Abstract
The development of an effective amphotericin B (AmB) topical formulation to replace the systemically toxic injections currently used in cutaneous leishmaniasis (CL) treatment is challenging due to poor absorption through the skin. Aiming at an effective local chemotherapy, we designed PLGA (poly(lactide-co-glycolide acid) microparticles loaded with deoxycholate amphotericin B (d-AmB) for both macrophage intracellular targeting and sustained extracellular release. For that, d-AmB/PLGA microparticles with sizes ranging from 0.5 μm to 20 μm were synthesized and tested both in vitro and in vivo. In vitro, d-AmB/PLGA was more selectively active against intracellular amastigotes of Leishmania amazonensis than free d-AmB (selectivity index = 50 and 25, respectively). In vivo, the efficacy of a single intralesional (i.l) injection with d-AmB/PLGA was determined in early and established BALB/c mouse ear lesions. In early lesions, a single injection given on day 10 of infection was more effective in controlling parasite growth than eight i.l. injections with free d-AmB, as measured on day 120. Such d-AmB/PLGA injection was also effective in established lesions (day 30), leading to 97% parasite burden reduction, as compared with d-AmB or liposomal AmB (Ambisome®) i.l. injection containing the same AmB dose. Pharmacokinetic studies showed that following d-AmB/PLGA injection, AmB leaked slower from non-infected than infected ears, yet remaining in the ear tissue for as long as 30 days. Of interest, AmB was not detectable in the circulating plasma for at least two weeks of d-AmB/PLGA injection, contrasting with the rapid and durable (2 days) detection after free d-AmB injection. Despite the transient ear swelling and local cell infiltration, no alterations in AST, ALT and creatinine serum levels was induced by d-AmB/PLGA. For its approved components, local efficacy, and single-dose applicability, this novel and safe AmB microparticle depot formulation has strong potential as a new therapy for human CL.
Keywords: Chemotherapy, Drug delivery systems, PLGA, Leishmania amazonensis
Graphical abstract
Highlights
-
•
d-AmB/PLGA was more selectively active against intracellular parasites than d-AmB.
-
•
A single ear injection effectively controlled established infections in mice.
-
•
Tissue and plasma pharmacokinetics confirmed the local depot effect.
-
•
Toxicological studies showed a transient local reaction, and no systemic toxicity.
-
•
d-AmB/PLGA has strong potential as a novel local and single-dose therapy for human CL.
1. Introduction
Cutaneous (CL) and visceral (VL) leishmaniasis are Neglected Tropical Diseases (NTDs) caused by intracellular protozoans of the genus Leishmania. CL is the most common form of leishmaniasis, annually affecting 1.2 million people worldwide (Alvar et al., 2012). The disease is characterized by chronic skin ulcers normally restricted to the site of infected sandfly bite. Unlike Old-World parasite species, like L. major and L. tropica that cause self-healing lesions, New World species such as L. braziliensis and L. amazonensis are not normally self-healing, and may evolve into disfiguring Mucosal and Diffuse CL forms.
Currently, even the more benign localized CL form is treated systemically with the same toxic drugs used with fatal VL. These include pentavalent antimonials, pentamidine and amphotericin B (AmB), given in one or more series of 20–30 parenteral injections that can cause serious adverse effects, and even death (Uliana et al., 2017). AmB is the most active antileishmanial drug available for clinical use. However, due to poor solubility, AmB must be formulated in deoxycholate (d-AmB) for intravenous injection, which improves solubility but contributes to its nephrotoxic effect. Liposomal formulations (e.g. Ambisome®) have considerably decreased AmB toxicity. Due to increased drug accumulation in the liver and spleen, less than 0.1% of the injected drug reaches the skin lesion (Wijnant et al., 2018b, 2018c), restricting Ambisome® to VL and more complicated CL cases. The only oral drug available for leishmaniasis is miltefosine, but this too has controversial efficacy in human CL (Kevric et al., 2015) and is not yet licensed in countries like Brazil.
There is a strong need for topical therapies for CL, particularly for the milder and most common cases with up to 4 ulcers not exceeding 3 cm in diameter (DNDi, 2017). Local thermotherapy is drug-free, but requires accurate equipment and trained personnel to avoid common burnings (López et al., 2012; Navin et al., 1990). However, drug formulations for topical application have not met the expected impact. Paromomycin-based ointment marketed in the Middle East is not effective against New World parasite species (Ben Salah et al., 2013; Sosa et al., 2013). Likewise, a cream containing 3% of AmB (Anfoleish) had its clinical study in Colombian CL patients discontinued because of poor efficacy (Asela et al., 2018). The development of an effective antileishmanial cream is a challenge since drugs must cross the strong stratum corneum barrier of the skin to reach the parasites within the phagolysosome of the infected macrophages in the deep layers of the skin. Epidermis thickening that normally accompanies lesion development is another factor that hinders drug absorption through the skin (Bocxlaer et al., 2016). Intralesional (i.l.) injections with antimonials aiming at bypassing epidermal permeation have proved a more effective measure for local drug delivery (Silva et al., 2016). However, periodical injections are needed to compensate for rapid drug absorption into the bloodstream and lymph vessels, which besides pain and repeated hospital visits, may also cause systemic side effects (Esfandiarpour et al., 2012). Besides, a study in Iran showed that weekly i.l. injections with 2 mg of d-AmB for up to twelve weeks can cure only 61% of CL patients (Goyonlo et al., 2014). In addition, there was a risk of local necrosis due to deoxycholate emulsifier. Another clinical study in Iran showed the low efficacy of intralesional injections with deoxycholate-free liposomal AmB (Ambisome®), which was probably caused by rapid diffusion of the small liposomes from the lesion (Yardley and Croft, 1997). Therefore, injections forming a local drug depot seem to be a better alternative for localized CL.
Controlled drug release systems using biodegradable PLGA microparticles have been marketed for some years to treat a variety of human diseases like cancer, diabetes, schizophrenia, and growth hormone disorders (Wang et al., 2016). Those systems are injected intramuscularly to form a local depot capable of releasing the drug during weeks or months, impressively reducing the frequency of doses and adverse effects as compared with conventional formulations. In leishmaniasis, we reported the synthesis and use of PLGA microparticles loaded with an antileishmanial chalcone (CH8) for treatment of experimental CL with a single s.c. injection (Sousa-Batista et al., 2018a,b ). In the present study, we describe the design and therapeutic use of PLGA microparticles loaded with AmB, aiming at a more rapid regulatory approval of this new antileishmanial treatment strategy.
