Abstract
A considerable number of human diseases have an inflammatory component, and a key mediator of immune activation and inflammation is inducible nitric oxide synthase (iNOS), which produces nitric oxide (NO) from L-arginine. Overexpressed or dysregulated iNOS has been implicated in numerous pathologies including sepsis, cancer, neurodegeneration, and various types of pain. Extensive knowledge has been accumulated about the roles iNOS plays in different tissues and organs. Additionally, X-ray crystal and cryo-EM structures have shed new insights on the structure and regulation of this enzyme. Many potent iNOS inhibitors with high selectivity over related NOS isoforms, neuronal NOS (nNOS) and endothelial NOS (eNOS), have been discovered, and these drugs have shown promise in animal models of endotoxemia, inflammatory and neuropathic pain, arthritis, and other disorders. A major issue in iNOS inhibitor development is that promising results in animal studies have not translated to humans; there are no iNOS inhibitors approved for human use. In addition to assay limitations, both the dual modalities of iNOS and NO in disease states (i.e., protective vs. harmful effects) and the different roles and localizations of NOS isoforms, create challenges for therapeutic intervention. This review summarizes the structure, function, and regulation of iNOS, with focus on the development of iNOS inhibitors (historical and recent). A better understanding of iNOS’ complex functions is necessary before specific drug candidates can be identified for classical indications such as sepsis, heart failure, and pain; however, newer promising indications for iNOS inhibition, such as depression, neurodegenerative disorders, and epilepsy, have been discovered.
Keywords: nitric oxide, inducible nitric oxide synthase, inflammation, reactive oxygen species, enzyme inhibition, sepsis, pain, cancer, neurodegeneration, nitrergic signaling, immune system activation, immune regulation, animal models
1. INTRODUCTION
Nitric oxide (NO) is an important cellular signaling molecule that participates in diverse physiological functions in mammals, including vasodilation, smooth muscle relaxation, neurotransmission, and the immune response. NO, a free radical, is produced by a family of enzymes called nitric oxide synthases (NOSs) by the oxidation of L-arginine (L-Arg) to L-citrulline. There are three isoforms of NOS. Two of them, neuronal NOS (nNOS) and endothelial NOS (eNOS), are constitutively expressed, while the third one is inducible and is thus termed iNOS. nNOS is primarily found in the nervous system and is necessary for neuronal signaling, while eNOS is localized to the endothelium and is essential for vasodilation and control of blood pressure.1 These two isoforms produce nanomolar amounts of NO for short periods of time (seconds to minutes) in a calcium/calmodulin (CaM)-dependent manner.2,3 iNOS, by contrast, is not constantly present in cells and is only expressed when the cell is induced or stimulated, typically by pro-inflammatory cytokines and/or bacterial lipopolysaccharide (LPS).3,4 Upon induction, iNOS generates significant amounts of NO (micromolar range), which lasts until the enzyme is degraded, sometimes for hours.5 The considerable amount of NO produced helps to defend against invading pathogens and is thus critical for the inflammatory response and the innate immune system. Inappropriately high NO concentrations from overexpression or dysregulation of iNOS, on the other hand, can result in toxic effects and is associated with a variety of human diseases, including septic shock, cardiac dysfunction, pain, diabetes, and cancers.4 The dual activity of iNOS-related NO (beneficial vs. detrimental) is highly concentration-dependent. Therefore, regulation of its production is important for both maintaining its proper physiological functions and controlling its deleterious effects. Because of its important role, iNOS research is a very popular field; a SciFinder search for “iNOS” reveals over 37,000 references. In this contribution, we present a general review of iNOS, with a focus on its structure, functions, and regulation, both physiologically and pathologically. Inhibition of iNOS as a potential therapeutic strategy and iNOS inhibition in clinical trials also will be discussed.6
2. STRUCTURE AND FUNCTIONS OF iNOS
iNOS is a 131 kDa mammalian protein composed of 1,153 amino acids, which are assembled into two major domains, a C-terminal reductase—containing a flavin mononucleotide (FMN) binding subdomain—and a N-terminal oxygenase.7 iNOS adopts a zinc-bridged, homodimeric quaternary structure that allows the enzyme to convert L-Arg to L-citrulline with the concomitant production of NO. This transformation is governed by an elaborate electron transport chain, involving the cofactors nicotinamide adenine dinucleotide phosphate (NADPH), flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), heme, and (6R)-5,6,7,8-tetrahydrobiopterin (H4B), which are essential for this reaction. This process is mediated by CaM, which binds in a hinge region between the oxygenase and reductase domains.8 While iNOS, nNOS, and eNOS share substantial gene alignment, cofactors, and overall function, differences in domain architecture allow for specialization of function and regulation. iNOS is the simplest mammalian NOS in terms of domain content, consisting solely of the reductase, oxygenase, and CaM-binding domains; it lacks the auto-inhibitory loop present in eNOS, as well as the PDZ domain of nNOS.9
All NOS enzymes have a catalytically inactive zinc at the dimer interface.10 One zinc atom per dimeric pair forms a zinc tetrathiolate cluster with each monomer donating two cysteine residues in a -CXXXXC- amino acid arrangement.11 Biochemical investigation has determined that the role of the zinc ion is to promote H4B binding.12
Although CaM is associated with all NOS isoforms, the tight binding of CaM to iNOS (because of sequence variation at the hinge region)13 allows it to activate at much lower physiological concentrations of calcium (40 nM in iNOS vs. 400 nM in nNOS and eNOS).14 Given that typical cytoplasmic calcium concentrations are near 100 nM,15 iNOS is effectively locked in an active position where regulation by calcium is no longer relevant. Post-translational modifications may also have functional or regulatory significance. For example, phosphotyrosine residues have also been detected in iNOS, and assays have shown that inhibition of cellular phosphatase activity by vanadate leads to increased iNOS activity.16
2.1. NO production mechanism
Although the driving force behind the production of NO is NADPH, the catalytic cycle proceeds through an elaborate intra- and inter-protein electron transport chain. The cycle begins when NADPH binds in the reductase domain and initiates the thermodynamically favored reduction (40 mV) of the adjacent FAD in a two-electron process (Fig. 1). Within the reductase domain, FAD is proximal to the FMN subdomain, which allows a one-electron reduction of FMN by FAD (6 mV).17 At this point in the cycle, the spatial transfer of electrons from one domain to another is unclear, as the FMN subdomain is distal to the heme-containing oxygenase domain. Studies have shown a great deal of flexibility within the protein and have elucidated the conformational changes during the catalytic cycle.18 Upon CaM and calcium sensitization, the enzyme contorts the FMN subdomain into proximity with the heme-containing oxygenase domain. Notably, the FMN domain does not transfer electrons to heme in its own monomer but rather into the dimeric protein’s other monomer.19 This explains why monomeric iNOS, and NOS enzymes in general, are inactive. Electrons received from FMN affect the reduction of heme iron from iron(III) to iron(II) and prime the active site by allowing the recruitment and activation of molecular oxygen. The exact mechanism of this activation is subject to debate.20 However, near the active site there is a binding region for H4B, which is believed to play a number of roles in the production of NO. H4B causes a high spin iron configuration in heme,21 stabilizes the dimeric form of the enzyme,22 and it may promote efficient NADPH coupling to NO production.23 Furthermore, during the oxidation of L-Arg it is proposed to play a vital role as an electron shuttle and reservoir within the active site.24 When L-Arg binds to an active site L-Glu residue, the heme cofactor executes a P450-like mechanism to oxidize L-Arg to Nω-hydroxy-L-arginine (L-NOHA). A second distinct step then further oxidizes L-NOHA to L-citrulline and NO. At two points in the transformation of L-Arg to NO, H4B provides stabilization of the charge during the activation of molecular oxygen.2 Donation of an electron from H4B to a heme iron(IV) hydroperoxyl species allows formation of the proposed catalytically active iron(IV) oxenoid radical cation.25 In the final step, which releases NO from heme, H4B recaptures an electron from iron, thereby polarizing the iron-nitrogen bond and allowing NO to diffuse out of the active site.26 During catalysis, H4B donates two electrons but recaptures only one. The source of the other electron is the FMN subdomain;27 however, the exact mechanism and spatial organization required to pass this electron remain unknown.
FIGURE 1.
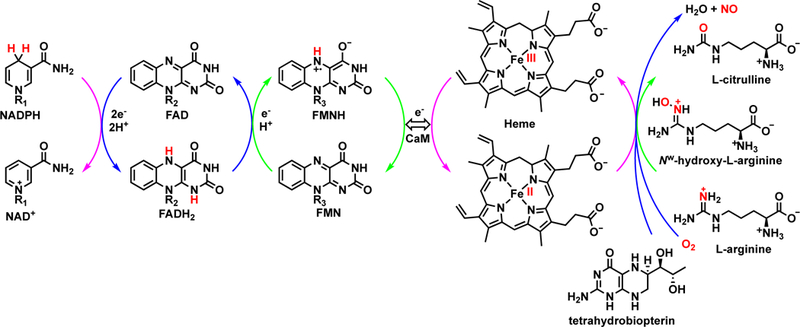
Schematic representation of electron transfers from NADPH to heme through FAD and FMN (via tetrahydrobiopterin). The double-headed arrow indicates a conformational change to bring FMN into proximity with heme. The exact catalytic mechanism is not shown. Figure adapted with permission195
2.2. Spatial organization of iNOS
A wealth of earlier biochemical evidence suggests that electrons are passed from the FMN subdomain into the heme in the oxygenase domain of the other monomer. However, until recently, there was no answer to fairly simple questions regarding the spatial organization of the domains and their movements to achieve catalysis. This problem largely stems from the inability to crystallize full-length dimeric NOS enzymes. In fact, crystal structures of the three isoforms’ dimeric oxygenase domains were only solved beginning in the late 1990s and completed in 2002 with the publication of the nNOS structure.11,28,29 Unlike nNOS or eNOS, the tight interaction between CaM and iNOS allowed their co-crystallization. This cemented the localization of CaM in NOS enzymes, but still left the overall organization unclear. In 2014, Marletta et al. published a cryo-EM study of the NOS enzymes that showed the three-dimensional domain architecture of the NOS family (Fig. 2) and visualized snapshots of the catalytic cycle in action.18 They found that iNOS folds almost in half to deliver an electron from FMN to heme. While the resolution of such images is limited to domain structure, computational fits of the observed three-dimensional shape have suggested a high degree of flexibility in the CaM binding region, as well the ability of the FMN subdomain to move long distances independently of the reductase domain it canonically resides in.
FIGURE 2.
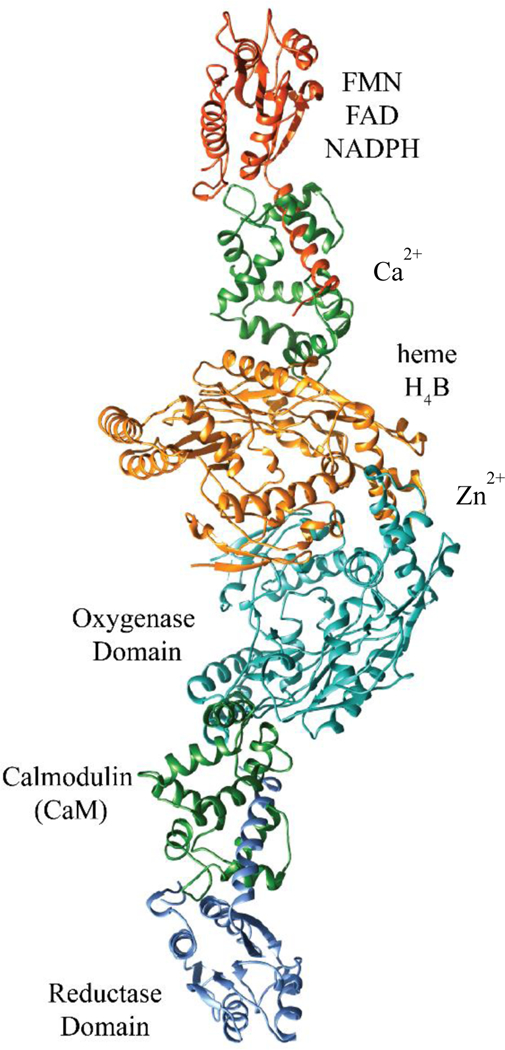
Assembled dimeric iNOS X-ray crystal structures organized based on cryo-EM data, from PDB IDs 3HR4 and 4CX7.29,38 The reductase domain shown is a 212-residue fragment of the full-length domain. Domains are annotated on the right side, cofactors on the left. The dimer interface is located between the orange- and cyan-colored domains.
2.3. Critical residues in iNOS
Mutational analysis of iNOS has identified a collection of amino acids that are critical for the function of the enzyme. When mutated to asparagine, Glu546 lowers the measured inter-domain electron transfer between the FMN subdomain and heme in the oxygenase domain. The rate constant was decreased by a third through a decrease in inter-domain binding affinity, as confirmed by electron paramagnetic resonance (EPR).19 In the H4B binding pocket in the oxygenase domain, residues Arg375, Trp455, Trp457, and Phe470 are crucial for H4B cofactor binding and subsequent dimerization and function of the enzyme.30 Arg375 is also implicated in modulation of the redox function of H4B.31 Phe831 and Leu832 in the reductase domain are integral to the hydrophobic core. When they are exchanged for polar residues—serine and proline, respectively—large decreases in iNOS activity result.32 Unsurprisingly, the mutation of heme-ligating Cys194 to alanine results in failure to bind heme.30 However, in addition to lacking heme, the enzyme fails to dimerize. The interaction of heme propionate and H4B is well known,32 and thus it is possible that heme-lacking monomers also lack H4B, which is crucial for the dimerization process.33 CaM binding is destabilized by the mutation of Ser562 and Cys563 in iNOS through the destruction of a crucial hydrogen bond network between CaM and iNOS.34
2.4. Biological function of NO produced by iNOS
The primary role of iNOS in human physiology is the destruction of invading pathogens.35 As discussed previously, iNOS and NO are produced as a part of the immune response and have a regulatory role. As NO is readily diffusible, it can have a variety of fates. NO’s high affinity for iron allows it to break up or inactivate heme-containing enzymes or iron-sulfur clusters.10 NO can be responsible for cysteine nitrosylation36 or can combine with superoxide to form ONOO- (peroxynitrite), a potent oxidant and nitrating agent. NO and ONOO- can cause DNA damage via oxidative deamination.37 The short biological half-lives of NO and ONOO- direct these damaging mechanisms preferentially to the invading pathogen, thus avoiding systemic toxicity. Finally, cyclic guanosine monophosphate (cGMP) production is increased through NO mediated activation of guanylate cyclase. cGMP is a soluble second messenger that participates in many downstream signaling cascades through activation of various protein kinases, ion channels, and phosphodiesterases.39–41
3. REGULATION OF iNOS ACTIVITY
The activity of expressed iNOS is regulated by different factors, including availability of its substrate and cofactors, interaction with other proteins, and auto-inactivation.
3.1. Substrate and cofactor availability
L-Arginine, the substrate for iNOS, is also essential for biosynthesis of proteins and other amino acids and removes toxic ammonia from the body during the urea cycle. In the urea cycle, arginase converts arginine to ornithine and urea, and this affects iNOS activity by competing for the same substrate (Fig. 3). Indeed, arginase inhibition has been found to increase NO synthesis in rabbit and rat macrophages42 and increases the amount of nitrated and S-nitrosylated protein in inflamed mouse lungs, possibly by enhancing NO production.43 On the other hand, arginase overexpression can inhibit iNOS activity and promote dysregulated inflammatory responses. For instance, arginase overexpression in human keratinocytes is implicated in the disease mechanism of psoriasis, an inflammatory skin disease, possibly as a result of decreased iNOS activity.44
FIGURE 3.
