Abstract
Delphinidin is a flavonoid belonging to dietary anthocyanidin family that has been reported to possess diverse anti-tumoral activities. However, the effects of delphinidin on colorectal cancer (CRC) cells and the underlying mechanisms are not fully understood. Thus, we aimed to investigate the anti-cancer activity of delphinidin in CRC cells and the underlying molecular mechanisms. The effects of delphinidin on the viability, metastatic characteristics, signaling, and microRNA (miR) profile of human CRC cell lines used were analyzed. In vivo metastasis was also evaluated using xenograft animal models. Our findings showed that delphinidin (<100 μM) inhibited the colony formation of DLD-1, SW480, and SW620 cells, but non-significantly affected cell viability. Delphinidin also suppressed the migratory ability and invasiveness of the tested CRC cell lines, downregulated integrin αV/β3 expression, inhibited focal adhesion kinase (FAK)/Src/paxillin signaling, and interfered with cytoskeletal construction. Analysis of the miR expression profile revealed a number of miRs, particularly miR-204-3p, that were significantly upregulated and downregulated by delphinidin. Abolishing the expression of one upregulated miR, miR-204-3p, with an antagomir restored delphinidin-mediated inhibition of cell migration and invasiveness in DLD-1 cells as well as the αV/β3-integrin/FAK/Src axis. Delphinidin also inhibited the lung metastasis of DLD-1 cells in the xenograft animal model. Collectively, these results indicate that the migration and invasion of CRC cells are inhibited by delphinidin, and the mechanism may involve the upregulation of miR-204-3p and consequent suppression of the αV/β3-integrin/FAK axis. These findings suggest that delphinidin exerts anti-metastatic effects in CRC cells by inhibiting integrin/FAK signaling and indicate that miR-204-3p may play an important role in CRC metastasis.
Subject terms: Gastrointestinal cancer, Cell signalling
Introduction
Colorectal cancer (CRC) is a leading cause of death in countries worldwide, including Taiwan, and it is the second and third most common cancer in women and men, respectively1. CRC originates from dysplastic adenomatous polyps that occasionally become cancerous, primarily due to genetic alterations that promote proliferation and inhibit apoptosis in colorectal epithelial cells2. Although chemotherapy, surgery, and novel anti-cancer drugs have been developed for the treatment of CRC, rapid and distant metastases lead to high mortality rates3. The acquisition of cell adhesion, migratory ability, and invasiveness plays a pivotal role in the metastasis of CRC4. Thus, these metastatic characteristics of CRC cells could be important treatment targets.
Cancer is a life-threatening malignant disease. Therefore, identifying molecules, including phytochemicals, with strong anti-tumoral potential and low toxicity is important for the development of effective treatments and novel drugs. Over the past decade, numerous natural compounds have been shown to possess chemopreventive and anti-tumoral activities in vitro or in vivo5–8, and accumulating evidence has shown that anthocyanins and anthocyanidins exhibit various biological activities, including anti-inflammatory9, anti-tumoral10, and anti-carcinogenic effects11. Delphinidin is the major anthocyanidin in many dietary fruits and vegetables, such as berries and tomatoes, and it has been shown to possess potent anti-oxidant, anti-inflammatory12, and anti-tumoral properties13.
MicroRNAs (miRs) are short (22–24 nucleotide) non-coding RNAs that bind to the 3′-untranslated region of coding RNAs by base pairing, which results in termination of protein translation or degradation of messenger RNA (mRNA)14. miRs have been implicated in embryonic development15,16, cellular homeostasis17,18, physiopathological conditions19,20, and dysregulated expression of oncogenic genes involved in various cancers, including renal cell carcinoma21, ovarian cancer22, and hepatocellular carcinoma23. miR dysregulation has also been shown to be involved in the induction of epithelial-mesenchymal transition (EMT) in various tumors24,25. This evidence indicates that uncontrolled miR expression may be associated with carcinogenesis, cancer progression, and tumor metastasis.
In this study, we aimed to investigate the effects of delphinidin on colon cancer metastasis, with an emphasis on the regulation of miR expression and integrin-associated signaling. Cell viability was assessed by the MTT assay. Carcinogenic properties were evaluated by adhesion and colony formation assays. Cell motility and invasiveness were assessed by transmigration and invasion assays. The related signaling cascades were evaluated by western blotting, and the miR expression profile was analyzed by microarray and quantitative real-time reverse transcription poly chain reaction (qRT-PCR).
Results
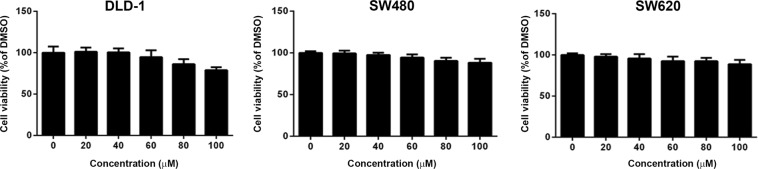
Delphinidin had non-significant effects on the viability of human CRC cells
To investigate the anti-tumoral effects of delphinidin in CRC, its effects on cell viability were first analyzed in three human CRC cell lines: DLD-1, SW480, and SW620. The results showed that delphinidin, at 20–100 μM, had non-significant effects on the viability of the three cell lines (Fig. 1, P > 0.05). These findings indicate that delphinidin does not influence the viability of CRC cells.
Figure 1.
Effects of delphinidin on the viability of human CRC cells. Cells were treated with delphinidin at serial concentrations for 24 h, and then the cell viability was assessed by MTT assay. Cell viability was presented as percentage of the DMSO control (0 μM) in mean ± standard deviation. No significant difference was observed among each treatment.