2. Material and methods
2.1. d-AmB/PLGA preparation
Microparticles were prepared by a double emulsion and solvent evaporation method (W1/O/W2) from a 1:10 w/w mixture of d-AmB (Fungizone®, Abbott) and PLGA 50:50 (poly(lactic-co-glycolic acid), Purac 504, Purasorb). Briefly, 500 mg of PLGA 50:50 was dissolved in 10 mL dichloromethane – DCM - (oil phase). Inner aqueous phase (w1) was prepared by dissolving 5 mg of d-AmB in 1 mL of distilled water. d-AmB, not pure AmB, was used for increased solubility in water. The w1 phase was emulsified into the oil phase using a high-shear homogenizer (Ultra-Turrax T25 Basic, Ika, Germany) at 7000 rpm for 10 min. The w1/O emulsion was added gradually dropped into 120 ml of aqueous (3%, w/w) polyvinyl alcohol (PVA, Sigma) solution under agitation. After that, DCM in the double emulsion was evaporated under low agitation on ice to yield d-AmB/PLGA microparticles. The obtained microparticles (d-AmB/PLGA) were washed 3x with water by centrifugation at 4000 rpm during 5 min, and dried by lyophilization (FreeZone 1 liophilizer, Labconco Corporation). Blank microparticles (PLGA) were prepared in the same way except that no d-AmB was added.
2.2. d-AmB/PLGA characterization
For total drug content, a dry d-AmB/PLGA sample was dissolved in a mixture (1:2) of dimethyl sulfoxide (DMSO, Sigma-Aldrich) and acetonitrile (Tedia). After centrifugation and 0.45 μM membrane filtration, AmB concentration was determined by UV-HPLC at 405 nm using a mobile phase containing acetonitrile:aqueous 0.01% phosphoric acid (Tedia) (60:40 v/v) flowing at a rate of 1 mL/min. A calibration curve ranging from 0.25 to 50 μg/mL AmB (r > 0.999) was done at each analysis. The encapsulation efficiency (EE %) was calculated as the ratio between measured and theoretical AmB amounts, multiplied by 100%. For in vitro release ratio, 2 g of d-AmB/PLGA was added in duplicate to 150 mL of 0.5% of polysorbate 80 and 0.1% sodium azide in phosphate buffered saline (PBS, pH 7.0), maintained at 37 °C, under 60 rpm agitation. At different time points (4 h–200 days), a sample (100 μL) was withdrawn, filtered and analyzed for AmB concentration, as above.
The particle size distribution was measured by laser diffraction (Beckman Coulter LS230) after resuspension of the solid microparticles in water and ultrasound dispersion. The zeta potential was measured using Zetamaster (Malvern Instruments). For scanning electron microscopy (SEM), dry microparticles and AmB powder were sputtered on double-face tape and these with gold (JFC-129 1300, JEOL) prior to analysis in electron microscope (JPC 1100, JEOL) at 15 kV.
2.3. Parasites, mice and ethics statement
Leishmania amazonensis (strain WHOM/BR/75/JOSEFA) promastigotes were maintained at 27 °C in medium M199 supplemented with 10% heat-inactivated fetal bovine serum (HIFBS, Cultilab), and used at the stationary phase of growth. BALB/c mice (female, 23 g) were maintained under controlled temperature, filtered air, filtered water, autoclaved bedding, and commercial food at our facilities at Federal University of Rio de Janeiro. All protocols using mice were approved by the Federal University of Rio de Janeiro institutional Animal Care and Use Committee (protocol number CAUAP118) under the CONCEA (National Council for control of Animal Experiments).
2.4. Cytotoxicity to intracellular amastigotes and macrophages
For antiamastigote activity, glass coverslip-adherent mouse peritoneal macrophages (5 × 105) were incubated with 5 × 106 L. amazonensis promastigotes for 4 h at 34 °C in M199 plus 10% HIFCS. Then, free parasites were removed by washing with PBS, and infected cells incubated for further 24 h for transformation and replication as amastigotes. Infected macrophages were treated with d-AmB, d-AmB/PLGA or PLGA in equivalent AmB concentrations ranging from 0.01 μM to 100 μM (n = 3) for 48 h at 37 °C. At the end of incubation time, cells were stained with Giemsa and the total number of amastigotes in 200 macrophages by coverslip was evaluated by direct counting. The results were analyzed by curve fitting regression and expressed as IC50 (concentration that inhibits parasite growth by 50%).
For macrophage cytotoxicity, adherent uninfected mouse peritoneal macrophages were treated for 48 h at 37 °C as for antiamastigote activity. Then, the cells were centrifuged (500 rpm/5 min) and the supernatants collected for quantification of released lactate dehydrogenase (LDH) using an assay kit (Doles, Brazil) and a microplate-reader spectrometer (SpectraMax M5) at 490 nm. Maximum and spontaneous release were obtained with cells cultured with 2% Triton X-100 or culture medium alone, respectively. The results calculated as % Specific Release = [(Maximum – Spontaneous)/(Drug – Spontaneous)] x 100, were analyzed by curve fitting regression, and expressed as CC50 (concentration that produces 50% of specific LDH release).
2.5. Efficacy against CL
Mice were infected in the ear pinnae with 2 × 106 promastigotes of L. amazonensis. On day 10 or 30 of infection, as indicated, they were injected intralesionally with d-AmB, d-AmB/PLGA or liposomal AmBisome® (5 μg of AmB/10 μL of PBS, 0.2 mg/Kg), using hypodermal needles. Controls received PLGA alone in equivalent dose (550 μg/10 μL, 25 mg/kg) or PBS alone (10 μL). Alternatively, animals treated on day 10 were given eight intralesional injections with free d-AmB (5 μg/10 μL each, 2x a week) on days 10–42. The eight-injection regimen was chosen not only for control maximization but also to mimic clinical studies in Iran where each CL lesion was injected with 2 mg of d-AmB (assuming 1 mL/injection) up to 12 times on a weekly basis, resulting in 61.4% of patients with full healing at the end of the 12th week (Goyonlo et al., 2014). Ear thicknesses were periodically measured during infection with a caliper gauge, and lesion sizes were expressed as the difference between infected and noninfected ears. At the end of experiments, the infected ears were photographed, and parasite burdens were measured by limiting dilution assay (Lima et al., 1997; Sousa-Batista et al., 2018b).
2.6. Local and systemic pharmacokinetics
For local pharmacokinetics, both uninfected and 7-day infected mice were given a single s.c. injection with d-AmB/PLGA (5 μg in 10 μL of PBS) in the ear pinnae. At different time points thereafter (30 min–90 days), the mice were killed, and the ears individually homogenized in 1 mL of acetonitrile. After centrifugation (10.000 rpm/10 min), the supernatants were dried, and assayed for AmB quantification using UV-HPLC, as described in item 2.2.
For systemic pharmacokinetics, uninfected mice were given a single s.c. injection with d-AmB/PLGA or d-AmB (5 μg in 10 μL of PBS) in the ear pinnae. At different time points (30 min–15 days), their blood was collected in heparinized tubes, centrifuged, and the obtained plasma treated with acetonitrile and phosphoric acid (pH 4). After centrifugation (1500 rpm/2 min), AmB concentration was quantified by UV-HPLC as above.