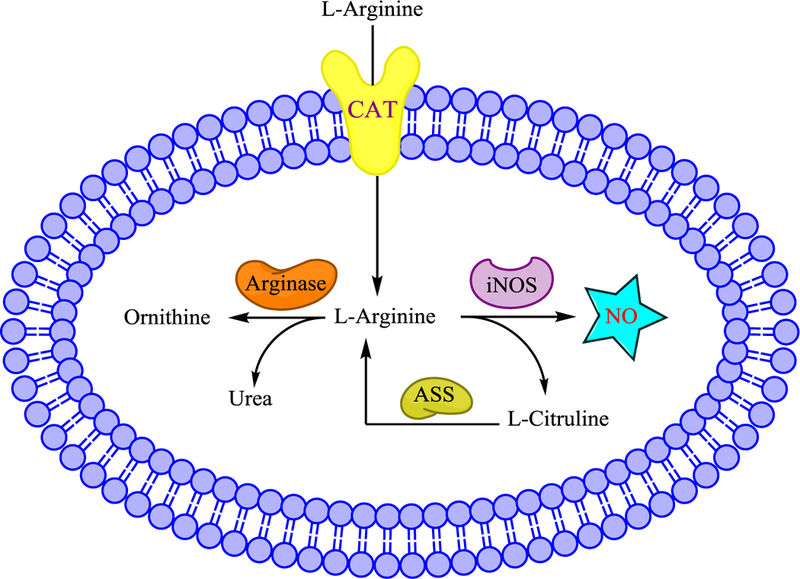
Regulation of iNOS activity by the availability of its substrate L-Arginine. CAT: cation amino acid transporters. ASS: Arginosuccinate synthase.
The availability of L-Arg is also controlled by the cation amino acid transporters, CAT1, CAT2 and CAT3,45 that transport it (Fig. 3). Conditions used to induce iNOS also help to increase expression of arginine transporters, thus increasing arginine uptake. In rodent macrophages, induction of iNOS by LPS is accompanied by an increase in CATs and increased L-Arg uptake.46 Likewise, deficiency in these transporters reduces iNOS activity. Deletion of the CAT2 arginine transporter gene impairs iNOS activity in stimulated astrocytes by 84% and also reduces NO synthesis in macrophages.47
Additionally, L-citrulline, the by-product of NO production, can be converted back to arginine through arginosuccinate synthase (Fig. 3). iNOS and arginosuccinate synthase are co-induced in macrophages, vascular smooth muscle cells, microglia, and neurons.48,49 The ability for cells to regenerate arginine from citrulline is necessary to ensure enough arginine for iNOS to maintain its role in homeostasis.
The availability of the essential cofactor H4B may also affect iNOS activity. H4B plays an essential role in the dimerization of iNOS to active enzyme.30,50 Cell lines producing little H4B have low iNOS activity, but supplementation with H4B increases iNOS dimerization; the H4B precursor sepiapterin can also rescue iNOS activity.51 Interestingly, during inflammation associated with wound healing, iNOS co-expresses and co-localizes with GTP-cyclohydrolase I, a key enzyme in the H4B biosynthetic pathway.52
3.2. Protein-protein interactions
Several proteins also interact with iNOS and regulate its activity. In the CNS, iNOS has been found in complex with a cytosolic protein called kalirin (mainly kalirin-7),53 in brains of mice treated with LPS. Studies have found that kalirin interacts with iNOS monomers but not dimers. In kalirin-expressing cells, most iNOS exists as a heterodimer with kalirin. There is little monomeric iNOS, indicating that kalirin inhibits iNOS activity by preventing iNOS homodimerization.54 Low expression of kalirin is correlated with increased iNOS activity in the hippocampus of Alzheimer’s patients.53 As excess NO produced during inflammation is neurotoxic, kalirin may be exerting neuroprotective effects by down-regulating iNOS.53 In murine macrophages, iNOS interacts with a 110-kDa protein called NAP110 (NOS-associated protein 110). NAP110 and iNOS complexes are found in the spleens and peritoneal macrophages of IFN-γ and LPS-treated mice. Co-expression of NAP110 and iNOS reduces the latter’s activity by 90%, although the amount of iNOS protein does not change. Similar to kalirin, NAP110 inhibits iNOS activity by interacting with iNOS monomers and preventing the enzyme’s dimerization. NAP110 may therefore be a regulatory switch to prevent NO levels from becoming toxic.55,56
iNOS has also been found to interact directly with Rho GTPase Rac 2, mainly through its oxygenase domain. Overexpression of Rac2 in RAW 264.7 cells potentiated LPS-induced iNOS activity.56 Another study demonstrated that iNOS and Rac2 interact in human neutrophils. This interaction may play a key role in anti-microbial defense,58 because cells treated with a Rac2 inhibitor generated less superoxide (which reacts with NO to produce toxic ONOO-). These results suggest that Rac2 can enhance NO generation through iNOS.57,58 Yoshida and Xia59 found that the chaperone heat shock protein 90 (hsp90) can also increase iNOS activity in a dose-dependent fashion, and the two proteins co-immunoprecipitate from activated macrophages.
3.3. Auto-inactivation
NO itself may negatively regulate iNOS activity. In stimulated murine macrophages, iNOS activity is increased when hemoglobin, a NO scavenger, was added. Addition of the NO donors S-nitrosoacetylpenicillamine or S-nitrosoglutathione significantly inhibits iNOS activity, suggesting that NO may be involved in some negative feedback mechanism. Furthermore, iNOS activity cannot be restored in activated cells after NO donors are removed, an indication of the irreversible binding of NO to iNOS.60 A kinetic study showed that the major mechanism for iNOS auto-inactivation is through S-nitrosation of the zinc-tetrathiolate cluster (vide infra), which causes zinc loss, irreversible iNOS dimer dissociation, and subsequent loss of activity. Reducing agents can protect iNOS from this inactivation, suggesting S-nitrosation was indeed the major inactivation pathway.61
4. iNOS EXPRESSION AND SIGNALING PATHWAYS
iNOS expression is chiefly governed by transcriptional regulation,62,63 which can differ depending on the cell type or species. In general, pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), interferon-γ (IFN-γ), and LPS, first bind to receptors on the cell surface and activate kinases, leading to the phosphorylation of various intracellular proteins and subsequent activation of specific transcription factors, including nuclear factor κB (NF-κB) transcription factors such as nuclear factor 1 transducer and activator of transcription 1a (STAT-1a).62 The active factors then translocate into the nucleus, where they bind to the promoter region of the iNOS gene and induce iNOS expression.
Different inducers trigger different signaling pathways. One of the major pathways involves activation of NF-κB, a major target of LPS, IL-1β, and TNF. In macrophages, LPS first activates a toll-like receptor (TLR4) on the cell membrane. The TLR4 complex contains several proteins including TNF-receptor associated factor 6 (TRAF6), myeloid differentiation primary response 88 (MyD88), and a kinase64. As NF-κB is a dimer in macrophages and is usually inactivated and bound by inhibitor κB (IκB), activation of this pathway induces a kinase cascade (one is via mitogen-activated protein kinase kinase, or MEKK), which leads to the phosphorylation of inhibitor of κB kinase (IKK) and subsequent phosphorylation of IκB. When phosphorylated, IκB is ubiquitinated and degraded in the proteasome, and NF-κB is released. NF-κB then translocates, interacts with the iNOS promoter, and triggers transcription of the iNOS genes64,65 (Fig. 4, left).
FIGURE 4.
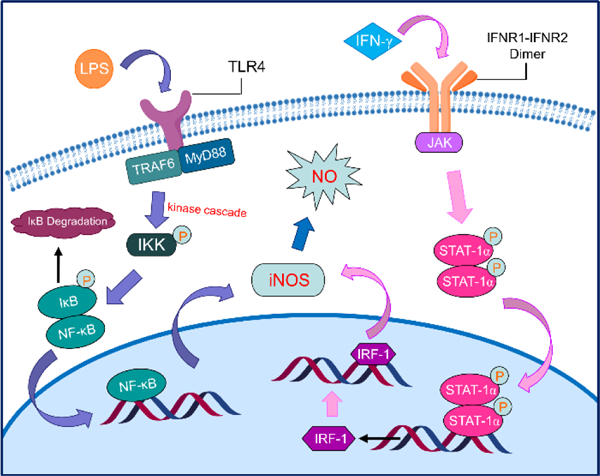
Different signaling pathways triggered by different iNOS inducers that regulate iNOS expression (left: activation of NF-κB pathway; right: activation of JAK/STAT-1α pathway).
By contrast, IFN-γ activates the JAK/STAT-1α pathway.62,66,67 Briefly, inducing cells with IFN-γ causes the dimerization of IFN-γ receptor and activation of Janus kinases (JAK, such as JAK2), which then phosphorylates STAT-1α. STAT-1α itself then dimerizes and translocates to the nucleus, where it facilitates the synthesis of interferon regulatory factor 1 (IRF-1). IRF-1 then binds to the iNOS gene promoter and induces iNOS expression (Fig. 4, right).
5. DISTRIBUTION AND INDUCTION OF iNOS
iNOS was initially purified from murine macrophages.14,68 Since then, different cell types, including hepatocytes,69,70 smooth muscle cells,71,72 chondrocytes,73 glial cells,74 astrocytes,75 neurons,76,77 and cardiac myocytes78 from different species, have been found to induce iNOS when stimulated. Successful cloning of full length iNOS cDNA isolated from RAW 264.7 murine macrophages has provided the amino acid sequence of the protein and insight into its structure and regulation.79,80 The murine macrophage iNOS amino acid sequence shares 51% homology with rat nNOS.80
Compared with other species, induction of iNOS in human cell types is limited. Human macrophages fail to produce sufficient amounts of NO under multiple different induction conditions,81 possibly the result of limited biosynthesis of H4B, an essential iNOS cofactor, in human macrophages.82 The first successful induction of human iNOS was in hepatocytes.70,83 Human liver iNOS cDNA shares 80% sequence homology with murine macrophage iNOS. Successful induction of human iNOS has also been achieved in human chondrocytes using IL-1β.73 cDNA cloned from this cell type shows 88% sequence similarity with murine macrophage iNOS, suggesting that iNOS structure and function is retained in different species and cell types.
In the CNS, iNOS is expressed in various glial cells, including astrocytes and microglia (the “macrophages of the brain”), in response to different stimuli.74,75 Compared to glial cells, iNOS expression in neurons is very limited, but there is still evidence for its expression in these cells. For instance, treatment of different human neuroblastoma cell lines (widely used as models of human neuronal cells) with cytokines results in expression of iNOS genes.84 Neuronal iNOS expression may also occur in Alzheimer’s disease or following hypoxia or ischemia.85,86
TNF, IL-1β, IFN-γ, and LPS are the main inducers of iNOS expression. Combinations of these inducers can generate synergistic effects in certain cells69,87 and are sometimes actually required to stimulate NO production, especially in nonimmune cells, where LPS only weakly induces iNOS.88 For instance, in mouse skeletal myocytes, none of these inducers alone can induce iNOS expression. However, stimulation of cells with both IFN-γ and TNF or IL-1 (or with all three cytokines) results in significant NO production.89 The stimuli and conditions that determine iNOS expression depend on the cell type and species. For example, LPS alone can stimulate iNOS expression in rat and mouse glial cells but not human ones.74,90
In addition to LPS and cytokines, invading viruses can also trigger iNOS induction in cells. Human immunodeficiency virus (HIV) induces iNOS expression in human glial cells,90–92 and high iNOS expression is also detected in hepatocytes of patients infected with hepatitis B virus (HBV). Transfection of hepatocytes with HBV genes results in iNOS expression, which indicates the virus can upregulate iNOS expression in human liver cells.93
6. iNOS IN DISEASE STATES
Although iNOS is necessary for normal physiology, excessive amounts of NO as a result of overexpression or dysregulation of iNOS are implicated in human diseases. There are many studies showing that excessive NO plays a role in sepsis. In animal models, high NO production contributes to vasodilation, hypotension, and cardiovascular malfunction during endotoxemia and cytokine-induced shock.65 High concentrations of nitrite and nitrate (the two stable products from NO production) are found in plasma of patients with septic shock,94 and iNOS activity is also detected in gangrenous tissues from sepsis patients, including fat, muscle, and arteries, but not skin.95 Additionally, increased iNOS activity is found in lung macrophages from patients with acute respiratory distress syndrome following sepsis.96 Because of the lethality of septic shock, this indication has spurred the development of numerous iNOS inhibitors.
High iNOS expression has also been found in human patients infected with different pathogens, particularly HIV,97 Mycobacterium tuberculosis,98 and Plasmodium falciparum.99 Indeed, both HIV and tuberculosis infection induce iNOS expression in brain-derived cells, and in the former case, this NO may contribute to the neuronal damage of AIDS-related dementia.92,97 iNOS induction may also be linked to diabetes and obesity-associated insulin resistance, as it is known that iNOS is expressed throughout insulin-sensitive tissues, and obesity increases iNOS levels in muscle, adipose tissue, and vasculature.100,101 Studies in rat models show that rat pancreatic islet cells are very susceptible to NO toxicity102 and iNOS overexpression is found in pancreatic macrophages of prediabetic rats.103 iNOS’ role in insulin resistance may in part be through S-nitrosylation or tyrosine nitration of insulin signaling protein.104,105
High levels of iNOS expression are also found in many cancerous tumors, including, colon, bladder, breast, and lung cancer.41 The role of iNOS in cancer is somewhat complex, as it has both tumor promoter and suppressor effects depending on the tumor and local environment. Nitrosation or nitrosylation (by NO and its products) in cancer cells can contribute to increased proliferation, metastasis, and even drug resistance.41 Most recently, high iNOS expression was correlated with poor survival in triple-negative breast cancer patients.106 In addition, NO produced by iNOS within the tumor itself can inhibit proliferation of T-lymphocytes (as seen in rat colon cancer models), which could explain how tumors can suppress host immune functions.107
Various studies have also indicated that iNOS (both centrally and peripherally expressed) may play a role in the development and sensation of inflammatory and neuropathic pain. Meller et al. found that LPS and cytokines induce thermal hyperalgesia (hypersensitivity to pain), possibly mediated via induction of iNOS in spinal cord glial cells.108 Similarly, in animal models of neuropathic pain, iNOS upregulation contributes to local inflammatory reactions.109 Recently, even cancer-associated pain was found to be associated with upregulated iNOS (and nNOS) in mice with bone cancer.110 As such, treatment of pain has also been a major driving force for development of iNOS inhibitory therapeutics.
The normal brain does not appear to express much iNOS, but following trauma, inflammatory damage or stimuli, or hypoxia (such as a from a stroke or ischemic event), iNOS expression in activated glial cells and neurons can occur.74,86,111 Some of these iNOS-producing cells build up around damaged sites in the brain, which could indicate some inflammatory link between NO originating from glial cells and neuronal injury.74 iNOS has been found in the brains of patients with Alzheimer’s disease,85,112 and high levels of iNOS have also been observed in multiple animal models of Parkinson’s disease.113 However, iNOS knock-out mice genetically cross-bred with transgenic mice bred to produce beta-amyloid plaques showed a much higher incidence of Alzheimer’s phenotypes than the transgenic mice bred to produce beta-amyloid plaques alone, suggesting iNOS could be protective and its inhibition might be detrimental.114 There is still some uncertainty, however, as to whether iNOS expression and activity are a direct cause of neuronal damage or are merely associated with certain stages of neurodegenerative disorders (i.e., correlation vs. causation), or might even be protective in some cases.
