Abstract
Loss of immunoreactivity in tissue sections has been shown to occur when slide sections are stored at room temperature for prolonged periods of time. We conducted a systematic investigation to determine the extent of staining loss in various storage conditions to determine an optimal storage method. We investigated 6 antibodies that are commonly used for breast cancer subtyping in research studies with immunohistochemistry (ER, PR, HER2, CK5/6, EGFR, and Ki67) in formalin-fixed paraffin-embedded breast tissue microarrays (TMAs) consisting of 148 patients. TMAs were sectioned at various time points: fresh, 1 week, 1 month, 6 months, and 12 months prior to staining. Slides sectioned at each time point were stored in 5 storage conditions: desiccator, paraffin dipped, 4°C, −20°C, and −80°C. IHC scores were assessed over time with McNemar’s test and Bowker’s Test of Symmetry. Desiccator storage was the only storage condition that did not show any loss in immunoreactivity for any antibody or time point in our study. Paraffin coated slides were the most difficult storage method operationally and also showed the most loss in immunoreactivity. Storing sections in a desiccator was the most effective method for minimizing immunoreactivity loss. Cold storage at 4°C is an intermediate option that is not as protective as a desiccator, but offers the advantage of being accessible to virtually all research labs.
Keywords: antigenicity, immunoreactivity, immunohistochemistry, stored sections, stored slides
Introduction
Immunohistochemistry (IHC) is a well-established method for the detection of protein expression in human tissues. It is quick, cost effective, and allows for evaluation within a tissue’s morphological context, justifying its routine use for the detection of biomarkers for cancer diagnosis, prognosis, staging, and therapeutic intervention, as well as research studies. The outcome of any given IHC assay is dependent on several conditions in a multistep process beginning with tissue acquisition in the operating room.
Several pre-analytical variables, such as cold ischemia time, type of fixative, fixation duration, processing and embedding, and post-sectioning storage have the potential to influence the final result of an IHC reaction1–4. While progress has been made over the years to standardize pre-analytic factors5,6 (College of American Pathologists, National Cancer Institute Best Practices for Biospecimen Resources, Clinical and Laboratory Standards Institute), one variable that is often overlooked in many research studies is the effect of storage on sectioned slides.7
It is common for sectioned slides to be stored for extended periods in research studies, especially in large multi-center epidemiological studies or clinical trials where a central laboratory amasses a large amount of slides over time to then evaluate in batch IHC assays once all materials are obtained. Given that many hospitals do not readily send patient blocks, sections are sent instead, and since the coordination of slide acquisition from many hospitals takes time, a situation arises in which the sections that were collected earlier are stored much longer than those that arrive later. This heterogeneous material quality may lead to spurious results if slides experience antigen degradation. If immunoreactivity is lost over time with slide storage, there could be a skew toward less positive results in older sections, with the worst case scenario being a false negative result.
The notion that storing tissue sections for a long period of time may negatively impact the quality of IHC results is not new. Reports of the problem date back to the early 1990s8 where it was reported in studies of breast cancer that immunoreactivity was weaker, and in some cases lost altogether, when slides were stored at room temperature prior to immunostaining.9 This general result has been both confirmed and disputed over the years, with an overall consensus that prolonged storage at room temperature is suboptimal7,10–14. Many alternatives to storage at room temperature have been proposed including vacuum storage, paraffin coating tissues after sectioning, storage at 4°C, adding antioxidants to paraffin, and storage in a nitrogen chamber, but an optimal alternative storage environment is not known or practiced consistently in the field.2
The purpose of this study was to evaluate, when storage is unavoidable in research studies, what storage conditions are best and minimize the loss of immunoreactivity for IHC assays. We included freezing storage conditions as previous reports that assess freezing on stored sections are uncommon and/or had small sample sizes.15,16 Moreover, freezer storage appears promising because immunoreactivity has been shown to decline with increasing storage temperature.4,15–17 We evaluated five storage conditions at 4 time points spanning one year and two freezing conditions, −20°C and −80°C; two 4°C conditions (paraffin coated sections vs. uncoated), and a desiccator at room temperature. We investigated six markers (ER, PR, HER2, Ki67, EGFR, CK5/6) that are commonly used for subtyping breast cancer and therefore have particular importance for epidemiological research studies of breast cancer.18,19
Methods
Study Population and specimens
Formalin-fixed, paraffin-embedded (FFPE) tumor samples from 148 breast cancer patients were evaluated for 6 common markers of breast cancer that are being evaluated for the African American Breast Cancer Epidemiology and Risk (AMBER) Consortium.20 The biospecimens used for this study were collected at Roswell Park Comprehensive Cancer Center following clinical protocols and the use of these specimens was approved by the Institutional Review Board at Roswell Park.