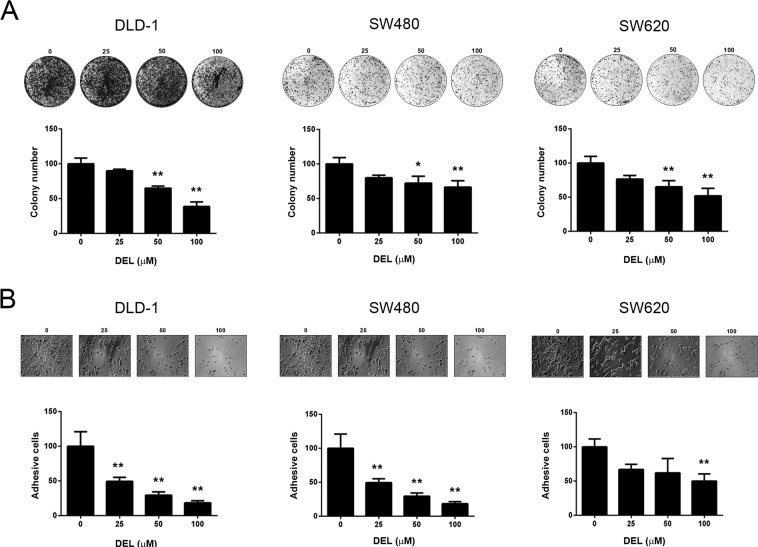
Delphinidin diminished the colony formation and adhesion of human CRC cells
Colonization on soft gel and attachment to extracellular matrix components, such as collagen, are notable characteristics of malignant cancer cells26. Since the viability of CRC cells was not affected by delphinidin, we explored its effects on colony formation and cell adhesion. As shown in Fig. 2A, 50 and 100 μM delphinidin significantly (P < 0.01) and dose-dependently decreased the number of colonies formed on soft agar by DLD-1, SW480, and SW620 cells. Similarly, the number of cells that adhered to culture plates coated with type I-collagen was also dose-dependently decreased by delphinidin treatment (P < 0.01; Fig. 2B). Interestingly, the number of attached SW620 cells was only significantly reduced following treatment with 100 μM delphinidin (Fig. 2B). Collectively, these results show that delphinidin diminishes the colony formation and ECM adhesion ability of CRC cells.
Figure 2.
Delphinidin inhibited colony formation and cell adhesion of human CRC cells. Cells were cultured in soft agar or collagen-coated plates, treated with delphinidin (DEL) at serial concentrations for 24 h, and then subject to (A) colony formation assay or (B) cell adhesion assessment, respectively. Quantitative data was acquired from three independent experiments and presented as mean ± standard deviation. * and **, P < 0.05 and P < 0.01 as compared to the DMSO control (0 μM).
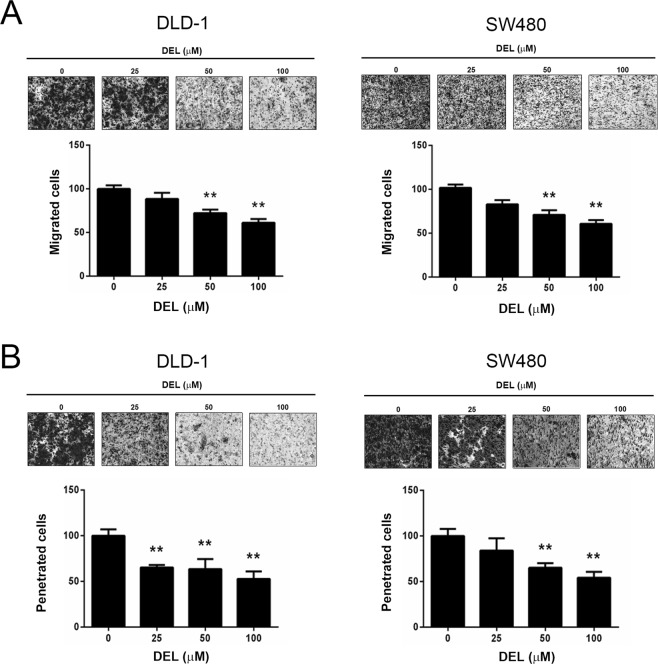
Delphinidin inhibited the migration and invasion of human CRC cells
The potent migratory and invasive potential of cancer cells plays pivotal roles in metastasis. Thus, we investigated whether delphinidin could inhibit the motility and invasiveness of CRC cells. As shown in Fig. 3A, 50 and 100 μM delphinidin significantly (P < 0.01) and dose-dependently decreased the transmigration of DLD-1 and SW480 cells. Similarly, the number of invaded DLD-1 and SW480 cells was also dose-dependently decreased following delphinidin treatment (50 and 100 μM, P < 0.01; Fig. 3B). These results demonstrate that delphinidin inhibits the motility and invasiveness of CRC cells.
Figure 3.
Delphinidin reduced cell migration and invasion of human CRC cells. Cells were cultured on membrane without or with Matrigel, treated with delphinidin (DEL) at serial concentrations for 24 h, and then subject to (A) transmigration assay or (B) invasion analysis. Quantitative data was acquired from three independent experiments and presented as mean ± standard deviation. **P < 0.01 as compared to the DMSO control (0 μM).
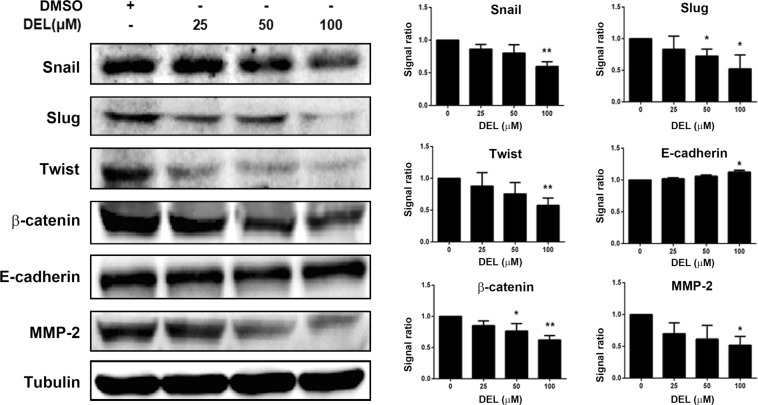
Delphinidin suppressed the epithelial-mesenchymal transition of human CRC cells
EMT is the primary process that transforms solid epithelial tumors into mesenchymal metastatic cancer cells27. Therefore, we assessed whether delphinidin regulates the expression of EMT markers in CRC cells. As shown in Fig. 4, the key EMT inducers Snail, Slug, Twist, and β-catenin were significantly downregulated in DLD-1 cells following treatment with 100 μM delphinidin (P < 0.05). Similarly, delphinidin also decreased the expression of matrix metalloproteinase-2 (MMP-2), a proteinase that is highly associated with tumor metastasis28. In contrast, the expression of the epithelial marker E-cadherin was upregulated following delphinidin (100 µM) treatment (P < 0.05; Fig. 4).
