Abstract
Background:
Helicobacter pylori infection produces progressive mucosal damage that may eventually result in gastric cancer. We studied the changes that occurred in the presence and severity of atrophic gastritis and the prevalence of H. pylori infection that occurred coincident with improvements in economic and hygienic conditions in Japan since World War II.
Materials and Methods:
The prevalence of H. pylori infection and histologic grades of gastric damage were retrospectively evaluated using gastric biopsy specimens obtained over a 40-year period. Gastric atrophy and intestinal metaplasia were scored using the updated Sydney classification system.
Results:
The prevalence of H. pylori and severity of atrophy were examined in 1381 patients including 289 patients examined in the 1970s (158 men; mean age, 44.9 years), 787 in the 1990s (430 men; 44.2 years), and 305 in the 2010s (163 men; 53.2 years). Overall, the prevalence of H. pylori infection decreased significantly from 74.7% (1970s) to 53% (1990s) and 35.1% (2010s) (p < .01). The prevalence of atrophy in the antrum and corpus was significantly lower in the 2010s (33, 19%, respectively) compared to those evaluated in either the 1970s (98, 82%) (p < .001) or 1990s (80, 67%) (p < .001). The severity of atrophy and intestinal metaplasia also declined remarkably among those with H. pylori infection.
Conclusions:
There has been a progressive and rapid decline in the prevalence of H. pylori infection as well a fall in the rate of progression of gastric atrophy among H. pylori-infected Japanese coincident with the westernization and improvements in economic and hygienic conditions in Japan since World War II.
Keywords: Gastric atrophy, intestinal metaplasia, smoking, salt intake, Helicobacter pylori, gastric cancer
In 1983, Warren and Marshall [1] cultured Helicobacter pylori and suggested that it was responsible for gastritis and the gastritis-related diseases, peptic ulcer disease, and gastric cancer [2,3]. In 1994, the World Health Organization classified H. pylori as a group I carcinogen [4] and confirmed that designation in 2012 [5]. Helicobacter pylori is now recognized as etiologically responsible for gastritis-associated peptic ulcer disease, the majority of gastric cancers as well as gastric MALT lymphoma. Proof of these etiological associations capped decades of work on the natural history of gastritis and its relation to disease [6–16].
Helicobacter pylori causes progressive gastric damage that is initially most prominent in the antrum and subsequently advances into the corpus [17,18]. Gastric cancer risk is associated with the extent and severity of atrophic injury, which is recognized by loss of normal glandular elements (atrophy), and the development of metaplastic epithelia (pseudopyloric or spasmolytic-polypeptide expressing type and intestinal type) [17,19]. The population risk of developing gastric cancer increases with the rate of development of these atrophic changers [17]. In Western countries such as the United States, the incidence of gastric cancer was noted to decline rapidly such that it fell from being the most common cancer in the first quarter of the 20th century to an uncommon disease by the beginning of the 21st century [20]. This change in incidence was initially associated with a marked increase in duodenal ulcer which then also declined as the prevalence of H. pylori infection declined [20]. The different H. pylori-related disease is associated with different patterns of gastritis (i.e., atrophic pangastritis or corpus predominant gastritis in gastric ulcer and gastric cancer and antral predominant with duodenal ulcer) suggesting that the rapid changes in disease manifestation were accompanied by similar changes in the rate of development of atrophic gastritis [17,19–22].
The incidence and mortality rate of gastric cancer have declined in the past several decades in Japan [23]. However, there have been few studies regarding the trends in H. pylori infection and the pattern and severity of gastritis over this same time period [24–28]. This study focused on correlating the long-term changes in the prevalence of H. pylori infection with the histologic expression of the infection in Japan over a 40-year period starting with the 1970s.
Methods
Patient Selection
We retrospectively analyzed records of patients undergoing upper gastrointestinal endoscopy based on three 3-year periods (i.e., 1975–1978, 1991–1994, and 2010–2013). The records were obtained from Hiroshima University Hospital (1975–1978 and 1991–1994) and Kawasaki Medical School (2010–2013) both located in the western portion of Honshu Island. The patients were among those who received upper endoscopy for investigation of dyspepsia or for screening of gastric cancer. Additional entry criteria included age older than 18 years at the time of endoscopy and no localized lesions in the upper gastrointestinal tract such as esophagitis, peptic ulcer, or malignancies. Exclusion criteria included: 1, history of previous H. pylori eradication therapy; 2, patients who, in the previous 8 weeks, had received drugs that may have affected the histologic evaluation before (e.g., nonsteroidal anti-inflammatory drug: NSAID, proton-pump inhibitor, or antibiotics). Written informed consent for the procedures was obtained from all patients. The study protocol was approved by the Ethics Committee of the Hospitals.
