Summary
Cochlear amplification denotes a boost to auditory sensitivity and selectivity that is dependent on outer hair cells from Corti's organ. Voltage-driven electromotility of the cell is believed to feed energy back into the cochlear partition via a cycle-by-cycle mechanism at very high acoustic frequencies. Here we show using wide-band macro-patch voltage-clamp to drive prestin, the molecular motor underlying electromotility, that its voltage-sensor charge movement is unusually low pass in nature, being incapable of following high-frequency voltage changes. Our data are incompatible with a cycle-by-cycle mechanism responsible for high-frequency tuning in mammals.
Subject Areas: Auditory Evoked Response, Bioelectronics, Mechanobiology
Graphical Abstract
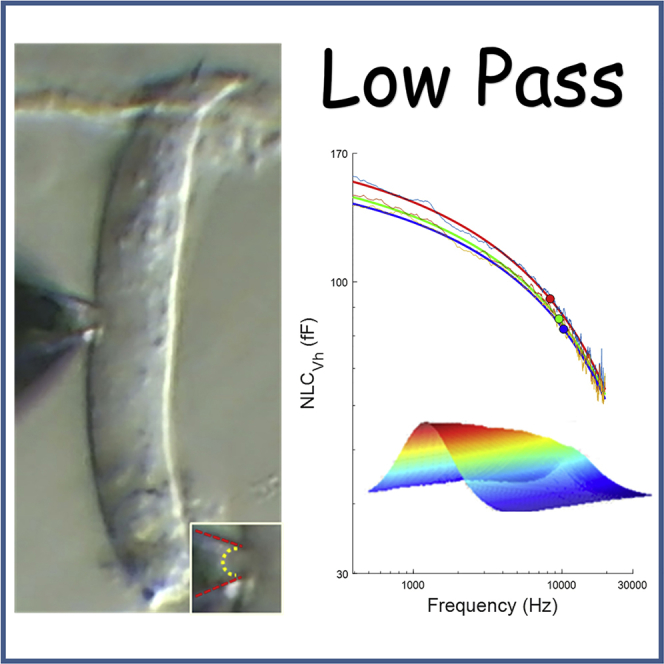
Highlights
-
•
Outer hair cells (OHC) boost auditory sensation for very high acoustic frequencies
-
•
We studied the frequency response of OHC's electromechanical nonlinear capacitance
-
•
The response is incommensurate with cycle-by-cycle feedback at very high frequencies
-
•
OHCs likely use another mechanism to drive cochlear amplification at high frequencies
Auditory Evoked Response; Bioelectronics; Mechanobiology
Introduction
In some mammalian species, hearing capabilities can extend out to 60–160 kHz (Heffner and Masterton, 1980, Castellote et al., 2014, Szymanski et al., 1999, Heffner et al., 2001). Indeed, tuned hair cell receptor potential-derived cochlear microphonic potentials have been measured beyond 60 kHz in the bat (Pollak et al., 1972). Underlying this electrical behavior are sharp frequency tuning and enhanced cochlear partition vibration, which are susceptible to outer hair cell (OHC) damage; studies in laboratory rodents provide such evidence, e.g., in the mouse sharp basilar membrane tuning is measurable beyond 60 kHz (MelladoLagarde et al., 2008). This enhancement over Bekesy's passive partition vibration (von Bekesy, 1960) is termed cochlear amplification (CA) (Davis, 1983) and amounts to an apparent gain of 100–1000 at best frequency locations along the cochlear duct. DC and AC trans-membrane voltages are sensed by the membrane protein prestin (SLC26a5) (Santos-Sacchi and Dilger, 1988, Iwasa and Kachar, 1989), a special member of the SLC26 family of anion transporters (Zheng et al., 2000), that populates the OHC lateral membrane at molecular densities up to 8,000/μm2 (Santos-Sacchi et al., 1997, Gale and Ashmore, 1997b). Voltage is thought to faithfully govern prestin's conformational state, evoking rapid switching between expanded and contracted molecular conformations that leads to rapid length changes (termed electromotility; eM) (Ashmore et al., 2010). Current dogma has it that evoked OHCeM provides mechanical feedback into the organ of Corti on a cycle-by-cycle basis to power CA. In fact, Frank et al. measured high-fidelity OHC AC eM responses beyond 80 kHz (Frank et al., 1999). During the last few years, however, the kinetics or frequency dependence of prestin's electromechanical capabilities have been reassessed in OHCs (Santos-Sacchi and Song, 2014, Santos-Sacchi, 2019). Both the frequency of prestin charge movement (measured as a nonlinear capacitance [NLC]) and that of its associated eM were found to be low-pass, precipitously rolling off within the bandwidth of human speech (200–8000 Hz) (Santos-Sacchi and Tan, 2018, Santos-Sacchi et al., 2019). However, those data must be viewed cautiously because measures were made in whole cell mode. In fact, being a piezoelectric-like protein (Iwasa, 1993, Gale and Ashmore, 1994, Kakehata and Santos-Sacchi, 1995), where reciprocal influences of mechanical load and voltage are at play, whole cell measures of OHC electromechanical activity may fail to establish the actual frequency capabilities of prestin. Here we address this issue by measuring the frequency response of prestin's NLC with on-cell and excised macro-patches from the cell's lateral membrane where isolation from external whole cell loads can be achieved. The macro-patch is the preferred methodology to gauge fast charge movement in voltage sensitive membrane proteins (Lu et al., 1995). Our patch data in mouse and guinea pig show low pass, voltage-driven electromechanical behavior of the protein, whose frequency response alters over time but still falls far short of its expected influence on cochlear amplification at very high frequencies, leading us to question prestin's role in this process.
