Abstract
Two environmental factors, crystalline silica (cSiO2), a toxic airborne particle encountered occupationally, and docosahexaenoic acid (DHA), a dietary omega-3 highly unsaturated fatty acid (HUFA), have the potential to influence the development of systemic lupus erythematosus (lupus). Using the NZBWF1 mouse, which spontaneously develops lupus, we found that intranasal exposure to cSiO2 significantly decreases latency and promotes rapid progression of the disease. Specifically, cSiO2 induces the development of ectopic lymphoid structures (ELS) containing germinal centers in the lungs that yield vigorous and diverse autoantibody responses locally and systemically. Transcriptomic analysis revealed that cSiO2 promotes a robust type I interferon gene signature that likely precipitates ELS neogenesis. Intriguingly, dietary supplementation with human-relevant doses of DHA impedes cSiO2-induced gene expression, ELS neogenesis, autoantibody elevation, and glomerulonephritis in this lupus-prone mouse model. Together our findings point to the feasibility of enhancing tissue omega-3 HUFAs as a personalized nutritional intervention to impede onset and progression of environment-triggered autoimmune disease.
Keywords: Autoimmunity, systemic lupus erythematosus, ectopic lymphoid structure, tertiary lymphoid organ, omega-3 fatty acid, silica, lung
Lupus results from gene-environment interactions
Systemic lupus erythematosus (lupus) is a prototypical autoimmune disease characterized by inflammation and a loss of self-tolerance. There are an estimated 250,000 patients in the US with lupus,1 and this is likely an underestimate, as lupus is frequently misdiagnosed.2 This devastating disease disproportionately impacts women of child-bearing age and of non-Caucasian descent. Lupus begins with benign but detectable elevations in plasma autoantibodies and inflammatory biomarkers, but eventually develops into full-scale systemic autoimmune disease. Rampant autoantibodies form immune complexes with their cognate autoantigens and can deposit in the kidney, triggering glomerulonephritis and ultimately renal failure if left untreated. While the genome is the primary predisposing factor for autoimmunity, environmental factors such as toxicants3 and diet4 modulate underlying hereditary susceptibilities, playing major roles in the development and/or exacerbation of symptoms. To illustrate the interactive actions of such factors, we review here investigations in a widely used preclinical mouse model of lupus demonstrating that 1) early autoimmune disease onset can be triggered by the respiratory toxicant crystalline silica (cSiO2) and 2) this triggering can be ameliorated by consumption of the dietary omega-3 fatty acid docosahexaenoic acid (DHA).
cSiO2 is an autoimmune trigger
SiO2 is the most abundant mineral on earth5 and an estimated 2.3 million Americans are exposed to cSiO2 occupationally.6 Exposure to cSiO2 is critical to the development of the human disease silicosis, which presents as chronic inflammation with severe fibrosis in the lungs and culminates in the loss of lung function.7 High levels of cSiO2 and other silicates are present in the lungs of miners exposed to high levels of coal dust containing cSiO2, indicating that the dust cannot be cleared from the lungs following chronic exposure.8 Clearance of inhaled cSiO2 from the lung has also been shown to be extremely slow in rodents,9 thus repeated inhalation of cSiO2 activates a persistent inflammatory response in the lungs.10 Continued over-activation of immune cells coupled with the release of cellular material following cSiO2-induced cell death results in production of antibodies against host antigens, contributing to the development of systemic autoimmunity.11,12
The etiological linkage between cSiO2 exposure and the development of autoimmunity is supported by human epidemiological studies, as reviewed by Parks et al.13 The review’s authors concluded that existing data met the Sir Bradford Hill criteria for establishing causality14; specifically, there was strength and consistency in the association between cSiO2 exposure and lupus. This was observed across different study designs and populations, there was an obvious exposure-response gradient, and epidemiological findings are consistent with mechanistic studies in animals. More recently, a National Institute for Environmental Health Sciences expert panel evaluated the role of environmental factors in autoimmune disease and concluded that cSiO2 contributes to multiple autoimmune diseases, including lupus, rheumatoid arthritis, systemic sclerosis, and ANCA vasculitis.3
Omega-3 HUFAs counter inflammation and autoimmunity
Consumption of omega-3 highly unsaturated fatty acids (HUFAs) has been shown to ameliorate chronic inflammatory and autoimmune diseases.15–17 The major omega-3 HUFAs eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA) are abundant in oily fish. Since the Western diet is lacking in fish and, in general, marine fatty acids,18,19 the most effective way to increase omega-3 HUFAs is through dietary supplementation.20,21 Indeed, after multivitamins, fish oil is the second most widely used supplement in the US.22 Crude fish oil or semi-purified mixtures of EPA, DPA, and DHA have been utilized in clinical trials for cardiovascular disease, inflammatory diseases, and autoimmune diseases,23 including lupus (reviewed elsewhere24). While there have been many positive findings, there are also inconclusive results, leaving many open questions about the dose required to see beneficial effects as well as the mechanisms behind omega-3 HUFAs’ protective actions.
