Abstract
We have tested the feasibility of tear glucose sensing using a daily, disposable contact lens embedded with boronic acid-containing fluorophores as a potential alternative to current invasive glucose-monitoring techniques. Our findings show that our approach may, indeed, be suitable for the continuous monitoring of tear glucose levels in the range 50–500 µM, which track blood glucose levels that are ~5–10-fold higher. We compare the response of the boronic acid probes in the contact lens to solution-based measurements and can conclude that both the pH and polarity within the contact lens need to be considered with respect to choosing/designing and optimizing glucose-sensing probes for contact lenses.
Diabetes results in long-term health disorders, including cardiovascular disease and blindness. One of the major challenges in the management of diabetes is the monitoring of glucose concentrations. A wide variety of methods have been proposed, including near-infrared spectroscopy,1,2 optical rotation,3,4 colorimetric,5,6 and fluorescence detection.7–11 The most commonly used technology for blood glucose determination is an enzyme-based method, which requires frequent blood sampling and, therefore, drawing. Although frequent “finger pricking” with a small needle to obtain the blood sample is a relatively painless process, this method does suffer from a few practical problems. The first one is inconvenience and the required compliance by patients, while the second is that this is not a continuous monitoring method. Despite intensive efforts, no method is presently available for the continuous noninvasive measurement of blood glucose.
Since the earliest reports of its presence in tears, glucose has remained a clinical and physiological curiosity.12,13 Although there is both general interest and agreement that tear glucose levels are low, actual glucose concentrations remain confusing and somewhat contradictory.13,14 For example, Giardini and Roberts have reported tear glucose concentrations to be ~3 mg/100 mL15 for subjects with normal glucose metabolism, and Ridley reported glucose concentrations of 65 mg/100 mL.16 These discrepancies are now thought to be due to inappropriate sampling methods.13,14 Elevated tear glucose during hyperglycemia was first demon-strated by Michail et al as early as 193717,18 as the tear glucose levels track blood levels in a manner analogous to the equilibrium that normally exists for glucose between blood and tissue fluid.19 Since that time, tear glucose has been used on occasion to assess patients for hyperglycemia in an invasive and noncontinuous manner.13
In this paper, we employ the notion of elevated tear glucose levels during hyperglycemia to investigate for the first time the possibility of monitoring tear glucose, and therefore blood glucose, using a disposable, off-the-shelf, contact lens. By incorporating monosaccharide-sensitive fluorescent probes within such a lens, we can indeed make progress toward this noninvasive approach for glucose monitoring.
As with any sensors, there are several issues that have to be addressed. The first is to identify suitable transduction elements, which in the presence of glucose, can report/produce suitable signals. The second is the design of the matrix to incorporate the transduction elements. For this, we have chosen an off-the-shelf disposable plastic contact lens, primarily because its physiological compatibility has already been assessed, and finally, the optimization of the sensor, with regard to sensitivity, response time, reversibility, shelf life, etc. The latter two issues will be discussed throughout much of this paper. For the identification of suitable transduction elements, boronic acid has been known to have a high affinity for diol-containing compounds, such as carbo-hydrates,20–22 in which the strong complexation has been used for the construction of carbohydrate sensors,23–30 transporters,31 and chromatographic materials.32 Naturally, boronic acid compounds have been used for the synthesis of glucose sensors.33–39 We note the work of Shinkai,33,34 Norrild,35 Lakowicz,36–39 and Drueckhammer,26 to name but just a few.
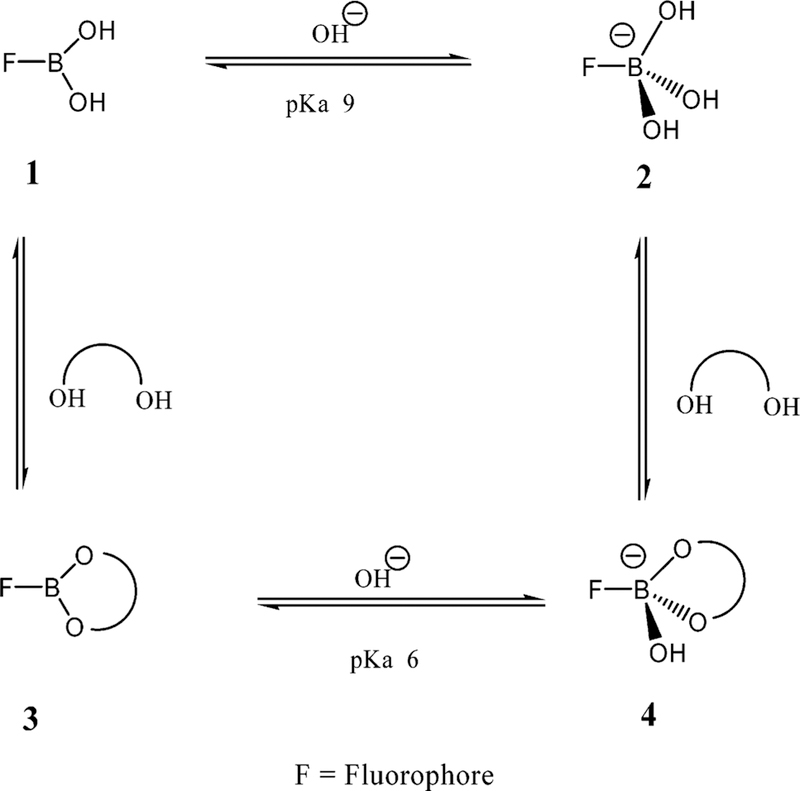
Boronic acids are weak Lewis acids composed of an electron-deficient boron atom and two hydroxyl groups, (1 in Figure 1), which can interact with strong bases such as OH– to from the anionic borate form (2 in Figure 1), showing a typically high pKa around 9.40,41 Boronic acids couple with diols to form a boronic acid diester group (3 in Figure 1). The diol is linked covalently, and the reaction is fast and completely reversible.41 In comparison to the boronic acid group, the boronic acid ester group shows higher acidity (pKa ~ 6) due to a more electrophilic boron atom. The monophenylboronic acid group shows higher affinity for D-fructose and a smaller affinity for D-glucose,41 with dissociation constants of ~0.5 and 10 mM respectively.41 The use of the boronic acid groups for sensing sugars is strongly dependent on the molecular geometry and the aromatic species where the boronic acid group is present; hence, glucose-sensitive probes can be made with a variety of affinities, in the millimolar range for blood glucose37–39 and in the micromolar range for tear glucose.
