Abstract
Most published studies addressing the role of HIFs in hypoxia-induced PH development employ models that may not recapitulate the clinical setting, including the use of animals having pre-existing lung/vascular defects secondary to embryonic HIF ablation or activation. Further, critical questions including how and when HIF-signaling contributes to hypoxia-induced PH remains unanswered.
Normal adult rodents in which global HIF1 or 2 was inhibited by inducible gene deletion or pharmacological inhibition (antisense oligonucleotides-ASO and small molecule inhibitors) were exposed to short-term (4 days) or chronic (4–5 weeks) hypoxia. Hemodynamic studies were performed, the animals euthanized and lungs and heart obtained for pathologic and transcriptomic analysis. Cell-type specific HIF signals for PH initiation were determined in normal pulmonary vascular cells in vitro and in mice (using cell-type specific HIF deletion).
Global HIF1α deletion in mice did not prevent hypoxia-induced PH at 5 weeks. Mice with global HIF2α deletion did not survive long-term hypoxia. Partial HIF2α gene deletion, or HIF2-ASO (but not HIF1-ASO) reduced vessel muscularization, rises in pulmonary artery pressures and right ventricular hypertrophy in mice exposed to 4–5 week hypoxia. A small molecule HIF2 inhibitor (PT2567) significantly attenuated early events (monocyte recruitment and vascular cell proliferation) in rats exposed to 4-day hypoxia as well as vessel musculization, tenascin C accumulation and PH development in rats exposed to 5 week hypoxia. In vitro, HIF2 induced a distinct set of genes in normal pulmonary vascular EC, mediating inflammation and proliferation of EC and SMC. EC HIF2α knockout prevented hypoxia-induced PH in mice.
Inhibition of HIF2, not HIF1 can provide a therapeutic approach to prevent the development of hypoxia-induced PH. Future studies are needed to investigate the role of HIFs in PH progression and reversal.
Summary:
Activation of HIF2 by hypoxia initiates vascular cell proliferation and recruitment of inflammatory cells at early stages of PH development through HIF2 dependent transcription of genes involved in these pathways in pulmonary vascular cells.
INTRODUCTION
Pulmonary hypertension due to lung disease and/or hypoxia (WHO group 3 PH) comprises nearly one million people worldwide making it the second largest group of patients suffering from this disease. Unfortunately, none of the currently approved drugs for group 1 PAH have been shown in randomized controlled trials to benefit patients with group 3 PH, emphasizing the need for a better understanding of disease mechanisms as they might aid in the discovery of new therapies.
There is abundant evidence supporting the central involvement of the hypoxia inducible factors (HIFs) in chronic hypoxia-induced PH (1–11). However, we believe the experimental approaches used in most previously published studies may not recapitulate the clinical settings. For example, some group 3 PH development is due to hypoxia exposure in post-natal humans and animals with normal pulmonary circulations. It is important to note that except for two studies in which Hif1 deletion in SMC or EC was initiated in adult mice (10, 11), in all other studies Hif1α or Hif2α deletion was initiated in developing embryos (1–9). It has been well-established that all these HIF knockout or activation models (PHD2 KO) exhibit vascular defects in developing embryos and likely in adult mice derived from these embryos (12–17). Also, due to the essential role of HIF in development, most published studies use cell-type specific HIF knockout models (2–7, 10, 11). We believe such cell-type specific HIF knockout approaches may have shortcomings for the purpose of determining the general function of a gene like HIF in a disease such as PH where interactions between multiple cell types (EC, SMC, and Fibs) are clearly necessary for disease progression and a specific gene may have different or opposite functions in different disease-involved cell types.
Beside inducible deletion, pharmacologic inhibition could be an important tool to study the general role of HIF in PH development in normal adult animals. Given the potential important role of HIF proteins and particularly HIF2 in PH, successful pharmacologic inhibition could also lay an important foundation for potential PH treatment. Recent studies have led to small molecules that specifically block HIF2, but not HIF1 activity (18, 19). These inhibitors have also been shown to exhibit strong antitumor activity in vivo and in vitro (18, 20). Additionally, antisense oligonucleotides (ASOs) to Hif1 and 2 have also been developed and shown to attenuate HIF expression in vivo and to abrogate HIF mediated disease pathology (21).
In the study presented here, we sought to re-evaluate the role of HIFs in hypoxia-induced PH using approaches that may better recapitulate the clinical settings. Specifically, HIF activity is inhibited by inducible gene deletion and pharmacologic inhibitors in which global (not cell-type specific) HIF1 or HIF2 activity inhibition is initiated in normal adult animals without any predisposed diseases resulting from embryonic deletion of HIFs. In addition, we sought to better understand the underlying molecular mechanisms concerning how HIFs promote PH development, by studying the role of HIF in early as well as late stages of PH development.
RESULTS
Hif1α is dispensable in establishing hypoxia-induced PH at 5 weeks in adult mice
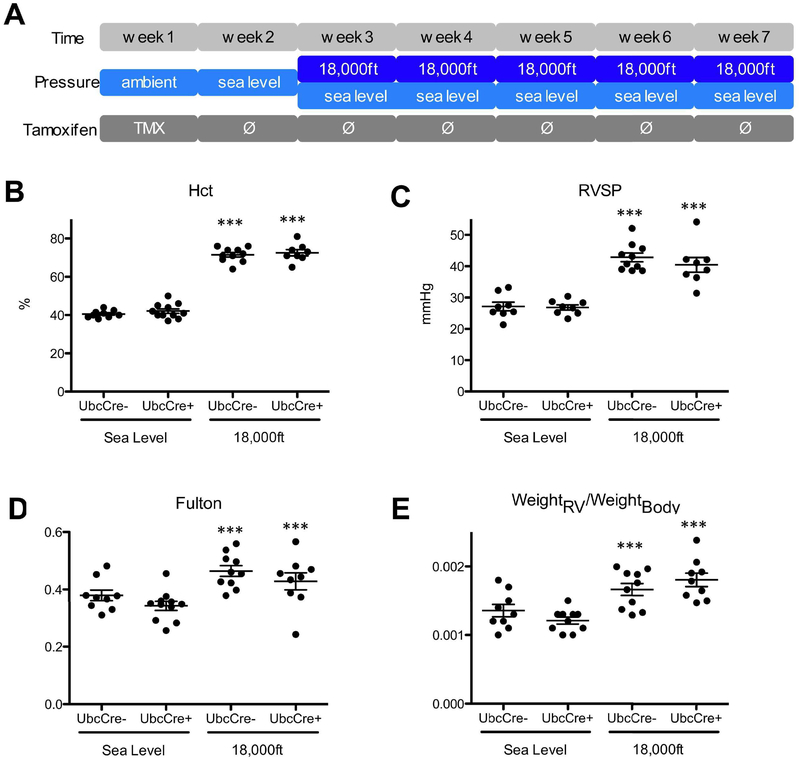
To delete the Hif1α gene in adult mice globally, Hif1αfl/fl;UbcCreERT+ and Hif1αfl/fl;UbcCreERT− (control) mice were injected with tamoxifen for 5 days. Mice were then allowed to rest for one week, before exposure to either hypobaric hypoxia (PB=370mmHg) or normobaric normoxia (PB=740mmHg) for 5 weeks (Fig 1A). After five weeks hypoxia exposure, readout parameters were measured and animals were euthanized. The efficiency of Hif1a gene deletion in UbcCreERT+ mice under hypoxic conditions is at 80% (Suppl. Table 1). Hypoxic exposure induced increases of hematocrit (Fig 1B), right ventricular systolic pressure, RVSP (Fig 1C), and right ventricular hypertrophy, as indicated by increases in Fulton index (Fig 1D) and in the ratio of the weight of the right ventricle (RV) versus body mass (weightRV/weightBody) (Fig 1E) in both Hif1αfl/fl;UbcCreERT− and Hif1αfl/fl;UbcCreERT+ mice. Hypoxia also similarily reduced body weights and increased heart rate in both UbcCreERT+ and UbcCreERT− animals, compared to normoxic animals (Suppl. Table 2). Thus, our data demonstrate that Hif1α expression is dispensable for development of PH and RV hypertrophy in normal adult mice after 5 weeks of hypoxia.
Figure 1: Global Hif1α is dispensable in establishing hypoxia-induced PH at 5 weeks in adult mice.
