Abstract
Background
Large numbers of tuberculosis (TB) patients seek care from private for-profit providers. This study aimed to assess and compare TB control activities in the private for-profit and public sectors in Kenya between 2013 and 2017.
Methods
We conducted a retrospective cross-sectional study using routinely collected data from the National Tuberculosis, Leprosy and Lung Disease Program.
Results
Of 421 409 patients registered and treated between 2013 and 2017, 86 894 (21%) were from the private sector. Data collection was less complete in the private sector for nutritional assessment and follow-up sputum smear examinations (p<0.001). The private sector notified less bacteriologically confirmed TB (43.1% vs 52.6%; p<0.001) and had less malnutrition (body mass index <18.5 kg/m2; 36.4% vs 43.3%; p<0.001) than the public sector. Rates of human immunodeficiency virus (HIV) testing and antiretroviral therapy initiation were >95% and >90%, respectively, in both sectors, but more patients were HIV positive in the private sector (39.6% vs 31.6%; p<0.001). For bacteriologically confirmed pulmonary TB, cure rates were lower in the private sector, especially for HIV-negative patients (p<0.001). The private sector had an overall treatment success of 86.3% as compared with the public sector at 85.7% (p<0.001).
Conclusions
The private sector is performing well in Kenya although there are programmatic challenges that need to be addressed.
Keywords: Kenya, operational research, private–public mix, SORT-IT, tuberculosis
Introduction
Many resource-poor countries have a large and expanding private health sector and there is growing evidence that increasing numbers of patients with tuberculosis (TB) seek care from private for-profit providers.1–3 In recent years the World Health Organization (WHO) has begun to address the issue of private for-profit providers in TB prevention and care through an evolving global strategy called the public–private mix (PPM). The PPM for TB care and control is defined as the involvement of all health care providers in TB control so as to promote the use of international standards for TB care in all health sectors and thereby achieve national and global TB control targets.3 In 2014 the WHO launched the End TB strategy, with an ambitious goal of ending the global TB epidemic by 2035. One of the components of the second pillar of the End TB strategy fully embraces PPM and emphasizes the importance of engaging communities, civil society organizations and all public and private care providers.4
The published literature contains conflicting reports about the impact of PPM programs on TB control efforts. In a systematic review that assessed 48 PPM TB programs worldwide and in another study from Myanmar, PPM was associated with significantly improved TB case detection, human immunodeficiency virus (HIV) testing uptake and treatment outcomes.5,6 However, in another study from Zambia, the majority of the facilities that diagnosed and managed TB in the private sector failed to report their TB activities to the National Tuberculosis Control Program.7 There are challenges and obstacles identified that need to be overcome to successfully scale up the PPM strategy. These challenges include weak collaboration between the TB programs and the private sector, limited funding, poor governance and lack of continuous training of private-sector health care providers.5,8
Kenya is one of the 14 countries in the world with a high burden of TB, multidrug-resistant TB (MDRTB) and TB/HIV. In 2017, the country notified 85 188 drug-sensitive TB cases, of which 22 992 (29%) were co-infected with HIV. In addition, 577 patients had MDRTB (TB that is resistant to at least isoniazid and rifampicin).9,10 Implementation of the PPM strategy started in 1997 as a joint collaboration between the National Tuberculosis, Leprosy and Lung Disease Program (NTLDP) and the Kenya Association for the Prevention of Tuberculosis and Lung Diseases (KAPTLD).11 In Kenya, the private sector includes both private not-for-profit and private for-profit health facilities. The private not-for-profit sector includes faith-based (FBO) and non-governmental organizations (NGOs).12 Both the FBO and the NGO health facilities function in a similar way to the public sector, and for TB control purposes their case finding and treatment outcomes are included in the public-sector TB program reports. The PPM strategy in Kenya has therefore focused predominately on private for-profit health facilities.
A previous assessment of PPM 10 years ago in Nairobi, the capital of Kenya, showed an improvement in TB case notifications from 469 to 1740 between 2002 and 2006.1 This increase in caseload was associated with an improvement in treatment success from 76% to 85%. However, HIV testing and provision of antiretroviral therapy (ART) and co-trimoxazole preventive therapy (CPT) to co-infected patients remained a challenge, with low reported uptake rates of 42%, 37% and 61%, respectively.1
Since then and following the WHO End TB strategy recommendations in 2015, there has been growing interest in improving the PPM strategy in Kenya. The NTLDP strategic plan for 2015–2018 included many activities for strengthening private-sector performance in TB control,11 and for several of these, the implementation is ongoing. The NTLDP was interested to know whether case finding, TB–HIV collaborative activities and treatment outcomes in the private sector have improved in recent years and whether the achievement of various indicators has kept pace with what has been achieved in the public sector.
