Abstract
Mild traumatic brain injury (mTBI) is a common diagnosis and approximately one third of mTBI patients experience a variety of cognitive, emotional, psychosocial, and behavioral post-concussion symptoms. When a cluster of these symptoms persists for more than 3 months they are often classified as post-concussion syndrome (PCS). The objective of this study was to determine prevalence rates, risk factors, and functional outcome associated with PCS 6 months after mTBI, applying divergent classification methods. Follow-up questionnaires at 6 months after mTBI included the Rivermead Post-Concussion Symptoms Questionnaire (RPQ) and the Glasgow Outcome Scale Extended (GOSE). The RPQ was analyzed according to different classification methods: the mapped International Classification of Diseases, 10th revision (ICD-10)/Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), the RPQ total score, the RPQ3 and the three-factor model using two different cutoff points (mild or worse and moderate or worse). Our results from a sample of 731 mTBI patients showed that prevalence rates of PCS ranged from 11.4% to 38.7% using divergent classification methods. According to all eight methods, 6.3% (n = 46) of mTBI patients experienced PCS. Applying the divergent classification methods resulted in a different set of predictors being statistically significantly associated with PCS, and a different percentage of overlap with functional impairment, measured with the GOSE. In conclusion, depending on the classification method and rating score used, prevalence rates of PCS deviated considerably. For future research, consensus regarding the diagnostic criteria for PCS and the analysis of the RPQ should be reached, to enhance comparability of studies regarding PCS after mTBI.
Keywords: : Glasgow outcome scale-extended, post-concussion syndrome, prevalence, Rivermead Post-Concussion Symptoms questionnaire, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide, with an annual incidence of 262 per 100,000 admitted TBI patients in Europe.1 The large majority (70–80%) of all TBI cases are evaluated as mild TBI (mTBI). In the first weeks following mTBI, many patients experience post-concussion symptoms comprising physical symptoms (e.g., headaches, dizziness, blurred vision, fatigue, and sleep disturbances), cognitive deficits (e.g., poor memory, and attention and executive difficulties), and behavioral/emotional symptoms (e.g., depression, irritability, anxiety-related disorders, and emotional lability).2 For most patients, these symptoms will diminish spontaneously,3 but for a subset of patients (estimated between 5%–43%4–9), symptoms last for months and sometimes even longer. When a set of symptoms persists for >3 months, it is often referred to as post-concussion syndrome (PCS).
It is challenging to define PCS, because there is no consensus as to the criteria for diagnosis.10 The most used criteria for diagnosis are those specified in the International Classification of Diseases, 10th revision (ICD-10)11 and the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV).2 Even though the ICD-10 and DSM-IV classifications deviate, they both include a brain injury with potential loss or alteration of consciousness, and the existence of certain symptoms. A frequently used instrument to assess the presence and severity of post-concussion symptoms is the Rivermead Post-Concussion Symptoms Questionnaire (RPQ).12 The RPQ was developed by King and colleagues, who proposed to use the total scale score for analyses.12 Subsequently, other evaluation methods have been applied. Potter and colleagues proposed a ≥12 cutoff for the total scale score.13 Eyres and colleagues suggested the use of a two subscale version, one scale containing three items (RPQ3) and one containing 13 items (RPQ13), because of a possible lack of unidimensionality for the RPQ total scale.14 Smith-Seemiller and colleagues recommended a modified scoring system with three subscales (cognitive, emotional, and somatic symptoms) or two subscales (collapsing somatic and emotional symptoms vs. cognitive symptoms) to be more sensitive.13,15 The majority of studies, however, mapped the ICD-10 or DSM-IV criteria to the RPQ.16–18 Patients are subsequently classified with PCS if they report at least three out of the following symptoms: headaches, dizziness, fatigue, irritability, impaired memory, impaired concentration, and insomnia. In addition to heterogeneity in classification methods, there is also no consensus on whether symptoms should be incorporated in the rating for PCS if they are rated as 2 (mild problem) or worse or only if they are rated as 3 (moderate problem) or worse.19,20
An abundance of studies are being done in the field of PCS regarding predictors and prediction modeling.20–22 We investigated whether classification methods have different predictors or have more predictive power, and expected that different risk factors would be significant depending on the classification method used. Advances and developments in prediction modeling are difficult, because an unambiguous definition for PCS is missing, and it is possible that different predictors are associated with PCS according to divergent classification methods.20
The application of different classification methods and cutoffs may lead to incomparability of studies assessing PCS. The main objective of this study was to examine how the four divergent classification methods and two different rating scores as cutoff defining PCS using the RPQ differ among patients 6 months after mTBI. First, descriptive analyses were done according to the four classification methods. Subsequently, the sample was analyzed on whether the risk factors predicting PCS differed across PCS classification methods, and lastly, the association with the clinically relevant Glasgow Outcome Scale Extended (GOSE) and different classification methods was observed. We expect differences in prevalence of PCS per classification method. We also hypothesize differences in predictors associated with PCS according to the divergent classification methods. Additionally, it was hypothesized that the functional outcome, measured by the GOSE, would differ, depending on the classification method used.
Methods
Study design
Data were obtained from the prospective observational Radboud University Brain Injury Cohort Study (RUBICS).23–26 All patients with mild, moderate or severe TBI admitted between January 1998 and December 2010 to the emergency department (ED) of the Radboud University Medical Centre (RUNMC), a level I trauma center in the Netherlands, were included in the database. The ethical standards committee of the RUNMC had approved this study.
