Abstract
Background: Electrophysiological measures are being increasingly utilized due to their ability to provide objective measurements with minimal bias and to detect subtle changes with quantitative data on neural function. Heterogeneous reporting of trial outcomes limits effective interstudy comparison and optimization of treatment. Objective: The objective of this systematic review is to describe the reporting of electrophysiological outcome measures in spinal cord injury (SCI) clinical trials in order to inform a subsequent consensus study. Methods: A systematic search of PubMed and EMBASE databases was conducted according to PRISMA guidelines. Adult human SCI clinical trials published in English between January 1, 2008 and September 15, 2018 with at least one electrophysiological outcome measure were eligible. Findings were reviewed by all authors to create a synthesis narrative describing each outcome measure. Results: Sixty-four SCI clinical trials were included in this review. Identified electrophysiological outcomes included electromyography activity (44%), motor evoked potentials (33%), somatosensory evoked potentials (33%), H-reflex (20%), reflex electromyography activity (11%), nerve conduction studies (9%), silent period (3%), contact heat evoked potentials (2%), and sympathetic skin response (2%). Heterogeneity was present in regard to both methods of measurement and reporting of electrophysiological outcome measures. Conclusion: This review demonstrates need for the development of a standardized reporting set for electrophysiological outcome measures. Limitations of this review include exclusion of non-English publications, studies more than 10 years old, and an inability to assess methodological quality of primary studies due to a lack of guidelines on reporting of systematic reviews of outcome measures.
Keywords: electromyography, electrophysiological outcome, motor evoked potential, outcome assessment (health care), spinal cord injuries, somatosensory evoked potential
Spinal cord injury (SCI) is a devastating neurologic event that results in significant sensory, motor, and autonomic dysfunction. There has been substantial effort in testing and developing treatments to improve recovery after SCI in animals, which often fail to translate to human studies. This may largely be due to the difference in outcome measures seen in animal studies compared to human studies. Outcome measures for preclinical studies show a range of measurements, including cellular and electrophysiological changes, whereas outcomes for human trials are largely functional and behavioral.1–3 Functional outcome measures are ultimately of most importance, but the failure to achieve impact of an intervention on a functional measure should not be fruitless. Understanding the effect of an intervention on the neurophysiology can guide future treatments that may subsequently achieve clinically meaningful outcomes. As long as this information remains unavailable, a major index in determining success or failure of a treatment will be absent, limiting the chance for a successful translation to individuals with SCI.
In contrast to the typical functional and behavioral primary outcome measures seen in human SCI clinical trials, electrophysiological (EP) measures are largely objective, independent of patient input, and unbiased in that results are not dependent on the subjective responses of patients.4 Used in conjunction with conventional clinical examinations, EP examinations have developed into a complement for assessing function after SCI.4 However, heterogeneity in reporting of EP measures (both units of measure and methods of collection) introduces bias and hampers interstudy analyses of intervention efficacy.
The methodology of collecting and reporting outcome measures is a recognized challenge in many clinical fields and has led to the development of guidelines and minimum data sets.1–3 A first step to create guidelines and data sets is often a systematic review to identify the range of outcomes used in the literature.1–3 The objectives of this study were to describe the range of EP outcome measures and the manner in which they are reported in clinical trials of human SCI prior to the development of guidelines on reporting of these outcomes.
Methods
The systematic review was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines and registered with the PROSPERO prospective register of systematic reviews (CRD42019127713). A systematic search of PubMed and EMBASE was performed to identify relevant articles to answer the above objectives.
The initial search strategy was constructed for PubMed and adapted to the EMBASE search. We used the MeSH term “spinal cord injuries.” The following search limitations (inclusion criteria) were used: clinical trials, publications between January 1, 2008 and September 15, 2018, humans, and English language. Due to the presence of many EP measures used in clinical trials and the use of various key words for such measures, the initial search intentionally used broad search terms to identify all SCI clinical trials. Electroencephalogram (EEG) and EP measures routinely performed in bowel and bladder studies were excluded because they were considered beyond the scope of this review.
Titles and abstracts retrieved by the search strategy were screened by two review authors (R.K. and M.S.) for full article review eligibility based on the identification of at least one EP outcome in response to a treatment or intervention in adult humans (>18 years age) with SCI. The full-text articles of the eligible abstracts were retrieved and assessed by R.K. and A.S. for final inclusion. A data extraction form was used for the desired information. Data elements included year, study design, population, sample size, intervention, comparison group, and outcome of interest (Table 1). We obtained detailed information on EP measures that included unit of measures, purpose of measurement, and methods of collection and reporting. Any discrepancies during this process were settled by consultation between two authors (R.K. and A.S.). There is no specific assessment tool or checklist available for appraisal of methodological quality of systematic reviews examining clinical outcome measures and their measurement properties. Risk of bias assessment was not performed as the primary goal was to evaluate EP outcome measure reporting. After data extraction the findings were reviewed by all authors. A descriptive synthesis narrative was prepared for each outcome measure. Descriptive statistics were used to report frequency and proportion of outcome measures. When considering the reporting method of a single instrument, proportions were presented as the percentage of studies that had used that instrument.
Table 1.
Clinical trials in SCI with neurophysiological outcome measures
| Year | Study design | Population | Sample size | Intervention | Comparison | Outcome of interest | |
|---|---|---|---|---|---|---|---|
| da Silva et al43 | 2018 | RCT | Chronic SCI AIS B-D | N=25 | Photomodulation + PT | Placebo + PT | EMG |
| (I=13, C=12) | |||||||
| Piazza et al25 | 2018 | Controlled trial | Chronic SCI AIS A-D + healthy controls | N=28 | ES cycling in SCI | ES cycling in healthy controls | H-reflex |
| (SCI=15, Healthy C=13) | |||||||
| Zhao et al44 | 2017 | Pre-post | Chronic SCI AIS A | N=8 | Human umbilical cord mesenchymal cells | NA | MEPs |
| Allison et al35 | 2017 | RCT | Chronic SCI AIS A-D | N=20 | Anti-inflammatory diet | No intervention | NCS |
| (I=12, C=8) | |||||||
| Nardone et al21 | 2017 | RCT | Chronic SCI AIS C-D | N=10 | Active rTMS | Sham rTMS | H-reflex, MEPs |
| (I=5, C=5) | |||||||
| Vaquero et al37 | 2017 | Pre-post | Chronic SCI AIS B-D | N=10 | Mesenchymal stromal stem cells | NA | EMG, MEPs, NCS, SSEPs |
| Radhakrishna et al45 | 2017 | RCT + crossover trial | Chronic SCI AIS A-B | N=45 | Spinalon™ (dose escalation study with 8 groups) | Placebo | EMG |
| Trumbower et al12 | 2017 | Randomized crossover design | Chronic SCI AIS C-D | N=6 | Hypoxia + hand opening practices | Normoxia + hand opening practices | EMG |
| Osuagwu et al46 | 2016 | RCT | Subacute SCI AIS B-C | N=12 | BCI+FES | FES | SSEPs |
| (I=7, C=5) | |||||||
| Vaquero et al11 | 2016 | Pre-post | Chronic SCI AIS A | N=12 | Mesenchymal stems cells | NA | EMG, MEPs, SSEPs |
| Oh et al47 | 2016 | Pre-post | Chronic SCI AIS B | N=16 | Mesenchymal stems cells | NA | MEPs, SSEPs |
| Lynch et al48 | 2016 | Randomized crossover trial | Chronic SCI | N=10 | Acute intermittent hypoxia (AIH) + ibuprofen | AIH + placebo | EMG |
| Khan et al28 | 2016 | Randomized crossover trial | Chronic SCI Incomplete motor | N=20 | Endurance gait training | Precision gait training | CMR |
| Chhabra et al49 | 2016 | RCT | Acute SCI AIS A | N=21 | Autologous bone marrow stem cells | No stem cells | MEPs, SSEPs |
| (I=14, C=7) | |||||||
| Hur et al36 | 2016 | Pre-post | Subacute & chronic SCI AIS A, B, D | N=14 | Adipose-derived mesenchymal stem cells | NA | EMG, MEPs, NCS, SSEPs |
| Wang et al50 | 2016 | RCT | Chronic SCI AIS A | N=12 | Surgical neural release and partial scar excision + OLP transplantation | Surgical neural release and partial scar excision | H-reflex and SSEPs |
| (I=8, C=4) | |||||||
| Shin et al19 | 2015 | Controlled trial | Acute, subacute, and chronic SCI AIS A-B | N=36 | Human fetal brain-derived neural stem cells | No stem cells | MEPs, SSEPs |
| (I=19, C=15) | |||||||
| Zewdie et al29 | 2015 | Randomized crossover trial | Chronic SCI AIS C-D | N=16 | Endurance training | Precision training | CMR, EMG, MEPs |
| Gomes-Osman et al9 | 2015 | Randomized crossover design | Chronic SCI AIS C-D | N=24 | Vibration, TENS, tDCS | Vibration, TENS, tDCS | MEPs |
| Gomes-Osman et al8 | 2015 | RCT+ crossover trial | Chronic SCI + healthy controls | N=21 | rTMS+ repetitive task practice (RTP) | sham-rTMS+RTP | MEPs |
| (I=11, healthy controls=10) |
Table 1.
