Abstract
Cytokines are essential regulatory components of the immune system, and their aberrant levels have been linked to many disease states. Despite increasing evidence that cytokines operate in concert, many of the physiological interactions between cytokines, and the shared genetic architecture that underlies them, remain unknown. Here, we aimed to identify and characterize genetic variants with pleiotropic effects on cytokines. Using three population-based cohorts (n = 9,263), we performed multivariate genome-wide association studies (GWAS) for a correlation network of 11 circulating cytokines, then combined our results in meta-analysis. We identified a total of eight loci significantly associated with the cytokine network, of which two (PDGFRB and ABO) had not been detected previously. In addition, conditional analyses revealed a further four secondary signals at three known cytokine loci. Integration, through the use of Bayesian colocalization analysis, of publicly available GWAS summary statistics with the cytokine network associations revealed shared causal variants between the eight cytokine loci and other traits; in particular, cytokine network variants at the ABO, SERPINE2, and ZFPM2 loci showed pleiotropic effects on the production of immune-related proteins, on metabolic traits such as lipoprotein and lipid levels, on blood-cell-related traits such as platelet count, and on disease traits such as coronary artery disease and type 2 diabetes.
Keywords: GWAS, multivariate analysis, colocalisation analysis, cytokines, eQTLs, protein QTLs, cardiometabolic diseases, blood cell traits, metabolites
Introduction
Cytokines are signaling molecules secreted by cells, and they are central to multiple physiological functions, especially immune regulation.1 Broadly speaking, cytokines include chemokines, which drive movement of cells, and growth factors, which drive cell growth and proliferation. Changes in circulating cytokine levels have been associated with infection,2 autoimmune diseases,3 and malignancies,4 as well as atherosclerosis and cardiovascular disease.5,6 The expression of cytokines can be strongly regulated by genetic variation,7 and several studies have identified cis-acting genetic variants associated with circulating levels of certain cytokines and their receptors under various conditions.8, 9, 10 These initial studies laid the foundation for genetic investigation of circulating cytokine levels at a scale and breadth that may improve our understanding of individual differences in immune response, inflammation, infection, and common disease susceptibility.
Despite cytokines operating in concert to facilitate immune regulation, genome-wide association studies (GWAS) have typically focused on individual cytokines.11, 12, 13, 14, 15, 16, 17, 18 The most extensive cytokine GWAS to date separately analyzed individual levels of 41 circulating cytokines in approximately 8,000 individuals, identifying 27 distinct loci each associated with at least one cytokine.19 Others have identified loci influencing cytokine production in response to pathogens.20,21 While these previous GWAS utilized a univariate framework, analyzing each cytokine separately, studies of related traits indicate that a multivariate framework can confer greater statistical power, for example by taking advantage of the tightly co-regulated nature of both pro- and anti-inflammatory cytokines.
Several methods for multivariate GWAS of correlated phenotypes have been developed.22, 23, 24, 25, 26, 27 Simulations have shown that multivariate analysis can result in increased power to detect genetic associations with small or pleiotropic effects across phenotypes.22,28, 29, 30 These have largely been conducted on metabolic traits where they have demonstrated a boost in statistical power. For example, multivariate analysis of four lipid traits led to a 21% increase in independent genome-wide significant variants compared to univariate analysis.23 Similar findings were shown for other metabolic traits.24,31 Moreover, complex genotype-phenotype dependencies have been revealed when variants were jointly tested with lipoprotein traits.32 Notably, a multivariate GWAS of networks of highly correlated serum metabolites was able to detect nearly twice the number of loci compared to univariate testing, with downstream tissue-specific transcriptional analyses showing that the top candidate genes from multivariate analysis were upregulated in atherosclerotic plaques.31
In this study, we focused on correlated immune traits by leveraging the correlation structure within a network of 11 cytokines to perform a multivariate genome-wide scan in 9,263 individuals from three population-based cohorts. We then investigated the colocalization of cytokine-associated variants with those regulating gene expression in numerous tissues and cell types, circulating protein and metabolite levels, hematological traits, and disease states. Finally, we highlighted and characterized variants as potential master regulators of the cytokine network, with pleiotropic effects on production of inflammatory proteins, immune cell function, lipoprotein and lipid levels, and cardiometabolic diseases.
Material and Methods
Study Populations
Approval for the study protocols for each cohort was obtained from their respective ethics committees, and all subjects enrolled in the study gave written informed consent.
The Cardiovascular Risk in Young Finns Study (YFS) is a longitudinal prospective cohort study that commenced in 1980, with follow-up studies carried out every three years. The purpose of this study was to monitor the risk factors of cardiovascular disease in children and adolescents from different regions of Finland. In the baseline study, which was conducted in five Finnish metropolitan areas (Turku, Helsinki, Kuopio, Tampere, and Oulu), a total of 3,596 children and adolescents were randomly selected from the national public register, the details of which were described by Raitakari, et al.33 Follow-up studies have been carried out every three years, in 1983, 1986, 1989, 2001, 2007, and 2011. For this current study, we utilized data from 2,204 participants (aged 30–45 years) who responded to the 2007 follow-up study (YFS07). Of these, 2,018 individuals had matched cytokine and genotype data available. Ethics were approved by the Joint Commission on Ethics of the Turku University and the Turku University Central Hospital.
The FINRISK cohorts were part of a cross-sectional population-based survey; such studies have been carried out every five years since 1972 in order to evaluate the risk factors of chronic diseases in the Finnish population.34 Each survey has recruited a representative random sample of 6,000–8,800 individuals, within the age group of 25–74 years, chosen from the national population information system. This study utilized samples from the 1997 (FINRISK97) and 2002 (FINRISK02) collections, which recruited individuals from five or six (for FINRISK02) major regional and metropolitan areas of Finland; the provinces of North Karelia, Northern Savo, Northern Ostrobothnia, Kainuu, and Lapland; the Turku and Loimaa region of southwestern Finland; and the Helsinki and Vantaa metropolitan area. In total, 8,444 (aged 24–74 years) and 8,798 (aged 51–74 years) individuals participated in the FINRISK97 and FINRISK02 studies, respectively. Importantly, each FINRISK survey is an independent cohort, each comprising a different set of participants. Ethics were approved by the coordinating ethical committee of the Helsinki and Uusimaa hospital district, Finland. For FINRSK97, cytokines profiles were measured for all participants where high-quality blood samples were still available. For FINRISK02, cytokine profiling was restricted to older participants (>50 years) due to budget constraints. Cytokine measurements and matched genotype data were available for a subset of 5,728 FINRISK97 participants and 2,027 FINRISK02 participants.
Blood Sample Collection
Blood samples and detailed information on various physical and clinical variables for the YFS and FINRISK cohorts were collected using similar protocols to those described previously.33,34 Venous blood was collected following an overnight fast for the YFS cohort, while non-fasting blood was collected for FINRISK. Samples were centrifuged, and the resulting plasma and serum samples were aliquoted into separate tubes and stored at −70°C for later analyses.
Genotype Processing and Quality Control
Genotyping in YFS and FINRISK cohorts was performed on whole blood genomic DNA. For YFS07 (n = 2,442), a custom 670K Illumina BeadChip array was used for genotyping. For FINRISK97 (n = 5,798), the Human670-QuadCustom Illumina BeadChip platform was used for genotyping. For FINRISK02 (n = 5,988), the Human670-QuadCustom Illumina BeadChip (n = 2,447) and the Illumina Human CoreExome BeadChip (n = 3,541) were used for genotyping. The Illuminus clustering algorithm was used for genotype calling,35 and quality control (QC) was performed using the Sanger genotyping QC pipeline. This included removal of SNPs and samples with >5% genotype missingness followed by removal of samples with gender discrepancies. Genotypes were then imputed with IMPUTE236 through the use of the 1000 Genomes Phase 1 version 3 as the reference panel followed by removal of SNPs with call rate < 95%, imputation “info” score < 0.4, minor allele frequency < 1%, and Hardy-Weinberg equilibrium p value < 5 × 10−6. In instances where data were generated using different genotyping platforms, overlapping SNPs were merged using PLINK version 1.90 software.37 A total of 6,664,959, 7,370,592, and 6,639,681 genotyped and imputed SNPs passed QC in YFS, FINRISK97, and FINRISK02, respectively. Cryptic relatedness was assessed using identity by descent (IBD) estimates, and in cases where the pi-hat relatedness was greater than 0.1, one of the two individuals was randomly removed (n = 44 for YFS, n = 291 for FINRISK97, and n = 39 for FINRISK02). Genetic principal components (PCs) were obtained through principal component analysis (PCA) using FlashPCA38 on ∼60,000 linkage disequilibrium (LD)-pruned SNPs. LD-based pruning was performed to remove SNPs that exceeded an r2 threshold of 0.05 through the use of PLINK’s –indep-pairwise command (SNP window = 100, SNPs shifted each time = 10, r2 threshold = 0.05).
Measurement of Cytokines
Concentrations of cytokines, chemokines, and growth factors (hereafter referred to as cytokines) were measured in serum (YFS07), EDTA plasma (FINRISK97), and heparin plasma (FINRISK02) using multiplex fluorescent bead-based immunoassays (Bio-Rad). A total of 48 cytokines were measured in YFS07 (n = 2,200) and FINRSK02 (n = 2,775) using two complementary array systems: the Bio-Plex ProTM Human Cytokine 27-plex assay and Bio-Plex ProTM Human Cytokine 21-plex assay. For FINRISK97, 19 cytokines were assayed on the Human Cytokine 21-plex assay system. All assays were performed in accordance with the manufacturer’s instructions, except that beads, detection antibodies, and streptavidin-phycoerythrin conjugate were used at half their recommended concentrations. Fluorescence intensity values determined using the Bio-Rad’s Bio-Plex 200 array reader were converted to concentrations from the standard curve generated by the Bio-Plex™ Manager 6.0 software. For each cytokine, a standard curve was derived by fitting a five-parameter logistic regression model to the curve obtained from standards provided by the manufacturer. Cytokines with concentrations at the lower and upper asymptotes of the sigmoidal standard curve were set to the concentration corresponding to the fluorescent intensity 2% above or below the respective asymptotes.
Cytokine Data Filtering, Normalization, and Clustering
The analysis was limited to 18 cytokines (Table S1) assayed in all three cohorts. Although Interleukin 1 receptor, type I (IL-1Ra) was assayed in all three cohorts, it was excluded from the analyses due to its inconsistent Pearson correlation pattern with the other 18 cytokines across the three datasets.