Study participants
In the current study, 797 patients were selected from the RUBICS database based on the following inclusion criteria: patients' age was ≥16 years, written informed consent was given by patients (or guardians), patients had mTBI and were admitted to the ED of RUNMC between January 2003 and June 2010. Diagnosis of mTBI was based on a Glasgow Coma Scale (GCS) score of 13–15 after initial resuscitation or followed by sedation and intubation during resuscitation for a non-neurological cause. Exclusion criteria were alcohol or drug abuse or dementia, unknown address, and not being able to speak or write Dutch. We selected 92% (n = 731) of mTBI patients who completed the RPQ (filled in all items) at 6 month follow-up for all analyses throughout this study.
Measurements
Clinical data were registered in the ED at admission by a neurologist and/or neurosurgeon and entered by a research nurse into the RUBICS databank. Demographic data (age, sex, and educational level), trauma mechanism, hospitalization, clinical variables, comorbidities, functional outcome (GOSE), and the RPQ were all collected with a postal questionnaire, which was self-rated by patients or guardians at 6 months after the trauma. Structured interviews during regular visits to the outpatient clinic or during consultation by telephone were used to determine GOSE scores.27
Assessment of persistent post-concussion symptoms and diagnosis of PCS
The prevalence rates and severity of persistent post-concussion symptoms were assessed with the postal RPQ at 6 month follow-up. Patients were asked to rate the severity of 16 different symptoms, commonly found after TBI, over the past 24 h. In each case, the symptoms were compared with how severe they had been before the injury occurred (premorbid). The patient was asked to rate the symptoms on a five point Likert scale: 0 (not experienced at all), 1 (not a problem), 2 (mild problem), 3 (moderate problem), and 4 (severe problem).
In the literature, there is not a gold standard concerning the use of the RPQ. Therefore, we used the following classification methods to classify patients as having PCS: mapped ICD-10/DSM-IV, RPQ total score,12 RPQ 3,14 and three-factor model (Table 1) .15 The mapped ICD-10/DSM-IV requires that three or more symptoms in the list in Table 1 reach cutoff, the RPQ3 requires that one or more symptoms in the list in Table 1 reach cutoff, the RPQ total score requires a sum score of all items of the RPQ of ≥12, and the three factor model requires that one or more items within each of the cognitive, emotional, and somatic scales reaches cutoff. For each classification method, we used two different rating scores as cutoff (≥ 2 and ≥3), resulting in eight different classification methods in total. Because no clear cutoff was found in the literature for the RPQ13, this scale was not taken into consideration. It should also be noted that the RPQ is based on self-report rather than clinical examination, and does not include information on the duration of the symptoms and clinically significant impairment. Therefore, it cannot accurately diagnose PCS.20
Table 1.
Classification Methods Regarding Post-Concussion Syndrome
| Classification methods | Mapped ICD-10/DSM-IV | RPQ Total score13 | RPQ314 | Three factor model15 | |
|---|---|---|---|---|---|
| At least 3 symptoms from the list below | All symptoms from the list below | At least 1 symptom from the list below | At least 1 symptom from each scale from the list below | ||
| Eligible symptoms from | Headache | Headache | Headache | Cognitive | Forgetfulness, poor memory |
| the RPQ | Dizziness | Dizziness | Dizziness | Poor concentration | |
| Sleep disturbance | Nausea and/or vomiting | Nausea and/or vomiting | Taking longer to think | ||
| Fatigue | Noise sensitivity | Emotional | Being irritable, easily angered | ||
| Being irritable, easily angered | Sleep disturbance | Feeling depressed or tearful | |||
| Forgetfulness, poor memory | Fatigue | Feeling frustrated or impatient | |||
| Poor concentration | Blurred vision | Restlessness | |||
| Light sensitivity | Somatic | Headache | |||
| Double vision | Dizziness | ||||
| Forgetfulness, poor memory | Nausea and/or vomiting | ||||
| Poor concentration | Noise sensitivity | ||||
| Taking longer to think | Sleep disturbance | ||||
| Being irritable, easily angered | Fatigue | ||||
| Feeling depressed or tearful | Blurred vision | ||||
| Feeling frustrated or impatient | Light sensitivity | ||||
| Restlessness | Double vision | ||||
| Cutoff; rating score 2 | Three items with score ≥2 | ≥ 12 (only symptoms ≥2)a | One item with score ≥2 | Each scale has one item ≥2 | |
| Cutoff; rating score 3 | Three items with score ≥3 | ≥ 12 (only symptoms ≥3) | One item with score ≥3 | Each scale has one item ≥3 | |
Example: Six symptoms with rating score 2 qualify as having PCS.
ICD, International Classification of Diseases; DSM, Diagnostic and Statistical Manual of Mental Disorders; RPQ, Rivermead Post-Concussion Symptoms Questionnaire; PCS, post-concussion syndrome.
Risk factors
Looking at the available data in our data set and using previous literature,20–22 the variables age, gender, level of education, injury mechanism (assault vs. other mechanisms), Injury Severity Scale (ISS), Abbreviated Injury Score of the Head (AISH), comorbidity, traumatic abnormalities on the head CT scan, and whether the patient was admitted to the hospital were considered as risk factors. We hypothesized that older age, female gender, lower years of education, higher ISS and AISH scores, comorbidity, abnormalities on CT, and being hospitalized would be associated with PCS.
Functional outcome
Functional outcome was assessed using the 6 month GOSE, which was completed as a postal questionnaire. The GOSE is a functional measurement scale specifically designed for TBI.28,29 The instrument evaluates functional outcome through eight categories encompassing consciousness, independence at home and outside the home, work, social and leisure activities, family and friendship, and return to normal life.30 After accumulating these categories an eight point scale ranging from 1 (dead) to 8 (completely recovered) is established, which has the ability to distinguish among functional outcomes. For 20 patients included in our study, the GOSE score was missing. When there was no available outcome at exactly 6 months, outcomes measured within a 2 month range were also approved. Functional impairment was classified as a GOSE score of ≤6.27
Statistical analysis
For demographic data (age, sex, and educational level), trauma mechanisms, hospitalization, clinical injury variables and comorbidities, descriptive analyses were performed. Patients included in the current study were compared with those having incomplete RPQ data on demographic (gender, age, educational level) and clinical variables using χ2 tests (categorical variables) and Student's t tests (continuous variables).