Clinical trials in SCI with neurophysiological outcome measures (CONT.)
| Year | Study design | Population | Sample size | Intervention | Comparison | Outcome of interest | |
|---|---|---|---|---|---|---|---|
| Zhai et al51 | 2015 | Controlled trial | Chronic SCI AIS A-D | N=62 | Mouse nerve growth factor (IM) + rehab training | GM-1(IV) + rehab training | SSEPs |
| (I=31, C=31) | |||||||
| Estigoni et al52 | 2014 | Pre-post | Chronic SCI AIS A-C | N=8 | FES | NA | EMG |
| Murray et al18 | 2014 | RCT + crossover trial | Chronic SCI AIS B-C | N=9 | Anodal tDCS 1mA and 2mA | sham | EMG, F-wave, MEPs |
| El-Kheir et al7 | 2014 | RCT | Chronic SCI AIS A-B | N=70 | Bone marrow stem cells + PT | PT only | MEPs, SSEPs |
| (I=50, C=20) | |||||||
| Mendonca et al10 | 2014 | Pre-post | Chronic SCI AIS A | N=14 | Bone marrow mesenchymal stem cells | NA | SSEPs |
| Chen et al53 | 2014 | RCT | Chronic SCI AIS A | N=7 | OEC, SC, OEC+SC | No stem cells | EMG, SSEPs |
| (I=5, C=2) | |||||||
| Leech et al54 | 2014 | Randomized crossover trial | Chronic SCI AIS D | N=10 | Gait training +SSRI | Gait training + 5HT antagonist | EMG |
| Knikou et al26 | 2014 | Pre-post | Chronic SCI AIS A-D | N=16 | Locomotor training | NA | EMG, H-reflex |
| Chu et al55 | 2014 | RCT + crossover trial | Chronic SCI AIS C-D | N=10 | baclofen, tizanidine | Placebo | EMG |
| Hofstoetter et al56 | 2014 | Pre-post | Chronic SCI AIS D | N=3 | Transcutaneous spinal cord stimulation | NA | EMG |
| Shapiro et al57 | 2014 | Pre-post | Acute SCI Complete injury | N=14 | Oscillating field stimulation | NA | SSEPs |
| Nardone et al58 | 2014 | Controlled trial + crossover trial | Chronic SCI AIS C-D + healthy controls | N=17 | rTMS | Sham stim in SCI and rTMS healthy controls | H-reflex |
| (SCI=9, healthy C=8) | |||||||
| Fenuta et al59 | 2014 | RCT + crossover trial | Chronic SCI AIS C-D + healthy controls | N=14 | Lokomat, manual treadmill, and ZeroG in SCI | Lokomat, manual treadmill, and ZeroG in control | EMG |
| (SCI=7, healthy C=7) | |||||||
| Tabakow et al22 | 2013 | Pre-post | Chronic SCI AIS A | N=6 | OEC | NA | EMG, MEPs, NCS |
| Dai et al60 | 2013 | RCT | Chronic SCI AIS A | N=40 | Bone marrow mesenchymal | No stem cells | EMG, SSEPs |
| (I=20, C=20) | |||||||
| Jette et al16 | 2013 | Randomized crossover design | Chronic SCI AIS A, C, D | N=16 | Active rTMS | Sham rTMS | MEPs |
| Hajela et al32 | 2013 | Pre-post | Chronic SCI AIS D | N=1 | Locomotor training | NA | Flexion reflex |
| D'Amico et al27 | 2013 | Controlled trial + crossover trial | Chronic SCI AIS A-B and non-SCI controls | N=13 | Zolmitriptan | Sugar pill | CMR, H-reflex |
| (I=6, C=7) | |||||||
| Chang et al6 | 2013 | RCT | Chronic SCI AIS A-B | N=14 | CPM of ankle joints | No CPM | H-reflex |
| (I=7, C=7) | |||||||
| Stetkarova et al20 | 2013 | Pre-post | Chronic SCI AIS A | N=9 | ITB | NA | H-reflex, silent period |
| Govil et al61 | 2013 | RCT | Chronic SCI AIS C-D | N=30 | EMG feedback to gluteus maximus + gait training | Gait training without EMG feedback | EMG |
| (I=15, C=15) |
Table 1.
Clinical trials in SCI with neurophysiological outcome measures (CONT.)
