Abstract
BACKGROUND & AIMS:
In 2017, the World Health Organization (WHO) designated clarithromycin-resistant Helicobacter pylori a high priority for antibiotic research and development. However, there are no clear data on the global distribution of resistance or its clinical effects. We performed a systematic review and meta-analysis to assess the distribution of H pylori resistance to commonly used antibiotics and to measure the association between antibiotic resistance and treatment failure.
METHODS:
We searched publication databases for studies that assessed rates of H pylori resistance to clarithromycin, metronidazole, levofloxacin, amoxicillin, or tetracycline. Pooled estimates of primary and secondary resistance and 95% confidence intervals (CIs) were grouped by WHO region. The association between antibiotic resistance and treatment failure was measured by extracting data on treatment efficacy in patients with resistant and susceptible isolates and pooling odds ratios with 95% CIs.
RESULTS:
We identified 178 studies, comprising 66,142 isolates from 65 countries. Primary and secondary resistance rates to clarithromycin, metronidazole, and levofloxacin were ≥15% in all WHO regions, except primary clarithromycin resistance in the Americas (10%; 95% CI, 4%–16%) and South-East Asia region (10%; 95% CI, 5%–16%) and primary levofloxacin resistance in the European region (11%; 95% CI, 9%–13%). There was considerable heterogeneity (I2 > 75%) among all analyses—this might have resulted from the grouping of resistance rates by country. Increasing antibiotic resistance was observed in most WHO regions. Resistance to clarithromycin was significantly associated with failure of clarithromycin-containing regimens (odds ratio, 6.97; 95% CI, 5.23–9.28; P < .001).
CONCLUSIONS:
Resistance of H pylori to antibiotics has reached alarming levels worldwide, which has a great effect on efficacy of treatment. Local surveillance networks are required to select appropriate eradication regimens for each region.
Keywords: Bacterial Infection, Drug, Epidemiology, Prevalence
Helicobacter pylori (HP) infection is one of the most common chronic bacterial infections in humans, affecting approximately 4.4 billion individuals worldwide.1 Reports of infection prevalence rates range widely among geographic regions, reaching the highest levels in developing countries and showing a well-established relationship with socioeconomic status and hygiene conditions.1,2 HP infection causes chronic progressive gastric inflammation and a variety of diseases, including gastric and duodenal ulcers and gastric cancer.3 In 1994 and 2009, the International Agency for Research on Cancer classified HP as a Group 1 carcinogen on the basis of a thorough review of relevant laboratory and epidemiologic studies.4 Gastric cancer is the fifth most common malignancy and the third leading cause of cancer-related morbidity globally, constituting 9% of all cancer-related mortality.5,6 Eradication of HP infection has been proven to reduce the incidence of gastric cancer.7,8 The efficacy of the HP eradication treatment has decreased dramatically because of antibiotic resistance.9,10 With rare exceptions, worldwide clarithromycin-containing regimens are no longer suitable for unconditional empiric use because of inadequate eradication rates (<80%).11–13 Of even more concern, the efficacy of available alternatives (such as quadruple, sequential, concomitant, and levofloxacin-containing triple regimens) has varied greatly,14,15 and, although the most recent international consensus reports strongly recommend the selection of treatment based on local resistance patterns, HP testing is rarely performed.3,16
The primary objective of this systematic review and meta-analysis was to assess the prevalence and 10-year trend of primary and secondary HP resistance to the commonly prescribed antibiotics for HP infection eradication in all World Health Organization (WHO) regions. The secondary objective was to measure the association between antibiotic resistance and failure to achieve eradication.
Materials and Methods
Search Strategy and Selection Criteria
The systematic review was developed partly as a component of the WHO priority exercise aimed at rating 25 antibiotic-resistant bacteria, including HP, according to 10 preselected criteria to provide indications for research and development of new effective antibiotics.17 The prevalence and 10-year trend of antibiotic-resistant pathogens were among the criteria. The protocol of this study is available online (PROSPERO registration number: CRD42017071054; www.crd.york.ac.uk/prospero). The systematic review and meta-analysis were conducted in accordance with the recommendations in the PRISMA-P (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols) statement.18 Literature published between January 2007 and June 2017 was systematically identified in PubMed using the following search terms: (Helicobacter pylori) AND (antibiotic OR antimicrobial OR antimicrobial OR antibacterial OR anti-bacterial OR drug)) AND (resistance OR resistant*). No restriction on study type or population type was applied. The search was restricted to studies published in English. References of included studies and previous systematic reviews on the topic were also inspected to include relevant publications. We included studies reporting data on prevalence of primary or secondary resistance of HP to the following antibiotics: clarithromycin, metronidazole, tetracycline, amoxicillin, levofloxacin, or ciprofloxacin. Exclusion criteria were studies reporting prevalence data on fewer than 50 isolates, studies reporting prevalence of resistance expressed only as a percentage with no mention of the total number of isolates, and studies not reporting the time frame or clustering data across a time period longer than 3 years. Data reported within guidelines, conference presentations, and letters without formal publication were also omitted. Duplicate publications or studies reporting data on the same cohort were used once by including only the most recently published data. Data on both single and combined resistance were extracted.
Definitions
Patients were defined as infected by HP if they tested positive by any of the following tests: histology, culture, serology, stool antigen, urea breath test, or rapid urease test. Prevalence of resistance was based on culture results and expressed as the number of resistant isolates divided by the total number of isolates tested. We selected 15% as the threshold for high resistant rate in accordance with the most recent international guidelines.3,16 Results from both phenotypic (E test, agar dilution) and genotypic (nucleic acid-based) tests were accepted for the definition of resistance. Primary resistance was defined as resistance to any antibiotic before the start of the first eradication treatment; secondary resistance was defined as resistance to any antibiotic in patients who had already undergone at least 1 unsuccessful eradication attempt. Response to treatment was evaluated on the basis of invasive (culture negative after gastric biopsy) and noninvasive tests.
Data Extraction
A 2-step selection process was conducted. The first selection of relevant studies was performed by inspection of titles and abstracts by a single reviewer (AS); full texts of relevant studies were retrieved and evaluated independently by 2 reviewers (AS, EC). Data from included studies were extracted independently, and any discrepancies and inconsistencies were discussed and resolved by consensus or involving a third reviewer (ET). The decision and reasons for inclusion/exclusion of the study and if arbitration about selection was required was reported on the data screening form.