Prevalence of PCS using the eight divergent classification methods was determined by computing the percentage of patients meeting the specific criteria of each classification method. We subsequently determined overlap between classification methods by calculating the number and percentage of patients diagnosed with PCS according to multiple classification methods.
The univariable associations between predictors and PCS according to multiple classification methods were explored by using χ2 tests (categorical variables) and an independent samples t test (continuous variables). All variables were included in a stepwise backwards multivariable logistical regression to identify significant risk factors (p < 0.05) of PCS. The association between PCS and functional impairment (GOSE ≤6) was determined by calculating the percentage of patients for each classification method of PCS that was functionally impaired. McNemar tests were used to see if the classification methods differed significantly in PCS/no PCS pattern at the population level, and a Cochran's Q test was used to see if the classification methods differed significantly (p < 0.05) at an individual level. Multiple imputation technique with five data sets was used to impute missing data for the following predictor variables: education (182 missing), comorbidity (237 missing), and hospital admission (2 missing).
All statistical analyses were performed using SPSS version 21 for Windows (IBM SPSS Statistics, SPSS Inc, Chicago, IL).
Results
Study population
In total, 731 mTBI patients were included in this study. Patients with a missing 6 month RPQ (n = 66) did not differ from those included in this study, except that their median age was 54.5 (interquartile range [IQR]: 42.75–68), which was significant on a p < 0.01 level. The characteristics of our study sample are shown in Table 2. The median age of the respondents was 44 years and 63% were male. Almost half (48%) of the patients were injured in road traffic accidents and a third were injured due to falls. One out of five people had one or more comorbid conditions and ∼13% showed abnormalities on the CT scan. Approximately 50% of the respondents were admitted to the hospital, and they were hospitalized for an average of 3 days. A total of 35 patients were admitted to the intensive care unit (ICU).
Table 2.
Characteristics of the Study Population
| n | 731 |
| Gender (male) | 463 (63.3%) |
| Age1 (years) | 44 (27-57) |
| Education | |
| Primary education | 21 (2.9%) |
| Secondary education | 336 (46.0%) |
| Higher professional education | 108 (14.8%) |
| Academic education | 84 (11.5%) |
| Unknown | 182 (24.9%) |
| Injury Mechanism | |
| Road traffic accident | 351 (48.0%) |
| Fall | 240 (32.8%) |
| Sports | 77 (10.5%) |
| Assault | 41 (5.6%) |
| Other/Unknown | 22 (3.0%) |
| Injury characteristics | |
| ISSa | 6 (4-14) |
| AISHa | 2 (2-2) |
| Head AIS 3 | 93 (12.7%) |
| Head AIS 4 | 57 (7.8%) |
| Head AIS 5 | 11 (1.5%) |
| Comorbidityb | |
| No pre-existing disease | 329 (45.0%) |
| 1 comorbid disease | 92 (12.6%) |
| 2 comorbid disease | 33 (4.5%) |
| 3 or more comorbidities | 40 (5.5%) |
| Unknown | 237 (32.4%) |
| CT scan | |
| No CT scan | 45 (6.2%) |
| CT scan, no abnormalities | 591 (81.0%) |
| CT scan, abnormalities | 94 (12.9%) |
| Hopsitalizationc | |
| Hospital admission | 373 (51.0%) |
| Number of days hospitalizeda | 3 (1-8) |
| ICU admission | 35 (4.8%) |
| GCSa | 15 (14-15) |
| 13 | 40 (5.5%) |
| 14 | 152 (20.8%) |
| 15 | 539 (73.7%) |
| GOSEa | 7 (6-8) |
| RPQ total scorea | 4 (4-15) |
Data are displayed as median, with the first and third quartile given in parentheses.
Comorbidity is defined as the presence of any co-existing diseases or disease processes additional to injury that the traumatic brain injury (TBI) patients sustained. The following diseases were assessed as comorbid disease: asthma, chronic bronchitis, chronic nonspecific lung disease (not asked about), heart disease, diabetes, back hernia or chronic backache, osteoarthritis, rheumatoid arthritis, and cancer.
Hospital or ICU admission for ≥1 day after arrival at emergency department.
ISS, Injury Severity Score; AISH, Abbreviated Injury Scale of the Head; AIS, Abbreviated Injury Scale; ICU, Intensive Care Unit; GOSE, Glasgow Outcome Scale Extended; RPQ, Rivermead Post-Concussion Symptoms Questionnaire.
Six-month persistent post-concussion symptoms
The three most frequently reported symptoms on the 6 month RPQ were fatigue, forgetfulness/poor memory, and poor concentration (Fig. S1) (see online supplementary material at http://www.liebertpub.com). Fatigue was experienced by 308 patients (42.1%), and 32 (4.4%) patients evaluated this as a severe problem. Nausea and/or vomiting was the least reported symptom (n = 42, 5.7%). Approximately one third of the patients (n = 242) experienced none of the symptoms (total RPQ score of 0), whereas three patients had an RPQ score of 59, which means that they experienced severe problems 6 months after the injury with almost every symptom on the list. Approximately 30% (n = 234) had a total RPQ score of ≥12. The median score on the RPQ for the study population was 4 (IQR, 4–15).