| Year | Study design | Population | Sample size | Intervention | Comparison | Outcome of interest | |
|---|---|---|---|---|---|---|---|
| Frolov et al62 | 2012 | Pre-post | Chronic SCI | N=20 | Hematopoietic autologous stem cell | NA | MEPs, SSEPs |
| Trumbower et al63 | 2012 | Pre-post | Chronic SCI AIS C-D | N=13 | Acute intermittent hypoxia | NA | EMG |
| Kumru et al40 | 2012 | RCT | Chronic SCI AIS A-D + healthy controls | N=52 | tDCS + visual illusion | None | CHEPs |
| (I=18, SCI C=20, healthy C=14) | |||||||
| Mazzoleni et al64 | 2011 | Controlled trial | Chronic SCI AIS C-D & healthy controls | N=10 | Locomotor training in SCI | Locomotor training in controls | EMG |
| (I= 5, C=5) | |||||||
| Chang et al24 | 2011 | Controlled trial | Chronic SCI AIS A & healthy controls | N=11 | Limb segment vibration in SCI | Limb segment vibration in controls | H-reflex |
| (I= 5, C=6) | |||||||
| Houldin et al65 | 2011 | Controlled trial | Chronic SCI AIS D + healthy controls | N=26 | Lokomat training | No training or Lokomat training | EMG |
| (SCI= 9, C=17) | |||||||
| Kuppuswamy et al17 | 2011 | Randomized crossover trial | Chronic SCI AIS A-D | N=15 | rTMS | Sham rTMS | MEPs, silent period, SSR |
| Theiss et al31 | 2011 | Randomized crossover | Chronic SCI AIS C-D | N=7 | Riluzole | Control | Flexion reflex, H-reflex |
| Murillo et al33 | 2011 | Controlled trial | Subacute and chronic SCI AIS A, C, D | N=28 | Vibration of rectus femoris in SCI | Vibration of rectus femoris in controls | H-reflex, T wave |
| (I=19, C=9) | |||||||
| Adams et al66 | 2011 | Randomized crossover design | Chronic SCI AIS A-C | N=7 | BWSTT | Tilt table standing | H-reflex |
| Grijalva et al67 | 2010 | RCT+ crossover trial | Chronic SCI AIS A | N=14 | 4 amino pyridine | Placebo | SSEPs |
| Hoffman et al15 | 2010 | RCT | Chronic SCI AIS B-D | N=13 | Bimanual MP + SS | Unimanual MP + SS | MEPs |
| (I= 7, C=6) | |||||||
| Kumru et al30 | 2010 | RCT + crossover | Chronic SCI AIS C-D | N=14 | rTMS | Sham stim | Flexion reflex, H-reflex, T reflex |
| (I= 8, C=7) | |||||||
| Lima et al68 | 2009 | Pre-post | Chronic SCI AIS A-B | N=20 | Olfactory mucosal cells | NA | EMG, SSEPs |
| Chhabra et al38 | 2009 | Pre-post | Chronic SCI AIS A-B | N=5 | Autologous olfactory mucosal transplantation | NA | EMG, MEPs, NCS, SSEPs, |
| Cotey et al69 | 2009 | Controlled trial | Chronic SCI AIS A, B, C + healthy controls | N=16 | Lokomat gait training + vibration to quads in SCI | Lokomat gait training + vibration to quads in healthy controls | EMG |
| (SCI=11, healthy C= 5) | |||||||
| Cristante et al70 | 2009 | Pre-post | Chronic SCI | N=39 | Stem cells | NA | SSEPs |
| Gorassini et al71 | 2009 | Controlled trial | Chronic SCI AIS C-D + healthy controls | N=25 | BWSTT in SCI | BWSTT in controls | EMG |
| (SCI=19, healthy C=6) | |||||||
| Adel et al72 | 2009 | Controlled trial | Chronic SCI AIS A, B, C | N=63 | Bone marrow stromal stem cells | No stem cells | SSEPs |
| (I=43, C=20) |
Table 1.
Clinical trials in SCI with neurophysiological outcome measures (CONT.)
| Year | Study design | Population | Sample size | Intervention | Comparison | Outcome of interest | |
|---|---|---|---|---|---|---|---|
| Lam et al73 | 2008 | Pre-post | Subacute and chronic SCI AIS D | N=9 | Gait training with resistance | NA | EMG |
| Mackay-Sim et al74 | 2008 | Controlled clinical trial | Chronic SCI AIS A | N=12 | OEC transplant | No transplant | MEPs, SSEPs |
| (I= 6, C=6) | |||||||
| Beekhuizen et al5 | 2008 | RCT | Chronic SCI AIS C-D | N=24 | Massed practice + SS | MP or SS or no intervention | MEPs |
| (6 in each group) | |||||||
| Kawashima et al75 | 2008 | Controlled trial | Chronic SCI AIS C-D in intervention and AIS A-B in controls | N=12 | UE activity effects on LE in cervical SCI | Upper extremity activity effects on LE in thoracic SCI | EMG |
| (cervical SCI=7, thoracic SCI controls=5 |
Note: AIS = American Spinal Cord Injury Association Impairment Scale; BCI = brain computer interface; BWSTT = body weight–supported treadmill training; C = control group; CHEP: contact heat evoked potential; CMR = cutaneomuscular reflex; CPM = continuous passive motion; EMG = electromyography; ES = electrical stimulation; FES = functional electrical stimulation; I = intervention group; ITB = intrathecal baclofen; LE = lower extremity; NA = not applicable; MEP = motor evoked potential; MP = massed practice; NCS = nerve conduction studies; OEC = olfactory ensheathing cells; OLP = olfactory lamina propria; PT = physical therapy; RCT = randomized control trial; rTMS = repetitive transcranial magnetic stimulation; SC = Schwann cells; SSEP = somatosensory evoked potentials; SSRIs = selective serotonin reuptake inhibitors; SSR = sympathetic skin response; SS = sensory stimulation; TENS = transcutaneous electrical nerve stimulation; tDCS = transcutaneous direct current stimulation; UE = upper extremity.
Results
Of the 2,030 articles identified, 64 articles met our eligibility criteria. Of these, 64 were included in this study (Figure 1), assessing 877 people with SCI who received various interventions and 324 people with and without SCI serving as controls. Mean sample size was 19 with standard deviation of 14. Only six studies5–10 (9%) reported sample size justification, and 18 studies (28%) reported small sample size as a limitation. In general, we defined participants as acute SCI with duration of injury of 1 to 2 weeks, subacute from 2 weeks to 6 months, and chronic if injury duration was greater than 6 months.
Figure 1.
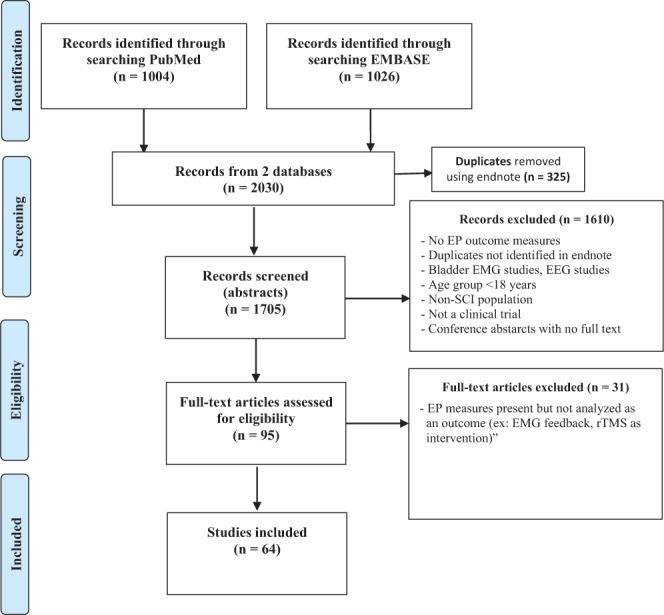
PRISMA flow diagram of the search strategy. EEG = electroencephalogram; EMG = electromyography; EP = electrophysiological; rTMS = repetitive transcranial magnetic stimulation.
Among 64 studies, we identified five types of clinical trial study designs (Figure 2). Hybrid study designs had both controls and crossover of interventions. Eleven (17%) controlled trials had people without SCI as controls.
Figure 2.
