Abstract
Summary
Cutaneous skeletal hypophosphatemia syndrome (CSHS), caused by somatic RAS mutations, features excess fibroblast growth factor-23 (FGF23) and skeletal dysplasia. Records from 56 individuals were reviewed and demonstrated fractures, scoliosis, and non-congenital hypophosphatemia that in some cases were resolved. Phosphate and calcitriol, but not skin lesion removal, were effective at controlling hypophosphatemia. No skeletal malignancies were found.
Purpose
CSHS is a disorder defined by the association of epidermal and/or melanocytic nevi, a mosaic skeletal dysplasia, and an FGF23-mediated hypophosphatemia. To date, somatic RAS mutations have been identified in all patients whose affected tissue has undergone DNA sequencing. However, the clinical spectrum and treatment are poorly defined in CSHS. The purpose of this study is to determine the spectrum of the phenotype, natural history of the disease, and response to treatment of hypophosphatemia.
Methods
Five CSHS subjects underwent prospective data collection at clinical research centers. A review of the literature identified 45 reports that included a total of 51 additional patients, in whom the findings were compatible with CSHS. Data on nevi subtypes, bone histology, mineral and skeletal disorders, abnormalities in other tissues, and response to treatment of hypophosphatemia were analyzed.
Results
Fractures, limb deformities, and scoliosis affected most CSHS subjects. Hypophosphatemia was not present at birth. Histology revealed severe osteomalacia but no other abnormalities. Skeletal dysplasia was reported in all anatomical compartments, though less frequently in the spine; there was no clear correlation between the location of nevi and the skeletal lesions. Phosphate and calcitriol supplementation was the most effective therapy for rickets. Convincing data that nevi removal improved blood phosphate levels was lacking. An age-dependent improvement in mineral abnormalities was observed. A spectrum of extra-osseous/extra-cutaneous manifestations that included both benign and malignant neoplasms was present in many subjects, though osteosarcoma remains unreported.
Conclusion
An understanding of the spectrum, natural history, and efficacy of treatment of hypophosphatemia in CSHS may improve the care of these patients.
Keywords: Epidermal nevus syndrome, FGF23, Hypophosphatemic rickets, RASopathies, Skeletal dysplasia
Introduction
Cutaneous skeletal hypophosphatemia syndrome (CSHS) refers to the association of epidermal and/or melanocytic nevi, mosaic skeletal dysplasia, and fibroblast growth factor 23 (FGF23)-mediated hypophosphatemia [1]. We reported the finding of somatic activating RAS mutations in nevoid skin and dysplastic bone in six CSHS subjects establishing a role of RAS hyperactivity in the syndrome’s pathogenesis [1, 2]. However, the clinical spectrum and natural history of this disease remain poorly described. This is due, in part, to its extreme rarity and diagnostic uncertainties in published reports, which have been variably reported as components of epidermal nevus syndromes (ENS). Optimal clinical management in CSHS has likewise not been established. Believing that skin lesions were the source of FGF23 excess, removal of nevi has been advocated as apotential treatment for the hypophosphatemia [3], but the efficacy of this approach has not been systematically investigated and remains unclear given uncertainty as to the tissue source of FGF23 in CSHS.
The purpose of this study is to describe the phenotype, clinical course, and response to treatment for mineral abnormalities in a series of CSHS subjects. Findings from this series were supported by an extensive review and analysis of all published reports likely to represent CSHS. Together, these data provide the first report on the spectrum, course, and treatment of the disease.
Subjects and methods
Subjects
Four subjects with CSHS were evaluated at the NIH Clinical Center and participated in an NIH IRB-approved protocol. Subjects are identified as CSHS101, 104–106. Clinical records and radiographs of an additional subject evaluated in another clinical center, CSHS102, were also reviewed. All subjects gave informed consent/assent. Selected clinical features of these subjects have been previously reported [1, 2]. All subjects underwent clinical phenotyping, including history and physical exam, biochemical evaluation, and imaging.
Genetic and histological evaluation of CSHS bone
A bone biopsy of radiographically appearing dysplastic bone was performed for clinical purposes from the anterior iliac crest of subject CSHS105 at the age of 17. The sample was divided in three similar pieces: one piece was fixed in 70 % ethanol and subsequently embedded undecalcified in methylmethacrylate. The specimen was sectioned, and the slides stained with Goldner’s trichrome to assess mineralization status. Analysis ofmicrostructural and kinetic indices was performed using OsteoMeasure (Osteometrics, Atlanta, GA, USA), and outcomes were expressed according to the ASBMR’s standardized nomenclature [4]. Another section of the biopsy was decalcified and subsequently embedded in paraffin, sectioned, and stained with hematoxylin-eosin. The third piece was used to assess for the presence of the HRAS G13R, which is the mutation found in the patient’s nevi. Bone marrow stromal cells (BMSCs) were isolated from the specimen and single colonies derived as previously reported [5]. Genomic DNA was isolated from colonies of clonal cells using the DNeasy Tissue Kit according to the manufacturer’s instructions (Qiagen). DNA was amplified in a GeneAmp PCR System 9700 (Applied Biosystems) with primers, HRAS forward: AGGTGGGGCAGGAGACCCTGTAG and HRAS reverse: AGCCCTATCCTGGCTGTGTCCTG. Sanger sequencing was performed on a 3730 DNA Analyzer (Applied Biosystems) on the PCR product with HRAS primer (TGGGCTCGCCCGCAGCAGCTGCTGGCACCTGG). Results were processed with the Chromas Lite 2.0 software.
Literature search strategy
A Medline search was performed in an effort to identify previously reported individuals with CSHS. The following term combinations were used without restrictions: “nevus rickets,” “nevus hypophosphatemia,” “nevus fibrous dysplasia,” and “phakomatosis pigmentokeratotica.” “Fibrous dysplasia” was used in the search, as there were some reports in which this term was used to describe individuals that in retrospect clearly had CSHS.
Report selection
Case reports of subjects with epidermal nevi (EN), congenital melanocytic nevi (CMN), or phakomatosis pigmentokeratotica (PPK) (coexistence of EN and speckled lentiginous nevi) were selected when rickets and/or focal dysplastic skeletal lesions were part of the subject’s medical history, and family history was negative for inherited skeletal disorders.
Results
CSHS cohort
Demographic information and clinical findings from the current cohort are summarized in Table 1. Biochemical data are displayed in Online Resource 1.
Table 1.