Figure 1.
Equilibrium for the boronic acid/diol (sugar) interaction.
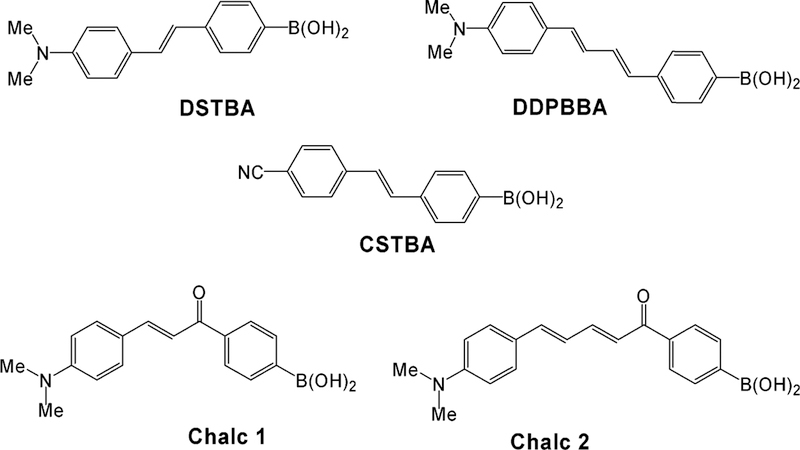
In this paper, we report on boronic acid containing fluorophores (BAFs) which employ different mechanisms to induce spectral changes in the presence of sugar,41 in particular, excited-state charge transfer (CT).41 CT is a versatile mechanism that can be applied to a large number of fluorophores where the boronic acid group and an electron donor group are present on the same fluorophore. Here, the BA group [–B(OH)2] acts as an electron-withdrawing group. However, in the presence of sugar and at an appropriate pH, the boronic acid group is present in its anionic form, namely, [–B(OH)(sugar)]–, and is no longer an electron-withdrawing group. Hence, spectral changes can be observed due to the perturbation of the charge-transfer nature of the excited state. Here we employ this mechanism with a range of probes developed in our laboratory (Figure 2)36–39,42–46 for glucose-sensing within a plastic contact lens polymer. These probes show both wavelength shifts and intensity changes toward glucose within the contact lens, demonstrating a strong future potential for the noninvasive monitoring of tear glucose and, therefore, blood glucose by our approach. In addition, we compare spectral data obtained from the contact lens with bulk solution-based measurements in an attempt to optimize sensor response with regard to leaching, pKa, and the dynamic range for sensing.
Figure 2.
Molecular structures of the CT probes studied in the contact lens.
EXPERIMENTAL SECTION
Materials.
All chemicals were purchased from Sigma. The preparation of the BAFs was in accordance with previous reports.39,41
The contact lenses were supplied by CIBA Vision, Atlanta, GA, and were stirred in 500 mL of water, 20 °C for 24 h before post-doping. The contact lens is a poly(vinyl alcohol)-type photocured polymer which swells slightly in water. Its hydrophilic character readily allows for the diffusion of the aqueous analytes in tears.
Doping was undertaken by incubating the lenses in a high concentration of the respective BAFs solution for 24 h before being rinsed in Millipore water. Lenses were used directly after being prepared.
Methods.
All solution fluorescence measurements were undertaken in 4 × 1 × 1-cm fluorometric plastic cuvettes using a Varian fluorometer.
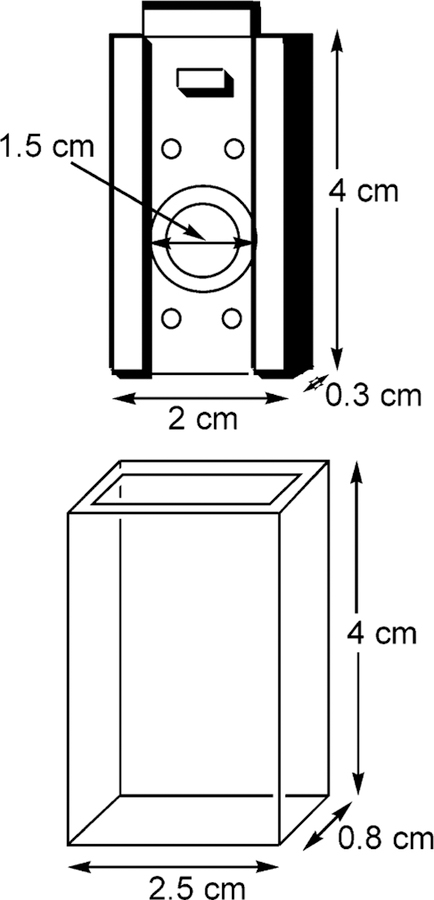
Doped contact lenses were mounted in a custom-made (CIBA Vision) lens holder, Figure 3, which was itself inserted into a quartz holder for fluorescence-sensing measurements. Excitation and emission was performed using a Varian fluorometer with the concave edge of the lens facing toward the excitation source. This geometry was employed to reduce any scattering of the excitation light. We additionally tested the lens excited from the convex edge, just as would be used in the eye, and found identical results.
