Abstract
Background
Global recognition of antimicrobial resistance (AMR) as an urgent public health problem has galvanized national and international efforts. Chief among these are interventions to curb the overuse and misuse of antibiotics. However, the impact of these initiatives is not fully understood, making it difficult to assess the expected effectiveness and sustainability of further policy interventions. We conducted a systematic review to summarize existing evidence for the impact of nationally enforced interventions to reduce inappropriate antibiotic use in humans.
Methods
We searched seven databases and examined reference lists of retrieved articles. To be included, articles had to evaluate the impact of national responsible use initiatives. We excluded studies that only described policy implementations.
Results
We identified 34 articles detailing interventions in 21 high- and upper-middle-income countries. Interventions addressing inappropriate antibiotic access included antibiotic committees, clinical guidelines and prescribing restrictions. There was consistent evidence that these were effective at reducing antibiotic consumption and prescription. Interventions targeting inappropriate antibiotic demand consisted of education campaigns for healthcare professionals and the general public. Evidence for this was mixed, with several studies showing no impact on overall antibiotic consumption.
Conclusions
National-level interventions to reduce inappropriate access to antibiotics can be effective. However, evidence is limited to high- and upper-middle-income countries, and more evidence is needed on the long-term sustained impact of interventions. There should also be a simultaneous push towards standardized outcome measures to enable comparisons of interventions in different settings.
Introduction
Antimicrobial resistance (AMR) is a major threat to global health. Without urgent action, an estimated 10 million annual deaths due to resistant pathogens are expected by 2050.1 Among antimicrobials, bacterial resistance to antibiotics is a major concern. Antibiotic consumption is a major driver of resistance, and global antibiotic consumption is rising; a recent comprehensive assessment of antibiotic consumption has shown a 65% increase in worldwide antibiotic consumption between 2000 and 2015, from 21.1 billion to 34.8 billion DDD.2
To counter the rise in AMR, the WHO’s Global Action Plan on Antimicrobial Resistance (2015)3 has identified responsible use as one of five key priority areas for urgent intervention, endorsing strategies to reduce both the supply of and demand for antibiotics. These include regulatory measures to reduce and optimize antimicrobial prescribing and dispensing, and campaigns to improve overall awareness and understanding of AMR, among both healthcare professionals and the general public.
Numerous interventions have been proposed and used to reduce antibiotic consumption in different settings, but the extent to which these have been implemented at a national level, and their impact, remain largely unknown. We conducted a systematic review to assess the impact of responsible use initiatives implemented at the national and/or subnational level to reduce unnecessary antibiotic consumption, in order to evaluate the available evidence on the impact of coordinated national implementations.
Methods
Inclusion and exclusion criteria
We conducted this review using PRISMA guidelines4 (Prospero registration number: CRD42017064629). We included studies describing any national-level and/or subnational-level responsible use initiatives to address antibiotic resistance in the community or at the primary, secondary or tertiary care levels. Studies with no reported outcomes relating to antibiotic consumption and/or prescription were excluded. The inclusion and exclusion criteria can be found in Table 1.
Table 1.
Inclusion and exclusion criteria
| Category | Criteria |
|---|---|
| Inclusion criteria | Peer-reviewed articles that described any national- and/or subnational-level responsible antibiotic use initiatives (i.e. interventions to reduce subtherapeutic consumption or prescription of antibiotics) |
| Setting | All healthcare settings (primary, secondary, tertiary care) as well as in the community |
| Outcomes | Any outcomes (externally measured, self-reported or observed changes) relating to antibiotic consumption and/or prescription |
| Exclusion criteria | Studies that only describe implementation of an intervention with no reported outcomes relating to antibiotic consumption and/or prescription |
| Studies that target antimicrobials other than antibiotics | |
| Studies that only describe interventions not related to antibiotic stewardship/responsible antibiotic use (e.g. infection control, vaccines, surveillance, etc.) | |
| Studies that only describe interventions for antibiotic use in animals and/or the environment |
Search strategy
The literature search focused on interventions related to responsible use of antibiotics (Table 2). The search strategy was developed with an information specialist and included Medical Subject Headings (MeSH) terms, keywords and free text (title and abstract) terms relating to AMR, national-level initiatives and their outcomes (Table 2).
Table 2.
Search terms
| MeSH terms | Keywords | |
|---|---|---|
| Antimicrobial | anti-infective agents | anti-infective agent* OR anti-bacterial agent* OR antibiotic* OR antimicrobial* OR anti-microbial* OR antibacterial* OR anti-bacterial* OR antiinfective* OR anti-infective* |
| anti-bacterial agents | ||
| Resistance | drug resistance | drug resistance OR drug resistance, multiple OR drug resistance, multiple, bacterial OR drug resistance, microbial OR resistance OR resistant OR resist |
| drug resistance, multiple | ||
| drug resistance, multiple, bacterial | ||
| drug resistance, microbial | ||
| Policy/programme | health policy | health policy OR health care reform OR national health policy OR policy making OR health promotion OR government program* OR policy OR policies OR program OR programme OR programs OR programmes OR campaign OR campaigns OR intervention OR interventions OR government OR governance OR govern OR governing OR national policy |
| health care reform | ||
| national health policy | ||
| policy making | ||
| health promotion | ||
| government programmes | ||
| Outcome | prescription drug misuse | consume OR consumption OR usage OR utilization OR utilisation OR stewardship OR rationale OR responsible OR guidance OR guideline OR guidelines prudent OR unnecessary OR underprescribe OR under-prescribe OR underprescribing OR under-prescribing OR underprescription OR under-prescription OR overprescribe OR over-prescribe OR overprescribing OR over-prescribing OR overprescription OR over-prescription OR prescribe OR prescribing OR prescription OR prescriptions OR practice pattern OR drug misuse OR reduce OR reduction OR decrease |
| prescription drug overuse | ||
| inappropriate prescribing | ||
| drug prescriptions | ||
| drug utilization | ||
| practice patterns, physicians |
The search strategy was used for the databases Medline, Embase and Global Health. To ensure coverage of low- and middle-income countries (LMICs), a simplified search strategy was used for the following databases: Latin American and Caribbean Health Sciences Literature (LILACS), Africa-Wide Information, Index Medicus for the South-East Asian Region (IMSEAR), Index Medicus for the Eastern Mediterranean Region (IMEMR) and Western Pacific Rim Region Index Medicus (WPRIM). In total, seven databases were searched from inception until May 2017. No date or language restrictions were applied; however, searches were conducted in English.
Search and retrieval of studies
Two reviewers (J. M. L. and S. R. S.) independently conducted the literature search and identified relevant articles based on title and/or abstract. If either reviewer considered a study potentially eligible, the two reviewers would independently assess the full text to determine whether it met the inclusion criteria. Full text articles in languages other than English were translated into English for screening. Any disagreements were resolved by discussion with a third reviewer (D. M. C.).
