Abstract
Background
Insomnia is a common and debilitating disorder experienced by cancer survivors. Although cancer survivors express a preference for using nonpharmacological treatment to manage insomnia, the comparative effectiveness between acupuncture and Cognitive Behavioral Therapy for Insomnia (CBT-I) for this disorder is unknown.
Methods
This randomized trial compared 8 weeks of acupuncture (n = 80) and CBT-I (n = 80) in cancer survivors. Acupuncture involved stimulating specific points on the body with needles. CBT-I included sleep restriction, stimulus control, cognitive restructuring, relaxation training, and education. We measured insomnia severity (primary outcome), pain, fatigue, mood, and quality of life posttreatment (8 weeks) with follow-up until 20 weeks. We used linear mixed-effects models for analyses. All statistical tests were two-sided.
Results
The mean age was 61.5 years and 56.9% were women. CBT-I was more effective than acupuncture posttreatment (P < .001); however, both acupuncture and CBT-I produced clinically meaningful reductions in insomnia severity (acupuncture: −8.31 points, 95% confidence interval = −9.36 to −7.26; CBT-I: −10.91 points, 95% confidence interval = −11.97 to −9.85) and maintained improvements up to 20 weeks. Acupuncture was more effective for pain at the end of treatment; both groups had similar improvements in fatigue, mood, and quality of life and reduced prescription hypnotic medication use. CBT-I was more effective for those who were male (P < .001), white (P = .003), highly educated (P < .001), and had no pain at baseline (P < .001).
Conclusions
Although both treatments produced meaningful and durable improvements, CBT-I was more effective and should be the first line of therapy. The relative differences in the comparative effectiveness between the two interventions for specific groups should be confirmed in future adequately powered trials to guide more tailored interventions for insomnia.
Nearly 60% of people with cancer experience insomnia symptoms (1), which, if not appropriately treated, can become chronic, leading to impaired psychological and physical health (2). Treatment for sleep difficulties in cancer patients is typically pharmacological (3), but sedative medications can have substantial side effects, including continued sleep difficulty, performance problems, memory disturbances, driving accidents, and falls (4) .
Acupuncture and Cognitive Behavioral Therapy for Insomnia (CBT-I) are two available nonpharmacological approaches. CBT-I has robust efficacy among cancer survivors (5) but is still not widely known by patients or oncology clinicians. In a survey of adult survivorship programs at National Cancer Institute-designated cancer centers, only 13% of cancer centers polled referred their patients to CBT-I (6). Poor adherence can also decrease its effectiveness in the real world (7). In comparison, research in noncancer populations demonstrates that acupuncture can improve subjective and objective insomnia compared with sham acupuncture (8,9). Acupuncture also has increasing evidence for pain and fatigue symptom management in cancer survivors (10). It is widely accessible, with 73% of cancer centers in the United States offering acupuncture (11).
To date, little research has evaluated acupuncture’s effectiveness for insomnia in cancer (12), and no head-to-head comparison between acupuncture and CBT-I has been conducted to our knowledge. Our objective was to evaluate the comparative effectiveness of acupuncture and CBT-I for insomnia severity among cancer survivors. Our primary hypothesis was that between acupuncture and CBT-I, one treatment would result in a greater overall reduction in insomnia. We also explored the relative comparative effectiveness in specific subgroups.
Methods
Pragmatic Trial Design
The Choosing Options for Insomnia in Cancer Effectively (CHOICE) trial protocol has been published (13). This dual-center, parallel group, randomized, comparative effectiveness trial was conducted at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia, PA, and at the Memorial Sloan Kettering Cancer Center in New York, NY (ClinicalTrials.gov Identifier: NCT02356575, https://clinicaltrials.gov/ct2/show/NCT02356575). Patient partners, not enrolled in the study, were involved in formulating the research question, selecting interventions and outcomes, recruitment (particularly minority outreach), and data interpretation (14). The pragmatic trial design sought to directly inform patient and clinician decision-making in the real world (15). Recruitment and treatment occurred from March 2015 to April 2017; follow-up assessments were completed in July 2017. Study procedures were approved by the institutional review boards, and all participants provided written informed consent.
Participants and Trial Procedures
All interested English-speaking individuals older than 18 years with a cancer diagnosis were eligible to participate. Participants were required to have completed active treatment (surgery, chemotherapy, radiotherapy) at least 1 month before study initiation (except for hormone treatment or maintenance targeted therapies), have a score higher than 7 on the primary outcome (Insomnia Severity Index [ISI]), and meet the criteria for insomnia disorder defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Patients were excluded if they had another sleep disorder not adequately treated, had previous experience with CBT-I or acupuncture for insomnia, had another psychiatric disorder not in remission or adequately treated, or were employed in a job requiring shift work. Participants using psychotropic medication (eg, antidepressants) and/or hypnotics or sedatives were eligible if the dose was stable over the previous 6 weeks. Sleep medication use was tracked for the duration of the study.
We recruited patients from cancer registry mailings and self and oncology provider referral. Eligible participants completed the baseline assessment and then received their treatment allocation details. Insomnia severity was assessed at baseline and at weeks 8, 12, 16, and 20. Participants completed all secondary measures at baseline and at weeks 8 and 20. Adverse events were identified during intervention sessions and follow-up interviews.
Randomization and Masking
Participants were sequentially randomly assigned and stratified by study site using permuted block random assignment. The study biostatistician generated the random assignment sequence before participant recruitment. Random assignment information was concealed in a letter inside a sealed envelope, which the participant opened after completing the baseline assessment. The primary investigators (ie, the primary investigators, co-investigators, and statistician) were blinded to random assignment. Patients, research staff, and treatment therapists were not blinded.