A) Experimental setup: Tamoxifen was injected into mice daily during week 1 to activate Cre and delete Hif1α gene, then all mice were moved to sea levels for one week, followed by exposing mice to either normoxia (PB-740mmHg) or hypoxia (simulated altitude of 18,000ft, PB=370 mmHg) for 5 weeks. After five weeks hypoxia exposure, readout parameters were measured and animals were euthanized. B) Hematocrit (Hct) levels. C) Right-ventricular systolic pressure (RVSP). D) Fulton index (ratio of weight of the RV to the weight of the LV and septum) and E) Ratio of RV-weight to bodyweight. Animal number in each group, under normoxia or hypoxia can be found in Fulton Index panel (Fig 1D). Due to technical difficulties, we were not able to obtain readings of Hct and/or RVSP for some mice, thus the animal number for these data was typically less than the animal number for Fulton index in this and other Figures of this study. * is for difference between hypoxia versus normoxia in the same genotype (or treatment) group in this and all other figures in the paper. Statistical analysis showed here is 2-way ANOVA analysis.
Mice with global genetic deletion of Hif2α do not survive long-term hypoxia
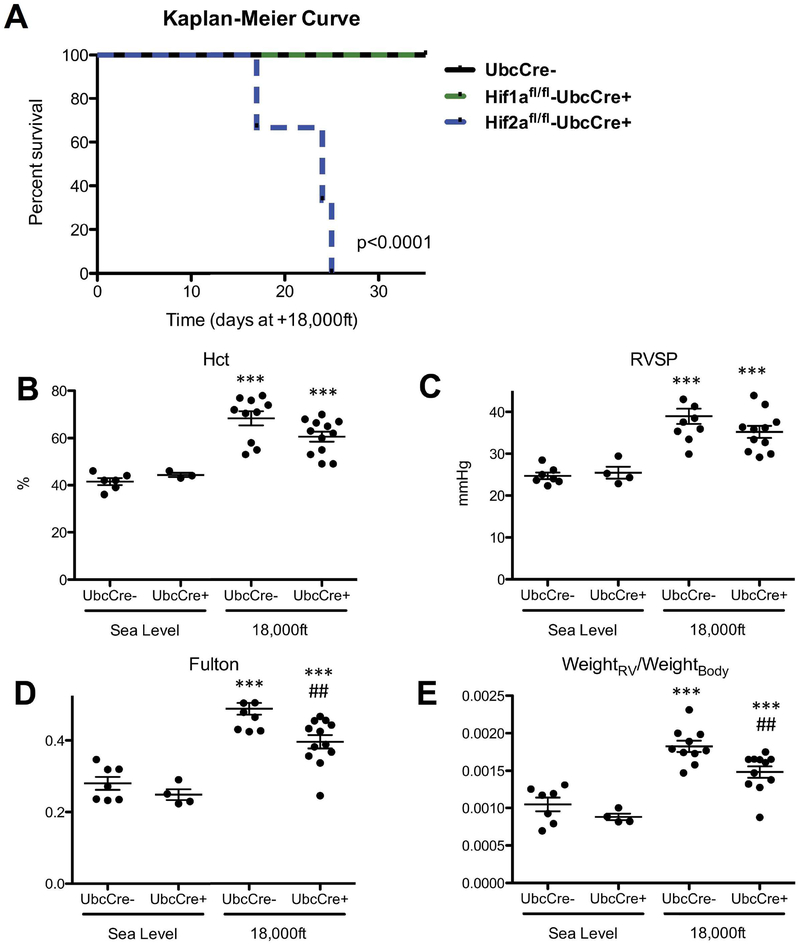
To investigate the role of Hif2α in hypoxia-induced PH development in normal adult mice, adult Hif2αfl/fl;UbcCreERT+ and Hif2αfl/fl;UbcCreERT− mice were similarly treated with tamoxifen and exposed to normoxia or hypoxia, as we did for Hif1α mice. The efficiency of Hif2a gene deletion in hypoxic Hif2αfl/fl;UbcCreERT+mice, at the end of the experiment was at 71% (Suppl. Table 1). While there was no difference in survival and PH development among Hif2αfl/fl;UbcCreERT− mice, Hif1αfl/fl;UbcCreERT+ mice and Hif1αfl/fl;UbcCreERT− animals (Fig 1 and Fig 2A), all Hif2αfl/fl;UbcCreERT+ mice died within 4 weeks of hypoxic exposure (Fig 2A). This data indicates that global Hif2α, but not Hif1α, deletion is not compatible with survival of mice exposed to chronic hypoxic conditions.
Figure 2: Global Hif2α gene deletion is incompatible for mouse survival under hypoxic conditions while global partial Hif2α gene deletion diminishes hypoxia-induced PH development at 5 weeks in adult mice.
Mice were treated with tamoxifen and exposed to normoxia or hypoxia as described in Fig 1A. A) Kaplan-Meier-Curve for survival of Hif2αfl/fl;UBC-CreERT+ mice during hypoxia exposure, compared to survival of Hif1αfl/fl;UBC-CreERT+ and Hif2αfl/fl;UBC-CreERT− mice during exposure to hypoxia. More than 9 mice in each group were used for this experiment. B–E: Hemodynamics of Hif2αfl/WT;UbcCreERT+ and Hif2αfl/WT;UbcCreER− mice after 5 week exposure to normoxia or hypoxia. B) Hematocrit (Hct) levels. C) RVSP. D) Fulton index. E) ratio of RV-weight to bodyweight. # is used to show the differences between genotypes or treatments under hypoxic condition in this and all other figures of this study. Statistical analysis showed here is 2-way ANOVA analysis (note in panel C there is a significant decrease in RVSP at 18,000 in UbcCre+ animals analyzed by unpaired T-test p<0.05).
Global partial Hif2α deletion diminishes development of hypoxia-induced PH at 5 weeks in adult mice
To prevent lethality, we generated Hif2αwt/fl;UbcCreERT+ and Hif2αwt/fl;UbcCreERT− mice, then treated them with tamoxifen and exposed them to hypoxia for 5 weeks. The efficiency of Hif2a gene deletion in hypoxic Hif2αwt/fl;UbcCreERT+mice at the end of the experiment was 36% (Suppl. Table 1). Cre-negative animals displayed increases in hematocrit (Fig 2B), RVSP (Fig 2C), Fulton index (Fig 2D), and weightRV/weightBody ratio (Fig 2E) in response to hypoxia. While there were still significant increases in these parameters in hypoxia-exposed Hif2αwt/fl;UbcCreERT+ mice, the level of increase for some parameters was significantly reduced in comparison to cre-negative littermates (Fig 2D–E). Thus, our data, generated from normal adult mice, demonstrate that reduction of Hif2α attenuates development of PH induced by 5 weeks of hypoxia exposure.
Knockdown of Hif2α utilizing antisense-oligonucleotides significantly reduces development of hypoxia-induced PH at 5 weeks in adult mice
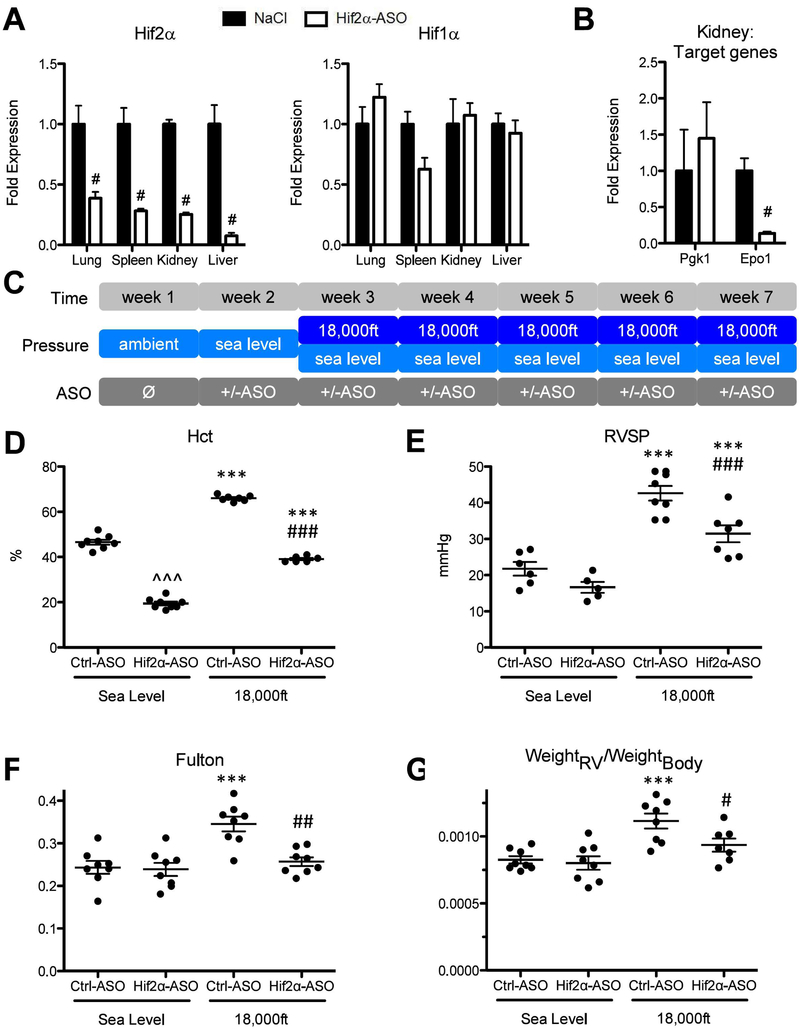
To further investigate the role of Hif2α in hypoxia-induced PH in normal adult mice and to examine whether targeting Hif2α therapeutically in adult mice under hypoxic conditions would be beneficial or lead to lethality, we used an antisense-oligonucleotide (ASO) approach. The effectiveness and feasibility of the Hif2α ASO in reducing Hif2α expression in adult mice maintained under normoxia, was tested in a pilot experiment, which showed an up to 90% reduction of Hif2α mRNA levels in lungs, spleens, kidneys and livers in mice treated with Hif2α-ASO two times per week for two weeks (Fig 3A left), without significant changes of Hif1α mRNA in all organs examined (Fig 3A right). Further, Hif2α-ASO also reduced expression of the HIF2 target gene Epo1, but not the HIF1 target gene Pgk1 in kidneys (Fig 3B).