Data were extracted and sorted by: study (author, year, country, city, study design), patients (sample size, age, sex, endoscopic, or histologic findings, underlying diseases and previous eradication regimens in case of secondary resistance), samples (total number of tested samples; number of resistant samples; type of tested samples, ie, stool, gastric or duodenal biopsy; antibiotic susceptibility test methodology; and breakpoint reference system), and rates of eradication failure in patients with available susceptibility testing before treatment.
Quality Assessment
The quality of the included studies was assessed by 2 reviewers (AS, EC) independently, using an adapted version of the tool proposed by the Joanna Briggs Institute for critical appraisal of prevalence studies (Supplementary Table 1).19 A score ranging from 0 to 8 points was attributed to each study (>5 points = high quality, 4–5 points = medium quality, <4 points = low quality). A third reviewer (ET) adjudicated in any case of disagreement. Need for arbitration and reason was reported in the data collection tool.
Data Analysis and Synthesis
Categorical variables were coded and recorded accordingly. Continuous numerical variables were reported as percentages or as means with SD, and skewed numerical variables as median with interquartile range. Meta-analysis of proportions was conducted by pooling primary and secondary prevalence data using a random-effect model and grouped according to WHO regions (www.who.org) (Supplementary Figure 1). We hypothesized the existence of substantial heterogeneity among studies due to different geographic origin and design. Between-study variability was measured with the I2 statistic. Considerable heterogeneity was reported for a value of I2 > 75%.20 Any source of variability was assessed by grouping results according to predefined categorical variables. Pooled estimates were stratified by study country, study period (2006–2008, 2009–2011, 2012–2016), and age groups (adults, children). Presence of publication bias was assessed by visually inspecting the funnel plot of the overall effect size (HP primary resistance to clarithromycin) against its SE and tested for significance with the Egger’s test. P value <.05 was regarded as significant. The association between treatment failure and selected antibiotic resistances was measured by computing the study odds of failing the treatment in patients with resistant strains compared to in patients with susceptible strains. Final estimates were computed for each antibiotic by pooling single odds ratios (ORs) with 95% confidence intervals (CIs) into meta-analysis with a random-effect model. All statistical analysis was performed using STATA, version 14.2 (StataCorp LLC, College Station, TX).
Results
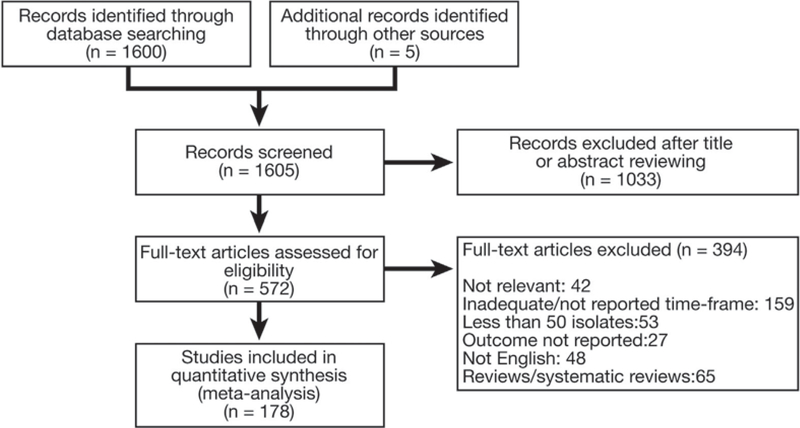
A total of 1600 citations were identified in the initial search, and 5 additional records were retrieved by inspecting the references of previous systematic reviews. By applying inclusion and exclusion criteria, 178 studies were selected for analysis (Figure 1). Publication bias was assessed for 104 studies (Supplementary Figure 6). The analysis showed visual asymmetry of the funnel plot and a significant Egger’s test (P < .01). Arbitration of the senior author was recorded only for 2 studies. Forty-two (24%) studies were randomized controlled trials, and 136 (76%) were observational studies (118 prospective and 18 retrospective). The studies were conducted in 65 countries, mainly in the Western Pacific Region (WPR) (n = 66 [37%]) and in the European Region (EUR) (n = 58 [33%]). Table 1 summarizes the geographic distribution of the studies.
Figure 1.
Flow chart of study selection.
Table 1.
Geographic Distribution of the Studies, Number of Tested Isolated, and Participants According to the World Health Organization Region
| WHO region (no. of studies) | Countries providing data (no. of studies) | No. of isolates | No. of patientsa |
|---|---|---|---|
| African (3) | Cameroon (1), Congo (1), Senegal (1) | 296 | 248 |
| Americas (13) | Argentina (1), Brazil (6), Canada (1), Colombia (2), Peru (1), USA (2) | 1647 | 1506 |
| Eastern Mediterranean (24) | Egypt (2), Iran (16), Morocco (1), Pakistan (4), Saudi Arabia (1) | 2668 | 3443 |
| European (58) | Austria (4), Belgium (3), Bulgaria (4), Croatia (3), France (4), Germany (4), Greece (3), Iceland (1), Ireland (3), Israel (4), Italy (4), Poland (5), Spain (7), The Netherlands (1), Turkey (3), United Kingdom (1), Multicentric/International (4) | 18,201 | 17,610 |
| South-East Asia (14) | Bangladesh (1), Bhutan (1), India (1), Indonesia (1), Thailand (10) | 1397 | 1830 |
| Western Pacific (66) | Australia (1), China (30), Japan (2), South Korea (6), Laos (1), Malaysia (3), New Zealand (1), Singapore (1), Taiwan (18), Vietnam (3) | 41,933 | 28,946 |
| Total (178) | 45 countries | 66,142 | 53,583 |
Six studies did not report number of patients.
The final analysis included 66,142 samples (99.5% endoscopic gastric or duodenal biopsies). Our analysis included 53,583 patients, with a median age of 48 years (interquartile range, 43–51 years) and approximately equal sex distribution (mean prevalence of males, 49% ± 9%). Six studies did not report the characteristics of the included population. The studies included mostly adults (45,021 patients were older than 18 years) with only 16% of the studies including children. The patients’ demographic and clinical features are summarized in Supplementary Table 2.
