Abstract
Background:
We aimed to characterize the impact of ART initiation on GALT at various sites along the gastrointestinal site.
Methodology:
Peripheral blood and duodenal and rectal biopsies were obtained from 12 HIV− and 33 treatment-naïve HIV+ subjects at baseline and after 9-months ART. Tissue was digested for immunophenotyping. Inflammatory, bacterial translocation and intestinal damage markers were measured in plasma.
Results:
Twenty-six HIV+ subjects completed follow-up. The lowest reconstitution of CD4+ T-cells and the lowest CD4/CD8 ratio during ART compared to blood were observed in the duodenum with the rectum being either intermediate or approaching blood levels. Regulatory T-cells (Treg) were in higher proportions in the duodenum than the rectum and neither declined significantly during ART. Several correlations with biomarkers of microbial translocation were observed including increases in LTA levels, which reflects gram-positive bacterial translocation, correlated with increases in %CD4+ T-cells in duodenum (Rho 0.773, P=0.033), and with decreases duodenal Treg populations (Rho −0.40, P=0.045).
Conclusions:
HIV-mediated immunological disruption is greater in the duodenum than rectum and blood before and during ART. Small intestine damage may represent a unique environment for T-cell depletion, which might be attenuated by interaction with gram positive bacteria.
Keywords: gastrointestinal associated lymphoid tissue, HIV, immune reconstitution, efavirenz, raltegravir, maraviroc, viral persistence, drug concentrations, rectum, duodenum, ART Tissue Penetration Ratio, small intestines, lipoteichoic acid, zonulin, sCD14, HIV DNA, HIV RNA, T reg cells, γδ intestinal T-cells
INTRODUCTION
Depletion of mucosal CD4+ T-cells and alterations in small intestinal function were recognized early in the HIV epidemic[1–4]. These functional defects are now believed to contribute to persistent systemic immune activation as a critical contributor to residual morbidity in chronic HIV infection successfully treated with antiretroviral therapy (ART)[5, 6]. Unexpectedly, the profound loss of mucosal CD4+ T-cells that occurs during acute infection in humans and experimental SIV infection is usually not restored by subsequent control of viremia and increased systemic CD4+ T-cell counts[7–10]. Indeed, as with systemic CD4+ T-cell recovery, there is broad variation in the mucosal recovery of CD4+ T-cells across patients following the initiation of ART that is independent of their systemic immune reconstitution[7, 8, 11].
There are several hypotheses to account for the discordant recovery of immune cell populations between compartments in the gut and peripheral blood. While low-level residual viral replication detected throughout the gastrointestinal tract could account for local CD4+ T-cell loss, the ratio of unspliced RNA to DNA is similarly low across all tissue and vascular compartments[12, 13]. Nonetheless, whether GALT tissue could represent a sanctuary for HIV replication remains a concern[14]. Systemically, an inflammation-induced reduction of critical mucosal homing receptor on CD4+ T-cells could blunt trafficking to tissue compartments[15, 16]. Gut bacteria may also assist the immune system by accumulating pro-inflammatory mediators or amplification it by, for example, cleaving sialic and dolichol components from enterocytes17]. Finally, lymphoid and lamina propria fibrosis has been demonstrated and is associated with the magnitude of immune reconstitution in clinical studies[18–20]. A unifying model to link residual morbidity, increased systemic inflammation/immune activation, and mucosal immune defects that persist despite chronic virologic suppression focuses on gut microbial antigen:mucosal immune function as a nidus for these events[5, 6, 21].
However, whether specific sites along the gastrointestinal tract contribute heterogeneously to this pathologic process is unknown. Several previous reports demonstrate that (a) the duodenum lags behind the rectal mucosa in immune reconstitution following initiation of ART when both sites are sampled cross-sectionally [12] or longitudinally[11, 22], (b) that the rectal mucosa achieves immune reconstitution comparable to normal controls over time[15, 23], and (c) that only patients treated during acute but not chronic infection experienced immune reconstitution similar to normal controls in duodenal tissue[10].
This trial was designed to identify possible diverse immunologic factors associated with this delay and to inform future investigations into the clinical consequences of delayed small intestinal immune reconstitution as well as therapeutic interventions to restore normal GALT immune functions.
METHODS
Study design
This pilot randomized controlled trial (RCT) enrolled chronically HIV-infected patients naive to ART with CCR5 tropism by Trofile ES™ and a cohort of healthy volunteers (HIV−) who were similar to the HIV-infected patients (HIV+) in age and lifestyle. HIV-infected participants were randomized to one of three ART regimens after completing baseline endoscopy as previously published[24]. In class switch to another NNRTI was permitted for subjects intolerant to EFV. All participants signed an informed consent form approved by the UC Davis Institutional Review Board prior to initiation of study procedures. (Clinicaltrials.gov:).
Sample preparation
At baseline and after 9 months of ART, rectal (10–15cm from the anal verge) and duodenal (second and third segments) biopsies were placed immediately in either liquid nitrogen, paraformaldehyde for paraffin-embedding or sterile media for single cell suspension by a single investigator (DMA). Peripheral blood mononuclear cells (PBMC) were isolated using Ficoll-Hypaque™ (Pfizer-Pharmacia, New York, NY). Single-cell suspensions generated by collagenase digestion were stained and analyzed by flow cytometry (Becton-Dickinson LSRII) as previously described[7, 24] and with the gating strategy as demonstrated in Figure S1. Similarly, immunofluorescent antibody tissue analysis was performed as previously reported[7, 24]. The numbers of positive cells were counted by a single observer (Z-MM) and presented as cells/mm2 of lamina propria.
