Abstract
We evaluated predictive factors for visual outcomes in patients with idiopathic epiretinal membrane (iERM) after pars plana vitrectomy (PPV). Clinical records for 114 eyes (114 patients, mean age: 70.6 years) with iERM treated by PPV between March 2012 and March 2018 were retrospectively reviewed. Overall, the mean postoperative best-corrected visual acuity (BCVA) and central retinal thickness measured by optical coherence tomography improved as early as 1 month after surgery, and further improved until 3 months (P < 0.01). Multiple linear regression analyses adjusted for the preoperative BCVA showed that older age (B, 0.010; 95% confidence interval, 0.003 to 0.016; P = 0.003) and a shorter axial length (AL; B, −0.059; 95% confidence interval, −0.099 to −0.019; P = 0.005) predicted worse postoperative BCVA. The Mann-Whitney U test showed that the postoperative BCVA was worse in eyes with AL < 23.6 mm than in eyes with AL ≥ 23.6 mm (P = 0.037), and in patients aged ≥69 years than in patients aged <69 years (P = 0.024). The findings may help in evaluating surgical indications for each patient to obtain satisfactory outcomes, irrespective of the preoperative BCVA.
Subject terms: Retinal diseases, Medical research
Introduction
Recent progress in medical technology has improved the safety of pars plana vitrectomy (PPV), which has led to expansion in its surgical indications. PPV is not only performed for vision-threatening retinal diseases, such as proliferative vitreoretinopathy and proliferative diabetic retinopathy, but also for improving the quality of vision in patients with idiopathic epiretinal membrane (iERM), which has a relative indication of surgery. Thus, in patients with iERM, predictive factors of treatment outcomes would be valuable in choosing the application and/or timing of surgery and achieving satisfying outcomes.
iERM is commonly observed in adults, and its incidence in the primary eye is 1.1% per year. The mean age of iERM diagnosis is 65 years1. It has been hypothesized that residual cortical vitreous secondary to a posterior vitreous detachment or partial separation are modified by glial cell proliferation on the surface, forming iERM. While metamorphopsia, blurred vision, monocular diplopia, and micropsia may be noted with any macular pathology due to the tractional force of iERM2–5 to the retina, majority of patients with iERM are asymptomatic. Thus, iERM could be occasionally detected using optical coherent tomography (OCT). In contrast, a subtle but clear impairment of visual function can be detected by measuring functional visual acuity and contrast visual acuity of eyes with iERM, even if eyes had best-corrected visual acuity (BCVA) better than 0 in LogMAR (1.0 in decimal in the Landolt C charts, equal to 20/20 in Snellen)6.
A previous report showed that better preoperative BCVA may lead to better postoperative BCVA, most likely due to less pathological changes in the retina before surgery7. Other reports have shown that disruptions of the integrity of the photoreceptor inner/outer segment layer8–11, and photoreceptor damage due to tractional forces of iERM were related to worse visual outcomes after PPV. Therefore, surgery should be performed before photoreceptor changes become evident on OCT images. However, surgeries for subclinical iERM with no clear symptoms may not be accepted, considering the risk-benefit ratio. Fundamental predictive factors related to eye characteristics, other than disease stage, such as the preoperative BCVA and photoreceptor damage detected by OCT findings, would help determine surgical indications in patients with iERM in the recent trend of early treatment than recommended previously.
iERM is formed in the vitreoretinal interface and the tractional force to the retina is most likely influenced by the change in the vitreous12,13. Previous observations in eyes with high myopia14,15 and posterior staphyloma16 show the differences in the vitreous conditions. Vitreous gel may be detached from the outermost layer of the cortex in the eye and observed as vitreous schisis with a longer axial length (AL). Thus, the vitreous condition may have differences according to the size of the eye.
In the present study, we analysed the effect of preoperative clinical data on the early postoperative BCVA in patients who underwent PPV for iERM. In particular, we focused on AL, which does not depend on the stage of the disease and may cause differences in the tractional force of vitreous on the retina.
Results
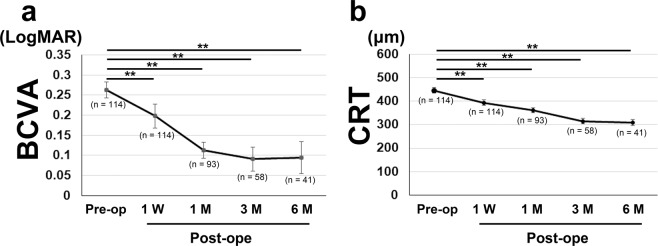
Of the 114 patients (114 eyes), 43 (37.7%) were males, and the mean age was 70.6 ± 0.7 years (range, 56–89 years) (Table 1). All patients visited the clinic 1 week after the surgery, and those with a good clinical course were referred back to their original clinics. Thus, the numbers of participants were 114, 93, 58, and 41 at 1 week and 1, 3, and 6 months after the surgery, respectively. The mean BCVA (LogMAR) before surgery, 1 week, and 1, 3, and 6 months after surgery were 0.26 ± 0.020 (range, −0.079 to 1.301; n = 114), 0.20 ± 0.032 (n = 114), 0.11 ± 0.022 (n = 93), 0.09 ± 0.030 (n = 58), and 0.09 ± 0.040 (n = 41), respectively (Fig. 1a). The mean central retinal thickness (CRT) before surgery, 1 week, and 1, 3, and 6 months after surgery were 446.40 ± 12.24 μm (n = 114), 393.81 ± 10.97 μm (n = 114), and 361.00 ± 10.91 μm (n = 93), 315.44 ± 12.01 μm (n = 58), and 309.37 ± 13.51 μm (n = 41), respectively (Fig. 1b). BCVA and CRT were significantly improved relative to the preoperative values at 1 week and 1, 3, and 6 months after surgery (P < 0.01 for all). There were no major complications during and after surgery, and no eyes underwent gas injections.