Results
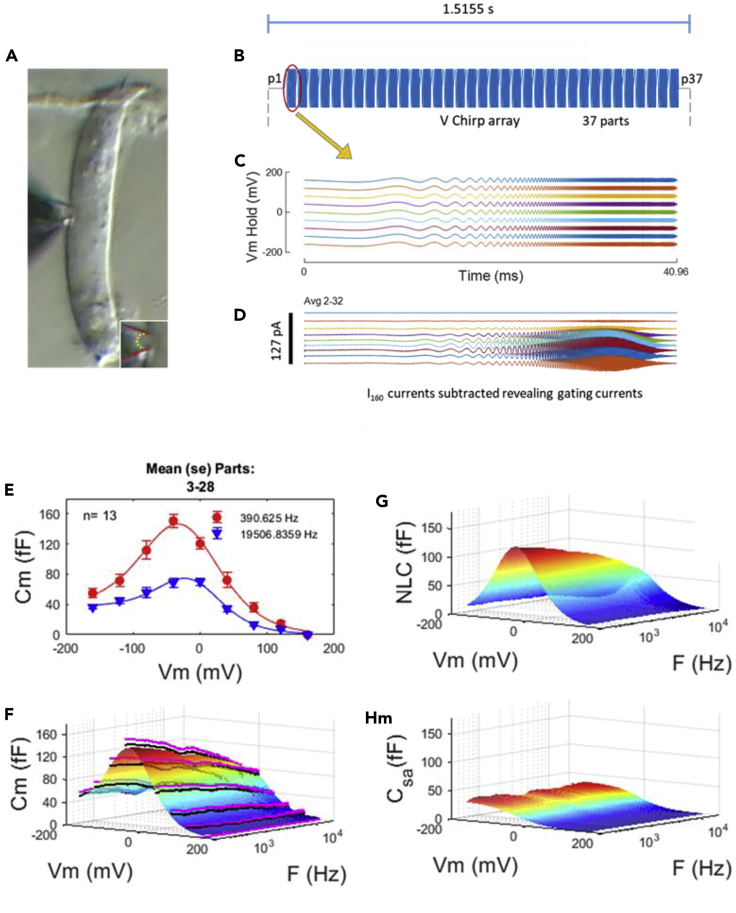
Our recording system response (Figures S1A andS1B), and results on an electrical model of a membrane patch (Figures S1C–S1F), confirming our capability to measure capacitance flat out to 20 kHz, is provided in the Transparent Methods section of the supplement. Figures1A–1D illustrate our basic experimental strategy. Macro-patches (3.5–4 μm inner diameter) were made on the lateral membrane of OHCs (Figure 1A) and a voltage chirp array delivered to the patch superimposed on stepped holding voltages (−160 to 160 mV) to extract prestin displacement currents (Figure 1D), following subtraction of currents at +160 mV where NLC is absent (Santos-Sacchi and Navarrete, 2002). Single-sine or dual-sine admittance analysis of the displacement currents provides estimates of NLC. Figure 1E shows average (+/− SE) cell capacitance data fitted with Equation 1 (see Methods) at 2 frequencies (390 and 19,500 Hz). Note the substantial drop in peak membrane capacitance at the higher frequency. Figure 1F displays in a 3D rendition all frequencies studied across holding voltage. Black lines are average data and pink lines are mean +SE, whereas the multicolored plot shows fits. The extracted NLC is shown in Figure 1G and illustrates the continuous roll-off of NLC with increasing frequency. Figure 1H shows the largely frequency independent component of membrane capacitance (Csa) at negative holding potentials that we previously attributed to membrane surface area/thickness changes during prestin's conformational changes between compact and expanded states (Santos-Sacchi and Navarrete, 2002). The clear roll-off differences between NLC and this Csa response (Figures 1G and 1H) indicate that our previous interpretation of the Csa component may need reevaluation.