7. DEVELOPMENT OF iNOS INHIBITORS
7.1. Assaying for iNOS activity and inhibition
Several in vitro assays are commonly used to measure iNOS activity during the development of iNOS inhibitors. Isolated enzyme-based systems are often initially used to determine the extent of iNOS inhibition. One common method is to measure the conversion of radiolabeled (3H or 14C)-L-Arg to L-citrulline115 using human or murine iNOS, although non-radioactive enzyme-based methods, such as the hemoglobin capture assay (where the oxidation of hemoglobin to methemoglobin by NO is measured spectrophotometrically) or are also employed.115 Cell-based systems (especially for dimerization inhibitors), where a macrophage cell line (such as RAW 264.7)116 is stimulated to express iNOS by action of added cytokines, have also been used. In these systems, NO production is monitored, for example, via the addition of Griess reagent to cell lysates, which detects nitrite production.
The most common in vivo assay for iNOS inhibition is the rodent endotoxemia model, which is roughly analogous to the systemic inflammatory phenotype observed in sepsis117. In this assay, an endotoxin (usually LPS) is administered to a rodent, which causes systemic inflammation with concomitant increases in cytokines, iNOS, and NO production, which can be monitored via plasma nitrate/nitrite levels.
Formalin or carrageenan models are often used to model inflammatory pain. A small amount of formalin solution is injected into the paw of a rodent to cause local inflammation118, and the frequency or duration of pain behaviors (flinching or biting and licking the paw) are measured in the presence or absence of the inhibitor of interest. Likewise, carrageenan injection119 causes localized edema, pain, and hyperalgesia. To assess iNOS’ possible role in neuropathic pain, surgical models such as the Chung model (pain is induced through surgical ligation of the spinal nerve)120 or the chronic constriction injury model (ligation of the sciatic nerve)121 are used. As iNOS inhibitors have also been investigated for arthritis, the adjuvant-induced arthritis model122 is employed to assess their efficacy. An adjuvant (such as Freund’s Complete Adjuvant) is administered to an animal to induce an arthritic phenotype. In the presence or absence of a drug, the animal is observed for arthritic symptoms and clinically scored; postmortem dissection and examination of the affected joint components are also sometimes employed.
7.2. Competitive iNOS inhibitors
There are two main modes of iNOS inhibition that act directly on the enzyme. First, the majority of iNOS inhibitors discovered are directly competitive with L-Arg (often acting by coordinating with the active-site L-Glu), and they either reversibly inhibit L-Arg binding or compete with L-Arg and then irreversibly inactivate the enzyme in a time-, concentration-, or NADPH-dependent manner. The activity and selectivity of iNOS inhibitors discussed in this section is summarized in Table 1.
Table 1.
Summary of NOS inhibition for relevant compounds
| Inhibition (μM) |
|||||
|---|---|---|---|---|---|
| Compound | |||||
| iNOS | eNOS | nNOS | Reference | Notes | |
| 1 | 1.4 (human, Ki) | 0.06 (human, Ki) | 0.09 (human, Ki) | 123 | For the acid form, L-NAME is only a weak inhibitor |
| 2 | 2.6 (human, Ki) | 0.7 (human, Ki) | 1.7 (human, Ki) | 123 | Values for competitive inhibition |
| 3 | 2.2 (murine, IC50) 3.9 (murine, Ki) |
3.9 (bovine, Ki) | 3.9 (rat, IC50) 1.7 (rat, Ki) |
123, 128 | Values for competitive inhibition; also a mechanism-based inactivator |
| 4 | 54±58 (murine, IC50) | Not reported; in vivo results suggest selectivity | 160 (rat, IC50) | 131,132 | Little selectivity later reported |
| 5 | 3.3 (murine, IC50) 0.9 (human, Ki) |
16 (human, Ki) | 92 (rat, IC50) 10 (human, Ki) |
128, 123 | Values for competitive inhibition |
| 6 | >1850 (human, IC50) | 2420 (human, IC50) | >1850 (human, IC50) | 138 | Is a prodrug; hydrolyzes to 5, little inhibitory activity |
| 7 | 2.19±0.23 (human, IC50) <40 nM (human, Kd) |
554±61 (human, IC50) | 177±44 (human, IC50) | 7 | Time and NADPH-dependent competitive inhibitors |
| 8 | 9.03±0.85 (human, IC50) <90 nM (human, Kd) |
>1000 (human, IC50) | 719±179 (human, IC50) | 7 | Time and NADPH-dependent competitive inhibitors |
| 9 | 2.9 (human, IC50) | Exact value not reported; around 25-fold weaker | Exact value not reported; around 5-fold weaker | 144 | Time-dependent, irreversible inhibitor |
| 10 | 100 (species not specified, IC50) | Exact value not reported; in vivo assays suggest no eNOS inhibition | Exact value not reported; selectivity is reported to be 8-fold | 149 | |
| 11 | 130 (species not specified, IC50) | Exact value not reported; in vivo assays suggest no eNOS inhibition | 180 (species not specified, IC50) | 148 | Molecular modeling suggests competitive inhibition. |
| 12 | 0.047 (human, Ki) | 9.0 (human, Ki) | 0.25 (human, Ki) | 150 | Proposed to be a competitive inhibitor |
| 13 | 0.007 (human, Kd) | 50 (human, Ki) | 2.0 (human, Ki) | 151 | Originally proposed to be irreversible or a very slow reversible inhibitor |
| 14 | 0.20±0.03 (presumably human, IC50) | 350±70 (presumably human, IC50) | Not reported | 154 | Competitive inhibitor |
| 15 | 1.0 (human, IC50) | 4.7 (human, IC50) | 1.1 (human, IC50) | 155 | Competitive inhibitor |
| 16 | 0.0019 (human, Ki) | 0.018 (human, Ki) | 0.0037 (human, Ki) | 156, 157 | Competitive inhibitor like 15. |
| 17 | 0.25 (human, IC50) | 226 (human, IC50) | 3.2 (human, IC50) | 160 | |
| 18 | 0.78 (human, IC50) | 1980 (human, IC50) | 27 (human, IC50) | 161 | |
| 19 | 0.16 (human, IC50) |
No significant effect at 100 μM (human) |
16 (rat, IC50) | 162 | |
| 20 | 0.037 (human, IC50) | >100 (human, IC50) | 1 (human, IC50) | 163 | Competitive inhibitors. |
| 21 | 0.4 (human, IC50) | 50 (human, IC50) | 1 (human, IC50) | 166 | X-ray crystallography indicates competitive inhibitors. |
| 22 | 0.17 (human, IC50) | 0.072 (human, IC50) | 0.074 (human, IC50) | 167 | |
| 23 | 0.028 (human, IC50) | 0.15 (human, IC50) | 0.10 (human, IC50) | 167 | |
| 24 | 0.071 (human, IC50) | >100 (human, IC50) | 6.6 (human, IC50) | 169 | |
| 25 | 0.086 (human, IC50) | 162 (human, IC50) | 17 (human, IC50) | 170, 171 | Competitive, time-dependent, irreversible inhibitor. |
| 26 | 7.3±1 (possibly murine, spectral binding constant for iNOS monomer) | Not reported | Not reported | 174 | Dimerization inhibitor |
| 27 | 0.028 (human, IC50, cell lysate) 0.0022 (human, Ki, purified iNOS monomer) |
32 (human, IC50, cell lysate) | 0.140 (human, IC50, cell lysate) | 175 | Dimerization inhibitor |
| 28 | 0.00029 (human, IC50, A172 cell lysate) | Selectivity reported to be >5000-fold (vaccinia-transfected cell lysate | Selectivity reported to be 62-fold (vaccinia-transfected cell lysate | 178 | Different selectivity values depending on whether vaccinia transfection or tetracycline induction is used |
| 29 | 1.3 (human, EC50, cell-based) 46 (murine, EC50, cell-based) |
>100 (human, EC50, cell-based) | >10 (human, EC50, cell-based) | 179 | Dimerization inhibitor |
| 30 | 0.011 (human, EC50, cell-based) 0.12 (murine, EC50, cell-based) |
26 (human, EC50, cell-based) | 2.3 (human, EC50, cell-based) | 179 | Dimerization inhibitor |
| 31 | 0.009 (human, EC50, cell-based) 0.034 (murine, EC50, cell-based) |
6.1 (human, EC50, cell-based) | 0.36 (human, EC50, cell-based) | 179 | Dimerization inhibitor |
| 32 | 0.091 (human, EC50, cell-based) | 16.5 (human, EC50, cell-based) | 0.30 (human, EC50, cell-based) | 181 | Dimerization inhibitor |
| 33 | ~0.003 (species not specified, IC50) | >30 (species not specified, IC50) | ~2.4 (species not specified, IC50) | 182 | Dimerization inhibitor |
| 34 | Not reported, but lowers iNOS expression in PC12 cell cultures | Not reported | Not reported | 56 | Exact mechanism of action unknown; possibly multiple targets |
| 35 | Inhibits NO production in LPS-stimulated macrophages at 20 μM | Not reported | Not reported | 200 | Exact mechanism of action unknown; proposed to be a competitive inhibitor |
Some of the earliest competitive iNOS inhibitors reported were arginine derivatives: L-nitroarginine, its more bioavailable methyl ester (L-NAME, 1, Fig. 5), and L-Nω-monomethylarginine (L-NMMA, 2).123 Although 2 is both a competitive inhibitor and slow, inactivator of iNOS (possibly through a hydroxylated intermediate and heme loss),124 simple arginine derivatives have little selectivity for iNOS over both of the other isoforms: 2 inactivates both iNOS and nNOS, and both 1 (in its hydrolyzed acid form, as L-NAME is only a weak inhibitor).123 and 2 are actually selective for eNOS. As such, both nitroarginines and 2 cause hypertension when administered to rats or humans,96,125 likely the result of inhibition of eNOS-mediated vasodilation.
FIGURE 5.
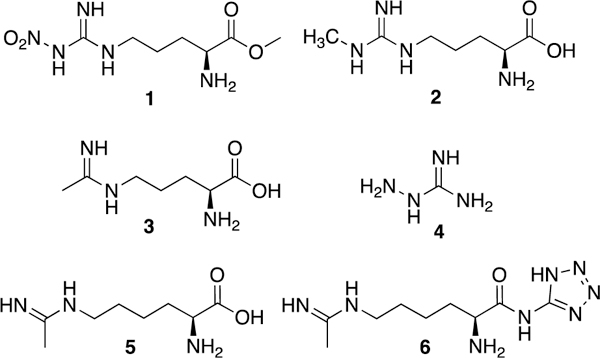
Arginine and amino acid amidine derivatives.
The amidine N5-(1-iminoethyl)-L-ornithine (L-NIO, 3), is a competitive, mechanism-based inactivator126,127 with ~1.8-fold selectivity for murine iNOS over rat nNOS,128 but little selectivity over bovine eNOS.123 In the rat endotoxemia model, László et al.129 examined the ability of iNOS inhibitors to prevent endotoxin-induced colonic microvessel damage and albumin leakage caused by excess NO. When administered during the period where iNOS was induced (~4 h past LPS insult), 3 significantly attenuated colonic leakage, but when administered earlier with LPS, 3 (along with 2) actually exacerbated vessel damage, presumably via inhibition of eNOS. Interestingly, bulky analogues of 3130 inactivate iNOS via a different mechanism than 3 (which induces heme loss) although they are less potent and their selectivity was not reported.
Aminoguanidine (4) is a time-dependent, competitive, mechanism-based inactivator of all NOS isoforms that may bind preferentially to iNOS.126 Corbett et al. reported that 4 was around 40 times less effective at raising mean arterial blood pressure in rats than 2, suggesting some selectivity for iNOS over eNOS in vivo.131 Misko et al.132 confirmed selectivity for iNOS over nNOS in both cell-based and cell-free extracts, but AstraZeneca later reported little selectivity.133 In vivo, 4 shows a variety of iNOS-mediated effects, including the ability to ameliorate negative cardiovascular changes in endotoxin-challenged rats, improve survival in the mouse endotoxemia model,134 and delay disease onset and severity in a murine autoimmune encephalomyelitis model (of multiple sclerosis.135
N6-(1-Iminoethyl)-L-lysine (L-NIL, 5), a competitive inactivator like 3,136 was reported to be more potent in vitro (IC50 = 3.3 μM for murine iNOS) than 2, 3, and nitroarginine, with 28-fold selectivity for iNOS over nNOS.128 Promisingly, 5 reduces early osteoarthritis progression in dogs without toxicity, possibly through reduction of apoptosis in cartilage.137 Nonetheless, 5 has undesirable physical properties, which prompted Hallinan and colleagues138 to devise a prodrug form of 5, the tetrazoleamide L-NIL-TA (or SC-51, 6). While inactive in vitro against human iNOS, the drug is orally bioavailable and hydrolyzes to 5 in vivo, and its activity is comparable to 5 in the LPS endotoxin model, the carrageenan inflammation model, and the adjuvant-induced arthritis model in rats without raising blood pressure. Interestingly, 6 also reduces exhaled NO in both asthmatic and healthy human patients,139 but it does not appear to have been further investigated clinically for this indication despite iNOS’ implication in asthma pathology (see asthma discussion in section on New Directions for iNOS Therapeutics).
Two other amino acid amidines of note are 7 and 8 (Fig. 6, developed by Glaxo-Wellcome; known as GW274150 and GW273629, respectively). These two compounds are potent (Kd <90 nM, IC50 <10 μM), selective (>80-fold i/n, >100-fold i/e), and time- and NADPH-dependent inhibitors (or inactivators) of both human and rodent iNOS. Effective at inhibiting plasma nitrate/nitrite increase in the mouse LPS model, these compounds also do not inhibit nNOS and eNOS in tissue slices or in vivo.7 Compound 7 showed analgesic activity in several rat pain models (adjuvant-induced paw inflammation and chronic constriction injury140 and reduced carrageenan-induced lung inflammation in rats in a dose-dependent fashion (with effects beginning at 2.5 mg/kg).141 Compound 7 progressed into Phase II clinical trials for rheumatoid arthritis and migraine. Although it showed some ability to reduce synovial thickness and vascularity in Phase IIa patients,142 it did not progress further. Additionally, 7 showed no ability to prevent migraines when dosed prophylactically;143 clinical development of 7 appears to have ceased ca. 2012.
FIGURE 6.
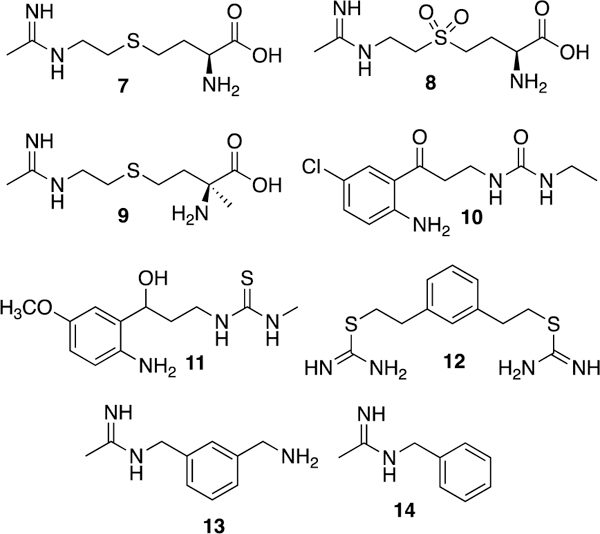
Aliphatic and aromatic amidines. When stereochemistry is unknown, a solid line is used (11).
