Abstract
Children with familial risk for major depressive disorder (MDD) have elevated risk for developing depression as adolescents. Here, we investigated longitudinally whether resting-state functional connectivity (RSFC) could predict the onset of MDD. In this pilot study, we followed a group of never-depressed children with familial risk for MDD and a group of age-matched controls without familial risk who had undergone an MRI study at 8–14 years of age. Participants were reassessed 3–4 years later with diagnostic interviews. We first investigated group differences in RSFC from regions in the emotion regulation, cognitive control, and default mode networks in the children who later developed MDD (converted), the children who did not develop MDD (nonconverted), and the control group. We then built a prediction model based on baseline RSFC that was independent of the group differences to classify the individuals who later developed MDD. Compared with the nonconverted group, the converted group exhibited hypoconnectivity between subgenual anterior cingulate cortex (sgACC) and inferior parietal lobule (IPL) and between left and right dorsolateral prefrontal cortices. The nonconverted group exhibited higher sgACC-IPL connectivity than did both the converted and control groups, suggesting a possible resilience factor to MDD. Classification between converted and nonconverted individuals based on baseline RSFC yielded high predictive accuracy with high sensitivity and specificity that was superior to classification based on baseline clinical rating scales. Intrinsic brain connectivity measured in healthy children with familial risk for depression has the potential to predict MDD onset, and it can be a useful neuromarker in early identification of children for preventive treatment.
Keywords: children, depression risk, machine learning, resilience, resting-state fMRI, subgenual ACC
Introduction
Major depressive disorder (MDD) is one of the most common psychiatric disorders, with an estimated lifetime prevalence of 16% (Kessler et al., 2003). It is associated with high comorbidity, social and occupational dysfunction (Godard et al., 2012), and high financial burden (Greenberg et al., 2015). A peak period of onset is adolescence and early adulthood (Merikangas and Avenevoli, 2002). An estimated 11% of adolescents are affected with MDD, which leads to significant distress, dysfunction, and mortality in these youth (Avenevoli et al., 2015). MDD is characterized by a negative feedback cycle in which individuals experience negative affect, perceive events negatively, and withdraw from social and pleasurable activities, leading to further exacerbation of negative mood, biased cognitions, and social withdrawal (Beck and Haigh, 2014).
People with MDD exhibit functional brain differences, as measured by neuroimaging, in default mode network (DMN), cognitive control, and emotion regulation networks. The DMN, anchored by the midline regions of medial prefrontal cortex (MPFC) and posterior cingulate cortex (PCC), is a set of brain regions that has been linked to self-referential processing and rumination in depression (Hamilton et al., 2015). The emotion regulation network, which involves the amygdala, the anterior cingulate cortex (ACC), and the MPFC, has shown functional brain differences in MDD (Hamilton et al., 2015; Sheline et al., 2009; Stuhrmann et al., 2011; Vilgis et al., 2018). The cognitive control network, which includes dorsolateral prefrontal cortex (DLPFC) and parietal regions, has shown abnormal activation and connectivity patterns in MDD (Fales et al., 2008; Ye et al., 2012). Within the emotion regulation network, subgenual ACC (sgACC) has been considered a core region in the pathophysiology of MDD (Drevets et al., 2008; Mayberg, 1997; Ongür et al., 1998; Siegle et al., 2012) and has been a successful target for deep brain stimulation treatment for MDD (Mayberg et al., 2005).
To identify possible neural risk factors for depression, several studies have examined brain function in children who are not themselves depressed but who have a parent with a history of MDD, which increases the risk of MDD in the offspring threefold to fivefold (Lieb et al., 2002; Williamson et al., 2004). Functional and anatomical brain differences between never-depressed children with familial risk for depression and children without familial risk have been reported in emotion processing regions (Chai et al., 2015; Foland-Ross et al., 2015; Monk et al., 2008), suggesting that these neural traits predispose children toward depression. Resting-state fMRI (rs-fMRI) has revealed altered patterns of functional connectivity in both the DMN and in emotion regulation and cognitive control networks in never-depressed at-risk children. At-risk children have exhibited hyperconnectivity between sgACC and DMN, and hypoconnectivity between sgACC and DLPFC and within the control network regions (Chai et al., 2016). Similar differences have been observed in adult (Greicius et al., 2003; Liston et al., 2014; Ye et al., 2012) and pediatric (Cullen et al., 2009; Gaffrey et al., 2010) patients with MDD. Finding these functional network differences among at-risk offspring who are not affected with MDD, before the period of risk for MDD onset, raises the hypothesis that the differences represent vulnerability factors for depression.
To examine this question, we conducted a follow-up study of the children imaged in our previous study (Chai et al., 2016, 2015). The children were 8–14 years old at baseline and 12–18 years old at follow-up. This age range at the follow-up is a heightened time for onset of MDD in at-risk youth, as rates of MDD from early to late adolescence increase as much as sixfold (Hankin et al., 1998). We investigated whether baseline resting-state connectivity in regions that had been previously associated with depression would predict the onset of MDD in these high-risk adolescents. We examined baseline resting-state functional connectivity (RSFC) data from seed regions in sgACC, DMN, amygdala, and DLPFC in children at familial risk for depression, and we tested whether they would predict which individuals would later develop MDD.
We performed two types of analyses. First, we examined group-level intrinsic connectivity differences. To understand risk factors for depression, we compared seed-to-voxel connectivity between the subset of children who later developed MDD (the converted group) and the subset of children who did not develop depression (the nonconverted group). To understand resilience to depression, we compared the nonconverted group with the control group in terms of sgACC connectivity, given the anatomical position of the sgACC that connects to the limbic and cortical structures (Johansen-Berg et al., 2008), and the link between sgACC connectivity and depression symptom in at-risk children (Chai et al., 2016). Second, we computed connectivity between the seed regions and regions from two different whole-brain atlases, defined independently from the regions that exhibited group differences, and trained machine learning models to classify which individuals would later develop depression. We hypothesized that (1) there would be differences in resting-state connectivity from these seed regions between the converted and nonconverted groups, and (2) models based on resting-state connectivity of these seed regions to other brain regions defined in the atlases could predict the onset of depression with higher accuracy than a model based on baseline clinical scores (the Child Behavior Checklist, CBCL) alone, even though depressive symptoms carry some prediction power for the onset of depression (Horwath et al., 1992).