Figure 4.
Delphinidin suppressed epithelial-to-mesenchymal transition in human CRC cells. Cells were treated with delphinidin (DEL) at serial concentrations for 24 h, then the cells were collected and lysed for the detection of epithelial and mesenchymal markers by using Western blotting. Quantitative data was acquired by using densitometric analysis from three independent experiments and presented as mean ± standard deviation. * and **P < 0.05 and P < 0.01 as compared to the DMSO control (0 μM).
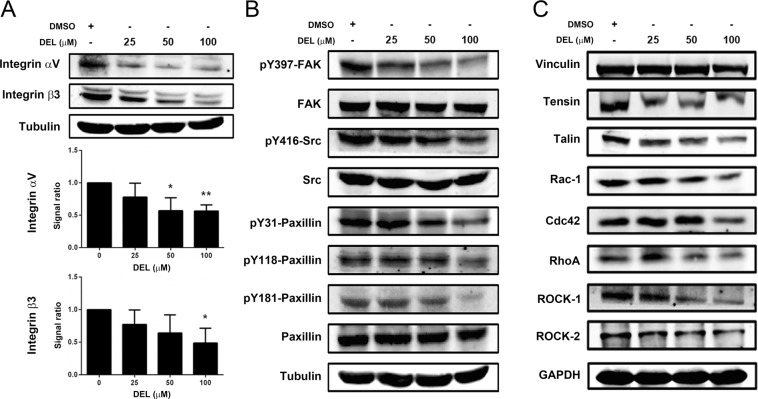
Delphinidin inhibited the integrin/FAK signaling cascade in DLD-1 cells
Integrin and downstream FAK signaling constitute a pivotal pathway in the regulation of cell adhesion and migration29,30. Since delphinidin diminished the adhesion, motility, and invasiveness of CRC cells, the effects of delphinidin on integrin and FAK signaling were investigated. As shown in Fig. 5A, delphinidin significantly and dose-dependently (P < 0.05) reduced the expression of integrin αV and β3 in DLD-1 cells. In parallel to the reduced integrin expression, the downstream signaling components, including FAK phosphorylation (Tyr397), Src phosphorylation (Tyr416), and Paxillin phosphorylation (Tyr31, Tyr118, Tyr181), were diminished in response to delphinidin treatment (Fig. 5B). In addition, the intracellular integrin-associated adaptor proteins Tensin and Talin were also downregulated in DLD-1 cells following exposure to delphinidin (Fig. 5C). The FAK-mediated small GTPases Rac-1, Cdc42, and Rho A and the downstream effectors of Rho A, ROCK1, and ROCK2 were also decreased (Fig. 5C). These findings indicate that delphinidin not only downregulates integrin expression and integrin-associated adaptor proteins but also inhibits FAK activation and the downstream small GTPases and effectors.
Figure 5.
Delphinidin reduced integrin expression and inhibited FAK cascade in DLD-1 cells. Cells were treated with delphinidin (DEL) at serial concentrations for 24 h, then the cells were collected and lysed for the detection of (A) integrins, (B) FAK signaling components, and (C) integrin-associated adaptor proteins by using Western blotting. Quantitative data was acquired by using densitometric analysis from three independent experiments and presented as mean ± standard deviation. * and **P < 0.05 and P < 0.01 as compared to the DMSO control (0 μM).
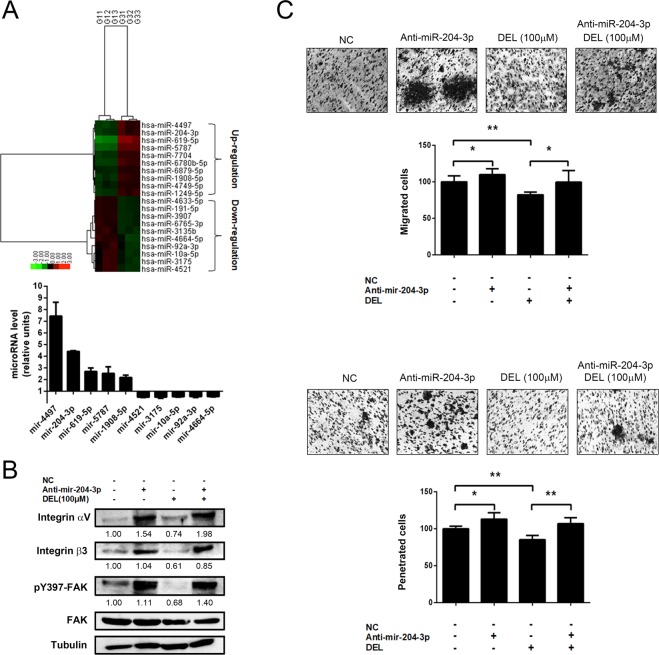
Delphinidin inhibited invasion and the integrin/FAK axis via the upregulation of miR-204-3p in DLD-1 cells
Accumulating evidence has shown that miRs play an important role in the motility and invasiveness of cancer cells and tumor metastasis31,32. Therefore, we evaluated whether delphinidin affects miR expression in CRC cells and explored the involvement of these miRs in the regulation of cell motility and integrin/FAK signaling. Using a microarray with 853 miRs, we observed that delphinidin treatment (100 μM for 24 h) altered the miR expression profile in DLD-1 cells. We identified 24 upregulated and 22 downregulated miRs with an expression ratio (delphinidin/control) log2 > 0.585 and < −0.585, respectively. The top ten most significantly up- and down-regulated miRs in response to delphinidin are presented in Fig. 6A (upper panel). Densitometric analysis showed that the expression of miR-204-3p was obviously upregulated, at ~4.5-fold (P < 0.01, Fig. 6A, lower panel). miR-204-3p has been reported to be involved in the invasion of renal cell carcinoma33. Therefore, the role of miR-204-3p in delphinidin-inhibited integrin/FAK signaling, transmigration, and invasion of DLD-1 cells was analyzed. As shown in Fig. 6B, the delphinidin-induced decreases in integrin αV/β3 levels and FAK phosphorylation (Tyr397) were restored in DLD-1 cells transfected with an Anti-mir-204-3p as compared to the values in cells transfected with a control vector (NC). Similarly, the delphinidin-induced inhibition of transmigration and invasion were also recovered in DLD-1 cells transfected with Anti-mir-204-3p when compared to control vector-transfected cells (NC, Fig. 6C, upper and lower panels). Collectively, these results show that delphinidin regulates miR expression in DLD-1 cells and that mir-204-3p plays an important role in the suppression of integrin/FAK signaling and the migration/invasion induced in response to delphinidin.