2.7. Systemic toxicity
Mice were infected with L. amazonensis. On day 30, they were given a single intralesional injection with PLGA, d-AmB, liposomal AmB (AmBisome®) or d-AmB/PLGA (5 μg in 10 μL of PBS). In the positive control group (n = 5), mice were intraperitoneally injected with 200 μL of 1% carbon tetrachloride (CCl4) in soybean oil three days before animal killing. On day 90 of infection, the sera were collected and assayed for AST, ALT, and creatinine as parameters of liver and kidney toxicity, using a colorimetric commercial kit (Doles, Brazil) adapted for microvolumes. The positive-control sera were taken from mice which received 200 μL of 1% carbon tetrachloride (CCl4) in soybean oil by the intraperitoneal route 3 days before serum collection (Otsuka et al., 2002).
2.8. Cutaneous sensitization
That was evaluated using an adapted mouse ear-swelling test (MEST) (Gad, 1994). Mice were sensitized 9, 7 and 5 days earlier with s.c. injections in the shaved hump with d-AmB, d-AmB/PLGA, or AmBisome® (5 μg of AmB/dose), or PBS alone (10 μL). Positive controls were topically applied with oxazolone (0.5% in acetone) in the same days. On day 0, mice were injected/applied in the ear pinnae with the same sensitizing dose. At different time points thereafter (20 min–72 h), the ear thicknesses were periodically measured with a caliper gauge, and edema was expressed as the difference between challenged and non-challenged ears. Edema was considered positive when 20% superior than PBS-injected ears (Gad, 1994).
2.9. Histopathology
Non-infected mice were given a single s.c. injection in the ear pinnae with d-AmB/PLGA (5 μg of AmB in 10 μL of PBS). After different time points (4 h, 1 day and 30 days), the ears were removed and preserved in Millonig fixative (pH 7.4). Longitudinal 4 μm-thick ear sections were stained with hematoxylin and eosin, and photographed under light microscope (Nikon eclipse - Ti).
2.10. Statistical analysis
One-way analysis of variance and the Bonferroni posttest were performed to demonstrate statistical differences (P < 0.05). To compare the difference between two groups, a nonparametric t-test was used. All statistical analyses used GraphPad Prism 6 software.
3. Results and discussion
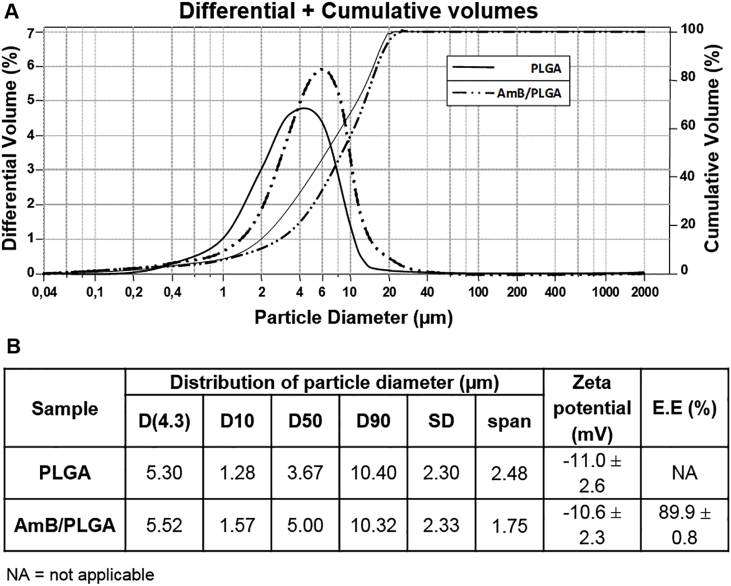
Currently, the conventional chemotherapy of human CL is based on multiple injections with systemically toxic drugs, urging safer and more active local treatments. Previously, we successfully used PLGA microparticles loaded with the active chalcone CH8 to promote local phagocytosis and sustained release for localized treatment of murine CL. Aiming at improving formulation efficacy and clinical licensing, we proposed here the use of PLGA microparticles loaded with AmB, the most active antileishmanial in clinical use. We sought to obtain PLGA particles with a size distribution that included both smaller particles (≤6 μm) for rapid macrophage internalization (Sousa-Batista et al., 2018a, Sousa-Batista et al., 2018b), and larger particles (≥10 μm) for extracellular depot (Gaumet et al., 2008). With finelly adjusted double emulsion and solvent evaporation conditions, we obtained blank PLGA and drug-loaded d-AmB/PLGA microparticles with adequate unimodal size distribution within the aimed range (Fig. 1A). d-AmB/PLGA sizes averaged 5.5 μm at D(4.3) and 10.3 μm at D(90) with 2.5 span value (Fig. 1B). The relatively weak d-AmB/PLGA negative charge (−10.6 mV) was also suitable to promote phagocytosis, prevent aggregation, and avoid cytotoxicity as observed with cationic particles (Fröhlich, 2012; Nicolete et al., 2011).
Fig. 1.
Microparticle physical characteristics. A) PLGA and d-AmB/PLGA microparticles were analysed by Coulter LS230 for differential (Gaussian lines) and cumulative (progressive lines) volumes. B) Data for diameter distribution, zeta potential, and encapsulation efficiency (E.E.). Means ± SD (n = 3).
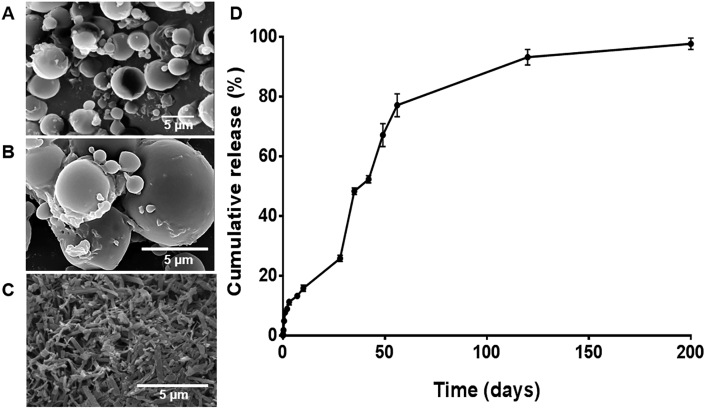
Different from chalcone CH8 that is readily entrapped in PLGA thanks to its high lipophilicity, AmB is amphiphilic, and more difficult to entrap. Despite the high process entrapment efficiency (89.9%), a maximum of 0.9% of drug loading in the microparticle was achieved. SEM analysis showed that the particles were spherical and smooth, with scarce external drug crystals, suggesting that most of the added AmB is inside the particle (Fig. 2 A and 2B vs free AmB crystals in 2C). The in vitro drug release kinetics occurred in biphasic mode (Fig. 2D). During the first 24 h, a fast release of 7% of the total drug was observed, probably due to external drug. Thereafter, a controlled drug release was observed, reaching 25% after one month, and 80% after two months, hypothetically adequate for CL treatment with a single dose. The observed in vitro AmB release rate is slower than reported with AmB-loaded PLGA nanoparticles aiming at oral delivery (Italia et al., 2009). In that study, AmB was released faster (∼60% of the drug in 25 days), suggesting that the larger particle size used here is important for a more prolonged release.
Fig. 2.