Demographic information and clinical findings from the CSHS cohort
| Subject | Sex | Ethnicity | Nevi type | Mutation [1], Lim et al. JAAD | Age of rachitic onset (years ) | Pathologic fractures | Scoliosis | Other skeletal defomrities | Laterality of skin vs. bone lesions | Response to oral phosphate and calcitriol | Spontaneous improvement of mineral alterations | Extra-osseous/ extra-cutaneous manifestations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSHS101 | F | Caucasian | EN | NRAS Q61R | 2 | Yes | No | Windswept deformity in lower extremities | Unilateral nevi; ipsilateral skeletal dysplasia | Healed rickets | No (f/u until age 9) | Brainstem lipoma; thyroid nodule; splenic hemangiomas |
| CSHS102 | F | Caucasian | EN | HRAS G13R | 1.5 | Yes | Yes | Coxa vara; lower extremities bowing and deformities secondary to fractures | Unilateral nevi; ipsilateral skeletal dysplasia | Limited effect during childhood; improved labs and ↓ fracture in adolescence | Yes. Decrease of fracture rate, age 12 (f/u until age 14) | Subaortic valve stenosis |
| CSHS104 | F | African American | CMN | NRAS Q61R | 1.3 | Yes | No | Upper and lower extremity bowing | Bilateral nevi; bilateral skeletal dysplasia | Initiation of ambulation; Improved labs | No (deceased at age 4 due to pericardial effusion) | CNS melanosis; ocular dermoid Pericardial effusion |
| CSHS105 | M | Caucasian | EN | HRAS G13R | 12 | Yes | Yes | Pronounced leg length discrepancy | Unilateral nevi; bilateral skeletal dysplasia | ↓ pain ↓ weakness | Yes. Resolution of mineral abnormalities at age 17 (f/u until age 19) | Colpocephaly body asymmetry |
| CSHS106 | F | Hispanic | PPK | HRAS G13R | 5 | Yes | Yes | Bowing of lower extremities; leg length discrepancy | Bilateral nevi; bilateral skeletal dysplasia | Improved labs | No (f/u until age 14) | Body asymmetry |
F female, M male, EN epidemral nevus, CMN congenital nrelanocytic nevus, PPK phakonratosis pignrentokeratotica, f/u follow-up, CNS central nervous system
Cutaneous nevi
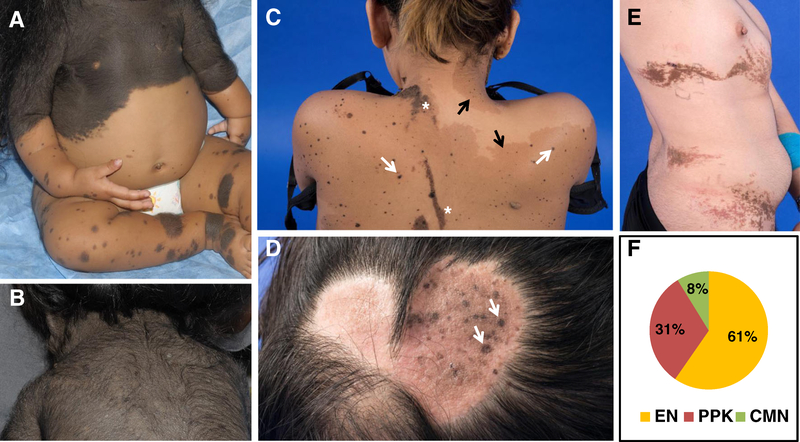
Dermatological findings in our series included EN (CSHS101, 102, 105), PPK (CSHS106), and giant CMN (CSHS104), the last defined as a CMN reaching ≥20 cm in adulthood [6]. Representative images are displayed in Fig. 1a–d.
Fig. 1.
Cutaneous nevi in CSHS. a Giant congenital melanocytic nevus (CMN) (CSHS104) composed of nevomelanocytes (neural crest origin). b Hair follicles may be enlarged and infiltrated by these cells, giving the observed hairy appearance. c Phakomatosis pigmentokeratotica (PPK) (CSHS106) characterized by speckled lentiginous nevi (white arrows), a subtype of melanocytic nevi (neural crest origin), and nevus sebaceous (NS) (asterisk), a subtype of epidermal nevus (epidermal origin). Superimposed café-au-lait macules are frequently present in these patients (black arrow). d NS (CSHS106) seen as a round waxy alopecic scalp plaque containing small speckled lentiginous nevi (arrows). e Epidermal nevus (EN) (CSHS 104) characterized by hyperplasia of elements of epidermal origin; e.g., sebaceous glands or keratinocytes. f Distribution of nevi types in 51 CSHS patients identified in published reports. EN in 31/51 (61 %) [1, 8, 10–12, 18–30, 32, 35, 36, 39–43, 46, 47], PPK in 16/51 (31 %), [3, 9, 11, 13, 14, 16,17, 31, 33, 37–39, 44, 45, 48, 49], and giant CMN in 4/51 subjects (8 %) [7, 12, 15, 34] (color figure online)
Features of mineral abnormalities and skeletal dysplasia
Hypophosphatemia and mosaic bone lesions coexisted in all of the subjects from our series. Hypophosphatemia was not present at birth and had a variable age of presentation ranging from 16 months in CSHS104 to 12 years in CSHS105. The most common initial symptoms attributed to hypophosphatemia were bone pain, limb length discrepancy, bone deformities, and impaired mobility.
Blood and urine biochemistries were consistent with FGF23-mediated hypophosphatemia in all the subjects from our series. These included elevated FGF23, renal phosphate wasting (as defined by a tubular reabsorption of phosphate <85 %), elevated alkaline phosphatase, and normocalcemia in the absence of evidence for intrinsic tubular dysfunction or kidney disease (Online Resource 1). PTH values were sometimes elevated in the setting of vitamin D deficiency. 1,25-dihydroxyvitamin D values were variable.
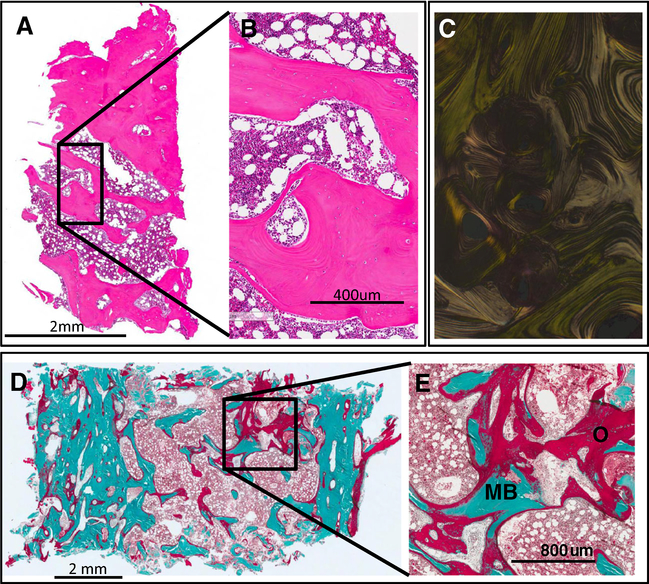
Radiographs demonstrated a broad skeletal phenotype that included dysplastic foci of mixed lytic and sclerotic bone, cortical irregularities, and classic rachitic features (Fig. 2). Marked osteoid accumulation was observed in the plastic-embedded bone specimen from the iliac crest in subject 105, although hematoxylin-eosin (H&E) staining of the same specimen did not demonstrate evidence other abnormal findings (Fig. 3). DNA isolated from BMSCs corroborated the presence of the HRAS G13R mutation in approximately 30 % of the colonies (Online Resource 2), suggesting that even at the tissue level the lesions are mosaic. 2D histomorphometry revealed severe osteomalacia in this area (mean, SD): osteoid volume/bone volume 59 % (1.48, 0.93), total osteoid surface 69.4 % (12.1, 4.64), bone volume/total volume 27 % (23.2, 4.37), trabecular thickness 125 μm (133, 22), trabecular separation 338 μm (570, 99), trabecular number 2.16 trabeculae/mm (1.75, 0.23), cortical thickness 783 μm (1202, 314), eroded surface 2.44 % (4.09, 2.33), and mineralizing surface 19 % (9.7, 4.9).