Before normalization, cytokine data were subset to individuals with matched genotype data in YFS07 (n = 2,018), FINRISK97 (n = 5,728), and FINRISK02 (n = 2,775). We excluded individuals in YFS07 who reported febrile infection in the two weeks prior to blood sampling (n = 92). To identify extreme outlier samples, PCA was performed on the log2 transformed cytokine values through the use of the missMDA R package.39 This method first imputed the missing cytokine values via a regularized iterative PCA algorithm implemented in the imputePCA function, then performed PCA. Three and two outlier samples were removed from FINRISK97 and FINRISK02, respectively. Based on IBD analysis described above, 44 (YFS07), 291 (FINRISK97), and 39 (FINRISK02) individuals were also removed. After filtering, a total of 1,843, 5,434, and 1,986 individuals passed QC in YFS07, FINRISK97, and FINRISK02, respectively, and these were used for downstream analysis.
Since all 18 cytokines displayed non-Gaussian distributions, we performed normalization of cytokine levels. For YFS07, the lower limit of detection (LOD) was available for each cytokine. Reported values that were below the LOD were indistinguishable from background noise signals or instrument error40 and were excluded and treated as missing. For FINRISK97 and FINRISK02, the detection limits were not available; however, it was observed that these two datasets exhibited a bimodal distribution, with the leftmost peak below the expected LOD when compared to the YFS dataset. Individuals in the leftmost peak were therefore set to missing. The log2-transformed cytokine values were then normalized to follow standard Gaussian distributions (with mean of 0 and SD of 1) using rank-based inverse normal transformation (rntransform) as implemented in the GenABEL R package.41 For each study group, residuals for all cytokines were calculated by regressing the normalized cytokine values on age, sex, BMI, lipid and blood pressure medication, pregnancy status (FINRISK97), and the first 10 genetic PCs through the use of a multiple linear regression model. Of note, information on pregnancy status was only available for the FINRISK97 cohort (n = 52; ∼2% of the women). The FINRISK02 is an older cohort (aged 51-74 years), so we do not expect any pregnant women in this cohort. Density distribution plots were generated to confirm that the resulting cytokine residuals were still normally distributed (data not shown).
Detection of groups of correlated cytokines was done in FINRISK97, the cohort with the largest sample size. Pairwise Pearson correlation was performed among residuals of 18 cytokines. These cytokines were then subjected to hierarchical clustering, with one minus the absolute correlation coefficient used as the dissimilarity metric. We then defined a cytokine network—a group of 11 cytokines that were moderately to highly correlated (r > 0.57)—for subsequent use in the multivariate analysis.
Statistical Analysis
Univariate association analysis was carried out with linear regression in PLINK,37 where the residuals of each cytokine were regressed on each SNP genotype. Summary statistics at each marker across three datasets were then combined in a meta-analysis using the METAL software program,42 which implemented a weighted Z-score method. Since 11 hypothesis tests were performed for each SNP, genome-wide significance was formally set at p value < 4.55 × 10−9, i.e., dividing the standard genome-wide significance threshold (p value < 5 × 10−8) by 11.
Multivariate testing (MV) was performed under the canonical correlation framework implemented in PLINK (MV-PLINK),22 which extracted the linear combination of traits most highly correlated with genotypes at a particular SNP. The test is based on Wilks’ Lambda (λ = 1−ρ2), where ρ is the canonical correlation coefficient between the SNP and the cytokine network. Corresponding p values were computed by transforming Wilks’ Lambda into a statistic that approximates an F distribution, and the loadings for each cytokine represented their individual contributions toward the multivariate association result.22 Since the multivariate beta-coefficients and standard errors were not calculated by MV-PLINK, the cohort-level multivariate p values were combined in a meta-analysis using the weighted Z-score method43,44 implemented in the metap R package. In brief, the p values for each dataset were transformed into unsigned Z-scores and weighted by their respective sample sizes, and the sum of each of these weighted Z-scores was then divided by the square root of the sum of squares of the sample size for each study. The combined weighted Z-scores obtained were then back-transformed into p values. Complete summary statistics from meta-analyses will be made available through the NHGRI-EBI GWAS Catalog.
To assess the inflation of the test statistics as a result of population structure, quantile-quantile (Q-Q) plots of observed-versus-expected log10 p values were generated from the multivariate analyses of the three datasets, both individually and meta-analyzed. Corresponding genomic inflation factor (λ) was calculated by taking the ratio of the median observed distribution of p values to the expected median.
To investigate the existence of additional independent signals within the significant multivariate loci, a conditional stepwise multivariate meta-analysis was performed within each locus. For each study cohort, the lead SNP at each locus (p value < 5 × 10−8), together with other covariates, was fitted in a linear regression model for each cytokine in the network. The resulting residuals were provided as an input for the multivariate test of the locus being assessed. The cohort-level conditional p values were then combined in a meta-analysis. The stepwise conditional analysis was repeated in the univariate model with the lead multivariate SNPs until no additional significant signal was identified.
Colocalization Analysis
Bayesian colocalization tests between cytokine-network-associated signals and the following trait- and disease-associated signals were performed using the COLOC R package.45 For whole blood cis expression quantitative trait loci (eQTLs), we downloaded publicly available summary data from the eQTLGen Consortium portal. The eQTLGen Consortium analysis is the largest meta-analysis of blood eQTLs to date and comprises of 31,684 blood and peripheral blood mononuclear cell (PBMC) samples from a total of 37 datasets.46 For immune cell cis-eQTLs, we either generated cis-eQTL summary data in resting B cells,47 resting monocytes,48 and stimulated monocytes with interferon-γ or lipopolysaccharide,48 or obtained publicly available cis-eQTL summary data generated by the BLUEPRINT consortium in neutrophils and CD4+ T cells.57 For cis-eQTL mapping in B cells and monocytes (resting and stimulated), information on accessing the raw gene expression and genotype data, data pre-processing, and cis-eQTL analysis has been described in a previous study.50 For protein QTLs (pQTLs), we used publicly available SomaLogic plasma protein GWAS summary statistics from the INTERVAL study.17 A colocalization test was performed for loci where the cytokine-network-associated variants (within 200 kb from the lead SNP) were also influencing protein levels, either in cis (cis-pQTLs) or trans (trans-pQTLs), at pQTL p value < 1 × 10−6. For disease or complex trait associations, we compiled summary statistics of 185 diseases and quantitative traits from GWAS studies conducted in European ancestry individuals, which were accessed from the UK Biobank (Table S2), or downloaded from either ImmunoBase, the NHGRI-EBI GWAS Catalog, or LD Hub. Here, we only considered immune-related and cardiometabolic diseases. For each cytokine network locus, we only tested traits or diseases with the minimum association p value < 1 × 10−6 at this locus. COLOC requires either beta-coefficients and its variance, or p values, for each SNP, in addition to MAF and sample size. Since PLINK multivariate did not produce beta values and standard errors, we instead used meta-analyzed p values for the multivariate cytokine GWAS summary data. For each association pair assessed for colocalization, SNPs within 200 kb of the lead multivariate cytokine GWAS SNP were considered. COLOC (coloc.abf) was run with default parameters and priors. COLOC computed posterior probabilities for the following five hypotheses: PP0, no association with trait 1 (cytokine GWAS signal) or trait 2 (e.g., eQTL signal); PP1, association with trait 1 only (i.e., no association with trait 2); PP2, association with trait 2 only (i.e., no association with trait 1); PP3, association with trait 1 and trait 2 by two independent signals; and PP4, association with trait 1 and trait 2 by shared variants. In practice, evidence of colocalization was defined by PP3 + PP4 ≥ 0.99 and PP4/PP3 ≥ 5, a cut off previously suggested.50 To further explore the possibility of colocalization with secondary multivariate cytokine-network- associated signals, we conducted colocalization analyses with conditional p values obtained from the stepwise conditional multivariate GWAS meta-analysis. A sensitivity analysis was further performed using the “sensitivity” function of the COLOC package. The sensitivity analysis takes the COLOC output and assesses whether the posterior inference is robust to the priors used in the colocalization analysis at a predefined rule, which was set to PP3 + PP4 ≥ 0.99 and PP4/PP3 ≥ 5 (threshold used as evidence for colocalization). The predefined decision rule determines the values of the posterior probabilities considered acceptable for the given priors. The sensitivity analysis demonstrated that the posterior for each colocalizing pair was robust to the priors chosen, in particular to the choice of p12 (1 × 10−5); hence, all colocalization analysis was performed using default priors. The output from the sensitivity analysis was indicated as “pass” in all the colocalization results reported.
Multi-trait colocalization analysis was performed with the MOLOC tool51 using default prior probabilities. This analysis was performed to assess whether the pairwise cytokine-to-disease and cytokine-to-molecular trait colocalizations, at the ABO locus, involved the same shared casual variant in a three-way colocalization analysis (e.g., CAD-to-cytokine network-to-protein). Only SNPs that were within 200 kb of the lead multivariate cytokine GWAS SNP and were common among all three datasets were assessed. We considered a posterior probability of associations (PPA) threshold of ≥80% as strong evidence that the disease, cytokine network, and complex trait (e.g., eQTL, proteins, metabolites, or blood cell traits) colocalized and shared a causal variant.
Results
Summary of Cohorts and Data
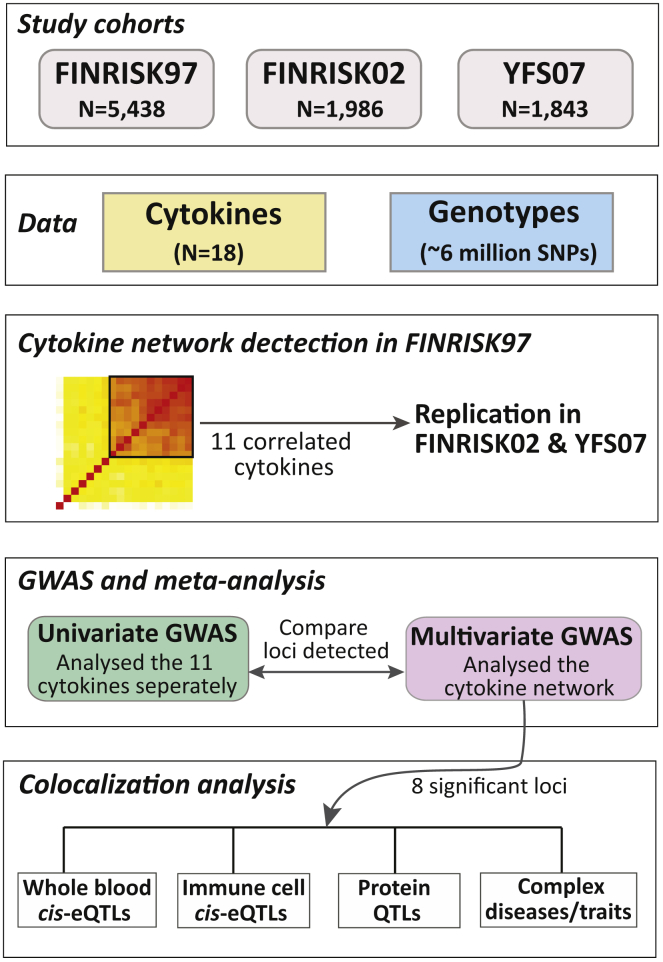
Our final dataset comprised a total of 9,267 individuals enrolled in three population-based studies, YFS07 (n = 1,843), FINRISK97 (n = 5,438), and FINRISK02 (n = 1,986), all of whom had available genome-wide genotype data and quantitative measurements of 18 cytokines (Table S1). Characteristics of the study cohorts are summarized in Table 1. Genotypes for the three datasets were imputed with IMPUTE236 using the 1000 Genomes Phase 1 version 3 of the reference panel. After QC, a total of 6,022,229 imputed and genotyped SNPs were available across all cohorts. Cytokine levels were measured in serum and plasma through the use of Bio-Plex ProTM Human Cytokine 27-plex and 21-plex assays, then subsequently normalized and adjusted for covariates, including age, sex, BMI, pregnancy status, blood-pressure-lowering medication, lipid-lowering medication, and population structure (see Material and Methods). An overview of the study is shown in Figure 1.