Microparticle SEM and in vitro release kinetics. A-C) Dried d-AmB/PLGA microparticles were imaged by SEM in lower (A) and higher (B) magnification to confirm absence external AmB. Dry AmB (C) is shown as control. D) d-AmB/PLGA microparticles were incubated in duplicate at 37 °C in PBS under mild agitation. At the indicated times, supernatant samples were removed for quantification of AmB by HPLC, followed by PBS volume replacement. Means ± SD (n = 2).
The in vitro antileishmanial activity of d-AmB/PLGA was investigated in L. amazonensis-infected macrophages. The anti-amastigote activity obtained with d-AmB/PLGA was similar to that of free d-AmB (IC50 = 0.05 μg/mL vs 0.08 μg/mL), and slightly higher than that of liposomal AmB (Ambisome®, IC50 = 1.4 μg/mL), as shown in Table 1. That contrasts with the lower activity of entrapped CH8/PLGA in relation to free CH8 (Sousa-Batista et al., 2018b), explained by the higher affinity of CH8 for PLGA than AmB, supported by higher entrapment ratio of the former (10% and 0.9% respectively). The indirect contribution of macrophage activation is unlike, since blank PLGA was devoid of antileishmanial activity. The lower Ambisome® activity may be due to its variable ability to be endocytosed by macrophages and/or the high AmB affinity for the liposomal distearoylphosphatidylglycerol and cholesterol (Legrand et al., 1996; Voak et al., 2018; Yardley and Croft, 1997). Since AmB activity is related to a disruptive effects in the cell membrane lipids (Grela et al., 2018), the slow rate of AmB transfer from the liposome to the cell membrane may also have accounted to reduced Ambisome® reduced activity. Anyway, the finding that d-AmB/PLGA was two-fold more selective than free d-AmB (SI = 50 and 25, respectively) is promising. A more prolonged in vitro assay to allow for greater intracellular drug release would possibly provide a greater differential effect between d-AmB/PLGA and free d-AmB.
Table 1.
In vitro cytotoxicity against intracellular amastigotes and macrophages.
| Samples | Amastigotes IC50 (μg/ml) | Macrophages CC50 (μg/ml) | SIb |
|---|---|---|---|
| d-AmB | 0.08 ± 0.2 | 2.0 ± 0.1 | 25.0 |
| PLGA | >100 | >100 | ND |
| Ambisomea | 1.4 ± 0.3 | 97.0 ± 2.0 | 69.3 |
| d-AmB/PLGAa | 0.05 ± 0.1 | 2.5 ± 0.1 | 50.0 |
Means ± SD(n = 3).
ND = not determined.
AmB equivalents.
SI (selectivity index) = CC50/IC50.
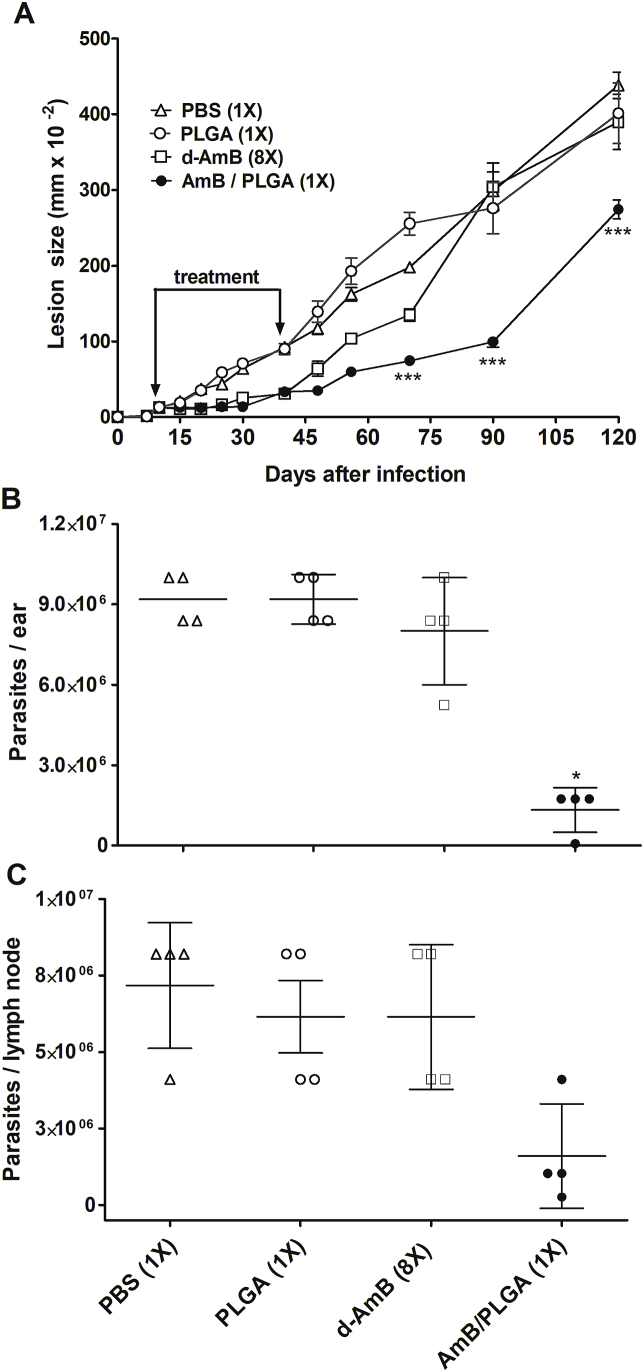
The efficacy of a single-dose treatment with d-AmB/PLGA was evaluated in the BALB/c mouse model of infection with L. amazonensis. Treatment was carried out either in the early stage of infection for maximization, or in established lesions for a more real situation. All formulations were tested intralesionally in the same AmB dosage (5 μg/animal, 0.2 mg/kg). For early treatment, on day 10 post infection mice were given a single dose of d-AmB/PLGA or the first of eight injections with free d-AmB. Multiple injections with free d-AmB effectively controlled lesion growth only during treatment (days 10–42 of infection) (Fig. 3). On the other hand, a single d-AmB/PLGA injection significantly delayed lesion growth for at least 80 days (day 90 of infection). Still, almost 4 months after treatment (day 120 of infection), lesions were 37% smaller, and the parasite burdens in the ear and draining lymph nodes were 85% and 78% smaller than PBS controls, respectively. The observed lesion recrudescence after 90 days of d-AmB/PLGA injection was possibly due to: i) After 90 days of injection, no more drug was locally available; ii) The BALB/c mouse model used is unable to mount an effective immune response. Although BALB/c mice are well-established models for antileishmanial drug evaluation, most studies tend to evaluate efficacy (parasite burden) soon after the end of treatment, because remaining parasites cannot be controlled by immunity (Mears et al., 2015; Mendonça et al., 2018).
Fig. 3.
