Abstract
Recent advances in cancer therapeutics, such as targeted therapy and immunotherapy, have raised the hope for cures for many cancer types. However, there are still ongoing challenges to the pursuit of novel therapeutic approaches, including high toxicity to normal tissue and cells, difficulties in treating deep tumor tissue, and the possibility of drug resistance in tumor cells. The use of live tumor-targeting bacteria provides a unique therapeutic option that meets these challenges. Compared with most other therapeutics, tumor-targeting bacteria have versatile capabilities for suppressing cancer. Bacteria preferentially accumulate and proliferate within tumors, where they can initiate antitumor immune responses. Bacteria can be further programmed via simple genetic manipulation or sophisticated synthetic bioengineering to produce and deliver anticancer agents based on clinical needs. Therapeutic approaches using live tumor-targeting bacteria can be applied either as a monotherapy or in combination with other anticancer therapies to achieve better clinical outcomes. In this review, we introduce and summarize the potential benefits and challenges of this anticancer approach. We further discuss how live bacteria interact with tumor microenvironments to induce tumor regression. We also provide examples of different methods for engineering bacteria to improve efficacy and safety. Finally, we introduce past and ongoing clinical trials involving tumor-targeting bacteria.
Subject terms: Cancer, Cancer therapy, Cancer, Cancer therapy
Cancer: tumor-targeting bacteria enter the anticancer arsenal
Live tumor-targeting bacteria can selectively induce cancer regression and, with the help of genetic engineering, be made safe and effective vehicles for delivering drugs to tumor cells. In a review article, Jung-Joon Min and colleagues from Chonnam National University Medical School in Hwasun, South Korea, discuss the clinical history of using natural or engineered bacterial strains to suppress cancer growth. Because bacteria such as Salmonella and Listeria preferentially home in on tumors or their surrounding microenvironments, researchers have harnessed these microbial agents to attack cancer cells without causing collateral damage to normal tissues. Bioengineers have also armed bacteria with stronger tumor-sensing and more targeted drug delivery capabilities, and improved control of off-target toxicities. An increasing number of therapeutic bacterial strains are now entering clinical testing, promising to enhance the efficacy of more conventional anticancer treatments.
Introduction
The challenges faced by current antitumor therapeutics, such as high toxicity to normal cells, the inability to treat deep tumor tissue, and the possibility of inducing drug resistance in tumor cells, have prompted the development of alternative approaches. Many facultative or obligate anaerobic bacteria, such as Clostridium, Bifidobacterium, Listeria, Escherichia coli, and Salmonella species, possess inherent tumor-targeting and tumor-killing activities. It has been > 100 years since William B. Coley used streptococcal cells and Coley’s toxin to cure patients with inoperable cancers1. Further clinical applications using bacteria for treating cancers were curtailed later mainly owing to the emergence of radiation therapy that came into vogue in medical fields since the 1920s. However, recent progress in the fields of immunology and biotechnology has generated new interest in the mechanism underlying the activity of Coley’s toxin, returning bacteria to the forefront for cancer researchers.
Live tumor-targeting bacteria can selectively colonize tumors or tumor-driven lymph nodes, inhibit tumor growth, and prolong survival after systemic infection in animal tumor models. For example, the most well-known attenuated Salmonella Typhimurium strain VNP20009 is attenuated by more than 10,000-fold compared with the wild-type strain and has a tumor:liver colonization ratio > 1000:1; furthermore, it exhibits robust inhibitory effects on tumor growth and metastasis in mouse models2,3. The use of tumor-targeting bacteria as delivery vectors can overcome penetration limitations and maximize the activities of chemotherapeutic drugs while reducing systemic toxicity to the host. Potential payloads for targeted cancer delivery include cytokines, cytotoxic agents, immunomodulators, prodrug-converting enzymes, and small interfering RNAs (siRNAs). By regulating bacterial gene expression, it is possible to further limit the accumulation of antitumor payloads at tumor sites as well as to control the timing of drug delivery.
In this review, we introduce and summarize the technologies underlying bacteria-based anticancer approaches as well as the potential benefits and challenges of these approaches. We also discuss how live bacteria interact with tumor microenvironments (TMEs) to induce tumor regression via colonization and proliferation. Finally, we introduce past and ongoing clinical trials involving tumor-targeting bacteria.
Mechanisms by which bacteria target and suppress tumors
Tumor targeting, penetration, and proliferation
The fundamental advantage of bacteria-based cancer therapy is the capability to specifically target tumors via unique mechanisms. For example, using light-emitting attenuated S. Typhimurium strains defective in ppGpp synthesis (∆ppGpp S. Typhimurium) and E. coli K-12 (MG1655), our group clearly demonstrated that bacteria accumulated exclusively in tumors after intravenous administration in various types of tumor-bearing mice4–7. Currently, it is thought that bacteria escape from the blood circulation into tumor tissue via both passive and active mechanisms. Bacteria may initially enter the tumor via passive entrapment in the chaotic tumor vasculature and then flow into the tumor owing to inflammation caused by a sudden increase in the amount of tumor necrosis factor-α (TNF-α) in the tumor vessels8. In the TME, the active mechanism likely involves chemotaxis toward molecules produced by dying tumor tissue and the low oxygen concentration in hypoxic tumors, the latter of which might be attractive to obligate anaerobes (e.g., Clostridium and Bifidobacterium9,10) and facultative anaerobes11,12. In fact, the active and passive mechanisms are not strain dependent or mutually exclusive, as bacteria might use both pathways to target tumors specifically. The tumor-targeting mechanism of Listeria spp. highlights the involvement of the host immune system. Listeria cells directly infect not only antigen-presenting cells, such as dendritic cells (DCs) or macrophages but also myeloid-derived suppressor cells (MDSCs), which can then deliver bacteria to TMEs. Through this unique mechanism, Listeria cells residing in MDSCs are protected from immune clearance, while Listeria cells in healthy tissue milieus are rapidly eliminated13,14.
Motility is a critical feature that enables bacteria to penetrate deeper into tumor tissue. Unlike the passive distribution and limited penetration intrinsic to chemotherapeutic drugs, bacteria are complex living organisms that can acquire energy from their surrounding environment; thus, their transport capacity is entropically unlimited. Theoretically, following systemic administration, bacteria can use their self-propulsion abilities to actively swim away from the vasculature to disperse themselves throughout tumor tissue. Forbes et al. observed that Salmonella cells started to accumulate in tumors as colonies and spread throughout the entire tumor tissue region within 3 days after injection15. Intratumoral Salmonella cells show three distinct colonization patterns in tumors: large proliferating colonies formed only near blood vessels and small colonies present both near (inactive) to and far (penetrating) from vessels16. Dynamic comparisons of bacterial distribution using in vitro models revealed that motility is critical for effective bacterial dispersion in tumor tissue17.
In addition to motility, the host immune response seems to affect the bacterial distribution in tumor tissue. According to a study by Strizker et al.18, enterobacterial (e.g., E. coli and S. Typhimurium) tumor colonization is likely influenced by both bacterial metabolism and the host TME, as macrophage depletion resulted in elevated bacterial tumor colonization, while bacterial strains defective for aromatic amino acid biosynthesis showed increased tumor specificity. In regard to the bacteria–host interaction, we previously used microscopy to demonstrate time-dependent changes in the intratumoral distribution of ∆ppGpp S. Typhimurium cells. The Salmonella cells initially spread widely within the tumors; however, as immune cells infiltrated, the Salmonella and immune cells interacted with one another, and the bacteria were ultimately surrounded by a neutrophil barrier7. Furthermore, as reported by other groups, neutrophil depletion increased the number of intratumoral bacteria and supported bacterial spreading throughout the tumor tissue8,19.
After successfully targeting and penetration of tumors, live bacteria can proliferate robustly. In one study using tumor-bearing mice, the number of ∆ppGpp S. Typhimurium cells reached greater than 1 × 1010 CFU/g of tumor tissue 3 days after intravenous administration, with a tumor:normal organ bacterial ratio exceeding 10,000:120; in addition, these bacteria remained countable even after 10 days21. The therapeutic S. Typhimurium strain VNP20009 propagates preferentially in tumors at a ratio of greater than 1000:1 compared with that in normal tissue2. Another study reported that S. Typhimurium strain A1-R selectively proliferates in tumors with a tumor:liver bacterial ratio as high as 2000–10,000:1, while the bacteria were completely cleared from the healthy tissue after 15 days22. Although additional studies are needed to clarify why it is beneficial for bacteria to target and grow in tumors, it is undeniable that the ability of therapeutic bacteria to target, penetrate, and proliferate in tumors is a promising advantage that overcomes some of the current limitations of conventional therapies.
Tumor suppression and microenvironmental changes
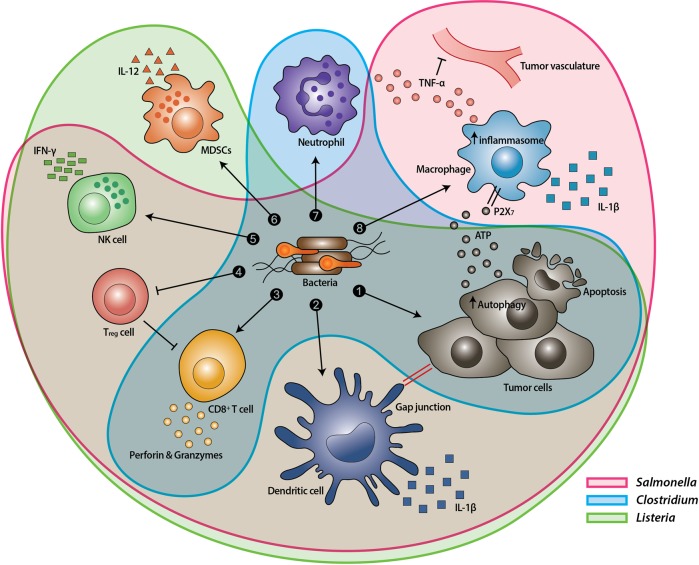
Bacterial overgrowth in tumors induces tumor regression via several different mechanisms (Fig. 1). Different bacterial strains display distinct mechanisms of tumor suppression in TMEs23–26. Salmonella spp. kill tumor cells directly by inducing apoptosis and/or autophagy via a variety of mechanisms, including toxin production or deprivation of nutrients from tumor cells15,27–30. Furthermore, Salmonella infection can induce upregulation of the ubiquitous protein Connexin 43 (Cx43) in tumor cells, promoting gap junction formation between tumor cells and dendritic cells (DCs). These functional connections allow cross-presentation of tumor antigens to DCs, leading to reduced expression of the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) in T cells and a consequential and specific increase in CD8+ T-cell activation31–33. From the perspective of the host–pathogen interaction, the bacterial components, including lipopolysaccharides (LPS) and flagellin, as well as dynamic bacterial proliferation in tumor masses induce significant migration of innate immune cells, such as macrophages, DCs, and neutrophils, to colonized tumors8,21. Subsequently, inflammasome activation leads to robust interleukin-1β (IL-1β) production by macrophages and DCs via two different mechanisms, direct activation by an interaction between Salmonella LPS and toll-like receptor 4 (TLR4) and indirect activation owing to the presence of tumor cells that were damaged by Salmonella34. LPS could be involved in the resulting elevation in TNF-α secretion via interactions with CD14 (a coreceptor of LPS), TLR4, and myeloid differentiation primary response 8835–37. Furthermore, flagellin and TLR5 signaling suppress tumor cell proliferation directly38 and decrease the number of CD4+ CD25+ regulatory T cells39. Intracellular Salmonella flagellin is also involved in NLRC4 inflammasome-driven secretion of IL-1β and IL-18, which serve as activators of IFN-γ-producing cytotoxic T cells and natural killer (NK) cells40. Based on accumulating evidence, it is thought that Salmonella spp. play a central role in orchestrating complex immune cell alterations during the host antitumor response.