Interventions
Acupuncture is a component of Traditional Chinese Medicine in which an acupuncturist inserts needles at specific locations on the body. Our manualized acupuncture protocol (Supplementary Materials, available online) included standardized points commonly used to address sleep problems with additional points to treat comorbid symptoms like pain and anxiety if needed. Licensed acupuncturists inserted 8–16 Seirin needles (0.16 mm−0.25 mm × 15 mm−40 mm) and manipulated the needles to achieve De Qi (a sensation of aching and soreness) (16). Participants received acupuncture twice weekly for 2 weeks, then weekly for 6 more weeks, for a total of 10 treatments for 8 weeks. The first acupuncture visit involved a detailed history and examination lasting 60 minutes, with each subsequent session lasting 30 minutes, for a total time of 330 minutes (total provider contact time was approximately 150 minutes). Four licensed acupuncturists with 11–14 years of experience delivered the interventions. They received training on the specific protocol, completed study checklists to ensure treatment fidelity, and J.J.M. checked documentation and provided feedback.
CBT-I is a manualized multi-component intervention (see Supplementary Materials, available online for the protocol) that includes sleep restriction, stimulus control, cognitive restructuring, relaxation training, and education. Sleep restriction and stimulus control are designed to break the conditioned association between the bed and not sleeping by limiting time and activities in bed. Cognitive restructuring addresses sleep-related anxiety, and relaxation training targets physiological arousal. Education was provided on healthy sleep behaviors. Participants received five weekly sessions of CBT-I followed by two biweekly sessions, for seven total sessions over 8 weeks. The first CBT-I session was 60 minutes and remaining sessions were 30 minutes each, for total contact time of 240 minutes. Four licensed therapists and five psychology trainees delivered CBT-I. All therapists were trained in CBT-I, and the research protocol received supervision from the study investigators (S.G., P.G., and K.D.) and completed study checklists to ensure treatment fidelity.
Measures
Primary Outcome
The primary outcome was the ISI. The ISI has demonstrated reliability, validity, and sensitivity to change (17). Items are scored on a 5-point scale (range 0–4) with higher scores representing more severe insomnia symptoms. An 8-point reduction on the ISI has been deemed clinically meaningful improvement (18). Our prespecified primary endpoint was 8 weeks from random assignment (end of treatment) with the secondary endpoint at 20 weeks from random assignment (3 months after the end of treatment).
Secondary Outcomes
The Pittsburgh Sleep Quality Index measures sleep quality in clinical populations, with higher scores indicating worse sleep quality (19). We used the Consensus Sleep Diary to calculate sleep efficiency (percentage of time in bed actually sleeping), sleep onset latency (time to fall asleep), wake after sleep onset (amount of time spent awake during the night), and total sleep time (20). Sleep medication usage was also recorded in the diary. Additional symptom measures included the Brief Pain Inventory (21), Multidimensional Fatigue Inventory-Short Form (22), the Hospital Anxiety and Depression Scale (23), and the Patient-Reported Outcomes Measurement Information System-Global Health Scale (24).
Treatment Expectancy
The four-item Mao Treatment Expectancy Scale was used to evaluate outcome expectancy related to acupuncture and CBT-I. The score range is 4–20, with a higher score indicating greater expected outcome for treatment (Cronbach’s alpha 0.82) (25).
Sample Size
In our previous research, CBT-I produced a 9.5-point reduction on the ISI with a 3.7 SD at the end of 8 weeks (26). Assuming the treatment difference between acupuncture and CBT-I would be no greater than 1.85 (0.5 SD of observed ISI in CBT-I group), 64 participants per treatment group (128 in total) were required to detect an effect size of 0.50 (moderate effect size) with 80% power using a two-sided alpha of 0.05. Accounting for 20% potential attrition, we estimated a need to enroll 160 participants.
Statistical Analysis
Intention-to-treat principles were followed. Our predefined primary endpoint was a two-group comparison of the mean change in ISI from baseline to end of treatment (week 8) between acupuncture and CBT-I. Because we repeated primary and secondary outcomes over time, we assessed differences in change from baseline to weeks 8 and 20 using a linear mixed-effects model. The fixed effects in the linear mixed-effects model for each outcome were treatment, time, site, treatment by time interaction, and the baseline outcome. We used subject-specific random intercepts to account for the correlation between repeated measures of each outcome. Missing data were minimal at 7.5%. We performed additional analyses by adjusting for baseline expectancy for either acupuncture or CBT-I in the model. All statistical tests were two-sided. Statistical significance was set at 0.05. We calculated Cohen’s Ds to interpret effect sizes between the two interventions.
Based on past literature and patient input, we selected four variables: sex, race, education, and presence of pain at four or greater as a priori subgroup analyses. We performed stratified analyses as done for the primary analyses and present the main effect along with 95% confidence intervals (CIs). Recognizing our trial is not powered to test statistically significant interactions between treatment arm and baseline variables, these results are exploratory rather than confirmatory. We used Bonferroni adjustments for statistical significance in the subgroup analyses. All statistical tests were two-sided. Statistical analyses were conducted using STATA (version 15.0; STATA Corporation, College Station, TX) and SAS (version 9.4; SAS Institute, Cary, NC).