Data synthesis
Three reviewers (J. M. L., S. R. S. and D. M C.) independently extracted data from included studies. Data were extracted on the following: (i) study characteristics (study design, setting); (ii) type of intervention; and (iii) results and type of outcome measure (e.g. antibiotic consumption, prescription, compliance with prescription guidelines, resistance rates). Differences in data extraction or study interpretation were resolved by discussion and consensus. Included studies were then grouped based on type of intervention conducted.
Risk of bias assessment
Three reviewers (J. M. L., S. R. S. and D. M. C.) independently assessed risk of bias. We used the Cochrane Risk of Bias Tool for Non-Randomized Studies of Interventions (ACROBAT-NRSI), since most of the intervention studies adopted a time-series design.5 We classified studies that had low risk of bias in all domains as low overall risk of bias. Studies that had high or unclear risk of bias in one or more domains were classified as overall high or unclear risk of bias.
Results
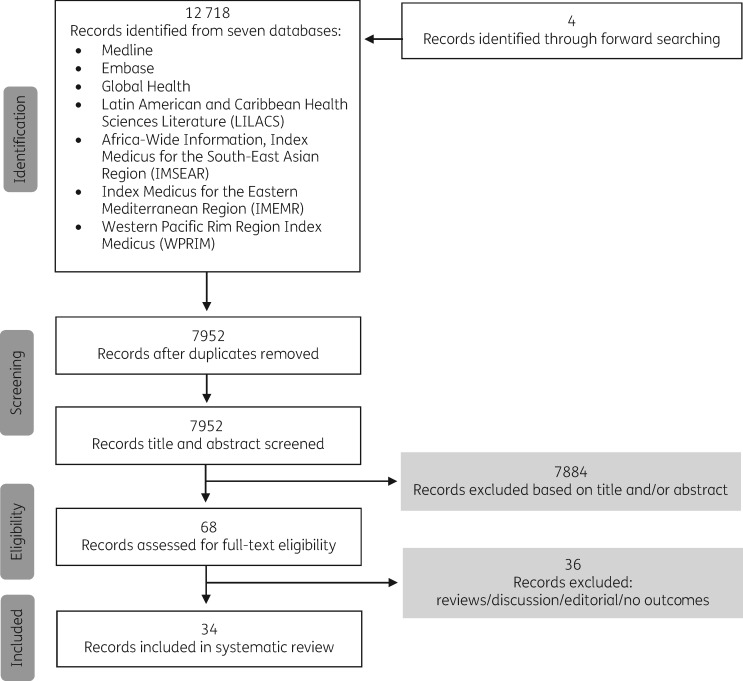
We identified 12 718 records from the database search, and two additional records from forward searching. After removing duplicate records, 7952 articles remained. These were screened for relevance by title and abstract, yielding 68 articles that were retrieved as full texts (Figure 1). Thirty-four articles met the inclusion criteria, all of which were journal articles and were published in English, Chinese, French, Croatian and Spanish. We did not conduct a meta-analysis owing to heterogeneity of study design, interventions, participants and outcomes, but instead present a qualitative summary of interventions, results and outcomes.
Figure 1.
Study flow diagram.
Countries and interventions (characteristics of included studies)
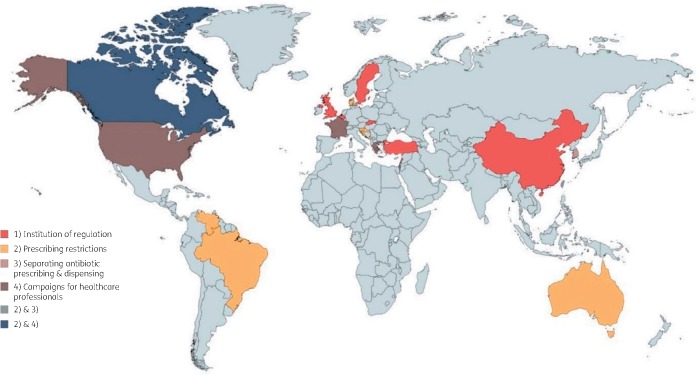
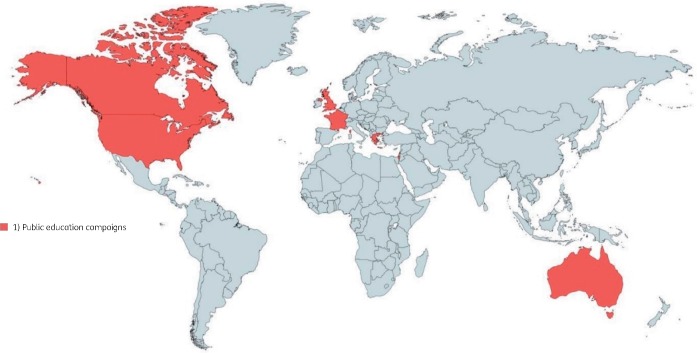
The 34 included studies used quantitative methods and were conducted in 21 countries (Figures 2 and 3). Twenty-two studies were carried out in high-income and 12 in upper-middle-income countries as defined by World Bank classification.6 Eight studies had high risk of bias, 20 studies had moderate risk of bias and 6 studies had low risk of bias. We classified studies into two broad domains: (i) interventions to reduce the inappropriate access to antibiotics; and (ii) interventions to reduce demand for antibiotics.
Figure 2.
Countries where interventions to reduce inappropriate access to antibiotics were implemented and evaluated. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 3.
Countries where interventions to decrease inappropriate demand for antibiotics were implemented and evaluated. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Full details of the included studies, including study design, setting and key findings, can be found in Table 3.
Table 3.
