Abstract
Background
Geriatric health charts that are similar to pediatric growth charts could facilitate monitoring health changes and predicting care needs in older adults. We aimed to validate an existing composite score (Health Assessment Tool [HAT]) and provide provisional age-specific reference curves for the general older population.
Methods
Data came from the Swedish National study on Aging and Care in Kungsholmen (N = 3,363 participants aged 60 years and over examined clinically at baseline and 3 years later). HAT was validated by exploring its relationship with health indicators (logistic regression) and comparing its ability to predict care consumption with that of two of its components, morbidity and disability (receiver operating characteristic curve areas). A flowchart was developed to obtain individual-level HAT scores (nominal response method). Sex-specific health charts were derived by graphing seven percentile curves of age-related HAT change (logistic quantile regression).
Results
HAT scores above the age- and sex-specific median were related to good performance in chair-stand tests (odds ratio [OR] = 2.62, 95% confidence interval [CI]: 2.07–3.31), balance and grip tests (interaction balance grip test, OR = 1.15, 95% CI: 1.05–1.25), and good self-rated health (OR = 2.19, 95% CI: 1.77–2.71). Receiver operating characteristic curve areas (HAT vs number of chronic disorders) were formal care, 0.76 versus 0.58 (p value < .001); informal care, 0.74 versus 0.59 (p value < .001); hospital admission, 0.70 versus 0.66 (p value < .001); primary care visits, 0.71 versus 0.69 (p value > .05); and specialty care visits, 0.62 versus 0.65 (p value < .001). HAT consistently predicted medical and social care service use better than disability.
Conclusions
HAT is a valid tool that predicts care consumption well and could be useful in developing geriatric health charts to better monitor health changes in older populations.
Keywords: Health assessment tool, Physical function, Cognitive function, Multimorbidity, Disability
Children’s development is followed up regularly from birth. At each visit, pediatricians record height and weight and compare them to percentile curves of growth to detect signs of possible problems. Like physical changes in children, clinical and functional changes can happen quickly in older adults. However, for older adults, no specific measures are recorded and compared over time, and what is more important, there are no geriatric health charts physicians can use to determine whether an individual’s aging process deviates from the most common trajectory. Moreover, whereas height and weight seem sufficient to capture early signs of health-related problems in children, it is more complex to capture such signs in older adults.
As people grow older, their health status becomes more heterogeneous, and multiple dimensions are needed to capture this heterogeneity (1). Most sexagenarians have good health and no chronic diseases or functional impairment (1–3). Indeed, in a study of Swedish urban older adults (1), most people did not develop functional deficits or disability until the age of 80 years, despite the presence of chronic disorders or even multimorbidity. In other words, morbidity started early in late adulthood, whereas severe disability surpassed a prevalence of 10% only after the age of 90 years. This implies that to develop a highly sensitive and predictive instrument, multiple health indicators must be integrated (1,4,5).
In a previous study (6), we proposed a new clinical measure, the Health Assessment Tool (HAT), to identify and follow health changes in older adults, detect unexpected health decline, and forecast care needs in a timely manner. HAT is an easy-to-use instrument based on five commonly used clinical indicators: physical function (gait speed), cognitive function (Mini-Mental State Examination [MMSE]), number of chronic disorders, dependence in instrumental activities of daily living (IADL), and dependence in personal activities of daily living (ADL). We found that HAT could monitor gradual health changes in older adults, mitigating the ceiling or floor effects of some of its component variables (eg, MMSE and ADL) and that it had a higher predictive validity for mortality and hospitalization than the Multidimensional Prognostic Index and self-rated health (6). In this study, we continued to explore the applicability of HAT in the general population. Specifically, we aimed to: (i) validate the tool by (a) verifying its association with other measures of health and (b) comparing its ability to predict medical and social care consumption with that of two of its components, morbidity and disability; (ii) implement an easy algorithm to compute HAT scores at the individual level; and (iii) provide provisional age-specific reference curves or geriatric health curves for the general older population.
Methods
Study Population
Data for this prospective study were gathered from the Swedish National study on Aging and Care in Kungsholmen (SNAC-K), a community-based longitudinal study (7). Participants were randomly selected from 11 age cohorts (ie, 60, 66, 72, 78, 81, 84, 87, 90, 93, 96, and 99 years and over) in the population of people aged 60 years and over, living at home or in institutions in Kungsholmen, Stockholm between 2001 and 2004. Of the 4,590 eligible participants, 1,227 declined to take part, leaving a study population of 3,363 (participation rate, 73.3%). Of the 3,363 participants in the SNAC-K study, 191 (5.7%) lived in institutions. SNAC-K participants aged 78 years and over were invited to participate again after 3 years. Of the 1,581 participants aged 78 years and over at baseline, 992 (87%) were alive and agreed to participate at the 3-year follow-up.
Data Collection and Health Assessment
At each examination, health status was assessed and information on health-related past events was collected by physicians, nurses, and psychologists via face-to-face interviews and examinations using extensive standardized protocols. All study nurses and doctors underwent training before data collection to ensure that they consistently followed the same standardized study protocols, procedures, and diagnostic criteria (8–10).