A related analogue, the selective, irreversible inhibitor cindunistat (SD6010, 9)144 was investigated by Pfizer for the treatment of osteoarthritis. Although it showed good efficacy in rodent models of inflammatory, neuropathic, and osteoarthritis-related pain,145 9 failed to reduce pain and joint-space narrowing in human patients, although other joint changes were not measured, and the drug was well-tolerated.146
Various ureas, thioureas, and isothioureas have also been investigated as iNOS inhibitors. Southan and colleagues published the first report of simple isothioureas inhibiting iNOS in 1995,147 but these molecules were still under investigation as of 2016. Recently, kynurenamine urea (such as 10)148,149 and thiourea (11) were shown to be both iNOS or dual iNOS and nNOS inhibitors with low eNOS inhibition and cytotoxicity.149 Although their potency is modest, molecular modeling supports the hypothesis that they are competitive with L-Arg. Bisisothiourea 12 (known as PBITU)150 is a very potent human iNOS inhibitor (Ki = 47 nM) with 190-fold selectivity for iNOS over eNOS, but it showed high acute toxicity in rodents.
Optimization of 12 led to 13 (1400W) a very potent and selective (~5000-fold i/e and >250-fold i/n) iNOS inhibitor.151 Mechanistically, 13 displays only weak competitive inhibition of eNOS and nNOS, but iNOS inhibition was found to be time- and NADPH-dependent, suggesting possible inactivation (with a measured Kd of 7 nM). Later studies showed that 13 was not modified by the enzyme or utilized as a substrate, but inactivated iNOS via a mechanism consistent with loss of heme as biliverdin (as 3 does).127,152 Administration of 13 reversed LPS-induced vascular injury in rats,151 and 13 also showed efficacy in animal pain models. Recent studies suggest that 1400W’s anti-inflammatory effects may be more complex than just iNOS inhibition, because in rat neuropathic pain models treated animals actually had increased levels of anti-pain cytokines.153 SAR studies of similar benzamidines performed by Amoroso and co-workers154 revealed that truncated compound 14 was more potent than 13, but less selective, suggesting that the aminomethyl group of 13 disfavors binding to eNOS and is necessary for full selectivity.
Although Merck, AstraZeneca, Searle/Pharmacia, and various academic groups have investigated both amino acid and aromatic amidines as competitive iNOS inhibitors, cyclic amidines (Fig. 7) are the most studied.133 Among other 2-iminoazaheterocycles investigated by Moore and co-workers, 2-iminopiperidine (15) was the most potent against human iNOS (IC50 = 1 μM). It showed ability to inhibit plasma NO increases in LPS-challenged rats when dosed orally, but did not have much selectivity over nNOS or eNOS.155 Ono Pharmaceutical Company discovered another iminopiperidine (ONO-1714, 16) with very potent iNOS inhibition (Ki = 1.88 nM) but weak selectivity (10-fold) for iNOS over eNOS.156 This compound also was later revealed as a very potent nNOS inhibitor.157 Compound 16 reduced lung injury in endotoxin-challenged rabbits158 and decreased lactic acidosis and hypotension in a canine endotoxic shock (LPS) model, but caused increased vascular resistance at higher doses in the latter animal model, possibly because of eNOS inhibition.159 2-Iminopyrrolidines (such as 17) also showed some promise as iNOS inhibitors (human iNOS IC50 = 0.25 μM) and activity in rodent endotoxemia models160 and had much higher i/e selectivity than 15 and 16, but low i/n selectivity (13-fold). Hydroxylation (as in 18) afforded a 3-fold increase in i/e selectivity and a 2.5-fold increase in i/n selectivity.161
FIGURE 7.
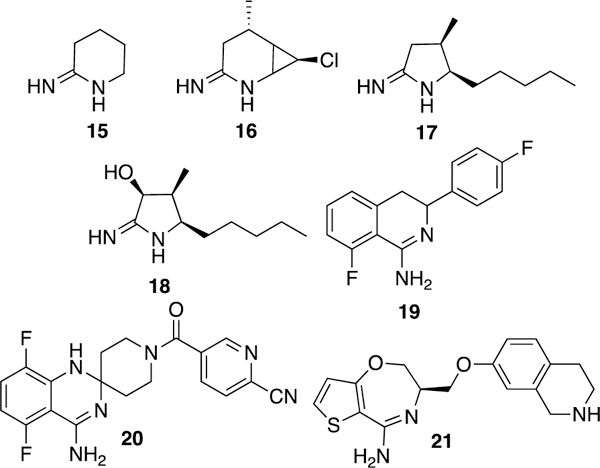
Cyclic amidine derivatives. When stereochemistry is unknown, a solid line is used (19).
In 2001, AstraZeneca reported that 3,4-dihydro-1-isoquinolinamines (especially 3-phenyl-3,4-dihydro-1-isoquinolinamines) inhibited iNOS.162 Compound 19 had a human iNOS IC50 of 160 nM, with i/n selectivity of 100-fold and i/e selectivity of approximately 1,000-fold. A derivative of 19, spirocyclic amide 20 (AR-C102222)163 maintains high i/e selectivity but has decreased i/n selectivity. Nonetheless, this compound displays high in vivo potency without adverse cardiovascular issues and had protective effects in rat models of adjuvant-induced arthritis. Compound 20 also had beneficial effects in a variety of mouse pain and inflammation models (ear inflammation and spinal nerve and hindpaw injury).164
Although X-ray crystal structures of iNOS have been available for over 20 years,28,165 most iNOS inhibitor design is not explicitly structure-based. A notable exception is the anchored plasticity method from AstraZeneca.166 In this method, a combination of X-ray crystallography and mutagenesis studies was used to elucidate the presence of an iNOS-specific pocket that opened upon inhibitor binding as a result of conformational changes in the enzyme. This area was then used to design new compounds, using a strategy where a known functional group capable of iNOS inhibition (such as an amidine) was “anchored” in the binding site, and then other portions were elaborated outwards to reach this induced-fit specificity pocket. Through this method, compound 21, with an IC50 of 400 nM (human iNOS) and n/e selectivity of 200, was designed. In the X-ray crystal structure of 21 bound to murine iNOS (Fig. 8), the thiophenecarboxamidine of 21 mimics L-Arg and forms a salt-bridge with the catalytic glutamate residue.
FIGURE 8.
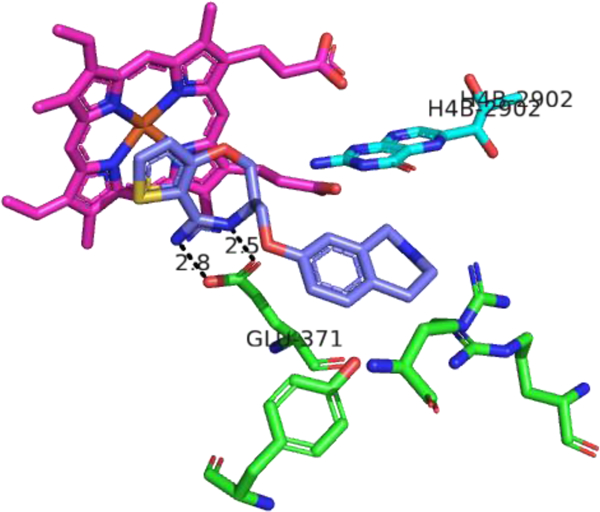
X-ray crystal structure of compound 21 (purple) bound in the murine iNOS active site (PDB ID: 3EBF166. Heme is depicted in magenta, H4B in cyan, and amino acid residues of the enzyme in green. Distances are measured in Ångstroms. Figure prepared using PyMol (www.pymol.org).
2-Aminopyridine is a more recently investigated L-Arg mimic for both iNOS and nNOS (Fig. 9). AstraZeneca reported that simple aminopyridines can antagonize L-Arg in iNOS,167 although most of these compounds are nonselective, weakly selective, or show lower activity in vitro and in vivo than the corresponding saturated cyclic amidines (such as 15). 4-Methylaminopyridine (22, 4-MAP)167,168 is a nonselective iNOS inhibitor that shows activity in rodent endotoxemia and pain models, although the dose required for analgesic effects is lower than those needed for efficacy in the endotoxic shock model, suggesting some of its analgesic properties are likely the result of nNOS inhibition.168
FIGURE 9.
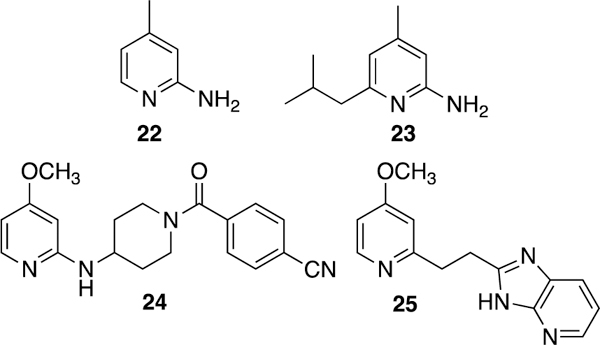
Pyridine and aminopyridine derivatives.
The disubstituted aminopyridine (23) is about six times more potent than 22 and has improved selectivity,167 but more complex aminopyridines are generally more effective. Through optimization, AR-C133057XX (24) was discovered.169 This piperidine amide derivative is potent in vivo and in vitro (IC50 = 71 nM against human iNOS) and extremely selective for iNOS over eNOS (<20% eNOS inhibition at 100 μM). X-ray crystallography revealed that the aminopyridine of 24 flips over 180˚ relative to that of 22, which the authors propose allows binding of the piperidine and benzonitrile to additional iNOS-selective regions.
An unconventional pyridine derivative is imidazopyridine 25 (BYK191023), which was discovered in 2005 in a high-throughput screen.170 Two years later it was determined to be a competitive, time-dependent, irreversible inhibitor in the presence of NADPH, likely causing the loss of heme.171 Highly selective for iNOS (in enzyme assays, cells, and isolated organ systems), 25 was also tested in several animal models. In addition to reduction of plasma nitrite/nitrate and maintenance of blood pressure in LPS-challenged rats,172 25 improved organ blood flow and reduced pulmonary hypertension and acidosis in septic sheep (relative to sheep treated with norepinephrine), but the drug did not increase overall survival.173
7.3. Dimerization inhibitors
The second mechanism upon which inhibitor development has focused is inhibiting formation of an active iNOS dimer. As the dimer interface is located at the far end of the active site, an inhibitor that anchors in the active site and blocks dimerization is desirable. It has long been known that imidazoles (Fig. 10) can coordinate the heme iron of NOS isoforms. While smaller imidazoles stabilize iNOS dimer formation, bulky imidazoles (such as the antifungal clotrimazole (26)) prevent dimer assembly.174
FIGURE 10.
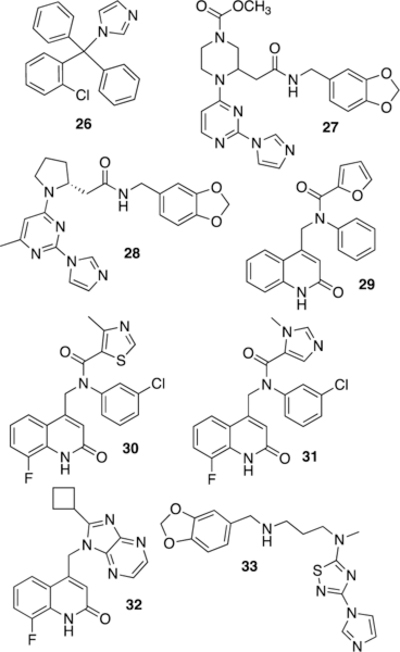
Various iNOS dimerization inhibitors. When stereochemistry is unknown, a solid line is used (27).
Following this discovery, pyrimidinylimidazoles (such as 27) were discovered by combinatorial chemistry at Berlex Bioscience.175 These compounds bind with high affinity to human iNOS monomers (Ki of 2.2 nM for 27) and show activity in rodent LPS models. X-ray crystallography of 27 bound to a murine iNOS monomer missing the N-terminal 114 residues (Fig. 11) suggested that these compounds act by coordinating iron, adopting a U-shaped conformation, and displacing the Glu371 residue from the active site. Disruption or disordering of this residue’s helix (helix 7a) then subsequently perturbs the dimerization interface. Mechanistic studies (spectroscopy and competition binding) performed by Blasko et al176 later confirmed that pyrimidinylimidazoles do act by coordinating the heme center of iNOS monomers and allosterically blocking dimerization, and it was proposed that the observed selectivity reflects different dimer assembly kinetics in eNOS and nNOS (vs. iNOS). It was shown more recently177 that pyrimidinylimidazoles can also break up active dimers into monomers in cells and that the monomer-inhibitor complex is irreversible and cannot be converted back into active dimers by added L-Arg or H4B.
FIGURE 11.
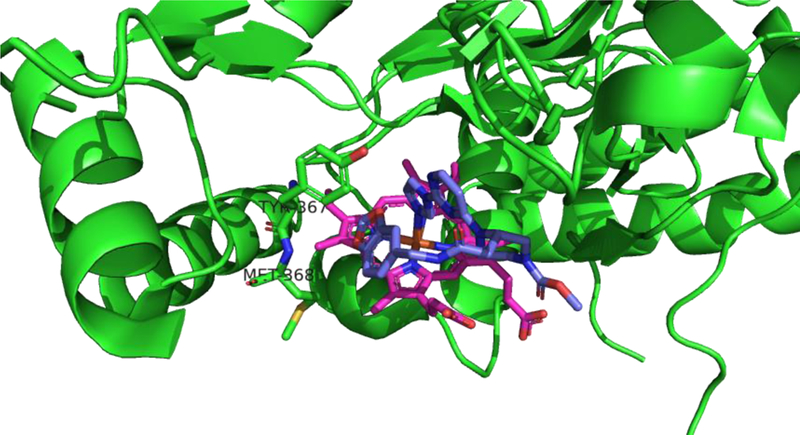
X-ray crystal structure of 27 (purple) bound to iNOS monomer (with N-terminal 114 residues truncated). (PDB ID: 1DD7)175. Note the coordination of the heme (magenta) iron by the inhibitors’s imidazole. The helix containing the catalytic Glu371 is disordered. Figure prepared using PyMol (www.pymol.org).
By X-ray crystallography, homoproline compound 28 also prevents dimerization by displacing iNOS’ helix 7a178. This compound is orally bioavailable and is an excellent inhibitor in cells (IC50 = 290 pM), presumably acting by binding to nascent iNOS monomers as, or shortly after, they are formed. Compound 28 also reduced clinical arthritis scores in adjuvant-induced arthritic rats.