Antibiotic resistance was assessed in 29,094 (54%) naïve patients and in 5676 (11%) previously treated ones. The presence of upper gastrointestinal symptoms was the most common indication for undergoing endoscopic procedure (97% of patients), and non-ulcer dyspepsia was the most frequent diagnosis after endoscopy (70% of patients). In most (n = 144 [81%]) studies, antibiotic resistance was detected by using culture-based methods (E test in 102 studies and agar dilution in 42). In 29 (16%) studies, antibiotic resistance was assessed by molecular-based tests, while in 5 studies the diagnostic test was not specified.
Most (n = 158 [89%]) studies specified the minimum inhibitory concentration values used for defining resistance. Clarithromycin breakpoint ranged from 0.5 to 1 mg/dL in 87% of the studies, metronidazole breakpoint was 8 mg/dL in 94% of the studies, levofloxacin breakpoint was 1 mg/dL in 68% of the studies and 2 mg/dL in 30% of the studies, and amoxicillin breakpoint ranged from 0.12 to 8 mg/dL (83% of the studies reported minimum inhibitory concentration between 0.25 and 1 mg/dL).
Quality Assessment
Disagreement on quality of the studies and need for arbitration was recorded for 3 studies. The quality was high in 53 (30%) studies, medium in 93 (52%) studies, and low in 31 (18%) studies. The main factors limiting overall study quality were the low representativeness of the included population (85% single-center studies with limited sample size) and poor reporting of patients’ demographic and endoscopic characteristics. Conversely, microbiologic methods for resistance testing were adequate in all studies reporting the information (93%). A full description of the diagnostic methods and specification of minimum inhibitory concentration breakpoints was provided in 88% (n = 158) of the studies.
Pooled Prevalence of Helicobacter pylori Primary Resistance
The prevalence of primary clarithromycin resistance was >15% in EUR (18%; 95% CI, 16%–20%), in the Eastern Mediterranean Region (EMR) (33%; 95% CI, 23%–44%), and the WPR (34%; 95% CI, 30%–38%). A resistance rate of 10% was recorded in the Americas Region (AMR) (95% CI, 4%–16%) and the South-East Asia Region (SEAR) (95% CI, 5%–16%). Primary resistance to metronidazole was detected at >15% in all WHO regions and ranged from 56% (95% CI, 46%–66%) in EMR to 23% (95% CI, 2%–44%) in AMR. Resistance to levofloxacin was ≥15% in all WHO regions, except EUR (11%; 95% CI, 9%–13%). Primary combined resistance to clarithromycin and metronidazole was 19% (95% CI, 0%–39%) in EMR and <10% in the other regions. Primary resistance to amoxicillin and tetracycline was ≤10% everywhere except in EMR, where amoxicillin resistance reached 14% (95% CI, 8%–20%). Only 3 studies provided prevalence data for the Africa Region (AFR), not allowing the stratification according to resistance type but only overall estimates. Pooled primary resistance prevalence by WHO region is shown in Table 2.
Table 2.
Pooled Prevalence of Primary and Secondary Antibiotic Resistance, Stratified by World Health Organization Region
| WHO region | Pooled prevalence of antibiotic resistance, % (95% CI) | |||||
|---|---|---|---|---|---|---|
| Africa region | Clarithromycin | Metronidazole | Levofloxacin | Cla+Met | Amoxicillin | Tetracycline |
| Overall | 15 (0–30) | 91 (87–94) | 14 (12–28) | — | 38 (32–45) | 13 (9–17) |
| Americas region | Clarithromycina | Metronidazole | Levofloxacin | Cla+Met | Amoxicillin | Tetracycline |
| Primary | 10 (4–16) | 23 (2–44) | 15 (5–16) | — | 10 (2–19) | — |
| Secondary | 18 (13–23) | 30 (19–41) | 22 (3–42) | — | 7 (1–13) | — |
| Not specifiedb | — | — | — | 3 (0–13)c | — | 4 (1–11)c |
| Overall | 14 (9–19) | 27 (14–39) | 14 (12–28) | 3 (0–13)c | 8 (3–13) | 4 (1–11)c |
| Eastern Mediterranean region | Clarithromycin | Metronidazole | Levofloxacin | Cla+Met | Amoxicillin | Tetracycline |
| Primary | 33 (23–44) | 56 (46–66) | 19 (10–29) | 19 (0–39) | 14 (8–20) | 10 (4–15) |
| Secondary | 17 (10–27) | 65 (54–74)c | 30 (14–46) | 11 (6–20) | 10 (5–18)c | 17 (8–26) |
| Not specifiedb | 25 (17–32) | 67 (61–72) | — | 8 (4–11) | 15 (8–22) | — |
| Overall | 29 (23–25) | 61 (55–67) | 23 (14–32) | 14 (5–23) | 14 (10–18) | 10 (8–13) |
| European region | Clarithromycina | Metronidazolea | Levofloxacina | Cla+Meta | Amoxicillin | Tetracycline |
| Primary | 18 (16–20) | 32 (27–36) | 11 (9–13) | 1 (0–2) | 0 (0–0) | 0 (0–0) |
| Secondary | 48 (38–57) | 48 (38–58) | 19 (14–24) | 18 (16–20) | 0 (0–0) | 0 (0–1) |
| Not specifiedb | 33 (26–39) | 47 (35–39) | 14 (10–18) | 7 (0–13) | 1 (0–2) | 1 (0–2) |
| Overall | 32 (25–31) | 38 (33–42) | 14 (12–16) | 15 (12–18) | 0 (0–0) | 0 (0–0) |
| Southeast Asia region | Clarithromycin | Metronidazolea | Levofloxacina | Cla+Met | Amoxicillin | Tetracycline |
| Primary | 10 (5–16) | 51 (26–76) | 30 (14–46) | — | 2 (0–5) | 0 (0–1) |
| Secondary | 15 (8–27)c | 44 (32–58)c | 24 (15–37) | — | — | — |
| Not specifiedb | 25 (0–55) | 80 (57–100) | 5 (3–11) | 6 (1–10) | 28 (0–62) | 1 (1–2) |
| Overall | 17 (6–28) | 59 (40–78) | 25 (13–28) | 6 (1–10) | 12 (6–17) | 0 (0–12) |
| Western Pacific region | Clarithromycina | Metronidazolea | Levofloxacin | Cla+Meta | Amoxicillin | Tetracyclinea |
| Primary | 34 (30–38) | 47 (37–57) | 22 (17–28) | 8 (6–10) | 1 (1–1) | 2 (1–2) |
| Secondary | 67 (54–80) | 62 (50–71) | 30 (20–39) | 13 (8–18) | 1 (1–2) | 0 (0–1) |
| Not specifiedb | 25 (21–29) | 69 (64–74) | 19 (17–21) | 14 (11–18) | 1 (1–2) | 10 (7–14) |
| Overall | 34 (30–38) | 55 (51–59) | 24 (21–26) | 11 (9–13) | 1 (1–1) | 2 (1–2) |
Cla+Met, combined resistance to clarithromycin and metronidazole.