Fig. 2.
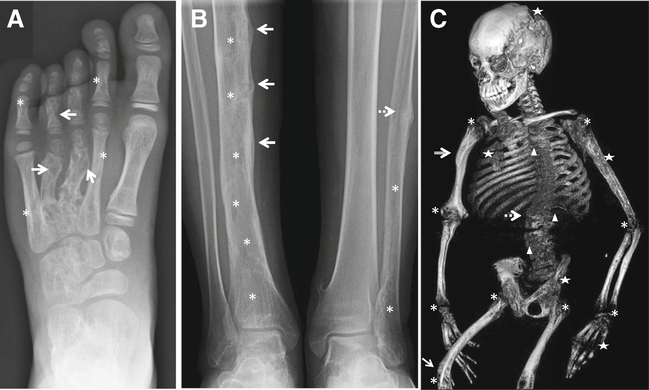
Representative radiographic skeletal features in CSHS. a Left foot radiograph (CSHS101) showing dysplastic lesions in phalanges and metacarpals of digits 2–5. In contrast, the bones of the first ray are spared from dysplasia, consistent with the mosaic nature of the syndrome. Dysplastic bone lesions are characterized by areas of mixed lysis/sclerosis. In this radiograph, the lytic component is more prominent in digits 3 and 4 (arrows), while the sclerotic component is more noticeable in digits 2 and 5 (asterisks). b Radiograph of mid and lower shafts of tibiae and fibulae (CSHS105). The right tibia shows cortical pseudofractures with periosteal reaction along the mid shaft (arrows) in addition to irregular lucent changes indicative of dysplasia (asterisks). A healing stress fracture is also observed in the mid left fibula (dashed arrow), with lucent changes in its mid and distal shaft (asterisks). The left tibia appears normal and unaffected, suggesting that dysplastic bones are more prone to fracture under the same systemic hypophosphatemic milieu than non-dysplastic bones. c Skeletal 3D CT reconstruction (CSHS106) showing severe rickets (asterisks) and lower limb bowing (arrow) secondary to osteomalacia. Patchy areas with lytic lesions are seen throughout the skeleton (stars) in addition to areas of sclerosis (compound arrow). This was the only patient in the cohort in which vertebral dysplasia was identified (triangles). Scoliosis, a frequent manifestation in CSHS, was severe in this patient (spotted arrow)
Fig. 3.
Bone histopathology. An iliac crest biopsy was performed in an area that appeared dysplastic on imaging in subject CSHS105. The same HRAS G13R mutation that had been identified in the skin was identified in bone marrow stromal cells extracted from this sample. a, b. H&E of the iliac crest does not exhibit any noticeable histopathological abnormality. c. Polarized light shows normal lamellar distribution of the collagen fibrils of the sample shown in a, b. d, e. Goldner’s trichrome stain of an undecalcified sample of the biopsy showing areas with excessive accumulation of osteoid indicative of severe osteomalacia. O (red) = osteoid; MB (green) = mineralized bone (color figure online)
The location and laterality of dysplastic skeletal lesions were compared with coexistent skin lesions. CSHS101 and 102 had unilateral nevi with ipsilateral bone lesions; CSHS105 had unilateral nevi with bilateral dysplasia; and CSHS104 and 106 had bilateral nevi and bilateral dysplasia. Both the appendicular and axial skeleton were affected in all subjects from our cohort. The pelvis and skull were the most commonly involved areas of the axial skeleton. Vertebral involvement was identified only in CSHS106.
Response to therapy
Oral phosphate and calcitriol were prescribed to all subjects in an effort to treat hypophosphatemia and related symptoms. When subjects adhered to the prescribed doses, all experienced significant symptomatic and/or biochemical improvements, e.g., healed rickets in CSHS101, decreased pain and weakness in CSHS105, initiation of ambulation in CSHS104, and improved biochemical parameters in CSHS106. In CSHS102, mineral abnormalities appeared less responsive to treatment during childhood but improved dramatically during adolescence.
Nevi removal has been postulated as a potential therapy for hypophosphatemia. However, no effects on mineral abnormalities were detected in either CSHS101 or 102 after undergoing extensive CO2 laser ablation and surgical excision, respectively.
Skeletal disease course
Resolution of biochemical abnormalities, alleviation of hypophosphatemia-related symptoms, and increase in BMD occurred in CSHS105 at age 17 (Fig. 4). Similarly, CSHS102 experienced a decrease in fracture rate with improved response to medication at age 12. The findings in these two subjects were suggestive of age-related regression of skeletal/mineral homeostasis abnormalities in CSHS, a finding which we sought to corroborate in the published literature.
Fig. 4.
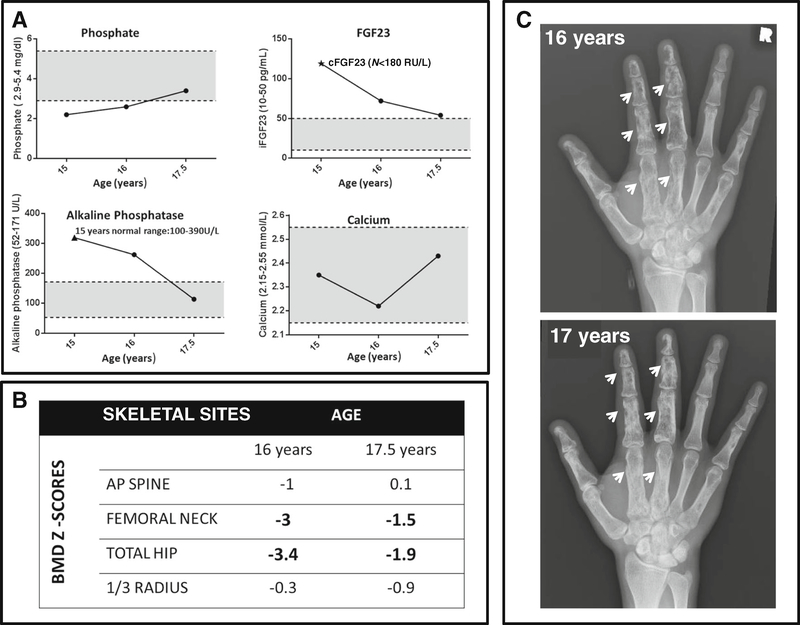
Changes in mineral metabolism and radiographic findings in CSHS105 following treatment with phosphate and calcitriol. a. Fasting biochemistries, measured when off medication for at least 48 h, had normalized by age 17.5 years. Initially FGF23 was measured using a “C-terminal” assay that detects both C-terminal fragments and intact molecule ((star) Immutopics, San Clemente, CA); subsequently, FGF23 was measured using an assay that measures only intact FGF23 assay (Kainos, Japan). Shaded areas denote normal ranges for a 17-year-old. b. Bone mineral density (BMD) Z-scores by DXA increased from baseline at age 16 to age 17.5. c. Dysplastic skeletal lesions appeared more radio dense at age 17 vs. age 16 (arrows), consistent with an increase in mineral deposition in the dysplastic bone
Extra-skeletal/extra-cutaneous manifestations
Findings, including benign tumors, were found in non-osseous/non-cutaneous tissues in all the subjects from the cohort. These are listed in Table 1, and representative images are displayed in Fig. 5.