Results
Participant Characteristics
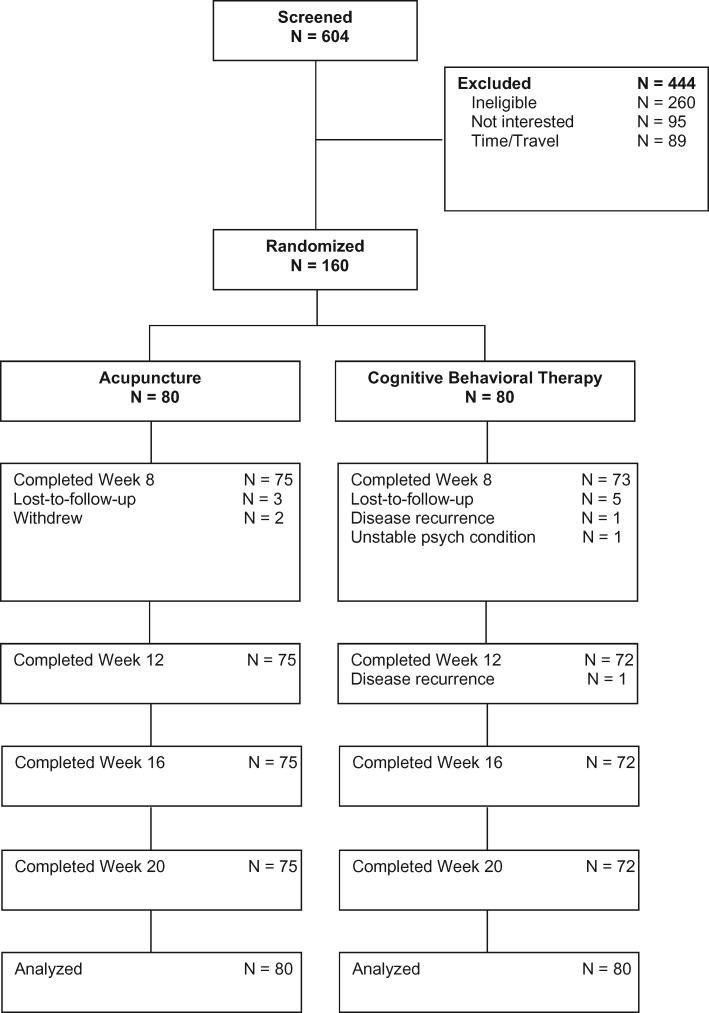
Participant flow is reported in Figure 1 . Of the 604 participants screened for eligibility, 444 were ineligible, not interested, did not have time, or were unable to travel for the study. We randomly assigned the remaining 160 participants to receive acupuncture (n = 80) or CBT-I (n = 80). Of those assigned to acupuncture, five (6.3%) withdrew. Among the 75 remaining, 73 (97.3%) completed eight or more acupuncture treatments. Of those assigned to CBT-I, eight (10.0%) withdrew. Among the 72 remaining, 62 (86.1%) attended six or more CBT-I sessions.
Figure 1.
CONSORT diagram.
Random assignment produced equivalent groups (Table 1). The mean (SD) age was 61.5 (11.7) years, 91 (56.9%) were women, 113 (70.6%) were white, 44 (27.5%) were black, and three (1.9%) were other races. The most common cancer type was breast (31.2%), followed by prostate (22.5%) and hematological cancers (8.1%).
Table 1.
Sociodemographic and clinical characteristics of participants
| Participant characteristic | Acupuncture no. (%) | CBT-I no. (%) | Total no. (%) |
|---|---|---|---|
| Age, mean (SD), y | 62.3 (11.4) | 60.7 (12.0) | 61.5 (11.7) |
| Sex | |||
| Male | 37 (46.2) | 32 (40.0) | 69 (43.1) |
| Female | 43 (53.8) | 48 (60.0) | 91 (56.9) |
| Race | |||
| White | 61 (76.2) | 52 (65.0) | 113 (70.6) |
| Black | 17 (21.2) | 27 (33.8) | 44 (27.5) |
| Other* | 2 (2.5) | 1 (1.2) | 3 (1.9) |
| Ethnicity | |||
| Hispanic | 3 (3.8) | 5 (6.2) | 8 (5.0) |
| Non-Hispanic | 77 (96.2) | 75 (93.8) | 152 (95.0) |
| Education | |||
| Some college or less | 24 (30.0) | 21 (26.2) | 45 (28.1) |
| College or graduate | 56 (70.0) | 59 (73.8) | 115 (71.9) |
| Marital status | |||
| Married or living with partner | 39 (48.8) | 42 (52.5) | 81 (50.6) |
| Single, divorced, separated, or widowed | 41 (51.2) | 38 (47.5) | 79 (49.4) |
| Employment | |||
| Full-time | 30 (38.0) | 24 (30.0) | 54 (34.0) |
| Part-time | 16 (20.2) | 11 (13.8) | 27 (17.0) |
| Not currently employed | 33 (41.8) | 45 (56.2) | 78 (49.1) |
| Income | |||
| <$20 000 | 9 (11.7) | 11 (14.1) | 20 (12.9) |
| $20 000–$34 999 | 12 (15.6) | 10 (12.8) | 22 (14.2) |
| $35 000–$64 999 | 14 (18.2) | 16 (20.5) | 30 (19.4) |
| $≥$65 000 | 42 (54.6) | 41 (52.6) | 83 (53.6) |
| Cancer type | |||
| Breast | 24 (30.0) | 26 (32.5) | 50 (31.2) |
| Prostate | 19 (23.8) | 17 (21.2) | 36 (22.5) |
| Colon/rectal | 5 (6.2) | 5 (6.2) | 10 (6.2) |
| Head/neck | 5 (6.2) | 6 (7.5) | 11 (6.9) |
| Hematologic | 7 (8.8) | 6 (7.5) | 13 (8.1) |
| GYN | 4 (5.0) | 3 (3.8) | 7 (4.4) |
| Other cancer† | 10 (12.5) | 13 (16.2) | 23 (14.4) |
| More than one cancer | 6 (7.5) | 4 (5.0) | 10 (6.2) |
| Cancer stage | |||
| 0 | 2 (2.5) | 3 (3.8) | 5 (3.1) |
| I | 35 (43.8) | 35 (43.8) | 70 (43.8) |
| II | 19 (23.8) | 20 (25.0) | 39 (24.4) |
| III | 15 (18.8) | 14 (17.5) | 29 (18.1) |
| IV | 7 (8.8) | 7 (8.8) | 14 (8.8) |
| Unknown‡ | 2 (2.5) | 1 (1.2) | 3 (1.9) |
| Cancer treatment§ | |||
| Surgery | 63 (78.7) | 52 (65.0) | 115 (71.9) |
| Chemotherapy | 39 (48.8) | 38 (47.5) | 77 (48.1) |