Figure 3: Knockdown of Hif2α but not Hif1α utilizing antisense-oligonucleotides significantly reduces development of hypoxia-induced PH at 5 weeks in adult mice.
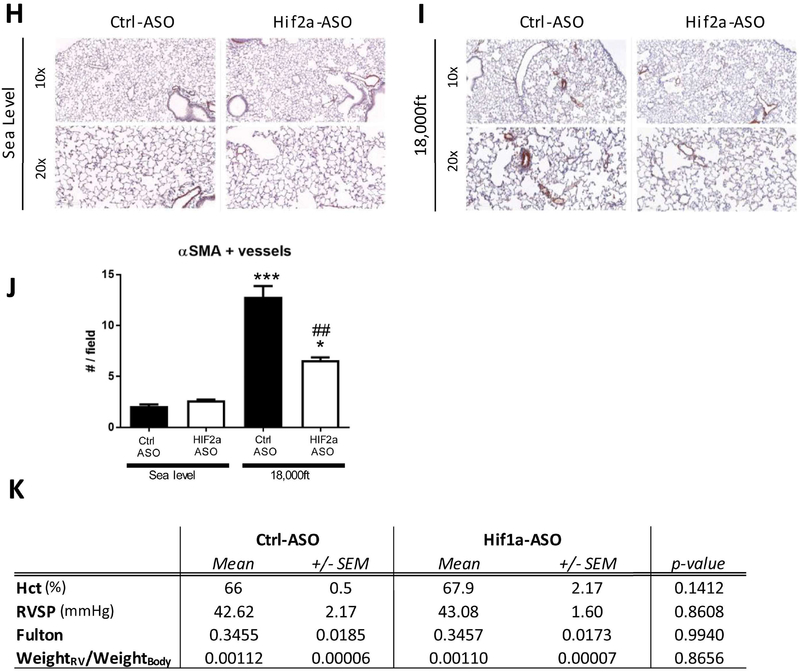
A and B) Testing the effectiveness and specificity of antisense-oligonucleotide in a pilot experiment. Wild-type C57bl/6J mice were treated either with injections of an antisense-oligonucleotide targeting Hif2α mRNA (Hif2α-ASO) or equal volumes of 0.9% NaCl (Ctrl) at days 1, 4, 8 and 11. At day 12, mice (N=3) were sacrificed and multiple organs were collected for RNA preparation. A) Levels of Hif1α and of Hif2α mRNA were quantified by qRT-PCR in indicated organs. B) Levels of Pgk1 (a HIF1 target gene) and Epo (a HIF2 target gene) in kidneys from mice targeted with NaCL or Hif2α-ASO, were quantified by qRT-PCR. C) Experimental setup. In week 2, mice were kept under sea level and began to receiving injection of Control, Hif1α-ASO, or Hif2α-ASO (two injections per week at Monday and Thursday). Starting week three, mice were exposed to either sea level or 18,000ft for 5 weeks, in which two injections per week were maintained. D–K) Endpoint measurements for the experimental animals in C. D) Hct. E) RVSP. F) Fulton index. G) ratio of RV-weight to bodyweight. H–J) alpha-SM-actin positive pulmonary vessels. K) Summary of Hct, RVSP, Fulton index, ratio of RV-weight to bodyweight for mice targeted with control or Hif1α-ASO under normoxia or hypoxia. Statistical significance as determined by t-test (A, B, J, and K) or 2-way ANOVA (D–G). ^ is used to show the differences between genotypes or treatments under normoxic condition in this and all other figures of this study.
To determine the general role of HIF in hypoxia-induced PH development, mice were injected with Hif2α-ASO, or a Control-ASO (unspecific, no known target) for one week with two injections per week and then exposed to either normoxia or to hypoxia for five weeks during which two injections of ASO per week were maintained (Fig 3C). The efficiency of Hif2a gene deletion in hypoxic mice targeted with Hif2a ASO was 72% at the end of the experiment (Suppl. Table 1). We did not observe any lethality in Hif2α-ASO treated mice under hypoxia. However, hypoxic Hif2α-ASO treated mice exhibited a trend towards increased weight loss (Suppl. Table 4). Further studies also revealed that levels of circulating catecholamines (epinephrine and norepinephrine) were reduced by HIF2α ASO treatment as were heart rate, cardiac output and dP/dt max (Suppl. Fig. 2B–E). In addition, two animals died during readout procedures. All these suggested an increased fragility in these mice.
The hematocrit in Hif2α-ASO treated mice was lower, both, under normoxia and under hypoxia (Fig 3D). Under hypoxic conditions, Hif2α-ASO-treated mice also exhibited reduction in RVSPs (Fig 3E), Fulton index (Fig 3F), and weightRV/weightBody ratio (Fig 3G), compared with hypoxic Ctrl-ASO mice. Consistent with hemodynamic data, hypoxia exposed Hif2α-ASO-treated animals exhibited a marked reduction in fully muscularized vessels in the lungs, compared to hypoxia exposed Ctrl-ASO mice (Fig 3H–J).
On the other hand, the Hif1α-ASO reduced Hif1α mRNA by 60% in lung tissues (Suppl. Table 1), but it had no effect on Hct, RVSP, or the Fulton index in hypoxic mice (Fig 3K), similar to the lack of effect observed in inducible Hif1α KO mice (Fig 1).
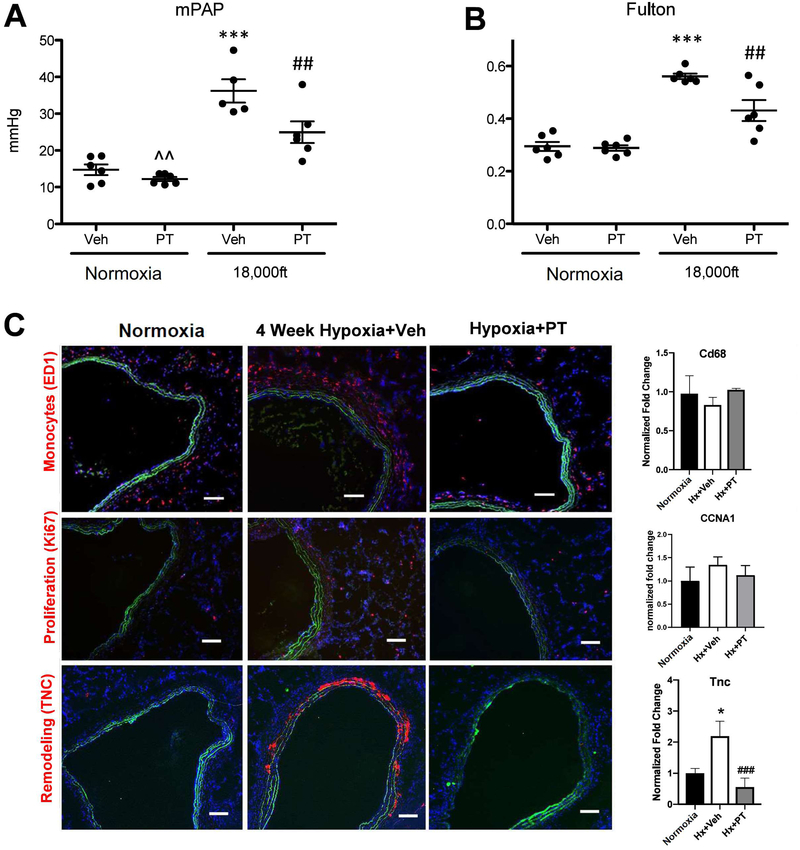
A small molecule Hif2 inhibitor PT2567 significantly reduces development of hypoxia-induced PH at 4 weeks in adult rats.