Figure 6.
Involvement of miR-204-3p in the inhibited integrin/FAK cascade and the suppressed cell migration and invasion of human CRC cell DLD-1 in response to delphinidin. (A) Cells were treated with delphinidin (DEL) at 100 μM for 24 h, and then the cells were lysed for RNA extraction and microRNA expression profiling by using microarray analysis. MicroRNAs with up-regulation and down-regulation in response to delphinidin were labeled with red and green color, respectively. (B) Cells were transfected with control vector (NC) or anti-miR-204-3p, treated with delphinidin for 24 h, and then lysed for the assessment of integrin expression and FAK activation by Western blotting. (C) Cells were transfected with control vector or anti-miR-204-3p, treated with delphinidin for 24 h, and then subject to the transmigration and invasion assay. Quantitative data was acquired from three independent experiments and presented as mean ± standard deviation. * and **P < 0.05 and P < 0.01 as compared to the DMSO control (0 μM).
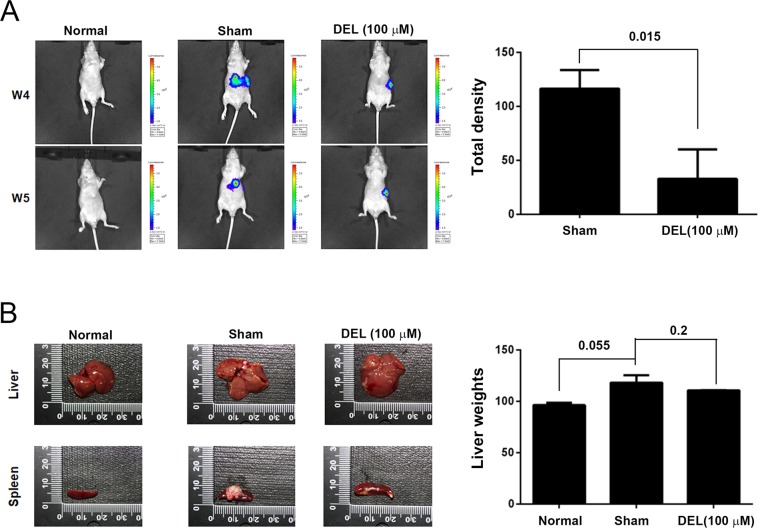
Delphinidin inhibited the in vivo metastasis of DLD-1 colon cancer cells
Based on the observation that delphinidin treatment suppressed the migration and invasion of CRC cells in vitro, we further investigated whether delphinidin inhibits the metastasis of a highly invasive CRC cell line (DLD-1) when compared to SW480 and SW620 cells. As shown in Fig. 7A, the metastasis of delphinidin-treated DLD-1 cells (100 μM for 24 h) to the lung was significantly reduced as compared to that of DMSO-treated DLD-1 cells (P < 0.05). In addition, the weights of the livers from DLD-1 model mice treated with DMSO or delphinidin (120.6 ± 5.6 mg and 110.6 ± 0.8 mg, respectively) were statistically the same (Fig. 7B, P = 0.2). These observations show that delphinidin treatment significantly attenuated the in vivo metastatic ability of DLD-1 cells but did not affect liver and spleen weights.
Figure 7.
Delphinidin in vivo attenuated the metastasis of human CRC cell DLD-1 in xenograft mice. DLD-1 cells stably expressing luciferase were intraperitoneally injected into mice, and (A) the metastasized DLD-1 cells were detected by using IVIS image system after two weeks, then (B) the mice were sacrificed to acquire liver samples for phenotyping and weighing. Quantitative data was acquired by using photodensitometric analysis from three independent experiments and presented as mean ± standard deviation. P values as compared to normal or sham control were indicated.
Discussion
Cell motility, EMT, and carcinogenicity are closely associated with the progression and metastasis of CRC, and early metastasis is the main cause of mortality in patients with CRC. Here, we found that delphinidin inhibited the adhesion, colony formation, motility, and invasion of CRC cells, which may be attributed to the inhibition of EMT, suppression of integrin/FAK signaling, and upregulation of miR-204-3p. These findings suggest that delphinidin has promising anti-metastatic potential in CRC.
Previous reports have demonstrated that several phenolic acids, including anthocyanins, protocatechuic acid (PCA), syringic acid, vanillic acid, phloroglucinol aldehyde, phloroglucinol acid, and gallic acid (GA), are metabolites of anthocyanins34, and the interplay between anthocyanins and the gastrointestinal microbiota plays a central role in producing these metabolites35. De Ferrars et al. analyzed the metabolite profile of cyanidin-3-glucoside in humans using high-sensitivity mass spectrometry, and identified 17 metabolites in serum, urine, and feces36, indicating that anthocyanins can be absorbed and metabolized by humans and the resulting metabolites are widely distributed in body fluids. In addition, a recent study reported that GA is a degradation product of delphinidin and both delphinidin and GA have potent biological activities, including anti-cancer and anti-inflammatory activities37–39. In our in vitro experiments, delphinidin treatments were conducted in a neutral pH condition; therefore, delphinidin may be partially transformed to the degradation products such as GA. Our in vitro findings clearly show that delphinidin has anti-metastatic effects on CRC cells. Our in vivo findings using a xenograft model also show that delphinidin attenuates the metastatic ability of xenografted DLD-1 cells in mice. Taken together, these observations indicate that delphinidin as well as its metabolites, such as GA, may directly and/or synergistically exert anti-metastatic effects on CRC cells.
