Abstract
Background:
Helicobacter pylori treatment recommendations often recommend use of double-dose PPI or greater. This is confusing because PPIs very markedly in relative potency such that a double dose of one may not even be equivalent to the single dose of another.
Objective:
To relate the concept of double-dose to specific amounts of the different PPIs
Methods:
We used data standardizing PPI potency in terms of the duration of intragastric pH >4/24 hours (pH4-time) to rank PPIs. Relative potency varies from 4.5 mg omeprazole equivalents (20 mg pantoprazole) to 72 mg omeprazole equivalents (40 mg rabeprazole).
Results:
We defined PPI dosing for H. pylori therapy as low dose (eg, approximately 20 mg omeprazole equivalents, b.i.d.), high or double dose as approximately 40 mg omeprazole equivalents, b.i.d.) and high dose as approximately 60 mg omeprazole equivalents, b.i.d.). For example, standard double dose PPI would thus be 40 mg of omeprazole, 20 mg of esomeprazole or rabeprazole, 45 mg of lansoprazole, or 120 mg of pantoprazole each given b.i.d.
Conclusions:
Simply doubling the dose of any PPI achieves markedly different effects on pH4-time. However, PPIs can be used interchangeably and cost effectively based on their omeprazole equivalency.
Keywords: Helicobacter pylori, potency, PPI-amoxicillin dual therapy, proton-pump inhibitor, resistance, susceptibility, therapy
1 |. INTRODUCTION
Reliably effective antimicrobial therapy is almost by definition susceptibility-based therapy.1 However, the majority of Helicobacter pylori eradication therapy studies are done and reported without susceptibility data, in part, because of a paucity of clinical laboratories that perform H. pylori susceptibility testing. This lack of susceptibility data prevents determining which component(s) of the regimen result in most treatment failures (ie, drugs used, doses, frequency of administration, and duration.). The lack of local resistance data has resulted in widespread use of empiric therapies utilizing antibiotics for which the infection is no longer susceptible. This has likely also contributed to the global increase in antimicrobial resistance.2
2 |. FACTORS THAT INFLUENCE CURE RATES OTHER THAN SUSCEPTIBILITY
Important factors influencing H. pylori eradication cure rates are shown in Table 1. One definition of an optimal therapy for susceptible infection is a regimen that will reliably achieve cure rates of ≥95% in adherent patients.3 Most regimens are acid dependent defined as becoming markedly less effective as the pH declines and with treatment success beings improved by the addition of an antisecretory drug. Bismuth triple therapy (bismuth, metronidazole, tetracycline) is an acid-independent therapy that can achieve high cure rates without antisecretory drugs (reviewed in Ref. 4). However, in the presence of metronidazole resistance, this regimen requires concomitant antisecretory drug therapy.4
TABLE 1.
Factors that influence cure rates
| Drugs: dose, formulation, frequency of administration, relation to meals, side effects |
| Antimicrobials: Susceptibility |
| Antisecretory drugs: Relative potency |
| Duration: in most instances 14 days has proven best |
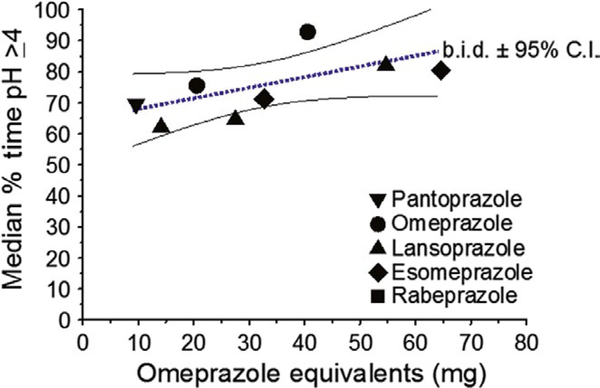
Proton-pump inhibitors (PPIs) are the most widely used antisecretory agents. However, PPIs vary greatly in relative potency assessed by their ability to maintain the intragastric pH at 4 or greater for a full 24 hours (pH4-time) (Table 2 and Figure 1).5,6 The new generation of PPIs, the reversible potassium inhibitors, have theoretical advantages over traditional PPI as they (a) achieve full effectiveness with a single day’s administration vs. requiring 3–5 days for traditional PPIs, (b) inhibit both active and non-secreting parietal cells, and (c) have a long half-life in the plasma making them as effective as twice a day PPI therapy.7,8 In western populations, the relative potency of 10 mg of vonoprazan once daily is approximately equivalent to 60–70 mg of an omeprazole given bid5 Typically, the best results with traditional PPI-containing therapies require a treatment duration of 14 days.9–12 This longer duration can help overcome the persister state which describes the fact that organisms can remain dormant without replicating allowing them to survive antibiotics effective during replications (eg, amoxicillin) if the duration of administration is shorter than the period of dormancy.13 In addition, because PPIs do not achieve full activity until after several days of administration, shorter therapies also limit the duration of effective antisecretory activity.13,14 Vonoprazan achieves full effectiveness on the first day of therapy and thus might allow for effective shorter duration therapy if it also helps overcome the persister effect.