Prevalence rates of PCS according to the different classification methods
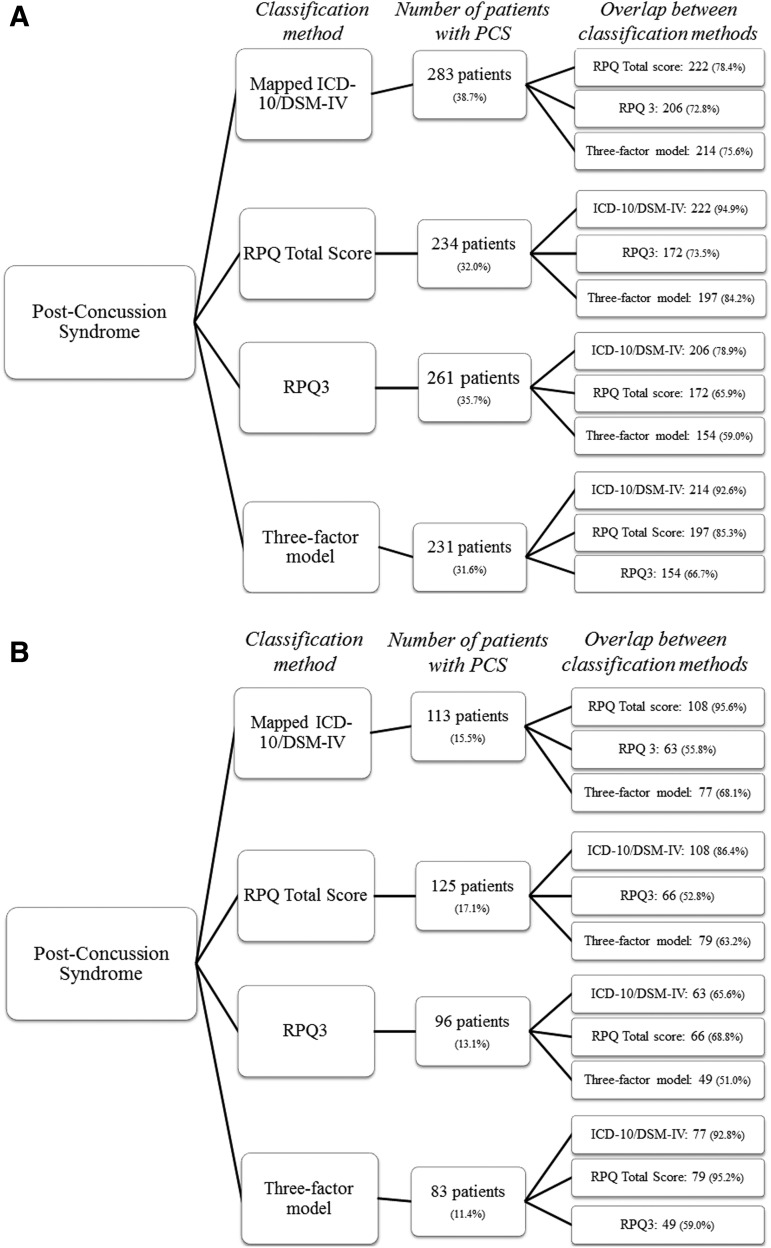
The use of divergent classification methods resulted in prevalence rates for 6 month PCS ranging from 11.4% (three factor model with rating score 3) to 38.7% (mapped ICD-10/DSM-IV with rating score 2; Fig 1 A and B). Classification methods overlapped substantially; for example, 95.6% (n = 108) of patients who met the criteria for PCS according to the mapped ICD-10/DSM-IV with rating score 2 also met the criteria for PCS according to the RPQ total score with rating score 2. The lowest amount of overlap was found for the classification methods RPQ3 and the three factor model with rating score 3 (n = 49, 51%) A total of 46 (6.3%) patients met the criteria for PCS according to all classification methods. When looking at the difference in PCS/no PCS pattern for the classification methods with rating score 2, a significant result was found for all classification methods, except for the mapped ICD-10/DSM-IV compared with the RPQ3 (p = 0.07) and the RPQ total score compared with the three factor model (p = 0.81). For the classification methods with rating score 3, all had significant differences in pattern, except for the mapped ICD-10/DSM-IV compared with the RPQ3 (p = 0.78) and the RPQ3 compared with the three factor model (p = 0.18). The lack of significant differences in PCS/no PCS pattern were characterized by the shared symptom overlap between the RPQ3 and the three factor model, and the mapped ICD-10/DSM-IV. A Cochran's Q test determined that all classification methods differed significantly from each other. These results demonstrated that the choice of classification method influenced PCS diagnosis both at a population level, and at an individual level.
FIG. 1.
(A) Number of mild traumatic brain injury (mTBI) patients with post-concussion syndrome (PCS) at 6 month follow-up per classification method with rating score 2, and the overlap between them. (B) Number of mTBI patients with PCS at 6 month follow-up per classification method with rating score 3, and the overlap between them.
Risk factors for PCS
Assault was significantly associated with 6 month PCS according to all classification methods, whereas traumatic abnormalities on the head CT scan and age were not statistically significantly associated with PCS according to any of the classification methods (Table 3, Tables S1–S4) (see online supplementary material at http://www.liebertpub.com). Female gender and lower education were significantly associated with all classification methods, except for the three factor model with rating score 3. The significance of the predictors ISS, AISH, comorbidity, and hospital admission, however, depended on the classification method used; for example, hospital admission was a significant predictor for PCS using six out of eight classification methods. Multivariable prediction models explained 6–14% (Nagelkerke R2) of the variation in PCS according to the different classification methods.
Table 3.
