Abstract
Repetitive transcranial magnetic stimulation (rTMS) has demonstrated antidepressant efficacy but has limited evidence in depression associated with traumatic brain injury (TBI). Here, we investigate the use of rTMS targeted with individualized resting-state network mapping (RSNM) of dorsal attention network (DAN) and default mode network (DMN) in subjects with treatment-resistant depression associated with concussive or moderate TBI. The planned sample size was 50 with first interim analysis planned at 20, but only 15 were enrolled before the study was terminated for logistical reasons. Subjects were randomized to 20 sessions of bilateral rTMS (4000 left-sided excitatory pulses, 1000 right-sided inhibitory pulses) or sham. Treatment was targeted to the dorsolateral prefrontal cluster with maximal difference between DAN and DMN correlations based on resting-state functional magnetic resonance imaging with individualized RSNM. Mean improvement in the primary outcome, Montgomery-Asberg Depression Rating Scale (MADRS), was 56% ± 14% (n = 9) with active treatment and 27% ± 25% (n = 5) with sham (Cohen's d = 1.43). One subject randomized to sham withdrew before starting treatment. There were no seizures or other significant adverse events. MADRS improvement was inversely correlated with functional connectivity between the right-sided stimulation site and the subgenual anterior cingulate cortex (sgACC; r = -0.68, 95% confidence interval 0.03-0.925). Active treatment led to increased sgACC-DMN connectivity (d = 1.55) and increased sgACC anti-correlation with the left- and right-sided stimulation sites (d = -1.26 and -0.69, respectively). This pilot study provides evidence that RSNM-targeted rTMS is feasible in TBI patients with depression. Given the dearth of existing evidence-based treatments for depression in this patient population, these preliminarily encouraging results indicate that larger controlled trials are warranted.
Keywords: depression, fMRI, rTMS, TBI
Introduction
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive technique for selective excitation or inhibition of focal brain regions.1 Excitatory rTMS over the left dorsolateral prefrontal cortex (DLPFC) has consistently demonstrated antidepressant efficacy, leading to its U.S. Food and Drug Administration (FDA) approval for medication-resistant major depressive disorder in 2008.2–5 Positive results have been reported with a wide variety of stimulation protocols, although the optimal treatment approach remains a topic of open debate.6 Similarly positive results have been reported for depression associated with various neurological disorders such as stroke and Parkinson's disease.7,8 Despite this established efficacy, rTMS has not been investigated in the setting of depression associated with traumatic brain injury (TBI),9 which affects nearly half of TBI patients.10
While traditional approaches to antidepressant pharmacotherapy and psychotherapy have failed to demonstrate benefit in randomized controlled trials for TBI-associated depression,11,12 rTMS may hold unique promise due to its structural neurorehabilitative effects. Excitatory stimulation also has been shown to accelerate stroke recovery when applied over the affected cortex.13 Contralateral inhibitory rTMS has demonstrated comparable efficacy both in stroke recovery14 and in major depression.6,15,16 The combination of excitatory treatment and contralateral inhibitory treatment can further improve efficacy in both neurorehabilitative17 and antidepressant settings.6 This is consistent with emerging evidence that rTMS modulates interhemispheric connectivity, which also is affected by TBI.18 Further, animal models have successfully used rTMS to restore cortical excitability,19 which is often compromised following TBI.20
A prevailing concern limiting the study of rTMS in the setting of TBI is that these patients may be at higher risk of seizures, which is a rare side effect of rTMS9 and a common complication of severe TBI.21 However, a growing body of literature has established the fact that this risk is not elevated in patients with diffuse/multi-focal axonal injury (which constitutes the majority of patients with TBI), and the elevated risk is unique to patients with a history of penetrating injury or intracranial hemorrhage.21,22 While the risk of headaches also has limited the use of rTMS for this indication, prior work suggests that the treatment may actually improve post-concussive headaches.23
Anatomical targeting of neuromodulation introduces additional challenges due to TBI-induced disruptions in functional connectivity (FC),24–26 which may affect functional network topography in these patients. rTMS is traditionally targeted to scalp regions overlying DLPFC, which shows substantial inter-individual topographic variability even in healthy individuals.27,28 This may explain the heterogeneity in rTMS response between patients and between studies.29 Functional imaging literature suggests that the treatment exerts its antidepressant effects via modulation of connectivity between DLPFC, default mode network (DMN), and subgenual anterior cingulate cortex (sgACC),30–32 regions which have been heavily implicated in major depression.30,33–35 Recent efforts to optimize rTMS targeting have focused on identifying DLPFC targets that are maximally anti-correlated with sgACC.29,31,36 This may approximate the mechanisms of sgACC deep brain stimulation, which has shown promising efficacy for treatment-resistant major depression.35
The antidepressant effects of sgACC deep brain stimulation appear to be associated with changes in externally-oriented attention-shifting and self-referential interoceptive awareness; this interaction has been proposed to represent a “depression switch” involving sgACC.37 These cognitive processes have been linked to the dorsal attention network (DAN) and DMN, respectively.38–40 DMN generally shows strong functional connectivity with sgACC and anti-correlation with DAN.39,41 DAN usually includes a node in DLPFC which was unrecognized until recently, possibly because its location shows substantial inter-individual variability.28,42,43 Interactions between these systems are likely affected after TBI, which has been associated with disrupted FC in anterior cingulate cortex, DLPFC, DAN, and DMN.24,25,44,45
Existing approaches for targeting rTMS have focused on connectivity with seed regions defined by standardized anatomical atlases.29,31 Such methods can predict efficacy of different rTMS targets at a group level, but have not been able to identify individualized treatment targets. A substantial emerging body of work has focused on developing and validating individual-level brain parcellation methods using resting-state functional magnetic resonance imaging (rsfMRI).46–48 These approaches can be used to generate individualized maps of DAN and DMN in an effort to identify rTMS targets for optimally modulating sgACC. Given the known disruptions in DAN and DMN that are inherent to TBI,24,25 individualized rTMS targeting may represent a promising approach for resolving the heterogeneity in this patient population. Thus, we hypothesized that rTMS targeted using an individualized rsfMRI approach expected to modulate sgACC would be feasible and improve mood in patients with treatment-resistant depression associated with TBI. We reasoned that if successful, this would set the stage for future direct comparisons between different rTMS targeting approaches.
Methods
Standard protocol approvals, registrations, and participant consents
The study protocol was approved by the Human Research Protection Office at Washington University School of Medicine in St. Louis, Missouri. All individuals gave informed written consent. The study was registered with ClinicalTrials.gov (NCT02980484). All procedures, outcome measures, and analyses were pre-specified and registered with the Open Science Foundation (osf.io/vjddq).49 The study was reviewed on a weekly basis by the investigators for safety.
Subjects
Referrals were solicited from the TBI clinics at Washington University in St. Louis and the Rehabilitation Institute of St. Louis. Participants included adults (age 18–65) with a primary diagnosis of TBI and a minimum score of 10 on the Montgomery-Asberg Depression Rating Scale (MADRS). All subjects met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for a major depressive episode secondary to TBI (ICD-10 F06.32—Mood disorder due to known physiological condition with major depressive-like episode) that persisted after at least one trial of antidepressant pharmacotherapy. Subjects with elevated seizure risk, including those with penetrating or hemorrhagic TBI, were excluded. TBI severity was classified according to the patient's existing clinical diagnosis, which was based on the consensus guidelines defined in 1993 by the American Congress of Rehabilitation Medicine.50 Other baseline clinical criteria were determined based on review of medical records. Intracranial metallic implants or neurostimulation devices were considered contraindications to treatment. Additional excluded diagnoses included dementia, moderate–severe autism spectrum disorder, bipolar I disorder, schizophrenia spectrum disorders, active psychosis, or dysphoria clearly explained by etiology other than TBI or major depressive disorder (MDD; such as a substance-induced mood disorder). No changes or recommendations were made for the subject's usual medical treatment regimen as part of the study.
Measures
The primary outcome measure was the MADRS.51 DSM-5 diagnostic assessment and MADRS were conducted by one of two senior psychiatry residents (SHS, NTT) who were blinded to the subjects' group assignments. For each subject, all MADRS assessments were administered by the same physician. MADRS was completed at baseline, after the 10th treatment, and after the final treatment.