Therefore the aim of this study was to assess, at the national level, TB control activities in the private for-profit health sector in Kenya from 2013 through 2017 and compare the findings with those reported in the public sector (including government, FBO and NGO sectors). The specific objectives were to determine and compare, between the private and public health sectors in Kenya, the completeness of data collection for selected key variables; notified TB cases in relation to key sociodemographic and clinical characteristics; TB–HIV collaborative activities, including HIV testing uptake, and the use of ART and CPT for those found to be co-infected; and treatment outcomes of notified TB cases in relation to HIV status.
Materials and methods
Study design
This was a retrospective cross-sectional study using routine surveillance case-based data collected by the NTLDP from 2013 to 2017.
Setting
General setting
Kenya is an East African country located along the Equator. The country is bordered by Somalia, Sudan, Ethiopia, Uganda and Tanzania. In 2017 the population was estimated at 49.7 million, with 32% living in urban areas, and life expectancy from birth was estimated at 58 y.13,14 The major drivers of the country’s economy have been agriculture, fishing, forestry, education, retail trade, wholesale, construction and financial insurance.13 In 2016, the gross domestic product per capita was approximately US$1580.15 In 2013, the number of physicians per 1000 population was estimated at 0.2.16
Health care system in Kenya
In Kenya, there are 9696 health facilities, of which 48% are government/public, 38% are private for-profit and 14% are private not-for-profit.12 The public sector includes all government health facilities (hospitals, clinics and dispensaries), public medical schools and the public pharmaceutical supply chain called KEMSA. The private not-for-profit sector comprises FBOs and NGOs, including mission health facilities (hospitals, clinics and dispensaries), medical schools and the faith-based pharmaceutical supply agency. The private for-profit sector includes private hospitals, privately owned clinics and private medical distributors/suppliers. Most of the Kenyan health workers work either full time or part time in the private sector, and this includes about 75% of the medical doctors and 66% of the clinical officers and nurses. In order to keep staff available for the government public sector, many doctors are allowed to work in the morning hours in public health facilities and in the afternoon in their private clinics.12
TB case management and reporting in Kenya
The diagnosis and treatment of TB in Kenya are the responsibility of the Ministry of Health through the NTLDP. Presumptive and confirmed TB cases are managed by the county departments of health through peripheral health facilities. Overall, the country has 3424 TB treatment facilities, of which 2654 are public, 688 are private for-profit and 82 are FBOs. The diagnosis and treatment of TB, HIV testing and ART and nutritional commodities are free of charge in the public sector and FBOs, while private-sector patients generally have to pay for TB services according to hospital policies. The private sector in Kenya does not currently treat drug-resistant TB and all diagnosed cases are referred to the public sector.
According to the NTLDP strategic plan, in areas where PPM is being implemented there is refresher training and on-the-job training about TB control for health care workers in the private sector, regular technical assistance provided by county TB coordinators, a networking of all private laboratories with the national External Quality Assurance System so that there is quality control of diagnosis and a system to ensure referral of samples between the different sectors if this is needed and a regular supply of updated guidelines for TB care and management to private-sector facilities.11
In order to ensure good quality data capture and prompt notification of cases, the NTLDP provides the private sector with paper-based reporting tools and, for every private facility, has linked the recording of results on case finding and treatment outcomes to the national electronic data reporting system (TIBU). Subcounty TB coordinators supervise each of the facilities (including private) in their zones once a month to ensure proper recording of cases and outcomes and adherence to guidelines. Once the data have been checked, the coordinators transfer the paper-based information into the TIBU using an electronic tablet. At the national level, a team of monitoring and evaluation officers do routine data checks and communicate any anomalies back to the subcounty TB coordinators, who then visit the facilities for on-the-job training to ensure data quality.
Every quarter, a review meeting is conducted between the national program officers and the county and subcounty TB coordinators to validate the data and ensure quality. Quarterly and annual reports are thus generated for all TB treatment units in the public and private sectors. The NTLDP also conducts annual data quality audits in selected public and private facilities to ensure the collected data are of high quality.17 In TIBU, some fields are marked as ‘must enter’ and therefore their data entry should be complete. These include variables that were collected in this study.
Study population
All patients diagnosed and treated for TB in the private and public sectors of Kenya from January 2013 through December 2017 were included.
