Abstract
Significance: Highly prevalent in Western cultures, obesity, metabolic syndrome, and diabetes increase the risk of cardiovascular morbidity and mortality and cost health care systems billions of dollars annually. At the cellular level, obesity, metabolic syndrome, and diabetes are associated with increased production of reactive oxygen species (ROS). Increased levels of ROS production in key organ systems such as adipose tissue, skeletal muscle, and the vasculature cause disruption of tissue homeostasis, leading to increased morbidity and risk of mortality. More specifically, growing evidence implicates the nicotinamide adenine dinucleotide phosphate oxidase (NOX) enzymes in these pathologies through impairment of insulin signaling, inflammation, and vascular dysfunction. The NOX family of enzymes is a major driver of redox signaling through its production of superoxide anion, hydrogen peroxide, and attendant downstream metabolites acting on redox-sensitive signaling molecules.
Recent Advances: The primary goal of this review is to highlight recent advances and survey our present understanding of cell-specific NOX enzyme contributions to metabolic diseases.
Critical Issues: However, due to the short half-lives of individual ROS and/or cellular defense systems, radii of ROS diffusion are commonly short, often restricting redox signaling and oxidant stress to localized events. Thus, special emphasis should be placed on cell type and subcellular location of NOX enzymes to better understand their role in the pathophysiology of metabolic diseases.
Future Directions: We discuss the targeting of NOX enzymes as potential therapy and bring to light potential emerging areas of NOX research, microparticles and epigenetics, in the context of metabolic disease.
Keywords: NADPH oxidases, metabolic disease, obesity, diabetes, ROS
Introduction
Obesity and diabetes have a profound effect on quality of life and place increasing financial and clinical burdens on our health care system (85, 194). In Western cultures where inactivity, high-fat intake, and nutrient excess are rampant, this poses a particular challenge. Considerable efforts have been channeled toward understanding metabolic disorders at the basic science and clinical levels.
Germane to this review, it is generally accepted that reactive oxygen species (ROS) play an important role in metabolic disorders and associated cardiovascular diseases (7, 86, 132, 136), including hypertension, hypercholesterolemia, coronary heart disease, and stroke (85). Nonetheless, as described recently by the COST Action BM1203 (EU-ROS) team (56), ROS and oxidative stress are all-too-commonly invoked on a casual basis for disease in an oversimplified way. Instead, complex redox biology should be viewed on the basis of the source of ROS, the amount and type of reactive species being produced, cellular location, and the redox-sensitive targets affected. Uncovering the source, localization, nature, and quantity of ROS, their redox modifiable targets, and downstream signaling during the development of obesity and metabolic disease could reveal important new therapeutic strategies to prevent or even halt disease progression.
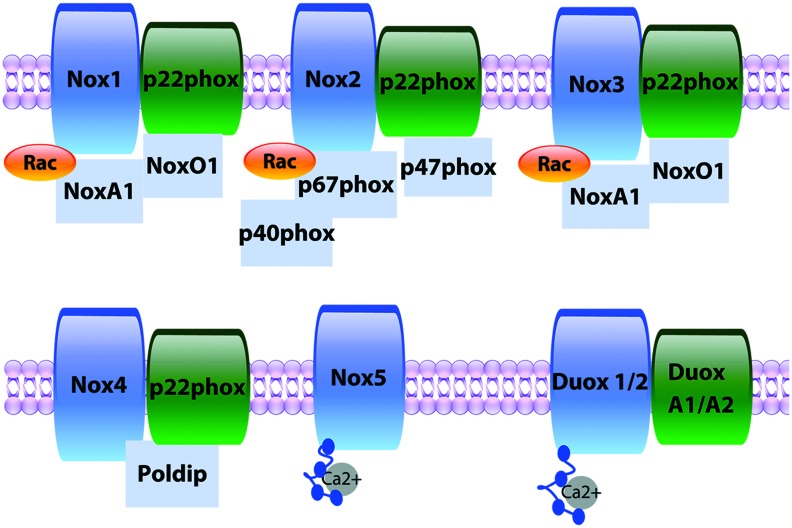
The NADPH (nicotinamide adenine dinucleotide phosphate) oxidase (NOX) family of enzymes (Nox 1–5 and dual oxygenase [Duox] 1 and 2) (Fig. 1) is a professional superoxide anion (O2•−) or hydrogen peroxide (H2O2) generator (13), whose structure and complex formation have been the topic of many reviews (13, 73, 142, 143). In brief, activation of NOXs 1–3 requires coordinated complexation of membrane and cytosolic subunits the same as or related to the classical phagocytic subunits p22phox, p47phox, and p67phox to produce O2•− (8, 13, 34, 51, 67, 146, 156, 169, 207). Nox4 associates with p22phox in the membrane and does not require classical cytosolic subunits or their homologs, rather, activity can be mediated by Poldip2 in the cytoplasm, with reports showing both O2•− and H2O2 generation (4, 126). Nox5 and Duoxs 1 and 2 reportedly to date do not require cytosolic subunits and are activated by calcium binding to intracellular N-terminal EF hand motifs to produce O2•− and one or both of O2•− and H2O2, respectively (9, 14, 43, 55). NOXs are found in virtually every cell type and serve important roles in physiological and pathological signaling (13). Indeed, NOX enzymes have been implicated in the pathogenesis of metabolic disorders. Nevertheless, the investigation of the specific NOX isoform or isoforms involved and their functional roles has been limited by, among other factors: (i) the use of the inhibitors diphenylene iodonium (DPI) and apocynin, which are NOX isoform nonspecific and also act to inhibit other ROS-producing enzymes or as an antioxidant; (ii) data from global (also noninducible) knockout (KO) animals may be confounded by effects on other tissues or compensation during development; and (iii) limitations of present experimental tools, including a lack of effective antibodies for all NOX subunits, continued use of suboptimal ROS probes, and limited methods for detecting NOX redox targets, although recent advancements in oxidative proteomics could greatly enhance the ability to identify these targets. In addition, the field faces the challenge of addressing the exact mechanisms through which redox signaling is propagated in the presence of an extensive antioxidant system, which has a significantly lower Km value for H2O2 than modifiable cysteines in proteins. Whether NOX redox signaling directly oxidizes kinase cysteines, requires local inactivation of antioxidants such as peroxiredoxin, or relies on peroxiredoxin-mediated oxidative relay is poorly understood and remain critical questions in the field.
FIG. 1.
NOX isozymes. NOX complexes consist of their eponymous membrane-spanning catalytic subunit, which transfers electrons from NADPH through C-terminal NADPH- and FAD-binding sites and heme groups and reduces oxygen to superoxide anion. Nox1, 2, 3, and 4 complex with the stabilizing p22phox membrane subunit. Canonical Nox2 requires assembly with cytosolic subunits (p47phox, p67phox, p40phox), while Nox1 and 3 require NoxA1 and NoxO1, the equivalent homologues for p47phox, and p67phox, respectively. Nox4 appears constitutively active and does not require cytosolic subunits but its activity can be modulated by Poldip2. In contrast, Nox5 and Duox 1/2 contain EF hand domains requiring calcium activation. NADPH, nicotinamide adenine dinucleotide phosphate; NOX, NADPH oxidase. Color images are available online.
Obesity and diabetes are complex conditions driven by a number of mechanisms, including inflammation, metabolic disruption, and ROS signaling. Numerous sources of ROS are implicated in the progression of metabolic diseases and resulting complications. The purpose of this review is to highlight recent advances and our present knowledge of specific NOX isoforms as to cell type and attendant signaling in the progression from normal metabolism to overweightness, obesity, metabolic syndrome, and diabetes. In this review, we present the gaps in knowledge and potential for future research endeavors. We focus on two key metabolic tissues (skeletal muscle and adipose) with additional emphasis placed on NOX in vascular pathologies, which are emerging as a leading cause of mortality in obese and diabetic patients. Notably, in this relatively nascent field, the literature is sparse in a number of areas.
Skeletal Muscle
In aggregate, skeletal muscle is a major metabolic organ in the human body. Reduced skeletal muscle energy consumption (i.e., physical inactivity) and consequential nutrient excess are major risk factors for the development of metabolic disorders (187). Skeletal muscle expresses Noxs1, 2, 4, and Duox1, and metabolic cues such as ATP, glucose, and fatty acids play a regulatory role in NOX expression and activity (49, 125, 160, 185). In response to exercise, NOX expression increases in a fiber-type-specific manner; specifically, Nox2 increases in type-2a fibers and Nox4 increases in type-1 fibers (125). In fact, Nox2 is activated in contracting muscle (demonstrated by Nox2-p47phox proximity ligation assay) (89) by the ATP-protein kinase C (PKC) pathway (49) as well as by mechanical forces, potentially suggesting that NOX enzymes play a beneficial role in sensing muscle activity and mediating adaptations to those stimuli. Beneficial roles could include exercise adaptation (69, 94, 123), Ca2+ handling (94), glucose uptake (189), and insulin signaling (58). On the contrary, Souto Padron de Figueiredo et al. found that global Nox2 (KO) mice were protected from high-fat diet (HFD)-induced insulin resistance, suggesting that Nox2 may contribute to obesity-associated skeletal muscle insulin resistance (185). Considering the silencing of Nox2 was not cell specific, preserved insulin sensitivity might also be attributed to effects in other cell types (e.g., immune and endothelial cells). For instance, metabolically activated endothelial Nox2 contributes to endothelial insulin resistance, which has been suggested to impair skeletal muscle interstitial insulin levels and promote insulin resistance (111). In addition, innate immune cell populations, especially macrophages enriched in the Nox2 system, are elevated in skeletal muscle in both obese humans and mice (61, 102, 158). Interestingly, metabolically activated macrophage-derived conditioned media induce myocyte insulin resistance via PKC ϴ and Ɛ (101), known mediators of redox signaling. It is thus plausible that localized activation of NOX enzymes in these other cell types under obese or diabetic conditions could be damaging to skeletal muscle insulin sensitivity (101, 111) and could potentially impair other immune cell-mediated events, such as muscle recovery after ischemic injuries (84).
Homing in on the myocyte, short hairpin RNA (shRNA) knockdown of Nox2 in immortalized mouse myoblasts blocked high glucose- and high fat-induced suppression of insulin signaling (measured by pAkt) (185), suggesting that Nox2 or its oxidative metabolites play a deleterious autocrine role in skeletal muscle insulin resistance. Yet, it should be pointed out that, at basal levels (unstimulated conditions), knockdown of Nox2 surprisingly lowered insulin-induced phosphorylation of protein kinase B (Akt) without affecting glucose uptake, suggesting that Nox2-p-Akt signaling may play different roles on insulin resistance in the presence and absence of high glucose/fat challenge (185).
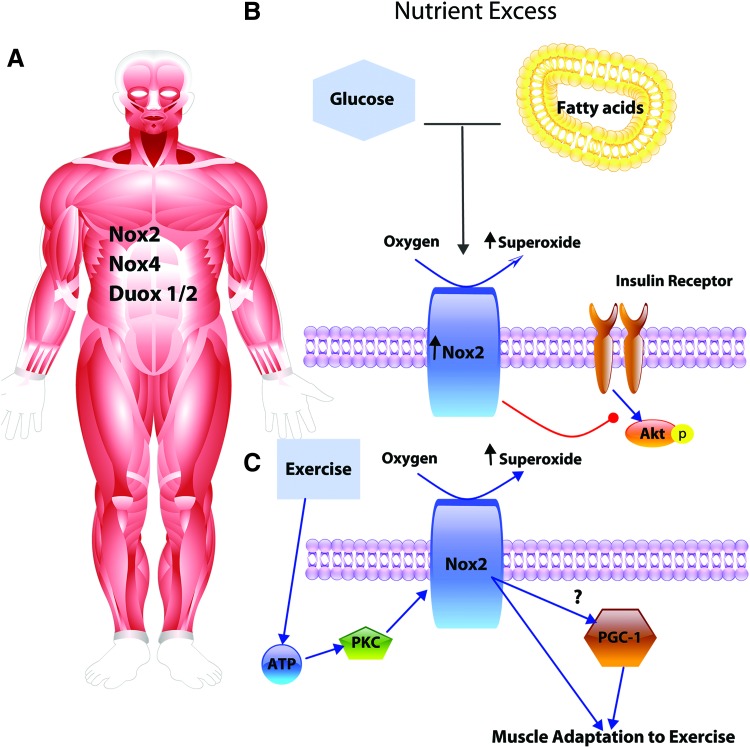
Taken together, those findings suggest that Nox2 signaling may mediate beneficial effects of exercise, and may also contribute to metabolic impairments associated with type II diabetes and obesity. Further investigation of these opposing effects is warranted and may depend on the quantity and location of ROS production (Fig. 2). In addition to its important metabolic functions, skeletal muscle interacts with cells in its milieu in a paracrine and perhaps even an endocrine manner. H2O2 produced during muscle contraction activates PGC-1α (69, 93), which regulates myokine production and muscle repair (52, 63, 139). These myokines play important endocrine roles throughout the body, such as in the regulation of adipose tissue phenotype and function as recently reviewed (21). Specifically, a number of myokines could potentially mediate vascular or adipose tissue NOXs; interleukin (IL)-6, BDNF, and IL-15 have been implicated to increase the expression or activity of NOX enzymes (98, 107, 200, 209), while irisin and IL-10 may act to downregulate NOX (97, 230). How the beneficial effects of exercise are transduced through myokine-mediated NOX expression and activity is relatively unknown. Therefore, future research aimed at understanding the role of NOX enzymes in skeletal muscle endocrine signaling and myokine impact on NOX expression and function in other tissues would be of great intrigue and interest.
FIG. 2.
Skeletal muscle Nox2 implicated in insulin resistance and exercise adaptation. (A) Skeletal muscle expresses Nox2, Nox4, and DUOX1/2. (B) In obesity and diabetes, Nox2 is upregulated. Increased Nox2 levels and ROS production induced by hyperglycemia and hyperlipidemia contribute to skeletal muscle insulin resistance, potentially through reduced insulin activation of Akt. (C) In response to exercise, an ATP-PKC pathway activates Nox2 leading to cellular adaptation, potentially mediated through PGC-1α. Akt, protein kinase B; PKC, protein kinase C; ROS, reactive oxygen species. Color images are available online.
Adipose Tissue (Visceral)
Adipose tissue is composed of a variety of cell types: adipocytes, preadipocytes, fibroblasts, vascular and lymphatic endothelial cells, and a number of resident immune cells, suggesting that a complex interaction between cell types sustains adipose tissue physiology and pathophysiology. Adipose depots act as the main storage sites for excess nutrients and, more specifically, visceral depots appear to represent an initial site of metabolic dysfunction on the road to obesity, metabolic syndrome, and type II diabetes. This is not mediated merely by global adipose depot expansion or increased adipocyte size (typified by the “healthy obese phenotype”), but requires visceral depot expansion and the disruption of autocrine, paracrine and endocrine adipose signaling (18, 135, 150). Further exemplifying this, lipodystrophy (loss of adipose tissue) induces metabolic derangements, such as insulin resistance, in the absence of obesity (106). This is potentially due to lipid accumulation in other tissues disrupting metabolic homeostasis and/or the loss of important adipokines such as adiponectin and leptin, which have been shown to modulate vascular NOX enzymes (96).
Adipocytes
A shift in adipose redox balance is a hallmark of metabolic disorders (86). Among the different NOXs, Nox4 is the most extensively studied in adipocytes (128, 175) while a few reports also examined changes in Nox 1 and 2 expression (174, 175). In healthy adipocytes, Nox4-mediated H2O2 production is increased following stimulation by insulin, amplifying its signaling through inhibition of the protein tyrosine phosphatase 1b (PTP1b), which promotes insulin receptor activation and glucose uptake (128). Along the same lines, in preadipocytes, Nox4-derived H2O2 is shown to augment insulin-mediated Akt activation and drive differentiation of preadipocytes into adipocytes (175). Nevertheless, the direct mechanisms and physiological implications of this Nox4-insulin cross talk in preadipocytes and mature adipocytes merit deeper investigation.
Likely an early occurrence in disease progression, nutrient excess leads to increased generation of intracellular H2O2 from Nox4 but not from mitochondria, causing expression of chemotactic factor genes and local inflammation that result in downstream insulin resistance in differentiated 3T3–L1 adipocytes (87). Importantly, in the same study, all of the NOX and Duox isoforms were probed and Nox4 was singularly and robustly induced at the RNA level, but less so at the protein level, perhaps suggesting a rapid turnover of Nox4 protein under glycolytic conditions. With little change in protein levels, the induction of Nox4-derived H2O2 is likely due to increased activity by posttranslational modification, potentially mediated by Poldip2 (126). Intriguingly, one recent study by Paredes et al. suggests that Poldip2 also plays a role in mitochondrial respiration; that is, downregulation of Poldip2 causes a shift toward glycolysis (155).