Histologic Assessment
Two biopsy specimens were each obtained from the lesser curvature of the middle antrum and the anterior and posterior regions of the corpus for evaluation of gastritis and H. pylori infection. Biopsy specimens were cut into 4-μm-thick slices and were stained using the hematoxylin and eosin, Giemsa, or Gimenez methods. Histologic slides were independently assessed by two gastroenterologists experienced in the evaluation and scoring of gastritis with no knowledge of the clinical findings of the patients (KH and TK). These two gastroenterologists are very versed in the pathology of alimentary tract, especially, the updated Sydney system. Disagreements were resolved by joint review. The presence of H. pylori was identified using Giemsa or Gimenez staining. Atrophy and intestinal metaplasia were scored as present or absent, and the severity of mucosal atrophy and intestinal metaplasia was scored on a scale of 0–3 according to the updated Sydney system of classification: 0 = normal, 1 = mild, 2 = moderate, and 3 = severe.
Smoking Rate and Salt Intake in Japanese Population
The Ministry of Health, Labour and Welfare has investigated the smoking prevalence every year from 1965 for men and woman over 20 years old [29]. We extracted the mean smoking rate according to gender from 1965 to 2012. Salt intake in the Japanese population has been investigated by the Ministry of Health, Labour and Welfare since 1975. Before that time, the salt intake was estimated from the consumption trend of salty foods. We used the data published from the years 1950 to 2010 [30].
Statistical Evaluation
The age-specific prevalence of H. pylori infection as well as the atrophic gastritis and intestinal metaplasia scores were separately calculated in the three periods (i.e., 1970s, 1990s, and 2010s) considering seven age groups: 18–19, 20–29, 30–39, 40–49, 50–59, 60–69, and 70–79 years. The statistical significance for each group was examined using the chi-square test.
p-values < .05 were considered statistically significant. 95% confidence intervals (CI) were also calculated. Results of gastritis scores are expressed as mean ± standard error and as mean and 95% CI, and age of subjects is expressed as mean ± standard deviation. The changes were also analyzed by ANOVA on ranks for the severity (Sydney score) of antral atrophy, antral intestinal metalasia, corpus atrophy, and corpus intestinal metaplasia for those with H. pylori infections both for the entire group from each of the three time periods and separately for three age groups (20–39, 40–59, and 60–79) comparing the three time periods. Pairwise multiple comparisons were performed using Dunn’s method (Sigma Stat California, USA 3.5). p < .05 was considered significant.
Results
The total number of patients underwent upper routine endoscopy from 2010 to 2013 was 5307, 5691, and 5089, respectively (We don’t have these data for 1975–1978, 1991–1994). Among these total patients, as not a random sample, we selected our study patients who gastroenterologists need to evaluate the histologic gastritis or H. pylori infection for taking gastric biopsy specimens or the patient wished to inspect. A total of 1381 subjects were entered including 289 (158 men, 131 women; mean age, 44.9 ± 16 years) in the 1970s group, 787 (430 men, 357 women; mean age, 44.2 ± 17 years) in the 1990s group, and 305 (163 men, 142 women; mean age, 53.2 ± 17 years) in the 2010s group. The overall prevalence of H. pylori infection was significantly lower (107/305) among those studied in the 2010s (35.1%; 95% CI = 29–40%) compared to those evaluated in either the 1970s (216/289, 74.7%; 95% CI = 69–79%; p < .001) or 1990s (417/787, 53%; 95% CI = 49–56%; p < .05). In the 1970s, the prevalence of H. pylori infection increased with age but reached a plateau by age 30 consistent with the notion that the disease is primarily acquired in childhood and the incidence for any birth cohort tends to be stable after about age 20. However, evaluation of the trends in 2010s shows that overall the rate of acquisition was low leading to a low overall prevalence which was relatively constant for the first two decades followed by an increase reflecting the higher rates of acquisition that had previously occurred among birth cohorts born before approximately 2000 (Fig. 1).