Data variables, data collection and sources of data
The data analysed was from the electronic records in the NTLDP (TIBU) which were downloaded into an Excel file (Microsoft, Redmond, WA, USA) between March and September 2018. The variables included in the analysis for both private and public sectors were recording of referral to the TB clinic; body mass index (BMI); follow-up sputum smear examination at months 2, 5 and 6 of treatment; HIV and ART uptake indicators (HIV testing and results, ART and CPT uptake and respective dates) and treatment outcomes with dates. Additional variables included the year of registration, demographic characteristics and the type and category of TB. Treatment outcomes (which were also analysed and presented by the type of TB and HIV status) were in line with WHO guidelines.18 To appreciate the nutritional status, all patients with a BMI <18.5 kg/m2 were considered ‘undernourished’.
Analysis and statistics
Data were exported from the Excel file into STATA version 14 (StataCorp, College Station, TX, USA) for analysis. A descriptive analysis was performed using frequencies and proportions. Demographic and clinical characteristics and treatment outcomes between the private and public sectors were compared using the χ2 test with levels of significance set at 5% (p<0.05).
Results
Between 2013 and 2017, there was a total of 421 409 TB patients registered in the NTLDP in Kenya, of whom 86 894 (21%) were notified from the private sector and 334 515 (79%) from the public sector. The completeness of data reporting in both sectors for the 5-year period is shown in Table 1. The private sector performed better with respect to documentation of referral status (p<0.02) and treatment outcomes (p<0.001), while the public sector performed better in nutritional assessment (BMI) (p<0.001) and in reporting follow-up sputum smear results at 2, 5 and 6 months of treatment (p<0.001). The recording of the date of HIV testing and date for ART initiation for HIV-positive TB patients was similarly poor between both sectors, at 51.9% and 45.8% in the private sector and 54.4% and 45.9% in the public sector, respectively.
Table 1.
Data completeness for TB patients registered for treatment in the private and public health sectors of Kenya between 2013 and 2017
| Variables | Private sector, n (%) | Public sector, n (%) | p-Value |
|---|---|---|---|
| Referral to the TB clinica | |||
| Total notified | 86 894 | 334 515 | |
| Referral status recorded | 85 256 (98.1) | 327 750 (98.0) | 0.02 |
| BMI | |||
| Total notified | 86 894 | 334 515 | |
| BMI recorded | 67 298 (77.4) | 275 875 (82.5) | <0.001 |
| Sputum smear examination month 2b | |||
| Total notified | 37 468 | 176 105 | |
| Smear results recorded | 28 921 (77.2) | 138 171 (78.5) | <0.001 |
| Sputum smear examination month 5b | |||
| Total notified | 37 468 | 176 105 | |
| Smear results recorded | 19 757 (52.7) | 94 982 (53.9) | <0.001 |
| Sputum smear examination month 6b | |||
| Total notified | 37 468 | 176 105 | |
| Smear results recorded | 25 920 (69.2) | 123 226 (70.0) | <0.001 |
| Treatment outcome | |||
| Total with treatment outcome | 86 894 | 334 515 | |
| Treatment outcome recorded | 79 391 (91.4) | 304 070 (90.9) | <0.001 |
| Date of treatment outcome | |||
| Total with treatment outcome | 86 894 | 334 515 | |
| Treatment outcome date recorded | 79 366 (91.3) | 303 938 (90.9) | <0.001 |
| Date of HIV testing | |||
| Total notified for HIV testing | 86 894 | 334 515 | |
| HIV testing date recorded | 45 127 (51.9) | 181 972 (54.4) | <0.001 |
| ART start datec | |||
| Total eligible for ARTd | 32 764 | 102 012 | |
| Total initiated on ART | 30 266 | 94 398 | |
| ART start date recorded | 13 854 (45.8)e | 43 320 (45.9)e | NS |
aAny referral by a health worker for TB investigation.
bAssessed for bacteriologically confirmed TB patients only.
cAssessed for the HIV co-infected patients only.
dTotal eligible for ART were only those who were positive for HIV on serological testing.
eThe denominator was the total initiated on ART.
Sociodemographic and clinical characteristics of the notified TB patients in both sectors for the 5-year period are compared in Table 2. The proportion of children was similar in both sectors but the private sector had more adults 15–64 years of age while the public sector had more elderly patients ≥65 years of age. Significantly more males were notified from the private sector. There was significantly more bacteriologically confirmed TB in the public sector (with >50% of cases bacteriologically confirmed), while the private sector notified more clinically diagnosed TB and extrapulmonary TB (EPTB). The proportion of patients previously treated for TB was higher in the public sector. There was significantly more malnutrition in the public sector, with a higher proportion of patients with a BMI <18.5 kg/m2.
Table 2.