Figure 3.
Contact lens mount and quartz holder.
The quartz lens holder had dimensions of 4 × 2.5 × 0.8 cm, all 4 sides being of optical quality. The contact lens was mounted onto a stainless steel mount of dimensions 4 × 2 × 0.3 cm, which fits tightly within the quartz outer holder. A circular hole in the center of the mount with a 1.5 cm i.d., had a raised quartz lip, which enabled the lens to be mounted. The mount and holder readily allow for ~1.5 cm3 of solution to be in contact with the front and back sides of the lens for the sugar-sensing experiments (Figure 3).
Leaching of the probes from the contact lens polymer was observed using the sample holder shown in Figure 3, which contained ~1.5 cm3 buffer, 20 °C. A Varian fluorometer measured the intensity change as a function of time to determine the percentage signal change, corresponding to dye leaching. It should be noted that with no sample present, no intensity fluctuations or drifts were observed, indicating stability of the fluorometer xenonarc source.
RESULTS
To determine the usefulness of BAFs with regard to tear glucose sensing in a contact lens, it is necessary to compare both solution and lens-based measurements. Subsequently, Figures 4 and 5 and Supporting Information Figures S1 and S2 show the solution responses of various probes shown in Figure 2 toward sugar, which is in accordance with previous reports from our laboratory.36–39,42–46
Figure 4.
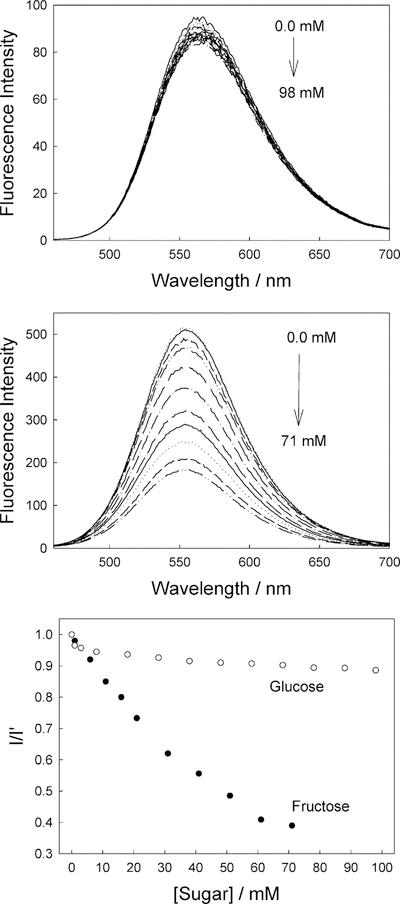
Emission spectra of DSTBA in pH 8.0 buffer/methanol (2:1) with increasing concentrations of fructose, λex = 340 nm (top), and ratiometric response to both fructose and glucose (bottom).
Figure 5.
Emission spectra of CSTBA in pH 8.0 buffer/methanol (2:1) with increasing concentrations of fructose, λex = 320 nm (top), and ratiometric response to both fructose and glucose (bottom).
Solution Sugar Sensing.
Stilbene Derivatives.
Figure 2 shows two stilbene derivatives which contain the boronic acid moiety. 4′-Dimethylaminostilbene-4-boronic acid (DSTBA) combines the electron-donating dimethylamino group with the electron-with-drawing boronic acid group, and 4′-cyanostilbene-4-boronic acid (CSTBA) combines the electron-withdrawing cyano group with the boronic acid, in essence, two probes demonstrating both reduced and increased CT respectively in the presence of sugar.
Figure 4 shows the effect of sugar on the emission properties of DSTBA in solution. The emission spectrum shows a hypso-chromic shift of ~30 nm and an increase in fluorescence intensity as the concentration of fructose increases (Figure 4, top). These dramatic and useful changes can simply be explained by the loss of the electron withdrawing property of the boronic acid group following the formation of the anionic form, as shown in Figure 1.
The CSTBA stilbene derivative possesses two electron-withdrawing groups. In the presence of sugar, we can observe a bathochromic shift, some 25 or so nm, and a decrease in the intensity at pH 8, Figure 5, which is opposite to that observed for DSTBA. This change has been attributed to an excited CT state present for the anionic form of CSTBA, in which no CT states are observed for the neutral form of the boronic acid group,41 suggesting that the anionic form of the boronic acid group can act as an electron donor group.
As previously mentioned, monoboronic acid derivatives show higher affinities for D-fructose, and the affinity decreases for D-galactose and D-glucose, as can be seen for both stilbene probes in Figures 4 and 5 bottom, respectively.
Polyene Derivative.
To test the suitability of longer-wavelength probes in the contact lens, we also considered a polyene derivative, DDPBBA, 1-(p-boronophenyl)-4-(p-dimethylaminophenyl)buta-1,2-deine,37,41,42 which combined a dimethylamino group and a boronic acid group in the para positions of each of the phenyl groups (Figure 2). As was observed for DSTBA, we observed a blue shift in the emission and an increase in the emission intensity for increasing sugar concentrations (see Supporting Information Figure S1).
Chalcone Derivatives.