Figure 1.
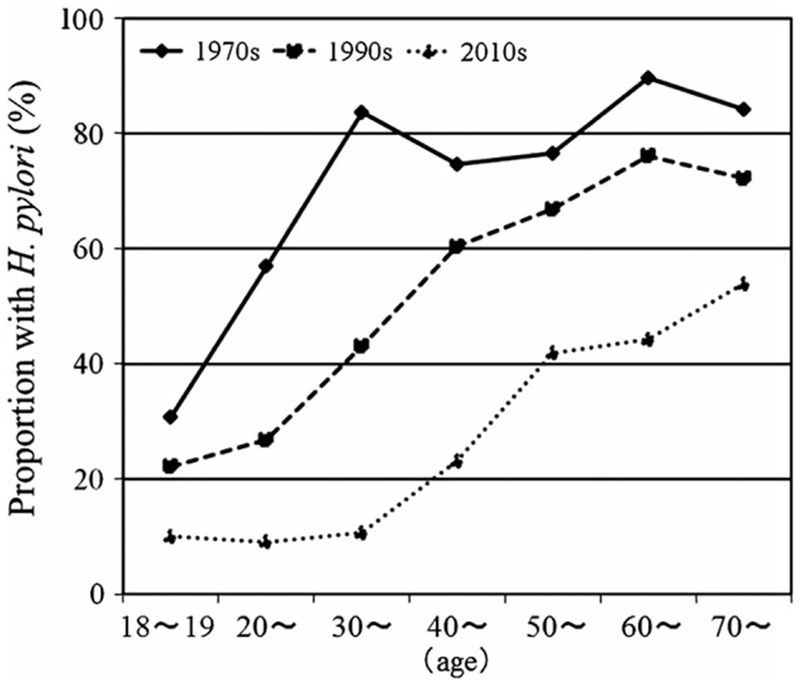
Age-specific prevalence of Helicobacter pylori from the 1970s to the 2010s in Japan. The pattern was typical for that of a developing country in the 1970s and then showed a progressive shift to the right as the rate of acquisition steadily declined in childhood. The base line prevalence currently appears to be stable at about 10%.
Prevalence of mucosal atrophy and intestinal metaplasia across the 1970s, 1990s, and 2010s in H. pylori-positive patients is shown in Table 1. The prevalence of atrophy in the antrum was significantly lower in the 2010s (33%, 35/107) compared to those evaluated in either the 1970s (98%, 212/216) (p < .001) or 1990s (80%, 333/417) (p < .001). Atrophy was also lower in the corpus and was significantly lower in the 2010s (19%, 20/107) compared to those evaluated in either the 1970s (82%, 177/216) (p < .001) or 1990s (67%, 280/417) (p < .001). Finally, the prevalence of intestinal metaplasia in the antrum was significantly lower in the 2010s (15%, 16/107) compared to those evaluated in the 1970s (63.9%, 138/216) (p < .001) and that in the corpus was also significantly lower in the 2010s (4.7%, 5/107) compared to those evaluated in the 1970s (32.4%, 70/216) (p < .05).
Table 1.
Prevalence of mucosal atrophy and intestinal metaplasia across the 1970s, 1990s, and 2010s in Helicobacter pylori-positive patients
| 1970s (n = 216) | 1990s (n = 417) | 2010s (n = 107) | |
|---|---|---|---|
| Atrophy (n) | |||
| Antrum | 98% (212) | 80% (333)* | 33% (35)**,## |
| Corpus | 82% (177) | 67% (280)* | 19% (20)**,## |
| Intestinal metaplasia (n) | |||
| Antrum | 63.9% (138) | 37.4% (156)* | 15% (16)** |
| Corpus | 32.4% (70) | 21.3% (89) | 4.7% (5)* |
p < .001 versus 1970s,
p < .05 versus 1970s,
p < .001 versus 1990s.
The severity and extent of mucosal damage were investigated by assessing the prevalence of gastric mucosal atrophy and intestinal metaplasia in relation to age. Among those with H. pylori infection, the severity of mucosal atrophy and intestinal metaplasia significantly declined during the 1970s, 1990s, and 2010s (Figs 2 and 3).
Figure 2.