Sociodemographic and clinical characteristics of notified TB patients in the private and public health sectors of Kenya between 2013 and 2017
| Characteristics | Private sector, n (%) | Public sector, n (%) | p-Value |
|---|---|---|---|
| Total notified TB patients | 86 894 | 334 515 | |
| Age (years) | |||
| <14 | 8191 (9.4) | 29 794 (8.9) | NS |
| 15–64 | 75 011 (86.3) | 286 939 (85.8) | <0.001 |
| ≥65 | 3692 (4.2) | 17 782 (5.3) | <0.01 |
| Gender | |||
| Male | 36 076 (41.5) | 124 614 (37.3) | <0.001 |
| Female | 50 818 (58.5) | 209 901 (62.7) | <0.001 |
| Type of TB | |||
| Bacteriologically confirmed PTB | 37 468 (43.1) | 176 105 (52.6) | <0.001 |
| Clinically diagnosed PTB | 32 002 (36.8) | 103 491 (30.9) | <0.001 |
| EPTB | 17 424 (20.1) | 54 919 (16.4) | <0.001 |
| Category of TB | |||
| New | 80 853 (93.0) | 306 010 (91.5) | <0.001 |
| Previously treated | 6041 (7.0) | 28 505 (8.5) | <0.001 |
| BMI (kg/m2) | |||
| <18.5 (malnutrition) | 31 613 (36.4) | 144 781 (43.3) | <0.001 |
| ≥18.5 (normal nutrition) | 55 281 (63.6) | 189 734 (56.7) | <0.001 |
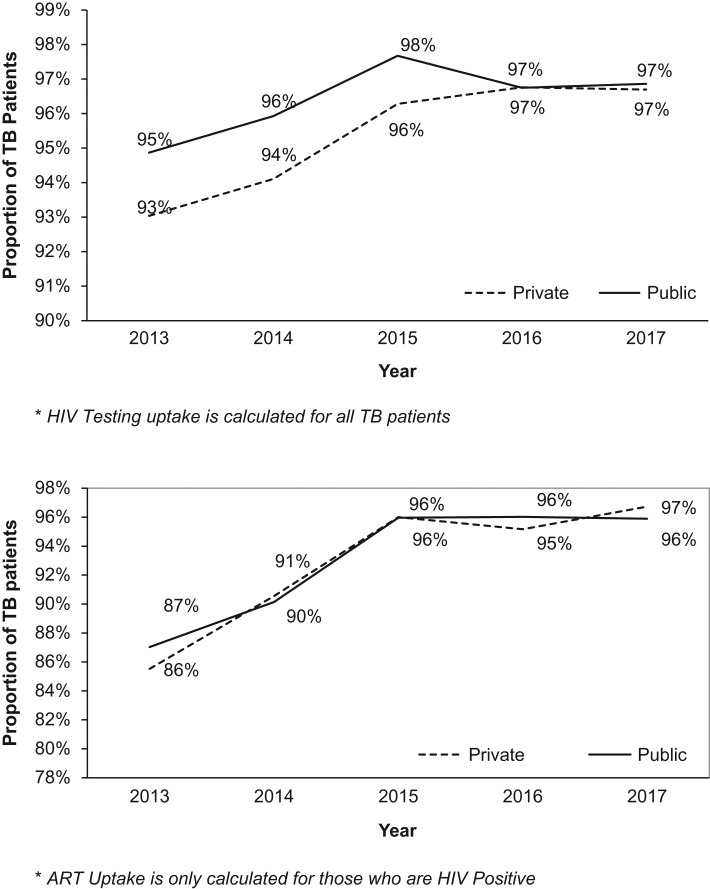
HIV-associated TB collaborative activities in both sectors for the 5-year period are compared in Table 3. HIV testing uptake was >95% in both sectors, although slightly better in the public sector. In those who were tested, HIV positivity was significantly higher in the private sector. For HIV-positive TB patients, uptake of ART was similar in both sectors at >92%, while that of CPT was at about 99%, with slightly better results in the public sector. HIV testing and ART uptake were also assessed annually between 2013 and 2017, with results shown in Figure 1A and B. HIV testing uptake in the private sector lagged behind the public sector for the first 3 years (2013–2015) and then caught up, and in both sectors HIV testing increased to plateau at 97% for 2016 and 2017. ART uptake for those who were HIV positive was fairly similar in both sectors and increased from 86–87% to 96–97%.
Table 3.