P value for subgroup comparison <.05.
Not specified: the study did not report the type of resistance.
Only one study contributed to analysis.
Pooled Prevalence of Helicobacter pylori Secondary Resistance
Secondary clarithromycin resistance was ≥15% all WHO regions: 18% (95% CI, 13%–23%) in AMR, 17% (95% CI, 10%–27%) in EMR, 48% (95% CI, 38%–57%) in EUR, 15% (95% CI, 8%–27%) in SEAR, and 67% (95% CI, 54%–80%) in WPR. Secondary metronidazole resistance was >15% in all WHO regions, showing the highest level in EMR (65%; 95% CI, 54%–74%) and WPR (62%; 95% CI, 50%–71%). Secondary resistance to levofloxacin was >15% in all WHO regions, showing the highest level in EMR (30%; 95% CI, 14%–46%) and WPR (30%; 95% CI, 20%–39%). Resistance to amoxicillin and tetracycline remained ≤10% in all WHO regions. Combined resistance to clarithromycin and metronidazole reached 18% (95% CI, 16%–20%) in EUR. Pooled secondary resistance prevalence by WHO region is shown in Table 2.
Analysis of Trend
In SEAR, clarithromycin resistance significantly increased from 13% (95% CI, 4%–22%) in 2006–2008 to 21% (95% CI, 1%–42%) in 2012–2016 (P < .001). Levofloxacin resistance in WPR significantly increased from 12% (95% CI, 8%–17%) in 2006–2008 to 31% (95% CI, 27%–36%) in 2012–2016 (P < .001). No statistically significant changes over time were observed for the other regions, but a general trend of increased resistance was observed in several WHO regions. The results of the trend analysis are shown in Table 3.
Table 3.
Subgroup Analysis of Antibiotic Resistance by Time Period, Stratified by World Health Organization Region
| WHO region, time period | Pooled prevalence of antibiotic resistance, % (95% CI) | |||
|---|---|---|---|---|
| Americas region | Clarithromycin | Metronidazole | Levofloxacin | Cla+Met |
| 2006–2008 | 11 (3–19) | 26 (10–42) | — | — |
| 2009–2011 | 9 (2–15)a | 21 (13–33) | 11 (5–16) | — |
| 2012–2016 | 20 (12–28) | 29 (0–59) | 19 (11–27) | — |
| Eastern Mediterranean region | Clarithromycin | Metronidazole | Levofloxacin | Cla+Metb |
| 2006–2008 | 29 (18–39) | 57 (47–68) | 12 (4–20) | 2 (0–5) |
| 2009–2011 | 25 (12–38) | 67 (56–68) | 32 (12–51) | 20 (4–37) |
| 2012–2016 | 32 (24–41) | 60 (49–71) | 24 (6–41) | 14 (8–21)a |
| European region | Clarithromycinb | Metronidazole | Levofloxacin | Cla+Met |
| 2006–2008 | 28 (24–32) | 38 (33–43) | 15 (12–18) | 15 (10–20) |
| 2009–2011 | 23 (20–27) | 33 (25–40) | 13 (9–17) | 12 (8–15) |
| 2012–2016 | 28 (25–31) | 46 (34–58) | 12 (8–15) | 23 (11–36) |
| Southeast Asia region | Clarithromycinb | Metronidazoleb | Levofloxacinb | Cla+Met |
| 2006–2008 | 13 (4–22) | 99 (98–100) | — | — |
| 2009–2011 | 0 (0–4)a | 63 (57–68) | 5 (3–11)a | — |
| 2012–2016 | 21 (1–42) | 53 (30–77) | 29 (16–42) | — |
| Western Pacific region | Clarithromycin | Metronidazole | Levofloxacinb | Cla+Metb |
| 2006–2008 | 32 (16–47) | 52 (29–76) | 12 (8–17) | 4 (2–6) |
| 2009–2011 | 34 (25–43) | 54 (44–64) | 16 (13–20) | 8 (5–11) |
| 2012–2016 | 35 (30–40) | 57 (52–62) | 31 (27–36) | 14 (11–17) |
NOTE. Data for amoxicillin and tetracycline were not pooled by time-period due to the lack of studies.
Cla+Met, combined resistance to clarithromycin and metronidazole.
Only one observation contributed to analysis.
P value for subgroup comparison <.05
Subgroup Analysis
Country.