Table 1.
Summary of Descriptive Characteristics of the Three Study Cohorts
| Characteristics | FINRISK97 | FINRISK02 | YFS07 |
|---|---|---|---|
| Collection year | 1997 | 2002 | 2007 |
| Number of individuals with matched cytokine and genotype data | 5,438 | 1,986 | 1,843 |
| Number of males (%) | 2,637 (48.5) | 991(49.9) | 841 (45.6) |
| Mean age in years (and range) | 47.6 (24–74) | 60.3(51–74) | 37.7 (30–45) |
| BMI (kg/m2); mean ± SD. | 26.6 ± 4.6 | 28.1 ± 4.5 | 25.9 ± 4.6 |
| Number of individuals on lipid lowering drugs (%) | 174 (3.2) | 284 (14.3) | 40 (2.2) |
| Number of individuals on blood pressure treatment drugs (%) | 698 (12.8) | 512 (25.8) | 127 (6.9) |
Abbreviations: BMI, body mass index; YFS, Young Finns Study
The numbers beside the cohort names refer to the calendar year (collection year) in which the samples and clinical information were obtained from each cohort.
Figure 1.
Overview of the Study Populations, Design, and the Analyses Conducted
A Correlation Network of Circulating Cytokines
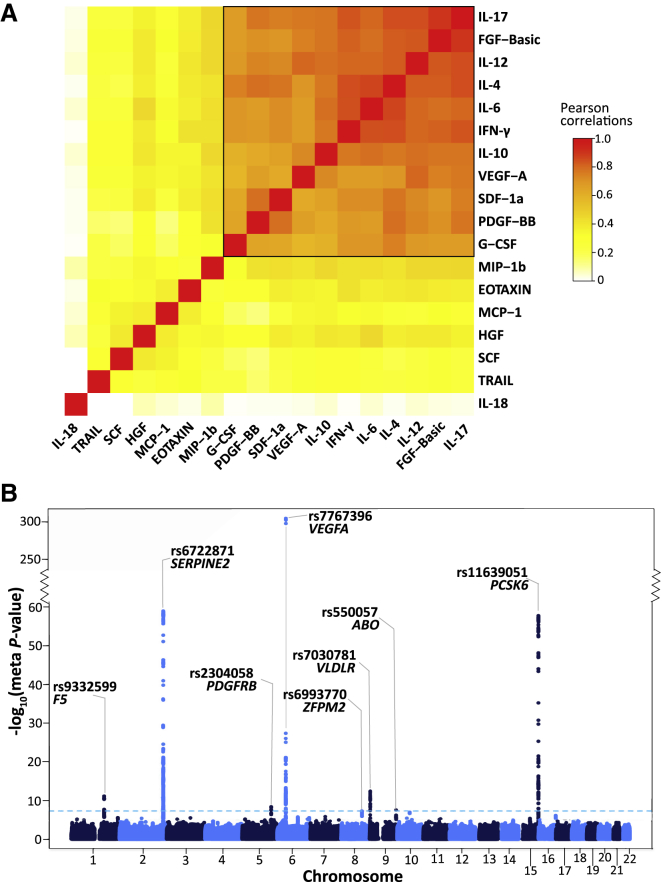
To characterize the correlation structure of circulating cytokines, we utilized the largest dataset available (FINRISK97) and the set of 18 cytokines overlapping all three cohorts. IL-18 was very weakly correlated with other cytokines (Figure 2A), while TRAIL, SCF, HGF, MCP-1, EOTAXIN, and MIP-1b showed moderate correlation with the others. A distinct set of 11 cytokines showed high correlation among themselves (median r = 0.75). In the smaller cohorts (YFS07 and FINRISK02), the cytokine correlation structure was similar but weaker (Figure S1), and the set of 11 cytokines also showed relatively high correlation (YFS07 median r = 0.42; FINRISK02 median r = 0.46). We used this set of 11 cytokines (denoted below as the cytokine network) for multivariate association analysis.
Figure 2.
Multivariate GWA Analysis of a Network of 11 Correlated Cytokines in Three Finnish Cohorts
(A) Correlation heatmap of the 18 cytokines in the FINRISK97 cohort. Each cell presents the pairwise Pearson’s correlation coefficient between the normalized cytokine residuals. The cytokines are ordered by hierarchical clustering, using 1 minus the absolute value of the correlations as the distance matrix. The color scale denotes the strength of the correlations, where red is a high positive correlation. The group of 11 tightly correlated cytokines (black box) was used for multivariate analysis.
(B) Manhattan plot for meta-analysis results from the multivariate GWAS of the cytokine network. The statistical strength of association (-log10 meta-p value; y axis) is plotted against all the SNPs ordered by chromosomal position (x axis). The sky-blue horizontal dashed line represents the genome-wide (meta-p value < 5 × 10−8) significance threshold. The lead SNP (lowest meta-p value) at each locus and the nearby genes are shown.
The cytokine network included both anti-inflammatory (IL-10, IL-4, IL-6) and pro-inflammatory (IL-12, IFN-γ, IL-17) cytokines as well as growth factors (FGF-basic, PDGF-BB, VEGF-A, G-CSF) and a chemokine (SDF-1a) involved in promoting leukocyte extravasation and wound healing.52, 53, 54 These cytokines were all positively correlated, which is likely indicative of counter-regulatory (negative-feedback) mechanisms among pro-inflammatory and anti-inflammatory pathways, such as those of IFN-γ and IL-10.55
Multivariate Genome-Wide Association Analysis for Cytokine Loci
We performed a multivariate GWAS on the cytokine network in each cohort separately, then cohort-level results were combined using meta-analysis (see Material and Methods). Since one hypothesis test (corresponding to the cytokine network) was performed for each SNP, a genome-wide significance threshold of p < 5 × 10−8 was used. Minimal inflation was observed for the cohort-level and meta-analysis test statistics with lambda (λ) inflation ranging from 1.00–1.02 (Figure S2A–S2D). To directly compare the statistical power of multivariate to univariate GWAS, we first performed univariate analysis in each dataset by regressing each of the cytokines in the cytokine network individually on each SNP, and we then combined the results in a meta-analysis. To account for the 11 cytokines tested, the genome-wide significance threshold was set at p < 4.55 × 10−9. For comparison, we selected the smallest univariate meta-analysis p value for any cytokine at a given locus.
We identified eight loci reaching genome-wide significance for the cytokine network (Figure 2B; Table 2). The strongest association was rs7767396 (meta-p value = 6.93 × 10−306), a SNP located 172 kb downstream of vascular endothelial growth factor A (VEGFA [MIM: 192240]) (Figure S3A). The VEGFA locus was previously identified in GWAS for individual cytokine levels, including VEGF-A, IL-7, IL-10, IL-12, and IL-13.14,19 Consistent with these earlier results, we found that VEGF-A, IL-10, and IL-12 were the top three cytokines based on their trait loadings (relative contribution of each cytokine to the multivariate association result) in each cohort and also significantly associated with this locus in the univariate scans (Figure S4A). Multivariate analysis also confirmed four other previously known associations,14,16,19 including loci harboring SERPINE2 (MIM: 177010) (rs6722871; meta-p value = 1.19 × 10−59), ZFPM2 (MIM: 603693) (rs6993770; meta-p value = 4.73 × 10−8), VLDLR (MIM: 192977) (rs7030781; meta-p value = 3.78 × 10−13), and PCSK6 (MIM: 167405) (rs11639051; meta-p value = 1.93 × 10−58) (Figure 2B; Table 2; Figure S3B–S3E). The cytokine with the highest loading at each of these loci was consistent with those previously identified in univariate analysis (Figure S4B–S4E).
Table 2.
Meta-Analyzed Results of Multivariate GWAS of Cytokine Network
| Locus | Locus Region | Top SNP | Average MAF | Top Multivariate Meta-p value |
Univariate Meta-p value (Top Cytokine) |
Detection |
|---|---|---|---|---|---|---|
| F5 | 1q24.2 | rs9332599 | 0.294 | 7.17 × 10−12 | 9.21 × 10−3 (SDF1-a) | multivariate |
| SERPINE2 | 2q36.1 | rs6722871 | 0.311 | 1.19 × 10−59 | 3.55 × 10−18 (PDGF-BB) | both |
| PDGFRB | 5q32 | rs2304058 | 0.379 | 4.06 × 10−9 | 1.52 × 10−5 (IL4) | multivariate |
| VEGFA | 6p21.1 | rs7767396 | 0.471 | 6.93 × 10−306 | 3.10 × 10−201 (VEGF-A) | both |
| ZFPM2 | 8q23.1 | rs6993770 | 0.221 | 4.73 × 10−8 | 1.01 × 10−7 (IL12p70) | multivariate |
| ABO | 9q34.2 | rs550057 | 0.306 | 2.75 × 10−8 | 4.9 × 10−3 (IL4) | multivariate |
| VLDLR | 9p24.2 | rs7030781 | 0.413 | 3.78 × 10−13 | 6.78 × 10−14 (VEGF-A) | both |
| PCSK6 | 15q26.3 | rs11639051 | 0.255 | 1.93 × 10−58 | 1.19 × 10−26 (PDGF-BB) | both |
| JMJD1C | 10q21.3 | rs9787438 | 0.374 | a1.30 × 10−7 | a8.96 × 10−12 (VEGF-A) | univariate |
The table shows the meta-analysis p values for the top SNP (lowest p value) at each locus associated with the cytokine network in the multivariate analysis at genome-wide significance threshold (p < 5 × 10−8). The corresponding lowest meta-p value for the same top SNP in the univariate analysis with any single cytokine present in the cytokine network, given in brackets beside the meta-p value, was also reported.