3T3–L1 adipocytes in culture exposed to high glucose and palmitate for 7 days exhibited increased glycolytic capabilities, NADPH content, and Nox4-derived H2O2 generation. Inhibition of the pentose phosphate pathway (PPP) or silencing of Nox4 both reduced H2O2 production and expression of monocyte chemoattractant protein 1 (MCP-1) (87), suggesting that PPP and Nox4 work in series to promote an inflammatory phenotype. Nox4 has been shown to activate NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), a transcriptional regulator of MCP-1, and thus, relocalization and increased Nox4 activity may mediate this action of NF-κB intracellularly contributing to the inflammatory phenotype. Interestingly, these effects of Nox4 appear to be dependent on their localization to lipid rafts (87), although the signaling pathways underlying the translocation of existing Nox4 or directed localization of newly translated Nox4 in response to nutrient excess are unknown. Furthermore, whether Nox4-mediated redox signaling, via its intracellular molecular targets or cell-to-cell communication, is altered with the translocation is also unknown. It is, however, essential to point out that adipose tissue is heterogeneous (discussed in Resident and infiltrating immune cells section). This study suggests that redistribution of Nox4 to the lipid rafts increases adipocyte ROS production, intracellular NF-κB activation, and chemotactic signaling. Nonetheless, the direct consequences of these on intracellular or extracellular ROS and neighboring nonadipocytes remain undetermined. One plausible consequence could be that excessive extracellular H2O2 may mediate shifts in resident macrophage polarization in adipose tissue, as H2O2 has been shown to induce production of tumor necrosis factor (TNF)-α (141).
In vivo experiments support some of the findings revealed in cell culture. Global KO of Nox4 accelerated HFD-induced insulin resistance in mouse adipose tissue compared with wild type (WT) HFD controls (117). This is consistent with a fundamental salutary role of Nox4 on adipocyte insulin signaling even under pathological conditions. Furthermore, Nox4 also plays an essential role in adipocyte differentiation (175) potentially causing reduced adipocyte numbers in these mice, which could result in greater adipocyte hypertrophy under HFD. Indeed, adipocyte hypertrophy is associated with insulin resistance even in lean or healthy weight individuals (2, 82). Taken together, this may suggest that deficient Nox4 signaling during development or childhood resulting in diminished adipocyte number could predispose individuals to adipocyte hypertrophy and type II diabetes.
In addition, on account of the ubiquitous deletion of Nox4, contribution of this NOX to insulin resistance appears more complex and exhibits cell-type dependence. For instance, Nox4 in endothelial cells promotes insulin signaling and likely contributes to the observed accelerated loss of insulin sensitivity in global Nox4 KO (137). On the contrary, an adipocyte-specific Nox4 KO resulted in delayed insulin resistance and inflammation during the development of obesity (45). This effect might be attributed to the role of adipocyte Nox4, per se, in insulin resistance via recruiting immune cells (87), which could suppress insulin signaling through inflammatory cytokines, such as TNF-α. Consistent with this line of reasoning, when adipocyte ROS are elevated or glutathione defense is depleted, HFD-induced adipose dysfunction is accelerated (149). Dysfunction in these animals reportedly relates to restricted adipose expansion and an unfavorable profile of adipose inflammation, fibrosis, and lipogenesis (45). Furthermore, similar to cell culture experiments, KO of glucose-6-phosphate dehydrogenase, a rate-limiting enzyme of the PPP, prevents insulin resistance and adipose tissue inflammation (81). These experiments strongly suggest a link between the PPP and Nox4 activity in mediating immune infiltration and diabetic disease progression. Thus, it appears that adipocyte Nox4-derived H2O2 is essential for adipocyte function under physiological conditions, but redistribution and exacerbated Nox4 activity in metabolic diseases may initiate adipose inflammation and insulin resistance. Use of time-inducible adipocyte-specific Nox4 KO mice and coculture (immune cells and adipocytes) in future studies could help clarify the role of Nox4 in early versus late stages of metabolic disorders.
Knowledge of a role for Nox2 in adipocyte function and pathology is available yet scarce. Elevated expression of adipocyte Nox2 has not been shown under high-glucose or high-palmitate conditions in cultured 3T3–L1 adipocytes, but was reported in response to uric acid exposure (174), which is commonly elevated in metabolic disorders (31, 133, 196). Adipocytes challenged with uric acid showed NOX-derived ROS (likely O2•−)-mediated impairment of nitric oxide signaling (174), however, the specific NOX isoform responsible was not identified.
Resident and infiltrating immune cells
In healthy adipose tissue, metabolic homeostasis is, in part, dependent on resident immune cells. That is, resident eosinophils release IL-4 and maintain an alternatively activated macrophage (AAM) population (212). AAMs appear to promote adipocyte insulin signaling, nutrient uptake gene expression, and adiponectin production (147, 212). Indeed, depletion of eosinophils greatly reduced adipose AAM populations, which exacerbated HFD-induced insulin resistance (212). In this regard, AAMs appear to be protective even under HFD conditions. Intriguingly, transgenic overexpression of IL-5, which is known to increase the levels of resident eosinophils in adipose tissue, preserves adipose insulin sensitivity and systemic glucose tolerance (170), potentially through eosinophil-IL-4-mediated AAM retention.
With respect to the role of ROS on eosinophils in the adipose tissue, excessive H2O2 causes eosinophil apoptosis (168) and eosinophil populations decrease in adipose tissue with obesity (15). Combined with the observation that exposure to excess nutrients increases adipocyte ROS production (87), previous studies suggest that adipocyte-derived ROS may contribute to the observed loss of eosinophil and AAM in adipose tissue during the development of obesity. Therefore, experimental evidence is needed to definitively confirm this and identify the potential source of H2O2 leading to the loss of homeostatic immune cells.
Obesity is not only associated with a loss of beneficial or homeostatic immune populations but is likely also characterized by increasing populations of inflammatory immune cells, specifically macrophages. As discussed in the Adipocytes section, increased activity of adipocyte Nox4 in obesity, per se, is linked to adipocyte MCP-1 production (87). Consequently, potentiated infiltration of inflammatory phagocytes (145) would be expected to increase vicinal Nox2, the well-accepted most abundant NOX in phagocytes. In particular, infiltrating immune cell populations are predominantly comprised of macrophages (145), which express high levels of Nox2. As a consequence of exposure to excess nutrients and adipocyte inflammatory cytokines, macrophages are driven toward a proinflammatory polarization (109).
Shifting to NOX signaling in macrophages, we now focus on the roles NOXs play in the propagation of macrophage populations in adipose tissue. In macrophages, Nox2 can be activated by glucose and fatty acids (92), leading to NF-κB-mediated activation of inflammation (138) and inhibition of IL-10 (10), promoting an inflammatory phenotype. Release of inflammatory cytokines from proinflammatory macrophages, such as TNF-α, can further augment Nox2 activation, reinforcing the deleterious proinflammatory polarization of the macrophage population (220). The growing population of proinflammatory macrophages in obese adipose tissue gives rise to a low-grade inflammatory and oxidative environment, which further disrupts tissue homeostasis.
A potential disruption mediated by vicinal inflammatory macrophages is the reduction of the adipokine adiponectin in adipocytes, which is decreased in a dose-dependent manner by exogenous H2O2 (64). Furthermore, adiponectin is a key inducer of nitric oxide, IL-10, and AAM polarization. Therefore, diminished production of adiponectin in obesity likely contributes to a vicious cycle of higher NOX expression, ROS production, and attendant inflammation (5, 32, 148). Indeed, the reduction in adipose adiponectin production represents a key event in the progression of metabolic diseases; as such it has been targeted as a therapeutic for obesity and type II diabetes (1). In addition, loss of adiponectin, increased ROS, and resultant inflammation appear to be key factors in decreased sensitivity to insulin.
In obesity, the accumulation of proinflammatory macrophages in adipose tissue interferes with insulin sensitivity. In culture, macrophages impair adipocyte insulin signaling (215) through a TNF-α- and NF-κB-mediated increase in adipocyte PTP1b (184). PTP1b negatively regulates insulin receptor phosphorylation and diminishes insulin signaling. In healthy subjects, the autocrine role of Nox4-driven oxidation and inactivation of PTP1b leads to augmented insulin signaling. This is potentially disrupted by glucose and palmitate (128). It is thus likely that obesity-related upregulation of PTP1b expression and reduced inactivation contribute to the associated insulin resistance in adipocytes. Furthermore, because NF-κB is redox sensitive at higher levels of ROS (likely H2O2), paracrine H2O2 derived from phagocytic Nox2 may also contribute to upregulation of PTP1b expression. This could mean that autocrine H2O2 directly inactivates adipocyte PTP1b, while potential paracrine H2O2 from macrophages increases adipocyte PTP1b expression through NF-κB.
In vitro work establishes the potential for deleterious Nox2 upregulation of inflammation in macrophages, contributing to adipose tissue dysfunction. However, there are scant data on the direct in vivo actions of macrophage (or other myeloid cells) Nox2 on adipose function. While little is known about the direct actions of adipose tissue phagocyte Nox2 in vivo, we can glean some information perhaps from studies in global Nox2 KO mice. First, it appears that deletion of Nox2 reduces immune cell (especially macrophage and T cell) recruitment (24) and infiltration into adipose tissue in obesity (159). Second, KO of Nox2 preferentially shifts macrophages toward the anti-inflammatory AAM phenotype (159), likely explained by the role of Nox2 in the activation of inflammatory transcription factors (AP1 and NF-κB), inhibition of IL-10, and glucose metabolism discussed above (138).
Interestingly, Coats et al. (37) showed that phagocytic Nox2 may have a dual role in adipose tissue from obese mice. Nox2 KO mice fed a HFD for 8 weeks maintained normal insulin signaling and exhibited fewer proinflammatory cytokines compared with HFD controls as would be expected since Nox2 activates proinflammatory signaling. Conversely, after prolonged (16 weeks) exposure to HFD, the protective effect of Nox2 KO was lost and Nox2 KO mice presented with adipose tissue insulin resistance and inflammation similar to HFD-fed WT mice. Moreover, at 16 weeks, HFD-fed Nox2 KO mice exhibited increased lipid deposition in the liver, corroborating a similar finding from an earlier article by Costford et al. (38).
To test for a more definitive role of myeloid cells, studies were recapitulated in mice with a myeloid-specific ablation of Nox2 (37). While myeloid deletion affects numerous cell types (basophil, neutrophil, etc.), the authors show that these effects are likely due to the decreased ability of macrophages lacking Nox2 to breakdown lipids. Mechanistically, in macrophages, Nox2-dependent exocytosis of lipases was needed to break down apoptotic adipocytes into free fatty acids for uptake. This finding highlights a potentially beneficial role of macrophage Nox2 in apoptotic cell clearance, especially since Nox2 can be activated by apoptotic by-products, such as ATP.
However, this finding was not corroborated in a similar study by Pepping et al. In that study, 16 weeks of HFD-induced macrophage infiltration, adipose inflammation, and insulin resistance were all abolished by myeloid-specific deletion of Nox2 (159). A major difference in that study, as pointed out by the authors, was the time of HFD onset. Pepping et al. started HFD feeding after adulthood, while the aforementioned study started HFD feeding on weaning. This could mean that there is an age-dependent difference in the development of visceral adiposity, with early induction by HFD requiring macrophage Nox2 for tissue maintenance, while at later onset, only the deleterious role of Nox2 is revealed. Potential clinically significant distinctions between the two stages of HFD exposure augur pivotal studies that would hone in on the age-dependent function of adipose macrophages in obesity. This appears to be especially important based on the increased prevalence of childhood obesity over the past decades (80).
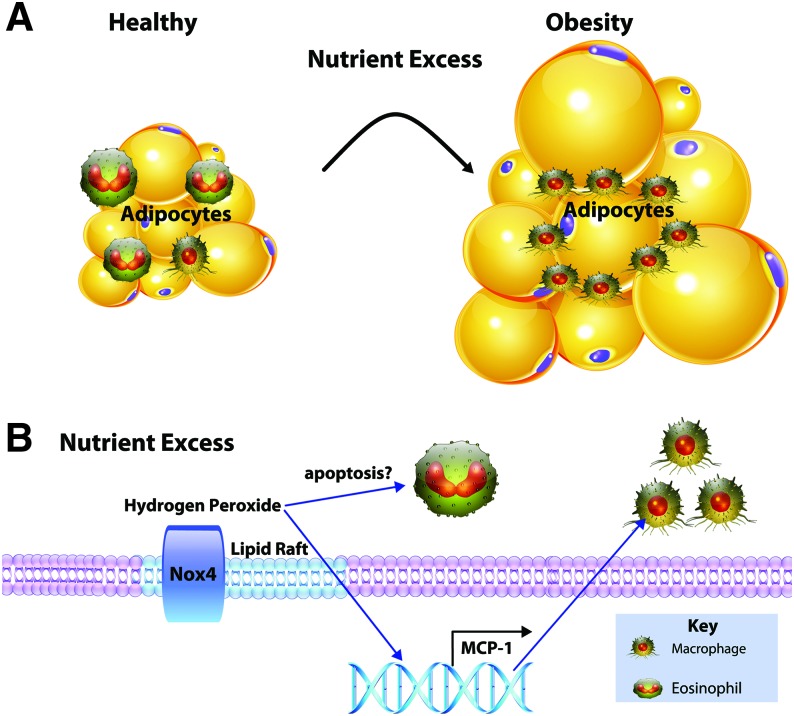
Based on this evidence, nutrient excess potentially leads to early alterations in adipocyte Nox4 signaling (Fig. 3). In response to nutrient excess, adipocytes augment chemoattractant release in an Nox4-dependent manner. This leads to increased proinflammatory phagocyte (predominantly macrophages) populations in visceral adipose tissue. Nox2-dependent signaling from macrophages promotes inflammation, insulin resistance, and altered adipocyte metabolism. However, some evidence supports that Nox2 is required for processing of apoptotic adipocytes and mitigating systemic lipid toxicity (38). Future work aimed at understanding the direct role of Nox2-derived O2•− or its downstream products on adipocyte function through coculture studies could uncover new pathways regulating metabolic disease progression.
FIG. 3.
Adipocyte Nox4 leads to a shift in adipose immune profile. (A) Nutrient excess leading to obesity causes a shift in immune cell populations. Adipose tissue comprised both adipocytes expressing mainly Nox4 and immune cells expressing Nox2. (B) Exposure to excess nutrients causes the localization of Nox4 to lipid rafts of adipocytes and increased H2O2. Elevated levels of adipose H2O2 increase MCP-1 and could potentially induce apoptosis of homeostatic immune cells, resulting in a shift toward insulin resistance and inflammation observed in obesity. H2O2, hydrogen peroxide; MCP-1, monocyte chemoattractant protein 1. Color images are available online.
Vasculature
While evidence clearly supports altered redox signaling early in adipose tissue contributing to metabolic disorders, disruptions in blood vessel NOX have also been reported early in obesity and may actually drive insulin resistance (105). This challenges, to some degree, the adipocentric view of metabolic disorders. Importantly, metabolic tissues and the vasculature are integrated in a number of ways, and more and more evidence illustrates that each depends on signaling from the other. Therefore, deciphering which organ is impaired by obesity first is a difficult task. Firstly, adipose tissue and skeletal muscle are perfused by capillaries that support the transport of (excess) nutrients, facilitate endocrine signals, and direct immune cell infiltration (105). Secondly, the vast majority of the vasculature is surrounded by specialized perivascular adipose tissue (PVAT) (17), which regulates vascular function and is, in turn, regulated by vascular signaling (5, 46). Finally, cytokines from adipose and skeletal muscle profoundly influence vascular function. In the context of NOX enzymes, adiponectin released from adipocytes was shown to suppress both Nox1 and 4 in vascular smooth muscle cells (VSMCs) and inhibit vascular NOX-derived O2•− (5, 96, 151), while the adipokine chemrin induces endothelial and VSMC NOX activity (144).