Health status
The health status was assessed with several indicators. Five were used in this study. (i) “Cognitive functioning” was measured with the MMSE (11). (ii) “Physical functional status” was assessed via gait speed, which was measured using standard procedures (1). (iii) “Chronic diseases” were diagnosed by physicians on the basis of their clinical examination, laboratory tests, and hospital records (ICD-10 diagnostic criteria) (1). (iv) “Instrumental disability” was defined as impairment in at least one IADL (grocery shopping, managing money, using the telephone, and using public transportation). People living in an institution were considered a priori dependent on others for grocery shopping. (v) “Personal disability” was defined as impairment in at least one ADL (bathing, dressing, toileting, transferring, and eating).
Other health measures
(i) “Balance” was measured as the time (in seconds) a participant could stand on one leg (up to 60 seconds). (ii) “Grip strength” was measured with a dynamometer and converted to kilograms. Participants were seated with their arm resting on a table and their elbow flexed at 90°. (iii) “Lower body strength” was measured with the chair-stand test: the ability to stand up from a chair five times without using the hands. (iv) “Self-rated health” was assessed with the question, “In general, how would you say your health is?”. The five possible answers were poor, fair, good, very good, and excellent.
Socioeconomic status and lifestyle
Information on socioeconomic factors and lifestyle variables was collected during the nurse interview at baseline or gathered via a self-administered questionnaire. “Educational level” was dichotomized into low (<8 years) and high (≥8 years). “Civil status” was divided into married (including cohabiting), widowed or divorced, and single. “Financial level” was considered low if the participant was unable to manage unplanned expenses. Participants were divided by “smoking habit” into never, former, and current smokers and by “alcohol consumption” into moderate drinkers (≤4 glasses per week for men and ≤2 glasses per week for women) and never/heavy drinkers (≤1 glass per month for men and women, ≥5 glasses per week for men, and ≥3 glasses per week for women). The categorization of alcohol consumption reflects the u-shape association often found between alcohol and health outcomes in older adults (12). “Physical activity” was divided into two categories: less than weekly light exercise and weekly light/intense exercise (light exercise: walks in the park, golf, or similar activities; intense exercise: jogging, fast long walks, swimming, or the equivalent).
Social care use
At the 3-year follow-up, the participant’s need for formal and/or informal care was reported by the participant or an informant (the person’s next of kin or a nurse). Only people aged 81 years and over were examined at the 3-year follow-up; hence, information on social care use was only available for those aged 78 years and over at baseline. “Formal care” included service (household chores), personal care, or medical care provided by the municipality or the county (in this case, Stockholm County), even if the care was provided through a private company. “Informal care” included service or care provided by relatives, friends, neighbors, or volunteer/nonprofit organizations. For both formal and informal care, the amount of care needed was recorded as hours per week and weeks per month. For the analyses, the two measures were combined and the total number of hours per month (calculated as hours per week multiplied by weeks per month) was used.
Medical care use
Medical care data were collected from inpatient and outpatient registries from the Stockholm County Council for the period 2001–2008 and were therefore available for the entire sample. These data included “hospital care use” and “outpatient care use.” For hospital care use, two outcome variables were computed for each participant: (i) the number of hospital admissions in the 3 years after baseline assessment and (ii) the number of days those who were hospitalized spent in the hospital during the 3 years after baseline assessment. The outpatient registry specifies not only the date of outpatient visits but also the specialty of the health care professional. We divided these data into primary care visits (codes 100s: paramedical professionals and 800s: nurses and general practitioners) and specialty care visits in the 3 years after baseline.
Statistical Analysis
HAT development
HAT measures the health status of people aged 60 years and over on a semicontinuous scale from 10 (good health) to 0 (bad health) (13). It was developed using the nominal response model (NRm). A more detailed description of the method used is found in a previous article by the same authors (6). In brief, using the regression coefficients from the NRm, two parameters were extracted for each health indicator included in the model. The difficulty parameter defined the level of health (eg, bad, medium, or good) at which the indicator divided people of different health status. The discrimination parameter defined how precisely the indicator divided people by health status. These parameters were used to determine the categorization of the indicators that yielded the HAT index with difficulty values covering the largest range of latent values (ie, it could differentiate the health status of people across the entire health continuum) and with as many discrimination values above 1 as possible to assure good precision (6). On the basis of the difficulty and discrimination values, we obtained the expected scores of the index. We derived the algorithm and coefficients to calculate HAT scores through a linear regression between the expected scores and the variables used in the final NRm. To account for the high discrimination power of both IADL and ADL, the linear regression analysis was stratified by no IADL or ADL impairment, at least one IADL impairment, and at least one ADL impairment.