Materials and Methods
Procedures
Children at risk for MDD based on lifetime history of parental depression, who had not themselves had mood episodes, had undergone rs-fMRI scans at baseline. At a 3- to 4-year follow-up, participants and their parents were invited back for diagnostic assessments to determine whether they had developed an episode of MDD at any point during the interval since the scan. The study was approved by the Institutional Review Boards at the Massachusetts General Hospital and at the Massachusetts Institute of Technology. Parents provided written informed consent for their and their child's participation, and youths provided written assent. Adolescents aged 18 years provided written consent for their own participation.
Participants
Baseline
Thirty-eight high-risk participants between 8 and 14 years old who were offspring of parents with a lifetime history of MDD (at-risk group) and 30 age-matched controls with no familial history of MDD were recruited in the baseline study (Chai et al., 2016, 2015). Exclusion criteria at baseline had included the presence of acute psychosis or suicidality in a parent or a child; the presence of bipolar disorder at any point in the lifespan in the parent; autism or intellectual disability in the child; a lifetime history of a traumatic brain injury or neurological disorder in the child; or any variables that would interfere with fMRI (e.g., braces, claustrophobia). At baseline, children had no current or prior history of major depressive disorder. The final sample included 46 participants (30 at-risk children and 16 control children). Six participants from the at-risk group and 12 participants from the control group were excluded from analysis due to excessive head movement during the functional scan (greater than 3 mm displacement in x, y, or z direction, or had more than 1/3 of the time points identified as outliers. Two participants from the at-risk group and two participants from the control group did not complete the resting-state scan.
Follow-up
We re-assessed 33 (25 at-risk and 8 controls) (72%) of the 46 participants who had useable baseline rs-fMRI data at a 3- to 4-year follow-up. Participants who had an incomplete resting scan or had too much head movement during the scan were not included in the present analysis. At the follow-up assessment, participants were between 12 and 18 years old (mean age = 15.3 years, SD = 1.7 years). Follow-up assessments were on average 205 weeks after the initial study (range 166–258 weeks, SD = 19.8 weeks). An overlapping set of participants was included in a separate report (Shapero et al., 2019), which examined whether structural and functional brain measures that differentiated children with familial risk for MDD from children without familial risk were related to symptom severity and MDD onset at follow-up.
Diagnostic assessment
Baseline
Each child and both parents in each family were assessed for current and lifetime mood disorders (MDD, bipolar disorder, and dysthymia), using structured clinical interviews in which the mother was the informant (Chai et al., 2016). Interviews about parents used the depression, mania, dysthymia, and psychosis modules from the Structured Interview for DSM-IV (First et al., 1995) and those about the child used the depression, mania, dysthymia, and psychosis modules from the Schedule of Affective Disorders and Schizophrenia for School-Aged Children–Epidemiological Version for DSM-IV (K-SADS-E) (Orvaschel, 1994). Parents completed the CBCL (Achenbach and Rescorla, 2001), which assessed symptom scores in the children. The CBCL includes a total problems score, as well as scores reflecting internalizing (affective and anxiety) and externalizing (attentional problems and disruptive behavior) symptoms. We also administered self-report depression symptom scores, the Child Depression Inventory (CDI) (Kovacs, 1985), to all children. More details on CBCL and CDI are included in Supplementary Data. We conducted two-sample t-tests in CBCL total problem scores, CBCL internalizing, externalizing, and anxiety scores, and CDI scores between the converted and nonconverted group, and between the control group and nonconverted group.
Follow-up
At follow-up, the child and one parent completed the K-SADS-E to report on the child's current history of psychopathology since the baseline assessment, to determine MDD conversion. The same interviewer administered the K-SADS-E first to the parent and then to the youth, and then created summary ratings. Currently, no consensus exists about how best to integrate discrepant information from multiple informants, despite the fact that parents and children often disagree in their reports of the child's symptoms (Braaten et al., 2001; Cantwell et al., 1997; Martel et al., 2017) To maintain fidelity to the K-SADS-E, we used the interviewer's summary ratings based on his/her “best-estimate” clinical judgment from interviewing both parent and child. The K-SADS-E diagnostic interviews have good inter-rater and retest reliability (Orvaschel et al., 1995). An advanced postdoctoral psychology fellow (B.G.S.) conducted all prospective interviews. He had extensive experience interviewing adolescents and parents with semi-structured diagnostic interviews, and was trained on reliability on the K-SADS-E with a perfect diagnostic reliability (K = 1.00, p < 0.001) and high item-level correlation (ICC = 0.88, p < 0.001). In addition, all diagnostic decisions were reviewed with a senior child and adolescent psychologist (D.R.H.B.) who has extensive expertise reliably administering, training, and supervising interviewers on the structured clinical interview for DSM-IV and K-SADS-E.
MRI procedure and image preprocessing
Data were acquired on a 3T TrioTim Siemens scanner by using a 32-channel head coil. T1-weighted whole-brain anatomical images and resting fMRI images were acquired. Rs-fMRI data were preprocessed by using standard procedure. The analysis was performed at 2 × 2 × 2 mm resolution. Functional connectivity analysis was carried out by using a seed-driven approach with in-house, custom software “CONN” (Chai et al., 2012; Whitfield-Gabrieli and Nieto-Castanon, 2012). We addressed head motion related artifacts by using a censoring method, which mitigates both motion artifact and distance-dependence artifacts in connectivity (Ciric et al., 2017). Head movement related noise was modeled by including additional regressors for outliers in the model. The converted, nonconverted, and control groups did not differ significantly in head movement parameters and the number of outlier scans in the resting scan. Details on the scanning procedure, image preprocessing, and noise estimation are described in Supplementary Data.