HIV-associated TB activities among patients notified in the private and public health sectors of Kenya between 2013 and 2017
| HIV-associated activities | Private sector, n (%) | Public sector, n (%) | p-Value |
|---|---|---|---|
| HIV testing | |||
| Total notified TB cases | 86 894 | 334 515 | |
| Number tested | 82 801 (95.3) | 322 394 (96.4) | <0.001 |
| HIV status | |||
| Positive | 32 764 (39.6) | 102 012 (31.6) | <0.001 |
| Negative | 50 037 (60.4) | 220 382 (68.4) | <0.001 |
| ART uptake | |||
| Eligible for ART | 32 764 | 102 012 | |
| Number started on ART | 30 266 (92.4) | 94 398 (92.5) | NS |
| CPT uptake | |||
| Eligible for CPT | 32 764 | 102 012 | |
| Number started on CPT | 32 410 (98.9) | 101 201 (99.2) | <0.001 |
Figure 1.
(A) Annual trends of HIV testing among TB patients registered for treatment in the private and public health sectors of Kenya between 2013 and 2017. *HIV testing uptake is calculated for all TB patients. (B) Annual trends of ART uptake among TB patients registered for treatment in the private and public health sectors of Kenya between 2013 and 2017. *ART uptake is calculated for only those who are HIV positive.
For all notified TB patients, the private sector had a treatment success of 86.3% (59 751/69 198) over the 4 years from 2013 to 2016, which was significantly better than the public sector at 85.7% (228 829/266 966) (p<0.001). The treatment success rate for HIV-positive TB patients was 82.3% in the private sector compared with 80.8% in the public sector (p<0.001), and for HIV-negative TB patients the treatment success was 89.2% in the private sector compared with 88.6% in the public sector (p<0.001) (see Table 4). Adverse outcomes such as death, lost to follow-up, failure and not evaluated were similar between the two sectors.
Table 4.
Treatment outcomes of notified TB patients in relation to HIV status in the private and public health sectors of Kenya between 2013 and 2016.
| Treatment outcomes | Private sector, n (%) | Public sector, n (%) | p-Value |
|---|---|---|---|
| HIV-positive TB | |||
| Total enrolled | 26 646 | 84 508 | |
| Treatment success | 21 951 (82.3) | 68 262 (80.8) | <0.001 |
| Died | 2580 (9.7) | 9166 (10.8) | NS |
| Lost to follow-up | 1100 (4.1) | 4010 (4.7) | NS |
| Failure | 116 (0.6) | 402 (0.5) | NS |
| Not evaluated | 899 (3.3) | 2668 (3.2) | NS |
| HIV-negative TB | |||
| Total enrolled | 39 044 | 172 462 | |
| Treatment success | 34 861 (89.2) | 152 894 88.6 | <0.001 |
| Died | 1215 (3.1) | 6426 (3.7) | NS |
| Lost to follow-up | 1597 (4.0) | 7822 (4.5) | NS |
| Failure | 181 (0.7) | 1078 (0.6) | NS |
| Not evaluated | 1190 (3.0) | 4242 (2.6) | NS |
| Overall treatment success | |||
| Total enrolled | 69 198 | 266 966 | |
| Treatment success | 59 751 (86.3) | 228 829 (85.7) | <0.001 |
NS: not significant.
More specifically, treatment success (cure and treatment completed) was analysed in relation to HIV status and the type of TB, with results shown in Table 5. For bacteriologically confirmed pulmonary TB (PTB), the cure rate was similar in HIV-positive patients in both the private and public sectors, but in HIV-negative patients the cure rate was significantly lower in the private compared with the public sector. In addition, irrespective of the patients’ HIV status, the treatment completion rate was significantly higher in the private sector compared with the public sector. For clinically diagnosed PTB and EPTB patients, the treatment completion rates were higher in the private sector irrespective of HIV status.
Table 5.
Treatment success (cure and treatment completed) in relation to type of TB for HIV-positive and HIV-negative patients in the private and public health sectors of Kenya between 2013 and 2016
| Successful treatment outcomes | Private sector, n (%) | Public sector, n (%) | p-Value |
|---|---|---|---|
| HIV positive | |||
| Bacteriologically confirmed PTB | |||
| Total enrolled | 8650 | 35 198 | |
| Cured | 6126 (70.8) | 25 100 (71.3) | NS |
| Treatment completed | 1015 (11.7) | 3567 (10.1) | <0.001 |
| Clinically diagnosed PTB and EPTB | |||
| Total enrolled | 17 996 | 49 310 | |
| Treatment completed | 14 798 (82.2) | 39 518 (80.1) | <0.001 |
| HIV negative | |||
| Bacteriologically confirmed PTB | |||
| Total enrolled | 19 303 | 96 697 | |
| Cured | 15 375 (79.7) | 78 176 (80.8) | <0.001 |
| Treatment completed | 1917 (9.9) | 8162 (8.4) | <0.001 |
| Clinically diagnosed PTB and EPTB | |||
| Total enrolled | 19 741 | 75 765 | |
| Treatment completed | 17 542 (88.9) | 66 418 (87.7) | <0.001 |
NS: not significant.
