probably exceeds losses from all other plant growth constraints. Water is very essential for plant growth and has an effect on all stages from germination to maturity. Drought-induced stress reduces leaf size, stem extension and root proliferation, modifies metabolic processes or the uptake of water and nutrients, disturbs plant–water relations, reduces water-use efficiency and affects plant photosynthesis (Ahanger et al. 2014). Plants display a variety of physiological and biochemical responses at cellular and whole-organism levels toward prevailing drought stress, thus making it a complex phenomenon. Injury caused by reactive oxygen species to biological macromolecules under drought stress is among the major deterrents to plant growth. Plants display a range of mechanisms to withstand drought stress. The major mechanisms include curtailed water loss by increased diffusive resistance, enhanced water uptake with prolific and deep root systems and its efficient use, and smaller and succulent leaves to reduce the transpirational loss. Among the nutrients, potassium ions help in osmotic adjustment; silicon increases root endodermal silicification and improves the cell water balance (Ahanger et al. 2015; Ahanger and Agarwal 2017; Ahanger et al. 2017). Low-molecular weight osmolytes, including glycine betaine, proline and other amino acids, organic acids, and polyols, are crucial to sustain cellular functions under drought (Ahanger et al. 2014; Ahanger and Agarwal 2017). To mitigate drought stress-induced challenges in crop production, understanding the effects of drought on plants, identification of drought stress adapted crop plants and understanding their morphological and physiological adaptation mechanisms are highly essential.
Sorghum [Sorghum bicolor (L.) Moench] is the world’s fifth most important cultivated cereal after wheat, rice, maize and barley, with a global production of 60 million tons (Dicko et al. 2006). Naturally, sorghum is a very hardy and drought-tolerant crop. However, interactions with the associated rhizospheric and endophytic microbes may also partly contribute to the adaptation and tolerance mechanisms. From the culture-dependent studies, various sorghum rhizospheric-associated bacteria have been isolated (Pedersen et al. 1978; Budi et al. 1999; Zinniel et al. 2002) and characterized for their PGP activities (Zinniel et al. 2002; Ali et al. 2009; Funnell-Harris et al. 2013). Also, many phylogenetically diverse root-endophytic bacteria, associated with the drought-tolerant sorghum genotypes, were identified and characterized for their potential PGP activities (Govindasamy et al. 2017). Thus, some of the essential mechanisms of IST by the plant colonizing microorganisms against abiotic stresses have been reported (Dimkpa et al. 2009; Govindasamy et al. 2017, 2018). Drought stress is one of the most important abiotic factors in reducing the growth, development and grain yield of sorghum in the semi-arid agro-ecologies. Functional characterization of sorghum-associated microbial resources and their field application can be a better agronomic option to mitigate drought stress. Hence, we hypothesize that rhizobacterial endophytes associated with the drought-tolerant genotype of sorghum would impart stress tolerance through their PGP activities. Accordingly, the objective of the present work was formulated to evaluate these rhizobacterial endophytes for their ability to improve the plant growth and induce/impart tolerance against imposed drought stress, and to study the physiological and molecular mechanisms underlying the prospective drought tolerance as imparted by the rhizobacterial endophytes in sorghum.
Materials and methods
Bacterial strains and growth conditions
Four root-tissue colonizing bacterial isolates with multi-PGP trait were isolated and characterized from the surface-sterilized roots of drought-tolerant sorghum cultivars, Maldandi 35-1 and Phule Maulee (Govindasamy et al. 2017). In the present study, these isolates were evaluated for their ability to induce drought stress tolerance in a drought susceptible sorghum genotype (R-16). Tryptic soy agar (TSA, Himedia, Mumbai, India) medium was used for culturing these isolates at the temperature of 28 ± 2 °C. Escherichia coli DH5α (Thermo Fisher Scientific, Massachusetts, USA) was cultured in Luria–Bertani broth (LB broth, Himedia, Mumbai, India) incubated at 37 °C. The pure cultures of root-endophytic bacterial isolates and E. coli DH5α were maintained at − 80 °C as 15% glycerol stocks.
16S rRNA gene PCR–RFLP and sequence analyses
The genomic DNA of endophytic bacterial isolates was extracted following the protocols of Charles and Nester (1993) and Sambrook and Russell (2001). The universal bacterial primers 8F (5′-AGAGTTTGATCCTTGGCTCAG-3′) and 1492R (5′- GGTTACCTTGTTACGACTT-3′) were used for the amplification of 16S rRNA gene (Lane 1991). PCR was performed in a BioRad thermocycler 1000 (Biorad, USA) and the resultant PCR-16S rRNA products were gel purified using GeneJET™ Gel Extraction Kit (Fermentas, USA). The purified 16S rRNA gene products were subjected to restriction digestion with three restriction endonucleases—MspI (Fermentas, USA), RsaI and HhaI (NEB, UK) individually by incubating the reaction mixture at 37° C for 4 h. Digested PCR products were resolved in a 1.3% agarose gel and the profile was digitized using the Gel imaging system (SynGene, USA). Restriction digestion banding patterns were scored manually. The scores were analyzed and a combined genetic diversity dendrogram was constructed based on SAHN method for UPGMA using NTSYS software version 2.02e (Applied Biostatistics Inc., New York). Purified 16S rRNA gene amplicons of each endophytic bacterium were sequenced at SciGenom (SciGenom Labs Pvt. Ltd., India). BLASTn search was performed for nucleotide sequence homology studies and 16S rRNA gene sequences were deposited at GenBank, NCBI.
In vivo studies in tissue culture media under control and induced drought stress conditions
Seeds of sorghum genotype (R-16, a non-stay green and senescent) were surface sterilized with 10% NaOCl for 5 min followed by 70% ethanol sterilization for 1 min. The surface sterilization steps were interspersed with several washings of seeds in sterile distilled water. Cultures of the bacterial endophytes were grown in nutrient broth kept in an orbital incubator shaker (200 rpm at 28 °C) for 48 h. Bacterial cells were harvested after centrifugation at 10,000 rpm for 10 min at room temperature and resuspended in sterile distilled water. The surface-sterilized seeds were imbibed in the endophytic bacterial suspension (A600 nm = 1.0 equal to 1.0–1.2 × 108 cfu/ml) for 4 h. The bacterized seeds were transferred and placed on ½Murashige and Skoog (MS) media containing 0.3% Gelzan as a solidifying agent (Sigma-Aldrich, USA) with and without 15% PEG 8000 (Sigma-Aldrich, USA). In the media tubes, 3 seeds per tube were incubated at 25 ± 2 °C for initial 12 h dark period, followed by 16 h light and 8 h dark cycle of photoperiod in a growth chamber. To induce drought stress, 15% PEG 8000 (w/v) was used in ½ MS media, which is equivalent to the water potential (ψ) of ~ − 1.21 MPa. All treatments, including unbacterized control and E. coli DH5α (negative control) bacterized plants, were maintained in five replicates. The root and shoot length of the seedlings was recorded on 15th day after incubation in a growth chamber.