HAT validation
First, we used logistic regression to study the association between HAT scores above the age- and sex-specific median (dependent variable) and self-rated health and physical tests not included in HAT (grip test, balance test, and chair-stand test). In the analysis, the continuous variables grip strength and balance were transformed into z scores (standardization) to facilitate comparison. Chair-stand test results were dichotomized into test passed (able to stand up from a chair five times without using the hands) and test not passed (unable to stand up from a chair five times or used the hands to do so). Self-rated health was dichotomized into poor, fair, or good health and very good or excellent health. The analysis was adjusted by socioeconomic status (education, financial level, and civil status) and lifestyle factors (smoking habits, alcohol consumption, and physical activity). Missing covariate values were imputed with multivariate imputation chained equations (14), which resulted in 50 new data sets. We included all the variables from the logistic model plus age at death and institutionalization status. The imputation was stratified by sex, mortality status during 13 years of follow-up, and outcome of interest. As a sensitivity analysis, we used linear regression models to examine the association between changes in HAT score (dependent variable) and self-rated health and physical tests not included in HAT. The results are reported in Supplementary Table 1.
Second, the ability of HAT to predict care consumption in the 3 years after baseline was compared to that of morbidity status (number of chronic diseases) and disability (total number of ADL and IADL impairments). All three variables (HAT, morbidity,and disability) were continuous in the receiver operating characteristic (ROC) curve analyses. Only people living in the community at baseline were included in these analyses because people living in institutions receive most of their medical and social care in the institution where they reside. The ability of each measure to predict medical and social care use was estimated by computing the area under the ROC curve. For social care use, only participants aged 78 years and over at baseline were included. For the derivation of the ROC curves for medical care use, all participants were included. Care consumption variables considered were at least 1 hospital admission, more than 11 primary care visits (median value), more than 5 specialty care visits (median value), and receipt of formal or informal care.
Individual HAT score and geriatric health curves
An algorithm composed of three subgroups was created to compute HAT score values for any person: the first for people without any disability (either ADL or IADL); the second for people with no ADL but some IADL, and the third for people with both ADL and IADL. Each algorithm subgroup represents one of the regression models between the expected scores and the health indicators included in HAT and was constructed to take the interaction terms present in the models into consideration. Finally, the change in the sex-specific HAT geriatric health charts by age was derived with logistic quantile regression (15). Seven percentiles were computed for each sex: 5th, 10th, 25th, 50th, 75th, 90th, and 95th.
Results
The demographic and clinical characteristics of the 3,363 participants in SNAC-K at baseline are reported in Table 1. Information on the clinical indicators is reported in accordance with the categorization used in the final NRm: two categories of IADL and two of ADL (0, 1+ impairments); four of MMSE scores (30, 29, 28–20, 19–0); four of gait speed (1.5 m/s or above, below 1.5 to 1 m/s, below 1 to 0.4 m/s, below 0.4 m/s); and three of chronic diseases (0, 1–2, 3+).
Table 1.
Characteristics of the Study Population at Baseline
| Age groups | |||
|---|---|---|---|
| Total | 60–78 years | 81 years and over | |
| N = 3,363 | n = 2,243 | n = 1,120 | |
| Age, mean (SD) | 74.7 (11.2) | 68.1 (6.7) | 87.9 (5.1) |
| Women, number (%) | 2,182 (65) | 1,333 (59) | 849 (76) |
| Living in an institution, number (%) | 191 (6) | 24 (1) | 167 (15) |
| ADL impairments* (1+), number (%) | 250 (7.5) | 25 (1.1) | 225 (20.5) |
| IADL impairments* (1+), number (%) | 618 (19.0) | 132 (6.0) | 486 (46.8) |
| MMSE*, number (%) | |||
| 30 | 1,123 (33.6) | 992 (44.4) | 131 (12.0) |
| 29 | 975 (29.1) | 745 (33.3) | 230 (20.7) |
| 28–20 | 1,018 (30.4) | 470 (21.0) | 548 (49.3) |
| 19–0 | 231 (6.9) | 29 (1.3) | 202 (18.2) |
| Gait speed* (m/s), number (%) | |||
| ≥1.5 | 690 (21.7) | 666 (30.4) | 24 (2.4) |
| 1.49–1 | 1258 (39.5) | 1081 (49.3) | 177 (17.8) |
| 0.99–0.4 | 861 (27.1) | 382 (17.4) | 479 (48.3) |
| 0.39–0 | 374 (11.8) | 62 (2.8) | 312 (31.5) |
| Number of chronic diseases*, number (%) | |||
| None | 712 (21.2) | 601 (26.8) | 111 (10.0) |
| 1–2 | 1,713 (51.1) | 1,163 (51.9) | 550 (49.4) |
| 3+ | 928 (27.7) | 475 (21.2) | 453 (40.7) |
Note: ADL = activities of daily living, IADL = instrumental activities of daily living, MMSE = Mini-Mental State Examination, NRm = nominal response model; SD = standard deviation.
*Categories for ADL, IADL, MMSE score, gait speed, and number of chronic diseases are those used in the best NRm to derive the Health Assessment Tool.
HAT development
Gait speed was the indicator with the highest precision and largest range of difficulty levels, which shows that this measure differentiated groups of people over a large spectrum of the health continuum. Because of the ceiling effect of the MMSE, most of the population had MMSE scores between 30 and 28. The contribution of the morbidity variable to differentiating health status was limited regardless of how the variable was categorized.