Chalcone derivatives, unlike the stilbenes and polyenes, have the advantage of much longer wavelength emission. This is particularly attractive because longer wavelength emission reduces the detection of any lens or eye autofluorescenceas well as scatter (λ−4 dependence) and also allows the use of cheaper and longer-wavelength laser or light-emitting diode excitation sources, reducing the need for UV excitation in the eye. Subsequently, we included in our contact lens feasibility studies two chalcone derivatives, which were previously synthesized in our laboratory,39,41 namely, Chalc 1 and Chalc 2 (Figure 2). For these probes, the boronic acid group does not produce resonance forms with the electron-donating amino group. The CT occurs between the dimethylamino group (electron-donating group) and the carbonyl group (electron-withdrawing group). Upon sugar’s binding to the boronic acid group, then a change in the electronic properties of the boron group, both when free and when complexed with sugar, leads to a change in the electronic density of the benzophenone moiety and, subsequently, the CT properties of the excited state of the fluorophore, noting that the boronic acid group is in resonance with the carbonyl group. Both chalcone derivatives show a similar response to sugar and pH.39,41 Figure S2 in the Supporting Information shows the response of Chalc 2 to increasing fructose concentrations.
Contact Lens Sugar Sensing.
Doped contact lenses, which were previously washed and allowed to leach excess dye for 1 h, were inserted in the contact lens holder (Figure 3). Buffered solutions of sugars were then added to the lens, which were also similarly buffered in the 1.5 cm3 cell volume. Fluorescence spectra were typically taken 15 min after each sugar addition to allow the lens to reach equilibrium.
Stilbene Derivatives.
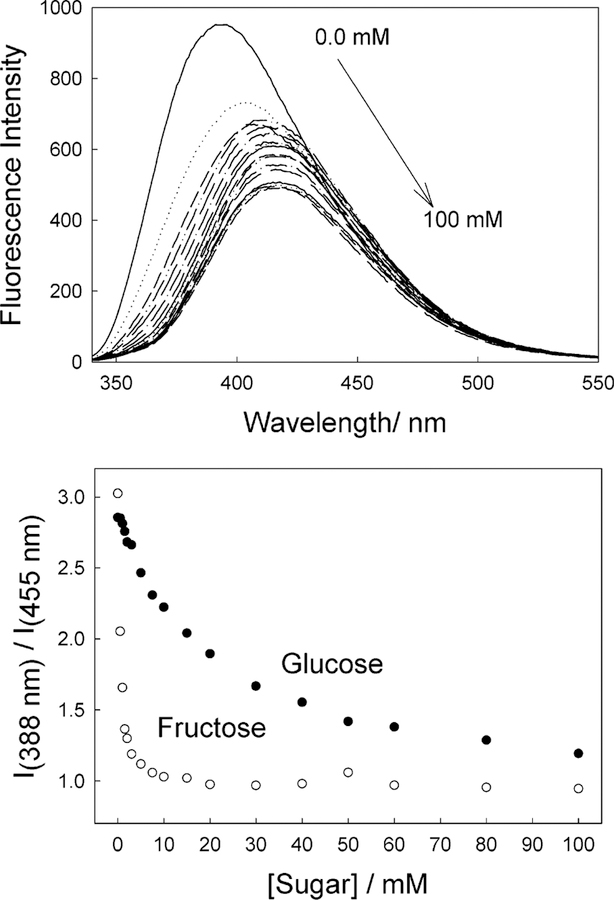
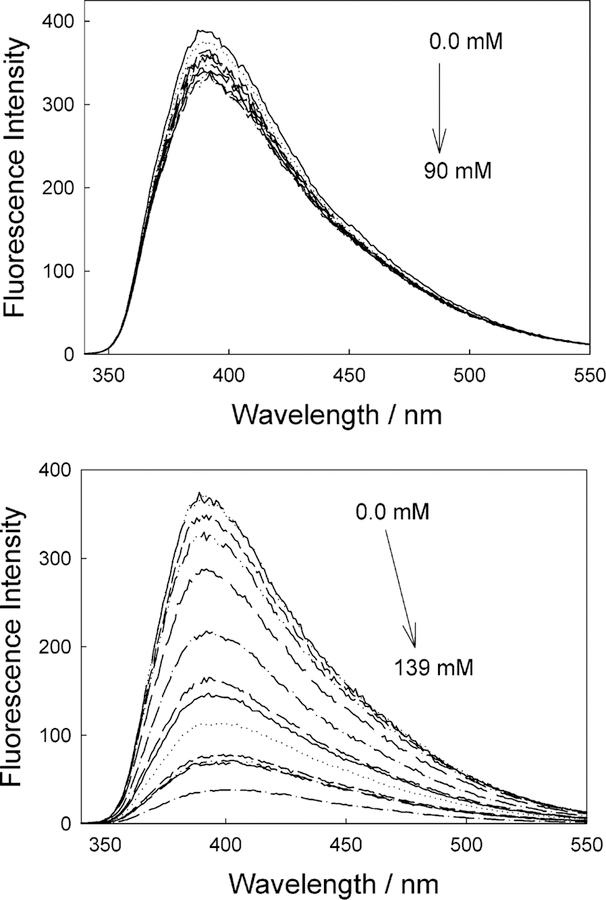
Figure 6 shows the emission spectra and response of a DSTBA-doped contact lens toward both glucose and fructose. As expected, the magnitude of the response toward fructose is greater, reflecting the higher affinity of monoboronic acids for fructose.41 Comparing the response of DSTBA in both solution and lens (Figure 4, top, and Figure 6, bottom, respectively), we can see that an opposite response is observed in the lens, where the emission spectra similarly shows a blue shift, accompanied by a decrease in intensity as the fructose concentration is increased. In addition, the sugar affinity is decreased slightly in the lens.
Figure 6.
Emission spectra of the DSTBA-doped contact lens, pH 8.0 buffer/methanol (2:1), with increasing concentrations of glucose, λex = 340 nm (top), and the emission spectra of the DSTBA-doped contact lens, pH 8.0 buffer/methanol (2:1), with increasing concentrations of fructose, λex = 340 nm (bottom).