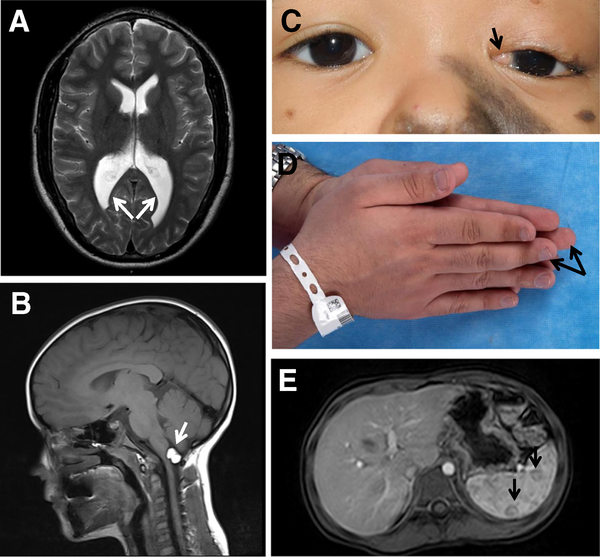
Fig. 5.
Representative extra-osseous/extra-cutaneous manifestations in CSHS. a. Enlarged posterior horns of the lateral ventricles in CSHS105 (arrows). b. Intracranial lipoma in CSHS101 (arrow). c. Limbal dermoid in CSHS104 (arrow). d. Hemibody asymmetry in CSHS105 (arrows). e. Splenic hemangiomas in CSHS101 (arrows)
Literature review
The search terms employed retrieved 39 reports that described 47 subjects with findings compatible with CSHS [1, 3, 7–42]. Seven additional reports were identified from references of the reports retrieved [43–49]. This resulted in a total of 45 reports with 51 subjects, which were included in the literature review. Sixty-one percent of the subjects were male. Ethnic backgrounds were diverse (Online Resource 3).
Cutaneous nevi
A similar spectrum of cutaneous findings to that of our CSHS cohort was identified in the literature review; EN affecting 57 %, PPK 32 %, and giant CMN 8 % of the patients (Fig. 1e).
Features of mineral abnormalities and skeletal dysplasia
Coexistence of hypophosphatemia and focal bone lesions was observed in 26/51 subjects described in the literature [1, 7–9, 11, 12, 14, 16, 17, 19, 22, 24, 25, 27–29, 32, 35–37, 41, 42, 45, 49]. In 18/51 reports, hypophosphatemia and/or rickets was reported without mention of skeletal dysplasia, and radiographs were either not provided or did not demonstrate dysplasia [3, 11–13, 15, 18, 20, 21, 23, 26, 31, 33, 34, 38, 39, 48]. Seven of fifty-one reports described dysplastic lesions on radiographs, but serum phosphate was either not reported [40, 43, 44, 46, 47] or was within the normal age-related normal range at the time of the report [10, 30].
Published reports, in which biochemistries were provided, showed similar results to that of our cohort, except for the aforementioned patients in whom phosphate was within the normal range (Online Resource 1). FGF23 levels were measured and elevated in all six subjects from the literature in whom it was assessed [1, 7, 8, 17, 18, 32].
The mean age of onset of hypophosphatemia in the published reports was 4 years (median 2.7 years, SD3.6, range 1–14years). The most common initial symptoms were similar to our cohort and included bone pain, limb length discrepancy, bone deformities, and impaired mobility. Four subjects [3, 19, 23, 27] had no evidence of rickets or hypophosphatemia in early childhood but developed these conditions later in childhood, indicating that systemic mineral abnormalities are not congenital.
Fractures and deformities are common in CSHS. Fractures were reported in 29/51 subjects (57 %) in the literature [1, 3, 8–14, 17–20, 22, 24–27, 29, 35, 36, 41–44, 47–49] and were frequently in areas of dysplastic bone. Limb deformities, predominantly bowing, were reported in 36/51 (70 %) [1, 3, 7, 9, 11–13, 15–17, 19–22, 24, 25, 27, 29–34, 37–39, 41–43, 47–49]. Scoliosis was described in 21/51 (41 %) [1, 3, 7, 9, 12–14, 16, 19, 20, 22, 24, 27, 30, 31, 34, 39–41, 44, 47, 48].
Literature reports of dysplastic bone lesions were similar to those in our cohort and were often described as “radiolucent,” “sclerotic,” “cyst-like,” “lytic,” and/or “fibrous dysplasia-like.”
Bone biopsy specimens were examined in four patients. Osteomalacia was identified in ¾, without any evidence of dysplasia [11, 14, 17]. “Hemangiomas” were described in the fourth biopsy, and it does not appear that the presence of osteomalacia was assessed [36].
In respect to the location and laterality of dysplastic bone and skin lesions, in patients with unilateral skin lesions (n = 15), skeletal lesions were ipsilateral in 10/15 (67 %) [10, 11, 14, 16, 17, 27, 35, 36, 40, 45] and bilateral in 5/15 (33 %) [1, 8, 29, 42, 44, 49]. Bilateral nevi and bilateral skeletal lesions were reported in five patients [9, 24, 32, 46, 47], while bilateral skin lesions with unilateral skeletal dysplasia were described in two patients [30, 37].
Skeletal dysplasia was reported in all skeletal compartments. Both the appendicular and axial skeletons were affected in 16 patients from the literature [1, 8–10, 14, 17, 28–30, 36, 42, 44–47, 49, 50]. Dysplasia limited to the appendicular skeleton was described in nine patients [11, 12, 19, 24, 27, 32, 35, 37, 40], while one report described dysplastic lesions exclusively in the axial skeleton [16]. The pelvis and skull were the most commonly involved areas of the axial skeleton, whereas vertebral involvement was identified only in two patients from the literature [8, 44].