TABLE 2.
Relative potency of PPIs as omeprazole equivalents and cure rate of PPI-amoxicillin dual therapy with PPIs according to omeprazole equivalents
| PPI | Dosage (mg) | Omeprazole equivalent (mg) |
Cure rate PPI-Amox |
|---|---|---|---|
| Pantoprazole | 20 | 4.5 | |
| Pantoprazole | 40 | 9.0 | ~10% |
| Esomeprazole | 10 | 16 | |
| Rabeprazole | 10 | 18 | |
| Omeprazole | 20 | 20 | ~20% |
| Lansoprazole | 30 | 27 | |
| Esomeprazole | 20 | 32 | |
| Rabeprazole | 20 | 36 | |
| Omeprazole | 40 | 40 | ~40% |
| Lansoprazole | 60 | 56 | |
| Esomeprazole | 40 | 64 | ~60% |
| Rabeprazole | 40 | 74 |
FIGURE 1.
The median pH4-time for different omeprazole equivalents administered twice a day for at least 5 days and the 95% confidence intervals (Adapted from Ref. 5, with permission)
3 |. WHICH PPI, WHICH DOSE, AND WHAT DOES DOUBLE-DOSE ACTUALLY MEAN?
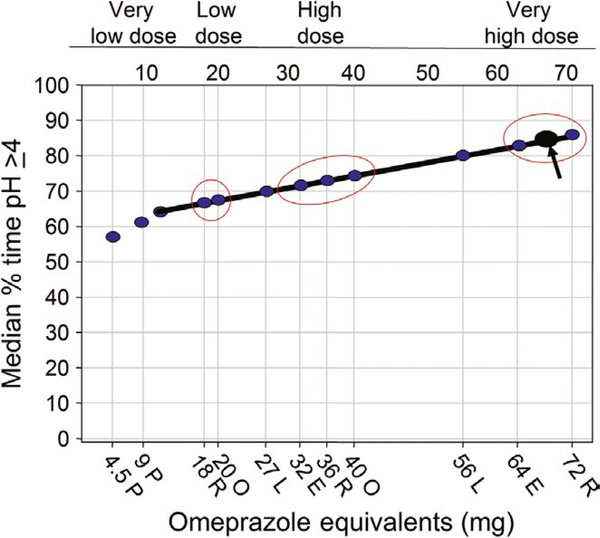
Recent treatment recommendations include use of a double dose of a PPI.15–17 The Maastricht V consensus states “increasing the dose of PPI from, for example, 20 mg omeprazole twice daily to 40 mg of esomeprazole or rabeprazole twice daily may increase cure rates by 8%−12%”.16 Here, we seek to unpack the concepts of drug especially PPI dosage and the important elements of therapy that critically influence effectiveness and put that statement into perspective. Overall, treatment of susceptible infections with 14-day duration therapies is associated with high cure rates.12 Since it would be impossible to improve cure rates by 8% to 12% if they were already ≥95%, any comparisons of PPIs derived from studies in populations in which resistance markedly reduced cure rates (eg, to 70% to 80%) are useless for clinical predictions.15,18 The treated population actually consists of two subpopulations, one with susceptible infections with high cure rates and one with resistant infections (which functionally eliminates clarithromycin, metronidazole or a fluoroquinolone) making treatment success entirely dependent on the PPI-amoxicillin component of the triple therapy. The outcome of the dual PPI-amoxicillin component is pH dependent (reviewed in Ref.19) which is related to relative PPI potency.5 Figure 2 groups PPIs in terms of what we propose to be very low-dose, low-dose, high-dose, and very