TMA construction and preparation of sections
Two TMAs were constructed, one to be stained with ER, PR, and HER2 (TMA 1); the 60 cases for this TMA were chosen because they were positive for these markers as indicated by the patients’ medical record. The second TMA (TMA 2) consisted of 88 patients (90 cases) who had triple negative breast cancer, and this TMA was stained with Cytokeratin 5/6, EGFR, and Ki67, markers for basal-like breast cancer.21 Intensity scores and percent positivity from the patient medical record are provided for all cases where it was reported in Supplemental Table 1. Three 0.6-millimeter FFPE tumor tissue cores per patient were arrayed into a recipient paraffin block guided by a pathologist (W.B.).
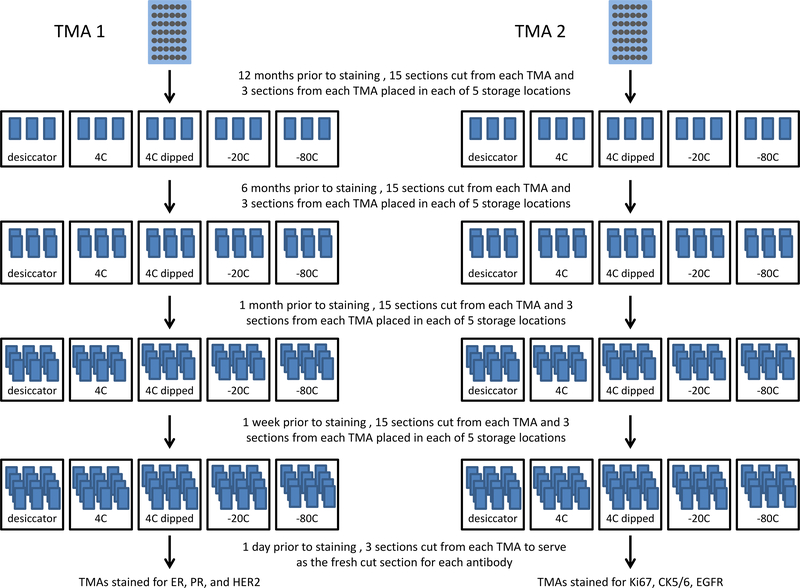
TMAs were sectioned at 4 μm and stored at different time points: 12 months, 6 months, 1 month, and 1 week prior to IHC staining. All stored sections were compared to a freshly cut TMA section that was sectioned the day before staining. For each time point, sets of paraffin slides were sectioned for each of six markers for each of the following storage conditions: 1) 4°C, 2) 4°C and paraffin dipped, 3) −20°C, 4) −80°C, and 5) desiccator at room temperature (Terra Universal Desiccator, Model 3950–06A, Fullerton, CA USA). Ameraffin® (Cardinal Health, Dublin, OH) embedding medium was used for paraffin dipping and the detailed procedure is provided in the supplemental materials. Exposure to light did not occur in any storage condition. All TMA sections were cut by the same person using the same microtome throughout the study. We did not repeat the room temperature (without desiccator) condition because TMA sections were limited, we wanted to maximize the number of storage conditions for comparison, and much previous work has addressed the room temperature storage condition specifically and shown that it is suboptimal7,10–14. An overall schematic of the study design for TMA section storage is shown in Figure 1.