Hypophosphatemia/rickets and response to treatment
Treatment approaches and efficacy differed among the reported patients from the literature. Of the 26 patients treated with a combination of phosphate supplements and calcitriol (or other active vitamin D analogs), 19/26 (73 %) experienced significant clinical improvement, e.g., resumed ambulation, decreased pain, and healing of radiographic rickets [11, 12, 14, 15, 19, 21, 25, 27, 29, 32, 35, 37, 38, 48]. In 4/26 (15 %) [17, 18, 20, 22], the initial response was not optimal but improved over time. In 2/26 (8 %), efficacy appeared limited [12, 13]. Of the 11 patients treated with non-active vitamin D (ergo- or cholecalciferol) [3, 7, 9, 11, 16, 24, 28, 31, 34, 38], symptomatic improvement was reported in only 2/11 (18 %) [16, 28]. However, some of these patients underwent nevi removal in addition to phosphate and active vitamin D replacement. In an effort to avoid the potential confounding effect of nevi removal on the efficacy of oral medication, response to medication was also analyzed in patients who did not undergo nevi removal. In these patients, including CSHS104–106, oral medication had an independent positive effect on symptoms and blood phosphate levels [11, 12, 14, 15, 21, 22, 27–29, 32, 35, 37, 39].
Twenty of fifty-one patients from the literature underwent surgical excision, dermabrasion, or CO2 laser ablation of the nevi in an effort to correct the hypophosphatemia. Serum phosphate and hypophosphatemic symptoms were unchanged in 7/20 (35 %) [12, 14, 22, 27, 29, 36] and improved in 12/20 (60 %) [16–20, 26, 31, 38, 41, 48]. However, attribution of improvement in blood phosphate levels to skin lesion removal was confounded by the fact that phosphate and calcitriol/ vitamin D were either not discontinued or even initiated at the time of nevi removal. In two patients, a direct association between nevi removal and a subsequent increase in serum phosphate was suggested [3, 20].
Skeletal disease course
Evidence of clinical improvement in mineral metabolism (e.g., drug dose reduction, fracture cessation, resolution of hypophosphatemia) was reported in ten patients from the literature, most of whom were over 17 years old (Online Resource 4). However, hypophosphatemia and/or osteomalacic manifestations persisted in five young adults (≤23 years) [11, 14, 17, 36, 44] and one older adult [9]. Of note, skeletal dysplasia remained apparent on imaging despite clinical improvement.
Extra-osseous/extra-cutaneous manifestations
A detailed list of extra-skeletal/extra-cutaneous abnormalities is included in Online Resource 5. Neurological abnormalities were reported in 23/51 (45 %) of the patients from the literature. Intellectual disability, developmental delay, ventricular enlargement, and EEG abnormalities were the most frequent findings. Ophthalmological disorders, predominantly colobomas, and ocular dermoids were identified in 13/51 patients (26 %). Body asymmetry was described (or observed in the images provided) in 11/51 patients (21 %). Angiomatous malformations and/or cardiac problems were also reported in 4/51 patients from the literature (8 %). Precocious puberty occurred in 4/51 (8 %) of the patients. Benign neoplasms were frequent, whereas malignant transformation was less common, albeit reported (5 malignant vs. 16 benign tumors) (Online Resource 6). Interestingly, osteosarcoma has not been reported to date in association with CSHS.
Discussion
The clinical features and natural history of CSHS were detailed through combining the findings from a cohort of subjects that underwent a thorough analysis with the findings from patients identified in an exhaustive literature review. Key features of the analysis include a broad spectrum of skeletal findings, the novel observation of age-related clinical improvement in skeletal disease, evidence that phosphate and active vitamin D treatment, but not nevi removal, are effective at healing rickets and improving hypophosphatemic symptoms, and the lack of reports of malignant transformation of skeletal lesions. Given that RAS mutations have been identified in the vast majority of epidermal and melanocytic nevi in which it has been assessed [51–54], as well as in recently identified reports of CSHS [1, 8], the findings presented here provide clinical context for RAS pathway activation in the pathogenesis of CSHS. However, since RAS-specific sequencing was not performed in the patients from the literature, some caution must be exercised in attributing all of the manifestations cited solely to RAS pathway activation.
As expected in patients with hypophosphatemic rickets and skeletal dysplasia, fractures and deformities affected the majority of CSHS subjects. Similar to other mosaic disorders, such as McCune-Albright syndrome [55], distribution and location of the dysplastic skeletal lesions varied, affected all skeletal compartments, and were not limited to the side of the body with the nevus. The spine was the skeletal site least affected by dysplasia. However, vertebral lesions are often difficult to discern on plain radiographs and the lower prevalence of spine involvement could be attributed to this. Scoliosis, on the other hand, was extremely common. The etiology of scoliosis in CSHS is not clear. It could be related to osteomalacia, but it should be noted that scoliosis has also been reported in association with other ENS in the absence of hypophosphatemia [39]. Hemibody asymmetry, impaired mineralization, and possible underlying dysplastic vertebrae could account for the high prevalence of scoliosis in CSHS.
In spite of detecting a high number of mutation-bearing colonies in cultured bone marrow stromal cells from the iliac crest biopsy performed on CSHS105, the H&E-stained histologic sections did not reveal any specific dysplastic findings. This could represent sampling error, but consistent with this observation were the findings from a patient with CSHS in which a bone biopsy was performed in a dysplastic-appearing area, and no histological abnormalities were noted beyond osteomalacia [17]. In contrast, in our prior report, small islands of dysplastic-appearing fibrous cells were observed in histologic sections from the rib of CSHS104 (obtained at autopsy), in which the presence of the RAS mutation was also verified [1]. Nonetheless, these fibrotic islands were small, thus they may not represent the mixed lytic/sclerotic lesions seen on imaging. Further study of additional bone samples from patients with CSHS is necessary to better characterize the cellular and tissue features of the CSHS skeletal dysplasia. Osteomalacia, on the other hand, has been confirmed in both bone samples from our cohort (CSHS104, 105), as well as in the literature [11, 14, 17]. Given that dysplastic appearing bones seem to be more prone to fracture, it may be possible that the most prominent effect of these RAS mutations on the skeleton, in addition to a hypothetical effect on FGF23 production, is to inhibit mineralization of mutation-bearing bone-cells. Comparison of bone from a non-dysplastic area vs. a dysplastic bone lesion within the same individual could be very informative for this matter.
While skeletal dysplasia is a defining feature of CSHS, it was not mentioned in a third of the published reports that we suspect or represent cases of CSHS. In some of the reports, in which there was no mention of skeletal dysplasia, it was clear from either the images in the paper [19, 22, 25, 41] or those confirmed by the authors [7] that dysplastic skeletal lesions were present. It is also likely that in the cases in which rachitic changes were reported, there was also an accompanying skeletal dysplasia. Similarly, abnormalities in phosphate were not reported in 7/51 subjects, although four of these subjects had a past medical history of numerous insufficiency fractures [10, 43, 44, 47], suggesting that hypophosphatemia had been present. Therefore, the overall estimation of the presence of skeletal disease likely represents an underestimation.
Unlike other genetic forms of FGF23-mediated hypophosphatemia, in which rickets develops in infancy (e.g., X-linked hypophosphatemic rickets), the onset of rachitic changes in CSHS is delayed. The range of the age of onset of hypophosphatemic changes in our cohort and in the literature review was broad (4 years (median 2.7 years, SD 3.6, range 1–14 years), emphasizing the importance of routine phosphate assessment in pediatric patients with ENS.