Additional measures collected at baseline and after the full treatment course included personality testing with the temperament and character inventory (TCI)52; self-report mood scales in the National Institutes of Health (NIH) Toolbox Emotion Battery53; cognitive testing with the NIH Toolbox Cognitive Battery (CB)54; self-report headache Likert scores and six-question Headache Impact Test (HIT-6)55; and an expert psychiatric evaluation based on DSM-5 diagnostic criteria. Structural and functional MRI scans were performed at baseline and at the end of the treatment course for all subjects. At the end of the treatment course, all subjects were asked to guess their group assignments to assess the adequacy of blinding.
Sample size
A recent meta-analysis of studies investigating rTMS for MDD demonstrated a weighted mean effect size of 0.86 for improvement in depressive symptom scores.56 However, this meta-analysis did not include the effects of structural MRI-guided neuronavigation, which has been shown to improve efficacy of rTMS with a relative effect size of 0.56.57 This suggests that the efficacy of neuronavigated rTMS in comparison with sham may be estimated as the sum of these two values, or 1.42. Further, bilateral rTMS is likely more effective than unilateral rTMS6. We thus conservatively anticipated an effect size of between 0.86 and 1.42.
These values were used to estimate the sample size required to achieve an 80% chance of detecting an effect at p < 0.05 using an unpaired two-tailed t-test. This yielded a required sample size between 20 and 46 subjects. We thus planned to conduct an interim analysis for sample size re-estimation after enrolling 20 subjects with total maximum enrollment of 50 subjects.
MRI acquisition and analysis
Acquisition
Pre- and post-treatment images were acquired with a 3T Siemens Magnetom Prisma magnetic resonance scanner (Siemens, Erlangen, Germany). Functional acquisition included 16.5 min of resting-state blood oxygen level–dependent (BOLD) scans in three runs (416 frames per run, 48 axial slices using multi-band acquisition factor of 4, 3 mm cubic voxel resolution, repetition time [TR] 800 msec, echo time [TE] 26.6 msec, flip angle 61°, imaging matrix 72 × 72). Structural acquisition included one T1 Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) structural sequence (176 frames, 0.9375 × 0.9375 × 1 mm voxel resolution, TR 2400 msec, TE 3.19 msec, flip angle 8°, imaging matrix 256 × 256). This T1 weighted scan required approximately 5 min. The complete imaging protocol required approximately 30 min per subject.
Processing
Spatial alignment, atlas registration, motion scrubbing at framewise displacement of 0.2 mm and frequency filter for respiratory artifact,58 nuisance regression, global signal regression, temporal filtering, spatial smoothing, and motion epoch interpolation were performed using in-house scripts by the methods described by Power and colleagues.59 Resulting time courses were used for all subsequent resting-state functional connectivity analyses.
Individualized resting-state network mapping
Processed time courses were used to construct individual-level resting-state functional connectivity maps via the multi-layer perceptron-based machine learning classifier as described in Hacker and colleagues.47 This method involves a machine learning algorithm that has been trained to recognize resting-state networks (RSNs) based on each voxel's seed-based connectivity profile. The algorithm classifies individual voxels in a patient's brain based on likelihood of membership in one of seven networks (dorsal attention, ventral attention, frontoparietal control, default mode, motor, language, and visual).
These seven RSNs were chosen meta-analytically based on task fMRI studies of 10 distinct networks, each containing several foci of activity. After refining these foci to maximize intra-network correlation and minimize inter-network correlation, three of the original networks (cingulo-opercular network, affective network, and distinct foveal and peripheral vision networks) were not clearly discriminable at the individual level.47 However, the optimal number of networks in the brain remains a topic of active debate due to improved granularity with newer RSN mapping procedures, which are outside the scope of the present work. The Yeo 2011 seven-network parcellation is still widely used as a consensus definition of network boundaries since it agrees with boundaries defined by task activation studies.41 This approach was chosen because it has been used successfully for preoperative neurosurgical mapping,60 while newer approaches to individualized RSN mapping have not been implemented clinically to our knowledge. Some recent techniques carry the advantage of further subclassifying certain RSNs,27,46 but we were unable to identify a theoretical basis for targeting a specific subdivision of DAN and DMN in this study.
rTMS target selection
Individualized rTMS treatment targets were identified as described in Siddiqi and colleagues, 2018 (preprint at biorxiv.org/content/early/2017/11/21/151696). The individualized RSN maps were used to calculate the absolute difference between likelihoods of DAN and DMN membership for each voxel. The image was masked to include only voxels within 6 mm of the outer surface of a standard Talairach atlas template brain in order to include only superficial gray matter regions,61 which are more readily accessible via rTMS.62 As an approximation of DLPFC, a second mask was applied to include only voxels within 20 mm of Brodmann areas 9 and 46, which was a smaller radius than described in previous retrospective work.29 A smaller size was chosen to avoid inclusion of excessively anterolateral regions (which are likely to produce substantial facial muscle contraction) and excessively posterior regions (which are associated with increased seizure risk due to possible inadvertent stimulation of motor cortex). Positive clusters in the resulting image were identified using FSL's cluster algorithm (FMRIB Software Library, Oxford, UK)63,64 with an image threshold of 75% of the maximum Z-score. The centers of gravity of the peak clusters in each hemisphere were chosen as optimal left- and right-sided rTMS stimulation sites (exemplars shown in Fig. 1). These coordinates were transformed from Talairach to native space using the 4dfp tool suite. In four subjects who volunteered to receive two additional resting-state fMRI scans for reliability assessment, test–retest variability of the target coordinates ranged between 0.9 and 8.3 mm (Fig. 1B).
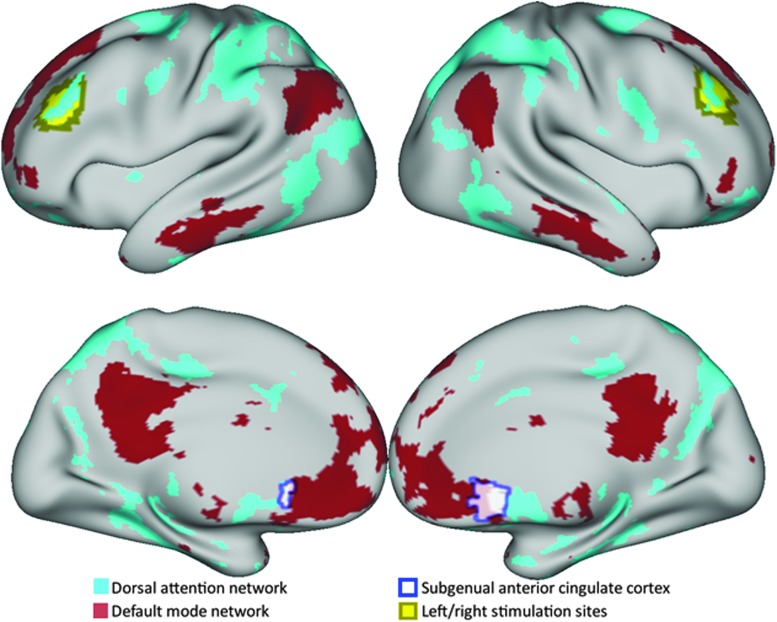
FIG. 1.
A priori regions of interest used for functional connectivity analysis depicted on a group-mean surface projection. Dorsal attention and default mode networks were identified using individualized winner-take-all maps for each subject. Left and right stimulation sites were identified using the individualized difference between dorsal attention and default mode network membership likelihoods. Subgenual anterior cingulate cortex, which was defined based on an a priori group seed, generally overlapped with the default mode network parcel. Color image is available online.
Study treatment
Subjects were randomized to receive active rTMS or sham treatment with a Magstim Rapid-2 stimulator. Active treatment was delivered using a double 70-mm air-cooled coil. Sham treatment was delivered using a double 70-mm Alpha sham coil, which produces a similar sound, but delivers only a weak magnetic pulse that is not adequate for substantial stimulation of deep or superficial nerves. All other study procedures, including neuronavigation, were identical for the two groups.
Treatment course included 20 daily sessions of bilateral rTMS using high-frequency left-sided stimulation (4000 pulses at 10 Hz frequency with 5-sec trains and 20-sec inter-train interval) followed by low-frequency right-sided stimulation (a single train of 1000 pulses with 1 Hz frequency). A dorsolateral prefrontal node, which was identified based on individual-level functional connectivity mapping as described above, was targeted using the Brainsight neuronavigation system (Rogue Research, Montreal, Canada). The intensity of rTMS stimulation was 120% of resting motor threshold (RMT). RMT was determined bilaterally by measuring electromyogram (EMG) activation in the contralateral abductor pollicis brevis muscle. An EMG activation of at least 50 μV was considered to be a positive test. The overall RMT was determined using the titration algorithm implemented by the TMS motor threshold assessment tool.65
Test–retest reliability
A subset of four subjects returned for two follow-up resting-state fMRI scans. To avoid bias from acute effects of treatment, these scans were completed at least 3 months after completion of study treatments. Parameters for each imaging sequence were identical to the initial and final study visits. The follow-up session included one structural T1 MPRAGE sequence and six 5.5-min BOLD runs following the same protocol as the pre-treatment and post-treatment imaging sessions. After the first three BOLD runs, each subject was removed from the scanner and asked to take a 5-min break. The subject was then placed back into the scanner for the final three BOLD runs. Connectivity results from these sessions were used for analysis of test–retest reliability.