HAT validation
Table 2 reports the association between HAT and other indicators of health. Very good or excellent self-rated health and the ability to perform the chair-stand test were associated with a HAT score above the median level (very good/excellent self-rated health: odds ratio [OR] = 2.19, 95% confidence interval [CI] = 1.77–2.71; passed chair test: OR = 2.62, 95% CI = 2.08–3.31). A combination of good balance and good grip strength was associated with better HAT scores (balance × grip strength OR = 1.15, 95% CI = 1.05–1.25).
Table 2.
Association Between Having a Health Assessment Tool (HAT) Score Above the Age- and Sex-Specific Median (Dependent Variable) and Having Good Scores on Indicators of Health Not Included in HAT (Independent Variables)
| Model 1 | Model 2 | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Balance (continuous variable in seconds) | 1.10* (1.00–1.20) | 1.08 (0.99–1.19) |
| Higher grip strength (continuous variable in kilogram) | 0.89* (0.81–0.98) | 0.91 (0.83–1.00) |
| Interaction between balance and grip strength | 1.15** (1.05–1.25) | 1.15** (1.05–1.25) |
| Chair-stand test (passed vs not passed) | 3.03*** (2.42–3.78) | 2.62*** (2.08–3.31) |
| Self-rated health (very good/excellent vs poor/fair/good) | 2.38*** (1.93–2.95) | 2.19*** (1.77–2.71) |
Note: Results expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Model 1: adjusted for the rest of the physical tests and self-rated health. Model 2: adjusted for the same variables as Model 1 + education level, financial level, civil status, smoking habits, alcohol consumption, and physical activity.
*p value < .05; **p value < .01; ***p value < .001.
HAT’s ability to predict hospital admissions and use of informal or formal care over 3 years was significantly greater than that of the count of morbidities (p values for all outcomes were <.001) and the count of disabilities (p values for all outcomes were <.001). HAT predicted whether participants made more than 11 primary care visits and more than 5 specialty care visits (ROC curve areas 0.70 and 0.63) better than disability count (ROC curve areas 0.56 and 0.52; all p values <.001). However, HAT was neither better nor worse than a count of morbidities at predicting number of primary care visits, and the count of morbidities outperformed HAT in predicting specialty care visits (Table 3).
Table 3.
Receiver Operating Characteristic (ROC) Curve Areas for the Prediction of Medical and Social Care Services Use in the 3 Years After Baseline Assessment Using the Health Assessment Tool (HAT) or Single Components of HAT (Morbidity and Disability). Only People Living in the Community at Baseline Were Included in the Analyses (n = 2,955)
| Predictors | |||||
|---|---|---|---|---|---|
| Care service | HAT | Morbidity* | p value | Disability† | p value |
| Hospital admissions (one or more) | 0.70 | 0.66 | <.001 | 0.59 | <.001 |
| Primary care visits (over the median value) | 0.71 | 0.69 | .056 | 0.57 | <.001 |
| Specialty care visits (over the median value) | 0.62 | 0.65 | <.001 | 0.51 | <.001 |
| Formal care (one or more hours/ month) | 0.76 | 0.58 | <.001 | 0.65 | <.001 |
| Informal care (one or more hours/ month) | 0.74 | 0.59 | <.001 | 0.62 | <.001 |
*Morbidity: number of chronic diseases.
†Disability: number of impairments in activities of daily living plus instrumental activities of daily living.
Individual HAT scores
To facilitate the computation of HAT scores at the individual level, we created two flowcharts that represent the algorithm (Supplementary Figure 1). Flowchart A is used for people without any ADL impairment who start with the maximum HAT score of 10. Three steps are needed to compute the final score. At each step, the points related to the particular test (gait speed, MMSE, and morbidity) are subtracted from the starting value of 10. Flowchart B is used for people with any ADL impairment who start with a maximum HAT score of 6.7. Four steps are needed. Table 4 shows two sample individual HAT scores calculated using the flowcharts. An online calculator of individual-level HAT scores is available at http://www.snac-k.se/research/results/.
Table 4.