In vivo studies in sterile soilrite mix under watered and imposed drought stress conditions
Soilrite containing a mixture of perlite, peat and exfoliated vermiculite in equal ratio (1:1:1) was used as a growth base for pot filling. Soilrite mix in biohazard bags was sterilized 3–4 times by intermittent autoclaving with 3 days interval at 121 °C for 1 h and aseptically air dried for 5 days. Around 200 g of sterile, completely dry soilrite mix was filled in the pots of 250 g capacity. The bacterized seeds were sown in sterile soilrite mix at the rate of 6 seeds per pot and incubated at 25 ± 2 °C, with dark treatment for initial 12 h, followed by the growth in greenhouse conditions. In total, six treatments were maintained including the unbacterized control and negative control seeds treated with E. coli DH5α. Two sets of five replications were kept for each treatment: one set was watered regularly at every 3–4 days interval. Another set of treatments was watered twice: first at the time of sowing and second after 4 days during initial establishment. Drought stress was imposed by no addition of water and maintained for the remaining period until 30 days after sowing. Root length and shoot length of the seedlings were recorded on 30 days after sowing. The harvested root samples were stored in hot air oven for 7 days at 60 °C for complete desiccation and dry weight of the roots was recorded.
Determination of root surface area
Sorghum seedlings from well-watered and drought stress treatments in tissue culture media (15th day after incubation) and sterile soilrite mix (25th day after sowing) were harvested. Total root surface area (cm2) of three plants from each replication was determined based on the adsorption and desorption of nitrite as suggested by Ansari et al. (1995). Harvested roots of individual seedlings were immersed in 0.05 M NaNO2 solution for 10 s. Prior to this, a thorough washing was done to remove the particles adhering to the roots. Excessive nitrite droplets adhering to roots were drained and the roots were transferred to a fresh beaker containing known volume of double distilled water. The roots were swirled in water for 2–3 times with 15 min incubation period and the aliquots were then spectrophotometrically analyzed at 540 nm for nitrite content after adding 1% sulphanilamide and 0.01 N NEDD. A standard curve was prepared using roots of known surface area and the total root surface area (cm2) of sorghum seedlings was calculated using this standard curve (Ansari et al. 1995).
Estimation of free proline accumulation in leaves
Leaves were harvested from all treatment seedlings grown in tissue culture media (15th day after incubation) and sterile soilrite mix (25th day after sowing). Harvested leaf samples from 3 seedlings under each replication were pooled. Individual pooled samples (200 mg) were homogenized with aqueous sulfosalicylic acid (3% w/v). Filtered homogenate (2 mL) was added with equal volume each of acid ninhydrin and acetic acid and the reaction mixture was kept at 100 °C for 1 h. The ice cooled reaction mixture was mixed vigorously with 4 mL of toluene followed by aspiration of aqueous phase that was warmed to room temperature. The absorbance of chromophore containing toluene was recorded at 520 nm using the toluene as a blank. Free proline concentration of leaves (µmoles g−1 FW) was estimated from a standard curve (Bates et al. 1973).
Estimation of cell Membrane Stability Index (MSI) of leaves
Leaves from three plants under each replication were pooled and harvested leaf samples of all the treatments were processed to estimate MSI. Matured leaves were cut into small pieces (1 cm2 discs) and then placed (0.5 g) in test tubes having 10 mL of double distilled water in two sets. One set was kept at 40 °C for 30 min and another set at 100 °C in boiling water bath for 15 min and their respective electrical conductivity C1 and C2 were measured by conductivity meter (Adawa-260, Thermo Scientific, USA) and MSI was calculated using the formula, MSI = 1 − C1/C2 × 100 (Sairam 1994).
Measurement of relative water content (RWC) of leaves
One leaf each from three plants under each replication was harvested and pooled. Individual pooled leaf samples of all the treatments were processed to estimate RWC. Leaves were wrapped in aluminum foil immediately and the leaves were put in a plastic bag and kept in a cool place. Fresh weight was determined after 2 hours. Then, the leaves were placed in distilled water at room temperature (approximately 20 °C) for 3–4 h, then they were carefully dried to measure their turgid weight. The same leaves were wrapped in paper cover and desiccated in the hot air oven at 60 °C for 48 h and dry weight was recorded. Finally, relative water content (RWC) of the leaves was calculated using the equation, RWC = (fresh weight − dry weight)/(turgid weight – dry weight) × 100 (Cornic 1994).
Expression analysis of drought responsive genes
Surface-sterilized seeds of sorghum genotype R-16 were inoculated separately with rhizobacterial endophytes viz., EB-14, EB-48, EB-65 and EB-165 and drought stress was imposed using 15% PEG 8000 in ½ MS basal medium + 0.3% Gelzan (solidifying agent), whereas another set of seedlings was maintained without 15% PEG as representing non-stressed controls. A total of six treatments in three replications each were maintained including the unbacterized control and E. coli DH5α bacterized plants are considered as negative control. Leaves of the sorghum plants were harvested at 15 days after seed inoculation and total RNA was extracted using QIAGEN RNeasy extraction kit following manufacturer’s protocol (QIAGEN, Germany). Extracted total RNA was treated with DNase I to remove the DNA contamination. DNase-treated RNA was reverse transcribed using RevertAid H minus Strand cDNA synthesis kit (Thermo Fisher Scientific, USA). Semi-quantitative RT-PCR analysis was carried out with cDNA templates and drought responsive genes-specific primers, viz., sbDREB2A (DROUGHT RESPONSIVE ELEMENT BINDING PROTEIN 2A), sbCBL01 (Calcineurin B-like protein 01), sbP5CS1 (Pyrroline-5-carboxylate synthase 1), sbP5CS2 (Pyrroline-5-carboxylate synthase 2), taERD1 (EARLY RESPONSIVE TO DEHYDRATION 1) (Table 1). Expression status of housekeeping gene Actin was kept as an internal control for comparison. Similarly, the expression levels of selected drought responsive genes (sbP5CS1 and sbP5CS2) under drought stress were ascertained through quantitative reverse transcription PCR (qRT-PCR). Gene-specific reverse and forward primers were designed (Table 1) and qRT-PCR was performed using QuantiTect SYBR Green PCR Kit (Qiagen). qRT-PCR was carried out in Light Cycler® 480 II (Roche, Germany) and the amplification conditions were as follows: 95 °C for 2 min, 40 cycles of 95 °C for 10 s, 55 °C for 30 s and 72 °C for 30 s. Actin was served as a reference gene for normalization of expression data. Relative fold change in the gene expression was calculated by 2−ΔΔCt method.
Table 1.
List of drought responsive genes and RT-PCR primer pairs used in the study
| Gene name | Forward primer sequence | Reverse primer sequence | GenBank-NCBI accession number | References |
|---|---|---|---|---|
| Semi-quantitative RT-PCR | ||||
| taERD1 | 5′-GGC TCT CCTC CTG GTT ATG TTG GC -3′ | 5′-TCC GGA GTG GTC GTG CAC CGT A -3′ | At5g51070 | Sherameti et al. (2008) |
| sbDREB2A | 5′-CGC CGA GAC CAT CAA GTG RTG GAA RGA RC -3′ | 5′-GA AGC CGT CGT CGC CYT CYT GRT A-3′ | DQ403725 | Nayak et al. (2009) |
| sbCBL01 | 5′-CCT CCC AAA CCG CCT TCA -3′ | 5′-GAC ACC CCT TTT CTT GAC ATC G -3′ | FJ901259 | Zhang et al. (2011) |
| sbP5CS1 | 5′-TAA TGT TGG AAG AGG TGG C -3′ | 5′-CAA GGC CCT CAC TCT TGT -3′ | ACU65226 | Su et al. (2011) |
| sbP5CS2 | 5′-GCT CTG GGT AGA TTA GG- 3′ | 5′-GTG CTG CAG ATA CAT CTA T -3′ | ACU65227 | Su et al. (2011) |
| Actin | 5′-TCA CCA TCG GGG CAG AG -3′ | 5′-GGG AGG CAA GGA TGG AC -3′ | – | Su et al. (2011) |
| Quantitative RT-PCR | ||||
| sbP5CS1 | 5′-TCT CTC TGC GGT GCC TAT TT -3′ | 5′-AAT TGC ACG GGA ACA GAT TC -3′ | – | This study |
| sbP5CS2 | 5′-CCT CTT CCC AGC TTC TTG TG -3′ | 5′TAG CCA GAA GAC CGG CTA AA -3′ | – | This study |
Statistical analysis
Statistical analysis was carried out using the SPSS statistical software package version 16.0 (IBM SPSS, USA). Data regarding plant growth measurements like root length, shoot length, root dry weight and root surface area; plant physiological responses in terms of free proline content, MSI and RWC of leaves were analyzed by the analysis of variance (ANOVA) and the treatment means were subjected to the least significant difference (LSD) and compared by Duncan’s Multiple-Range Test (DMRT). All the hypotheses were tested at the 95% confidence interval (α = 0.05).