high-dose regimens.5,8 The relative potency of PPIs shown in Figure 2 and Table 2 is based on data from western populations and represents the median for relatively large groups.3 Nonetheless, individual results will differ around that median. Asian populations more often have corpus gastritis, smaller parietal cell masses, and reduced PPI metabolism compared to western populations making PPIs more effective in raising intragastric pH and thus in improving the effectiveness of PPI-amoxicillin dual therapy.5 Note that in this characterization 30 and 60 mg of lansoprazole occupies intermediate positions with 30 mg falling between low- and high-dose regimens and 60 mg between high- and very high-dose regimens.
FIGURE 2.
Median pH4 time for different omeprazole equivalents showing different doses of PPI given bid and 10 mg of vonoprazan given once daily in terms of relative effectiveness as adjuvants for improving the efficacy of a triple therapy containing amoxicillin. P, pantoprazole; R, rabeprazole; O, omeprazole; E, esomeprazole; L, lansoprazole. Arrow shows vonoprazan 10 mg
4 |. PPI POTENCY AND CURE RATE WITH PPI-AMOXICILLIN DUAL THERAPY
Table 2 also shows the expected cure rates with different omeprazole equivalents in western populations with PPI-containing triple therapies ranging from very weak PPI (≤10 mg omeprazole equivalent to maximum (64–72 mg omeprazole equivalents).5 Figure 2 illustrates the fallacy and inappropriateness of lumping different PPIs and of conclusions such as triple therapy with drug “X” was superior to PPI-based triple therapy or statements such as triple therapy with rabeprazole 40 mg bid (eg, 72 omeprazole equivalents bid) was superior to triple therapy with pantoprazole 40 mg bid (eg, nine omeprazole equivalents bid). While literally true, the comparisons are actually studies using markedly different antisecretory effects and not differences in the individual PPIs (eg, while results with 40 mg of esomeprazole bid might be superior to 20 mg of omeprazole bid, they would be expected to yield identical results if the comparison was made with 60 mg of omeprazole bid. A PPI is not a PPI and authors should disclose when drugs with different potency are compared.
5 |. WHAT DOES DOUBLE-DOSE MEAN IN TERMS OF PPI, PPI DOSE, AND RELATIVE PPI POTENCY?