Similarly, Figure 7 shows the response of CSTBA in the lens for both glucose and fructose. Although a similar reduction in intensity is observed as compared to solution, no red shift in the emission is observed, indicative of a reduction in the electron-donating capability of the anionic sugar-bound form.
Figure 7.
Emission spectra of the CSTBA-doped contact lens, pH 8.0 buffer/methanol (2:1), with increasing concentrations of glucose, λex = 320 nm (top), and the emission spectra of the CSTBA-doped contact lens, pH 8.0 buffer/methanol (2:1), with increasing concentrations of fructose, λex = 320 nm (bottom).
The lack of suitable spectral shifts in the presence of sugar eliminates at this stage the possibility of wavelength ratiometric sensing, as shown for the solution-based measurements in Figures 4 and 5, bottom. Subsequently, Supporting Information Figure S3 compares the responses of the stilbene probes based on a simple intensity ratio measurement. It is interesting to see the much greater response for fructose for CSTBA in the lens, as compared DSTBA, for which notable changes in intensity occur at <20 mM [fructose]. However, the glucose response of DSTBA in the contact lens appears more promising for [glucose] < 10 mM, for which a 10% fluorescence intensity change is observed for ~10 mM glucose at pH 8.0.
Polyene Derivative.
The spectral response of DDPBBA in the contact lens is also different from that observed in solution (cf. Supporting Information Figure S1 and Figure 8), where a decrease in intensity is typically observed for increasing sugar concentration, and a slight blue shift is evident for fructose binding. This is in contrast to solution-based responses, which show both a blue-shifted and increased emission (Supporting Information Figure S1). Although the general spectral changes observed for both DSTBA (Figure 6) and DDPBBA (Figure 8) are similar, a greater dynamic response to sugar is observed for DSTBA, as compared to DDPBBA (cf. Supporting Information Figure S3, top, and Figure 8, bottom). In addition, the response of DDPBBA to both glucose and fructose is similar over the sugar concentration range studied (Figure 8, bottom), as compared to the significantly different responses observed for both sugars for DSTBA and CSTBA (Supporting Information Figure S3)
Figure 8.
Emission spectra of the DDPBBA-doped contact lens, pH 8.0 buffer/methanol (2:1), with increasing concentrations of glucose, λex = 340 nm (top); the emission spectra of the DDPBBA-doped contact lens, pH 8.0 buffer/methanol (2:1), with increasing concentrations of fructose, λex = 340 nm (middle); and the intensity ratio plot for the DDPBBA-doped lens toward both glucose and fructose, where I and I0 are the intensities in the presence and absence of sugar, respectively, at λem max (bottom).
Chalcone Derivatives.
The responses of Chalc 1- and Chalc 2-doped contact lenses toward sugar are shown in Figure 9 and Supporting Information Figure S4, respectively. Both chalcone-doped lenses display similar responses to sugar (Figures 9 and S4, bottom, respectively); only their respective emission wave-lengths differ. Chalc 1 shows an emission centered around 560 nm in the lens, as compared to 580 nm in solution (not shown), whereas Chalc 2 shows an emission centered at ~630 nm, as compared to 665 nm in solution (Supporting Information Figure S2). In contrast to the responses observed in solution, a reduction in fluorescence intensity is observed for both Chalc 1- and 2-doped contact lenses (Figures 9 and S4, respectively). Interestingly, the solution response for Chalc 2 toward 100 mM fructose at pH 8.0 produces an ~3-fold increase in fluorescence emission (Supporting Information Figure S2), as compared to the 2.6-fold reduction for the same fructose concentration in the contact lens.
Figure 9.
Emission spectra of the Chalc 1-doped contact lens, pH 8.0 buffer/methanol (2:1), with increasing concentrations of glucose, λex = 430 nm (top); the emission spectra of the Chalc 1-doped contact lens, pH 8.0 buffer/methanol (2:1), with increasing concentrations of fructose, λex = 430 nm (middle); and the intensity ratio plot for the Chalc 1-doped lens toward both glucose and fructose, where I and I0 are the intensities in the presence and absence of suga, respectively, at λem,max (bottom).
Probe Leaching from the Contact Lens.
To ascertain the practical use of a glucose-sensing contact lens, leaching studies of the probes from within the lens were undertaken, Figure 10. Due to the very low concentration of probes within the lens, absorbance measurements could not be used to track the amount of unleached dye. As a result, we tracked the percent loss of fluorescence emission from the lens as a function of time. Although it could be argued that this method is problematic—for example, dye could have a different quantum yield inside and outside the lens—this method was used to simply give an indication of how long we needed to preleach the lenses before use, as well as to provide general information on the dye—lens compatibility.
Figure 10.
Static leaching of the various doped contact lenses as a function of time, inferred from the change in total fluorescence intensity of the lens vs time. 1, DSTBA; 2, CSTBA; 3, DDPBBA; 4, Chalc 1; and 5, Chalc 2.
Leaching experiments were also performed in the presence of sugar, and we can report similar leaching rates for the BAFs-sugar complexes.
DISCUSSION
It is generally found that when designing and fabricating plastic sensors, both the transduction element and polymeric support are either chosen simultaneously on the basis of the properties of both, or a support is found that is compatible with the sensing probes and the environment to be sensed. Seldom do we see a sensor whose transduction elements are chosen on the basis of the merits of the polymeric support alone. However, because the physiological compatibility of disposable plastic contact lenses has already been assessed and optimized with regard to vision correction, size, and oxygen/analyte permeability, etc., we are encouraged to design and fabricate a glucose-sensing contact lens starting with the unmodified polymeric support. Consequently, we chose a range of water-soluble BAFs that have been previously synthesized and well-characterized by our laboratory and others36–39,42–46 to assess the feasibility of a glucose-sensing contact lens based on BAFs.
