Abstract
Extracellular ATP (eATP) activates T cells by engaging the P2X7R receptor. We identified two loss-of-function P2X7R mutations that are protective against type 1 diabetes (T1D) and thus hypothesized that eATP/P2X7R signaling may represent an early step in T1D onset. Specifically, we observed that in patients with newly diagnosed T1D, P2X7R is upregulated on CD8+ effector T cells in comparison with healthy control subjects. eATP is released at high levels by human/murine islets in vitro in high-glucose/inflammatory conditions, thus upregulating P2X7R on CD8+ T cells in vitro. P2X7R blockade with oxidized ATP reduces the CD8+ T cell–mediated autoimmune response in vitro and delays diabetes onset in NOD mice. Autoreactive CD8+ T-cell activation is highly dependent upon eATP/P2X7R-mediated priming, while a novel sP2X7R recombinant protein abrogates changes in metabolism and the autoimmune response associated with CD8+ T cells. eATP/P2X7R signaling facilitates the onset of autoimmune T1D by fueling autoreactive CD8+ cells and therefore represents a novel targeted therapeutic for the disorder.
Introduction
Understanding of the immunological mechanisms underlying type 1 diabetes (T1D) development has broadened dramatically (1,2), which has aided in design of potential immunoregulatory treatments capable of preventing and/or curing the disease (3,4). A key goal of these approaches is to revert hyperglycemia in T1D patients or to prevent the onset of disease in individuals at high risk (5). However, despite much effort, an immune-based cure for T1D does not exist, and concern remains due to the increased risk of mortality associated with the disorder (6). Reversal of diabetes can currently be obtained only with pancreatic islet (7,8) or whole-pancreas transplantation, which confers a different set of complications and suboptimal long-term outcomes (9).
The purine ATP is a small molecule (10) present in high concentrations within cells that can be released into the extracellular compartment as extracellular ATP (eATP) by damaged or necrotic cells (11) and by activated immune cells (12,13). Once in the extracellular space, eATP can be sensed by ionotropic purinergic P2X receptors (seven receptors named P2X1–P2X7 receptors, or P2XRs) (14–16) as a fundamental step in immune cell activation (17–20). In particular, P2X7R (12,16,21,22) has been linked to T-cell activation, serving as a signal amplification mechanism for antigen recognition (17), Th1/Th17 generation (1,23), and allograft rejection (24). Interestingly, ATP is cosecreted with insulin by pancreatic β-cells (25,26). We hypothesize that eATP-driven/P2X7R-mediated immunity may be activated by pancreatic β-cell injury (e.g., viruses, stresses) when β-cells release eATP and prime passenger leukocytes (25,26). During the autoimmune response, eATP can also be released by cytotoxic T cells, thus creating a feedback loop that sustains the autoimmune response and inflammation (1,23,24). Understanding the potential role of the purinergic system in the priming of the T cell–mediated anti-islet immune response in the pathogenesis of T1D will contribute to design of potential therapies for T1D, especially considering P2X7R inhibitors are available for human use (27,28).
Research Design and Methods
Genetic Studies
A detailed description of the Joslin Diabetes Center study of Genetics of Kidneys in Diabetes (GoKinD) was recently published (1). Unrelated European American individuals from GoKinD (n = 3,410) and the Exome Sequencing Project (ESP6500; n = 8,600) cohort databases were interrogated for P2X7R genetic variants. The FREQ Procedure of the SAS system was used to determine the frequency of each single nucleotide polymorphism (SNP) of human P2X7R. Odds ratios (OR) for each SNP were calculated, and Bonferroni correction was applied to each P value.
Patients
Blood samples were obtained from patients with new-onset T1D, patients with long-standing T1D, patients with type 2 diabetes (T2D), and healthy control subjects, who were enrolled under institutional review board committee approval (Table 1). Peripheral blood mononuclear cell (PBMC) fractions were isolated from 20 mL whole blood by Ficoll density gradient centrifugation.
Table 1.
Baseline demographic characteristics of patients enrolled
| Healthy donors (n = 10) | Long-standing T1D (n = 10) | Newly diagnosed T1D (n = 10) | T2D (n = 5) | P values | |
|---|---|---|---|---|---|
| Sex, male/female | 4/6 | 4/6 | 5/5 | 4/1 | NS |
| Age, years | 42.0 ± 5.0 | 42.0 ± 12.3 | 10.2 ± 3.2 | 57.2 ± 1.7 | P < 0.001 |
| Diabetes duration, years | — | 27.1 ± 8.5 | — | 3.4 ± 0.9 | N/A |
| HbA1c, % (mmol/mol) | 5.3 ± 1.0 (34 ± 10.9) | 8.3 ± 1.1 (67 ± 12.0) | 12.2 ± 1.4 (110 ± 14.8) | 6.3 ± 0.2 (45.4 ± 2.9) | P < 0.001 (P < 0.001) |
| EIR, IU | — | 39.8 ± 10.0 | — | — | N/A |
Data are expressed as n or mean ± SEM. HbA1c, glycated hemoglobin A1c; EIR, exogenous insulin requirement; NS, not significant; N/A, not applicable.
Human Antibodies
The following antibodies were used for flow cytometric analysis: phycoerythrin (PE)-Cy7–conjugated anti-human CCR7, allophycocyanin (APC)-labeled anti-human CD45RO, Alexa Fluor 700–conjugated CD4, V500-conjugated anti-human CD8, APC-labeled anti-human CD11c, and PE-Cy7–conjugated anti-human CD19 (purchased from BD Biosciences [San Jose, CA], eBioscience [San Diego, CA], or Life Technologies [Carlsbad, CA]). FITC-conjugated anti-human P2X7R was purchased from Alomone Labs (Jerusalem, Israel).
Human Flow Cytometric Analysis
To characterize P2X7R expression on T cells, human PBMCs isolated from healthy control subjects, new-onset T1D patients, long-standing T1D patients, and T2D patients were stained with anti-human CD4, CD8, CD11c, or CD19 and anti-human P2X7R. Human P2X7R expression on CD4+ and CD8+ effector and central memory T cells was determined by staining for P2X7R, CD45RO, CCR7, and CD4 or CD8, respectively. The number of cells was calculated by acquiring 105 events in the lymphocyte gate (SSC-FSC) by flow cytometric analysis.
Intracellular Staining for Flow Cytometry
Anti-human CD4, CD8, CD25, interferon-γ (IFN-γ), and interleukin-17 (IL-17) were purchased from BD Biosciences, Becton Dickinson (Franklin Lakes, NJ), or Life Technologies, and anti-mouse FITC-labeled P2X7R was purchased from Alomone Labs. Resting cells were activated with PMA (5 ng/mL), ionomycin (500 ng/mL), and brefeldin A (2 μmol/L) (Sigma-Aldrich, St. Louis, MO) for 3 h. Cells were harvested in FACS tubes and placed on ice. Cells were then washed with FACSFlow (BD Biosciences) and processed for intracellular cytokine staining with 0.5% saponin (Sigma-Aldrich) and 2% formaldehyde for fixation and permeabilization. Antibodies used for intracellular staining (BD Biosciences unless stated otherwise) were PE-conjugated anti–IL-17 and APC-conjugated anti–IFN-γ.
Human ELISpot Assay
An ELISpot assay was used to measure the number of IFN-γ–producing cells according to the manufacturer’s protocol (BD Biosciences) as previously shown by our group (2). A total of 2 × 106 PBMCs, isolated from T1D patients, were cultured for 48 h in the presence of IA-2 (100 µg/mL) peptide. At day 1 after stimulation, 500 µL media was added to the culture. Cells were collected at day 2 and plated in a human IFN-γ ELISpot assay with or without oxidized ATP (oATP) or CE-224,535 at different concentrations (1, 10, or 100 μmol/L). Spots were counted using an A.EL.VIS ELISpot Reader (A.EL.VIS GmbH, Hannover, Germany).
In another assay, we isolated CD8+ T cells using microbeads from PBMCs obtained from healthy subjects, and 5 × 105 CD8+ T cells were incubated with a mixture of vaccine antigens (Diftavax) (tetanus toxoid, diptheria toxin, and haemophilus) for 24 h with/without adding the receptor antagonists oATP or CE-224,535. Using an ELISpot assay, we tested the ability of both P2X7R receptor antagonists (oATP or CE-224,535) to halt the activation of CD8+ T cells when exposed to vaccination antigens.
eATP Measurements
Supernatants were collected from a β-cell line (beta-lox5, provided by Clayton Mathews, University of Florida) previously cultured and exposed to IFN-γ (2 ng/mL, R&D Systems [Minneapolis, MN]) with or without IL-1β (1,000 units/mL, PeproTech [Rocky Hill, NJ]) for 3 days to replicate proinflammatory/diabetogenic conditions as previously described (3,4) under high-glucose conditions (35 mmol/L) for eATP quantification. We further compared eATP levels measured in supernatants of β-cells with that of other cell lines (Caco-2) and of PBMCs isolated from T1D subjects (2 × 106 cells/well), cultured in the same conditions. Finally, we also collected supernatants from PBMCs of T1D subjects previously exposed to IA-2 or Pediacel (Sanofi Pasteur), Diftavax, or anti-CD3/CD28 (as described above) to quantify eATP. Briefly, supernatant samples were resuspended in ATP assay buffer (300–400 mL) on ice and spun at 14,000g for 5 min at 4°C, after which the supernatant was transferred to a 10-kDa molecular weight cutoff spin filter and spun at 14,000g for 5 min at 4°C to be deproteinized. The samples were transferred to a 96-well plate and the fluorescence (lex 535 nm, lem 587 nm) was measured using a SpectraMax Paradigm Multi-Mode Detection Platform (Molecular Devices, Sunnyvale, CA). The background was subtracted from the measurement, and the amount of ATP present in the samples was determined from the standard curve. Results obtained were normalized to the number of cells.