Resting-state connectivity analysis
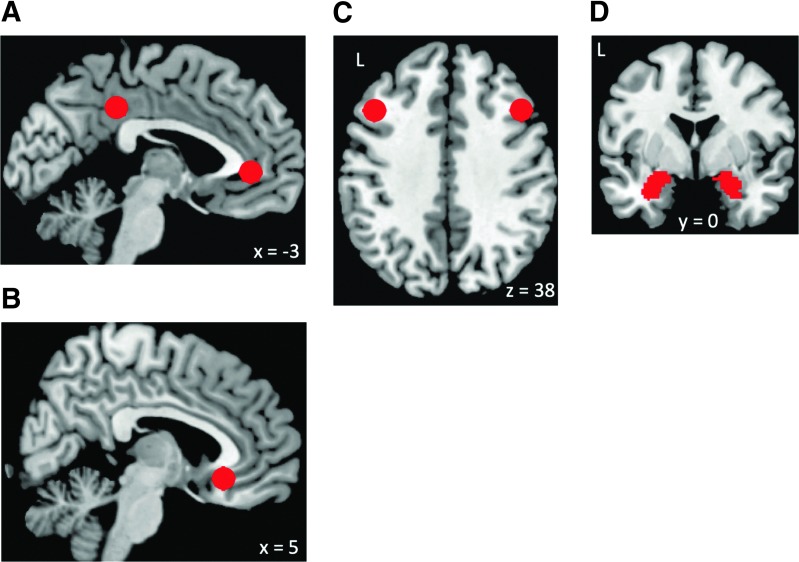
We computed whole-brain seed-to-voxel resting-state connectivity from six regions of interests (seeds) that are core regions in the DMN, emotion regulation, and cognitive control networks (Drevets et al., 2008; Hamilton et al., 2015; Stuhrmann et al., 2011): DMN, sgACC, left and right DLPFC, and left and right amygdala (Fig. 1). The DMN (MPFC and PCC), left and right DLPFC, and sgACC seeds were defined as 6-mm spheres around peak coordinates from literature (Fair et al., 2009; Kelly et al., 2009). DMN connectivity was calculated from the averages of the time series from MPFC and PCC seeds (Fox et al., 2005; Whitfield-Gabrieli et al., 2009), given their similar connectivity patterns. The amygdala seeds were defined from the WFU Pick Atlas (Maldjian et al., 2003). Time series of all the voxels within each seed were averaged, and first-level correlation maps were produced by extracting the residual BOLD time course from each seed and computing Pearson's correlation coefficients between that time course and the time course of all other voxels. Correlation coefficients were converted to normally distributed z-scores by using the Fisher transformation to allow for second-level General Linear Model analyses. To determine connectivity differences between the converted group and nonconverted group, first-level connectivity maps for each participant in the converted group and nonconverted group were entered into a between-group t-test for each seed. Two layers of multiple comparison correction were used, one for correcting the number of clusters and one for correcting the number of seeds used. We first used a cluster-forming threshold for voxel-level statistics (p < 0.001), and we corrected for multiple comparison at the cluster level by using false discovery rate correction (Genovese et al., 2002), as implemented in SPM. The cluster-level corrected p-values were further corrected for the number of seeds used (six), using Bonferroni correction. Regions that showed significant connectivity differences (survived Bonferroni correction for the number of seeds tested) between groups were further examined for their connectivity values (significantly above or below zero) by using one-sample t-tests in each group.
FIG. 1.
Seeds (regions of interest) used in the study. (A) DMN (posterior cingulate cortex and medial prefrontal cortex), (B) sgACC, (C) left and right DLPFC, (D) left and right amygdala. Images are presented in neurological convention in all figures (left side of the brain is on the left side of the image). DLPFC, dorsolateral prefrontal cortex; DMN, default mode network; L, left hemisphere; R, right hemisphere; sgACC, subgenual anterior cingulate cortex.
To investigate resilience to depression, we performed an additional analysis to compare the nonconverted and control groups on sgACC connectivity, given the pivotal role of sgACC in emotion regulation and treatment in depressed patients (Drevets et al., 1997; Mayberg et al., 2005; Siegle et al., 2012). First-level sgACC connectivity maps were entered into a between-group t-test to determine sgACC connectivity differences between the control and nonconverted groups. The same procedure for multiple comparison correction was applied. Due to the low sample size (n = 8) of the control group who were later assessed at follow-up, we conducted this test twice, once with the entire control group from Chai et al. (2016) (n = 16) and once with the subset of controls who were assessed at the follow-up visit (0 conversion to depression). We performed an additional exploratory analysis comparing RSFC of the amygdala and DLPFC seeds between the nonconverted and control groups (Supplementary Data). To account for the effect of sex, we performed additional group analyses with sex included as a covariate. We also examined age-related changes in connectivity for the seeds that showed significant group differences, given the range of the participants' age (8–14 at the time of the scan) (Supplementary Data).
Prediction models for onset of depression
We trained three linear classification models with support vector machine, implemented in machine-learning software Weka (Hall et al., 2009), to categorize individual participants into those who later developed depression and those who did not, based on their rs-fMRI data (first two models) or clinical scores (third model) at the baseline visit. To create robust prediction models that can be generalized to new cases, we performed leave-one-out cross-validation so that each individual was classified on the basis of data from the other individuals. Specifically, data from all participants except one were used as the training set to build a classification model, and the remaining participant was classified with the model and used as the validation case. This procedure was iterated for each participant and used to estimate specificity/sensitivity from the out-of-sample predictions. In the first model, connectivity values between each of the six seed regions and 116 anatomically defined areas from the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) were estimated and used in the prediction model. The matrix of connectivity values for all participants were used in feature selection. Feature selection was carried out before training the classification model. We used information gain (IG) (Mitchell, 1997; Yang and Pedersen, 1997), a filter approach for feature selection. IG ranks the features by the IG (entropy) for each feature that measures how important and relevant it is to the group label. Based on Austin and Steyerberg (2015), we used 13 features in the model, which is roughly half of the number of participants (n = 25). The top 13 features that carried the most information (calculated by the feature's contribution on decreasing overall entropy) based on the IG method were then used in the classification procedure to classify between converted and nonconverted at-risk participants. In the second model, connectivity values between the six seed regions and all areas in the Human Connectome Project (HCP) atlas, an atlas based on multimodal MRI data (Glasser et al., 2016), were estimated and used in the prediction model. The same feature selection procedure was applied in this model. We constructed a third model based on baseline CBCL scores (Total, Internalizing, Externalizing, Anxiety/Depressed symptoms, and Affective Problem Scale) to examine how well these baseline scores predict the onset of MDD.
Results
Depression conversion
Ten out of the 25 at-risk children developed MDD by the time of follow-up. None of the control participants assessed at follow-up developed MDD. The converted and nonconverted group did not differ significantly in age [t(23) = 0.96, p = 0.34], IQ [t(23) = 0.73, p = 0.47], or baseline clinical questionnaire measures [CBCL total: t(23) = 1.03, p = 0.31; CBCL internalizing: t(23) = 0.42, p = 0.68; CBCL externalizing: t(23) = 1.9, p = 0.07; CDI: t(21) = 0.86, p = 0.40] (Table 1). Gender distribution was only marginally different in the converted group compared with the converted group (Fisher's exact test, p = 0.05, Table 1). The nonconverted group and the control group also did not differ significantly in age [t(29) = 0.57, p = 0.57], IQ [t(29) = 0.73, p = 0.47], or baseline clinical questionnaire measures [CBCL total: t(24) = 1.3, p = 0.20; CBCL internalizing: t(24) = 1.4, p = 0.18; CBCL externalizing: t(24) = 0.06, p = 0.95; CDI: t(21) = 0.03, p = 0.97] (Table 1). The converted group had elevated baseline CBCL total scores [t(19) = 2.4, p = 0.03] compared with the control group, but it did not differ significantly from the control group in age [t(24) = 0.42, p = 0.68], IQ [t(24) = 0.098, p = 0.92], and other baseline clinical measures [CBCL internalizing: t(19) = 1.99, p = 0.06; CBCL externalizing: t(19) = 1.79, p = 0.09; CDI: t(16) = 0.80, p = 0.44] (Table 1).