| Radiation | 42 (52.5) | 37 (46.2) | 79 (49.4) |
| Hormonal | 19 (23.8) | 18 (22.5) | 37 (23.1) |
| Years since cancer diagnosis, mean (SD) | 6.4 (5.1) | 5.7 (5.6) | 6.1 (5.4) |
| Years since insomnia onset, mean (SD) | 9.7 (9.6) | 8.6 (8.6) | 9.2 (9.1) |
| Insomnia Severity Index Total, mean (SD) | 17.6 (4.1) | 18.5 (4.4) | 18.0 (4.3) |
| Pittsburgh Sleep Quality Index Total, mean (SD) | 11.8 (3.2) | 12.1 (3.7) | 12.0 (3.4) |
| Sleep diary variables | |||
| Minutes sleep onset latency, mean (SD) | 32.1 (29.3) | 47.1 (62.1) | 39.2 (48.2) |
| Minutes awake after sleep onset, mean (SD) | 58.0 (38.8) | 53.6 (42.1) | 55.9 (40.4) |
| Minutes total sleep time, mean (SD) | 346.1 (82.1) | 340.8 (99.2) | 343.6 (90.4) |
| Sleep efficiency percentage, mean (SD) | 73.2 (13.5) | 72.1 (18.3) | 72.7 (15.9) |
| Brief Pain Inventory | |||
| Pain severity, mean (SD) | 2.4 (2.4) | 2.1 (2.0) | 2.3 (2.2) |
| Multidimensional Fatigue Symptom Inventory−Short Form Total, mean (SD) | 19.8 (21.3) | 21.9 (22.3) | 20.9 (21.8) |
| Hospital Anxiety and Depression Scale | |||
| Anxiety, mean (SD) | 7.8 (4.0) | 7.8 (4.3) | 7.8 (4.2) |
| Depression, mean (SD) | 4.6 (3.1) | 5.1 (3.4) | 4.9 (3.3) |
| PROMIS Global Health | |||
| Global Physical Health Score, mean (SD) | 45.2 (8.4) | 43.4 (8.7) | 44.3 (8.6) |
| Global Mental Health Score, mean (SD) | 45.0 (8.0) | 44.1 (8.2) | 44.6 (8.1) |
| Treatment expectancy | |||
| Expectancy for acupuncture treatment | 13.3 (4.0) | 13.2 (3.2) | 13.3 (3.6) |
| Expectancy for CBT-I treatment | 12.8 (3.5) | 13.2 (2.9) | 13.0 (3.2) |
Other includes Asian and more than one race. CBT-I = Cognitive Behavioral Therapy for Insomnia; PROMIS = Patient-Reported Outcomes Measurement Information System; SD = standard deviation.
Other cancer includes skin, lung, other gastrointestinal, other genito-urinary, etc.
Some cancer types do not have staging systems.
Subjects can have more than one type of cancer treatment.
Primary Outcome
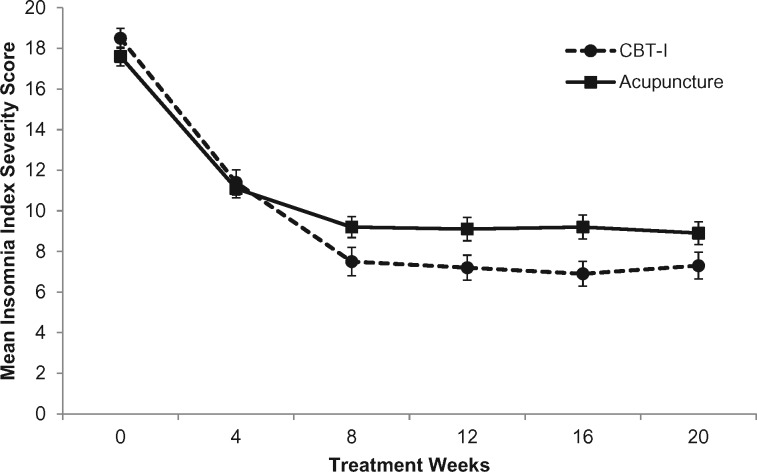
Results are presented in Table 2. At the 8-week primary endpoint, there was a statistically significant group difference with regards to change in insomnia severity from baseline (Figure 2). Participants randomly assigned to CBT-I reported greater improvement than those assigned to acupuncture over time (P < .001 for treatment and time interaction). Immediately posttreatment (primary endpoint), the acupuncture group reported a reduction of −8.31 (95% CI = −9.36 to −7.26) points compared with −10.91 (95% CI = −11.97 to −9.85) points in the CBT-I group; the between-group difference was 2.60 points (95% CI = 1.11 to 4.09, P < .001, Cohen’s D = 0.32) favoring CBT-I. Both groups exceeded the minimally important change value of 8 points. The improvements for both groups were maintained at the 20-week assessment with a group difference of 2.53 points (95% CI = 1.03 to 4.02, P = .001, Cohen’s D = 0.31). Further analyses adjusting for baseline expectancy for acupuncture and CBT-I did not change the magnitude of effect or statistical significance.
Table 2.
