Abstract
NGLY1 deficiency is a rare inherited disorder caused by mutations in the NGLY1 gene encoding N-glycanase 1 that is a hydrolase for N-linked glycosylated proteins. An induced pluripotent stem cell (iPSC) line was generated from the dermal fibroblasts of a 16-year-old patient with homozygous mutation of p.R401X (c.1201 A >T) in the NGLY1 gene. Our iPSC model offers a useful resource to study the disease pathophysiology and to develop therapeutics for treatment of NGLY1 patients.
Resource utility
This TRNDi010-C iPSC line presents a patient-specific disease model for studies of NGLY1 deficiency phenotype and pathophysiology and can be used as a cell-based model for drug discovery and therapeutic development to treat NGLY1 patients.
Resource details
NGLY1 deficiency, also known as NGLY1-related congenital disorder of deglycosylation, is a rare autosomal recessive disorder caused by mutations in the NGLY1 gene which encodes a specialized enzyme called N-glycanase that removes N-linked glycan from glycosylated proteins within the body. Deficiency of this protein can lead to malfunctions of cellular functions and accumulation of misfolded proteins within cells in specific tissues or organs. The symptoms and severity of this disease can dramatically vary among affected individuals, who may have developmental delays, intellectual disability, movement disorders, seizures, liver disease, and alacrima (Enns et al., 2014; Lam et al., 2017; Caglayan et al., 2015).
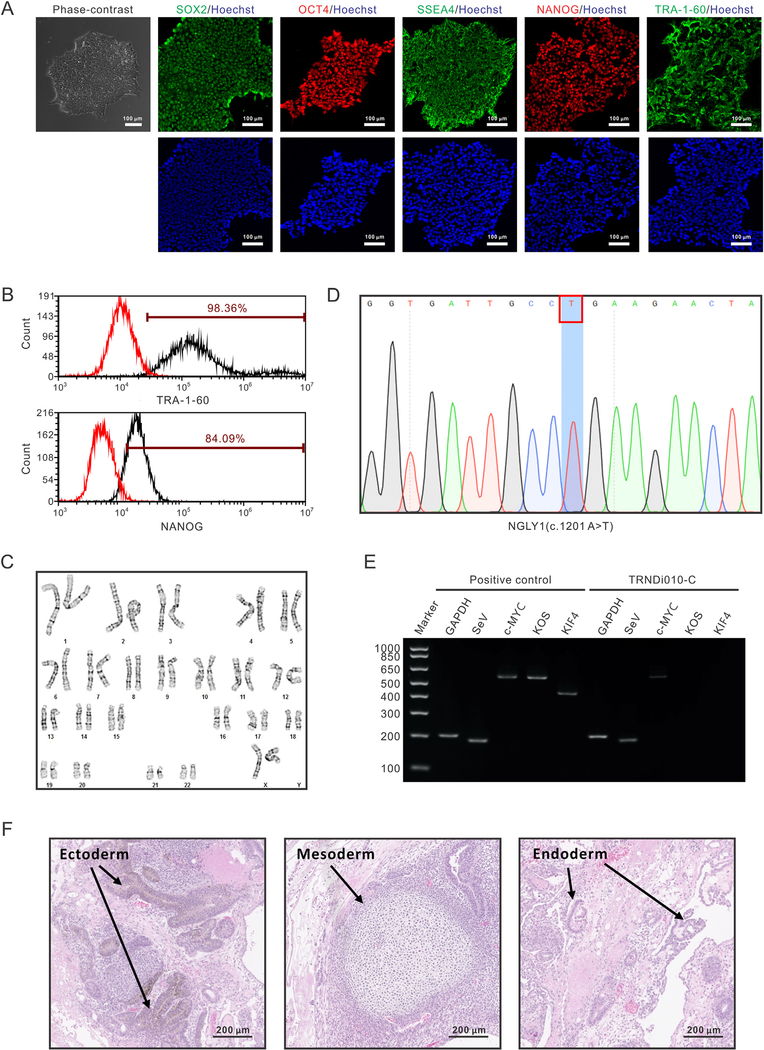
In this study, a human dermal fibroblast was derived from a 16-year-old female patient (GM26612, Coriell Institute) with a homozygous nonsense mutation of p.R401X (c.1201A > T) in exon 8 of the NGLY1 gene (3p24.2) (Enns et al., 2014; Need et al., 2012). The iPSC line, TRNDi010-C, was reprogramed from the fibroblasts using the non-integrating CytoTune-Sendai viral vector kit (A16517, Thermo Fisher Scientific) containing four pluripotency transcription factors, OCT3/4, KLF4, SOX2, and c-MYC (Beers et al., 2015). Individual colonies were picked, expanded and further analyzed at the cellular and genetic level to confirm successful reprogramming (Table 1). The TRNDi010-C iPS cells displayed the standard pluripotent stem cell morphology under phase contrast microscopy and expressed pluripotency markers OCT4, NANOG and SOX2 in the nuclei and SSEA4 and TRA-1–60 on the plasma membrane (Fig. 1A). The quantitative analysis by flow cytometry revealed 98.36% and 84.09% expression rate of TRA-1–60 and NANOG, respectively (Fig. 1B). G-banded karyotyping analysis was used to confirm the karyotype, which showed the normal diploid 46, XX, without any detectable abnormalities (Fig. 1C). The genetic mutation, c.1201A > T (p.R401X), was validated by Sanger sequencing of the PCR product harboring the single nucleotide variant (Fig. 1D), consistent with the description of Coriell Institute. After passage 30, the exogenous reprogramming factors were eliminated from TRNDi010-C iPSCs, despite the remaining low level of SeV (Fig. 1E). To further test the pluripotency of this iPSC line, teratoma formation experiment was performed. As shown in Fig. 1F, the imaging data identified its ability to generate derivative of three germ layers, ectoderm, mesoderm and endoderm in vivo. Furthermore, this iPSC was negative for mycoplasma contamination (Supplementary Fig. S1). The STR DNA profile of the TRNDi010-C matched with its parental GM26612 fibroblast at all 18 loci (information available with the authors).
Table 1.
Characterization and validation.
| Classification | Test | Result | Data |
|---|---|---|---|
| Morphology | Photography | Normal | Fig. 1 Panel A |
| Phenotype | Immunocytochemistry | SOX2, OCT4, NANOG, SSEA-4, TRA-1–60 | Fig. 1 Panel A |
| Flow cytometry | TRA-1–60 (98.36%); NANOG (84.09%) | Fig. 1 Panel B | |
| Genotype | Karyotype (G-banding) and resolution | 46XX Resolution: 350–400 |
Fig. 1 Panel C |
| Identity | Microsatellite PCR (mPCR) OR | Not performed | N/A |
| STR analysis | 18 sites tested, all sites matched | Available with the authors | |
| Mutation analysis (IF APPLICABLE) | Sequencing | Homozygous mutation NGLY1, p.R401X |
Fig. 1 Panel D |
| Southern Blot OR WGS | N/A | N/A | |
| Microbiology and virology | Mycoplasma | Mycoplasma testing by luminescence. Negative | Supplementary Fig. S1 |
| Differentiation potential | Teratoma formation | Teratoma with three germ layers formation, ectoderm, mesoderm and endoderm. | Fig. 1 Panel F |
| Donor screening (OPTIONAL) | HIV 1 + 2 Hepatitis B, Hepatitis C | N/A | N/A |
| Genotype additional info (OPTIONAL) | Blood group genotyping | N/A | N/A |
| HLA tissue typing | N/A | N/A |
Fig. 1.
Materials and methods
Cell culture and reprogramming
Patient skin fibroblasts were obtained from Coriell Cell Repositories (GM26612), and cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/mL penicillin and 100 μg/mL streptomycin in a humidified incubator with 5% CO2 at 37 °C. Patient fibroblasts were reprogrammed into iPSCs using the non-integrating Sendai virus technology (Beers et al., 2015). Human iPSCs were cultured in mTeSR™1 (STEMCELL Technologies) on Matrigel (Corning, 354277)-coated plates at 37 °C in humidified air with 5% CO2 and 5% O2. The cells were passaged with ReLeSR™ (STEMCELL Technologies) at generally 1:10 ratio when they reached around 70% confluency.