Efficacy of single-dose treatment with d-AmB/PLGA in early CL. Mice were infected with L. amazonensis in the ear pinna. On day 10 of infection, they were given a single intralesional injection with d-AmB/PLGA (5 μg of AmB), PLGA (500 μg) or PBS vehicle (10 μL). d-AmB (5 μg/dose) was given by the same route twice a week (total 8 doses). Lesion sizes were measured on the indicated days (A). Parasite loads were determined on day 120 post infection in the ear (B) and draining lymph nodes (C) by limiting dilution assay. Means ± SD (n = 5). ***p < 0.01 and *p < 0.5 in relation to d-AmB 8X.
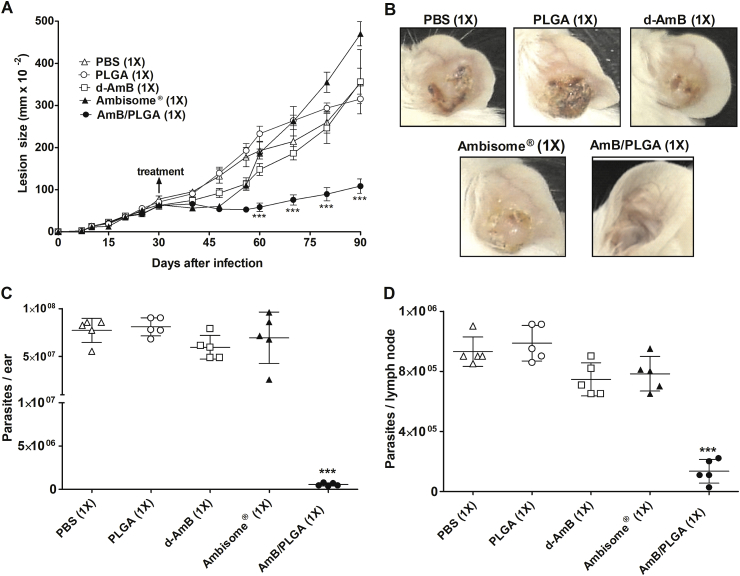
Treatment was also evaluated in established 30-day lesions, when animals received a single injection with d-AmB/PLGA, Ambisome®, or d-AmB, all containing 5 μg of AmB for comparison. Controls received blank PLGA or PBS alone. No pure AmB could be included due to AmB precipitation in aqueous solutions. As seen in early treatment, d-AmB/PLGA effectively controlled lesion growth for at least 60 days (Fig. 4). At that stage when lesions reached 400 (mm x 10−2) in thickness, the experiments ended in accordance with the ethical terms of our animal license. The lesion sizes were 69% smaller than PBS controls, while the parasite burdens in the ear and draining lymph nodes were significantly reduced (97% and 87% reduction, respectively). Unlike all the other treatments, ears given d-AmB/PLGA were non-ulcerated and healed. Ambisome® was only transiently effective, possibly due to rapid outflow of the small liposomes from the site of infection (Yardley and Croft, 1997; Wijnant et al., 2018c). As observed with eight injections (Fig. 3), a single injection with free d-AmB was ineffective. In both the early and late treatment models, empty PLGA was inert against parasite growth, indicating that parasite killing is due to AmB, but a synergistic PLGA effect leading to macrophage activation cannot be discarded (Nicolete et al., 2011).
Fig. 4.
Efficacy of single-dose treatment with d-AmB/PLGA in established CL. Mice were infected in the ear with L. amazonensis. On day 30 of infection, they were given a single intralesional subcutaneous injections with d-AmB, AmBisome® or d-AmB/PLGA (5 μg of AmB each). Controls were given PLGA (500 μg) or PBS vehicle (10 μL). A) Lesion sizes were measured on the indicated days. B) Representative lesion pictures on day 90. C) Parasite loads in the ear, and D) draining lymph nodes, measured on day 90 by limiting dilution assay. Means ± SD (n = 5). ***p < 0.01 in relation to d-AmB 1X.
d-AmB rather than pure AmB was incorporated into PLGA microparticles due to the poor AmB solubility in the process-compatible solvents. The 5 μg AmB dose (0.22 mg/kg) was chosen for safety reasons since local necrosis was produced with 10 μg and 50 μg of d-AmB injected twice a week for 2 weeks (our unpublished observation). Such toxicity was likely due to deoxycholate (Dayan et al., 2018), and warns against local therapy with d-AmB (eg Fungizone®, Anforicin B®). To reduce potential toxicity, we are presently adjusting the synthesis process to increase drug encapsulation rate in the absence of deoxycholate. A previous study used a polymethacrylic acid (PMA) hydrogel as a vehicle to inject deoxycholate-free AmB into mouse lesions caused by L. major to promote accelerated healing (Corware et al., 2011). However, considering the high anti-amastigote activity of the formulation (IC50 = 0.08 μg of AmB/mL), much higher individual doses containing 135 μg of AmB (total of 400 μg) were needed compared with the 5 μg single dose reported here.
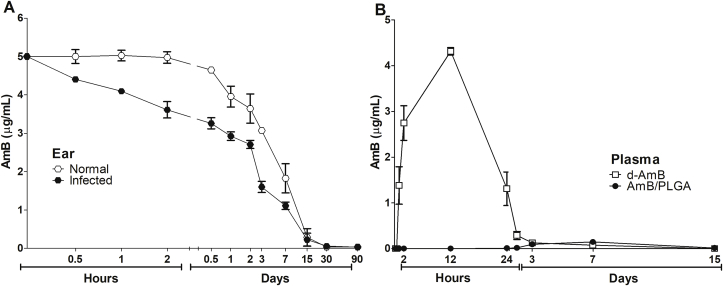
The d-AmB/PLGA local pharmacokinetics was studied in both uninfected and 7-day infected mouse ears. Independently of infection, AmB was released from the ears within 30 days (Fig. 5A), compatible with the PLGA 50:50 degradation time (Makadia and Steve, 2011). Drug release was faster in vivo than in vitro (80 days, Fig. 2), likely due to degrading enzymes in the living tissue. We observed lower AmB retention in the infected ears than healthy ears, possibly caused by the higher vascular permeability of infected tissue allowing faster leakage. This is compatible with a recent report showing that the stage of murine CL has profound impact on skin access of intravenously delivered d-AmB (Wijnant et al., 2018a). In that study, 24 h after administration, d-AmB accumulation in established lesions was higher than in healthy skin. Thus, in a reverse way we demonstrate that local tissue inflammation caused by infection may facilitate drug permeation out of CL skin lesion. Therefore, it will be important to take into account the stage of lesion development to calculate treatment duration.
Fig. 5.
Local and systemic pharmacokinetics. (A) Infected and non-infected mice were treated in the ear with a single dose of d-AmB/PLGA (5 μg of AmB). B) Non-infected mice were treated with d-AmB or d-AmB/PLGA (5 μg of AmB each). After the indicated time points, AmB was quantified in the ear or plasma by HPLC/UV at 405 nm. Means ± SD (n = 3).