Statistical analysis
Clinical measures
The primary analysis of the primary outcome measure was mean percentage change in MADRS, which was compared between the active and sham groups. The original plan was to perform unpaired two-tailed t-tests at the interim and final analyses. Because the study stopped before the planned interim analyses, no formal test of statistical significance was performed for treatment-induced changes. Instead, effect sizes are reported for the purposes of planning future trials. Equality of variances was assessed via F-tests.
Secondary analyses of the primary outcome included mean percentage change in individual MADRS subscales
Secondary outcomes included changes in NIH Toolbox cognitive composite scores; NIH Toolbox emotional composite scores; temperament and character inventory scales; and HIT-6 and headache Likert scores.
Imaging analyses
A whole–brain “winner-take-all” parcellated map was generated by assigning each voxel to the network with which it demonstrated the highest likelihood of membership. The networks identified by this parcellated map were used as regions of interest (ROIs) for seed-based connectivity analysis.
BOLD time courses were analyzed for seed-based functional connectivity by determining covariance matrices between five a priori specified regions of interest (ROIs). ROIs included the individualized winner-take-all maps of DAN and DMN; sgACC as defined by the meta-analysis in Fox and colleagues29; and 24-mm diameter spherical ROIs at the left and right-sided stimulation sites (Fig. 1A). Effect sizes were calculated to compare treatment-induced changes in connectivity between DAN, DMN, and sgACC in active and sham groups.
To examine connectivity-based predictors of response, the connectivity of both stimulation sites with sgACC was compared with antidepressant response. A least squares regression model was constructed using baseline target-sgACC connectivity and baseline MADRS as predictors of post-treatment MADRS. Because antidepressant response could not be assumed to be normally distributed in this small sample, all data were rank-transformed, which is consistent with prior methods described in Weigand and colleagues.31
Imaging test–retest reliability
Test–retest reliability of the targeting method was assessed for the four subjects who received a pair of follow-up scan sessions several months after treatment. The target stimulation coordinates were computed separately for each session. Euclidean distance between this pair of coordinates was calculated in order to confirm the intrinsic test–retest reliability of the targeting method.
Test–retest reliability was also assessed for functional connectivity measures. For each ROI, seed-based connectivity was computed with the five a priori ROIs used for functional connectivity analysis. This generated 15 connectivity values for each of the four subjects. Pearson correlations were calculated for these 60 connectivity values between pre-treatment and post-treatment scans. The resulting correlation values were used as estimates of test–retest reliability of the a priori ROI pairs.
Reliability of individualization
To assess whether individualization was reliable, FC values and target coordinates were compared within individuals and between individuals. We hypothesized that inter-individual variability would significantly exceed intra-individual variability, suggesting that the observed variability results from genuine differences between individuals rather than methodological limitations.
For the four subjects who received additional test–retest scans, individualization was assessed for ROI-ROI connectivity values. For each ROI pair, intra-individual variability was calculated as the mean change in connectivity between the two separate scan sessions across these four subjects. To estimate inter-individual variability, this calculation was modified such that each subject's first scan was compared with the second scan for the other three subjects. Mean inter-individual variability was compared with mean intra-individual variability using paired t-test.
To determine whether the target remained stable during the course of treatment, optimal stimulation sites were computed separately for the pre-treatment scan and the post-treatment scan for all subjects in the study. The Euclidean distance between these two coordinates was used as an estimate of intra-individual change. To estimate inter-individual variability, this calculation was modified such that each subject's pre-treatment scan was compared with the post-treatment scan for all other subjects, which is representative of the variability expected by chance. To confirm whether individualized targets were more stable than expected by chance, inter-individual and intra-individual variability were compared for both targets using a permutation test.
Results
Allocation and study flow
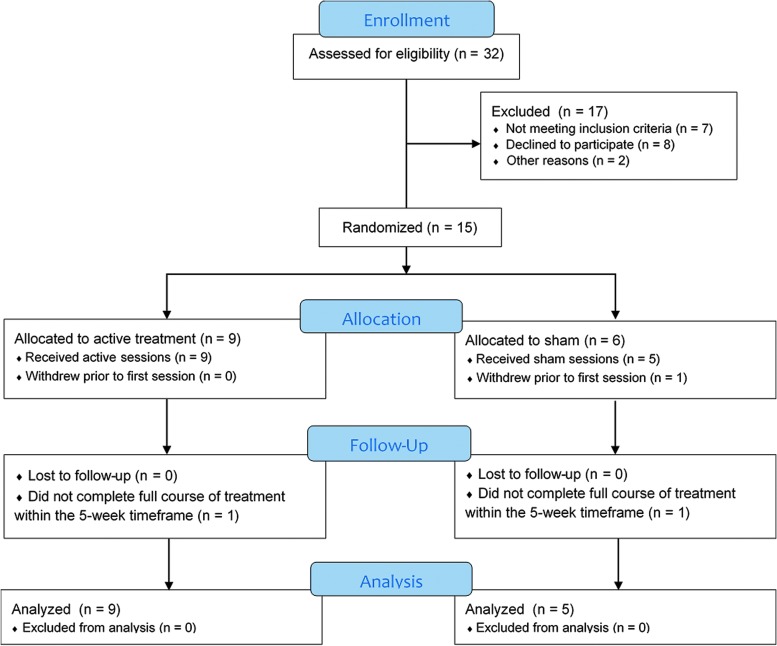
A total of 32 subjects were assessed for eligibility with a phone screen, of which 15 subjects were consented and randomized to the study. The flow of participants is depicted as a Consolidated Standards of Reporting Trials (CONSORT) diagram in Figure 2. After randomization, one subject in the sham group withdrew prior to starting treatment. Two subjects (one active, one sham) were unable to complete the full course of treatments within the 5-week timeframe; in both cases, the subjects missed several visits due to headaches. One subject in the sham group opted out of the post-treatment MRI scanning session. Baseline clinical characteristics (Table 1) were comparable between the active and sham groups.
FIG. 2.
Consolidated Standards of Reporting Trials diagram depicting flow of participants through the trial. A total of 32 subjects were screened by phone, of which 15 were randomized. After randomization, one subject withdrew prior to starting treatment, while two others (one active, one sham) withdrew during the course of treatment but were included in intent-to-treat analysis. Color image is available online.
Table 1.
Clinical and Demographic Variables between the Two Groups
| Active | Sham | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (A) Key clinical and demographic variables were comparable between the two groups | |||||||||
| Age (years) | 43 ± 13 | 50 ± 18 | |||||||
| Sex | 7 M, 2 F | 4 M, 2 F | |||||||
| Duration since TBI (years) | 8.4 ± 8.2 | 8.1 ± 11.3 | |||||||
| TBI mechanism | 4/9 MVC 2/9 military/fire 1/9 sports 3/9 other |
3/6 MVC 3/6 sports |
|||||||
| Duration of depression (years) | 4.8 ± 4.2 | 7.7 ± 9.9 | |||||||
| Treatment trials (antidepressants, augmentation, or CBT) | 4.8 ± 3.0 | 5.4 ± 3.4 | |||||||
| Comorbid PTSD | 4/9 | 3/6 | |||||||
| Age | Gender | TBI Mechanism | TBI severity | Structural MRI abnormalities | Duration since TBI (year) | Duration of depression (year) | Antidepressant trials | Augmentation trials | Psychiatric comorbidities |
|---|---|---|---|---|---|---|---|---|---|
| (B) Baseline clinical characteristics for each subject | |||||||||
| Active | |||||||||
| 20 | M | Assault | Concussive | None | 1 | 3-4 | 5 | 3 | N/A |
| 44 | M | IED blast | Concussive | None | 6 | 5-6 | 8 | 3 | PTSD |
| 38 | M | Sports | Concussive (multiple) | None | 10 to 20 | 2-3 | 3 | 0 | Anxiety |
| 58 | M | Firefighting injury | Concussive | None | 15 | 15 | 5 | 0 | PTSD |
| 34 | M | MVC | Concussive | None | 0.5 | 0.5 | 4 | 0 | PTSD |
| 64 | M | Multiple (accident, MVC, fall) | Concussive (multiple) | None | 2 to 50 | 2.5 | 4 | 0 | N/A |
| 55 | F | MVC | Concussive | None | 2 | 3 | 3 | 0 | PTSD |
| 38 | M | Accident | Moderate | Focal atrophy | 6 | 6 | 1 | 0 | N/A |
| 39 | F | MVC | Moderate | Focal atrophy | 5 | 5 | 3 | 1 | Anxiety |
| Sham | |||||||||
| 61 | F | MVC | Concussive | None | 0.5 | 0.5 | 1 | 1 | N/A |
| 36 | F | MVC | Concussive | None | 2.5 | 2.5 | 3 | 1 | PTSD |
| 65 | M | MVC | Concussive | None | 3 | 3 | 6 | 0 | PTSD, resolved |
| 19 | M | Sports | Concussive (multiple) | None | 4 | 4 | 3 | 1 | Anxiety; cannabis use disorder |
| 51 | M | Sports | Concussive (multiple) | None | 20 to 30 | 25 | 9 | 2 | N/A |
| 56 | M | Sports | Concussive (multiple) | None | 20 to 40 | 6 | 3 | 1 | PTSD |
M, male; F, female; TBI, traumatic brain injury; MVC, motor vehicle collision ; CBT, cognitive-behavioral therapy; PTSD, post-traumatic stress disorder; MRI, magnetic resonance imaging; N/A, not applicable; IED, improvised explosive device.