Instance where the top SNP at a locus crossed only the univariate significance threshold (p < 4.55 × 10−9), then the corresponding meta-p value for that SNP in the multivariate was also given. The univariate significance threshold was calculated from a Bonferroni correction for 11 cytokines tested (p < 5 × 10−8/11).
The multivariate GWAS also detected novel cytokine associations not identified in any previous univariate tests of these cytokines. These were three loci with genic lead SNPs in the candidate genes F5 (MIM: 612309), PDGFRB (MIM: 173410), and ABO (MIM: 110300). The lead variant at the F5 locus (rs9332599; meta-p value = 7.17 × 10−12) is located in intron 12 of F5 (Figure S3F). At the platelet-derived growth factor receptor-beta (PDGFRB) locus, the lead variant rs2304058 (meta-p value = 4.06 × 10−9) is within intron 10 of PDGFRB (Figure S3G). At the ABO locus, the lead variant rs550057 (meta-p value = 2.75 × 10−8) is within the first intron of ABO (Figure S3H); furthermore, rs550057 is located ∼1.6 kb upstream of the erythroid cell specific enhancer, which contains a GATA-1 transcription factor binding site and has been shown to enhance the transcription of the ABO gene.56
To investigate the presence of multiple independently associated variants at each of the eight loci, we performed stepwise conditional multivariate meta-analysis. Three loci (SERPINE2, VEGFA, and PCSK6) exhibited evidence of multiple independent signals (Table S3). In addition to the lead variants (rs6722871, rs7767396, and rs11639051) at each of these three loci, we identified additional association signals (rs55864163, SERPINE2, meta-pcond= 9.03 × 10−29; rs112215592, SERPINE2, meta-pcond = 2.10 × 10−12; rs4714729, VEGFA, meta-pcond = 7.49 × 10−10; and rs6598475, PCSK6, meta-pcond = 2.63 × 10−17), which were independently associated with the cytokine network. We also performed conditional univariate analysis that adjusted for the lead multivariate SNPs, which were either the same lead univariate SNPs or in high LD (r2 = 0.99). This univariate analysis also uncovered the same secondary signal at the VEGFA locus in association with VEGF-A cytokine levels (rs4714729; meta-pcond = 8.8 × 10−13) (Table S3).
Colocalization of Cytokine Variants with cis-eQTLs in Whole Blood
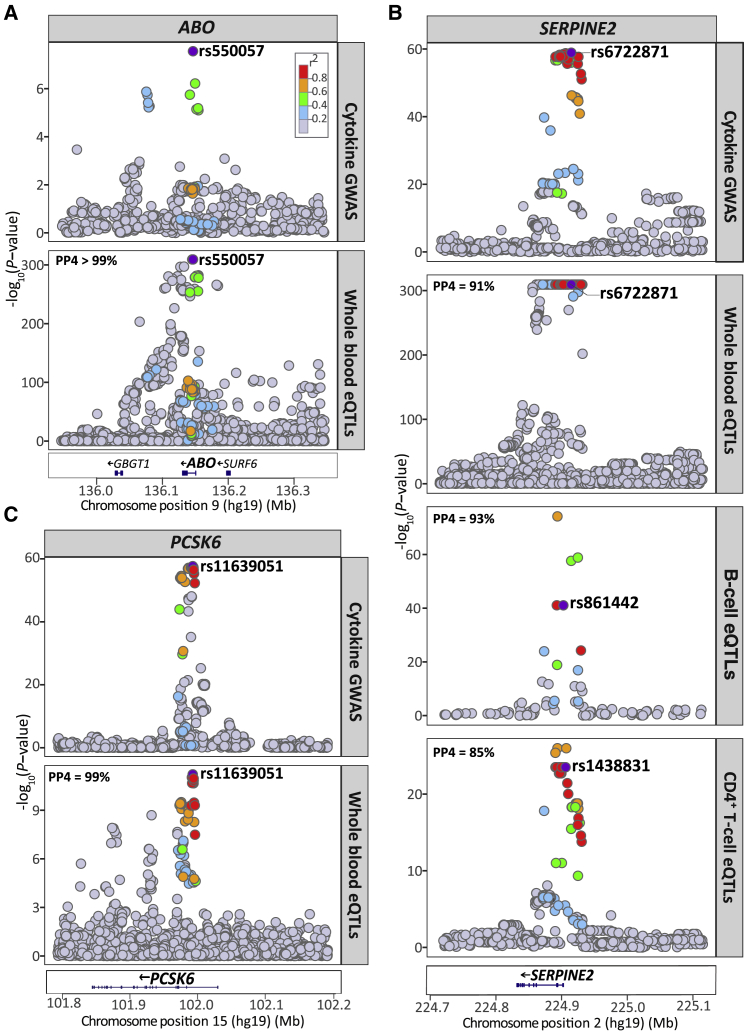
To characterize the regulatory effects of the multivariate cytokine-associated loci, we queried the largest publicly available set of results for whole blood cis-eQTLs, a meta-analysis of 31,684 individuals, which was obtained from the eQTLGen Consortium database.46 We found SNPs, lead or LD-proxy (r2>0.5), at seven of the eight cytokine loci (ABO, F5, PCSK6, PDGFRB, SERPINE2, VEGFA, and VLDLR) with cis-regulatory effects (p value < 1 × 10−6) on gene expression (for a total of 17 unique genes) in blood (Table S4). Using Bayesian colocalization analysis, we further demonstrated that associations at three of these loci colocalized with cis-eQTLs for ABO, PCSK6, and SERPINE2 expression (Figure 3A–3C; Table S5). We did not observe colocalization with the secondary multivariate GWAS signals at the PCSK6 and SERPINE2 loci.
Figure 3.
Regional Plots for the Cytokine Network Association and Whole Blood and Immune Cell cis-eQTL Association Signals at the ABO, PCSK6, and SERPINE2 Locus
(A) The cytokine network GWAS signal (top) colocalizes with the whole blood cis-eQTLs signal for ABO (bottom) at the ABO locus on chromsome 9; (B) colocalizes with whole blood cis-eQTLs for PCSK6 expression (bottom) at the PCSK6 locus on chromosome 15; (C) colocalizes with the cis-eQTL signals for SERPINE2 expression in whole blood (middle), B cells (middle), and CD4+ T cells (bottom) at the SERPINE2 locus on chromosome 2. For each plot, the circles represent the -log10 association p values (y axis) of SNPs plotted against their chromosomal position (x axis). The eQTL association plots show the lead cytokine network GWAS SNP tested in the colocalization analysis. The lead cytokine network GWAS SNP rs6722871 was not present in the B cell and CD4+ T cell eQTL dataset, instead, the next top GWAS SNP present in each of the eQTL dataset (rs861442, B cell; rs1438831, CD4+ T cell) is shown. For all regional plots, pairwise LD (r2) in the region is colored with respect to the lead cytokine network GWAS SNP. LD was calculated from the 1000 Genomes European population.
Colocalization of Cytokine Variants with Immune Cell-Specific cis-eQTLs
Next, we investigated the cell-type- or context-dependent regulatory effects of genetic variants associated with the cytokine network by interrogating previously published cis-eQTLs specific to resting B cells,47 resting monocytes,48 stimulated monocytes with interferon-γ or lipopolysaccharide,48 resting neutrophils,57 naive CD4+ T cells49,57 and CD8+ T cells,49 all isolated from healthy donors of European ancestry (Table S6). Three out of the eight cytokine network loci harbored cis-eQTLs (p value < 1 × 10−6) in at least one immune cell type, in either a stimulated or a non-stimulated state (Table S7). For example, SNPs at the SERPINE2 locus were reported to have cis-eQTL effects across multiple immune cell types, including B cells, CD4+, and CD8+ T cells (Table S7).
Further, colocalization analysis showed that the cytokine network variants at SERPINE2 had strong evidence of sharing a causal variant with SERPINE2 cis-eQTLs in CD4+ T cells and B cells, similar to the colocalization we observe in whole blood (Figure 3B; Table S8). Evidence of colocalization was not observed with the secondary multivariate GWAS signals at this locus.
Colocalization of Cytokine Variants with Plasma Protein QTLs
To investigate protein-level effects of cytokine network variants, we utilized plasma protein QTLs (pQTLs) from the INTERVAL study.17 Colocalization analysis, considering only proteins with both cis- and trans-pQTLs, at the cytokine network loci, with association p value < 1 × 10−6, showed that all the eight cytokine network loci had strong evidence of shared causal variants with plasma levels of a total of 146 proteins (out of the 215 tested) (Table S9). Of these, the ABO and ZFPM cytokine network loci strongly colocalized with trans-pQTL signals for 55 (out of 81) and 87 (out of 98) proteins, respectively (Table 3; Table S9). Of these, 14 and 75 proteins shared the same causal lead pQTLs with the lead cytokine network variants at the ABO (rs550057) and ZFPM2 (rs6993770) loci, respectively, suggesting these variants have extensive pleiotropic effects on multiple cytokines and proteins—which potentially have shared underlying pathophysiology and/or biology.
Table 3.