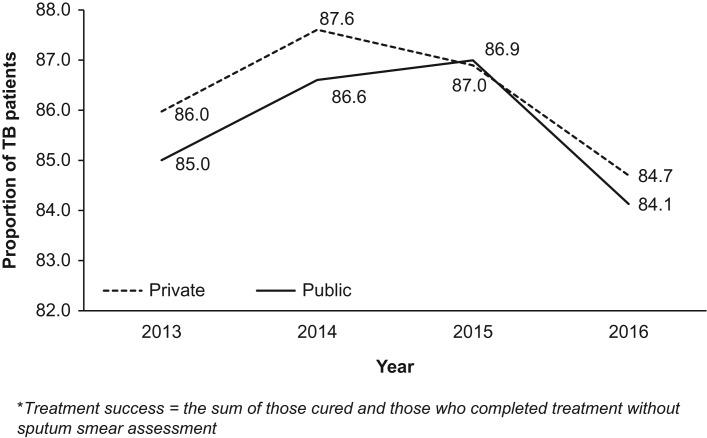
Figure 2 shows annual trends over 4 years in overall treatment success between the two sectors. The treatment success rate decreased in both sectors between 2014 and 2016, from 88% to 85% in the private sector and from 87% to 84% in the public sector.
Figure 2.
Annual trends of treatment success of notified TB patients in the private and public health sectors of Kenya between 2013 and 2016. Treatment success = the sum of those cured and those who completed treatment without sputum smear assessment.
Discussion
This is the first study in Kenya assessing TB control activities in the private for-profit and public health sectors at a national scale. The previous study in Kenya, conducted >10 years ago, included only one county, Nairobi, the capital city.11 There were a number of important findings.
Overall, one-fifth of all TB patients in the country were managed in the private sector, a notable increase from the situation in 2006 when only 9% of TB patients in Nairobi received private-sector care.1 Indeed, the proportion of TB patients managed in the private sector in Kenya was two times higher than in Lagos state, Nigeria, which has had a strong PPM program.19 Reasons for the increase in private-sector involvement in Kenya may be due to the political and technical determination to implement the PPM strategy countrywide. However, it could also be due to poor reception in the public health sector, which makes patients prefer going to private health facilities.
In terms of comparisons, the private sector performed variably with respect to the public sector. For data completion, there was better documentation about referral status and treatment outcomes in the private sector, but for baseline nutritional status and follow-up sputum smear examination, the documentation was not as good. The reasons for this are not clear.
The Kenya NTLDP guidelines in 2013 stipulated the importance of nutritional assessment at the start of treatment,20 and it is possible that some private-sector clinics only measured weight and not weight and height, thereby missing the opportunity to calculate BMI. The underreporting of sputum smear follow-up may have been due to a lack of microscopy access or patients not willing to pay for these examinations. This deficiency needs correction, as it offers the opportunity for earlier diagnosis of treatment failure and switching to a more appropriate treatment regimen.18 The lower rates of sputum smear follow-up may also have been responsible for the lower cure rates and higher treatment completion rates in the private sector among bacteriologically confirmed TB patients, both HIV positive and HIV negative.
In both sectors, documentation of the date of HIV testing and the date for ART initiation for HIV-positive TB patients was poor. Even as early as 2011, Kenya ART guidelines stipulated that all HIV-positive patients should be started on treatment immediately after diagnosis,21 and knowledge of start dates is important in monitoring adherence to this guideline and assessing how well HIV-positive patients respond to ART.
In terms of demographic characteristics and types of TB, there was a higher proportion of males diagnosed in the private sector, which is contrary to that reported elsewhere.2 The reasons may relate to males being more occupied with income-generating activities and preferring private-sector access because of its flexibility and shorter patient waiting time. There were also fewer elderly patients in the private sector, which may relate to financial constraints and more elderly people living in rural areas where most health facilities are public.
The lower rate of bacteriological confirmation and the higher rate of clinical diagnosis of TB in the private sector was an important finding. This may relate to less use of laboratory technology, greater confidence among private-sector doctors to diagnose TB on symptoms, signs and chest radiography or a higher rate of true smear-negative TB because of HIV co-infection. It will be important to further assess this finding through qualitative research, as there are a number of conditions that can be mistaken for TB, such as asthma, chronic lung disease and congestive cardiac failure,22 and there is the risk of possible false TB diagnosis and incorrect treatment.
HIV–TB collaborative activities were excellent in both sectors, with a notable improvement in the private sector compared with 10 years earlier.1 HIV testing uptake was above the national target of 95% in both sectors and ART uptake among HIV-positive patients was at similar levels, with rates continuing to improve over the 4 years of the study.