Integrins are well-characterized cell surface receptors that are composed of non-covalent, heterodimeric complexes with an α subunit and a β subunit. The major signaling pathway downstream of integrin is the FAK cascade, which has been widely reported to be involved in EMT, a process that leads to the invasion and metastasis of various tumors40. During EMT, dynamic changes in the cytoskeleton lead to a loss of cell-cell contacts and epithelial cell polarity, accompanied with enhanced cell motility. Thus, potent EMT inducers, including Snail, Slug, Twist, and ZEB2, have been implicated in tumor progression and metastasis41,42. Snail and ZEB2 have also been reported to affect cell-matrix adhesion by modulating integrins and basement membrane proteins43. In this study, we found that delphinidin downregulated the expression of the EMT inducers Snail, Slug, Twist, and β-catenin in DLD-1 cells. In addition, delphinidin also decreased the expression of integrin and its adaptor proteins Vinculin, Talin, and Tensin and suppressed proteins in the FAK signaling cascade, including Src, Paxillin, Rac-1, Cdc42, Rho A, and ROCK-1/2. These findings indicate that delphinidin exerts both anti-EMT and anti-metastatic activities in CRC cells through inhibition of the integrin/FAK cascade.
miRs have been shown to play important roles in carcinogenesis, tumor progression, and metastasis44–46. Through the miRNA array analysis, we demonstrated that delphinidin can alter the miR expression profile in DLD-1 cells; 24 miRs upregulated and 22 miRs were downregulated (data not shown). The biological functions of several of the identified miRs have been investigated; however, most of the miRs that were significantly changed in response to delphinidin are first found to be associated with the motility and invasiveness of CRC cells. For example, miR-4497 has been reported as a tumor suppressor in laryngeal squamous cell carcinoma that functions through inhibition of Gastrulation Brain Homeobox 2, a homeobox gene involved in the normal development of rhombomeres47. In addition, miR-204-3p was shown to attenuate high glucose-induced apoptosis of podocytes by suppressing Bradykinin B2 Receptor48. Finally, overexpression of miR-92a-3p in the peripheral blood mononuclear cells of patients with chronic lymphocytic leukemia has been reported as a potential independent biomarker associated with prolonged overall survival49. However, further investigation is required to determine the roles of these miRs in regard to the anti-metastatic activity of delphinidin in CRC cells.
In conclusion, our results show that miR-204-3p is upregulated in response to delphinidin and that miR-204-3p plays an important role in the regulation of integrin/FAK signaling and the invasion of DLD-1 cells. These findings reveal that delphinidin inhibits the motility and invasiveness of CRC cells by regulating the miR-204-3p-mediated integrin/FAK cascade. In vivo metastasis analysis also showed similar inhibitory effects of delphinidin on DLD-1 cells. Taken together, the study results suggest that miR-204-3p may be a potential molecular target for CRC therapy.
Methods
Chemicals and antibodies
All general chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), including delphinidin (≥95%, no. 43725); Giemsa; crystal violet; 2-propanol; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT); 1-butanol; dimethyl sulfoxide (DMSO); phosphate-buffered saline (PBS); sodium chloride; sodium dodecyl sulfate (SDS); Tris-HCl; type I collagen (no. C7661); and trypsin/EDTA. Primary antibodies specific binding to human Snail (SC-393172), Slug (SC-166902), Twist (SC-81417), E-cadherin (SC-71007), β-catenin (SC-59737), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, SC-47724), matrix metalloproteinase-2 (MMP-2, SC-13594), tubulin (SC-134237), integrin αV (SC-376156), integrin β3 (SC-365679), focal adhesion kinase (FAK, SC-271126), phospho-FAK (pY397-FAK, SC-81493), Src (SC-32789), phospho-Src (pY416-Src, SC-24621, CS#2101), paxillin (SC-136297), and phospho-paxillin (pY118-Paxillin, SC-365020), and peroxidase-conjugated secondary antibodies specific binding to mouse IgG and rabbit IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against human phospho-paxillin (pY31-Paxillin and pY181-Paxillin) were purchased from BioSource International (Camarillo, CA, USA).
Cell culture and treatments
The human colon cancer cell lines DLD-1, SW480, and SW620 were acquired from ATCC and maintained in RPMI-1640 medium and Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich) containing 10% v/v fetal bovine serum (FBS; Biological Industries, Cromwell, CT, USA) and incubated at 37 °C with 5% CO2. For delphinidin treatment, cells reached 80% confluence were incubated with serial concentrations of delphinidin (20–100 μM). DMSO alone (final concentration, 0.1%) was used as the solvent control (presented as 0 μM delphinidin). After the 24 hour (h)-treatment, the cells were collected, washed with PBS, and used for the following experiments.
Cell viability assay
Viable cells were assessed by using the MTT assay. In brief, 2 × 105 cells were seeded into a 24-well plate containing 10% v/v FBS culture medium for 24 h to allow cells attached to the plate, then the attached cells were treated with serial concentrations of delphinidin (20–100 μM) for 24 h. The treated cells were washed with PBS twice and incubated with MTT reagent (5 mg/mL; Sigma-Aldrich) at 37 °C for 2 h. After removing the supernatant, isopropanol was added and the solubilized formazan was determined by measuring the absorbance at 563 nm using a spectrophotometer (U-2900; Hitachi, Tokyo, Japan). The percentage of viable cells was estimated by comparison to the absorbance of DMSO-treated cells. Three independent experiments were performed for statistical analysis.