Significant Predictors in a Multivariable Model of 6 Month PCS Using Divergent Classification Methods on a p < 0.05 Level
| Mapped ICD-10/DSM-IV | RPQ total score | RPQ3 | Three factor model | |||||
|---|---|---|---|---|---|---|---|---|
| Predictor | ≥ 2* | ≥ 3** | ≥ 2 | ≥ 3 | ≥ 2 | ≥ 3 | ≥ 2 | ≥ 3 |
| Gender | 0.53 | 0.48 | 0.53 | 0.54 | 0.38 | 0.40 | 0.59 | |
| Age | ||||||||
| Education (Primary/Secondary) | 1.73 | 1.80 | 1.69 | 1.82 | 1.55 | 1.80 | 1.62 | |
| Injury mechanism (Assault vs. other mechanisms) | 0.38 | 0.26 | 0.25 | 0.34 | 0.34 | 0.21 | 0.27 | 0.29 |
| ISS | 1.03 | |||||||
| AISH | 1.21 | 1.24 | 1.29 | 1.22 | 1.29 | |||
| CT abnormalities | ||||||||
| Comorbidity | 0.54 | 0.51 | 0.60 | 0.65 | 0.59 | 0.52 | ||
| Hospital admission | 0.45 | 0.45 | 0.61 | 0.43 | 0.52 | 0.53 | ||
| R2 | 0.13 | 0.09 | 0.14 | 0.11 | 0.10 | 0.10 | 0.11 | 0.06 |
Cells in gray indicate that the predictor is statistically significantly (p < 0.05) associated with PCS in multivariable logistic regression analysis and in the cells are the odds ratios. Cells in white indicate that the predictor is not statistically significantly associated with PCS.
For each classification method, we used two different rating scores as cutoff: rating score 2 (* ≥ 2) and rating score 3 (** ≥3).
PCS, post-concussion syndrome; ICD, International Classification of Diseases; DSM, Diagnostic and Statistical Manual of Mental Disorders; RPQ, Rivermead Post-Concussion Symptoms Questionnnaire; ISS, Injury Severity Scale; AISH, Abbreviated Injury Severity Scale of the Head.
PCS and functional outcome
A total of 198 (27.1%) patients were functionally impaired (GOSE ≤6) 6 months post-injury. There was a significant association between PCS according to all classification methods and functional impairment (p < 0.01). The highest percentage of functional impairment for patients with PCS was found for the RPQ total scale with rating score 3 (72.8%, n = 91), whereas the RPQ3 with rating score 2 recorded the lowest percentage (46.0%, n = 120) (Table 4).
Table 4.
mTBI Patients with PCS and Functionally Impaired (GOSE ≤6)
| Mapped ICD-10/DSM-IV | RPQ total score | RPQ3 | Three factor model | |
|---|---|---|---|---|
| Rating score 2a | 51.6% (146) | 58.1% (136) | 46.0% (120) | 54.5% (126) |
| Rating score 3b | 71.7% (81) | 72.8% (91) | 67.7% (65) | 71.7% (59) |
Mild or worse.
Moderate or worse.
p < 0.01 on all associations.
mTBI, mild traumatic brain injury; GOSE, Glasgow Outcome Scale Extended; ICD, International Classification of Diseases; DSM, Diagnostic and Statistical Manual of Mental Disorders; PCS, post-concussion syndrome; RPQ, Rivermead Post-Concussion Symptoms Questionnaire.
Discussion
The prevalence of PCS 6 months following mTBI ranged from 11.4% to 38.7%, depending on the classification method and rating score applied. The divergent classification methods in this study additionally influenced the statistical significance of predictors and the association with functional outcome, as measured with the GOSE.
The prevalence rates of PCS in our study are in line with preceding studies, which reported that prevalence rates of PCS after mTBI fluctuate and are estimated to range from 5% to 43%.4–9 The prevalence rates that were found in the literature were dependent on many aspects, such as the case mix of the sample and setting, but they were also dependent on the rating score applied and the classification method used to identify mTBI patients with PCS. Yeates has pointed out that the inconsistency in definition and classification criteria interferes with the righteous classification and identification of patients with PCS,31 which ultimately leads to incommensurable prevalence rates and outcomes. Additionally, Waljas and colleagues have also stated that the rate of PCS diagnosis varies greatly based on which rating scale is being used,19 which substantiates the decision during the writing of this article to research two different rating scores as cutoff points. Recently, the DSM criteria for PCS have been revised substantially. As this definition deviates significantly from the DSM-IV (e.g. the term mild neurocognitive impairment [MNI] from TBI was introduced instead of PCS),32 it is likely that this will result in even more heterogeneity in prevalence rates. Tator and colleagues have recently emphasized “a refinement of the definition of PCS,”33 and also the lack of consensus with regard to the definitions of PCS has previously been identified as a problem.8 This problem presented itself as an opportunity in our study to explore and compare prevalence rates, risk factors, and functional outcome when divergent cutoff rating scores and classification methods of the RPQ are applied.