Colocalization of Cytokine-Network-Associated Variants at the ABO and ZFPM2 Loci with Those of Plasma Protein Levels, Quantitative Traits, and Disease Risk
| Group/ Functions | Evidence | Names |
|---|---|---|
| ABO Locus (Chromosome 9) | ||
| Diseases | ||
| Cardiometabolic diseases | strong | pulmonary embolism, ischemic stroke, coronary artery disease, type 2 disease, |
| Cardiometabolic diseases | none | deep vein thrombosis |
| Blood Cell Traits | ||
| Blood cell counts | Strong | white blood cell, granulocytes, basophils + eosinophils, basophils + neutrophils, eosinophils + neutrophils, eosinophils, neutrophils, hematocrit (%), haemoglobin, myeloid, red blood cells, platelet distribution width |
| Blood cell counts | Suggestive | basophils, reticulocytes |
| Blood cell counts | None | monocyte, platelet, plateletcrit (%), red cell distribution width |
| Metabolites | ||
| IDL particle constituents | strong | Total cholesterol (IDL-C), free cholesterol (IDL-FC), total lipids (IDL-L), total particle concentration (IDL-P), phospholipids (IDL-PL), triglycerides (IDL-TG) |
| LDL subclass particle constituents | strong | For large particles: total cholesterol (L-LDL-C), cholesterol esters (L-LDL-CE), free cholesterol (L-LDL-FC), total lipids (L-LDL-L), total particle concentration (L-LDL-P), phospholipids (L-LDL-PL), For medium particles: total cholesterol (M-LDL-C), cholesterol esters (M-LDL-CE), total lipids (M-LDL-L), total particle concentration (M-LDL-P), phospholipids (M-LDL-PL) For small particles: total cholesterol (S-LDL-C), total lipids (S-LDL-L), total particle concentration (S-LDL-P) |
| VLDL subclass particle constituents | strong | For small particles: total cholesterol (S-VLDL-C), For extra-small particles: total lipids (XS-VLDL-L), phospholipids (XS-VLDL-PL) |
| Other | strong | HbA1c, Apolipoprotein B, total LDL cholesterol, total serum cholesterol |
| Proteins | ||
| Chemokine activity | strong | FAM3B, FAM3D, MIP-5, TECK, |
| Chemokine activity | suggestive | CCL28 |
| Chemokine receptors | strong | IL-3RA, HGF receptor, sGP130, VEGF-R2, VEGF-R3 |
| Chemokine receptors | none | TCCR |
| Receptor function and/or signaling | strong | F177A, GP116, IGF-1R, IR, JAG1, MBL, PEAR1, PYY, SECTM1, SEMA6A, TLR4 |
| Receptor function and/or signaling | suggestive | PLXB2 |
| Receptor function and/or signaling | none | CD109, CD209, GFRAL, GPIV, LIF-R, Notch-1, PEAR1, sTIE1, sTIE2 |
| Cell adhesion | strong | Cadherin-1, E-selectin, Endoglin, ICAM-4, ISLR2, Laminin, NCAM-L1, OX2G, P-selectin, sICAM-1, sICAM-2, sICAM-5 |
| Cell adhesion | none | ADAM23, BCAM, Cadherin-5, Desmoglein-2, ESAM |
| Enzyme function | strong | B3GN2, B4GT1, B4GT2, Cathepsin S, CLIC5, DPEP2, FA20B, FUT10, GLCE, GNS, IAP, LPH, MA1A2, NDST1, QSOX2, ST4S6, TPST2, XXLT1 |
| Enzyme function | none | ATS13, BGAT, CEL, CHSTB, DYR, MINP1, TLL1 |
| Miscellaneous | strong | C1GLC, CASC4, GOLM1, KIN17, THSD1, TUFT1, |
| Miscellaneous | none | Factor VIII, OBP2B |
| ZFPM2 Locus (Chromosome 8) | ||
| Blood Cell Traits | ||
| Blood cell counts | strong | white blood cells, granulocytes, basophils + neutrophils, neutrophils + eosinophils, basophils, neutrophils, myeloid, platelets, plateletcrit (%), platelet distribution width, mean platelet volume |
| Proteins | ||
| Cytokine/chemokine activity | strong | EDA, IL-7, PDGF-AA, PDGF-BB, PDGF-D, VEGF-A, NAP-2, RANTES, TARC |
| Immune response | strong | CLM2, COCH, CYTF, DB119 |
| Receptor function and/or signaling | strong | ANG-1, APP, BDNF, CD44, CGB2, CRIM1, Dkk-1, Dkk-4, EDAR, EPHB2, EPHB3, GI24, GRP, LIRB4, Mammaglobin-2, OBP2A, P2RX6, PAP1, PTPRD, RGS10, RGS3, RHOG, THA, MESD2 |
| Receptor function and/or signaling | suggestive | Ephrin-A3 |
| Receptor function and/or signaling | none | UNC5H4, sRAGE |
| Cell adhesion | strong | Galectin-7, KIRR2, MADCAM-1, MFGM, ON, P-Selectin, PCDG8, SCF, SPARCL1 |
| Enzyme activity | strong | Arylsulfatase A, ASM3A, B4GT7, Cathepsin A, CHSTB, CPXM1, FUT8, GSTM1-1, INP5E, MMEL2, MYSM1, PAI-1, PDIA5, RIFK, SIRT5, SPTC1, UD2A1 |
| Enzyme activity | none | PDE3A, ZFP91, LAML2, HECW1 |
| Enzyme inhibitor | strong | SERPINE2, SPINK5, TICN3, WFD13 |
| Transcription/translation | strong | APBB1, CENPW, HIF-1a, PAIP1 |
| Transcription/translation | suggestive | ID2 |
| Miscellaneous | strong | 4EBP2, APLP2, ARL1, ASIC4, CA063, Coactosin-like protein, CQ089, DJB11, MPP7, NSG2, PROL1, RBM28, SATB1, SYT11, SYT17, TXNDC4 |
| Miscellaneous | none | CNA2 |
Evidence: Evidence of colocalization, Strong = PP3+PP4 ≥ 0.99 and PP4/PP3 ≥ 5; Suggestive = PP3 + PP4 > 0.75 and PP4/PP3 > 3; None = association signal for the trait at the locus, but no evidence of colocalization
Refer to Table S9 for full descriptions of the proteins. The proteins have been grouped into broad functional categories using the Uniprot database.85
The ABO locus colocalized with trans-pQTLs for several membrane proteins (B3GN2, endoglin, GOLM1, OX2G, and TPST2) and cell surface receptors (IL-3RA, LIFR, IGF-I R, and HGF receptors). ABO colocalization was also observed with trans-pQTLs for adhesion and immune-related molecules involved in leukocyte recruitment, cell adhesion, and transmigration, including sGP130, sICAM-1, sICAM-2, LIRB4, and P-selectin (Table 3; Table S9). At the ZFPM2 locus, colocalization was seen with trans-pQTLs for proteins generally found in platelet granules (e.g., VEGF-A, PDGF-AA, PDGF-BB, PDGF-D, angiopoietin, and P-selectin). At the SERPINE2 locus, we observed that, in addition to colocalizing with the cis-eQTL signal for SERPINE2 expression, the cytokine-network-associated variants colocalized with the cis-pQTL variants for SERPINE2 protein levels (Table S9). Likewise, the VEGFA locus colocalized with a cis-pQTL for VEGF-A, and the PDGFRB locus with a cis-pQTL for PDGFRB.
Relationships of Cytokine Network Variants with Complex Traits and Diseases
Using the NHGRI GWAS Catalog,58,59 we found that, across all eight cytokine network loci, 55 SNPs matched SNPs previously associated with quantitative traits and diseases (Table S10). The lead cytokine network variant at ZFPM2 (rs6993770) has previously been associated with various platelet traits, including platelet count, distribution width, plateletcrit (total platelet mass), and mean volume17,60 (Table S10).
Next, GWAS summary statistics from a broad range of traits and diseases (Table S2), including hematopoietic traits, circulating metabolites, and immune- and cardiometabolic-related diseases, were compiled for colocalization analysis with the cytokine network loci. The two cytokine-network-associated loci, ABO and ZFPM2, exhibited strong evidence of colocalization for several traits and diseases. The ZFPM2 locus colocalized not only with signals for several platelet trait associations, but also with other hematological trait-associated signals, including white blood cell counts and specifically neutrophil and basophil counts (Table 3; Table S11). The ABO locus showed colocalization with various QTLs for hematological traits, including red blood cell traits (haemoglobin concentration, red blood cell count, and hematocrit) and white blood cell counts, including granulocyte count and specifically eosinophil count (Table 3; Table S11). This is consistent with the ABO locus being identified as a pQTL for proteins involved in leukocyte activation as identified previously. Cytokine network variants at the ABO locus colocalized with those of intermediate-density, low-density, and very-low-density lipoprotein subclasses as well as glycosylated haemoglobin (HbA1c) (Table 3; Table S11), suggesting both inflammatory and metabolic effects. Notably, the same cytokine network variants at the ABO locus also strongly colocalized with signals associated with coronary artery disease (CAD), pulmonary embolism, ischemic stroke (MIM: 601367), and type 2 diabetes (T2D [MIM: 125853]) (Table 3, Table S11).
Multi-Trait Colocalization at the ABO Locus
Given its extensive pleiotropy and disease relevance, we performed three-way multi-trait colocalization (MOLOC)51 at the ABO locus to assess shared genetic etiology between disease traits, the cytokine network, and various molecular traits. Consistent with our pairwise COLOC analysis, the majority of these colocalizing proteins, lipids and lipoproteins, and blood cell traits also showed evidence of multi-trait colocalization (PPA ≥ 80%) with CAD-cytokine network, T2D-cytokine network, pulmonary embolism-cytokine network, and ischemic stroke-cytokine network pairs (Tables S12–S15). Overall, this suggested that the ABO locus contributes to the shared genetic architecture among several known cardiometabolic disease risk factors, which includes multiple inflammatory, haemostatic, and metabolic processes.
Discussion
In this study, we first identified a network of 11 correlated cytokines which are known to participate in a broad array of immune responses in circulation. These cytokines include those involved in the classical TH1 (IL-12, IFN-γ), TH2 (IL-4, IL-6, and IL-10), TH17 (IL-6, IL-17, and G-CSF), and Treg (IL-10) responses52,53 as well as the promotion of angiogenesis, tissue repair, and remodelling typically coinciding with inflammatory and post-inflammatory states (VEGF-A, FGF-basic, and PDGF-BB).54 Although previous in vitro challenge studies20,21 indicate antagonistic relationships among selected cytokines in the network, our analyses in >9,000 individuals are consistent with a previous study which utilized similar data,19 showing that these 11 circulating cytokines are positively correlated in the general population. Therefore, at the population level, it is more likely that an equilibrium in circulating levels of disparate cytokines exists, possibly maintained by counter-regulatory mechanisms.
Our multivariate GWAS meta-analysis identified eight loci associated with the cytokine network, confirming six previously reported associations for circulating cytokine levels14,16,19 as well as uncovering two additional signals (PDGFRB and ABO), empirically demonstrating that jointly modeling correlated traits in a multivariate GWAS can increase statistical power to detect additional associations compared to the univariate approach. This contributes to the growing body of literature which shows, through both simulation and empirical analyses, that multivariate outperforms the univariate analysis, leading to the identification of novel pleiotropic loci.22,28, 29, 30 On the other hand, we and others have also noted that in certain circumstances, the multivariate approach may suffer from power loss; for example, when the SNP influences nearly all the traits equally or the direction of genetic and cross-trait correlation is the same.22,23,61
Further, integrative genetic analyses revealed evidence for shared genetic influences between these loci, molecular QTLs, and complex trait and disease associations. This study identified several regions harboring cytokine-associated signals that colocalize with whole blood and/or immune cell-specific cis-eQTLs for a number of genes, including SERPINE2, ABO, and PCSK6, suggesting that these genes are possible candidates underlying the collective expression of cytokines in the cytokine network—or vice versa. Our findings also highlight the fact that the cytokine network associations at the pleiotropic loci, ABO and ZFPM2, overlap with signals associated with multiple traits, including cardiometabolic diseases, immune-related proteins, and platelet traits.