Understanding the physiological and pathophysiological roles of vascular NOX in the development and progression of metabolic disease is of high importance as cardiovascular mortality increasingly involves the risks associated with obesity and type II diabetes. Around 70% percent of diabetics die from some form of heart disease, with atherosclerosis likely subserving much of this disease risk (74). Furthermore, clarifying the role of specific isoforms in the context of specific vascular beds is crucial as their function and signaling targets may vary (118).
Perivascular adipose and adventitia
Similar to other adipose depots, PVAT immune cells contribute to normal function (211), but shifts in immune cell phenotypes and infiltration of proinflammatory immune cells (expressing Nox2) are a hallmark of dysfunctional adipose tissue arising in obesity, which was recently reviewed extensively (79). In rodents challenged with obesity, PVAT increases chemoattractant release (23, 46, 214). It is tempting to speculate that, similar to other adipose depots, Nox4 mediates MCP-1 expression in PVAT adipocytes as well. However, adipocytes in PVAT plausibly arise from different precursor cell lines compared with visceral depots (22). This warrants investigation into Nox4-mediated MCP-1 expression in PVAT adipocytes. Whether or not it is mediated by adipocyte Nox4, PVAT and adventitia show immune infiltration and increased expression of Nox2 in metabolic and vascular disorders (77, 223). Our understanding of specific NOX isoform functions in PVAT is significantly impeded not only by the diversity of PVAT along the vasculature (17) but also the use of apocynin or DPI, which are isoform nonspecific NOX inhibitors and have other NOX-independent side effects. Still, these studies in general highlight the role of PVAT immune infiltration (mainly macrophages), increased NOX expression (29), and exacerbated ROS production in obesity-induced impairment of vascular function (19, 100, 131).
Similar to visceral adipose tissue, increased ROS in PVAT promote the expression of proinflammatory cytokines, such as TNF-α, which can in turn activate vascular NOX enzymes (46, 204). Utilizing the Nox2-specific inhibitor Nox2ds-tat peptide in vitro on thoracic aorta PVAT from obese Zucker rats (a rodent model of metabolic syndrome), it was shown that Nox2 is responsible for a majority of oxidant production (46). In addition, vascular reactivity experiments revealed that PVAT Nox2 and TNF-α were essential for PVAT-mediated metabolic syndrome impairment of endothelial function, suggesting that PVAT Nox2 is dependent on inflammatory mediators to negatively influence vascular function.
While most evidence with respect to PVAT implies an “outside-in” mechanism of metabolic disease progression, recent evidence suggests that the PVAT both receives and sends signals to the vascular wall (5). Antonopoulos et al. showed that PVAT gene expression of adiponectin was inversely related to vascular O2•− in diabetic patients. In addition, using organ coculture experiments, activation of internal mammary artery with NADPH resulted in a similar upregulation of PVAT adiponectin gene expression potentially through a 4-hydroxynonenal-dependent pathway (5). This study provides evidence that vascular NOX signaling potentially activates compensatory gene expression in PVAT, but whether this change in gene expression corresponds to changes in protein level or changes in vascular function remains unexplored. While most studies support a detrimental role of PVAT ROS on vascular function in metabolic disease, the transmission of PVAT signals to the endothelium and VSMC is not well understood and may involve inflammatory mediators and/or intermediary cells such as adventitial fibroblasts.
Adventitial fibroblasts lie just below the PVAT and are recognized for their expression of Nox2 and 4 and contribution to vascular function (42, 48, 153). Fibroblasts are shown to be early responders in atherosclerosis progression (216). Signaling interactions between the PVAT and adventitial fibroblasts have been demonstrated (120, 171, 228) and may promote atherosclerosis-related proliferation and migration of adventitial fibroblasts (231). However, the role of NOX enzymes mediating this interaction in metabolic disease has not been studied and could represent an area ripe for exploration. Since H2O2 can induce NOX activity in a feed-forward manner in other cell types, it is plausible Nox2 activity in PVAT would start a chain reaction of increasing NOX activity throughout the vascular wall. Therefore, studies aimed at uncovering temporal and causal relationships between NOX isoform expression and functionality in the vascular cell types is expected to add to our understanding of the progression of vascular dysfunction in metabolic disease.
Endothelium and smooth muscle
The human endothelium expresses Nox1, Nox2, Nox4, and Nox5 at both membrane and intercellular locations, but a thorough understanding of the contribution of distinct cellular NOXs in metabolism and progression of metabolic dysfunction is lacking (115, 199). Delineating the roles of NOX isoforms in endothelial and smooth muscle cells is complicated by potential differences among vascular beds (118, 157). Metabolic disease is associated with endothelial dysfunction, VSMC remodeling, and loss of vascular compliance, all of which lead to the development of cardiovascular diseases such as hypertension and atherosclerosis. The NOX family of enzymes has been strongly implicated in the pathogenesis of metabolic disease-associated vascular dysfunction. Below, each vascular NOX isoform is discussed with regard to its cell type-specific role in the vascular complications of metabolic disease.
Nox4
In general, Nox4 is recognized for its role in physiological function. Nox4 promotes a differentiated contractile VSMC phenotype (36, 128), potentially dependent on subcellular localization of Nox4 to actin filaments. In the endothelium, similar to its function in adipocytes, Nox4 is essential to maintaining insulin signaling through Akt (137). In addition to regulating insulin signaling in the endothelium, Nox4 also appears to be an important player in the regulation of vascular tone. Importantly, Nox4-derived H2O2 does not react with nitric oxide. Endothelium-targeted overexpression of Nox4 lowers basal blood pressure and enhances aortic endothelial-dependent dilation ex vivo (165), potentially through NO-independent alterations in VSMC ion channels and membrane potential (165). Moreover, another study showed that laminar shear stress may promote endothelial nitric oxide synthase (eNOS) activation through Nox4-induced sulfenylation of SHP2 (tyrosine-protein phosphatase nonreceptor type 11) in the endothelium (173). These data support multiple roles for endothelial Nox4 in blood flow regulation. Further supporting the beneficial role of endothelial Nox4, Hu et al. reported that endothelial-specific, Nox4 dominant-negative mutant mice exhibited increased pulse wave velocity, a measure of aortic stiffness (an independent risk factor for cardiovascular events), in hyperglycemic apolipoprotein E (ApoE)−/− mice, suggesting a key role of endothelial Nox4 in preventing the progression of arterial stiffness and atherosclerosis (91).
In addition to Nox4 regulation of vascular tone, some protective effects elicited by Nox4 may be mediated through repression of Nox2 and inflammation. In particular, global KO of Nox4 exacerbated aortic inflammation in ApoE−/− mice, and had no effects on nitrotyrosine levels. Moreover, shRNA against Nox4 in human aortic endothelial cells increased Nox2 expression at both the basal level and after glucose insults, and exacerbated the expression of MCP-1, which was abrogated by Nox2 siRNA (71). These data suggest that there is a mechanism through which Nox4 downregulates Nox2 in the endothelium, resulting in blunted inflammation and vascular dysfunction. Conversely, isolated mouse coronary endothelial cells exposed to high glucose and insulin showed reduced Nox4 expression, which was prevented in cells isolated from Nox2 KO mice (59). From these studies, it appears Nox4-mediated downregulation of Nox2 (at least in the endothelium) is a key aspect of Nox4's vasoprotective effects. Based on this evidence, altered or diminished Nox4 in obesity may be an early event triggering endothelial dysfunction and vascular stiffness. Vascular stiffening before clinical manifestations of cardiovascular disease increases the risk of cardiovascular events and end-organ damage (205) and may represent a key pathological event for therapeutic intervention. Understanding Nox4-mediated pathways promoting vascular compliance may uncover novel targets to halt vascular complications of obesity before the development of deleterious cardiovascular pathologies, such as atherosclerosis.
Indeed, in the context of diabetic atherosclerosis, global Nox4 KO mice showed exaggerated disease progression associated with increased aortic macrophage infiltration and endothelial dysfunction (40, 47, 71, 113). These effects may, in part, be explained by increased expression of other NOXs, specifically Nox2 in the endothelium (71) and Nox1 in the VSMC (47). Moreover, disruption of endothelial Nox4 signaling in mice accelerated atherosclerotic progression (91) potentially through promotion of adhesion molecule expression, immune cell accumulation, and intima thickening (176). From global and endothelial-targeted Nox4 KO mice, it appears Nox4-mediated signaling is protective against diabetic atherosclerosis. However, global Nox4 KO may be dominated by its more pronounced effects on endothelial cells, while diverging influence of Nox4 on the VSMC may be masked. For instance, hyperglycemia has been reported to increase Nox4 expression and binding to p22phox, essential for mediating IGF-1-stimulated signaling in VSMC (213), including oxidation/activation of sarcoma viral oncogene (Src) and SMC proliferation in vivo (213). These findings highlight a potential role for VSMC Nox4 in the development of atherosclerosis and vascular remodeling.
In aged ApoE−/− mice, VSMC Nox4 expression is enhanced and associated with mitochondrial-ROS production as well as mitochondrial dysfunction. Furthermore, the same study also showed that aortic Nox4 expression correlates with age and atherosclerotic severity (201). Along the same line of reasoning, Tong et al. reported that targeting a nonfunctional Nox4 mutant to VSMC protected against atherosclerotic progression through downregulation of MCP-1, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule (VCAM)-1 (192). In addition, Nox4 was found to promote epoxide hydrolase 2 expression, an enzyme that metabolizes epoxyeicosatrienoic acids. Activity of epoxide hydrolase 2 is strongly associated with VSMC proliferation as well as human coronary heart disease (114). However, the mechanism of Nox4-mediated upregulation of epoxide hydrolase 2 in rodents remains unclear as it appears to be independent of H2O2 production (192). In the study of VSMC nonfunctional Nox4 mice, left common carotid ligation-mediated induction of turbulent blood flow was used as the model for atherosclerosis, which likely disrupted endothelial function.
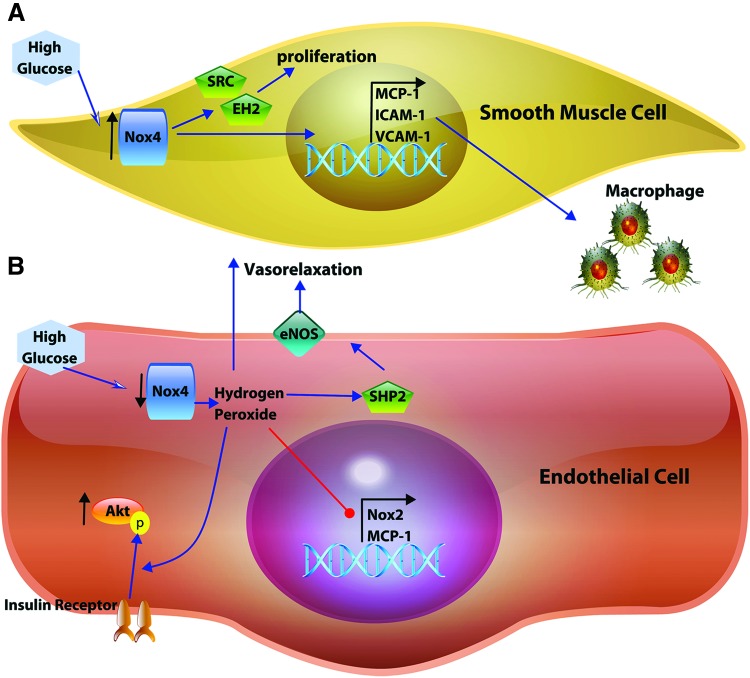
The contributions of endothelial signaling to VSMC Nox4 or vice versa have not been well defined. It is interesting that Nox4 has seemingly opposing effects in the two cell types (Fig. 4), where disturbed flow downregulates endothelial Nox4 leading to upregulation of VSMC Nox4 (91, 192). Uncovering endothelial and VSMC cross talk involving Nox4 expression or activity could elucidate mechanisms of metabolic disease-related atherosclerosis. While the studies above indicate that VSMC Nox4 is involved in lesion formation in mice and that Nox4 expression is correlated with atherosclerotic severity (201), human atherosclerotic lesions show decreased Nox4 expression (71), which may be instrumental for plaque instability and rupture (218). Temporal mechanisms underlying the expression and actions of Nox4 in atherosclerotic lesions are likely complex, but are expected to provide key insight into therapeutics aiming to reduce plaque rupture and thrombolytic events.
FIG. 4.
Diverging roles of NADPH oxidase 4 in the vascular smooth muscle and endothelium. (A) In vascular smooth muscle cells, elevated glucose levels induce increased expression of Nox4, which promotes proliferation and macrophage infiltration, both crucial components of atherosclerotic progression. (B) In the endothelium, exposure to elevated glucose diminishes Nox4 expression leading to the loss of Nox4-mediated salutary homeostatic signaling, enhancing insulin signaling activation of Akt, vasorelaxation (directly by H2O2 and indirectly through SHP2 and eNOS), and potentially repression of Nox2 and MCP-1 expression. eNOS, endothelial nitric oxide synthase; SHP2, tyrosine-protein phosphatase nonreceptor type 11. Color images are available online.
Nox4 has also been implicated in angiogenesis. Nox4 H2O2 production from vascular endothelial cells and cardiomyocytes has been shown to induce angiogenesis (30, 39, 227) and increase capillary density (39). In accordance with this, global Nox4 KO mice exhibited blunted exercise-induced angiogenesis (206). Mechanisms are expected to involve upregulation of vascular endothelial growth factor (VEGF) and repression of antiangiogenic factors. Peripheral capillary rarefaction has been previously described in early metabolic syndrome associated with elevated oxidant products (62), but the direct mechanisms remain unclear. It is possible that loss of Nox4 signaling in obesity contributes to vessel rarefaction through upregulation of other NOX isozymes. Determining the role of Nox4 in the maintenance of capillary density in adipose and skeletal muscle warrants investigation and may shed light on metabolic disease progression.
Nox2
In metabolic disease, endothelial Nox2 is generally considered to be deleterious as it impairs endothelial-dependent vasodilation. For instance, patients with hereditary deficiency of Nox2 exhibited enhanced flow-mediated vasodilation compared with healthy subjects (203). Supporting work in rodents also showed that Nox2 KO or treatment with Nox2ds-tat (aka gp91ds-tat) was protective against obesity and insulin resistance-associated vascular dysfunction (54, 186). During the development of metabolic syndrome and type II diabetes, Nox2 is highly active in the vessel wall and contributes extensively to vascular O2•− production (78) not only in endothelial cells but also in VSMCs (161).
Activation of endothelial Nox2 can be mediated by metabolic factors, including glucose, palmitate, and oxidized low-density lipoprotein (59, 112, 198). Evidence suggests that Nox2-mediated impairment of endothelial function is, in part, mediated by extracellular signal-regulated kinase (Erk)1/2 activation. Erk1/2 signaling may downregulate eNOS and insulin receptor signaling (54, 59). However, p-eNOS was only partially restored on Erk1/2 inhibition, suggesting that other mechanisms leading to endothelial dysfunction may also be at play. In addition, nitric oxide is directly inactivated by O2•− from Nox2, which may be mediated through colocalization of Nox2-eNOS in lipid rafts resulting in peroxynitrite formation (124, 226). Obese patients (regardless of sex) showed increased expression of endothelial p47phox (needed for canonical Nox2 activation), suggesting Nox2 contributes to increased vascular O2•− and oxidative stress in these patients (178). Impaired nitric oxide and insulin signaling likely induces increased vascular tone as well as insulin resistance, leading to higher risk for cardiovascular pathologies and events.
Isolated primary coronary endothelial cells showed robust Nox2 activation, increased senescence, and apoptosis following 24 h of high glucose and insulin challenge (59), all of which potentially predispose the vessels to atherosclerosis and cardiac events (217). Even in isolated human adipose microvascular endothelial cells from healthy adults, Nox2 O2•− was induced by insulin, which appeared to inhibit insulin-induced nitric oxide production (129). Interestingly, Nox2 expression, as well as activity, was potentiated at what could be considered normal physiological levels of insulin (1 nM). This potentially suggests an important physiological role of Nox2 in mediating insulin signaling, which could provide negative feedback and fine tuning.
Although involvement of Nox2 under physiological circumstances requires further investigation, contribution of this isozyme to metabolic disease progression is validated by multiple studies. For instance, knocking out Nox2 abolished HFD-induced ROS production and Erk1/2 activation, and also reduced weight gain leading to improved insulin sensitivity and endothelial function in middle-aged mice (54). In addition, selective Nox2 inhibitor Nox2ds-tat effectively ameliorated insulin resistance-induced oxidative stress and vascular dysfunction independent of a change in body mass (186).