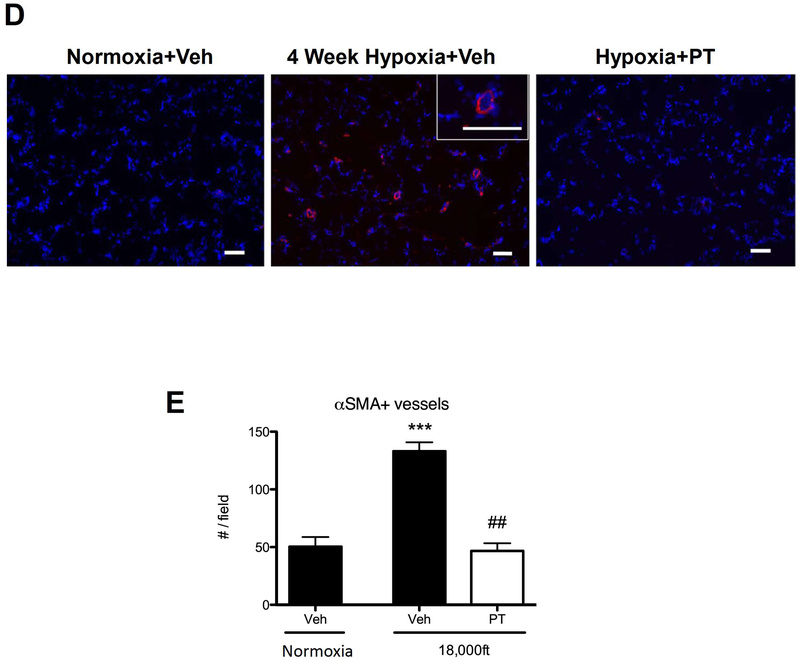
To further address the safety of HIF2 inhibition and the role of HIF2 in initiation of hypoxia-induced PH, we treated rats (which develop more severe PH than mice) with a small molecule inhibitor (Peloton, PT2567) that specifically blocks HIF2 activity (18, 19). 4 week hypoxia exposure led to PH development in rats treated with the control reagent (0.5% methylcellulose and 0.5% Tween-80), as demonstrated by increased mean Pulmonary Arterial Pressure (mPAP) (Fig 4A) and increased Fulton Index (Fig 4B). The HIF2 inhibitor, PT2567, reduced the mPAP (Fig 4A) and attenuated RV remodeling (Fig 4B). To better understand the molecular mechanisms underlying HIF2’s role in PH development in adult animals, we examined multiple pathways and genes that are important in PH development (3–7, 22–24). Indeed, increased inflammatory cell accumulation (monocytes) (Fig 4C, top panel), slight increased number of proliferating cells (Ki67) (Fig 4C, middle panel) and increased Tenascin C (TNC) expression (Fig 4C, lower panel) were observed in hypoxia-exposed rats, which were reduced by the HIF2 inhibitor (PT2567) in adventitial areas of pulmonary vessels from hypoxia exposed rats. The changes in proliferation and TNC expression in hypoxic rats and rats treated with HIF2 inhibitor using immunostaining were confirmed by CCNA1 (a cyclin, marker for cell proliferation) and TNC mRNA levels in lung tissues of the corresponding rats (Fig. 4C middle and bottom right). However, the increased monocyte accumulation in hypoxic rats using immunostaining was not confirmed by their mRNA levels in lung tissues of the corresponding rats (Fig 4C, top right), likely due to the possibility that macrophages/monocytes are attracted to pulmonary vessels, but the total cell numbers are not significantly altered in the whole lungs of rats exposed to hypoxia for 4 weeks. In addition, more muscularized vessels were observed in hypoxia-exposed rats, which were reduced by the HIF2 inhibitor (PT2567) (Fig 4D and E), data consistent with our HIF2α ASO observations in mice. Further, consistent with previous reports (3–7, 23), a number of functionally important genes in PH such as Icam1, Sdf1, Arg1, Arg2, Ccnd1, Edn1, Pai1, Tgfa and Tsp1 exhibited increased levels in lungs of hypoxia exposed rats (Suppl. Fig 3). The increase in the above genes, but not genes such as Adm, Glut1, and Ndrg1 was significantly reduced in hypoxia exposed rats treated with the HIF2 inhibitor PT2567 (Suppl. Fig 3), suggested that the first group of genes likely represents target (direct or indirect) genes unique to HIF2, while the HIF2 inhibitor insensitive genes are likely to be regulated in a Hif2-independent manner at this time point. These data indicate that HIF2 inhibition significantly reduces development of hypoxia-induced PH, by preventing induction of genes involved inflammation (Suppl Fig 3B) and cell signaling (proliferation and fibrotic responses) (Suppl Fig 3C).
Figure 4: Small molecule Hif2 inhibitor PT2567 significantly attenuates development of hypoxia-induced PH at 4 weeks in adult rats.
Sprague Dawley (SD) male rats weighing 210–245 grams (Charles River Laboratories) were housed in chambers under normoxia or hypoxic (high altitude ~ 18,000 feet) conditions for four weeks. Rats were dosed with vehicle methylcellulose (0.5%)/Tween-80 (0.5%) or PT2567 (300 mg/kg/day), beginning the day they were placed in chambers. After 4 weeks, endpoint measurements for the experimental animals were conducted (A–C). A) Mean Pulmonary Arterial Pressure. B) Ratio of weight of right ventricle versus weight of left ventricle. C) Representative images of pulmonary vessels stained with anti-macrophages/monocytes antibody, clone ED-1 (Top), anti-Ki67 antibody (Middle), or anti-Tenascin C antibody (Bottom). The levels of CD68, CCNA1, and TNC mRNAs in the whole lung tissues of indicated rats were also shown (Right). D, E) α-SM-actin positive pulmonary vessels, scale bar: 100μm. Statistical significance determined by 2-way ANOVA (A, B) or by t-test (C–E).
Hif2 activity is required for increased accumulation of monocytes and increased cell proliferation observed in hypoxia-exposed adult rats at 4 days.
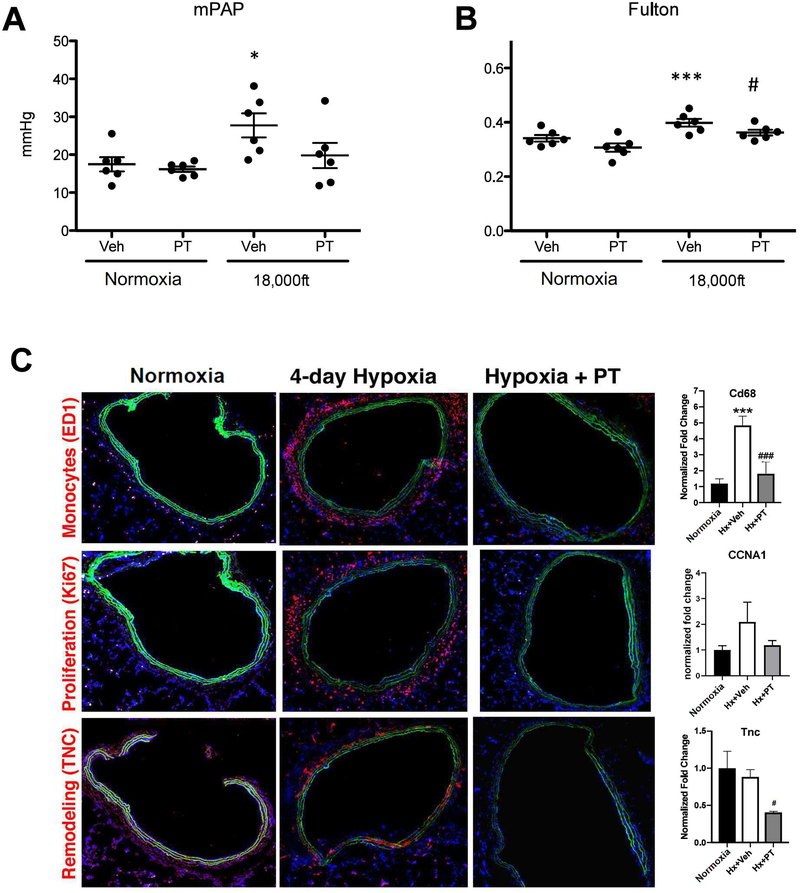
Using both genetic and pharmacological approaches, our studies shown above demonstrated that inhibtion of HIF2 is able to attenuate the development of PH at 4–5 weeks of hypoxia using normal adult mice and rats as models. Studies below are intended to address multiple critical remaining questions concerning how HIF2 promotes PH initiation, but not to determine the general role of HIF2 in hypoxia-induced PH. The first question we wanted to answer is at which stage of PH development HIF2 signaling has an impact. To help address this question, we performed short-term hypoxia experiments. 4-day hypoxia exposure led to moderate increases of mPAP (Fig 5A) and Fulton index (Fig 5B) in rats treated with control reagent, in which only the Fulton index was reduced by the HIF2 inhibitor PT2567 (Fig 5B). Consistent with our previous findings (25, 26), in short-term hypoxia exposed rats, changes in TNC expression were minimal (Fig 5C), but there was a significant increase in accumulation of monocytes and cell proliferation (Fig 5C). The HIF2 inhibitor PT2567 completely abolished these changes (Fig 5C). The changes of Cd68, CCNA1 and TNC expression in hypoxic rats and rats treated with HIF2 inhibitor examined by immunostaining, were confirmed by their mRNA levels in lungs of the corresponding rats (Fig. 5C right side). Examination of gene expression in lungs demonstrated that most HIF2 regulated genes (Icam1, Sdf1, Arg1, Arg2, Ccnd1, Edn1, Pai1, but not Tgfa and Tsp1) that were observed in 4 week hypoxia rats (Suppl. Fig 3), also exhibited induction in 4 day hypoxia exposed rats (Suppl. Fig 4). Importantly, the Hif2 inhibitor PT2567 reduced expression of these genes (Suppl Fig 4). However, Pdgfb and CXCR4 were induced, and Id1 was reduced, only in rats exposed to short-term hypoxia (Suppl. Fig 4). The effectiveness of HIF2 inhibitor in preventing monocyte/macrophage accumulation and vascular cell proliferation, and the fact that changes in gene expression were largely overlapping in rats exposed to short-term hypoxia and to long-term hypoxia, support the idea that HIF2 activity is essential in initiating hypoxia PH at a very early stage (4 days).