Mice
Female BALB/c, NOD/ShiLtJ (NOD) at 4 and 8 weeks of age and female NOD/SCID, BDC2.5 TCR NOD (NOD-BDC2.5), NOD.Tcr NY8.3 (8.3-NOD), and NOD-P2X7R knockout mice at 8 weeks of age were obtained from the The Jackson Laboratory (Bar Harbor, ME). Female pmeLUC transgenic mice were kindly provided by collaboration with the Danish Genetically Modified Animal Resource, represented by Ernst-Martin Fuchtbauer, Department of Molecular Biology, Aarhus University. Transgenic mice were generated by injection of the pmeLUC DNA construct into B6D2F1/J mouse pronuclei. All mice were cared for and used in accordance with guidelines approved by the Institutional Animal Care and Use Committee.
Results
P2X7R Loss-of-Function Mutation Protects Against T1D
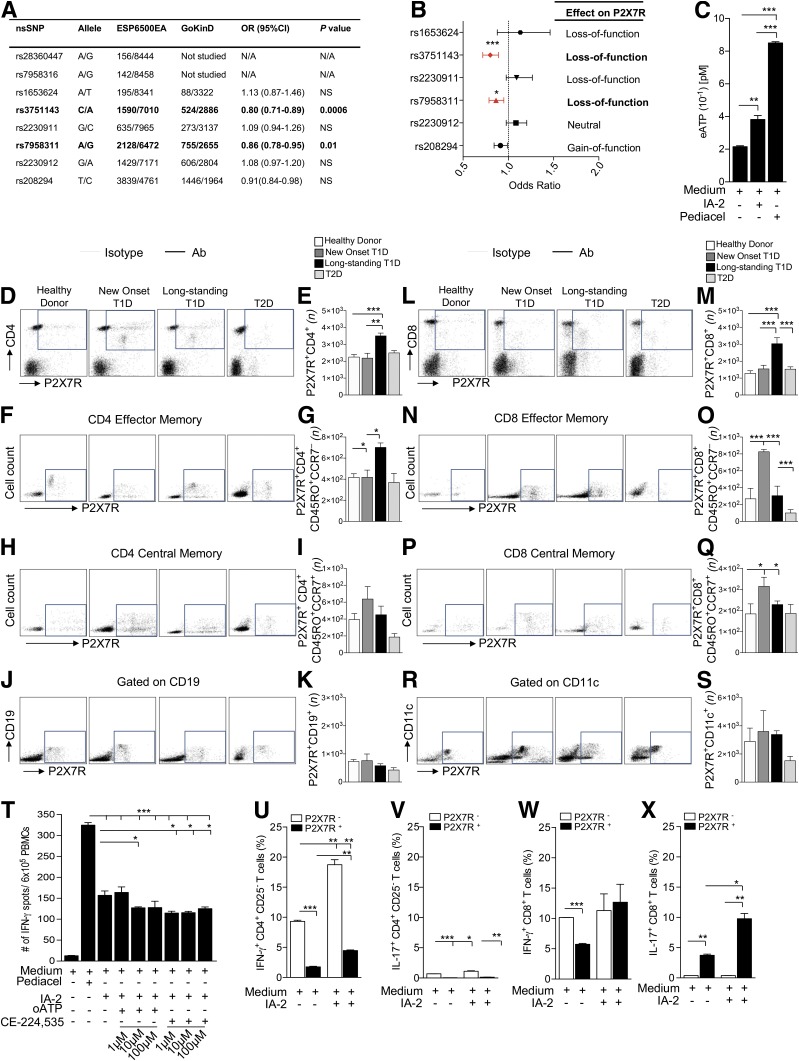
We first analyzed unrelated European American patients from GoKinD (n = 1,705) and the Exome Sequencing Project (ESP6500; n = 4,300) cohorts in order to identify any associations between P2X7R genetic variants and the T1D condition. The human P2X7R gene is a highly polymorphic gene that spans 52 kb and maps to the chromosomal region 12q24.31. Interrogation of the GoKinD and ESP6500 databases revealed eight SNPs in the human P2X7R gene (Fig. 1A). Among these SNPs, the rs3751143 and rs7958311 point mutations are nonsynonymous SNPs (nsSNPs) with an alanine substitution for glutamate at amino acid position 496 (1513A>C, E496A) and an arginine substitution for histidine at position 270 (835G>A, R270H), respectively, leading to P2X7R loss of function. Both rs3751143 and rs7958311 appeared to be protective against the development of T1D (rs3751143: OR 0.8, P = 0.0006; rs7958311: OR 0.86, P = 0.01) (Fig. 1A and B), even after a conservative Bonferroni correction.
Figure 1.
The eATP/P2X7R axis plays a role in human T1D onset. A and B: The human P2X7R SNP distribution among the GoKinD and ESP6500EA cohorts is shown. Two loss-of-function mutations (rs3751143 and rs7958311) appeared to be protective toward the onset of T1D (rs3751143: OR 0.8; rs7958311: OR 0.86) even after a conservative Bonferroni correction. C: Increased eATP release by PBMCs from T1D patients was observed following IA-2 challenge as compared with PBMCs alone; Pediacel was used as a positive control. D–S: Greater numbers of P2X7R+CD4+ T cells (D and E), P2X7R+CD4+CD45RO+CCR7− effector memory T cells (F and G), P2X7R+CD8+ T cells (L and M) and, to a lesser extent, P2X7R+CD8+CD45RO+CCR7+ central memory T cells (P and Q) were evident in long-standing T1D patients as compared with healthy control subjects, T2D patients, and newly diagnosed T1D patients. Increased numbers of P2X7R+CD4+CD45RO+CCR7+ central memory T cells (H and I), P2X7R+CD8+CD45RO+CCR7− effector memory T cells (N and O), and P2X7R+CD8+CD45RO+CCR7+ central memory T cells (P and Q) were observed in newly diagnosed T1D patients as compared with healthy control subjects, T2D patients, and long-standing T1D patients. No difference or only slight differences were observed in the numbers of P2X7R+CD19+ (J and K) and P2X7R+CD11c+ (R and S) cells among groups. T: oATP and CE-224,535 inhibited the anti-islet immune response following culture of PBMCs from new-onset T1D patients with the IA-2 peptide; Pediacel was used as a positive control. U–X: While within the CD4+CD25– T-cell population, the increased percentage of IFN-γ+ and IL-17+ cells during the autoimmune response to IA-2 peptide was observed in the P2X7R− subpopulation (U and V); for CD8+ T cells, most IL-17+ cells generated during the autoimmune response to IA-2 peptide were within the P2X7R+ subpopulation (W and X). Data are expressed as mean ± SEM. Data are representative of at least n = 3 samples. *P < 0.05; **P < 0.01; ***P < 0.001. ESP6500EA; Exome Sequencing Project (ESP6500) European American.
Elevated P2X7R+ T Cells Are Evident in Newly Diagnosed T1D Patients, and P2X7R Inhibitors Reduce the Human Autoimmune Response In Vitro
In order to understand the role of eATP/P2X7R signaling in T1D, we first analyzed eATP production during an autoimmune response in vitro. When PBMCs obtained from new-onset T1D patients were challenged with the islet peptide IA-2, increased levels of eATP were evident in the supernatants (Fig. 1C). We then determined the P2X7R expression profile of different lymphocyte subpopulations in PBMCs obtained from patients with newly diagnosed and long-standing T1D as well as healthy control subjects and T2D patients (Fig. 1D–S and Supplementary Fig. 1A). A higher number of P2X7R+CD4+ T cells (Fig. 1D and E), P2X7R+CD4+CD45RO+CCR7+ effector memory T cells (Fig. 1F and G), and P2X7R+CD8+ T cells (Fig. 1L and M) were evident in long-standing T1D patients as compared with healthy control subjects, T2D patients, and newly diagnosed T1D patients. Increased numbers of P2X7R+CD4+CD45RO+CCR7– central memory T cells (although not statistically significant) (Fig. 1H and I), P2X7R+CD8+CD45RO+CCR7+ effector memory T cells (Fig. 1N and O), and P2X7R+CD8+CD45RO+CCR7– central memory T cells (Fig. 1P and Q) were observed in newly diagnosed T1D patients as compared with healthy control subjects, T2D patients, and long-standing T1D patients. While there was no difference in the numbers of P2X7R+CD19+ cells (Fig. 1J and K) between groups, increased numbers of P2X7R+CD11c+ cells (although not statistically significant) (Fig. 1R and S) were observed in newly diagnosed and long-standing T1D patients as compared with healthy control subjects and T2D patients. We further observed reduced expression of P2X7R+CD4+CD45RO+CCR7– central memory T cells (although not statistically significant) (Fig. 1H and I) and a significant reduction in the expression of P2X7R+CD8+CD45RO+CCR7+ effector memory T cells (Fig. 1N and O) in T2D patients as compared with all other groups. To evaluate the benefit of targeting the eATP/P2X7R axis during the autoimmune response, two P2X7R inhibitors, oATP and CE-224,535, were used in human autoimmune assays in vitro. In a 24-h ELISpot assay, the T-cell response against the islet peptide IA-2, measured as number of IFN-γ–producing cells, was reduced by the addition of the P2X7R inhibitors oATP and CE-224,535 (Fig. 1T).