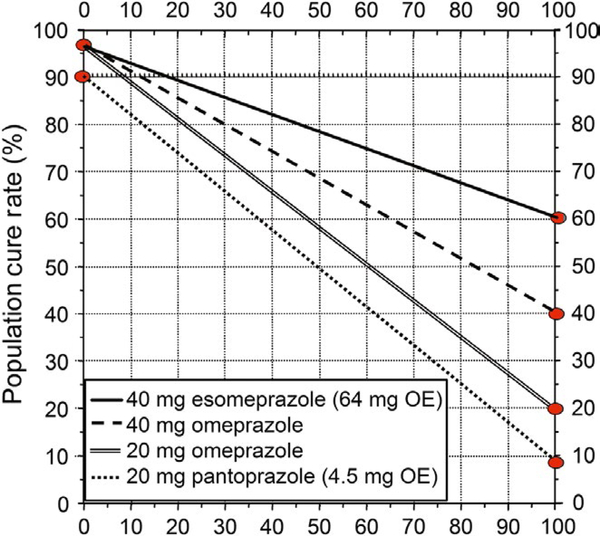
As noted above, the enhanced effectiveness of triple therapy with double-dose PPI is related to the ability to increase the intragastric pH. The linear increase in pH4 time increases with an increase in omeprazole equivalent dose up to approximately 70 mg of omeprazole and then plateaus such that increasing the PPI dose higher would be unlikely to provide further improvement in outcome. For example, doubling the dose of pantoprazole from 20 to 40 mg results in omeprazole equivalent of 9 mg whereas doubling 20 mg of rabeprazole produces the effect of 72 mg of omeprazole.3,5 It should also be clear that similar effects using equivalent doses of any PPI and thus cost effectiveness may be improved by using higher doses of cheaper drugs such as 60 mg of omeprazole or lansoprazole bid rather than 40 mg of esomeprazole or rabeprazole. As shown in Figure 3, increasing the pH4-time increases the effectiveness of the PPI-amoxicillin dual therapy and thus the success rate as some patients with clarithromycin resistance who would otherwise fail therapy will now be cured.5,8,10 This improved outcome for the individual results in unintended misuse of the drug with resistance such as clarithromycin or levofloxacin. For example, in Japan, the cure rate with a 7-day course of 20 mg of vonoprazan plus 750 mg of amoxicillin bid of those with clarithromycin resistance is approximately 80%.8 More recent studies using vonoprazan and amoxicillin without clarithromycin have confirmed the effectiveness of vonoprazan-amoxicillin dual therapy with 95% cure rate with amoxicillin 500 mg tid20 and consistent with the data with high-dose PPI plus amoxicillin.19,21 One can therefore conclude that at least 80% of those receiving that combination would have been cured had clarithromycin been omitted. Currently, there are approximately 1.4 million H. pylori treatments/year in Japan mostly using this regimen which results in more than 3000 kg of unnecessary antibiotic use/year in for just this indication which serves only to increase global antimicrobial resistance.2,22,23
FIGURE 3.
Plot of the effect of increasing the omeprazole equivalent on triple therapies given for 14 days in the presence of increasing clarithromycin resistance. Very low-dose PPI such as 4.5 mg omeprazole equivalent is estimated to achieve an overall cure rate of approximately 90% with susceptible infections and 10% with PPI-amoxicillin (~1 gram bid). The cure rate improves to ≥95% as omeprazole equivalent dose is increased to 20 mg or greater. As the omeprazole equivalent dose is increased, the proportion cured in the clarithromycin resistant population also progressively improves and results in the “double-dose” phenomenon
The minimum dose (omeprazole equivalent) of PPI to reliable cure ≥95% of cases with clarithromycin triple therapy and clarithromycin-susceptible infections is unknown but is not greater than approximately 20 mg of omeprazole or an equivalent given bid On a practical note, we recommend 40 mg of omeprazole or its equivalent bid10 If clarithromycin resistance is high (eg, more than 10%), clarithromycin should not be used unless susceptibility is confirmed. If there is no other choice, then an omeprazole equivalent of at least 60 mg bid should be used or use vonoprazan, if it is available.
To date, H. pylori has rarely developed resistance to tetracycline or amoxicillin. The cure rate with amoxicillin alone is low24 but the addition of a PPI can increase cure rates that theoretically can achieve 100% (reviewed in Ref. 19). There have been some recent successes with dual therapy21 but it has become clear that it is very difficult to reliably achieve a sustained high intragastric pH with PPI oral therapy.25 Vonoprazan is currently the most effective PPI and preliminary studies have shown that while 7-day therapy is promising no one has yet reliably achieved 95% or greater cure rates. Longer duration or different dosing will likely be required if the goal of a highly effective therapy is to be achieved.8 Use of an amoxicillin-antisecretory dural therapy has a potential advantage in that H. pylori culture and susceptibility testing are not required until or unless the pattern of resistance changes.