Results
Genetic distinctness and phylogenetic identity of multi-trait PGP rhizobacterial endophytes
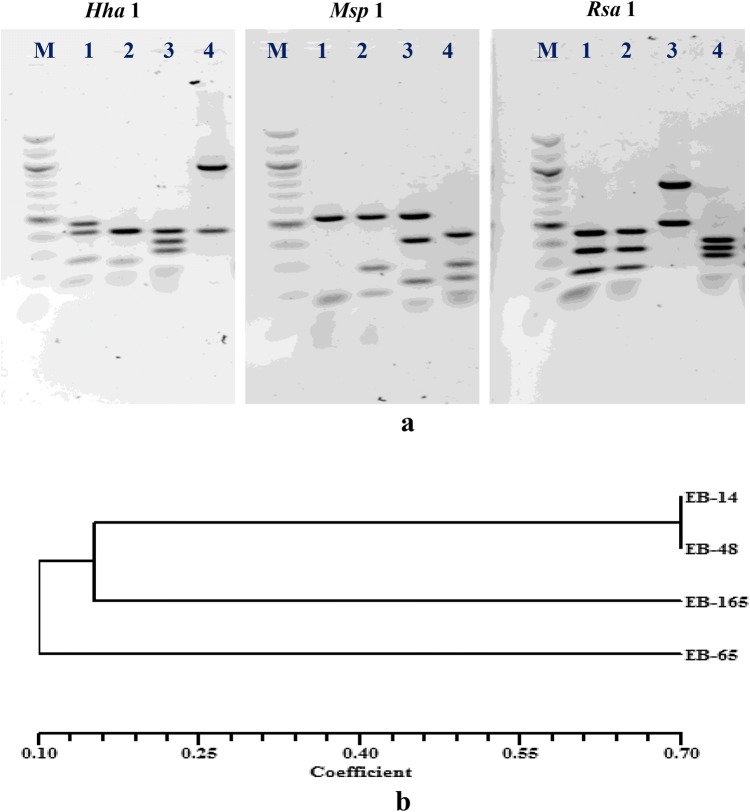
The four endophytic rhizobacteria were selected on the basis of their PGP traits from a total of 280 isolates characterized from the roots of drought-tolerant sorghum genotypes (Govindasamy et al. 2017). Isolate EB-48 showed three PGP traits such as P-solubilization, siderophore and IAA production. Isolate EB-65 showed PGP traits like N-fixation, IAA production and ACC deaminase activities. Isolates EB-14 and EB-165 showed four and five PGP traits, respectively (Table 2). A combined genetic diversity dendrogram was generated based on 16S rRNA gene PCR–RFLP and similarity coefficient analysis (Fig. 1a, b). The isolates EB-14 and EB-48 clustered together with similarity coefficient of 0.70 showing their close genetic relatedness, whereas isolate EB-165 formed a separate cluster with similarity coefficient of 0.20, thus showing its genetic distinctness. Isolate EB-65 was an outgroup showing genetic distinctness from all other three isolates. Partial 16S rRNA gene sequencing of these bacterial endophytes (GenBank Accessions KU258056–KU258059) and BLAST analysis further confirmed their distinctness as shown in RFLP dendrogram (Fig. 1b) and helped in tracing their phylogenetic identity/taxonomic position (Table 2). 16S rRNA gene sequence of isolate EB-14 was identical to many sequences, belonging to the genera Enterobacter, Klebsiella and Leclercia (100% identity and 100% coverage), whereas the isolate EB-48 showed 99% sequence similarity with several strains of Enterobacter cloacae (for example, 99.75% with Enterobacter cloacae strain KNR2.7) which showed the phylogenetic identity of γ-Proteobacteria and Gram-negative bacteria. 16S rRNA gene sequence of isolate EB-165 was 99% identical to the genus Ochrobactrum (for example, 99.22% with strain of Ochrobactrum sp. P14P1), which belonged to the phylogenetic group of α-Proteobacteria and Gram-negative bacteria. Whereas 16S rRNA gene sequence of EB-65 was in close relationship with the genus Microbacterium (for example, 99.87% with strain of Microbacterium sp. 8 SY-2016) belonging to the group of Actinobacteria with high-G + C containing Gram-positive bacteria (Table 2).
Table 2.
Potential plant growth-promoting traits of four rhizobacterial endophytes isolated from drought-tolerant sorghum cultivars and phylogenetic identity based on their partial 16S rRNA gene sequence analysis
| Isolate | Sorghum cultivar | Plant growth promoting traits† | Closest match (NCBI acc. no.); % sequence similarity | Phylogenetic group; Gram reaction | GenBank submission (NCBI acc. no.) | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | P | S | I | A | |||||
| EB-14 | Maldandi 35-1 | + | + | + | + | − | 100% with many strains of genera Enterobacter, Klebsiella and Leclercia. | γ-Proteobacteria; Gram negative | KU258056 |
| EB-48 | Maldandi 35-1 | − | + | + | + | − | Many strains of the genus Enterobacter; 99.75% with Enterobacter cloacae strain KNR2.7 (MG274281) | γ-Proteobacteria; Gram negative | KU258057 |
| EB-65 | Maldandi 35-1 | + | − | − | + | + | Many strains of the genus Microbacterium; 99.87% with Microbacterium sp. 8 SY-2016 (LT009507) | Actinobacteria; High-G + C Gram positive | KU258058 |
| EB-165 | Phule Maulee | + | + | + | + | + | Many strains of the genus Ochrobactrum; 99.22% with Ochrobactrum sp. strain P14P1 (KX673853) | α2-Proteobacteria; Gram negative | KU258059 |
N nitrogen fixation, P phosphorus solubilization, S- siderophore production, I indole acetic acid production, A 1-amino cyclopropane-1-carboxylate deaminase activity (data from Govindasamy et al. 2017)
†+ Positive for the trait; − negative for the trait
Fig. 1.
Genetic distinctness of multi-PGP root-bacterial endophytes as revealed from PCR–RFLP gel profile of 16S rRNA genes digested with restriction enzymes Hha1, Msp1 and Rsa1 (a) and combined genetic diversity dendrogram constructed using NTSYS software version 2.02e (b)
Rhizobacterial endophytes enhance plant growth and impart tolerance to PEG-induced drought stress
Studies using tissue culture media showed that all these four rhizobacterial endophytes improved the shoot length (cm) of bacterized seedlings, but were on par with the negative and absolute controls under normal (½-MS media alone) conditions. Inoculation with EB-165 resulted in maximum shoot length followed by EB-14, which was on par with other two isolates EB-48 and EB-65. During drought stress (½ MS-media + 15% PEG), however, a significant improvement in the shoot length was noticed due to these endophytic rhizobacteria, compared to the seedlings that were un-inoculated or those inoculated with E. coli strain DH5α (Fig. 2a). Rhizobacterial endophytes-treated sorghum seedlings, when grown in ½ MS media (non-stressed conditions), showed increase in root length, but were statistically on par with both the controls. However, under drought stress (½-MS media + 15% PEG), a significant increase in root length (of more than 6 cm/plant) was observed with all the four endophytic rhizobacterial treatments over controls. The highest root length was observed in EB-14 (6.85 ± 1.05 cm/plant) followed by EB-65 (6.30 ± 0.34 cm/plant) and EB-165 (6.22 ± 0.50 cm/plant) bacterized seedlings (Table 3).