Table 1.
Participant Demographic and Clinical Scores at Baseline
| Group mean (SD) | Between-group test | |||||
|---|---|---|---|---|---|---|
| Converted | Nonconverted | Controls | pa | pb | pc | |
| Age | 11.5 ± 1.41 | 10.9 ± 1.51 | 11.3 ± 1.84 | 0.34 | 0.57 | 0.72 |
| Gender | 7 F, 3 M | 4 F, 11 M | 8 F, 8 M | 0.05 | 0.27 | 0.42 |
| IQ (KBIT) | 115.3 ± 13.2 | 119.3 ± 13.5 | 115.8 ± 12.8 | 0.47 | 0.47 | 0.92 |
| CBCL total | 52.5 ± 9.8 | 47.5 ± 12.8 | 41.0 ± 11.8 | 0.31 | 0.20 | 0.03 |
| CBCL internalizing | 52.7 ± 10.9 | 50.6 ± 13.2 | 44.3 ± 8.50 | 0.68 | 0.18 | 0.06 |
| CBCL externalizing | 52.4 ± 7.8 | 45.3 ± 9.8 | 45.1 ± 10.5 | 0.07 | 0.95 | 0.09 |
| CDI | 46.6 ± 5.9 | 44.0 ± 7.6 | 43.0 ± 8.1 | 0.40 | 0.97 | 0.44 |
Mean ± SD where appropriate. All group tests were t-tests except for Gender (Fisher's exact test).
p-Value, between-group test p-value (converted vs. nonconverted groups).
p-Value, between-group test p-value (control group vs. nonconverted group).
p-Value, between-group test p-value (control group vs. converted group).
CBCL, Child Behavior Checklist; CDI, total score on the Child Depression Inventory; F, female; M, male; SD, standard deviation.
Connectivity differences between converted and nonconverted groups
Decreased DLPFC connectivity in children who later developed depression
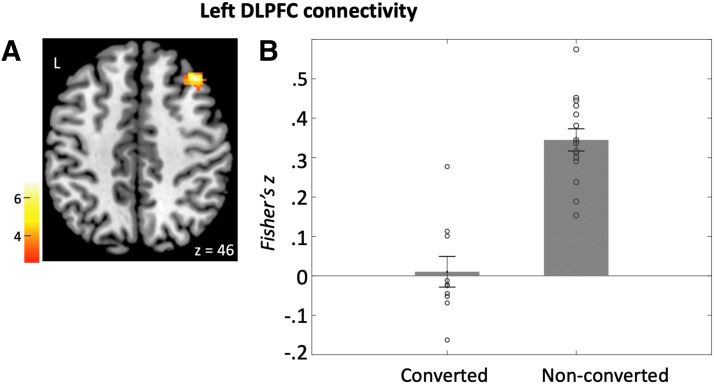
Compared with the nonconverted group, the converted group exhibited decreased baseline connectivity between the left DLPFC seed and a region in the right DLPFC (BA46) (left DLPFC seed; Fig. 2 and Table 2A). The nonconverted group had significant positive connectivity between left DLPFC seed and right DLPFC [t(14) = 12.2, p < 0.001], whereas the converted group did not have significant connectivity between left and right DLPFC regions [t(9) = 0.26, p = 0.8].
FIG. 2.
Left DLPFC connectivity. (A) Left DLPFC seed exhibited higher connectivity with the right DLPFC in the nonconverted group compared with the converted group. (B) Bars represent mean connectivity (Fisher's z) between the left and right DLPFC in each group.
Table 2.
Between-Group Connectivity Differences from (A) Left DLPFC, and (B) Subgenual Anterior Cingulate Cortex Seeds
| BA | Volume | x, y, z | t | df | p | |
|---|---|---|---|---|---|---|
| (A) Left DLPFC connectivity | ||||||
| Nonconverted>converted | ||||||
| R DLPFC | 9/8 | 968 | 38, 28, 46 | 6.51 | 23 | 0.007 |
| Converted>nonconverted | ||||||
| None | ||||||
| BA | Volume | x, y, z | t | df | p | |
|---|---|---|---|---|---|---|
| y | ||||||
| z | ||||||
| (B) sgACC connectivity | ||||||
| Nonconverted>converted | ||||||
| L IPL/postcentral gyrus | 40/2 | 896 | −40, −30, 52 | 5.22 | 23 | 0.005 |
| Converted>nonconverted | ||||||
| None | ||||||
| Nonconverted>control | ||||||
| L IPL/postcentral gyrus | 40/2 | 1128 | −42, −30, 46 | 5.54 | 29 | 0.004 |
Peak coordinates (x y z) based on MNI brain.
t, peak t-value from the cluster; p, FDR-corrected cluster-level p-value.
BA, Brodmann area; DLPFC, dorsolateral prefrontal cortex; FDR, false discovery rate; IPL, inferior parietal lobule; MNI, Montreal Neurologic Institute; sgACC, subgenual anterior cingulate cortex; volume, cluster size in microliter.