Figure 5: Small molecule Hif2 inhibitor PT2567 significantly attenuates early events in hypoxia-exposed adult rats at 4 days.
Sprague Dawley rats were housed in chambers under normoxia or hypoxic conditions for four days. Rats were dosed with vehicle or PT2567 as described in Figure 4. After 4 days, endpoint measurements for the experimental animals were conducted (A–C). A) Mean Pulmonary Arterial Pressure. B) Ratio of weight of right ventricle versus weight of left ventricle. C) Representative images of pulmonary vessels stained with anti-macrophages/monocytes antibody, clone ED-1 (Top), anti-Ki67 antibody (Middle), or anti-Tenascin C antibody (Bottom). The levels of CD68, CCNA1, and TNC mRNAs in lungs of indicated rats were also shown (right). Statistical significance determined by 2-way ANOVA (A, B) or by t-test (C).
Normal pulmonary artery endothelial cells display unique responses to acute hypoxia in a Hif2α dependent manner
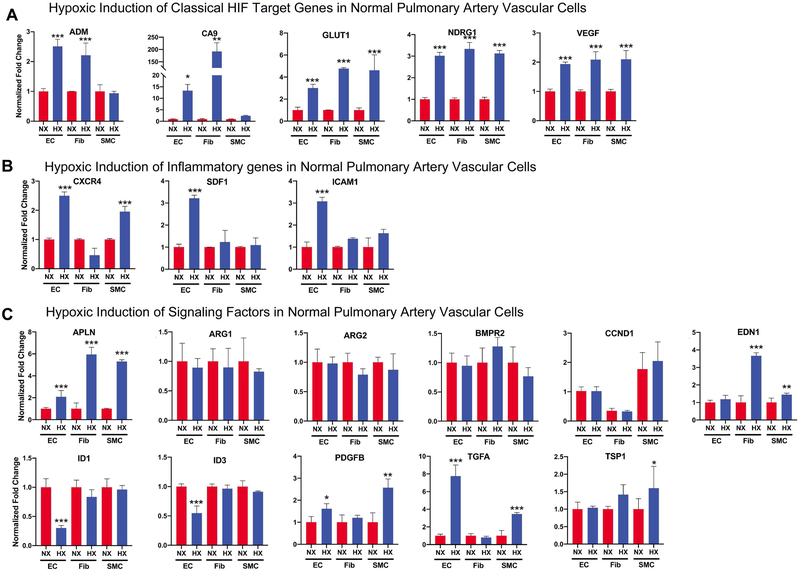
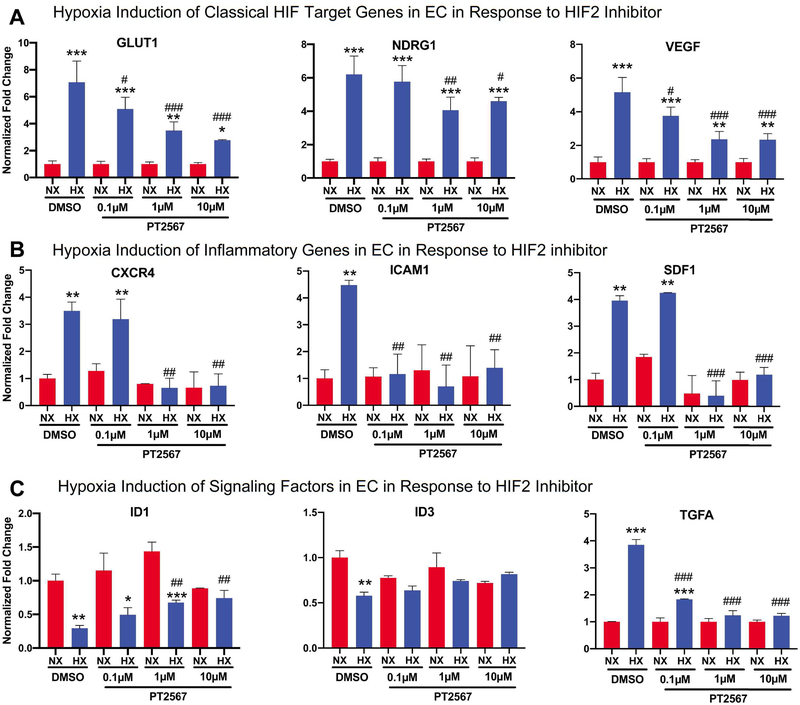
Our short-term in vivo hypoxia studies support the hypothesis that HIF2 activity is activated by hypoxia very early in one or multiple pulmonary vascular cells (EC, SMC, and Fibs), initiating PH development. Here, we attempted to determine the main cell type(s) whose activation by acute hypoxia may explain the observed events in 4 day hypoxia rats (Fig 5) by first comparing and contrasting the response of normal human pulmonary artery derived EC, SMC, and Fibs to acute hypoxia. We chose to use normal vascular cells, as we were studying the function of HIF in PH initiation. Acute hypoxia (1.5% for 16 hours) significantly activated expression of several classic HIF target genes including Adm, Ca9, Glut1, Ndrg1, and Vegfa in all three cell types, suggesting activation of these classical HIF target genes lacks cell-type specificity (Fig 6A). Hypoxic induction of the pro-inflammatory genes Sdf1, Cxcr4, and Icam1 was most prominent in ECs (Fig 6B). Additional unique responses of ECs to acute hypoxia was evidenced by reduced expression of Id1 and Id3, and increased expression of the growth factor Tgfa, a ligand of Egfr (Fig 6C), all of which can promote cell proliferation and survival. However, induction of Apln, Edn1, Pdgfb, and Tsp1 was mainly observed in SMC and Fibs while Arg1 and Arg2 were not induced by acute hypoxia in any of the three cell types (Fig. 6C). These data support a unique role of EC in response to acute hypoxia, to increase genes involved in inflammation and cell migration and proliferation. Thus, we determined if HIF2 is responsible for increased expression of inflammatory and proliferative genes in EC under hypoxia. Interestingly, HIF2 inhibitor PT2567 completely abolished hypoxia-mediated changes of inflammatory (Cxcr4, Icam1 and Sdf1) (Fig 7B) and signaling/proliferation (Id1, Id3 and Tgfa) (Fig 7C) genes in control ECs, but only attenuated hypoxic induction of classical HIF target genes (Fig 7A). The essential role of HIF2, but not HIF1 in regulating inflammatory and signaling/proliferation genes in control ECs was further confirmed using an siRNA approach as HIF2a siRNA, but not HIF1a siRNA significantly attenuated changes of inflammatory and signaling/proliferation gene expression, induced by hypoxia in ECs (Suppl Fig 5C and D). While the direct function of proteins such as ID1 and ID3 is likely intracellular, the increased production of genes/proteins such as SDF1 and TGFA in EC could also involve paracrine signaling. To assess the role of activated ECs in regulating other pulmonary vascular cells, we prepared conditional-medium from normal ECs (EC-CM) cultured under normoxia or hypoxia, in the presence or absence of a HIF2 inhibitor PT2567. We found that HIF2 mediated activation of control EC also increased the expression of genes involved in cell proliferation (CCNE1 and CCNE2), pro-inflammation (CCL2), and anti-apoptosis (BCL2, BCL2L1, and BIRC5) in control SMC. This suggests a role for HIF2-signaling in activating EC, which promote SMC activation (Suppl. Fig 6). Thus, our studies support a hypothesis that activation of EC by hypoxia in a HIF2 dependent manner (but not HIF1), activates EC, also initiating proliferation of other vascular cells (SMC) and recruitment of monocytes/macrophages via increased expression of inflammatory cytokines (SDF1) and growth factors (Tgfa).