Table 1.
Baseline Characteristics.
| Mean Age (yrs, range) | 70.6 ± 0.7 (56 to 89) |
| Gender (male, %) | 43 (37.7) |
| Mean BCVA (logMAR, range) | 0.26 ± 0.02 (−0.079 to 1.301) |
| Mean CRT (μm, range) | 446.40 ± 12.24 (139 to 738) |
| Mean IOP (mmHg, range) | 13.50 ± 0.30 (7.0 to 21.0) |
| Mean Axial Length (mm, range) | 24.01 ± 0.12 (21.78 to 26.45) |
|
Cystoid changes in inner layer (eyes, [%]) |
74 (64.9) |
|
Cystoid changes in outer layer (eyes, [%]) |
33 (28.9) |
|
Cotton Ball Sign at the fovea (eyes, [%]) |
34 (29.8) |
| Complications | |
| Glaucoma (eyes, [%]) | 9 (7.9) |
| Hypertension (patients, [%]) | 13 (11.4) |
| Diabetes (patients, [%]) | 9 (7.9) |
Data are shown in mean ± SE with range for continuous valuable.
Figure 1.
Mean best-corrected visual acuity (BCVA) and mean central retinal thickness (CRT) at each time point. (a,b) Generalized mixed model analysis was performed for comparing the preoperative data with the 1-week and 1-, 3-, and 6-month postoperative data. Overall, the mean BCVA (a) and CRT (b) were significantly improved after surgery. Data are shown in mean ± standard error. **P < 0.01.
Simple linear regression analyses showed that older age (respective P values at the 1 and 3 months were 0.007 and 0.007), worse preoperative BCVA (P < 0.001 and P < 0.001), shorter AL (P = 0.005 and P = 0.018), and the preoperative presence of cystoid changes in the inner retinal layer (P = 0.031 and P = 0.044) were related to worse BCVA at 1 and 3 months after surgery (Table 2). Greater preoperative CRT was related to worse visual outcome after 1 month (P = 0.036); however, there was no significant association at 3 months (P = 0.059) despite a trend. Older age (P = 0.002), worse preoperative BCVA (P < 0.001), greater preoperative CRT (P = 0.037), and shorter AL (P < 0.001) were also related to worse visual outcome, 1 week after the surgery (data not shown).
Table 2.
Correlation Between Baseline Parameters and postoperative best-corrected visual acuity at Month 1 and 3.
| Variables | Month 1 | Month 3 | ||
|---|---|---|---|---|
| B (95% CI) |
P Value | B (95% CI) |
P Value | |
| Age |
0.008 (0.002 to 0.014) |
0.007** |
0.011 (0.003 to 0.018) |
0.007** |
| Gender |
−0.003 (−0.103 to 0.097) |
0.958 |
0.032 (−0.090 to 0.155) |
0.602 |
| Preoperative BCVA |
0.578 (0.426 to 0.730) |
<0.001** |
0.426 (0.236 to 0.615) |
<0.001** |
| Preoperative CRT |
0.000 (0.000 to 0.001) |
0.036* |
0.000 (0.000 to 0.001) |
0.059 |
| Preoperative IOP |
0.010 (−0.007 to 0.028) |
0.242 |
0.015 (−0.006 to 0.037) |
0.159 |
| Axial Length |
−0.053 (−0.090 to −0.017) |
0.005** |
−0.058 (−0.106 to −0.011) |
0.018* |
| Cystoid changes in inner layer |
0.113 (0.011 to 0.215) |
0.031* |
0.136 (0.004 to 0.276) |
0.044* |
| Cystoid changes in outer layer |
−0.021 (−0.127 to 0.084) |
0.688 |
0.004 (−0.132 to 0.141) |
0.948 |
| Cotton Ball Sign |
−0.030 (−0.132 to 0.072) |
0.561 |
−0.055 (−0.177 to 0.067) |
0.370 |
Simple linear regression analyses. BCVA, best-corrected visual acuity; CRT, central retinal thickness; IOP, intraocular pressure. *P < 0.05, **P < 0.01.
Multiple linear regression analyses adjusted for the preoperative BCVA were performed by referring to the results of the simple linear regression analyses (Table 3). The selected factors that were statistically significant in univariate analyses are shown in Table 2. After adjusting for the preoperative BCVA (Table 3, Supplementary Fig. 1), we found that worse visual outcomes at 1 month and 3 months were associated with older age (respective P values at 1 month and 3 months were 0.005 and 0.003), and shorter AL (P = 0.002 and P = 0.005). The other factors did not show significant relationships. The same results were also observed at 1 week (P = 0.001 for both, data not shown).
Table 3.
Predictors of visual outcome at Month 1 and 3 after pars plana vitrectomy in idiopathic epiretinal membrane.
| Variables | Month 1 | Month 3 | ||||
|---|---|---|---|---|---|---|
| B (95% CI) |
β | P Value | B (95% CI) |
β | P Value | |
| Age |
0.007 (0.002 to 0.012) |
0.228 | 0.005** |
0.010 (0.003 to 0.016) |
0.328 | 0.003** |
| Axial Length |
−0.046 (−0.075 to −0.017) |
−0.248 | 0.002** |
−0.059 (−0.099 to −0.019) |
−0.315 | 0.005** |
Multiple linear regression analyses adjusted for preoperative BCVA. The selected factors were age, axial length, preoperative CRT and cystoid changes in inner layer. The R square values for Months 1 and 3 were 0.44 and 0.37, respectively. **P < 0.01.