Systemic pharmacokinetics was also studied, where AmB plasma concentration following a single intralesional injection with d-AmB/PLGA was compared with free d-AmB (Fig. 5B). While AmB was readily detectable in the plasma within 1 h following d-AmB injection, reaching a peak accumulation within 12 h, it remained undetectable following d-AmB/PLGA injection throughout the 15-day follow up. The observation that d-AmB/PLGA does not detectably leaks to the circulation is compatible with a sustained release depot together with phagocytosis by local macrophages. On the other hand, the rapid leakage to the blood following d-AmB injection corroborates with the low efficacy of i.l. injections (Fig. 3, Fig. 4). Although the pharmacokinetics of locally injected liposomal AmB (Ambisome®) was not studied here, it is likely that the small liposomes (∼80 nm) will also escape from the lesion to the blood either directly or through lymphatic drainage (Wijnant et al., 2018c). These local and systemic pharmacokinetic findings confirm the sustained local release and systemic safeness.
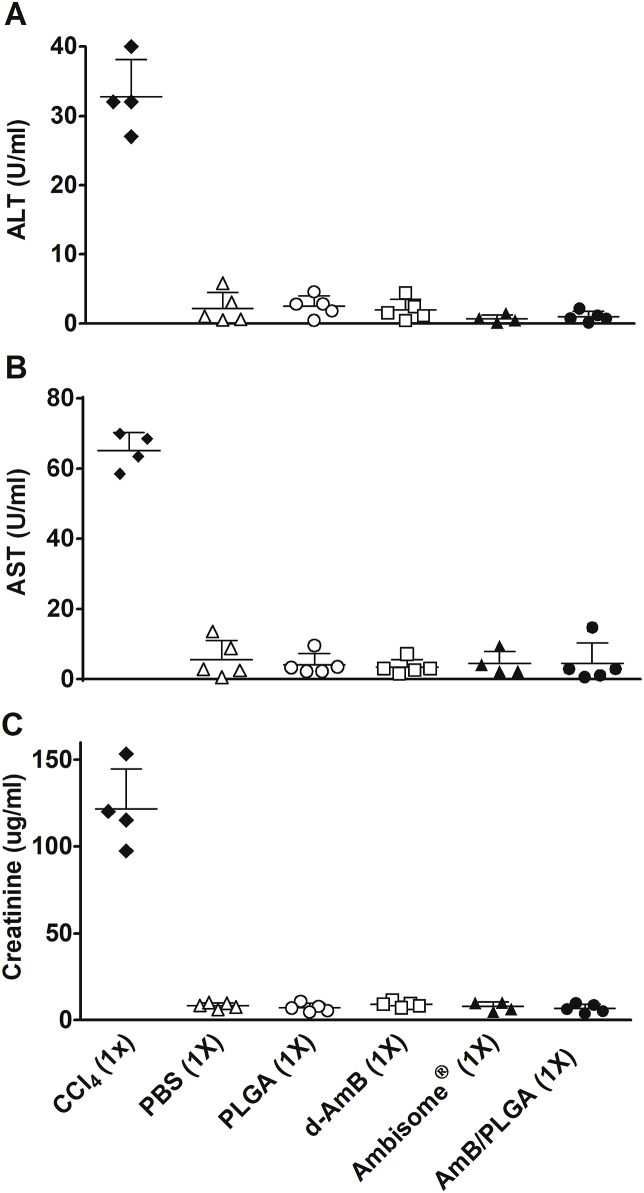
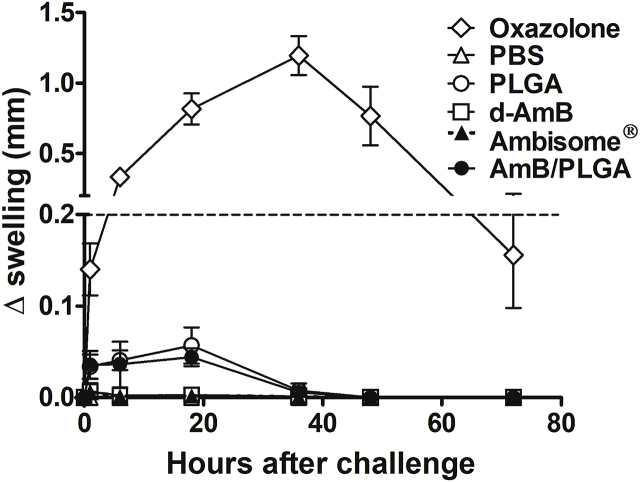
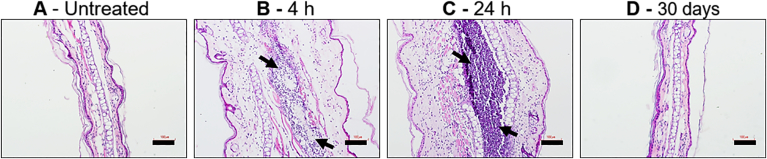
Despite its approved components, the new AmB formulation and use demands safety assessment (Wang et al., 2016). Thus, all AmB formulations used here were compared for increased plasma ALT, AST and creatinine levels after a single intralesional injection. No treatment including d-AmB/PLGA resulted in detectable plasma levels, indicative of lack of liver, heart and kidney toxicity (Fig. 6). For skin reactivity following a single s.c. injection with d-AmB/PLGA, two tests were used: i) the mouse ear-swelling test (MEST) indicative of cutaneous sensitization, and ii) histological analysis. Non-infected animals were used to avoid dual treatment and infection effects. For MEST, mice were sensitized with three weekly s.c. injections in the back, and challenged 5 days later in the ear. A transient swelling response starting after 2 h and decreasing after 20 h was seen in mice receiving Ambisome®, d-AmB/PLGA and PLGA (Fig. 7). The reaction, maximized by sequential pre-sensitization, is considered mild in comparison with the reference agent oxazolone. For histological analysis, ear tissue slices were prepared after 4 h, 24 h and 30 days of d-AmB/PLGA injection. Fig. 8 shows representative slides of 3 mice in each time point. As early as 4 h, the ears were thickened with cellular infiltrate and edema, compatible with the swelling seen in Fig. 7. The inflammatory infiltrate increased at 24 h, as seen by increased numbers of purple nuclei. Thirty days after injection, the ears presented normal morphological structure, indicating that the inflammatory response waned. Similar findings were found in ears injected with PLGA alone (not shown), in accordance with the swelling response in Fig. 7, and previous studies demonstrating the slight inflammatory effect of PLGA microparticles (Barsoum et al., 1997; Xie et al., 2015). No microparticles could be seen in the histological sections, because the polymer degrades with the tissue fixatives.
Fig. 6.
Systemic toxicology. Thirty-day infected mice were given a single dose of the indicated AmB formulations or controls, as for Fig. 4. After sixty days of treatment, sera were collected and individually assayed for AST (A), ALT (B), and creatinine (C). Positive control (1% of CCl4) was given by intraperitoneal injection on day 87. Means ± SD (n = 5).
Fig. 7.
Cutaneous hypersensitivity. Mice were pre-sensitized in the back by 3 s.c. injections with d-AmB, d-AmB/PLGA (5 μg of AmB) or PBS (10 μL) alone, on days −9, −7 and −5. Oxazolone was applied topically on the same days. On day 0, their ear pinnae was challenged in the same way, and the resulting swelling measured in the indicated days. The dashed line indicates sensitization threshold, that is 20% of achieved with 0.5% oxazolone. Means ± SD (n = 5).