Colony formation assay
Cells were resuspended in the agarose medium consisting RPMI-1640 medium, 10% v/v FBS, 0.3% w/v agarose, and delphinidin, and plated in a 6-well plate pre-coated with agarose (RPMI medium containing 10% v/v FBS and 0.6% w/v agarose). After incubated in a humidified atmosphere at 37 °C containing 5% CO2 for 7 days, the cells were fixed, stained with crystal violet, and then photographed under a microscope (Nikon Eclipse TE2000-U equipped with a Nikon Digital Camera DXM1200; Nikon, Japan). Colonies greater than 0.1 mm in diameter were counted.
Cell adhesion assay
Cells were incubated with serial concentrations of delphinidin for 24 h, and then transferred to 12-well plates coated with type I collagen (105 cells per well). After incubated at 37 °C for 8 h, non-adherent cells were removed by washing with PBS. Then, the adhered cells were visualized with crystal violet staining, photographed, and quantitated using a cell counter.
Transmigration and invasion assays
The cell migratory ability was assessed by transmigration assay. In brief, cells pretreated with serial concentrations of delphinidin for 24 h were seeded onto 24-well Millicell® Hanging Cell Culture inserts (8 μm pore size; Millipore, Bedford, MA, USA). FBS (20%) was added to the lower compartment as a chemoattractant, and then the cells were incubated at 37 °C for 12 h. The cells that transmigrated to the lower surface of the insert were fixed with 4% paraformaldehyde, visualized using Giemsa staining (Sigma-Aldrich), and then photographed. The numbers of transmigrated cells in five random fields under the microscope were counted for the quantitative analysis.
The cell invasiveness was assessed by invasion assay. In brief, cells were seeded onto the insert which was pre-coated with 100 μL of Matrigel (20× diluted with PBS; BD Biosciences, San Jose, CA, USA), and then incubated for 16 h. Cells that invaded the lower surface of the insert were visualized using Giemsa staining and quantitated as described in the transmigration assay.
Western blotting
After treatment, the cells were detached by trypsinization, collected by centrifugation, and then lysed in the PBS lysis buffer containing protease and phosphatase inhibitor cocktail (Sigma-Aldrich). The cell lysates were centrifuged at 20,000 x g at 4 °C for 10 min, and then the supernatant was collected and used as a crude extract. The protein concentration in the extracts was assessed by Bradford protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Extracts containing equal protein (30 µg) were separated by electrophoresis on an SDS-polyacrylamide gel. The electrophoresed proteins were transferred to a nitrocellulose membrane (PROTRAN BA85, 0.45 μm; Sigma) with a Transphor Unit (Bio-Rad Laboratories, Hercules, CA, USA). The transferred membrane was blocked with 1% (w/v) BSA in PBS followed by a 1-h incubation with the primary antibodies and then the secondary antibodies. The signal was developed with ECL chemiluminescence reagent (SuperSignal West Dura HRP Detection Kit; Pierce Biotechnology, Rockford, IL, USA), and the signals were acquired and quantitated with an image analysis system (Fujifilm, Tokyo, Japan).
Microarray analysis for miR profiling
DLD-1 cells treated with 100 μM delphinidin or 0.1% DMSO for 24 h were used for the miRNA microarray analysis. In brief, total RNA from DLD-1 cells was extracted using the miRNeasy mini kit (QIAGEN, Mainz, Germany) and labeled with Cy3 during reverse transcription to cDNA using an Agilent miRNA Labeling Kit (Agilent, UK) and Spike Kit (Agilent, UK). The labeled cDNAs were hybridized (in duplicate) to Human miRNA Microarray, Release 19.0, 8 × 60 K (v19) microarray slides (Agilent, UK) according to the microRNA Hybridization Kit protocol (Agilent, UK) and scanned using a Nimblegen MS200 array scanner. Data were normalized using the Plier algorithm and were log transformed and analyzed using GeneSpring GX 13.0 (Agilent, UK). miRs with P values less than 0.05 and fold changes >2 or <−2 between control (DMSO) and delphinidin-treated cells were considered significantly changed.
miR transfection
The Anti-miR-204-3p and corresponding negative control (NC) vectors were obtained from System Biosciences (Palo Alto, CA, USA). DLD-1 cells were transfected with Anti-miR-204-3p or Anti-miR-NC using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Xenograft animal model and cancer cell imaging
Male Balb/c nude mice were obtained from the National Laboratory Animal Center of Taiwan (Taipei City, Taiwan) and maintained under the supervision of the Institutional Animal Care and Use Committee (IACUC) of Chung Shan Medical University. All the protocols for animal experiments have been approved by the IACUC (No. 1947), and all the experiments were performed in accordance with the approved guidelines. After a 1-week acclimation period, the mice were randomly divided into the following three groups (n = 6 per group): Normal (no cancer cell implantation), Sham (implantation of cancer cells treated with 0.1% DMSO/PBS for 24 h), and Delphinidin (implantation of cancer cells treated with 100 μM delphinidin for 24 h). Cancer cell implantation was performed by intraperitoneal injection of luciferase-transfected DLD-1 cells (2 × 106 cells in 50 μL of PBS), and the animals were maintained for two weeks. After the maintenance period, the mice were anesthetized and intraperitoneally injected with luciferin (at 150 mg/kg in 100 μL). Images were captured at 20 min after injection using an IVIS-200 Imaging System (Xenogen, Alameda, CA, USA) and processed using Living Image software (Xenogen) by region-of-interest analysis of the total photons per second for each tumor, with background subtraction. After the image analysis was completed, the mice were sacrificed to examine the organs.
Statistical analysis
The data represent the mean ± SD of three independent experiments, except where indicated. Student’s t test was used to analyze the significance of differences. P values less than 0.05 were considered statistically significant.
Supplementary information
Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (grant no. MOST 108-2320-B-040-001, 108-2320-B-040-026-MY3, 107-2320-B-040-006-MY2 and 106-2320-B-040-017).