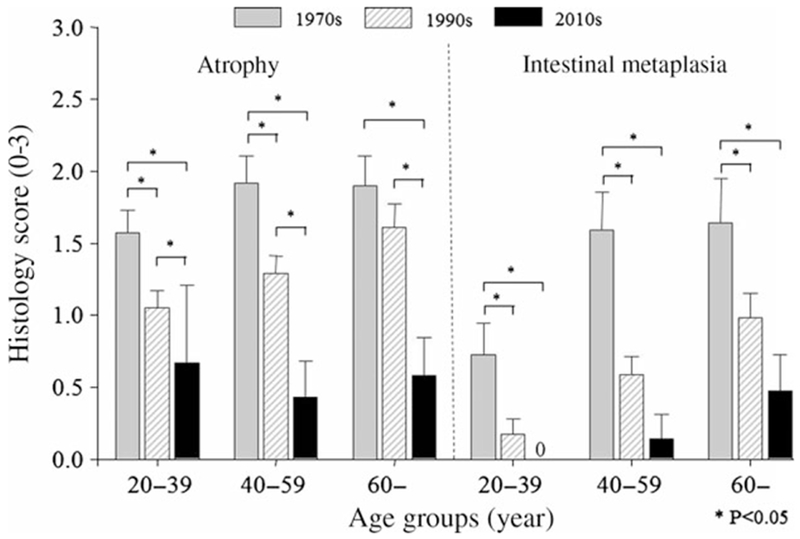
Mean ± 95% CI of atrophy and intestinal metaplasia scores of antral mucosal biopsies in Helicobacter pylori-positive patients according to age group. Both mucosal atrophy and metaplasia in the antrum significantly decreased in time period setting in all age group.
Figure 3.
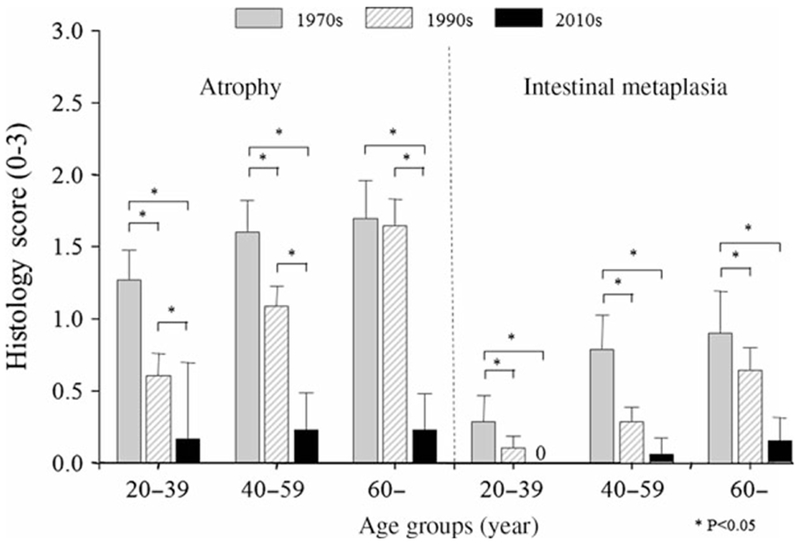
Mean ± 95% CI of atrophy and intestinal metaplasia scores of corpus mucosal biopsies in Helicobacter pylori-positive patients according to age group. Both mucosal atrophy and metaplasia in the corpus significantly decreased in time period setting in all age group.
Discussion
A significant decline in the prevalence of H. pylori infection in recent years in Japan has been documented previously and corresponds to improvements in national hygienic conditions and a trend toward nuclear families [24–28]. To our knowledge, this is the first report describing the recent trends while separately addressing the reduction in the prevalence of H. pylori infection in addition to the decline in severity of gastritis among H. pylori-infected Japanese adults. We show that among H. pylori-infected individuals, the age-specific severity of atrophy and intestinal metaplasia has declined remarkably over 40 years of observation. Sipponen et al. [31] reported the prevalence of gastritis in Finland over 15 years using biopsy specimens of patients obtained in 1977 (702 patients), 1985 (1309 patients), and 1992 (1447 patients). They reported a decreasing prevalence of gastritis associated with a decreasing rate of H. pylori infections. Valle et al. [32] had previously reported the long-term course and consequences of H. pylori gastritis in a 32-year follow-up study (1952–1983) in Finland. They showed that the appearance of parietal cell antibodies during follow-up was associated with progression of severe corpus atrophy which was accompanied by disappearance of H. pylori infection. Imai and Murayama [33] examined autopsies and resected stomachs of Japanese patients in two different periods (1957–1962 and 1978–1980) and showed that although there was a downward tendency in the prevalence of H. pylori infection among middleaged individuals, the prevalence of intestinal metaplasia generally remained high. In contrast, our more recent data clearly show a long-term trend toward a reduction in the prevalence of H. pylori infection as well as a change in the pattern of damage among those with H. pylori infection. This trend was evident despite the introduction of proton-pump inhibitors whose use would tend to enhance the rate of progression among those with H. pylori infection [34,35].