Plasma Interleukin-6 (IL-6; high-sensitivity kit, R&D Systems), soluble CD14 (sCD14; R&D Systems), high-sensitivity C Reactive Protein (CRP; CardioPhase high-sensitivity kit,-CRP assay, Siemens), and zonulin-1 (ALPCO) levels were assessed according to manufactures’ recommendations. Lipoteichoic acid (LTA) was measured as previously published[25]. IL-4 was measured by ELISA using the Vascular Injury-II kit (MesoScale Discovery, Gaithersburg, MD)
Statistical Methods
Paired comparisons assessed using Wilcoxon matched-pairs signed-ranks test and cross-sectional pairwise comparisons between groups were performed using the non-parametric Kruskal-Wallis and Mann-Whitney U tests. Analysis of the magnitude of changes in each parameter across the different compartments were calculated by comparing the ratios between the post-treatment and baseline measurements using Wilcoxon rank tests. We used linear regression analysis to adjust the effect of the study group (independent variable) on the % of activated T-cells (dependent variable) for the %CD4+ T-cells and to determine the slope of %CD4+ T-cell and CD4/CD8 ratio change in each compartment. The Spearman-rank correlation coefficient was used to analyze correlations between continuous variables. Results are expressed as estimated means (95% CI) unless stated otherwise. A P value of <0.05 was considered statistically significant. As a pilot project designed to explore hypotheses for future investigations, no adjustments for multiple comparisons were included[26]. All statistical analyses were conducted with Stata v.13.0 (StataCorpLP College Station, Texas).
RESULTS
Thirty-two HIV-infected patients naïve to ART underwent endoscopy for duodenal and rectal biopsies and peripheral blood collection at baseline and 9 months after randomization to efavirenz (EFV) versus maraviroc (MVC) versus MVC plus raltegravir (RAL) (1:1:1), each in combination with a fixed-dose combination of emtricitabine and tenofovir disoproxil fumarate (FTC/TDF) as described in the results of the parent clinical trial[24]. Twelve HIV− controls with similar demographics to the HIV+ subjects underwent identical procedures at a single time point, permitting the analysis to examine immunologic reconstitution with respect to what is expected in normal controls. It is noteworthy that these normal controls yielded similar results to our previous study with a different cohort of normal controls[7]. Six patients were excluded from the analyses due to loss of follow-up (n=5) or ART change to another class (n=1). Twenty-six patients completed the 9 months of treatment as recently reported in an analysis that focused on the impact of different antiretroviral regimens on immune reconstitution (Table 1)[24]. Clinically, all individuals rapidly achieved and maintained HIV suppression (plasma HIV RNA≤20 copies/mL).
TABLE 1.
General characteristics of the study population.
| HIV-uninfected controls (n=12) | HIV-infected (n=26) | |
|---|---|---|
| Age (years, IQR) | 35 (29, 42) | 37 (25, 42) |
| Male gender (No., %) | 7 (58%) | 22 (85%) |
| Race (No., %) | ||
| Asian | 1 (8.3%) | 2 (7.7%) |
| Black | 1 (8.3%) | 10 (38.4%) |
| Caucasian | 9 (55.0%) | 12 (46.1%) |
| Pacific Islander | 1 (8.3%) | 1 (3.8%) |
| Hispanic | 0 (0%) | 1 (3.8%) |
| CD4+ T-cell count (cells/mm3, IQR) | NA | 436 (283, 572) |
| CD4/CD8 ratio (IQR) | 1.6 (1.2, 2.6) | 0.43 (0.33, 0.60) |
| HIV RNA Level (copies/mL, IQR) | NA | 14130 (4059, 34009) |
Greater impairment in recovery of CD4+ and CD8+ T-cells in duodenum compared to blood and rectum
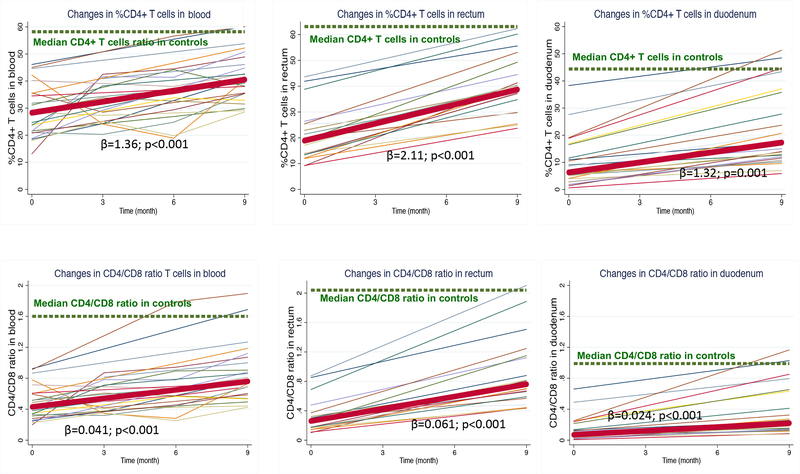
The percent CD4+ T-cells was depleted in all compartments in HIV+ vs. HIV− individuals (in blood, 28.3% vs. 58.2%; in rectum, 18.9% vs. 63.1%; and in duodenum 6.2% vs. 44.4%, all P values <0.001), and this difference was particularly striking in the duodenum where the ratio with HIV− was lowest (0.49, 0.30, and 0.14 for blood, rectum and duodenum respectively)(Table S1). The lowest reconstitution of percent CD4+ T-cells (median ratio of HIV+ to HIV− after treatment was observed in duodenum: 0.69, 0.62, 0.37 for blood, rectum, and duodenum; P=0.001 for the comparison across compartments), where the slope of increase was the smallest (Figure 1A).