Fig. 1. Mechanisms by which bacteria target tumors.
After systemic administration, bacteria localize to the tumor microenvironment. The interactions between bacteria, cancer cells, and the surrounding microenvironment cause various alterations in tumor-infiltrating immune cells, cytokines, and chemokines, which further facilitate tumor regression. ① Bacterial toxins from S. Typhimurium, Listeria, and Clostridium can kill tumor cells directly by inducing apoptosis or autophagy15,24,27–30,41. Toxins delivered via Salmonella can upregulate Connexin 43 (Cx43), leading to bacteria-induced gap junctions between the tumor and dendritic cells (DCs), which allow cross-presentation of tumor antigens to the DCs31–33. ② Upon exposure to tumor antigens and interaction with bacterial components, DCs secrete robust amounts of the proinflammatory cytokine IL-1β, which subsequently activates CD8+ T cells21,33. ③ The antitumor response of the activated CD8+ T cells is further enhanced by bacterial flagellin (a protein subunit of the bacterial flagellum) via TLR5 activation39. The perforin and granzyme proteins secreted by activated CD8+ T cells efficiently kill tumor cells in primary and metastatic tumors33,41. ④ Flagellin and TLR5 signaling also decreases the abundance of CD4+ CD25+ regulatory T (Treg) cells, which subsequently improves the antitumor response of the activated CD8+ T cells39. ⑤ S. Typhimurium flagellin stimulates NK cells to produce interferon-γ (IFN-γ), an important cytokine for both innate and adaptive immunity31. ⑥ Listeria-infected MDSCs shift into an immune-stimulating phenotype characterized by increased IL-12 production, which further enhances the CD8+ T and NK cell responses14. ⑦ Both S. Typhimurium and Clostridium infection can stimulate significant neutrophil accumulation8,20,21,44. Elevated secretion of TNF-α8,21 and TNF-related apoptosis-inducing ligand (TRAIL)45 by neutrophils enhances the immune response and kills tumor cells by inducing apoptosis. ⑧ The macrophage inflammasome is activated through contact with bacterial components (LPS and flagellin) and Salmonella-damaged cancer cells, leading to elevated secretion of IL-1β and TNF-α into the tumor microenvironment21,34. NK cell: natural killer cell. Treg cell: regulatory T cell. MDSCs: myeloid-derived suppressor cells. P2X7 receptor: purinoceptor 7-extracellular ATP receptor. LPS: lipopolysaccharide
Considering their intrinsic pathogenic properties, Listeria spp. can kill tumor cells directly via activation of nicotinamide adenine dinucleotide phosphate oxidase and increased intracellular calcium levels, both of which lead to high reactive oxygen species (ROS) levels41. More recently, another study demonstrated a dual mode of action for Listeria spp.; Listeria cells can enter tumor cells directly or indirectly affect tumors via immunosuppressive effects on MDSCs. Listeria-infected tumor cells are then targeted by activated immune cells, whereas an immune-stimulating phenotype is simultaneously induced in a subpopulation of Listeria-carrying MDSCs via increased IL-12 production that then supports enhanced T and NK cell responses14. Both studies showed that CD8+ T cells can efficiently kill tumor cells in primary and metastatic tumors.
During infection by Clostridium spp., a variety of secreted bacterial toxins (such as hemolysins and phospholipases) can perturb cellular membrane structure or interfere with intracellular functions24,42,43. Like Salmonella and Listeria spp. infections, clostridial infection can also recruit granulocytes and cytotoxic lymphocytes to TMEs, and such recruitment leads to significant increases in the levels of various cytokines and chemokines at the infected sites that can then promote tumor elimination44,45.
In summary, it is speculated that in addition to its intrinsic antitumor effects, bacterial infection makes its most critical contribution to tumor regression by activating a complex immune cell population in TMEs. Although the primary mechanism varies, it is clear that bacteria likely offer a unique immunotherapy strategy that can be potentiated through sophisticated genetic engineering of bacterial strains.
Engineering of bacteria
Virulence attenuation
Engineering bacteria to minimize their virulence against the host immune system is also essential46,47. It should be noted that some bacterial virulence factors may be responsible for their intrinsic antitumor activity. Therefore, attenuation must be achieved without ablation of their antitumor activity. For example, in human pathogens, fatally toxic strains have been converted into largely safe strains via deletion of major virulence genes2,10,21. VNP20009, an attenuated S. Typhimurium strain, was generated via deletion of the msbB and purI genes; this strain has been extensively studied in tumor-bearing mice and shows promising tumor-targeting specificity and tumor inhibitory effects28,48–50. Deletion of msbB in the Salmonella genus results in myristoylation of the lipid A component of LPS, which results in a significant reduction in LPS-driven induction of TNF and can reduce the risk of septic shock51. VNP20009 was subsequently tested in phase I trials in human cancer patients51–54. Disappointingly, VNP20009 lacked tumor specificity and had no clear value in tumor treatment in the patients52,54,55. This failure might be attributed to the penta-acylated lipid A produced by VNP20009, which is a TLR4 antagonist56. To modify the LPS structure to sustain antitumor activity, mutant Salmonella strains were generated that produce a homologous hexa-acylated lipid A, which has a high affinity for TLR4, via deletion of the pagP, pagL, and lpxR genes47,57,58. These mutations did not affect virulence in the msbB mutant background59. Mutations in rfaG and rfaD result in the production of truncated LPS, which results in attenuated toxicity and tumor specificity; however, the antitumor effects of these bacteria were also decreased. Chromosomal integration of the LPS biosynthetic genes in the araBAD locus overcame these limitations, and the mutant strain showed attenuated virulence and therapeutic effects60.
Another nontoxic Salmonella strain was engineered by downregulating the expression of endotoxin-associated genes or by inhibiting their functional activity. Salmonella relA- and spoT-mutant strains defective in the synthesis of ppGpp, a signaling molecule involved in toxin gene expression, exhibited negligible toxicity. The LD50 value of the ΔppGpp strain was increased up to 105–106-fold compared with those of wild-type strains61. The ΔppGpp strain had excellent antitumor activity via its ability to activate the inflammasome (NLRP3, IPAF) and induce the expression of several proinflammatory cytokines (IL-1β, IL-18, and TNF-α)21. Deletion of the phoP and phoQ genes did not affect the antitumor activity of Salmonella, while the deletions reduced its virulence in normal tissue62. Strains bearing these mutations have been used to produce an excellent vaccine and have recently been used as a delivery system for tumor therapeutics63–67. Oral administration of a S. Typhimurium mutant strain deficient in synthesizing the ZnuABC zinc transport system can induce an effective immune response that protects mice against intestinal infections68. Mutations in the genes encoding DNA adenine methylase, adenylate cyclase, and cyclic adenosine monophosphate receptor protein, which are involved in various pathogenic gene regulation pathways, could reduce bacterial toxicity in vivo69. Deletion of the gmd gene in Salmonella inhibited biofilm formation and induced an immune response at an early stage of infection28,57. Furthermore, deletion of htrA, STM3120, and slyA significantly reduced bacterial survival in macrophages as well as the anticancer effects of the bacteria70. Deletion of genes involved in cell invasion can attenuate Listeria monocytogenes cytotoxicity. Hly deletion causes defects in phagolysosome release52,71,72. L. monocytogenes mutant strains lacking actA or ActA PEST-like sequences also lack intercellular diffusion ability73,74, and mutant strains lacking inlA and inlB are invasion defective75,76.
Introduction of specific nutrient-dependent mutations in bacteria is an additional approach to improving tumor-specific proliferation with virulence attenuation. The A1-R Salmonella strain, which is auxotrophic for leucine and arginine, preferentially colonizes tumors, exhibits antitumor effects and increases the susceptibility of tumors to chemotherapy22,77–79. A L. monocytogenes strain was engineered to be auxotrophic for the cell wall component d-alanine via inactivation of the dal/dat locus. This mutant strain was highly attenuated and could induce cytotoxic T lymphocytes22,80. The Salmonella strains SL3261 and SL7207, which carry an aroA deletion, and BRD509, which is an aroA/aroD double mutant strain that is auxotrophic for aromatic amino acids, are highly attenuated and do not disperse freely in the host29,57,81–85. Another S. Typhimurium strain, YB1, which was derived from SL7207 by placing the essential gene asd under the control of a hypoxia-induced promoter, could not survive in normal tissue without an exogenous supply of diaminopimelic acid, although it could still colonize hypoxic tumors; thus, this strain causes reduced damage to normal tissue while retaining its tumor-targeting ability21,47,86,87. Furthermore, deletion of the purI and purD genes resulted in the need for exogenous adenine, which increased the ability of the bacteria to efficiently proliferate in purine-rich regions, such as tumor tissue2,88. The attenuated bacterial strains used for cancer therapy are listed in Table 1.
Table 1.