Treatment success rates were generally good and slightly better in the private compared with the public sector. However, in both sectors, the treatment success rate decreased over the 4 years. A possible reason is the new NTLDP directive to use all diagnosed patients as the denominator for treatment outcomes rather than only those registered for treatment. This directive was based on a Kenya study that looked at underreporting of smear-positive TB in 2014–2015 and the 2015–2016 national prevalence survey showing underreporting of TB cases by 21% and 50%, respectively.23,24 A study in Ghana showed that while treatment success of registered patents was high, this decreased when diagnosed patients were used as the denominator, because some diagnosed patients did not make it to treatment.25 It was reassuring to see that adverse outcomes were no different between the two sectors, in contrast to findings reported elsewhere.2
The strengths of this study include the large number of patients and the nationwide coverage, making the results representative for Kenya. The conduct and reporting of the study also followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.26 Limitations included the retrospective nature of the study, the use of programmatic data that might have had some inaccuracies and the non-inclusion of other TB control indicators such as isoniazid preventive therapy in children <5 years of age and HIV-infected persons and the application of directly observed therapy for bacteriologically confirmed PTB patients.
The study has some important implications. There is a need to further train and supervise private-sector staff on the importance of data completeness and provide updates on new guidelines, as has been suggested elsewhere.5 Regular meetings that involve both sectors and offer opportunities to discuss respective performances should be arranged and would be a source of motivation for improvement. With the new directive on using diagnosed patients as the denominator for treatment outcomes and treatment success rates declining, it will be important for both sectors to monitor pretreatment loss to follow-up (namely diagnosed patients not being registered for treatment). This can be substantial27 and needs to be kept as low as possible. Finally, the Kenya NTLDP could learn about strengths and limitations of the PPM model by reviewing and learning from other countries.28
Conclusions
This study shows that between 2013 and 2017, the PPM model in Kenya contributed substantially to TB case detection, with the private sector providing good TB diagnostic and treatment services for one-fifth of all TB patients in the country. In the private sector, there is room for improvement in terms of better recording of some key variables and paying more attention to bacteriological confirmation of cases and follow-up of sputum smears. Some focused qualitative research would help to understand some of these deficiencies. Implications for moving forward and further improving the Kenya PPM model were discussed.
Authors’ contributions: EWM, ADH, SAD and POO conceived and designed the study and all the authors read and approved the study protocol. EWM collected the data. All the authors contributed to the analysing and interpretation of the data. EWM drafted the manuscript and all authors critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Acknowledgements: This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization. The training model is based on a course developed jointly by the International Union against Tuberculosis and Lung Disease (The Union) and Medécins Sans Frontières (MSF). The specific SORT IT program that resulted in this publication was implemented by MSF, Brussels Operational Centre, Luxembourg and the Centre for Operational Research, The Union. Mentorship and the coordination/facilitation of these SORT IT workshops were provided through the Centre for Operational Research, The Union; the Luxembourg Operational Research Unit (LuxOR); AMPATH, Eldoret, Kenya; the Institute of Tropical Medicine, Antwerp, Belgium; the Centre for International Health, University of Bergen, Norway; the University of Washington, Seattle, WA, USA; the Luxembourg Institute of Health, Luxembourg; the Institute of Medicine, University of Chester, Chester, UK; and the National Institute for Medical Research, Muhimbili Medical Research Centre, Dar es Salaam, Tanzania. The authors are grateful to the head of the Kenya NTLDP for providing permission to use the routine surveillance data in this study.
Funding: The SORT IT program was funded by the UK Department for International Development (DFID); La Fondation Veuve Emile Metz-Tesch supported open access publication costs. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Ethical approval: This study was approved by the Ethics Review Committee of Moi University/Moi Teaching and Referral Hospital, Kenya (approval number 0003047) and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France (EAG number 05/18). Permission was sought from the head of the NTLDP to use the case-based data for this study and confidentiality was assured by not including any personal identifiers during the analysis, report writing and dissemination.