Fig. 2.
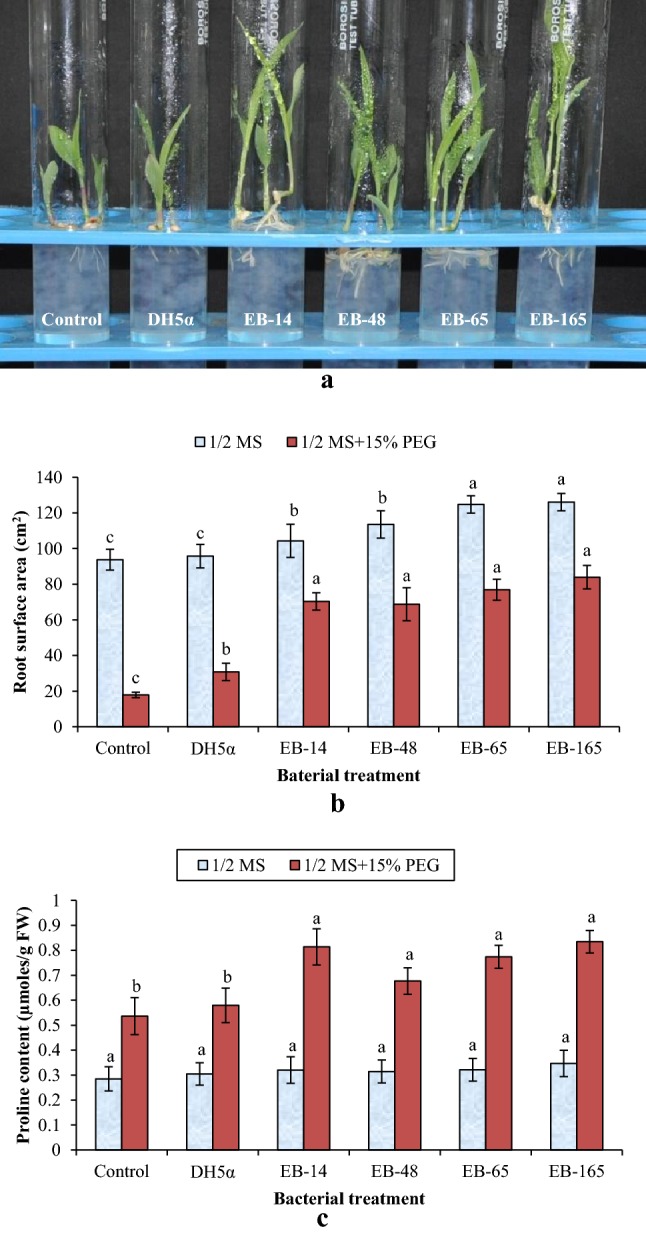
The effect of multi-trait PGP rhizobacterial endophytes on the shoot growth of sorghum (genotype R-16) seedlings (at 5th day after incubation in the growth chamber) under drought stress condition (a). Effect of multi-trait PGP rhizobacterial endophytes on root surface area (b) and free proline accumulation in leaves (c) of sorghum grown under ½ MS and ½ MS + 15% PEG 8000 (induced drought stress) conditions. Based on Duncan’s Multiple-Range Test (DMRT), the bars in graph with the same alphabet letter indicate non-significance at P ≥ 0.05
Table 3.
Effect of multi-PGP trait containing endophytic bacterial isolates for their growth enhancing ability in sorghum genotype (R-16, a non-stay green and senescent genotype) under normal and induced drought stress conditions
| Treatment | Plant growth parameters in tissue culture medium† | Plant growth parameters in sterile soilrite base† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shoot length (cm/plant) | Root length (cm/plant) | Shoot length (cm/plant) | Root length (cm/plant) | Root dry weight (g/plant) | ||||||
| ½ MS | ½ MS + 15% PEG | ½ MS | ½ MS + 15% PEG | Watered | Drought stressed | Watered | Drought stressed | Watered | Drought stressed | |
| Control | 15.56 ± 0.98ab | 11.31 ± 0.50b | 10.68 ± 0.92b | 4.34 ± 0.68b | 30.70 ± 1.86a | 25.18 ± 1.40b | 25.25 ± 1.30b | 22.43 ± 1.00c | 1.13 ± 0.01a | 1.11 ± 0.01b |
| E.coli DH5α | 14.33 ± 1.52b | 11.03 ± 0.65b | 10.26 ± 0.86b | 4.21 ± 0.50b | 31.73 ± 2.23a | 26.19 ± 1.45b | 25.43 ± 1.14b | 25.10 ± 1.60b | 1.13 ± 0.02a | 1.12 ± 0.01b |
| EB-14 | 16.93 ± 1.60a | 13.39 ± 0.34a | 12.31 ± 1.00a | 6.85 ± 1.05a | 33.50 ± 2.14a | 29.40 ± 1.00a | 26.33 ± 1.60b | 34.27 ± 3.57a | 1.14 ± 0.01a | 2.22 ± 0.04a |
| EB-48 | 16.70 ± 2.14ab | 12.93 ± 0.50a | 11.63 ± 0.98ab | 6.06 ± 0.34a | 33.65 ± 2.52a | 28.86 ± 1.14a | 26.50 ± 1.45b | 34.63 ± 2.23a | 1.13 ± 0.04a | 2.22 ± 0.03a |
| EB-65 | 16.46 ± 1.76a | 13.64 ± 0.58a | 11.85 ± 1.30ab | 6.30 ± 0.34a | 34.27 ± 4.57a | 29.67 ± 1.30a | 27.16 ± 1.57b | 35.10 ± 2.25a | 1.14 ± 0.02a | 2.23 ± 0.03a |
| EB-165 | 16.53 ± 1.45a | 13.81 ± 0.42a | 12.20 ± 1.14a | 6.22 ± 0.50a | 34.00 ± 3.05a | 29.10 ± 1.76a | 27.88 ± 2.00a | 35.83 ± 1.52a | 1.15 ± 0.03a | 2.24 ± 0.02a |
| P value | 0.052 | 0.036 | 0.051 | 0.015 | 0.063 | 0.043 | 0.040 | 0.046 | 0.059 | 0.011 |
Based on Duncan’s multiple-range test (DMRT), values with the same superscripts (Alphabet letter) within column indicate no significant differences at P ≥ 0.05
†Values are the means of five replications ± standard error
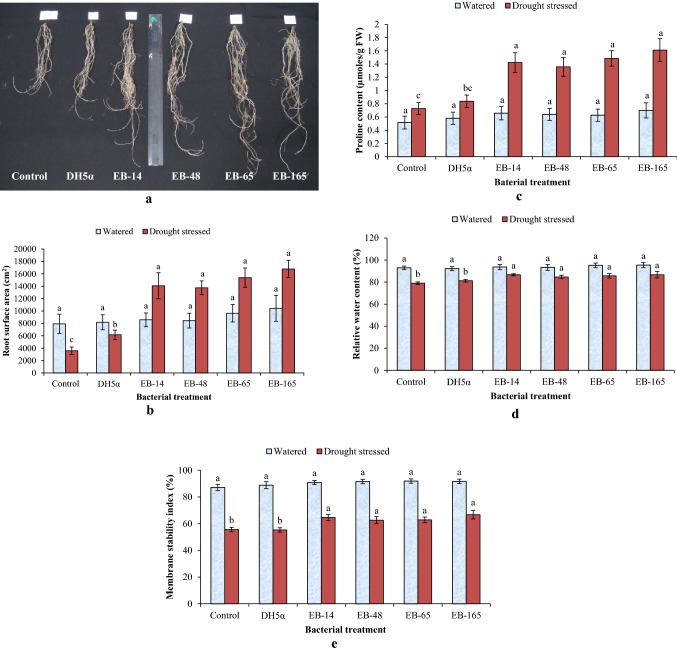
In addition to the increased root length, a significant improvement in the root surface area (cm2) was also observed in sorghum plants treated with rhizobacterial endophytes containing multiple PGP traits. The highest total root surface area of 83.6 ± 6.11 cm2 and 76.89 ± 5.88 cm2 was noticed in plants treated with EB-165 and EB-65, respectively, under drought stress (Fig. 2b). A significant increase in total root surface area was observed with all the four endophytic rhizobacterial treatments as compared to control. Under non-stressed conditions (½-MS media alone), endophytic isolates EB-48 and EB-14-treated seedlings recorded lower root surface area when compared to isolates EB-65 and EB-165. However, the total root surface area increased significantly in all the four endophytic rhizobacterial treatments as compared to both controls (negative control bacterized with the strain of E. coli DH5α and un-inoculated control). The proline accumulation in shoots, particularly on leaves, is an important physiological indicator of the level of stress tolerance in plants. Under non-stressed conditions (½-MS media alone), seedlings treated with all the four rhizobacterial endophytes showed increase in proline content of > 0.31 ± 0.05 μmol/g fresh weight of leave tissues (Fig. 2c). However, proline accumulation in leaves of these treatments was not significant over negative and absolute controls. Under drought stress (½-MS media + 15% PEG), free proline accumulation in leaves of all four endophytic rhizobacteria-treated seedlings was statistically on par with each other. It was also found to be significantly higher as compared to the negative control and un-inoculated control seedlings.
Rhizobacterial endophytes enhance growth and impart tolerance to drought stress in sorghum grown in sterile soilrite mix
In the in vivo experiments using sterile soilrite mix, all the four rhizobacterial endophytes showed an increase in shoot length of bacterized seedlings, but were on par with the negative and absolute control seedlings under regularly watered conditions. Isolate EB-65 showed increased shoot length followed by EB-165, which was on par with other two isolates EB-48 and EB-14. However, under drought stress the improvement in shoot length by these rhizobacterial endophytes was significant compared to the negative control seedlings bacterized with E. coli strain DH5α and un-inoculated control (Table 3). Under sufficiently watered conditions, root length of sorghum seedlings treated with endophytic rhizobacteria EB-165 showed significant increase, whereas increase in the root length of seedlings treated