Figure 1. (A) Changes in %CD4+ T-cells. (B) Changes in CD4/CD8 ratio.
Cross-sectional values in controls are represented by a dashed green line). Individual trajectories of values derived from HIV-infected participants are represented by thin color lines, and mean values are illustrated by a thick red line. The coefficient of slope (β) ant its P value was calculated by regressing the %CD4+ T-cells over time.
The CD8+ T-cell subset was expanded in all compartments in HIV+ vs. HIV−, but to a larger extent in the gut (blood, 63.4% vs. 36.7%, rectum, 72.2% vs. 30.9% and duodenum 88.7% vs. 44.2%, all P values <0.001)(Table S1). The percent CD8+ T-cells significantly decreased during ART, but at the end of follow-up remained more expanded in the gut with respect to blood (median ratio in blood, rectum and duodenum compared with HIV− : 1.4, 1.7 and 1.7, P=0.007 for the comparison across compartments). As shown in Figure S2, changes in %CD4+ T cells in gut tissue were not predictive of changes in blood, but significantly correlated between rectum and duodenum. In contrast, changes in duodenal, but not rectal, %CD8+ T cells were more predictive of changes in peripheral CD8+ T cells, and did not correlated between compartments. These findings support the importance of the duodenum in the immunopathogenesis of HIV infection and underline the relevance of addressing different gastrointestinal sites in studies aimed at understanding the mucosal immunology implications of HIV.
Persistence of low circulating CD4/CD8 ratio during ART predicts immune activation and increased mortality in the setting of peripheral CD4+ T-cell immune reconstitution [27]. The greatest CD4/CD8 ratio imbalance in HIV+ vs. HIV−individuals was observed in the duodenum (in blood, 0.43 vs.1.59; in rectum 0.26 vs. 2.04 and in duodenum 0.07 vs. 0.99, all P values <0.001). The CD4/CD8 ratio increased slower in duodenum than in rectum and blood, but still remained substantially decreased in duodenum after 9 months of ART (median CD4/CD8 ratio of HIV+/HIV−0.5, 0.4 and 0.2, P<0.001 for the comparison across compartments)(Figure 1B). These data indicate that the gut, especially the small intestines, serves as a site for more severe CD4+ T-cell depletion and CD4/CD8 ratio imbalance during ART.
T-cell activation is slow to decrease in duodenum compared to blood and rectum and not correlated with systemic inflammation
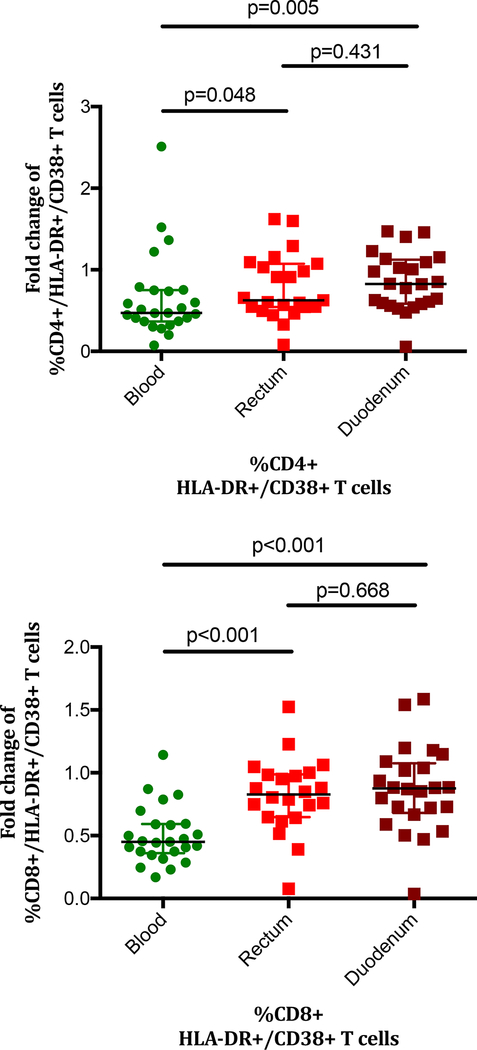
The subset of activated T-cells is considered of crucial importance as it is targeted for ongoing HIV infection/replication (CD4+ subset), fuels the inflammatory response and promotes immunosenescence[28]. We observed a parallelism between the dynamics of activated T-cells in blood and rectum that was not replicated in the duodenum. In duodenum, the %HLA-DR+/CD38+/CD4+ T-cells was not increased at baseline in HIV+ vs. HIV− individuals (52.2% vs. 58.6%, P=0.192), but significantly declined to levels lower than observed in HIV− (38.6%, P=0.029)(Table S1). This difference at the end of follow-up between HIV+ and HIV− subjects, however, was not significant when adjusted by the percentage of duodenal CD4+ T-cells in a linear regression analysis (Coef. −1.09, P=0.852). Likewise, duodenal %HLA-DR+/CD38+/CD8+ showed non-statistically significant higher values in HIV+ vs. HIV− individuals (64.0% vs. 57.0%, P=0.192), but significantly decreased to normal levels during ART (58.3%, P=0.028). Thus, not only did the CD8+ T-cell frequency decline, but the proportion with an activated phenotype also decreased. T-cell activation reductions were significantly greater in blood compared to rectum and duodenum, with the duodenum being the compartment with the smallest changes during treatment (median fold month 9/month 0 change in blood, rectum and duodenum: CD4+ T-cells; 0.5, 0.6 and 0.8, P=0.016; CD8+ T-cells; 0.5, 0.8 and 0.9, P<0.001)(Figure 2). These data indicate that peripheral T-cell activation reductions as measured by CD38+/HLA-DR+ co-expression, exceed those in duodenum or rectum. Indeed, the correlation with peripheral CD8+ T-cell activation at month 9 was weaker in duodenum (Rho 0.49) than in colon (Rho 0.70) suggesting perhaps that the antigenic stimulation driving the high constitutive levels of mucosal activation are more diverse and less influenced by ART in the duodenum than the rectum.