Attenuated bacterial strains used for cancer therapy
| Bacteria | Strains | Mutated/modified genes | Phenotype description | References |
|---|---|---|---|---|
| Salmonella Typhimurium | A1-R | ∆leu/∆arg | Auxotrophic strain defective in leucine and arginine synthesis | 22 |
| VNP20009 | ∆msbB/∆purI | Lipid A structure modification, reduced ability to induce TNF-α production; deficiency in adenine synthesis | 2 | |
| SHJ2037 | ∆relA/∆spoT | Unable to produce ppGpp (a global regulator involved in bacterial adaptation to extreme environments); reduction in bacterial invasion | 6,20,21 | |
| SL3261 | aro- | Defective in aromatic amino-acid biosynthesis | 83 | |
| SL7207 | 84 | |||
| BRD509 | 85 | |||
| YB1 | 105 | |||
| LH430; VNP (Pho/Q-) | ∆phoP/∆phoQ | Reduced bacterial survival in macrophages | 60,163 | |
| MvP728 | ∆purD/∆htrA | Defective in purine biosynthesis and heat-shock protein production in response to stress stimuli | 88 | |
| YB1; ST8 | ∆asd | Defective in diaminopimelic acid (DAP) synthesis, leading to bacterial lysis during growth without an exogenous DAP supply | 105,164 | |
| c4550 | ∆cya/∆crp | Disabled production of cAMP (cyclic adenosine monophosphate) synthetase and cAMP receptor protein | 165 | |
| SF200; S364 | ∆pagP/∆pagL/∆lpxR | Homogenous hexa-acylated lipid A, triggers immune stimulation in the host | 57,58 | |
| RE88 | ∆dam | Defective in DNA adenine methylase production | 69 | |
| SB824 | ∆sptP | Defective in pathogenicity island 1 (SPI-1) | 166 | |
| ST8 | ∆gmd | Limited ability to spread beyond the anaerobic regions of tumors | 164 | |
| SF200 | rfa- | Highly truncated LPS and attenuated bacterial virulence | 167 | |
| MPO378 | ∆purD/∆upp | Defective in purine biosynthesis and uracil phosphoribosyl transferase | 168 | |
| Listeria monocytogenes | DP-L4027 | ∆LLO (hly) | Defective phagolysosome release | 71 |
| DP-L4029 | ∆actA | Defective surface-bound ActA polypeptide, constitutive LLO activity at physiologic pH | 73 | |
| DP-L4017 | LLO L461T, LLOD26 | Cytotoxic, defective cell-to-cell spreading | 52 | |
| DP-L4042 | ∆PEST | Cytotoxic, defective cell-to-cell spreading | 74 | |
| DP-L4097 | LLO S44A | Cytotoxic, defective cell-to-cell spreading | 72 | |
| DP-L4364 | ∆lplA | Unable to produce lipoate protein ligase, limited ability to proliferate intracellularly | 169 | |
| DP-L4405 | ∆inlA | Impaired InlA-mediated infection | 75 | |
| DP-L4406 | ∆nlB | Impaired InlB-mediated infection | ||
| CS-L0001 | ∆actA/∆inlB | No host actin nucleation, defective cell-to-cell spreading | 76 | |
| CS-L0002 | ∆actA/∆lplA | |||
| CS-L0003 | L461T/∆lplA | Unable to produce lipoate protein ligase, limited ability to proliferate intracellularly; abortive infection: defective cell-to-cell infection | ||
| DP-L4038 | ∆actA/L461T LLO | Defective surface-bound ActA polypeptide, constitutive LLO activity at physiologic pH | 52 | |
| DP-L4384 | S44A/L461T | Defective cell-to-cell spreading |
Enhancement of tumor targeting
The engineering approaches used to improve bacterial tumor targeting can also increase both safety and antitumor efficacy. In one approach to achieve these outcomes, the ppGpp-deficient strain SHJ2037 was genetically engineered to display tumor-specific ligands on the cell surface. An Arg-Gly-Asp peptide that binds to αvβ3 integrin was fused to outer membrane protein A to drive its expression on the bacterial surface89. The resulting strain showed enhanced tumor specificity and markedly increased antitumor activity in MDA-MB-231 breast cancer cells and MDA-MB-435 melanoma xenografts overexpressing αvβ3 integrin. Bacteria were also engineered to target tumor-associated antigens, such as carcinoembryonic antigen or the lymphoma-associated antigen CD20. These strains had effective anticancer effects and reduced nonspecific bacterial accumulation in the liver and spleen90,91. By exploiting biotin-streptavidin binding, an L. monocytogenes strain was constructed in which the cells were coated with plasmid-loaded nanoparticles that expressed a bioluminescence gene. This strain, called a microrobot, delivered functional nucleic acid molecules to solid tumors and could be traced via bioluminescence imaging60,92,93. An intriguing alternative that can increase tumor selectivity is to display synthetic adhesins (SAs) on the surface of E. coli. SAs have a modular structure consisting of a stable β-domain required for outer membrane anchoring and surface-exposed immunoglobulin domains with high affinity and specificity that can be selected from large libraries94. Probiotic strains displayed improved tumor specificity by increasing the injection capacity of the bacteria without attenuating their intrinsic properties95–99. Probiotic E. coli Symbioflor-2 cells were very rapidly removed from the spleen and liver and survived only in a tumor, indicating efficient tumor targeting. Mice infected with a probiotic Salmonella strain tolerated a large bacterial load without any pathological symptoms; however, improvements are needed in the payload delivery system owing to the strain’s inferior therapeutic efficacy, despite its excellent safety in vivo57.
Drug expression strategies
Most payloads delivered by tumor-targeting bacteria are toxic to both tumor cells and normal cells; thus, strict control of their production is preferred over constitutive expression. Precise triggering of payload expression can maximize its therapeutic effects while minimizing systemic toxicity. A controllable gene expression system can theoretically be constructed by inserting a specific promoter sequence upstream of a drug-encoding gene, thereby conferring transcriptional control via external signals. Such a system makes it possible to manage the timing and location of drug production in vivo. The strategies for this type of gene regulation, or triggering, belong to mostly following three categories: internal triggering, self triggering (quorum sensing-QS), and external triggering100.
Unlike normal tissue, TMEs have special properties, including hypoxia, acidosis, and necrosis, which bacteria can sense and utilize to improve tumor specificity101. For example, hypoxia-inducible promoters such as those of HIP-1 and pepT are activated by fumarate and nitrate reduction in the hypoxic environment within tumor tissue102–104. This hypoxia-inducible expression system was designed to function under only anaerobic conditions for expression of essential genes such as asd105. Flentie et al. identified five promoter sequences specifically activated by the acidic microenvironments associated with cancer cells in vitro and with tumors in vivo. Acidosis-specific promoters were identified by using a custom-designed promoterless transposon reporter encoding bacterial luciferase to screen a library of 7400-independent Salmonella transposon insertion mutants in coculture with melanoma or colon carcinoma cells. An attenuated Salmonella strain expressing Shiga toxin under the control of a promoter induced by low pH showed strong tumor selectivity and antitumor activity106. A glucose sensor was engineered in E. coli via synthetic fusion of the Trg chemoreceptor with the EnvZ osmosensor. In this construct, Trg contributes the periplasmic and transmembrane domains as well as a short cytoplasmic segment, and EnvZ contributes the cytoplasmic kinase/phosphatase domain. The engineered bacteria sensed the glucose levels in tumor cell masses and responded to the tumor’s metabolic activity, possibly leading to its therapeutic effect. These features are likely conserved in other members of this sensor family107.
Bacteria can colonize and proliferate in TMEs at a tumor-to-normal tissue ratio exceeding 10,000; thus, QS can be used as a gene expression switch108,109. One useful QS system is regulated by an autoinducer, the synthetic LuxI protein, and the transcriptional regulatory protein LuxR. The acylhomoserine lactone (AHL) produced by LuxI, which depends on bacterial density, activates LuxR and promotes transcription of its target genes. AHL concentration-dependent QS systems have been used successfully to highly express heterologous proteins in bacteria-colonizing tumors90,109–111. The QS approach has been used to introduce a variety of gene circuits. For example, introduction of a synchronized lysis circuit into bacteria improved anticancer efficacy by allowing drug release via periodic introduction of the autoinducer (positive feedback) and the resulting activation of a bacteriophage lysis gene (negative feedback)108.
In addition to internal and self triggering, the expression of gene circuits can also be controlled by external inducers, including chemicals such as l-arabinose, salicylic acid (ASA), and tetracycline. Transcription from the PBAD promoter can be controlled via an interaction between the AraC repressor and l-arabinose5,112. In an attenuated Salmonella strain, pBAD-driven expression of therapeutic payloads from a plasmid could be regulated via intravenous or intraperitoneal l-arabinose administration20,21,113. A Salmonella strain with a mutation in the Ara operon, which results in impaired l-arabinose metabolism, exhibits strong activation of the PBAD promoter114. In the ASA expression system, gene regulation is controlled by the XylS2-dependent Pm promoter115–117. An attenuated Salmonella strain harboring an ASA expression system on a plasmid or on the chromosome allowed efficient regulation of genes encoding prodrug-converting enzymes (see below) and led to a marked reduction in tumor growth115. The pTet expression system is simultaneously regulated by the PtetA and PtetR bidirectional promoters, which are induced by tetracycline or doxycycline118. In a preclinical study, a reporter gene and a therapeutic gene were inserted under these bidirectional promoters to visualize the targeting process and deliver therapeutic drugs, respectively7. This chemically inducible system is regulated in a dose- and time-dependent manner119; therefore, inaccuracies in the timing of inducer administration or the dose of the inducer can lead to nonspecific or suboptimal expression of the target molecules in TMEs. An alternative inducible system uses the radiation-inducible recA promoter48. Radiation causes DNA damage that activates transcription of the genes under the control of the recA promoter. This method combines the therapeutic effects of radiation therapy with radiation-dependent induction of anticancer gene expression120. Induction of TNF-related apoptosis-inducing ligand (TRAIL) expression via a 2 Gy dose of γ-irradiation 48 hours after administration of the engineered Salmonella strain significantly delayed the growth of 4T1 breast cancer cells48. Radiation is advantageous because it can penetrate the tumor tissue and be used for localized treatment. However, radiation can also cause toxicity by inducing DNA damage in the healthy cells around the tumor and likely causes fatal mutations in the therapeutic bacteria that could attenuate their therapeutic effect101.
Drug delivery
Despite studies on the antitumor effects of bacteria, bacteria alone are often insufficient to suppress tumors completely. To enhance the positive outcomes of bacterial cancer therapy, the usefulness of eukaryotic and prokaryotic expression systems for the delivery of therapeutic payloads, including cytotoxic agents, prodrug-converting enzymes, immune regulators, tumor stroma-targeting molecules, and siRNAs, has been explored (listed in Table 2). The prokaryotic expression systems discussed above are the most commonly used approach, and these systems depend on transforming bacteria with prokaryotic plasmids encoding target genes70,121,122; by contrast, eukaryotic expression systems involve transduction of host cells, such as immune cells or tumor cells, with eukaryotic plasmids encoding the cDNAs of the target genes123.
Table 2.