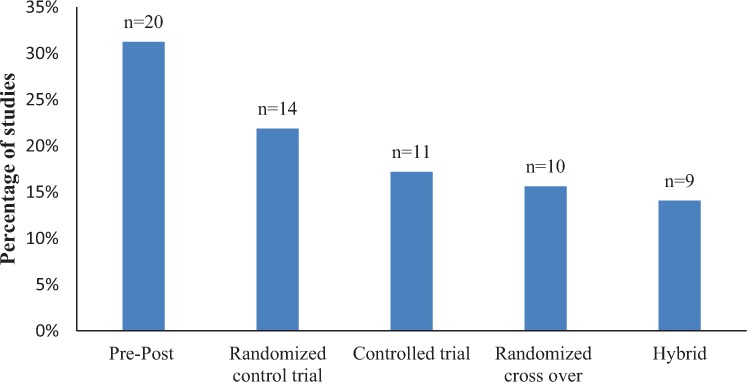
Types of study designs identified in this systematic review with numbers of studies above the columns
Identified EP measures are presented in Figure 3. The most commonly used EP measures were electromyography (EMG) (n and %), motor evoked potentials (MEPs), somatosensory evoked potentials (SSEPs), and H-reflex. Other infrequently reported EP measures include reflex EMG activity, nerve conduction studies (NCS), silent period, contact heat evoked potentials (CHEPs), and sympathetic skin response (SSR). Only twenty-six studies (41%) considered more than one EP outcome measure.
Figure 3.
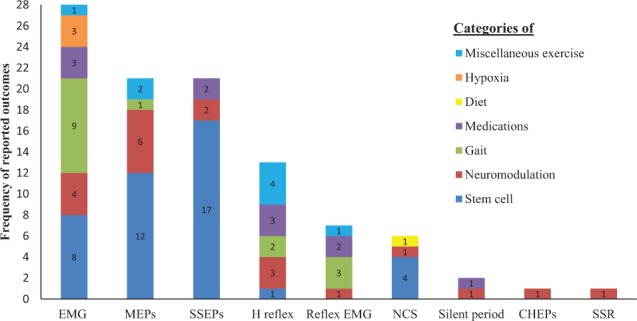
Frequency of electrophysiological (EP) outcomes identified. Each EP outcome has color-coded columns to illustrate the categories of research using the EP outcome. CHEPs = contact heat evoked potentials; CMR = cutaneomuscular reflex; EMG = electromyography; MEPs = motor evoked potentials; NCS = nerve conduction studies; SSEPs = somatosensory evoked potentials; SSR = sympathetic skin response.
Clinical trials that reported EP outcomes (Table 1) were frequently studying effects of the interventions categorized in Figure 4. Among 16 studies with neuromodulation intervention, seven trials studied repetitive transcranial magnetic stimulation (rTMS). Gait training interventions involved training with Lokomat, body weight–supported treadmill training, and over ground training.
Figure 4.
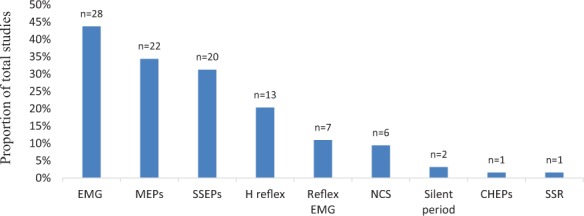
Types of interventions investigated in clinical trials using electrophysiologic outcomes. CHEPS = contact heat evoked potentials; EMG = electromyography; MEPs = motor evoked potentials; NCS = nerve conduction studies; SSEPs= somatosensory evoked potentials; SSR = sympathetic skin response.
Electromyography
The EMG signal is a biomedical signal that measures electrical currents generated in muscles in various neuromuscular settings, such as resting state and voluntary and involuntary contractions. The motor unit action potential (MUAP) waveform and motor unit firing behavior derived from EMG signals provide an important source of information on motor neuron and muscle pathophysiology. Invasive and/or surface electrodes are used to acquire this muscle signal. EMG signals/activity were acquired from surface electrodes placed directly on the skin in all but one study that used a concentric needle electrode.11 The signal is picked up by the surface electrode, filtered, and amplified, and it can then be analyzed in different ways.
EMG activity was clinically assessed in 28 (44%) studies. EMG activity of muscles obtained via surface electrodes was frequently reported as mean EMG amplitude in 14 studies, followed by peak EMG amplitude in five studies and presence or absence of any EMG activity pre-post intervention in four studies. Other infrequent methods of reporting EMG results include EMG area, median frequency, and recruitment. In one study, coactivity ratio was calculated from EMG activity of agonist and antagonist muscles.12 Three studies reported EMG activity using a combination of the above measures. Units of measures and details on EMG outcomes were absent in two studies. Given the variability in the reporting of EMG outcomes, no further analysis can be provided.
Motor evoked potentials
MEPs are muscle action potentials elicited by transcranial brain stimulation or trans–spinal cord stimulation. MEPs elicited by transcranial stimulation (invasive and noninvasive) can monitor the functional integrity of the motor pathways. Development of noninvasive methods of eliciting MEPs has become popular as a diagnostic and prognostic tool for neurological disorders.13 Noninvasively MEPs can be produced by transcranial electrical stimulation (TES) and transcranial magnetic stimulation (TMS). The clinical usefulness of TES is limited due to local discomfort produced by the high-voltage electrical stimulation to the scalp to elicit MEPs.14 The development of TMS13 in 1985, a new type of cortical magnetic stimulator to elicit MEPs, opened up opportunities to use it as an outcome measure. The investigator holds the stimulating coil tangentially over the motor cortex of the target area after mapping to stimulate the cortex. MEPs are recorded with surface electrodes, which are placed over the contralateral target muscles.
MEPs were reported in 21 (33%) studies included in this systematic review. Most commonly MEPs were obtained from upper limbs; this was followed by lower limbs and infrequently from trunk in two studies. There was heterogeneity in methods and reporting of MEPs as an outcome measure. Twelve studies reported MEP amplitudes obtained at resting state or during voluntary contraction of muscle. Other MEP measures reported were MEP latencies in three studies, cortical map area in three studies, and stimulator intensity to elicit MEPs in three studies. Presence and absence of MEPs pre-post intervention were reported in five studies. Four studies only reported no change in MEPs without any further information of their methods or units of measure. Seven studies used more than one method to report changes in MEPs.
Methods of obtaining MEPs were reported in 14 (60%) studies. Seven studies obtained resting MEPs at stimulator intensity, which ranged from 1.1 to 1.4 times resting motor threshold.5,9,15–20 Resting motor threshold was defined as lowest stimulator intensity required to produce MEPs with amplitude of at least 50 μV. Three studies reported active MEPs obtained at various percentages of maximum voluntary contraction (MVC), which ranged from 10% to 70% of MVC.9,21 Definition of active MEPs and stimulator intensities to produce active MEPs also differed in each study. In another three studies, MEPs were elicited at intensities ranging from 40% to 100% of maximum stimulator output.5,10,22
Somatosensory evoked potentials
A somatosensory evoked potential (SSEP) is an evoked potential recorded with surface electrodes over the extremities, spine, and scalp, following the electrical stimulation of peripheral nerves. They provide a means for assessment of ascending somatosensory pathways. SSEPs are often performed with stimulation of the median nerve at the wrist and the posterior tibial nerve at the ankle. SSEPs evaluate the integrity of the somatosensory pathways from all levels of the nervous system: peripheral nerve, spinal cord, and brain.