Figure 1.
Overall study design for tissue microarray (TMA) section storage. TMA 1, stained for ER, PR, and HER2, contained 60 patients that were chosen because they were positive for these markers in the patients’ medical record. TMA 2 consisted of 88 patients with triple negative breast cancer, and this TMA was stained with Cytokeratin 5/6, EGFR, and Ki67. All stored sections were compared to a fresh section that was cut one day prior to staining.
Immunohistochemistry
Prior to immunostaining, TMAs were removed from storage and dried at 60°C for one hour with the exception of the paraffin dipped TMAs that were dried overnight so that the excess paraffin was completely removed. For each antibody stain, slides stored at different time points and fresh cut slides were stained for each antibody in a single batch at the end of the study. All slides were stained on the Dako Omnis autostainer (Dako, Glostrup, Denmark), which has temperature and humidity controls, and is a fully automated staining process. On the Omnis, an onboard antigen retrieval step was used for all markers (Table 1). Endogenous peroxidases were quenched with EnVision™ FLEX peroxidase-blocking reagent (Dako), slides were rinsed with wash buffer (Dako), and then monoclonal primary antibody (Table 1) was applied. The EnVision™ FLEX DAB+ Chromogen System (Dako) was used for visualization.
Table 1.
Antibody and staining information. Abbreviations: HIER - heat-induced epitope retrieval.
| Antibody name | Clone | Vendor | Catalog No. | Concentration (μg/ml) | TMA tested | Antigen Retrieval |
|---|---|---|---|---|---|---|
| Cytokeratin 5/6 | D5/16 B4 | Dako | M7237 | 0.9 | TMA #2 | HIER, pH 9 |
| EGFR | 31G7 | Invitrogen | 28–0005 | 0.6 | TMA #2 | Proteinase K |
| ER | SP1 | Cell Marque | 249R-15 | 0.5 | TMA #1 | HIER, pH 9 |
| HER2 | CB11 | BioGenex | MU134-UC | 0.2 | TMA #1 | HIER, pH 9 |
| Ki67 | MIB-1 | Dako | GA626 | Ready to use | TMA #2 | HIER, pH 6 |
| PR | Y85 | Cell Marque | 323R-15 | 0.4 | TMA #1 | HIER, pH 9 |
Positive control tissues consisted of tissues of cases at Roswell Park with a known score, determined for each antibody with a regimented series of testing and validation prior to this study. These control tissues were run alongside the TMAs and then compared with our reference control slides (cataloged from original antibody validation) to ensure that staining was accurate. Positive control slides matched the corresponding reference slides in intensity for each antibody in our study. A duplicate slide with control tissue that had all reagents applied except the primary antibody served as a negative control.
Slide Scanning and Digital Image Analysis
Slides were digitally scanned using Aperio ScanScope XT (Leica Biosystems, Inc., Buffalo Grove, IL) with 20x bright-field microscopy and then Aperio ImageScope version 12.2.1.5005 (Leica Biosystems, Inc., Buffalo Grove, IL) was used to review images for quality. Cores were excluded if they were folded or >75% of the tissue was absent22. An annotation layer was created for each core of interest in the TMA. Under the guidance of a pathologist, representative areas of invasive tumor cell‐only regions were manually circled using the pen tool.
The Aperio platform was used to develop quantitative image analysis algorithm macros for the quantification of IHC slides. Briefly, these algorithms used color de‐convolution to separate diaminobenzidine (DAB) from the hematoxylin counterstain thereby providing stain separation. Each algorithm was tailored to fine-tune the cell feature detection using cellular, nuclear, and stain parameters, creating an algorithm macro based on the cell compartment location of the target protein.