We subsequently divided the patients by their AL to find the cut-off value, which reflects the visual outcome (Fig. 2). In patients with AL < 23.6 mm, the postoperative mean BCVA was significantly worse than that in patients with AL ≥ 23.6 mm at 1 week and 1 and 3 months after surgery (P < 0.001, P = 0.001, P = 0.037 respectively), whereas the preoperative BCVA was not different between the two groups (P = 0.203). The mean postoperative BCVA in patients with AL ≥ 23.6 mm was better than the preoperative value at all time points. Other characteristics of patients with AL < 23.6 mm included an older age at the time of surgery and female predominance. However, there were no differences in the presence of preoperative OCT findings such as the cotton ball sign between patients with AL < 23.6 mm and those with AL ≥ 23.6 mm (Table 4, Supplementary Fig. 1). Gender was reanalysed using multiple linear regression analyses adjusted for the preoperative BCVA; however, it showed no association with the postoperative BCVA at 1 (P = 0.278) and 3 (P = 0.138) months (data not shown).
Figure 2.
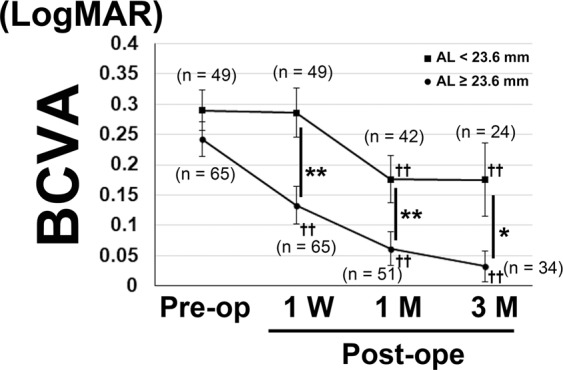
Mean best-corrected visual acuity (BCVA) in the patients with axial length (AL) < and ≥23.6 mm. Mann-Whitney U test was performed for comparing mean BCVA at each time point between the groups. In patients with AL <23.6 mm, the mean BCVAs were significantly worse at 1 week and 1- and 3-months postoperatively. Generalized mixed model analysis was performed for comparing the preoperative data with the 1-week and 1- and 3-month postoperative data. BCVA improved at 1 week after surgery in patients with AL ≥23.6 mm, while it was better than the preoperative value at 1 and 3 months after surgery in both groups. Data are shown as mean ± standard error. *P < 0.05, **P < 0.01 for comparisons between the groups. ††P < 0.01 for comparisons between preoperative and postoperative values at each time point.
Table 4.
Comparison of Parameters in Patients with Axial Length <23.6 mm and ≥23.6 mm.
| Variables | AL < 23.6 mm | AL ≥ 23.6 mm | P value |
|---|---|---|---|
| Baseline | |||
| Age | 74.31 ± 0.99 | 67.74 ± 0.87 | <0.001** |
| Gender (male, [%]) | 8 (16.3) | 35 (53.8) | <0.001** |
| Preoperative BCVA | 0.290 ± 0.03 | 0.242 ± 0.03 | 0.273 |
| Preoperative CRT | 444.55 ± 20.42 | 447.80 ± 15.09 | 0.896 |
| Cystoid changes in inner layer (eyes, [%]) | 35 (71.4) | 39 (60.0) | 0.206 |
| Cystoid changes in outer layer (eyes, [%]) | 15 (30.6) | 18 (27.7) | 0.734 |
| Cotton Ball Sign at the fovea (eyes, [%]) | 12 (24.5) | 22 (33.8) | 0.280 |
| Intra- and Post-operative Complications | |||
| Retinal breaks | 2 (4.1) | 7 (10.8) | 0.296 |
| Retinal hemorrhage | 3 (6.1) | 1 (1.5) | 0.313 |
| Vitreous hemorrhage | 1 (2.0) | 0 (0) | 0.430 |
| Postoperative refractive error | −0.73 ± 0.09 | −1.49 ± 0.21 | 0.003** |
Data are shown in mean ± SE. Student’s t-test, chi-square test, and Fisher’s exact test. Data of posterior vitreous detachment was obtained from surgical record at the beginning of the surgery. BCVA, best-corrected visual acuity; CRT, central retinal thickness. **P < 0.01.
With regard to age, patients aged ≥69 years exhibited worse postoperative BCVA than did those aged <69 years at 1 week (P = 0.003), and 1 (P = 0.010) and 3 (P = 0.024) months (number of patients with age ≥69 years and <69 years: 50 and 43 at 1 month and 30 and 28 at 3 months; Supplementary Fig. 2).
We also divided the patients into 4 groups on the basis of AL (<23.6 mm or ≥23.6 mm) and age (<69 or ≥69), and found significant differences among the four groups (Table 5). Although the preoperative BCVA showed no significant differences, the postoperative BCVA was worse in patients with AL < 23.6 mm and age ≥69 years than in those with AL ≥23.6 mm and age <69 years at 1 (P = 0.016) and 3 (P = 0.031) months.
Table 5.