SERPINE2 encodes protease nexin-1, an inhibitor of serine proteases such as thrombin and plasmin, and is therefore implicated in coagulation, fibrinolysis, and tissue remodelling.62 It shares similar functions with its better-known homolog SERPINE1 (MIM: 173360), or plasminogen activator inhibitor-1 (PAI-1), the elevation of which is associated with thrombosis and cardiovascular risk.62 However, there is also evidence that SERPINE2 has pleiotropic roles in immune and inflammatory regulation, roles that could be either dependent or independent of its function as a serine protease. It is expressed in many tissue types, and its expression can be induced by pro-inflammatory cytokines such as IL-1α.63,64 Conversely, SERPINE2 can itself influence inflammatory status: SERPINE2 is a candidate susceptibility gene for chronic obstructive pulmonary disease, and SERPINE2-knockout mice exhibited extensive accumulation of lymphocytes in the lungs, through a mechanism linked to thrombin and NFκB activation.64 We observed in our data that the cytokine network associations overlapped with the SERPINE2 pQTL signal. Moreover, using immune cell-specific cis-eQTL data, we further demonstrated colocalization between the cytokine network and SERPINE2 cis-eQTL signals specifically in CD4+ T cells and B cells. This suggests that the association between SERPINE2 and the cytokine network at this locus is at least partially driven by lymphocytic expression—consistent with SERPINE2 itself influencing chemotaxis and recruitment of lymphocytes.64 Our analyses demonstrate that the importance of SERPINE2 in regulating immune and inflammatory processes is potentially greater than previously anticipated, and warrants further targeted research.
Like SERPINE2, the ABO locus has widespread pleiotropic effects. The most well-known function of ABO is its determination of blood group. The human ABO gene has three major alleles (A, B, and O) that determine ABO blood type. The A and B alleles encode for distinct “A” versus “B” glycosyltransferases that add specific sugar residues to a precursor molecule (H antigen) to form A versus B antigens, respectively.65 The O allele results in a protein without glycosyltransferase activity.65 The lead cytokine-associated variant rs550057 and its proxies in moderate LD (r2 = 0.6; rs507666, rs687289) have been previously shown to determine the ABO allele,66 but they have also been associated with circulating levels of inflammatory proteins such as sICAM-1, P-selectin, and ALP.17,67,68 Our study showed that cytokine network associations at the ABO locus share colocalized signals with a host of other proteins and traits, including lipoproteins (IDL, LDL, and VLDL), proteins of immune function, immune cell subsets, and cardiometabolic diseases (Table 3); these results highlight the potential for shared molecular etiology among these traits. Our analyses highlight the potential genetic basis for numerous previous observations linking ABO blood group to an array of similar traits and phenotypes.18,69, 70, 71, 72, 73, 74 We also observed multi-trait colocalization among cardiometabolic diseases, cytokine network, and other features relating to multiple inflammatory (e.g., inflammatory proteins, cytokines, and cytokine receptors), haemostatic (blood cell traits), and metabolic processes (lipids and metabolites); this further strengthens the evidence for a shared causal variant. Altogether, these results suggest that certain genetic variants, e.g., at the ABO locus, influence the risk of cardiometabolic disease through a constellation of pleiotropic effects.
It could therefore be speculated, due to its involvement in multiple inflammatory, haemostatic, and metabolic processes, that the ABO gene influences the risk of cardiometabolic disease; however, our current understanding of the mechanisms behind this remains unclear. For instance, non-O blood groups have been associated with increased risk of cardiovascular disease, venous thromboembolism, stroke, and T2D.70,75 However, the O blood group has itself been linked to elevated IL-10 and worse outcomes given existing coronary disease (risk of cardiovascular death, of recurrent myocardial infarction, and of all-cause mortality).66 Other studies have suggested a role for von Willebrand factor (VWF), a coagulative factor which also expresses ABO antigens—in particular, the O phenotype is associated with lower VWF, which may explain reduced thrombotic and cardiovascular risk.66,76 It has been suggested that the link between ABO blood group type and venous thromboembolism (VTE) is potentially driven by VWF and Factor VIII—non-O blood group individuals presented a higher risk of venous thromboembolism and had elevated levels of both VWF and Factor VIII.77,78 Also relevant is the link between ABO and adhesion molecules such as E-selectin and sICAM-1 which are overexpressed in inflammatory states.18,68,72,73 sICAM-1 is a known positive correlate with cardiovascular disease; however, it is the A blood group, not O, that is associated with reduced sICAM-1 levels, again complicating the picture.72 Inferring the exact causal relationships among all these entities will require intricate follow-up experimental investigation, involving simultaneous examination of all key players. It is particularly unclear whether the link with cardiometabolic diseases may be due to its direct modification of H antigen, or of the glycosyltransferase activity of the encoded enzyme on other proteins, or some combination of both. In our study, formal causal inference (e.g., with Mendelian randomization) was not possible because the corresponding multivariate beta-coefficients and standard errors are not currently calculable and the locus itself has extensive pleiotropy.
The ZFPM2 locus has been associated with platelet traits,60 and our findings highlight its importance as a determinant of platelet and angiogenic cytokine activity. ZFPM2 encodes a zinc finger cofactor that regulates the activity of GATA4, a transcription factor reported to play a critical role not only in heart development79 but also in modulation of angiogenesis. In particular, GATA4 directly binds to the promoter of angiogenic factor VEGFA and regulates its expression,80 and it has been shown that disruption of ZFPM2-GATA4 interaction alters the expression of VEGFA and other angiogenesis-related genes.81 VEGF-A and PDGFR-BB, which are part of the cytokine network, have been found to be released via alpha granules of activated platelets, and serum VEGF-A levels correlate closely with blood platelet counts.82, 83, 84 In our study, we show that the cytokine-associated signal at the ZFPM2 locus colocalized with GWAS signals for platelet traits (platelet count, platelet distribution width, and mean platelet volume) and platelet proteins (e.g., VEGF-A, PDGF-AA, PDGF-BB, PDGF-D, angiopoietin, and P-selectin). Our findings provide additional insights into the relationships between the ZFPM2 locus, cytokines and various platelet-associated proteins, and their role in platelet biology. The lead cytokine network SNP rs6993770 has been reported to be a trans-eQTL in whole blood for gene products typically found in platelets and their receptors (e.g., CXCL5, GP9, MYL9, and VWF).46 Collectively, these findings suggest that this locus regulates the number and/or cytokine activity of circulating platelets, and that this potentially occurs via interaction with GATA4 (MIM: 600576) and regulation of VEGFA.
In conclusion, our study illustrates the utility of multivariate analysis of correlated immune traits and highlights potentially fruitful avenues of biological investigation for multivariate genetic signals. Our results highlight the fact that certain gene loci drive the expression of a cytokine network with immune, inflammatory, and tissue repair functions; and, simultaneously, these loci are implicated in the regulation of other haemostatic and metabolic functions, with relevance to human health and disease. This stresses the fact that the processes of inflammation, haemostasis, and repair often run concurrent with each other after injury, and that biological systems often feature ample redundancy and feedback loops within individual effectors.
Declaration of Interests
Veikko Salomaa has consulted for Novo Nordisk and Sanofi and received honoraria from these companies. He also has ongoing research collaboration with Bayer Ltd. (All unrelated to the present study). The other authors declare no conflicts of interest.
Acknowledgments
This work was supported partially by the Victorian Government’s OIS Program and by core funding from: the UK Medical Research Council (MR/L003120/1), the British Heart Foundation (RG/13/13/30194, RG/18/13/33946), and the NIHR (Cambridge BRC at the Cambridge University Hospitals NHS Foundation Trust). The YFS was supported by the Academy of Finland: 322098, 286284, 134309 (Eye), 126925, 121584, 124282, 129378 (Salve), 117787 (Gendi), and 41071 (Skidi); the Social Insurance Institution of Finland; Competitive State Research Financing of the Expert Responsibility area of Kuopio, Tampere and Turku University Hospitals (X51001); Juho Vainio Foundation; Paavo Nurmi Foundation; Finnish Foundation for Cardiovascular Research; Finnish Cultural Foundation; The Sigrid Juselius Foundation; Tampere Tuberculosis Foundation; Emil Aaltonen Foundation; Yrjö Jahnsson Foundation; Signe and Ane Gyllenberg Foundation; Diabetes Research Foundation of Finnish Diabetes Association; EU Horizon 2020 (755320—TAXINOMISIS); ERC (742927—MULTIEPIGEN project); and Tampere University Hospital Supporting Foundation. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, or DHSC. The authors acknowledge support from: A.P.N., Australian Postgraduate Award; M.I. and S.C.R., NIHR (Cambridge BRC at the Cambridge University Hospitals NHS Foundation Trust); G.A., NHMRC Early Career Fellowship (1090462); Q.Q.H., Melbourne Research Scholarship; H.H.T., NHMRC Postgraduate Scholarship; N.F.G. and C.W., Wellcome Trust (WT107881); C.W., Medical Research Council (MC_UU_00002/4); J.K., Sigrid Juselius Foundation, Academy of Finland (297338 and 307247) and Novo Nordisk Foundation (NNF17OC0026062); P.W., Novo Nordisk Foundation (15998) and Academy of Finland (312476 and 312477); T.L., Academy of Finland (322098); A.S.H., Academy of Finland (321356); and V.S., Finnish Foundation for Cardiovascular Research.
Published: October 31, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.10.001.
Contributor Information
Artika P. Nath, Email: artika.nath@baker.edu.au.
Michael Inouye, Email: mi336@medschl.cam.ac.uk.