Figure 1.
Measures of NLC in Macro-Patches from Outer Hair Cells (OHC)
(A) An image of an OHC (10 μm wide) showing a patch pipette sealed to the lateral membrane. Inset: enlarged view of patch. Red lines outline pipette, dotted yellow line outlines membrane patch.
(B) Section of command voltage stimulus, composed of 35 repeats of a chirp stimulus of 10 mV peak.
(C) Expanded view of individual chirp stimuli at the range of holding potential delivered to the patch (−160 to 160 mV).
(D) Extracted AC displacement (“gating”) currents, representative of voltage sensor charge movements during conformational changes in prestin. Evoked raw currents at each holding potential were averaged from parts 2–32, then the averaged current at 160 mV, where prestin activity is absent, was subtracted from all raw currents at each holding potential, revealing prestin displacement currents. Here, for illustration, we plot the average of currents from all cells, but for subsequent data analysis each cell was individually analyzed to provide statistics. The magnitude of AC currents peaks near Vh, where prestin charge movement vs. voltage is maximal. The displacement currents were used to analyze for nonlinear capacitance (NLC).
(E) Extracted membrane capacitance at two stimulating frequencies, (390 Hz [red circles]) and 19,500 Hz [blue triangles]). Mean ± SE. Solid lines are fits based on Equation 1 (see Methods).
(F) Membrane capacitance as a function of voltage and frequency. Black lines are averages from 13 patches, and pink lines show mean +SE. Smooth multicolored surface is fit to averaged data.
(G) Extracted NLC. Note the roll-off in peak NLC magnitude as frequency of stimulation increases from 390 to 20,000 Hz.
(H) Csa component of fit. Note frequency independence.
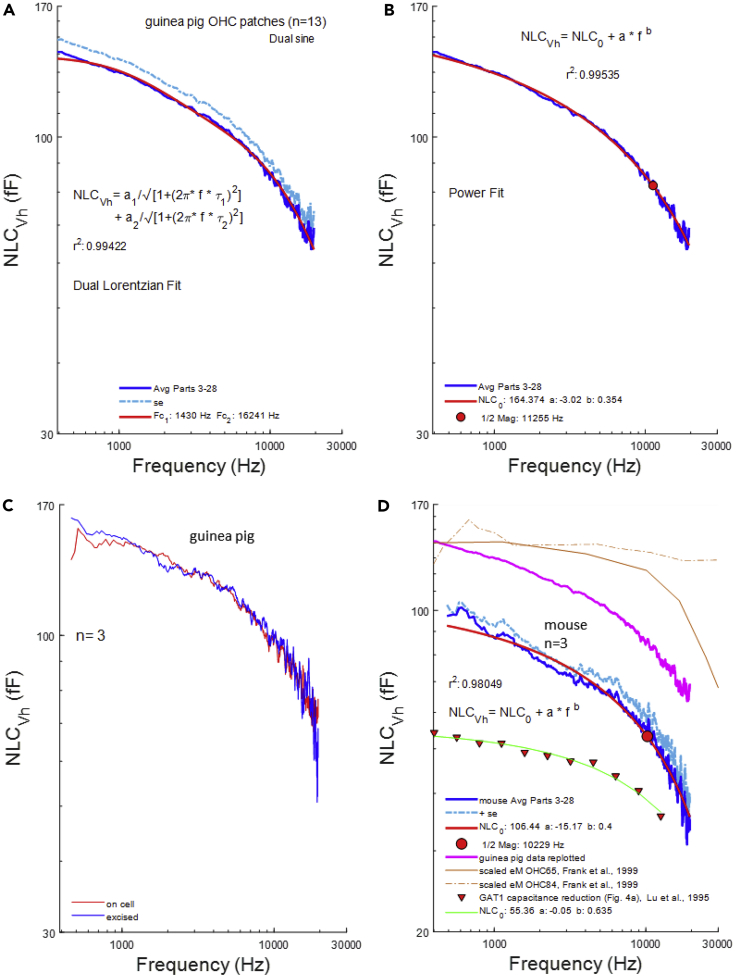
NLC at Vh reflects the charge distribution where an equal amount of voltage sensor charge resides on either side of the plasma membrane. Furthermore, that characteristic voltage (Vh) is a reflection of the ratio of forward and backward rates of prestin transitions, providing a voltage at which to measure the characteristic rate or frequency of sensor charge movement. The coupling of charge movement and eM (Santos-Sacchi and Tan, 2018) also indicates that at this voltage OHCeM gain (cell length change/membrane potential) is maximal. In Figure 2A, we plot the frequency response of NLC at Vh (mean: blue line; mean +SE: light blue dotted line). The roll-off is precipitous commencing at the lowest measured frequency. It is clear that our data, collected at 24.4 Hz resolution, requires a multi-Lorentzian (double in this case), or a power function fit (Figure 2B). The double Lorentzian fit indicates a slow and fast component with frequency cut-offs (Fc) at about 1.5 and 16.2 kHz, respectively. For the power fit, we estimate a cut-off at one-half magnitude of 11.3 kHz. Figure 2C indicates that the frequency response of NLC is the same with on-cell or immediately excised macro-patches in the guinea pig. In Figure 2D, we show that the frequency response of NLC in mouse patches is similar to that of the guinea pig. Half-magnitude cut-off of the power fit is at 10.5 kHz. For comparison, we plot the chloride dependent capacitance frequency response of GAT1 (triangles) (Lu et al., 1995), and its power function fit (green line), indicating that it is somewhat faster than the frequency response of prestin. The guinea pig response (pink line) is replotted in the figure along with scaled eM data (OHC 65: magenta solid line; OHC84: dashed magenta line) from Frank et al. (1999) (Figure 2A), showing that the speed of prestin conformational transitions falls far below OHC mechanical responses. In other words, the drive for eM (voltage-dependent conformational changes in prestin) does not correspond to their measured eM. Can NLC frequency response be augmented?