Mean best-corrected visual acuity for patients stratified by age and axial length.
| Group | 1 | 2 | 3 | 4 | P value |
|---|---|---|---|---|---|
| Axial Length (mm) | <23.6 | <23.6 | ≥23.6 | ≥23.6 | |
| Age (yrs) | <69 | ≥69 | <69 | ≥69 | |
| Preoperative BCVA | 0.341 ± 0.10 | 0.276 ± 0.03 | 0.206 ± 0.03 | 0.286 ± 0.05 | 0.288 |
| Eyes | 11 | 38 | 36 | 29 | |
| Postoperative BCVA | |||||
| Month 1 | 0.196 ± 0.10 | 0.170 ± 0.04 | 0.001 ± 0.02 | 0.170 ± 0.06 | 0.006** |
| Eyes | 10 | 32 | 33 | 18 | (0.016*) |
| Month 3 | 0.134 ± 0.15 | 0.192 ± 0.06 | −0.016 ± 0.02 | 0.110 ± 0.05 | 0.038* |
| Eyes | 7 | 17 | 21 | 13 | (0.031*) |
Data are shown as mean ± SE. P values for differences among the four groups were derived by one-way analysis of variance. Post hoc Bonferroni correction analyses were applied for differences between groups 2 and 3 (P values in parentheses). *P < 0.05, **P < 0.01.
Representative data for patients with shorter and longer ALs are shown in Fig. 3(a–d). A 75-year-old woman exhibited an AL of 22.6 mm and worse postoperative BCVA relative to the average value (preoperative: 0.155, postoperative: 0.699 at 1 month and 0.523 at 3 months; Fig. 3a,b). In contrast, a 63-year-old woman exhibited an AL of 25.6 mm and good postoperative BCVA (preoperative: 0.097, postoperative: −0.079 at 1 and 3 months; Fig. 3c,d).
Figure 3.
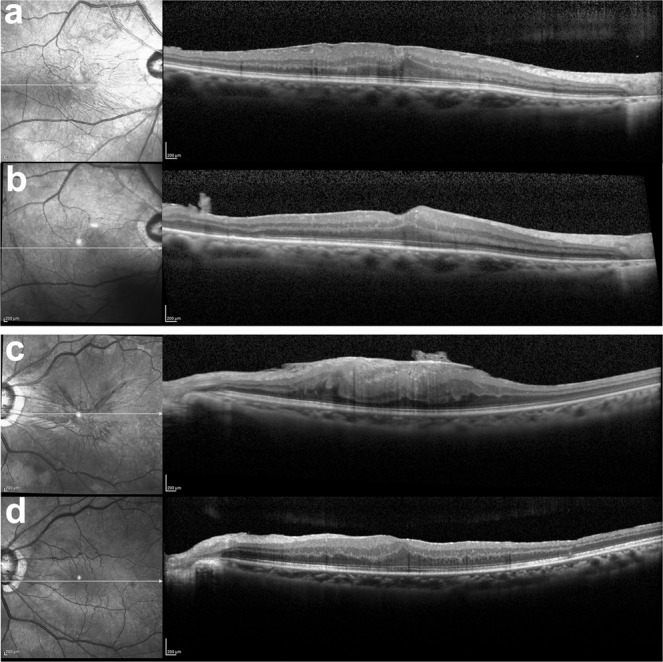
Representative preoperative optical coherent tomography (OCT) images of the eyes with idiopathic epiretinal membrane and an axial length (AL) < and ≥23.6 mm. (a,b) A 75-year-old woman exhibited an AL of 22.6 mm and worse postoperative BCVA relative to the average value (preoperative: 0.155, postoperative: 0.699 at 1 month and 0.523 at 3 months). (c,d) A 63-year-old woman exhibited an AL of 25.6 mm and good postoperative BCVA (preoperative: 0.097, postoperative: −0.079 at 1 and 3 months). Preoperative (a,c) and postoperative (b,d) OCT images at 1 month.
Discussion
In the present study, we found that patients with iERM exhibited an overall improvement in BCVA after PPV; however, the mean outcome value was worse for patients with a shorter AL and an older age after adjustment for the preoperative BCVA. Eyes with AL ≥ 23.6 mm and patients aged <69 years exhibited a significantly better postoperative BCVA than did eyes with AL < 23.6 mm and patients aged ≥69 years, respectively.
Simple linear regression analysis showed that better preoperative BCVA was related to better postoperative BCVA, which is consistent with the findings of previous reports7,9,17–24. Similarly, better pretreatment BCVA leading to better posttreatment BCVA is often reported in patients with retinal diseases such as diabetic macular oedema25,26. This is most likely because better pretreatment BCVA reflects lower levels of retinal neural damage that may be irreversible after treatment. Although the improvement in BCVA may be less in the eyes that had better preoperative BCVA due to the ceiling effect7,9,20,27, to obtain better visual outcomes, patients with iERM may benefit more if they undergo surgery before substantial BCVA loss. However, early intervention may not always be successful and lead to overtreatment, considering the risk of surgical complications. However, such complications have become rare with the recent advances in technology.
After adjusting for the preoperative BCVA, older age and a shorter AL affected the visual outcomes. The worse recovery of the retinal condition in older patients may be related to the fundamental vulnerability of the retinal neurons. Both photoreceptor cells28 and ganglion cell nerve fibres21 are reduced with age under physiological conditions. The tractional force of the iERM before surgery and/or the force at the time of removing iERM during surgery, may easily damage the retinal neurons in older patients. In fact, there is a report that eyes of older patients had a trend of exhibiting postoperative intraretinal cystoid changes, which could reflect neurodegeneration29–35 more frequently36. Patients with iERM may benefit by undergoing surgery at a relatively younger age. However, older patients also visit the clinics and require surgery. For older patients, when obtaining informed consent, it should be made clear to the patients to not have very high expectations; nonetheless, the patients may still undergo the surgery.