Preclinical evidence that dietary DHA consumption prevents cSiO2-triggered lupus
To investigate contributions of the exposome to onset and progression of lupus, we have employed the NZBWF1 mouse, a widely used animal model that mimics human lupus.25,26 As in humans, lupus development in NZBWF1 mice primarily occurs in females. These mice develop robust autoantibody responses and lupus nephritis around 32 weeks of age and typically will die within a year. Intriguingly, when these mice are intranasally instilled with cSiO2, they develop lupus about 3 months earlier than vehicle-treated animals.12 Our lab has focused on understanding the early inflammatory events that lead to cSiO2-triggered onset of lupus in this model, as well as the potential to block cSiO2-triggered inflammation using dietary intervention with the omega-3 HUFA DHA.12,27,28 In our most recently published study, 6-week old female NZBWF1 mice were introduced to diets supplemented with DHA- enriched algal oil, providing 0, 0.4 or 1% DHA per day, which are calorically equivalent to human doses of 0, 2 or 5 grams/day, respectively.29 These mice were intranasally instilled with 1 mg cSiO2 at 8, 9, 10, and 11 weeks of age. Cohorts were then sacrificed at 1, 5, 9, and 13 weeks after the final instillation and bronchoalveolar lavage fluid (BALF), lungs, kidneys, and blood were collected for analysis. Mice exposed to cSiO2 developed glomerulonephritis at 13 weeks post-instillation, but this response was blocked by DHA feeding.
DHA’s protective effects against cSiO2-induced kidney inflammation were specifically linked to reduced pathogenesis in the lung.29 Cell counts from the BALF of cSiO2–treated NZBWF1 mice revealed a robust increase in immune cells at 9 and 13 weeks post-instillation, with the main cell types being macrophages and neutrophils; at 13 weeks, lymphocytes were also present in the BALF. cSiO2–triggered elevations of these cells were significantly suppressed by feeding DHA, both at the low and high doses. Relatedly, following H&E staining, we observed perivascular and peribronchiolar lymphoid cell infiltration in cSiO2-instilled mice, suggesting the development of ectopic lymphoid structures (ELS).12,30,31 This was confirmed by immunohistochemical staining for T cells, B cells, and follicular dendritic cells. The infiltrating lymphoid cells clearly adopted a germinal center-like formation, with lymphoid cells surrounding aggregated follicular dendritic cells and yielding IgG- producing plasma cells.32 Organized lymphoid structures in the lungs of mice have also been termed inducible bronchus-associated lymphoid tissue, or iBALT, as they develop only in response to environmental exposures or infections.33,34 Intriguingly, DHA supplementation impeded formation of cSiO2-induced ectopic lymphoid structures and germinal centers in the lung at all time points.
In lupus and other autoimmune diseases, ELS can serve as a platform for the development of autoantibodies.31,35 Using an autoantigen microarray, we identified numerous autoantibodies in the BALF and plasma that were upregulated in response to cSiO2, beginning at 5 weeks post-instillation and becoming highly robust at 9 and 13 weeks.36 These included IgG, IgM, and IgA isotypes. In mice that consumed diets supplemented with DHA, cSiO2-induced autoantibody production was suppressed. Importantly, known human lupus markers, namely anti-DNA, anti-RNP, anti-SM, anti- histone, anti-dsDNA, anti-Ro/SSA, anti-La/SSB, and anti-complement autoantibodies,37 were suppressed by feeding DHA.
Type 1 interferon response is induced by cSiO2 but suppressed by DHA supplementation.
Recently, we analyzed gene expression in the lungs and the kidneys using a platform targeting 770 immune-related genes to assess the effects cSiO2 exposure and DHA consumption on mRNA signatures over time in female NZBWF1 mice (publication in press).38 These analyses were performed on samples from the experiment detailed above, as well as in animals sacrificed either one day following a single cSiO2 exposure or one day after the fourth exposure. Just 1 day after the last of four weekly cSiO2 doses, genes related to inflammation as well as innate and adaptive immunity were dramatically increased in lungs of animals fed the control diet but were significantly reduced in mice fed DHA diets. Importantly, mRNA signatures in lungs of cSiO2-treated mice fed the control diet over 13 weeks reflected progressive amplification of type 1 interferon (IFN)- and chemokine-related gene pathways, in addition to other immune-related pathways. At all timepoints, these lung signatures were suppressed in mice fed DHA.
IFN signaling is strongly implicated in the development of autoimmune diseases, with type 1 IFN – and more specifically IFNα – being associated with the development of lupus.39 Early studies using the lupus-prone NZBWF1 mouse model showed that induction of type I IFN using polyinosinic:polycytidylic acid accelerated the disease onset.40 In 1979, it was reported that patients with autoimmune disease had increased levels of circulating IFN, and in lupus patients both type I and type II IFN were observed.41 In the late 1990s and early 2000s, there were multiple reports that patients administered IFNα to treat certain malignancies and hepatitis C developed lupus-like symptoms, further strengthening the connection between type I IFN and the development of lupus. 42,43,44 In 2003, Baechler et al. showed that expression of IFN target genes was closely correlated with disease activity and severity.45 Of high relevance to our model, it was recently reported that cSiO2-induced cell death and self-dsDNA release could induce a type I IFN response in mice via the cytosolic receptor STING (stimulator of interferon genes).46,47 These findings were extended to humans, showing that patients with silicosis had an increase in circulating dsDNA as well as activation of the pathway sensing DNA and inducing a type I IFN response in the lung tissue.46
Proposed role for type 1 IFN in cSiO2-induced autoimmunity
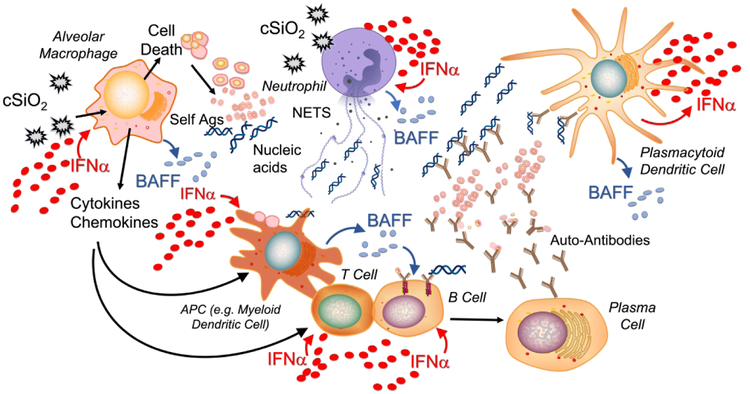
When cSiO2 is inhaled it can induce cell death (e.g. apoptosis, pyroptosis, and necroptosis) in multiple cell types in the lung, including alveolar macrophages and neutrophils (Figure 1). If dead cells are not properly cleared, cellular debris accrues. Of note, impaired efferocytosis (i.e. phagocytosis of dead/dying cells) is implicated in lupus and other autoimmune diseases, resulting in insufficient clearance of cellular debris, including host nucleic acids.48 In addition, cSiO2 has been shown to induce formation of neutrophil extracellular traps via a mechanism known as NETosis, leading to the extrusion of nuclear contents that further contribute to extracellular DNA accumulation.49 While it is well established that viral nucleic acids can trigger an IFN response, endogenous nucleic acids released by dying and NETosing cells can as well.50,51 Many cell types can recognize double stranded and single stranded nucleic acids through various receptors, including toll like receptors and cytosolic nucleic acid sensors such as STING,47 ultimately leading to the release of type I interferons. In lupus, one of the key cell types implicated in this response is the plasmacytoid dendritic cell (pDC), which primarily releases IFNα.39
Figure 1: Putative role for type 1 IFN in cSiO2-induced lupus.
