Abstract
Background:
Perioperative hypothermia is linked to multiple postoperative complications including increased surgical bleeding, surgical site infection, myocardial events, and increased length of hospital stay. The purpose of this study is to determine the effects of forced-air warming blanket position, above the shoulders versus under the trunk/legs, on intraoperative core body temperature and perioperative complications in elective lumbar spine surgery.
Methods:
After IRB approval, patients were enrolled in a consecutive fashion and randomized to either upper body (Group I) or lower body (Group II) groups. Primary outcomes were intraoperative body temperature, incidence of hypothermia, postoperative complications, and infection. Secondary outcomes included blood loss, operative time, and length of stay.
Results:
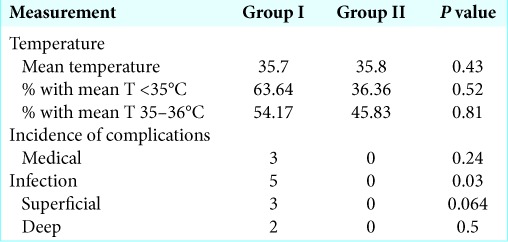
Seventy-four patients were included (Group I, 38; Group II, 36, mean age 60.7 years, 54% of male). Average patient follow-up was 69 ± 33.6 days in Group I and 67 ± 34.6 days in Group II. Average intraoperative body temperature was 35.7 in Group I and 35.8 in Group II (P = 0.27). Incidence of critical hypothermia (T < 35°C) was 18.4% and 11.1% in Groups I and II, respectively (P = 0.52). Incidence of mild hypothermia (T: 35°C–36°C) was 34.2% and 30.56% in Groups I and II, respectively (P = 0.81). Separately, pooled analysis comparing average body temperature and incidence infection demonstrated a relationship between mild hypothermia and infection (P = 0.03).
Conclusion:
Compared to using a lower body Bair Hugger under the patient, using standard upper body Bair Hugger may be associated with increased surgical site infection. Given equivalent body warming, we recommend using the lower body Bair Hugger to avoid infection.
Keywords: Elective lumbar surgery, Intraoperative warmer, Perioperative hypothermia

INTRODUCTION
Perioperative hypothermia is defined as a core temperature below 36°C and may occur during surgery for a number of reasons. In the setting of low operating temperature, anesthesia interferes with the body’s natural thermoregulatory processes.[2] Cold-induced vasoconstriction is inhibited, and loss of sympathetic tone causes heat to move from the core to the periphery. This may result in a decrease in core temperature up to 6.[2,5] Interestingly, perioperative hypothermia is linked to multiple postoperative complications, including increased surgical bleeding, surgical site infection, myocardial events, and increased length of hospital stay.[2]
Potential preventative measures utilized in many operating rooms include warming the patient preoperatively, increasing the temperature in the operating room, active warming during surgery, passive warming during surgery, prewarming intravenous fluids and blood products, prewarming irrigation fluids, and prewarming administered airway gases.[5] Active warming through forced-air warming blankets, such as Bair Hugger®, is an effective method for combating intraoperative hypothermia.[1,5] Despite the wide use of forced-air warming blankets, there are no guidelines for where to position the blanket during spine surgery, either below the lower body or over the upper body [Figure 1]. We aimed to determine the effects of Bair Hugger positioning during elective lumbar spine surgery on intraoperative body temperature and perioperative complications.
Figure 1:
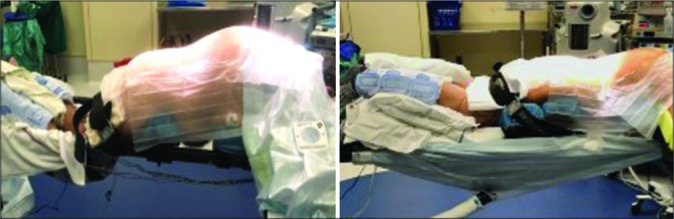
Image showing proper Bair Hugger placement. Left image shows upper body Bair Hugger (Group I), right image shows lower body Bair Hugger (Group II).