Decreased sgACC-parietal connectivity in children who later developed depression
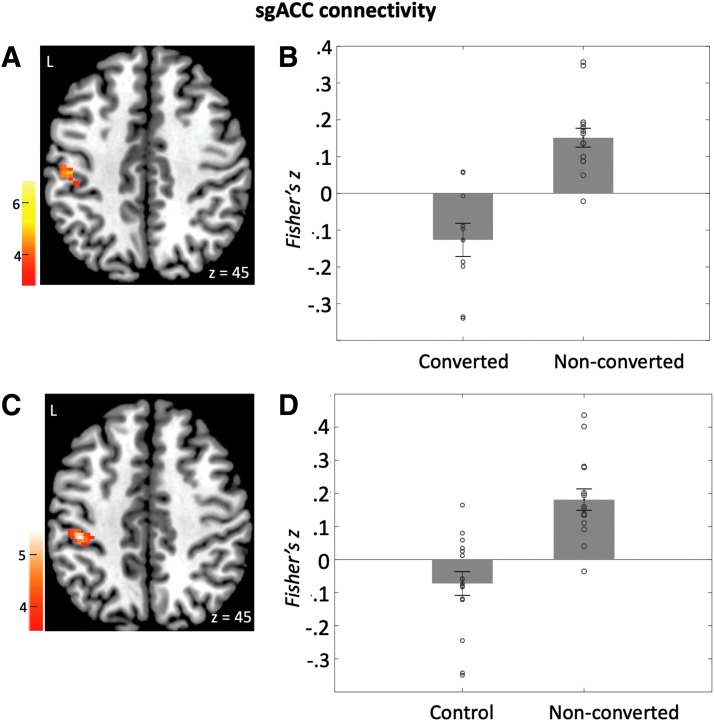
Compared with the nonconverted group, the converted group exhibited decreased connectivity between the sgACC seed and right inferior parietal lobule (IPL)/precentral gyrus (Fig. 3 and Table 2B). The nonconverted group had significant positive connectivity [t(14) = 5.9, p < 0.001], whereas the converted group had negative connectivity [t(9) = −2.8, p = 0.02]. All the group differences described earlier for DLPFC connectivity and sgACC-parietal connectivity remained when sex was included as a covariate (Supplementary Table S1).
FIG. 3.
sgACC connectivity. (A) sgACC seed exhibited higher connectivity with the left inferior parietal lobule/postcentral gyrus in the nonconverted group compared with the converted group. (B) Bars represent mean sgACC-parietal connectivity (Fisher's z) in the converted and nonconverted groups in cluster shown in (A). (C) sgACC exhibited higher connectivity with the left inferior parietal lobule/postcentral gyrus in the nonconverted group compared with the control group. (D) Bars represent mean sgACC-parietal connectivity (Fisher's z) in the nonconverted group and control group in the cluster shown in (C). Error bars represent standard errors of the means.
Connectivity differences between nonconverted and control groups
Compared with the entire control group (including those who did not return for the follow-up), the nonconverted group exhibited greater baseline connectivity between the sgACC seed and right IPL/precentral gyrus (Fig. 3 and Table 2B). The nonconverted group had significant positive connectivity [t(14) = 5.6, p < 0.001], whereas the control group did not show connectivity significantly different from zero [t(15) = −2.0, p = 0.06]. This difference remains if only those who were assessed at follow-up (0 conversion to MDD) were included in the analysis or when sex was included as a covariate (Supplementary Data).
Classification of at-risk children and controls
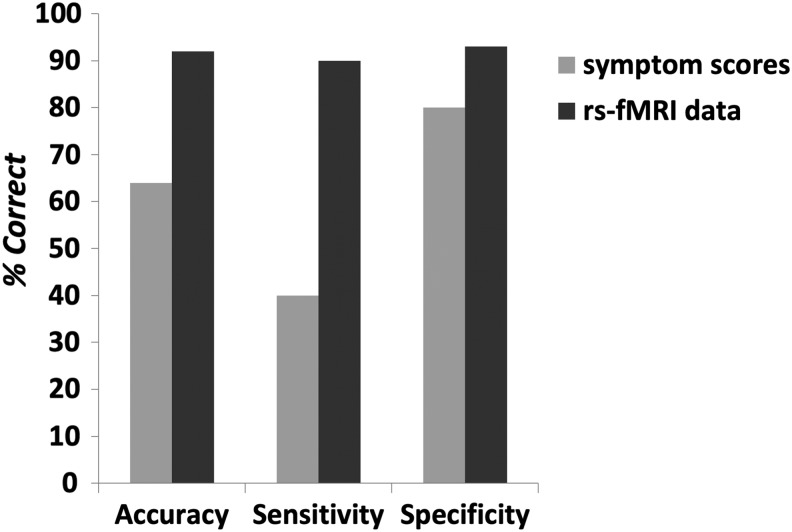
The classification model based on connectivity data between the seeds and areas from the AAL atlas yielded 92% accuracy, 90% sensitivity, and 93% specificity (Fig. 4). Nine out of the 10 individuals who later developed depression were correctly classified. Fourteen out of the 15 nonconverted subjects were correctly classified. Features used in the prediction model and normalized attribute weights are listed in Table 3. Attribute weights represent the hyperplane separating the two classes. The absolute magnitudes of the weights represent the importance of each feature in classifying the data points: Features with higher weights are more relevant than lower weights in discriminating the two classes.
FIG. 4.
Performance of the prediction models based on baseline rs-fMRI data from the AAL atlas (dark gray bars) or baseline symptom scores (light gray bars). AAL, Automated Anatomical Labeling; rs-fMRI, resting-state fMRI.
Table 3.
List of Features (Connectivity Values Between Seeds and Brain Areas from the Automated Anatomical Labeling Atlas) Used in the Support Vector Machine Prediction Model Ordered by Normalized Attribute Weights from the Model
| Seed | AAL area | SVM attribute weight |
|---|---|---|
| DMN | L IFG (pars opercularis) | 0.9906 |
| R DLPFC | R Mid cingulum | 0.8846 |
| L Amygdala | R Hippocampus | −0.8647 |
| R DLPFC | L Parahippocampal gyrus | 0.8394 |
| DMN | L Middle cingulate area | 0.7858 |
| sgACC | L Postcentral gyrus | 0.783 |
| L Amygdala | R Cerebellum lobule IV, V | −0.7769 |
| DMN | L IPL | 0.7289 |
| sgACC | L Superior frontal gyrus | −0.6393 |
| R DLPFC | L Superior parietal lobule | 0.5375 |
| DMN | R Hippocampus | −0.3475 |
| R Amygdala | R Superior parietal lobule | 0.1328 |
| DMN | R Middle cingulate area | −0.106 |
AAL, Automated Anatomical Labeling; DMN, default mode network; SVM, support vector machine.
The parietal cluster that showed different sgACC connectivity between the converted and nonconverted groups (from Table 2) overlapped with the AAL parietal region that contributed to the classification model (Table 3): 77% of the left postcentral gyrus/IPL cluster derived from the group analysis lies within the AAL left postcentral gyrus region; 21% of the group analysis cluster lies within the AAL left inferior parietal region.