Figure 2. Changes in proportions of (A) CD4+ and (B) CD8+ T-cells with the activated phenotype compared with baseline activation levels.
No change from baseline to the 9-month measurement would result in a fold change of 1. ART had minimal impact on T-cell activation level changes in GALT P values were calculated using the Mann Whitney’s U test.
Comparison of gamma delta (γδ) and regulatory T-cells subsets in HIV-infected patients at baseline with respect to normal controls and changes during treatment
γδ T-cells encompass a lymphoid subset with a distinct T-cell receptor on its surface and are critical to the protection of the host to a variety of outside challenges. This population is most abundant in gut mucosa and mainly resides within the intraepithelial T-cell subset in closest proximity to luminal antigen. We observed three-fold higher frequencies of γδ T-cells in blood in HIV+ at baseline vs. HIV− (3.4% vs. 0.9%, P<0.001), but not in rectum or duodenum (Table S1). Further, %γδ T-cells only differed from levels observed in HIV− after ART in blood but not in the gut (Figure S3).
We further investigated the role of γδ T-cells by exploring the Vδ1+ and Vδ2+ subpopulations. This was accomplished by gating γδ subpopulations by mean fluorescence intensity (MFI), as it has been shown that Vδ1+ show low, while Vδ2+ display high MFI signal[29]. While Vδ2+ cells predominate in blood, the γδT-cell population of the gut mucosa is mainly comprised of Vδ1+ cells[29]. Vδ1+ T-cells play well-documented roles in enterocyte barrier homeostasis and tumor surveillance[30]. In contrast, circulating Vδ2+ T-cells display enhanced gut-homing potential upon microbial activation and populate intestinal mucosa[31]. We observed a striking difference in peripheral blood from predominance of Vδ1+ T-cells in HIV− to Vδ2+ T-cells in HIV-infected subjects, with three-fold increased frequencies in HIV+ vs. HIV−, (between group comparison, P=0.005)(Figure S4a). ART did not affect this Vδ2+/Vδ1+ imbalance and no differences were observed in the tissue frequencies of these subsets, suggesting that if this subset plays any role in HIV-related gut mucosal immunopathogenesis, it does so primarily at a functional level.
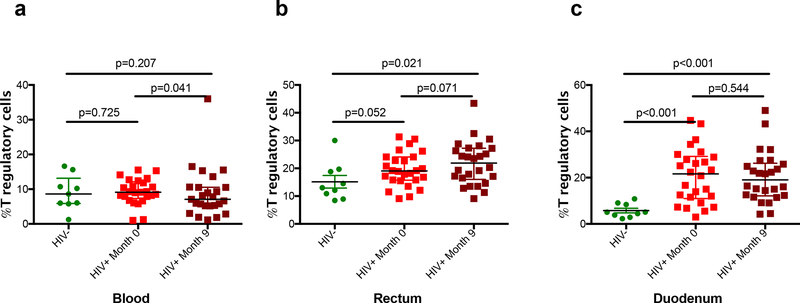
Regulatory T-cells (Treg) are increased in HIV infection, but their mechanism for contributing to HIV immunopathogenesis is controversial[32, 33]. While the baseline frequency of peripheral Treg was similar in HIV+ cohorts versus HIV−, we observed 1.5-fold and 4-fold increased levels at baseline in rectum and duodenum in HIV+ compared to HIV− (p=0.052 and p<0.001, respectively)(Figure 3). During treatment, there was a significant decline of the Tregs in the blood from 9.1% to 7.5% (p=0.04) but not in the rectum or duodenum. GALT levels remained significantly higher after 9-months of ART relative to HIV−. As with other immunologic parameters, this appeared more discordant in the duodenum.
Figure 3. Percentage of T regulatory cells across compartments.
P values between HIV- and HIV+ groups were calculated using the Mann Whitney’s U test. P values between month 0 and month 9 time-points in the HIV+ group were calculated using the Wilcoxon signed-rank test.