The eventual outcome of an H. pylori infection is related to the interaction between the virulence of the H. pylori strain, the genetic background of the host, and environmental factors such as diet [20]. Both smoking and salt intake have been shown to promote more rapid development of atrophic gastritis and intestinal metaplasia [36–38]. In a previous study, we investigated the relationship between H. pylori infection, smoking, atrophic gastritis, and intestinal metaplasia and found that atrophy and the histologic grade of intestinal metaplasia grades were higher in H. pylori-positive smokers than among nonsmokers [36]. Figure 4 shows the change in the fall in prevalence of smoking in the Japanese population over the past 40 years. High salt intake has long been established as risk factors for gastric cancer [39], and the daily salt intake in Japanese population has been decreasing over the past 60 years (Fig. 5). In addition, the reduction in salt intake and smoking, the Japanese diet has changed markedly since World War II such that the fat and protein intake are now similar to that Western countries [40]. The introduction of refrigeration and improvements in transportation have also resulted in a more varied diet and elimination of seasonal variation in availability of fruits and vegetables.
Figure 4.
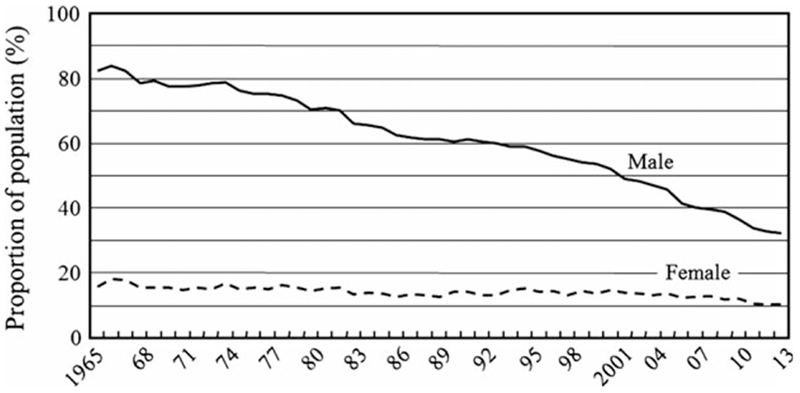
Time trend over 40 years showing the prevalence of smoking according to the sex in Japanese population. The smoking rate in the Japanese population has decreased remarkably over the past 40 years [29].
Figure 5.
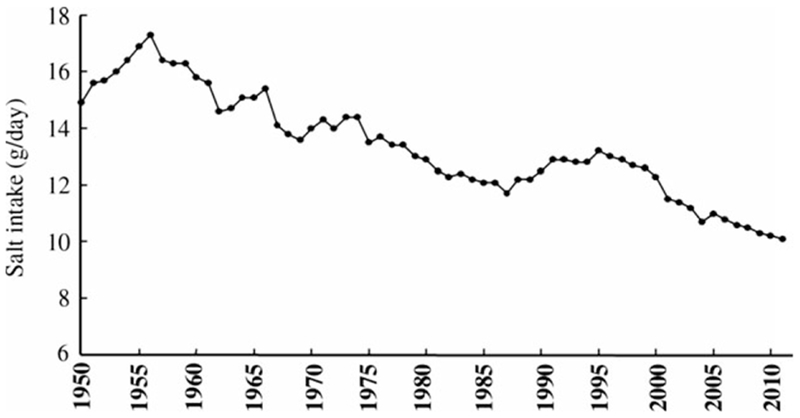
Time trend of daily salt intake levels in the past 60 years in the Japanese population. Salt intake in the Japanese population has decreased remarkably in the past 60 years [30].