Figure 6. Normal human pulmonary artery vascular cells exhibit unique properties in response to acute hypoxia.
Normal human pulmonary artery vascular cells (EC, Fibs, SMCs, N=5 for each cell type) were cultured under normoxia or hypoxia (1.5% O2) for 16 hours, and then cells were collected for RNA preparation. The same set of genes that were examined in vivo were studied here. Results were from at least 5 different cell populations for each cell type, in which result for a specific cell population was from three independent N or H experiments here or other similar experiments in this paper. A) Classical HIF target genes. B) Genes involved in inflammation. C) Genes involved in signaling. Statistical significance determined by t-test.
Figure 7. HIF2 inhibitor PT2567 significantly attenuates altered production of genes involved in inflammation and signaling in normal pulmonary endothelial cells in response to acute hypoxia.
To determine if HIF2 activity is responsible for hypoxia-mediated gene expression changes in EC, normal human pulmonary artery EC cells (N=3) were cultured under normoxia or hypoxia (1.5% O2) for 16 hours, in the presence of DMSO (control) or different concentration of HIF2 inhibitor PT2567, and then cells were collected for RNA preparation and qRT-PCR. A) Select classical HIF target genes. B) Genes involved in inflammation that are significantly induced by hypoxia in EC (Fig 6B). C) Genes involved in signaling that are significantly altered by hypoxia in EC (Fig 6C). Statistical significance determined by t-test.
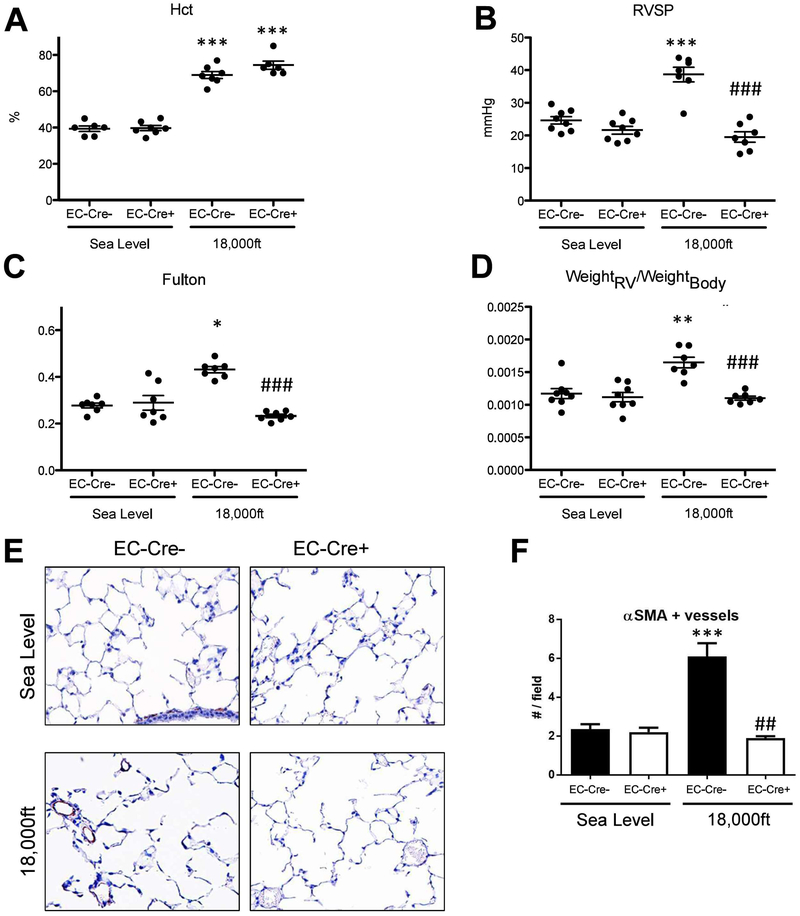
Hif2α expression in endothelial cells is required for hypoxia-induced PH and vascular remodeling in mice
To confirm the essential role of EC Hif2α in PH development in vivo, and to better understand the role of EC HIF2 in PH development, we generated and exposed EC Hif2α knockouts and their controls to normoxia or hypoxia for 5 weeks. As expected, Hif2αfl/fl-EC-Cre− animals subjected to hypoxia displayed an increase in hematocrit (Fig 8A) and PH development (Fig 8B–D). We observed a similar increase of hematocrit in the hypoxia-exposed Hif2αfl/fl-EC-Cre+ mice, confirming previous reports that endothelial Hif2α is not required for hypoxia-induced erythropoiesis and erythrocytosis (4). However, hypoxia exposed Hif2αfl/fl-EC-Cre+ mice exhibited no signs of PH (Fig 8B–D). The increase in the number of αSMA-positive pulmonary vessels in hypoxia-exposure HIF2αfl/fl-EC-Cre− mice was also abolished in hypoxia-exposure Hif2αfl/fl-EC-Cre+ mice (Fig 8E–F). We also examined the same set of genes that we studied in rats treated with the HIF2 inhibitor (Suppl Fig 3). As expected, genes induced by hypoxia in the lungs of control rats (Suppl Fig 3) were partially overlapping with the genes induced in mice by chronic hypoxia (Suppl Fig 7). The list included Ndrg1, Sdf1, Arg1, Edn1. However, the only genes that were reduced in both hypoxia exposed rats treated with HIF2 inhibitor (Suppl Fig 3) and in mice with EC Hif2α KO were Sdf1, Arg1, and Edn1 (Suppl Fig 7). This indicates the particular importance of these genes in the development of hypoxia-induced PH. Interestingly, we found that Acta1 and Myh7 gene expression was markedly reduced in the RV of hypoxic Hif2αfl/fl-EC-Cre+ mice (Suppl Fig 8), compared to RV of hypoxic Hif2αfl/fl-EC-Cre− mice, confirming reduced RV remodeling.
Figure 8. Hif2α expression in endothelial cells is required for development of hypoxia-induced PH.
Endothelial Hif2α knockout or Hif2α WT mice were exposed to either sea level atmosphere or to simulated 18,000ft of altitude for 5 weeks. A–G are the endpoint measurements in these mice. A) Hct. B) RVSP. C) Fulton index. D) ratio of RV-weight to bodyweight; E–F) α-SM-actin positive pulmonary vessels. Statistical significance determined by 2-way ANOVA (A–D) or t-test (F).
DISCUSSION
Using both genetic and pharmacological approaches to inhibit global HIF1 or HIF2 activity, we found that inhibition of HIF2α, but not HIF1α, attenuates the development of PH in normal adult animals exposed to chronic (4–5 weeks) hypoxia. Importantly, in addition to addressing the role of HIF1 and HIF2 in hypoxia-induced PH development using normal adult animals, we also uncovered several novel roles of HIF2 in adult animals including its requirement for animals to survive under chronic hypoxic conditions. Our in vitro studies (pulmonary vascular cell’s response to acute hypoxia), in combination with short-term hypoxia in vivo studies, support a hypothesis that EC HIF2 is “essential” in PH initiation because, only ECs can increase the production of diffusible cytokines (SDF1), that may recruit monocytes/macrophages and other blood/bone marrow derived cells to lung vessels, at early stages of PH development. This result is also consistent with an earlier study showing that EC-derived Sdf1 contributes to PH in PHD2-deficient mice (3). Our studies also support the hypothesis that HIF2 in SMC or Fibs is “not essential” in PH initiation. This might be due to the fact that more than one cell type (among SMC, Fibs, and EC) can induce the expression of the PH-related genes such as Apln, Edn1, Pdgfb, Tgfa, and Tsp1. Thus, our studies contribute to a better understanding of the role of HIF2 in PH initiation.