Health Assessment Tool (HAT) Scores of Two People With Different Health Characteristics. HAT Scores and the Corresponding Percentile Were Computed Using the Flowcharts (Supplementary Figure 1)
| Characteristics | Person 1 | Person 2 |
|---|---|---|
| Sex | Woman | Man |
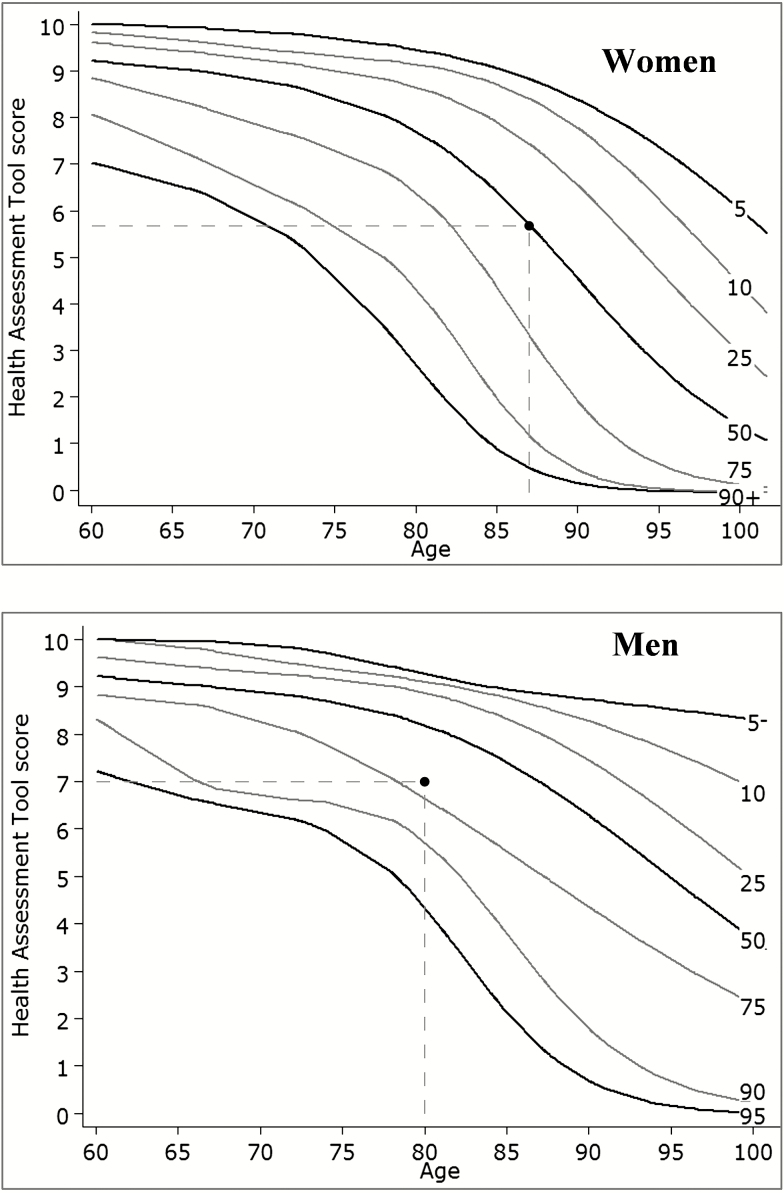
| Age | 87 | 80 |
| ADL disabilities | 0 | 0 |
| IADL disabilities | 1 | 0 |
| Gait speed (m/s) | 1.1 | 0.9 |
| MMSE score | 27 | 29 |
| Number of chronic diseases | 3 | 2 |
| HAT score | 5.7 | 7.0 |
| Percentile* | 50th | 70th |
Note: ADL = activities of daily living; IADL = instrumental activities of daily living; MMSE = Mini-Mental State Examination.
*See Figure 1 for graphical representation.
Geriatric health charts
Individual HAT scores can be plotted on the provisional geriatric health charts that we have created on the basis of our SNAC-K population; there are separate geriatric health charts for men and women (Figure 1). Figure 1 shows how the individual scores in Table 4 compare with the percentile distributions derived from the general older SNAC-K sample. In addition to calculating an individual’s HAT score, the online calculator also plots the score on the appropriate geriatric health chart.
Figure 1.
Reference curves (5th to 90th percentiles) for women and men. The dots in the graphs represent the Health Assessment Tool scores of the two people in Table 4.
Discussion
Public health strategies focus on maintaining and prolonging people’s mental and physical capacities as long as possible by acting in a timely and proper manner. To this end, health systems need to detect and control health changes at the right time and in the right place. This article presents a novel approach to monitoring changes in older adults’ health via geriatric health charts that can aid in planning ad hoc personalized and public health actions.
The findings of this study suggest that HAT is a good candidate for use in developing geriatric health charts. HAT is a composite measure that can be used to trace health changes as people age. Most of older adults present with several concurrent health problems, as previously shown by others and by us. By considering several health indicators, we can better capture the complexity of health in old age. HAT assembles these health indicators into a single score, reducing data dimensionality and providing a comprehensive picture of a person’s health status. Moreover, the tool predicts care needs better than a single count of morbidities or disabilities, which confirms that reducing the dimensions to a single score does not eliminate important information. HAT scores are also strongly associated with other common health indicators. Screening older adults with this multidimensional tool may thus facilitate patient-centered care and treatment.
The NRm, linked to the item response theory frame (13), was used to determine the cross-sectional relationship among the five health measures and to create HAT. This statistical technique allowed us to select cutoffs for each measure that maximized the information related to a person’s health contained in the individual variables (6). In line with the results of a previous article (1), the NRm identified the two ADL measures and the MMSE score as the indicators that could optimally differentiate between the best and worst health status in people aged 60 years and over. On the other hand, gait speed was informative over a large range of the health continuum. Indeed, in older adults, gait speed is associated not only with survival and functioning, but also with well-being (16–20). The number of chronic diseases a person had was the least informative variable, although it was still useful in people with relatively good functioning.