When comparing divergent classification methods, different patients were identified as having PCS. There was a difference of almost 30% in prevalence rates between the classification method with the highest (mapped ICD-10/DSM-IV with rating score 2; 38.7%) and lowest (three factor model with rating score 3; 11.4%) percentage. Forty-six patients experienced PCS according to all eight classification methods. The most overlap in identifying the same patients experiencing PCS was found between the mapped ICD-10/DSM-IV and the RPQ total score (95.6%), both with rating score 3. This can be explained by the overlap between symptoms included in both classification methods and by the fact that six out of seven eligible symptoms from the RPQ enclosed in the mapped ICD-10/DSM-IV are in the top eight most reported symptoms in this population. The lowest percentage of overlap was found between the RPQ3 and the three-factor model (51.0%) when a rating score of 3 was used as a cutoff. This can be explained by the fact that the RPQ3 only defines three somatic symptoms, whereas four out of the five most reported symptoms (forgetfulness/poor memory, poor concentration, taking longer to think, feeling frustrated or impatient) in this study population are cognitive or emotional, which are captured in the three factor model. This is also in line with the thought that the RPQ3 measures symptoms that occur more often in the acute phase after mTBI.18
In this study, we found that the classification method used influenced the statistical significance of predictors; that is, several predictors were statistically significantly associated with PCS using some classification methods but not using others. This might be one of the reasons for the substantial heterogeneity in studies on predictors and prediction modelling for PCS,21,22 hampering prognostic research. However, the results also showed that assault was associated with all classification methods, and female gender and lower education were associated with all but one classification method.
Although PCS was statistically significantly associated with functional impairment (GOSE ≤6), there was variation in the amount of overlap between PCS and functional impairment dependent on the classification methods applied, ranging from 46.0% to 72.8%. Restricting PCS to only those symptoms that were reported as being “moderate or worse” resulted in higher overlap between PCS and functional impairment. This may indicate that symptoms reported as moderate or worse are more likely to represent clinically relevant symptomatology than symptoms reported as mild. This is in line with the findings by Waljas and colleagues,19 who reported that when using rating score 3 as a cutoff, patients with head injury were successfully distinguished from healthy controls, whereas when rating score 2 was used as cutoff, this resulted in a substantial proportion of healthy controls being diagnosed with PCS.
The present study is unique because eight divergent classification methods for PCS were applied, and the statistical effect this might have had on predictors associated with PCS and the different percentages seen as functionally impaired, measured by the GOSE, were assessed.
Our study had several limitations. First, Ruff declared that PCS concerns a complex interplay of biological, psychological, and social factors that include prior health, life stressors, and compensation/litigation issues.8 This implies that an overview of many aspects of a patient's current, but also previous life before the trauma, is required for correct assessment.
Our study was a post-hoc analysis of prospectively collected data of individuals after mTBI, and there were no pre-injury data available except for pre-existing comorbidity. Additionally, post-concussion symptoms in our study were self-reported, which might have led to more or fewer reported symptoms on the questionnaire than if the respondents had been interviewed by a physician.34 Response bias might also have played a role during our study. Respondents with symptoms may have been more likely to participate in the 6 month follow-up questionnaires than patients who were currently not experiencing/or had never experienced any symptoms.
Further, it has been argued that the RPQ is not the most ideal instrument to use in an mTBI population,35 but there is currently no consensus on what would be a better instrument to use. Looking at the RPQ total scale, one should keep in mind that even though the total RPQ score has been proposed by the developer of the instrument and is used in most articles until now, Eyres and colleagues have revalidated the RPQ, and have pointed out that the various items of the RPQ have very low construct validity and in consequence of this, should not be computed into a sum score,14 but into two subscales. During this study, we have decided to not take the RPQ13 into consideration, because no clear cutoff was found in the literature. For future studies, it would be interesting to look at the RPQ13 and establish a cutoff in view of the fact that a large number of the reported symptoms at 6 months are considered cognitive, provided that enough clinical data and concurrent evidence are available to define and diagnose TBI.
A limitation concerning the use of mapped ICD-10/DSM-IV in this study was that we imposed them as the same, because we do not have the required data to differentiate between the ICD-10 and DSM-IV. This might have led to over/under-reporting of prevalence rates, and limited the ability to report about the differences among the most applied definitions. Previous studies have shown that DSM-IV usually leads to lower prevalence rates, because the diagnostic criteria seem to be more stringent,36,37 yet McCauley and colleagues have stated that there should be no clinical preference for any one of the diagnostic criteria.38 In previous research, the variability in instruments used to diagnose mTBI has also been considered a difficulty. Depending on the diagnosis criteria used, different individuals will be classified as having mTBI, which may lead to inconsistencies and might influence the results.22,39–42 In our study, this could possibly mean that we have included patients who would not have been diagnosed with mTBI using other diagnostic criteria, which could affect the risk factors and functional outcome of this population.
In our study we used a relatively low threshold (p = 0.05) for the inclusion of predictors in the backward selection procedure. Higher levels (e.g., p = 0.20 or p = 0.15743,44) as well as advanced statistical methods such as shrinkage and bootstrap validation are usually recommended to enhance the internal and external validity of prediction models.43 Therefore, the results on predictors in our study should be interpreted as a proof of principle (there are different predictors associated with PCS according to different definitions) rather than considered applicable for clinical practice. Regarding the results of the regression, these could have been weakened by the fact that we looked at assault compared with all other injury mechanisms combined. More detailed information on the circumstances of the injury is essential to comprehend the real effect of the injury mechanism on the outcome.
Additionally, lower education and comorbidity were considered significant risk factors for PCS, which could have been impacted by the large number of imputed values.
A final limitation of our study is that data were collected in one academic hospital, which limits the generalizability of the results, because of differences in the case mix and because patients with severe trauma are more likely to be admitted to the ED of an academic hospital.
During the last decade, a shift from identifying PCS and interpreting it as an exclusive syndrome to recognizing it as being a highly complex and ever-changing condition in different settings/populations, can be observed. This development leads to more and more specific research in the area of PCS or, as now suggested, persistent post-concussive symptoms. This debate and inconsistency concerning definitions, diagnostic criteria, assessment, and evaluation of PCS hampers its research and therapy. Standardizing and improving diagnosis and assessment of PCS will facilitate to identify opportunities for intervention when patients experience the disabling PCS symptoms, or even prevent mTBI patients from developing PCS. In addition, it is recommended to perform sensitivity and specificity analyses on the different classification methods for the RPQ to evaluate their classification accuracy.18
Conclusion
Our study showed that prevalence rates of PCS 6 months after mTBI deviated considerably, depending on the classification method and rating score used. In addition, applying divergent classification methods resulted in a different set of predictors being statistically significantly associated with PCS, and a different percentage of overlap with functional impairment, measured with the GOSE.