Human CD8+ Cell Function Appears Highly Linked to eATP/P2X7R Signaling During the Autoimmune Response
To further delineate the role of eATP/P2X7R signaling in CD4+ and CD8+ naive T cells, we cultured CD4+CD25– and CD8+ T cells in the presence of IA-2 and measured the percentage of IFN-γ+ and IL-17+ cells generated during an autoimmune response by flow cytometry. The CD4+ T cell–associated autoimmune response appeared to be primarily mediated by IFN-γ+ cells, with few IL-17+ cells being generated (Fig. 1U and V); in fact, in both cases cytokines appeared to be predominantly produced by the P2X7R−CD4+CD25− subpopulation and thus appeared independent of eATP/P2X7R signaling (Fig. 1U and V). In contrast, the CD8+ cell autoimmune response was mostly associated with IL-17 production, principally from the P2X7R+CD8+ subpopulation, and therefore was strictly dependent upon eATP/P2X7R signaling (Fig. 1W–X).
eATP Is Highly Released by Human/Murine Pancreatic Islets In Vitro During High-Glucose/Inflammatory Conditions
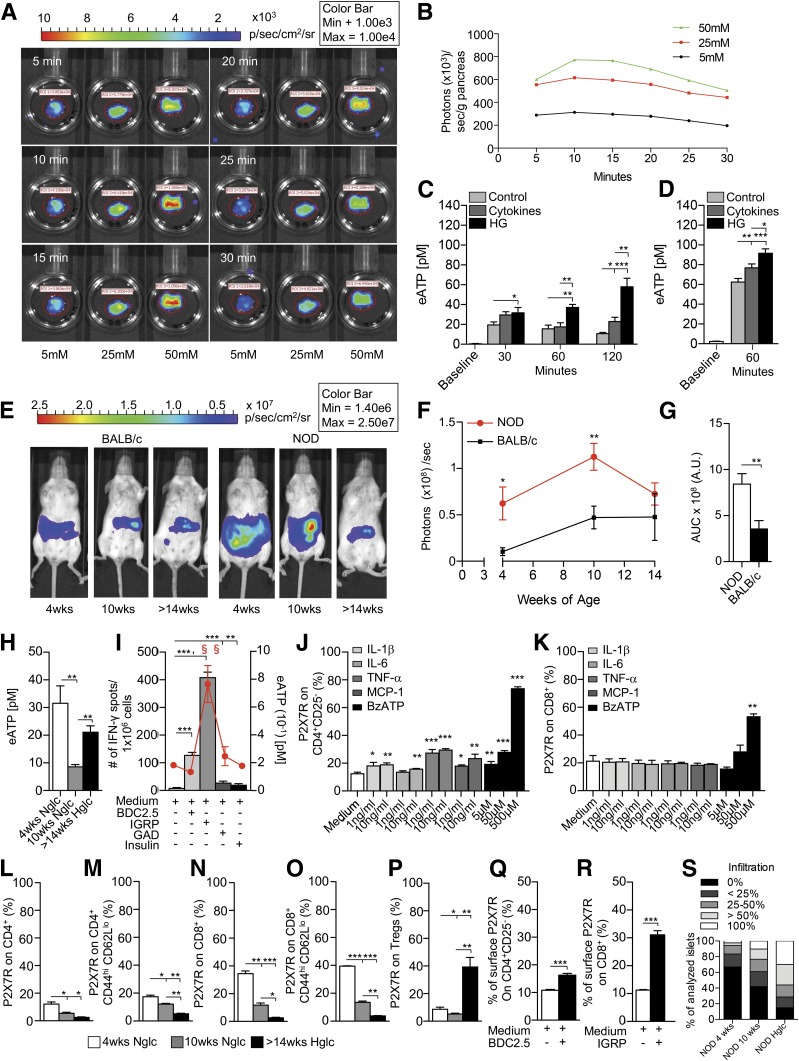
We then explored the ability of pancreatic islets to release eATP in response to various stimuli. First, pancreatic eATP release was evaluated in vitro during challenge with high glucose and with proinflammatory cytokines. Pancreata were harvested from pmeLUC transgenic mice, which express luciferase on their cell membrane, and were cultured in the presence of different glucose concentrations. Luminescence was monitored at different time points during high-glucose challenge using an IVIS Caliper luminometer (Fig. 2A). In this assay, the levels of bioluminescence observed are proportional to eATP concentration (29). An increase in the extent of luminescence dependent on glucose level was evident (Fig. 2A and B and Supplementary Fig. 1B). We then purified islets from 10-week-old NOD/SCID mice and from deceased human donors and cultured them with either 1) normal glucose (control), 2) a cocktail of cytokines composed of IL-1β, TNF-α, and IFN-γ (known to be involved in islet damage), or 3) high glucose (25 mmol/L) (Fig. 2C and D). Both the cytokine cocktail and high-glucose conditions increased the release of eATP by murine and human pancreatic islets; indeed, the highest eATP release was observed following exposure to high glucose (Fig. 2C and D). We next challenged a human β-cell line (beta-lox5) in the presence of different stimuli (i.e., high glucose, IFN-γ, or IL-1β) and quantified eATP release (Supplementary Fig. 1C). The same experiment was performed on a human intestinal Caco-2 cell line and on human PBMCs from T1D using all the aforementioned conditions including Diftavax and anti-CD3/anti-CD28 stimulation (Supplementary Fig. 1D and E). Beta-lox5 cells released high levels of eATP, measured in the culture supernatant, as compared with other cell lines (Supplementary Fig. 1C–E).
Figure 2.
β-Cells release eATP during stress. A and B: A glucose-dependent increase in the extent of luminescence (A) was evident in pancreata harvested from pmeLUC transgenic mice, cultured in the presence of different glucose concentrations and quantified as photons/seconds/grams of pancreas (B). C: Higher levels of eATP were found in the supernatants of murine islets obtained from 10-week-old NOD/SCID mice and cultured in high glucose (HG) at different time points as compared with controls. The same effect was evident to a lesser extent with a cocktail of cytokines (IL-1β, TNF-α, and IFN-γ), with high glucose being the more robust inducer of eATP. D: High-glucose conditions and cytokines (IL-1β, TNF-α, and IFN-γ) caused release of eATP by human islets cultured for 60 min. E–G: A stable luminescence signal, as a proxy for in vivo eATP release, was observed in BALB/c mice injected i.p. with HEK293-pmeLUC transgenic cells and d-luciferin at different ages, while in NOD mice the luminescence signal appeared higher than in BALB/c mice. A gradual disappearance of bioluminescence was evident over time in NOD mice, paralleling the autoimmune-mediated destruction of pancreatic islets. A.U., arbitrary units; AUC, area under the curve. H: Higher levels of eATP in peripheral serum were detected in 4-week-old NOD mice, and in hyperglycemic (Hglc) NOD mice to a lesser extent, as compared with 10-week-old NOD mice. Nglc, normoglycemic. I: During islet peptide (BDC2.5, IGRP, GAD65, and insulin) stimulation of splenocytes from NOD mice, IGRP (a CD8-restricted islet peptide) caused the highest (represented by §) release of eATP in the supernatant represented by the red line that reflects eATP release during islet peptide (BDC2.5, IGRP, GAD65, and insulin) stimulation of splenocytes from NOD mice; and the highest increase in the number of IFN-γ+ cells (represented by §) as compared with controls. BDC2.5, GAD65, and insulin peptide stimulation induced an increase in the number of IFN-γ+ spots as compared with controls as well, but to a lesser extent. J and K: CD4+CD25– T cells cultured in the presence of different concentrations of IL-1β, IL-6, TNF-α, MCP-1, or BzATP (benzoyl-ATP) showed a slight but significant upregulation of P2X7R expression on CD4+CD25– T cells, while in CD8+ T cells, BzATP only was capable of upregulating P2X7R expression. L–P: In 4-week-old NOD mice, the percentages of P2X7R+ on CD4+/CD4+ effector T cells and CD8+/CD8+ effector T cells were increased as compared with 10-week-old normoglycemic or >14-week-old hyperglycemic NOD mice. P2X7R+CD4+ effector, P2X7R+CD8+, and CD8+ effector T cells were increased in 10-week-old mice as compared with hyperglycemic NOD mice. The percentage of P2X7R+ Tregs was increased in hyperglycemic as compared with 4-week-old and 10-week-old NOD mice and in 4-week-old mive as compared with 10-week-old NOD mice (Q and R). P2X7R expression was increased in CD8+ T cells and in CD4+CD25– T cells, to a lesser extent, after culture with BDC2.5 and IGRP peptides, respectively. S: Insulitis score in 4-week-old, 10-week-old, and hyperglycemic NOD mice is shown; n = 9 sections per group were analyzed. Data are expressed as mean ± SEM. Data are representative of at least n = 3 mice. *P < 0.05; **P < 0.01; ***P < 0.001.
eATP Is Released by Pancreatic Islets In Vivo During the Early Phase of Diabetes Onset in NOD Mice
To assess the in vivo release of eATP from pancreatic islets during the onset of autoimmune diabetes in NOD mice, HEK293-pmeLUC transgenic cells and d-luciferin were injected into the peritoneal cavity of NOD and BALB/c mice. HEK293-pmeLUC transgenic cells function as reporters of eATP concentration in vivo (16). Please see research design and methods for a description of the system. A stable luminescence signal was observed in BALB/c mice at different ages (Fig. 2E and F), while in NOD mice, the luminescence signal appeared higher than that which was obtained in BALB/c mice (Fig. 2E and F). A gradual disappearance of bioluminescence was evident over time in NOD mice, paralleling insulitis progression and the autoimmune-mediated destruction of pancreatic islets (Fig. 2E and F). The area under the curve of bioluminescence confirmed the increased eATP release in NOD mice as compared with BALB/c mice over time (Fig. 2G). Peripheral serum levels of eATP were then measured and were confirmed to be highest in 4-week-old NOD mice (Fig. 2H).