The initial reports and binding constants of these BAFs suggested the possibility of detecting submillimolar glucose concentrations,36–39,42–46 which is required for tear glucose monitoring.12–14,19,47 However, we have found the response of the BAFs was notably different in the lens as compared to the solution, although given their spectral differences alone, then this does not preclude their use in a glucose-sensing contact lens.
For most of the BAFs studied, the response to sugars in the lens was opposite to that observed in solution. To understand these changes and, therefore, characterize the contact lens environment for further sensor development, we assessed the response of the BAFs in solution in different pH and polarity media.
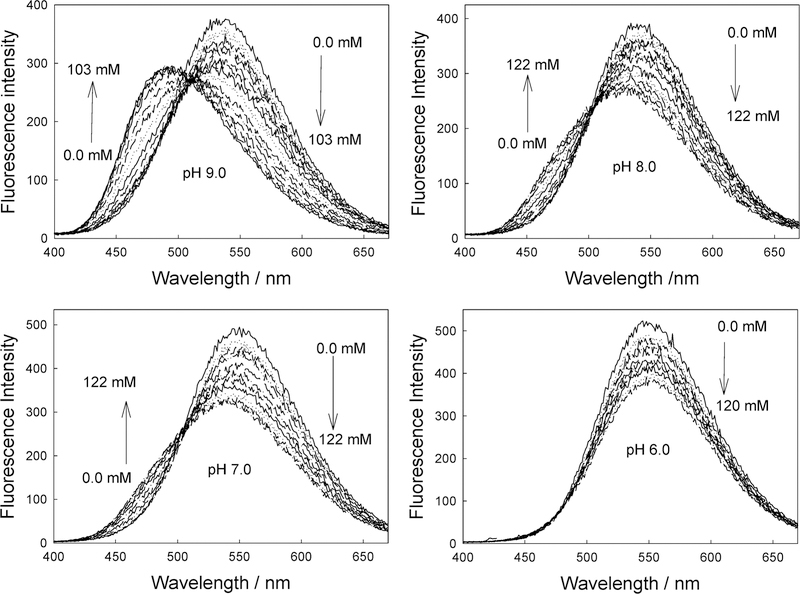
Figure 11 shows the emission spectra of DDPBBA in different pH media in the presence of increasing glucose concentrations. Interestingly, we were able to observe a similar spectral response to glucose in pH 6.0 media as compared to the contact lens (cf. Figure 11 and Figure 8, top), although the wavelength emission maximums are somewhat different, suggesting more than just a pH effect. At this pH, it is thought that the sugar-bound form would be dominant, pKa ~ 6. Indeed, in a study of all the probes in both solution and in the contact lens, we observed lens responses that were not identical to those observed in any pH solution, again suggesting an additional effect is also playing a role on the BAFs spectral response to sugar in the contact lens. To investigate lens pH further, we doped a contact lens with the well-known pH-sensitive probe, fluorescein,48 and measured the fluorescein lifetime(s) in the lens, determined using the time-correlated single photon counting technique.48 A comparison with the lifetimes obtained for fluorescein in different pH buffers led us to choose a lens pH in the range 5.5–6.5. Surprisingly, externally buffering the lens had little effect on the fluorescein lifetime and, therefore, lens pH. Although it could be argued that fluorescein could sample a different microregion of the lens not accessible to external buffer, our fluorescein pH lens results are also consistent with the BAFs lens results, which all have different molecular structures and are also likely to probe different lens microdomains. Indeed, the leaching results have shown different BAF diffusion rates (Figure 10), suggesting diffusion from different microdomains, given that the molecular structures, and therefore solution diffusion rates, are likely to be similar. Hence, there is strong evidence for a contact lens pH in the range 5.5–6.5.
Figure 11.
Emission spectra of DDPBBA in different pH media (buffer/methanol 2:1) with increasing glucose concentrations.
To assess the polarity within the contact lens also, we measured the intensity ratio of the 0,0 (or I1) and 0,2 (or I3) bands of a pyrene-doped lens, in which the intensity ratio of pyrene fluorescence bands is widely used to estimate the polarity of media, such as in micelles.49,50 The lens was postdoped with pyrene by immersing the lens in a pyrene buffer methanol solution (pH 8.0, 2:1 v/v) for 1 h, then rinsed extensively with Millipore water. The estimated value of I1/I3 was ~1.28, indicating the polarity within the lens is not different from that of methanol (I1/I3 for MeOH ) 1.33).50 In retrospect, this was not surprising, given that the contact lens is PVA-based.
By determining both the pH and polarity within the contact lens, it is possible to rationalize the different spectral responses we have observed as compared to solution. As the solution pH increases, the emission spectrum of DDPBBA displays a large blue shift (Figure 11). These spectral changes induced by the pH are due to the formation of the anionic form of the boronic acid group (form 2 in Figure 1). As the anionic boronate species is formed, the boron group is no longer an electron-withdrawing group, resulting in the removal, perturbation, or both of the charge-transfer nature of the excited state. An important feature here is the change in acidity (electrophilicity) of the boron group between the uncomplexed and complexed forms. Indeed, this acidity change is the driving force enabling the use of the boronic acid moiety for sugar sensing. At a lower pH (such as in the contact lens), the simple complexation of the boronic acid with sugar (the equilibrium between species 1 and 3 in Figure 1) does not fully result in a perturbation of the fluorophore; hence, DDPBBA is not suitable as a wavelength ratiometric probe in the contact lens. The same, however, would be true at a much higher pH also, that is, the equilibrium between species 2 and 4, Figure 1. To induce a spectral change of the fluorophore, the complexation of the BAFs with sugar should result in a perturbation of the electronic properties of the fluorophore, that is, from the neutral (1 in Figure 1) to the anionic form (4 in Figure 4). As was briefly mentioned in the Introduction, these BAFs typically display pKa around 9,41 with a pKa ~ 6 for the sugar-complexed form41 (Figure 1). Hence, these probes are ideal for solution sugar sensing in the pH range 6.5–8.5, which for blood glucose levels is ideal,47 in which the maximum spectral change is usually observed in the pH range 7–7.5. However, the low-pH nature of the contact lens limits the spectral changes and, therefore, the dynamic range for tear glucose sensing. In addition, these probes are polarity-sensitive. For DSTBA and DDPBBA, as the polarity of the solvent increases, a red-shifted emission band can be observed (see Figure 3 in ref 41), which accounts for the emission maximum difference between DDPBBA in the contact lens (Figure 8, top, and in pH 6 solution, Figure 11). A similar rationale can also be drawn for the other BAFs considered here.