When inhaled, cSiO2 induces cell death in multiple cell types present in the lung (e.g. neutrophils and macrophages) leading to the extracellular release of host nucleic acids. cSiO2 also stimulates release of neutrophil extracellular traps (NETs) which contribute to the accumulation of extracellular DNA. Extracellular nucleic acids, either viral or innate, induce the release of type I IFN from many immune cell types. In lupus, plasmacytoid dendritic cells (pDCs) are recognized as a primary producer of IFNα, the major type I IFN. Extracellular DNA along with other uncleared cellular debris can be presented by antigen presenting cells, promoting the development of autoreactive B and T cells. This process is enhanced directly by IFNα and by other cytokines and chemokines, such as B-cell activating factor (BAFF), released from immune cells in response to IFNα. Autoreactive plasma cells will produce autoantibodies against host antigens, forming DNA-containing immune complexes that can be recognized by pDCs to further drive this cycle. Adapted from Chasset F & Arnaud L, 2018.50
Based on published studies and our findings, we propose that the type I IFN response plays a central role in cSiO2-triggered autoimmunity. IFNα can act on multiple cell types and many of its actions promote a feed-forward loop to drive the type I IFN response. For example, IFNα can induce additional NETosis52 and can also act on macrophages, leading to the release of proinflammatory chemokines and cytokines that stimulate antigen presentation and T and B cell activation. Additionally, IFNα can act directly on myeloid dendritic cells to promote antigen presentation. In the case of cSiO2-triggered autoimmunity, host antigens are likely be presented due to the impaired clearance of cellular debris. IFNα can further promote B cell maturation into plasma cells. In lupus, these may be autoreactive plasma cells producing autoantibodies against host antigens, including host DNA. These DNA-containing immune complexes can be recognized by pDCs, leading to additional release of IFNα.53
Of high relevance to human autoimmunity, IFNα elicits the release of B-cell activating factor (BAFF) from multiple cell types.54,55 The main role of BAFF is to promote B-cell maturation, increasing the release of autoantibodies and driving this feed-forward cycle. BAFF is the target of the drug Belimumab (Benlysta™), which was approved by the FDA in 2011, making it the first drug in 52 years approved to treat lupus in adults.56 In April 2019, Benlysta was also approved for treatment of pediatric lupus.57 We have found that cSiO2 induces elevated BAFF in the BALF and plasma of NZBWF1 mice and that elevation of this critical B-cell maturation factor is dose-dependently suppressed by DHA supplementation.28
Can omega-3 HUFA supplementation be used as a personalized nutrition tool against lupus?
cSiO2 treatment markedly increases expression of several cytokines and receptors that are pathogenic in the progression of lupus. Importantly, dietary DHA reversed this effect (Table 1).28,29 Relevant to the treatment of lupus, several of the cytokines identified in our preliminary and published studies are pharmacological targets for drugs already approved or currently in clinical trials for the treatment of lupus and other autoimmune diseases.53,56,58–62 Notably, IFNα is a target of ongoing lupus clinical trials using multiple strategies to impede different steps of the IFN pathway, as reviewed previously.53 Many of the therapies in development are monoclonal antibodies, which are extremely expensive.63 For example, a Belimumab dosing regimen costs approximately $35,000 per year, placing a substantial financial burden on uninsured patients and their families.56,64 While omega-3 HUFA supplementation might not eliminate the need for these drugs, it has the potential to interfere with many of these targets simultaneously for less than few dollars a day, reducing the need for high doses of a drug over prolonged periods of time.
Table 1.