Our prior animal studies using the H. pylori-infected Mongolian gerbil showed that excessive salt enhanced gastric corpus gastritis [37] and that long-term administration of a high-protein or casein diets suppressed corpus atrophic gastritis [41]. In that study, we proposed that the high-protein diet enhanced gastrin secretion which stimulated G cells to increase acid secretion which in turn limited the H. pylori infection to the antrum similar to what is seen in patients with duodenal ulcer [42]. Whatever the mechanism, the change in the Japanese diet to one with an increase in the intake of protein, fresh fruits, and vegetable and decreased use of salt likely contributed greatly to the observed changes in the development of atrophic gastritis demonstrated among H. pylori-infected Japanese.
As noted earlier, the most common H. pylori strains circulating in Japan are CagA positive of the East Asian CagA genotype [43]. These strains are thought to be highly virulent, and recent studies [44–46] have confirmed that these strains remain most common. The fact that the extent and severity of atrophic changes have declined rapidly despite the presence of these highly virulent H. pylori strains emphasizes the predominate importance of environmental factors in determining the outcome of H. pylori infections. The time-related decline in the rate of acquisition of atrophic changes among Japanese with H. pylori infection has occurred despite no change in host genetics or the prevalence of what is considered a highly virulent H. pylori strain [43]. As in Western countries, the change in the velocity in the development of atrophic damage among those with H. pylori has occurred coincident with changes in the environmental factors associated with decreased severity of gastritis such as westernization of the diet and use of refrigeration [20].
There are two limitations to this study. Firstly, our study is a retrospective analysis what reviewed biopsy specimens collected from a nonrandom sample. Secondly, considerable difficulties persist in the classification and grading of gastric atrophy with a substantial interobserver variability. In this study, the presence and severity of gastric atrophy and intestinal metaplasia were assessed by gastroenterologists experienced in gastric histopathology. The two gastroenterologists in this study are very versed in the pathology of alimentary tract, especially, the updated Sydney system. Two assessments were made one for atrophy and the other for the presence of intestinal metaplasia. Intestinal metaplasia is not subject to difficulties with intraobserver variation and served as a separate measure of the presence of corpus and antral atrophy making misclassification bias less likely.
In conclusion, our data clearly showed a trend of a fall in the prevalence of H. pylori infection of the last 40 years that was additional coupled with a decrease in the extent and severity of mucosal atrophy and intestinal metaplasia among those with H. pylori infections. These results are consistent with prior experience that decline in the incidence of gastric cancer may occur more rapidly than the decline in H. pylori infection.
Acknowledgements and Disclosures
Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grants R01 DK062813 and DK56338 which funds the Texas Medical Center Digestive Diseases Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH.
Competing interests: Dr. Graham is an unpaid consultant for Novartis in relation to vaccine development for treatment or prevention of H. pylori infection. Dr. Graham is a paid consultant for RedHill Biopharma regarding novel H. pylori therapies and has received research support for culture of H. pylori. He is a consultant for Otsuka Pharmaceuticals regarding diagnostic breath testing. Dr. Graham has received royalties from Baylor College of Medicine patents covering materials related to 13C-urea breath test.