6 |. SUMMARY
The lessons from these analyses on the effects of PPI relative potency on treatment outcome of triple therapy include: (a) the need to express PPI effectiveness in terms of relative potency (ie, in omeprazole equivalents), (b) the marked effect of resistance on treatment outcome, (c) that improved effectiveness of dual PPI-amoxicillin therapy is pH, dose, and duration dependent, (d) the importance of cure rates with the susceptible and resistant sub-populations for comparing the effect of drugs and regimens, (e) that claimed difference in treatment results are study population-specific which prevents valid comparisons and meta-analyses, and (f) PPI potency and susceptibility data are required for valid comparisons.3
Acknowledgments
Funding information
Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center.
Dr Graham is a consultant for RedHill Biopharma regarding novel H. pylori therapies. He has received research support for culture of H. pylori and is the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease. He is also a consultant for BioGaia in relation to probiotic therapy for H. pylori infection and for Takeda in relation to H. pylori therapies. Dr Dore has received unrelated and unrestricted grants from BioGaia, Stockholm, Sweden in relation to probiotic therapy for H. pylori infection.
Footnotes
CONFLICTS OF INTEREST
Dr Lu has no conflicts to report.
REFERENCES
- 1.Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc. 2011;86:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dang BN, Graham DY. Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat Rev Gastroenterol Hepatol. 2017;7:383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham DY. Illusions regarding Helicobacter pylori clinical trials and treatment guidelines. Gut. 2017;66:2043–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: The good, the bad, and the ugly. Gastroenterol Clin North Am. 2015;44:537–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;6:800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. EurJ Clin Pharmacol. 2009;65:19–31. [DOI] [PubMed] [Google Scholar]
- 7.Sachs G, Shin JM, Munson K, et al. Review article: the control of gastric acid and Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:1383–1401. [DOI] [PubMed] [Google Scholar]
- 8.Graham DY, Dore MP. Update on the use of vonoprazan: a competitive acid blocker. Gastroenterology. 2018;154:462–466. [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence. Consensus Report. Gut. 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- 10.Shiotani A, Lu H, Dore MP, Graham DY. Treating Helicobacter pylori effectively while minimizing misuse of antibiotics. Cleve Clin J Med. 2017;84:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham DY. Helicobacter pylori update: Gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: Evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5:48–56. [DOI] [PubMed] [Google Scholar]
- 15.Vallve M, Vergara M, Gisbert JP, Calvet X. Single vs. double dose of a proton pump inhibitor in triple therapy for Helicobacter pylori eradication: a meta-analysis. Aliment Pharmacol Ther. 2002;16:1149–1156. [DOI] [PubMed] [Google Scholar]
- 16.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the. Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. [DOI] [PubMed] [Google Scholar]
- 17.Mahachai V, Vilaichone RK, Pittayanon R, et al. Helicobacter pylori management in ASEAN: The Bangkok consensus report. J Gastroenterol Hepatol. 2018;33:37–56. [DOI] [PubMed] [Google Scholar]
- 18.Chey WD, Leontiadis Gl, Howden CW, Moss SF ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239. [DOI] [PubMed] [Google Scholar]
- 19.Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016;65:870–878. [DOI] [PubMed] [Google Scholar]
- 20.Furuta T, Yamade M, Uotani T, et al. Tul299 - Vonoprazan-based dual therapy with amoxicillin is as effectve as the triple therapy for the eradication of H. pylori. Gastroenterology 2018;154:S-927. [Google Scholar]
- 21.Yang JC, Lin CJ, Wang HL, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2015;13:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang BN, Graham DY. It is time to rethink H. pylori therapy. J Gastrointestin Liver Dis. 2017;26:115–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albrich WC, Monnet DL, Harbarth S. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg Infect D/s. 2004;10:514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axon AT. Helicobacter pylori therapy: effect on peptic ulcer disease. J Gastroenterol Hepatol. 1991;6:131–137. [DOI] [PubMed] [Google Scholar]
- 25.Graham DY, Lu H, Shiotani A. Failure of optimized dual proton pump inhibitor amoxicillin therapy: What now? Saudi J Gastroenterol. 2017;23:265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]