The model based on connectivity values between the seeds and areas in the HCP atlas yielded similar discrimination performance (96% accuracy, 100% sensitivity, and 93% specificity). Features used in the prediction are listed in Table 4. In contrast, the model based on baseline CBCL scores (Total, Internalizing, Externalizing, Anxiety/Depressed symptoms, and Affective Problem Scale) yielded only 64% accuracy with 40% sensitivity and 80% specificity. Only 4 out of the 10 converted subjects were correctly classified. Twelve out of the 15 nonconverted subjects were correctly classified.
Table 4.
List of Features (Connectivity Values Between Seeds and Brain Areas from the Human Connectome Project Atlas) Used in the Support Vector Machine Prediction Model, Ordered by Normalized Attribute Weights from the Model
| Seed | HCP atlas area | SVM attribute weight |
|---|---|---|
| sgACC | L Area 23d | 1.285 |
| DMN | R Area 5m ventral | 1.0665 |
| DMN | R Area PFcm | 0.9983 |
| L Amygdala | R PreSubiculum | −0.8381 |
| LDLPFC | L Area 1 | −0.8373 |
| sgACC | L Area 2 | 0.7733 |
| R Amygdala | L Area STGa | −0.7497 |
| DMN | L Area 23c | 0.6657 |
| DMN | L Area 7PC | 0.5974 |
| R Amygdala | R Medial IntraParietal area | 0.5053 |
| DMN | L Area IFJa | 0.4971 |
| DMN | R Area 2 | 0.4719 |
| DMN | L Area 8C | 0.2409 |
HCP, Human Connectome Project.
Discussion
In this study, we investigated the potential of using rs-fMRI for early identification of high-risk children who are likely to develop MDD over the subsequent 3–4 years. First, in regards to neurobiological risk, there were marked differences in intrinsic functional connectivity in the emotion regulation and cognitive control networks between previously never-depressed children with familial risk for MDD who either converted or did not convert to MDD. The children who later developed MDD, compared with the children who did not develop MDD, showed lower baseline bilateral DLPFC connectivity and lower baseline sgACC-IPL connectivity. Second, in regards to resilience, compared with the control group who had no familial risk for MDD, the nonconverted group exhibited higher baseline sgACC-IPL connectivity. Lastly, machine-learning models based on baseline resting-state connectivity in brain regions defined from two different atlases (independent from the clusters that exhibited group differences) accurately predicted the onset of MDD, with high sensitivity and specificity that was superior to a model based on baseline behavioral scores.
Risk for MDD onset
Reduced baseline connectivity between sgACC and a region in the frontoparietal control network (IPL) was observed in at-risk children who later developed MDD compared with those who did not develop MDD. We previously reported reduced baseline connectivity between sgACC and another frontoparietal network region (left DLPFC) in this same sample of at-risk children compared with control children (Chai et al., 2016). Altered sgACC connectivity has been linked with symptom severity scores in children and adolescents with MDD (Connolly et al., 2013; Gaffrey et al., 2010). Given these findings and the unique anatomical position of sgACC that connects to both limbic subcortical regions and cortical regions (Johansen-Berg et al., 2008), it is possible that the reduced coupling of sgACC and the cognitive control network in at-risk children contributes to dysfunction of emotion regulation, and it appears to place children at risk for onset of MDD.
Compared with at-risk children who did not develop MDD, connectivity within the cognitive control network—between left and right DLPFC—was reduced in the children who developed MDD 3–4 years later. A similar pattern of connectivity difference was found in these never-depressed children with familial risk for depression when they were contrasted to children without familial risk for depression: At baseline, these at-risk children showed lower connectivity within the frontal-parietal control network (Chai et al., 2016). Similar patterns of reduced connectivity in cognitive control regions have been observed in adults with MDD (Alexopoulos et al., 2012; Veer et al., 2010; Ye et al., 2012), and this reduced connectivity may contribute to difficulty in cognitive control and emotion regulation in patients (Gotlib and Hamilton, 2008). The present results suggest that weakened connectivity in cognitive control regions is present in the brain years before the onset of depression and greater weakness is a possible risk factor for the development of depression in offspring of depressed parents.
Resilience to depression
The group of at-risk children who did not develop MDD 3–4 years later had greater connectivity between sgACC and left IPL compared with both control children and at-risk children who later developed MDD. This suggests that elevated sgACC-IPL connectivity may be protective and represent a neuromarker for resilience to depression in children at familial risk for MDD. Our finding is consistent with another study that found greater limbic control network connectivity in resilient high-risk adolescents compared with high-risk adolescents who developed depression and low-risk control adolescents (Fischer et al., 2018). The sgACC and frontoparietal network are typically anticorrelated in healthy adults and children (Kelly et al., 2009; Margulies et al., 2007), but the resilient group in this study showed positive connectivity between these regions. These compensatory functional connectivity patterns in the emotion regulation network in at-risk children and adolescents may represent resilience to depression, although the origin and development of such patterns are unknown.
Prediction of MDD onset
The machine-learning models based on baseline resting-state connectivity predicted the subset of at-risk children who later converted to depression with higher accuracy and sensitivity compared with the model based on baseline clinical CBCL scores alone. The resting-state connectivity that carried the most predictive power included connections within or between three important networks for depression: the emotion regulation network, the DMN, and the cognitive control network. Abnormal patterns of connectivity in these networks have been previously reported in adult (Ye et al., 2012) and pediatric (Gaffrey et al., 2010) MDD, as well as in never-depressed children at familial risk for depression (Chai et al., 2016; Clasen et al., 2014). Here, we demonstrated that using a relatively easy and reliable brain measure (rs-fMRI), it is possible to predict the onset of MDD in adolescents with high accuracy.
Among the features that carried the most predictive power in the AAL model, sgACC-postcentral gyrus connectivity overlapped with the group difference results: Connectivity between sgACC and a cluster in the postcentral gyrus/IPL was higher in the nonconverted than the converted group. We did not find other overlaps in the brain for baseline differences between converted and nonconverted groups versus the most predictive features in the machine-learning models. This could be because the atlas regions included in the machine-learning model were much larger than the clusters that were found in the direct group comparison, which represented the clusters with maximal differences between the groups. We used well-established atlas regions in the prediction model to avoid dependency on the group difference results and showed high prediction accuracy from atlas regions, which are more generalizable to new cases. It is also possible that our small sample size was not enough to detect significant group differences in some of the features that carried predictive power in the classification model.