Change in secondary study outcomes from baseline by treatment
| Outcome variable | Change from baseline, mean (95% CI) |
Between- group difference, mean (95% CI) |
||
|---|---|---|---|---|
| Acupuncture | CBT-I | Acupuncture versus CBT-I | P | |
| Primary outcome | ||||
| Insomnia Severity Index Score† | <.001* | |||
| 8 weeks | −8.31 (−9.36 to −7.26) | −10.91 (−11.97 to −9.85) | 2.60 (1.11 to 4.09) | |
| 20 weeks | −8.59 (−9.65 to −7.55) | −11.12 (−12.19 to −10.06) | 2.53 (1.03 to 4.02) | |
| Secondary sleep outcomes | ||||
| Pittsburgh Sleep Quality Index Total† | .009 | |||
| 8 weeks | −4.39 (−5.10 to −3.67) | −5.90 (−6.61 to −5.18) | 1.51 (0.50 to 2.52) | |
| 20 weeks | −4.62 (−5.33 to −3.91) | −5.84 (−6.56 to −5.13) | 1.22 (0.21 to 2.23) | |
| Sleep onset latency, min† | <.001 | |||
| 8 weeks | −10.80 (−16.46 to −5.14) | −27.13 (−33.12 to −21.14) | 16.33 (8.09 to 24.57) | |
| 20 weeks | −10.91 (−16.70 to −5.13) | −24.37 (−30.36 to −18.38) | 13.46 (5.13 to 21.79) | |
| Wake after sleep onset, min† | .002 | |||
| 8 weeks | −26.89 (−34.16 to −19.61) | −35.83 (−43.51 to −28.16) | 8.95 (−1.63 to 19.52) | |
| 20 weeks | −22.01 (−29.45 to −14.58) | −29.51 (−37.16 to −21.87) | 7.50 (−3.16 to 18.16) | |
| Total sleep time, min‡ | .003 | |||
| 8 weeks | 61.81 (45.79 to 77.84) | 35.01 (18.12 to 51.91) | 26.80 (3.51 to 50.09) | |
| 20 weeks | 50.71 (34.39 to 67.03) | 45.23 (28.33 to 62.12) | 5.48 (−18.01 to 28.97) | |
| Sleep efficiency, %‡ | <.001 | |||
| 8 weeks | 0.11 (0.08 to 0.13) | 0.16 (0.13 to 0.18) | −0.05 (−0.09 to −0.01) | |
| 20 weeks | 0.10 (0.07 to 0.12) | 0.13 (0.10 to 0.16) | −0.04 (−0.07 to 0.00) | |
| Secondary comorbid symptoms and quality-of-life measures | ||||
| Brief Pain Inventory Severity† | .20 | |||
| 8 weeks | −0.49 (−0.81 to −0.16) | 0.00 (−0.33 to 0.33) | −0.49 (−0.95 to −0.02) | |
| 20 weeks | −0.46 (−0.79 to −0.14) | −0.35 (−0.68 to −0.02) | −0.11 (−0.58 to 0.35) | |
| HADS-Anxiety† | .94 | |||
| 8 weeks | −2.21 (−2.82 to −1.60) | −1.93 (−2.56 to −1.30) | −0.28 (−1.16 to 0.60) | |
| 20 weeks | −1.94 (−2.55 to −1.33) | −1.83 (−2.46 to −1.20) | −0.10 (−0.98 to 0.78) | |
| HADS-Depression† | .75 | |||
| 8 weeks | −1.06 (−1.57 to −0.55) | −1.45 (−1.98 to −0.92) | 0.39 (−0.35 to 1.13) | |
| 20 weeks | −0.91 (−1.42 to −0.40) | −1.22 (−1.75 to −0.69) | 0.31 (−0.43 to 1.05) | |
| MFSI-SF total score† | .53 | |||
| 8 weeks | −10.82 (−13.94 to −7.70) | −12.48 (−15.69 to −9.27) | 1.65 (−2.82 to 6.12) | |
| 20 weeks | −12.20 (−15.32 to −9.08) | −11.19 (−14.38 to −8.00) | −1.01 (−5.46 to 3.44) | |
| PROMIS-Physical‡ | .40 | |||
| 8 weeks | 2.19 (0.92 to 3.46) | 3.73 (2.44 to 5.02) | −1.54 (−3.36 to 0.28) | |
| 20 weeks | 3.29 (2.02 to 4.56) | 4.49 (3.20 to 5.78) | −1.20 (−3.02 to 0.62) | |
| PROMIS-Mental‡ | .36 | |||
| 8 weeks | 3.34 (1.99 to 4.69) | 3.39 (2.02 to 4.76) | −0.06 (−1.98 to 1.87) | |
| 20 weeks | 4.08 (2.73 to 5.43) | 3.41 (2.04 to 4.78) | 0.67 (−1.25 to 2.59) | |
Two-sided P: overall treatment and time interaction from the mixed-effects models. CBT-I = Cognitive Behavioral Therapy for Insomnia; CI = confidence interval; HADS = Hospital Anxiety and Depression Scale; MFSI-SF = Multidimensional Fatigue Symptom Inventory-Short Form; min = minutes; PROMIS = Patient-Reported Outcomes Measurement Information System; SD = standard deviation.
A greater negative value represents improvement in symptoms.
A greater positive value represents improvement in symptoms.
Figure 2.
Effects of intervention over time. This figure shows the between- and within-group change in insomnia severity at the primary and secondary end- points for participants assigned to acupuncture (n = 80) or Cognitive Behavioral Therapy for Insomnia (CBT-I; n = 80). Error bar represents the standard error of the mean.