Silica enhances (↑) and DHA suppresses (↓) many inflammatory endpoints targeted by drugs either approved or in clinical trials for treatment of autoimmune disease.
| Target | Silica | DHA | Treatment | Drug | |
|---|---|---|---|---|---|
| IFNα | ↑ | ↓ | IFNα mAb | Rontalizumab, Sifalimumab50 | |
| IFNAR1 mAb | Anifrolumab50 | ||||
| IFNα vaccine | Interferon-α-kinoid (IFN-K)50 | ||||
| BAFF | ↑ | ↓ | BAFF mAb | Belimumab (Benlysta™)53 | |
| IL-6 | ↑ | ↓ | IL-6R mAb | Tocilizumab55 | |
| IL-6 mAb | Sirukumab56 | ||||
| TNF-α | ↑ | ↓ | TNF-α mAb | Infliximab57 | |
| MCP-1 | ↑ | ↓ | Neutralize MCP-1 | Emapticap58 | |
| CD3 | ↑ | ↓ | CD3 mAb | Muromonab-CD359 |
Recently, a population study conducted under the aegis of the Michigan Lupus Epidemiology & Surveillance (MILES) Program reported that higher dietary intake of omega-3 fatty acids, and lower dietary omega-6:omega-3 ratios, were favorably associated with patient-reported outcomes, particularly self-reported lupus activity and sleep quality.4 To harness the protective effects of DHA and other omega-3 HUFAs, it is important to understand how they act. When consumed, fatty acids are incorporated into the cell membrane,21 and the resulting levels of omega-3 and omega-6 HUFAs in the membrane can have a direct effect on inflammatory pathways in the cell. One way that membrane HUFAs can influence inflammatory responses is by impacting lipid raft formation. It has been reported that DHA and EPA can impede lipid raft formation and prevent the aggregation and activation of transmembrane receptors involved in inflammatory signaling pathways.65 HUFAs can also be cleaved from the membrane, both extracellularly and intracellularly by phospholipases to generate free HUFAs.66,67 Free EPA and DHA have been shown to activate transmembrane receptors involved in blocking pro-inflammatory signaling pathways68,69 and are also reported to be ligands for PPARγ, which can inhibit NF-kB dependent transcription.70,71 Additionally, both EPA and DHA can be metabolized to downstream specialized pro-resolving mediators, known as SPMs, which include resolvins, protectins, maresins, and anti-inflammatory epoxide metabolites.72,73 These have also been shown to inhibit inflammatory signaling pathways74,75 and promote both phagocytosis of bacteria and efferocytosis of dead cells.76,77 Finally, EPA and DHA can directly compete with omega-6 HUFAs for incorporation into the cell membrane and for metabolism to downstream eicosanoids. This is of biological importance, as downstream metabolites of the omega-6 HUFA arachidonic acid (i.e. prostaglandins, thromboxanes, and leukotrienes) are well known to promote an inflammatory response.78 Accordingly, altering the HUFA balance of the membrane to favor omega-3 HUFAs can have a direct impact on promoting resolution over inflammation.
Because the levels of omega-6 and omega-3 HUFAs may have profound and counteracting effects on inflammation, resolution, and disease status, it is critical to gauge their content in the phospholipid membrane. Assessing the phospholipid content of one’s red blood cells as a biomarker of HUFA status in tissues is now quite simple with several companies providing relatively inexpensive, at-home finger prick tests.79 From just one drop of blood collected and stabilized on filter paper, dozens of major fatty acids, including omega-3 and −6 HUFAs, can be quantified. We favor using the omega-3 HUFA score over other alternative approaches to reflect the omega-3 content in the red blood cells (RBCs) of our mice. The HUFA score represents the major omega-3 HUFAs as a percent of total HUFAs,80 while other widely used indices present EPA and DHA as a percent of total fatty acids. There are a few advantages to presenting the omega-3 content using the HUFA score. First, HUFAs have similar chemical properties and will be modified – or degraded – at similar rates. Therefore, the HUFA score should remain relatively constant despite methodological differences.81 Second, the major omega-6 and omega-3 HUFAs are the primary substrates for known proinflammatory and anti-inflammatory lipid mediators.21,81 Limiting analysis to the HUFA pool allows the clinician to better estimate potential competition between omega-6- and omega-3-derived metabolites. Finally, the HUFA score is predictable from dietary fat intake, which would be important for developing a personalized nutritional intervention.82
In a preliminary investigation,83 using data from a previously published study that assessed dose response of DHA on cSiO2-triggered lupus in NZBWF1 mice,28 we showed that an increased omega-3 HUFA score in RBCs was associated with decreased autoimmune biomarkers, such as anti-dsDNA IgG antibodies and BAFF. The HUFA score was also negatively correlated with B and T cell infiltration in the lungs and levels of pro-inflammatory cytokines and chemokines, such as TNF-α and MCP-1, respectively. Similar associations were found between these endpoints and HUFA scores in the lung, spleen, and kidney. The high omega-3 HUFA scores (~70%) associated with reduced autoimmune disease in mice are similar to those reported for humans living in communities predominately consuming fish and other marine fats.84 This is substantially higher than HUFA scores observed in individuals consuming a Western diet (~30%),82 which consists of foods rich in omega-6 fatty acids, such as vegetable oils, poultry, and beef.85 These findings suggest that the omega-3 content of the RBCs, which is decidedly influenced by diet, may be leveraged to ameliorate the inflammatory status of patients with lupus and other autoimmune diseases. These personalized interventions are in step with the goals of the NIH’s Precision Medicine Initiative, which defines precision medicine as “an innovative approach to disease prevention and treatment that takes into account individual differences in people’s genes, environments, and lifestyles”.86
Conclusion
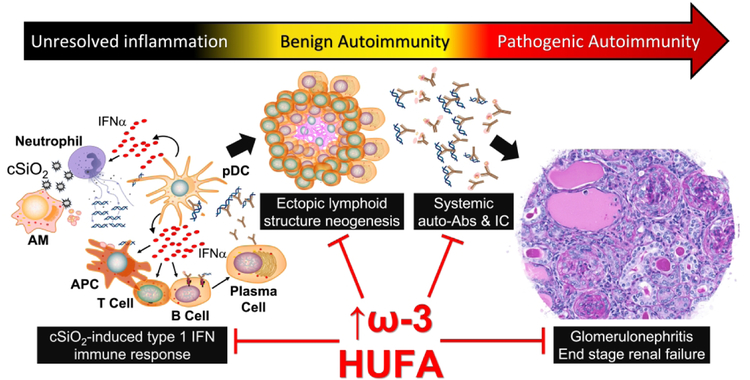
Taken together, intranasal exposure of lupus-prone mice to cSiO2 induces a type I IFN gene signature that precedes ELS induction in the lung, with lymphoid cell infiltration occurring as early as 1 week post-instillation and worsening over the course of the disease. ELS neogenesis evokes vigorous elevations of diverse autoantibodies in the BALF and plasma that form immune complexes with their cognate antigens and deposit in the kidneys, ultimately resulting in glomerulonephritis. When animals are fed DHA, the IFN-related genes are suppressed, ELS development is suppressed, autoantibodies are reduced, and animals present much less severe glomerulonephritis (Figure 2). Because DHA consumption results in an enrichment of omega-3 HUFAs in the phospholipids, measuring the HUFA score of patients could be ideal for developing strategies to effectively combat inflammatory and autoimmune diseases. While it is critical to validate these preclinical findings in humans, our assessment provides insight into the range of omega-3 HUFA scores that might be required in humans to achieve protective effects. Ultimately, a precision health strategy for an individual would require at least two tactics to potentiate the omega-3 HUFA score: 1) increasing omega-3 HUFA intake by dietary supplementation and 2) reducing consumption of foods that containing high amounts of omega-6 HUFAs. This strategy may protect and benefit patients with lupus or other autoimmune diseases, individuals genetically predisposed to these diseases, or individuals environmentally exposed to cSiO2 and other autoimmune disease triggers.