Study characteristics
| Authors | Country | Title | Intervention | Study design | Year | Outcome measure | Risk of bias | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Krcmery and Gould (1999)8 | Slovakia | Antibiotic policies in Central/Eastern Europe (CEE) after 1990 | Institution of regulation (antibiotic committees) | Time series analysis | 1994–7 | Antibiotic consumption (number of antibiotic packages/year) | Moderate | Overall decrease of antibiotic consumption: ∼11 000 packages/year in 1994 to ∼ 9000 packages/year in 1997 |
| 2 | Altunsoy et al. (2011)10 | Turkey | The impact of a nationwide antibiotic restriction programme on antibiotic usage and resistance against nosocomial pathogens in Turkey | Institution of regulation (antibiotic committees) | Pre–post study | 2001–5 |
|
Moderate | 11.3% decrease in antibiotic consumption (g) and cost
|
| Negative correlation between ceftriaxone consumption (−36.8%) and the prevalence of ceftriaxone-resistant Escherichia coli and Klebsiella spp. (ρ −0.395, P=0.332 and ρ −0.627, P=0.037); all non-significant | |||||||||
| Decreased use of carbapenems was correlated with decreased carbapenems-resistant Pseudomonas spp. and Acinetobacter spp (ρ 0.155, P=0.712; ρ 0.180, P=0.668) | |||||||||
| Methicillin resistance rates of S. aureus decreased from 44% to 41% | |||||||||
| 3 | Nathwani et al. (2011)9 | Scotland, UK | Scottish Antimicrobial Prescribing Group (SAPG): development and impact of the Scottish National Antimicrobial Stewardship Programme | Institution of regulation (antibiotic committees) | Time series analysis | 2005–9 |
|
Moderate | 44 500 fewer prescriptions in 2009 compared with 2008
|
| Increased number of NHS boards achieving ≥95% compliance with the empirical prescribing policy (range: 65%–89%) | |||||||||
| 4 | Tao et al. (2013)11 | China | Analysis of the current situation of antibiotics use in China: a hospital-based perspective | Institution of regulation (antibiotic committees) | Time series analysis | 2008–11 |
|
Moderate | Percentage of drug sales for antimicrobials decreased from 23.8% (2009) to 19.4% (2011) |
| Sales volume for second- (24.51% to 9.46%) and third-line (21.54% to 4.78%) antibiotics decreased from 2010 to 2011, while sales volume for first-line antibiotics increased from 2010 to 2011 (7.96% to 13.94%) | |||||||||
| 5 | Xiao et al. (2013)12 | China | Changes in Chinese policies to promote the rational use of antibiotics | Institution of regulation (antibiotic committees) | Time series analysis | 2009–12 |
|
Moderate | Percentage of drug sales for antimicrobials decreased from 25% (2011) to 17% (2012) |
| Percentage of antimicrobial prescriptions decreased in both inpatient settings (68% versus 58%) and outpatient settings (25% versus 15%) | |||||||||
| 6 | Malmvall et al. (2007)17 | Sweden | Reduction of antibiotics sales and sustained low incidence of bacterial resistance: report on a broad approach during 10 years to implement evidence-based indications for antibiotic prescribing in Jönköping County, Sweden | Institution of regulation (antibiotic committees) | Time series analysis | 1993–2005 |
|
Moderate | 31% decrease in overall antibiotic consumption: 15.9 DDD in 1993 to 11.0 DDD in 2005
|
| No increase in the prevalence of resistant pneumococci or Haemophilus influenzae in the county | |||||||||
| 7 | Zhang et al. (2017)13 | Tianjin, China | Effectiveness of antibiotic use management in Tianjin (2011–2013): a quasi-experimental study | Institution of regulation (antibiotic committees) | Quai-experimental study | 2011–13 | Antibiotic consumption (percentage of antibiotic use in inpatients; DDD per 100 patient days) | Moderate | Decrease in percentage of antibiotic use by inpatients (%): 60.38% in 2011 to 46.88% in 2013, P<0.000 |
| Decrease in DDD/100 patient days: 51.60 DDD in 2011 to 35.37 DDD in 2013, P<0.000 | |||||||||
| 8 | Mölstad et al. (1999)14 | Sweden | Major change in the use of antibiotics following a national programme: Swedish Strategic Programme for the Rational Use of Antimicrobial Agents and Surveillance of Resistance (STRAMA) | Institution of regulation (antibiotic committees) | Time series analysis | 1980–97 | Antibiotic consumption (DDD per 1000 inhabitants/day) | Moderate | Decrease in DDD per 1000 inhabitants/day: 16.3 DDD in 1993 to 13.0 DDD in 1997
|
| 9 | Mölstad et al. (2008)15 | Sweden | Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish STRAMA programme | Institution of regulation (antibiotic committees) | Time series analysis | 1987–2004 |
|
Moderate | Decrease in DDD per 1000 inhabitants/day: 15.7 DDD in 1995 to 12.6 DDD in 2004 |
Decrease in number of prescriptions per 1000 inhabitants per year: 536 prescriptions in 1995 to 410 prescriptions in 2004
| |||||||||
| National frequency of penicillin-resistant S. pneumoniae increased from 4% to 6% despite decrease in antibiotic use among children | |||||||||
| Resistance in S. pneumoniae also increased to erythromycin, tetracyclines and co-trimoxazole between 1994 and 2004 | |||||||||
| Rate of ampicillin-resistant E. coli in urinary cultures increased from 17% to 24%, trimethoprim-resistant E. coli increased from 8% to 15% from 1997–2004 | |||||||||
| 10 | Zou et al. (2014)16 | China | Is nationwide special campaign on antibiotic stewardship programme effective on ameliorating irrational antibiotic use in China? Study on the antibiotic use of specialized hospitals in China in 2011–2012 | Institution of regulation (antibiotic committees) | Pre–post study | 2011–12 | Antibiotic consumption (DDD per 100 inpatient days; percentage of antibiotic use in outpatient and inpatient cases) | High | Decrease in DDD per 100 patient days: 39.37 DDD in 2011 to 26.54 DDD in 2012, P<0.001 |
| Decrease in percentage of antibiotic use in outpatient cases: 24.12% in 2011 to 18.71% in 2012, P=0.109 | |||||||||
| Decrease in percentage of antibiotic use in inpatient cases: 64.85% in 2011 to 60.10% in 2012, P=0.006 | |||||||||
| 11 | Allouch et al. (2016)7 | Lebanon | Antibiotic use, cost, and consumption in tertiary hospitals in Lebanon: a comparative study before and after an implementation of antibiotic-restriction program (ARP) | Institution of regulation (clinical guidelines) | Retrospective cohort study | March–June 2013 |
|
Moderate | Decreases in proportional consumption of third-generation cephalosporins (19% to 12%, P<0.001), increase in the consumption of penicillin derivatives (24% to 28%, P<0.001). |
| Decrease in rate of restricted antibiotic use: 37.1% versus 26.1%, P<0.0001 | |||||||||
| 22.3% decrease in the expenditure on all antibiotics (P<0.001). | |||||||||
| 12 | Goosens et al. (2008)19 | Belgium | Achievements of the Belgian Antibiotic Policy coordination committee (BAPCOC) | Institution of regulation (clinical guidelines); public education campaigns | Time series analysis | 1999–present |
|
High | 90% of hospitals had key structural resources and tools in place for effective antibiotic management and infection control |
| 35% relative reduction from 2004 to 2008 in the incidence of nosocomial acquisition of MRSA among patients admitted to acute care hospitals | |||||||||
| 36% decrease in number of reimbursed packages per 1000 inhabitants per day | |||||||||
| Increased compliance with hand hygiene: 49% to 69% in 2005; 53% to 69% in 2007 | |||||||||
Decrease in resistance rates from 2000 to 2007
| |||||||||