Interestingly, the eyes with a shorter AL had worse postoperative visual outcomes compared with those with a longer AL in iERM. This was significant 1 week after surgery (data not shown), and clearer at 1 and 3 months after surgery, adjusting for the preoperative BCVA. For patients with iERM, if the patient has a shorter AL, clinicians should be more prudent in recommending the surgery.
The mechanism of a shorter AL causing worse visual outcomes may be related to the tractional forces at the vitreoretinal interface caused by iERM. A previous study has shown that posterior vitreous detachment (PVD) is less frequently seen in eyes with a shorter AL in diabetic retinopathy patients37. Eyes with a shorter AL may have no PVD or macula-sparing PVD, which directly mediates vitreous tractional forces to the macula chronically (Fig. 4a). Moreover, iERM is gradually modified by the migration of Müller glial cells and cytokines such as transforming growth factor-beta from the retina, as reported previously38. The cell migration becomes easier when the tractional force is greater, and cytokines are reported to be concentrated in eyes with a shorter AL39, most probably because vitreous liquefaction has not progressed much; this slows the turnover of soluble factors40. Thus, iERM may have undergone severe modifications in eyes with a shorter AL, and these iERMs may have exhibited stronger adhesion to the retina with greater tractional force. In addition, under these conditions, the surgical procedure for intentionally detaching the vitreous and/or iERM may have acutely exerted a greater tractional force on the retina. These chronic and/or acute tractional forces may damage the neural retina in the macular region substantially by the end of the surgery. This could occur easily in the eyes with a shorter AL. In fact, the sticky adhesion of iERM to the retina or unintended en bloc resection of iERM and the internal limiting membrane (ILM) because of strong adhesion was often observed in the surgical records of patients with a shorter AL. We found these intraoperative findings in the surgical records of 53% eyes with a shorter AL and 20% eyes with a longer AL (as far as we could find the comments by the surgeons). In contrast, eyes with a relatively longer AL may easily develop PVD or vitreous schisis with vitreous liquefaction, which causes lesser traction on the retina and/or lesser modification of the iERM, thus resulting in lesser damage to the macula (Fig. 4b).
Figure 4.
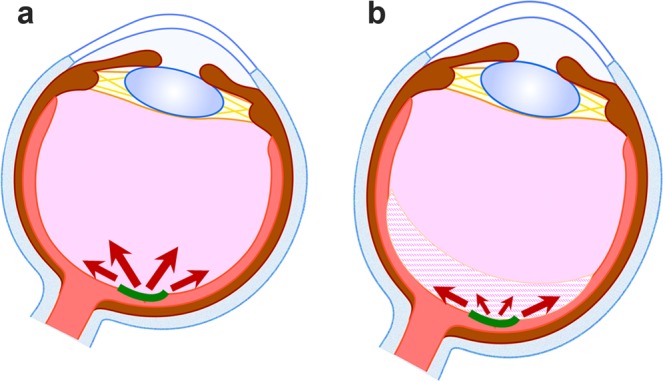
A hypothetical mechanism for the differences related to the tractional force of the retina according to the axial length (AL). (a) Eyes with a shorter AL may have undetached or incompletely detached posterior vitreous, which directly mediates vitreous tractional force to the macula chronically (arrows). Under this condition, idiopathic epiretinal membrane (iERM) modification by glial cells and cytokines may occur easily; this further strengthens the adhesion of iERM to the retina and increases the tractional force. In addition, the surgical procedure to intentionally detach the vitreous and/or iERM may mediate greater tractional force to the retina acutely, which induces more damage to the macula. (b) In contrast, eyes with relatively longer AL may already have posterior vitreous detachment or vitreous schisis (shaded area), which causes less traction (arrows) and less damage to the macula. iERM is shown in green.
The shorter AL group in our study involved older patients; this could be a confounding factor for a worse visual outcome. However, this also indicated that surgery for iERM may be required after a certain age in patients with a shorter AL, probably because of differences in the vitreous condition. Eyes with a shorter AL may take longer to develop iERM related to later PVD occurrence. Given that iERM is of two different types based on the extracellular matrix41, differences in the developmental course may result in differences in the components and characteristics of iERM.
The findings related to the outer retina and photoreceptor cells, such as the cystoid changes in the outer retinal layer and cotton ball sign42 were not included in the predictive factors of visual outcomes in the current study, although were analysed. This would be because the surgical application for iERM in the current study was early and most eyes had not yet developed these findings by the time of surgery. Therefore, AL, which is not related to the disease stage, could be a valuable factor in determining the surgery considering the recent trend of giving more importance to a satisfaction of the patients. Eyes with a shorter AL may have retinal damages and/or may have been damaged at the time of surgery, making them less likely to have better outcomes than expected. Given that good outcomes cannot be anticipated with the current surgical applications, whether the eyes with a shorter AL should be treated earlier (i.e. before having retinal damage) or treated later (i.e. after BCVA impairment has progressed and intervention has become unavoidable) would be the topic for future discussion.