with three other endophytic isolates was not as significant compared to both the controls. Under drought stress, there was a significant increase in root length (of more than 34 cm/plant) in all four endophytic rhizobacterial treatments as compared to the control seedlings (Fig. 3a). The highest root length was observed when sorghum seeds treated with isolate EB-165 (35.83 ± 1.52 cm/plant) followed by EB-65 (35.10 ± 2.25 cm/plant) and EB-48 (34.63 ± 2.23 cm/plant). A slight increase in root dry weight of harvested seedlings was observed in the treatments of endophytic rhizobacteria EB-165. It was statistically on par with the seedlings treated with other three isolates and does not show any significant difference over both the controls under regularly watered conditions. However, under drought stress, the improvement in root biomass/root dry weight by these rhizobacterial endophytes in sorghum seedlings was significantly high compared to the seedlings of negative control and un-inoculated control (Table 3).
Fig. 3.
The effect of multi-PGP trait root-bacterial endophytes on root growth of sorghum (genotype R-16) seedlings under drought stress condition (a). Effect of multi-trait PGP rhizobacterial endophytes on root surface area (b) and free proline accumulation in leaves (c) relative water content or RWC (d) and membrane stability index or MSI (e) of the sorghum grown in soilrite mix under watered and drought stress conditions. Based on Duncan’s multiple-range test (DMRT), the bars in graph with the same alphabet letter indicate non-significance at P ≥ 0.05
A significant improvement in the total root surface area was also observed in sorghum plants inoculated with all these four rhizobacterial endophytes. Under drought stress, higher root surface area of 16,786 ± 1402 cm2 and 15,378 ± 1562 cm2 was found in plants inoculated with endophytic isolates EB-165 and EB-65, respectively. These treatments were highly significant as compared to both the controls. However, under regularly watered conditions, the total root surface area of all four endophytic isolates-treated seedlings grown in soilrite mix has not shown any significant increase as compared to both the controls (Fig. 3b). The proline accumulation in the leaves of treated seedlings was quite low under regularly watered conditions and showed non-significant values (Fig. 3c). However, the proline accumulation increased in rhizobacterial endophytes-treated seedlings (> 1.35 ± 0.14 μmol/g fresh weight of leave tissues) under drought stress. These values of proline increase were highly significant (p ≥ 0.05) as compared to control seedlings (Fig. 3c).
In addition to free proline content, the results of other physiological parameters such as relative water content (RWC) and cell membrane stability index (MSI) showed significant improvement in the seedlings treated with rhizobacterial endophytes under imposed drought stress. Increased RWC was observed with all the four endophytic rhizobacteria-treated sorghum leaves as compared to the leaves of negative (E. coli strain DH5α) and un-inoculated control seedlings (Fig. 3d). Similarly, improvement in cell Membrane Stability Index (MSI) was recorded in all the four endophytic rhizobacteria-treated sorghum leaves (Fig. 3e). However, under regularly watered conditions, both the RWC and MSI did not show any significant difference between endophytic rhizobacteria-treated sorghum leaves and leaves of both the controls.
Differential expression of drought responsive genes in sorghum
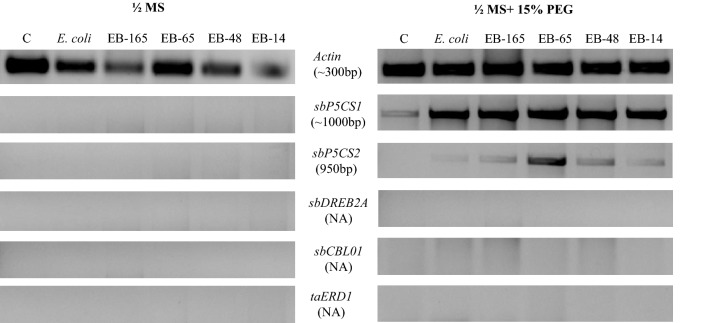
In the absence of moisture stress, transcripts of drought responsive genes were not detected or invisible in sorghum (R-16) seedlings of control and bacterized treatments. However, under the influence of drought stress, low level of expression of sbP5CS1 was recorded in the untreated control and relatively high level of expression (up-regulation) was observed in sorghum seedlings treated with rhizobacterial endophytes (Fig. 4). Relatively high levels of expression (up-regulation) of sbP5CS 2 were observed when sorghum was inoculated with Microbacterium sp. EB-65 followed by Ochrobactrum sp. EB-165, Enterobacter cloacae strain EB-48 and Enterobacter sp. EB-14 under drought. However, the expression of this gene was conspicuously less or invisible in the seedlings inoculated with E. coli DH5α and in the un-inoculated control seedlings. Interestingly, other drought responsive genes such as sbDREB2A, sbCBL01 and taERD1 showed no perceptible expression in the sorghum seedlings grown under control and drought conditions. From the qRT-PCR results, the relative quantification of differentially expressed genes showed that 12-fold increased expression of sbP5CS 2 in EB-65 inoculated seedlings, followed by ninefold high expression in EB-48 inoculated seedlings under drought stress (Fig. 5). However, among the various endophytic bacterial treatments, sbP5CS 1 exhibited relatively high expression of eightfold in EB-165-inoculated seedlings under drought stress (Fig. 5). Both genes, sbP5CS 1 and sbP5CS 2, showed significantly much higher expression levels in the endophytes with PGP traits-inoculated seedlings as compared to E. coli strain DH5α-inoculated seedlings (negative control).