| 13 | Tambić-Andršević (2009)20 | Croatia | Antibiotic resistance control in Croatia | Prescribing restrictions | Time series analysis | 2003–8 | Antibiotic consumption (DDD per 1000 inhabitants/day) | High | Decrease in DDD per 1000 inhabitants/day (outpatient): 23.6 DDD in 2003 to 22.6 DDD in 2008 |
| Decrease in DDD per 1000 inhabitants/day (inpatient): 2.5 DDD in 2002 to 1.5 DDD in 2008 | |||||||||
| 14 | Conly (2012)18 | Canada | Antimicrobial resistance programs in Canada 1995–2010: a critical evaluation | Prescribing restrictions; public education campaigns | Time series analysis | 1995–2010 | Antibiotic consumption (oral antimicrobial prescriptions per 1000 inhabitants) | High | Decrease in oral antimicrobial prescriptions per 1000 inhabitants: 25.3% decrease in prescriptions, driven by decreases in β-lactams, sulphonamides and tetracyclines |
| 15 | Sørensen and Monnet (2000)21 | Denmark | Control of antibiotic use in the community: the Danish experience | Prescribing restrictions | Time series analysis | 1995–6 | Antibiotic consumption (DDD per 1000 inhabitants; percentage of antimicrobial use) | High | Decrease in DDD per 1000 inhabitants: 4620 DDD per 1000 inhabitants in 1995 to 4122 DDD in 1996 |
| Decrease in tetracycline use: 578 DDD in 1995 to 391 DDD in 1996 | |||||||||
| Decrease in percentage of antimicrobial use: 4.5% reduction in the use of antimicrobials in the primary healthcare sector in 1998 to 1999 | |||||||||
| 16 | Fürst et al. (2015)22 | Slovenia | The influence of a sustained multifaceted approach to improve antibiotic prescribing in Slovenia during the past decade: findings and implications | Prescribing restrictions; public education campaigns | Time series analysis | 1995–2012 |
|
Moderate | Decrease in prescriptions per 1000 inhabitants: 791.29 prescriptions in 1999 to 525.97 prescriptions in 2012 |
| Decrease in DDD per 1000 inhabitants/day 20.38 DDD in 1999 to 14.01 DDD in 2012 (P<0.0001); driven by significant decreases in tetracyclines, phenoxymethylpenicillin, cephalosporins and macrolides | |||||||||
| S. pneumoniae resistance to penicillin decreased from 14.5% to 10%. | |||||||||
| S. pneumoniae resistance to macrolides increased from 5.4% to 21% | |||||||||
| 17 | Liou et al. (2015)23 | Taiwan, China | The primary resistance of Helicobacter pylori in Taiwan after the national policy to restrict antibiotic consumption and its relation to virulence factors—a nationwide study | Prescribing restrictions | Time series analysis | 1997–2008 |
|
Low | Decrease in DDD per 1000 inhabitants/day (1997 to 2008)
|
| Primary H. pylori levofloxacin resistance rose from 4.9% in 2000–7 to 8.3% in 2008–10 and to 13.4% in 2011–12, P=0.001 | |||||||||
| 18 | Duborija-Kovačević (2006)24 | Montenegro | Antibiotic prescribing policy of the Republic Health Insurance Fund of Montenegro in the period 2000–2004: effects of drug utilization reform strategy | Prescribing restrictions | Pre–post study | 2000–4 | Antibiotic consumption (DDD per 1000 inhabitants/day; percentage of antibiotic prescriptions) | High | Decrease in percentage of antibiotic prescription: lower by 12% in 2004 compared with 2000 |
Decrease in DDD per 1000 inhabitants/day: 14.57 DDD in 2004 to 2.80 DDD in 2000
| |||||||||
| 19 | Cheng et al. (2012)25 | Australia | Control of fluoroquinolone resistance through successful regulation | Prescribing restrictions | Time series analysis | 1992–2010 | Resistance rates | High | Quinolone resistance rates in disease-causing isolates of E. coli increased from 0.4% in 1992 to 4.9% in 2006. Since 2006, surveillance of isolates from community-acquired infections showed a decrease in resistance rates to 4.1% in 2008. |
| 20 | Marshall et al. (2006)26 | Canada | Impact of administrative restrictions on antibiotic use and expenditure in Ontario: time series analysis | Prescribing restrictions | Time series analysis | 1999–2002 | Antibiotic consumption (number of prescriptions per week) | Low | Changes in number of antibiotic prescriptions per week
|
| 21 | Kliemann et al. (2016)27 | Brazil | Socioeconomic determinants of antibiotic consumption in the state of São Paulo, Brazil: the effect of restricting over-the-counter sales | Prescribing restrictions | Time series analysis | 2008–12 | Antibiotic consumption (DDD per 1000 inhabitants/day) | Moderate | Decrease in DDD per 1000 inhabitants/day: 8.44 DDD in 2008 to 8.06 DDD in 2012 |
| 22 | Rivas and Alonso (2011)28 | Venezuela | Regulation of dispensing drugs and their effect on the consumption of antibiotics in Venezuela | Prescribing restrictions | Retrospective study | 2005–8 | Antibiotic consumption (DDD per 1000 inhabitants/day) | Moderate | Decrease in DDD per 1000 inhabitants/day (2005 to 2008)
|
| 23 | Chou et al. (2003)29 | Taiwan | Impact of separating drug prescribing and dispensing on provider behaviour: Taiwan’s experience | Separation of antibiotic prescribing from dispensing | Time series with control group | 1996–9 | Antibiotic prescription (probability of non-prescription) Average drug dispensing expenditure per visit | Low | No significant difference between control and experimental cities: 7% increase in non-prescription probability in antibiotics immediately after the policy was in place, but the effect diminished over time |
| Non-significant changes in average drug dispensing expenditure per visit | |||||||||
| 24 | Kim et al. (2016)30 | South Korea | Antibiotic control policies in South Korea, 2000–2013 | Separation of antibiotic prescribing from dispensing | Time series analysis | 1998–2008 | Antibiotic consumption (DDD per 1000 inhabitants/day) | High | Decrease in DDD per 1000 inhabitants/day: 28.8 DDD in 1998 to 22.8 DDD in 2008 |
| 25 | Belongia et al. (2005)31 | Wisconsin and Minnesota, USA | Impact of statewide programme to promote appropriate antimicrobial drug use | Campaigns for healthcare professionals; public education campaigns | Pre–post study with control | 1998–2003 | Antibiotic prescription; annual primary care prescriptions of antibiotics per physician | Low | Decrease in percentage of annual primary care prescriptions of antibiotics per physician (dividing the number of new filled prescriptions by the number of prescribers per year):
|
| 26 | Weiss et al. (2011)32 | Quebec, Canada | Impact of a multipronged education strategy on antibiotic prescribing in Quebec, Canada | Campaigns for healthcare professionals; public education campaigns | Time series analysis | 2003–7 | Antibiotic consumption (number of outpatient antibiotic prescriptions per 1000 inhabitants/day) | Moderate | Decrease in the number of outpatient antibiotic prescriptions per 1000 inhabitants/day (2003 to 2007):
|
| 27 | Chahwakilian et al. (2011)33 | France | Impact of the French campaign to reduce the inappropriate ambulatory antibiotic use on the prescription and consultation rates for respiratory tract infections | Campaigns for healthcare professionals; public education campaigns | Retrospective cohort study | 2001–9 |
|
Moderate | Decrease in DDD per 1000 inhabitants/day: 35.7 DDD in 2001 to 30.2 DDD in 2009 |
Decrease in the number of ambulatory antibiotic prescriptions per 1000 inhabitants/year
| |||||||||
| 23% decrease in the number of consultations for RTIs between 2001 and 2009 | |||||||||
| Decrease in the proportion of consultations resulting in antibiotic prescriptions: 58% in 2001 to 46% in 2009 | |||||||||