In addition to Berlex Biosciences’ work, Kalypsys Inc. was also active in the area of iNOS dimerization inhibitor development. In 2009, an ultra high-throughput cell-based screen was performed on non-L-Arg and non-imidazole compounds (the latter out of concern over cytochrome inhibition), and quinolinone hit 29 was identified.179 Optimization was performed to improve murine iNOS activity (for animal use), and new leads 30 (KLYP596) and 31 were found to have improved potency and selectivity, with 30 having an EC50 of 11 nM and the higher selectivity of the two (i/e of 2300 and i/n of 210) in cell-based assays. Gel electrophoresis and immunoblotting of enzyme isolated from treated cells revealed dose-dependent loss of dimeric iNOS.180 Biochemical characterization revealed that inhibition was insensitive to addition of L-Arg and H4B, but binding of 30 did not result in spectral shifts consistent with imidazole-like heme coordination. Although 30 and 31 have good oral bioavailability, they have high clearance and a short half-life in vivo. Nonetheless, both compounds reduce plasma nitrate levels in LPS-challenged mice and reduce pain behaviors in mouse formalin and chronic nerve constriction injury models.179
To improve pharmacokinetics, researchers at Kalypsys also investigated quinolinone-benzimidazoles and imidazopyrazines as conformationally restricted analogues of 29, and after SAR analysis, cyclobutyl analogue 32 (KD7332) was discovered.181 A potent dual iNOS/nNOS inhibitor, 32 displayed reduced clearance in vivo, and showed anti-pain activity in the mouse formalin model and the Chung model of neuropathic pain, with a large therapeutic index. Another potent (IC50 = 3 nM) and selective (nNOS IC50 = 2.4 μM, eNOS = >30 μM) Kalypsys molecule (33, KD7040)182,183 was described as being a skin-permeable selective iNOS dimerization inhibitor. It was investigated (Phase I) for topical use in the treatment of post-herpetic neuralgia, but its development appears to have ceased ca. 2008.
8. CLINICAL FAILURES
The classical indications for iNOS inhibition are sepsis (systemic inflammation) and pain. However, attempts to treat sepsis and septic shock with iNOS inhibitors in a clinical setting have been met with limited success. Although some beneficial effects have been shown, nonselective iNOS inhibition (such as with 2) can actually increase cardiac dysfunction and mortality in septic shock patients.184 It is proposed that eNOS could have some protective role in sepsis (as has been demonstrated in murine eNOS overexpression studies)185 and thus nonselective inhibition could be harmful. Selective iNOS inhibition results in fewer side effects, but is still controversial for this indication.173,186 Additionally, iNOS knockout mice (or mice treated with 1, 2, or 3) are not protected from developing endotoxemic phenotype;187 this effect has also been reported to occur in liver cells).40 Bredan and Cauwels also suggest that agents that scavenge NO or mediate the downstream effects of NO could be more effective than iNOS inhibition itself .188
Despite efficacy in animal models, no iNOS inhibitors have been approved for pain treatment; arthritis trials have repeatedly failed, and anti-nociceptive activity in animal models does not translate to humans in clinical trials. First, it is possible that the role of NO in pain is more complicated than simply “too much NO is bad” (as is proposed to be the case for sepsis). Second, it is possible that iNOS inhibition could have real benefits, but the development of pain therapeutics (not exclusive to iNOS) is fraught with failure, in part because of possible assay limitations, and in part because pain is subjective and both affects - and is affected by – other complex behaviors in both animals and humans.189
Another area where iNOS inhibition seems to have failed is treatment of cardiogenic shock related to myocardial infarction. Early studies suggested that iNOS plays a major role in heart failure, as upregulation of iNOS in mouse heart cells results in cardiac death.190 However, in the best-known clinical trial for cardiogenic shock in infarction patients (the TRIUMPH trial),191 compound 2 failed to reduce severity of heart failure or mortality and also resulted in hypertension. It was hypothesized that lack of selectivity192 or too low a dose193 could have been responsible for these failures. A recent study (2015) using resuscitated swine found that neither global NOS inhibition (using 1) nor more selective iNOS inhibition (using 4) improves survival or myocardial dysfunction following ventricular fibrillation in cardiac arrest (when iNOS levels increase).194 Additionally, the observation that nonselective inhibition adversely affects blood pressure and cardiac function in this study also suggests that NO may be actually important for proper recovery from cardiac events.
9. NEW DIRECTIONS FOR iNOS THERAPEUTICS
Despite the focus on septic shock and various types of pain, it is conceivable that other diseases with a sizeable inflammatory component could benefit from iNOS inhibitors. Indeed, many studies indicate that iNOS inhibition could have broader therapeutic use in other diseases, including neurologic disease, diabetes, cancer, and respiratory disease; these uses are summarized briefly below.
Although the NOS isoform most investigated as a target for anti-neurodegenerative agents is nNOS,195 evidence suggests that iNOS inhibition also might be beneficial for treating or preventing neurodegenerative disorders, such as Parkinson’s disease or the neuronal damage associated with stroke. For example, iNOS silencing (with siRNA) reduces the Parkinsonism symptoms in animals treated with the neurotoxin 6-OHDA.196 Compound 4 shows beneficial effects in animal models of stroke, such as ability to reduce infarct volume in hypoxic-ischemic rat pups197 and in adult rats,198 although iNOS inhibition is believed to be responsible for only part of the observed effects in the latter study. Another mechanism, potentially polyamine oxidase inhibition by 4, also may be responsible. Selective iNOS inhibition (such as with 7) has also been found to be neuroprotective, preventing neuroinflammation in the 6-OHDA model of Parkinsonism in rodents at 10 mg/kg, although higher doses seem to have little effect.199 The naturally occurring flavonol quercetin (34, Figure 12) prevents both 6-OHDA-induced apoptosis in neuron-like PC12 cell cultures and neuronal death in zebrafish. This phenomenon was only observed in early zebrafish larvae, suggesting that this compound may not cross the blood-brain barrier effectively. Quercetin also lowers iNOS expression in PC12 cultures (and it is proposed that some of its neuroprotective effect may come from reduction of iNOS expression), but 34 also can lower the expression of other pro-inflammatory cytokines; therefore, its effects may be multifaceted.55
FIGURE 12.
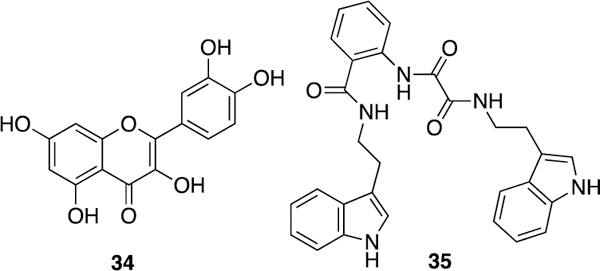
Neuroprotective iNOS inhibitors.
A 2014 report200 discusses a series of natural product-like compounds discovered via high-throughput virtual screening. One compound (bis-indole 35) is proposed to be a competitive inhibitor of NO production in stimulated macrophages. Compound 35 also displayed partial neuroprotective effects in zebrafish larvae treated with the neurotoxin MPTP, although these effects were not correlated specifically with iNOS inhibition.
iNOS inhibition has also been investigated more recently as a target of interest in epilepsy. Uzüm et al. demonstrated in rats that epilepsy (represented by long-term clonic-tonic seizures), like sepsis, is associated with systemic inflammation (including increased iNOS levels in the liver and kidney).201 Other studies have shown iNOS inhibition could be beneficial in epilepsy. For example, aminoguanidine (4) suppresses the development of chemically induced kindled and spontaneous recurrent seizures in mice in a dose-dependent fashion.202 Furthermore, a selective iNOS inhibitor, 13, reduces the development of temporal lobe epilepsy in rats treated with the neurotoxin kainate.203 In this study, treated animals had reduced neuronal hyperexcitability (epileptiform spiking) and displayed a 90% reduction in spontaneous recurrent seizures (relative to control) in the six-month period following the kainate insult.
Overall, iNOS’ role in the brain may be more complicated than originally believed. Recent studies suggest iNOS may also play some role in depression. Montezuma et al. report that both iNOS deletion and selective iNOS inhibition (using either 4 or 13) reduce immobility time in mice in the forced swimming test in the same way that antidepressant drugs do, but nNOS-specific inhibitors (at the same doses relative to their Ki values) do not.204 Compound 13 also exhibits an ability to reverse stress-induced depressive behaviors in mice consistent with prior results that indicate chronic stress may lead to an increase in general inflammation.205
A full summary of the role of iNOS in cancer is beyond the scope of this review,41,206 although iNOS inhibitors have demonstrated a variety of chemopreventive and anticancer effects in animal models. Both 4 and 6, for example, reduce the incidence and number of preneoplastic lesions in rats fed a selective colon carcinogen; administration of the iNOS inhibitor with the COX-2 inhibitor celecoxib further enhanced chemopreventive potential, suggesting that overexpressed iNOS could be regulating inflammation through COX-2.207 Positive effects from iNOS inhibition were also observed in mice implanted with human melanoma xenografts; compound 5 slowed tumor growth and showed synergistic activity when administered with cisplatin.208 Similarly, 5, 22, and a variety of isothioureas prevented transformation of rat tracheal cells into cancer cells.209 Both iNOS knockdown and the use of compounds, 1, 2, and 13 inhibit breast-derived cancer cell proliferation, cancer stem-cell self-renewal, and migration in vitro. Compounds 1 and 2 also reduced tumor volume and lung metastases in triple-negative breast cancer mouse xenograft models, where their activity also synergized with docetaxel.106
iNOS inhibition has also been investigated for the treatment of the chronic, low-grade inflammation associated with type II diabetes, obesity-associated insulin resistance, and related conditions. Perrault and Marette reported that iNOS-knockout mice, while not protected from diet-induced obesity, are protected from associated insulin resistance and show improved glucose tolerance and normal glucose uptake in muscle.210 Noronha and colleagues observed similar results on insulin tolerance, but obese iNOS knockout mice still were hypertensive with increased vascular reactive oxygen species.100 Similarly, 5 improved insulin sensitivity and fasting hyperglycemia in obese and diabetic mice (which have iNOS overexpressed in the liver, muscle, and adipose tissue.211 Obesity can also impair lymphatic function (possibly via the accumulation of perilymphatic inflammatory cells that produce iNOS), and treatment of obese mice with 13 resulted in decreased perilymphatic inflammatory cell localization and modest improvements in lymphatic function.212
Finally, iNOS inhibition has potential therapeutic indications in the treatment of certain respiratory conditions. Mice lacking iNOS (but not eNOS) are protected from tobacco smoke-induced chronic obstructive pulmonary disorde.213 Treatment with 5 reversed eight months of tobacco-induced lung injury after three months of administration. According to Nathan,214 for this and other conditions, iNOS may be “beginning to smoke.”
10. CONCLUDING REMARKS
iNOS has been shown to have both beneficial and detrimental consequences. NO produced by iNOS is essential for the normal inflammatory response, while dysregulation of iNOS is implicated in a variety of chronic and acute diseases. Recent advances in structural characterization and new insights into regulation of iNOS expression have allowed the design and development of highly selective and potent iNOS inhibitors. Although iNOS inhibitors show significant promise in animal models for septic shock, pain, and other conditions, to date they have failed in clinical trials. Further efforts in the field should focus on understanding the complex role of iNOS in biological systems (both its harmful and protective roles) and refinement of both animal models and clinical trials for inflammatory diseases. New indications that iNOS inhibition could be useful for include treating cancer, neurodegenerative diseases, and depression (among others); these indications have revitalized the field and may lead to novel therapeutic avenues.
ACKNOWLEDGMENTS
Research on NOS inhibitors in our laboratory is generously supported by the National Institutes of Health, via R01 GM049725 to R.B.S. and F32 GM109667 to M.A.C. G. M. is supported by a Chemistry of Life Processes Predoctoral Training Program Grant (5T32 GM105538).
Abbreviations Used
- 6-OHDA
6-hydroxydopamine
- CaM
calmodulin
- CAT
cationic amino acid transporter
- cDNA
complementary DNA
- cGMP
cyclic guanosine monophosphate
- CNS
central nervous system
- COX-2
cyclooxygenase 2
- EC50
concentration of drug that gives half maximal response
- eNOS
endothelial nitric oxide synthase
- EPR
electron paramagnetic resonance
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- GTP
guanosine triphosphate
- H4B
(6R)-5,6,7,8-tetrahydrobiopterin
- HBV
hepatitis B virus
- HIV
human immunodeficiency virus
- HSP
heat shock protein
- IC50
concentration to achieve 50% inhibition
- i/e
selectivity of a compound for iNOS versus eNOS
- i/n
selectivity of a compound for iNOS versus nNOS
- IFN-γ
interferon γ
- IRF-1
interferon regulatory factor 1
- IκB
inhibitory κB
- IKK
inhibitor of IκB kinase
- iNOS
inducible nitric oxide synthase
- IL-1
interleukin 1
- JAK
Janus kinase
- Kd
dissociation constant
- Ki
equilibrium constant for dissociation of an enzyme–inhibitor complex (used as a measure of potency where lower numbers indicate higher potency)
- L-Arg
L-arginine
- L-NOHA
Nω-hydroxy-L-arginine
- LPS
lipopolysaccharide
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NAP110
NOS associated protein 110
- NF-κB
nuclear factor κB
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- ONOO
peroxynitrite anion
- SAR
structure-activity relationship
- siRNA
small interfering RNA
- STAT-1α
signal transducer and activator of transcription 1α
- TNF
tumor necrosis factor alpha
AUTHORS’ BIOGRAPHIES
Maris A. Cinelli received her B.S. degree in physiology from Northern Michigan University (2006) and her Ph.D. in medicinal chemistry from Purdue University (2011) under the mentorship of Prof Mark Cushman, where she worked on the design and synthesis of topoisomerase I poisons. She did her postdoctoral research at Northwestern University (Prof. Richard Silverman), where she developed 2-aminoquinolines as selective inhibitors of neuronal nitric oxide synthase as potential antineurodegenerative agents and was a recipient of a Ruth S. Kirchstein National Research Service Award (F32, NIH). She currently works as a senior scientist at Michigan State University, where she studies fatty acid metabolites and analogs as drug leads. Her research interests include organic synthesis, structure‐based and computer‐aided drug design, and enzyme inhibition.
Ha T. Do obtained her B.Sc. degree in chemistry (honor program) from University of Science- Ho Chi Minh City, Vietnam in 2008. She then obtained her PhD degree in organic chemistry from University of Houston in 2015 under the supervision of Professor Scott R. Gilbertson, where she worked on the syntheses of the natural product dysiherbaine and small molecules for breast cancer treatment. After a short training with Professor Zhengqiang Wang at the Center for Drug Design at University of Minnesota, Ha joined the laboratory of Professor Richard B. Silverman at Northwestern University as a postdoctoral fellow and worked on optimizing the blood brain barrier permeability of neuronal nitric oxide synthase inhibitors for the development of new drugs for neurodegenerative diseases. She now holds a scientist position at Mersana Therapeutics, Inc. Her research interests are in organic synthesis, medicinal chemistry, and drug development to treat cancers and CNS disorders.
Galen Miley received his Bachelor of Chemistry degree from Reed College in Portland, Oregon in 2013. Following graduation, he worked as a research chemist at the Portland Veterans Affairs Research Foundation/OHSU under Michael Riscoe studying Endochin-like quinolones for the treatment and prevention of malaria. He is currently pursuing his PhD degree at Northwestern University as a NIH T32/CLP and PPG fellow under the joint supervision of Richard Silverman and Neil Kelleher. His dissertation work integrates LC/MS based chemical biology techniques into the study of neuronal nitric oxide synthase (nNOS) as a treatment for melanoma. He is interested in a career in drug discovery and evaluation in the fields of infectious disease, virology and immune modulation.