Fig. 4.
Semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of various drought responsive genes from the leaves of sorghum (genotype R-16) under ½ MS and ½ MS + 15% PEG 8000 (drought stress) conditions (Lane M: 100 bp marker; Lane 1 Un-inoculated control; Lane 2 inoculated with E. coli DHα; Lane 3 inoculated with Ochrobactrum sp. EB-165; Lane 4 inoculated with Microbacterium sp. EB-65; Lane 5 inoculated with Enterobacter cloacae strain EB-48; Lane 6 inoculated with Enterobacter sp. EB-14). NA not amplified
Fig. 5.
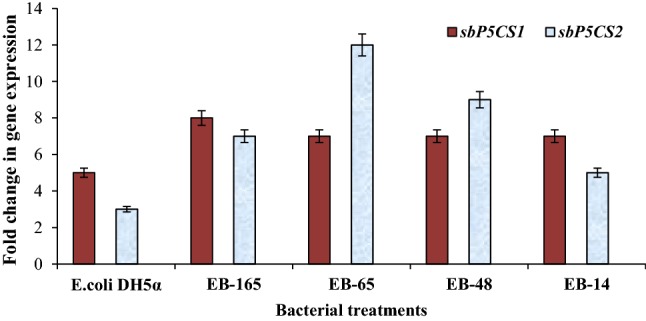
qRT-PCR analysis for relative quantification of expression (fold changes in the expression levels) of two drought responsive genes, sbP5CS 1 and sbP5CS 2 in the leaves of sorghum (genotype R-16) under drought stress (½ MS + 15% PEG 8000) conditions
Discussion
Utilization of plant-associated microbial communities is one of the potential ways to improve the productivity of food crops. Endophytic bacterial communities play key roles in improving the plant’s nutrition and growth (Rosenblueth and Martínez-Romero 2006; Berg 2009). Root-endophytic bacterial diversity and their respective metabolic functions are influenced by soil characteristics, plant species and its genetic makeup (Hirsch and Mauchline 2012; Govindasamy et al. 2017). Understanding the plant–endophyte interactions is very essential because these microbes are selectively recruited by each plant species to colonize their interior tissues, which in turn provides tolerance through IST (Dimkpa et al. 2009; Yang et al. 2009; Reinhold-Hurek and Hurek 2011; Marasco et al. 2012; Govindasamy et al. 2018). In the present study, sorghum rhizobacterial endophytes, characterized with multi-PGP traits, were evaluated for their ability to promote plant growth and to provide drought stress tolerance in a non-stay green and senescent (drought susceptible) genotype (R-16) of sorghum. These rhizobacterial endophytes belong to phylogenetically different groups, namely, α-& γ-Proteobacteria and Actinobacteria (Govindasamy et al. 2017). Previous studies have shown significant PGP activities of isolated endophytic rhizobacteria of the genus Enterobacter and Microbacterium (Zinniel et al. 2002; Rosenblueth and Martínez-Romero 2006; Berg 2009; Funnell-Harris et al. 2013). Another important endophytic rhizobacterial genus is Ochrobactrum sp. EB-165 which also possesses multi-PGP activity. In desert soil where the availability of moisture is a major limiting factor, Ochrobactrum has been identified as one of the most abundant genus within Proteobacteria. This bacterium is known for its ambivalent functional interactions along with the potential plant growth promotion effects (Berg 2009; Marasco et al. 2012).
Early plant establishment and optimal plant growth under the stress conditions can contribute to improved tolerance and successful adaptation. In the plant tissue culture media, seedlings treated with rhizobacterial endophytes showed significant increase in shoot length (~ 15.71%–23.63% over controls), root length (~ 41.75%–60.20% over controls) and root surface area (~ more than 1-fold over controls) under PEG-induced drought stress (1/2-MS + 15% PEG media) over negative control and un-inoculated control seedlings. Hence, measurement of cellular level osmolytes such as proline, betaine and/or sugars is pertinent for the identification of crop genotypes that are tolerant to abiotic stresses (Shahid et al. 2012). The proline accumulation in shoots, particularly in leaves, is an important physiological indicator of stress tolerance. More than twofold increase in free proline content in the leaves of seedlings inoculated with rhizobacterial endophytes under PEG-induced drought stress was observed as compared to the non-stressed seedlings. Under induced drought stress conditions, proline content in the leaves of seedlings inoculated with endophytic rhizobacteria showed 0.5- to 1-fold increase over control seedlings. Hmida-Sayari et al. (2005) observed that increased proline accumulation in leaves provided salt tolerance in transgenic tomato plants. Plant growth-promoting endophyte Burkholderia phytofirmans PsJN interacts with grapevine plantlets and increases the levels of proline, starch and phenolics leading to enhanced cold tolerance (Barka et al. 2006). Similar increase in free proline content in the leaves of sweet pepper plants inoculated with endophytic bacteria due to PEG-induced water deficit stress has also been reported (Sziderics et al. 2007).