For ER, PR and Ki67 stains, the Aperio Nuclear V9 algorithm macro was used. For ER and PR antibodies, cell nuclei were individually classified as 0 -no staining, 1+ -weak, 2+ -moderate, and 3+ -strong. The analysis results provided the total number of detected cells, the percentage of cells per class (0, 1+, 2+ and 3+) and the percentage of positive stained cells along with the average staining intensity of the positive nuclei as a score of 0, 1+, 2+ and 3+. H-scores, which measure the extent of immunoreactivity, were calculated for ER and PR. H-scores were obtained by the formula 1×(%1+) + 2×(%2+) + 3×(%3+) and ranges from 0 to 300, where 300 represents 100% of cells being 3+. For Ki67, nuclear intensity was calculated as either negative 0 or positive 1+; the total number of detected cells and the percentage of positively stained cells were reported.
The Aperio Membrane V9 algorithm macro was used for HER2 and EGFR to detect membrane staining for individual tumor cells and to quantify the intensity and completeness of membrane staining. Tumor cells were individually classified as 0, 1+, 2+, and 3+ based on their membrane staining intensity and completeness, using the HER2 scoring scheme: score 0 = no staining or unspecific staining of tumor cells; score 1 = weak (intensity) and incomplete staining (quality) for more than 10% of tumor cells (quantity); score 2 = moderate and complete staining for more than 10% of tumor cells; score 3 = strong and complete staining for more than 10% of tumor cells. The analysis results provided the total number of detected cells, the percentage of cells per class (0, 1+, 2+ and 3+), and the percentage and number of cells with complete membrane staining.
The Aperio Cytoplasmic V2 algorithm macro was used for CK5/6 to detect and quantify the positive staining intensity and the percentage of cells that stained. Cells were individually classified using the scoring scheme 0 -no staining, 1+ -weak, 2+ -moderate, and 3+ -strong. The algorithm results included the total number of cells, percentage per scoring class, average positive intensity (as RGB value) and H-score.
TMA Core-to-case collapsing and positivity thresholds
We used a tumor cellularity-weighted approach to determine a single IHC score for cases that had at least two TMA cores; this method has been shown to diminish the influence of small discordant regions.22,23 The weighted approach sums the product of percent positivity and core weight for all cores per case, where core weight is the number of tumor nuclei in a given core divided by the total number of tumor nuclei for all cores for that case.
Thresholds for positivity for ER and PR adhered to current clinical guidelines that use 1% as a cutoff for determining positivity for these markers.24 Using clinical scoring criteria, case-level HER2 status was defined as positive (3+; ≥10% of tumor cells staining at the 3+ intensity level), equivocal (2+; <10% of tumor cells staining at the 3+ intensity level and ≥10% of tumor cells staining at the 2+ intensity level), or negative25 (0/1+; all other cases); this was also applied to EGFR that stains in a manner similar to HER2. A 14% cutoff was applied for Ki67,22 and 1% cutoff was used for CK5/6. Stains were also evaluated with H-score treated as a continuous variable but the overall results were unchanged (data not shown).
Statistical analysis
Statistical analyses were conducted with SAS version 9.3 (SAS Institute). Following Fergenbaum et al. 2004, for ER, PR, CK5/6, and Ki67, immunoreactivity in stored and fresh sections was compared in paired 2 × 2 tables using McNemar’s test.10 For a given storage condition, agreement between each time point and fresh sections was assessed using unweighted K statistics and corresponding 95% confidence intervals. Because three categories of staining intensity were scored for HER2 and EGFR, 3 × 3 tables were used, and the p-values correspond to a generalization of McNemar’s test called Bowker’s Test of Symmetry. All p-values were two sided.