T Cells Release eATP During an Autoimmune Response In Vitro and eATP Stimulates P2X7R Expression on T Cells
We then assessed eATP release during an autoimmune response in vitro by incubating splenocytes from hyperglycemic NOD mice for 24 h in the presence of different islet-derived peptides (BDC2.5, IGRP, GAD65, and insulin) in an ELISpot assay (Fig. 2I). eATP levels in the supernatant were increased during the autoimmune response against islet peptides and were observed to be highest following stimulation with the CD8-restricted peptide IGRP (Fig. 2I, red line). Interestingly, eATP levels paralleled the number of IFN-γ+ T cells generated during the autoimmune response (Fig. 2I, bar graph). To evaluate the effect of eATP on P2X7R expression in naive T cells in in vitro studies, we used BzATP, a prototypic P2X7R agonist that exhibits 5- to 10-fold greater potency than ATP. CD4+CD25– and CD8+ T cells, isolated from normoglycemic NOD mice, were cultured for 24 h in the presence of varying concentrations of activating cytokines, such as IL-1β, IL-6, TNF-α, MCP-1, ATP, or BzATP, with P2X7R expression then quantified by flow cytometry. Almost all cytokines induced a slight but significant upregulation of P2X7R expression on CD4+CD25– T cells as compared with medium alone; in addition, a threefold increase in P2X7R expression was evident when culturing CD4+CD25– T cells with BzATP and ATP (Fig. 2J and Supplementary Fig. 2G). While no cytokines induced a significant increase in P2X7R expression in CD8+ T cells, ATP and BzATP at its highest concentration significantly induced an increase in P2X7R expression as compared with medium alone (Fig. 2K and Supplementary Fig. 2H). It is likely that inflammation triggered by cytokines and cell necrosis involve two different pathways, such that only inflammation due to cell necrosis is eATP/P2X7R dependent.
P2X7R+ T Cells Increase In Vivo and In Vitro During the Murine Autoimmune Response
Flow cytometric analysis of splenic CD4+ and CD8+ T cells in NOD mice revealed an increased percentage of P2X7R+CD4+ T cells, P2X7R+CD4+ effector T cells, and particularly P2X7R+CD8+ T cells and P2X7R+CD8+ effector T cells, both in 4-week-old and partially in 10-week-old NOD mice as compared with hyperglycemic NOD mice (Fig. 2L–O). Conversely, the percentage of P2X7R+CD4+CD25+Foxp3+ regulatory T cells (Tregs) was significantly increased in hyperglycemic NOD mice as compared with other groups (Fig. 2P). This observation led us to investigate the function of P2X7R+ and P2X7R− Tregs. We thus compared the ability of P2X7R+ and P2X7R− Tregs to suppress the autoimmune response generated by diabetogenic T cells isolated from NOD BDC2.5 mice and stimulated with BDC2.5 peptide. P2X7R+ Tregs showed impaired suppressive function (Supplementary Fig. 5A and B), reduced metabolic activity, and lower oxygen consumption rates (OCR) as compared with P2X7R− Tregs (Supplementary Fig. 5D and E). Moreover, P2X7R+ Tregs produced more proinflammatory cytokines (i.e., IFN-γ and IL-17) compared with P2X7R− Tregs (Supplementary Fig. 5C). P2X7R stimulation with BzATP during Treg generation also induced a decrease in the percentage of newly generated Tregs and an overall reduction in FoxP3 expression (Supplementary Fig. 5F–H). Interestingly, P2X7R signaling with BzATP increased the production of proinflammatory cytokines (Supplementary Fig. 5I) as well as the percentage of apoptotic cells (Supplementary Fig. 5G).
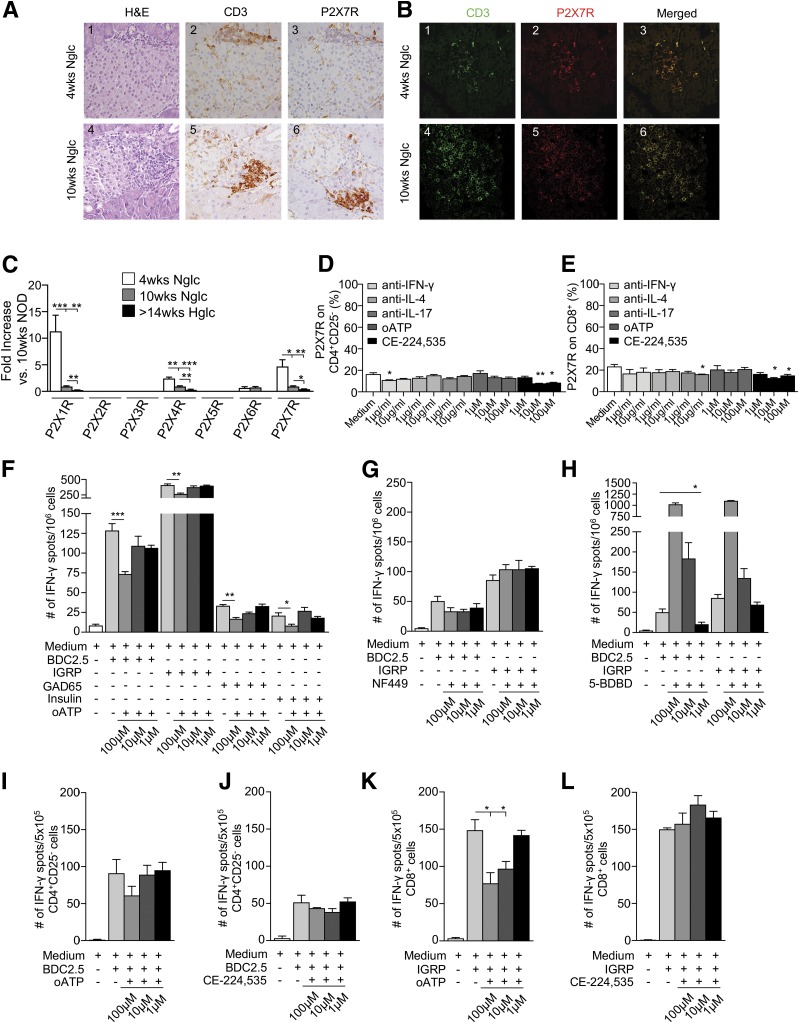
When isolated CD4+CD25– and CD8+ T cells were cultured for 24 h in the presence of BDC2.5/IGRP peptides and CD11c+ cells, a significant increase in P2X7R+ cells, particularly in those that were CD8+, was evident (Fig. 2Q and R). Interestingly, islets from 4-week-old NOD mice showed preserved architecture and scanty T-cell infiltrate with very few P2X7R+ cells, while islets from 10-week-old and hyperglycemic NOD exhibited substantial CD3+ T cell infiltrate, which appeared to be predominantly P2X7R+ (Fig. 3A and B and Supplementary Fig. 2A and B). We then evaluated the expression of P2XsR mRNA in the whole pancreas and in CD4+CD25– and CD8+ T cells obtained from spleen of NOD mice at different ages. P2X1R and P2X7R mRNA expression appeared highest among P2X receptors in the pancreas of NOD mice in the early phase of the disease and decreased following islet infiltration and insulitis (Fig. 3C). Conversely, mRNA P2X expression in splenic CD4+CD25– and CD8+ T cells is broader and more diverse (Supplementary Fig. 2C and D). These data suggest that islet inflammation and damage that occurred during the autoimmune response contribute to the engagement of ATP/P2X7R immunity by stimulating P2X7R expression on T cells and thus creating a proinflammatory environment through generation of a pool of highly activated T cells committed to the Th1/Th17 lineage, which thus facilitates T1D onset.
Figure 3.
P2X7R+ T cells make up the vast majority of pancreas-infiltrating cells, while P2X7R blockade reduces the autoimmune response in vitro. A and B: Islets from 10-week-old and, to a lesser extent, 4-week-old NOD mice showed CD3+ T-cell (green) infiltrate, which appeared to be predominantly P2X7R+ (red). H&E, hematoxylin and eosin; Nglc, normoglycemic. C: P2X1R, P2X4R, and P2X7R mRNA pancreatic expression was evident in 4-week-old and, to a lesser extent, 10-week-old NOD mice. Hglc, hyperglycemic. D: Significant downregulation of P2X7R expression on CD4+CD25– T cells was observed when cells were cultured with anti–IFN-γ or the P2X7R inhibitor CE-224,535 as compared with medium alone. E: P2X7R downregulation was observed on CD8+ T cells cultured with anti–IL-17 or CE-224,535 as compared with medium alone. F: Splenocytes from hyperglycemic NOD mice cultured with the CD4/CD8-restricted islet mimotope peptides BDC2.5/IGRP, respectively, or with GAD65 and insulin in the presence of oATP showed a reduction in the number of IFN-γ+ T cells as compared with controls. G and H: Splenocytes from hyperglycemic NOD mice cultured with BDC2.5 or IGRP peptides in the presence of different concentrations of the P2X1R antagonist NF449 or the P2X4R antagonist 5-BDBD did not show any effect or showed an increase in the number of IFN-γ+ T cells, respectively. I–L: oATP but not CE-224,535 reduced the number of IFN-γ+ CD8+ but not CD4+ T cells when CD4+CD25– cells isolated from BDC2.5 TCR Tg NOD mice (I and J) or CD8+ T cells isolated from 8.3 TCR Tg NOD mice (K and L) were cultured with BDC2.5 or IGRP peptides, respectively, in the presence of different concentrations of oATP or CE-224,535. Data are expressed as mean ± SEM. Data are representative of at least n = 3 mice. *P < 0.05; **P < 0.01; ***P < 0.001.