References
- 1.Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983;1:1273–5. [PubMed] [Google Scholar]
- 2.Marshall BJ, Royce H, Annear DI, et al. Original isolation of Campylobacter pyloridis from human gastric mucosa. Microbios Lett 1984;25:83–8. [Google Scholar]
- 3.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1:1311–5. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer, World Health Organization. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum 1994;61:177–241. [PMC free article] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer. Helicobacter pylori. Biologic Agents: A Review of Human Carcinogens (vol. 100B). Leon: International Agency for Research on Cancer, 2012;385–435. [Google Scholar]
- 6.Comfort MW. Gastric acidity before and after development of gastric cancer: its etiologic, diagnostic and prognostic significance. Ann Intern Med 1951;36:1331–48. [DOI] [PubMed] [Google Scholar]
- 7.Comfort MW, Vanzant FR. Gastric acidity in carcinoma of the stomach. Am J Surg 1934;26:447–56. [Google Scholar]
- 8.Kekki M, Saukkonen M, Sipponen P, Varis K, Siurala M. Dynamics of chronic gastritis in the remnant after partial gastrectomy for duodenal ulcer. Scand J Gastroenterol 1980;15:509–12. [DOI] [PubMed] [Google Scholar]
- 9.Kekki M, Sipponen P, Siurala M, Laszewicz W. Peptic ulcer and chronic gastritis: their relation to age and sex, and to location of ulcer and gastritis. Gastroenterol Clin Biol 1990;14:217–23. [PubMed] [Google Scholar]
- 10.Kekki M, Sipponen P, Siurala M. Progression of antral and body gastritis in patients with active and healed duodenal ulcer and duodenitis. Scand J Gastroenterol 1984;19:382–8. [PubMed] [Google Scholar]
- 11.Siurala M, Kekki M, Varis K, Isokoski M, Ihamäki T. Gastritis and gastric cancer. Br Med J 1972;3:530–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siurala M. Gastritis, its fate and sequelae. Ann Clin Res 1981;13:111–3. [PubMed] [Google Scholar]
- 13.Siurala M, Varis K, Wiljasalo M. Studies of patients with atrophic gastritis: a 10–15-year follow- up. Scand J Gastroenterol 1966;1:40–8. [DOI] [PubMed] [Google Scholar]
- 14.Villako K, Siurala M. The behaviour of gastritis and related conditions in different population samples. Ann Clin Res 1981;13:114–8. [PubMed] [Google Scholar]
- 15.Correa P A human model of gastric carcinogenesis. Cancer Res 1988;48:3554–60. [PubMed] [Google Scholar]
- 16.Correa P, Schmidt BA. The relationship between gastric cancer frequency and the ratio of gastric to duodenal ulcer. Aliment Pharmacol Ther 1995;9(Suppl. 2):13–9. [PubMed] [Google Scholar]
- 17.Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol 2013;23:492–501. [DOI] [PubMed] [Google Scholar]
- 18.Graham DY. Campylobacter pylori and peptic ulcer disease. Gastroenterology 1989;96:615–25. [DOI] [PubMed] [Google Scholar]
- 19.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784–9. [DOI] [PubMed] [Google Scholar]
- 20.Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol 2014;20:5191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai YC, Hsiao WH, Yang HB, Cheng HC, Chang WL, Lu CC, Sheu BS. The corpus-predominant gastritis index may serve as an early marker of Helicobacter pylori-infected patients at risk of gastric cancer. Aliment Pharmacol Ther 2013;37:969–78. [DOI] [PubMed] [Google Scholar]
- 22.Rugge M, Fassan M, Pizzi M, Farinati F, Sturniolo GC, Plebani M, Graham DY. Operative link for gastritis assessment vs operative link on intestinal metaplasia assessment. World J Gastroenterol 2011;17:4596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marugame T, Matsuda T, Kamo K, Katanoda K, Ajiki W, Sobue T, Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2001 based on the data from 10 population-based cancer registries. Jpn J Clin Oncol 2007;37:884–91. [DOI] [PubMed] [Google Scholar]
- 24.Haruma K, Okamoto S, Kawaguchi H, Gotoh T, Kamada T, Yoshihara M, Sumii K, Kajiyama G. Reduced incidence of Helicobacter pylori infection in young Japanese persons between the 1970s and the 1990s. J Clin Gastroenterol 1997;25:583–6. [DOI] [PubMed] [Google Scholar]
- 25.Fujisawa T, Kumagai T, Akamatsu T, Kiyosawa K, Matsunaga Y. Changes in seroepidemiological pattern of Helicobacter pylori and hepatitis A virus over the last 20 years in Japan. Am J Gastroenterol 1999;94:2094–9. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima S, Nishiyama Y, Yamaoka M, Yasuoka T, Cho E. Changes in the prevalence of Helicobacter pylori infection and gastrointestinal diseases in the past 17 years. J Gastroenterol Hepatol 2010;25(Suppl. 1):S99–110. [DOI] [PubMed] [Google Scholar]
- 27.Akamatsu T, Ichikawa S, Okudaira S, Yokosawa S, Iwaya Y, Suga T, Ota H, Tanaka E. Introduction of an examination and treatment for Helicobacter pylori infection in high school health screening. J Gastroenterol 2011;46:1353–60. [DOI] [PubMed] [Google Scholar]
- 28.Ueda J, Gosho M, Inui Y, et al. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter 2014;19:105–10. [DOI] [PubMed] [Google Scholar]
- 29.2013. [WWW document.] URL http://www.health-net.or.jp/tobacco/product/pd090000.html (accessed: 1 October 2013).