The five health indicators included in HAT were chosen on the basis of previous research (6) and for their strong associations with a variety of health outcomes and other physical functioning measures (1,5,21–27). The importance of holistically assessing older individuals’ health, beyond the simple absence of diseases, has also been highlighted by the World Health Organization (28), which defines healthy aging as “the process of developing and maintaining the functional ability that enables well-being in older age.” Of the physical tests typically used to measure health in older adults, we included only the test of gait speed, as it is quick and easy to perform. Other objective measures of physical functioning (balance, grip strength, and the chair-stand test) are also good indicators of health status (29), and age- and sex-specific HAT scores were associated with those indicators even after adjustment for lifestyle factors and socioeconomic status. Age- and sex-specific HAT scores were also associated with self-rated health, a powerful proxy of objective health (30).
Furthermore, in a previous study, HAT adequately predicted adverse outcomes such as shorter survival. It captured heterogeneity in the health of men and women of different ages at single points in time and over time (6). All these findings highlight HAT’s ability to capture the multidimensional and complex nature of health in older adults, both at the individual and population level. For example, although morbidity and disability are major determinants of poor health as we age (28), this study shows that these measures alone are less helpful in explaining the wide variation of care needs in older adults than HAT. All these properties make HAT suitable for use in creating reference curves for the general older population that are similar to the growth charts used by pediatricians to illustrate the distribution of selected body measurements in children.
Several other existing health indices also include subjective measures of health and well-being (31). Some of these tools have been developed for specific groups of people (eg, chronically ill patients), for specific clinical settings, or for nursing homes (32). Furthermore, other concepts such as successful aging (33) and frailty (34,35) have also emerged to characterize the healthier or sicker parts of the older population. The FRAIL scale (36,37), for example, is based on a limited number of self-reported questions and no objective measures of health, which may limit its use among cognitively impaired people or people living in nursing homes. Other frailty scores as the EASY-Care (38) rely on unmeasurable concepts such as “clinical reasoning and tacit knowledge of the frailty phenotype”. Other indices, such as the short-form surveys SF-36 and SF-12 (39,40), have mostly been used for research purposes. Both instruments measure overall physical and mental health but also rely on self-reported measures of health and well-being. Certain indices already available in the literature include both objective and subjective measures of health (eg, perceived health and emotional health), as well as proxies for social and care support (41). Although measures of subjective health and need for social and care support contribute to the person-centeredness of health definitions and care provision, their inclusion in health indices may preclude future research on the association between objective and subjective measures of health and on the assessment of their determinants. Our aim was to create an easy-to-use tool based on objective measures of health with clear clinical significance and applicability. Although we have only compared HAT to a few of the many available indices, HAT proved to have better predictive ability than its components and a predictor of short- or long-term survival (ie, the Multidimensional Prognostic Index) (42).
An added value of HAT is that it was constructed on the basis of information that is usually available or easily collected in various settings such as primary care, hospitals, and social care, which will enhance its applicability. Although HAT was constructed using advanced statistical methods, interpreting HAT scores is quite straightforward: values below 5 indicate mild to severe ADL limitations, values below 3 indicate severe disability, and values from 5 to 10 indicate a gradual increase in the number of chronic diseases and cognitive decline and an overall decline in physical functioning. The geriatric health charts can help health care professionals interpret individual scores in the context of the person’s age and sex. For instance, a score of 4.5 in a 60-year-old man should be considered a clear sign of compromised health (96% of 60-year-old men have HAT scores >4.5), whereas the same HAT score in a 90-year-old woman could be considered acceptable (4.5 is the median score for 90-year-old women). The geriatric health curves can be used not only as reference curves for assessing the present health status of older adults but also to forecast future trajectories. This can be useful information for health care professionals, and it can also be useful for individuals and their family members in planning social care. Geriatric health curves can also help policy makers allocate resources and quantify the amount and type of prevention needed in the older population. In this regard, HAT has the advantage of being a good predictor of care needs. In fact, it may be a more reliable guide for allocating resources than information such as current resource utilization, which is more directly affected by care-seeking behaviors, policies, and care availability. Finally, the HAT index can help researchers better describe the process of aging and better understand the diverse pathways that lead from determinants to functional ability and well-being.
We consider one of the major strengths of this study to be the method used to analyze the information provided by the single indicators and incorporate it into the latent construct of health. There are different approaches to deriving latent measures of health; all are part of the same analytic framework of mixed-effect models (43). Factor analysis is a data-reduction tool that is often used when the observed data are continuous, whereas latent class analysis is used for categorical variables. The latter has been commonly used to combine different health measures and define clusters of health patterns (44,45). However, when the observed data are a combination of categorical and continuous variables, either bounded or not, the item response theory method (13) is preferable because it provides more insight into the relationships among the individual indicators. An additional strength of the study was the practical representation of how an assessment tool such as HAT could be used to trace individual health changes in routine clinical practice.