PH is observed in post-natal humans and animals with normal pulmonary circulations in response to chronic hypoxia stress. However, to study the role of HIF in PH development, most published studies used models in which HIF deletion or HIF activation (in PHD2 knockout) was initiated in embryonic life (1, 3–8, 10). We think results from such approaches needed to be re-evaluated because 1) all these models are known to exhibit vascular defects in developing embryos and likely in adult mice derived from these embryos (12–17) and 2) there are examples demonstrating differences in phenotype (baseline as well as stressed) when gene deletion is initiated in the embryo versus in the adult (27–29). For example, inhibition of monocyte chemoattractant protein 1 (MCP1) initiated in adult animals reduces hypoxia- or MCT-induced PH (27, 28). In contrast, MCP-1 or MCP-1 receptor knock-out mice (initiated in the embryo) exhibit spontaneous PH (29). Our data showing the effects of HIF2 inhibition in normal adult animals demonstrates the functional importance of HIF2 in PH development, eliminating the concerns that HIF2’s function in most previous reported studies is due to the defects in the lung vasculature and heart by Hif2α-deletion or activation in developing embryos. Although we also used mice with constitutive EC-specific knockout of Hif2α, initiated in the embryo, we recognize that results from the EC HIF2a KO study have limitations. However, the purpose of our EC HIF2a knockout study is not to determine the general function of HIF2 in hypoxia-induced PH, but to provide a molecular explanation for the function of HIF2 in EC in PH development. Results from in vivo studies in these mice confirmed a) results obtained from mice with an inducible, global knockout, b) results from animals treated two different inhibitors and c) results from in vitro studies.
The role of Hif1α in the development of hypoxia-induced PH is controversial: Studies demonstrated either a partial amelioration of PH (10, 11), or a temporary slowing of PH progression (1) or even elevated PAPs (2), in mice with reduced HIF1a expression. Additional in vivo studies in which HIF1 is activated by genetic manipulation of PHDs or the VHL protein, demonstrated Hif1α was not required for PH development (3, 4, 9). We speculate that the different results reported could be due to using hypoxia or pseudo-hypoxia (PHD or VHL knockout animals) approaches or deletion of Hif1a gene in different cell types or deletion of Hif1a initiated in embroys or adult mice. Our studies using global (not cell-type specific) inhibition of HIF1a in normal adult animals (not initiated in embryo) and using hypoxia to activate HIF (not pseudo-hypoxia), do not support an indespensible role of Hif1α for PH in animals after 4–5 weeks of hypoxia exposure. However, we cannot exclude a transient role of Hif1α in the earlier stage of PH development, which we did not investigate. Indeed, a transient role of Hif1α in hypoxia-induced PH has been reported (1). Also, we cannot exclude disease-relevant Hif1α-signaling in SMC in vivo, as we did not confirm sufficient knockdown in this cell type in our ASO and our inducible knockout system. Last, our in vivo studies were performed using hypobaric hypoxia, while previous studies were performed using normobaric hypoxia. Since some physiological responses differ depending on the mode of hypoxia (30), the different experimental setting might also contribute to some of the differences between our findings and previous studies on the role of Hif1α in hypoxic PH.
Another distinct feature of this study is to inhibit global, not cell type specific HIF activity for purpose of determining HIF function in PH development. Cell-type specific knockout is a powerful method to understand the contribution of the targeted gene in a specific cell type to the disease. However, we believe conclusions derived solely from cell-type specific knockout experiments may have shortcomings for the purpose of determining the general function of a gene in a disease such as PH where interactions between multiple cell types are clearly necessary for disease progression and a specific gene may have different or opposite functions in different disease-involved cell types. For example, caveolin-1 is reduced in PH EC and its reduction in EC promotes PH development (31); however, caveolin-1 is over-expressed in PH SMC and its over-expression in SMC also promotes PH development (31, 32). Using global HIF gene deletion or pharmacological inhibition, we concluded that HIF2, not HIF1 is important for hypoxia-induced PH development. However, EC Hif2a gene deletion (Fig 8) appears to be more effective than global HIF2 reduction (ASO and HIF2 inhibitor PT2567) in preventing development of hypoxia-induced PH, suggesting the true role of HIF2 in hypoxia-induced PH development is less important than what are reported in EC HIF2a deletion models.
Cell type specific knockout approaches may also miss the possible side effects of therapeutic inhibition of a gene such as Hif2α, especially if Hif2α also plays important roles in other cell types for other processes. Indeed, complete global deletion of Hif2α is detrimental for mice’s survival under chronic hypoxia, which is a novel and important finding. Although the underlying causes for this lethality and the different tolerance to chronic hypoxia between Hif2α KO mice and mice/rats treated with Hif2α inhibitor are beyond the scope of this study, we speculate that mice with significant loss of Hif2α might have succumbed to cardiogenic shock under hypoxic conditions. This is based on our findings that Hif2α-ASO-treatment reduced levels of circulating catecholamines that were significantly increased in hypoxia in control-ASO mice (Suppl. Fig. 2B). Hif2a-ASO also abolished the increase in heart rate that was observed in control mice with hypoxia-exposure (Suppl. Fig. 2C), resulting in reduced cardiac output (Suppl. Fig. 2D). Hif2α knockdown also resulted in lower dP/dtmax (RV), a parameter of ventricular systolic function (Suppl. Fig. 2E). These effects could be mediated by loss of Hif2α in heart tissue (Suppl. Fig. 2A) or by loss in other organs (e.g., the adrenal glands). In conclusion, we speculate that lower hematocrits in Hif2α-ASO-treated animals in addition to the observed changes in cardiac function, could result in critically low delivery of oxygen (DO2) and finally death of Hif2α KO mice. This interpretation of our data is consistent with previous studies, demonstrating Hif2α-dependent changes in catecholamines (8), heart rate and cardiac output and physiology (33–35). Importantly, we did not observe changes in heart rate in mice with EC-specific Hif2α knockout, demonstrating that the observed reduction in RVSPs in Hif2α-ASO-treated animals was not merely due to impaired cardiac function. In summary, our study suggests that we need to exercise caution, particularly for patients residing at high altitude, if a Hif2α inhibitor is going to be used in clinic in future.
Most published studies have evaluated the end effect of HIF inhibition in animals exposed to chronic hypoxia, without knowledge of the role of HIF in early stage of PH initiation. We performed studies in animals exposed to chronic as well as short-term hypoxia. We revealed that HIF2 is essential in several early events (macrophage recruitment and vascular cell proliferation) of PH development, likely by activating genes such as Sdf1 (inflammation) and Tgfa (cell proliferation) in vivo.
To our knowledge, this is the first time that the responses of the three primary normal human pulmonary vascular cell types (EC, SMC and Fibs) to acute hypoxia have been examined concurrently for a set of genes that have been reported (3, 4, 6, 9, 22–24) and demonstrated in our current studies (Suppl. Figs 3, 4 and 7), to be important for PH development. Our studies lead to novel findings that EC is the primary cell type that can be activated by short-term hypoxia (Id reduction), in a HIF2 dependent manner to produce inflammatory (Sdf1) and growth promoting factors (Tgfa) (Fig 7), to activate other vascular cells (Suppl Fig. 6). Our findings from short-term in vivo and in vitro studies are consistent with previous studies by us and others that recruitment of bone marrow/blood derived cells such as monocytes/macrophages is an early and critical event in PH development (25, 36–39) and cytokines/chemokines including Sdf1 are among the earliest inflammatory factors increased in the pulmonary arteries of hypoxia-exposed animals and whose increase precedes monocyte/macrophage accumulation (26). Our in vitro studies also indicated that normal SMC and Fibs can be acutely activated by hypoxia to produce factors such as Apln, Edn1, and Tsp1 that have been shown to be involved in vessel constriction (4, 6). While knockout of Hif2α in SMC does not prevent hypoxic PH (7), studies on the effect of Hif2α inhibition in Fibs, in combination of in other pulmonary cell types, such as SMC, are needed. Further, our data indicated that there is no hypoxic induction of Arg1 and Arg2 in normal EC, SMC and Fibs (Fig 6) although we consistently observed increased expression of Arg1 and Arg2 in lungs of chronic and short-term hypoxia exposed rodents and whose expression is reduced by HIF2 inhibition (Suppl Figs 3, 4, 7). These data suggested that increased expression of genes such as Arg1 and Arg2 could be mainly from recruited cells.
The critical role of HIF2 in the development of hypoxia-induced PH has raised significant interest in targeting HIF2 for treatment of PH patients. In fact, one study has already demonstrated a quite effective role of a HIF2 inhibitor in reversing PH in animal models (40). However, small animal models for PH have well-recognized limitations (41). More importantly, targets that have been demonstrated to be effective in animal models are often failed in clinical trials (42, 43). Thus, we believe further studies are needed before initiation of a HIF2 inhibitor clinical trial in humans. Accumulating data indicates that vascular cells established from PH patients and large animals (cows) exhibit and maintain their unique phenotypes in vitro (44). We believe these cells could provide an excellent platform to further determine the role of HIF2 for PH treatment. In addition, although Hif1 is not essential in hypoxia-induced PH development, the role of Hif1 in PH disease maintanace is also possible.