In an elegantly designed temporal study, endothelial function was assessed at 8, 12, 30, and 36 weeks of HFD in cerebral arterioles and carotid arteries. HFD for 12 weeks reduced cerebral arteriolar dilation to acetylcholine, which was prevented in the HFD-fed Nox2 KO mice despite the fact that no changes in fat mass, body mass, or basal glucose levels were observed (127). Nevertheless, since the study utilized global Nox2 KO mice, the direct contribution of endothelial Nox2 to the observed phenotype cannot be delineated as Nox2 in other cell types may also play a crucial role (e.g., macrophages and VSMC). It would be of great significance to conduct these experiments in cell-specific, inducible Nox2 KO mice, which were recently developed (172). Using these genetically constructed mice, it was shown that while Nox2 KO in myeloid cell reduced basal blood pressure, Nox2 KO in endothelial cells reduced blood pressure elevation in response to AngII (172). To what extent this observation can be recapitulated in obesity or diabetes studies is unknown. Future investigation using the cell-type-specific, inducible Nox2 KO mice would be essential to delineate the contribution of this isozyme in the progression of metabolic diseases.
Nox1
A number of studies in recent years have been conducted to investigate the specific role of vascular Nox1 in metabolic disorders. Qiu et al. reported that restoration of glycemic control via genetic deletion of myostatin in a rodent model of metabolic syndrome improved microvascular function and reduced Nox1 expression (162), implicated in eNOS uncoupling (180, 222) and may be regulated by Toll-like receptors (119). This contention is supported by a recent study from the same group showing a protective effect of global Nox1 KO on microvessel function (mesenteric arteries) in db/db mice (191). As Nox1 is expressed in both endothelial cells and VSMCs, global Nox1 KO-mediated improvements on both endothelium-dependent (acetylcholine-induced dilation) and smooth muscle-dependent responses (NO donor-induced dilation and myogenic tone) indicate that Nox1 present in both cell types likely contributes to metabolic vascular pathology. These findings are further supported in a Nox1 KO hyperglycemia mouse model. Alves-Lopes et al. showed that the contractile dysfunction of internal pudendal arteries was normalized in Nox1 KO hyperglycemic mice in comparison with the hyperglycemic WT counterparts, through reduced activation of Rho kinase and VSMC contractile state (3). Again, that study utilized global Nox1 KO and should be confirmed utilizing VSMC-specific KO of Nox1.
Studies investigating the contribution of Nox1 to atherosclerosis have shown conflicting results. Nox1 was originally shown to play a detrimental role in both HFD-induced and diabetic atherosclerosis (72, 177). Genetic deletion of Nox1 in the ApoE−/− mice reduced carotid lesion severity, macrophage infiltration, and inflammatory cytokine levels, suggesting Nox1-derived O2•− production promotes atherosclerosis. However, a more recent study using the same mouse model (Nox1 and ApoE double KO) showed that Nox1 deficiency is associated with elevated levels of cholesterol, LDL, and plasma triacylglycerol compared with ApoE−/− alone. Moreover, O2•− production in atherosclerotic aortas was surprisingly twofold higher in Nox1/ApoE double-KO versus ApoE−/− mice, suggesting a protective role of Nox1-derived O2•− against atherosclerosis (183). Nevertheless, these results may potentially be revealed by the increased lipid burden in these animals and may in fact highlight an important role of Nox1 in nonvascular tissue in controlling metabolic processes in the ApoE−/− model. Interestingly, in this study, atherosclerotic pathology only differed in severity at the aortic sinus between the Nox1 KO ApoE−/− and the ApoE−/− mice. This could be due to differences in cellular lineages along the aorta.
Possible explanations for opposing findings in the Sheehan et al. and Sobey et al. studies using similar animal models may include different diet fat content or induced vascular injury. In mice fed with higher fat content (42%), Nox1 KO seems protective (177), whereas in mice treated with lower fat content (22%), deleting Nox1 appears detrimental (183). The adverse vascular effects of Nox1 KO may be secondary to a role of Nox1 in controlling cholesterol and lipid circulation in other tissues. Finally, Nox1 KO was found to be protective when carotid ligation was implemented in concert with HFD to induce atherosclerosis (177). It is therefore possible that artery ligation-induced turbulent blood flow is a trigger for the detrimental activation of Nox1 in atherosclerosis development.
While recent advances have been made to understand the role of Nox1 in obesity- and diabetes-associated vasculopathy, many questions remain, especially cell type-specific involvement of Nox1. Development of endothelial- and VSMC-specific inducible KO of Nox1 would greatly assist in deciphering Nox1 mechanisms in various cell types and would also be well suited to answer the temporal contribution of Nox1 to obesity-associated metabolic disorders.
Nox5
Nox5 is not expressed in murine models and, therefore, robust in vivo data are lacking. Little is known about Nox5 in obesity and metabolic diseases, but a role in diabetes is implicated as high glucose increases endothelial calcium concentration (208), which is needed for Nox5 activation. In addition, high glucose-treated human pulmonary endothelial cells showed increased activity of PKCα. PKCα was subsequently shown to phosphorylate Nox5 and increase its activity in response to calcium stimulated by ionomycin in endothelial cells overexpressing Nox5 (26). In human VSMC, expression of Nox5 can be induced by factors elevated in obesity/type II diabetes, such as TNF-α, endothelin-1, and angiotensin II (154). Interestingly, overexpression of both catalytically active and inactive Nox5 variants in VSMC induces proliferation (154). This suggests a potential role for Nox5, independent of O2•− production, in the hyperproliferative VSMC phenotype seen in obesity/diabetic-induced atherosclerosis. In human subjects, Nox5 expression and activity are increased in coronary arteries of patients with coronary artery disease (76). Interestingly, Nox5 expression showed temporal and cell-specific differences with increased endothelial expression preceding upregulation in the vascular smooth muscle. The significance in timing of Nox5 expression is not known but may suggest that damaged endothelium and vascular inflammation promote VSMC Nox5 and atherosclerosis progression.
In summary, a dearth of data from vascular cell-specific KO mice limits present appreciation of cell-specific contributions of NOXs in metabolic disease-associated vascular pathologies. Based on present evidence, it is speculated that disruption of physiological endothelial Nox4 signaling is an early event in metabolic disease pathology. Downregulation of endothelial Nox4 leads to upregulation of other NOX isoforms, potentially in both endothelium and VSMCs. Eventually, loss of endothelial Nox4 signaling and potentiation of other vascular NOX enzymes lead to endothelial and VSMC dysfunction that ultimately result in the progression of atherosclerosis and increases in cardiovascular mortality. Future research utilizing temporal study design and cell-specific, inducible KO mice of vascular NOX enzymes should bring to light more exciting discoveries.
Emerging New Areas of Investigation
Microparticles
Microparticles are small (0.1–1 μm) diameter membrane vesicles, which display receptors, membrane components, and contain proteins along with nucleic acids from the cell of origin (134). Microparticles are released from virtually every cell type, including endothelium in response to stress, especially altered blood flow and apoptosis (134). Release of endothelial microparticles increases following the ingestion of a high-fat meal (197) and the response is exaggerated in diabetic subjects (197). A more recent study found that the increased levels of circulating microparticles in diabetic patients correlate with cardiovascular death (179). Together, the studies suggest a clinical link between microparticles, type II diabetes, and cardiovascular complications. Underlying mechanisms for these observations are not well understood, but may involve microparticle-induced endothelial inflammation and atherosclerosis, as injection of microparticles from injured endothelial cells impaired endothelial cell function and promoted macrophage infiltration of lesions in ApoE−/− mice (95). In that study, microparticles released by cultured human coronary endothelial cells in response to high glucose showed elevated NOX-derived ROS, but the specific isoform was not identified (95). It would be intriguing to replicate the study with microparticles obtained from endothelial cells following NOX gene silencing and/or ex vivo treatment of microparticles with NOX inhibitors to probe a role for NOX-containing microparticles in metabolic disease-induced atherosclerosis. Recently, both Nox2 and Nox4 were shown to be expressed in circulating microparticles in lean and obese patients (50). It was observed that microparticles derived from obese patients showed higher expression of Nox4 and reduced expression of Nox2 compared with lean patients (50). Although limited, these intriguing findings suggest there is a potential for microparticles containing NOX to participate in diabetic-induced atherosclerosis.
Epigenetics
Epigenetics, in general, refers to post-translation modifications made to histones, various species of RNA, and DNA methylation that influence the structure and function of chromatin and gene transcription. Redox regulation of the epigenetic programming of gene transcription has been the topic of recent reviews (104, 110). Still, the direct role of NOX enzymes in epigenetic gene regulation, and in the context of metabolic disease and atherosclerosis, has been insufficiently explored, and represents a fertile field for future investigation. As discussed above, atherosclerosis is a common consequence of metabolic diseases and recent advancements have been made in the potential cross talk between redox signaling and epigenetics in the disease progression resulting in atherogenesis.
Original observations identified H2O2 being an instigator that promotes hypomethylation of DNA through increased expression of the ten-eleven translocation enzymes (TET) and depletion of upstream precursor S-adenosyl methionine, which correlates with reported diminished methylation in human tissues in metabolic disease (90). However, this notion has recently been challenged in VSMCs. In atherosclerotic lesions, Liu et al. (122) demonstrated that reduction in the transcriptional activating “mark” 5-hydroxymethylcytosine (5hmC), essential for VSMC contractile gene expression, was coupled with a decrease in TET2 expression. Therefore, loss of this marker and modulator may contribute to lesion progression. In agreement with a potential role for ROS in this process, Delatte et al. (44) found dedifferentiation and reduced 5hmC in VSMC in an animal model of oxidative stress. These observations may suggest that redox signaling and potentially NOX participate in the epigenetic reprogramming of VSMC in atherosclerosis.
In addition to modulation of DNA methylation, post-translational modifications to histone 3 may regulate VSMC proliferation. Redox modification or methylation of histone 3 was suggested to lead to enhanced proliferation. Garcia et al. (65a) showed enhanced glutathionylation of histone 3 in physiological proliferating cells and cancer cell lines, establishing a link between ROS production and an epigenetic marker of proliferation. However, the source of ROS and potential mechanistic relevance to atherosclerosis still remain unclear. In concert with histone 3 modifications and cell phenotype, DNA methylation lysine 4 appears to regulate the contractile VSMC phenotype. Indeed, multiple isoforms of histone demethylases have been shown to be upregulated in type II diabetes and contribute to atherosclerosis (28, 229). With that said, to our knowledge direct links between NOX and histone demethylase enzyme expression/activity have not been established. In addition, how redox modification of histone 3 promotes or interferes with histone 3 methylation is yet to be determined. Even though a direct role of NOX enzymes in these redox pathways has not been revealed, this is highly plausible based on the roles of NOX enzymes in atherosclerosis discussed in the Vasculature section.
Aside from NOX-derived ROS putatively driving epigenetic changes in metabolic disease, evidence exists linking epigenetic enzymes in the regulation of NOX expression. Two important families of enzymes regulating DNA accessibility through histone modification are the histone acetyl transferases (HATs) and histone deacetylases (HDACs). Canonical activity of HATs uses acetyl–CoA to add an acetyl group to lysine (K) residues at histone tails inducing chromatin structural remodeling allowing access to gene promoter regions. On the contrary, HDAC enzymes remove acetyl groups from histones and are generally associated with transcriptional repression. Paradoxically, some reports have shown that HDAC inhibitors reduce NOX expression and consequently NOX-derived ROS production in the vasculature (25, 130, 181).
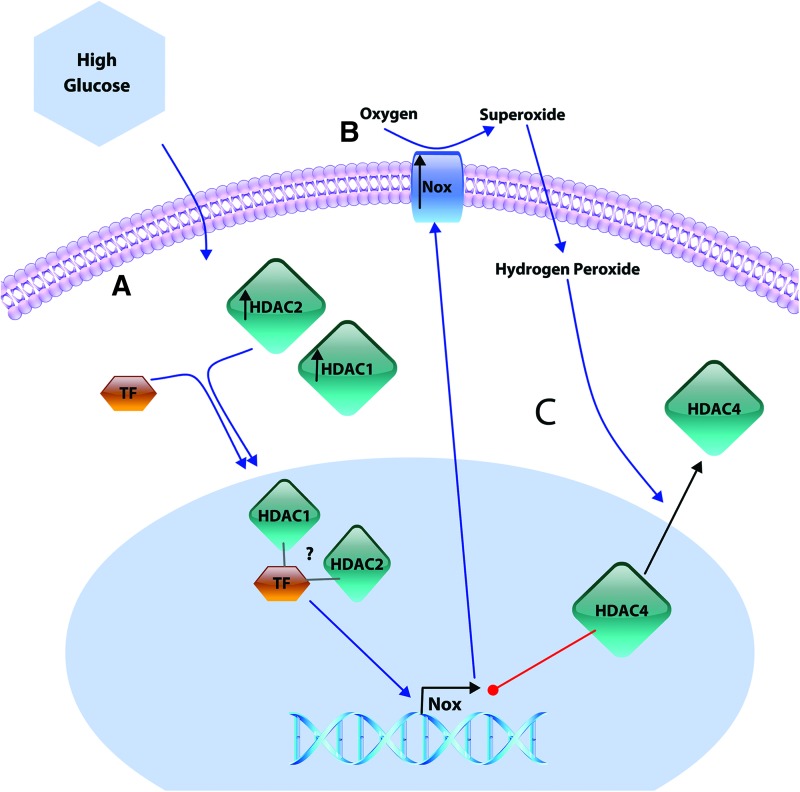
It is proposed that vascular HDACs 1 and 2 increase in experimental diabetes (130) and may form a complex with transcription factors to regulate NOX transcription. This notion is supported by evidence of class 1 HDACs 1 and 2, at NOX promoter regions in VSMC (130) and HDAC inhibition reducing RNA polymerase 2 enrichment at NOX promoters in macrophages and lung fibroblasts (25). In opposition to this, the transcription of VSMC NOX was repressed by overexpression of class 2a HDAC4, suggesting an HDAC class-specific regulation of NOX expression. While little is known about HDAC-NOX pathways, indirect evidence suggests potential complex feedback and feed-forward pathways in hyperglycemia. Along these lines, it was demonstrated that H2O2 inhibits enzymatic activity of class 1 HDACs (53), setting up a potential feedback loop where NOX-derived ROS inhibit or activate (directionality remains to be determined) HDAC-mediated NOX transcription. In addition, class 2a HDAC4 has been shown to negatively regulate NOX expression in the VSMC (130), which is exported from the nucleus of myocytes under oxidative stress conditions (123). Thus, these new concepts suggest a potential role for NOX-derived H2O2 to facilitate HDAC4 export from the nucleus and remove its repression of NOX transcription (Fig. 5). While limited data exist to date, future investigation into the epigenetic regulations of NOX transcription and activity in the vasculature and, in turn, the impact of NOX-derived ROS on epigenetic regulation will enhance our knowledge of mechanisms governing vascular complications of obesity and type II diabetes.
FIG. 5.
Potential HDAC-mediated Nox expression in hyperglycemia. (A) Hyperglycemia may increase the expression of some class 1 HDACs (HDAC 1 and 2), which are suggested to complex with transcription factors and facilitate Nox expression. (B) The increased expression of Nox isozymes and increased oxidative stress induced by hyperglycemia have been implicated in the export of the class 2a HDAC4 from the nucleus. (C) HDAC4 is suggested to repress Nox expression suggesting a potential feed-forward signaling pathway where Nox-derived ROS remove HDAC4 repression of Nox transcription. HDAC, histone deacetylase. Color images are available online.
Therapies
A number of therapies (metformin, nitro fatty acids, and exercise) have been shown to alter NOX expression and activity. In some ways, these therapies may be more advantageous than targeted inhibition of a specific isoform as they might restore physiological NOX signaling across all isoforms instead of inhibiting one isozyme. Exercise benefits are multifaceted, although mechanisms of these beneficial effects are not completely understood. Evidence suggests exercise training suppresses p47phox membrane localization (193) and decreased Nox2 expression (75) in rodent aortas, and inhibited Nox2 activity (20) in human subjects. In obese rats, exercise-triggered reduction in vascular NOX O2•− production, restoration of nitric oxide bioactivity, and suppression of vascular inflammation all contributed to increased vascular compliance (193). Exercise also exerts an anti-inflammatory effect, reducing cytokines known to enhance NOX expression and activity, such as TNF-α (195) and IL-6 (83). These exercise-mediated adaptations of vascular function in metabolic disease can conceivably reduce the risk of cardiovascular mortality in humans.