Web Resources
BLUEPRINT immune cell summary statistics,
ftp://ftp.ebi.ac.uk/pub/databases/blueprint/blueprint_Epivar/
eQTLGen Consortium portal, http://www.eqtlgen.org/
GWAS Catalog, https://www.ebi.ac.uk/gwas/
ImmunoBase, https://www.immunobase.org/
OMIM, https://www.omim.org/
Summary statistics from the multivariate GWAS meta-analyses, https://www.ebi.ac.uk/gwas/downloads/summary-statistics
Supplemental Data
References
- 1.Dinarello C.A. Historical insights into cytokines. Eur. J. Immunol. 2007;37(Suppl 1):S34–S45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vignali D.A., Kuchroo V.K. IL-12 family cytokines: immunological playmakers. Nat. Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Shea J.J., Ma A., Lipsky P. Cytokines and autoimmunity. Nat. Rev. Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 4.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 5.Dranoff H., Taleb S., Mallat Z., Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb. Vasc. Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 6.Kaptoge S., Seshasai S.R., Gao P., Freitag D.F., Butterworth A.S., Borglykke A., Di Angelantonio E., Gudnason V., Rumley A., Lowe G.D. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur. Heart J. 2014;35:578–589. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Craen A.J., Posthuma D., Remarque E.J., van den Biggelaar A.H., Westendorp R.G., Boomsma D.I. Heritability estimates of innate immunity: an extended twin study. Genes Immun. 2005;6:167–170. doi: 10.1038/sj.gene.6364162. [DOI] [PubMed] [Google Scholar]
- 8.Rafiq S., Stevens K., Hurst A.J., Murray A., Henley W., Weedon M.N., Bandinelli S., Corsi A.M., Guralnik J.M., Ferruci L. Common genetic variation in the gene encoding interleukin-1-receptor antagonist (IL-1RA) is associated with altered circulating IL-1RA levels. Genes Immun. 2007;8:344–351. doi: 10.1038/sj.gene.6364393. [DOI] [PubMed] [Google Scholar]
- 9.Interleukin 1 Genetics Consortium Cardiometabolic effects of genetic upregulation of the interleukin 1 receptor antagonist: a Mendelian randomisation analysis. Lancet Diabetes Endocrinol. 2015;3:243–253. doi: 10.1016/S2213-8587(15)00034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollegaard M.V., Bidwell J.L. Cytokine gene polymorphism in human disease: on-line databases, Supplement 3. Genes Immun. 2006;7:269–276. doi: 10.1038/sj.gene.6364301. [DOI] [PubMed] [Google Scholar]
- 11.Larsen M.H., Albrechtsen A., Thørner L.W., Werge T., Hansen T., Gether U., Haastrup E., Ullum H. Genome-wide association study of genetic variants in LPS-stimulated IL-6, IL-8, IL-10, IL-1ra and TNF-α cytokine response in a Danish cohort. PLoS ONE. 2013;8:e66262. doi: 10.1371/journal.pone.0066262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matteini A.M., Li J., Lange E.M., Tanaka T., Lange L.A., Tracy R.P., Wang Y., Biggs M.L., Arking D.E., Fallin M.D. Novel gene variants predict serum levels of the cytokines IL-18 and IL-1ra in older adults. Cytokine. 2014;65:10–16. doi: 10.1016/j.cyto.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tekola Ayele F., Doumatey A., Huang H., Zhou J., Charles B., Erdos M., Adeleye J., Balogun W., Fasanmade O., Johnson T. Genome-wide associated loci influencing interleukin (IL)-10, IL-1Ra, and IL-6 levels in African Americans. Immunogenetics. 2012;64:351–359. doi: 10.1007/s00251-011-0596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debette S., Visvikis-Siest S., Chen M.H., Ndiaye N.C., Song C., Destefano A., Safa R., Azimi Nezhad M., Sawyer D., Marteau J.B. Identification of cis- and trans-acting genetic variants explaining up to half the variation in circulating vascular endothelial growth factor levels. Circ. Res. 2011;109:554–563. doi: 10.1161/CIRCRESAHA.111.243790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He M., Cornelis M.C., Kraft P., van Dam R.M., Sun Q., Laurie C.C., Mirel D.B., Chasman D.I., Ridker P.M., Hunter D.J. Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels. Arterioscler. Thromb. Vasc. Biol. 2010;30:885–890. doi: 10.1161/ATVBAHA.109.199422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S.H., Ruggiero D., Sorice R., Song C., Nutile T., Vernon Smith A., Concas M.P., Traglia M., Barbieri C., Ndiaye N.C. Six novel loci associated with circulating VEGF levels identified by a meta-analysis of genome-wide association studies. PLoS Genet. 2016;12:e1005874. doi: 10.1371/journal.pgen.1005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun B.B., Maranville J.C., Peters J.E., Stacey D., Staley J.R., Blackshaw J., Burgess S., Jiang T., Paige E., Surendran P. Genomic atlas of the human plasma proteome. Nature. 2018;558:73–79. doi: 10.1038/s41586-018-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sliz E., Kalaoja M., Ahola-Olli A., Raitakari O., Perola M., Salomaa V., Lehtimäki T., Karhu T., Viinamäki H., Salmi M. Genome-wide association study identifies seven novel loci associating with circulating cytokines and cell adhesion molecules in Finns. J. Med. Genet. 2019;56:607–616. doi: 10.1136/jmedgenet-2018-105965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahola-Olli A.V., Würtz P., Havulinna A.S., Aalto K., Pitkänen N., Lehtimäki T., Kähönen M., Lyytikäinen L.P., Raitoharju E., Seppälä I. Genome-wide Association Study Identifies 27 Loci Influencing Concentrations of Circulating Cytokines and Growth Factors. Am. J. Hum. Genet. 2017;100:40–50. doi: 10.1016/j.ajhg.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., Oosting M., Deelen P., Ricaño-Ponce I., Smeekens S., Jaeger M., Matzaraki V., Swertz M.A., Xavier R.J., Franke L. Inter-individual variability and genetic influences on cytokine responses to bacteria and fungi. Nat. Med. 2016;22:952–960. doi: 10.1038/nm.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., Oosting M., Smeekens S.P., Jaeger M., Aguirre-Gamboa R., Le K.T.T., Deelen P., Ricaño-Ponce I., Schoffelen T., Jansen A.F.M. A functional genomics approach to understand variation in cytokine production in humans. Cell. 2016;167:1099–1110.e14. doi: 10.1016/j.cell.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira M.A., Purcell S.M. A multivariate test of association. Bioinformatics. 2009;25:132–133. doi: 10.1093/bioinformatics/btn563. [DOI] [PubMed] [Google Scholar]
- 23.O’Reilly P.F., Hoggart C.J., Pomyen Y., Calboli F.C.F., Elliott P., Jarvelin M.R., Coin L.J.M. MultiPhen: joint model of multiple phenotypes can increase discovery in GWAS. PLoS ONE. 2012;7:e34861. doi: 10.1371/journal.pone.0034861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X., Stephens M. Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nat. Methods. 2014;11:407–409. doi: 10.1038/nmeth.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turley P., Walters R.K., Maghzian O., Okbay A., Lee J.J., Fontana M.A., Nguyen-Viet T.A., Wedow R., Zacher M., Furlotte N.A., 23andMe Research Team. Social Science Genetic Association Consortium Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat. Genet. 2018;50:229–237. doi: 10.1038/s41588-017-0009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cichonska A., Rousu J., Marttinen P., Kangas A.J., Soininen P., Lehtimäki T., Raitakari O.T., Järvelin M.R., Salomaa V., Ala-Korpela M. metaCCA: summary statistics-based multivariate meta-analysis of genome-wide association studies using canonical correlation analysis. Bioinformatics. 2016;32:1981–1989. doi: 10.1093/bioinformatics/btw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mägi R., Suleimanov Y.V., Clarke G.M., Kaakinen M., Fischer K., Prokopenko I., Morris A.P. SCOPA and META-SCOPA: software for the analysis and aggregation of genome-wide association studies of multiple correlated phenotypes. BMC Bioinformatics. 2017;18:25. doi: 10.1186/s12859-016-1437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Q., Wang Y. Methods for analyzing multivariate phenotypes in genetic association studies. J. Probab. Stat. 2012;2012:652569. doi: 10.1155/2012/652569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S., Xing E.P. Statistical estimation of correlated genome associations to a quantitative trait network. PLoS Genet. 2009;5:e1000587. doi: 10.1371/journal.pgen.1000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Sluis S., Posthuma D., Dolan C.V. TATES: efficient multivariate genotype-phenotype analysis for genome-wide association studies. PLoS Genet. 2013;9:e1003235. doi: 10.1371/journal.pgen.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inouye M., Ripatti S., Kettunen J., Lyytikäinen L.P., Oksala N., Laurila P.P., Kangas A.J., Soininen P., Savolainen M.J., Viikari J. Novel Loci for metabolic networks and multi-tissue expression studies reveal genes for atherosclerosis. PLoS Genet. 2012;8:e1002907. doi: 10.1371/journal.pgen.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marttinen P., Pirinen M., Sarin A.P., Gillberg J., Kettunen J., Surakka I., Kangas A.J., Soininen P., O’Reilly P., Kaakinen M. Assessing multivariate gene-metabolome associations with rare variants using Bayesian reduced rank regression. Bioinformatics. 2014;30:2026–2034. doi: 10.1093/bioinformatics/btu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raitakari O.T., Juonala M., Rönnemaa T., Keltikangas-Järvinen L., Räsänen L., Pietikäinen M., Hutri-Kähönen N., Taittonen L., Jokinen E., Marniemi J. Cohort profile: the cardiovascular risk in Young Finns Study. Int. J. Epidemiol. 2008;37:1220–1226. doi: 10.1093/ije/dym225. [DOI] [PubMed] [Google Scholar]
- 34.Borodulin K., Vartiainen E., Peltonen M., Jousilahti P., Juolevi A., Laatikainen T., Männistö S., Salomaa V., Sundvall J., Puska P. Forty-year trends in cardiovascular risk factors in Finland. Eur. J. Public Health. 2015;25:539–546. doi: 10.1093/eurpub/cku174. [DOI] [PubMed] [Google Scholar]
- 35.Teo Y.Y., Inouye M., Small K.S., Gwilliam R., Deloukas P., Kwiatkowski D.P., Clark T.G. A genotype calling algorithm for the Illumina BeadArray platform. Bioinformatics. 2007;23:2741–2746. doi: 10.1093/bioinformatics/btm443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howie B.N., Donnelly P., Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang C.C., Chow C.C., Tellier L.C., Vattikuti S., Purcell S.M., Lee J.J. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham G., Inouye M. Fast principal component analysis of large-scale genome-wide data. PLoS ONE. 2014;9:e93766. doi: 10.1371/journal.pone.0093766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Josse J., Husson F. missMDA: A package for handling missing values in multivariate data analysis. J. Stat. Softw. 2016;70:1–31. [Google Scholar]
- 40.Whitcomb B.W., Schisterman E.F. Assays with lower detection limits: implications for epidemiological investigations. Paediatr. Perinat. Epidemiol. 2008;22:597–602. doi: 10.1111/j.1365-3016.2008.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aulchenko Y.S., Ripke S., Isaacs A., van Duijn C.M. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23:1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- 42.Willer C.J., Li Y., Abecasis G.R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitlock M.C. Combining probability from independent tests: the weighted Z-method is superior to Fisher’s approach. J. Evol. Biol. 2005;18:1368–1373. doi: 10.1111/j.1420-9101.2005.00917.x. [DOI] [PubMed] [Google Scholar]