Author contributions
C.C.H., C.H.H. and Y.C.L. performed cell viability assay, transmigration assay, invasion assay, and Western blotting. C.H.H. and T.W.H. carried out the microRNA array analysis and microRNA experiments. C.H.H., Y.C.L. and S.H.K. conducted the in vivo xenograft experiments. S.H.K. and C.J.W. participated in the study design, scientific criticism, statistical analysis, and article writing. All authors critically reviewed the manuscript and approved the final draft to be published.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chi-Chou Huang and Chia-Hung Hung.
Contributor Information
Chau-Jong Wang, Email: wcj@csmu.edu.tw.
Shao-Hsuan Kao, Email: kaosh@csmu.edu.tw.
Supplementary information
is available for this paper at 10.1038/s41598-019-55505-z.
References
- 1.Yu J. PUMA Kills Stem Cells to Stall Cancer? Molecular and cellular pharmacology. 2009;1:112–118. doi: 10.4255/mcpharmacol.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu W, Leibowitz B, Zhang L, Yu J. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene. 2010;29:1622–1632. doi: 10.1038/onc.2009.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faerden AE, et al. Lymph node micrometastases and isolated tumor cells influence survival in stage I and II colon cancer. Diseases of the colon and rectum. 2011;54:200–206. doi: 10.1007/DCR.0b013e3181fd4c7c. [DOI] [PubMed] [Google Scholar]
- 4.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Radhakrishnan EK, et al. [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling. PloS one. 2014;9:e104401. doi: 10.1371/journal.pone.0104401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee M, et al. Curcumin Downregulates Human GM3 Synthase (hST3Gal V) Gene Expression with Autophagy Induction in Human Colon Carcinoma HCT116 Cells. Evidence-based complementary and alternative medicine: eCAM. 2018;2018:6746412. doi: 10.1155/2018/6746412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park HJ, Choi YJ, Lee JH, Nam MJ. Naringenin causes ASK1-induced apoptosis via reactive oxygen species in human pancreatic cancer cells. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2017;99:1–8. doi: 10.1016/j.fct.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Lee HL, Lin CS, Kao SH, Chou MC. Gallic acid induces G1 phase arrest and apoptosis of triple-negative breast cancer cell MDA-MB-231 via p38 mitogen-activated protein kinase/p21/p27 axis. Anti-cancer drugs. 2017;28:1150–1156. doi: 10.1097/CAD.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 9.Tancharoen Salunya, Shakya Prana, Narkpinit Somphong, Dararat Pornpen, Kikuchi Kiyoshi. Anthocyanins Extracted from Oryza sativa L. Prevent Fluorouracil-Induced Nuclear Factor-κB Activation in Oral Mucositis: In Vitro and In Vivo Studies. International Journal of Molecular Sciences. 2018;19(10):2981. doi: 10.3390/ijms19102981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou L, et al. Anti-tumor properties of anthocyanins from Lonicera caerulea ‘Beilei’ fruit on human hepatocellular carcinoma: In vitro and in vivo study. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;104:520–529. doi: 10.1016/j.biopha.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 11.Ouanouki A, Lamy S, Annabi B. Anthocyanidins inhibit epithelial-mesenchymal transition through a TGFbeta/Smad2 signaling pathway in glioblastoma cells. Molecular carcinogenesis. 2017;56:1088–1099. doi: 10.1002/mc.22575. [DOI] [PubMed] [Google Scholar]
- 12.Afaq F, et al. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. The Journal of investigative dermatology. 2007;127:222–232. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- 13.Yun JM, Afaq F, Khan N, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Molecular carcinogenesis. 2009;48:260–270. doi: 10.1002/mc.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 15.Klattenhoff CA, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–583. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bronnum H, et al. miR-21 promotes fibrogenic epithelial-to-mesenchymal transition of epicardial mesothelial cells involving Programmed Cell Death 4 and Sprouty-1. PloS one. 2013;8:e56280. doi: 10.1371/journal.pone.0056280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonello ZA, Reiff T, Ballesta-Illan E, Dominguez M. Robust intestinal homeostasis relies on cellular plasticity in enteroblasts mediated by miR-8-Escargot switch. The EMBO journal. 2015;34:2025–2041. doi: 10.15252/embj.201591517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ke J, et al. Role of microRNA221 in regulating normal mammary epithelial hierarchy and breast cancer stem-like cells. Oncotarget. 2015;6:3709–3721. doi: 10.18632/oncotarget.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng XG, et al. Overexpression of miR-122 promotes the hepatic differentiation and maturation of mouse ESCs through a miR-122/FoxA1/HNF4a-positive feedback loop. Liver international: official journal of the International Association for the Study of the Liver. 2014;34:281–295. doi: 10.1111/liv.12239. [DOI] [PubMed] [Google Scholar]
- 20.Kolesnikoff N, et al. Specificity protein 1 (Sp1) maintains basal epithelial expression of the miR-200 family: implications for epithelial-mesenchymal transition. The Journal of biological chemistry. 2014;289:11194–11205. doi: 10.1074/jbc.M113.529172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dasgupta P, et al. MicroRNA-203 Inhibits Long Noncoding RNA HOTAIR and Regulates Tumorigenesis through Epithelial-to-mesenchymal Transition Pathway in Renal Cell Carcinoma. Molecular cancer therapeutics. 2018;17:1061–1069. doi: 10.1158/1535-7163.MCT-17-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, et al. Long noncoding RNA H19 promotes transforming growth factor-beta-induced epithelial-mesenchymal transition by acting as a competing endogenous RNA of miR-370-3p in ovarian cancer cells. OncoTargets and therapy. 2018;11:427–440. doi: 10.2147/OTT.S149908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu D, et al. MiR-488 suppresses cell proliferation and invasion by targeting ADAM9 and lncRNA HULC in hepatocellular carcinoma. American journal of cancer research. 2017;7:2070–2080. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Suzuki HI, Miyazono K. Emerging complexity of microRNA generation cascades. Journal of biochemistry. 2011;149:15–25. doi: 10.1093/jb/mvq113. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki HI, Katsura A, Matsuyama H, Miyazono K. MicroRNA regulons in tumor microenvironment. Oncogene. 2015;34:3085–3094. doi: 10.1038/onc.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan PC, et al. Synergistic effect of focal adhesion kinase overexpression and hepatocyte growth factor stimulation on cell transformation. The Journal of biological chemistry. 2002;277:50373–50379. doi: 10.1074/jbc.M204691200. [DOI] [PubMed] [Google Scholar]