At present, metformin is a frontline drug for type II diabetes, in part, because it has widespread benefits dependent and independent of its glucose-lowering effects. Among its many effects, metformin has displayed inhibition of NOX activity through reducing p47phox membrane localization in endothelial cells (11). Another therapeutic approach, presently in clinical trials, to treat metabolic disorders, is nitro-fatty acids. Nitro-fatty acids trigger post-translational modifications of reactive protein thiols resulting in activation or inactivation of the targeted protein. Biologically, nitro-fatty acids increase antioxidant defenses (12, 103) in human cells, and can even potentially inhibit NOX directly (70). Moreover, in rats, nitro-fatty acids accumulate in the adipose tissue (60), which may direct treatment to a key site of oxidative stress and inflammation. In addition, nitro-fatty acids have been shown to improve glucose tolerance and reverse pulmonary hypertension in a mouse model of obesity (99).
NOX inhibitors
Direct targeting of NOX enzymes has garnered much attention over recent decades. The oldest and most widely used inhibitors are DPI and apocynin, which are not isoform specific and have other non-NOX-related actions. Briefly, DPI inhibits flavoproteins, including eNOS and xanthine oxidase, while apocynin is an ROS scavenger and Rho kinase inhibitor. While apocynin has been shown to improve vascular function in obese mice (54), its broad profile of inhibition may interfere with salutary redox pathways and yield unwanted side effects. The more recent development of inhibitors, which show increased specificity within the NOX family may be more advantageous for understanding the roles of NOX enzymes in metabolic disease.
A number of compounds have been developed to target NOX enzymes, specifically GenKyoTex compounds (GKT-137831 and GKT-136901), and triazolo pyrimidine compounds (VAS2870 and VAS3947). In general, these compounds demonstrate inhibitory action on NOX enzymes, while having no or limited inhibitory action on xanthine oxidase or other flavoproteins.
The VAS compounds are postulated to interfere with complex formation and/or conformational changes of all the NOX enzymes with an IC50 in the low μM range (66, 190). These attributes make VAS compounds potentially useful pan-NOX inhibitors, but limit their use in determining isoform-specific functions and therefore can only be used to draw a general conclusion about NOX involvement in a physiological or pathological process. Furthermore, experimental interpretation could be confounded by VAS compounds' off-target effects involving thiol alkylation that may replicate redox effects of ROS (188). Despite this, VAS compounds have been shown to reduce ex vivo constriction of aortas from mice with experimental diabetes (167) and improve endothelial-dependent dilation in fructose-fed rats (57).
The GKT compounds show considerable advantages in potency and isoform specificity over the VAS compounds. Pharmacokenetic studies demonstrate similar ability to inhibit Nox1 and Nox4 (<170 nM ki), but less potency with respect to inhibiting Nox2 (>1530 nM ki) and Nox5 (>410 nM ki) (6, 65). Therefore, bioavailability should be monitored for in vivo application to ensure Nox1/Nox4 targeting. Although experimentally these compounds could be contrasted with gene-silencing techniques to determine isoform-specific effects, in vivo application in treating metabolic diseases may yield unwanted side effects especially in the vascular endothelial cells where Nox4 has been demonstrated to have vasodilatory and antiatherogenic functions as described in the Nox4 section. With that said, GKT-137831 has been used to inhibit VSMC inflammation and proliferation stimulated by adipokine chemerin in db/db mice (144) and to abrogate endothelin-1 signaling in cultured endothelial cells exposed to elevated glucose (152).
Another small molecule with NOX inhibitor actions is the synthetic organoselenium ebselen along with its congeners. Ebselen inhibits Nox1, Nox2, and Nox5 in the nM range (150, 500, and 700 nM, respectively) with Nox2 selectivity improved in subsequent analogs, such as that named Thr101, which has an IC50 for Nox2 of 300 nM and IC50 for Noxs1,4, and 5 above 3 μM (182). Other biological actions have been reported for low μM concentrations of ebselen such as inhibition of eNOS (225), suppression of amino acid metabolism (224), and action as a glutathione peroxidase mimetic (140). These effects, however, have not yet been assessed for ebselen congeners. Despite those widespread actions, which may complicate interpretation of ebselen-generated experimental results, the oral bioavailability, antioxidant actions, and ability to inhibit both Nox1 and Nox2 make ebselen a potential therapeutic option to treat metabolic disorders as long as in vivo concentrations are kept in a therapeutic window above the IC50 for Nox2 (500 nM) and below the IC50 for eNOS (8500 nM). In particular, ebselen was shown to reduce vascular dysfunction and improve insulin signaling in obese and diabetic rodents (16, 27, 33, 140, 219). In comparison, ebselen and its congeners appear to hold higher therapeutic potential than VAS or GKT compounds in the context of cardiometabolic diseases because they target Nox1, Nox2, and Nox5, which, as discussed previously, play key roles in the detrimental progression of metabolic disorders, but elicit little to no effects on the beneficial Nox4-mediated functions in vascular endothelial cells. Nevertheless, off-target inhibitory actions on eNOS still likely limit its clinical application. As such, a more rational approach strives to selectively target a single NOX isoform. Over the past decade, our laboratory has developed a number of isoform-specific NOX inhibitors, both for Nox2 and for Nox1.
Nox2-specific inhibitors
As discussed throughout the review, Nox2 is often linked to insulin resistance, inflammation, and oxidative damage; therefore, specific targeting of Nox2 in obesity and type II diabetes is expected to hold therapeutic value. The original peptide inhibitor of Nox2 (Nox2ds-tat, aka gp91ds-tat) interferes with p47phox docking to Nox2 and is widely used in various disease models (41) (Fig. 6). Importantly, the peptide is specific to the Nox2-p47phox interaction and shows no inhibitory effect on Nox4, conical Nox1, or hybrid Nox1 (Nox1-p47phox) activity (41). This Nox2ds-tat peptide inhibitor has been shown to reverse atherosclerotic progression (163), block PVAT-mediated vascular impairment (46), and restore insulin signaling (186) in metabolic disease. Historically, peptidic drugs are viewed as not being advantageous due to concerns of bioavailability and membrane permeability. However, evolving technologies and strategies (166, 202) render the utility of a peptidic NOX inhibitor more likely. Recently, the laboratory has developed Nox2-selective tetrahydroisoquinoline small-molecule inhibitors CPP11g and CPP11h (35, 116). Similar to the Nox2ds-tat, these small molecules show no inhibition of other NOX isoforms and exhibit no known antioxidant properties (35). These compounds have been tested in vivo in an acute inflammatory mouse model, where they effectively ameliorated endothelial dysfunction in response to TNF-α (116). Thus, they may demonstrate promising therapeutic potential in obesity and type II diabetes similar to or even better than that observed with Nox2ds-tat.
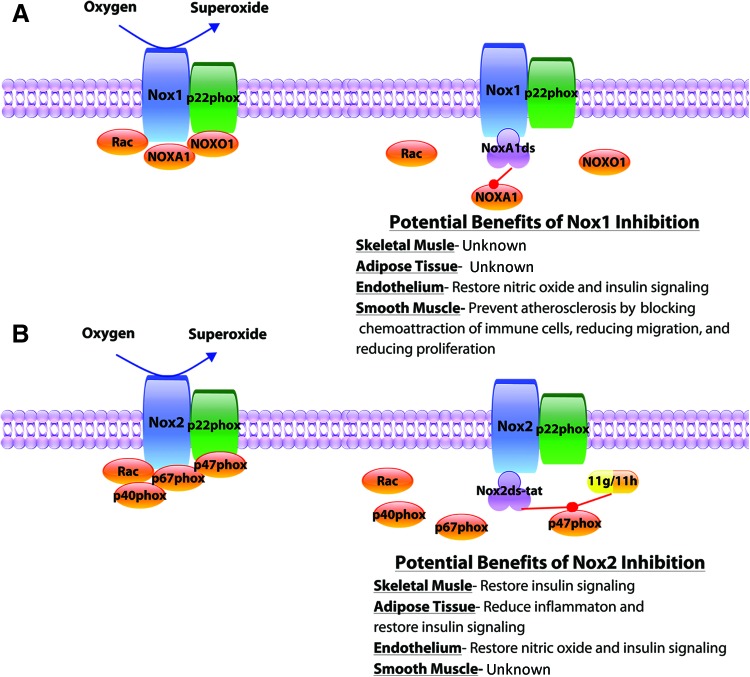
FIG. 6.
Selective Nox inhibitors. Over the past two decades our laboratory has developed 4 Nox isoform-specific inhibitors. (A) The peptide NoxA1ds specifically interrupts the complexation between NoxA1 and Nox1, with potential benefits of selective Nox1 inhibition in metabolic diseases listed. (B) At present, one peptide and two small-molecule inhibitors exist for Nox2. Nox2ds-tat mimics the Nox2-p47phox docking site inhibiting assembly and Nox2 superoxide production. The small-molecule inhibitors 11g and 11h are projected to fit into a p47phox binding pocket disrupting the interaction with p22phox and the assembly of the Nox2 isozyme specifically. Potential benefits of selectively inhibiting Nox2 in metabolic diseases are listed. Color images are available online.
Nox1-specific inhibitors
Similar to Nox2, Nox1, as discussed throughout the review, has been linked to a number of complications in metabolic disease, particularly atherosclerosis. Previously, the small molecule inhibitor ML171 was shown to inhibit Nox1 in a submicromolar range, while inhibition of other isoforms was only observed at significantly higher concentrations (68). This observation would render ML171 a relative selective inhibitor of Nox1. Even though this compound to date has been used sparingly, the therapeutic potential is promising due to the low IC50 for Nox1. Our laboratory has recently developed an Nox1-selective peptide inhibitor, NoxA1ds (164), which blocks the interaction between NoxA1 and Nox1 (Fig. 6). Importantly, NoxA1ds showed no inhibitory effects on Nox2, Nox4, Nox5, or xanthine oxidase. In vitro administration of NoxA1ds inhibited hypoxia-induced superoxide production in human pulmonary endothelial cells (43a). Based on the literature discussed in this review, use of NoxA1ds may prove efficacious in reducing vascular resistance (3, 191) and preventing atherosclerotic development (72, 177) in metabolic disease.
In summary, these selective inhibitors are expected to be useful tools in determining isoform-specific signaling in cell culture and animal models of metabolic disease. Clinically, these selective inhibitors or their successors could have far-reaching indications and implications, including improving nitric oxide signaling, enhancing insulin sensitivity and mitigating inflammation. Nevertheless, it is plausible that global inhibition of even a specific isozyme could yield unintended side effects due to the diverse physiological and pathological roles of NOX in different systems and cell types. Therefore, the next frontier of NOX therapies (and many other therapies, for that matter) will be delivering specific NOX inhibitors to a specific tissue location or cell type. Emerging technologies such as microbubbles may be useful in achieving targeting specificity. Experiments have shown microbubbles carrying a drug payload can be targeted to release the drug in a specific area by ultrasound excitation (88, 108). Such therapies would likely reduce drug concentrations needed and avoid associated systemic side effects. In addition, manufacturing microbubbles to recognize specific cell surface marks may increase the delivery to sites of inflammation and injury (121, 210). In a similar manner, utilizing phagocytic cell biology to take up drugs and to preferentially deliver them in high inflammatory regions (221) may hold promise. Coupling NOX isoform-specific inhibitors with site- or tissue-specific drug delivery technologies is an exciting notion with a conceivably high therapeutic potential.
Conclusion
NOX enzymes play distinct isoform- and cell-type-specific functions, which are still not completely understood. The progression of metabolic dysfunction from nutrient excess to obesity and type II diabetes appears to be related to a loss of beneficial NOX signaling followed by increased expression of specific NOX isoforms and deleteriously exaggerated O2•− production. In metabolic disease, excessive ROS production from NOX enzymes contributes to inflammation, increased vascular tone, insulin resistance, and the progression of atherosclerosis. However, with NOX isozymes displaying important roles in both physiology and pathophysiology, it is likely that pan inhibition of NOX enzymes will not be a plausible therapeutic strategy. Targeting specific NOX isoforms potentially in a tissue- or cell-type-controlled manner may provide an advantageous therapeutic strategy in addressing metabolic disease pathologies.
Acknowledgments
We thank Sanghamitra Sahoo, PhD, for her constructive criticism of this review. This work was supported by NIH grants R01HL112914, R01HL079207, P01HL103455, and T32 GM008424 (to P.J.P.); T32 HL007563 (E.D); and DK114012 (M.J.J).
Abbreviations Used
- AAM
alternatively activated macrophages
- Akt
protein kinase B
- ApoE
apolipoprotein E
- DPI
diphenylene iodonium
- Duox
dual oxygenase
- eNOS
endothelial nitric oxide synthase
- Erk
extracellular signal-regulated kinase
- H2O2
hydrogen peroxide
- HATs
histone acetyl transferases
- HDAC
histone deacetylase
- HFD
high-fat diet
- IL
interleukin
- KO
knockout
- MCP-1
monocyte chemoattractant protein 1
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NOX
NADPH oxidase
- Nox
NADPH oxidase (specific isoform, denoted by subsequent number)
- O2•−-
superoxide anion
- PKC
protein kinase C
- PPP
pentose phosphate pathway
- PTP1b
protein tyrosine phosphatase 1b
- PVAT
perivascular adipose tissue
- ROS
reactive oxygen species
- shRNA
short hairpin RNA
- TNF
tumor necrosis factor
- VSMC
vascular smooth muscle cells
- WT
wild type
Author Disclosure Statement
P.J.P. is named on a filed patent (U.S. patent No. 9,187,528 B2) for NoxA1ds. All other authors declare that they have no conflicts of interest pertaining to the contents of this article.