Figure 2: cSiO2-induced autoimmune pathogenesis is correlated with the omega-3 HUFA score.
The cSiO2-induced type I IFN gene signature promotes ectopic lymphoid structure neogenesis in the lungs, and production of pathogenic autoantibodies which form immune complexes (IC) with host antigens. This culminates in systemic autoimmunity and glomerulonephritis. When mice are fed diets rich in DHA, autoimmune pathogenesis is suppressed. Improved disease status and decreased inflammatory endpoints are associated with an increase in omega-3 HUFAs in the cell phospholipid membrane. Biomarkers that evaluate membrane fatty acids, such as the HUFA score, are crucial to developing omega-3 supplementation strategies to prevent or ameliorate inflammatory and autoimmune disease.
Acknowledgments
We thank Dr. Melissa Bates for her exceptional animal studies, which provide the underpinning of this review and the current work in our lab. We also thank Dr. Abby Benninghoff and Dr. Lichchavi Rajasinghe for their research insights, data analysis and technical support.
Funding
Research was funded by NIH ES027353 (JJP, JRH), NIEHS Training Grant T32ES007255 (KAW), Ruth L. Kirschstein Individual Predoctoral NRSA F31ES030593 (KAW), Lupus Foundation of America (KAW, JJP), the Dr. Robert and Carol Deibel Family Endowment (JJP).
Footnotes
Conflicting Interests Statement
The authors declare no real, perceived or potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Dorner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet 2019;393(10188):2344–2358. doi: 10.1016/S0140-6736(19)30546-X [DOI] [PubMed] [Google Scholar]
- 2.Durcan L, O’Dwyer T, Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 2019;393(10188):2332–2343. doi: 10.1016/S0140-6736(19)30237-5 [DOI] [PubMed] [Google Scholar]
- 3.Parks CG, Miller FW, Pollard KM, et al. Expert panel workshop consensus statement on the role of the environment in the development of autoimmune disease. Int J Mol Sci 2014;15(8):14269–14297. doi: 10.3390/ijms150814269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charoenwoodhipong P, Harlow SD, Marder W, et al. Dietary omega polyunsaturated fatty acid intake and patient-reported outcomes in systemic lupus erythematosus: The Michigan Lupus Epidemiology & Surveillance (MILES) Program. Arthritis Care & Research 0(ja). doi: 10.1002/acr.23925 [DOI] [PMC free article] [PubMed]
- 5.Parks CG, Cooper GS, Nylander-French LA, et al. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: a population-based, case-control study in the southeastern United States. Arthritis Rheum 2002;46(7):1840–1850. doi: 10.1002/art.10368 [DOI] [PubMed] [Google Scholar]
- 6.Workers’ exposure to respirable crystalline silica: Final rule overview. Occupational Safety and Health Administration; 2016. https://www.osha.gov/Publications/OSHA3683.pdf. Accessed June 25, 2019.