Since the availability of FGF23 assays, elevations in FGF23 have consistently been reported in CSHS. Complete biochemical panels from reported individuals, in which FGF23 was not assessed, also displayed a profile compatible with FGF23-mediated hypophosphatemia. Therefore, excess FGF23 is the most probable cause of hypophosphatemia in CSHS, although secondary hyperparathyroidism due to vitamin D insufficiency, as observed in some cases, cannot be eliminated as the cause, or at least a contributing factor to lower serum phosphate and impaired mineralization. We speculate that hyperactive RAS signaling is responsible for FGF23 overproduction, but the precise mechanisms, by which this would lead to an increase in FGF23, and its tissue source, remain unclear. The skin, as the pathological source of FGF23, was initially proposed by Aschinberg et al. who reported a boy with EN and severe rickets, in whom removal of fibroangiomas (not EN) normalized blood phosphate [3]. The excised tissue was infused into a dog and was reported to result in increased renal phosphate excretion. However, a careful analysis of data revealed that serum phosphate did not decrease in the dog, making it unlikely that the excised tissue led to dysregulated phosphate homeostasis. Similarly, Ivker et al. described a girl with severe hypophosphatemia, despite being appropriately treated with oral medication, in which nevi removal was eventually associated with an increase in serum phosphate; however, in that report, it was not clear if oral treatment supplementation had been discontinued and/or if compliance with treatment prior to surgery had been adequate [20]. Outcomes in other subjects, in whom nevi removal was reported to be associated with an increase in serum phosphate, were also confounded by concomitant treatment of hypophosphatemia with oral medications. Importantly, with careful observation in subjects CSHS101 and CSHS102, phosphate status was unchanged after nevi removal. Therefore, it is unlikely that in CSHS, the source of excess FGF23 is overproduction by the skin. Supporting this conclusion is the fact that the vast majority of syndromic and non-syndromic cases of EN and CMN are not associated with hypophosphatemia. Furthermore, it is improbable that skin cells of very different origins (e.g., keratinocytes, melanocytes, sebocytes), which are differentially affected in patients with CSHS, are capable of secreting FGF23. In addition, when directly investigated, FGF23 was not found to be expressed in nevi [1, 26, 32]. Taking into account that normal bone is the physiological source of FGF23 [56] and that skeletal dysplasia is identified in most subjects with CSHS; it is most likely that dysplastic bone is the source of pathologically secreted FGF23.
The best, currently approved, medical therapy for the hypophosphatemia of CSHS is the combination of oral phosphate and active vitamin D (e.g., calcitriol). Active vitamin D is necessary given that FGF23 suppresses the action of 1-α-hydroxylase, which is necessary for the conversion of inactive 25-hydroxy vitamin D to active 1,25-dihydroxyvitamin D. Emerging therapies that may prove effective in the treatment of CSHS include cinacalcet, which diminishes the phosphaturic effects of FGF23 [57], and an anti-FGF23 monoclonal antibody, which blocks the action of FGF23 [58]. In addition, the selective FGF receptor inhibitor, BGJ398, which has action in the RAS pathway and which is associated with an increase in serum phosphate, may be a potential treatment for CSHS [59].
Delayed onset and age-related remission of mineral abnormalities in CSHS is an important observation of our study and similar to what is seen in patients with fibrous dysplasia of bone. In fibrous dysplasia, which like CSHS is a disease of skeletal stem cells caused by somatic mosaic expression of gain-of-function mutations in GTPases (Gsα) [60], delayed onset is attributed to the time it takes for patients to develop a sufficient mass of dysplastic tissue and the loss of phosphate wasting attributed to age-dependent apoptosis of mutation-bearing skeletal stem cells [61]. It is intriguing to speculate that a similar mechanism, age-dependent apoptosis, and/or oncogene-induced senescence [62] may be responsible for the age-related waning of symptoms in CSHS. Prospective long-term follow-up of our cohort and other CSHS patients will help determine the likelihood of skeletal disease resolution in these patients.
The most common cutaneous findings in our cohort and in the literature were EN. However, given that CSHS is quite rare and that EN occurs in approximately 1/1000 births [63], it is likely that the association of CSHS with PPK is much higher, given that PPK is very rare (only ~30–40 reports in the literature [64]), yet it was found in 16/51 of the published cases and in CSHS106. The skin lesions that characterize PPK have different embryonic origins (epidermis and neural crest) but bear the same mutation [52]. This implies mutagenesis in an earlier cellular progenitor, in contradistinction to EN, which is exclusively of epidermal origin. This may explain the higher incidence of abnormalities in tissues derived from non-ectodermal layers (such as the skeleton) in PPK vs. most cases of EN.
Neoplastic growth was frequent in our cohort and published reports, which is not unexpected given that RAS mutations are known to lead to unchecked cell proliferation and tumor development [65]. Surprisingly, despite abundance of dysplastic and mutated skeletal tissue in CSHS, there were no reports of osteosarcoma or other skeletal malignancies an observation that parallels the scarcity of RAS mutations in sporadic bone cancer (http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/).
Our study has two major limitations: (1) long-term follow-up of our CSHS cohort is lacking, as well as (2) the retrospective nature of the literature review and the possibility of misclassifying some of the reported subjects as having CSHS. Despite this, our report offers insight into clinical features and natural history of the syndrome. This provides guidance for clinicians who evaluate and treat patients with CSHS and suggests a developmental, physiologic framework within which to understand and study CSHS going forward.
Summary
CSHS is a rare mosaic and sporadic disorder. Somatic RAS mutations have been identified in the affected tissues from all patients that have undergone sequencing. The incidence of CSHS is proportionally much higher in PPK than in other nevi subtypes, but it is more frequently associated with EN, owing to the relatively higher incidence of EN vs. PPK in general population. Concomitant rickets and skeletal dysplasia are reported in most CSHS subjects. Skeletal dysplasia may affect all skeletal sites and its distribution is not dependent on nevi location. Although skeletal dysplasia is less frequent in the spine, scoliosis is very common and likely multifactorial. Histological analysis of bone with dysplastic appearance on imaging did not reveal any particular features beyond osteomalacia. FGF23-mediated hypophosphatemia and attendant symptoms are typically detected during childhood and not present shortly after birth. Calcitriol (or analogs) and phosphate supplementation may be the best treatment currently available, but more directed therapies are in development. Convincing evidence supporting nevi removal as an effective treatment for hypophosphatemia is lacking, but fortunately, mineral abnormalities may remit spontaneously with age. Similarly to other ENS, neurological and ophthalmological abnormalities are the most common extra-osseous/extra-cutaneous abnormalities, followed by body asymmetry. Risk of developing tumors appears increased in CSHS, but malignant transformation of the bone has not been reported.