References
- 1. Achari AE. and Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci 18: 1321, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Acosta JR, Douagi I, Andersson DP, Backdahl J, Ryden M, Arner P, and Laurencikiene J. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia 59: 560–570, 2016 [DOI] [PubMed] [Google Scholar]
- 3. Alves-Lopes R, Neves KB, Montezano AC, Harvey A, Carneiro FS, Touyz RM, and Tostes RC. Internal pudental artery dysfunction in diabetes mellitus is mediated by NOX1-derived ROS-, Nrf2-, and Rho kinase-dependent mechanisms. Hypertension 68: 1056–1064, 2016 [DOI] [PubMed] [Google Scholar]
- 4. Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, and Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935–45941, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L, Sanna F, De Silva R, Petrou M, Sayeed R, Krasopoulos G, Lee R, Digby J, Reilly S, Bakogiannis C, Tousoulis D, Kessler B, Casadei B, Channon KM, and Antoniades C. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 64: 2207–2219, 2015 [DOI] [PubMed] [Google Scholar]
- 6. Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, and Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aroor AR. and DeMarco VG. Oxidative stress and obesity: the chicken or the egg? Diabetes 63: 2216–2218, 2014 [DOI] [PubMed] [Google Scholar]
- 8. Banfi B, Clark RA, Steger K, and Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 278: 3510–3513, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, and Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J Biol Chem 279: 18583–18591, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Barrett JP, Henry RJ, Villapol S, Stoica BA, Kumar A, Burns MP, Faden AI, and Loane DJ. NOX2 deficiency alters macrophage phenotype through an IL-10/STAT3 dependent mechanism: implications for traumatic brain injury. J Neuroinflammation 14: 65, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Batchuluun B, Inoguchi T, Sonoda N, Sasaki S, Inoue T, Fujimura Y, Miura D, and Takayanagi R. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis 232: 156–164, 2014 [DOI] [PubMed] [Google Scholar]
- 12. Bates DJ, Smitherman PK, Townsend AJ, King SB, and Morrow CS. Nitroalkene fatty acids mediate activation of Nrf2/ARE-dependent and PPARgamma-dependent transcription by distinct signaling pathways and with significantly different potencies. Biochemistry 50: 7765–7773, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Bjorkman U. and Ekholm R. Generation of H2O2 in isolated porcine thyroid follicles. Endocrinology 115: 392–398, 1984 [DOI] [PubMed] [Google Scholar]
- 15. Bolus WR, Peterson KR, Hubler MJ, Kennedy AJ, Gruen ML, and Hasty AH. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments. Mol Metab 8: 86–95, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, Gross SS, Nasjletti A, and Goligorsky MS. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res 94: 377–384, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, and Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol 34: 1621–1630, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, Mannino S, Crosignani P, Bosi E, Luzi L, Ruotolo G, and Perseghin G. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care 34: 210–215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Candela J, Wang R, and White C. Microvascular endothelial dysfunction in obesity is driven by macrophage-dependent hydrogen sulfide depletion. Arterioscler Thromb Vasc Biol 37: 889–899, 2017 [DOI] [PubMed] [Google Scholar]
- 20. Cangemi R, Loffredo L, Battaglia S, Perri L, Santulli M, Carnevale R, Nocella C, Muscolo M, Masala D, and Violi F. Does regular physical exercise preserve artery dilation by lowering Nox2-related oxidative stress? Antioxid Redox Signal 28: 1576–1581, 2018 [DOI] [PubMed] [Google Scholar]
- 21. Carson BP. The potential role of contraction-induced myokines in the regulation of metabolic function for the prevention and treatment of type 2 diabetes. Front Endocrinol (Lausanne) 8: 97, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, and Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 126: 1067–1078, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, and Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res 104: 541–549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaubey S, Jones GE, Shah AM, Cave AC, and Wells CM. Nox2 is required for macrophage chemotaxis toward CSF-1. PLoS One 8: e54869, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen F, Li X, Aquadro E, Haigh S, Zhou J, Stepp DW, Weintraub NL, Barman SA, and Fulton DJR. Inhibition of histone deacetylase reduces transcription of NADPH oxidases and ROS production and ameliorates pulmonary arterial hypertension. Free Radic Biol Med 99: 167–178, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen F, Yu Y, Haigh S, Johnson J, Lucas R, Stepp DW, and Fulton DJ. Regulation of NADPH oxidase 5 by protein kinase C isoforms. PLoS One 9: e88405, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen J, Li H, Addabbo F, Zhang F, Pelger E, Patschan D, Park HC, Kuo MC, Ni J, Gobe G, Chander PN, Nasjletti A, and Goligorsky MS. Adoptive transfer of syngeneic bone marrow-derived cells in mice with obesity-induced diabetes: selenoorganic antioxidant ebselen restores stem cell competence. Am J Pathol 174: 701–711, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen J, Zhang J, Yang J, Xu L, Hu Q, Xu C, Yang S, and Jiang H. Histone demethylase KDM3a, a novel regulator of vascular smooth muscle cells, controls vascular neointimal hyperplasia in diabetic rats. Atherosclerosis 257: 152–163, 2017 [DOI] [PubMed] [Google Scholar]
- 29. Chen JY, Tsai PJ, Tai HC, Tsai RL, Chang YT, Wang MC, Chiou YW, Yeh ML, Tang MJ, Lam CF, Shiesh SC, Li YH, Tsai WC, Chou CH, Lin LJ, Wu HL, and Tsai YS. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler Thromb Vasc Biol 33: 839–846, 2013 [DOI] [PubMed] [Google Scholar]
- 30. Chen L, Xiao J, Kuroda J, Ago T, Sadoshima J, Cohen RA, and Tong X. Both hydrogen peroxide and transforming growth factor beta 1 contribute to endothelial Nox4 mediated angiogenesis in endothelial Nox4 transgenic mouse lines. Biochim Biophys Acta 1842: 2489–2499, 2014 [DOI] [PubMed] [Google Scholar]
- 31. Chen MY, Zhao CC, Li TT, Zhu Y, Yu TP, Bao YQ, Li LX, and Jia WP. Serum uric acid levels are associated with obesity but not cardio-cerebrovascular events in Chinese inpatients with type 2 diabetes. Sci Rep 7: 40009, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, and Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 56: 1387–1394, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Chew P, Yuen DY, Koh P, Stefanovic N, Febbraio MA, Kola I, Cooper ME, and de Haan JB. Site-specific antiatherogenic effect of the antioxidant ebselen in the diabetic apolipoprotein E-deficient mouse. Arterioscler Thromb Vasc Biol 29: 823–830, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Cifuentes ME, Rey FE, Carretero OA, and Pagano PJ. Upregulation of p67(phox) and gp91(phox) in aortas from angiotensin II-infused mice. Am J Physiol Heart Circ Physiol 279: H2234–H2240, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Cifuentes-Pagano E, Saha J, Csanyi G, Ghouleh IA, Sahoo S, Rodriguez A, Wipf P, Pagano PJ, and Skoda EM. Bridged tetrahydroisoquinolines as selective NADPH oxidase 2 (Nox2) inhibitors. Medchemcomm 4: 1085–1092, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, and Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 27: 42–48, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, Peris E, Cui C, Hoffman A, Zhou G, Fernandez S, Zhai L, Hall BA, Haka AS, Shah AM, Reardon CA, Brady MJ, Rhodes CJ, Maxfield FR, and Becker L. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep 20: 3149–3161, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Costford SR, Castro-Alves J, Chan KL, Bailey LJ, Woo M, Belsham DD, Brumell JH, and Klip A. Mice lacking NOX2 are hyperphagic and store fat preferentially in the liver. Am J Physiol Endocrinol Metab 306: E1341–E1353, 2014 [DOI] [PubMed] [Google Scholar]
- 39. Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, and Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 124: 731–740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Craige SM, Kant S, Reif M, Chen K, Pei Y, Angoff R, Sugamura K, Fitzgibbons T, and Keaney JF., Jr Endothelial NADPH oxidase 4 protects ApoE−/−- mice from atherosclerotic lesions. Free Radic Biol Med 89: 1–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, and Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Csanyi G, Taylor WR, and Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med 47: 1254–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, and Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275: 23227–23233, 2000 [DOI] [PubMed] [Google Scholar]
- 43a. de Jesus DS, DeVallance E, Li Y, Falabella M, Guimaraes D, Shiva S, Kaufman BA, Gladwin MT, and Pagano PJ. Nox1/Ref-1-mediated activation of CREB promotes Gremlin1-driven endothelial cell proliferation and migration. Redox Biol 22: 2213–2317, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Delatte B, Jeschke J, Defrance M, Bachman M, Creppe C, Calonne E, Bizet M, Deplus R, Marroqui L, Libin M, Ravichandran M, Mascart F, Eizirik DL, Murrell A, Jurkowski TP, and Fuks F. Genome-wide hydroxymethylcytosine pattern changes in response to oxidative stress. Sci Rep 5: 12714, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Den Hartigh LJ, Omer M, Goodspeed L, Wang S, Wietecha T, O'Brien KD, and Han CY. Adipocyte-specific deficiency of NADPH oxidase 4 delays the onset of insulin resistance and attenuates adipose tissue inflammation in obesity. Arterioscler Thromb Vasc Biol 37: 466–475, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. DeVallance E, Branyan KW, Lemaster K, Olfert IM, Smith DM, Pistilli EE, Frisbee JC, and Chantler PD. Aortic dysfunction in metabolic syndrome mediated by perivascular adipose tissue TNFalpha and NOX2 dependent pathway. Exp Physiol 103: 590–603, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Di Marco E, Gray SP, Chew P, Kennedy K, Cooper ME, Schmidt HH, and Jandeleit-Dahm KA. Differential effects of NOX4 and NOX1 on immune cell-mediated inflammation in the aortic sinus of diabetic ApoE−/− mice. Clin Sci (Lond) 130: 1363–1374, 2016 [DOI] [PubMed] [Google Scholar]
- 48. Di Wang H, Ratsep MT, Chapman A, and Boyd R. Adventitial fibroblasts in vascular structure and function: the role of oxidative stress and beyond. Can J Physiol Pharmacol 88: 177–186, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Diaz-Vegas A, Campos CA, Contreras-Ferrat A, Casas M, Buvinic S, Jaimovich E, and Espinosa A. ROS production via P2Y1-PKC-NOX2 is triggered by extracellular ATP after electrical stimulation of skeletal muscle cells. PLoS One 10: e0129882, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dimassi S, Chahed K, Boumiza S, Canault M, Tabka Z, Laurant P, and Riva C. Role of eNOS- and NOX-containing microparticles in endothelial dysfunction in patients with obesity. Obesity (Silver Spring) 24: 1305–1312, 2016 [DOI] [PubMed] [Google Scholar]
- 51. Dinauer MC, Orkin SH, Brown R, Jesaitis AJ, and Parkos CA. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature 327: 717–720, 1987 [DOI] [PubMed] [Google Scholar]
- 52. Dinulovic I, Furrer R, Beer M, Ferry A, Cardel B, and Handschin C. Muscle PGC-1alpha modulates satellite cell number and proliferation by remodeling the stem cell niche. Skelet Muscle 6: 39, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Doyle K. and Fitzpatrick FA. Redox signaling, alkylation (carbonylation) of conserved cysteines inactivates class I histone deacetylases 1, 2, and 3 and antagonizes their transcriptional repressor function. J Biol Chem 285: 17417–17424, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Du J, Fan LM, Mai A, and Li JM. Crucial roles of Nox2-derived oxidative stress in deteriorating the function of insulin receptors and endothelium in dietary obesity of middle-aged mice. Br J Pharmacol 170: 1064–1077, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, and Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem 274: 37265–37269, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Egea J, Fabregat I, Frapart YM, Ghezzi P, Gorlach A, Kietzmann T, Kubaichuk K, Knaus UG, Lopez MG, Olaso-Gonzalez G, Petry A, Schulz R, Vina J, Winyard P, Abbas K, Ademowo OS, Afonso CB, Andreadou I, Antelmann H, Antunes F, Aslan M, Bachschmid MM, Barbosa RM, Belousov V, Berndt C, Bernlohr D, Bertran E, Bindoli A, Bottari SP, Brito PM, Carrara G, Casas AI, Chatzi A, Chondrogianni N, Conrad M, Cooke MS, Costa JG, Cuadrado A, My-Chan Dang P, De Smet B, Debelec-Butuner B, Dias IHK, Dunn JD, Edson AJ, El Assar M, El-Benna J, Ferdinandy P, Fernandes AS, Fladmark KE, Forstermann U, Giniatullin R, Giricz Z, Gorbe A, Griffiths H, Hampl V, Hanf A, Herget J, Hernansanz-Agustin P, Hillion M, Huang J, Ilikay S, Jansen-Durr P, Jaquet V, Joles JA, Kalyanaraman B, Kaminskyy D, Karbaschi M, Kleanthous M, Klotz LO, Korac B, Korkmaz KS, Koziel R, Kracun D, Krause KH, Kren V, Krieg T, Laranjinha J, Lazou A, Li H, Martinez-Ruiz A, Matsui R, McBean GJ, Meredith SP, Messens J, Miguel V, Mikhed Y, Milisav I, Milkovic L, Miranda-Vizuete A, Mojovic M, Monsalve M, Mouthuy PA, Mulvey J, Munzel T, Muzykantov V, Nguyen ITN, Oelze M, Oliveira NG, Palmeira CM, Papaevgeniou N, Pavicevic A, Pedre B, Peyrot F, Phylactides M, Pircalabioru GG, Pitt AR, Poulsen HE, Prieto I, Rigobello MP, Robledinos-Anton N, Rodriguez-Manas L, Rolo AP, Rousset F, Ruskovska T, Saraiva N, Sasson S, Schroder K, Semen K, Seredenina T, Shakirzyanova A, Smith GL, Soldati T, Sousa BC, Spickett CM, Stancic A, Stasia MJ, Steinbrenner H, Stepanic V, Steven S, Tokatlidis K, Tuncay E, Turan B, Ursini F, Vacek J, Vajnerova O, Valentova K, Van Breusegem F, Varisli L, Veal EA, Yalcin AS, Yelisyeyeva O, Zarkovic N, Zatloukalova M, Zielonka J, Touyz RM, Papapetropoulos A, Grune T, Lamas S, Schmidt H, Di Lisa F, and Daiber A. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol 13: 94–162, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. El Assar M, Angulo J, Santos-Ruiz M, Moreno P, Novials A, Villanueva-Penacarrillo ML, and Rodriguez-Manas L. Differential effect of amylin on endothelial-dependent vasodilation in mesenteric arteries from control and insulin resistant rats. PLoS One 10: e0120479, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Espinosa A, Garcia A, Hartel S, Hidalgo C, and Jaimovich E. NADPH oxidase and hydrogen peroxide mediate insulin-induced calcium increase in skeletal muscle cells. J Biol Chem 284: 2568–2575, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Fan LM, Cahill-Smith S, Geng L, Du J, Brooks G, and Li JM. Aging-associated metabolic disorder induces Nox2 activation and oxidative damage of endothelial function. Free Radic Biol Med 108: 940–951, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fazzari M, Khoo NK, Woodcock SR, Jorkasky DK, Li L, Schopfer FJ, and Freeman BA. Nitro-fatty acid pharmacokinetics in the adipose tissue compartment. J Lipid Res 58: 375–385, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fink LN, Costford SR, Lee YS, Jensen TE, Bilan PJ, Oberbach A, Bluher M, Olefsky JM, Sams A, and Klip A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring) 22: 747–757, 2014 [DOI] [PubMed] [Google Scholar]
- 62. Frisbee JC, Goodwill AG, Frisbee SJ, Butcher JT, Brock RW, Olfert IM, DeVallance ER, and Chantler PD. Distinct temporal phases of microvascular rarefaction in skeletal muscle of obese Zucker rats. Am J Physiol Heart Circ Physiol 307: H1714–H1728, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Furrer R, Eisele PS, Schmidt A, Beer M, and Handschin C. Paracrine cross-talk between skeletal muscle and macrophages in exercise by PGC-1alpha-controlled BNP. Sci Rep 7: 40789, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, and Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752–1761, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gaggini F, Laleu B, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg Med Chem 19: 6989–6999, 2011 [DOI] [PubMed] [Google Scholar]
- 65a. Garcia-Gimenez JL, Olaso G, Hake SB, Bonisch C, Wiedemann SM, Markovic J, Dasi F, Gimeno A, Perez-Quilis C, Palacios O, Capdevila M, Vina J, and Pallardo FV. Histone h3 glutathionylation in proliferating mammalian cells destabilizes nucleosomal structure. Antioxid Redox Signal 19: 1305–1320, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gatto GJ, Jr., Ao Z, Kearse MG, Zhou M, Morales CR, Daniels E, Bradley BT, Goserud MT, Goodman KB, Douglas SA, Harpel MR, and Johns DG. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J Enzyme Inhib Med Chem 28: 95–104, 2013 [DOI] [PubMed] [Google Scholar]
- 67. Geiszt M, Lekstrom K, Witta J, and Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem 278: 20006–20012, 2003 [DOI] [PubMed] [Google Scholar]
- 68. Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, and Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol 5: 981–993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, and Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142–149, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Gonzalez-Perilli L, Alvarez MN, Prolo C, Radi R, Rubbo H, and Trostchansky A. Nitroarachidonic acid prevents NADPH oxidase assembly and superoxide radical production in activated macrophages. Free Radic Biol Med 58: 126–133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gray SP, Di Marco E, Kennedy K, Chew P, Okabe J, El-Osta A, Calkin AC, Biessen EA, Touyz RM, Cooper ME, Schmidt HH, and Jandeleit-Dahm KA. Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler Thromb Vasc Biol 36: 295–307, 2016 [DOI] [PubMed] [Google Scholar]