Secondary Outcomes
Secondary sleep outcomes are also presented in Table 2. During treatment and follow-up, CBT-I was more effective than acupuncture at improving overall sleep quality (P = .009 for treatment and time interaction), shortening sleep onset latency (P < .001), reducing wake after sleep onset (P = .002), and increasing sleep efficiency (P < .001); however, acupuncture was more effective at increasing total sleep time (P = .003). Although baseline pain was low, individuals in the acupuncture group had greater decreases in pain severity than the CBT-I group at week 8 (−0.49 in BPI severity score, 95% CI = −0.95 to −0.02); however, the difference was no longer statistically significant at week 20. Both groups had similar improvements in fatigue, anxiety, depression, and quality of life (mental and physical health).
Sleep Medications
At baseline, 23.8% of participants used at least one prescription medication for sleep during the prior week. Compared to baseline, there was a statistically significant reduction in the proportion of participants still using prescription medication with 18.2% at week 8 (P<.001) and 17.0% at week 20 (P<.001) reporting having used at least one prescription sleep aid. The frequency of medication usage decreased from 0.92 to 0.81 (P = .08) and 0.66 (P = .02) nights per week at weeks 8 and 20. No group differences were seen.
Adverse Events
Of the 78 participants attending at least one acupuncture treatment, nine reported an adverse event (AE). Most were related to the needling site and included soreness, itchiness, and pain. Of the 73 participants attending at least one CBT-I session, five reported an AE, mostly related to increased drowsiness and daytime fatigue. All AEs were mild to moderate. Two participants in the CBT-I group experienced cancer progression during the study unrelated to the intervention.
Subgroup Analyses
Using Bonferroni adjustments (P < .01), CBT-I was statistically significantly more effective for improving insomnia than acupuncture for men (P < .001) and those who were white (P = .003), highly educated (P < .001), and without clinically meaningful pain at baseline (P < .001). The magnitude of differences in ISI score between acupuncture and CBT-I for women, non-whites (mostly black), lower education, and those with pain were small (see Table 3).
Table 3.
Subgroup analyses
| Subgroup | Total no. | Insomnia severity index week 8 change from baseline, mean (95% CI) |
Difference between treatments at week 8,* mean (95% CI) |
P † | |
|---|---|---|---|---|---|
| Acupuncture | CBT-I | Acupuncture versus CBT-I | |||
| Sex | |||||
| Male | 69 | −8.03 (−9.69 to −6.37) | −11.94 (−13.73 to −10.15) | 3.91 (1.46 to 6.36) | <.001 |
| Female | 91 | −8.56 (−10.00 to −7.12) | −10.21 (−11.59 to −8.83) | 1.66 (−0.34 to 3.66) | .20 |
| Race | |||||
| White | 113 | −7.92 (−9.15 to −6.69) | −10.88 (−12.21 to −9.55) | 2.95 (1.13 to 4.77) | .003 |
| Non-white | 47 | −9.60 (−11.97 to −7.22) | −10.90 (−12.95 to −8.85) | 1.30 (−1.84 to 4.41) | .70 |
| Education | |||||
| Some college or less | 45 | −11.72 (−13.77 to −9.67) | −11.76 (−13.94 to −9.58) | 0.05 (−2.93 to 3.02) | .75 |
| College or graduate | 115 | −7.06 (−8.31 to −5.81) | −10.59 (−11.84 to −9.34) | 3.54 (1.76 to 5.32) | <.001 |
| Baseline Brief Pain Inventory Worst pain | |||||
| <4 | 90 | −6.83 (−8.20 to −5.46) | −10.53 (−11.90 to −9.16) | 3.70 (1.77 to 5.63) | <.001 |
| ≥4 | 70 | −10.32 (−12.03 to −8.61) | −11.33 (−13.08 to −9.58) | 1.01 (−1.44 to 3.45) | .59 |
Positive number represents that the Insomnia Severity Index score at week 8 is higher in the acupuncture group than in the CBT-I group. CBT-I = Cognitive Behavioral Therapy for Insomnia; CI = confidence interval.
Mixed-effect P for treatment and time interaction in each subgroup. All tests were two-sided.
Discussion
In this comparative effectiveness, randomized trial of cancer survivors with insomnia, CBT-I was more effective than acupuncture in reducing insomnia severity immediately after treatment. However, both treatments reduced insomnia severity by 8 points or more, a value established to represent clinically meaningful improvement (18), and these were well maintained at the 3-month follow-up. In subgroup analyses, CBT-I was statistically significantly more effective than acupuncture for men and those who identified as white, with higher education, and without pain. Acupuncture was more effective than CBT-I for pain in the short term. There were no group differences on measures of fatigue, depression, anxiety, and quality of life. Both treatments reduced sleep medication use.
It is important to interpret this trial in the context of prior literature. Compared with other CBT-I studies, we enrolled more minority patients and included cancer types other than breast, yet the effects of CBT-I were robust and consistent. A systematic review and meta-analysis included eight RCTs comparing CBT-I to control subjects with a total of 752 cancer survivors (5). The control groups represented in these studies included treatment as usual, wait lists, and sleep education. The pooled difference between pre- and post-intervention means for insomnia severity in the meta-analysis was −7.83 for CBT-I compared with −3.51 in the control groups (5). The differences we observed were −10.91 and −8.31 in the CBT-I and acupuncture groups, respectively. While our findings add to the evidence that CBT-I is efficacious, they also demonstrate that the improvements in the acupuncture group are twice the magnitude of what other studies have reported for inactive or attention matched control groups.
Despite the effectiveness of CBT-I, awareness of its benefits among patients and oncology clinicians remains low, and access to qualified CBT-I therapists is limited (27). During our patient and stakeholder engagement, several barriers were identified. First, there remains a stigma associated with receiving psychological treatment such as CBT-I. Second, there is a lack of institutional resources and inadequate insurance coverage for CBT-I training and delivery. Third, geography may inhibit many cancer patients from attending weekly visits to see a qualified CBT-I provider. Despite recognition of CBT-I as the gold standard and first-line therapy (5,28,29), systematic dissemination and implementation efforts are required to make CBT-I available to patients from diverse backgrounds and those who live in more rural communities. Increasingly, efforts are focused on expanding access to CBT-I through telemedicine (30) and online interventions (31).