Payloads for bacteria-mediated drug delivery
| Antitumor agents | References |
|---|---|
| Strategies | |
| Hypoxia-inducible promoter | 102–104 |
| Acidosis-specific promoter | 106 |
| Glucose-dependent hybrid receptor Trz1 | 107,170 |
| Quorum sensing | 90,108–111 |
| l-arabinose-inducible pBAD promoter | 5,20,21,95,112,114 |
| Salicylic acid-inducible Pm promoter | 115–117 |
| Tetracycline-inducible Tet promoter | 7,118 |
| Radiation-inducible RecA promoter | 48,120 |
| Cytotoxic agents | |
| ClyA | 5,7 |
| Apoptin | 65 |
| TNF-α | 120,127 |
| TRAIL | 48,78,126,171 |
| FasL | 49 |
| Invasin | 128 |
| Azurin | 29 |
| Prodrug-converting enzymes | |
| CD | 54 |
| HSV1-TK/GCV | 131 |
| β-glucuronidase | 129 |
| Carboxypeptidase G2 | 130 |
| Immunomodulator | |
| IL-18 | 49 |
| IL-2 | 23,29 |
| FlaB | 20,141 |
| PSA | 84,139,144 |
| HER-2/neu | 143 |
| NY-ESO-1 | 83 |
| Survivin | 88 |
| Mage-b | 145 |
| Tumor stroma | |
| Endostatin | 148,149 |
| VEGFR2 | 121,145 |
| Endoglin | 150,151 |
| siRNA | |
| Stat3 | 63,122,149,152 |
| IDO | 153 |
| Survivin | 155 |
| Sox2 | 30 |
| PLK1 | 57 |
ClyA cytolysin A, TNF-α tumor necrosis factor-α, TRAIL TNF-related apoptosis-inducing ligand, FasL Fas ligand, CD cytosine deaminase, HSV1-TK/GCV herpes simplex virus type I thymidine kinase/ganciclovir, IL-18 interleukin-18, IL-2 interleukin-2, FlaB flagellin, PSA prostate-specific antigen, VEGFR2 vascular endothelial growth factor receptor, Stat3 signal transducer and activator of transcription 3, IDO immunosuppressor indoleamine 2,3-dioxygenase, PLK1 cell cycle-associated polo-like kinase 1
Cytotoxic agents
Cytotoxic agents carried by tumor-targeting bacteria can have intrinsic antitumor activity. Combined with the use of inducible promoters, the expression of cytotoxic agents can be tightly controlled to reduce their toxic effects on normal tissue. Cytolysin A (ClyA), a 34 kDa pore-forming hemolytic protein produced by E. coli, S. Typhimurium, and Paratyphi A, can be transported to the bacterial surface and secreted without posttranslational modification. Several bacterial strains, such as E. coli and attenuated S. Typhimurium, have been engineered to express ClyA from a constitutive promoter5 or from inducible promoters activated by arabinose5 or doxycycline7, and these strains have shown excellent tumor-inhibiting effects.
Induction of apoptosis in tumor cells is a promising cancer therapy strategy. Apoptin, a chicken anemia virus-derived protein, selectively induces apoptosis in a large number of human cancer cell types through a p53-independent, Bcl-2-insensitive pathway, with no effects on normal tissue124. By transforming an apoptin-encoding eukaryotic expression plasmid (pCDNA3.1) into an attenuated S. Typhimurium strain, Wen et al. observed significant tumor regression with minimal systemic toxicity in human laryngeal cancer-bearing mice65. Other cytotoxic agents that can be similarly used to induce apoptosis include three members of the TNF-α family (TNF-α, TRAIL, and the Fas ligand); however, owing to their short half-life and hepatotoxicity, the usefulness of these cytotoxic ligands is limited by their insufficient tumor exposure and detrimental effects on liver function125. To improve the bioavailability and sustainability of these proteins, bacteria have been used to deliver them directly to the tumor region48,78,126,127. Forbes et al. engineered a nonpathogenic S. Typhimurium strain expressing murine TRAIL under the control of the radiation-inducible recA promoter. After irradiation, TRAIL was secreted, whereupon it delayed mammary tumor growth significantly and reduced the risk of death48.
Invasin, a Yersinia surface protein, can bind to β1 integrin selectively to trigger bacterial entry into host cells. Using a nonpathogenic, recombinant invasive E. coli strain coexpressing invasin and the model antigen ovalbumin as well as LLO, Critchley-Thorne et al. showed that the engineered strain could invade β1 integrin-expressing cells and deliver proteins to tumors to produce therapeutic effects in mice128. Azurin, a low-molecular-weight redox protein, can be internalized efficiently to initiate cancer cell apoptosis by raising the intracellular p53 and Bax levels to induce the release of mitochondrial cytochrome c into the cytosol. The effectiveness of E. coli-based azurin delivery in suppressing the growth of B16 mouse melanoma and 4T1 mouse breast cancer was demonstrated by Nissle in 1917; furthermore, this approach also prevented pulmonary metastasis and stimulated inflammatory responses29.
Prodrug-converting enzymes
The expression of prodrug-converting enzymes can convert prodrugs into cytotoxic agents specifically in the tumor region. The usefulness of this strategy to improve cancer treatment efficacy and reduce the side effects associated with systemic administration has been explored. Several prodrug-converting enzymes have been delivered by bacteria96,129–131. Cytosine deaminase (CD) converts nontoxic 5-fluorocytosine (5-FC) into the chemotherapeutic agent 5-fluorouracil (5-FU). 5-FU is highly toxic because it is further metabolized into a product that interferes with DNA and RNA synthesis132–135. Upon coadministration of an attenuated S. Typhimurium (VNP20009) strain expressing E. coli CD and 5-FC into patients, conversion of 5-FC to 5-FU was observed, indicating bacterial production of functional CD in the tumor54.
The herpes simplex virus type I thymidine kinase/ganciclovir (HSV1-TK/GCV) system is another prodrug-converting enzyme/prodrug combination that has been widely studied for use in tumor therapy. Tumor tissue-specific HSV1-TK expression can convert the nontoxic precursor ganciclovir into ganciclovir-3-phosphate, a toxic substance that kills tumor cells. Liu et al. tested the in vivo efficacy of a Bifidobacterium infantis strain expressing HSV1-TK and GCV for prodrug therapy in a rat bladder tumor model. The results showed that this targeted approach could effectively inhibit rat bladder tumor growth by increasing caspase 3 expression and inducing apoptosis131. Another prodrug-converting enzyme delivery strain, E. coli DH5α expressing β-glucuronidase, which hydrolyzes the glucuronide prodrug 9ACG into the topoisomerase I inhibitor 9-aminocamptothecin (9AC), showed efficient tumor inhibition129. The use of attenuated S. Typhimurium VNP20009 as a vector to deliver carboxypeptidase G2 showed enhanced antitumor efficacy in conjunction with prodrug administration130.
Immunomodulators
Cytokines can achieve antitumor effects by facilitating the proliferation, activation, and differentiation of immune cells, by inducing apoptosis in tumor cells, and via antiangiogenesis effects on tumor vasculature. Several cytokines, including GM-CSF, IL-12, and IL-18, have entered clinical trials for cancer therapy136. Cytokines expressed by tumor-targeting bacteria were delivered specifically to the tumor region, where they augmented the antitumor immune response in the TME20,29,49,62,137–140. Intravenous administration of an IL-18-expressing attenuated S. Typhimurium strain inhibited primary tumor growth in mice, induced massive leukocyte infiltration (mainly granulocytes), and increased NK and CD4+ T cell but not CD8+ T-cell recruitment. Furthermore, this approach also increased cytokine production in the tumor region, including that of IL-1β, TNF-α, IFN-γ, and GM-CSF49. IL-2 is the most widely studied cytokine in the context of bacterial delivery systems. Oral administration of a S. Typhimurium Ty21a strain expressing IL-2 inhibited hepatocellular carcinoma (HCC) in mouse models23,29. Flagellin, which activates the innate immune system via TLR5, has been established as an excellent immunotherapy adjuvant141. Our group treated colon cancer-bearing mice with an attenuated ΔppGpp S. Typhimurium strain expressing heterologous flagellin and showed that it enhanced antitumor immunity via cooperation with the TLR4 and TLR5 signaling pathways. This approach also promotes an M2-to-M1 shift in macrophages and increases nitric oxide levels in tumors20.
Engineered bacteria expressing tumor-associated antigens may sensitize TMEs and overcome the self tolerance aroused by regulatory T cells, thereby eliciting effector and memory T-cell responses toward antigen-producing tumor cells142,143. A number of prostate cancer-associated antigens have been reported. Bacteria-based vaccines against prostate-specific antigen (PSA) have been tested in several mouse models139,144. Endogenous PSA gene delivery using attenuated S. Typhimurium SL7207-alleviated immune tolerance to murine prostate stem cell antigens and significantly retarded tumor growth84. Gene therapy approaches using antigens against HER-2/neu143, NY-ESO-183, survivin88, and Mage-b145 have also shown promising tumor inhibition effects.
Great interest has developed in the field of immune checkpoint blockade (ICB) cancer therapy. Despite the success of ICB therapy in clinical trials, only some patients benefit from this treatment. There are several reasons for the host resistance underlying this effect, of which the immunosuppressive TME is the most important146,147. Studies demonstrate that tumor colonization by bacteria can induce proinflammatory reactions involving elevated expression of IL-1β, TNF-α, and IFN-γ, as well as NK and T-cell activation; therefore, a combination of ICB and bacterial therapies may overcome host resistance131.
Targeting the tumor stroma
Angiogenesis plays important role in tumor growth and metastasis. Targeting tumor neovascularization provides a promising direction for cancer therapy. Endostatin is a 20 kDa C-terminal fragment from type XVIII collagen that can inhibit tumor vessel generation in a dose-dependent manner without obvious side effects or drug resistance148,149. Xu et al. cloned endostatin and an siRNA against signal transducer and activator of transcription 3 (Stat3) in an attenuated S. Typhimurium strain. They then tested the strain’s therapeutic efficacy in orthotropic HCC and showed that it could inhibit tumor proliferation and metastasis, reduce the amount of tumor microvasculature, increase the CD4+/CD8+ T-cell populations and the expression levels of several inflammatory cytokines (including IFN-γ and TNF-α), and downregulate TGF-β, regulatory T cells, and vascular endothelial growth factor (VEGF) expression149. VEGF and its receptor (VEGFR) regulate tumor angiogenesis121,145. Oral administration of attenuated S. Typhimurium SL3261 expressing the extracellular VEGFR2 domain inhibited tumor growth, neovascularization, and pulmonary metastasis. Furthermore, the percentages of CD4+ and CD8+ T cells in the tumor region also increased significantly121. Endoglin (CD105) is a member of the TGF-β receptor family. TGF-β1 and hypoxia can upregulate the endoglin gene promoter, and this promoter is highly active in tumoral endothelial cells. Therefore, endoglin has been considered a target for cancer therapy150. Paterson et al. used Listeria-based vaccines against CD105, Lm-LLO-CD105A, and Lm-LLO-CD105B to treat breast cancer in a mouse model. The vaccines stimulated a robust antiangiogenesis effect and an antitumor immune response that inhibited primary and metastatic tumors151.
Gene silencing
siRNAs, a class of 20–25 base pair-long double-stranded RNAs that mediate silencing of specific target genes, have provided a promising approach to cancer therapy. However, the largest barrier to RNA interference therapy is the need for specific delivery of siRNAs to the tumor region. Bacteria-based delivery systems for siRNAs against Stat363,122,149,152, IDO153,154, survivin155, Sox230, and the cell cycle-associated polo-like kinase 1 (PLK1)57 have been tested in mouse tumor models. Oral administration of an attenuated S. Typhimurium strain harboring a eukaryotic expression plasmid encoding siRNA-Stat3 enhanced NK cell activity and T-lymphocyte function and elevated the percentage of CD8+ T cells, whereas it decreased the number of CD4+ CD25+ regulatory T cells in the tumor; these effects led to inhibition of tumor growth and prolonged survival of tumor-bearing mice122. Silencing host IDO expression using a S. Typhimurium VNP20009 strain expressing shIDO elicited significant tumor infiltration by ROS-generating polymorphonuclear neutrophils, which promoted intratumoral cell death and substantial control of B16F10 melanomas153 and CT26 or MC38 colorectal cancers156.