Results
Percentage of Positive Results in Stored and Fresh TMA Sections
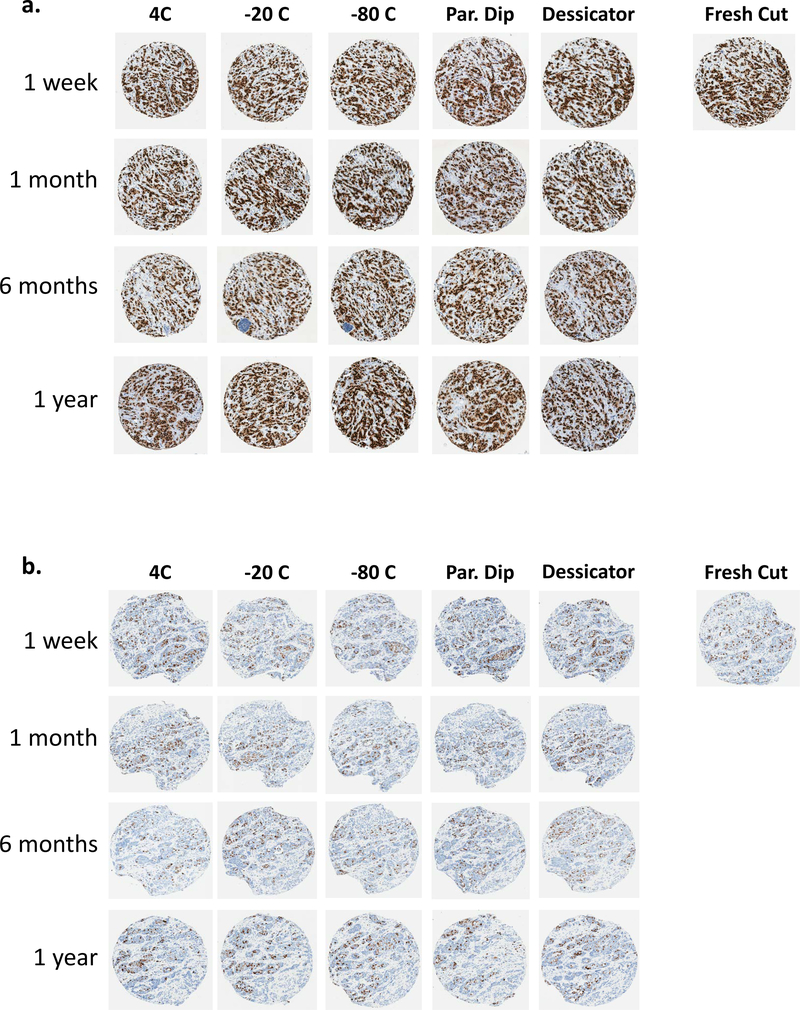
Fresh cut sections were used as the reference to compare all stored slides. The cross-classification of stored and fresh sections stained for all six antibodies is shown fully in Supplemental Tables 2 and 3. For ER, PR, and HER2, the number of positive cases was similar for fresh cut and stored slides, regardless of the storage condition or duration of storage. Significantly fewer cases were classified as Ki67 positive for the paraffin dipped condition stored for 1 month than the fresh cut (57.8% versus 73.3%, P = 0.02, Table 2) and paraffin dipped slides were also shown to have significantly less immunoreactivity for EGFR at the 1 week, 1 month, and 6 month time points (Table 2). However, at the 12 month storage time point, where a continuation of this trend is expected for EGFR, no significant difference was observed between the stored and fresh sections (Supp. Table 3). Unexpectedly, the slides stored for only one week showed more negativity than all other longer storage time points for CK5/6 (Table 2). Representative cores from TMA 1 and TMA 2 for two antibodies (ER and Ki67) are shown in Figure 2.
Table 2.
Significant differences between stored slides and the fresh cut slide. Only storage conditions and durations with a significant difference are listed. For ER, PR, and HER2, there were no significant differences between the number of positive cases for fresh cut and stored slides, regardless of the storage condition or duration of storage. Kappa, K, measures agreement between the stored and fresh sections.
| Storage condition | Duration of storage | P | K (95% CI) | |
|---|---|---|---|---|
| Ki67 | paraffin dipped | 1 month | 0.02 | 0.57 (0.33–0.81) |
| CK5/6 | 4C | 1 week | 0.03 | 0.78 (0.61–0.96) |
| −20C | 1 week | 0.03 | 0.65 (0.44–0.86) | |
| −80C | 1 week | 0.03 | 0.67 (0.47–0.87) | |
| EGFR | paraffin dipped | 1 week | 0.005 | 0.51 (0.31–0.71) |
| paraffin dipped | 1 month | <0.0001 | 0.27 (0.09–0.46) | |
| paraffin dipped | 6 months | 0.001 | 0.39 (0.19–0.59) | |
| −20C | 6 months | 0.02 | 0.61 (0.43–0.79) |
Figure 2.