Genome analysis
The genome analysis of variants in NGLY1 was conducted through Applied StemCell (Milpitas, California, USA). Briefly, genomic DNA was extracted from iPSC line TRNDi010-C using QuickExtract™ DNA Extraction Solution (Lucigen) followed by PCR amplification using MyTaq™ Red Mix (Bioline, Taunton, MA). Amplifications were carried out on T00 Thermal Cycler from Bio-Rad (#1861096) using the following program: 95 °C, 2 min; 35 cycles of [95 °C, 15 s; 60 °C, 15 s; 72 °C, elongation duration varies by amplicon size], 72 °C 5 min; 4 °C, indefinite. Genotyping of the homozygous for the p.R401X variant (c.1201 A > T) in exon 8 of the NGLY1 gene was performed using Sanger sequencing analysis. The specific primers for gene amplification and sequencing are listed in Table 2.
Table 2.
Reagents details.
| Antibodies used for immunocytochemistry/flow-cytometry | |||
|
| |||
| Antibody | Dilution | Company Cat # and RRID | |
|
| |||
| Pluripotency Markers | Mouse anti-SOX2 | 1:50 | R & D systems, Cat# MAB2018, RRID: AB_358009 |
| Pluripotency Markers | Rabbit anti-NANOG | 1:400 | Cell signaling, Cat# 4903, RRID: AB_10559205 |
| Pluripotency Markers | Rabbit anti-OCT4 | 1:400 | Thermo Fisher, Cat# A13998, RRID: AB_2534182 |
| Pluripotency Markers | Mouse anti-SSEA4 | 1:1000 | Cell signaling, Cat# 4755, RRID: AB_1264259 |
| Pluripotency Markers | Mouse anti- TRA-1–60 | 1:1000 | Cell signaling, Cat# 4746, RRID: AB_2119059 |
| Secondary Antibodies | Donkey anti-Mouse IgG (Alexa Fluor 488) | 1:400 | Thermo Fischer, Cat# A21202, RRID: AB_141607 |
| Secondary Antibodies | Donkey anti-Rabbit IgG (Alexa Fluor 594) | 1:400 | Thermo Fischer, Cat# A21207, RRID: AB_141637 |
| Flow Cytometry Antibodies | Anti-Tra-1–60-DyLight 488 | 1:50 | Thermo Fischer, Cat# MA1–023-D488X, RRID: AB_2536700 |
| Flow Cytometry Antibodies | Anti-Nanog-Alexa Fluor 488 | 1:50 | Millipore, Cat# FCABS352A4, RRID: AB_10807973 |
| Flow Cytometry Antibodies | Mouse-IgM-DyLight 488 | 1:50 | Thermo Fischer, Cat# MA1–194-D488, RRID: AB_2536969 |
| Flow Cytometry Antibodies | Rabbit IgG-Alexa Fluor 488 | 1:50 | Cell Signaling, Cat# 4340S, RRID: AB_10694568 |
|
| |||
| Primers | |||
|
| |||
| Target | Forward/Reverse primer (5′–3′) | ||
|
| |||
| Sev specific primers (RT-PCR) | Sev/181 bp | F: GGA TCA CTA GGT GAT ATC GAG C R: ACC AGA CAA GAG TTT AAG AGA TAT GTA TC |
|
| Sev specific primers (RT-PCR) | KOS/528 bp | F: ATG CAC CGC TAC GAC GTG AGC GC R: ACC TTG ACA ATC CTG ATG TGG |
|
| Sev specific primers (RT-PCR) | Klf4/410 bp | F: TTC CTG CAT GCC AGA GGA GCC C R: AAT GTA TCG AAG GTG CTC AA |
|
| Sev specific primers (RT-PCR) | C-Myc/523 bp | F: TAA CTG ACT AGC AGG CTT GTC G R: TCC ACA TAC AGT CCT GGA TGA TGA TG |
|
| House-Keeping gene (RT-PCR) | GAPDH/197bp | F: GGA GCG AGA TCC CTC CAA AAT R: GGC TGT TGT CAT ACT TCT CAT GG |
|
| Targeted mutation analysis (PCR) | NGLY1 (c.1201A > T)/258bp | F: GAC AAC AGA GCG AGA CTT C R: AAA AAG ATA GCC ACA CCA TAC C |
|
Immunocytochemistry