These findings highlight the need for a universal guideline with respect to diagnostic criteria for PCS, and a gold standard for analysis of the RPQ, to enhance comparability of studies regarding PCS after mTBI.
Supplementary Material
Acknowledgments
This article was written in the context of the CENTER-TBI project. CENTER-TBI has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 602150. TRACK-TBI has received funding from the National Institutes of Neurologic Disorders and Stroke, grant no U01 NS086090. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Disclosure Statement
No competing interests exist. The ethical standards committee of the Radboud University Medical Center has approved the RUBICS study. Written informed consent was obtained from all patients in this study.
References
- 1.Peeters W., van den Brande R., Polinder S., Brazinova A., Steyerberg E.W., Lingsma H.F., and Maas A.I. (2015). Epidemiology of traumatic brain injury in Europe. Acta Neurochir. (Wien) 157, 1683–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th Ed. (DSM-IV). American Psychiatric Association: Washington, DC [Google Scholar]
- 3.Levin H.S., and Diaz-Arrastia R.R. (2015). Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 14, 506–517 [DOI] [PubMed] [Google Scholar]
- 4.Binder L.M. (1986). Persisting symptoms after mild head injury: a review of the postconcussive syndrome. J. Clin. Exp. Neuropsychol. 8, 323–346 [DOI] [PubMed] [Google Scholar]
- 5.Leddy J.J., Sandhu H., Sodhi V., Baker J.G., and Willer B. (2012). Rehabilitation of concussion and post-concussion syndrome. Sports Health 4, 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinos P., Sakellaropoulos G., Georgiopoulos M., Stavridi K., Apostolopoulou K., Ellul J., and Constantoyannis C. (2010). Postconcussion syndrome after mild traumatic brain injury in Western Greece. J. Trauma 69, 789–794 [DOI] [PubMed] [Google Scholar]
- 7.King N.S., and Kirwilliam S. (2013). The nature of permanent post-concussion symptoms after mild traumatic brain injury. Brain Impair. 14, 235–242 [DOI] [PubMed] [Google Scholar]
- 8.Ruff R.M. (2011). Mild traumatic brain injury and neural recovery: rethinking the debate. NeuroRehabilitation 28, 167–180 [DOI] [PubMed] [Google Scholar]
- 9.Hiploylee C., Dufort P.A., Davis H.S., Wennberg R.A., Tartaglia M.C., Mikulis D., et al. (2016). Longitudinal study of postconcussion syndrome: not everyone recovers. J. Neurotrauma [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll L.J., Cassidy J.D., Peloso P.M., Borg J., von Holst H., Holm L., Paniak C., Pepin M., and Injury WHOCCTFoMTB. (2004). Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 43, Suppl., 84–105 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (1993). The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. World Health Organization: Geneva [Google Scholar]
- 12.King N.S., Crawford S., Wenden F.J., Moss N,.E., and Wade D.T. (1995). The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 242, 587–592 [DOI] [PubMed] [Google Scholar]
- 13.Potter S., Leigh E., Wade D., and Fleminger S. (2006). The Rivermead Post Concussion Symptoms Questionnaire: a confirmatory factor analysis. J. Neurol. 253, 1603–1614 [DOI] [PubMed] [Google Scholar]
- 14.Eyres S., Carey A., Gilworth G., Neumann V., and Tennant A. (2005). Construct validity and reliability of the Rivermead Post-Concussion Symptoms Questionnaire. Clin. Rehabil. 19, 878–887 [DOI] [PubMed] [Google Scholar]
- 15.Smith-Seemiller L., Fow N.R., Kant R., and Franzen M.D. (2003). Presence of post-concussion syndrome symptoms in patients with chronic pain vs mild traumatic brain injury. Brain Inj. 17, 199–206 [DOI] [PubMed] [Google Scholar]
- 16.Hou R., Moss-Morris R., Peveler R., Mogg K., Bradley B.P., and Belli A. (2012). When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 83, 217–223 [DOI] [PubMed] [Google Scholar]
- 17.Lagarde E., Salmi L.R., Holm L.W., Contrand B., Masson F., Ribereau-Gayon R., Laborey M., and Cassidy J.M. (2014). Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs. postconcussion syndrome. JAMA Psychiatry 71, 1032–1040 [DOI] [PubMed] [Google Scholar]
- 18.Thompson C., Davies P., Herrmann L., Summers M., and Potter S. (2016). Approaches to establishing validated cut-off scores on the Rivermead Post Concussion Symptoms Questionnaire (RPQ). The Eleventh World Congress on Brain Injury; March 2nd–5th, The Hague: Brain Inj [Google Scholar]
- 19.Waljas M., Iverson G.L., Lange R.T., Hakulinen U., Dastidar P., Huhtala H., et al. (2015). A prospective biopsychosocial study of the persistent post-concussion symptoms following mild traumatic brain injury. J. Neurotrauma 32, 534–547 [DOI] [PubMed] [Google Scholar]
- 20.Cnossen M.C., Winkler E.A., Yue J.K., Okonkwo D.O., Valadka A., Steyerberg E.W., et al. (2017). Development of a prediction model for post-concussive symptoms following mild traumatic brain injury: a TRACK-TBI Pilot Study. J. Neurotrauma [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverberg N.D., Gardner A.J., Brubacher J.R., Panenka W.J., Li J.J., and Iverson G.L. (2015). Systematic review of multivariable prognostic models for mild traumatic brain injury. J. Neurotrauma 32, 517–526 [DOI] [PubMed] [Google Scholar]