Representative tissue microarray (TMA) cores of breast cancer tissue for slides stored over time and in five storage conditions. a) ER antibody. b) Ki67 antibody.
Discussion
Desiccator storage was the only storage condition that did not show any loss in immunoreactivity for any antibody or time point in our study. Sections that were stored in the cold (4°C, −20°C, or −80°C) showed some loss of immunoreactivity for the CK5/6 and EGFR markers, and changes in immunoreactivity for paraffin dipped slides were observed for two antibodies - Ki67 and EGFR (Table 2). One unexpected finding is that slides stored for only one week showed more negativity for the CK5/6 antibody in all three cold storage conditions than slides that were stored for much longer; we hypothesize that this is a spurious association due to the small number of cores available for comparison in the 1 week and fresh TMA sections for this antibody. Because we wanted to perform staining in a single batch at the end of the study, our experimental design necessitated that the 1 week and fresh sections were among the last slides cut from the TMA block (Figure 1) and therefore experienced the most core dropout. With core-to-case collapsing (described in methods), the combination of cores that are left for a case after dropout may influence the composite score for that case and this is exacerbated as core dropout increases. This unexpected finding may also be explained by core heterogeneity between TMA slices.
We focused on testing various storage arrangements that were likely to ameliorate antigenicity decline. Early studies of cold and desiccator storage have shown that the duration of slide storage influences IHC assay results.11,16,17,26,27 While three of our antibodies show some change in immunoreactivity in cold storage (Table 2), three antibodies did not show any change in immunoreactivity over time in cold storage (ER, PR, HER2) and we did not observe any immunoreactivity loss for any storage duration for slides that were stored in a desiccator. We speculate some reasons for the discrepancy between our findings and those from earlier reports. First, it is possible that pre-analytic factors may have played a bigger role in some earlier studies, before it was well known how these factors can influence IHC outcome and so they were less controlled.1,2 Second, we used a state-of-the-art automated staining platform with temperature and humidity controls, clinical-grade reagents, and antigen retrieval for all antibody staining protocols with slides sectioned at different time points stained in a single antibody batch at the end of the study. Third, digital pathology analysis served to reduce inter- and intra-observer subjectivity for pathologist interpretation.28 Because automated scoring cannot reliably distinguish between tumor and non-tumor cells,23 we manually circled the tumor to demarcate specific regions for automated analysis, adding to the thoroughness of our scoring analysis. A limitation of our study is that we only assessed the effects of slide storage for one year. In reality, the storage of slides for many clinical trials or other large studies is likely to occur for longer time periods.
While contrasting with some early studies, our results are consistent with recent and more thorough studies of antigenicity decline12,29,30. Grillo et al. 2015 found that loss of antigenicity was proportional to tissue section age and was dependent on storage conditions with cold storage being the least affected and maintaining immunoreactivity for at least two years (desiccator storage was not evaluated in this study).12 Similarly, Blows et al. 2016 reported that PR staining on breast TMAs was unaffected after one year of storage - even for slides at room temperature without protective modifications.29 Immunohistochemistry is prone to issues associated with lack of reproducibility and variability among staining batches, 31,32 and generalizations across IHC studies is complicated by the use of different antibody clones, lots, and procedural differences like antigen retrieval. This may explain why different studies of antigenicity loss show differing results for the same markers or storage conditions. Nevertheless, taken collectively, our results are consistent with an overall trend that antigen preservation is maximized by storing sections in dry, cold, and dark conditions.