- 30.2010. [WWW document.] URL http://www2.ttcn.ne.jp/~honk-awa/2173.html (accessed: 1 October 2010).
- 31.Sipponen P, Helske T, Järvinen P, Hyvärinen H, Seppälä K, Siurala M. Fall in the prevalence of chronic gastritis over 15 years: analysis of outpatient series in Finland from 1977, 1985, and 1992. Gut 1994;35:1167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valle J, Kekki M, Sipponen P, Ihamäki T, Siurala M. Long-term course and consequences of Helicobacter pylori gastritis. Results of a 32-year follow-up study. Scand J Gastroenterol 1996;31:546–50. [DOI] [PubMed] [Google Scholar]
- 33.Imai T, Murayama H. Time trend in the prevalence of intestinal metaplasia in Japan. Cancer 1983;52:353–61. [DOI] [PubMed] [Google Scholar]
- 34.Yang HB, Sheu BS, Wang ST, Cheng HC, Chang WL, Chen WY. H. pylori eradication prevents the progression of gastric intestinal metaplasia in reflux esophagitis patients using long-term esomeprazole. Am J Gastroenterol 2009;104:1642–9. [DOI] [PubMed] [Google Scholar]
- 35.Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996;334:1018–22. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura M, Haruma K, Kamada T, Mihara M, Yoshihara M, Sumioka M, Fukuhara T, Chayama K. Cigarette smoking promotes atrophic gastritis in Helicobacter pylori-positive subjects. Dig Dis Sci 2002;47:675–81. [DOI] [PubMed] [Google Scholar]
- 37.Shinozaki K, Kamada T, Sugiu K, Kusunoki K, Manabe N, Shiotani A, Hata J, Teramoto F, Haruma K. High-salt and high-fat diets promote corpus atrophic gastritis in Mongolian gerbils. Kawasaki Med J 2010;36:97–105. [DOI] [PubMed] [Google Scholar]
- 38.Nomura A, Yamakawa H, Ishidate T, Kamiyama S, Masuda H, Stemmermann GN, Heilburn LK, Hankin JH. Intestinal metaplasia in Japan: association with diet. J Natl Cancer Inst 1982;68:401–5. [PubMed] [Google Scholar]
- 39.Shikata K, Kiyohara Y, Kubo M, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer 2006;119:196–201. [DOI] [PubMed] [Google Scholar]
- 40.Furukawa N, Iwakiri R, Koyama T, Okamoto K, Yoshida T, Kashiwagi Y, Ohyama T, Noda T, Sakata H, Fujimoto K. Proportion of reflux esophagitis in 6010 Japanese adults: prospective evaluation by endoscopy. J Gastroenterol 1999;34:441–4. [DOI] [PubMed] [Google Scholar]
- 41.Shinozaki K, Kamada T, Sugiu K, Kusunoki H, Manabe N, Shiotani A, Hata J, Teramoto F, Haruma K. High-protein diet suppresses corpus atrophic gastritis in Helicobacter pylori infected Mongolian gerbils. Nutr Cancer 2010;62:1067–73. [DOI] [PubMed] [Google Scholar]
- 42.Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology 1997;113:1983–91. [DOI] [PubMed] [Google Scholar]
- 43.Matsunari O, Shiota S, Suzuki R, Watada M, Kinjo N, Murakami K, Fujioka T, Kinjo F, Yamaoka Y. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. J Clin Microbiol 2012;50:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahara S, Sugimoto M, Vilaichone RK, Mahachai V, Miyajima H, Furuta T, Yamaoka Y. Role of Helicobacter pylori cagA EPIYA motif and vacA genotypes for the development of gastrointestinal diseases in Southeast Asian countries: a meta-analysis. BMC Infect Dis 2012;12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujiya K, Nagata N, Uchida T, et al. Different gastric mucosa and CagA status of patients in India and Japan infected with Helicobacter pylori. Dig Dis Sci 2014;59:631–7. [DOI] [PubMed] [Google Scholar]
- 46.Shiota S, Suzuki R, Yamaoka Y. The significance of virulence factors in Helicobacter pylori. J Dig Dis 2013;14:341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