The initial protocol specified that interim sample size re-estimation would be conducted after enrolling 20 subjects. The interim analysis was completed earlier than initially planned because the lead investigator and the senior investigator both left the institution for unrelated reasons. Due to the subsequent logistical challenges, recruitment was discontinued after enrolling 15 subjects.
Clinical outcomes
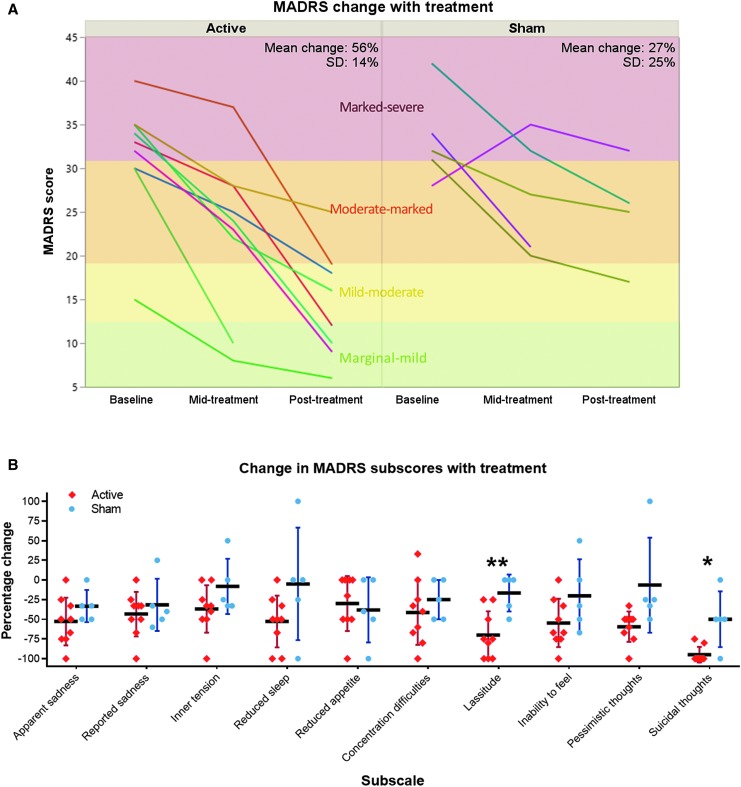
The primary study outcome was mean percentage improvement in MADRS over the course of the full study period (Fig. 3A). A Shapiro-Wilk test revealed that this parameter was normally distributed in both the active group (W = 0.91, p = 0.32) and the sham group (W = 0.82, p = 0.11). An F-test showed no significant difference in variances between the two groups (p = 0.38). MADRS improvement was greater in the active treatment group than in the sham group (Cohen's d = 1.43). Because the clinical trial was discontinued early, statistical hypothesis confirmation testing was not conducted. Subscores of the MADRS with the largest differences in improvement between active treatment and sham (Fig. 3B) were lassitude (Cohen's d = 2.0) and suicidal thoughts (Cohen's d = 1.75).
FIG. 3.
Change in Montgomery-Asberg Depression Rating Scale (MADRS) with treatment. (A) Overall MADRS improvement with treatment was greater in the active group than the sham group. Severity grades are depicted as delineated by Muller and colleagues.70 (B) Change in individual MADRS subscores in the treatment and sham groups. **Cohen's d = 2.0, *Cohen's d = 1.75. Color image is available online.
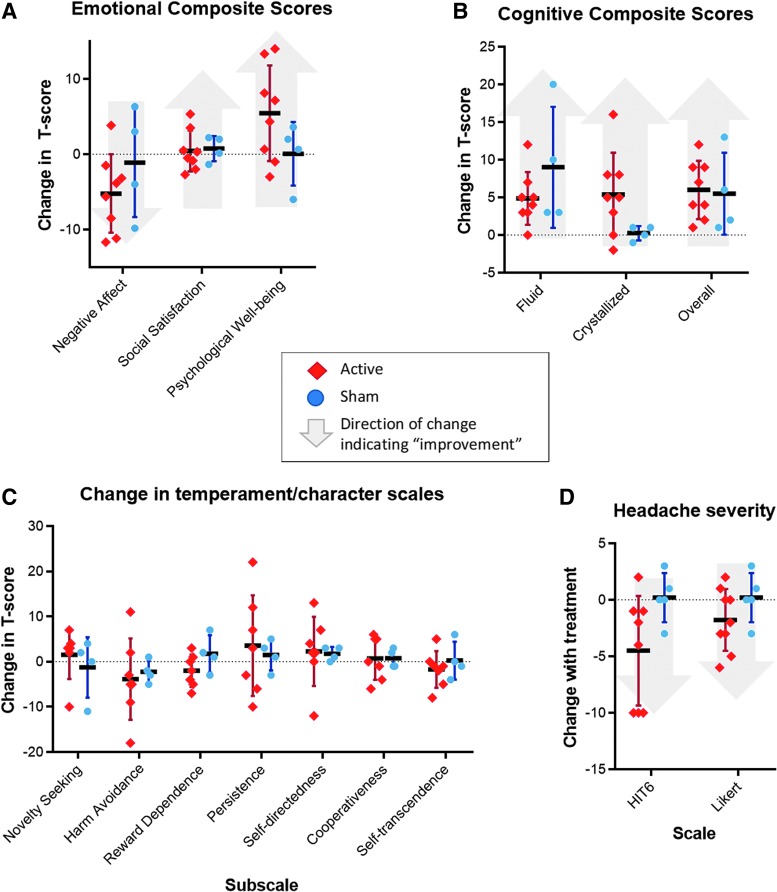
Additional secondary clinical outcomes included changes in NIH Toolbox cognitive/emotional composite scores, headache scales, and temperament/character scales (Fig. 4). Each of these outcomes showed no clear difference between active and sham. Of note, HIT6 headache scales suggested a trend toward improvement in the active group, but this was confounded by the fact that these scales were not completed by two of the subjects who withdrew prematurely due partly to headaches.
FIG. 4.
Secondary clinical outcomes. There was no significant change in secondary clinical outcome variables. (A) Active treatment was associated with a trend toward decrease in negative affect and increase in psychological well-being. (B) Change in overall cognition showed wide variance between subjects. (C) Active treatment did not clearly affect personality measures. (D) Likert scales and HIT6 suggested a trend in the direction of improvement in headaches; of note, HIT6 was not completed by two subjects who withdrew early due partly to headaches. Color image is available online.
The most common adverse effect was transient twitching and discomfort in the facial muscles, which was reported by seven of nine subjects in the active group and zero of five subjects in the sham group. Worsening headaches were reported by one subject in the active group and one in the sham group. One subject in the active group experienced a presyncopal episode during the first treatment, but chose to continue with the remainder of the study. There were no seizures or events concerning for seizure auras.