Clinical Trials
Since Dr. William B. Coley first used the live infectious agent erysipelas (Streptococcus pyogenes) for cancer treatment in 1891157, several bacterial strains have been studied and selected for testing in human patients (Table 3). Among the bacterial species selected for human studies, Listeria vaccine strains (with or without combination agents) have shown very promising outcomes, and some strains are now being tested in phase II and III clinical trials158.
Table 3.
Previous and ongoing clinical trials
| Bacterial strain | Phase | Cancer type | n | References |
|---|---|---|---|---|
| S. Typhimurium VNP20009 | I | Metastatic melanoma; metastatic renal cell carcinoma | 25 | 159 |
| S. Typhimurium VNP20009 | I | Melanoma | 4 | 53 |
| S. Typhimurium VNP20009 expressing TAPET-CD (cytosine deaminase) | I | Head and neck or esophageal adenocarcinoma | 3 | 54 |
| S. Typhimurium VNP20009 | I | Patients with advanced or metastatic solid tumors | Not provided | http://www.clinicaltrials.gov/ct2/show/NCT00004216 |
| S. Typhimurium VNP20009 | I | Unspecified adult solid tumors | Not provided | https://www.clinicaltrials.gov/ct2/show/NCT00006254 |
| S. Typhimurium VNP20009 | I | Neoplasm or neoplasm metastatic tumors | 45 | http://www.clinicaltrials.gov/ct2/show/NCT00004988 |
| S. Typhimurium expressing human IL-2 | I | Liver cancer | 22 | https://www.clinicaltrials.gov/ct2/show/NCT01099631 |
| S. Typhimurium Ty21a VXM01 | I | Pancreatic cancer | 26 | 172 |
| Clostridium novyi-NT | I | Colorectal cancer | 2 | https://www.clinicaltrials.gov/ct2/show/NCT00358397 |
| Clostridium novyi-NT | I | Solid tumor malignancies | 5 | https://www.clinicaltrials.gov/ct2/show/NCT01118819 |
| Clostridium novyi-NT | I | Solid tumor malignancies | 24 | https://www.clinicaltrials.gov/ct2/show/NCT01924689 |
| Clostridium novyi-NT | Ib | Refractory advanced solid tumors | 18- recruiting | https://clinicaltrials.gov/ct2/show/NCT03435952 |
| Listeria monocytogenes | II | Metastatic pancreatic tumors | 90 | 173 |
| Listeria monocytogenes | II | Cervical cancer | 109 | 174 |
| Listeria monocytogenes | III | Cervical cancer | 450- recruiting | https://clinicaltrials.gov/ct2/show/record/NCT02853604 |
In 1999, the attenuated S. Typhimurium VNP20009 strain designed by Vion Pharmaceutics, Inc., was the first Salmonella strain to enter phase I human clinical trials. The effectiveness of this strain was tested in 24 patients with metastatic melanoma and in one patient with metastatic renal carcinoma. Analysis of increasing doses (1 × 106–1 × 109 CFU/m2 delivered via intravenous injection) revealed that the maximum-tolerated dose) was 3.0 × 108 CFU/m2. Although increased levels of several proinflammatory cytokines (such as IL-1β, TNF-α, IL-6, and IL-12) and tumor colonization were found in some patients, no objective tumor regression was observed, even in patients with colonized tumors159. Another clinical trial with S. Typhimurium VNP20009 was performed with four additional metastatic melanoma patients, but there was no objective tumor response, and only minor and transient side effects were observed53. To improve its therapeutic efficacy, VNP20009 was modified to express E. coli CD, which converts 5-FC to toxic 5-FU. Three patients with head and neck squamous carcinoma and esophageal adenocarcinoma were treated by intratumoral injection of these bacteria (at three increasing doses: 3 × 106, 1 × 107, or 3 × 107 CFU/m2) for multiple cycles; 100 mg/kg/day 5-FC was delivered orally three times daily for multiple cycles. Two patients showed tumor colonization for at least 15 days after the initial administration, with a tumor-to-plasma ratio of 3:1; this ratio was <1.0 in the noncolonized patient. No significant adverse responses were observed after six treatment cycles. More recently, there have been four other unpublished and completed phase I clinical trials using S. Typhimurium (three clinical trials with attenuated VNP20009 and one clinical trial with S. Typhimurium χ4550 expressing IL-2, as summarized in Table 3). The results of these clinical trials revealed that discrepancies between the outcomes in preclinical animal models and human patients might be owing to differences in tumor structure and growth rates that could alter bacterial penetration, proliferation, and clearance within tumors as well as in the peripheral circulation. Another lesson learned from the clinical trials using Salmonella spp. is that TLR4-mediated signaling might be important for tumor colonization and antitumor activity, as a VNP20009 strain lacking lipid A function failed to colonize tumors sufficiently enough to suppress tumor growth (as discussed above).
Based on the tumor colonization and tumor lysis studies initiated by Möse and colleagues in the 1950s160,161, the oncolytic Clostridium butyricum M-55 strain (also known as Clostridium sporogenes ATCC 13732) has entered phase I clinical trials with a large number of patients. Robert et al.162 demonstrated promising antitumor responses in both canine and human clinical studies after intratumoral injection of Clostridium novyi-NT spores. In this study, the data from lesion biopsies and computed tomography imaging clearly showed extensive tumor destruction owing to gas pockets produced by C. novyi-NT. Although tumor colonization and objective tumor responses were observed using both intravenous and intratumoral administration in these clinical trials, the Clostridium cells alone failed to eradicate all of the cancer cells, resulting in tumor relapse. Currently, a phase Ib clinical trial using a C. novyi-NT strain in combination with an anti-PD1 antibody (pembrolizumab) to treat refractory advanced solid tumor patients is underway (summarized in Table 3).
Although limited, these clinical data have revealed many important obstacles as well as encouraging challenges that must be overcome for successful human application in the future. Perhaps engineering bacteria to specifically target tumors or the use of combinations of bacteria-based approaches and other immunotherapies in conjunction with advanced diagnostic approaches will enable better intratumoral bacterial colonization and enhance the resulting therapeutic outcomes.
Conclusions and future perspectives
Tumor-targeting bacteria possess unique features, including tumor selectivity and unlimited gene packaging capability, that make them ideal vehicles for delivering therapeutic payloads in a cancer-specific manner. This unlimited gene packaging capability not only allows the expression of large or multiple target genes but also supports engineering signaling networks that can enable bacteria to perform sophisticated tasks in cancer treatment. Despite the great therapeutic potential of engineered tumor-targeting bacteria, successful cancer therapy will likely require combinatorial approaches, as cancer heterogeneity (at both the molecular and histologic levels) makes it very difficult to achieve a cure with single anticancer agents. In addition to chemotherapy and radiotherapy, whose anticancer effects can be synergistic with those of bacteria, intratumoral bacterial infection is attractive as an amendment to other immunotherapeutic approaches. For example, some natural or engineered bacterial strains can induce a tumor-specific T-cell response in TMEs or lymphoid tissue via activation of multiple TLR pathways, induction of bacteria-specific CD4+ T cells, and generation of proinflammatory TMEs. This approach may have unique characteristics that enable the bacteria to induce TLR-mediated CD8+ and CD4+ T-cell infiltration of poorly infiltrated tumors, thereby leading to a synergistic effect with ICB treatment. More-sophisticated engineering of tumor-targeting bacteria may allow stronger tumor sensing, finer tuning of drug production, and improved control of bacterial toxicity and genetic instability. Clinical studies with such “smart” bacteria will hopefully establish this approach as another powerful weapon in the arsenal in our fight against cancer in the near future.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2017R1A2B3012157).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. IOWA Orthop. J. 2006;26:154–158. [PMC free article] [PubMed] [Google Scholar]
- 2.Clairmont C, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J. Infect. Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 3.Luo X, et al. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models. Oncol. Res. 2001;12:501–508. doi: 10.3727/096504001108747512. [DOI] [PubMed] [Google Scholar]
- 4.Min JJ, et al. Noninvasive real-time imaging of tumors and metastases using tumor-targeting light-emitting Escherichia coli. Mol. Imaging Biol. 2008;10:54–61. doi: 10.1007/s11307-007-0120-5. [DOI] [PubMed] [Google Scholar]
- 5.Jiang SN, et al. Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy. Mol. Ther. 2010;18:635–642. doi: 10.1038/mt.2009.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen VH, et al. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res. 2010;70:18–23. doi: 10.1158/0008-5472.CAN-09-3453. [DOI] [PubMed] [Google Scholar]
- 7.Jiang SN, et al. Engineering of bacteria for the visualization of targeted delivery of a cytolytic anticancer agent. Mol. Ther. 2013;21:1985–1995. doi: 10.1038/mt.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leschner S, et al. Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha. PLoS ONE. 2009;4:e6692. doi: 10.1371/journal.pone.0006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malmgren RA, Flanigan CC. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955;15:473–478. [PubMed] [Google Scholar]
- 10.Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl. Acad. Sci. USA. 2001;98:15155–15160. doi: 10.1073/pnas.251543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasinskas RW, Forbes NS. Salmonella typhimurium specifically chemotax and proliferate in heterogeneous tumor tissue in vitro. Biotechnol. Bioeng. 2006;94:710–721. doi: 10.1002/bit.20883. [DOI] [PubMed] [Google Scholar]
- 12.Kasinskas RW, Forbes NS. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res. 2007;67:3201–3209. doi: 10.1158/0008-5472.CAN-06-2618. [DOI] [PubMed] [Google Scholar]
- 13.Quispe-Tintaya W, et al. Nontoxic radioactive Listeria(at) is a highly effective therapy against metastatic pancreatic cancer. Proc. Natl. Acad. Sci. USA. 2013;110:8668–8673. doi: 10.1073/pnas.1211287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra D, Jahangir A, Quispe-Tintaya W, Einstein MH, Gravekamp C. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br. J. Cancer. 2013;108:2281–2290. doi: 10.1038/bjc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganai S, Arenas RB, Sauer JP, Bentley B, Forbes NS. In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene Ther. 2011;18:457–466. doi: 10.1038/cgt.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M, Forbes NS. Trg-deficient Salmonella colonize quiescent tumor regions by exclusively penetrating or proliferating. J. Control Release. 2015;199:180–189. doi: 10.1016/j.jconrel.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toley BJ, Forbes NS. Motility is critical for effective distribution and accumulation of bacteria in tumor tissue. Integr. Biol. (Camb.) 2012;4:165–176. doi: 10.1039/c2ib00091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stritzker J, et al. Enterobacterial tumor colonization in mice depends on bacterial metabolism and macrophages but is independent of chemotaxis and motility. Int J. Med. Microbiol. 2010;300:449–456. doi: 10.1016/j.ijmm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Westphal K, Leschner S, Jablonska J, Loessner H, Weiss S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res. 2008;68:2952–2960. doi: 10.1158/0008-5472.CAN-07-2984. [DOI] [PubMed] [Google Scholar]