IHC is a mainstay both in cancer research and clinical diagnostics. While the issue of antigen degradation is not as critical in clinical practice because sectioned slides are typically stained immediately, slide storage is a common occurrence in research studies, and is nearly unavoidable in large multicenter research efforts. Here, we find that paraffin coating showed a decline in immunoreactivity for two markers that we examined, being the least effective and most cumbersome storage method in practice, requiring additional steps to remove the wax prior to staining. Similar findings for paraffin coating have been noted in other studies.12,13,30,33 Desiccator storage preserved antigenicity with no observation of declining immunoreactivity over time in any of the six markers that we examined. Cold storage is an intermediate option that is not as protective as a desiccator, but offers the advantage of being accessible to virtually all research labs.
Supplementary Material
Acknowledgements
This work was supported by the Breast Cancer Research Foundation, the National Center for Research Resources [S10OD019977], and the National Cancer Institute [P01CA151135, P30CA016056], and used Roswell Park Comprehensive Cancer Center’s Pathology Network Shared Resource. Alexis Billups, Cassi Versaggi, and Kristin Gawera provided assistance for this project.
Sources of support: Breast Cancer Research Foundation, National Center for Research Resources [S10OD019977], and the National Cancer Institute [P01CA151135, P30CA016056].
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Bass BP, Engel KB, Greytak SR, Moore HM. A Review of Preanalytical Factors Affecting Molecular, Protein, and Morphological Analysis of Formalin-Fixed, Paraffin-Embedded (FFPE) Tissue: How Well Do You Know Your FFPE Specimen? Archives of Pathology & Laboratory Medicine 2014;138:1520–30. [DOI] [PubMed] [Google Scholar]
- 2.Engel KB, Moore HM. Effects of Preanalytical Variables on the Detection of Proteins by Immunohistochemistry in Formalin-Fixed, Paraffin-Embedded Tissue. Archives of Pathology & Laboratory Medicine 2011;135:537–43. [DOI] [PubMed] [Google Scholar]
- 3.Khoury T Delay to formalin fixation alters morphology and immunohistochemistry for breast carcinoma. Appl Immunohistochem Mol Morphol 2012;20:531–42. [DOI] [PubMed] [Google Scholar]
- 4.Xie R, Chung JY, Ylaya K, et al. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2011;59:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robb JA, Bry L, Sluss PM, Wagar EA, Kennedy MF. A Call to Standardize Preanalytic Data Elements for Biospecimens, Part II. Archives of Pathology & Laboratory Medicine 2015;139:1125–8. [DOI] [PubMed] [Google Scholar]
- 6.Robb JA, Gulley ML, Fitzgibbons PL, et al. A Call to Standardize Preanalytic Data Elements for Biospecimens. Archives of Pathology & Laboratory Medicine 2013;138:526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Economou M, Schöni L, Hammer C, Galván JA, Mueller D-E, Zlobec I. Proper paraffin slide storage is crucial for translational research projects involving immunohistochemistry stains. Clinical and Translational Medicine 2014;3:4-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond WA, Leong AS. Oestrogen receptor staining of paraffin-embedded breast carcinomas following short fixation in formalin: a comparison with cytosolic and frozen section receptor analyses. J Pathol 1990;160:295–303. [DOI] [PubMed] [Google Scholar]
- 9.Prioleau J, Schnitt SJ. p53 antigen loss in stored paraffin slides. N Engl J Med 1995;332:1521–2. [DOI] [PubMed] [Google Scholar]
- 10.Fergenbaum JH, Garcia-Closas M, Hewitt SM, Lissowska J, Sakoda LC, Sherman ME. Loss of Antigenicity in Stored Sections of Breast Cancer Tissue Microarrays. Cancer Epidemiology Biomarkers & Prevention 2004;13:667. [PubMed] [Google Scholar]
- 11.Gelb AB, Freeman VA, Astrow SH. Evaluation of methods for preserving PTEN antigenicity in stored paraffin sections. Appl Immunohistochem Mol Morphol 2011;19:569–73. [DOI] [PubMed] [Google Scholar]