Targeting the eATP/P2X7R Axis In Vitro Reduced T-Cell P2X7R Expression as Well as the CD8+ T Cell–Dependent Autoimmune Response
To evaluate whether targeting of eATP/P2X7R-driven immunity had any effect on the expression of P2X7R, isolated CD4+CD25– and CD8+ T cells were cultured for 24 h with different anticytokine monoclonal antibodies (mAbs) (i.e., anti–IFN-γ, anti–IL-4, or anti–IL-17) or with P2X7R inhibitors (oATP and CE-224,535). Compared with baseline, CE-224,535 but not oATP significantly reduced P2X7R expression on both CD4+CD25– and CD8+ T cells (Fig. 3D and E). A slight decrease was also observed in CD4+CD25– T cells cultured with anti–IFN-γ mAb (Fig. 3D) and in CD8+ T cells cultured with anti–IL-17 mAb (Fig. 3E). To test the effect of blocking eATP/P2X7R-driven immunity during an autoimmune response, we cultured splenocytes from hyperglycemic NOD mice with BDC2.5, IGRP, GAD65, and insulin peptides for 24 h in the presence of different concentrations of oATP (1, 10, 100µM) in an ELISpot assay. Blocking the eATP/P2X7R axis reduced the production of IFN-γ by T cells in this autoimmune assay (Fig. 3F). Interestingly, the effect was specific to P2X7R, as the P2X1R inhibitor NF449 and the P2X4R inhibitor 5-BDBD were ineffective or induced IFN-γ production by T cells, respectively (Fig. 3G and H). We then cultured isolated CD4+CD25– and CD8+ T cells in the same islet peptide–based ELISpot assay in the presence of different concentrations of oATP or CE-224,535, with islet peptides and CD11c+ cells to present antigen. oATP but not CE-224,535 reduced the production of IFN-γ by CD8+ T cells (Fig. 3K and L) but not by CD4+CD25– T cells (Fig. 3I and J) following stimulation with IGRP or BDC2.5 peptides, respectively. Interestingly, in the same aforementioned autoimmune assay, BzATP significantly reduced the number of IFN-γ+ T cells by inducing T-cell apoptosis when used at higher concentrations (Supplementary Fig. 2E and F). In order to understand the effect of P2X7R targeting on a broader CD8-restricted immune response, CD8+ T lymphocytes isolated from peripheral blood of healthy control individuals were incubated with a mixture of vaccine antigens (Diftavax) for 24 h in the presence or absence of P2X7R antagonists (oATP or CE224,535). The number of IFN-γ–producing CD8+ T cells was significantly reduced after addition of both antagonists, reaffirming the effect of P2X7R targeting on CD8 activation (Supplementary Fig. 1F).
Targeting the eATP/P2X7R Axis Delays Diabetes Onset in NOD Mice In Vivo
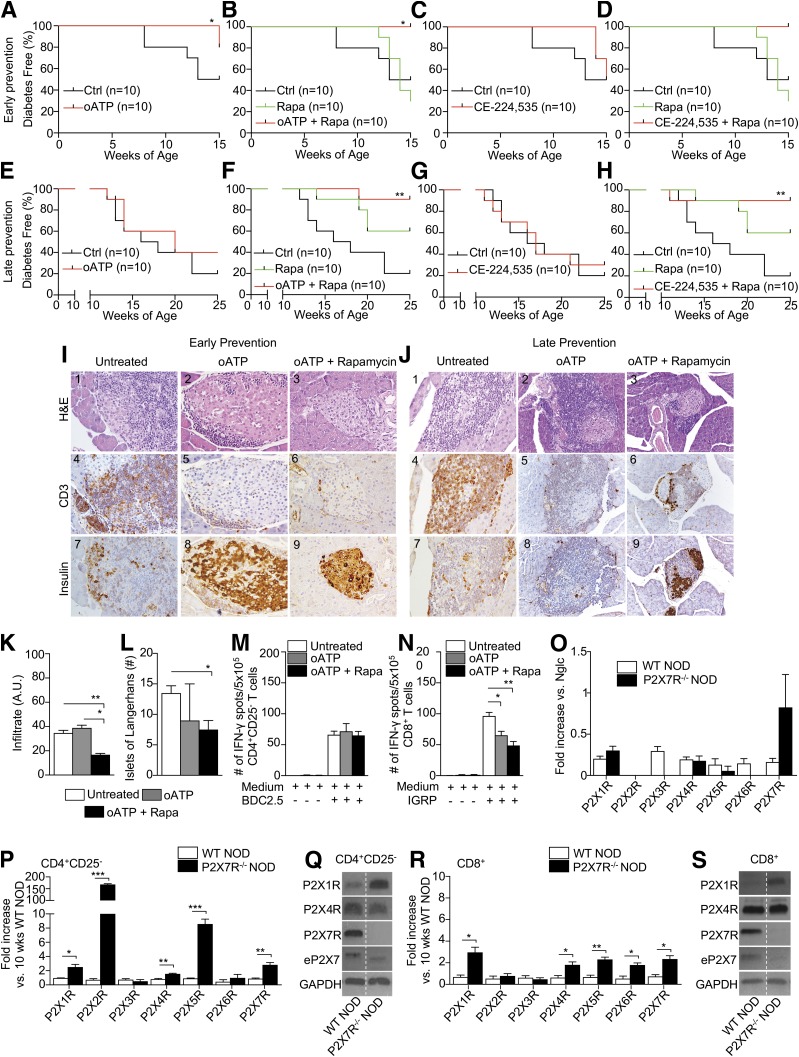
We then tested the effect of targeting the eATP/P2X7R axis in NOD mice in vivo in early and late prevention of diabetes as well as in reversal studies, using 1) oATP, 2) CE-224,535, and 3) a combination of oATP or CE-224,535 with clinical-grade-dose rapamycin. A significant delay in diabetes onset in early prevention studies was observed in NOD mice treated with oATP or the combination of oATP and rapamycin but not in untreated control subjects or those treated with CE-224,535 alone or in combination with rapamycin (Fig. 4A–D). In late prevention studies, both oATP and CE-224,535 successfully delayed diabetes onset when used in combination with rapamycin but not when used alone (Fig. 4E–H). Treatment with rapamycin alone did not show any effect on diabetes onset in either early or late prevention studies. In reversal studies, NOD mice treated with oATP alone, rapamycin alone, or the combination of CE-224,535 and rapamycin remained hyperglycemic (Supplementary Fig. 3A–E). The combination of oATP and rapamycin or CE-224,535 alone resulted in 2 out of 5 mice becoming normoglycemic for several days, then reverting to hyperglycemia (Supplementary Fig. 3B and C). Rapamycin alone did not reverse hyperglycemia in NOD mice (Supplementary Fig. 3E). Histopathological analysis of 4-week-old NOD mice treated with oATP alone or in combination with rapamycin demonstrated better preserved islet architecture and insulin staining as compared with untreated 4-week-old NOD mice (Fig. 4I). Diminished lymphoid infiltration was observed in the islets of mice treated with the combination of oATP plus rapamycin as indicated by staining for the T-cell marker CD3 when compared with both control animals and mice treated with oATP alone (Fig. 4I: 4–6). Semiquantitative analysis confirmed the diminished islet infiltrate (Fig. 4K) despite a slight reduction in the number of islets of Langerhans in mice treated with either oATP alone or oATP and rapamycin. The aforementioned effect may be related to rapamycin, which has been reported to be capable of impairing islet cell function (Fig. 4L). Amelioration of islet architecture and of preserved insulin staining was also observed in 10-week-old NOD mice treated with the combination of oATP and rapamycin in comparison with untreated and oATP-treated mice, despite the presence of lymphoid infiltrate (Fig. 4J). Thus, targeting the eATP/P2X7R axis delays diabetes onset in the early stages of the disease but does not revert established autoimmune diabetes, possibly because in the earlier disease stages, due to mild infiltration and greater available β-cell mass, higher release of eATP by the islets plays a role (Fig. 4A–D). At later stages of the disease, loss of β-cell mass contributes to a decline in eATP release, which could explain the lack of effect when using eATP/P2X7R blocking agents (Fig. 4E–H). We then performed a series of autoimmune in vitro assays to mimic the onset of autoimmune diabetes observed in NOD mice. CD4+CD25– and CD8+ T cells thus were isolated from the spleens of NOD mice untreated or treated with oATP alone or in combination with rapamycin and cultured in the presence of BDC2.5 or IGRP peptides. The number of IFN-γ+CD8+ but not IFN-γ+CD4+ T cells was reduced in mice treated with oATP alone or in combination with rapamycin as compared with untreated mice, suggesting an effect of P2X7R blockade on the CD8+ T cell–dependent autoimmune response only (Fig. 4M and N). Interestingly, when studying the P2X7R−/− NOD mouse, which is not protected against autoimmune diabetes (30), we observed substantial mRNA expression of P2X7R, while Western blot analysis confirmed the absence of P2X7R expression on CD4+CD25– and CD8+ T cells (Fig. 4Q and S). Upregulation of other P2XR expression on T cells was also evident (Fig. 4O–S). We then examined expression of CD38, a cyclic ADP ribose hydrolase that when absent in the NOD mouse, rapidly accelerates onset of hyperglycemia. Genetic deletion of P2X7R in CD38-deficient NOD mice was shown to prevent this rapid T1D onset (30). Indeed, immunophenotypic expression analysis of splenocytes from P2X7R−/− NOD revealed significant upregulation of CD38 expression on CD4+ and CD8+ T cells as compared with wild-type (WT) NOD (Supplementary Fig. 4A–D).