Supplementary Material
Acknowledgments
This study was supported in part by the Division of Intramural Research, National Institute of Dental and Craniofacial Research, National Institutes of Health, and Department of Health and Human Services, Bethesda, MD (D.O., A.M.B., R.I.G., L.G.C., and M.T.C.). K.A.C. was supported by a Doris Duke Charitable Foundation Clinical Scientist Development Award, and Y.H.L. by a Doris Duke Charitable Foundation Medical Student Research Fellowship and the Yale Center for Mendelian Genomics (NIH U54 HG006504). YHL is also supported by the Medical Scientist Training Program at Yale University.
Footnotes
Conflicts of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
Electronic supplementary material The online version of this article (doi:10.1007/s00198-016-3702-8) contains supplementary material, which is available to authorized users.
References
- 1.Lim YH et al. (2014) Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Hum Mol Genet 23(2):397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim YH, Ovejero D, Derrick KM; Yale Center for Mendelian Genomics, Collins MT, Choate KA (2016) Cutaneous skeletal hypophosphatemia syndrome (CSHS) is a multilineage somatic mosaic RASopathy. J Am Acad Dermatol 75(2):420–427. doi: 10.1016/j.jaad.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aschinberg LC et al. (1977) Vitamin D-resistant rickets associated with epidermal nevus syndrome: demonstration of a phosphaturic substance in the dermal lesions. J Pediatr 91(1):56–60 [DOI] [PubMed] [Google Scholar]
- 4.Dempster DW et al. (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28(1):2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuznetsov SA et al. (1997) Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res 12(9):1335–47 [DOI] [PubMed] [Google Scholar]
- 6.Castilla EE, da Graca Dutra M, Orioli-Parreiras IM (1981) Epidemiology of congenital pigmented naevi: I. Incidence rates and relative frequencies. Br J Dermatol 104(3):307–15 [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal S et al. (2013) Hypophosphatemic rickets associated with giant hairy nevus. Indian J Endocrinol Metab 17(Suppl 1):S188–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avitan-Hersh E et al. (2014) Postzygotic HRAS mutation causing both keratinocytic epidermal nevus and thymoma and associated with bone dysplasia and hypophosphatemia due to elevated FGF23. J Clin Endocrinol Metab 99(1):E132–6 [DOI] [PubMed] [Google Scholar]
- 9.Bouthors J et al. (2006) Phacomatosis pigmentokeratotica associated with hypophosphataemic rickets, pheochromocytoma and multiple basal cell carcinomas. Br J Dermatol 155(1):225–6 [DOI] [PubMed] [Google Scholar]
- 10.Cabanillas M et al. (2009) Epidermal nevus syndrome associated with polyostotic fibrous dysplasia, CNS lipoma, and aplasia cutis. Dermatol Online J 15(10):7. [PubMed] [Google Scholar]
- 11.Carey DE et al. (1986) Hypophosphatemic rickets/osteomalacia in linear sebaceous nevus syndrome: a variant of tumor-induced osteomalacia. J Pediatr 109(6):994–1000 [DOI] [PubMed] [Google Scholar]
- 12.Chou YY et al. (2005) Hypophosphatemic rickets associated with epidermal nevus syndrome and giant hairy nevus. J Pediatr Endocrinol Metab 18(1):93–5 [DOI] [PubMed] [Google Scholar]
- 13.de Morais OO et al. (2013) Phacomatosis pigmentokeratotica—a patient with hypophosphatemic rickets. Skinmed 11(2): 125–8 [PubMed] [Google Scholar]
- 14.Feldmann JL et al. (1990) Solomon’s syndrome associated with fibrous dysplasia of bone and vitamin-resistant rickets. Rev Rhum Mal Osteoartic 57(12):881–4 [PubMed] [Google Scholar]
- 15.Gathwala G et al. (2013) Giant congenital melanocytic nevi: a rare association with hypophosphatemic rickets. Indian J Pediatr 80(5): 430–1 [DOI] [PubMed] [Google Scholar]
- 16.Goldblum JR, Headington JT (1993) Hypophosphatemic vitamin D-resistant rickets and multiple spindle and epithelioid nevi associated with linear nevus sebaceus syndrome. J Am Acad Dermatol 29(1):109–11 [DOI] [PubMed] [Google Scholar]
- 17.Heike CL et al. (2005) Skeletal changes in epidermal nevus syndrome: does focal bone disease harbor clues concerning pathogenesis? Am J Med Genet A 139(2):67–77 [DOI] [PubMed] [Google Scholar]
- 18.Hoffman WH et al. (2005) Elevated fibroblast growth factor-23 in hypophosphatemic linear nevus sebaceous syndrome. Am J Med Genet A 134(3):233–6 [DOI] [PubMed] [Google Scholar]
- 19.Hosalkar HS et al. (2003) Linear sebaceous naevus syndrome and resistant rickets. J Bone Joint Surg Br 85(4):578–83 [DOI] [PubMed] [Google Scholar]
- 20.Ivker R, Resnick SD, Skidmore RA (1997) Hypophosphatemic vitamin D-resistant rickets, precocious puberty, and the epidermal nevus syndrome. Arch Dermatol 133(12): 1557–61 [PubMed] [Google Scholar]
- 21.John M, Shah NS (2005) Hypophosphatemic rickets with epidermal nevus syndrome. Indian Pediatr 42(6):611–2 [PubMed] [Google Scholar]
- 22.Kishida ES et al. (2005) Epidermal nevus syndrome associated with adnexal tumors, spitz nevus, and hypophosphatemic vitamin D-resistant rickets. Pediatr Dermatol 22(1):48–54 [DOI] [PubMed] [Google Scholar]
- 23.Klein GL et al. (1998) Congenital linear sebaceous nevus syndrome. J Bone Miner Res 13(6):1056–7 [DOI] [PubMed] [Google Scholar]
- 24.Moorjani R, Shaw DG (1976) Feuerstein and Mims syndrome with resistant rickets. Pediatr Radiol 5(2):120–2 [DOI] [PubMed] [Google Scholar]
- 25.Moreira AI et al. (2010) Epidermal nevus syndrome associated with hypophosphatemic rickets. Dermatol Online J 16(9):14. [PubMed] [Google Scholar]
- 26.Narazaki R et al. (2012) Linear nevus sebaceous syndrome with hypophosphatemic rickets with elevated FGF-23. Pediatr Nephrol 27(5):861–3 [DOI] [PubMed] [Google Scholar]
- 27.Olivares JL et al. (1999) Epidermal naevus syndrome and hypophosphataemic rickets: description of a patient with central nervous system anomalies and review of the literature. Eur J Pediatr 158(2):103–7 [DOI] [PubMed] [Google Scholar]
- 28.O’Neill EM (1993) Linear sebaceous naevus syndrome with oncogenic rickets and diffuse pulmonary angiomatosis. J R Soc Med 86(3): 177–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oranje AP et al. (1994) Solomon’s epidermal nevus syndrome (type: linear nevus sebaceus) and hypophosphatemic vitamin D-resistant rickets. Arch Dermatol 130(9):1167–71 [PubMed] [Google Scholar]