The limitations of the current study were the small sample size, retrospective study design, and relatively short follow-up duration. However, patients with iERM may have rapid recovery of BCVA under the recent early indication of the surgery. In fact, the mean BCVA reached a plateau at 3 months after surgery, with no change between 3 and 6 months (paired t-test, P = 0.394; data not shown). Also, patients who showed a good clinical course were referred back to their original clinics soon after surgery; thus, the number of participants decreased with time. This may have resulted in selection bias. Furthermore, the presence or absence of PVD was not determined because of the retrospective design. A recent report showed PVD by focusing on the vitreous at the time of scanning using a wide-angle OCT system43; thus, a future prospective study is warranted. Although we found surgical records regarding iERM adhesion in many of the patients, they were not from all patients. Although ILM was peeled as far as possible, it may have remained in some patients. Cataract surgery was performed at the same time as PPV as is usual in Japan, although cataract was mild and of the same grade in all patients and most likely caused no differences in the outcomes of the current study.
Early treatment for iERM may be accepted universally considering the significant progress of surgical technology and the better visual outcomes in patients with better preoperative BCVA7, as previously reported. However, patients with a shorter AL may not necessarily show the expected postoperative BCVA and may have a limited outcome, most likely because patients with iERM may be susceptible to iERM-related retinal damage. The current study may help in evaluating surgical indications in each patient to obtain better and satisfactory outcomes irrespective of the pathological stage of iERM reflected in the preoperative BCVA, although further long-term studies with an increased number of patients are warranted.
Methods
Patients
This was a retrospective study performed at the Vitreo-Retina Surgical Division Clinic of the Department of Ophthalmology at Keio University Hospital. Informed consent was obtained from all subjects after providing them with an explanation of the procedures to be used in the present study. The procedures adhered to the tenets of the Declaration of Helsinki, and approval to perform this study was obtained from the Keio University School of Medicine Ethics Committee (Approval number: 20100003).
Inclusion and exclusion criteria
One hundred fourteen eyes of 114 patients who underwent the surgery between March 2012 and March 2018 due to subjective visual disturbances resulting from iERM were studied. The eyes with (1) media opacities, which result in the acquisition of poor OCT images; (2) a secondary ERM caused by conditions such as diabetic retinopathy, retinal vein occlusion, retinal tear and detachment, uveitis, and trauma; (3) a severe cataract of more than grade 3 nuclear sclerosis or cortical opacity; and (4) a high myopia (AL ≥ 26.5 mm) were excluded. High myopia was excluded to avoid the inclusion of pathological myopia.
Eye examinations
All patients underwent complete ophthalmologic examinations, including measurement of BCVA using a Landolt C chart, biomicroscopy of the fundus, fundus photography, and OCT (see below), pre- and post-operatively. An IOL Master (Carl Zeiss Meditec, CA, USA) was used for preoperative AL measurement.
OCT
The OCT images were obtained with spectral domain-OCT (Heidelberg Spectralis OCT, Dossenheim, Germany). After dilatation of the pupils, the patients were asked to fixate on a target, and both horizontal and vertical images were recorded at baseline (before the surgery), as well as 1 week and 1, 3, and 6 months after the surgery. The CRT was defined as the distance between the inner retinal surface and the inner border of the retinal pigment epithelium and was measured with the built-in scale in the OCT system. Cystoid changes were defined in the vertical and horizontal OCT images within 1500 μm from the fovea. The inner layer of the retina represents the layers from the internal limiting membrane to the inner nuclear layer, and the outer layer represents the outer plexiform layer to the inner border of the retinal pigment epithelium. The cotton ball sign42,44 was defined in the OCT images according to previous reports. The findings were defined by two retina specialists (SM and YO).
Surgery
In all patients, phacoemulsification with intraocular lens implantation was performed for mild cataract, followed by PPV for iERM with 25-gauge 3-port vitrectomy using forceps by experienced surgeons (HS and YO). Intentional ILM peeling was performed in occasional cases where en bloc resection of ERM and ILM was not performed.
Statistical analyses
Data are expressed as mean ± SE. Generalized mixed model analyses, Mann-Whitney U test, simple linear regression analyses, multiple linear regression analyses adjusted for the preoperative BCVA using the forced input method, Student’s t-test, chi-square test, Fisher’s exact test, and Pearson correlation coefficient were performed using SPSS software (version 25.0, SPSS Japan, Tokyo, Japan). A P value of < 0.05 was considered statistically significant.
Approval, accordance and informed consent statements
The study adhered to the tenets of the Declaration of Helsinki, and approval to perform this study was obtained from the Keio University School of Medicine Ethics Committee (Approval number: 20100003). Informed consent was obtained from all the participants.
Supplementary information
Acknowledgements
The authors thank all the clinical staff members for assistance at the Medical Retina Clinic.
Author contributions
Conception and designs: S.M. and Y.O. Data collection: S.M., H.S., Y.S., K.W. and Y.O. Analysis and interpretation: S.M. Review the manuscript: N.N., T.K., H.S., H.T. and K.T. Overall responsibility: Y.O.
Data availability
The protocol and the datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-55544-6.