Richard B. Silverman obtained his B.S. degree in chemistry from The Pennsylvania State University and A.M. and Ph.D degrees in organic chemistry with David Dolphin from Harvard University. With a two-year NIH-supported postdoctoral fellowship, he studied enzymology and enzyme inhibitors with Robert Abeles at Brandeis University prior to beginning his independent career as an Assistant Professor of Chemistry at Northwestern University in 1976. Currently, he is the Patrick G. Ryan/Aon Professor at Northwestern University. He is the inventor of pregabalin, marketed by Pfizer as Lyrica for fibromyalgia, neuropathic pain, and epilepsy. Current research involves the design, synthesis, and mechanism of selective enzyme inhibitors, inactivators, and activators for the treatment of a variety of neurodegenerative diseases (Parkinson’s disease, ALS, Alzheimer’s disease, cerebral palsy), neurological disorders (epilepsy and addiction), cancer (hepatocellular carcinoma and melanoma), and lysosomal storage diseases (Gaucher’s disease).
REFERENCES
- 1.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Soud HM, Gachhui R, Raushel FM, et al. The ferrous-dioxy complex of neuronal nitric oxide synthase: divergent effects of l-arginine and tetrahydrobiopterin on its stability. J Biol Chem. 1997;272:17349–17353. [DOI] [PubMed] [Google Scholar]
- 3.Kone BC, Kuncewicz T, Zhang W, et al. Protein interactions with nitric oxide synthases: controlling the right time, the right place, and the right amount of nitric oxide. Am J Physiol - Renal. 2003;285:F178–F190. [DOI] [PubMed] [Google Scholar]
- 4.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252–259. [DOI] [PubMed] [Google Scholar]
- 5.MacMicking J, Xie Q-w, Nathan C Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Yu T, Lian YJ, et al. Nitric oxide synthase inhibitors: a review of patents from 2011 to the present. Expert Opin Ther Pat 2015; 25: 49–68. [DOI] [PubMed] [Google Scholar]
- 7.Alderton WK, Angell ADR, Craig C, et al. GW274150 and GW273629 are potent and highly selective inhibitors of inducible nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 2005;145:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Soud HM, Stuehr DJ. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci USA. 1993;90:10769–10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenman JE, Chao DS, Gee SH, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. [DOI] [PubMed] [Google Scholar]
- 10.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raman CS, Li H, Martásek P, et al. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. [DOI] [PubMed] [Google Scholar]
- 12.Chreifi G, Li H, McInnes CR, et al. Communication between the zinc and tetrahydrobiopterin binding sites in nitric oxide synthase. Biochemistry. 2014;53:4216–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venema RC, Sayegh HS, Kent JD, et al. Identification, characterization, and comparison of the calmodulin-binding domains of the endothelial and inducible nitric oxide synthases. J Biol Chem. 1996;271:6435–6440. [DOI] [PubMed] [Google Scholar]
- 14.Hemmens B, Mayer B. Enzymology of nitric oxide synthases In: Titheradge MA, editor. Nitric oxide protocols. Totowa, NJ: Humana Press; 1998. p. 1–32. [DOI] [PubMed] [Google Scholar]
- 15.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. [DOI] [PubMed] [Google Scholar]
- 16.Pan J, Burgher KL, Szczepanik AM, et al. Tyrosine phosphorylation of inducible nitric oxide synthase: implications for potential post-translational regulation. Biochem J. 1996;314:889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noble MA, Munro AW, Rivers SL, et al. Potentiometric analysis of the flavin cofactors of neuronal nitric oxide synthase. Biochemistry. 1999;38:16413–16418. [DOI] [PubMed] [Google Scholar]
- 18.Campbell MG, Smith BC, Potter CS, et al. Molecular architecture of mammalian nitric oxide synthases. Proc Natl Acad Sci USA. 2014;111:E3614–E3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Chen L, Lu C, et al. Regulatory role of Glu546 in flavin mononucleotide — heme electron transfer in human inducible nitric oxide synthase. Inorg Chem. 2013;52:4795–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giroud C, Moreau M, Mattioli TA, et al. Role of arginine guanidinium moiety in nitric oxide synthase mechanism of oxygen activation. J Biol Chem. 2010;285:7233–7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer B, Wu C, Gorren ACF, et al. Tetrahydrobiopterin binding to macrophage inducible nitric oxide synthase: heme spin shift and dimer stabilization by the potent pterin antagonist 4-amino-tetrahydrobiopterin. Biochemistry. 1997;36:8422–8427. [DOI] [PubMed] [Google Scholar]
- 22.Crane BR, Arvai AS, Ghosh DK, et al. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science. 1998;279:2121–2126. [DOI] [PubMed] [Google Scholar]
- 23.Vásquez-Vivar J, Hogg N, Martásek P, et al. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem. 1999;274:26736–26742. [DOI] [PubMed] [Google Scholar]
- 24.Hurshman AR, Krebs C, Edmondson DE, et al. Ability of tetrahydrobiopterin analogues to support catalysis by inducible nitric oxide synthase: formation of a pterin radical is required for enzyme activity. Biochemistry. 2003;42:13287–13303. [DOI] [PubMed] [Google Scholar]
- 25.Daff S No synthase: Structures and mechanisms. Nitric Oxide. 2010;23:1–11. [DOI] [PubMed] [Google Scholar]
- 26.Wei C-C, Wang Z-Q, Hemann C, et al. A tetrahydrobiopterin radical forms and then becomes reduced during Nω-hydroxyarginine oxidation by nitric oxide synthase. J Biol Chem. 2003;278:46668–46673. [DOI] [PubMed] [Google Scholar]
- 27.Wei C-C, Wang Z-Q, Tejero J, et al. Catalytic reduction of a tetrahydrobiopterin radical within nitric oxide synthase. J Bio Chem. 2008;283:11734–11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischmann TO, Hruza A, Niu XD, et al. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat Struct Mol Biol. 1999;6:233–242. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Shimizu H, Flinspach M, et al. The novel binding mode of N-alkyl-N’-hydroxyguanidine to neuronal nitric oxide synthase provides mechanistic insights into no biosynthesis. Biochemistry. 2002;41:13868–13875. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh S, Wolan D, Adak S, et al. Mutational analysis of the tetrahydrobiopterin-binding site in inducible nitric oxide synthase. J Biol Chem. 1999;274:24100–24112. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z-Q, Tejero J, Wei C-C, et al. Arg375 tunes tetrahydrobiopterin functions and modulates catalysis by inducible nitric oxide synthase. J Inorg Biochem. 2012;108:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naureckiene S, Kodangattil SR, Kaftan EJ, et al. Identification of critical amino acid residues for human iNOS functional activity. Protein J. 2008;27:309–318. [DOI] [PubMed] [Google Scholar]
- 33.Panda K, Rosenfeld RJ, Ghosh S, et al. Distinct dimer interaction and regulation in nitric oxide synthase types I, II, and III. J Biol Chem. 2002;277:31020–31030. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Fan W, Chen L, et al. Role of an isoform-specific serine residue in FMN-heme electron transfer in inducible nitric oxide synthase. J Biol Inorg Chem. 2012;17:675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakravortty D, Hensel M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microb Infect. 2003;5:621–627. [DOI] [PubMed] [Google Scholar]
- 36.Fernhoff NB, Derbyshire ER, Marletta MA. A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase. Proc Natl Acad Sci USA. 2009;106:21602–21607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burney S, Caulfield JL, Niles JC, et al. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res Fund Mol M. 1999;424:37–49. [DOI] [PubMed] [Google Scholar]
- 38.Xia C, Misra I, Iyanagi T, et al. Regulation of interdomain interactions by calmodulin in inducible nitric oxide synthase. J Biol Chem. 2009;284:30708–30717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. [DOI] [PubMed] [Google Scholar]
- 40.Chanthaphavong RS, Loughran PA, Lee TYS, et al. A role for cGMP in inducible nitric oxide synthase (iNOS)-induced tumor necrosis factor (TNF) α-converting enzyme (TACE/ADAM17) activation, translocation, and TNF receptor 1 (TNFR1) shedding in hepatocytes. J Biol Chem. 2012;287:35887–35898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vannini F, Kashfi K, Nath N. The dual role of iNOS in cancer. Redox Biol. 2015;6:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hey C, Boucher J-L, Vadon-Le Goff S, et al. Inhibition of arginase in rat and rabbit alveolar macrophages by Nω-hydroxy-D,L-indospicine, effects on L-arginine utilization by nitric oxide synthase. Br J Pharmacol. 1997;121:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ckless K, Lampert A, Reiss J, et al. Inhibition of arginase activity enhances inflammation in mice with allergic airway disease, in association with increases in protein S-nitrosylation and tyrosine nitration. J Immunol. 2008;181:4255–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruch-Gerharz D, Schnorr O, Suschek C, et al. Arginase 1 overexpression in psoriasis: limitation of inducible nitric oxide synthase activity as a molecular mechanism for keratinocyte hyperproliferation. Am J Pathol. 2003;162:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidlin A, Wiesinger H. Transport of L-arginine in cultured glial cells. Glia. 1994;11:262–268. [DOI] [PubMed] [Google Scholar]
- 46.Bogle RG, Baydoun AR, Pearson JD, et al. L-arginine transport is increased in macrophages generating nitric oxide. Biochem J. 1992;284:15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manner CK, Nicholson B, MacLeod CL. CAT2 arginine transporter deficiency significantly reduces iNOS-mediated NO production in astrocytes. J Neurochem. 2003;85:476–482. [DOI] [PubMed] [Google Scholar]
- 48.Kawahara K, Gotoh T, Oyadomari S, et al. Co-induction of argininosuccinate synthetase, cationic amino acid transporter-2, and nitric oxide synthase in activated murine microglial cells. Mol Brain Res. 2001;90:165–173. [DOI] [PubMed] [Google Scholar]
- 49.Mori M, Gotoh T. Arginine metabolic enzymes, nitric oxide and infection. J Nutr. 2004;134:2820S–2825S. [DOI] [PubMed] [Google Scholar]
- 50.Cho HJ, Martin E, Xie QW, et al. Inducible nitric oxide synthase: identification of amino acid residues essential for dimerization and binding of tetrahydrobiopterin. Proc Natl Acad Sci USA. 1995;92:11514–11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tzeng E, Billiar TR, Robbins PD, et al. Expression of human inducible nitric oxide synthase in a tetrahydrobiopterin (H4B)-deficient cell line: H4B promotes assembly of enzyme subunits into an active dimer. Proc Natl Acad Sci USA. 1995;92:11771–11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank S, Pfeilschifter J, Madlener M, et al. Induction of inducible nitric oxide synthase and its corresponding tetrahydrobiopterin-cofactor-synthesizing enzyme GTP-cyclohydrolase I during cutaneous wound repair. J Invest Dermatol. 1998;111:1058–1064. [DOI] [PubMed] [Google Scholar]
- 53.Youn H, Ji I, Ji HP, et al. Under-expression of kalirin-7 increases iNOS activity in cultured cells and correlates to elevated iNOS activity in Alzheimer’s disease hippocampus. J Alzheimer’s Dis. 2007;12:271–281. [DOI] [PubMed] [Google Scholar]
- 54.Ratovitski EA, Alam MR, Quick RA, et al. Kalirin inhibition of inducible nitric oxide synthase. J Biol Chem. 1999;274:993–999. [DOI] [PubMed] [Google Scholar]
- 55.Ratovitski EA, Bao C, Quick RA, et al. An inducible nitric oxide synthase (NOS)-associated protein inhibits NOS dimerization and activity. J Biol Chem. 1999;274:30250–30257. [DOI] [PubMed] [Google Scholar]
- 56.Zhang ZJ, Cheang LCV, Wang MW, et al. Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. Int J Mol Med. 2011;27:195. [DOI] [PubMed] [Google Scholar]
- 57.Kuncewicz T, Balakrishnan P, Snuggs MB, et al. Specific association of nitric oxide synthase-2 with Rac isoforms in activated murine macrophages. Am J Physiol - Renal. 2001;281:F326–F336. [DOI] [PubMed] [Google Scholar]
- 58.Jyoti A, Singh AK, Dubey M, et al. Interaction of inducible nitric oxide synthase with Rac2 regulates reactive oxygen and nitrogen species generation in the human neutrophil phagosomes: Implication in microbial killing. Antioxid Redox Sign. 2013;20:417–431. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Zhang J, Liu Y, et al. Role of nitric oxide synthase in the development of bone cancer pain and effect of L-NMMA. Mol Med Rep. 2016;13:1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Assreuy J, Cunha FQ, Liew FY, et al. Feedback inhibition of nitric oxide synthase activity by nitric oxide. Br J Pharmacol. 1993;108:833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith BC, Fernhoff NB, Marletta MA. Mechanism and kinetics of inducible nitric oxide synthase auto-S-nitrosation and inactivation. Biochemistry. 2012;51:1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kleinert H, Schwarz PM, Förstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol Chem. 2003;384:1343–1364. [DOI] [PubMed] [Google Scholar]
- 63.Pautz A, Art J, Hahn S, et al. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010;23:75–93. [DOI] [PubMed] [Google Scholar]
- 64.Zhou X, Yang W, Li J. Ca2+- and protein kinase C-dependent signaling pathway for nuclear factor-κb activation, inducible nitric oxide synthase expression, and tumor necrosis factor-α production in lipopolysaccharide-stimulated rat peritoneal macrophages. J Biol Chem. 2006;281:31337–31347. [DOI] [PubMed] [Google Scholar]
- 65.Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta. 1999;1411:437–455. [DOI] [PubMed] [Google Scholar]
- 66.Ganster RW, Taylor BS, Shao L, et al. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-κB. Proc Natl Acad Sci USA. 2001;98:8638–8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dell’Albani P, Santangelo R, Torrisi L, et al. Jak/stat signaling pathway mediates cytokine-induced iNOS expression in primary astroglial cell cultures. J Neurosci Res. 2001;65:417–424. [DOI] [PubMed] [Google Scholar]
- 68.Hevel JM, White KA, Marletta MA. Purification of the inducible murine macrophage nitric oxide synthase. Identification as a flavoprotein. J Biol Chem. 1991;266:22789–22791. [PubMed] [Google Scholar]
- 69.Geller DA, Nussler AK, Di Silvio M, et al. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci USA. 1993;90:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nussler AK, Billiar TR, Hoffman RA, et al. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992;176:261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koide M, Kawahara Y, Tsuda T, et al. Cytokine-induced expression of an inducible type of nitric oxide synthase gene in cultured vascular smooth muscle cells. FEBS Lett. 1993;318:213–217. [DOI] [PubMed] [Google Scholar]