- 12.Grillo F, Pigozzi S, Ceriolo P, Calamaro P, Fiocca R, Mastracci L. Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem Cell Biol 2015;144:93–9. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ. Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst 1996;88:1054–9. [DOI] [PubMed] [Google Scholar]
- 14.Songun I, van der Velde CJ, Gramer-Knijnenburg G, van Krieken JH. Detectability of retinoblastoma (Rb) gene product and p53 antigen expression decreases in stored paraffin sections. Histopathology 1997;30:604–5. [DOI] [PubMed] [Google Scholar]
- 15.Grabau D A, Hansen O Sr, et al. Influence of Storage Temperature and High-Temperature Antigen Retrieval Buffers on Results of Immunohistochemical Staining in Sections Stored for Long Periods1998. [Google Scholar]
- 16.Wester K, Wahlund E, Sundstrom C, et al. Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol 2000;8:61–70. [PubMed] [Google Scholar]
- 17.van den Broek LJ, van de Vijver MJ. Assessment of problems in diagnostic and research immunohistochemistry associated with epitope instability in stored paraffin sections. Appl Immunohistochem Mol Morphol 2000;8:316–21. [PubMed] [Google Scholar]
- 18.Allott EH, Cohen SM, Geradts J, et al. Performance of Three-Biomarker Immunohistochemistry for Intrinsic Breast Cancer Subtyping in the AMBER Consortium. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2016;25:470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allott EH, Geradts J, Cohen SM, et al. Frequency of breast cancer subtypes among African American women in the AMBER consortium. Breast cancer research : BCR 2018;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer JR, Viscidi E, Troester MA, et al. Parity, Lactation, and Breast Cancer Subtypes in African American Women: Results from the AMBER Consortium. JNCI Journal of the National Cancer Institute 2014;106:dju237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367–74. [DOI] [PubMed] [Google Scholar]
- 22.Khoury T, Zirpoli G, Cohen SM, et al. Ki-67 Expression in Breast Cancer Tissue Microarrays: Assessing Tumor Heterogeneity, Concordance With Full Section, and Scoring Methods. Am J Clin Pathol 2017;148:108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allott EH, Geradts J, Sun X, et al. Intratumoral heterogeneity as a source of discordance in breast cancer biomarker classification. Breast cancer research : BCR 2016;18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 2010;6:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 2014;138:241–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirlacher M, Kasper M, Storz M, et al. Influence of slide aging on results of translational research studies using immunohistochemistry. Modern Pathology 2004;17:1414. [DOI] [PubMed] [Google Scholar]
- 27.DiVito KA, Charette LA, Rimm DL, Camp RL. Long-term preservation of antigenicity on tissue microarrays. Laboratory Investigation 2004;84:1071. [DOI] [PubMed] [Google Scholar]
- 28.Stalhammar G, Fuentes Martinez N, Lippert M, et al. Digital image analysis outperforms manual biomarker assessment in breast cancer. Mod Pathol 2016;29:318–29. [DOI] [PubMed] [Google Scholar]
- 29.Blows FM, Ali HR, Dawson S-J, et al. Decline in Antigenicity of Tumor Markers by Storage Time Using Pathology Sections Cut From Tissue Microarrays. Applied Immunohistochemistry & Molecular Morphology 2016;24:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson C, Karlsson MG. Effects of long-term storage on the detection of proteins, DNA, and mRNA in tissue microarray slides. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2011;59:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker M Reproducibility crisis: Blame it on the antibodies. Nature 2015;521:274–6. [DOI] [PubMed] [Google Scholar]
- 32.Bordeaux J, Welsh A, Agarwal S, et al. Antibody validation. Biotechniques 2010;48:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blind C, Koepenik A, Pacyna-Gengelbach M, et al. Antigenicity testing by immunohistochemistry after tissue oxidation. J Clin Pathol 2008;61:79–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.