In the sterile soilrite mix, improvement in plant growth parameters mainly root length (~ 44.2 to 50.8% over controls), root surface area (~ 1–1.5 fold over controls) and root dry mater (~ 91.3 to 99.8% over controls) were significant in the seedlings inoculated with rhizobacterial endophytes. Our findings are supported by the observations of Ali et al. (2009) who observed that sorghum rhizosphere-associated bacteria Pseudomonas spp. increased the early establishment and plant growth promotion, and enhanced stress tolerance of the seedlings to the elevated temperatures. Similarly, it was reported that stress-tolerant genotypes of crops were successful in maintaining higher root growth and producing maximum root biomass. This in turn improved water uptake and mineral nutrient utilization in these genotypes growing under drought and salt stress (Sairam 1994; Shahid et al. 2012; Singh et al. 2013). In addition to the root growth, cellular osmotic adjustment through accumulation of proline in the leaves of seedlings treated with rhizobacterial endophytes increased more than 1–1.5-fold under drought stress. However, this was more than 2–2.5-fold increase over negative control and un-inoculated plants grown under regularly watered conditions. Interestingly, proline content in leaves of stressed seedlings bacterized with the strain of E. coli DH5α increased over control as well as unstressed E. coli DH5α-inoculated control. Previously, it has been shown that proline accumulated in leaf tissues of Arabidopsis thaliana upon recognition of a virulent races of Pseudomonas syringae pv. tomato (Fabro et al. 2004). Therefore, proline accumulation in unstressed E. coli DH5α may be the result of initial biotic stress caused during plant–bacterial interactions. Proline increase in the stress imposed E. coli DH5α-treated leaves could be attributed to the additive effect of biotic stress and drought stress. However, increased proline accumulation in leaves at later stages could result from potential plant–endophytic bacteria interactions contributing to improved stress tolerance (Hmida-Sayari et al. 2005; Barka et al. 2006; Sziderics et al. 2007). Physiological responses to stress like relative water content (RWC) and cell membrane stability index (MSI) showed significant improvement in the seedlings inoculated with rhizobacterial endophytes under drought. It’s in accordance with previous reports on improvement of RWC under gradual drought stress and during stress recovery in two of the medicinal species, Plantago ovata and P. psyllium (Rahimi et al. 2010). Physiological traits like high MSI and increased accumulation of osmolytes were used in the screening of stress-tolerant genotypes of wheat and pea to drought and salt stress, respectively (Sairam 1994; Shahid et al. 2012). Furthermore, to corroborate the changes in physiological drought tolerance of sorghum R-16 seedlings (non-stay green and senescent genotype), gene expression study was undertaken with drought imposed plants attributable to endophytic rhizobacteria inoculations. Plants inoculated with rhizobacterial endophytes showed high expression levels (up-regulation) of sbP5CS 1 and sbP5CS 2 genes which may be responsible for proline accumulation in the leaves of sorghum seedlings under drought stress. It also appears that inoculation of endophytes per se did not activate any drought tolerance trait in the control sorghum seedlings; nevertheless, upon drought, expressions of drought responsive genes are activated/up-regulated by the rhizobacterial endophytes which may conferred drought stress tolerance in the senescent sorghum seedlings. From the present study, it is evident that multi-trait PGP rhizobacterial endophytes viz, Ochrobactrum sp. EB-165, Microbacterium sp. EB-65, Enterobacter cloacae strain EB-48, and Enterobacter sp. EB-14 triggers stress-responsive gene expression only under stress induced conditions. Similar molecular responses of differential expression of abiotic stress related genes were observed in sweet pepper inoculated with the endophytic bacteria and wheat inoculated with endophytic fungus Piriformospora indica (Sziderics et al. 2007; Sherameti et al. 2008). Thus, in the present study, in vivo plant experiments clearly establish the potential of specific plant-endophytic rhizobacterial interactions in improving growth promotion. Favorable physiological responses/changes caused by multi-PGP traits containing rhizobacterial endophytes in a genotype susceptible to stress can contribute to induced stress tolerance and better adaptation of plants to drought stress.
Conclusion
Four rhizobacterial endophytes isolated from the drought-tolerant sorghum cultivars were characterized with various functional attributes that promote plant growth. These endophytes are genetically distinct presenting different phylogenetic identity and affinity toward α and γ-Proteobacteria and Actinobacteria. Our results indicated that these endophytes enhanced plant growth and potentially modulate the physiological responses of the sorghum R-16 seedlings (non-stay green and senescent genotype), to induce drought tolerance. In addition to the changes in plant physiological drought tolerance mechanisms, up-regulation of specific abiotic stress-responsive genes was observed only when the inoculated sorghum seedlings were under imposed drought stress. This work thus helps us to understand the molecular mechanisms of multi-trait PGP rhizobacterial endophyte-mediated tolerance to drought stress. As a future perspective, this study has also generated valuable information for the development of rhizobacterial endophytes or its consortia-based bio-inoculants to mitigate the drought stress in sorghum which is generally cultivated in resource poor, rain-fed and dry land regions.
Acknowledgements
The first author expresses his gratitude to Dr. H S Talwar, ICAR-Indian Institute of Millets Research, Hyderabad, India for providing seeds of sorghum stay green and non-stay green genotypes. Thanks are due to Dr. S. V. Ramesh, ICAR-CPCRI, Kasaragod, India and Dr. P. Panneerselvam, ICAR-NRRI, Cuttack, India for critically going through the manuscript. We are grateful to the Director, ICAR-National Institute of Abiotic Stress Management, Baramati for providing necessary laboratory facilities. The authors are also thankful to the Indian Council of Agricultural Research, New Delhi for funding this study (Project Ref. No.: IXX08578).
Author contributions
VG conceived and designed the study, performed the experiments and data analysis, and wrote the manuscript. PG maintained the endophyte seed stocks, grew plants, helped in data recording, growth analysis of plant samples and wrote the manuscript. LA grew plants, helped in data recording and molecular analysis of plant samples. MK and SKR helped in physiological measurements and data analysis. KA helped in editing the manuscript. JR supervised the study and revised the manuscript. PSM coordinated the project. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they do not have any conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Ahanger MA, Agarwal RM. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L) Protoplasma. 2017;254:1471–1486. doi: 10.1007/s00709-016-1037-0. [DOI] [PubMed] [Google Scholar]
- Ahanger MA, Tyagi SR, Wani MR, Ahmad P. Drought tolerance: role of organic osmolytes, growth regulators, and mineral nutrients. In: Ahmad P, Wani M, editors. physiological mechanisms and adaptation strategies in plants under changing environment. New York: Springer; 2014. pp. 25–55. [Google Scholar]
- Ahanger MA, Agarwal RM, Tomar NS, Shrivastava M. Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L. cultivar Kent) J Plant Int. 2015;10:211–223. [Google Scholar]
- Ahanger MA, Tittal M, Mir RA, Agarwal RM. Alleviation of water and osmotic stress-induced changes in nitrogen metabolizing enzymes in Triticum aestivum L. cultivars by potassium. Protoplasma. 2017;254:1953–1963. doi: 10.1007/s00709-017-1086-z. [DOI] [PubMed] [Google Scholar]
- Ali SZ, Sandhya V, Grover M, Kishore N, Rao LV, Venkateswarlu B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol Fert Soils. 2009;46:45–55. [Google Scholar]
- Ansari SA, Kumar P, Gupta BN. Root surface measurements based on adsorption and desorption of nitrite. Plant Soil. 1995;175:133–137. [Google Scholar]