Blinding integrity
Integrity of blinding was assessed based on a post-treatment survey of suspected group assignment. The group assignment was correctly guessed by three of the nine subjects in the active group and one of the five subjects in the sham group. One of the nine subjects in the active group guessed incorrectly. The remaining nine subjects reported that their suspicion was “50-50” in either direction.
Functional connectivity
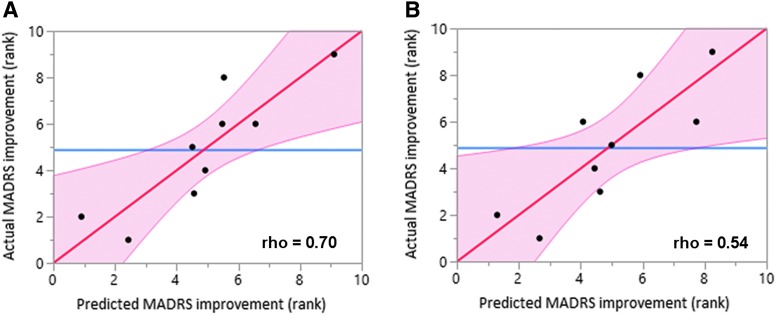
Within the active group, baseline functional connectivity (FC) and baseline MADRS were used as predictors of post-treatment MADRS in an effort to identify predictors of treatment response (Fig. 5). Antidepressant response was negatively correlated with baseline FC between the right-sided stimulation site and sgACC (Fig. 5A; Spearman rho = 0.70, p = 0.04). Patients stimulated at sites with stronger sgACC anti-correlation had stronger antidepressant responses. The left-sided stimulation site showed a trend in the same direction, but did not reach significance (Fig. 5B).
FIG. 5.
Functional connectivity as a predictor of antidepressant response. (A) Connectivity between the right-sided stimulation site and the a priori subgenual anterior cingulate cortex (sgACC) showed a significant relationship with antidepressant response (p = 0.04) (B) Antidepressant response was inversely related to connectivity between the left-sided stimulation site and the a priori sgACC, but this did not reach significance. Color image is available online.
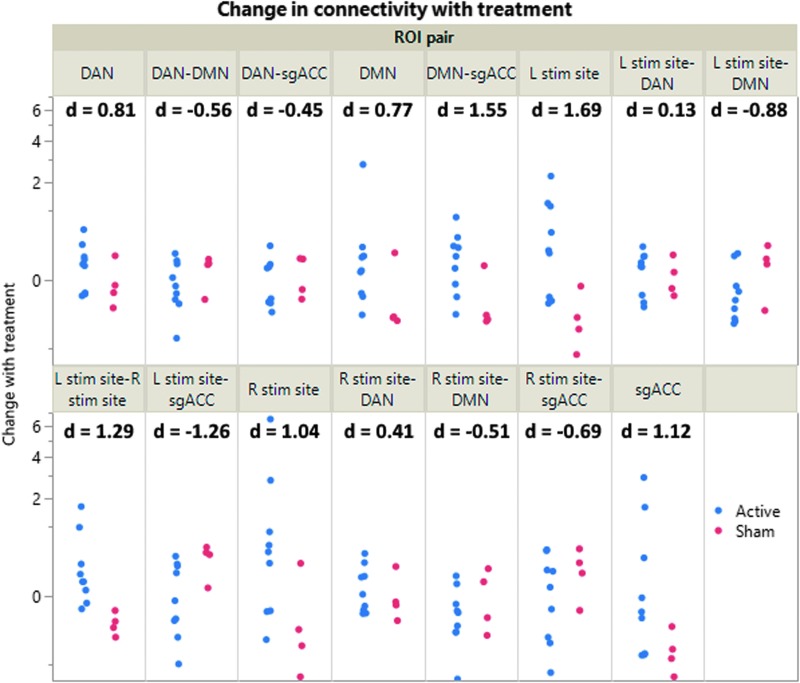
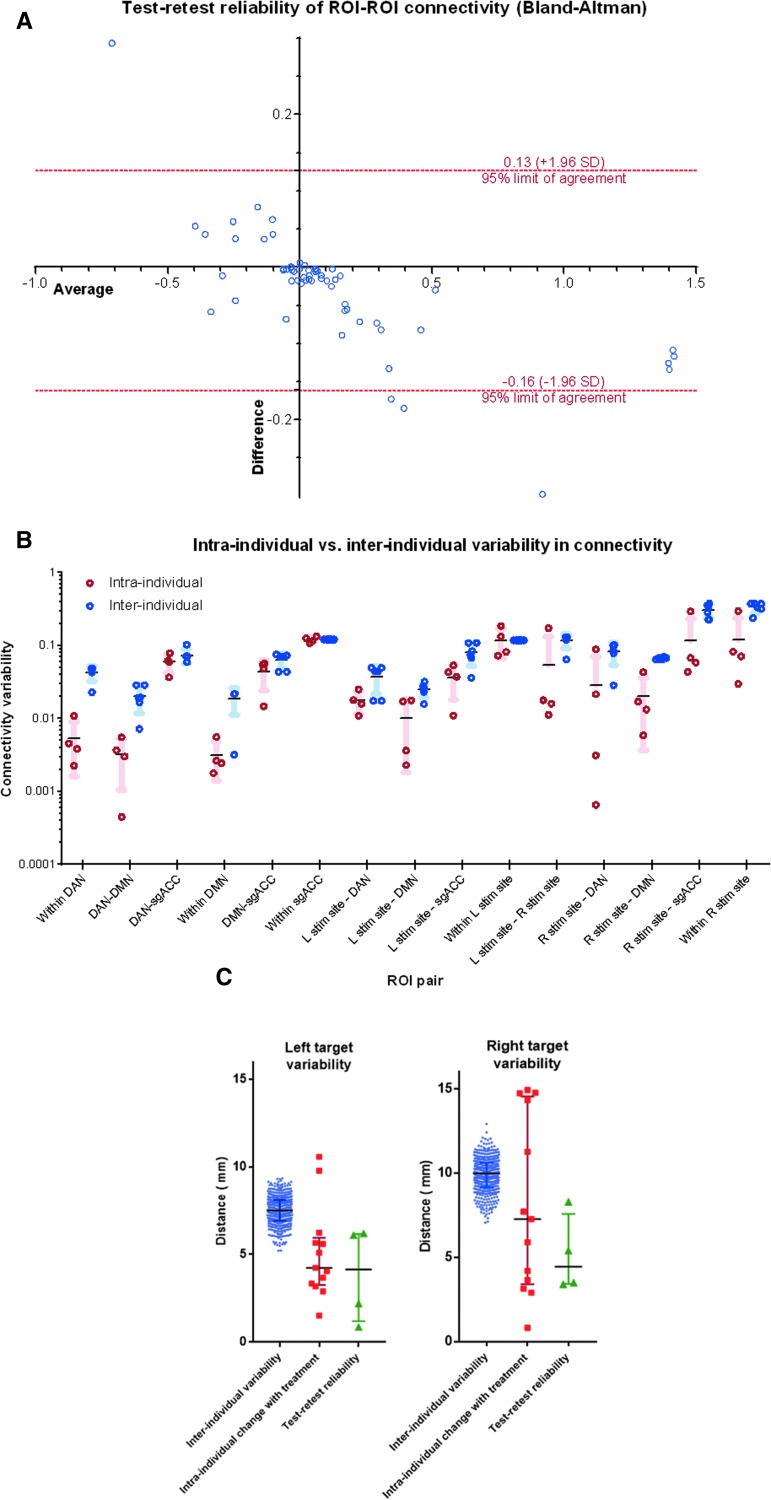
Substantial changes in connectivity were observed within and between the a priori ROIs (Fig. 6). These differences were most prominent for sgACC connectivity with the left stimulation site (Cohen's d = -1.26) and DMN (Cohen's d = 1.55). Within-ROI connectivity also demonstrated substantial changes within the left stimulation target (Cohen's d = 1.55), the right stimulation target (Cohen's d = 1.04), DAN (Cohen's d = 0.81), and DMN (Cohen's d = 0.77). These connectivity measures showed high test–retest reliability with some suggestion of regression to the mean (Fig. 7A). Despite these treatment-induced changes, within-subject connectivity changes were significantly lower than between-subject differences (p = 0.0007 across all measures, Fig. 7B).
FIG. 6.