On the basis of these findings it appears that to observe suitable spectral responses in the presence of sugars within the contact lens and, therefore, to maximize the dynamic range for tear glucose sensing, then either a method of controlling the pH within the lens must be adopted, noting that our attempts of external buffering were unsuccessful, or probes with a lower pKa need to be used. Given that BAFs have mostly been designed for blood glucose measurements at pH ~ 7.5,41 it is likely that we will need to both design and synthesize suitable BAFs. Work is currently underway in our laboratory and will be reported in due course.
5. CONCLUSIONS
We have tested the feasibility of physiological glucose sensing in tears, which is known to track blood glucose levels ~10-fold, using a disposable and off-the-shelf plastic contact lens. By embedding boronic acid-containing fluorophores within a contact lens, whose physiological compatibility has been predetermined by the fact that they are readily available in most pharmacies, we are able to readily determine glucose concentrations <10 mM, in which the blood glucose level is 3–8 mM for a healthy person and increases to between 2 and 40 mM in diabetics. For tear glucose, these values are typically 10-fold lower.
Although the solution response of the boronic acid-containing fluorophores shows the feasibility of determining the required low glucose concentrations, the low contact lens pH and methanol-like polarity significantly reduce the dynamic range for sensing. Subsequently, we can conclude that this approach for the potential continuous monitoring of glucose is feasible if suitable boronic acid-containing fluorophores can be designed to respond well in the contact lens microenvironment. We also believe that this approach may well offer an alternative sensing platform to current invasive glucose monitoring techniques, in which simple spectroscopy can be used to determine the response of suitable fluorescent probes toward glucose in the contact lens within the eye. In addition, the notion of using a contact lens to sense glucose is likely to be received well by diabetics, because many have eye disorders and require vision correction, which is thought to be due to glycosylation of protein in blood vessels.48
In addition to tear glucose, other tear analytes could also be determined by the incorporation of suitable transduction elements within contact lenses, such as chloride or even cholesterol. Further reports of physiological analyte monitoring using this noninvasive contact lens approach will be reported by our laboratory in due course.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge financial support from CIBA Vision, Atlanta, GA, and informative discussions with Dawn Smith and Larry Chappoy, also of CIBA Vision.
ACRONYMS AND SYMBOLS
- BA
boronic acid
- BAFs
boronic acid containing fluorophores
- CT
excited-state charge transfer
- Chalc 1
3-[4′(dimethylamino)-phenyl]-1-(4′-boronophenyl)-prop-2-en-1-one
- Chalc 2
5-[4′-(dimethylamino)phenyl]-1-(4′-boronophenyl)-pent-2,4-dien-1-one
- DSTBA
4′-dimethylaminostilbene-4-boronic acid
- CSTBA
4′-cyanostilbene-4-boronic acid
- DDPBBA
1-(p-boronophenyl)-4-(p-dimethylaminophenyl)buta-1,2-deine
- PVA
polyvinyl alcohol
- TCSPC
time-correlated single photon counting
Footnotes
SUPPORTING INFORMATION AVAILABLE
Emission spectra and intensity ratio plots. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Robinson MR; Eaton RP; Haaland DM; Koepp GW; Thomas EV; Stallard BR; Robinson PL Clin. Chem 1992, 38, 1618–1622. [PubMed] [Google Scholar]
- (2).Heise HM; Marbach R; Koschinsky TH; Gries FA Ann. Occup. Hyg 1994, 18, 439–447. [DOI] [PubMed] [Google Scholar]
- (3).March WF; Rabinovitch B; Adams R; Wise JR; Melton M Trans. Am. Soc. Artif. Intern. Organs 1982, 28, 232–235. [PubMed] [Google Scholar]
- (4).Rabinovitch B; March WF; Adams RL Diabetes Care 1982, 5, 254–258. [DOI] [PubMed] [Google Scholar]
- (5).Schier GM; Moses RG; Gan IET; Blair SC Diabetes Res. Clin. Pract 1988, 4, 177–181. [DOI] [PubMed] [Google Scholar]
- (6).Clarke W; Becker DJ; Cox D; Santiago JV; White NH; Betschart J; Eckenrode K; Levandoski LA; Prusinki EA; Simineiro LM; Snyder AL; Tideman AM; Yaegar T Diabetes Res. Clin. Pract 1988, 4, 209–214. [DOI] [PubMed] [Google Scholar]
- (7).Trettnak W; Wolfbeis OS Anal. Chim. Acta 1989, 221, 195–203. [Google Scholar]
- (8).Meadows D; Schultz JS Talanta 1988, 35, 145–150. [DOI] [PubMed] [Google Scholar]