Figure 4.
P2X7R blockade delays diabetes in NOD mice and reduces islet infiltration and the autoimmune response by CD8+ T cells. A–H: The effect of P2X7R blockade was tested in early (4-week-old NOD mice) and late (10-week-old NOD mice) prevention studies in vivo using oATP, CE-224,535, and a combination of oATP or CE-224,535 with clinical-grade-dose rapamycin (Rapa). The treatment with oATP and oATP plus rapamycin (A and B), but not with CE-224,535 alone or with rapamycin (C and D), significantly delayed the onset of diabetes in the early prevention study setting as compared with untreated animals; data are representative of n = 10 mice per group. In the late prevention study, while oATP and CE-224,535 alone did not show any effect on the onset of diabetes (E and G), their combination with rapamycin successfully delayed diabetes onset (F and H); data are representative of n = 10 mice per group. I and J: Representative hematoxylin and eosin (H&E) staining and immunohistochemical analysis of pancreatic islet tissue sections from the different groups of treated and untreated NOD mice. K and L: Semiquantitative analysis of islet infiltrate in 4-week-old treated NOD mice revealed that oATP plus rapamycin is more effective in reducing islet infiltration, although it is associated with an overall reduced number of islets. A.U., arbitrary units. M and N: CD4+CD25– and CD8+ T cells isolated from the spleens of 10-week-old treated and untreated NOD mice and cultured in the presence of BDC2.5 or IGRP peptides, respectively, showed a reduced number of IFN-γ+ CD8+ but not CD4+ T cells in mice treated with oATP alone or in combination with rapamycin as compared with untreated mice. O: The pancreata of 10-week-old normoglycemic (Nglc) P2X7R−/− and WT NOD mice contain P2X7R mRNA. P: mRNA expression of P2X1R, P2X2R, P2X4R, P2X5R, and P2X7R in splenic CD4+CD25– cells was significantly increased in P2X7R−/− NOD mice as compared with WT NOD mice. Q: Western blot analysis of P2X1R, P2X4R, P2X7R, and eP2X7 (extracellular P2X7) in splenic CD4+CD25– T cells confirmed the absence of P2X7R protein in P2X7R−/− NOD mice as compared with WT NOD mice. R: mRNA expression of P2X1R, P2X4R, P2X5R, and P2X7R in splenic CD4+CD25– and in CD8+ T cells (except for P2X2R) were significantly increased in P2X7R−/− NOD mice as compared with WT NOD mice. S: Western blot analysis of P2X1R, P2X4R, and P2X7R in splenic CD8+ T cells confirming the absence of P2X7R protein in P2X7R−/− NOD mice as compared with WT NOD mice. Data are expressed as mean ± SEM. Data are representative of at least n = 3 mice. *P < 0.05; **P < 0.01; ***P < 0.001.
Characterization of P2X7R− and P2X7R+ T Cells
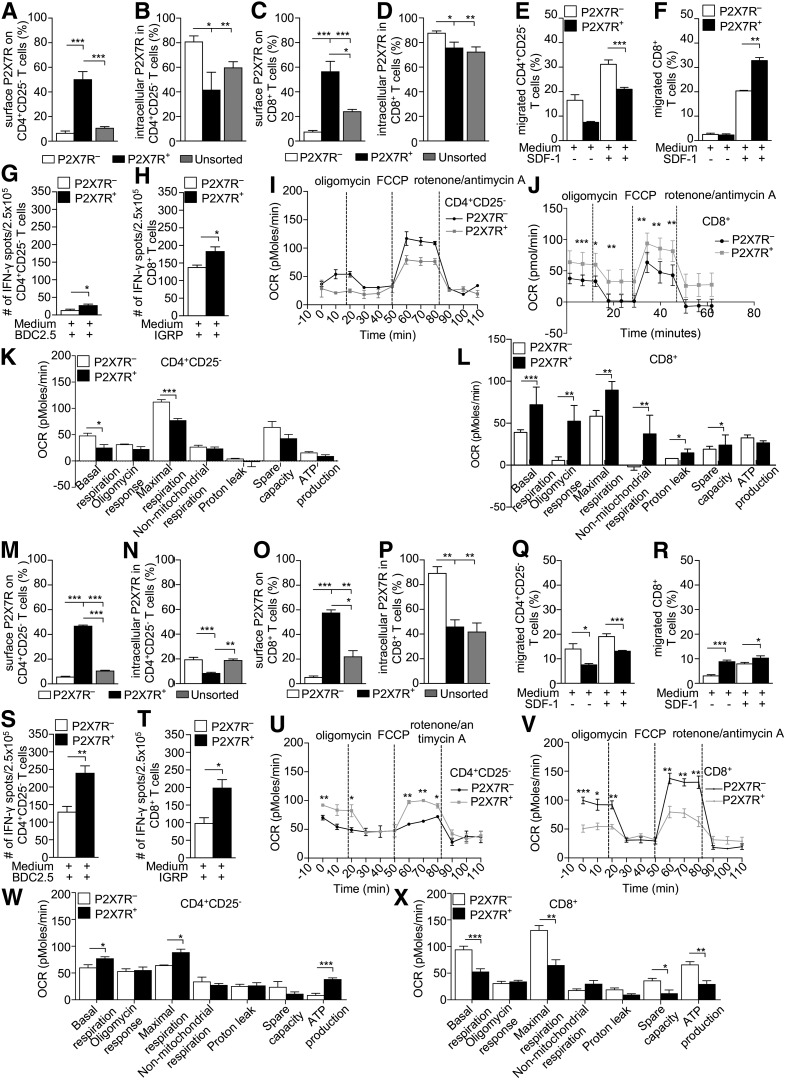
In order to further explore the role of the eATP/P2X7R pathway in T1D onset, we characterized P2X7R− and P2X7R+ cells for migratory ability, autoimmune response, and metabolic profile. Surprisingly, in NOD mice, T cells expressed a significant amount of intracellular P2X7R, which may serve as a P2X7R reservoir (Fig. 5A–D). Among CD4+CD25– T cells, those that were P2X7R− seemed to display enhanced migratory ability in response to SDF-1 as compared with P2X7R+ (Fig. 5E). The opposite effect was observed in CD8+ T cells; those that were P2X7R+ showed a significantly greater migratory ability as compared with P2X7R− (Fig. 5F), suggesting a differential impact of P2X7R signaling on CD4+ and CD8+ T-cell migration ability. Moreover, P2X7R+CD4+CD25– T cells and P2X7R+CD8+ T cells showed a significantly greater autoimmune response compared with P2X7R−CD4+CD25– T cells and P2X7R−CD8+ T cells, as demonstrated by a substantially greater number of IFN-γ+ T cells generated by P2X7R+CD4+CD25– T cells and P2X7R+CD8+ T cells during an islet peptide–based ELISpot assay (Fig. 5G and H). In addition, metabolic profiles were significantly altered between P2X7R+CD4+CD25– T cells, P2X7R+CD8+ T cells, and their P2X7R− counterparts. Upon examination of bioenergetic changes in the aforementioned four populations, the OCR, an indicator of oxidative phosphorylation, was significantly decreased in the basal state in P2X7R+CD4+CD25– T cells when compared with P2X7R− CD4+CD25– T cells and P2X7R+CD8+ T cells (Fig. 5I–L). P2X7R+CD4+ T cells showed significantly reduced maximal respiration in response to the uncoupling agent FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone) as compared with P2X7R−CD4+ T cells (Fig. 5I and J). While P2X7R+CD8+ T cells appeared hypermetabolic given that they showed significantly higher maximal respiration in response to the uncoupling agent FCCP, OCR values significantly increased in response to oligomycin (ATP synthase inhibitor) and to rotenone (complex I inhibitor) and antimycin A (complex III inhibitor) (Fig. 5J and L). The latter suggests differential behavior when mitochondrial respiration is augmented as spare respiratory capacity (SRC) increased in P2X7R+CD8+ T cells in comparison with their P2X7R− counterparts (Fig. 5J and L). We have extended our investigation to assess the glycolytic capacity of P2X7R− and P2X7R+ CD8+ T cells. P2X7R−CD8+ T cells did not display a robust glycolytic response when exposed to glucose or oligomycin (Supplementary Fig. 3F and G) and therefore exhibited reduced glucose uptake, while P2X7R+CD8+ T cells displayed an increased glycolytic reserve, likely to enhance their activity during an autoimmune response (Supplementary Fig. 3F and G).
Figure 5.