iPSC colonies, cultured in the 96-well plate, were washed with Dulbecco’s phosphate-buffered saline (DPBS) without Ca2+ and Mg2+ and fixed in 4% paraformaldehyde for 15 min at room temperature. Fixed cells were washed with DPBS twice, and permeabilized with 0.1% Triton X-100 in DPBS for 15 min. After 1 h of blocking, the cells were incubated with primary antibodies, diluted in the blocking buffer, for overnight at 4 °C. Cells were washed twice with DPBS and a corresponding secondary antibody conjugated with Alexa Fluor 488 or Alex Fluor 647 was added to the cells and incubated for 1 h at room temperature (Antibodies used are listed in Table 2). Cells were then stained with Hoechst 33342 for 15 min and imaged using an INCell Analyzer 2500 imaging system (GE Healthcare) with Cy5, FITC and DAPI filter sets.
Flow cytometry analysis
The iPSCs were dissociated by TrypLE Express enzyme (Thermo Fisher Scientific). After washing once with DPBS, cells were fixed with 4% paraformaldehyde for 10 min and were permeabilized with 0.2% Tween-20 in DPBS for another 10 min at room temperature, followed by staining with fluorophore-conjugated antibodies (Table 2) for 1 h at 4 °C. The cells were then analyzed on a BD AccuriC6 FlowCytometry system (BD Biosciences).
G-banded karyotyping
The G-banded karyotyping analysis was performed by the WiCell Research Institute (Madison, WI) using the iPS cells at passage 6. Twenty randomly selected metaphase cells were selected for the standard cytogenetic analysis.
Short tandem repeat (STR) analysis
The STR analysis of patient fibroblasts and iPSCs was performed by the Johns Hopkins University Genetic Resources Core Facility using the Promega PowerPlex 18D Kit. The ABI Prism® 3730xl Genetic Analyzer was used to electrophorese the PCR products and GeneMapper® v 4.0 software (Applied Biosystems) was used to analyze the data.
Mycoplasma detection
The Lonza MycoAlert kit was used to assess the mycoplasma according to the instructions from the company. B/A ratio > 1.2 indicates the positive sample; 0.9–1.2 indicates the ambiguous result; < 0.9 indicates the negative sample.
Sendai virus detection
Using the RNeasy Plus Mini Kit (Qiagen), the total RNA was extracted. The cDNA was reverse-transcribed from 1 μg RNA by SuperScript™ III First-Strand Synthesis SuperMix (Thermo Fisher Scientific). The Platinum II Hot-Start PCR Master Mix (Thermo Fisher Scientific) was used to amplify the target sequence with a PCR program: 94 °C, 2 min; 30 cycles of 94 °C, 15 s, 60 °C, 15 s, and 68 °C, 15 s on Mastercycler pro S (Eppendorf) with the specific primers (Table 2). The human fibroblasts (GM05659, Coriell Institute) transfected with Sendai virus for 4 days was used as the positive control.
Teratoma formation assay
Patient iPSCs were dissociated with 0.5 mM EDTA in PBS and were resuspended approximately 1 × 107 cells in 400 μL culture medium supplied with 25 mM HEPES (pH 7.4) and stored on ice. Then, 50% volume (200 μL) of cold Matrigel (Corning, 354277) was added and mixed with the cells. The mixture was injected subcutaneously into NSG mice (JAX No. 005557) at 150 μL per injection site. Visible tumors were removed 6–8 weeks post-injection and were immediately fixed in 10% Neutral Buffered Formalin. The fixed tumors were embedded in paraffin and stained with hematoxylin and eosin.