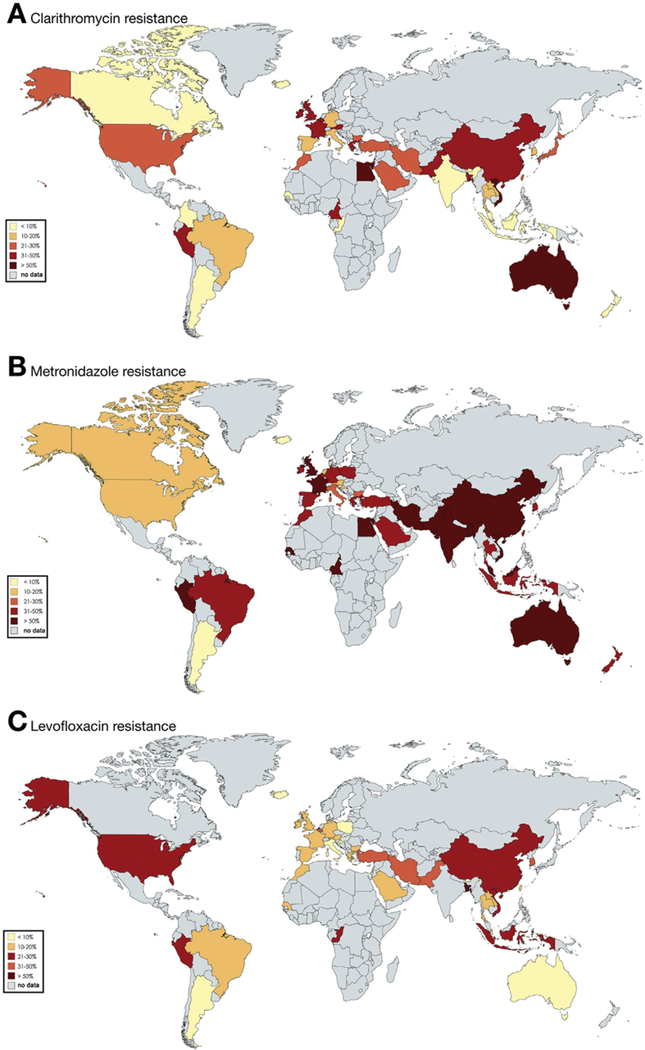
Fifteen countries provided data for EUR. Clarithromycin resistance was ≥15% in 11 of 15 countries, with the highest rates in Israel (47%; 95% CI, 39–56) and France (43%; 95% CI, 28–57). Metronidazole resistance was ≥15% in 12 of 15 countries, with the highest rate in Israel (57%; 95% CI, 48%–65%). Levofloxacin resistance was ≥15% in 5 of 15 countries: 30% (95% CI, 21%–39%) in Turkey, 29% (95% CI, 18%–41%) in Belgium, 18% (95% CI, 15%–22%) in Germany, 16% (95% CI, 14%–18%) in Spain, and 15% (95% CI, 12%–18%) in France. Resistance to amoxicillin or tetracycline was negligible (<5%) in most of the countries. Pooled estimates of antibiotic resistance by country are shown in Figure 2 and Supplementary Table 6.
Figure 2.
Pooled prevalence (2006–2016) of resistance to clarithromycin (A), metronidazole (B), and levofloxacin (C) by country.
Ten countries provided data for WPR. Clarithromycin resistance was ≥15% in 7 of 10 countries; the highest rates were recorded in Australia (96%; 95% CI, 92%–99%) and Vietnam (63%; 95% CI, 37%–88%). Metronidazole resistance was >15% in all countries. The highest rates were recorded in Malaysia (82%; 95% CI, 75%–88%) and in China (77%; 95% CI, 74%–79%). Levofloxacin resistance was ≥15% in 4 countries: China (33%; 95% CI, 29%–38%), Vietnam (32%; 95% CI, 20%–43%), South Korea (28%; 95% CI, 21%–35%), and Taiwan (15%; 95% CI, 13%–17%). The prevalence of resistance to amoxicillin or tetracycline was <5% in all countries except Vietnam, where tetracycline resistance was 17% (95% CI, 4%–31%). Pooled estimates of antibiotic resistance by country are shown in Figure 2 and Supplementary Table 8.
In AFR, AMR, EMR, and SEAR, fewer data were available, and the data were not distributed homogeneously across the countries (Table 1). Only 3 studies provided data for AFR. In SEAR, most studies originated from Thailand. In AMR, the most observations came from South America, while North America was scarcely represented. In EMR, almost all studies originated from Iran. A summary of pooled prevalence of antibiotic resistance by country in AFR, AMR, EMR, and SEAR is shown in Figure 2 and in Supplementary Tables 3, 4, 5, and 7.
Age.
Subgroup analysis by age group showed higher rates of antibiotic resistance in the adult population compared to children. An opposite tendency was detected in AMR for metronidazole resistance (40% in children vs 22% in adults), in EMR for metronidazole resistance (81% vs 61%) and levofloxacin resistance (29% vs 18%), and in WPR for clarithromycin resistance (85% vs 32%). Details of subgroup analysis by age group are shown in Table 4.
Table 4.
Prevalence of Antibiotic Resistance By Age Group, Stratified by World Health Organization Region
| WHO region, patient population | Pooled prevalence of antibiotic resistance, % (95% CI) | ||
|---|---|---|---|
| Americas region | Clarithromycin | Metronidazolea | Levofloxacin |
| Adults | 13 (8–18) | 22 (8–36) | 18 (11–14) |
| Children | 19 (13–26)b | 40 (33–48)b | — |
| Not specified | — | — | — |
| Eastern Mediterranean region | Clarithromycina | Metronidazolea | Levofloxacina |
| Adults | 29 (23–36) | 61 (55–67) | 18 (12–24) |
| Children | 10 (3–29)b | 81 (60–92)b | 29 (14–50)b |
| Not specified | 34 (28–40) | 67 (61–72) | 9 (7–12) |
| European region | Clarithromycin | Metronidazolea | Levofloxacina |
| Adults | 28 (25–31) | 40 (34–42) | 11 (9–13) |
| Children | 24 (19–30) | 20 (17–24) | 4 (1–7) |
| Not specified | 39 (24–54) | 49 (41–57) | 22 (17–26) |
| Southeast Asia region | Clarithromycin | Metronidazole | Levofloxacin |
| Adults | 16 (5–27) | 59 (40–78) | 25 (13–28) |
| Children | 29 (22–38)b | — | — |
| Not specified | — | — | — |
| Western Pacific region | Clarithromycina | Metronidazolea | Levofloxacina |
| Adults | 32 (25–38) | 53 (45–61) | 27 (22–32) |
| Children | 85 (80–90)b | 43 (37–50)b | 17 (12–22)b |
| Not specified | 12 (4–21) | 95 (94–96) | 17 (12–21) |
NOTE. Data for amoxicillin and tetracycline were not pooled by age group due to the lack of studies.
P value for subgroup comparison <.05
Fewer than 3 observations contributed to analysis.
Diagnostic method.
Clustering results by diagnostic method (genotypic vs phenotypic) showed no significant difference in antibiotic resistance prevalence (data not shown).
Heterogeneity Assessment
Considerable heterogeneity was detected between the studies in all the analyses pooling clarithromycin, metronidazole, and levofloxacin resistance rates (I2 > 75%). Within all the subgroup analyses conducted, we detected a relevant reduction (<75%) of I2 when grouping the studies by country of origin. The results of the heterogeneity assessment by country are displayed in Supplementary Table 9. No relevant reduction of heterogeneity was detected when grouping results according to other relevant variables, such as patient age, diagnostic method, or study quality.