Characterization of P2X7R− and P2X7R+ cells reveals dependence of CD8+ T cells on eATP/P2X7R signaling. A–D: P2X7R− CD4+CD25–/CD8+ T cells isolated from 10-week-old hyperglycemic or normoglycemic NOD or BDC2.5/8.3 TCR Tg NOD mice (n = 3 samples per group) showed no surface expression of P2X7R, while significant intracellular expression was detected. E and F: The percentage of P2X7R−CD4+CD25– T cells migrating to SDF-1 was significantly higher as compared with P2X7R+CD4+CD25– T cells, while the opposite was observed in CD8+ T cells, with a higher percentage of migrating P2X7R+CD8+ T cells as compared with P2X7R−CD8+ T cells. G and H: P2X7R+CD4+CD25–/CD8+ T cells from hyperglycemic NOD mice cultured with the CD4/CD8-restricted islet mimotope peptides BDC2.5 and IGRP, respectively, generated more IFN-γ+ T cells. I and K: Higher OCR in basal conditions and after FCCP addition was observed in P2X7R− CD4+CD25– T cells as compared with P2X7R+CD4+CD25– T cells. J and L: In P2X7R+CD8+ T cells, OCR was increased after FCCP addition and reduced in response to rotenone/antimycin A as compared with P2X7R−CD8+ T cells. While the maximal respiration capacity and SRC of P2X7R+CD8+ T cells were significantly increased, following addition of oligomycin and in response to rotenone/antimycin A, the OCR was significantly reduced as compared with P2X7R+CD8+ T cells. M–P: P2X7R−CD4+CD25–/CD8+ T cells from NOD.BDC2.5 and NOD.8.3 mice showed significant intracellular P2X7R expression as well. Q and R: Among CD4+CD25– T cells from NOD.BDC2.5 mice, the percentage of migrating P2X7R− cells was significantly higher as compared with P2X7R+ cells, while among CD8+ T cells from NOD.8.3 mice, the percentage of P2X7R+ cells migrating was higher as compared with P2X7R− cells. S and T: Among both CD4+CD25– and CD8+ T cells, the number of IFN-γ+ T cells generated during an islet peptide-based autoimmune assay was significantly higher among P2X7R+ as compared with the P2X7R− cells. U and W: Among CD4+CD25– T cells from NOD.BDC2.5 mice, an increase in OCR was observed in P2X7R+ cells in basal conditions and after FCCP injection as compared with P2X7R− cells. Among CD4+CD25– T cells from NOD.BDC2.5 mice, the basal, maximal respiration, and ATP-related OCR were significantly increased in P2X7R+ as compared with P2X7R− cells. V and X: Among CD8+ T cells from NOD.8.3 mice, OCR was increased in basal conditions and after FCCP injection in P2X7R− as compared with P2X7R+ cells. Among CD8+ T cells from NOD.8.3 mice, the basal, maximal respiration, SRC, and ATP-related OCR increased in P2X7R− as compared with P2X7R+ cells. Data are expressed as mean ± SEM. Data are representative of at least n = 3 mice. *P < 0.05; **P < 0.01; ***P < 0.001.
We then characterized P2X7R− and P2X7R+ cells within CD4+ T cells from NOD.BDC2.5 mice and CD8+ T cells from NOD.8.3 mice. Immunophenotypic analysis, examination of migratory properties, results of ELISpot assays, and bioenergetic profiling confirmed what was observed for WT NOD mice (Fig. 5M–X), with minor differences. P2X7R+CD4+CD25– T cells from NOD.BDC2.5 mice exhibited higher OCR at basal conditions and in response to FCCP (Fig. 5U and W), as well as higher ATP production (Fig. 5W) overall.
Novel Recombinant Protein sP2X7R Reduces CD8-Restricted Autoimmune Responses
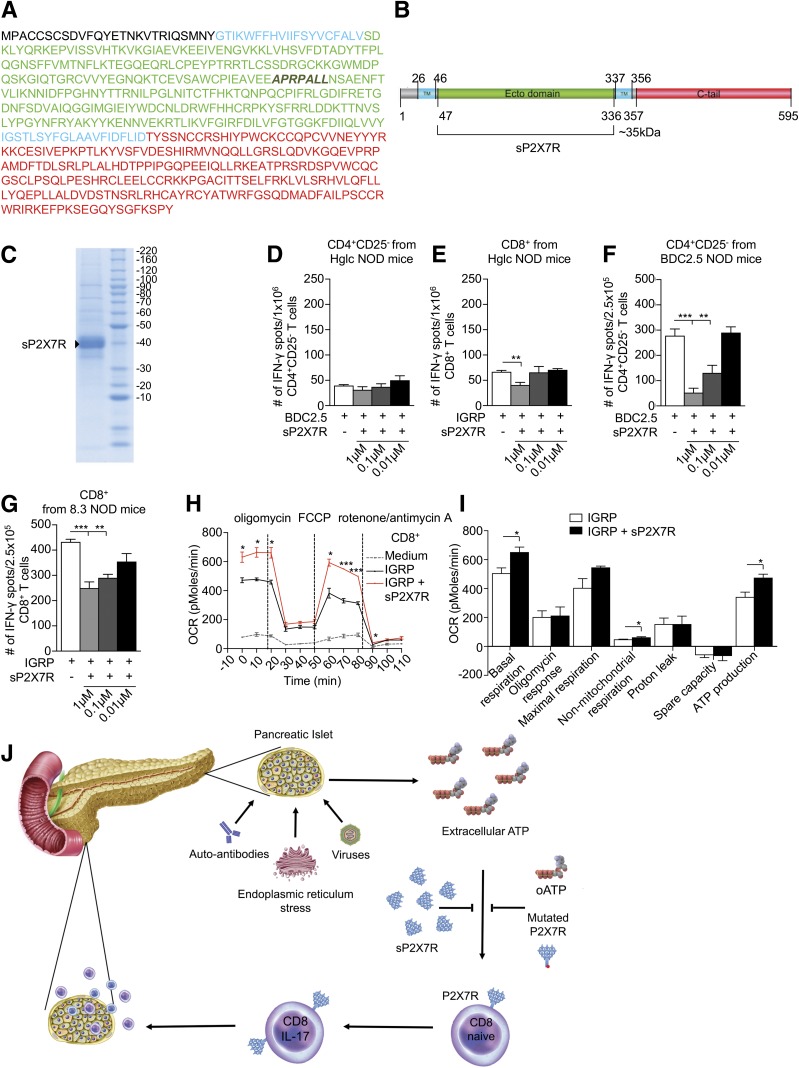
We generated a recombinant protein based on the 595-amino-acid P2X7R extracellular domain (sP2X7R) (Fig. 6A–C), thus offering a novel therapeutic option able to quench eATP (Supplementary Video). The addition of sP2X7R to an anti–islet peptide–based ELISpot reduced the number of IFN-γ+CD8+ but not CD4+ T cells from NOD mice (Fig. 6D and E). The same effect was observed when using transgenic CD8+/CD4+ T cells (Fig. 6F and G). CD8+ T cells that were cultured with IGRP peptide and sP2X7R exhibited higher OCR at basal conditions and in response to FCCP at all time points (Fig. 6H and I). ATP production was also higher in cells that were cultured with sP2X7R as compared with CD8+ T cells cultured with IGRP peptide alone, suggesting interference of our recombinant protein sP2X7R with the eATP/P2X7R axis and with the bioenergetic activity of CD8+ T cells (Fig. 6I). We can now propose a working hypothesis in which eATP released by β-cells during stress/injury mediated by viruses, autoantibodies, and other factors induces P2X7R expression on T cells (particularly on CD8+ cells) and facilitates their activation, leading to T-cell priming. Pharmacological targeting of the eATP/P2X7R signaling using oATP or the novel recombinant sP2X7R protein prevents the deleterious signaling that would be generated outright in CD8+ autoreactive T cells, thus delaying the onset of diabetes (Fig. 6J).
Figure 6.
The novel recombinant protein sP2X7R containing the extracellular P2X7R domain reduces the in vitro autoimmune response. A and B: A schematic representation of the sequence and domain arrangements of human P2X7R and sP2X7R. C: SDS-PAGE of sP2X7R after purification by gel filtration and staining with Coomassie blue is shown. D and E: CD4+CD25– and CD8+ T cells isolated from hyperglycemic (Hglc) NOD mice were cultured with the BDC2.5 and the IGRP peptides respectively; 1 µM sP2X7R reduces the number of IFN-γ+CD8+ but not CD4+ T cells. F and G: CD4+CD25– and CD8+ T cells isolated from NOD.BDC2.5/8.3 mice were cultured with BDC2.5 and IGRP peptides and sP2X7R. sP2X7R reduces the number of IFN-γ+CD4+/CD8+ T cells dose dependently. H and I: Metabolic profile of CD8+ T cells at steady state and during an autoimmune response with or without sP2X7R. T cells cultured with sP2X7R showed higher OCR at basal and after FCCP as compared with controls. Basal and nonmitochondrial respiration as well as ATP production levels were higher in cells cultured with sP2X7R as compared with controls. J: Working hypothesis summarizing the role of the eATP/P2X7R axis in the onset of T1D and the effects of the novel sP2X7R recombinant protein. Data are expressed as mean ± SEM. Data are representative of at least n = 3 mice. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
The emerging role of eATP as an initiator of the immune response, aside from its metabolic function, has been the focus of intense investigation (1,24). eATP levels can increase as a consequence of release from damaged cells or by activated immune cells and can be sensed by P2X7R (1,24), which is expressed on T cells, thereby triggering their activation. Our initial observation described herein, that two P2X7R loss-of-function nsSNPs appear to be protective against T1D, prompted us to hypothesize that eATP/P2X7R signaling may be involved in the onset of T1D, despite the lack of protection observed in P2X7R−/− NOD mice. To this end, we reported an upregulation of CD38 and of other P2XsR on T cells from P2X7R−/− NOD mice, which may explain why the incidence of T1D was not altered by genetic deletion of P2X7R. In fact, lack of CD38 may impair calcium mobilization and immune function while accelerating T1D onset in NOD mice, whereas lack of P2X7R may counteract this effect and stabilize calcium efflux and prevent the acceleration of T1D onset. Notably, the rs7958311 loss-of-function mutation was shown to be associated with insulin resistance and impaired glucose tolerance in a cohort of T2D patients (31). Because eATP is secreted with insulin (25,26), we also hypothesize that β-cells may participate in their own demise by secreting eATP as a consequence of injury and thus may activate leukocytes in an initial step of the T cell–mediated anti-islet autoimmune response. ATP release is not restricted to leukocytes, as it has been described that eATP is also secreted by platelet-dense granules, therefore providing a positive feedback mechanism (32). It is possible that this pathway that potentiates platelet activation through nucleotide receptors (P2X1, P2Y1, and P2Y12) expressed on the platelet surface may be affected as well (32).