Supplementary Material
Key resources table.
| Unique stem cell line identifier | TRNDi010-C |
| Alternative name(s) of stem cell line | HT592C |
| Institution | National Institutes of Health National Center for Advancing Translational Sciences Bethesda, Maryland, USA |
| Contact information of distributor | Dr. Wei Zheng, Wei.Zheng@nih.gov |
| Type of cell line | iPSC |
| Origin | Human |
| Additional origin info | Age: 16-year-old Sex: Female Ethnicity: Caucasian |
| Cell Source | Skin fibroblasts |
| Clonality | Clonal |
| Method of reprogramming | Integration-free Sendai viral vectors |
| Genetic Modification | Yes |
| Type of Modification | Hereditary |
| Associated disease | NGLY1 Deficiency |
| Gene/locus | NGLY1R401X |
| Method of modification | N/A |
| Name of transgene or resistance | N/A |
| Inducible/constitutive system | N/A |
| Date archived/stock date | 04–23–2018 |
| Cell line repository/bank | N/A |
| Ethical approval | NIGMS Informed Consent Form was obtained from patient at time of sample submission. Confidentiality Certificate: CC-GM-15–004 |
Acknowledgment
We want to thank Dr. Zu-xi Yu of the Pathology Core of National Heart, Lung and Blood Institute, National Institutes of Health for sectioning and staining the teratoma. We also would like to thank Research Services Section at National Center for Advancing Translational Sciences for coordinating the STR DNA analysis and mycoplasma testing service. This work was supported by the Intramural Research Program of the National Center for Advancing Translational Sciences, National Institutes of Health, and was a CRADA collaboration between NCATS, NGLY1.org, and Retrophin.
Footnotes
Declaration of Competing Interests
No potential conflicts of interest were disclosed.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scr.2019.101496.
References
- Beers J, Linask KL, Chen JA, Siniscalchi LI, Lin Y, Zheng W, Rao M, Chen G, 2015. A cost-effective and efficient reprogramming platform for large-scale production of integration-free human induced pluripotent stem cells in chemically defined culture. Sci. Rep 5, 11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglayan AO, Comu S, Baranoski JF, Parman Y, Kaymakcalan H, Akgumus GT, Caglar C, Dolen D, Erson-Omay EZ, Harmanci AS, Mishra-Gorur K, Freeze HH, Yasuno K, Bilguvar K, Gunel M, 2015. NGLY1 mutation causes neuromotor impairment, intellectual disability, and neuropathy. Eur. J. Med. Genet 58, 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns GM, Shashi V, Bainbridge M, Gambello MJ, Zahir FR, Bast T, Crimian R, Schoch K, Platt J, Cox R, Bernstein JA, Scavina M, Walter RS, Bibb A, Jones M, Hegde M, Graham BH, Need AC, Oviedo A, Schaaf CP, Boyle S, Butte AJ, Chen R, Chen R, Clark MJ, Haraksingh R, Consortium FC, Cowan TM, He P, Langlois S, Zoghbi HY, Snyder M, Gibbs RA, Freeze HH, Goldstein DB, 2014. Mutations in NGLY1 cause an inherited disorder of the endoplasmic reticulum-associated degradation pathway. Genet. Med 16, 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C, Ferreira C, Krasnewich D, Toro C, Latham L, Zein WM, Lehky T, Brewer C, Baker EH, Thurm A, Farmer CA, Rosenzweig SD, Lyons JJ, Schreiber JM, Gropman A, Lingala S, Ghany MG, Solomon B, Macnamara E, Davids M, Stratakis CA, Kimonis V, Gahl WA, Wolfe L, 2017. Prospective phenotyping of NGLY1-CDDG, the first congenital disorder of deglycosylation. Genet. Med 19, 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need AC, Shashi V, Hitomi Y, Schoch K, Shianna KV, McDonald MT, Meisler MH, Goldstein DB, 2012. Clinical application of exome sequencing in undiagnosed genetic conditions. J. Med. Genet 49, 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.