Both strengths and limitations of this study can be noted. Strengths include prospective examination of connectivity in regions of interest believed to be associated with MDD for their potential to predict onset of MDD in adolescents, as well as state-of-the art neuroimaging, diagnostic assessment, and data analytic methods. However, our study should be viewed in light of several limitations. The small sample size limited our power to detect group differences in this early pilot study. Nevertheless, this study provides preliminary evidence that intrinsic functional connectivity may reflect vulnerability for and resilience to depression. All testing procedures and data analyses were carried out with careful and rigorous methods. Our findings are largely consistent with results in adult and pediatric MDD patients and children with familial risk for MDD. Future studies with larger samples are needed to confirm the results. Participants had a higher than average mean IQ, so future studies will need to examine more representative samples. In addition, participants were not asked after the study whether they fell asleep during the scan. We communicated with the participants via the scanner intercom after each scan. All participants were alert and responding after the resting-state scan. Although we did not specifically ask whether they fell asleep during part of the resting-state scan, we believe that sleepiness was unlikely since the resting-state scan was conducted in the beginning of the session, right after MPRAGE. Lastly, we restricted our sample to offspring at risk for depression. We chose these participants because we hypothesized that they would have an elevated rate of MDD emergence during adolescence, and indeed, consistent with the larger literature (Lieb et al., 2002; Williamson et al., 2004), more than a third of the participants did develop MDD. Studies using similar methods in large samples of children who do not have familial risk for depression will be needed to determine whether these predictors operate in the population in general or only in offspring at risk.
Psychosocial approaches to preventing onset of depression in adolescence have shown promise (Hetrick et al., 2016; Merry et al., 2012), including cognitive-behavioral interventions similar to those used to treat depression (Garmy et al., 2017; Perry et al., 2017; Rohde et al., 2018, 2014). Our findings suggest that reduced connectivity within the cognitive control network and connectivity between emotion/limbic regions and control regions may indicate susceptibility for MDD onset. Future research on cognitive-behavioral interventions targeting emotion regulation and cognitive risk factors for depression is needed to evaluate the effectiveness of such interventions in reducing the risk for MDD onset. If confirmed, our findings offer the exciting prospect of being able to use fMRI studies to identify asymptomatic children who are at elevated risk to develop MDD as adolescents, so that these youths can be targeted for preventive cognitive-behavioral interventions to reduce this risk.
Supplementary Material
Acknowledgments
The authors thank Christian Hoover, Lauren Jacobs, Flavia Vaz de Souza, Gretchen Reynolds, Daniel O'Young and Jiahe Zhang, Elana Kagen and Tara Kenworthy for their help in data collection. This research was carried out in the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at the Massachusetts Institute of Technology and in the Child Cognitive Behavioral Therapy Program at the Massachusetts General Hospital. The follow-up study was supported by a Harvard University Catalyst Award, which draws its funding from the National Institutes of Health (NIH 5UL1TR001102-03). The baseline study was carried out in the MGH Clinical and Research Program in Pediatric Psychopharmacology and was supported by the Tommy Fuss Fund, the Poitras Center for Psychiatric Disorders Research, and the MGH Pediatric Psychopharmacology Council Fund. The funding sources had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the article; or in the decision to submit the article for publication.
Disclaimer
This article was prepared while B.G.S. was employed at the Massachusetts General Hospital/Harvard Medical School. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. government.
Author Disclosure Statement
The authors report no conflicts of interest. Dr. Joseph Biederman is currently receiving research support from the following sources: The Department of Defense, AACAP, Alcobra, Forest Research Institute, Ironshore, Lundbeck, Magceutics, Inc., Merck, PamLab, Pfizer, Shire Pharmaceuticals, Inc., SPRITES, Sunovion, Vaya Pharma/Enzymotec, and NIH. In 2014, Dr. Joseph Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He has a U.S. Patent Application pending (Provisional No. #61/233,686) through MGH corporate licensing, on a method to prevent stimulant abuse. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Ingenix, Prophase, Shire, Bracket Global, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH.
Supplementary Material
References
- Achenbach TM, Rescorla LA. 2001. Manual for ASEBA School-Age Forms & Profile. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. 2012. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord 139:56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC, Steyerberg EW. 2015. The number of subjects per variable required in linear regression analyses. J Clin Epidemiol 68:627–636 [DOI] [PubMed] [Google Scholar]
- Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. 2015. Major Depression in the National Comorbidity Survey–Adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry 54:37–44.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Haigh EAP. 2014. Advances in cognitive theory and therapy: the generic cognitive model. Annu Rev Clin Psychol 10:1–24 [DOI] [PubMed] [Google Scholar]
- Braaten EB, Biederman J, Dimauro A, Mick E, Monuteaux MC, Muehl K, et al. . 2001. Methodological complexities in the diagnosis of major depression in youth: an analysis of mother and youth self-reports. J Child Adolesc Psychopharmacol 11:395–407 [DOI] [PubMed] [Google Scholar]
- Cantwell DP, Lewinsohn PM, Rohde P, Seeley JR. 1997. Correspondence between adolescent report and parent report of psychiatric diagnostic data. J Am Acad Child Adolesc Psychiatry 36:610–619 [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castañán AN, Öngür D, Whitfield-Gabrieli S. 2012. Anticorrelations in resting state networks without global signal regression. Neuroimage 59:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Hirshfeld-Becker D, Biederman J, Uchida M, Doehrmann O, Leonard JA, et al. . 2016. Altered intrinsic functional brain architecture in children at familial risk of major depression. Biol Psychiatry 80:849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Hirshfeld-Becker D, Biederman J, Uchida M, Doehrmann O, Leonard JA, et al. . 2015. Functional and structural brain correlates of risk for major depression in children with familial depression. Neuroimage Clin 8:398–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, et al. . 2017. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage 154:174–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen PC, Beevers CG, Mumford JA, Schnyer DM. 2014. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Dev Cogn Neurosci 7:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, et al. . 2013. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry 74:898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, et al. . 2009. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett 460:227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. . 1997. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386:824–827 [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. 2008. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13:663–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair Da, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, et al. . 2009. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol 5:14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. . 2008. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry 63:377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. 1995. Structured Clinical Interview for DSM-IV Axis I Disorders (Clinician Version). New York: New York State Psychiatric Institute Biometrics Department [Google Scholar]