MATERIALS AND METHODS
Data collection
Following IRB approval, a prospective, randomized control trial was undertaken at a single institution. Consecutive patients 18–75 years of age undergoing elective lumbar spine surgery between December 2017 and May 2018 were enrolled in this study. Exclusion criteria included: (1) history of arterial or venous thromboembolic disease, (2) known heritable hypercoagulable state, (3) active infection at the surgical site, (4) intravascular clotting disorder, (5) active malignancy, and (6) receipt of intraoperative blood products. Patient was randomized to either having an upper body Bair Hugger placed on top of upper back and arms (Group I) or to having a lower body Bair Hugger secured under the torso and legs (Group II) using an online random integer generator (Random.org). Our primary outcomes were average intraoperative body temperature, incidence of hypothermia, postoperative medical complications, and postoperative infection. Secondary outcomes included operative blood loss and length of stay. Demographic information, medical comorbidities, number of procedure levels, procedure type (decompression vs. decompression and fusion), intraoperative temperature data, estimated blood loss, and postoperative complications, specifically medical complications and infection, were retrieved from the patients’ electronic medical record. Intraoperative temperature was measured by temperature sensing Foley catheter at 15-min intervals per institutional anesthesia protocol.
Statistical analysis
All data were entered into a Microsoft Excel spreadsheet (Microsoft Excel, Microsoft Office, Redmond, Washington). The alpha level for statistical significance was set a priori at 0.05. All continuous data were assessed for normality through observation of plots and with Shapiro–Wilk tests. In cases, where normality assumptions were not met, nonparametric testing was conducted. Descriptive statistics including mean, range, and standard deviation were calculated for all continuous variables; ratios and percentages were calculated for categorical variables. For continuous variables, an independent t-test was used to determine differences between subjects who had the warmer placed over the upper body or below the lower body. For categorical variables, Fisher’s exact test was used to determine associations between subjects who had their warmer placed over the upper body versus below the lower body. For those distributions that did not demonstrate a normal distribution, Kruskal–Wallis test was used as the nonparametric alternative for an independent t-test. All analyses were performed using JMP pro statistical software (JMP pro, Version 13, SAS Institute Inc., Cary, North Carolina).
RESULTS
Randomization
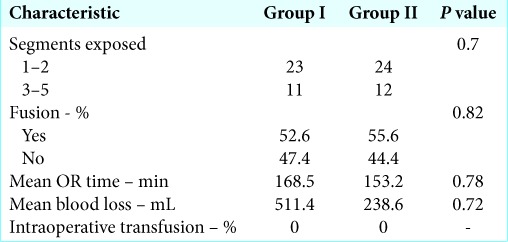
40 patients were enrolled into Group I and 38 were enrolled into Group II. Two patients were excluded from Group I because anterior approaches were performed, and two patients were excluded from Group II due to an anterior approach in one and the presence of a pathologic fracture in the other. 38 patients and 36 patients were included for analysis in Groups I and II, respectively. There were no statistically significant differences between Groups I and II in terms of patient age, body mass index, sex, race, smoking status, or preoperative medical comorbidities, except for higher prevalence of coronary artery disease in Group I (21.1% vs. 2.8%, P = 0.03). There were no statistically significant differences in the prevalence of DM, hypertension, COPD, CHF, renal disease, coagulopathy, or HIV [Table 1]. Patients were categorized by number of levels operated, 1–2 versus 3–5 versus >5 (P = 0.70), as well as categorized by procedure type, laminectomy alone vs. laminectomy, and fusion (P = 0.82). There were no statistically significant differences between Groups I and II in terms of levels of surgery or procedure type [Table 2]. There were no statistically significant differences between Groups I and II in terms of operative time (P = 0.87) or estimated operative blood loss (P = 0.33). Average patient follow-up was 69 days in Group I and 67 days in Group II.
Table 1:
Demographic data for patient in Groups I and II.
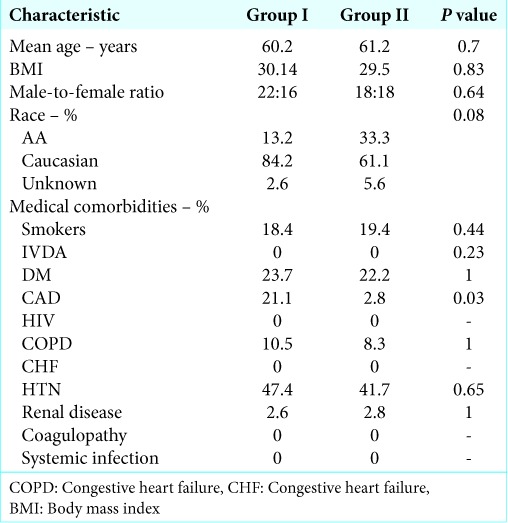
Table 2:
Perioperative surgical data including segments exposed, fusion status, OR time, blood loss, and intraoperative transfusion rate.