Although we believe that HAT could be a good candidate measure for use in monitoring patients over time, we acknowledge that it might not be the only tool for which geriatric health curves could be computed. Another limitation worth considering is that the sample used to develop and derive HAT scores represented a healthy part of the general older population, at least in the sample below 90 years. Indeed, the sample population had higher MMSE scores and a lower prevalence of ADL disability than people of the same age in Sweden and in other countries. Although this could be a drawback for the use of HAT as an absolute scale, it is a strength if we wish to compare the health status of older adults with that of a reference population that has free access to good quality care. HAT scores can be difficult to calculate by hand; we therefore developed an online calculator that allows any user to easily compute a person’s HAT score and compare it to provisional health curves. The score’s ability to capture and describe health in older adults might improve with the introduction of more health components; however, this would lengthen the time it takes to compute the score. The health charts provided in this article were derived from cross-sectional data and do not represent a longitudinal change in the score. Moreover, the reproducibility of HAT should be tested in other populations.
Conclusions
It is critical to monitor health changes in people as they age to capture deviations from normal ranges as soon as possible. Public health initiatives to improve older adults’ health should be based on individuals’ health trajectories, because different points in the trajectory require different responses from health care systems. During the period of life in which health is usually good and stable, the goal will be to promote healthy behaviors and detect chronic conditions and physical decline in a timely manner. In the stage of life when health starts to decline, improvements can be achieved by removing barriers that limit participation and by finding strategies such as rehabilitation that reverse or slow the decline in capacities. Finally, when health starts to be significantly worse and people become functionally dependent, health systems may intervene to compensate for the loss of capacity and, at the extreme end, to support palliative care for a dignified end of life (28). A fundamental prerequisite for this social and medical care delivery framework is the ability to measure individual-level health status and trajectories pragmatically and comprehensively. HAT and its derived age- and sex-specific geriatric health charts are valid measures that can contribute to better understanding the dynamic and heterogeneous aging process, to monitoring individuals’ health over time, and to planning for the future care needs of older adults.
Ethics Approval
SNAC-K received ethical permission for baseline and follow-ups from the Ethics Committee at Karolinska Institutet and the Regional Ethics Review Board in Stockholm (Dnrs: 01-114, 04-929/3, 2007/279-31). Written informed consent was obtained from all participants.
Funding
This work was supported by the Swedish Research Council for Health, Working Life and Welfare (2017-01764).
Conflict of Interest
The authors declare that they have no competing interests.
Supplementary Material
References
- 1. Santoni G, Angleman S, Welmer AK, Mangialasche F, Marengoni A, Fratiglioni L. Age-related variation in health status after age 60. PLoS One. 2015;10:e0120077. doi: 10.1371/journal.pone.0120077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collerton J, Davies K, Jagger C, et al. Health and disease in 85 year olds: baseline findings from the Newcastle 85+ cohort study. BMJ. 2009;339:b4904. doi: 10.1136/bmj.b4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacobs JM, Maaravi Y, Cohen A, Bursztyn M, Ein-Mor E, Stessman J. Changing profile of health and function from age 70 to 85 years. Gerontology. 2012;58:313–321. doi: 10.1159/000335238 [DOI] [PubMed] [Google Scholar]
- 4. Sierra F. Moving geroscience into uncharted waters. J Gerontol A Biol Sci Med Sci. 2016;71:1385–1387. doi: 10.1093/gerona/glw087 [DOI] [PubMed] [Google Scholar]
- 5. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.M255 [DOI] [PubMed] [Google Scholar]
- 6. Santoni G, Marengoni A, Calderón-Larrañaga A, et al. Defining health trajectories in older adults with five clinical indicators. J Gerontol A Biol Sci Med Sci. 2017;72:1123–1129. doi: 10.1093/gerona/glw204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lagergren M, Fratiglioni L, Hallberg IR, et al. A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res. 2004;16:158–168. doi: 10.1007/BF03324546 [DOI] [PubMed] [Google Scholar]
- 8. Qiu C, von Strauss E, Bäckman L, Winblad B, Fratiglioni L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology. 2013;80:1888–1894. doi: 10.1212/WNL.0b013e318292a2f9 [DOI] [PubMed] [Google Scholar]
- 9. Laukka EJ, Lovden M, Herlitz A, et al. Genetic effects on old-age cognitive functioning: a population-based study. Psychol Aging. 2013;28:262–274. doi: 10.1037/a0030829 [DOI] [PubMed] [Google Scholar]
- 10. Welmer A-K, Kåreholt I, Angleman S, Rydwik E, Fratiglioni L. Can chronic multimorbidity explain the age-related differences in strength, speed and balance in older adults?Aging Clin Exp Res. 2012;24:480–489. doi: 10.3275/8584 [DOI] [PubMed] [Google Scholar]