Secondary Outcome
Forty-five studies provided data for the secondary analysis. Among these, 30 (68%) were randomized controlled trials and 15 (32%) had an observational design. The secondary analysis included 13,707 isolates cultured from the same number of patients before starting HP eradication. Sixty percent of the included patients (n = 8296) were naïve to HP therapy. Administered therapies varied widely according to antibiotic associations, dosages, anti-acid combination, treatment schemes, and duration. Among these, 35 (38%) were triple regimens (proton pump inhibitor plus 2 of the following antibiotics: clarithromycin, metronidazole, amoxicillin, and levofloxacin), 23 (25%) were concomitant regimens (with an equal distribution between bismuth quadruple and non-bismuth quadruple regimens), and 31 (34%) were sequential regimens. Four (3%) regimens were dual therapy. Due to the high number of treatment schemes, a subgroup analysis was not possible.
A statistically significant association between eradication treatment failure and resistance detected before treatment was observed for all the antibiotics. Patients with clarithromycin-resistant HP infections had a risk of failing eradication 7-fold (OR, 6.97; 95% CI, 5.23–9.28; P < .001) higher than patients with susceptible strains when treated with a clarithromycin-containing regimen. A strong association was also observed for levofloxacin (OR, 8.18; 95% CI,3.81–17.56; P < .001), metronidazole (OR, 2.52; 95% CI, 1.82–3.48; P = .004) and combined clarithromycin and metronidazole resistance (OR, 9.40; 95% CI, 5.48–16.12; P < .001); (Supplementary Figures 2–5).
Discussion
Our study found that in most WHO regions, pooled prevalence of both primary and secondary resistance of HP to clarithromycin, metronidazole, and levofloxacin is >15%, the common threshold for choosing alternative empiric regimens.3–16
Metronidazole resistance is the most prevalent pattern of resistance worldwide. Both primary and secondary resistance are, in fact, well above the threshold, with the highest level recorded in the Eastern areas of the world (EMR 56% and 65%, SEAR 51% and 44%, WPR 47% and 62%). The resistance rates to clarithromycin, metronidazole, and levofloxacin have increased over time in all WHO regions. In particular, a significant increase of resistance to clarithromycin occurred in SEAR (from 13% in 2006–2008 to 21% in 2012–2016) and to levofloxacin in WPR (from 12% in 2006–2008 to 31% in 2012–2016), crossing the intervention threshold in 10 years. We also observed that the resistance rates are higher in previously treated individuals than in patients who never received eradication treatment and higher in adults than in children. We described a clear significant association between antibiotic resistance and treatment failure as secondary outcome.
A meta-analysis by De Francesco et al21 in 2010 previously provided a global picture of HP antibiotic primary resistance. They retrieved resistance data between 1997 and 2007 from 31 studies, mostly from Europe. The main findings, clustered according to the different continental regions, showed clarithromycin resistance ranging from 11.1% in Europe to 29.3% in America. Metronidazole resistance had already been detected at rates >15% on all the continents (America 44%, Africa 92%, Asia 37%, and Europe 17%), and levofloxacin resistance rate was >15% in Europe (24%). Tetracycline and amoxicillin resistance were both <10% worldwide.21 Although the different geographic data aggregation allows only a rough comparison, our study shows 10 years later, a further increase of primary resistance to clarithromycin, levofloxacin, and metronidazole worldwide.
Pooled antibiotic primary resistance data in Latin America were published by Camargo et al22 in 2014. That meta-analysis collected data between 1988 and 2011 from 59 studies. The pooled prevalence of resistance rates for clarithromycin, metronidazole, fluoroquinolones, amoxicillin, and tetracycline were 12%, 53%, 15%, 4%, and 6%, respectively. Taking into account that almost all studies contributing to our pooled data for AMR derived from South America, our findings confirm these resistance rates.
Kuo et al13 provided an overview of primary antibiotic resistance prevalence in the past 25 years in the Asia-Pacific region. This meta-analysis, published in 2017, included 162 studies analyzing data from 37,219 patients mainly from WPR. In the time period 2006–2015, clarithromycin and metronidazole primary resistance reached 20% and 47%, respectively; the levofloxacin primary resistance rate was 21%, and both tetracycline and amoxicillin primary resistance rates were 3%.13 In WPR, we reported the same resistance rate to metronidazole (47%), similar resistance rates to levofloxacin (22%), amoxicillin (1%), and tetracycline (2%), and a higher resistance rate to clarithromycin (34%).
In the subgroup analysis by time period, we detected, in general, a trend of increased resistance to all the antibiotics. A significant increase in resistance over time was recorded for all antibiotics in SEAR and for levofloxacin in WPR. The same tendency was described by Kuo et al13 for clarithromycin, metronidazole, and levofloxacin in the time period of 2006–2015 in the Asia-Pacific region.
Antibiotic resistance prevalence data in children are scarce. In our meta-analysis, 23 studies could provide clustered data by age group. In a meta-analysis of 6 studies published in 2017 in the Iranian pediatric population, the prevalence of resistance to clarithromycin, metronidazole, and fluoroquinolones was 12%, 71%, and 16%, respectively.23 Our pooled estimates on children in the EMR region confirm similar rates of resistance. Although fluoroquinolones are contraindicated for the treatment of any infection in children, the resistance rate to levofloxacin was remarkable in EMR (29%) and WPR (17%), possibly reflecting resistance in the infecting strain and intrafamilial spread of quinolone-resistant isolates from adults.24,25 As for the high rate of metronidazole resistance in children from AMR, it is important to emphasize that 2 of 3 studies contributing to the analysis for this region were conducted in Brazil, where high prevalence of self-medication of antibiotics has been observed in children and adolescents.26
The alarming global levels of HP resistance in treatment-naïve patients can be correlated with the increasing and uncontrolled consumption of antibiotics that are commonly used in HP empirical therapy and also used to treat other common infections in the general population (eg, respiratory, genital, and urinary infections, parasite infestation).27 For example, in the time period 2000–2010, global macrolide consumption increased by 19% and fluoroquinolone consumption by 64%.28 The highest primary resistance rate registered for metronidazole and levofloxacin in the Western areas of the world likely relates to the massive use of metronidazole to treat parasite infestations in developing countries and to the increased consumption of fluoroquinolones in these regions.13,27 Beyond the widely proven relationship between antibiotic consumption and development of resistance, it has been recently demonstrated that the previous use of macrolides correlates directly to HP eradication failure. In one study in which a clarithromycin-containing regimen was used, the eradication failure rate was significantly higher in patients with a history of previous macrolide use for longer than 2 weeks than in patients with a shorter antibiotic duration.29 A recent study from Korea demonstrated that the regions that experienced the most significant fail in eradication rates were those with the highest macrolide prescriptions.30 Antibiotic resistance is the major driver of eradication failure. The expected efficacy of standard triple regimen has dropped to insufficient levels in many areas of the world, causing this regimen to no longer be considered appropriate for unconditional empiric use.11,12 Moreover, the alternative regimens suggested for overcoming clarithromycin resistance have shown heterogeneous efficacy results strongly correlated to the resistance profile of the antibiotics included.15,31,32 In the analysis of the secondary outcome, our meta-analysis summarized data from 45 randomized controlled trials, and we confirmed a clear association between HP resistance and treatment failure. In particular, patients harboring clarithromycin-resistant strains had a 7-fold probability of failing eradication with a clarithromycin-containing regimen. For levofloxacin resistance and combined (clarithromycin plus metronidazole) resistance, the risks are 8- and 9-fold, respectively.