Fig. 8.
- Histopathological analysis. Mice were given a single s.c. injection in the ear pinna with d-AmB/PLGA (5 μg of AmB), or were left untreated (A). After 4 h (B), 1 day (C) or 30 days (D) post injection, longitudinal ear sections were obtained and stained with H&E. The inflammatory infiltrate is shown with arrows. Representative photographs of triplicate samples are shown. Bars = 100 μm.
The single subcutaneous injection contained 5 μg of AmB and 550 μg of PLGA (0.23 mg of AmB/Kg and 25 mg of PLGA/Kg of mouse body weight, 22 g). Dose translation from mouse to human using the body surface area normalization method (Nair and Jacob, 2016) provides 0.02 mg of AmB/Kg and 2.1 mg of PLGA/Kg of human body weight (60 Kg). Such translated AmB dose is compatible with its clinical use, based on studies conducted in Iran and India, where CL patients received weekly i.l. injections with 0.04 mg of d-AmB/Kg (Goyonlo et al., 2014; Mushtaq et al., 2016). As to the polymer, PLGA-based drug products like Risperdal Consta® (131 mg), Lupron Depot® (120 mg) and Trelstar Depot® (170 mg) used to treat schizophrenia, prostate cancer and endometriosis, respectively, are given as single doses of approximately 2.2 mg of PLGA/Kg, which is similar to used here. Therefore, both AmB and PLGA doses used here are clinically compatible. For human administration, it is expected that treatment will be accomplished by perilesional injections with small volumes of d-AmB/PLGA a single time, different from the present infiltration with 1–3 mL of Glucantime in different times.
In summary, this study describes the safe and effective use of AmB for one-dose localized treatment of CL in mice, not achievable with marketed deoxycholate and liposomal formulations. Such d-AmB/PLGA microparticles platform has strong potential for ready licensing, and revolutionizing the treatment of localized and uncomplicated CL, the commonest form of leishmaniasis. Besides, the d-AmB/PLGA platform is potentially transposed to systemic treatment of VL and systemic mycosis.
Acknowledgements
This research was funded by the National Council for Scientific and Technological Development (CNPq) and the Rio de Janeiro State Research Foundation (FAPERJ).
References
- Alvar J., Vélez I.D., Bern C., Herrero M., Desjeux P., Cano J., Jannin J., de Boer M. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asela C., Cruz C., Alves F., Robledo S., Arana B. A phase II study to evaluate the safety and efficacy of topical 3 % amphotericin B cream ( Anfoleish ) for the treatment of uncomplicated cutaneous leishmaniasis in. PLoS Neglected Trop. Dis. 2018;13:1–12. doi: 10.1371/journal.pntd.0006653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum I.S., Kopydlowski K.M., Cuenin P., Setterstrom J.A. Evaluation of hypersensitivity to microencapsulated ampicillin in Guinea pigs. J. Antimicrob. Chemother. 1997;39:63–69. doi: 10.1093/jac/39.1.63. [DOI] [PubMed] [Google Scholar]
- Ben Salah A., Ben Messaoud N., Guedri E., Zaatour A., Ben Alaya N., Bettaieb J., Gharbi A., Belhadj Hamida N., Boukthir A., Chlif S., Abdelhamid K., El Ahmadi Z., Louzir H., Mokni M., Morizot G., Buffet P., Smith P.L., Kopydlowski K.M., Kreishman-Deitrick M., Smith K.S., Nielsen C.J., Ullman D.R., Norwood J. a, Thorne G.D., McCarthy W.F., Adams R.C., Rice R.M., Tang D., Berman J., Ransom J., Magill A.J., Grogl M. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N. Engl. J. Med. 2013;368:524–532. doi: 10.1056/NEJMoa1202657. [DOI] [PubMed] [Google Scholar]
- Bocxlaer K. Van, Yardley V., Murdan S., Croft S.L. Drug permeation and barrier damage in Leishmania-infected mouse skin. J. Antimicrob. Chemother. 2016;71:1578–1585. doi: 10.1093/jac/dkw012. [DOI] [PubMed] [Google Scholar]
- Corware K., Harris D., Teo I., Rogers M., Naresh K., Müller I., Shaunak S. Accelerated healing of cutaneous leishmaniasis in non-healing BALB/c mice using water soluble amphotericin B-polymethacrylic acid. Biomaterials. 2011;32:8029–8039. doi: 10.1016/j.biomaterials.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan S.H., Schlessinger J., Beer K., Donofrio L.M., Jones D.H., Humphrey S., Carruthers J., Lizzul P.F., Gross T.M., Beddingfield F.C., 3rd Treatment session: pooled analysis of Data from the phase 3 REFINE trials. Aesthet. Surg. J. 2018;38:998–1010. doi: 10.1093/asj/sjy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DNDi . 2017. DNDi's Strategy for Cutaneous Leishmaniasis [WWW Document]https://www.dndi.org/diseases-projects/leishmaniasis/dndi-strategy-cl/ accessed 11.16.17. [Google Scholar]
- Esfandiarpour I., Farajzadeh S., Rahnama Z., Fathabadi E.A., Heshmatkhah A. Adverse effects of intralesional meglumine antimoniate and its influence on clinical laboratory parameters in the treatment of cutaneous leishmaniasis. Int. J. Dermatol. 2012;51:1221–1225. doi: 10.1111/j.1365-4632.2012.05460.x. [DOI] [PubMed] [Google Scholar]
- Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad S.C. The mouse ear swelling test (MEST) in the 1990s. Toxicology. 1994;93:33–46. doi: 10.1016/0300-483X(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Gaumet M., Vargas A., Gurny R., Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008;69:1–9. doi: 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Goyonlo V.M., Vosoughi E., Kiafar B., Nahidi Y., Momenzadeh A., Taheri A.R. Efficacy of intralesional amphotericin B for the treatment of cutaneous leishmaniasis. Indian J. Dermatol. 2014;59:631–636. doi: 10.4103/0019-5154.143571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grela E., Piet M., Luchowski R., Grudzinski W., Paduch R. Imaging of human cells exposed to an antifungal antibiotic amphotericin B reveals the mechanisms associated with the drug toxicity and cell defence. Sci. Rep. 2018:1–7. doi: 10.1038/s41598-018-32301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italia J.L., Yahya M.M., Singh D., Kumar M.N.V.R. Biodegradable nanoparticles improve oral bioavailability of amphotericin B and show reduced nephrotoxicity compared to intravenous Fungizone ®. Pharm. Res. (N. Y.) 2009;26:1324–1331. doi: 10.1007/s11095-009-9841-2. [DOI] [PubMed] [Google Scholar]
- Kevric I., Cappel M.A., Keeling J.H. New world and old world Leishmania infections: a practical review. Dermatol. Clin. 2015;33:579–593. doi: 10.1016/j.det.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Legrand P., Vertut-Doï a., Bolard J. Comparative internalization and recycling of different amphotericin B formulations by a macrophage-like cell line. J. Antimicrob. Chemother. 1996;37:519–533. doi: 10.1093/jac/37.3.519. [DOI] [PubMed] [Google Scholar]