Association of changes in immune cell populations with biomarkers of inflammation and intestinal damage
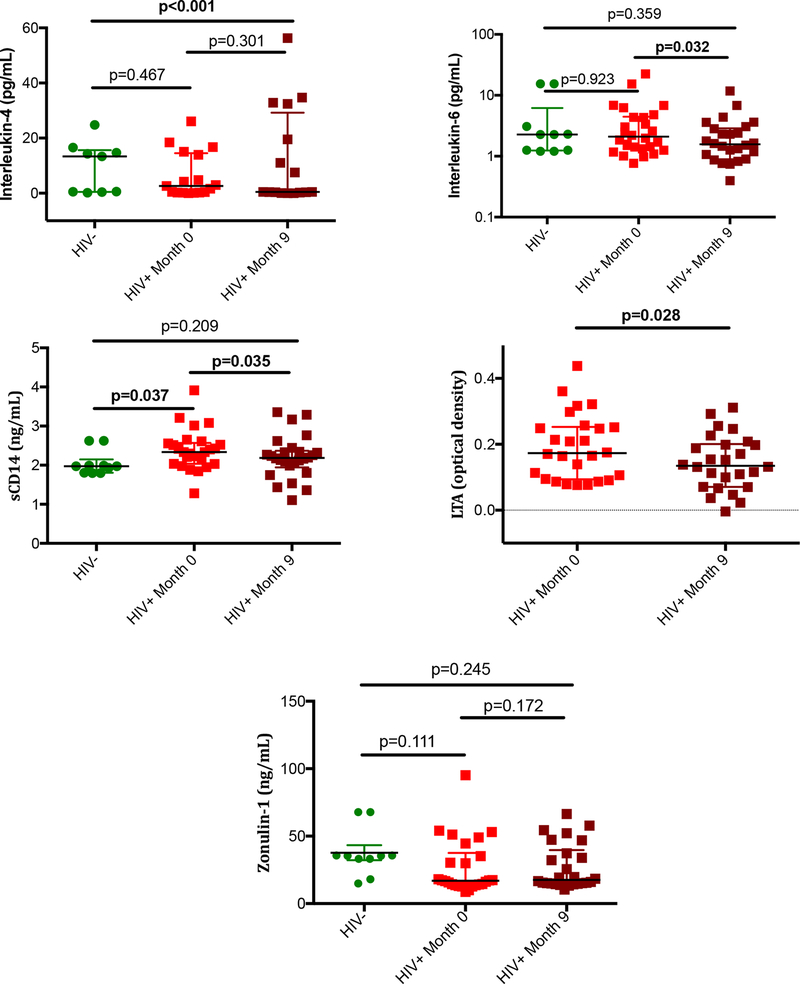
Biomarkers of inflammation, intestinal damage, and microbial translocation were measured on plasma samples. IL-6, sCD14, and LTA declined under ART from baseline to 9-months of treatment (P=0.032, 0.035, 0.028 and <0.001, respectively)(Figure 4). Zonulin-1, a tight junction protein that reflects enterocyte integrity, and interleukin-4, a mediator of TH2 response did not change significantly from baseline[34]. To determine whether circulating and intestinal immune restoration were associated with less inflammation and gut damage, we then calculated the correlations between changes in CD4+ and CD8+ T-cells in each compartment and changes in the plasma biomarkers (IL-4, IL-6, sCD14, LTA, and zonulin-1) in the HIV+ group. Increases in %CD4+ T-cells in rectum were associated with decreases in sCD14 levels (Rho −0.636, P=0.047), reflective of lipopolysaccharide (LPS)-induced monocyte activation. Increases in LTA levels, which reflects gram-positive bacterial antigen translocation, correlated with increases in %CD4+ T-cells in duodenum (Rho 0.773, P=0.033), and with decreases in rectal Vδ2+ populations (Rho −0.717, P=0.029) and duodenal Treg populations (Rho −0.40, P=0.045) with no significant correlations detected for changes in mucosal %CD8+ T-cells. These exploratory findings suggest that regulation of duodenal mucosal immune cells may be positively influenced by translocation of by-products from gram-positive bacteria, indicating that the consequences of bacterial translocation might be determined by the nature of the translocated products.
Figure 4. Soluble biomarkers of inflammation and intestinal damage.
Plasma levels of IL-4, IL-6, sCD14, LTA, and zonulin-1 are represented. P values between HIV- and HIV+ groups were calculated using the Mann Whitney’s U test. P values between month 0 and month 9 time-points in the HIV+ group were calculated using the Wilcoxon signed-rank test. LTA levels could not be measured in controls due to insufficient plasma volume.
Zonu, w0 N=26
We did not detect significant correlations between changes in T-cell activation phenotypes in blood or GALT and changes in IL-6, sCD14, LTA or zonulin-1 concentrations. In sum, CD4+ T-cell recovery in the rectum correlates with decreased sCD14 consistent with less Gram-negative bacterial translocation, yet CD4+ T-cell recovery in the duodenum is associated with Gram-positive bacterial translocation, which suggests that the gut microbiome may be shifting towards anti-inflammatory Gram-positive organisms.
DISCUSSION
These results demonstrate that a more blunted reconstitution in the duodenum than the rectum is observed after 9-months of ART achieving only 38.9% compared to 61.3% of normal control proportions of CD4+ T-cells in GALT tissue from the duodenum and rectum, respectively. The ‘hyper-infiltration’ of CD8+ T-cells into the duodenum that we identified in a previous clinical trial was again observed here[7]. CD8+ T-cell percentages declined significantly in all three compartments, but the changes were in parallel in the blood and rectum from baseline to 9-months on ART, while the decline in the duodenum was to a lesser degree. This suggests that the diverse antigens driving the homing of CD8+ T-cells to mucosal tissue are modestly impacted by viral suppression in the duodenal tissue while rectal and the peripheral blood compartments appear in equilibrium with each other.