In summary, our studies have demonstrated a positive role of HIF2 in PH development in response to chronic hypoxia in normal adult animals. However, more research is needed to determine if HIF2 is truly a good PH treatment target because HIF2 activity is likely to be one of the many factors that are required, acting in different cell types, at different stages, to initiate PH (45). In addition, there are data from cancer research that factors/pathways that initiate cancer often fail to be good treatment tragets because there are many additional changes occur during cancer progression.
Supplementary Material
Acknowledgements:
Ionis Pharmaceuticals supplied compounds HIF1 ASOs and technical support.
Footnotes
Publisher's Disclaimer: This manuscript has recently been accepted for publication in the European Respiratory Journal. It is published here in its accepted form prior to copyediting and typesetting by our production team. After these production processes are complete and the authors have approved the resulting proofs, the article will move to the latest issue of the ERJ online.
REFERENCES
- 1.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103(5):691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YM, Barnes EA, Alvira CM, Ying L, Reddy S, Cornfield DN. Hypoxia-inducible factor-1alpha in pulmonary artery smooth muscle cells lowers vascular tone by decreasing myosin light chain phosphorylation. Circ Res. 2013;112(9):1230–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2alpha. Circulation. 2016;133(24):2447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapitsinou PP, Rajendran G, Astleford L, Michael M, Schonfeld MP, Fields T, et al. The Endothelial Prolyl-4-Hydroxylase Domain 2/Hypoxia-Inducible Factor 2 Axis Regulates Pulmonary Artery Pressure in Mice. Mol Cell Biol. 2016;36(10):1584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labrousse-Arias D, Castillo-Gonzalez R, Rogers NM, Torres-Capelli M, Barreira B, Aragones J, et al. HIF-2alpha-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodelling and vasoconstriction. Cardiovasc Res. 2016;109(1):115–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowburn AS, Crosby A, Macias D, Branco C, Colaco RD, Southwood M, et al. HIF2alpha-arginase axis is essential for the development of pulmonary hypertension. Proc Natl Acad Sci U S A. 2016;113(31):8801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang H, Babicheva A, McDermott KM, Gu Y, Ayon RJ, Song S, et al. Endothelial HIF-2alpha contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol. 2018;314(2):L256–L75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, et al. Heterozygous deficiency of hypoxia-inducible factor-2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest. 2003;111(10):1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickey MM, Richardson T, Wang T, Mosqueira M, Arguiri E, Yu H, et al. The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest. 2010;120(3):827–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, et al. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1alpha. Am J Respir Crit Care Med. 2014;189(3):314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheikh AQ, Saddouk FZ, Ntokou A, Mazurek R, Greif DM. Cell Autonomous and Non-cell Autonomous Regulation of SMC Progenitors in Pulmonary Hypertension. Cell Rep. 2018;23(4):1152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A. 1997;94(9):4273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Developmental biology. 1999;209(2):254–67. [DOI] [PubMed] [Google Scholar]
- 14.Favier J, Kempf H, Corvol P, Gasc JM. Coexpression of endothelial PAS protein 1 with essential angiogenic factors suggests its involvement in human vascular development. Dev Dyn. 2001;222(3):377–88. [DOI] [PubMed] [Google Scholar]
- 15.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8(7):702–10. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol. 2006;26(22):8336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, et al. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci U S A. 1997;94(17):9102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace EM, Rizzi JP, Han G, Wehn PM, Cao Z, Du X, et al. A Small-Molecule Antagonist of HIF2alpha Is Efficacious in Preclinical Models of Renal Cell Carcinoma. Cancer Res. 2016;76(18):5491–500. [DOI] [PubMed] [Google Scholar]
- 19.Wehn PM, Rizzi JP, Dixon DD, Grina JA, Schlachter ST, Wang B, et al. Design and Activity of Specific Hypoxia-Inducible Factor-2alpha (HIF-2alpha) Inhibitors for the Treatment of Clear Cell Renal Cell Carcinoma: Discovery of Clinical Candidate ( S)-3-((2,2-Difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1 H-inden-4-yl)oxy)-5-fluorobenzonitrile (PT2385). J Med Chem. 2018;61(21):9691–721. [DOI] [PubMed] [Google Scholar]
- 20.Courtney KD, Infante JR, Lam ET, Figlin RA, Rini BI, Brugarolas J, et al. Phase I Dose-Escalation Trial of PT2385, a First-in-Class Hypoxia-Inducible Factor-2alpha Antagonist in Patients With Previously Treated Advanced Clear Cell Renal Cell Carcinoma. J Clin Oncol. 2018;36(9):867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin MK, Drager LF, Yao Q, Bevans-Fonti S, Yoo DY, Jun JC, et al. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1alpha. PLoS One. 2012;7(10):e46562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99(7):675–91. [DOI] [PubMed] [Google Scholar]
- 23.Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR. The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol. 2015;308(3):L229–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011;183(2):152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006;168(2):659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke DL, Frid MG, Kunrath CL, Karoor V, Anwar A, Wagner BD, et al. Sustained hypoxia promotes the development of a pulmonary artery-specific chronic inflammatory microenvironment. Am J Physiol Lung Cell Mol Physiol. 2009;297(2):L238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosanovic D, D B, Vroom C, Bieniek E, Ardeschir Ghofrani H, Weissmann N, Seeger W, Grimminger F, Klussmann S, Eulberg D, Schermuly RT. Selective inhibition of chemokine CCL2/MCP-1 reduces experimental pulmonary hypertension. Pneumologie. 2013;67:P10. [Google Scholar]
- 28.Ikeda Y, Yonemitsu Y, Kataoka C, Kitamoto S, Yamaoka T, Nishida K, et al. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2002;283(5):H2021–8. [DOI] [PubMed] [Google Scholar]
- 29.Yu YR, Mao L, Piantadosi CA, Gunn MD. CCR2 deficiency, dysregulation of Notch signaling, and spontaneous pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2013;48(5):647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coppel J, Hennis P, Gilbert-Kawai E, Grocott MP. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: a systematic review of crossover trials. Extreme physiology & medicine. 2015;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathew R. Cell-specific dual role of caveolin-1 in pulmonary hypertension. Pulmonary medicine. 2011;2011:573432–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, et al. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. 2007;21(11):2970–9. [DOI] [PubMed] [Google Scholar]
- 33.Formenti F, Beer PA, Croft QP, Dorrington KL, Gale DP, Lappin TR, et al. Cardiopulmonary function in two human disorders of the hypoxia-inducible factor (HIF) pathway: von Hippel-Lindau disease and HIF-2alpha gain-of-function mutation. FASEB J. 2011;25(6):2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koeppen M, Lee JW, Seo SW, Brodsky KS, Kreth S, Yang IV, et al. Hypoxia-inducible factor 2-alpha-dependent induction of amphiregulin dampens myocardial ischemia-reperfusion injury. Nat Commun. 2018;9(1):816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith TG, Brooks JT, Balanos GM, Lappin TR, Layton DM, Leedham DL, et al. Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology. PLoS Med. 2006;3(7):e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gambaryan N, Perros F, Montani D, Cohen-Kaminsky S, Mazmanian M, Renaud JF, et al. Targeting of c-kit+ haematopoietic progenitor cells prevents hypoxic pulmonary hypertension. Eur Respir J. 2011;37(6):1392–9. [DOI] [PubMed] [Google Scholar]
- 37.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, et al. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation. 2011;123(18):1986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G, et al. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med. 2013;5(200):200ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pugliese SC, Kumar S, Janssen WJ, Graham BB, Frid MG, Riddle SR, et al. A Time- and Compartment-Specific Activation of Lung Macrophages in Hypoxic Pulmonary Hypertension. J Immunol. 2017;198(12):4802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai Z, Zhu MM, Peng Y, Machireddy N, Evans CE, Machado R, et al. Therapeutic Targeting of Vascular Remodeling and Right Heart Failure in Pulmonary Arterial Hypertension with a HIF-2alpha Inhibitor. Am J Respir Crit Care Med. 2018;198(11):1423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297(6):L1013–32. [DOI] [PubMed] [Google Scholar]
- 42.Lythgoe MP, Rhodes CJ, Ghataorhe P, Attard M, Wharton J, Wilkins MR. Why drugs fail in clinical trials in pulmonary arterial hypertension, and strategies to succeed in the future. Pharmacol Ther. 2016;164:195–203. [DOI] [PubMed] [Google Scholar]
- 43.Wilkins MR. Apoptosis Signal-Regulating Kinase 1 Inhibition in Pulmonary Hypertension. Too Much to ASK? Am J Respir Crit Care Med. 2018;197(3):286–8. [DOI] [PubMed] [Google Scholar]
- 44.Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, et al. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol. 2011;187(5):2711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu CJ, Zhang H, Laux A, Pullamsetti SS, Stenmark KR. Mechanisms contributing to persistently activated cell phenotypes in pulmonary hypertension. J Physiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.