Treatment-induced changes in functional connectivity. Active treatment led to substantial connectivity changes within and between the five a priori regions of interest (ROIs). Statistical hypothesis testing was not conducted due the early termination of the trial, but the magnitude and direction of effects were consistent with the a priori hypotheses. On average, there was an increase in the magnitude of default mode network (DMN)-subgenual anterior cingulate cortex (sgACC) correlation, dorsal attention network (DAN)-sgACC anti-correlation, and DAN-DMN anti-correlation. Connectivity generally increased within sgACC, DMN, and DAN. The left-sided stimulation site showed an increase in magnitude of anti-correlation with sgACC and DMN, but no substantial change in correlation with DAN. The right-sided stimulation site showed an increase in magnitude of anti-correlation with DMN and sgACC as well as correlation with DAN. Connectivity increased within and between the two stimulation sites. Color image is available online.
FIG. 7.
Test–retest reliability. (A) Bland-Altman analysis showed an apparent inverse relationship between test–retest mean and test–retest difference, suggesting likely regression to the mean. In general, the magnitude of test–retest differences was greatest for region of interest (ROI) pairs with high baseline positive or negative connectivity. Two outliers were both related to right stimulation site connectivity in the same subject. (B) For each ROI pair, within-subject differences in ROI-ROI connectivity were significantly lower than between-subject differences (p < 0.0007 across all measures). (C) Reliability and consistency of individualized repetitive transcranial magnetic stimulation (rTMS) targets. The calculated “optimal” left and right targets remained relatively consistent over the course of treatment, with mean variability of 5.1 mm and 9.9 mm, respectively. For the left stimulation site, permutation testing showed that these distances were significantly smaller than those expected by random inter-individual variability (p = 0.009). For the right stimulation site, this difference did not reach significance (p = 0.095). The intrinsic test–retest reliability of the targeting method ranged between 0.9 and 8.3 mm. Color image is available online.
Each subject's individualized rTMS targets remained relatively consistent over the course of treatment (Fig. 7C). The spatial distance between pre-treatment and post-treatment targets (intra-individual change) was generally lower than the mean distance between targets across all subjects (inter-individual variability). On average, the left-sided target showed intra-individual change of 5.1 mm ±2.6 mm and inter-individual variability of 7.7 ± 1.1 mm (p = 0.009). The right-sided target showed within-subject change of 9.9 mm ±8.0 mm and inter-individual variability of 11.7 mm ±1.41 mm (p = 0.095). The magnitude of inter-individual variability appeared to exceed the intrinsic test–retest reliability of the scans; targets identified using two back-to-back scanning sessions were centered 0.9-8.3 mm apart. Treatment-induced changes appeared to be within the range of the intrinsic test–retest reliability.
Discussion
Clinical findings
The results of this pilot study provide preliminary evidence for efficacy of rTMS in treatment-resistant depression associated with TBI. Treatment was associated with an apparent improvement in clinician-rated depression scales despite substantial prior treatment resistance. On average, subjects receiving active treatment had failed more than four conventional antidepressant treatments over a clinical course that included over 8 years of TBI symptoms and nearly 5 years of depressive symptoms. Secondary outcome measures suggested a trend toward improvement in self-report emotional measures and crystallized cognition. The treatments were generally well-tolerated. Significant treatment-induced adverse effects were limited to a single presyncopal episode in one subject and possible transient worsening of baseline headaches in a different subject.
While rTMS is widely accepted as an effective antidepressant treatment and a promising neurorehabilitative treatment, this study represents the first pilot double-blind randomized, controlled trial of rTMS in TBI-associated depression to our knowledge. This adds to the growing literature supporting the use of rTMS for neuropsychiatric applications. Recent literature suggests that rTMS is as effective as pharmacotherapy for depression associated with Parkinson's disease7 and stroke.8 However, this carries particular significance for TBI patients given that several trials have failed to demonstrate efficacy of conventional antidepressant pharmacotherapy in this population.11,12
The most important limitation to this study is the premature termination when only 75% of the desired minimum sample size had been recruited. While this decision was made due to external circumstances unrelated to the study, it may introduce chronological bias due to unobserved time trends in patient selection.68 Further, the small sample size limits the generalizability of the findings. Replication is particularly important in context of recent observations that rTMS may be associated with a strong placebo effect in military veterans with major depression.69 The study also was limited by the use of an imperfect sham device—while the Magstim Alpha sham coil can produce a similar auditory sensation, it is potentially distinguishable from active treatment because it does not cause facial twitches. Due to these limitations, these results should be considered preliminary and require replication in a larger multi-site setting.
Physiological findings
While the study was not initially powered for analysis of imaging data, some robust relationships were observed nonetheless. Notably, antidepressant efficacy of rTMS was related to the degree of functional anti-correlation between the right-sided stimulation site and sgACC. This is consistent with prior reports showing that antidepressant efficacy of rTMS is related to anti-correlation between the stimulation site and sgACC.29,31
Evaluation of treatment-induced changes in functional connectivity demonstrated several changes consistent with the pre-registered a priori hypotheses, suggesting successful engagement of the intended targets. Treatment-induced changes were consistent with the predicted directionality for nearly all a priori ROI pairs, including DAN to DMN, DMN to sgACC, both stimulation sites to DMN, stimulation sites to sgACC, and within-network connectivity for all five ROIs. Surprisingly, DAN connectivity with both stimulation sites was the only parameter that did not change as predicted, indicating that the observed network dynamics were not simply driven by changes within the stimulated site and the stimulated network. Given the high test–retest reliability of connectivity values observed in the study, it appears unlikely that treatment-induced changes were attributable to random chance. This suggests that rTMS can potentially modulate specific brain systems in a partially predictable manner.
In addition to the small sample size, investigation of physiological changes is limited by the use of combined left-sided excitatory and right-sided inhibitory treatment. This approach was chosen in order to maximize the likelihood of treatment efficacy, given that bilateral treatment may be more effective than unilateral treatment for primary major depression.6 However, this may affect our ability to reliably interpret physiological changes due to unpredictable interactions between the two treatments. This not only introduces confounds when investigating the direction and laterality of treatment effects, but also limits the development of clear a priori hypotheses given the lack of prior literature surrounding the physiological effects of bilateral treatment. Despite this key limitation, these data provide additional support for the notion that rTMS can modulate interactions involving DLPFC, sgACC, and DMN, which have been heavily implicated in major depression.37,70
The imaging findings also provide further support for the notion that functional connectivity-based treatment targeting may have played a role in the clinical effects observed in this study. Our protocol specifically targeted DAN, which has been implicated in the chronic sequelae of TBI25 and in major depression.70 This targeting appeared to be reliable across sessions and stable with treatment. Treatment-induced changes and baseline predictors of antidepressant response showed effects in the stimulated networks. Prior literature on the topic, by contrast, implicates frontoparietal control/central executive network.30 This discordance may be explained by the fact that group-based targeting tends to approximate the frontoparietal control network, which is the largest network in DLPFC at the group level.42,43 The most important future direction includes replication of these findings in a larger sample across multiple centers.
Using the preliminary data generated by this pilot study, a larger replication study is already underway. An upcoming follow-up study will directly compare the effects of rTMS with functional connectivity-based targeting, other rTMS targeting approaches, and effects of laterality and/or other stimulation parameters. Meanwhile, data collection is ongoing for a physiological study investigating the immediate functional connectivity effects of targeting different individualized resting-state network maps other than DAN and/or DMN, which will hopefully reveal potential targets for other TBI-associated neuropsychiatric syndromes. Such analyses are also being conducted retrospectively on rsfMRI data that incidentally targeted different RSNs in different individuals due to lack of individualized targeting. Together, these results will hopefully converge to conclusively clarify the role of rTMS in TBI and the relevance of individualized resting-state network mapping for targeting neuromodulation.
Conclusions
This pilot study represents a novel neuromodulatory approach to management of treatment-resistant depression associated with traumatic brain injury. Treatment was associated with improvement in depressive symptoms and neurophysiological changes in the targeted brain networks. Given the dearth of evidence-based treatments for this patient population, these preliminary findings suggest a clear need for larger studies to investigate different treatment approaches. If successfully replicated, this study paves the way for the use of neurostimulation to target a wide variety of behavioral manifestations of traumatic brain injury.
Acknowledgments
We would especially like to thank the experimental participants. We thank Dr. Abraham Snyder for logistical support with implementation of individualized RSN mapping. We thank Dr. Benjamin Srivastava for assisting with clinical assessments. We thank Linda Hood for technical support with MRI equipment. We thank Drs. Sindhu Jacob and Martin Wice for referring participants. We additionally acknowledge valuable discussions with Drs. Michael Fox, Steven Petersen, Charles Conway, Eric Wasserman, Sarah Lisanby, Bruce Luber, Andrew Drysdale, Irving Reti, Vani Rao, Maurizio Corbetta, Gordon Shulman, and Abraham Snyder, which led to the conception and refinement of the study design. The first author would additionally like to thank Drs. Kevin Black, Pilar Cristancho, Charles Zorumski, and C. Robert Cloninger for extensive longitudinal guidance leading to the conception of this study.