References
- 1. Chakaya J, Uplekar M, Mansoer J, et al. Public-private mix for control of tuberculosis and TB-HIV in Nairobi, Kenya: outcomes, opportunities and obstacles. Int J Tuberc Lung Dis. 2008;12(11):1274–8. [PubMed] [Google Scholar]
- 2. Nwe TT, Saw S, Le Win L, et al. Engagement of public and private medical facilities in tuberculosis care in Myanmar: contributions and trends over an eight-year period. Infect Dis Poverty. 2017;6(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization Global tuberculosis report, 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 4. World Health Organization The End TB Strategy. Global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 5. Lei X, Liu Q, Escobar E, et al. Public–private mix for tuberculosis care and control: a systematic review. Int J Infect Dis. 2015;34:20–32. [DOI] [PubMed] [Google Scholar]
- 6. Thet Lwin ZM, Sahu SK, Owiti P, et al. Public-private mix for tuberculosis care and control in Myanmar: a strategy to scale up? Public Health Action. 2017;7(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chongwe G, Kapata N, Maboshe M, et al. A survey to assess the extent of public-private mix DOTS in the management of tuberculosis in Zambia. Afr J Prim Health Care Fam Med. 2015;7(1):692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uplekar M. Public-private mix for tuberculosis care and prevention. What progress? What prospects? Int J Tuberc Lung Dis. 2016;20(11):1424–9. [DOI] [PubMed] [Google Scholar]
- 9. National Tuberculosis, Leprosy and Lung Disease Program National Tuberculosis, Leprosy and Lung Disease Program 2017 annual report. Nairobi: Ministry of Health; 2018. [Google Scholar]
- 10. World Health Organization Global tuberculosis report 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 11. National Tuberculosis, Leprosy and Lung Disease Program National strategic plan for tuberculosis, leprosy and lung health, 2015–2018. Nairobi: Ministry of Health; 2014. [Google Scholar]
- 12. Netherlands Enterprise Agency Kenyan healthcare sector. Opportunities for the Dutch life sciences & health sector. RVO-138-1601/RP-INT. The Hague: Netherlands Enterprise Agency; 2016. [Google Scholar]
- 13. Kenya National Bureau of Statistics, Ministry of Health Kenya demographic and health survey 2014. Nairobi: Kenya National Bureau of Statistics; 2014. [Google Scholar]
- 14. World Health Organization Countries. Kenya statistics. Available from: http://www.who.int/countries/ken/en/ [accessed 15 February 2018].
- 15. Kenya National Bureau of Statistics Economic survey 2017. Nairobi: Kenya National Bureau of Statistics; 2017. [Google Scholar]
- 16. United Nations Statistics Division World statistics pocketbook. Kenya. Available from: http://data.un.org/CountryProfile.aspx?crName=kenya [accessed 14 November 2018].
- 17. National Tuberculosis, Leprosy and Lung Disease Program National strategic plan for tuberculosis, leprosy and lung health, 2015–2018. Monitoring and evaluation framework. Nairobi: Ministry of Health; 2014. [Google Scholar]
- 18. World Health Organization Definitions and reporting framework for tuberculosis. Available from: http://www.who.int/tb/publications/definitions/en/ [accessed 11 November 2018].
- 19. Daniel OJ, Adedeji Adejumo O, Abdur-Razzaq HA, et al. Public-private mix for TB and TB-HIV care in Lagos, Nigeria. Int J Tuberc Lung Dis. 2013;17(9):1195–8. [DOI] [PubMed] [Google Scholar]
- 20. National Tuberculosis, Leprosy and Lung Disease Program National Tuberculosis, Leprosy and Lung Disease Program treatment guidelines. Nairobi: Ministry of Health; 2013. [Google Scholar]
- 21. National AIDS and STI Control Program Guidelines for antiretroviral therapy in Kenya. 4th ed.Nairobi: Ministry of Health; 2011. [Google Scholar]
- 22. Harries AD, Hargreaves NJ, Kwanjana JH, et al. Clinical diagnosis of smear-negative pulmonary tuberculosis: an audit of diagnostic practice in hospitals in Malawi. Int J Tuberc Lung Dis. 2001;5(12):1143–7. [PubMed] [Google Scholar]
- 23. National Tuberculosis, Leprosy and Lung Disease Program Kenya tuberculosis prevalence survey. Nairobi: Ministry of Health; 2018. [Google Scholar]
- 24. Tollefson D, Ngari F, Mwakala M, et al. Under-reporting of sputum smear-positive tuberculosis cases in Kenya. Int J Tuberc Lung Dis. 2016;20(10):1334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Afutu FK, Zachariah R, Hinderaker SG, et al. High initial default in patients with smear-positive pulmonary tuberculosis at a regional hospital in Accra, Ghana. Trans R Soc Trop Med Hyg. 2012;106(8):511–3. [DOI] [PubMed] [Google Scholar]
- 26. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7. [DOI] [PubMed] [Google Scholar]
- 27. MacPherson P, Houben RMGJ, Glynn JR, et al. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Org. 2014;92(2):126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naqvi SA, Naseer M, Kazi A, et al. Implementing a public-private mix model for tuberculosis treatment in urban Pakistan: lessons and experiences. Int J Tuberc Lung Dis. 2012;16(6):817–21. [DOI] [PubMed] [Google Scholar]