| 28 | Plachouras et al. (2014)34 | Corinth, Greece | Promoting prudent use of antibiotics: the experience from a multifaceted regional campaign in Greece | Campaigns for healthcare professionals; public education campaigns | Pre–post study with control | January–February 2009 | Antibiotic consumption (DDD per 1000 inhabitants/year; percentage of antibiotic use) | Moderate | Increase in DDD per 1000 inhabitants/year: 26 DDD (January–February 2009) to 32 DDD (March 2009) |
Changes in percentage of antibiotic use, P=0.02:
| |||||||||
| 29 | Bernier et al. (2014)35 | France | Outpatient antibiotic use in France between 2000 and 2010: after the nationwide campaign, it is time to focus on the elderly | Campaigns for healthcare professionals; public education campaigns | Time series analysis | 2000–10 | Antibiotic prescription (number of weekly antibiotic prescriptions per 1000 inhabitants) | Moderate | 30% (95% CI −36.3 to −23.8) decrease in weekly antibiotic prescriptions during campaign period; no significant differences during non-campaign period |
| 21% increase (95% CI 12.9%–29.6%) antibiotic consumption in seniors | |||||||||
| 30 | Hemo et al. (2009)36 | Israel | Can a nationwide media campaign affect antibiotic use? | Campaigns for healthcare professionals; public education campaigns | Pre–post study | 2004–5; 2005–6 | Antibiotic consumption (purchasing rates for upper respiratory infection, otitis media and pharyngitis) | Low | Decrease in antibiotic purchasing rates for:
|
| 31 | Lambert et al. (2007)37 | England | Can mass media campaigns change antimicrobial prescribing? A regional evaluation study | Public education campaigns | Pre–post study | 2004, 2005 | Antibiotic prescription; antibiotic prescriptions per 1000 STAR-PU (Specific Therapeutic group Age-sex Related Prescribing Units) | Moderate | 21.7 fewer items prescribed per 1000 population (P<0.0005); 5.8% absolute reduction in prescribing |
| 32 | Sabuncu et al. (2009)38 | France | Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007 | Public education campaigns | Time series analysis | 2002–7 | Antibiotic prescription; number of antibiotic prescriptions per 100 inhabitants | Low | 26.5% (95% CI −33.5 to −19.6) decrease in the number of antibiotic prescriptions per 100 inhabitants:
|
| 33 | Parsons et al. (2004)40 | England | Did local enhancement of a national campaign to reduce high antibiotic prescribing affect public attitudes and prescribing rates? | Public education campaigns | Time series analysis | 1995/6–99/2000 |
|
Moderate | Decrease in number of antibiotic prescriptions per 1000 patients: 686 1995/6 to 431 in 1999/2000; not significant |
| Decrease in the proportion of responders who believed that children should be prescribed antibiotics for a fever: 56% in 1995/6 to 49% in 1999/2000 | |||||||||
| 34 | Wutzke et al. (2006)39 | Australia | Evaluation of a national programme to reduce inappropriate use of antibiotics for upper respiratory tract infections: effects on consumer awareness, beliefs, attitudes and behaviour in Australia | Public education campaigns | Time series analysis | 2001–4 (June to August) | Antibiotic prescription (total antibiotic prescriptions dispensed in the community; total antibiotic prescriptions dispensed for upper respiratory tract infections | Moderate | Decrease in total antibiotic prescriptions in the community: 23.08 million antibiotic prescriptions in 1998/9 to 21.44 million in 2001/2 |
| Decrease in total antibiotic prescriptions for upper respiratory tract infections: 216 000 fewer prescriptions for upper respiratory tract infections from 2001 to 2003 |
Reducing inappropriate access to antibiotics
Interventions within this domain included the institution of antibiotic-specific regulations (12 studies), prescribing restrictions (10 studies), separation of drug prescribing from dispensing (2 studies) and educational campaigns for healthcare professionals (6 studies).
Institution of regulation (antibiotic committees and clinical guidelines)
Eleven studies in seven countries described establishment of national hospital antibiotic management teams as an intervention to address AMR. Responsibilities of hospital antibiotic management teams included training of healthcare professionals, surveillance of antibiotic consumption, prescription review, evaluation of hospital resources for infection control and development of clinical guidelines for antibiotic prescription. Additionally, two studies in two countries described the strengthening and dissemination of clinical guidelines for the prescription of antibiotics in both inpatient and outpatient settings.
Of the 11 studies, 10 found overall reductions in antibiotic use, while 1 found mixed results in terms of consumption levels; a decrease in consumption of third-generation cephalosporins after implementation of an antibiotic restriction programme was accompanied by an increase in consumption of penicillins.7 Other outcome measures included reductions in the number of antibiotic packages consumed and/or prescribed,8,9 seasonal variation in fluoroquinolone use,9 grams consumed per year,10 antibiotic sales value and volume,11,12 percentage of antibiotic use by inpatients13 as well as DDD.13–16 Three studies evaluated the link between antibiotic consumption and antibiotic resistance rates, calculated as the percentage of bacterial isolates displaying resistance,10,15,17 and found mixed results. At the institutional level, two studies found increased compliance with national antibiotic prescribing guidelines as well as better key structural resources for antibiotic management and infection control in hospitals.18–20
Restriction of prescribing practices
Two types of prescribing restrictions were described in 11 studies representing nine countries. The first was restrictions on reimbursement of antibiotic prescription (e.g. when antibiotics were prescribed as first-line treatment, or reduced reimbursement in general). These were described in six countries and covered the following antibiotics: methicillin,21 amoxicillin/clavulanic acid,22,23 third- and fourth-generation cephalosporins,22,24 fluoroquinolones,22,24–26 aminoglycosides, macrolides21–24 and tetracyclines.21,23 Although all six studies found significant overall decreases in antibiotic consumption (as expressed by DDD and number of weekly antibiotic prescriptions), one study24 found increases in macrolides and penicillin prescriptions. Of the six studies, two investigated the link between antibiotic consumption with resistance rates of Streptococcus pneumoniae and Helicobacter pylori, and found mixed results.
The remaining five studies evaluated the restriction of antibiotic purchases without a prescription. Four of the studies found reductions in antibiotic consumption after implementation,11,12,16,27 while one study found no changes in antibiotic consumption.28 All results were expressed in DDD.
Separation of antibiotic prescribing from dispensing
Two studies reported the impact of separating drug prescribing from drug dispensing in primary care to disincentivize profit making from prescribing.29,30 After implementation of the separation policy in both countries, reductions in antibiotic use, measured in DDD, and prescription were observed. However, in one study, this effect diminished over time and there was no significant reduction in overall antibiotic expenditure.