Our data in the Asia-Pacific region were consistent with those from Kuo et al,13 who reported efficacy of clarithromycin-based triple therapy <80% in countries where clarithromycin resistance was >20%. In addition, the efficacy of sequential therapy and of concomitant therapy was also <80% in most countries with clarithromycin resistance >20%. The meta-analysis of Nyssen et al15 highlighted that in the subgroup of clarithromycin-resistant strains, both sequential treatment and standard triple regimen did not achieve satisfactory eradication rates (mean efficacy: 75% vs 43%, respectively). Levofloxacin resistance also seems to play an important role in determining treatment failure. In the meta-analysis by Chen et al,32 the efficacy of levofloxacin triple regimen was significantly higher for levofloxacin-susceptible strains than for resistant strains (eradication rate: 81.1% vs 36.3%; risk ratio, 2.38; 95% CI, 1.6–3). Furthermore, we showed a slightly lower risk of treatment failure in patients with metronidazole-resistant HP infection treated with metronidazole-containing regimens, consistent with the previously described data suggesting that the impact of metronidazole resistance is less than that of clarithromycin and levofloxacin resistance because it can be partially overcome by increasing the dose and duration of treatment, especially when used as part of a bismuth-containing quadruple therapy.3,33–35 The resistance in previously treated individuals is generally higher than in naïve patients for almost all included antibiotics. No previous pooled data on secondary antibiotic resistance are available in the literature for making a comparison.
The results of our meta-analysis should be also considered for the possible impact on the incidence of gastric cancer. Based on GLOBOCAN estimates, the highest incidence of gastric cancer is in Western Asia (China, Japan, and Korea), where antibiotic resistance rates are proven to be very high. On the other hand, regions with lower level of antibiotic resistance (such as the region of Americas) are showing lower incidence of gastric cancer.5 Despite this association, a causal relationship remains hard to infer, mainly because the lack of surveillance systems for antibiotic-resistant HP does not allow defining of actual incidence of antibiotic-resistant infections. In countries where HP is highly endemic, the role of eradication treatment in reducing the incidence of gastric cancer has been widely acknowledged.7,8 Based on our data, we can hypothesize that this trend in reduction is expected to revert soon because available treatment can no longer guarantee a satisfactory eradication rate.
Our meta-analysis has limitations. First, antibiotic-resistance prevalence data were limited in several countries. An attempt to fill the gap in knowledge is being conducted by the Pan-European Registry on Helicobacter pylori Management, a multicenter, prospective, noninterventional register systematically collecting data from 30 European countries on prescribed regimen, compliance, outcome, and antibiotic resistance in order to produce descriptive studies of HP infection management.35 Second, between-results heterogeneity among WHO regions and among different countries in the same WHO region is considerable. Sensitivity analysis by sorting the studies according to the countries provided a partial explanation of heterogeneity and further underlines the importance of implementing local antibiotic resistance surveillance. Another possible cause of study heterogeneity is different patterns of antibiotic consumption, depending on the variable infectious disease burden in different geographic areas. A potential bias in data reporting, as demonstrated by the detection of significant publication bias, also constitutes a limitation in drawing overall pooled estimates from heterogeneous data. Third, several medium-to-high quality studies were conducted in a single medical center with a small sample size and might not be representative of the general population within a region. Fourth, although studies suggest that culture-based tailored treatment are likely the best approach to minimize eradication failures,36,37 HP susceptibility testing is rarely performed, despite the almost universal availability of culture facilities for other common pathogens.
This meta-analysis provides a comprehensive overview of global antibiotic resistance of HP in the last 10 years, shows worrisome levels of resistance rates in several areas of the world, and suggests that the development of resistance is associated with an increased risk of treatment failure. Repeated antibiotic treatment also increases the ecological pressure of antibiotic usage in the community and further contributes to the antibiotic resistance burden, especially in low- and lower-middle income countries. Therefore, the implementation of local and national surveillance system networks and the development of new noninvasive techniques in clinical practice are urgently needed to improve treatment effectiveness and consequently limit the malignant and nonmalignant burden of HP chronic infection.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
Current evidence recommends selection of alternative regimens for Helicobacter pylori eradication in settings where antibiotic resistance is higher than 15–20%. However, the distribution of antibiotic resistance is not well reported worldwide.
NEW FINDINGS
In a comprehensive assessment of global H pylori antibiotic resistance patterns over ten years, resistance to clarithromycin, metronidazole and levofloxacin was found to cross the threshold of 15% in the majority of WHO regions.
LIMITATIONS
This systematic review encompassed 178 studies from 65 countries, with data lacking in numerous developing countries.
IMPACT
This study reports alarming rates of antibiotic resistance worldwide, underlining the need to implement surveillance networks in order to improve the eradication rate and, consequently, limit the burden of H pylori-induced diseases.
Acknowledgments
Anne McDonough provided writing assistance and editorial support. She is an independent clinical writer with no university affiliation. She was partly supported by World Health Organization Priority pathogen list project, grant number 3021017.
Funding
This work has been partially funded by the German Center for Infection Research, Clinical Research Unit (grant number 08701) and the World Health Organization Priority Pathogen List project (grant number 3021017).