- 7.Leung CC, Yu IT, Chen W. Silicosis. Lancet 2012;379(9830):2008–2018. doi: 10.1016/S0140-6736(12)60235-9 [DOI] [PubMed] [Google Scholar]
- 8.Cohen RA, Petsonk EL, Rose C, et al. Lung pathology in U.S. coal workers with rapidly progressive pneumoconiosis implicates silica and silicates. Am J Respir Crit Care Med 2016;193(6):673–680. doi: 10.1164/rccm.201505-1014OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawasaki H A review of the fate of inhaled alpha-quartz in the lungs of rats. Inhal Toxicol 2019:1–10. doi: 10.1080/08958378.2019.1597218 [DOI] [PubMed]
- 10.Kawasaki H A mechanistic review of silica-induced inhalation toxicity. Inhalation Toxicology 2015;27:363–377. doi: 10.3109/08958378.2015.1066905 [DOI] [PubMed] [Google Scholar]
- 11.Brown JM, Pfau JC, Holian A. Immunoglobulin and lymphocyte responses following silica exposure in New Zealand mixed mice. Inhal Toxicol 2004;16(3):133–139. doi: 10.1080/08958370490270936 [DOI] [PubMed] [Google Scholar]
- 12.Bates MA, Brandenberger C, Langohr I, et al. Silica triggers inflammation and ectopic lymphoid neogenesis in the lungs in parallel with accelerated onset of systemic autoimmunity and glomerulonephritis in the lupus-prone NZBWF1 mouse. PLoS ONE 2015;10(5):e0125481. doi: 10.1371/journal.pone.0125481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parks CG, Cooper GS. Occupational exposures and risk of systemic lupus erythematosus: a review of the evidence and exposure assessment methods in population- and clinic-based studies. Lupus 2006;15(11):728–736. doi: 10.1177/0961203306069346 [DOI] [PubMed] [Google Scholar]
- 14.Hill AB. The environment and disease: Association or causation? Proc R Soc Med 1965;58:295–300. https://www.ncbi.nlm.nih.gov/pubmed/14283879. Accessed May 29, 2019. [PMC free article] [PubMed] [Google Scholar]
- 15.Gioxari A, Kaliora AC, Marantidou F, Panagiotakos DP. Intake of omega-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018;45:114–124 e114. doi: 10.1016/j.nut.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 16.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015;1851(4):469–484. doi: 10.1016/j.bbalip.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 17.Pestka JJ. n-3 polyunsaturated fatty acids and autoimmune-mediated glomerulonephritis. Prostaglandins Leukot Essent Fatty Acids 2010;82(4–6):251–258. doi: 10.1016/j.plefa.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papanikolaou Y, Brooks J, Reider C, Fulgoni VL 3rd. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003–2008. Nutr J 2014;13:31. doi: 10.1186/1475-2891-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thuppal SV, von Schacky C, Harris WS, et al. Discrepancy between knowledge and perceptions of dietary omega-3 fatty acid intake compared with the omega-3 index. Nutrients 2017;9(9). doi: 10.3390/nu9090930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Micha R, Khatibzadeh S, Shi P, et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ 2014;348:g2272. doi: 10.1136/bmj.g2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browning LM, Walker CG, Mander AP, et al. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr 2012;96(4):748–758. doi: 10.3945/ajcn.112.041343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report 2015(79):1–16. doi: [PMC free article] [PubMed] [Google Scholar]
- 23.Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans 2017;45(5):1105–1115. doi: 10.1042/BST20160474 [DOI] [PubMed] [Google Scholar]
- 24.Akbar U, Yang M, Kurian D, Mohan C. Omega-3 fatty acids in rheumatic diseases: A critical review. J Clin Rheumatol 2017;23(6):330–339. doi: 10.1097/RHU.0000000000000563 [DOI] [PubMed] [Google Scholar]
- 25.Dubois EL, Horowitz RE, Demopoulos HB, Teplitz R. NZB/NZW mice as a model of systemic lupus erythematosus. JAMA 1966;195(4):285–289. https://www.ncbi.nlm.nih.gov/pubmed/4159181. Accessed Jan 24, 2019. [PubMed] [Google Scholar]
- 26.Crampton SP, Morawski PA, Bolland S. Linking susceptibility genes and pathogenesis mechanisms using mouse models of systemic lupus erythematosus. Dis Model Mech 2014;7(9):1033–1046. doi: 10.1242/dmm.016451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pestka JJ, Vines LL, Bates MA, He K, Langohr I. Comparative effects of n-3, n-6 and n-9 unsaturated fatty acid-rich diet consumption on lupus nephritis, autoantibody production and CD4+ T cell-related gene responses in the autoimmune NZBWF1 mouse. PLoS One 2014;9(6):e100255. doi: 10.1371/journal.pone.0100255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bates MA, Brandenberger C, Langohr II, et al. Silica-triggered autoimmunity in lupus-prone mice blocked by docosahexaenoic acid consumption. PLoS One 2016;11(8):e0160622. doi: 10.1371/journal.pone.0160622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bates MA, Akbari P, Gilley KN, et al. Dietary docosahexaenoic acid prevents silica-induced development of pulmonary ectopic germinal centers and glomerulonephritis in the lupus-prone NZBWF1 mouse. Front Immunol 2018;9:2002. doi: 10.3389/fimmu.2018.02002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bates MA P, Gilley KN., Jackson-Humbles DN, Wagner JG, Li N, Kopec AK, Wierenga KM, Brandenberger C, Holian A, Benninghoff AD, Harkema JR, and Pestka JJ Dietary docosahexaenoic acid prevents silica-induced development of pulmonary ectopic germinal centers and glomerulonephritis in the lupus-prone NZBWF1 mouse. Frontiers Immunol (in press) 2018. doi: [DOI] [PMC free article] [PubMed]