- 20.Zheng JH, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017;9:eaak9537. doi: 10.1126/scitranslmed.aak9537. [DOI] [PubMed] [Google Scholar]
- 21.Kim JE, et al. Salmonella typhimurium suppresses tumor growth via the pro-inflammatory cytokine interleukin-1beta. Theranostics. 2015;5:1328–1342. doi: 10.7150/thno.11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao M, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc. Natl Acad. Sci. USA. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer. 2010;10:785–794. doi: 10.1038/nrc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middlebrook JL, Dorland RB. Bacterial toxins: cellular mechanisms of action. Microbiol. Rev. 1984;48:199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staedtke V, Roberts NJ, Bai RY, Zhou S. Clostridium novyi-NT in cancer therapy. Genes Dis. 2016;3:144–152. doi: 10.1016/j.gendis.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flickinger, J. C., Rodeck, U. & Snook, A. E. Listeria monocytogenes as a vector for cancer immunotherapy: current understanding and progress. Vaccines (Basel)6, pii: E48 (2018). [DOI] [PMC free article] [PubMed]
- 27.Uchugonova A, et al. Imaging the different mechanisms of prostate cancer cell-killing by tumor-targeting saalmonella typhimurium A1-R. Anticancer Res. 2015;35:5225–5229. [PubMed] [Google Scholar]
- 28.Lee CH, et al. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene Ther. 2014;21:309–316. doi: 10.1038/gt.2013.86. [DOI] [PubMed] [Google Scholar]
- 29.Uchugonova A, et al. Cancer-cell killing by engineered Salmonella imaged by multiphoton tomography in live mice. Anticancer Res. 2012;32:4331–4337. [PubMed] [Google Scholar]
- 30.Liu B, et al. Blockage of autophagy pathway enhances Salmonella tumor-targeting. Oncotarget. 2016;7:22873–22882. doi: 10.18632/oncotarget.8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saccheri F, et al. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci. Transl. Med. 2010;2:44ra57. doi: 10.1126/scitranslmed.3000739. [DOI] [PubMed] [Google Scholar]
- 32.Chang WW, et al. Salmonella enhance chemosensitivity in tumor through connexin 43 upregulation. Int J. Cancer. 2013;133:1926–1935. doi: 10.1002/ijc.28155. [DOI] [PubMed] [Google Scholar]
- 33.Lin HC, et al. The inhibition of indoleamine 2, 3-dioxygenase 1 by connexin 43. Int J. Med. Sci. 2017;14:1181–1188. doi: 10.7150/ijms.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phan TX, et al. Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol. Immunol. 2015;59:664–675. doi: 10.1111/1348-0421.12333. [DOI] [PubMed] [Google Scholar]
- 35.Beutler B, Cerami A. The biology of cachectin/TNF—a primary mediator of the host response. Annu Rev. Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 36.Kocijancic D, et al. Therapeutic benefit of Salmonella attributed to LPS and TNF-alpha is exhaustible and dictated by tumor susceptibility. Oncotarget. 2017;8:36492–36508. doi: 10.18632/oncotarget.16906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903–914. doi: 10.1016/S1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 38.Cai Z, et al. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. 2011;71:2466–2475. doi: 10.1158/0008-5472.CAN-10-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sfondrini L, et al. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J. Immunol. 2006;176:6624–6630. doi: 10.4049/jimmunol.176.11.6624. [DOI] [PubMed] [Google Scholar]
- 40.Kupz A, Curtiss R, 3rd, Bedoui S, Strugnell RA. In vivo IFN-gamma secretion by NK cells in response to Salmonella typhimurium requires NLRC4 inflammasomes. PLoS ONE. 2014;9:e97418. doi: 10.1371/journal.pone.0097418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SH, Castro F, Paterson Y, Gravekamp C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009;69:5860–5866. doi: 10.1158/0008-5472.CAN-08-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chagnon A, Hudon C, McSween G, Vinet G, Fredette V. Cytotoxicity and reduction of animal cell growth by Clostridium M-55 spores and their extracts. Cancer. 1972;29:431–434. doi: 10.1002/1097-0142(197202)29:2<431::AID-CNCR2820290226>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 43.Cheong I, et al. A bacterial protein enhances the release and efficacy of liposomal cancer drugs. Science. 2006;314:1308–1311. doi: 10.1126/science.1130651. [DOI] [PubMed] [Google Scholar]
- 44.Agrawal N, et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc. Natl Acad. Sci. USA. 2004;101:15172–15177. doi: 10.1073/pnas.0406242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shinnoh M, et al. Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int. J. Oncol. 2013;42:903–911. doi: 10.3892/ijo.2013.1790. [DOI] [PubMed] [Google Scholar]
- 46.Ozdemir T, Fedorec AJH, Danino T, Barnes CP. Synthetic biology and engineered live biotherapeutics: toward increasing system complexity. Cell Syst. 2018;7:5–16. doi: 10.1016/j.cels.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Felgner S, et al. Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. Oncoimmunology. 2018;7:e1382791. doi: 10.1080/2162402X.2017.1382791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganai S, Arenas RB, Forbes NS. Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br. J. Cancer. 2009;101:1683–1691. doi: 10.1038/sj.bjc.6605403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loeffler M, Le’Negrate G, Krajewska M, Reed JC. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol. Immunother. 2009;58:769–775. doi: 10.1007/s00262-008-0555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thamm DH, et al. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clin. Cancer Res. 2005;11:4827–4834. doi: 10.1158/1078-0432.CCR-04-2510. [DOI] [PubMed] [Google Scholar]
- 51.Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Hum. Gene Ther. 2001;12:1594–1596. [PubMed] [Google Scholar]
- 52.Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 2002;156:1029–1038. doi: 10.1083/jcb.200201081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heimann DM, Rosenberg SA. Continuous intravenous administration of live genetically modified salmonella typhimurium in patients with metastatic melanoma. J. Immunother. 2003;26:179–180. doi: 10.1097/00002371-200303000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemunaitis J, et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003;10:737–744. doi: 10.1038/sj.cgt.7700634. [DOI] [PubMed] [Google Scholar]
- 55.Chorobik P, Czaplicki D, Ossysek K, Bereta J. Salmonella and cancer: from pathogens to therapeutics. Acta Biochim. Pol. 2013;60:285–297. doi: 10.18388/abp.2013_1984. [DOI] [PubMed] [Google Scholar]
- 56.Teghanemt A, Zhang D, Levis EN, Weiss JP, Gioannini TL. Molecular basis of reduced potency of underacylated endotoxins. J. Immunol. 2005;175:4669–4676. doi: 10.4049/jimmunol.175.7.4669. [DOI] [PubMed] [Google Scholar]
- 57.Felgner, S. et al. aroA-deficient Salmonella enterica saerovar Typhimurium is more than a metabolically attenuated mutant. MBio7, pii: e01220-16 (2016). [DOI] [PMC free article] [PubMed]
- 58.Liang K, et al. Endostatin gene therapy delivered by attenuated Salmonella typhimurium in murine tumor models. Cancer Gene Ther. 2018;25:167–183. doi: 10.1038/s41417-018-0021-6. [DOI] [PubMed] [Google Scholar]
- 59.Kong Q, et al. Palmitoylation state impacts induction of innate and acquired immunity by the Salmonella enterica serovar typhimurium msbB mutant. Infect. Immun. 2011;79:5027–5038. doi: 10.1128/IAI.05524-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frahm, M. et al. Efficiency of conditionally attenuated Salmonella enterica serovar Typhimurium in bacterium-mediated tumor therapy. MBio6, pii: e00254-15 (2015). [DOI] [PMC free article] [PubMed]
- 61.Fujimori M. Genetically engineered bifidobacterium as a drug delivery system for systemic therapy of metastatic breast cancer patients. Breast Cancer. 2006;13:27–31. doi: 10.2325/jbcs.13.27. [DOI] [PubMed] [Google Scholar]
- 62.Cheng X, Zhang X, Zhou Y, Zhang C, Hua ZC. A Salmonella Typhimurium mutant strain capable of RNAi delivery: higher tumor-targeting and lower toxicity. Cancer Biol. Ther. 2014;15:1068–1076. doi: 10.4161/cbt.29185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu YF, et al. A new expression plasmid in Bifidobacterium longum as a delivery system of endostatin for cancer gene therapy. Cancer Gene Ther. 2007;14:151–157. doi: 10.1038/sj.cgt.7701003. [DOI] [PubMed] [Google Scholar]
- 64.Chen Y, et al. Development of a Listeria monocytogenes-based vaccine against hepatocellular carcinoma. Oncogene. 2012;31:2140–2152. doi: 10.1038/onc.2011.395. [DOI] [PubMed] [Google Scholar]
- 65.Guan GF, et al. Salmonella typhimurium mediated delivery of sapoptin in human laryngeal cancer. Int J. Med. Sci. 2013;10:1639–1648. doi: 10.7150/ijms.6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galan JE, Curtiss R., 3rd Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Micro. Pathog. 1989;6:433–443. doi: 10.1016/0882-4010(89)90085-5. [DOI] [PubMed] [Google Scholar]
- 67.Angelakopoulos H, Hohmann EL. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect. Immun. 2000;68:2135–2141. doi: 10.1128/IAI.68.4.2135-2141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chirullo B, et al. Attenuated mutant strain of Salmonella Typhimurium lacking the ZnuABC transporter contrasts tumor growth promoting anti-cancer immune response. Oncotarget. 2015;6:17648–17660. doi: 10.18632/oncotarget.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewen S, et al. A Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol. Immunother. 2008;57:507–515. doi: 10.1007/s00262-007-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, et al. Salmonella VNP20009-mediated RNA interference of ABCB5 moderated chemoresistance of melanoma stem cell and suppressed tumor growth more potently. Oncotarget. 2016;7:14940–14950. doi: 10.18632/oncotarget.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones S, Portnoy DA. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect. Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glomski IJ, Decatur AL, Portnoy DA. Listeria monocytogenes mutants that fail to compartmentalize listerolysin O activity are cytotoxic, avirulent, and unable to evade host extracellular defenses. Infect. Immun. 2003;71:6754–6765. doi: 10.1128/IAI.71.12.6754-6765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Camilli A, Tilney LG, Portnoy DA. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Decatur AL, Portnoy DA. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science. 2000;290:992–995. doi: 10.1126/science.290.5493.992. [DOI] [PubMed] [Google Scholar]
- 75.Bakardjiev AI, Stacy BA, Fisher SJ, Portnoy DA. Listeriosis in the pregnant guinea pig: a model of vertical transmission. Infect. Immun. 2004;72:489–497. doi: 10.1128/IAI.72.1.489-497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brockstedt DG, et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc. Natl Acad. Sci. USA. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoffman RM, Zhao M. Whole-body imaging of bacterial infection and antibiotic response. Nat. Protoc. 2006;1:2988–2994. doi: 10.1038/nprot.2006.376. [DOI] [PubMed] [Google Scholar]