In the only previous clinical trial that simultaneously sampled both upper and lower intestinal compartments but in a cohort with symptomatic gastrointestinal disease, Cassol et al. observed that the percentage of CD4+ T-cells increased in parallel in the blood and rectum after 6 months of NNRTI/NRTI treatment in a cohort of AIDS patients in South Africa[11]. However, the proportion of CD4+ T-cells in the duodenum did not change over that same 6-month period. The additional immunologic cellular subsets examined here provide insights into potential interactions between these parameters.
The present study was able to expand on the profile of GALT lymphoid populations to include an analysis of the CD4/CD8 ratio, lymphocytes with an activated phenotype, γδ cells, and Treg populations. CD4/CD8 ratio has regained attention as an indicator of immune reconstitution[27]. The CD4/CD8 ratio increased the most in the blood and rectum and achieved the same ratio of 0.76 after 9 months of treatment. However, the recovery of CD4/CD8 ratio follows a separate trajectory in the duodenum, and still remains remarkably depressed at 0.22 after 9 months of ART. This pattern of recovery suggests that over time the large intestines immune populations achieve equilibrium with the peripheral blood as has been reported previously in patients on long-term suppressive ART (mean of 28 months)[23]. The activated CD8+ T-cell proportions fell in all three compartments with the largest declines in the blood and rectum and least in the duodenum. The small decline in the duodenum suggests ongoing immune stimulation in the duodenum. These data suggest that homing pathways stimulating CD8+ T-cell migration to the lamina propria may be important targets of investigation.
γδ T-cells are located primarily in the intraepithelial layer on the luminal side of the basement membrane and are believed to be an important source of innate antimicrobial factors that protect against invasion by bacterial pathogens[35]. Interestingly, statistically significant higher levels were observed in the peripheral blood before and after HIV treatment compared with normal controls. The increased proportion of γδ cells in the peripheral blood may represent a persistent state of proinflammatory hyperactivity with IL-4 and IL-10 production seen in other chronic infections such as malaria where persistently elevated γδ cells is associated with symptomatic clinical disease during reinfection[36]. Indeed, we observed increased frequencies of the Vδ2+ γδ subset, which have been demonstrated to increase in response to intestinal bacterial species activation and enhances gut-homing potential[31].
In contrast, analysis of Treg subsets in peripheral blood and intestinal mucosa revealed a much more provocative insight into the immunopathology of these compartments, especially the duodenum. There exists significant literature exploring the role of Tregs in the pathogenesis of HIV disease[21, 37]. ‘Classically’, Treg CD4+ cells are believed to exert an anti-inflammatory role but in HIV, they are elevated and are associated with disease progression. In the intestines in particular, they exist in a reciprocal relationship with TH17 cells that participate in epithelial integrity such that high Treg/TH17 ratios correlate with microbial translocation. Perhaps due to persistent stimuli from viral, bacterial or other processes, suppressive ART did not normalize Treg populations in either tissue compartment. Future studies will need to address the cytokine production and functional status of this population of cells to better understand their potential role in HIV mucosal immune deficiency.
The clinical implications of delayed CD4/CD8 recovery in the small intestines are unknown. However, different sites across the body represent unique niches for HIV biology. For example, the sequestered sites protected by B-cell follicles may represent a focus for the HIV reservoir[38, 39]. Similarly, the data presented here supports the hypothesis that the small intestine represents a unique site with respect to immune deficiency and the dynamics of immune reconstitution. The mechanisms behind the severe CD4+ T-cell depletion, other perturbations in immune populations and the delayed recovery following ART initiation are unknown, but important clues are provided by the research reported from this clinical trial. Given the exploratory study design, our results must be interpreted cautiously. A number of potential uncontrolled confounders, including the extent of pre-treatment of immune suppression or the effects of the ART regimen used could have affected our results[24].
In the same cohort of patients, we have recently described the correlations between antiretroviral drug tissue penetration and HIV RNA/DNA persistence[40]. We did not find evidence for a significant contribution of residual HIV replication in the tissue compartments for persistent CD4+ T-cell depletion even though drug tissue distribution was similar for the multiple classes of ART measured in this RCT. While our negative data do not exclude a potential contribution to ongoing HIV replication as others have reported in mucosal tissues [12, 41], the robust penetration of these antiretroviral drugs into these highly vascularized tissues does weighs in favor of other local factors playing a more dominant role, namely, bacterial translocation or mesenteric lymph node fibrosis, which may explain the heterogeneous immune restoration along the GI tract.
While plasma levels of LPS, the pro-inflammatory gram-negative cell wall antigen have been well characterized in HIV literature with inconsistent results, the gram-positive cell wall component, LTA has not been explored in response to HIV treatment up to now. The observation that LTA positively correlated with increases duodenal %CD4+ T-cells, peripheral %CD8+ T-cells and with duodenal %Treg decline is very provocative and will require further characterization to fully understand. Our findings, coupled with systemic CD4+ T-cell immune reconstitution correlating with zonulin levels as a measure of enterocyte integrity, further support that hypothesis that restoration systemic immune function depends on restored mucosal immune function. However, given the exploratory nature of our study, these correlations must be interpreted as hypothesis-generating. Given the wide spectrum of pathogenicity of gram-positive bacteria in the gut, including bacteria with known beneficial immune properties such as bifidobacterial, to clear pathobionts, such as Staphylococcus aureus, defining the associations of specific mucosal-adherent bacteria with mucosal T cell subsets is one of our future directions.