Campaigns for healthcare professionals
Six studies described campaigns for healthcare professionals in five countries. Campaigns included disseminating flyers and toolkits,18,22,31,32 conducting workshops and seminars on antibiotic prescription,22,31,33,34 providing individual prescribing feedback,31–33 promoting antibiotic prescribing guidelines in medical school education32 and sharing experiences among healthcare professionals.34 Outcome measures to evaluate the effectiveness of these campaigns included the number of annual primary care antibiotic prescriptions per physician,31 antibiotic prescriptions per 1000 inhabitants per year or month,32,33 DDD,22,34 percentage of patients receiving antibiotic prescriptions32,33 and expenditure on antibiotic prescriptions per 1000 inhabitants per month.32
Four studies showed overall decreases in the number of antibiotic prescriptions per physician at the primary care level,22,31–33 especially in prescribing for respiratory tract infections. One study found no significant changes in overall physician antibiotic prescribing post intervention, although there were increases in prescriptions for both amoxicillin and penicillin.34
Decreasing the demand for antibiotics
We found 13 studies from 10 countries describing national public education campaigns to increase awareness about appropriate antibiotic use, hand hygiene and vaccination. These included media campaigns using booklets, exhibits, flyers, posters, websites, newspapers, television or radio spots.18,19,31,32,35–39 Other platforms were also used to raise awareness about antibiotics, such as health information workshops and seminars in the community,18,22,32,40 childcare centres, primary schools and nursing homes.31,34 In addition to messages about antibiotic awareness, there were also hand hygiene campaigns for the prevention of nosocomial infections,19 and introduction of influenza vaccines for children.33
Outcome measures used to evaluate these campaigns included the number of reimbursed antibiotic packages, number of prescriptions per physician, volume of antibiotics distributed to retail outlets per capita, percentage of consultations resulting in antibiotic prescriptions and DDD. Using these measures, 10 studies found overall decreases in antibiotic consumption. Of these, two studies found notable decreases only during and immediately after the intervention period,35,40 two studies found decreases in antibiotic prescriptions for upper respiratory tract infections,39 including nasopharyngitis and influenza,33 and one study showed that the overall decrease was driven mostly by penicillin, macrolides and cephalosporins.38
One study34 found no overall change in antibiotic consumption (in DDD), but reported decreased consumption of macrolides, offset by increased consumption of amoxicillin and penicillin. Only one study19 linked antibiotic consumption with resistance rates and found reductions in penicillin-, tetracycline- and macrolide-resistant S. pneumoniae.
Heterogeneity in outcomes and outcome measures
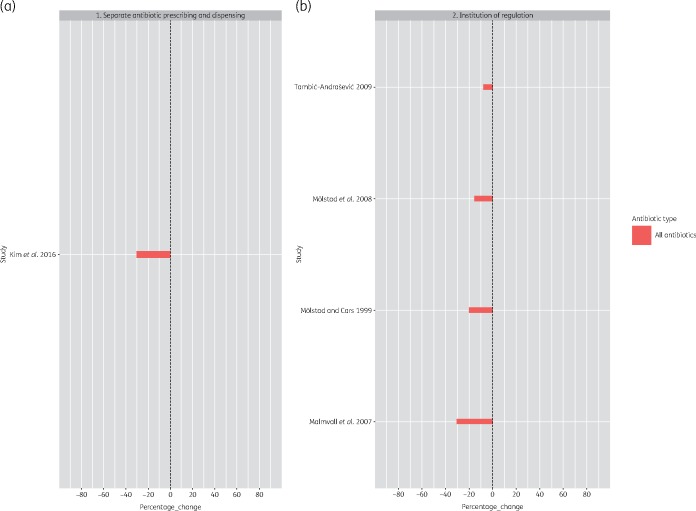
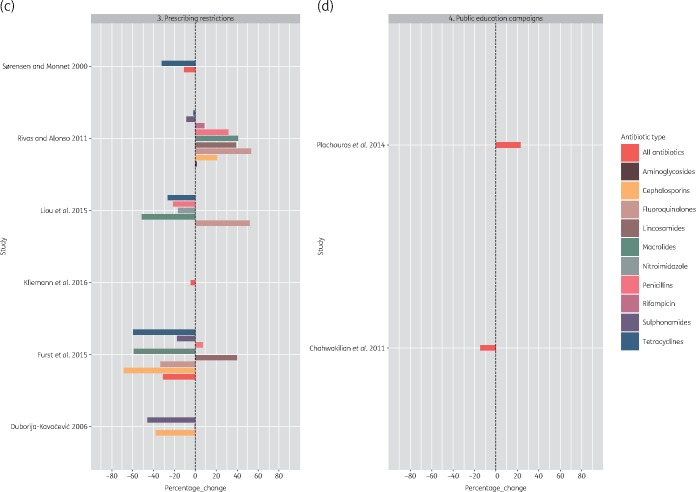
While individual studies in our review showed that national programmes had a positive impact on antibiotic use, it was challenging to evaluate the impact across time and geography owing to the heterogeneity in outcome measures used to quantify the impact of the programmes. Differences in programme outcomes between settings could stem from differences in study designs, data collection methods and the diverse number of outcome indicators used. The WHO recommends use of DDD to assess and compare trends in drug consumption between population groups.41 However, only 10 studies used this outcome measure (Figure 4).
Figure 4.
Percentage change in DDD per 1000 inhabitants per day post intervention. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 4.
Continued.
Discussion
In our systematic review of responsible use initiatives that have been implemented at the national and/or subnational level to address antibiotic resistance, we identified 34 articles that detailed interventions carried out in 21 high- and upper-middle-income countries. These interventions addressed both access to and demand for antibiotics, with the most common outcome measure of impact being changes in antibiotic consumption. Heterogeneity of study designs, populations, analytical strategies and effect measures meant it was not possible to conduct a meta-analysis. However, overall evidence suggested that interventions were effective in reducing antibiotic consumption in different countries to varying degrees.
However, summarizing evidence of the impact of national interventions from our review was challenging, because studies used a wide range of evaluation and outcome measures, such as compliance rates, resistance rates, general knowledge and perceptions surrounding antibiotic use and antibiotic resistance, and population levels of antibiotic consumption. The heterogeneous outcome measures used to evaluate the effectiveness of the intervention made comparison of results challenging and could pose potential difficulties in decision making or implementation of interventions.
While antibiotic consumption was the most common type of outcome reported, it was also measured in a number of different ways, including prescription rates, sales value or volume, number of prescriptions and DDD. Due to variability in specific outcome measures used, only 10 comparable studies evaluating the impact of national programmes on DDD were available. Standardized outcomes and outcome assessment tools would greatly enhance capacity to monitor global progress in addressing AMR, and allow better assessments of intervention effectiveness in different settings.