References
- 1.Fraser-Bell S, Guzowski M, Rochtchina E, Wang JJ, Mitchell P. Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology. 2003;110:34–40. doi: 10.1016/S0161-6420(02)01443-4. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe NS. Vitreous traction at the posterior pole of the fundus due to alterations in the vitreous posterior. Transactions - American Academy of Ophthalmology and Otolaryngology. American Academy of Ophthalmology and Otolaryngology. 1967;71:642–652. [PubMed] [Google Scholar]
- 3.Wise GN. Relationship of idiopathic preretinal macular fibrosis to posterior vitreous detachment. American journal of ophthalmology. 1975;79:358–362. doi: 10.1016/0002-9394(75)90606-6. [DOI] [PubMed] [Google Scholar]
- 4.SMIDDY WILLIAM E., MAGUIRE ALBERT M., GREEN W RICHARD, MICHELS RONALD G., DE LA CRUZ ZENAIDA, ENGER CHERYL, JAEGER MICHELLE, RICE THOMAS A. Idiopathic Epiretinal Membranes. Retina. 2005;25(Supplement):811–821. doi: 10.1097/00006982-200507001-00012. [DOI] [PubMed] [Google Scholar]
- 5.Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vitreomacular traction syndrome. The British journal of ophthalmology. 2002;86:902–909. doi: 10.1136/bjo.86.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishi Y, et al. Detection of early visual impairment in patients with epiretinal membrane. Acta ophthalmologica. 2013;91:e353–357. doi: 10.1111/aos.12060. [DOI] [PubMed] [Google Scholar]
- 7.Scheerlinck LM, van der Valk R, van Leeuwen R. Predictive factors for postoperative visual acuity in idiopathic epiretinal membrane: a systematic review. Acta ophthalmologica. 2015;93:203–212. doi: 10.1111/aos.12537. [DOI] [PubMed] [Google Scholar]
- 8.Mitamura Y, Hirano K, Baba T, Yamamoto S. Correlation of visual recovery with presence of photoreceptor inner/outer segment junction in optical coherence images after epiretinal membrane surgery. The British journal of ophthalmology. 2009;93:171–175. doi: 10.1136/bjo.2008.146381. [DOI] [PubMed] [Google Scholar]
- 9.Inoue M, et al. Preoperative inner segment/outer segment junction in spectral-domain optical coherence tomography as a prognostic factor in epiretinal membrane surgery. Retina (Philadelphia, Pa.) 2011;31:1366–1372. doi: 10.1097/IAE.0b013e318203c156. [DOI] [PubMed] [Google Scholar]
- 10.Cobos E, et al. Preoperative study of the inner segment/outer segment junction of photoreceptors by spectral-domain optical coherence tomography as a prognostic factor in patients with epiretinal membranes. Clinical ophthalmology (Auckland, N.Z.) 2013;7:1467–1470. doi: 10.2147/opth.S44837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Kim YM, Chung EJ, Lee SY, Koh HJ. Structural and functional predictors of visual outcome of epiretinal membrane surgery. American journal of ophthalmology. 2012;153:103–110.e101. doi: 10.1016/j.ajo.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Sonoda KH, et al. Residual vitreous cortex after surgical posterior vitreous separation visualized by intravitreous triamcinolone acetonide. Ophthalmology. 2004;111:226–230. doi: 10.1016/j.ophtha.2003.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Peyman GA, Cheema R, Conway MD, Fang T. Triamcinolone acetonide as an aid to visualization of the vitreous and the posterior hyaloid during pars plana vitrectomy. Retina (Philadelphia, Pa.) 2000;20:554–555. doi: 10.1097/00006982-200005000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Ng DS, et al. Advances of optical coherence tomography in myopia and pathologic myopia. Eye (London, England) 2016;30:901–916. doi: 10.1038/eye.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchida A, et al. Vitrectomy for myopic foveoschisis with internal limiting membrane peeling and no gas tamponade. Retina (Philadelphia, Pa.) 2014;34:455–460. doi: 10.1097/IAE.0b013e3182a0e477. [DOI] [PubMed] [Google Scholar]
- 16.Spaide RF, Fisher Y. Removal of adherent cortical vitreous plaques without removing the internal limiting membrane in the repair of macular detachments in highly myopic eyes. Retina (Philadelphia, Pa.) 2005;25:290–295. doi: 10.1097/00006982-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Fernandez M, Castro Navarro J, Gonzalez Castano C, Garcia Alonso A, Fonolla Gil M. Epiretinal membrane surgery: anatomic and functional outcomes. Archivos de la Sociedad Espanola de Oftalmologia. 2013;88:139–144. doi: 10.1016/j.oftal.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Shiono A, et al. Photoreceptor outer segment length: a prognostic factor for idiopathic epiretinal membrane surgery. Ophthalmology. 2013;120:788–794. doi: 10.1016/j.ophtha.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 19.Brito PN, et al. Possible role for fundus autofluorescence as a predictive factor for visual acuity recovery after epiretinal membrane surgery. Retina (Philadelphia, Pa.) 2014;34:273–280. doi: 10.1097/IAE.0b013e3182999a02. [DOI] [PubMed] [Google Scholar]
- 20.Kunikata H, Abe T, Kinukawa J, Nishida K. Preoperative factors predictive of postoperative decimal visual acuity>/= 1.0 following surgical treatment for idiopathic epiretinal membrane. Clinical ophthalmology (Auckland, N.Z.) 2011;5:147–154. doi: 10.2147/opth.S15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asaria R, Garnham L, Gregor ZJ, Sloper JJ. A prospective study of binocular visual function before and after successful surgery to remove a unilateral epiretinal membrane. Ophthalmology. 2008;115:1930–1937. doi: 10.1016/j.ophtha.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Falkner-Radler CI, Glittenberg C, Hagen S, Benesch T, Binder S. Spectral-domain optical coherence tomography for monitoring epiretinal membrane surgery. Ophthalmology. 2010;117:798–805. doi: 10.1016/j.ophtha.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita T, et al. Time course of changes in metamorphopsia, visual acuity, and OCT parameters after successful epiretinal membrane surgery. Investigative ophthalmology & visual science. 2012;53:3592–3597. doi: 10.1167/iovs.12-9493. [DOI] [PubMed] [Google Scholar]