- 72. Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C, El-Osta A, Andrews KL, Chin-Dusting JP, Touyz RM, Wingler K, Cooper ME, Schmidt HH, and Jandeleit-Dahm KA. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127: 1888–1902, 2013 [DOI] [PubMed] [Google Scholar]
- 73. Groemping Y. and Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J 386: 401–416, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gu K, Cowie CC, and Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care 21: 1138–1145, 1998 [DOI] [PubMed] [Google Scholar]
- 75. Guizoni DM, Dorighello GG, Oliveira HC, Delbin MA, Krieger MH, and Davel AP. Aerobic exercise training protects against endothelial dysfunction by increasing nitric oxide and hydrogen peroxide production in LDL receptor-deficient mice. J Transl Med 14: 213, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, and Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52: 1803–1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, and Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, and Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105: 1656–1662, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Guzik TJ, Skiba DS, Touyz RM, and Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res 113: 1009–1023, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hales CM, Carroll MD, Fryar CD, and Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief 288: 1–8, 2017 [PubMed] [Google Scholar]
- 81. Ham M, Choe SS, Shin KC, Choi G, Kim JW, Noh JR, Kim YH, Ryu JW, Yoon KH, Lee CH, and Kim JB. Glucose-6-phosphate dehydrogenase deficiency improves insulin resistance with reduced adipose tissue inflammation in obesity. Diabetes 65: 2624–2638, 2016 [DOI] [PubMed] [Google Scholar]
- 82. Hammarstedt A, Graham TE, and Kahn BB. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetol Metab Syndr 4: 42, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hammerback K, Felias-Christensen G, and Phelan EA. Evaluation of a telephone-based physical activity promotion program for disadvantaged older adults. Prev Chronic Dis 9: E62, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hammers DW, Rybalko V, Merscham-Banda M, Hsieh PL, Suggs LJ, and Farrar RP. Anti-inflammatory macrophages improve skeletal muscle recovery from ischemia-reperfusion. J Appl Physiol (1985) 118: 1067–1074, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hammond RA. and Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes 3: 285–295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Han CY. Roles of reactive oxygen species on insulin resistance in adipose tissue. Diabetes Metab J 40: 272–279, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Han CY, Umemoto T, Omer M, Den Hartigh LJ, Chiba T, LeBoeuf R, Buller CL, Sweet IR, Pennathur S, Abel ED, and Chait A. NADPH oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes. J Biol Chem 287: 10379–10393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Helfield B, Chen X, Qin B, and Villanueva FS. Individual lipid encapsulated microbubble radial oscillations: effects of fluid viscosity. J Acoust Soc Am 139: 204–214, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Henriquez-Olguin C, Diaz-Vegas A, Utreras-Mendoza Y, Campos C, Arias-Calderon M, Llanos P, Contreras-Ferrat A, Espinosa A, Altamirano F, Jaimovich E, and Valladares DM. NOX2 inhibition impairs early muscle gene expression induced by a single exercise bout. Front Physiol 7: 282, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hiltunen MO, Turunen MP, Hakkinen TP, Rutanen J, Hedman M, Makinen K, Turunen AM, Aalto-Setala K, and Yla-Herttuala S. DNA hypomethylation and methyltransferase expression in atherosclerotic lesions. Vasc Med 7: 5–11, 2002 [DOI] [PubMed] [Google Scholar]
- 91. Hu P, Wu X, Khandelwal AR, Yu W, Xu Z, Chen L, Yang J, Weisbrod RM, Lee KSS, Seta F, Hammock BD, Cohen RA, Zeng C, and Tong X. Endothelial Nox4-based NADPH oxidase regulates atherosclerosis via soluble epoxide hydrolase. Biochim Biophys Acta 1863: 1382–1391, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Huang X, Sun M, Li D, Liu J, Guo H, Dong Y, Jiang L, Pan Q, Man Y, Wang S, and Li J. Augmented NADPH oxidase activity and p22phox expression in monocytes underlie oxidative stress of patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 91: 371–380, 2011 [DOI] [PubMed] [Google Scholar]
- 93. Irrcher I, Ljubicic V, and Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol 296: C116–C123, 2009 [DOI] [PubMed] [Google Scholar]
- 94. Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki Y, and Takeda S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med 19: 101–106, 2013 [DOI] [PubMed] [Google Scholar]
- 95. Jansen F, Yang X, Franklin BS, Hoelscher M, Schmitz T, Bedorf J, Nickenig G, and Werner N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc Res 98: 94–106, 2013 [DOI] [PubMed] [Google Scholar]
- 96. Jiang F, Lim HK, Morris MJ, Prior L, Velkoska E, Wu X, and Dusting GJ. Systemic upregulation of NADPH oxidase in diet-induced obesity in rats. Redox Rep 16: 223–229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kassan M, Galan M, Partyka M, Trebak M, and Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol 31: 2534–2542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kaur N, Naga OS, Norell H, Al-Khami AA, Scheffel MJ, Chakraborty NG, Voelkel-Johnson C, Mukherji B, and Mehrotra S. T cells expanded in presence of IL-15 exhibit increased antioxidant capacity and innate effector molecules. Cytokine 55: 307–317, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kelley EE, Baust J, Bonacci G, Golin-Bisello F, Devlin JE, St Croix CM, Watkins SC, Gor S, Cantu-Medellin N, Weidert ER, Frisbee JC, Gladwin MT, Champion HC, Freeman BA, and Khoo NK. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc Res 101: 352–363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ketonen J, Shi J, Martonen E, and Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J 74: 1479–1487, 2010 [DOI] [PubMed] [Google Scholar]
- 101. Kewalramani G, Fink LN, Asadi F, and Klip A. Palmitate-activated macrophages confer insulin resistance to muscle cells by a mechanism involving protein kinase C theta and epsilon. PLoS One 6: e26947, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Khan IM, Perrard XY, Brunner G, Lui H, Sparks LM, Smith SR, Wang X, Shi ZZ, Lewis DE, Wu H, and Ballantyne CM. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int J Obes (Lond) 39: 1607–1618, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Khoo NKH, Li L, Salvatore SR, Schopfer FJ, and Freeman BA. Electrophilic fatty acid nitroalkenes regulate Nrf2 and NF-kappaB signaling: A medicinal chemistry investigation of structure-function relationships. Sci Rep 8: 2295, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kietzmann T, Petry A, Shvetsova A, Gerhold JM, and Gorlach A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br J Pharmacol 174: 1533–1554, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, and Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 28: 1982–1988, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim JK, Gavrilova O, Chen Y, Reitman ML, and Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem 275: 8456–8460, 2000 [DOI] [PubMed] [Google Scholar]
- 107. Kim SH, Won SJ, Sohn S, Kwon HJ, Lee JY, Park JH, and Gwag BJ. Brain-derived neurotrophic factor can act as a pronecrotic factor through transcriptional and translational activation of NADPH oxidase. J Cell Biol 159: 821–831, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kopechek JA, Carson AR, McTiernan CF, Chen X, Klein EC, and Villanueva FS. Cardiac gene expression knockdown using small inhibitory RNA-loaded microbubbles and ultrasound. PLoS One 11: e0159751, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, and Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab 20: 614–625, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kreuz S. and Fischle W. Oxidative stress signaling to chromatin in health and disease. Epigenomics 8: 843–862, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, and Kadowaki T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 13: 294–307, 2011 [DOI] [PubMed] [Google Scholar]
- 112. Kumar B, Kowluru A, and Kowluru RA. Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 56: 2985–2992, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Langbein H, Brunssen C, Hofmann A, Cimalla P, Brux M, Bornstein SR, Deussen A, Koch E, and Morawietz H. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur Heart J 37: 1753–1761, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lee CR, North KE, Bray MS, Fornage M, Seubert JM, Newman JW, Hammock BD, Couper DJ, Heiss G, and Zeldin DC. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet 15: 1640–1649, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Li JM. and Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem 277: 19952–19960, 2002 [DOI] [PubMed] [Google Scholar]
- 116. Li Y, Cifuentes-Pagano E, DeVallance ER, de Jesus DS, Sahoo S, Meijles DN, Koes D, Camacho CJ, Ross M, St Croix C, and Pagano PJ. NADPH oxidase 2 inhibitors CPP11G and CPP11H attenuate endothelial cell inflammation & vessel dysfunction and restore mouse hind-limb flow. Redox Biol 22: 101143, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Li Y, Mouche S, Sajic T, Veyrat-Durebex C, Supale R, Pierroz D, Ferrari S, Negro F, Hasler U, Feraille E, Moll S, Meda P, Deffert C, Montet X, Krause KH, and Szanto I. Deficiency in the NADPH oxidase 4 predisposes toward diet-induced obesity. Int J Obes (Lond) 36: 1503–1513, 2012 [DOI] [PubMed] [Google Scholar]
- 118. Li Y. and Pagano PJ. Microvascular NADPH oxidase in health and disease. Free Radic Biol Med 109: 33–47, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liang CF, Liu JT, Wang Y, Xu A, and Vanhoutte PM. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler Thromb Vasc Biol 33: 777–784, 2013 [DOI] [PubMed] [Google Scholar]
- 120. Lin S, Ma S, Lu P, Cai W, Chen Y, and Sheng J. Effect of CTRP3 on activation of adventitial fibroblasts induced by TGF-beta1 from rat aorta in vitro. Int J Clin Exp Pathol 7: 2199–2208, 2014 [PMC free article] [PubMed] [Google Scholar]
- 121. Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, and Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 104: 2107–2112, 2001 [DOI] [PubMed] [Google Scholar]
- 122. Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, Hwa J, Yu J, and Martin KA. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 128: 2047–2057, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Liu Y, Hernandez-Ochoa EO, Randall WR, and Schneider MF. NOX2-dependent ROS is required for HDAC5 nuclear efflux and contributes to HDAC4 nuclear efflux during intense repetitive activity of fast skeletal muscle fibers. Am J Physiol Cell Physiol 303: C334–C347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lobysheva I, Rath G, Sekkali B, Bouzin C, Feron O, Gallez B, Dessy C, and Balligand JL. Moderate caveolin-1 downregulation prevents NADPH oxidase-dependent endothelial nitric oxide synthase uncoupling by angiotensin II in endothelial cells. Arterioscler Thromb Vasc Biol 31: 2098–2105, 2011 [DOI] [PubMed] [Google Scholar]
- 125. Loureiro AC, do Rego-Monteiro IC, Louzada RA, Ortenzi VH, de Aguiar AP, de Abreu ES, Cavalcanti-de-Albuquerque JP, Hecht F, de Oliveira AC, Ceccatto VM, Fortunato RS, and Carvalho DP. Differential expression of NADPH oxidases depends on skeletal muscle fiber type in rats. Oxid Med Cell Longev 2016: 6738701, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lynch CM, Kinzenbaw DA, Chen X, Zhan S, Mezzetti E, Filosa J, Ergul A, Faulkner JL, Faraci FM, and Didion SP. Nox2-derived superoxide contributes to cerebral vascular dysfunction in diet-induced obesity. Stroke 44: 3195–3201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, and Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol 24: 1844–1854, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mahmoud AM, Ali MM, Miranda ER, Mey JT, Blackburn BK, Haus JM, and Phillips SA. Nox2 contributes to hyperinsulinemia-induced redox imbalance and impaired vascular function. Redox Biol 13: 288–300, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Manea SA, Antonescu ML, Fenyo IM, Raicu M, Simionescu M, and Manea A. Epigenetic regulation of vascular NADPH oxidase expression and reactive oxygen species production by histone deacetylase-dependent mechanisms in experimental diabetes. Redox Biol 16: 332–343, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Marchesi C, Ebrahimian T, Angulo O, Paradis P, and Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 54: 1384–1392, 2009 [DOI] [PubMed] [Google Scholar]
- 132. Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, Di Rosa G, Gitto E, and Arrigo T. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci 16: 378–400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Matsuura F, Yamashita S, Nakamura T, Nishida M, Nozaki S, Funahashi T, and Matsuzawa Y. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 47: 929–933, 1998 [DOI] [PubMed] [Google Scholar]
- 134. Mause SF. and Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 107: 1047–1057, 2010 [DOI] [PubMed] [Google Scholar]
- 135. McLaughlin T, Abbasi F, Lamendola C, and Reaven G. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med 167: 642–648, 2007 [DOI] [PubMed] [Google Scholar]
- 136. McMurray F, Patten DA, and Harper ME. Reactive oxygen species and oxidative stress in obesity-recent findings and empirical approaches. Obesity (Silver Spring) 24: 2301–2310, 2016 [DOI] [PubMed] [Google Scholar]
- 137. Meng D, Mei A, Liu J, Kang X, Shi X, Qian R, and Chen S. NADPH oxidase 4 mediates insulin-stimulated HIF-1alpha and VEGF expression, and angiogenesis in vitro. PLoS One 7: e48393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Morgan MJ. and Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21: 103–115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mormeneo E, Jimenez-Mallebrera C, Palomer X, De Nigris V, Vazquez-Carrera M, Orozco A, Nascimento A, Colomer J, Lerin C, and Gomez-Foix AM. PGC-1alpha induces mitochondrial and myokine transcriptional programs and lipid droplet and glycogen accumulation in cultured human skeletal muscle cells. PLoS One 7: e29985, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nakamura Y, Feng Q, Kumagai T, Torikai K, Ohigashi H, Osawa T, Noguchi N, Niki E, and Uchida K. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. J Biol Chem 277: 2687–2694, 2002 [DOI] [PubMed] [Google Scholar]
- 141. Nakao N, Kurokawa T, Nonami T, Tumurkhuu G, Koide N, and Yokochi T. Hydrogen peroxide induces the production of tumor necrosis factor-alpha in RAW 264.7 macrophage cells via activation of p38 and stress-activated protein kinase. Innate Immun 14: 190–196, 2008 [DOI] [PubMed] [Google Scholar]
- 142. Nauseef WM. Biological roles for the NOX family NADPH oxidases. J Biol Chem 283: 16961–16965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nauseef WM. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim Biophys Acta 1840: 757–767, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Neves KB, Nguyen Dinh Cat A, Lopes RA, Rios FJ, Anagnostopoulou A, Lobato NS, de Oliveira AM, Tostes RC, Montezano AC, and Touyz RM. Chemerin regulates crosstalk between adipocytes and vascular cells through Nox. Hypertension 66: 657–666, 2015 [DOI] [PubMed] [Google Scholar]
- 145. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, and Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914–920, 2009 [DOI] [PubMed] [Google Scholar]
- 146. Nunoi H, Rotrosen D, Gallin JI, and Malech HL. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science 242: 1298–1301, 1988 [DOI] [PubMed] [Google Scholar]
- 147. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, and Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447: 1116–1120, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, and Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 285: 6153–6160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Okuno Y, Fukuhara A, Hashimoto E, Kobayashi H, Kobayashi S, Otsuki M, and Shimomura I. Oxidative stress inhibits healthy adipose expansion through suppression of SREBF1-mediated lipogenic pathway. Diabetes 67: 1113–1127, 2018 [DOI] [PubMed] [Google Scholar]
- 150. Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, and Blair SN. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J 34: 389–397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K, Hough K, Scalia R, and Goldstein BJ. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes 55: 1840–1846, 2006 [DOI] [PubMed] [Google Scholar]