- 31.Corsiero E, Nerviani A, Bombardieri M, Pitzalis C. Ectopic lymphoid structures: Powerhouse of autoimmunity. Front Immunol 2016;7:430. doi: 10.3389/fimmu.2016.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nacionales DC, Weinstein JS, Yan XJ, et al. B cell proliferation, somatic hypermutation, class switch recombination, and autoantibody production in ectopic lymphoid tissue in murine lupus. J Immunol 2009;182(7):4226–4236. doi: 10.4049/jimmunol.0800771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 2004;10(9):927–934. doi: 10.1038/nm1091 [DOI] [PubMed] [Google Scholar]
- 34.Hwang JY, Randall TD, Silva-Sanchez A. Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung. Front Immunol 2016;7:258. doi: 10.3389/fimmu.2016.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones GW, Jones SA. Ectopic lymphoid follicles: inducible centres for generating antigen-specific immune responses within tissues. Immunology 2016;147(2):141–151. doi: 10.1111/imm.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajasinghe L, Li Q, Bates M, Akbari P, Harkema J, Pestka J. Docosahexaenoic acid (DHA) suppresses broad spectrum of pathogenic autoantibodies elicited in murine model of lupus flaring (OR12–03-19). Current developments in nutrition 2019;3(Suppl 1). doi: 10.1093/cdn/nzz049.OR12-03-19 [DOI] [Google Scholar]
- 37.Li QZ, Zhou J, Wandstrat AE, et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol 2007;147(1):60–70. doi: 10.1111/j.1365-2249.2006.03251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benninghoff AD, Bates MA, Wierenga KA, et al. Docosahexaenoic acid consumption impedes early interferon- and chemokine-related gene expression during silica-triggered flaring of murine lupus. Front Immunol In Press [DOI] [PMC free article] [PubMed]
- 39.Crow MK, Olferiev M, Kirou KA. Type I interferons in autoimmune disease. Annu Rev Pathol 2019;14:369–393. doi: 10.1146/annurev-pathol-020117-043952 [DOI] [PubMed] [Google Scholar]
- 40.Steinberg AD, Baron S, Talal N. The pathogenesis of autoimmunity in New Zealand mice, I. Induction of antinucleic acid antibodies by polyinosinic-polycytidylic acid. Proc Natl Acad Sci U S A 1969;63(4):1102–1107. doi: 10.1073/pnas.63.4.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med 1979;301(1):5–8. doi: 10.1056/NEJM197907053010102 [DOI] [PubMed] [Google Scholar]
- 42.Gota C, Calabrese L. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity 2003;36(8):511–518. https://www.ncbi.nlm.nih.gov/pubmed/14984028. Accessed Apr 15, 2019. [DOI] [PubMed] [Google Scholar]
- 43.Rizvi R, Hojjati M. Interferon-alpha induced lupus in a patient with chronic hepatitis C virus. J Clin Rheumatol 2011;17(3):152–153. doi: 10.1097/RHU.0b013e31821557e7 [DOI] [PubMed] [Google Scholar]
- 44.Ronnblom LE, Alm GV, Oberg KE. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med 1991;115(3):178–183. doi: 10.7326/0003-4819-115-3-178 [DOI] [PubMed] [Google Scholar]
- 45.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 2003;100(5):2610–2615. doi: 10.1073/pnas.0337679100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benmerzoug S, Rose S, Bounab B, et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat Commun 2018;9(1):5226. doi: 10.1038/s41467-018-07425-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity 2013;38(5):870–880. doi: 10.1016/j.immuni.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdolmaleki F, Farahani N, Gheibi Hayat SM, et al. The Role of Efferocytosis in Autoimmune Diseases. Front Immunol 2018;9:1645. doi: 10.3389/fimmu.2018.01645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rizzi M, Carniato F, Tonello S, et al. Charged molecular silica trigger in vitro NETosis in human granulocytes via both oxidative and autophagic pathways. Eur Rev Med Pharmacol Sci 2018;22(20):7058–7068. doi: 10.26355/eurrev_201810_16178 [DOI] [PubMed] [Google Scholar]
- 50.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol 2015;15(2):87–103. doi: 10.1038/nri3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crow MK, Ronnblom L. Type I interferons in host defence and inflammatory diseases. Lupus Sci Med 2019;6(1):e000336. doi: 10.1136/lupus-2019-000336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Romo GS, Caielli S, Vega B, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011;3(73):73ra20. doi: 10.1126/scitranslmed.3001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chasset F, Arnaud L. Targeting interferons and their pathways in systemic lupus erythematosus. Autoimmun Rev 2018;17(1):44–52. doi: 10.1016/j.autrev.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 54.Jacob N, Guo S, Mathian A, et al. B Cell and BAFF dependence of IFN-alpha-exaggerated disease in systemic lupus erythematosus-prone NZM 2328 mice. J Immunol 2011;186(8):4984–4993. doi: 10.4049/jimmunol.1000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez P, Scheel-Toellner D, Rodriguez-Carrio J, Caminal-Montero L, Gordon C, Suarez A. Interferon-alpha-induced B-lymphocyte stimulator expression and mobilization in healthy and systemic lupus erthymatosus monocytes. Rheumatology (Oxford) 2014;53(12):2249–2258. doi: 10.1093/rheumatology/keu249 [DOI] [PubMed] [Google Scholar]
- 56.Lamore R 3rd, Parmar S, Patel K, Hilas O Belimumab (benlysta): a breakthrough therapy for systemic lupus erythematosus. P T 2012;37(4):212–226. https://www.ncbi.nlm.nih.gov/pubmed/22593633. Accessed Apr 20, 2018. [PMC free article] [PubMed] [Google Scholar]
- 57.FDA approves first treatment for pediatric patients with lupus. Arnold N; 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-pediatric-patients-lupus. Accessed Apr 26, 2019.