- 27.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes & development. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang F, Wang YG, Wang C. Metformin Inhibited Growth, Invasion and Metastasis of Esophageal Squamous Cell Carcinoma in Vitro and in Vivo. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2018;51:1276–1286. doi: 10.1159/000495539. [DOI] [PubMed] [Google Scholar]
- 29.Wu JI, Lin YP, Tseng CW, Chen HJ, Wang LH. Crabp2 Promotes Metastasis of Lung Cancer Cells via HuR and Integrin beta1/FAK/ERK Signaling. Scientific reports. 2019;9:845. doi: 10.1038/s41598-018-37443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tai YL, Lai IR, Peng YJ, Ding ST, Shen TL. Activation of focal adhesion kinase through an interaction with beta4 integrin contributes to tumorigenicity of colon cancer. FEBS letters. 2016;590:1826–1837. doi: 10.1002/1873-3468.12215. [DOI] [PubMed] [Google Scholar]
- 31.Li W, et al. miR-199a-5p regulates beta1 integrin through Ets-1 to suppress invasion in breast cancer. Cancer science. 2016;107:916–923. doi: 10.1111/cas.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ungewiss C, et al. The microRNA-200/Zeb1 axis regulates ECM-dependent beta1-integrin/FAK signaling, cancer cell invasion and metastasis through CRKL. Scientific reports. 2016;6:18652. doi: 10.1038/srep18652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han Z, et al. ERbeta-Mediated Alteration of circATP2B1 and miR-204-3p Signaling Promotes Invasion of Clear Cell Renal Cell Carcinoma. Cancer research. 2018;78:2550–2563. doi: 10.1158/0008-5472.CAN-17-1575. [DOI] [PubMed] [Google Scholar]
- 34.Tsuda T. Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Molecular nutrition & food research. 2012;56:159–170. doi: 10.1002/mnfr.201100526. [DOI] [PubMed] [Google Scholar]
- 35.Faria A, Fernandes I, Norberto S, Mateus N, Calhau C. Interplay between anthocyanins and gut microbiota. Journal of agricultural and food chemistry. 2014;62:6898–6902. doi: 10.1021/jf501808a. [DOI] [PubMed] [Google Scholar]
- 36.de Ferrars RM, et al. The pharmacokinetics of anthocyanins and their metabolites in humans. British journal of pharmacology. 2014;171:3268–3282. doi: 10.1111/bph.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goszcz K, Deakin SJ, Duthie GG, Stewart D, Megson IL. Bioavailable Concentrations of Delphinidin and Its Metabolite, Gallic Acid, Induce Antioxidant Protection Associated with Increased Intracellular Glutathione in Cultured Endothelial. Cells. Oxidative medicine and cellular longevity. 2017;2017:9260701. doi: 10.1155/2017/9260701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao CC, Chen SC, Huang HP, Wang CJ. Gallic acid inhibits bladder cancer cell proliferation and migration via regulating fatty acid synthase (FAS) Journal of food and drug analysis. 2018;26:620–627. doi: 10.1016/j.jfda.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyun KH, Gil KC, Kim SG, Park SY, Hwang KW. Delphinidin Chloride and Its Hydrolytic Metabolite Gallic Acid Promote Differentiation of Regulatory T cells and Have an Anti-inflammatory Effect on the Allograft Model. Journal of food science. 2019;84:920–930. doi: 10.1111/1750-3841.14490. [DOI] [PubMed] [Google Scholar]
- 40.Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Current opinion in cell biology. 2005;17:542–547. doi: 10.1016/j.ceb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Science signaling. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nature reviews. Molecular cell biology. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nam EH, Lee Y, Park YK, Lee JW, Kim S. ZEB2 upregulates integrin alpha5 expression through cooperation with Sp1 to induce invasion during epithelial-mesenchymal transition of human cancer cells. Carcinogenesis. 2012;33:563–571. doi: 10.1093/carcin/bgs005. [DOI] [PubMed] [Google Scholar]
- 44.Guan H, et al. MicroRNA-93 promotes proliferation and metastasis of gastric cancer via targeting TIMP2. PloS one. 2017;12:e0189490. doi: 10.1371/journal.pone.0189490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teoh SL, Das S. The Role of MicroRNAs in Diagnosis, Prognosis, Metastasis and Resistant Cases in Breast Cancer. Current pharmaceutical design. 2017;23:1845–1859. doi: 10.2174/1381612822666161027120043. [DOI] [PubMed] [Google Scholar]
- 46.Guo L, et al. MicroRNA-143-3p inhibits colorectal cancer metastases by targeting ITGA6 and ASAP3. Cancer science. 2019;110:805–816. doi: 10.1111/cas.13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Zhang L, Tang S. MicroRNA-4497 functions as a tumor suppressor in laryngeal squamous cell carcinoma via negatively modulation the GBX2. Auris, nasus, larynx. 2019;46:106–113. doi: 10.1016/j.anl.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Han Xu, Li Qiaobei, Wang Chunyan, Li Yinyan. MicroRNA-204-3p Attenuates High Glucose-Induced MPC5 Podocytes Apoptosis by Targeting Braykinin B2 Receptor. Experimental and Clinical Endocrinology & Diabetes. 2018;127(06):387–395. doi: 10.1055/a-0630-0173. [DOI] [PubMed] [Google Scholar]
- 49.Papageorgiou Sotirios G., Diamantopoulos Marios A., Kontos Christos K., Bouchla Anthi, Vasilatou Diamantina, Bazani Efthymia, Scorilas Andreas, Pappa Vasiliki. MicroRNA-92a-3p overexpression in peripheral blood mononuclear cells is an independent predictor of prolonged overall survival of patients with chronic lymphocytic leukemia. Leukemia & Lymphoma. 2018;60(3):658–667. doi: 10.1080/10428194.2018.1461861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.