Additionally, although establishing a direct link between antibiotic consumption and resistance rates is a crucial aspect of evaluating national initiatives, we found only five studies in our review linking population-level antibiotic consumption to resistance levels, and these reported mixed results; in some studies, decreased antibiotic consumption did not lead to lowered resistance rates. Establishing an association between antibiotic consumption and resistance rates could help to determine the extent to which reducing antibiotic consumption is an effective intervention, as well as to assess how this compares with other types of interventions, such as improving vaccination coverage and reducing animal antibiotic use. However, the evidence on this remains limited.
There were other challenges in evaluating the success of national- or subnational-level AMR interventions. First, most of the studies included in our review were before–after evaluations. As such, secular changes independent of the intervention might have affected outcomes, such as changes in vaccination policies, or improvements in medical education. Second, reductions in overall population-level antibiotic consumption may not be synonymous with reductions in inappropriate antibiotic use. It was not possible from these data to determine how much inappropriate use was reduced by specific interventions. Further, comparison across countries was challenging as studies were done over different timescales and at different timepoints. Lastly, as most of the evaluations included in our review were not long term, it was difficult to assess whether reduction in antibiotic consumption from the intervention was sustained over time, whether it led to other adverse outcomes (e.g. increased prescribing of third-line antibiotics) or whether antibiotic consumption bounced back after a certain time period.
Strengths and limitations
In interpreting evidence from these national interventions, several limitations should be borne in mind. We were unable to exclude publication bias or reporting bias, which might have impacted the validity and generalization of our conclusions. Very few studies included in our review showed limited or no impact post intervention; the majority of them were examples of interventions that had a positive impact. We were also unable to identify evaluations of national programmes that were not published. Additionally, all the published studies evaluating national implementation of AMR interventions came from high- and upper-middle-income countries, despite the fact that increases in antibiotic consumption are largely driven by increased demand in LMICs.2 Previous evidence has shown that LMICs have different priorities and contextual issues, such as health system processes, patient demand, varying cultures of care, availability of universal access to quality antimicrobials, laboratory infrastructure and surveillance systems.42,43 In these settings, multipronged interventions combining different restrictive and enabling strategies are more likely to be effective.44
Although we were unable to review unpublished evidence, we sought to minimize potential biases due to language and geography as much as possible, by not applying any language restrictions in our review, and searching smaller, more regionally focused databases (e.g. WPRIM) to capture regional or geographically focused research that may not have been indexed in larger, standard academic databases. Similar to a preceding study,44 this review sought to bring together evidence on the types of government policy interventions and options in addressing AMR. One notable strength of this review is the focus on the population impact of evaluated national interventions to address AMR. This has important policy implications, because although the efficacy of many interventions to address AMR may have been determined in idealized trial conditions in specific settings, it is difficult to generalize their impact, even if they can be scaled up nationally. Published evaluations of national interventions using standardized methods should form a key component of national action plans to address AMR, so that information on what types of interventions work in different settings can be readily shared and adapted by other countries.
Conclusions
Based on the available evidence from primarily high-income countries, our systematic review highlights that strategies to reduce inappropriate demand and access to antibiotics appear to have a quantifiable impact primarily on antibiotic consumption, but more evidence is needed on the long-term impacts of these interventions, such as increases in the consumption of antibiotic subtypes, impacts on prevalence of antibiotic-resistant organisms, as well as the health and economic burden of these infections. It is also challenging to generalize interventions such as restricting access to non-prescription antibiotics to other settings where there may not currently be an adequate number of qualified prescribers in primary care, and where there may be adverse consequences in terms of restricting access to necessary antibiotics. More evidence is needed on the types of interventions that are relevant and effective in these settings. In addition, harmonizing the use of standardized outcome measures in the evaluation of national programmes addressing AMR is crucial to enable comparisons of interventions that are carried out in different settings, longer term and across different populations.
Funding
This work was supported by the Saw Swee Hock School of Public Health (SSHSPH) Antimicrobial Resistance Programme, National Medical Research Council Centre Grant (CG) Programme – Collaborative Solutions Targeting Antimicrobial Resistance Threats in Health System (CoSTAR-HS) AMR research grant (CGAug16C005).
Transparency declarations
None to declare.
Author contributions
All authors attest that they meet the ICMJE criteria for authorship. J. M. L. and C. C. T. conceived the idea for the manuscript and drafted the paper. All authors contributed to critically revise the manuscript and approved the final article.
References
- 1. Review on Antimicrobial Resistance. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations: Review on Antimicrobial Resistance 2016. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf.
- 2. Klein EY, Van Boeckel TP, Martinez EM. et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA 2018; 115: E3463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Action Plan on Antimicrobial Resistance 2015. https://www.who.int/antimicrobial-resistance/global-action-plan/en/. [DOI] [PubMed]
- 4. Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sterne JAC, Higgins JPT, Reeves BC; development group for ACROBAT-NRSI. A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI), Version 1.0.0, 24 September 2014. http://www.bristol.ac.uk/population-health-sciences/centres/cresyda/barr/riskofbias/robins-i/acrobat-nrsi/.