In conclusion, this study indicates that HIV-mediated immunological disruption is greater in the duodenum than rectum and blood before and during ART. Small intestine damage may represent a unique environment for T-cell depletion possibly related to gut microbe:mucosal immunological interactions. Future studies should examine both the tissue adherent microbial communities as well as their metabolic products in order to define novel strategies to enhance GALT reconstitution.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to acknowledge all the study participants who contributed to this work as well as the clinical research staff of the UC Davis CTSC Clinical Research Center who made this research possible.
Funding: This research was made possible by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), NIH Roadmap for Medical Research, and by a grant from Pfizer Investigator Initiated Research Program, the UCSF/ Gladstone Institute of Virology & Immunology CFAR (grant number P30 AI027763), and the UNC CFAR (P30 AI50410). SSV is funded by a grant from the Spanish Ministry of Health and Innovation. SSV and TS received grants to perform this work from the Spanish Network on AIDS Research (RIS Cohort, CoRIS), which is funded by the Instituto de Salud Carlos III through the Red Temática de Investigación Cooperativa en Sida (RD06/006). The results and views expressed in this work do not represent those of Pfizer or the US Federal Government/Veterans Administration Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing Interests: In addition to the grant to the University of California Davis funding the clinical trial, DMA serves on advisory/speakers boards for ViiV, Gilead, Janssen, Merck and Napo Pharmaceuticals and has conducted clinical trials for Pfizer, ViiV, Gilead, Merck, BioNor and EnteraHealth. The remaining authors have declared that no competing interests exist.
Bibliography
- 1.Gillin JS, Shike M, Alcock N, Urmacher C, Krown S, Kurtz RC, et al. Malabsorption and mucosal abnormalities of the small intestine in the acquired immunodeficiency syndrome. Ann Intern Med 1985; 102(5):619–622. [DOI] [PubMed] [Google Scholar]
- 2.Jarry A, Cortez A, Rene E, Muzeau F, Brousse N. Infected cells and immune cells in the gastrointestinal tract of AIDS patients. An immunohistochemical study of 127 cases. Histopathology 1990; 16(2):133–140. [DOI] [PubMed] [Google Scholar]
- 3.Ullrich R, Zeitz M, Heise W, L’Age M, Hoffken G, Riecken EO. Small intestinal structure and function in patients infected with human immunodeficiency virus (HIV): evidence for HIV-induced enteropathy. Ann Intern Med 1989; 111(1):15–21. [DOI] [PubMed] [Google Scholar]
- 4.Kotler DP, Gaetz HP, Lange M, Klein EB, Holt PR. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med 1984; 101(4):421–428. [DOI] [PubMed] [Google Scholar]
- 5.Utay NS, Hunt PW. Role of immune activation in progression to AIDS. Current opinion in HIV and AIDS 2016; 11(2):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George MD, Asmuth DM. Mucosal immunity in HIV infection: what can be done to restore gastrointestinal-associated lymphoid tissue function? Curr Opin Infect Dis 2014; 27(3):275–281. [DOI] [PubMed] [Google Scholar]
- 7.Asmuth DM, Ma ZM, Mann S, Knight TH, Yotter T, Albanese A, et al. Gastrointestinal-associated lymphoid tissue immune reconstitution in a randomized clinical trial of raltegravir versus non-nucleoside reverse transcriptase inhibitor-based regimens. AIDS 2012; 26(13):1625–1634. [DOI] [PubMed] [Google Scholar]
- 8.Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, et al. Lack of Mucosal Immune Reconstitution during Prolonged Treatment of Acute and Early HIV-1 Infection. PLoS Med 2006; 3(12):e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005; 434(7037):1093–1097. [DOI] [PubMed] [Google Scholar]
- 10.Allers K, Puyskens A, Epple HJ, Schurmann D, Hofmann J, Moos V, et al. The effect of timing of antiretroviral therapy on CD4(+) T-cell reconstitution in the intestine of HIV-infected patients. Mucosal immunology 2016; 9(1):265–274. [DOI] [PubMed] [Google Scholar]
- 11.Cassol E, Malfeld S, Mahasha P, Bond R, Slavik T, Seebregts C, et al. Impaired CD4+ T-cell restoration in the small versus large intestine of HIV-1-positive South Africans receiving combination antiretroviral therapy. The Journal of infectious diseases 2013; 208(7):1113–1122. [DOI] [PubMed] [Google Scholar]
- 12.Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. The Journal of infectious diseases 2010; 202(10):1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Marle G, Gill MJ, Kolodka D, McManus L, Grant T, Church DL. Compartmentalization of the gut viral reservoir in HIV-1 infected patients. Retrovirology 2007; 4(87): doi: 10.1186/1742-4690-1184-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafeuillade A. Eliminating the HIV reservoir. Curr HIV/AIDS Rep 2012; 9(2):121–131. [DOI] [PubMed] [Google Scholar]