- 22.Cassidy J.D., Cancelliere C., Carroll L.J., Cote P., Hincapie C.A., Holm L.W., Hartvigsen J., Donovan J., Nygren-de Boussard C., Kristman V.L., and Borg J. (2014). Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 95, 3 Suppl., S132–151 [DOI] [PubMed] [Google Scholar]
- 23.Stulemeijer M., van der Werf S., Borm G.F., and Vos P.E. (2008). Early prediction of favourable recovery 6 months after mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 79, 936–942 [DOI] [PubMed] [Google Scholar]
- 24.Jacobs B., Beems T., Stulemeijer M., van Vugt A.B., van der Vliet T.M., Borm G.F., et al. (2010). Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J. Neurotrauma 27, 655–668 [DOI] [PubMed] [Google Scholar]
- 25.Vos P.E., Jacobs B., Andriessen T.M., Lamers K.J., Borm G.F., Beems T., Edwards M., Rosmalen C.F., and Vissers J.L. (2010). GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology 75, 1786–1793 [DOI] [PubMed] [Google Scholar]
- 26.Haagsma J.A., Scholten A.C., Andriessen T.M., Vos P.E., Van Beeck E.F., and Polinder S. (2015). Impact of depression and post-traumatic stress disorder on functional outcome and health-related quality of life of patients with mild traumatic brain injury. J. Neurotrauma 32, 853–862 [DOI] [PubMed] [Google Scholar]
- 27.Wilson J.T., Pettigrew L.E., and Teasdale G.M. (1998). Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma 15, 573–585 [DOI] [PubMed] [Google Scholar]
- 28.Shukla D., Devi B.I., and Agrawal A. (2011). Outcome measures for traumatic brain injury. Clin. Neurol. Neurosurg. 113, 435–441 [DOI] [PubMed] [Google Scholar]
- 29.Nichol A.D., Higgins A.M., Gabbe B.J., Murray L.J., Cooper D.J., and Cameron P.A. (2011). Measuring functional and quality of life outcomes following major head injury: common scales and checklists. Injury 42, 281–287 [DOI] [PubMed] [Google Scholar]
- 30.Jennett B., Snoek J., Bond M.R., and Brooks N. (1981). Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J. Neurol. Neurosurg. Psychiatry 44, 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeates K.O. (2010). Mild traumatic brain injury and postconcussive symptoms in children and adolescents. J. Int. Neuropsychol. Soc. 16, 953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Ed. (DSM-5). American Psychiatric Association: Washington, DC [Google Scholar]
- 33.Tator C.H., Davis H.S., Dufort P.A., Tartaglia M.C., Davis K.D., Ebraheem A., and Hiploylee C. (2016). Postconcussion syndrome: demographics and predictors in 221 patients. J. Neurosurg. 125, 1206–1216 [DOI] [PubMed] [Google Scholar]
- 34.Iverson G.L., Brooks B.L., Ashton V.L., and Lange R.T. (2010). Interview versus questionnaire symptom reporting in people with the postconcussion syndrome. J. Head Trauma Rehabil. 25, 23–30 [DOI] [PubMed] [Google Scholar]
- 35.Lannsjo M, Borg J., Bjorklund G., Af Geijerstam J.L., and Lundgren-Nilsson A. Internal construct validity of the Rivermead Post-Concussion Symptoms Questionnaire. J. Rehabil. Med. 43, 997–1002 [DOI] [PubMed] [Google Scholar]
- 36.Boake C., McCauley S.R., Levin H.S., Pedroza C., Contant C.F., Song J.X., Brown S.A., Goodman H., Brundage S.I., and Diaz-Marchan P.J. (2005). Diagnostic criteria for postconcussional syndrome after mild to moderate traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 17, 350–356 [DOI] [PubMed] [Google Scholar]
- 37.Dean P.J., O'Neill D., and Sterr A. (2012). Post-concussion syndrome: prevalence after mild traumatic brain injury in comparison with a sample without head injury. Brain Inj. 26, 14–26., [DOI] [PubMed] [Google Scholar]
- 38.McCauley S.R., Boake C., Pedroza C., Brown S.A., Levin H.S., Goodman H.S., and Merrit S.G. (2005). Postconcussional disorder: are the DSM-IV criteria an improvement over the ICD-10? J. Nerv. Ment. Dis.193, 540–550 [DOI] [PubMed] [Google Scholar]
- 39.De Kruijk J.R., Twijnstra A., and Leffers P. (2001). Diagnostic criteria and differential diagnosis of mild traumatic brain injury. Brain Inj., 15, 99–106 [DOI] [PubMed] [Google Scholar]
- 40.De Kruijk J.R., Twijnstra A., Meerhoff S., and Leffers P. (2001). Management of mild traumatic brain injury: lack of consensus in Europe. Brain Inj. 15, 117–23 [DOI] [PubMed] [Google Scholar]
- 41.Williams D.H., Levin H.S., and Eisenberg H.M. (1990). Mild head injury classification. Neurosurgery 27, 422–428 [DOI] [PubMed] [Google Scholar]
- 42.Culotta V.P., Sementilli M.E., Gerold K., and Watts C.C. (1996). Clinicopathological heterogeneity in the classification of mild head injury. Neurosurgery 38, 245–250 [DOI] [PubMed] [Google Scholar]
- 43.Steyerberg E.W. (2008). Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. Springer Science & Business Media, New York, NY [Google Scholar]
- 44.Akaike H. (1974). A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19, 716–723 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.