These authors disclose the following: Alessia Savoldi’s and Elena Carrara’s work was supported in part by the World Health Organization Priority Pathogen List project, grant 3021017. David Y. Graham’s work is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center. Dr Graham is a consultant for RedHill Biopharma regarding novel H pylori therapies. He has received research support for culture of H pylori and is the Principal Investigator of an international study of the use of antimycobacterial therapy for Crohn’s disease. He is also a consultant for BioGaia in relation to probiotic therapy for H pylori infection and for Takeda in relation to H pylori therapies. Evelina Tacconelli reports research grants from the Innovative Medicines Initiative Brussels, European Commission 7th Framework, World Health Organization, and the German Center for Infection Research.
Abbreviations used in this paper:
- AFR
African Region
- AMR
Americas Region
- CI
confidence interval
- EMR
Eastern Mediterranean Region
- EUR
European Region
- HP
Helicobacter pylori
- OR
odds ratio
- SEAR
South-East Asian Region
- WPR
Western Pacific Region
- WHO
World Health Organization
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2018.07.007.
Conflicts of interest
The remaining author discloses no conflicts.
References
- 1.Hooi JKY, Lai WJ, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017;153:420–429. [DOI] [PubMed] [Google Scholar]
- 2.Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther 1995;9(Suppl 2):33–39. [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum; 2012;100(Pt B):1–441. [PMC free article] [PubMed] [Google Scholar]
- 5.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:359–386. [DOI] [PubMed] [Google Scholar]
- 7.Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology 2016;150:1113–1124. [DOI] [PubMed] [Google Scholar]
- 8.Rokkas T, Rokka A, Portincasa P, et al. A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Ann Gastroenterol 2017;30:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SY, Choi DJ, Chung JW. Antibiotic treatment for Helicobacter pylori: is the end coming? World J Gastrointest Pharmacol Ther 2015;6:183–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang CS, Baik GH. Attempts to enhance the eradication rate of Helicobacter pylori infection. World J Gastroenterol 2014;20:5252–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007;12: 275–278. [DOI] [PubMed] [Google Scholar]
- 12.Arslan N, Yilmaz N, Demiray-Gurbuz E. Importance of antimicrobial susceptibility testing for the management of eradication in Helicobacter pylori infection. World J Gastroenterol 2017;23:2854–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo YT, Liou MJ, El Omar EM, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:707–715. [DOI] [PubMed] [Google Scholar]
- 14.Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 2016;43:514–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyssen OP, McNicholl AG, Megraud F, et al. Sequential versus standard triple first-line therapy for Helicobacter pylori eradication. Cochrane Database Syst Rev 2016; 6:CD009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallone CA, Chiba N, van Zanten SV, et al. The Toronto Consensus for the Treatment of Helicobacter pylori infection in adults. Gastroenterology 2016;151:51–69. [DOI] [PubMed] [Google Scholar]
- 17.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018;18:318–327. [DOI] [PubMed] [Google Scholar]
- 18.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Br Med J 2015;354:i4086. [DOI] [PubMed] [Google Scholar]
- 19.Munn Z, Moola S, Riitano D, et al. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag 2014;3:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews. Version 5.1.0 [updated March 2011]. Available at: http://handbook-5-1.cochrane.org/. Accessed December 14, 2017.
- 21.De Francesco V, Giorgio F, Hassan C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis 2010;19:409–414. [PubMed] [Google Scholar]
- 22.Camargo MC, García A, Riquelme A, et al. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am J Gastroenterol 2014;109:485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousefi-Avarvand A, Vaez H, Tafaghodi M, et al. Antibiotic resistance of Helicobacter pylori in Iranian children: a systematic review and meta-analysis. Microb Drug Resist 2018;24:980–986. [DOI] [PubMed] [Google Scholar]
- 24.Lopes AI, Oleastro M, Palha A, et al. Antibiotic-resistant Helicobacter pylori strains in Portuguese children. Pediatr Infect Dis J 2005;24:404–409. [DOI] [PubMed] [Google Scholar]
- 25.Liu G, Xu X, He L, et al. Primary antibiotic resistance of Helicobacter pylori isolated from Beijing children. Helicobacter 2011;16:356–362. [DOI] [PubMed] [Google Scholar]
- 26.Pereira FS, Bucaretchi F, Stephan C, et al. Self-medication in children and adolescents. J Pediatr (Rio J) 2007; 83:453–458. [DOI] [PubMed] [Google Scholar]
- 27.Megraud FH pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 2004;53: 1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14:742–750. [DOI] [PubMed] [Google Scholar]
- 29.Lim SG, Park RW, Shin SJ, et al. The relationship between the failure to eradicate Helicobacter pylori and previous antibiotics use. Dig Liver Dis 2016;48:385–390. [DOI] [PubMed] [Google Scholar]
- 30.Shin WG, Lee SW, Baik GH, et al. Eradication rates of Helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: nationwide survey. Helicobacter 2016;21:266–278. [DOI] [PubMed] [Google Scholar]
- 31.Marin AC, Nyssen OP, McNicholl AG, et al. Efficacy and safety of quinolone-containing rescue therapies after the failure of non-bismuth quadruple treatments for Helicobacter pylori eradication: systematic review and meta-analysis. Drugs 2017;77:765–776. [DOI] [PubMed] [Google Scholar]
- 32.Chen PY, Wu MS, Chen CY, et al. Systematic review with meta-analysis: the efficacy of levofloxacin triple therapy as the first- or second-line treatments of Helicobacter pylori infection. Aliment Pharmacol Ther 2016;44:427–437. [DOI] [PubMed] [Google Scholar]
- 33.Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan, 2009 revised edition. Helicobacter 2010;15:1–20. [DOI] [PubMed] [Google Scholar]
- 34.Bardhan K, Bayerdörffer E, Veldhuyzen Van Zanten SJ, et al. The HOMER study: the effect of increasing the dose of metronidazole when given with omeprazole and amoxicillin to cure Helicobacter pylori infection. Helicobacter 2000;5:196–201. [DOI] [PubMed] [Google Scholar]
- 35.McNicholl AG, Gasbarrini A, Tepes B, et al. Pan-European registry on H. pylori management (HP-EuReg): bacterial resistance. Gastroenterology 2015;148(Suppl 1):S417. [Google Scholar]
- 36.Shiotani A, Lu H, Dore MP, et al. Treating Helicobacter pylori effectively while minimizing misuse of antibiotics. Cleve Clin J Med 2017;84:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dang BN, Graham DY. Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat Rev Gastroenterol Hepatol 2017;14:383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.