- Barka AE, Nowak J, Clément C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium Burkholderia phytofirmans strain PsJN. Appl Environ Microbiol. 2006;72:7246–7252. doi: 10.1128/AEM.01047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates L, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Berg G. Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotech. 2009;84:11–18. doi: 10.1007/s00253-009-2092-7. [DOI] [PubMed] [Google Scholar]
- Budi SW, van Tuinen D, Martinotti G, Gianinazzi S. Isolation from the Sorghum bicolor mycorrhizosphere of a bacterium compatible with arbuscular mycorrhiza development and antagonistic towards soil borne fungal pathogens. Appl Environ Microbiol. 1999;65:5148–5550. doi: 10.1128/aem.65.11.5148-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles TC, Nester EW. A chromosomally encoded two-component sensory transduction system is required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1993;175:6614–6625. doi: 10.1128/jb.175.20.6614-6625.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S, Clément C, Sessitsch A. Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem. 2010;42:669–678. [Google Scholar]
- Cornic G. Drought stress and high light effects on leaf photosynthesis. In: Baker NR, Bowyer JR, editors. Photo inhibition of Photosynthesis: from molecular mechanisms to the field. Oxford: BIOS Oxford publications; 1994. pp. 297–313. [Google Scholar]
- Dicko MH, Gruppen H, Traoré AS, Voragen AG, van Berkel WJ. Sorghum grain as human food in Africa: relevance of starch content and amylase activities. Afr J Biotechnol. 2006;5:384–395. [Google Scholar]
- Dimkpa C, Weinand T, Asch F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009;32:1682–1694. doi: 10.1111/j.1365-3040.2009.02028.x. [DOI] [PubMed] [Google Scholar]
- Fabro G, Kovacs I, Pavet V, Szabados L, Alvarez ME. Proline accumulation and AtP5CS2 gene activation are induced by plant–pathogen incompatible interactions in Arabidopsis. Mol Plant Microbe Interact. 2004;17:343–350. doi: 10.1094/MPMI.2004.17.4.343. [DOI] [PubMed] [Google Scholar]
- Funnell-Harris DL, Sattler SE, Pedersen JF. Characterization of fluorescent Pseudomonas spp. associated with roots and soil of two sorghum genotypes. Eur J Plant Pathol. 2013;136:469–481. [Google Scholar]
- George P, Gupta A, Gopal M, Thomas L, Thomas GV. Multifarious beneficial traits and plant growth promoting potential of Serratia marcescens KiSII and Enterobacter sp. RNF 267 isolated from the rhizosphere of coconut palms (Cocos nucifera L.) World J Microbiol Biotechnol. 2013;29:109–117. doi: 10.1007/s11274-012-1163-6. [DOI] [PubMed] [Google Scholar]
- Govindasamy V, Senthilkumar M, Magheshwaran V, Kumar U, Bose P, Sharma V, Annapurna K. Bacillus and Paenibacillus spp.: potential PGPR for sustainable agriculture. In: Maheshwari DK, editor. Plant growth and health promoting bacteria. Microbiology monographs. Berlin: Springer; 2011. pp. 333–364. [Google Scholar]
- Govindasamy V, Franco CMM, Gupta VV. Endophytic actinobacteria: diversity and ecology. In: Verma VC, Gange A, editors. Advances in endophytic research. Berlin: Springer; 2014. pp. 27–59. [Google Scholar]
- Govindasamy V, Raina SK, George P, Kumar M, Rane J, Minhas PS, Vittal KPR. Functional and phylogenetic diversity of cultivable rhizobacterial endophytes of sorghum [Sorghum bicolor (L.) Moench] Antonie Van Leeuwenhoek. 2017;110:925–943. doi: 10.1007/s10482-017-0864-0. [DOI] [PubMed] [Google Scholar]
- Govindasamy V, George P, Raina SK, Kumar M, Rane J, Annapurna K. Plant-associated microbial interactions in the soil environment: role of endophytes in imparting abiotic stress tolerance to crops. In: Bal SK, Mukherjee J, Choudhury BU, Dhawan AK, editors. Advances in crop environment interaction. Heidelberg, Germany: Springer, GmbH; 2018. pp. 245–284. [Google Scholar]
- Hirsch PR, Mauchline TH. Who’s who in the plant root microbiome? Nat Biotechnol. 2012;30:961–962. doi: 10.1038/nbt.2387. [DOI] [PubMed] [Google Scholar]
- Hmida-Sayari A, Gargouri-Bouzid R, Bidani A, Jaoua L, Savoure A, Jaoua S. Over expression of D1-pyrroline-5-carboxylate synthase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci. 2005;169:746–750. [Google Scholar]
- Kloepper JW, Ryu CM, Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94:1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester: Wiley Publications; 1991. pp. 115–176. [Google Scholar]
- Marasco R, Rolli E, Ettoumi B, Vigani G, Mapelli F, Borin S, Ayman FA, El-Behairy UA, Sorlini C, Cherif A, Zocchi G, Daffonchio DA. Drought resistance-promoting microbiome is selected by root system under desert farming. PLoS One. 2012;7(10):e48479. doi: 10.1371/journal.pone.0048479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak SN, Balaji J, Upadhyaya HD, Hash CT, Kishor PK, Chattopadhyay D, Rodriquez LM, Blair MW, Baum M, McNally K, This D. Isolation and sequence analysis of DREB2A homologues in three cereal and two legume species. Plant Sci. 2009;177(5):460–467. [Google Scholar]
- Pedersen WL, Chakrabarty K, Klucas RV, Vidaver AK. Nitrogen fixation (acetylene reduction) associated with roots of winter wheat and sorghum in Nebraska. Appl Environ Microbiol. 1978;35:129–135. doi: 10.1128/aem.35.1.129-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker PAHM. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol. 2014;52:347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- Rahimi A, Hosseini MS, Pooryoosef M, Fateh I. Variation of leaf water potential, relative water content and SPAD under gradual drought stress and stress recovery in two medicinal species of Plantago ovate and P. psyllium. Plant Ecophysiol. 2010;2:53–60. [Google Scholar]
- Reinhold-Hurek B, Hurek T. Living inside plants: bacterial endophytes. Curr Opin Plant Biol. 2011;14:435–443. doi: 10.1016/j.pbi.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Rosenblueth M, Martínez-Romero E. Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact. 2006;19:827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- Sairam RK. Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian J Exp Biol. 1994;3:584–593. [Google Scholar]
- Sambrook J, Russell RW. Molecular cloning: a laboratory manual. 3. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Senthilkumar M, Swarnalakshmi K, Govindasamy V, Lee YK, Annapurna K. Biocontrol potential of soybean bacterial endophytes against charcoal rot fungus, Rhizoctonia bataticola. Curr Microbiol. 2009;58(4):288–293. doi: 10.1007/s00284-008-9329-z. [DOI] [PubMed] [Google Scholar]
- Shahid MA, Balal RM, Pervez MA, Abbas T, Ashfaq M, Ghazanfar U, Afzal M, Rashid A, Garcia-Sanchez F, Mattson NS. Differential response of pea (Pisum sativum L.) genotypes to salt stress in relation to the growth, physiological attributes, antioxidant activity and organic solutes. AJCS. 2012;6:828–838. [Google Scholar]
- Sherameti I, Tripathi S, Varma A, Oelmüller R. The root-colonizing endophyte Piriformospora indica confers drought tolerance in Arabidopsis by stimulating the expression of drought stress-related genes in leaves. Mol Plant Microbe Interact. 2008;21(6):799–807. doi: 10.1094/MPMI-21-6-0799. [DOI] [PubMed] [Google Scholar]
- Singh B, Ahuja S, Singhal RK, Babu VP. Effect of gamma radiation on wheat plant growth due to impact on gas exchange characteristics and mineral nutrient uptake and utilization. J Radioanal Nuclear Chem. 2013;298:249–257. [Google Scholar]
- Su M, Li XF, Ma XY, Peng XJ, Zhao AG, Cheng LQ, Chen SY, Liu GS. Cloning two P5CS genes from bioenergy sorghum and their expression profiles under abiotic stresses and MeJA treatment. Plant Sci. 2011;181(6):652–659. doi: 10.1016/j.plantsci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Sziderics AH, Rasche F, Trognitz F, Sessitsch A, Wilhelm E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.) Can J Microbiol. 2007;53:1195–1202. doi: 10.1139/W07-082. [DOI] [PubMed] [Google Scholar]
- Yang J, Kloepper JW, Ryu CM. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14:1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bian M, Yu H, Liu Q, Yang Z. Identification of alkaline stress-responsive genes of CBL family in sweet sorghum (Sorghum bicolor L.) Plant Physiol Biochem. 2011;49(11):1306–1312. doi: 10.1016/j.plaphy.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Zinniel DK, Lambrecht P, Harris NB, Feng Z, Kuczmarski D, Higley P, Ishimaru CA, Arunakumari A, Barletta RG, Vidaver AK. Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl Environ Microbiol. 2002;68:2198–2208. doi: 10.1128/AEM.68.5.2198-2208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]