- 78.Nagakura C, et al. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 2009;29:1873–1878. [PubMed] [Google Scholar]
- 79.Hayashi K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J. Cell Biochem. 2009;106:992–998. doi: 10.1002/jcb.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thompson RJ, Bouwer HGA, Portnoy DA, Frankel FR. Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires D-alanine for growth. Infect. Immun. 1998;66:3552–3561. doi: 10.1128/iai.66.8.3552-3561.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin CH, et al. Recombinant Salmonella-based CEACAM6 and 4-1BBL vaccine enhances T-cell immunity and inhibits the development of colorectal cancer in rats: In vivo effects of vaccine containing 4-1BBL and CEACAM6. Oncol. Rep. 2015;33:2837–2844. doi: 10.3892/or.2015.3901. [DOI] [PubMed] [Google Scholar]
- 82.Yoon W, Choi JH, Kim S, Park YK. Engineered Salmonella typhimurium expressing E7 fusion protein, derived from human papillomavirus, inhibits tumor growth in cervical tumor-bearing mice. Biotechnol. Lett. 2014;36:349–356. doi: 10.1007/s10529-013-1370-8. [DOI] [PubMed] [Google Scholar]
- 83.Meng JZ, et al. Oral vaccination with attenuated Salmonella enterica strains encoding T-cell epitopes from tumor antigen NY-ESO-1 induces specific cytotoxic T-lymphocyte responses. Clin. Vaccin. Immunol. 2010;17:889–894. doi: 10.1128/CVI.00044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ahmad S, et al. Induction of effective antitumor response after mucosal bacterial vector mediated DNA vaccination with endogenous prostate cancer specific antigen. J. Urol. 2011;186:687–693. doi: 10.1016/j.juro.2011.03.139. [DOI] [PubMed] [Google Scholar]
- 85.al-Ramadi BK, et al. Potent anti-tumor activity of systemically-administered IL2-expressing Salmonella correlates with decreased angiogenesis and enhanced tumor apoptosis. Clin. Immunol. 2009;130:89–97. doi: 10.1016/j.clim.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 86.Chandra D, et al. 32-Phosphorus selectively delivered by listeria to pancreatic cancer demonstrates a strong therapeutic effect. Oncotarget. 2017;8:20729–20740. doi: 10.18632/oncotarget.15117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murakami T, et al. Adjuvant treatment with tumor-targeting Salmonella typhimurium A1-R reduces recurrence and increases survival after liver metastasis resection in an orthotopic nude mouse model. Oncotarget. 2015;6:41856–41862. doi: 10.18632/oncotarget.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong G, et al. Novel cancer vaccine based on genes of Salmonella pathogenicity island 2. Int J. Cancer. 2010;126:2622–2634. doi: 10.1002/ijc.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park SH, et al. RGD peptide cell-surface display enhances the targeting and therapeutic efficacy of attenuated salmonella-mediated cancer therapy. Theranostics. 2016;6:1672–1682. doi: 10.7150/thno.16135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai YM, Toley BJ, Swofford CA, Forbes NS. Construction of an inducible cell-communication system that amplifies Salmonella gene expression in tumor tissue. Biotechnol. Bioeng. 2013;110:1769–1781. doi: 10.1002/bit.24816. [DOI] [PubMed] [Google Scholar]
- 91.Bereta M, et al. Improving tumor targeting and therapeutic potential of Salmonella VNP20009 by displaying cell surface CEA-specific antibodies. Vaccine. 2007;25:4183–4192. doi: 10.1016/j.vaccine.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akin D, et al. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat. Nanotechnol. 2007;2:441–449. doi: 10.1038/nnano.2007.149. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y, et al. Smart bacterial magnetic nanoparticles for tumor-targeting magnetic resonance imaging of HER2-positive Breast cancers. ACS Appl Mater. Interfaces. 2019;11:3654–3665. doi: 10.1021/acsami.8b15838. [DOI] [PubMed] [Google Scholar]
- 94.Pinero-Lambea C, et al. Programming controlled adhesion of E. coli to target surfaces, cells, and tumors with synthetic adhesins. ACS Synth. Biol. 2015;4:463–473. doi: 10.1021/sb500252a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stritzker J, et al. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J. Med. Microbiol. 2007;297:151–162. doi: 10.1016/j.ijmm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 96.Weibel S, Stritzker J, Eck M, Goebel W, Szalay AA. Colonization of experimental murine breast tumours by Escherichia coli K-12 significantly alters the tumour microenvironment. Cell Microbiol. 2008;10:1235–1248. doi: 10.1111/j.1462-5822.2008.01122.x. [DOI] [PubMed] [Google Scholar]
- 97.Secher T, Samba-Louaka A, Oswald E, Nougayrede JP. Escherichia coli producing colibactin triggers premature and transmissible senescence in mammalian cells. Plos ONE. 2013;8:e77157. doi: 10.1371/journal.pone.0077157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bereswill S, et al. Pro-inflammatory potential of Escherichia Coli astrains K12 and Nissle 1917 in a murine model of sacute Ileitis. Eur. J. Microbiol. Immunol. 2013;3:126–134. doi: 10.1556/EuJMI.3.2013.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y, et al. E. coli Nissle 1917-derived minicells for targeted delivery of chemotherapeutic drug to hypoxic regions for cancer therapy. Theranostics. 2018;8:1690–1705. doi: 10.7150/thno.21575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liang K, et al. Genetically engineered salmonella typhimurium: recent advances in cancer therapy. Cancer Lett. 2019;448:168–181. doi: 10.1016/j.canlet.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 101.Chien T, Doshi A, Danino T. Advances in bacterial cancer therapies using synthetic biology. Curr. Opin. Syst. Biol. 2017;5:1–8. doi: 10.1016/j.coisb.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mengesha A, et al. Development of a flexible and potent hypoxia-inducible promoter for tumor-targeted gene expression in attenuated Salmonella. Cancer Biol. Ther. 2006;5:1120–1128. doi: 10.4161/cbt.5.9.2951. [DOI] [PubMed] [Google Scholar]
- 103.Ryan RM, et al. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther. 2009;16:329–339. doi: 10.1038/gt.2008.188. [DOI] [PubMed] [Google Scholar]
- 104.Javan, B. Shahbazi, M. Hypoxia-inducible tumour-specific promoters as a dual-targeting transcriptional regulation system for cancer gene therapy. Ecancermedicalscienc11, 10.3332/ecancer.2017.751 (2017). [DOI] [PMC free article] [PubMed]
- 105.Yu B, et al. Explicit hypoxia targeting with tumor suppression by creating an “obligate” anaerobic Salmonella Typhimurium strain. Sci. Rep. 2012;2:436. doi: 10.1038/srep00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Flentie K, et al. A bioluminescent transposon reporter-trap identifies tumor-specific microenvironment-induced promoters in Salmonella for conditional bacterial-based tumor therapy. Cancer Discov. 2012;2:624–637. doi: 10.1158/2159-8290.CD-11-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baumgartner JW, et al. Transmembrane signalling by a hybrid protein: communication from the domain of chemoreceptor Trg that recognizes sugar-binding proteins to the kinase/phosphatase domain of osmosensor EnvZ. J. Bacteriol. 1994;176:1157–1163. doi: 10.1128/jb.176.4.1157-1163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Din MO, et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature. 2016;536:81–85. doi: 10.1038/nature18930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Swofford CA, Van Dessel N, Forbes NS. Quorum-sensing Salmonella selectively trigger protein expression within tumors. Proc. Natl Acad. Sci. USA. 2015;112:3457–3462. doi: 10.1073/pnas.1414558112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim K, et al. Cell mass-dependent expression of an anticancer protein drug by tumor-targeted Salmonella. Oncotarget. 2018;9:8548–8559. doi: 10.18632/oncotarget.24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anderson JC, Clarke EJ, Arkin AP, Voigt CA. Environmentally controlled invasion of cancer cells by engineered bacteria. J. Mol. Biol. 2006;355:619–627. doi: 10.1016/j.jmb.2005.10.076. [DOI] [PubMed] [Google Scholar]
- 112.Loessner H, et al. Remote control of tumour-targeted Salmonella enterica serovar Typhimurium by the use of L-arabinose as inducer of bacterial gene expression in vivo. Cell Microbiol. 2007;9:1529–1537. doi: 10.1111/j.1462-5822.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 113.Olino K, et al. Tumor-associated antigen expressing Listeria monocytogenes induces effective primary and memory T-cell responses against hepatic colorectal cancer metastases. Ann. Surg. Oncol. 2012;19:S597–S607. doi: 10.1245/s10434-011-2037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hong H, et al. Targeted deletion of the ara operon of Salmonella typhimurium enhances L-arabinose accumulation and drives PBAD-promoted expression of anti-cancer toxins and imaging agents. Cell Cycle. 2014;13:3112–3120. doi: 10.4161/15384101.2014.949527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Royo JL, et al. In vivo gene regulation in Salmonella spp. by a salicylate-dependent control circuit. Nat. Methods. 2007;4:937–942. doi: 10.1038/nmeth1107. [DOI] [PubMed] [Google Scholar]
- 116.Becker PD, Royo JL, Guzman CA. Exploitation of prokaryotic expression systems based on the salicylate-dependent control circuit encompassing nahR/P(sal)::xylS2 for biotechnological applications. Bioeng. Bugs. 2010;1:244–251. doi: 10.4161/bbug.1.4.11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Medina C, Camacho EM, Flores A, Mesa-Pereira B, Santero E. Improved expression systems for regulated expression in Salmonella infecting eukaryotic cells. PLoS ONE. 2011;6:e23055. doi: 10.1371/journal.pone.0023055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baron U, Freundlieb S, Gossen M, Bujard H. Co-regulation of two gene activities by tetracycline via a bidirectional promoter. Nucleic Acids Res. 1995;23:3605–3606. doi: 10.1093/nar/23.17.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Padidam M. Chemically regulated gene expression in plants. Curr. Opin. Plant Biol. 2003;6:169–177. doi: 10.1016/S1369-5266(03)00005-0. [DOI] [PubMed] [Google Scholar]
- 120.Nuyts S, et al. Increasing specificity of anti-tumor therapy: cytotoxic protein delivery by non-pathogenic clostridia under regulation of radio-induced promoters. Anticancer Res. 2001;21:857–861. [PubMed] [Google Scholar]
- 121.Zuo SG, et al. Orally administered DNA vaccine delivery by attenuated salmonella typhimurium targeting fetal liver kinase 1 inhibits murine lewis lung carcinoma growth and metastasis. Biol. Pharm. Bull. 2010;33:174–182. doi: 10.1248/bpb.33.174. [DOI] [PubMed] [Google Scholar]
- 122.Berger E, et al. Salmonella SL7207 application is the most effective DNA vaccine delivery method for successful tumor eradication in a murine model for neuroblastoma. Cancer Lett. 2013;331:167–173. doi: 10.1016/j.canlet.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 123.Yoshimura K, et al. Selective targeting of antitumor immune responses with engineered live-attenuated Listeria monocytogenes. Cancer Res. 2006;66:1096–1104. doi: 10.1158/0008-5472.CAN-05-2307. [DOI] [PubMed] [Google Scholar]