This is one of the very few and largest acupuncture studies for insomnia conducted in cancer survivors. Basic science research in animal models has demonstrated that acupuncture affects the synthesis, release, and action of several neurotransmitters involved in sleep (eg, catecholamine, glutamate), thus providing biological plausibility for its treatment of insomnia (32–34). Two recent trials in noncancer populations demonstrated acupuncture was more efficacious than sham acupuncture for improving insomnia severity and objective outcomes of sleep efficiency and total sleep time measured by polysomnography and actigraphy (8,9). With most US cancer centers offering acupuncture as a complementary therapy (11), our results add to the evidence that acupuncture may be a reasonable choice when CBT-I is not available or desirable. Further, acupuncture was more effective than CBT-I for short-term pain reduction; therefore, it may be a reasonable approach for survivors experiencing both insomnia and pain. Adequately powered and controlled trials of acupuncture will be needed to establish the specific efficacy of acupuncture for insomnia in cancer survivors.
Choosing the best treatment for individual patients will rely on patients’ own beliefs and preference, availability of therapeutic options, and clinical evidence (35). We observed statistically nonsignificant differences in rates of withdrawal (6.3% in acupuncture versus 10.0% in CBT-I) and adherence to treatment (97.3% in acupuncture versus 86.1% in CBT-I) slightly favoring acupuncture; however, both treatments were well received. The subgroup differences in the relative effects between acupuncture and CBT-I based on sex, race, education, and pain need further exploration to help inform individualized treatment decisions for diverse groups of cancer survivors. In clinical care, it is unlikely one size fits all; our research findings will build a foundation for future patient-centered sleep management.
Our trial was designed as a head-to-head comparative effectiveness trial rather than a noninferiority trial. Although we found a small effect size difference between the two interventions (Cohen’s D of 0.32), the clinical importance of this difference may not be relevant for some patients. Second, the two interventions had different time-commitment requirements with increased overall treatment time for acupuncture but less face-to-face contact time with a provider. Third, although we screened patients for other sleep disorders, they did not undergo a formal polysomnogram and we did not include an objective outcome measure of sleep (eg, actigraphy). However, neither of these are recommended for the diagnosis or evaluation of insomnia treatment in clinical practice (36). Lastly, our study was conducted in tertiary cancer centers, and although the sample was more diverse than prior research, participants were still highly educated.
Although both acupuncture and CBT-I resulted in clinically meaningful and sustained reductions in insomnia, CBT-I was more effective overall. The variations in the relative effectiveness between CBT-I and acupuncture based on sex, race, education, and pain require confirmation in future studies. These findings and future research have the potential to improve outcomes by helping cancer survivors and their caregivers make informed and evidence-based decisions leading to patient-centered, individualized care for cancer survivors with insomnia.
Funding
This work was supported by a Patient-Centered Outcomes Research Institute (PCORI) award (grant number CER-1403-14292). This work was also supported in part by a National Institutes of Health/National Cancer Institute Cancer Center grant (number P30 CA008748).
Notes
Affiliations of authors: Departments of Psychology and Oncology, Memorial University of Newfoundland, St. John’s, NL, Canada (SNG); Department of Biostatistics, Epidemiology, and Informatics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (SXX); Department of Psychiatry and Behavioral Sciences (KD), and Department of Medicine (TB, QL, PK, JJM), Memorial Sloan Kettering Cancer Center, New York, NY; Department of Family Medicine and Community Health, Penn Presbyterian Medical Center, Andrew Mutch Building, Philadelphia, PA (FKB, SS); Department of Psychiatry, University of Pennsylvania, Philadelphia, PA (PG).
The statements presented in this article are solely the responsibility of the authors and do not necessarily represent the views of PCORI, its Board of Governors, or Methodology Committee. The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The authors report no conflicts of interest.
Sincere thanks go to Winifred Chain, Linda Geiger, Donna-Lee Lista, Jodi MacLeod, Alice McAllister, Hilma Maitland, Edward Wolff, and the other CHOICE Study Patient Advisory Board members; the study participants; the study therapists, and the research staff for their support of this study. We also thank our Data Safety and Monitoring Board members, Tavis Campbell, PhD, Roger Cohen, MD, and Sarah Ratcliffe, PhD, whose time and expertise were uncompensated.
Part of the content from the CHOICE study was presented at the ASCO annual meeting as an oral presentation in June 2018 and featured in ASCO Press Cast. It also was selected as part of the Best of ASCO program.
After deidentification, individual participant data that underlie the results reported in this article, the study protocol, and the statistical analysis plan will be available beginning 3 months and ending 36 months following article publication to investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose for individual participant data meta-analysis. Proposals should be directed to liq2@mskcc.org; to gain access, data requestors will need to sign a data access agreement.
SNG contributed to conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of the manuscript. SXX contributed to data analysis and interpretation, manuscript writing, and final approval of the manuscript. KD contributed to data analysis and interpretation, manuscript writing, and final approval of the manuscript. TB contributed to data analysis and interpretation, manuscript writing, and final approval of the manuscript. QL contributed to the collection and assembly of data, analysis and interpretation, manuscript writing, and final approval of the manuscript. FKB contributed to conception and design, data analysis and interpretation, manuscript writing, and final approval of the manuscript. SS contributed to collection and assembly of data, manuscript writing, and final approval of the manuscript. PK contributed to data analysis and interpretation, manuscript writing, and final approval of the manuscript. PG contributed to the collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of the manuscript. JJM contributed to conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of the manuscript.