- Lima H.C., Bleyenberg J.A., Titus R.G. A simple method for quantifying Leishmania in tissues of infected animals. Parasitol. Today Off. 1997;13:80–82. doi: 10.1016/S0169-4758(96)40010-2. [DOI] [PubMed] [Google Scholar]
- López L., Robayo M., Vargas M., Vélez I.D. Thermotherapy. An alternative for the treatment of American cutaneous leishmaniasis. Trials. 2012;13:1–7. doi: 10.1186/1745-6215-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadia H.K., Steve S. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377.Poly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears E.R., Modabber F., Don R., Johnson G.E. A Review: the current in vivo models for the discovery and utility of new anti-leishmanial drugs targeting cutaneous leishmaniasis. PLoS Neglected Trop. Dis. 2015;9:1–23. doi: 10.1371/journal.pntd.0003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça V.C., Martins V.T., Lage D.P., Dias D.S., Ribeiro P.A.F., Carvalho R.S.A.M., Dias T.A.L., Miyazaki C.K., Menezes-souza D., Roatt B.M., Tavares C.A.P., Barichello J.M., Duarte M.C., Coelho E.A.F. Comparing the therapeutic efficacy of different amphotericin B- carrying delivery systems against visceral leishmaniasis. Exp. Parasitol. 2018;186:24–35. doi: 10.1016/j.exppara.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Mushtaq S., Dogra D., Dogra N. Clinical Response with intralesional Amphotericin B in the treatment of old world cutaneous leishmaniasis : a preliminary report. Dermatol. Ther. 2016;29:398–405. doi: 10.1111/dth.12377. [DOI] [PubMed] [Google Scholar]
- Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navin T.R., Arana B.A., Arana F.E., de Mérida A.M., Castillo A.L., Pozuelos J.L. Placebo-controlled clinical trial of meglumine antimonate (glucantime) vs . localized controlled heat in the treatment of cutaneous leishmaniasis in Guatemala. Am. J. Trop. Med. Hyg. 1990;42:43–50. doi: 10.4269/ajtmh.1990.42.43. [DOI] [PubMed] [Google Scholar]
- Nicolete R., Santos D.F. Dos, Faccioli L.H. The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response. Int. Immunopharmacol. 2011;11:1557–1563. doi: 10.1016/j.intimp.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Otsuka T., Takagi H., Horiguchi N., Toyoda M., Sato K., Takayama H., Mori M. CCl4-induced acute liver injury in mice is inhibited by hepatocyte growth factor overexpression but stimulated by NK2 overexpression. FEBS Lett. 2002;532:391–395. doi: 10.1016/S0014-5793(02)03714-6. [DOI] [PubMed] [Google Scholar]
- Silva R.E., Júnior A.T., Senna M.C., Rabello A., Cota G. Intralesional meglumine antimoniate for the treatment of localised cutaneous leishmaniasis : a retrospective review of a Brazilian referral centre. Mem. Inst. Oswaldo Cruz. 2016;111:512–516. doi: 10.1590/0074-0276016018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa N., Capitán Z., Nieto J., Nieto M., Calzada J., Paz H., Spadafora C., Kreishman-Deitrick M., Kopydlowski K., Ullman D., McCarthy W.F., Ransom J., Berman J., Scott C., Grogl M. Randomized, double-blinded, phase 2 trial of WR 279,396 (paromomycin and gentamicin) for cutaneous leishmaniasis in Panama. Am. J. Trop. Med. Hyg. 2013;89:557–563. doi: 10.4269/ajtmh.12-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Batista A.J., Arruda-Costa N., Rossi-Bergmann B., Ré M.I. Improved drug loading via spray drying of a chalcone implant for local treatment of cutaneous leishmaniasis. Drug Dev. Ind. Pharm. 2018;18:1–8. doi: 10.1080/03639045.2018.1461903. [DOI] [PubMed] [Google Scholar]
- Sousa-Batista A.J., Pacienza-Lima W., Arruda-Costa N., Falcão C.A.B., Ré M.I., Rossi-Bergmann B. Depot subcutaneous injection with chalcone CH8-loaded poly(Lactic-Co-Glycolic Acid) microspheres as a single-dose treatment of cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.01822-17. e01822-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliana S.R.B., Trinconi C.T., Coelho A.C. Chemotherapy of leishmaniasis: present challenges. Parasitology. 2017;20:1–17. doi: 10.1017/S0031182016002523. [DOI] [PubMed] [Google Scholar]
- Voak A.A., Standing J.F., Sepu N., Harris A., Croft S.L., Seifert K. Pharmacodynamics and cellular accumulation of amphotericin B and miltefosine in Leishmania donovani -infected primary macrophages. J. Antimicrob. Chemother. 2018;73:1314–1323. doi: 10.1093/jac/dky014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Qu W., Choi S.H. 2016. FDA's Regulatory Science Program for Generic PLA/PLGA - Based Drug Products [WWW Document]. Am. Pharm. Rev.http://www.americanpharmaceuticalreview.com/Featured-Articles/188841-FDA-s-Regulatory-Science-Program-for-Generic-PLA-PLGA-Based-Drug-Products/ accessed 7.17.2016. [Google Scholar]
- Wijnant G., Bocxlaer K., Van Francisco A.F., Yardley V., Harris A., Alavijeh M., Murdan S., Croft S.L. Local skin inflammation in cutaneous leishmaniasis as a source of variable pharmacokinetics and therapeutic efficacy of liposomal amphotericin B. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.00631-18. e00631-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnant G., Bocxlaer K. Van, Yardley V., Harris A., Alavijeh M., Silva-pedrosa R., Antunes S., Mauricio I., Murdan S., Croft S.L. Comparative efficacy, toxicity and biodistribution of the liposomal amphotericin B formulations Fungisome ® and AmBisome ® in murine cutaneous leishmaniasis. IJP Drugs Drug Resist. 2018;8:223–228. doi: 10.1016/j.ijpddr.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnant G., Bocxlaer K. Van, Yardley V., Harris A., Murdan S., Croft S.L. AmBisome® treatment of murine cutaneous leishmaniasis: relation between skin pharmacokinetics and efficacy. Antimicrob. Agents Chemother. 2018;62:e02009–e02017. doi: 10.1128/AAC.02009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Lin W., Xing C., Yang Y., Chi Q., Zhang H., Li Y., Li Z., Yang Y., Yang Z., Li M. In vitro and in vivo evaluations of PLGA microspheres containing nalmefene. PLoS One. 2015;10:1–19. doi: 10.1371/journal.pone.0125953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley V., Croft S.L. Activity of liposomal amphotericin B against experimental cutaneous leishmaniasis. Antimicrob. Agents Chemother. 1997;41:752–756. doi: 10.1128/AAC.41.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]