Temperature
Patient body temperature date is summarized in Table 3. Average body temperature was 35.7 in Group I and 35.8 in Group II (P = 0.27). Subgroup analysis of average body temperature <35°C (critical hypothermia) showed an incidence of critical hypothermia of 18.4% and 11.1% in Groups I and II, respectively (P = 0.52). Subgroup analysis performed for average body temperature between 35°C and 36°C (mild hypothermia) showed an incidence of mild hypothermia of 34.2% and 30.56% in Groups I and II, respectively (P = 0.81).
Table 3:
Core body temperature measurements for patients in Groups I and II.
Complications
Three patients in Group I or more had a medical complication while inpatient. These included pulmonary embolism, acute kidney injury, symptomatic anemia, and delirium. No patient in Group II had an inpatient medical complication (P = 0.11). 5 of 38 patients in Group I and 0 of 36 patients in Group II had either late superficial or deep postoperative infection (P = 0.02).
Pooled patient analysis
Interestingly, when data from Groups I and II were pooled to compare operative blood loss among patient with any hypothermia versus normothermia (<36°C vs. ≥36°C), patient with normothermia had an increased likelihood of having EBL >300 cc (0.049) [Figure 2]. In similar analysis, patient with mild hypothermia was not more or less likely to have EBL >300. Patient critical hypothermia was less likely than other patients to have EBL <300. Separately, pooled analysis comparing average body temperature and incidence infection demonstrated a relationship between mild hypothermia and infection (P = 0.03), but not critical hypothermia (P = 1.0).
Figure 2:
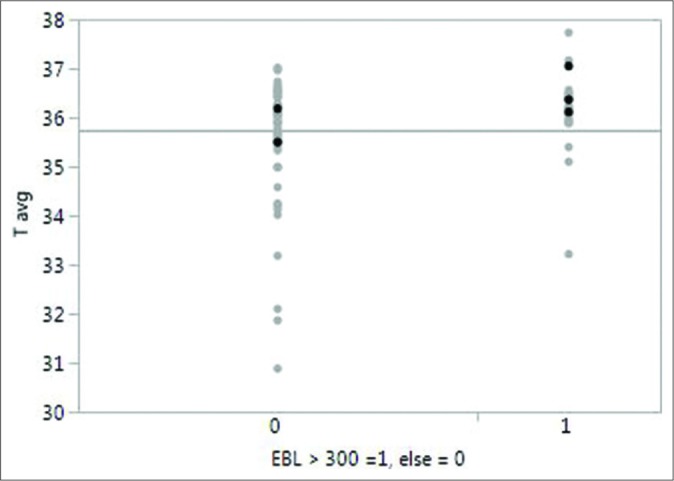
Graph showing the distribution of core body temperature measurements in patients with greater than and less than 300 cc of operative blood loss.
DISCUSSION
To the best of our knowledge, this is the first study to evaluate the effects of Bair Hugger position on intraoperative body temperature during and postoperative complications after elective lumbar spine surgery. We found a higher incidence of postoperative infection in patients warmed with a standard upper body warmer compared to those warmed with a lower body warmer placed under the patient. Secondary analysis of our data demonstrated a relationship between mild hypothermia and infection, as well as an unexpected correlation between normothermia and increased blood loss. There was no difference in incidence of hypothermia between Group I and Group II.
In recent years, there has been a concern that forced-air warming blankets may be a source of surgical site infection. Literature on the subject is limited and overall suggests that the benefit of maintaining euthermia outweighs the risk of infection. To date, literature has suggested that Bair Hugger does not cause postoperative infections. Reed et al. analyzed the forced-air warming hoses and found them to have a high concentration of microbes.[6] Moretti et al. conducted a comparative study of 20 hip arthroplasty patients warmed with Bair Hugger versus 10 patients who underwent surgery without forced-air warms.[4] They found an increased bacterial burden in operating room air with the Bair Hugger but no difference in postoperative incidence infection. We did find a correlation between upper body Bair Hugger use and surgical site infection in elective posterior lumbar spine surgery compared to using a lower body warmer under the trunk and legs. This may be related to proximity of the blanket to the surgical field in combination with the unique physiology of prone position and the fact that patient lay on lumbar wounds, particularly those patients who are slow to mobilize. All of our procedures we performed using traditional midline open approaches.