Author contributions: SHS, DLB, ARC, and NTT designed the clinical protocol. ARC provided rTMS expertise and equipment as well as functional connectivity expertise. CDH and TOL provided expertise and novel analytical scripts for individualized RSN mapping and functional connectivity analysis. ECL provided expertise for clinical implementation of RSN mapping techniques. SK provided expertise and developed scripts for functional connectivity processing. SHS coordinated the study, recruited subjects, administered treatments, processed MRI data, conducted functional connectivity analyses, and conducted all statistical analyses. NTT, CDL, and XH also administered study treatments. SHS, NTT, LT, and PS acquired MRI scans and conducted clinical assessments. SHS and DLB wrote the manuscript with intellectual contributions from all authors.
Author Disclosure Statement
SHS serves as a scientific consultant for SigNeuro LLC. DLB has served as a consultant for Pfizer Inc, Intellectual Ventures, Signum Nutralogix, Kypha Inc, Sage Therapeutics, iPerian Inc, Navigant, Avid Radiopharmaceuticals (Eli Lilly and Co.), the St Louis County Public Defender, the United States Attorney's Office, the St Louis County Medical Examiner, GLG, and Stemedica. DLB holds equity in the company Inner Cosmos. DLB receives royalties from sales of Concussion Care Manual (Oxford University Press). No conflicts of interest with the presented work. ECL holds equity in the companies Neurolutions and Inner Cosmos.
References
- 1. Pascual-Leone A., Tormos J.M., Keenan J., Tarazona F., Canete C., and Catala M.D. (1998). Study and modulation of human cortical excitability with transcranial magnetic stimulation. J. Clin. Neurophysiol. 15, 333–343 [DOI] [PubMed] [Google Scholar]
- 2. George M.S., Lisanby S.H., Avery D., McDonald W.M., Durkalski V., Pavlicova M., Anderson B., Nahas Z., Bulow P., Zarkowski P., Holtzheimer 3rd P.E., Schwartz T., and Sackeim H.A. (2010). Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch. Gen. Psychiatry 67, 507–516 [DOI] [PubMed] [Google Scholar]
- 3. George M.S., Taylor J.J., and Short E.B. (2013). The expanding evidence base for rTMS treatment of depression. Curr. Opin. Psychiatry 26, 13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perera T., George M.S., Grammer G., Janicak P.G., Pascual-Leone A., and Wirecki T.S. (2016). The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 9, 336–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Connolly K.R., Helmer A., Cristancho M.A., Cristancho P., and O'Reardon J.P. (2012). Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J. Clin. Psychiatry 73, e567–e573 [DOI] [PubMed] [Google Scholar]
- 6. Brunoni A.R., Chaimani A., Moffa A.H., Razza L.B., Gattaz W.F., Daskalakis Z.J., and Carvalho A.F. (2017). Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry 74, 143–152 [DOI] [PubMed] [Google Scholar]
- 7. Xie C.L., Chen J., Wang X.D., Pan J.L., Zhou Y., Lin S.Y., Xue X.D., and Wang W.W. (2015). Repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression in Parkinson disease: a meta-analysis of randomized controlled clinical trials. Neurol. Sci. 36, 1751–1761 [DOI] [PubMed] [Google Scholar]
- 8. Shen X., Liu M., Cheng Y., Jia C., Pan X., Gou Q., Liu X., Cao H., and Zhang L. (2017). Repetitive transcranial magnetic stimulation for the treatment of post-stroke depression: a systematic review and meta-analysis of randomized controlled clinical trials. J. Affect. Disord. 211, 65–74 [DOI] [PubMed] [Google Scholar]
- 9. Reti I.M., Schwarz N., Bower A., Tibbs M., and Rao V. (2015). Transcranial magnetic stimulation: a potential new treatment for depression associated with traumatic brain injury. Brain Inj. 29, 789–797 [DOI] [PubMed] [Google Scholar]
- 10. Kreutzer J.S., Seel R.T., and Gourley E. (2001). The prevalence and symptom rates of depression after traumatic brain injury: a comprehensive examination. Brain Inj. 15, 563–576 [DOI] [PubMed] [Google Scholar]
- 11. Fann J.R., Hart T., and Schomer K.G. (2009). Treatment for depression after traumatic brain injury: a systematic review. J. Neurotrauma 26, 2383–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fann J.R., Bombardier C.H., Temkin N., Esselman P., Warms C., Barber J., and Dikmen S. (2017). Sertraline for major depression during the year following traumatic brain injury: a randomized controlled trial. J. Head Trauma Rehabil. 32, 332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khedr E.M., Ahmed M.A., Fathy N., and Rothwell J.C. (2005). Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology 65, 466–468 [DOI] [PubMed] [Google Scholar]
- 14. Mansur C.G., Fregni F., Boggio P.S., Riberto M., Gallucci-Neto J., Santos C.M., Wagner T., Rigonatti S.P., Marcolin M.A., and Pascual-Leone A. (2005). A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 64, 1802–1804 [DOI] [PubMed] [Google Scholar]
- 15. Klein E., Kreinin I., Chistyakov A., Koren D., Mecz L., Marmur S., Ben-Shachar D., and Feinsod M. (1999). Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch. Gen. Psychiatry 56, 315–320 [DOI] [PubMed] [Google Scholar]
- 16. Eche J., Mondino M., Haesebaert F., Saoud M., Poulet E., and Brunelin J. (2012). Low- vs high-frequency repetitive transcranial magnetic stimulation as an add-on treatment for refractory depression. Front. Psychiatry 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sasaki N., Kakuda W., and Abo M. (2014). Bilateral high- and low-frequency rTMS in acute stroke patients with hemiparesis: a comparative study with unilateral high-frequency rTMS. Brain Inj. 28, 1682–1686 [DOI] [PubMed] [Google Scholar]
- 18. Shin S.S. and Pelled G. (2017). Novel neuromodulation techniques to assess interhemispheric communication in neural injury and neurodegenerative diseases. Front Neural Circuits 11, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li N., Yang Y., Glover D.P., Zhang J., Saraswati M., Robertson C., and Pelled G. (2014). Evidence for impaired plasticity after traumatic brain injury in the developing brain. J. Neurotrauma 31, 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chistyakov A.V., Soustiel J.F., Hafner H., Trubnik M., Levy G., and Feinsod M. (2001). Excitatory and inhibitory corticospinal responses to transcranial magnetic stimulation in patients with minor to moderate head injury. J. Neurol. Neurosurg. Psychiatry 70, 580–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pitkanen A. and Immonen R. (2014). Epilepsy related to traumatic brain injury. Neurotherapeutics 11, 286–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scheid R. and von Cramon D.Y. (2010). Clinical findings in the chronic phase of traumatic brain injury: data from 12 years' experience in the Cognitive Neurology Outpatient Clinic at the University of Leipzig. Dtsch. Arztebl. Int. 107, 199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koski L., Kolivakis T., Yu C., Chen J.K., Delaney S., and Ptito A. (2015). Noninvasive brain stimulation for persistent postconcussion symptoms in mild traumatic brain injury. J. Neurotrauma 32, 38–44 [DOI] [PubMed] [Google Scholar]
- 24. Han K., Mac Donald C.L., Johnson A.M., Barnes Y., Wierzechowski L., Zonies D., Oh J., Flaherty S., Fang R., Raichle M.E., and Brody D.L. (2014). Disrupted modular organization of resting-state cortical functional connectivity in U.S. military personnel following concussive 'mild' blast-related traumatic brain injury. Neuroimage 84, 76–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han K., Chapman S.B., and Krawczyk D.C. (2016). Disrupted intrinsic connectivity among default, dorsal attention, and frontoparietal control networks in Individuals with chronic traumatic brain injury. J. Int. Neuropsychol. Soc. 22, 263–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caeyenberghs K., Verhelst H., Clemente A., and Wilson P.H. (2016). Mapping the functional connectome in traumatic brain injury: what can graph metrics tell us? Neuroimage [DOI] [PubMed] [Google Scholar]