- 15.Shacklett BL, Cox CA, Sandberg JK, Stollman NH, Jacobson MA, Nixon DF. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J Virol 2003; 77(10):5621–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girard A, Vergnon-Miszczycha D, Depince-Berger AE, Roblin X, Lutch F, Lambert C, et al. Brief Report: A High Rate of beta7+ Gut-Homing Lymphocytes in HIV-Infected Immunological Nonresponders is Associated With Poor CD4 T-Cell Recovery During Suppressive HAART. J Acquir Immune Defic Syndr 2016; 72(3):259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano-Villar S, Rojo D, Martinez-Martinez M, Deusch S, Vazquez-Castellanos JF, Bargiela R, et al. Gut Bacteria Metabolism Impacts Immune Recovery in HIV-infected Individuals. EBioMedicine 2016; 8:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asmuth DM, Pinchuk IV, Wu J, Vargas G, Chen X, Mann S, et al. Role of intestinal myofibroblasts in HIV-associated intestinal collagen deposition and immune reconstitution following combination antiretroviral therapy. AIDS 2015; 29(8):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin Immunol 2008; 20(3):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. The Journal of clinical investigation 2002; 110(8):1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epple HJ, Schneider T, Zeitz M. Chapter 77 - Microbial Translocation and the Effects of HIV/SIV Infection on Mucosal Barrier Function In: Mucosal Immunology (Fourth Edition). Lambrecht JMSWRLKCN (editor). Boston: Academic Press; 2015. pp. 1521–1530. [Google Scholar]
- 22.Hayes TL, Asmuth DM, Critchfield JW, Knight TH, McLaughlin BE, Yotter T, et al. Impact of highly active antiretroviral therapy initiation on CD4(+) T-cell repopulation in duodenal and rectal mucosa. AIDS 2013; 27(6):867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheth PM, Chege D, Shin LY, Huibner S, Yue FY, Loutfy M, et al. Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal immunology 2008; 1(5):382–388. [DOI] [PubMed] [Google Scholar]
- 24.Serrano-Villar S, Sainz T, Ma ZM, Utay NS, Chun T-W, Mann S, et al. Effects of Combined CCR5/Integrase Inhibitors-Based Regimen on Mucosal Immunity in HIV-Infected Patients Naive to Antiretroviral Therapy: A Pilot Randomized Trial. PLoS pathogens 2016; 12(1):e1005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siewe B, Keshavarzian A, French A, Demarais P, Landay A. A role for TLR signaling during B cell activation in antiretroviral-treated HIV individuals. AIDS research and human retroviruses 2013; 29(10):1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1(1):43–46. [PubMed] [Google Scholar]
- 27.Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS pathogens 2014; 10(5):e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mudd JC, Lederman MM. CD8 T cell persistence in treated HIV infection. Current opinion in HIV and AIDS 2014; 9(5):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deusch K, Luling F, Reich K, Classen M, Wagner H, Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the gamma/delta T cell receptor, the CD8 accessory molecule and preferentially uses the V delta 1 gene segment. European journal of immunology 1991; 21(4):1053–1059. [DOI] [PubMed] [Google Scholar]
- 30.Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the complexity of gammadelta T-cell subsets in mouse and human. Immunology 2012; 136(3):283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy NE, Bashir Z, Vossenkamper A, Hedin CR, Giles EM, Bhattacharjee S, et al. Proinflammatory Vdelta2+ T cells populate the human intestinal mucosa and enhance IFN-gamma production by colonic alphabeta T cells. J Immunol 2013; 191(5):2752–2763. [DOI] [PubMed] [Google Scholar]
- 32.Imamichi H, Lane HC. Regulatory T cells in HIV-1 infection: the good, the bad, and the ugly. The Journal of infectious diseases 2012; 205(10):1479–1482. [DOI] [PubMed] [Google Scholar]
- 33.Seddiki N, Kelleher AD. Regulatory T cells in HIV infection: who’s suppressing what? Curr HIV/AIDS Rep 2008; 5(1):20–26. [DOI] [PubMed] [Google Scholar]
- 34.Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol 2012; 10(10):1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, et al. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proceedings of the National Academy of Sciences of the United States of America 2011; 108(21):8743–8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jagannathan P, Kim CC, Greenhouse B, Nankya F, Bowen K, Eccles-James I, et al. Loss and dysfunction of Vdelta2(+) gammadelta T cells are associated with clinical tolerance to malaria. Science translational medicine 2014; 6(251):251ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanwar B, Favre D, McCune JM. Th17 and regulatory T cells: implications for AIDS pathogenesis. Current opinion in HIV and AIDS 2010; 5(2):151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bednar MM, Sturdevant CB, Tompkins LA, Arrildt KT, Dukhovlinova E, Kincer LP, et al. Compartmentalization, Viral Evolution, and Viral Latency of HIV in the CNS. Curr HIV/AIDS Rep 2015; 12(2):262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohler SL, Pham MN, Folkvord JM, Arends T, Miller SM, Miles B, et al. Germinal Center T Follicular Helper Cells Are Highly Permissive to HIV-1 and Alter Their Phenotype during Virus Replication. J Immunol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asmuth DM, Thompson CG, Chun TW, Ma ZM, Mann S, Sainz T, et al. Tissue Pharmacologic and Virologic Determinants of Duodenal and Rectal Gastrointestinal-Associated Lymphoid Tissue Immune Reconstitution in HIV-Infected Patients Initiating Antiretroviral Therapy. The Journal of infectious diseases 2017; 216(7):813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J InfectDis 2008; 197(5):714–720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.