- 11. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 12. Doll R, Peto R, Hall E, Wheatley K, Gray R. Mortality in relation to consumption of alcohol: 13 years’ observations on male British doctors. BMJ. 1994;309:911–918. doi: 10.1136/bmj.309.6959.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hambleton RK, Swaminathan H, Rogers HJ.. Fundamentals of Item Response Theory. Newbury Park, CA: Sage Press; 1991. [Google Scholar]
- 14. van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. doi:10.1002/(SICI)1097-0258(19990330)18:6<681::AID-SIM71>3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- 15. Bottai M, Cai B, McKeown RE. Logistic quantile regression for bounded outcomes. Stat Med. 2010;29:309–317. doi: 10.1002/sim.3781 [DOI] [PubMed] [Google Scholar]
- 16. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ostir GV, Kuo YF, Berges IM, Markides KS, Ottenbacher KJ. Measures of lower body function and risk of mortality over 7 years of follow-up. Am J Epidemiol. 2007;166:599–605. doi: 10.1093/aje/kwm121 [DOI] [PubMed] [Google Scholar]
- 18. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z [DOI] [PubMed] [Google Scholar]
- 19. Hall WJ. Update in geriatrics. Ann Intern Med. 2006;145:538–543. doi: 10.7326/0003-4819-127-7-199710010-00007 [DOI] [PubMed] [Google Scholar]
- 20. Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-related change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol A Biol Sci Med Sci. 2016;71:1184–1194. doi: 10.1093/gerona/glw043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yancik R, Ershler W, Satariano W, Hazzard W, Cohen HJ, Ferrucci L. Report of the national institute on aging task force on comorbidity. J Gerontol A Biol Sci Med Sci. 2007;62:275–280. doi: 10.1093/gerona/62.3.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galenkamp H, Braam AW, Huisman M, Deeg DJ. Somatic multimorbidity and self-rated health in the older population. J Gerontol B Psychol Sci Soc Sci. 2011;66:380–386. doi: 10.1093/geronb/gbr032 [DOI] [PubMed] [Google Scholar]
- 23. Atkinson HH, Rosano C, Simonsick EM, et al. ; Health ABC study. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62:844–850. doi: 10.1093/gerona/62.8.844 [DOI] [PubMed] [Google Scholar]
- 24. Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161 [DOI] [PubMed] [Google Scholar]
- 25. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 26. Marengoni A, Angleman S, Fratiglioni L. Prevalence of disability according to multimorbidity and disease clustering: a population-based study. J Comorb. 2011;1:11–18. doi: 10.15256/joc.2011.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cooper R, Kuh D, Cooper C, et al. ; FALCon and HALCyon Study Teams. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40:14–23. doi: 10.1093/ageing/afq117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. WHO. World Report on Ageing and Health. Geneva: World Health Organization; 2015. [Google Scholar]
- 29. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rutledge T, Linke SE, Johnson BD, et al. Self-rated versus objective health indicators as predictors of major cardiovascular events: the NHLBI-sponsored women’s ischemia syndrome evaluation. Psychosom Med. 2010;72:549–555. doi: 10.1097/PSY.0b013e3181dc0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McDowell I. Measuring Health: A Guide to Rating Scales and Questionnaires. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 32. Rubenstein LZ, Stuck AE, Siu AL, Wieland D. Impacts of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J Am Geriatr Soc. 1991;39:8S–16S. doi: 10.1111/j.1532-5415.1991.tb05927.x [DOI] [PubMed] [Google Scholar]
- 33. Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14:6–20. doi: 10.1097/01.JGP.0000192501.03069.bc [DOI] [PubMed] [Google Scholar]
- 34. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 35. Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9:71–72. doi: 10.1016/j.jamda.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 36. Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Woo J, Yu R, Wong M, Yeung F, Wong M, Lum C. Frailty screening in the community using the FRAIL scale. J Am Med Dir Assoc. 2015;16:412–419. doi: 10.1016/j.jamda.2015.01.087 [DOI] [PubMed] [Google Scholar]
- 38. van Kempen JA, Schers HJ, Melis RJ, Olde Rikkert MG. Construct validity and reliability of a two-step tool for the identification of frail older people in primary care. J Clin Epidemiol. 2014;67:176–183. doi: 10.1016/j.jclinepi.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 39. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. doi: 10.1007/BF03260127 [DOI] [PubMed] [Google Scholar]
- 40. Ware J Jr, Kosinski M, Keller SD. A 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.2307/3766749 [DOI] [PubMed] [Google Scholar]
- 41. Aliberti MJR, Apolinario D, Suemoto CK, et al. Targeted geriatric assessment for fast-paced healthcare settings: development, validity, and reliability. J Am Geriatr Soc. 2018;66:748–754. doi: 10.1111/jgs.15303 [DOI] [PubMed] [Google Scholar]
- 42. Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11:151–161. doi: 10.1089/rej.2007.0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCulloch CE, Neuhaus JM.. Generalized Linear Mixed Models. New York, NY: John Wiley & Sons, Ltd; 2001. [Google Scholar]
- 44. Heinen T. Latent Class and Discrete Latent Trait Models: Similarities and Differences Thousand Oaks, CA: Sage; 1996. [Google Scholar]
- 45. Lafortune L, Béland F, Bergman H, Ankri J. Health state profiles and service utilization in community-living elderly. Med Care. 2009;47:286–294. doi: 10.1097/MLR.0b013e3181894293 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.