- 44.Zaykin D.V. Optimally weighted Z-test is a powerful method for combining probabilities in meta-analysis. J. Evol. Biol. 2011;24:1836–1841. doi: 10.1111/j.1420-9101.2011.02297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giambartolomei C., Vukcevic D., Schadt E.E., Franke L., Hingorani A.D., Wallace C., Plagnol V. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10:e1004383. doi: 10.1371/journal.pgen.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Võsa U., Claringbould A., Westra H.J., Bonder M.J., Deelen P., Zeng B., Kirsten H., Saha A., Kreuzhuber R., Kasela S. Unraveling the polygenic architecture of complex traits using blood eQTL meta-analysis. bioRxiv. 2018;447367 [Google Scholar]
- 47.Fairfax B.P., Makino S., Radhakrishnan J., Plant K., Leslie S., Dilthey A., Ellis P., Langford C., Vannberg F.O., Knight J.C. Genetics of gene expression in primary immune cells identifies cell type-specific master regulators and roles of HLA alleles. Nat. Genet. 2012;44:502–510. doi: 10.1038/ng.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fairfax B.P., Humburg P., Makino S., Naranbhai V., Wong D., Lau E., Jostins L., Plant K., Andrews R., McGee C., Knight J.C. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science. 2014;343:1246949. doi: 10.1126/science.1246949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kasela S., Kisand K., Tserel L., Kaleviste E., Remm A., Fischer K., Esko T., Westra H.J., Fairfax B.P., Makino S. Pathogenic implications for autoimmune mechanisms derived by comparative eQTL analysis of CD4+ versus CD8+ T cells. PLoS Genet. 2017;13:e1006643. doi: 10.1371/journal.pgen.1006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo H., Fortune M.D., Burren O.S., Schofield E., Todd J.A., Wallace C. Integration of disease association and eQTL data using a Bayesian colocalisation approach highlights six candidate causal genes in immune-mediated diseases. Hum. Mol. Genet. 2015;24:3305–3313. doi: 10.1093/hmg/ddv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giambartolomei C., Zhenli Liu J., Zhang W., Hauberg M., Shi H., Boocock J., Pickrell J., Jaffe A.E., Pasaniuc B., Roussos P., CommonMind Consortium A Bayesian framework for multiple trait colocalization from summary association statistics. Bioinformatics. 2018;34:2538–2545. doi: 10.1093/bioinformatics/bty147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu J., Paul W.E. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 54.Barrientos S., Brem H., Stojadinovic O., Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569–578. doi: 10.1111/wrr.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu X., Ivashkiv L.B. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sano R., Nakajima T., Takahashi K., Kubo R., Kominato Y., Tsukada J., Takeshita H., Yasuda T., Ito K., Maruhashi T. Expression of ABO blood-group genes is dependent upon an erythroid cell-specific regulatory element that is deleted in persons with the B(m) phenotype. Blood. 2012;119:5301–5310. doi: 10.1182/blood-2011-10-387167. [DOI] [PubMed] [Google Scholar]
- 57.Chen L., Ge B., Casale F.P., Vasquez L., Kwan T., Garrido-Martín D., Watt S., Yan Y., Kundu K., Ecker S. Genetic drivers of epigenetic and transcriptional variation in human immune cells. Cell. 2016;167:1398–1414.e24. doi: 10.1016/j.cell.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welter D., MacArthur J., Morales J., Burdett T., Hall P., Junkins H., Klemm A., Flicek P., Manolio T., Hindorff L., Parkinson H. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–D1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacArthur J., Bowler E., Cerezo M., Gil L., Hall P., Hastings E., Junkins H., McMahon A., Milano A., Morales J. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog) Nucleic Acids Res. 2017;45(D1):D896–D901. doi: 10.1093/nar/gkw1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Astle W.J., Elding H., Jiang T., Allen D., Ruklisa D., Mann A.L., Mead D., Bouman H., Riveros-Mckay F., Kostadima M.A. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell. 2016;167:1415–1429.e19. doi: 10.1016/j.cell.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galesloot T.E., van Steen K., Kiemeney L.A., Janss L.L., Vermeulen S.H. A comparison of multivariate genome-wide association methods. PLoS ONE. 2014;9:e95923. doi: 10.1371/journal.pone.0095923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouton M.C., Boulaftali Y., Richard B., Arocas V., Michel J.B., Jandrot-Perrus M. Emerging role of serpinE2/protease nexin-1 in hemostasis and vascular biology. Blood. 2012;119:2452–2457. doi: 10.1182/blood-2011-10-387464. [DOI] [PubMed] [Google Scholar]
- 63.Santoro A., Conde J., Scotece M., Abella V., Lois A., Lopez V., Pino J., Gomez R., Gomez-Reino J.J., Gualillo O. SERPINE2 inhibits IL-1α-induced MMP-13 expression in human chondrocytes: Involvement of ERK/NF-κB/AP-1 pathways. PLoS ONE. 2015;10:e0135979. doi: 10.1371/journal.pone.0135979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solleti S.K., Srisuma S., Bhattacharya S., Rangel-Moreno J., Bijli K.M., Randall T.D., Rahman A., Mariani T.J. Serpine2 deficiency results in lung lymphocyte accumulation and bronchus-associated lymphoid tissue formation. FASEB J. 2016;30:2615–2626. doi: 10.1096/fj.201500159R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto F., Cid E., Yamamoto M., Saitou N., Bertranpetit J., Blancher A. An integrative evolution theory of histo-blood group ABO and related genes. Sci. Rep. 2014;4:6601. doi: 10.1038/srep06601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johansson Å., Alfredsson J., Eriksson N., Wallentin L., Siegbahn A. Genome-wide association study identifies that the ABO blood group system influences interleukin-10 levels and the risk of clinical events in patients with acute coronary syndrome. PLoS ONE. 2015;10:e0142518. doi: 10.1371/journal.pone.0142518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masuda M., Okuda K., Ikeda D.D., Hishigaki H., Fujiwara T. Interaction of genetic markers associated with serum alkaline phosphatase levels in the Japanese population. Hum. Genome Var. 2015;2:15019. doi: 10.1038/hgv.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yao C., Chen G., Song C., Keefe J., Mendelson M., Huan T., Sun B.B., Laser A., Maranville J.C., Wu H. Genome-wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat. Commun. 2018;9:3268. doi: 10.1038/s41467-018-05512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meo S.A., Rouq F.A., Suraya F., Zaidi S.Z. Association of ABO and Rh blood groups with type 2 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2016;20:237–242. [PubMed] [Google Scholar]
- 70.Chen Z., Yang S.H., Xu H., Li J.J. ABO blood group system and the coronary artery disease: an updated systematic review and meta-analysis. Sci. Rep. 2016;6:23250. doi: 10.1038/srep23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larson N.B., Bell E.J., Decker P.A., Pike M., Wassel C.L., Tsai M.Y., Pankow J.S., Tang W., Hanson N.Q., Alexander K. ABO blood group associations with markers of endothelial dysfunction in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2016;251:422–429. doi: 10.1016/j.atherosclerosis.2016.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paré G., Chasman D.I., Kellogg M., Zee R.Y., Rifai N., Badola S., Miletich J.P., Ridker P.M. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet. 2008;4:e1000118. doi: 10.1371/journal.pgen.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qi L., Cornelis M.C., Kraft P., Jensen M., van Dam R.M., Sun Q., Girman C.J., Laurie C.C., Mirel D.B., Hunter D.J. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum. Mol. Genet. 2010;19:1856–1862. doi: 10.1093/hmg/ddq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suhre K., Arnold M., Bhagwat A.M., Cotton R.J., Engelke R., Raffler J., Sarwath H., Thareja G., Wahl A., DeLisle R.K. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun. 2017;8:14357. doi: 10.1038/ncomms14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fagherazzi G., Gusto G., Clavel-Chapelon F., Balkau B., Bonnet F. ABO and Rhesus blood groups and risk of type 2 diabetes: evidence from the large E3N cohort study. Diabetologia. 2015;58:519–522. doi: 10.1007/s00125-014-3472-9. [DOI] [PubMed] [Google Scholar]
- 76.O’Donnell J., Boulton F.E., Manning R.A., Laffan M.A. Amount of H antigen expressed on circulating von Willebrand factor is modified by ABO blood group genotype and is a major determinant of plasma von Willebrand factor antigen levels. Arterioscler. Thromb. Vasc. Biol. 2002;22:335–341. doi: 10.1161/hq0202.103997. [DOI] [PubMed] [Google Scholar]
- 77.Tirado I., Mateo J., Soria J.M., Oliver A., Martínez-Sánchez E., Vallvé C., Borrell M., Urrutia T., Fontcuberta J. The ABO blood group genotype and factor VIII levels as independent risk factors for venous thromboembolism. Thromb. Haemost. 2005;93:468–474. doi: 10.1160/TH04-04-0251. [DOI] [PubMed] [Google Scholar]
- 78.Schleef M., Strobel E., Dick A., Frank J., Schramm W., Spannagl M. Relationship between ABO and Secretor genotype with plasma levels of factor VIII and von Willebrand factor in thrombosis patients and control individuals. Br. J. Haematol. 2005;128:100–107. doi: 10.1111/j.1365-2141.2004.05249.x. [DOI] [PubMed] [Google Scholar]
- 79.Svensson E.C., Tufts R.L., Polk C.E., Leiden J.M. Molecular cloning of FOG-2: a modulator of transcription factor GATA-4 in cardiomyocytes. Proc. Natl. Acad. Sci. USA. 1999;96:956–961. doi: 10.1073/pnas.96.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heineke J., Auger-Messier M., Xu J., Oka T., Sargent M.A., York A., Klevitsky R., Vaikunth S., Duncan S.A., Aronow B.J. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J. Clin. Invest. 2007;117:3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou B., Ma Q., Kong S.W., Hu Y., Campbell P.H., McGowan F.X., Ackerman K.G., Wu B., Zhou B., Tevosian S.G., Pu W.T. Fog2 is critical for cardiac function and maintenance of coronary vasculature in the adult mouse heart. J. Clin. Invest. 2009;119:1462–1476. doi: 10.1172/JCI38723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pal E., Korva M., Resman Rus K., Kejžar N., Bogovič P., Strle F., Avšič-Županc T. Relationship between circulating vascular endothelial growth factor and its soluble receptor in patients with hemorrhagic fever with renal syndrome. Emerg. Microbes Infect. 2018;7:89. doi: 10.1038/s41426-018-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Banks R.E., Forbes M.A., Kinsey S.E., Stanley A., Ingham E., Walters C., Selby P.J. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br. J. Cancer. 1998;77:956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Graff J., Klinkhardt U., Schini-Kerth V.B., Harder S., Franz N., Bassus S., Kirchmaier C.M. Close relationship between the platelet activation marker CD62 and the granular release of platelet-derived growth factor. J. Pharmacol. Exp. Ther. 2002;300:952–957. doi: 10.1124/jpet.300.3.952. [DOI] [PubMed] [Google Scholar]
- 85.UniProt Consortium UniProt: a hub for protein information. Nucleic Acids Res. 2015;43:D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.