- (9).Tolosa L; Malak H; Rao G; Lakowicz JR Sens. Actuators, B 1997, 45, 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Tolosa L; Gryczynski I; Eichorn LR; Dattelbaum JD; Castellano FN; Rao G; Lakowicz JR Anal. Biochem 1999, 267, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).D’Auria S; Dicesare N; Gryczynski Z; Gryczynski I; Rossi M; Lakowicz JR Biochem. Biophys. Res. Commun 2000, 274, 727–731. [DOI] [PubMed] [Google Scholar]
- (12).Daum KM; Hill RM Invest. Ophthalmol. Vis. Sci 1982, 22 (4), 509–514. [PubMed] [Google Scholar]
- (13).Gasser AR; Braverman LE; Fleming MC; Arky RA; Alter BR Am. Ophthalmol. J 1968, 65 (3), 414–420. [DOI] [PubMed] [Google Scholar]
- (14).Das BN; Sengupta S; Das BK; Goswami NR J. Indian Med. Assoc 1995, 93 (4), 127–128. [PubMed] [Google Scholar]
- (15).Giardiai A; Roberts JR E. Br. Ophthalmol. J 1950, 34, 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Ridley F Br. J. Exp. Pathol 1930, 11, 217–222. [Google Scholar]
- (17).Michail D; Zolog NCR Soc. Biol., Paris 1937, 126, 1042. [Google Scholar]
- (18).Michail D; Vancea P; Zolog NCR Soc. Biol., Paris 1937, 125, 1095. [Google Scholar]
- (19).Van Haeringen NJ Surv. Ophthalmol 1981, 29 (2), 84–96. [DOI] [PubMed] [Google Scholar]
- (20).Sugihara JM; Bowman CM J. Am. Chem. Soc 1958, 80, 2443. [Google Scholar]
- (21).Lorand JP; Edwards JO J. Org. Chem 1959, 24, 769. [Google Scholar]
- (22).Spingsteen G; Wang B Tetrahedron 2002, 38, 5291. [Google Scholar]
- (23).James TD; Sandanayake KRAS; Shinkai S Nature 1995, 374, 345. [Google Scholar]
- (24).Norrild JC; Eggert HJ Am. Chem. Soc 1995, 117, 1479. [Google Scholar]
- (25).Eggert H; Frederiksen J; Morin C; Norrild JC J. Org. Chem 1999, 64, 3846. [Google Scholar]
- (26).Yang W; He H; Drueckhammer DG Angew. Chem., Int. Ed 2001, 40, 1714. [PubMed] [Google Scholar]
- (27).Wang W; Gao S; Wang B Org. Lett 1999, 1, 1209. [DOI] [PubMed] [Google Scholar]
- (28).Gao S; Wang W; Wang B Bioorg. Chem 2001, 29, 308. [DOI] [PubMed] [Google Scholar]
- (29).Lavigne JJ; Anslyn EV Angew. Chem., Int. Ed 1999, 38, 3666. [PubMed] [Google Scholar]
- (30).Yoon J; Czarnik AW J. Am. Chem. Soc 1992, 114, 5874. [Google Scholar]
- (31).Smith BD; Gardiner SJ; Munro TA; Paugam MF; Riggs JA J. Inclusion Phenom. Mol. Recognit. Chem 1998, 32, 121. [Google Scholar]
- (32).Soundararajan S; Badawi M; Kohlrust CM; Hagerman JH Anal. Biochem 1989, 178, 125. [DOI] [PubMed] [Google Scholar]
- (33).James TD; Sandanayake KRAS; Shinkai S Angew Chem. Int. Ed., Engl 1994, 33, 2207. [Google Scholar]
- (34).James TD; Sandanayake KRAS; Iguchi R; Shinkai SJ Am. Chem. Soc 1995, 117, 8982. [Google Scholar]
- (35).Bielecki M; Eggert H; Norrild JCJ Chem. Soc., Perkin Trans 1999, 2, 449. [Google Scholar]
- (36).Dicesare N; Lakowicz JR Anal. Biochem 2001, 294, 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Dicesare N; Lakowicz JR J. Photochem. Photobiol., A 2001, 143, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Dicesare N; Lakowicz JR Org. Lett 2001, 3 (24), 3891–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Dicesare N; Lakowicz JR Tetrahedron Lett 2002, 43, 2615–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Karnati VV; Gao X; Gao S; Yang W; Ni W; Sankar S; Wang B Bioorg. Med. Chem. Lett 2002, 12, 3373–3377. [DOI] [PubMed] [Google Scholar]
- (41).Dicesare N; Lakowicz JR Biomed. Opt. J 2002, 7 (4), 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Dicesare N; Lakowicz JR J. Phys. Chem. A 2001, 105, 6834–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Dicesare N; Lakowicz JR Chem. Commun, 2001, 2022–2023. [DOI] [PMC free article] [PubMed]
- (44).Diceasre N; Pinto MR; Schanze KS; Lakowicz JR Langmuir 2002, 18, 7785–7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Dicesare N; Adhikari DP; Heynekamp JJ; Heagy MD; Lakoiwcz JR Fluoresc. J 2002, 12 (5), 147–154. [PMC free article] [PubMed] [Google Scholar]
- (46).Cao H; Diaz DL; Dicesare N; Lakowicz JR; Heagy MD Org. Lett 2002, 4 (9), 1503–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Chen R; Jin Z; Colon LA J. Capillary Electrophor 1996, 5, 243–248. [PubMed] [Google Scholar]
- (48).Lakowicz JR Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer/ Academic Plenum Publishers: New York, 1997. [Google Scholar]
- (49).Turro NJ; Baretz BH; Kuo PI Macromolecules 1984, 17, 1321. [Google Scholar]
- (50).Kalyanasundaram K; Thomas JK J. Am. Chem. Soc 1977, 99, 2039. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.