- 6. Fantom N, Serajuddin U.. The World Bank's Classification of Countries by Income: The World Bank 2016. 10.1596/1813-9450-7528. [DOI]
- 7. Allouch A, Sabbah H, Hassan S. et al. Antibiotic use, cost, and consumption in tertiary hospitals in Lebanon: a comparative study before and after an implementation of antibiotic-restriction program (ARP). BJMMR 2016; 12: 1–15. [Google Scholar]
- 8. Krcmery V, Gould IM.. Antibiotic policies in Central/Eastern Europe (CEE) after 1990. J Hosp Infect 1999; 43: S269–74. [DOI] [PubMed] [Google Scholar]
- 9. Nathwani D, Sneddon J, Malcolm W. et al. Scottish Antimicrobial Prescribing Group (SAPG): development and impact of the Scottish National Antimicrobial Stewardship Programme. Int J Antimicrob Agents 2011; 38: 16–26. [DOI] [PubMed] [Google Scholar]
- 10. Altunsoy A, Aypak C, Azap A. et al. The impact of a nationwide antibiotic restriction program on antibiotic usage and resistance against nosocomial pathogens in Turkey. Int J Med Sci 2011; 8: 339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tao J, Zhang T, Xu J. et al. Analysis of the current situation of antibiotics use in China: a hospital-based perspective. Drug Inf J 2013; 47: 23–31. [DOI] [PubMed] [Google Scholar]
- 12. Xiao Y, Zhang J, Zheng B. et al. Change in Chinese policies to promote the rational use of antibiotics. PLOS Med 2013; 10: e1001556.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang HH, Du Y, Liu W. et al. Effectiveness of antibiotic use management in Tianjin (2011–2013): a quasi-experimental study. Med Sci Monit 2017; 23: 725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mölstad S, Cars O.. Major change in the use of antibiotics following a national programme: Swedish Strategic Programme for the Rational Use of Antimicrobial Agents and Surveillance of Resistance (STRAMA). Scand J Infect Dis 1999; 31: 191–5. [DOI] [PubMed] [Google Scholar]
- 15. Mölstad S, Erntell M, Hanberger H. et al. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish Strama programme. Lancet Infect Dis 2008; 8: 125–32. [DOI] [PubMed] [Google Scholar]
- 16. Zou X, Fang Z, Min R. et al. Is nationwide special campaign on antibiotic stewardship program effective on ameliorating irrational antibiotic use in China? Study on the antibiotic use of specialized hospitals in China in 2011–2012. J Huazhong Univ Sci Technol [Med Sci] 2014; 34: 456–63. [DOI] [PubMed] [Google Scholar]
- 17. Malmvall BE, Mölstad S, Darelid J. et al. Reduction of antibiotics sales and sustained low incidence of bacterial resistance: report on a broad approach during 10 years to implement evidence-based indications for antibiotic prescribing in Jönköping County, Sweden. Qual Manag Health Care 2007; 16: 60–7. [PubMed] [Google Scholar]
- 18. Conly JM. Antimicrobial resistance programs in Canada 1995–2010: a critical evaluation. Antimicrob Resist Infect Control 2012; 1: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goosens H, Coenen S, Costers M. et al. Achievements of the Belgian antibiotic policy coordination committee (BAPCOC). Euro Surveill 2008; 13: pii=19036. [PubMed] [Google Scholar]
- 20. Tambić-Andrašević A. Antibiotic resistance control in Croatia. Infektološki Glasnik 2009; 29: 145–50. [Google Scholar]
- 21. Sørensen TL, Monnet D.. Control of antibiotic use in the community: the Danish experience. Infect Control Hosp Epidemiol 2000; 21: 387–9. [DOI] [PubMed] [Google Scholar]
- 22. Fürst J, Čižman M, Mrak J. et al. The influence of a sustained multifaceted approach to improve antibiotic prescribing in Slovenia during the past decade: findings and implications. Expert Rev Anti Infect Ther 2015; 13: 279–89. [DOI] [PubMed] [Google Scholar]
- 23. Liou JM, Chang CY, Chen MJ. et al. The primary resistance of Helicobacter pylori in Taiwan after the national policy to restrict antibiotic consumption and its relation to virulence factors—a nationwide study. PLoS One 2015; 10: e0124199.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duborija-Kovačević N. Antibiotic prescribing policy of the Republic Health Insurance Fund of Montenegro in the period 2000–2004: effects of drug utilization reform strategy. Medicinski Pregled 2006; 59: 235–40. [DOI] [PubMed] [Google Scholar]
- 25. Cheng AC, Turnidge J, Collignon P. et al. Control of fluoroquinolone resistance through successful regulation. Emerg Infect Dis 2012; 18: 1453.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marshall D, Gough J, Grootendorst P. et al. Impact of administrative restrictions on antibiotic use and expenditure in Ontario: time series analysis. J Health Serv Res Policy 2006; 11: 13–20. [DOI] [PubMed] [Google Scholar]
- 27. Kliemann BS, Levin AS, Moura ML. et al. Socioeconomic determinants of antibiotic consumption in the state of São Paulo, Brazil: the effect of restricting over-the-counter sales. PLoS One 2016; 11: e0167885.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rivas P, Alonso G.. Regulación de la dispensación de medicamentos y su efecto en el consumo de antibióticos en Venezuela. Rev Panam Salud Publica 2011; 30: 592–7. [DOI] [PubMed] [Google Scholar]
- 29. Chou Y, Yip WC, Lee CH. et al. Impact of separating drug prescribing and dispensing on provider behaviour: Taiwan’s experience. Health Policy Plan 2003; 18: 316–29. [DOI] [PubMed] [Google Scholar]
- 30. Kim BN, Kim BH, Oh MD.. Antibiotic control policies in South Korea, 2000–2013. Infect Chemother 2016; 48: 151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belongia EA, Knobloch MJ, Kieke BA. et al. Impact of statewide program to promote appropriate antimicrobial drug use. Emerg Infect Dis 2005; 11: 912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weiss K, Blais R, Fortin A. et al. Impact of a multipronged education strategy on antibiotic prescribing in Quebec, Canada. Clin Infect Dis 2011; 53: 433–9. [DOI] [PubMed] [Google Scholar]
- 33. Chahwakilian P, Huttner B, Schlemmer B. et al. Impact of the French campaign to reduce inappropriate ambulatory antibiotic use on the prescription and consultation rates for respiratory tract infections. J Antimicrob Chemother 2011; 66: 2872–9. [DOI] [PubMed] [Google Scholar]
- 34. Plachouras D, Antoniadou A, Giannitsioti E. et al. Promoting prudent use of antibiotics: the experience from a multifaceted regional campaign in Greece. BMC Public Health 2014; 14: 866.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bernier A, Elisabeth DA, Ligier C. et al. Outpatient antibiotic use in France between 2000 and 2010: after the nationwide campaign, it is time to focus on the elderly. Antimicrob Agents Chemother 2014; 58: 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hemo B, Shamir-Shtein NH, Silverman BG. et al. Can a nationwide media campaign affect antibiotic use? Am J Manag Care 2009; 15: 529–34. [PubMed] [Google Scholar]
- 37. Lambert MF, Masters GA, Brent SL.. Can mass media campaigns change antimicrobial prescribing? A regional evaluation study. J Antimicrob Chemother 2007; 59: 537–43. [DOI] [PubMed] [Google Scholar]
- 38. Sabuncu E, David J, Bernède-Bauduin C. et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLoS Med 2009; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wutzke SE, Artist MA, Kehoe LA. et al. Evaluation of a national programme to reduce inappropriate use of antibiotics for upper respiratory tract infections: effects on consumer awareness, beliefs, attitudes and behaviour in Australia. Health Promot Int 2006; 22: 53–64. [DOI] [PubMed] [Google Scholar]
- 40. Parsons S, Morrow S, Underwood M.. Did local enhancement of a national campaign to reduce high antibiotic prescribing affect public attitudes and prescribing rates? Eur J Gen Pract 2004; 10: 18–23. [DOI] [PubMed] [Google Scholar]
- 41. Patrick DM, Marra F, Hutchinson J. et al. Per capita antibiotic consumption: how does a North American jurisdiction compare with Europe? Clin Infect Dis 2004; 39: 11–7. [DOI] [PubMed] [Google Scholar]
- 42. Tomson G, Ioana V.. The need to look at antibiotic resistance from a health systems perspective. Ups J Med Sci 2014; 119: 117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wilkinson A, Ebata A, MacGregor H.. Interventions to reduce antibiotic prescribing in LMICs: a scoping review of evidence from human and animal health systems. Antibiotics 2019; 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van Katwyk SR, Grimshaw JM, Nkangu M. et al. Government policy interventions to reduce human antimicrobial use: a systematic review and evidence map. PLoS Med 2019; 16: e1002819. [DOI] [PMC free article] [PubMed] [Google Scholar]