SSEPs were obtained in 21 (33%) studies reported in this systematic review. Most frequently, SSEPs were measured in clinical trials by stimulation of the median nerve (n = 11) followed by the tibial nerve (n = 10). Infrequently SSEPs were obtained from ulnar nerve and para-vertebral area. Para-vertebral SSEPs were obtained to report changes in sensory level pre-post intervention. There was no information on site of stimulation to obtain SSEPs in seven studies. Reporting SSEPs included percentage of subjects with presence of SSEPs pre-post intervention in 10 studies, latencies in seven studies, and amplitudes along with latencies in two studies. SSEPS were mentioned as outcome measures in two studies, but no results were reported.
H-reflex
The Hoffmann reflex (H-reflex) is an electrically induced reflex that bypasses the muscle spindle.23 H-reflex is a useful measure to assess modulation of monosynaptic reflex activity in the spinal cord and is used as an estimate of alpha motoneuron (α MN) excitability. It is elicited by selectively stimulating the Ia fibers of the posterior tibial or median nerve. H-reflex can be used to evaluate various neurologic conditions, musculoskeletal injuries, and application of therapeutic intervention.23 The H-reflex amplitude is highly variable under different conditions; therefore different methods have been used and recommended for normalization such that comparisons can be made within and between subjects.
In this systematic review, 13 SCI clinical trial studies (20%) obtained H-reflex as one of the outcome measures. All but one study reported H-reflex obtained from the soleus muscle by tibial nerve stimulation. The one exception did not provide any information on which nerve and muscle were used to obtain the H-reflex. The most common method of reporting H-reflex was the ratio of maximum H-reflex to maximum compound muscle action potential (CMAP) amplitude, known as Hmax/Mmax (n = 8, 62%). Other methods of reporting included conditioned H-reflex amplitude, homosynaptic depression of H-reflex, and recruitment curves. Four studies6,24–26 reported H-reflex post activation depression at various frequencies by comparing H-reflex amplitudes pre and post intervention.
Methods to normalize and collect H-reflex varied as well. In addition to the Hmax/Mmax ratio for normalization, the following alternatives were used for normalization. Soleus H-reflex conditioned by peroneal nerve stimulation and plantar stimulation was obtained respectively in studies by Knikou et al26 and Piazza et al.25 Stimulation intensities to obtain H-reflex were adjusted with reference to Mmax for normalization in four studies.6,24,26,27 Only one study obtained H-reflex at an intensity to evoke 50% of Hmax for normalization.25 In all of the 13 studies, H-reflex amplitudes were compared.
Reflex EMG activity: In seven studies (11%), reflex EMG activity was measured from target muscles using surface electrodes in response to cutaneous stimulation. We found that authors used different nomenclature to report this reflex activity.
Cutaneous muscular reflex (CMR): Three studies27–29 reported it as CMR, which was evoked by electrical stimulation of tibial nerve behind the medial malleolus or median arch of foot.27–29 EMG activity was collected from target muscles using surface electrodes. Withdrawal reflex activity or flexion reflex was evoked by electrical stimulation of the medial arch of the foot to produce a TA EMG response in two studies30,31 and by stimulation of sural nerve in another study.32 T-reflex or T-wave was recorded from soleus muscle after tapping the Achilles tendon in two studies.30,33 Reflex EMG was commonly reported as mean EMG amplitude.
Nerve conduction studies (NCS): NCS are obtained by electrical stimulation of sensory and/or motor nerves, with responses collected from surface electrodes or cutaneous or muscle targets, respectively. NCS provides information on conduction properties of examined nerves and assists in diagnosis of peripheral nerve disorders.34
Six studies (9%) included in this systematic review reported NCS including F-waves as an outcome measure. Sensory and motor NCS were obtained from various peripheral nerves. Conduction velocities were compared in four studies,22,35–37 and additionally CMAP amplitudes were compared in two of these studies.22,35 In one study, the NCS was reported as no change in NCS pre-post intervention without any further details.38
F-wave is a late response that follows the motor response and is elicited by supramaximal electrical stimulation of a mixed or a motor nerve.34 Various F-wave parameters are used for diagnostic evaluation of peripheral nerve disorder. Only one study reported F-waves. In this study, F-wave was obtained from extensor carpi radialis muscle by radial nerve stimulation to study the effects of transcranial direct current stimulation (tDCS) on spinal excitability. Results were reported as the number of times F-wave was present during 20 stimuli.18
Cortical and cutaneous silent period: Cortical silent period (SP) is the interruption of EMG activity following a suprathreshold cortical magnetic stimulation. The duration of the cortical SP is a measure of intracortical inhibition due to gamma-aminobutyric acid B (GABA B) receptor-mediated inhibition of cortical excitability.39 There is strong evidence that the mechanisms responsible for the cortical SP have functional relevance.
One study obtained cortical SP from right abductor pollicis brevis muscle during near-maximum voluntary contraction following contralateral TMS at 140% of resting motor threshold.20 In another study17 cortical SP was obtained at an intensity 20% below active motor threshold during a 10% MVC from first dorsal interossei, thenar, and ECR. In these studies, duration and latency of cortical SP were compared. The cutaneous SP is a brief transient suppression of the voluntary muscle contraction that follows noxious cutaneous nerve stimulation due to suppression of activity in spinal motor nuclei. Only one study20 reported cutaneous SP obtained from stimulating cutaneous nerves in the index fingers and collected from abductor pollicis brevis. Onset latency, end latency, and duration were measured.
Contact heat evoked potentials (CHEPs): CHEPs provide an objective evaluation of small fiber function and have been used to study neuropathic pain.40,41 CHEPs are obtained by a heat stimulus of 32°C to 51°C over the skin. The resulting evoked potentials can be recorded and measured from sensory cortex Thus, amplitudes and latencies are obtained, being utilized to detect small fiber alterations in neuropathic pain patients. CHEPs were reported in a study to record response of tDCS combined with visual illusion on neuropathic pain by Kumru et al.40 Thermal stimuli were delivered at the fastest available ramp rate of 70°C/s from a baseline temperature of 32°C to a maximum of 51°C over C4 sensory dermatome. Latencies and amplitudes were compared.
Sympathetic skin response (SSR): SSR represents a potential generated in the skin sweat glands; it originates by activation of the reflex arch with different kinds of stimuli. SSR is most frequently used in diagnosing the functional impairment of nonmyelinated postganglionic sudomotor sympathetic fibers in peripheral neuropathies. SSR has been proposed as a noninvasive approach to investigate the function of the sympathetic system.42 Only one study reported SSR in response to rTMS.17 In this study, SSR was obtained from surface electrodes from the palm and dorsum of the hand. SSR was elicited by applying magnetic stimulation to the back of neck at 65% of maximum stimulator output. The waveform, frequency of occurrence, latency, and amplitude of SSR potentials were measured.