Phenotypic analysis of P2X7R expression on different T-cell populations in the periphery of patients with newly diagnosed T1D revealed many perturbations of the P2X7R-related phenotype. Interestingly, we demonstrated that during an autoimmune response in vitro, eATP is released in high amounts and that this release may upregulate P2X7R on T cells (particularly on CD8+ cells), thus creating a vicious cycle that sustains the autoimmune response and making P2X7R an attractive target in T1D. Pancreatic islets exposed to high glucose or to inflammatory cytokines increasingly released eATP as well; these conditions are found frequently in prediabetes. Peripheral levels of eATP are increased in the early phase of T1D onset in NOD mice, and, similarly, P2X7R expression is increased on CD8+ T cells. Most if not all of the T cells infiltrating the pancreas in NOD mice appeared to be P2X7R+, and P2X7R mRNA was increased in the pancreata of NOD mice. Of note, P2X7R blockade was effective in reducing CD8-restricted but not CD4-restricted autoimmune responses in vitro. Targeting of eATP/P2X7- driven immunity with oATP delayed diabetes onset in early prevention studies in NOD mice in vivo, with pathology confirming reduced lymphoid infiltrate and preserved insulin staining in treated NOD mice. Characterization of the P2X7R− and P2X7R+ cellular compartments revealed that P2X7R+CD8+ T cells migrate more efficiently and produce more IFN-γ/IL-17 when challenged with islet peptides as compared with P2X7R−CD8+ T cells and, generally, as compared with CD4+CD25– cells. The bioenergetic profiles of P2X7R− or P2X7R+CD4+CD25– and CD8+ T cells were significantly different. Although it has been reported that NOD mice develop an aggressive T cell–mediated destruction of β-cells with limited similarity to human disease and also show poor predictive value in screening for effective intervention therapies for T1D, the NOD mouse remains the most reliable T1D model, as it shares many genetic and physiopathological features with human T1D (33).
The secretion of eATP and insulin constitute, per se, a potential driving force for T-cell activation. Any perturbation of insulin secretion, or any overexertion by or stress of β-cells, may favor increased release of eATP, which in turn may activate passenger leukocytes. Viral infection and endoplasmic reticulum stress may result in perturbation of the eATP-releasing machinery within β-cells as well. Second, it appears that autoreactive CD8+ T cells are more prone to be affected by and possibly more dependent upon eATP/P2X7R signaling as compared with autoreactive CD4+ T cells. Third and finally, the novel recombinant protein sP2X7R, which we created and produced based on the extracellular domain of P2X7R, reduced CD8+ T-cell activity and autoimmune responses and thus may represent a novel therapeutic tool for the prevention of T1D. In conclusion, eATP/P2X7R signaling facilitates the onset of autoimmune diabetes by fueling autoreactive CD8+ cells and may represent a novel therapeutic target for T1D.
Supplementary Material
Article Information
Funding. This work was supported by Fondazione Romeo ed Enrica Invernizzi. P.F. is the recipient of a European Foundation for the Study of Diabetes/Sanofi European Research Programme grant and is supported by an American Heart Association Grant-in-Aid.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.T. and M.B.N. designed and performed experiments, analyzed data, and wrote the paper. F.D., A.V., V.U., S.F., R.B., S.D., C.Ro., A.M., and E.-M.F. performed experiments and analyzed data. C.F. and A.P. designed and performed research, analyzed the data, and edited the paper. A.S., D.C., E.G., C.M., F.B., M.G.P., and C.H.W. helped with sample collection, pathology, and analysis of data. C.H.W., M.A.A., C.Ri., F.F., F.D.V., A.P., S.D.-P., and G.V.Z. coordinated research. P.F. conceived the study, designed research, and wrote and edited the paper. All authors reviewed and edited the paper. P.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-1227/-/DC1.
A.P. is currently affiliated with the Center for Scientific Review, National Institutes of Health, Bethesda, MD.
The opinions expressed in this article are the authors' own and do not necessarily reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government.
References
- 1.Vergani A, Tezza S, D’Addio F, et al. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation 2013;127:463–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guleria I, Gubbels Bupp M, Dada S, et al. Mechanisms of PDL1-mediated regulation of autoimmune diabetes. Clin Immunol 2007;125:16–25 [DOI] [PubMed] [Google Scholar]
- 3.McClymont SA, Putnam AL, Lee MR, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol 2011;186:3918–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiorina P, Jurewicz M, Vergani A, et al. Targeting the CXCR4-CXCL12 axis mobilizes autologous hematopoietic stem cells and prolongs islet allograft survival via programmed death ligand 1. J Immunol 2011;186:121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naserke HE, Bonifacio E, Ziegler AG. Prevalence, characteristics and diabetes risk associated with transient maternally acquired islet antibodies and persistent islet antibodies in offspring of parents with type 1 diabetes. J Clin Endocrinol Metab 2001;86:4826–4833 [DOI] [PubMed] [Google Scholar]
- 6.Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014;371:1972–1982 [DOI] [PubMed] [Google Scholar]
- 7.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant 2008;8:1990–1997 [DOI] [PubMed] [Google Scholar]
- 8.D’Addio F, Maffi P, Vezzulli P, et al. Islet transplantation stabilizes hemostatic abnormalities and cerebral metabolism in individuals with type 1 diabetes. Diabetes Care 2014;37:267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorina P, Secchi A. Pancreatic islet cell transplant for treatment of diabetes. Endocrinol Metab Clin North Am 2007;36:999–1013, ix [DOI] [PubMed] [Google Scholar]
- 10.Novak I. ATP as a signaling molecule: the exocrine focus. News Physiol Sci 2003;18:12–17 [DOI] [PubMed] [Google Scholar]
- 11.Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009;461:282–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schenk U, Westendorf AM, Radaelli E, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal 2008;1:ra6. [DOI] [PubMed] [Google Scholar]
- 13.Piccini A, Carta S, Tassi S, Lasiglié D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A 2008;105:8067–8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 1998;50:413–492 [PubMed] [Google Scholar]
- 15.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature 2006;442:527–532 [DOI] [PubMed] [Google Scholar]
- 16.Wilhelm K, Ganesan J, Müller T, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med 2010;16:1434–1438 [DOI] [PubMed] [Google Scholar]
- 17.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 2011;11:201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casati A, Frascoli M, Traggiai E, Proietti M, Schenk U, Grassi F. Cell-autonomous regulation of hematopoietic stem cell cycling activity by ATP. Cell Death Differ 2011;18:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabel CA. P2 purinergic receptor modulation of cytokine production. Purinergic Signal 2007;3:27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria TH17 cell differentiation. Nature 2008;455:808–812 [DOI] [PubMed] [Google Scholar]
- 21.Yip L, Woehrle T, Corriden R, et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J 2009;23:1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrari D, Pizzirani C, Adinolfi E, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 2006;176:3877–3883 [DOI] [PubMed] [Google Scholar]
- 23.Vergani A, Fotino C, D’Addio F, et al. Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection. Diabetes 2013;62:1665–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vergani A, Tezza S, Fotino C, et al. The purinergic system in allotransplantation. Am J Transplant 2014;14:507–514 [DOI] [PubMed] [Google Scholar]
- 25.Richards-Williams C, Contreras JL, Berecek KH, Schwiebert EM. Extracellular ATP and zinc are co-secreted with insulin and activate multiple P2X purinergic receptor channels expressed by islet beta-cells to potentiate insulin secretion. Purinergic Signal 2008;4:393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geisler JC, Corbin KL, Li Q, Feranchak AP, Nunemaker CS, Li C. Vesicular nucleotide transporter-mediated ATP release regulates insulin secretion. Endocrinology 2013;154:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem 1993;268:8199–8203 [PubMed] [Google Scholar]
- 28.Rizzo R, Ferrari D, Melchiorri L, et al. Extracellular ATP acting at the P2X7 receptor inhibits secretion of soluble HLA-G from human monocytes. J Immunol 2009;183:4302–4311 [DOI] [PubMed] [Google Scholar]
- 29.Falzoni S, Donvito G, Di Virgilio F. Detecting adenosine triphosphate in the pericellular space. Interface Focus 2013;3:20120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YG, Scheuplein F, Driver JP, et al. Testing the role of P2X7 receptors in the development of type 1 diabetes in nonobese diabetic mice. J Immunol 2011;186:4278–4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todd JN, Poon W, Lyssenko V, et al. Variation in glucose homeostasis traits associated with P2RX7 polymorphisms in mice and humans. J Clin Endocrinol Metab 2015;100:E688–E696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahner BN, Shankar H, Murugappan S, Prasad GL, Kunapuli SP. Nucleotide receptor signaling in platelets. J Thromb Haemost 2006;4:2317–2326 [DOI] [PubMed] [Google Scholar]
- 33.Roep BO, Atkinson M. Animal models have little to teach us about type 1 diabetes: 1. In support of this proposal. Diabetologia 2004;47:1650–1656 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.