- 72.Macnaul KL, Hutchinson NI. Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem Biophys Res Commun. 1993;196:1330–1334. [DOI] [PubMed] [Google Scholar]
- 73.Charles IG, Palmer RM, Hickery MS, et al. Cloning, characterization, and expression of a cDNA encoding an inducible nitric oxide synthase from the human chondrocyte. Proc Natl Acad Sci USA. 1993;90:11419–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nomura Y, Kitamura Y. Inducible nitric oxide synthase in glial cells. Neurosci Res. 1993;18:103–107. [DOI] [PubMed] [Google Scholar]
- 75.Galea E, Reis DJ, Feinstein DL. Cloning and expression of inducible nitric oxide synthase from rat astrocytes. J Neurosci Res. 1994;37:406–414. [DOI] [PubMed] [Google Scholar]
- 76.Koprowski H, Zheng YM, Heber-Katz E, et al. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci USA. 1993;90:3024–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Minc-Golomb D, Tsarfaty I, Schwartz JP. Expression of inducible nitric oxide synthase by neurones following exposure to endotoxin and cytokine. Br J Pharmacol. 1994;112:720–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balligand JL, Ungureanu-Longrois D, Simmons WW, et al. Cytokine-inducible nitric oxide synthase (iNOS) expression in cardiac myocytes. Characterization and regulation of iNOS expression and detection of iNOS activity in single cardiac myocytes in vitro. J Biol Chem. 1994;269:27580–27588. [PubMed] [Google Scholar]
- 79.Lyons CR, Orloff GJ, Cunningham JM. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992;267:6370–6374. [PubMed] [Google Scholar]
- 80.Xie Q, Cho H, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. [DOI] [PubMed] [Google Scholar]
- 81.Albina JE. On the expression of nitric oxide synthase by human macrophages. Why no NO? J Leukocyte Biol. 1995;58:643–649. [DOI] [PubMed] [Google Scholar]
- 82.Schneemann M, Schoedon G, Hofer S, et al. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993;167:1358–1363. [DOI] [PubMed] [Google Scholar]
- 83.Geller DA, Lowenstein CJ, Shapiro RA, et al. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci USA. 1993;90:3491–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Obregón E, Punzón MC, González-Nicolás J, et al. Induction of adhesion/differentiation of human neuroblastoma cells by tumour necrosis factor-α requires the expression of an inducible nitric oxide synthase. Eur J Neurosci. 1997;9:1184–1193. [DOI] [PubMed] [Google Scholar]
- 85.Vodovotz YLM, Flanders KC, Chesler L, Xie QW, Smith TW, Weidner J, Mumford R, Webber R, Nathan C, Roberts AB, Lippa CF, and Sporn MB. Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer’s disease. J Exp Med. 1996;184:1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vannucchi MG, Corsani L, Gianfriddo M, et al. Expression of neuronal and inducible nitric oxide synthase in neuronal and glial cells after transient occlusion of the middle cerebral artery. Neuroscience. 2005;136:1015–1026. [DOI] [PubMed] [Google Scholar]
- 87.Sparrow JR, Nathan C, Vodovotz Y. Cytokine regulation of nitric oxide synthase in mouse retinal pigment epithelial cells in culture. Exp Eye Res. 1994;59:129–139. [DOI] [PubMed] [Google Scholar]
- 88.Huang H, Rose JL, Hoyt DG. P38 mitogen-activated protein kinase mediates synergistic induction of inducible nitric oxide synthase by lipopolysaccharide and interferon-γ through signal transducer and activator of transcription 1 Ser727 phosphorylation in murine aortic endothelial cells. Mol Pharmacol. 2004;66:302–311. [DOI] [PubMed] [Google Scholar]
- 89.Williams G, Brown T, Becker L, et al. Cytokine-induced expression of nitric oxide synthase in C2C12 skeletal muscle myocytes. Am J Physiol-Reg I. 1994;267:R1020–R1025. [DOI] [PubMed] [Google Scholar]
- 90.Saha RN, Pahan K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Sign. 2006;8:929–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu S, Ali H, Sheng WS, et al. Gp-41-mediated astrocyte inducible nitric oxide synthase mrna expression: Involvement of interleukin-1β production by microglia. J Neurosci. 1999;19:6468–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu X, Jana M, Dasgupta S, et al. Human immunodeficiency virus type 1 (HIV-1) tat induces nitric oxide synthase in human astroglia. J Biol Chem. 2002;277:39312–39319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Majano PL, García-Monzón C, López-Cabrera M, et al. Inducible nitric oxide synthase expression in chronic viral hepatitis. Evidence for a virus-induced gene upregulation. J Clin Invest. 1998;101:1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ochoa JB, Udekwu AO, Billiar TR, et al. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg. 1991;214:621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Annane D, Sanquer S, Sébille V, et al. Compartmentalised inducible nitric oxide synthase activity in septic shock. Lancet. 2000;355:1143–1148. [DOI] [PubMed] [Google Scholar]
- 96.Kobayashi Y, Ikeda K, Shinozuka K, et al. L-nitroarginine increases blood pressure in the rat. Clin Exp Pharmacol Physiol. 1991;18:397–399. [DOI] [PubMed] [Google Scholar]
- 97.Adamson DC, McArthur JC, Dawson TM, et al. Rate and severity of HIV-associated dementia (HAD): correlations with Gp41 and iNOS. Mol Med. 1999;5:98–109. [PMC free article] [PubMed] [Google Scholar]
- 98.Persichini T, Mancino G, Cappelli G, et al. Mycobacterium tuberculosis enhances iNOS mRNA expression and HIV replication in human astrocytoma cells. Neuroreport. 1997;8:1897–1901. [DOI] [PubMed] [Google Scholar]
- 99.Cramer JP, Mockenhaupt FP, Ehrhardt S, et al. iNOS promoter variants and severe malaria in Ghanaian children. Trop Med Int Health. 2004;9:1074–1080. [DOI] [PubMed] [Google Scholar]
- 100.Noronha BT, Li J-M, Wheatcroft SB, et al. Inducible nitric oxide synthase has divergent effects on vascular and metabolic function in obesity. Diabetes. 2005;54:1082–1089. [DOI] [PubMed] [Google Scholar]
- 101.Soskić SS, Dobutović BD, Sudar EM, et al. Regulation of inducible nitric oxide synthase (iNOS) and its potential role in insulin resistance, diabetes and heart failure. Open Card Med J. 2011;5:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kröncke K-D, Brenner H-H, Rodriguez M-L, et al. Pancreatic islet cells are highly susceptible towards the cytotoxic effects of chemically generated nitric oxide. BBA - Molecular Basis Dis. 1993;1182:221–229. [DOI] [PubMed] [Google Scholar]
- 103.Kleemann R, Rothe H, Kolb-Bachofen V, et al. Transcription and translation of inducible nitric oxide synthase in the pancreas of prediabetic BB rats. FEBS Lett. 1993;328:9–12. [DOI] [PubMed] [Google Scholar]
- 104.Carvalho-Filho MA, Ueno M, Carvalheira JBC, et al. Targeted disruption of iNOS prevents LPS-induced S-nitrosation of IR beta/IRS-1 and Akt and insulin resistance in muscle of mice. Am J Physiol - Endoc M. 2006;291:E476–E482. [DOI] [PubMed] [Google Scholar]
- 105.Pilon G, Charbonneau A, White PJ, et al. Endotoxin mediated-iNOS induction causes insulin resistance via ONOO− induced tyrosine nitration of IRS-1 in skeletal muscle. PLOS ONE. 2011;5:e15912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Granados-Principal S, Liu Y, Guevara ML, et al. Inhibition of iNOS as a novel effective targeted therapy against triple-negative breast cancer. Breast Cancer Res. 2015;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lejeune P, Lagadec P, Onier N, et al. Nitric oxide involvement in tumor-induced immunosuppression. J Immunol. 1994;152:5077–5083. [PubMed] [Google Scholar]
- 108.Meller ST, Dykstra C, Grzybycki D, et al. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33:1471–1478. [DOI] [PubMed] [Google Scholar]
- 109.Levy D, Höke A, Zochodne DW. Local expression of inducible nitric oxide synthase in an animal model of neuropathic pain. Neurosci Lett. 1999;260:207–209. [DOI] [PubMed] [Google Scholar]
- 110.Yoshida M, Xia Y. Heat shock protein 90 as an endogenous protein enhancer of inducible nitric oxide synthase. J Biol Chem. 2003;278:36953–36958. [DOI] [PubMed] [Google Scholar]
- 111.Kawase M, Kinouchi H, Kato I, et al. Inducible nitric oxide synthase following hypoxia in rat cultured glial cells. Brain Res. 1996;738:319–322. [DOI] [PubMed] [Google Scholar]
- 112.Haas J, Storch-Hagenlocher B, Biessmann A, et al. Inducible nitric oxide synthase and argininosuccinate synthetase: co-induction in brain tissue of patients with Alzheimer’s dementia and following stimulation with β-amyloid 1–42 in vitro. Neurosci Lett. 2002;322:121–125. [DOI] [PubMed] [Google Scholar]
- 113.Koppula S, Kumar H, Kim IS, et al. Reactive oxygen species and inhibitors of inflammatory enzymes, NADPH oxidase, and iNOS in experimental models of Parkinson’s disease. Mediat Inflamm. 2012;2012:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilcock DM, Lewis MR, Van Nostrand WE, et al. Progression of amyloid pathology to Alzheimer’s disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci. 2008;28:1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hevel JM, Marletta MA. Nitric oxide synthase assays. Methods Enzymol. 1994;233:250–258. [DOI] [PubMed] [Google Scholar]
- 116.Naureckiene S, Edris W, Ajit SK, et al. Use of a murine cell line for identification of human nitric oxide synthase inhibitors. J Pharmacol Toxicol Methods. 2007;55:303–313. [DOI] [PubMed] [Google Scholar]
- 117.Nemzek JA, Hugunin KMS, Opp MR. Modeling sepsis in the laboratory: merging sound science with animal well-being. Comparative Med. 2008;58:120–128. [PMC free article] [PubMed] [Google Scholar]
- 118.Wang LX, Wang ZJ. Animal and cellular models of chronic pain. Adv Drug Del Rev. 2003;55:949–965. [DOI] [PubMed] [Google Scholar]
- 119.Winter C, Risley EA, and Nuss GW Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. [DOI] [PubMed] [Google Scholar]
- 120.Kim S, and Chung JM An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. PAIN. 1992;50:355–363. [DOI] [PubMed] [Google Scholar]
- 121.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. [DOI] [PubMed] [Google Scholar]
- 122.Whiteley PE, Dalrymple SA. Models of inflammation: adjuvant-induced arthritis in the rat. Current protocols in pharmacology: John Wiley & Sons, Inc; 2001. [DOI] [PubMed] [Google Scholar]
- 123.Vítacek J, Lojek, A, Valacchi, G, and Kubala, L. Arginine-based inhibitors of nitric oxide synthase: therapeutic potential and challenges. Mediat Inflamm. 2012;2012:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Olken NM, Osawa Y, Marletta MA. Characterization of the inactivation of nitric oxide synthase by NG-methyl-L-arginine: evidence for heme loss. Biochemistry. 1994;33:14784–14791. [DOI] [PubMed] [Google Scholar]
- 125.Haynes WG, Noon JP, Walker BR, et al. Inhibition of nitric oxide synthesis increases blood pressure in healthy humans. J Hypertens. 1993;11:1375–1380. [DOI] [PubMed] [Google Scholar]
- 126.Wolff DJ, Lubeskie A. Aminoguanidine is an isoform-selective, mechanism-based inactivator of nitric oxide synthase. Arch Biochem Biophys. 1995;316:290–301. [DOI] [PubMed] [Google Scholar]
- 127.Fast W, Nikolic D, Van Breemen RB, et al. Mechanistic studies of the inactivation of inducible nitric oxide synthase by N5-iminoethyl-L-ornithine. J Am Chem Soc. 1999;121:903–916. [Google Scholar]
- 128.Moore WM, Webber RK, Jerome GM, et al. L-N6-(1-Iminoethyl)lysine: A selective inhibitor of inducible nitric oxide synthase. J Med Chem. 1994;37:3886–3888. [DOI] [PubMed] [Google Scholar]
- 129.László F, Whittle BJR. Actions of isoform-selective and non-selective nitric oxide synthase inhibitors on endotoxin-induced vascular leakage in rat colon. Eur J Pharmacol. 1997;334:99–102. [DOI] [PubMed] [Google Scholar]
- 130.Tang W, Li H, Poulos TL, et al. Mechanistic studies of inactivation of inducible nitric oxide synthase by amidines. Biochemistry. 2015;54:2530–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Corbett JA, Tilton RG, Chang K, et al. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992;41:552–556. [DOI] [PubMed] [Google Scholar]
- 132.Misko TP, Moore WM, Kasten TP, et al. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993;233:119–125. [DOI] [PubMed] [Google Scholar]
- 133.Tinker A, and Wallace AV Selective inhibitors of inducible nitric oxide synthase: potential agents for the treatment of inflammatory diseases? Curr Top Med Chem. 2006;6:77–92. [DOI] [PubMed] [Google Scholar]
- 134.Wu CC, Chen SJ, Szabó C, et al. Aminoguanidine attenuates the delayed circulatory failure and improves survival in rodent models of endotoxic shock. Br J Pharmacol. 1995;114:1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cross AH, Misko TP, Lin RF, et al. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J Clin Invest. 1994;93:2684–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wolff DJ, Lubeskie A, Gauld DS, et al. Inactivation of nitric oxide synthases and cellular nitric oxide formation by N6-iminoethyl-L-lysine and N5-iminoethyl-L-ornithine. Eur J Pharmacol. 1998;350:325–334. [DOI] [PubMed] [Google Scholar]
- 137.Pelletier J-P, Jovanovic DV, Lascau-Coman V, et al. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: Possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 2000;43:1290–1299. [DOI] [PubMed] [Google Scholar]
- 138.Hallinan EA, Tsymbalov S, Dorn CR, et al. Synthesis and biological characterization of L-N6-(1-iminoethyl)lysine 5-tetrazole-amide, a produg of a selective iNOS inhibitor. J Med Chem. 2002;45:1686–1689. [DOI] [PubMed] [Google Scholar]
- 139.Hansel TT, Kharitonov SA, Donnelly LE, et al. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 2003. [DOI] [PubMed] [Google Scholar]
- 140.De Alba J, Clayton NM, Collins SD, et al. GW274150, a novel and highly selective inhibitor of the inducible isoform of nitric oxide synthase (iNOS), shows analgesic effects in rat models of inflammatory and neuropathic pain. Pain. 2006;120:170–181. [DOI] [PubMed] [Google Scholar]
- 141.Dugo L, Marzocco S, Mazzon E, et al. Effects of GW274150, a novel and selective inhibitor of iNOS activity, in acute lung inflammation. Br J Pharmacol. 2004;141:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Seymour M, Petavy F, Chiesa F, et al. Ultrasonographic measures of synovitis in an early phase clinical trial: a double-blind, randomized, placebo and comparator controlled phase IIa trial of GV274150 (a selective inducible nitric oxide synthase inhibitor) in rheumatoid arthritis. Clin Exp Rheumatol 2012;30:254–261. [PubMed] [Google Scholar]
- 143.Høivik HO, Laurijssens BE, Harnisch LO, et al. Lack of efficacy of the selective iNOS inhibitor GW274150 in prophylaxis of migraine headache. Cephalalgia. 2010;30:1458–1467. [DOI] [PubMed] [Google Scholar]
- 144.Dunstan RR Khan RW NK Skin wound healing in the SKH-1 female mouse following inducible nitric oxide synthase inhibition. Br J Dermatol 2007;157:656–61 [DOI] [PubMed] [Google Scholar]