- 24.Sheales MP, Kingston ZS, Essex RW. Associations between preoperative OCT parameters and visual outcome 3 months postoperatively in patients undergoing vitrectomy for idiopathic epiretinal membrane. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2016;254:1909–1917. doi: 10.1007/s00417-016-3326-x. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, et al. Predictive factors of better outcomes by monotherapy of an antivascular endothelial growth factor drug, ranibizumab, for diabetic macular edema in clinical practice. Medicine. 2017;96:e6459. doi: 10.1097/md.0000000000006459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sophie R, Lu N, Campochiaro PA. Predictors of Functional and Anatomic Outcomes in Patients with Diabetic Macular Edema Treated with Ranibizumab. Ophthalmology. 2015;122:1395–1401. doi: 10.1016/j.ophtha.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 27.Nitta E, et al. Displacement of the retina and its recovery after vitrectomy in idiopathic epiretinal membrane. American journal of ophthalmology. 2013;155:1014–1020.e1011. doi: 10.1016/j.ajo.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Investigative ophthalmology & visual science. 1993;34:3278–3296. [PubMed] [Google Scholar]
- 29.Zur D, et al. Disorganization of Retinal Inner Layers as a Biomarker for Idiopathic Epiretinal Membrane After Macular Surgery-The DREAM Study. American journal of ophthalmology. 2018;196:129–135. doi: 10.1016/j.ajo.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 30.Frisina R, et al. Cystoid macular edema after pars plana vitrectomy for idiopathic epiretinal membrane. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2015;253:47–56. doi: 10.1007/s00417-014-2655-x. [DOI] [PubMed] [Google Scholar]
- 31.Gaudric A. Macular cysts, holes and cavitations: 2006 Jules Gonin lecture of the Retina Research Foundation. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2008;246:1071–1079. doi: 10.1007/s00417-008-0818-3. [DOI] [PubMed] [Google Scholar]
- 32.Johnson MW. Tractional cystoid macular edema: a subtle variant of the vitreomacular traction syndrome. American journal of ophthalmology. 2005;140:184–192. doi: 10.1016/j.ajo.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Koizumi H, et al. Three-dimensional evaluation of vitreomacular traction and epiretinal membrane using spectral-domain optical coherence tomography. American journal of ophthalmology. 2008;145:509–517. doi: 10.1016/j.ajo.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Burggraaff MC, Trieu J, de Vries-Knoppert WA, Balk L, Petzold A. The clinical spectrum of microcystic macular edema. Investigative ophthalmology & visual science. 2014;55:952–961. doi: 10.1167/iovs.13-12912. [DOI] [PubMed] [Google Scholar]
- 35.Reichenbach A, et al. Muller cells as players in retinal degeneration and edema. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2007;245:627–636. doi: 10.1007/s00417-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 36.Leisser C, et al. Risk factors for postoperative intraretinal cystoid changes after peeling of idiopathic epiretinal membranes among patients randomized for balanced salt solution and air-tamponade. Acta ophthalmologica. 2018;96:e439–e444. doi: 10.1111/aos.13635. [DOI] [PubMed] [Google Scholar]
- 37.Song WK, et al. Axial length and intraoperative posterior vitreous detachment as predictive factors for surgical outcomes of diabetic vitrectomy. Eye (London, England) 2010;24:1273–1278. doi: 10.1038/eye.2009.332. [DOI] [PubMed] [Google Scholar]
- 38.Kanda A, Noda K, Hirose I, Ishida S. TGF-beta-SNAIL axis induces Muller glial-mesenchymal transition in the pathogenesis of idiopathic epiretinal membrane. Scientific reports. 2019;9:673. doi: 10.1038/s41598-018-36917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Q, et al. Intravitreal vascular endothelial growth factor concentration and axial length. Retina (Philadelphia, Pa.) 2015;35:435–439. doi: 10.1097/iae.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 40.Kim MS, Park SJ, Park KH, Woo SJ. Different Mechanistic Association of Myopia with Rhegmatogenous Retinal Detachment between Young and Elderly Patients. BioMed research international. 2019;2019:5357241. doi: 10.1155/2019/5357241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kritzenberger M, et al. Different collagen types define two types of idiopathic epiretinal membranes. Histopathology. 2011;58:953–965. doi: 10.1111/j.1365-2559.2011.03820.x. [DOI] [PubMed] [Google Scholar]
- 42.Tsunoda K, Watanabe K, Akiyama K, Usui T, Noda T. Highly reflective foveal region in optical coherence tomography in eyes with vitreomacular traction or epiretinal membrane. Ophthalmology. 2012;119:581–587. doi: 10.1016/j.ophtha.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 43.Tsukahara M, Mori K, Gehlbach PL, Mori K. Posterior Vitreous Detachment as Observed by Wide-Angle OCT Imaging. Ophthalmology. 2018;125:1372–1383. doi: 10.1016/j.ophtha.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 44.Govetto A, et al. Tractional Abnormalities of the Central Foveal Bouquet in Epiretinal Membranes: Clinical Spectrum and Pathophysiological Perspectives. American journal of ophthalmology. 2017;184:167–180. doi: 10.1016/j.ajo.2017.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The protocol and the datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.