Discussion
To our knowledge, this is the first systematic review of the SCI clinical trials literature that used EP outcome measures. Although EP outcome measures provide objective evaluation, we found substantial variation in the types of outcomes assessed, methods to collect data, and how they are reported. Key EP measures in SCI clinical trials include EMG activity, MEPs, SSEPs and H-reflex. Very few studies considered them all. The review demonstrates heterogeneity with regard to methods of measurement and reporting of EP outcome measures. Sample sizes in these studies were small, which was often reported as a limitation. Only 6 of 64 studies reported sample size justification. Heterogeneity of outcome reporting is recognized to challenge interstudy comparison and is likely to lead to bias in the dissemination of knowledge. To overcome these challenges, the development of standardized reporting of EP outcome measures is critically important. Beyond demonstrating heterogeneity, this study aimed to collate current reporting practice to assist stakeholders in developing guidelines for reporting EP outcome measures. To this end, the findings from this study provide a starting point for the development of standardized reporting of EP outcome measures. It is alarming that in many studies neither details on methods of EP outcome assessment nor EP data elements were reported. To overcome these challenges, we propose the development of standardized reporting guidelines. The findings of this study provide a basis for the development of a standardized reporting of EP outcomes measures in SCI clinical trials. We propose the use of Delphi method to develop consensus on standardized guidelines for collecting and reporting of EP outcomes in SCI clinical trials. The Delphi method is a process of arriving at group consensus by providing experts with rounds of questionnaires and the group response before each subsequent round. The results of this study could be used in the development of such a questionnaire, for example, What parameters (amplitude, latency) should be reported for a given neurophysiological test? What should be the optimal stimulator intensity to obtain MEPs? After standardizing EP collecting and reporting, further studies could help determine the effect size for each EP outcome to reflect meaningful changes.
This review also provides information on currently available EP measures that can complement clinical exam and provide more accurate objective changes in the nervous system after SCI. No single outcome measure can be applied to all people with SCI to detect changes or monitor progress. Currently available functional outcome measures are limited, especially during early recovery of SCI. Thus, carefully tracking neuromuscular changes using EP assessments after SCI is important in assessing clinical treatment effect. EP measures could help assess changes in the spinal cord that cannot be assessed with clinical exam. Thus, EP measures may play a role in SCI outcome predictions. However, the first step is to standardize collecting and reporting methods in order to make clinically meaningful assessments.
Limitations
The search strategy excluded non-English articles. The global representation of included studies suggests that the non-English exclusion is unlikely significant. Publications older than 10 years were not included based on the argument that there has been a paradigm shift regarding the evaluation of the EP outcome measures. The authors propose that assessment of the last 10 years of published data is representative of current practice. We also noted that some studies were not captured by this search when we used the “clinical trial” filters in PubMed and EMBASE. To allow replicability and consistency of methodology of our search strategy, we did not include those studies that we found randomly. In this systematic review, we did not assess methodological quality of primary studies due to lack of standardized guidelines and check lists for systematic review on outcome measures.
Conclusion
This systematic review provides a comprehensive synthesis of evidence regarding the utility of EP measures in SCI clinical trials. EP outcomes are applicable to every type of patient regardless of their neurological level and type of injury. EP outcomes are a valuable, sensitive, and objective outcome measures in SCI clinical trials that can be applied to all levels of SCI to measure both upper and lower extremity function as well as outcomes that are currently limited. However, significant heterogeneity exists in the outcome reporting of studies assessing treatment of SCI. The development of standardized reporting of EP outcome measures would support the field in the future. The findings from this study should be used to inform a larger consensus process to define the core EP outcomes and data elements in SCI research. The continued understanding and development of existing EP measures will ensure the field of SCI research will be well-positioned to assess the efficacy of emerging interventions and the impact on function.
Acknowledgments
The authors declare no conflicts of interest.
REFERENCES
- 1.Jones LAT, Bryden A, Wheeler TL et al. Considerations and recommendations for selection and utilization of upper extremity clinical outcome assessments in human spinal cord injury trials. Spinal Cord. 2018;56(5):414–425. doi: 10.1038/s41393-017-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolliger M, Blight AR, Field-Fote EC et al. Lower extremity outcome measures: Considerations for clinical trials in spinal cord injury. Spinal Cord. 2018;56(7):628–462. doi: 10.1038/s41393-018-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steeves JD, Lammertse D, Curt A et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: Clinical trial outcome measures. Spinal Cord. 2007;45(3):206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- 4.Xie J, Boakye M. Electrophysiological outcomes after spinal cord injury. Neurosurg Focus. 2008;25(5):E11. doi: 10.3171/FOC.2008.25.11.E11. [DOI] [PubMed] [Google Scholar]
- 5.Beekhuizen KS, Field-Fote EC. Sensory stimulation augments the effects of massed practice training in persons with tetraplegia. Arch Phys Med Rehabil. 2008;89(4):602–608. doi: 10.1016/j.apmr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Chang YJ, Liang JN, Hsu MJ, Lien HY, Fang CY, Lin CH. Effects of continuous passive motion on reversing the adapted spinal circuit in humans with chronic spinal cord injury. Arch Phys Med Rehabil. 2013;94(5):822–828. doi: 10.1016/j.apmr.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 7.El-Kheir WA, Gabr H, Awad MR et al. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23(6):729–745. doi: 10.3727/096368913X664540. [DOI] [PubMed] [Google Scholar]
- 8.Gomes-Osman J, Field-Fote EC. Improvements in hand function in adults with chronic tetraplegia following a multiday 10-Hz repetitive transcranial magnetic stimulation intervention combined with repetitive task practice. J Neurol Phys Ther. 2015;39(1):23–30. doi: 10.1097/NPT.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes-Osman J, Field-Fote EC. Cortical vs. afferent stimulation as an adjunct to functional task practice training: A randomized, comparative pilot study in people with cervical spinal cord injury. Clin Rehabil. 2015;29(8):771–782. doi: 10.1177/0269215514556087. [DOI] [PubMed] [Google Scholar]