- 145.Connor J, Rogers K, Sunyer T, et al. Efficacy of the selective inducible nitric oxide synthase inhibitor SD-6010 in nonclinical inflammatory, neuropathic, and osteoarthritis pain. Osteoarthr Cartilage. 2012;20:S54–S296. [Google Scholar]
- 146.Hellio le Graverand M-P, Clemmer RS, Redifer P, et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann Rheum Dis. 2013;72:187–195. [DOI] [PubMed] [Google Scholar]
- 147.Southan GJ, Szabó C, Thiemermann C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br J Pharmacol. 1995;114:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chayah M, Camacho ME, Carrión MD, et al. N,N-disubstituted thiourea and urea derivatives: design, synthesis, docking studies, and biological evaluation against nitric oxide synthase. MedChemComm. 2016;7:667–678. [Google Scholar]
- 149.Chayah M, Carrión MD, Gallo MA, et al. Development of urea and thiourea kynurenamine derivatives: Synthesis, molecular modeling, and biological evaluation as nitric oxide synthase inhibitors. ChemMedChem. 2015;10:874–882. [DOI] [PubMed] [Google Scholar]
- 150.Garvey EP, Oplinger JA, Tanoury GJ, et al. Potent and selective inhibition of human nitric oxide synthases. Inhibition by non-amino acid isothioureas. J Biol Chem. 1994;269:26669–26676. [PubMed] [Google Scholar]
- 151.Garvey EP, Oplinger JA, Furfine ES, et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–4963. [DOI] [PubMed] [Google Scholar]
- 152.Zhu Y, Nikolic D, Van Breemen RB, et al. Mechanism of inactivation of inducible nitric oxide synthase by amidines. Irreversible enzyme inactivation without inactivator modification. J Am Chem Soc. 2005;127:858–868. [DOI] [PubMed] [Google Scholar]
- 153.Staunton C, Djouhri L, Barrett-Jolley R, and Thippeswami T 1400W alleviates pain and increases the expression levels of anti-nociceptive and neuromodulatory mediators. P Physiol Soc. 2014:31: p126P, PCA151, poster communications. [Google Scholar]
- 154.Maccallini C, Patruno A, Bešker N, et al. Synthesis, biological evaluation, and docking studies of N-substituted acetamidines as selective inhibitors of inducible nitric oxide synthase. J Med Chem. 2009;52:1481–1485. [DOI] [PubMed] [Google Scholar]
- 155.Moore WM, Webber RK, Fok KF, et al. 2-iminopiperidine and other 2-iminoazaheterocycles as potent inhibitors of human nitric oxide synthase isoforms. J Med Chem. 1996;39:669–672. [DOI] [PubMed] [Google Scholar]
- 156.Naka M, Nanbu T, Kobayashi K, et al. A potent inhibitor of inducible nitric oxide synthase, ONO-1714, a cyclic amidine derivative. Biochem Biophys Res Commun. 2000;270:663–667. [DOI] [PubMed] [Google Scholar]
- 157.Segikuchi F, Mita Y, Kamanaka Y, Kawao N, Matsuya H, Taga C, and Kawabata A The potent inducible nitric oxide synthase inhibitor ONO-1714 inhibits neuronal NOS and exerts antinociception in rats. Neurosci. Lett. 365 (2), 111–115. [DOI] [PubMed] [Google Scholar]
- 158.Mikawa K, Nishina K, Takao Y, et al. ONO-1714, a nitric oxide synthase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesth Analg. 2003;97:1751–1755. [DOI] [PubMed] [Google Scholar]
- 159.Mitaka C, Hirata Y, Yokoyama K, et al. A selective inhibitor for inducible nitric oxide synthase improves hypotension and lactic acidosis in canine endotoxic shock. Crit Care Med. 2001;29:2156–2161. [DOI] [PubMed] [Google Scholar]
- 160.Hagen TJ, Bergmanis AA, Kramer SW, et al. 2-Iminopyrrolidines as potent and selective inhibitors of human inducible nitric oxide synthase. J Med Chem. 1998;41:3675–3683. [DOI] [PubMed] [Google Scholar]
- 161.Tsymbalov S, Hagen TJ, Moore WM, et al. 3-Hydroxy-4-methyl-5-pentyl-2-iminopyrrolidine: A potent and highly selective inducible nitric oxide synthase inhibitor. Bioorg Med Chem Lett. 2002;12:3337–3339. [DOI] [PubMed] [Google Scholar]
- 162.Beaton H, Hamley P, Nicholls DJ, et al. 3,4-Dihydro-1-isoquinolinamines: a novel class of nitric oxide synthase inhibitors with a range of isoform selectivity and potency. Bioorg Med Chem Lett. 2001;11:1023–1026. [DOI] [PubMed] [Google Scholar]
- 163.Tinker AC, Beaton HG, Boughton-Smith N, et al. 1,2-Dihydro-4-quinazolinamines: potent, highly selective inhibitors of inducible nitric oxide synthase which show antiinflammatory activity in vivo. J Med Chem. 2003;46:913–916. [DOI] [PubMed] [Google Scholar]
- 164.LaBuda CJ, Koblish M, Tuthill P, et al. Antinociceptive activity of the selective iNOS inhibitor AR-C102222 in rodent models of inflammatory, neuropathic and post-operative pain. Eur J Pain. 2006;10:505–505. [DOI] [PubMed] [Google Scholar]
- 165.Crane BR, Arvai AS, Gachhui R, et al. The structure of nitric oxide synthase oxygenase domain and inhibitor complexes. Science. 1997;278:425–431. [DOI] [PubMed] [Google Scholar]
- 166.Garcin ED, Arvai AS, Rosenfeld RJ, et al. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat Chem Biol. 2008;4:700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hagmann WK, Caldwell CG, Chen P, et al. Substituted 2-aminopyridines as inhibitors of nitric oxide synthases. Bioorg Med Chem Lett. 2000;10:1975–1978. [DOI] [PubMed] [Google Scholar]
- 168.Pettipher E, Hibbs T, Smith M, et al. Analgesic activity of 2-amino-4-methylpyridine, a novel NO synthase inhibitor. Inflammation Res. 1997;46:135–136. [DOI] [PubMed] [Google Scholar]
- 169.Connolly S, Aberg A, Arvai A, et al. 2-aminopyridines as highly selective inducible nitric oxide synthase inhibitors. Differential binding modes dependent on nitrogen substitution. J Med Chem. 2004;47:3320–3323. [DOI] [PubMed] [Google Scholar]
- 170.Strub A, Ulrich W-R, Hesslinger C, et al. The novel 2-[2-(4-methoxy-pyridin-2-yl)-ethyl]-3H-imidazo[4,5-b]pyridine (BYK191023) is a highly selective inhibitor of the inducible nitric oxide synthase. Mol Pharmacol. 2006;69:328–337. [DOI] [PubMed] [Google Scholar]
- 171.Tiso M, Strub A, Hesslinger C, et al. BYK191023 (2-[2-(4-methoxy-pyridin-2-yl)-ethyl]- 3H-imidazo[4,5-b]pyridine is an NADPH- and time-dependent irreversible inhibitor of inducible nitric oxide synthase. Mol Pharmacol. 2008;73:1244–1253. [DOI] [PubMed] [Google Scholar]
- 172.Lehner MD, Marx D, Boer R, et al. In vivo characterization of the novel imidazopyridine BYK191023 (2-[2-(4-methoxy-pyridin-2-yl)-ethyl]-3H-imidazo[4,5-b]pyridine), a potent and highly selective inhibitor of inducible nitric oxide synthase. J Pharmacol Exp Ther. 2006;317:181–187. [DOI] [PubMed] [Google Scholar]
- 173.Su F, Huang H, Akieda K, et al. Effects of a selective iNOS inhibitor versus norepinephrine in the treatment of septic shock. Shock. 2010;34:243–249. [DOI] [PubMed] [Google Scholar]
- 174.Sennequier N, Wolan D, Stuehr DJ. Antifungal imidazoles block assembly of inducible NO synthase into an active dimer. J Biol Chem. 1999;274:930–938. [DOI] [PubMed] [Google Scholar]
- 175.McMillan K, Adler M, Auld DS, et al. Allosteric inhibitors of inducible nitric oxide synthase dimerization discovered via combinatorial chemistry. Proc Natl Acad Sci USA. 2000;97:1506–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Blasko E, Glaser CB, Devlin JJ, et al. Mechanistic studies with potent and selective inducible nitric oxide synthase dimerization inhibitors. J Biol Chem. 2002;277:295–302. [DOI] [PubMed] [Google Scholar]
- 177.Nagpal L, Haque MM, Saha A, et al. Mechanism of inducible nitric oxide synthase dimerization inhibition by novel pyrimidine imidazoles. J Biol Chem. 2013;288:19685–19697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Davey DD, Adler M, Arnaiz D, et al. Design, synthesis, and activity of 2-imidazol-1-ylpyrimidine derived inducible nitric oxide synthase dimerization inhibitors. J Med Chem. 2007;50:1146–1157. [DOI] [PubMed] [Google Scholar]
- 179.Bonnefous C, Payne JE, Roppe J, et al. Discovery of inducible nitric oxide synthase (iNOS) inhibitor development candidate KD7332, part 1: identification of a novel, potent, and selective series of quinolinone iNOS dimerization inhibitors that are orally active in rodent pain models. J Med Chem. 2009;52:3047–3062. [DOI] [PubMed] [Google Scholar]
- 180.Symons KT, Massari ME, Nguyen PM, et al. KLYO956 is a non-imidazole-based orally active inhibitor of nitric oxide synthase dimerization. Mol Pharmacol. 2009;76:153–162. [DOI] [PubMed] [Google Scholar]
- 181.Payne JE, Bonnefous C, Symons KT, et al. Discovery of dual inducible/neuronal nitric oxide synthase (iNOS/nNOS) inhibitor development candidate 4-((2-cyclobutyl-1h-imidazo[4,5-b]pyrazin-1-yl)methyl)-7,8-difluoroquinolin-2(1h)-one (kd7332) part 2: Identification of a novel, potent, and selective series of benzimidazole-quinolinone iNOS/nNOS dimerization inhibitors that are orally active in pain models. J Med Chem. 2010;53:7739–7755. [DOI] [PubMed] [Google Scholar]
- 182.Kalypsys I iNOS inhibitor, topical pain product, KD7040, http://www.kalypsys.com/pipeline/clinical/kd7040.pdf. accessed April 12th, 2017, also available at: https://web.archive.org/web/20070327050804/http://www.kalypsys.com/pipeline/clinical/kd7040.pdf).
- 183.Cowans B, Mougin-Andres P, Wheeler P, et al. Salts of inducible nitric oxide synthase dimerization inhibitors. Pat Appl US20070197609. August, 2007. [Google Scholar]
- 184.Lopez A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: Effect on survival in patients with septic shock. Crit Care Med 2004;32:21–30. [DOI] [PubMed] [Google Scholar]
- 185.Yamashita T, Kawashima S, Ohashi Y, et al. Resistance to endotoxin shock in transgenic mice overexpressing endothelial nitric oxide synthase. Circulation. 2000;101:931–937. [DOI] [PubMed] [Google Scholar]
- 186.Stahl W, Matejovic M, Radermacher P. Inhibition of nitric oxide synthase during sepsis: revival because of isoform selectivity? Shock. 2010;34:321–322. [DOI] [PubMed] [Google Scholar]
- 187.Cauwels A, Van Molle W, Janssen B, et al. Protection against TNF-induced lethal shock by soluble guanylate cyclase inhibition requires functional inducible nitric oxide synthase. Immunity. 2000;13:223–231. [DOI] [PubMed] [Google Scholar]
- 188.Bredan AS, Cauwels A. Is there no treatment for severe sepsis? Libyan J Med. 2008;3:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. [DOI] [PubMed] [Google Scholar]
- 190.Mungrue IN, Gros R, You X, et al. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Inv. 2002;109:735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alexander J, Reynolds HR, Stebbins AL, et al. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: The TRIUMPH randomized controlled trial. JAMA. 2007;297:1657–1666. [DOI] [PubMed] [Google Scholar]
- 192.Bailey A, Pope TW, Moore SA, et al. The tragedy of TRIUMPH for nitric oxide synthesis inhibition in cardiogenic shock. Am J Cardiovasc Drugs. 2007;7:337–345. [DOI] [PubMed] [Google Scholar]
- 193.Howes LG, Brillante DG. Expert opinion on tilarginine in the treatment of shock. Expert Opin Inv Drugs. 2008;17:1573–1580. [DOI] [PubMed] [Google Scholar]
- 194.Dokken BB, Gaballa MA, Hilwig RW, et al. Inhibition of nitric oxide synthases, but not inducible nitric oxide synthase, selectively worsens left ventricular function after successful resuscitation from cardiac arrest in swine. Acad Emerg Med. 2015;22:197–203. [DOI] [PubMed] [Google Scholar]
- 195.Mukherjee P, Cinelli MA, Kang S, et al. Development of nitric oxide synthase inhibitors for neurodegeneration and neuropathic pain. Chem Soc Rev. 2014;43:6814–6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li M, Dai F-r, Du X-p, et al. Neuroprotection by silencing iNOS expression in a 6-OHDA model of Parkinson’s disease. J Mol Neurosci. 2012;48:225–233. [DOI] [PubMed] [Google Scholar]
- 197.Tsuji M, Higuchi Y, Shiraishi K, et al. Protective effect of aminoguanidine on hypoxic-ischemic brain damage and temporal profile of brain nitric oxide in neonatal rat. Pediatr Res. 2000;47:79–79. [DOI] [PubMed] [Google Scholar]
- 198.Cockroft KM, Meistrell M, Zimmerman GA, et al. Cerebroprotective effects of aminoguanidine in a rodent model of stroke. Stroke. 1996;27:1393–1398. [DOI] [PubMed] [Google Scholar]
- 199.Broom L, Marinova-Mutafchieva L, Sadeghian M, et al. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radical Biol Med. 2011;50:633–640. [DOI] [PubMed] [Google Scholar]
- 200.Zhong H-J, Liu L-J, Chong C-M, et al. Discovery of a natural product-like iNOS inhibitor by molecular docking with potential neuroprotective effects in vivo. PLOS ONE. 2014;9:e92905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Üzüm G, Akgün-Dar K, Kandil A, et al. Similarity of inflammatory response in epileptic seizures and sepsis: Does the sensitivity to sepsis in epileptic patients increase? Crit Care. 2014;18:P56–P56. [Google Scholar]
- 202.Rehni AK, Singh TG, Kalra R, et al. Pharmacological inhibition of inducible nitric oxide synthase attenuates the development of seizures in mice. Nitric Oxide. 2009;21:120–125. [DOI] [PubMed] [Google Scholar]
- 203.Puttachary S, Sharma S, Verma S, et al. 1400W, a highly selective inducible nitric oxide synthase inhibitor is a potential disease modifier in the rat kainate model of temporal lobe epilepsy. Neurobiol Dis. 2016;93:184–200. [DOI] [PubMed] [Google Scholar]
- 204.Montezuma K, Biojone C, Lisboa SF, et al. Inhibition of iNOS induces antidepressant-like effects in mice: pharmacological and genetic evidence. Neuropharmacology. 2012;62:485–491. [DOI] [PubMed] [Google Scholar]
- 205.Peng Y-L, Liu Y-N, Liu L, et al. Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. J Neuroinflamm. 2012;9:75–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Janakiram NB, Rao CV. iNOS-selective inhibitors for cancer prevention: promise and progress. Future Med Chem. 2012;4:2193–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rao CV, Indranie C, Simi B, et al. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–170. [PubMed] [Google Scholar]
- 208.Sikora AG, Gelbard A, Davies MA, et al. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin Cancer Res. 2010;16:1834–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sharma S, Wilkinson BP, Gao P, et al. Differential activity of NO synthase inhibitors as chemopreventive agents in a primary rat tracheal epithelial cell transformation system. Neoplasia (New York, NY). 2002;4:332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138–1143. [DOI] [PubMed] [Google Scholar]
- 211.Fujimoto M, Shimizu N, Kunii K, et al. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes. 2005;54:1340–1348. [DOI] [PubMed] [Google Scholar]
- 212.Torrisi JS, Hespe GE, Cuzzone DA, et al. Inhibition of inflammation and iNOS improves lymphatic function in obesity. Sci Rep. 2016;6:19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Seimetz M, Parajuli N, Pichl A, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305. [DOI] [PubMed] [Google Scholar]
- 214.Nathan C Is iNOS beginning to smoke? Cell 147:257–258. [DOI] [PubMed] [Google Scholar]