- Fischer AS, Camacho C, Ho TC, Whitfield SG, Gotlib IH. 2018. Neural markers of resilience in adolescents at familial risk for major depressive disorder. JAMA Psychiatry 75:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Gilbert BL, Joormann J, Gotlib IH. 2015. Neural markers of familial risk for depression: an investigation of cortical thickness abnormalities in healthy adolescent daughters of mothers with recurrent depression. J Abnorm Psychol 124:476–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Repovš G, Belden AC, Botteron KN, Luking KR, et al. . 2010. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport 21:1182–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmy P, Clausson EK, Berg A, Steen Carlsson K, Jakobsson U. 2017. Evaluation of a school-based cognitive–behavioral depression prevention program. Scand J Public Health [Epub ahead of print]; DOI: 10.1177/1403494817746537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. 2002. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878 [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. . 2016. A multi-modal parcellation of human cerebral cortex. Nature 536:171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard J, Baruch P, Grondin S, Lafleur MF. 2012. Psychosocial and neurocognitive functioning in unipolar and bipolar depression: a 12-month prospective study. Psychiatry Res 196:145–153 [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP. 2008. Neuroimaging and depression: current status and unresolved issues. Curr Dir Psychol Sci 17:159–163 [Google Scholar]
- Greenberg PE, Fournier A-A, Sisitsky T, Pike CT, Kessler RC. 2015. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76:155–162 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, National H, Frank E, Holmes G, Pfahringer B, Reutemann P, et al. . 2009. The WEKA data mining software: an update. SIGKDD Explor 11:10–18 [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH. 2015. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry 78:224–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Angell KE, Silva PA, McGee R. 1998. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol 107:128–140 [DOI] [PubMed] [Google Scholar]
- Hetrick SE, Cox GR, Witt KG, Bir JJ, Merry SN. 2016. Cognitive behavioural therapy (CBT), third-wave CBT and interpersonal therapy (IPT) based interventions for preventing depression in children and adolescents. Cochrane Database Syst Rev CD003380; DOI: 10.1002/14651858.CD003380.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwath E, Johnson J, Klerman GL, Weissman MM. 1992. Depressive symptoms as relative and attributable risk factors for first-onset major depression. Arch Gen Psychiatry 49:817–823 [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, et al. . 2008. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex 18:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. . 2009. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex 19:640–657 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. . 2003. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095–3105 [DOI] [PubMed] [Google Scholar]
- Kovacs M. 1985. The Children's Depression, Inventory (CDI). Psychopharmacol Bull 21:995–998 [PubMed] [Google Scholar]
- Lieb R, Isensee B, Höfler M, Pfister H, Wittchen H-U. 2002. Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Arch Gen Psychiatry 59:365–374 [DOI] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. . 2014. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 76:517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19:1233–1239 [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. 2007. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37:579–588 [DOI] [PubMed] [Google Scholar]
- Martel MM, Markon K, Smith GT. 2017. ResearchrReview: multi-informant integration in child and adolescent psychopathology diagnosis. J. Child Psychol Psychiatry Allied Discip 58:116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. 1997. Limbic-cortical dysregulation: a proposed model of depression. J. Neuropsychiatry Clin. Neurosci 9:471–481 [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. . 2005. Deep brain stimulation for treatment-resistant depression. Neuron 45:651–660 [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Avenevoli S. 2002. Epidemiology of mood and anxiety disorders in children and adolescents. In: Tsuang MT, Tohen M. (eds.) Textbook in Psychiatric Epidemiology, 2nd ed. Hoboken, NJ: John Wiley & Sons; pp. 657–704 [Google Scholar]
- Merry SN, Hetrick SE, Cox GR, Brudevold-Iversen T, Bir JJ, McDowell H. 2012. Cochrane review: psychological and educational interventions for preventing depression in children and adolescents. Cochrane Database Syst Rev 7:1409–1685 [DOI] [PubMed] [Google Scholar]
- Mitchell TM. 1997. Machine learning. Annu Rev Comput Sci DOI: 10.1145/242224.242229 [DOI] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, et al. . 2008. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry 165:90–98 [DOI] [PubMed] [Google Scholar]
- Ongür D, Drevets WC, Price JL. 1998. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A 95:13290–13295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvaschel H. 1994. Schedule for Affective Disorder and Schizophrenia for School-Age children–Epidemiologic Version, 5th ed. Fort Lauderdale, FL: Nova Southeastern University, Center for Psychological Studies [Google Scholar]
- Orvaschel H, Lewinsohn PM, Seeley JR. 1995. Continuity of psychopathology in a community sample of adolescents. J Am Acad Child Adolesc Psychiatry 34:1525–1535 [DOI] [PubMed] [Google Scholar]
- Perry Y, Werner-Seidler A, Calear A, Mackinnon A, King C, Psych MC, et al. . 2017. Preventing depression in final year secondary students: school-based randomized controlled trial. J Med Internet Res 19:e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Brière FN, Stice E. 2018. Major depression prevention effects for a cognitive-behavioral adolescent indicated prevention group intervention across four trials. Behav Res Ther 100:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Stice E, Shaw H, Brière FN. 2014. Indicated cognitive behavioral group depression prevention compared to bibliotherapy and brochure control: acute effects of an effectiveness trial with adolescents. J Consult Clin Psychol 82:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapero BG, Chai XJ, Vangel M, Biederman J, Hoover C, Whitfield-Gabrieli S, et al. . 2019. Neural markers of depression risk predict the onset of depression. Psychiatry Res Neuroimaging 285:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. . 2009. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A 106:1942–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, et al. . 2012. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Arch Gen Psychiatry 69:913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U. 2011. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. . 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289 [DOI] [PubMed] [Google Scholar]
- Veer IM, Beckmann CF, van Tol M-J, Ferrarini L, Milles J, Veltman DJ, et al. . 2010. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci 4:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgis V, Gelardi KL, Helm JL, Forbes EE, Hipwell AE, Keenan K, et al. . 2018. Dorsomedial prefrontal activity to sadness predicts later emotion suppression and depression severity in adolescent girls. Child Dev 89:758–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. . 2009. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A 106:1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DE, Birmaher B, Axelson DA, Ryan ND, Dahl RE. 2004. First episode of depression in children at low and high familial risk for depression. J Am Acad Child Adolesc Psychiatry 43:291–297 [DOI] [PubMed] [Google Scholar]
- Yang Y, Pedersen JO. 1997. A Comparative Study on Feature Selection in Text Categorization. In: Proceedings of the Fourteenth International Conference on Machine Learning. San Fransisco, CA, pp. 412–420
- Ye T, Peng J, Nie B, Gao J, Liu J, Li Y, et al. . 2012. Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. Eur J Radiol 81:4035–4040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.