- 27. Laumann T.O., Gordon E.M., Adeyemo B., Snyder A.Z., Joo S.J., Chen M.Y., Gilmore A.W., McDermott K.B., Nelson S.M., Dosenbach N.U., Schlaggar B.L., Mumford J.A., Poldrack R.A., and Petersen S.E. (2015). Functional system and areal organization of a highly sampled individual human brain. Neuron 87, 657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mueller S., Wang D., Fox M.D., Yeo B.T., Sepulcre J., Sabuncu M.R., Shafee R., Lu J., and Liu H. (2013). Individual variability in functional connectivity architecture of the human brain. Neuron 77, 586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fox M.D., Buckner R.L., White M.P., Greicius M.D., and Pascual-Leone A. (2012). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry 72, 595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liston C., Chen A.C., Zebley B.D., Drysdale A.T., Gordon R., Leuchter B., Voss H.U., Casey B.J., Etkin A., and Dubin M.J. (2014). Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol. Psychiatry 76, 517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weigand A., Horn A., Caballero R., Cooke D., Stern A.P., Taylor S.F., Press D., Pascual-Leone A., and Fox M.D. (2017). Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol. Psychiatry. 84, 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Philip N.S., Barredo J., van 't Wout-Frank M., Tyrka A.R., Price L.H., and Carpenter L.L. (2018). Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol. Psychiatry 83, 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dougherty D.D., Weiss A.P., Cosgrove G.R., Alpert N.M., Cassem E.H., Nierenberg A.A., Price B.H., Mayberg H.S., Fischman A.J., and Rauch S.L. (2003). Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J. Neurosurg. 99, 1010–1017 [DOI] [PubMed] [Google Scholar]
- 34. Mayberg H.S., Liotti M., Brannan S.K., McGinnis S., Mahurin R.K., Jerabek P.A., Silva J.A., Tekell J.L., Martin C.C., Lancaster J.L., and Fox P.T. (1999). Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry 156, 675–682 [DOI] [PubMed] [Google Scholar]
- 35. Mayberg H.S., Lozano A.M., Voon V., McNeely H.E., Seminowicz D., Hamani C., Schwalb J.M., and Kennedy S.H. (2005). Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 [DOI] [PubMed] [Google Scholar]
- 36. Fox M.D., Liu H., and Pascual-Leone A. (2013). Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. NeuroImage 66, 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi K.S., Riva-Posse P., Gross R.E., and Mayberg H.S. (2015). Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 72, 1252–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., and Raichle M.E. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U. S. A. 103, 10046–10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gao W. and Lin W. (2012). Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum. Brain Mapp. 33, 192–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Raichle M.E. (2015). The brain's default mode network. Annu. Rev. Neurosci. 38, 433–447 [DOI] [PubMed] [Google Scholar]
- 41. Gordon E.M., Laumann T.O., Adeyemo B., Huckins J.F., Kelley W.M., and Petersen S.E. (2016). Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex 26, 288–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gordon E.M., Laumann T.O., Adeyemo B., and Petersen S.E. (2015). Individual variability of the system-level organization of the human brain. Cereb. Cortex 27, 386–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gordon E.M., Laumann T.O., Adeyemo B., Gilmore A.W., Nelson S.M., Dosenbach N.U., and Petersen S.E. (2016). Individual-specific features of brain systems identified with resting state functional correlations. Neuroimage 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharp D.J., Scott G., and Leech R. (2014). Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 10, 156–166 [DOI] [PubMed] [Google Scholar]
- 45. van der Horn H.J., Liemburg E.J., Scheenen M.E., de Koning M.E., Spikman J.M., and van der Naalt J. (2017). Graph analysis of functional brain networks in patients with mild traumatic brain injury. PLoS One 12, e0171031–e0171031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang D., Buckner R.L., Fox M.D., Holt D.J., Holmes A.J., Stoecklein S., Langs G., Pan R., Qian T., Li K., Baker J.T., Stufflebeam S.M., Wang K., Wang X., Hong B., and Liu H. (2015). Parcellating cortical functional networks in individuals. Nat. Neurosci. 18, 1853–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hacker C.D., Laumann T.O., Szrama N.P., Baldassarre A., Snyder A.Z., Leuthardt E.C., and Corbetta M. (2013). Resting state network estimation in individual subjects. Neuroimage 82, 616–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Glasser M.F., Coalson T.S., Robinson E.C., Hacker C.D., Harwell J., Yacoub E., Ugurbil K., Andersson J., Beckmann C.F., Jenkinson M., Smith S.M., and Van Essen D.C. (2016). A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Siddiqi S.H. (2016). rTMS for major depression associated with TBI. Open Science Framework [Google Scholar]
- 50. (1993) Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 8, 86–87 [Google Scholar]
- 51. Asberg M., Montgomery S.A., Perris C., Schalling D., and Sedvall G. (1978). A comprehensive psychopathological rating scale. Acta Psychiatr. Scand. Suppl, 5–27 [DOI] [PubMed] [Google Scholar]
- 52. Svrakic D.M., Whitehead C., Przybeck T.R., and Cloninger C.R. (1993). Differential diagnosis of personality disorders by the seven-factor model of temperament and character. Arch. Gen. Psychiatry 50, 991–999 [DOI] [PubMed] [Google Scholar]
- 53. Salsman J.M., Butt Z., Pilkonis P.A., Cyranowski J.M., Zill N., Hendrie H.C., Kupst M.J., Kelly M.A., Bode R.K., Choi S.W., Lai J.S., Griffith J.W., Stoney C.M., Brouwers P., Knox S.S., and Cella D. (2013). Emotion assessment using the NIH Toolbox. Neurology 80, S76–S86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tulsky D.S., Carlozzi N.E., Holdnack J., Heaton R.K., Wong A., Goldsmith A., and Heinemann A.W. (2017). Using the NIH Toolbox Cognition Battery (NIHTB-CB) in individuals with traumatic brain injury. Rehabil. Psychol. 62, 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dowson A.J. (2001). Assessing the impact of migraine. Curr. Med. Res. Opin. 17, 298–309 [PubMed] [Google Scholar]
- 56. Liu B., Zhang Y., Zhang L., and Li L. (2014). Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry 14, 342–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fitzgerald P.B., Hoy K., McQueen S., Maller J.J., Herring S., Segrave R., Bailey M., Been G., Kulkarni J., and Daskalakis Z.J. (2009). A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology 34, 1255–1262 [DOI] [PubMed] [Google Scholar]
- 58. Siegel J.S., Mitra A., Laumann T.O., Seitzman B.A., Raichle M., Corbetta M., and Snyder A.Z. (2016). Data quality influences observed links between functional connectivity and behavior. Cereb. Cortex 2, 4492–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., and Petersen S.E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 84, 320–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee M.H., Miller-Thomas M.M., Benzinger T.L., Marcus D.S., Hacker C.D., Leuthardt E.C., and Shimony J.S. (2016). Clinical resting-state fMRI in the preoperative setting: are we ready for prime time? Top. Magn. Reson. Imaging 25, 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fischl B. and Dale A.M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 97, 11050–11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Klomjai W., Katz R., and Lackmy-Vallee A. (2015). Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 58, 208–213 [DOI] [PubMed] [Google Scholar]
- 63. Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., and Smith S.M. (2012). FSL. Neuroimage 62, 782–790 [DOI] [PubMed] [Google Scholar]
- 64. Bullmore E.T., Suckling J., Overmeyer S., Rabe-Hesketh S., Taylor E., and Brammer M.J. (1999). Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans. Med. Imaging. 18, 32–42 [DOI] [PubMed] [Google Scholar]
- 65. Awiszus F. (2011). Fast estimation of transcranial magnetic stimulation motor threshold: is intrinsic functional connectivity MRI. Cereb. Cortex 1–20 [DOI] [PubMed] [Google Scholar]
- 66. Slotnick S.D. (2017). Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cogn. Neurosci. 8, 150–155 [DOI] [PubMed] [Google Scholar]
- 67. Greenland S. (1987). Interpreting time-related trends in effect estimates. J. Chronic Dis. 40 Suppl 2, 17S–24S [DOI] [PubMed] [Google Scholar]
- 68. Yesavage J.A., Fairchild J.K., Mi Z., Biswas K., Davis-Karim A., Phibbs C.S., Forman S.D., Thase M., Williams L.M., Etkin A., O'Hara R., Georgette G., Beale T., Huang G.D., Noda A., and George M.S. (2018). Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kaiser R.H., Andrews-Hanna J.R., Wager T.D., and Pizzagalli D.A. (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Müller M.J., et al. (2003). Differentiating moderate and severe depression using the Montgomery-Asberg depression rating scale (MADRS). J Affect Disord 77, 255–260 [DOI] [PubMed] [Google Scholar]