- 30.Pierini AM, Ortonne JP, Floret D (1981) Cutaneous manifestations of McCune-Albright syndrome: report of a case (author’s transl). Ann Dermatol Venereol 108(12):969–76 [PubMed] [Google Scholar]
- 31.Saraswat A et al. (2003) Phakomatosis pigmentokeratotica associated with hypophosphataemic vitamin D-resistant rickets: improvement in phosphate homeostasis after partial laser ablation. Br J Dermatol 148(5):1074–6 [DOI] [PubMed] [Google Scholar]
- 32.Sethi SK, Hari P, Bagga A (2010) Elevated FGF-23 and parathormone in linear nevus sebaceous syndrome with resistant rickets. Pediatr Nephrol 25(8):1577–8 [DOI] [PubMed] [Google Scholar]
- 33.Shahgholi E et al. (2011) Congenital rhabdomyosarcoma, central precocious puberty, hemihypertrophy and hypophosphatemic rickets associated with epidermal nevus syndrome. J Pediatr Endocrinol Metab 24(11–12):1063–6 [DOI] [PubMed] [Google Scholar]
- 34.Shieh CC, Wang PJ (1991) Giant nevocellular nevi with rickets and brainstem tumor. Pediatr Neurol 7(6):452–4 [DOI] [PubMed] [Google Scholar]
- 35.Skovby F, Svejgaard E, Moller J (1987) Hypophosphatemic rickets in linear sebaceous nevus sequence. J Pediatr 111(6 Pt 1):855–7 [DOI] [PubMed] [Google Scholar]
- 36.Stosiek N et al. (1994) Extensive linear epidermal nevus associated with hemangiomas of bones and vitamin-D-resistant rickets. Dermatology 189(3):278–82 [DOI] [PubMed] [Google Scholar]
- 37.Sukkhojaiwaratkul D, Mahachoklertwattana P, Poomthavorn P (2014) Epidermal nevus syndrome with hypophosphatemic rickets in a young girl. J Paediatr Child Health 50(7):566–9 [DOI] [PubMed] [Google Scholar]
- 38.Tokatli A, Coskun T, Ozalp I (1997) Hypophosphatemic vitamin-D resistant rickets associated with epidermal nevus syndrome. A case report. Turk J Pediatr 39(2):247–51 [PubMed] [Google Scholar]
- 39.Vidaurri-de la Cruz H et al. (2004) Epidermal nevus syndromes: clinical findings in 35 patients. Pediatr Dermatol 21(4):432–9 [DOI] [PubMed] [Google Scholar]
- 40.Yu AC et al. (1995) Epidermal naevus syndrome associated with polyostotic fibrous dysplasia and central precocious puberty. Eur J Pediatr 154(2):102–4 [DOI] [PubMed] [Google Scholar]
- 41.Zutt M et al. (2003) Schimmelpenning-Feuerstein-Mims syndrome with hypophosphatemic rickets. Dermatology 207(1):72–6 [DOI] [PubMed] [Google Scholar]
- 42.Rustin MH et al. (1989) Polyostotic fibrous dysplasia associated with extensive linear epidermal naevi. Clin Exp Dermatol 14(5): 371–5 [DOI] [PubMed] [Google Scholar]
- 43.Bouwes Bavinck JN, van de Kamp JJP (1985) Organoid naevus phakomatosis: Schimmelpenning-Feuerstein-Mims syndrome. Br J Dermatol 113:491–492 [Google Scholar]
- 44.Camacho Martinez F, Moreno Gimenez JC (1985) Epidermal nevus syndrome (of Solomon, Fretzin and Dewald). Ann Dermatol Venereol 112(2):143–7 [PubMed] [Google Scholar]
- 45.Grun G, Didier MF (1972) Albright’s syndrome (apropos of 2 cases). Bull Soc Fr Dermatol Syphiligr 79(2):184–5 [PubMed] [Google Scholar]
- 46.Kaplan I, Metzker A, Calderon S (1993) Epidermal nevus syndrome with maxillary involvement. Int J Oral Maxillofac Surg 22(5):298–300 [DOI] [PubMed] [Google Scholar]
- 47.Muhle C et al. (1998) Skeletal involvement and follow-up in linear nevus sebaceous syndrome. Eur Radiol 8(4):606–8 [DOI] [PubMed] [Google Scholar]
- 48.Sanmaneechai O, Wisuthsarewong W, Sawathiparnich P (2006) Epidermal nevus syndrome presenting as hypophosphatemic rickets. Endocrinologist 16(3):145–149 [Google Scholar]
- 49.Sugarman GI, Reed WB (1969) Two unusual neurocutaneous disorders with facial cutaneous signs. Arch Neurol 21(3):242–7 [DOI] [PubMed] [Google Scholar]
- 50.Shpall S et al. (1994) Risk of malignant transformation of congenital melanocytic nevi in blacks. Pediatr Dermatol 11(3):204–8 [DOI] [PubMed] [Google Scholar]
- 51.Groesser L et al. (2013) Phacomatosis pigmentokeratotica is caused by a postzygotic HRAS mutation in a multipotent progenitor cell. J Invest Dermatol 133(8):1998–2003 [DOI] [PubMed] [Google Scholar]
- 52.Groesser L et al. (2012) Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet 44(7):783–U211 [DOI] [PubMed] [Google Scholar]
- 53.Hafner C et al. (2012) Keratinocytic epidermal nevi are associated with mosaic RAS mutations. J Med Genet 49(4):249–253 [DOI] [PubMed] [Google Scholar]
- 54.Kinsler VA et al. (2013) Multiple congenital melanocytic nevi and neurocutaneous melanosis are caused by postzygotic mutations in codon 61 of NRAS. J Investig Dermatol 133(9):2229–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyce AM, Collins MT (1993) Fibrous dysplasia/McCune-Albright syndrome. In: Pagon RA et al. (ed) GeneReviews(R) Seattle (WA) [Google Scholar]
- 56.Lorenz-Depiereux B et al. (2006) DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38(11 ): 1248–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geller JL et al. (2007) Cinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res 22(6):931–7 [DOI] [PubMed] [Google Scholar]
- 58.Carpenter TO et al. (2014) Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest 124(4): 1587–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wohrle S et al. (2013) Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatémie rickets. J Bone Miner Res 28(4):899–911 [DOI] [PubMed] [Google Scholar]
- 60.Boyce AM, Bhattacharyya N, Collins MT (2013) Fibrous dysplasia and fibroblast growth factor-23 regulation. Curr Osteoporos Rep 11(2): 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuznetsov SA et al. (2008) Age-dependent demise of GNAS-mutated skeletal stem cells and “normalization” of fibrous dysplasia of bone. J Bone Miner Res 23(11):1731–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192(4):547–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paller AS et al. (1994) Genetic and clinical mosaicism in a type of epidermal nevus. N Engl J Med 331(21):1408–15 [DOI] [PubMed] [Google Scholar]
- 64.Chantorn R, Shwayder T (2011) Phacomatosis pigmentokeratotica: a further case without extracutaneous anomalies and review of the condition. Pediatr Dermatol 28(6):715–9 [DOI] [PubMed] [Google Scholar]
- 65.Shaw RJ, Cantley LC (2006) Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441(7092):424–30 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.