- 124.Danen-Van Oorschot AA, et al. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc. Natl Acad. Sci. USA. 1997;94:5843–5847. doi: 10.1073/pnas.94.11.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu X, et al. Nanocarriers for TRAIL delivery: driving TRAIL back on track for cancer therapy. Nanoscale. 2017;9:13879–13904. doi: 10.1039/C7NR04959E. [DOI] [PubMed] [Google Scholar]
- 126.Cao HD, et al. Attenuated Salmonella typhimurium carrying TRAIL and VP3 genes inhibits the growth of gastric cancer cells in vitro and in vivo. Tumori. 2010;96:296–303. doi: 10.1177/030089161009600218. [DOI] [PubMed] [Google Scholar]
- 127.Yoon. WS, Chae YS, Hong J, Park YK. Antitumor therapeutic effects of a genetically engineered Salmonella typhimurium harboring TNF-α in mice. Appl. Microbiol. Biotechnol. 2011;89:1807–1819. doi: 10.1007/s00253-010-3006-4. [DOI] [PubMed] [Google Scholar]
- 128.Critchley-Thorne RJ, Stagg AJ, Vassaux G. Recombinant Escherichia coli expressing invasin targets the Peyer’s patches: the basis for a bacterial formulation for oral vaccination. Mol. Ther. 2006;14:183–191. doi: 10.1016/j.ymthe.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 129.Cheng CM, et al. Tumor-targeting prodrug-activating bacteria for cancer therapy. Cancer Gene Ther. 2008;15:393–401. doi: 10.1038/cgt.2008.10. [DOI] [PubMed] [Google Scholar]
- 130.Friedlos F, et al. Attenuated Salmonella targets prodrug activating enzyme carboxypeptidase G2 to mouse melanoma and human breast and colon carcinomas for effective suicide gene therapy. Clin. Cancer Res. 2008;14:4259–4266. doi: 10.1158/1078-0432.CCR-07-4800. [DOI] [PubMed] [Google Scholar]
- 131.Tang W, He Y, Zhou S, Ma Y, Liu G. A novel Bifidobacterium infantis-mediated TK/GCV suicide gene therapy system exhibits antitumor activity in a rat model of bladder cancer. J. Exp. Clin. Cancer Res. 2009;28:155. doi: 10.1186/1756-9966-28-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu SC, Minton NP, Giaccia AJ, Brown JM. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther. 2002;9:291–296. doi: 10.1038/sj.gt.3301659. [DOI] [PubMed] [Google Scholar]
- 133.Dresselaers T, et al. Non-invasive 19F MR spectroscopy of 5-fluorocytosine to 5-fluorouracil conversion by recombinant Salmonella in tumours. Br. J. Cancer. 2003;89:1796–1801. doi: 10.1038/sj.bjc.6601345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barbe S, et al. Secretory production of biologically active rat interleukin-2 by Clostridium acetobutylicum DSM792 as a tool for anti-tumor treatment. FEMS Microbiol. Lett. 2005;246:67–73. doi: 10.1016/j.femsle.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 135.Sasaki T, et al. Genetically engineered Bifidobacterium longum for tumor-targeting enzyme-prodrug therapy of autochthonous mammary tumors in rats. Cancer Sci. 2006;97:649–657. doi: 10.1111/j.1349-7006.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Le UN, et al. Engineering and visualization of bacteria for targeting infarcted myocardium. Mol. Ther. 2011;19:951–959. doi: 10.1038/mt.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yoon W, et al. Application of genetically engineered Salmonella typhimurium for interferon-gamma-induced therapy against melanoma. Eur. J. Cancer. 2017;70:48–61. doi: 10.1016/j.ejca.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 138.Yuhua L, et al. Prophylaxis of tumor through oral administration of IL-12 GM-CSF gene carried by live attenuated salmonella. Chin. Sci. Bull. 2001;46:1107–1111. doi: 10.1007/BF02900689. [DOI] [Google Scholar]
- 139.Shahabi V, et al. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol. Immunother. 2008;57:1301–1313. doi: 10.1007/s00262-008-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xiang R, et al. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res. 2005;65:553–561. [PubMed] [Google Scholar]
- 141.Coakley M, et al. Intestinal bifidobacteria that produce trans-9, trans-11 conjugated linoleic acid: a fatty acid with antiproliferative activity against human colon SW480 and HT-29 cancer cells. Nutr. Cancer. 2006;56:95–102. doi: 10.1207/s15327914nc5601_13. [DOI] [PubMed] [Google Scholar]
- 142.Buonaguro L, Petrizzo A, Tornesello ML, Buonaguro FM. Translating tumor antigens into cancer vaccines. Clin. Vaccin. Immunol. 2011;18:23–34. doi: 10.1128/CVI.00286-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Singh R, Paterson Y. In the FVB/N HER-2/neu transgenic mouse both peripheral and central tolerance limit the immune response targeting HER-2/neu induced by Listeria monocytogenes-based vaccines. Cancer Immunol. Immunother. 2007;56:927–938. doi: 10.1007/s00262-006-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hannan R, et al. Combined immunotherapy with Listeria monocytogenes-based PSA vaccine and radiation therapy leads to a therapeutic response in a murine model of prostate cancer. Cancer Immunol. Immunother. 2012;61:2227–2238. doi: 10.1007/s00262-012-1257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Arrach N, Zhao M, Porwollik S, Hoffman RM, McClelland M. Salmonella promoters preferentially activated inside tumors. Cancer Res. 2008;68:4827–4832. doi: 10.1158/0008-5472.CAN-08-0552. [DOI] [PubMed] [Google Scholar]
- 146.Pitt JM, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44:1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 147.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 148.Zhang HY, et al. Tumor-targeted delivery of biologically active TRAIL protein. Cancer Gene Ther. 2010;17:334–343. doi: 10.1038/cgt.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jia H, et al. Antitumor effects of Stat3-siRNA and endostatin combined therapies, delivered by attenuated Salmonella, on orthotopically implanted hepatocarcinoma. Cancer Immunol. Immunother. 2012;61:1977–1987. doi: 10.1007/s00262-012-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nassiri F, et al. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283–2290. [PubMed] [Google Scholar]
- 151.Wood LM, et al. Targeting tumor vasculature with novel Listeria-based vaccines directed against CD105. Cancer Immunol. Immunother. 2011;60:931–942. doi: 10.1007/s00262-011-1002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Manuel ER, et al. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella-based STAT3 shRNA suppresses the growth of established melanoma tumors. Cancer Res. 2011;71:4183–4191. doi: 10.1158/0008-5472.CAN-10-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blache CA, et al. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res. 2012;72:6447–6456. doi: 10.1158/0008-5472.CAN-12-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Manuel ER, et al. Salmonella-based therapy targeting indoleamine 2,3-dioxygenase coupled with enzymatic depletion of tumor hyaluronan induces complete regression of aggressive pancreatic tumors. Cancer Immunol. Res. 2015;3:1096–1107. doi: 10.1158/2326-6066.CIR-14-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kong Q, et al. Phosphate groups of lipid A are essential for Salmonella enterica serovar Typhimurium virulence and affect innate and adaptive immunity. Infect. Immun. 2012;80:3215–3224. doi: 10.1128/IAI.00123-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Phan, T. et al. Salmonella-mediated therapy targeting indoleamine 2, 3-dioxygenase 1 (IDO) activates innate immunity and mitigates colorectal cancer growth. Cancer Gene Ther.10.1038/s41417-019-0089-7 (2019). [DOI] [PMC free article] [PubMed]
- 157.Coley WB., II Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891;14:199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou S, Gravekamp C, Bermudes D, Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer. 2018;18:727–743. doi: 10.1038/s41568-018-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Toso JF, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 2002;20:142–152. doi: 10.1200/JCO.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Carey RW, Holland JF, Whang HY, Neter E, Bryant B. Clostridial oncolysis in man. Eur. J. Cancer (1965) 1967;3:43–46. doi: 10.1016/0014-2964(67)90060-6. [DOI] [Google Scholar]
- 161.Heppner F, Mose JR. The liquefaction (oncolysis) of malignant gliomas by a non pathogenic Clostridium. Acta Neurochir. (Wien.) 1978;42:123–125. doi: 10.1007/BF01406639. [DOI] [PubMed] [Google Scholar]
- 162.Roberts NJ, et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 2014;6:249ra111. doi: 10.1126/scitranslmed.3008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Russmann H, et al. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science. 1998;281:565–568. doi: 10.1126/science.281.5376.565. [DOI] [PubMed] [Google Scholar]
- 164.Shi L, Yu B, Cai CH, Huang JD. Angiogenic inhibitors delivered by the type III secretion system of tumor-targeting Salmonella typhimurium safely shrink tumors in mice. AMB Express. 2016;6:56. doi: 10.1186/s13568-016-0226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sorenson BS, Banton KL, Frykman NL, Leonard AS, Saltzman DA. Attenuated Salmonella typhimurium with IL-2 gene reduces pulmonary metastases in murine osteosarcoma. Clin. Orthop. Relat. Res. 2008;466:1285–1291. doi: 10.1007/s11999-008-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jellbauer S, Panthel K, Hetrodt JH, Russmann H. CD8 T-cell induction against vascular endothelial growth factor receptor 2 by Salmonella for vaccination purposes against a murine melanoma. PLoS ONE. 2012;7:e34214. doi: 10.1371/journal.pone.0034214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kocijancic D, et al. Local application of bacteria improves safety of Salmonella -mediated tumor therapy and retains advantages of systemic infection. Oncotarget. 2017;8:49988–50001. doi: 10.18632/oncotarget.18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mesa-Pereira B, Medina C, Camacho EM, Flores A, Santero E. Improved cytotoxic effects of Salmonella-producing cytosine deaminase in tumour cells. Micro. Biotechnol. 2015;8:169–176. doi: 10.1111/1751-7915.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.O’Riordan M, Moors MA, Portnoy DA. Listeria intracellular growth and virulence require host-derived lipoic acid. Science. 2003;302:462–464. doi: 10.1126/science.1088170. [DOI] [PubMed] [Google Scholar]
- 170.Panteli JT, Forbes NS. Engineered bacteria detect spatial profiles in glucose concentration within solid tumor cell masses. Biotechnol. Bioeng. 2016;113:2474–2484. doi: 10.1002/bit.26006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kimura H, et al. Targeted therapy of spinal cord glioma with a genetically modified Salmonella typhimurium. Cell Prolif. 2010;43:41–48. doi: 10.1111/j.1365-2184.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schmitz-Winnenthal FH, et al. A phase 1 trial extension to assess immunologic efficacy and safety of prime-boost vaccination with VXM01, an oral T cell vaccine against VEGFR2, in patients with advanced pancreatic cancer. Oncoimmunology. 2018;7:e1303584. doi: 10.1080/2162402X.2017.1303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Le DT, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 2015;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Basu P, et al. A randomized phase 2 satudy of ADXS11-001 listeria monocytogenes-Listeriolysin O immunotherapy with or without cisplatin in treatment of advanced cervical cancer. Int J. Gynecol. Cancer. 2018;28:764–772. doi: 10.1097/IGC.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]