Supplementary Material
References
- 1. Savard J, Ivers H, Villa J, et al. Natural course of insomnia comorbid with cancer: an 18−month longitudinal study. J Clin Oncol. 2011;29(26):3580–3586. [DOI] [PubMed] [Google Scholar]
- 2. Stepanski EJ, Walker MS, Schwartzberg LS, et al. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J Clin Sleep Med. 2009;5(2):132–136. [PMC free article] [PubMed] [Google Scholar]
- 3. Berger AM, Matthews EE, Kenkel AM.. Management of sleep-wake disturbances comorbid with cancer. Oncology (Williston Park). 2017;31(8):610–617. [PubMed] [Google Scholar]
- 4. Kripke DF. Hypnotic drug risks of mortality, infection, depression, and cancer: But lack of benefit. F1000Res. 2016;5:918.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. [DOI] [PubMed] [Google Scholar]
- 6. Zhou ES, Partridge AH, Syrjala KL, et al. Evaluation and treatment of insomnia in adult cancer survivorship programs. J Cancer Surviv. 2017;11(1):74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matthews EE, Schmiege SJ, Cook PF, et al. Adherence to cognitive behavioral therapy for insomnia (CBTI) among women following primary breast cancer treatment: a pilot study. Behav Sleep Med. 2012;10(3):217–229. [DOI] [PubMed] [Google Scholar]
- 8. Fu C, Zhao N, Liu Z, et al. Acupuncture improves peri-menopausal insomnia: a randomized controlled trial. Sleep. 2017;(11):40. [DOI] [PubMed] [Google Scholar]
- 9. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193–200. [DOI] [PubMed] [Google Scholar]
- 10. Zia FZ, Olaku O, Bao T, et al. The National Cancer Institute's Conference on Acupuncture for Symptom Management in Oncology: State of the Science, Evidence, and Research Gaps. J Natl Cancer Inst Monogr. 2017;2017(52):68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yun H, Sun L, Mao JJ.. Growth of integrative medicine at leading cancer centers between 2009 and 2016: a systematic analysis of NCI-designated comprehensive cancer center websites. J Natl Cancer Inst Monogr. 2017;2017(52):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi TY, Kim JI, Lim HJ, et al. Acupuncture for managing cancer-related insomnia: a systematic review of randomized clinical trials. Integr Cancer Ther. 2017;16(2):135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garland SN, Gehrman P, Barg FK, et al. CHoosing Options for Insomnia in Cancer Effectively (CHOICE): design of a patient centered comparative effectiveness trial of acupuncture and cognitive behavior therapy for insomnia. Contemp Clin Trials. 2016;47:349–355. [DOI] [PubMed] [Google Scholar]
- 14. MacLeod J, Wolff E, McAllister A, et al. Including the patient voice in patient-centered outcomes research in integrative oncology. J Natl Cancer Inst Monogr. 2017;2017(52):46–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sedgwick P. Explanatory trials versus pragmatic trials. BMJ. 2014;349:g6694.. [DOI] [PubMed] [Google Scholar]
- 16. Mao JJ, Farrar JT, Armstrong K, et al. De qi: Chinese acupuncture patients' experiences and beliefs regarding acupuncture needling sensation-An exploratory survey. Acupunct Med. 2007;25(4):158–165. [DOI] [PubMed] [Google Scholar]
- 17. Savard MH, Savard J, Simard S, et al. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14(6):429–441. [DOI] [PubMed] [Google Scholar]
- 18. Morin CM, Belleville G, Belanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 20. Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cleeland CS, Ryan KM.. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 22. Stein KD, Jacobsen PB, Blanchard CM, et al. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. [DOI] [PubMed] [Google Scholar]
- 24. Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mao JJ, Xie SX, Bowman MA.. Uncovering the expectancy effect: the validation of the acupuncture expectancy scale. Altern Ther Health Med. 2010;16(6):22–27. [PMC free article] [PubMed] [Google Scholar]
- 26. Garland SN, Carlson LE, Stephens AJ, et al. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32(5):449–457. [DOI] [PubMed] [Google Scholar]
- 27. Fleming L, MacMahon K.. ‘CBT-I in cancer: we know it works, so why are we waiting?’ Curr Sleep Med Rep. 2015;1(3):177–183. [Google Scholar]
- 28. Medalie L, Cifu AS.. Management of chronic insomnia disorder in adults. JAMA. 2017;317(7):762–763. [DOI] [PubMed] [Google Scholar]
- 29. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD.. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 30. McCarthy MS, Matthews EE, Battaglia C, Meek PM.. Feasibility of a telemedicine-delivered cognitive behavioral therapy for insomnia in rural breast cancer survivors. Oncol Nurs Forum. 2018;45(5):607–618. [DOI] [PubMed] [Google Scholar]
- 31. Zachariae R, Amidi A, Damholdt MF. Internet-delivered cognitive-behavioral therapy for insomnia in breast cancer survivors: a randomized controlled trial. J Natl Cancer Inst. 2018; 110(8):880–887. [DOI] [PMC free article] [PubMed]
- 32. Soligo M, Nori SL, Protto V, et al. Acupuncture and neurotrophin modulation. Int Rev Neurobiol. 2013;111:91–124. [DOI] [PubMed] [Google Scholar]
- 33. Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26(1):17–22. [DOI] [PubMed] [Google Scholar]
- 34. Manni L, Albanesi M, Guaragna M, et al. Neurotrophins and acupuncture. Auton Neurosci. 2010;157(1–2):9–17. [DOI] [PubMed] [Google Scholar]
- 35. Garland SN, Eriksen W, Song S, et al. Factors that shape preference for acupuncture or cognitive behavioral therapy for the treatment of insomnia in cancer patients. Support Care Cancer. 2018;26(7):2407–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.