- 152. Padilla J, Carpenter AJ, Das NA, Kandikattu HK, Lopez-Ongil S, Martinez-Lemus LA, Siebenlist U, DeMarco VG, and Chandrasekar B. TRAF3IP2 mediates high glucose-induced endothelin-1 production as well as endothelin-1-induced inflammation in endothelial cells. Am J Physiol Heart Circ Physiol 314: H52–H64, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pagano PJ. and Gutterman DD. The adventitia: the outs and ins of vascular disease. Cardiovasc Res 75: 636–639, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Pandey D, Patel A, Patel V, Chen F, Qian J, Wang Y, Barman SA, Venema RC, Stepp DW, Rudic RD, and Fulton DJ. Expression and functional significance of NADPH oxidase 5 (Nox5) and its splice variants in human blood vessels. Am J Physiol Heart Circ Physiol 302: H1919–H1928, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Paredes F, Sheldon K, Lassegue B, Williams HC, Faidley EA, Benavides GA, Torres G, Sanhueza-Olivares F, Yeligar SM, Griendling KK, Darley-Usmar V, and San Martin A. Poldip2 is an oxygen-sensitive protein that controls PDH and alphaKGDH lipoylation and activation to support metabolic adaptation in hypoxia and cancer. Proc Natl Acad Sci U S A 115: 1789–1794, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Parkos CA, Dinauer MC, Walker LE, Allen RA, Jesaitis AJ, and Orkin SH. Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc Natl Acad Sci U S A 85: 3319–3323, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Patel H, Chen J, Das KC, and Kavdia M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc Diabetol 12: 142, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, and Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 8: 301–309, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Pepping JK, Vandanmagsar B, Fernandez-Kim SO, Zhang J, Mynatt RL, and Bruce-Keller AJ. Myeloid-specific deletion of NOX2 prevents the metabolic and neurologic consequences of high fat diet. PLoS One 12: e0181500, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pinho RA, Sepa-Kishi DM, Bikopoulos G, Wu MV, Uthayakumar A, Mohasses A, Hughes MC, Perry CGR, and Ceddia RB. High-fat diet induces skeletal muscle oxidative stress in a fiber type-dependent manner in rats. Free Radic Biol Med 110: 381–389, 2017 [DOI] [PubMed] [Google Scholar]
- 161. Qin Z, Hou X, Weisbrod RM, Seta F, Cohen RA, and Tong X. Nox2 mediates high fat high sucrose diet-induced nitric oxide dysfunction and inflammation in aortic smooth muscle cells. J Mol Cell Cardiol 72: 56–63, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Qiu S, Mintz JD, Salet CD, Han W, Giannis A, Chen F, Yu Y, Su Y, Fulton DJ, and Stepp DW. Increasing muscle mass improves vascular function in obese (db/db) mice. J Am Heart Assoc 3: e000854, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Quesada IM, Lucero A, Amaya C, Meijles DN, Cifuentes ME, Pagano PJ, and Castro C. Selective inactivation of NADPH oxidase 2 causes regression of vascularization and the size and stability of atherosclerotic plaques. Atherosclerosis 242: 469–475, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ranayhossaini DJ, Rodriguez AI, Sahoo S, Chen BB, Mallampalli RK, Kelley EE, Csanyi G, Gladwin MT, Romero G, and Pagano PJ. Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. J Biol Chem 288: 36437–36450, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, and Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011 [DOI] [PubMed] [Google Scholar]
- 166. Raza F, Zafar H, Zhu Y, Ren Y, Ullah A, Khan AU, He X, Han H, Aquib M, Boakye-Yiadom KO, and Ge L. A review on recent advances in stabilizing peptides/proteins upon fabrication in hydrogels from biodegradable polymers. Pharmaceutics 10: 16, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Rehman AU, Dugic E, Benham C, Lione L, and Mackenzie LS. Selective inhibition of NADPH oxidase reverses the over contraction of diabetic rat aorta. Redox Biol 2: 61–64, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Reis AC, Alessandri AL, Athayde RM, Perez DA, Vago JP, Avila TV, Ferreira TP, de Arantes AC, Coutinho Dde S, Rachid MA, Sousa LP, Martins MA, Menezes GB, Rossi AG, Teixeira MM, and Pinho V. Induction of eosinophil apoptosis by hydrogen peroxide promotes the resolution of allergic inflammation. Cell Death Dis 6: e1632, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Rey FE, Cifuentes ME, Kiarash A, Quinn MT, and Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 170. Rozenberg P, Reichman H, Zab-Bar I, Itan M, Pasmanik-Chor M, Bouffi C, Qimron U, Bachelet I, Fulkerson PC, Rothenberg ME, and Munitz A. CD300f:IL-5 cross-talk inhibits adipose tissue eosinophil homing and subsequent IL-4 production. Sci Rep 7: 5922, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ruan CC, Zhu DL, Chen QZ, Chen J, Guo SJ, Li XD, and Gao PJ. Perivascular adipose tissue-derived complement 3 is required for adventitial fibroblast functions and adventitial remodeling in deoxycorticosterone acetate-salt hypertensive rats. Arterioscler Thromb Vasc Biol 30: 2568–2574, 2010 [DOI] [PubMed] [Google Scholar]
- 172. Sag CM, Schnelle M, Zhang J, Murdoch CE, Kossmann S, Protti A, Santos CXC, Sawyer G, Zhang X, Mongue-Din H, Richards DA, Brewer AC, Prysyazhna O, Maier LS, Wenzel P, Eaton PJ, and Shah AM. Distinct regulatory effects of myeloid cell and endothelial cell NAPDH oxidase 2 on blood pressure. Circulation 135: 2163–2177, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Sanchez-Gomez FJ, Calvo E, Breton-Romero R, Fierro-Fernandez M, Anilkumar N, Shah AM, Schroder K, Brandes RP, Vazquez J, and Lamas S. NOX4-dependent hydrogen peroxide promotes shear stress-induced SHP2 sulfenylation and eNOS activation. Free Radic Biol Med 89: 419–430, 2015 [DOI] [PubMed] [Google Scholar]
- 174. Sautin YY, Nakagawa T, Zharikov S, and Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 293: C584–C596, 2007 [DOI] [PubMed] [Google Scholar]
- 175. Schroder K, Wandzioch K, Helmcke I, and Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol 29: 239–245, 2009 [DOI] [PubMed] [Google Scholar]
- 176. Schurmann C, Rezende F, Kruse C, Yasar Y, Lowe O, Fork C, van de Sluis B, Bremer R, Weissmann N, Shah AM, Jo H, Brandes RP, and Schroder K. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur Heart J 36: 3447–3456, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, and Miller FJ., Jr Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 216: 321–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, and Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation 115: 627–637, 2007 [DOI] [PubMed] [Google Scholar]
- 179. Sinning JM, Losch J, Walenta K, Bohm M, Nickenig G, and Werner N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J 32: 2034–2041, 2011 [DOI] [PubMed] [Google Scholar]
- 180. Siu KL, Gao L, and Cai H. Differential roles of protein complexes NOX1-NOXO1 and NOX2-p47phox in mediating endothelial redox responses to oscillatory and unidirectional laminar shear stress. J Biol Chem 291: 8653–8662, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Siuda D, Zechner U, El Hajj N, Prawitt D, Langer D, Xia N, Horke S, Pautz A, Kleinert H, Forstermann U, and Li H. Transcriptional regulation of Nox4 by histone deacetylases in human endothelial cells. Basic Res Cardiol 107: 283, 2012 [DOI] [PubMed] [Google Scholar]
- 182. Smith SM, Min J, Ganesh T, Diebold B, Kawahara T, Zhu Y, McCoy J, Sun A, Snyder JP, Fu H, Du Y, Lewis I, and Lambeth JD. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem Biol 19: 752–763, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Sobey CG, Judkins CP, Rivera J, Lewis CV, Diep H, Lee HW, Kemp-Harper BK, Broughton BR, Selemidis S, Gaspari TA, Samuel CS, and Drummond GR. NOX1 deficiency in apolipoprotein E-knockout mice is associated with elevated plasma lipids and enhanced atherosclerosis. Free Radic Res 49: 186–198, 2015 [DOI] [PubMed] [Google Scholar]
- 184. Song DD, Chen Y, Li ZY, Guan YF, Zou DJ, and Miao CY. Protein tyrosine phosphatase 1B inhibits adipocyte differentiation and mediates TNFalpha action in obesity. Biochim Biophys Acta 1831: 1368–1376, 2013 [DOI] [PubMed] [Google Scholar]
- 185. Souto Padron de Figueiredo A, Salmon AB, Bruno F, Jimenez F, Martinez HG, Halade GV, Ahuja SS, Clark RA, DeFronzo RA, Abboud HE, and El Jamali A. Nox2 mediates skeletal muscle insulin resistance induced by a high fat diet. J Biol Chem 290: 13427–13439, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Sukumar P, Viswambharan H, Imrie H, Cubbon RM, Yuldasheva N, Gage M, Galloway S, Skromna A, Kandavelu P, Santos CX, Gatenby VK, Smith J, Beech DJ, Wheatcroft SB, Channon KM, Shah AM, and Kearney MT. Nox2 NADPH oxidase has a critical role in insulin resistance-related endothelial cell dysfunction. Diabetes 62: 2130–2134, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, and Hill JO. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000–2002. Diabetes Care 28: 1599–1603, 2005 [DOI] [PubMed] [Google Scholar]
- 188. Sun QA, Hess DT, Wang B, Miyagi M, and Stamler JS. Off-target thiol alkylation by the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine (VAS2870). Free Radic Biol Med 52: 1897–1902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Sylow L, Moller LL, Kleinert M, Richter EA, and Jensen TE. Stretch-stimulated glucose transport in skeletal muscle is regulated by Rac1. J Physiol 593: 645–656, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, and Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006 [DOI] [PubMed] [Google Scholar]
- 191. Thompson JA, Larion S, Mintz JD, Belin de Chantemele EJ, Fulton DJ, and Stepp DW. Genetic deletion of NADPH oxidase 1 rescues microvascular function in mice with metabolic disease. Circ Res 121: 502–511, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Tong X, Khandelwal AR, Wu X, Xu Z, Yu W, Chen C, Zhao W, Yang J, Qin Z, Weisbrod RM, Seta F, Ago T, Lee KS, Hammock BD, Sadoshima J, Cohen RA, and Zeng C. Pro-atherogenic role of smooth muscle Nox4-based NADPH oxidase. J Mol Cell Cardiol 92: 30–40, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Touati S, Montezano AC, Meziri F, Riva C, Touyz RM, and Laurant P. Exercise training protects against atherosclerotic risk factors through vascular NADPH oxidase, extracellular signal-regulated kinase 1/2 and stress-activated protein kinase/c-Jun N-terminal kinase downregulation in obese rats. Clin Exp Pharmacol Physiol 42: 179–185, 2015 [DOI] [PubMed] [Google Scholar]
- 194. Tsai AG, Williamson DF, and Glick HA. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obes Rev 12: 50–61, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Tsukui S, Kanda T, Nara M, Nishino M, Kondo T, and Kobayashi I. Moderate-intensity regular exercise decreases serum tumor necrosis factor-alpha and HbA1c levels in healthy women. Int J Obes Relat Metab Disord 24: 1207–1211, 2000 [DOI] [PubMed] [Google Scholar]
- 196. Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, Shirakura T, Kato K, Imaizumi K, Takahashi H, Tamura M, Maeda N, Funahashi T, and Shimomura I. Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem 288: 27138–27149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Tushuizen ME, Nieuwland R, Rustemeijer C, Hensgens BE, Sturk A, Heine RJ, and Diamant M. Elevated endothelial microparticles following consecutive meals are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care 30: 728–730, 2007 [DOI] [PubMed] [Google Scholar]
- 198. Valente AJ, Irimpen AM, Siebenlist U, and Chandrasekar B. OxLDL induces endothelial dysfunction and death via TRAF3IP2: inhibition by HDL3 and AMPK activators. Free Radic Biol Med 70: 117–128, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Van Buul JD, Fernandez-Borja M, Anthony EC, and Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7: 308–317, 2005 [DOI] [PubMed] [Google Scholar]
- 200. Vazquez N, Walsh TJ, Friedman D, Chanock SJ, and Lyman CA. Interleukin-15 augments superoxide production and microbicidal activity of human monocytes against Candida albicans. Infect Immun 66: 145–150, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Vendrov AE, Vendrov KC, Smith A, Yuan J, Sumida A, Robidoux J, Runge MS, and Madamanchi NR. NOX4 NADPH oxidase-dependent mitochondrial oxidative stress in aging-associated cardiovascular disease. Antioxid Redox Signal 23: 1389–1409, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Verdine GL. and Hilinski GJ. Stapled peptides for intracellular drug targets. Methods Enzymol 503: 3–33, 2012 [DOI] [PubMed] [Google Scholar]
- 203. Violi F, Sanguigni V, Carnevale R, Plebani A, Rossi P, Finocchi A, Pignata C, De Mattia D, Martire B, Pietrogrande MC, Martino S, Gambineri E, Soresina AR, Pignatelli P, Martino F, Basili S, and Loffredo L. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation 120: 1616–1622, 2009 [DOI] [PubMed] [Google Scholar]
- 204. Virdis A, Duranti E, Rossi C, Dell'Agnello U, Santini E, Anselmino M, Chiarugi M, Taddei S, and Solini A. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur Heart J 36: 784–794, 2015 [DOI] [PubMed] [Google Scholar]
- 205. Vlachopoulos C, Aznaouridis K, and Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010 [DOI] [PubMed] [Google Scholar]
- 206. Vogel J, Kruse C, Zhang M, and Schroder K. Nox4 supports proper capillary growth in exercise and retina neo-vascularization. J Physiol 593: 2145–2154, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Volpp BD, Nauseef WM, and Clark RA. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science 242: 1295–1297, 1988 [DOI] [PubMed] [Google Scholar]
- 208. Wang S, Peng Q, Zhang J, and Liu L. Na+/H+ exchanger is required for hyperglycaemia-induced endothelial dysfunction via calcium-dependent calpain. Cardiovasc Res 80: 255–262, 2008 [DOI] [PubMed] [Google Scholar]
- 209. Wang Y, Nie W, Yao K, Wang Z, and He H. Interleukin 6 induces expression of NADPH oxidase 2 in human aortic endothelial cells via long noncoding RNA MALAT1. Pharmazie 71: 592–597, 2016 [DOI] [PubMed] [Google Scholar]
- 210. Weller GE, Lu E, Csikari MM, Klibanov AL, Fischer D, Wagner WR, and Villanueva FS. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation 108: 218–224, 2003 [DOI] [PubMed] [Google Scholar]
- 211. Withers SB, Forman R, Meza-Perez S, Sorobetea D, Sitnik K, Hopwood T, Lawrence CB, Agace WW, Else KJ, Heagerty AM, Svensson-Frej M, and Cruickshank SM. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci Rep 7: 44571, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, and Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332: 243–247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Xi G, Shen X, Maile LA, Wai C, Gollahon K, and Clemmons DR. Hyperglycemia enhances IGF-I-stimulated Src activation via increasing Nox4-derived reactive oxygen species in a PKCzeta-dependent manner in vascular smooth muscle cells. Diabetes 61: 104–113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, Mikhed Y, Munzel T, Daiber A, Forstermann U, and Li H. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Arterioscler Thromb Vasc Biol 36: 78–85, 2016 [DOI] [PubMed] [Google Scholar]
- 215. Xie L, Ortega MT, Mora S, and Chapes SK. Interactive changes between macrophages and adipocytes. Clin Vaccine Immunol 17: 651–659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Xu F, Ji J, Li L, Chen R, and Hu WC. Adventitial fibroblasts are activated in the early stages of atherosclerosis in the apolipoprotein E knockout mouse. Biochem Biophys Res Commun 352: 681–688, 2007 [DOI] [PubMed] [Google Scholar]
- 217. Xu F, Sun Y, Chen Y, Sun Y, Li R, Liu C, Zhang C, Wang R, and Zhang Y. Endothelial cell apoptosis is responsible for the formation of coronary thrombotic atherosclerotic plaques. Tohoku J Exp Med 218: 25–33, 2009 [DOI] [PubMed] [Google Scholar]
- 218. Xu S, Chamseddine AH, Carrell S, and Miller FJ., Jr Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol 2: 642–650, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, and Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke 29: 12–17, 1998 [DOI] [PubMed] [Google Scholar]
- 220. Yarilina A, Park-Min KH, Antoniv T, Hu X, and Ivashkiv LB. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat Immunol 9: 378–387, 2008 [DOI] [PubMed] [Google Scholar]
- 221. Yong SB, Song Y, Kim HJ, Ain QU, and Kim YH. Mononuclear phagocytes as a target, not a barrier, for drug delivery. J Control Release 259: 53–61, 2017 [DOI] [PubMed] [Google Scholar]
- 222. Youn JY, Gao L, and Cai H. The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia 55: 2069–2079, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Youn JY, Siu KL, Lob HE, Itani H, Harrison DG, and Cai H. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes 63: 2344–2355, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Yu Y, Jin Y, Zhou J, Ruan H, Zhao H, Lu S, Zhang Y, Li D, Ji X, and Ruan BH. Ebselen: mechanisms of glutamate dehydrogenase and glutaminase enzyme inhibition. ACS Chem Biol 12: 3003–3011, 2017 [DOI] [PubMed] [Google Scholar]
- 225. Zembowicz A, Hatchett RJ, Radziszewski W, and Gryglewski RJ. Inhibition of endothelial nitric oxide synthase by ebselen. Prevention by thiols suggests the inactivation by ebselen of a critical thiol essential for the catalytic activity of nitric oxide synthase. J Pharmacol Exp Ther 267: 1112–1118, 1993 [PubMed] [Google Scholar]
- 226. Zhang AY, Yi F, Zhang G, Gulbins E, and Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension 47: 74–80, 2006 [DOI] [PubMed] [Google Scholar]
- 227. Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, and Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Zhang ZB, Ruan CC, Lin JR, Xu L, Chen XH, Du YN, Fu MX, Kong LR, Zhu DL, and Gao PJ. Perivascular adipose tissue-derived PDGF-D contributes to aortic aneurysm formation during obesity. Diabetes 67: 1549–1560, 2018 [DOI] [PubMed] [Google Scholar]
- 229. Zhong Q. and Kowluru RA. Epigenetic modification of Sod2 in the development of diabetic retinopathy and in the metabolic memory: role of histone methylation. Invest Ophthalmol Vis Sci 54: 244–250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Zhu D, Wang H, Zhang J, Zhang X, Xin C, Zhang F, Lee Y, Zhang L, Lian K, Yan W, Ma X, Liu Y, and Tao L. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol 87: 138–147, 2015 [DOI] [PubMed] [Google Scholar]
- 231. Zhu X, Zhang HW, Chen HN, Deng XJ, Tu YX, Jackson AO, Qing JN, Wang AP, Patel V, and Yin K. Perivascular adipose tissue dysfunction aggravates adventitial remodeling in obese mini pigs via NLRP3 inflammasome/IL-1 signaling pathway. Acta Pharmacol Sin 40: 46–54, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]