Few studies have linked intraoperative hypothermia to postoperative infection. The colorectal surgery literature has shown hypothermia to correlate with a 3-fold increase in surgical site.[2,3,7] Our study found a correlation between mild hypothermia and postoperative infection, corroborating the current literature. Similar to spine surgery, the surgical site in colorectal surgery is in the body’s core. A 0.5–1.5°C decrease in core body temperature typically occurs during surgery because anesthesia-related peripheral vasodilation and core to periphery redistribution of body heat, as well as loss of normal thermoregulation mechanism.[1,9] Forced-air warmers are a common and effective for maintaining core body temperature.[10] If given the option between using a standard upper body Bair Hugger versus lower body Bair Hugger, we recommend using the lower body Bair Hugger to obviate any increased risk of infection.
Literature has also shown mild hypothermia to correlate with increased blood loss.[7,9] Mild hypothermia results in platelet dysfunction and impaired clotting. In our study, patient with normothermia had increased blood loss; patients with hypothermia did not. The reason for this discrepancy is not clear. It may be due to the location of surgery, periphery versus core. The previous research found high blood loss with hypothermia in hip arthroplasty.[8] Higher body temperature in spine surgery might decrease peripheral vasodilatation and increase core blood loss.
Strengths of our study include its prospective randomized study design and its evaluation of Bair Hugger position during lumbar spine surgery, which has not been studied in literature. Limitations of this study include small sample size and relatively short follow-up (<1 year). Future research will delineate the true relationship between blood loss and core body temperature. Future research will also compare the placement of lower body Bair Hugger under the legs versus on top of the legs for efficacy and complication profile.
CONCLUSION
Compared to using a lower body Bair Hugger under the patient, the use of a standard upper body Bair Hugger may be associated with increased surgical site infection. Given equivalent body warming, we commend using the lower body Bair Hugger to avoid infection.
Acknowledgment
We thank Mohammad Farooq Usmani for his assistance in preparation and submission of the manuscript.
Footnotes
How to cite this article: Buraimoh MA, Nash A, Howard B, Yousaf I, Koh E, Banagan K, et al. Effect of forced-air warming blanket position in elective lumbar spine surgery: Intraoperative body temperature and postoperative complications. Surg Neurol Int 2019;10:229.
Contributor Information
Morenikeji Ayodele Buraimoh, Email: aburaimoh@gmail.com.
Alysa Nash, Email: umdorthospine@gmail.com.
Bailey Howard, Email: bailey.howard@som.umaryland.edu.
Imran Yousaf, Email: imranyousaf@gmail.com.
Eugene Koh, Email: ekoh@som.umaryland.edu.
Kelley Banagan, Email: kbanagan@som.umaryland.edu.
Daniel Gelb, Email: dgelb@som.umaryland.edu.
David Schreibman, Email: dschreibman@som.umaryland.edu.
Steven C. Ludwig, Email: sludwig@som.umaryland.edu.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Allen MW, Jacofsky DJ. Normothermia in arthroplasty. J Arthroplasty. 2017;32:2307–14. doi: 10.1016/j.arth.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Hart SR, Bordes B, Hart J, Corsino D, Harmon D. Unintended perioperative hypothermia. Ochsner J. 2011;11:259–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of wound infection and temperature group. N Engl J Med. 1996;334:1209–15. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 4.Moretti B, Larocca AM, Napoli C, Martinelli D, Paolillo L, Cassano M, et al. Active warming systems to maintain perioperative normothermia in hip replacement surgery: A therapeutic aid or a vector of infection? J Hosp Infect. 2009;73:58–63. doi: 10.1016/j.jhin.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Park FD, Park S, Chi SI, Kim HJ, Seo KS, Kim HJ, et al. Clinical considerations in the use of forced-air warming blankets during orthognathic surgery to avoid postanesthetic shivering. J Dent Anesth Pain Med. 2015;15:193–200. doi: 10.17245/jdapm.2015.15.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed M, Kimberger O, McGovern PD, Albrecht MC. Forced-air warming design: Evaluation of intake filtration, internal microbial buildup, and airborne-contamination emissions. AANA J. 2013;81:275–80. [PubMed] [Google Scholar]
- 7.Reynolds L, Beckmann J, Kurz A. Perioperative complications of hypothermia. Best Pract Res Clin Anaesthesiol. 2008;22:645–57. doi: 10.1016/j.bpa.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–92. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- 9.Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109:318–38. doi: 10.1097/ALN.0b013e31817f6d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torossian A. Thermal management during anaesthesia and thermoregulation standards for the prevention of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2008;22:659–68. doi: 10.1016/j.bpa.2008.07.006. [DOI] [PubMed] [Google Scholar]