- 10.Mendonca MV, Larocca TF, de Freitas Souza BS et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5(6):126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaquero J, Zurita M, Rico MA et al. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy. 2016;18(8):1025–1036. doi: 10.1016/j.jcyt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Trumbower RD, Hayes HB, Mitchell GS, Wolf SL, Stahl VA. Effects of acute intermittent hypoxia on hand use after spinal cord trauma: A preliminary study. Neurology. 2017;89(18):1904–1907. doi: 10.1212/WNL.0000000000004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 14.Rossini PM, Burke D, Chen R et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman LR, Field-Fote EC. Functional and corticomotor changes in individuals with tetraplegia following unimanual or bimanual massed practice training with somatosensory stimulation: A pilot study. J Neurol Phys Ther. 2010;34(4):193–201. doi: 10.1097/NPT.0b013e3181fbe692. [DOI] [PubMed] [Google Scholar]
- 16.Jette F, Cote I, Meziane HB, Mercier C. Effect of single-session repetitive transcranial magnetic stimulation applied over the hand versus leg motor area on pain after spinal cord injury. Neurorehabil Neural Repair. 2013;27(7):636–643. doi: 10.1177/1545968313484810. [DOI] [PubMed] [Google Scholar]
- 17.Kuppuswamy A, Balasubramaniam AV, Maksimovic R et al. Action of 5 Hz repetitive transcranial magnetic stimulation on sensory, motor and autonomic function in human spinal cord injury. Clin Neurophysiol. 2011;122(12):2452–2461. doi: 10.1016/j.clinph.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Murray LM, Edwards DJ, Ruffini G et al. Intensity dependent effects of transcranial direct current stimulation on corticospinal excitability in chronic spinal cord injury. Arch Phys Med Rehabil. 2015;96(4 suppl):S114–121. doi: 10.1016/j.apmr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin JC, Kim KN, Yoo J et al. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. 2015;2015 doi: 10.1155/2015/630932. 630932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stetkarova I, Kofler M. Differential effect of baclofen on cortical and spinal inhibitory circuits. Clin Neurophysiol. 2013;124(2):339–345. doi: 10.1016/j.clinph.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Nardone R, Langthaler PB, Orioli A et al. Effects of intermittent theta burst stimulation on spasticity after spinal cord injury. Restor Neurol Neurosci. 2017;35(3):287–294. doi: 10.3233/RNN-160701. [DOI] [PubMed] [Google Scholar]
- 22.Tabakow P, Jarmundowicz W, Czapiga B et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013;22(9):1591–1612. doi: 10.3727/096368912X663532. [DOI] [PubMed] [Google Scholar]
- 23.Palmieri RM, Ingersoll CD, Hoffman MA. The Hoffmann reflex: Methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004;39(3):268–277. [PMC free article] [PubMed] [Google Scholar]
- 24.Chang SH, Tseng SC, McHenry CL, Littmann AE, Suneja M, Shields RK. Limb segment vibration modulates spinal reflex excitability and muscle mRNA expression after spinal cord injury. Clin Neurophysiol. 2012;123(3):558–568. doi: 10.1016/j.clinph.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piazza S, Torricelli D, Gomez-Soriano J et al. Assessing sensorimotor excitability after spinal cord injury: A reflex testing method based on cycling with afferent stimulation. Med Biol Eng Comput. 2018;56(8):1425–1434. doi: 10.1007/s11517-018-1787-2. [DOI] [PubMed] [Google Scholar]
- 26.Knikou M, Mummidisetty CK. Locomotor training improves premotoneuronal control after chronic spinal cord injury. J Neurophysiol. 2014;111(11):2264–2275. doi: 10.1152/jn.00871.2013. [DOI] [PubMed] [Google Scholar]
- 27.D'Amico JM, Li Y, Bennett DJ, Gorassini MA. Reduction of spinal sensory transmission by facilitation of 5-HT1B/D receptors in noninjured and spinal cord-injured humans. J Neurophysiol. 2013;109(6):1485–1493. doi: 10.1152/jn.00822.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan AS, Patrick SK, Roy FD, Gorassini MA, Yang JF. Training-specific neural plasticity in spinal reflexes after incomplete spinal cord injury. Neural Plast. 2016;2016 doi: 10.1155/2016/6718763. 6718763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zewdie ET, Roy FD, Yang JF, Gorassini MA. Facilitation of descending excitatory and spinal inhibitory networks from training of endurance and precision walking in participants with incomplete spinal cord injury. Prog Brain Res. 2015;218:127–155. doi: 10.1016/bs.pbr.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Kumru H, Murillo N, Samso JV et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair. 2010;24(5):435–441. doi: 10.1177/1545968309356095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theiss RD, Hornby TG, Rymer WZ, Schmit BD. Riluzole decreases flexion withdrawal reflex but not voluntary ankle torque in human chronic spinal cord injury. J Neurophysiol. 2011;105(6):2781–2790. doi: 10.1152/jn.00570.2010. [DOI] [PubMed] [Google Scholar]
- 32.Hajela N, Mummidisetty CK, Smith AC, Knikou M. Corticospinal reorganization after locomotor training in a person with motor incomplete paraplegia. Biomed Res Int. 2013;2013 doi: 10.1155/2013/516427. 516427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murillo N, Kumru H, Vidal-Samso J et al. Decrease of spasticity with muscle vibration in patients with spinal cord injury. Clin Neurophysiol. 2011;122(6):1183–1189. doi: 10.1016/j.clinph.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Mallik A, Weir AI. Nerve conduction studies: Essentials and pitfalls in practice. J Neurol Neurosurg Psychiatry. 2005;76(suppl 2):ii23–31. doi: 10.1136/jnnp.2005.069138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allison DJ, Gabriel DA, Klentrou P, Josse AR, Ditor DS. The influence of chronic inflammation on peripheral motor nerve conduction following spinal cord injury: A randomized clinical trial. Top Spinal Cord Inj Rehabil. 2017;23(4):377–385. doi: 10.1310/sci16-00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hur JW, Cho TH, Park DH, Lee JB, Park JY, Chung YG. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J Spinal Cord Med. 2016;39(6):655–664. doi: 10.1179/2045772315Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaquero J, Zurita M, Rico MA et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. 2017;19(3):349–359. doi: 10.1016/j.jcyt.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Chhabra HS, Lima C, Sachdeva S et al. Autologous mucosal transplant in chronic spinal cord injury: An Indian pilot study. Spinal Cord. 2009;47(12):887–895. doi: 10.1038/sc.2009.54. [DOI] [PubMed] [Google Scholar]
- 39.Poston B, Kukke SN, Paine RW, Francis S, Hallett M. Cortical silent period duration and its implications for surround inhibition of a hand muscle. Eur J Neurosci. 2012;36(7):2964–2971. doi: 10.1111/j.1460-9568.2012.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumru H, Soler D, Vidal J et al. The effects of transcranial direct current stimulation with visual illusion in neuropathic pain due to spinal cord injury: An evoked potentials and quantitative thermal testing study. Eur J Pain. 2013;17(1):55–66. doi: 10.1002/j.1532-2149.2012.00167.x. [DOI] [PubMed] [Google Scholar]
- 41.Kumru H, Soler D, Vidal J, Tormos JM, Pascual-Leone A, Valls-Sole J. Evoked potentials and quantitative thermal testing in spinal cord injury patients with chronic neuropathic pain. Clin Neurophysiol. 2012;123(3):598–604. doi: 10.1016/j.clinph.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 42.Gutrecht JA. Sympathetic skin response. J Clin Neurophysiol. 1994;11(5):519–524. doi: 10.1097/00004691-199409000-00006. [DOI] [PubMed] [Google Scholar]
- 43.da Silva FC, Gomes AO, da Costa Palacio PR et al. Photobiomodulation improves motor response in patients with spinal cord injury submitted to electromyographic evaluation: randomized clinical trial. Lasers Med Sci. 2018;33(4):883–890. doi: 10.1007/s10103-018-2447-3. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, Tang F, Xiao Z et al. Clinical study of NeuroRegen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017;26(5):891–900. doi: 10.3727/096368917X695038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radhakrishna M, Steuer I, Prince F et al. Double-blind, placebo-controlled, randomized phase i/iia study (safety and efficacy) with buspirone/levodopa/carbidopa (Spinalon™) in subjects with complete AIS A or motor-complete AIS B spinal cord injury. Curr Pharm Des. 2017;23(12):1789–1804. doi: 10.2174/1381612822666161227152200. [DOI] [PubMed] [Google Scholar]