- 58.Sheppard M, Laskou F, Stapleton PP, Hadavi S, Dasgupta B. Tocilizumab (Actemra). Hum Vaccin Immunother 2017;13(9):1972–1988. doi: 10.1080/21645515.2017.1316909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pelechas E, Voulgari PV, Drosos AA. Sirukumab: a promising therapy for rheumatoid arthritis. Expert Opin Biol Ther 2017;17(6):755–763. doi: 10.1080/14712598.2017.1315099 [DOI] [PubMed] [Google Scholar]
- 60.Blair HA, Deeks ED Infliximab Biosimilar (CT-P13; Infliximab-dyyb): A review in autoimmune inflammatory diseases. BioDrugs 2016;30(5):469–480. doi: 10.1007/s40259-016-0193-2 [DOI] [PubMed] [Google Scholar]
- 61.Menne J, Eulberg D, Beyer D, et al. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol Dial Transplant 2017;32(2):307–315. doi: 10.1093/ndt/gfv459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatenoud L CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol 2003;3(2):123–132. doi: 10.1038/nri1000 [DOI] [PubMed] [Google Scholar]
- 63.Shaughnessy AF. Monoclonal antibodies: magic bullets with a hefty price tag. BMJ 2012;345:e8346. doi: 10.1136/bmj.e8346 [DOI] [PubMed] [Google Scholar]
- 64.Panopalis P, Yazdany J, Gillis JZ, et al. Health care costs and costs associated with changes in work productivity among persons with systemic lupus erythematosus. Arthritis Rheum 2008;59(12):1788–1795. doi: 10.1002/art.24063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem 2009;284(40):27384–27392. doi: 10.1074/jbc.M109.044065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc Natl Acad Sci U S A 2012;109(22):8517–8522. doi: 10.1073/pnas.1200189109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Norris PC, Gosselin D, Reichart D, Glass CK, Dennis EA. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc Natl Acad Sci U S A 2014;111(35):12746–12751. doi: 10.1073/pnas.1404372111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, Yu Y, Funk CD. Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). FASEB J 2013;27(12):4987–4997. doi: 10.1096/fj.13-235333 [DOI] [PubMed] [Google Scholar]
- 69.Yan Y, Jiang W, Spinetti T, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013;38(6):1154–1163. doi: 10.1016/j.immuni.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 70.Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta 2007;1771(8):926–935. doi: 10.1016/j.bbalip.2007.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang HY, Lee HN, Kim W, Surh YJ. Docosahexaenoic acid induces M2 macrophage polarization through peroxisome proliferator-activated receptor gamma activation. Life Sci 2015;120:39–47. doi: 10.1016/j.lfs.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 72.Serhan CN. Systems approach to inflammation resolution: identification of novel anti-inflammatory and pro-resolving mediators. J Thromb Haemost 2009;7 Suppl 1:44–48. doi: 10.1111/j.1538-7836.2009.03396.x [DOI] [PubMed] [Google Scholar]
- 73.Ostermann AI, Schebb NH. Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids. Food Funct 2017;8(7):2355–2367. doi: 10.1039/c7fo00403f [DOI] [PubMed] [Google Scholar]
- 74.Sham HP, Walker KH, Abdulnour RE, et al. 15-epi-Lipoxin A4, Resolvin D2, and Resolvin D3 Induce NF-kappaB Regulators in Bacterial Pneumonia. J Immunol 2018;200(8):2757–2766. doi: 10.4049/jimmunol.1602090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Titos E, Rius B, Lopez-Vicario C, et al. Signaling and immunoresolving actions of resolvin D1 in inflamed human visceral adipose tissue. J Immunol 2016;197(8):3360–3370. doi: 10.4049/jimmunol.1502522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiang N, Fredman G, Backhed F, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012;484(7395):524–528. doi: 10.1038/nature11042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fredman G, Hellmann J, Proto JD, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun 2016;7:12859. doi: 10.1038/ncomms12859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lands B Highly unsaturated fatty acids (HUFA) mediate and monitor food’s impact on health. Prostaglandins Other Lipid Mediat 2017;133:4–10. doi: 10.1016/j.prostaglandins.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 79.Johnston DT, Deuster PA, Harris WS, Macrae H, Dretsch MN. Red blood cell omega-3 fatty acid levels and neurocognitive performance in deployed U.S. Servicemembers. Nutr Neurosci 2013;16(1):30–38. doi: 10.1179/1476830512Y.0000000025 [DOI] [PubMed] [Google Scholar]
- 80.Stark KD. The percentage of n-3 highly unsaturated fatty acids in total HUFA as a biomarker for omega-3 fatty acid status in tissues. Lipids 2008;43(1):45–53. doi: 10.1007/s11745-007-3128-3 [DOI] [PubMed] [Google Scholar]
- 81.Lands B, Bibus D, Stark KD. Dynamic interactions of n-3 and n-6 fatty acid nutrients. Prostaglandins Leukot Essent Fatty Acids 2018;136:15–21. doi: 10.1016/j.plefa.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 82.Strandjord SE, Lands B, Hibbeln JR. Validation of an equation predicting highly unsaturated fatty acid (HUFA) compositions of human blood fractions from dietary intakes of both HUFAs and their precursors. Prostaglandins Leukot Essent Fatty Acids 2018;136:171–176. doi: 10.1016/j.plefa.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wierenga KA, Bates MA, Lock AL, et al. Percent n-3 in highly unsaturated fatty acids (HUFAs) is predictive of disease outcomes in environmental toxicant-triggered autoimmunity. In The Toxicologist. Vol 168. Toxicol Sci 168(1): Society of Toxicology, 2019; 2019. [Google Scholar]
- 84.Yamada T, Strong JP, Ishii T, et al. Atherosclerosis and omega-3 fatty acids in the populations of a fishing village and a farming village in Japan. Atherosclerosis 2000;153(2):469–481. https://www.ncbi.nlm.nih.gov/pubmed/11164437. Accessed June 12, 2019. [DOI] [PubMed] [Google Scholar]
- 85.USDA Food Composition Database. United States Department of Agriculture; 2018. https://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=675&nutrient2=620&nutrient3=&subset=0&sort=c&measureby=g. Accessed July 1, 2019.
- 86.FACT SHEET: President Obama’s Precision Medicine Initiative. The White House; 2015. https://obamawhitehouse.archives.gov/the-press-office/2015/01/30/fact-sheet-president-obama-s-precision-medicine-initiative. Accessed August 6, 2019.