- 46.Osuagwu BC, Wallace L, Fraser M, Vuckovic A. Rehabilitation of hand in subacute tetraplegic patients based on brain computer interface and functional electrical stimulation: A randomised pilot study. J Neural Eng. 2016;13(6) doi: 10.1088/1741-2560/13/6/065002. 065002. [DOI] [PubMed] [Google Scholar]
- 47.Oh SK, Choi KH, Yoo JY, Kim DY, Kim SJ, Jeon SR. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery. 2016;78(3):436–447. doi: 10.1227/NEU.0000000000001056. discussion 447. [DOI] [PubMed] [Google Scholar]
- 48.Lynch M, Duffell L, Sandhu M et al. Effect of acute intermittent hypoxia on motor function in individuals with chronic spinal cord injury following ibuprofen pretreatment: A pilot study. J Spinal Cord Med. 2017;40(3):295–303. doi: 10.1080/10790268.2016.1142137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chhabra HS, Sarda K, Arora M et al. Autologous bone marrow cell transplantation in acute spinal cord injury--an Indian pilot study. Spinal Cord. 2016;54(1):57–64. doi: 10.1038/sc.2015.134. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Lu J, Li YA et al. Autologous olfactory lamina propria transplantation for chronic spinal cord injury: Three-year follow-up outcomes from a prospective double-blinded clinical trial. Cell Transplant. 2016;25(1):141–157. doi: 10.3727/096368915X688065. [DOI] [PubMed] [Google Scholar]
- 51.Zhai HW, Gong ZK, Sun J et al. Ganglioside with nerve growth factor for the recovery of extremity function following spinal cord injury and somatosensory evoked potential. Eur Rev Med Pharmacol Sci. 2015;19(12):2282–2286. [PubMed] [Google Scholar]
- 52.Estigoni EH, Fornusek C, Hamzaid NA, Hasnan N, Smith RM, Davis GM. Evoked EMG versus muscle torque during fatiguing functional electrical stimulation-evoked muscle contractions and short-term recovery in individuals with spinal cord injury. Sensors (Basel) 2014;14(12):22907–22920. doi: 10.3390/s141222907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen L, Huang H, Xi H et al. A prospective randomized double-blind clinical trial using a combination of olfactory ensheathing cells and Schwann cells for the treatment of chronic complete spinal cord injuries. Cell Transplant. 2014;23(suppl 1):S35–44. doi: 10.3727/096368914X685014. [DOI] [PubMed] [Google Scholar]
- 54.Leech KA, Kinnaird CR, Hornby TG. Effects of serotonergic medications on locomotor performance in humans with incomplete spinal cord injury. J Neurotrauma. 2014;31(15):1334–1342. doi: 10.1089/neu.2013.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu VW, Hornby TG, Schmit BD. Effect of antispastic drugs on motor reflexes and voluntary muscle contraction in incomplete spinal cord injury. Arch Phys Med Rehabil. 2014;95(4):622–632. doi: 10.1016/j.apmr.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Hofstoetter US, McKay WB, Tansey KE, Mayr W, Kern H, Minassian K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med. 2014;37(2):202–211. doi: 10.1179/2045772313Y.0000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shapiro S. A review of oscillating field stimulation to treat human spinal cord injury. World Neurosurg. 2014;81(5–6):830–835. doi: 10.1016/j.wneu.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 58.Nardone R, Holler Y, Thomschewski A et al. rTMS modulates reciprocal inhibition in patients with traumatic spinal cord injury. Spinal Cord. 2014;52(11):831–835. doi: 10.1038/sc.2014.136. [DOI] [PubMed] [Google Scholar]
- 59.Fenuta AM, Hicks AL. Metabolic demand and muscle activation during different forms of bodyweight supported locomotion in men with incomplete SCI. Biomed Res Int. 2014;2014 doi: 10.1155/2014/632765. 632765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai G, Liu X, Zhang Z, Yang Z, Dai Y, Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73–79. doi: 10.1016/j.brainres.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Govil K, Noohu MM. Effect of EMG biofeedback training of gluteus maximus muscle on gait parameters in incomplete spinal cord injury. NeuroRehabilitation. 2013;33(1):147–152. doi: 10.3233/NRE-130939. [DOI] [PubMed] [Google Scholar]
- 62.Frolov AA, Bryukhovetskiy AS. Effects of hematopoietic autologous stem cell transplantation to the chronically injured human spinal cord evaluated by motor and somatosensory evoked potentials methods. Cell Transplant. 2012;21(suppl 1):S49–55. doi: 10.3727/096368912x633761. [DOI] [PubMed] [Google Scholar]
- 63.Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26(2):163–172. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- 64.Mazzoleni S, Boldrini E, Laschi C, Carrozza MC, Stampacchia G, Rossi B. Changes on EMG activation in healthy subjects and incomplete SCI patients following a robot-assisted locomotor training. IEEE Int Conf Rehabil Robot. 2011;2011 doi: 10.1109/ICORR.2011.5975467. 5975467. [DOI] [PubMed] [Google Scholar]
- 65.Houldin A, Luttin K, Lam T. Locomotor adaptations and aftereffects to resistance during walking in individuals with spinal cord injury. J Neurophysiol. 2011;106(1):247–258. doi: 10.1152/jn.00753.2010. [DOI] [PubMed] [Google Scholar]
- 66.Adams MM, Hicks AL. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. J Spinal Cord Med. 2011;34(5):488–494. doi: 10.1179/2045772311Y.0000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grijalva I, Garcia-Perez A, Diaz J et al. High doses of 4-aminopyridine improve functionality in chronic complete spinal cord injury patients with MRI evidence of cord continuity. Arch Med Res. 2010;41(7):567–575. doi: 10.1016/j.arcmed.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Lima C, Escada P, Pratas-Vital J et al. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair. 2010;24(1):10–22. doi: 10.1177/1545968309347685. [DOI] [PubMed] [Google Scholar]
- 69.Cotey D, Hornby TG, Gordon KE, Schmit BD. Increases in muscle activity produced by vibration of the thigh muscles during locomotion in chronic human spinal cord injury. Exp Brain Res. 2009;196(3):361–374. doi: 10.1007/s00221-009-1855-9. [DOI] [PubMed] [Google Scholar]
- 70.Cristante AF, Barros-Filho TE, Tatsui N et al. Stem cells in the treatment of chronic spinal cord injury: Evaluation of somatosensitive evoked potentials in 39 patients. Spinal Cord. 2009;47(10):733–738. doi: 10.1038/sc.2009.24. [DOI] [PubMed] [Google Scholar]
- 71.Gorassini MA, Norton JA, Nevett-Duchcherer J, Roy FD, Yang JF. Changes in locomotor muscle activity after treadmill training in subjects with incomplete spinal cord injury. J Neurophysiol. 2009;101(2):969–979. doi: 10.1152/jn.91131.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adel N, Gabr H, Hamdy SI, Afifi L, Mahmoud HA. Stem cell therapy in chronic spinal cord injuries. Egypt J Neurol Psychiat Neurosurg. 2009;46(2):467–478. [Google Scholar]
- 73.Lam T, Wirz M, Lunenburger L, Dietz V. Swing phase resistance enhances flexor muscle activity during treadmill locomotion in incomplete spinal cord injury. Neurorehabil Neural Repair. 2008;22(5):438–446. doi: 10.1177/1545968308315595. [DOI] [PubMed] [Google Scholar]
- 74.Mackay-Sim A, Feron F, Cochrane J et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain. 2008;131(pt 9):2376–2386. doi: 10.1093/brain/awn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawashima N, Nozaki D, Abe MO, Nakazawa K. Shaping appropriate locomotive motor output through interlimb neural pathway within spinal cord in humans. J Neurophysiol. 2008;99(6):2946–2955. doi: 10.1152/jn.00020.2008. [DOI] [PubMed] [Google Scholar]