Figure 2.
Frequency Response of NLC at Vh
(A) Blue line indicates mean NLC, dotted light blue line shows mean +SE. We require at least a double Lorentzian fit that indicates low and high pass components.
(B) Power fit of our data illustrating the continuous roll-off of NLC across frequency. One-half magnitude (red circle) is near 10.5 kHz.
(C) Average of three patches before and after inside-out patch excision, illustrating similar frequency response for each.
(D) Mouse NLC frequency response (mean, mean +SE, blue line, dotted light blue line, respectively) is similarly low pass compared with guinea pig response (pink line). Red circle shows one-half magnitude at 10.5 kHz. For comparison, we plot the capacitance function of Lu et al. (1995) (Figure 4A) for GAT1, fitted with a power function (solid green line). Also, for comparison, the scaled electromotility for the two presented cells from Frank et al. (1999) (Figure 2A) is shown. Note prestin's voltage-driven charge movements are remarkably lower-pass than eM.
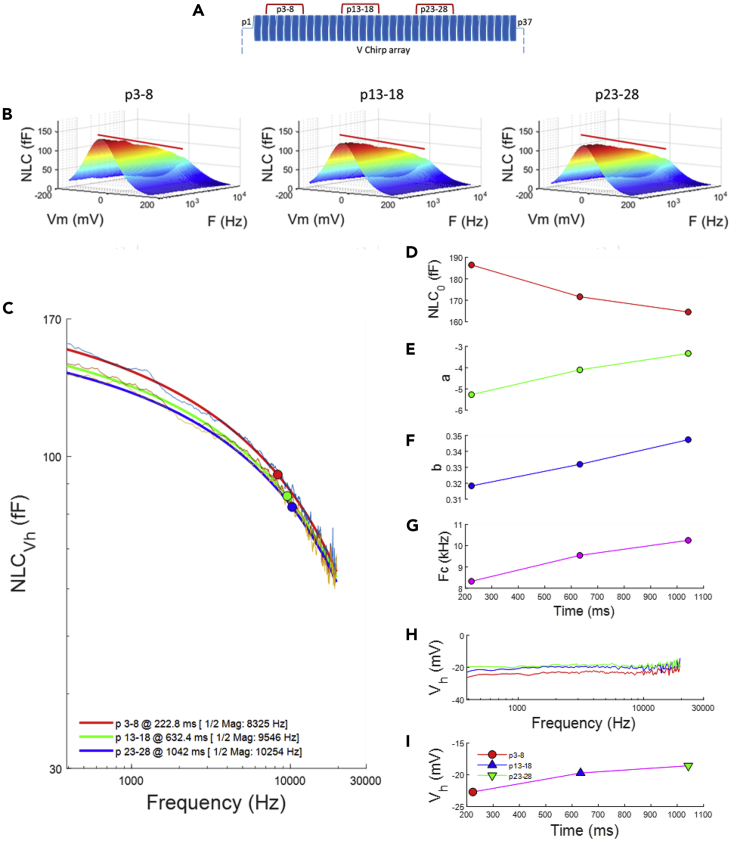
We have previously shown that NLC is not stationary in time (Santos-Sacchi et al., 2009). Step changes in voltage evoke initial rapid changes in NLC, which immediately alter in magnitude in a stretched exponential fashion, accompanied by shifts in Vh. In Figure 3, we explore the frequency response of NLC along the duration of our holding potential steps. Figure 3A shows the regions of chirp currents that were averaged for comparison. The extracted NLC from the three regions are shown in Figure 3B, and for the first region (p3-8) a red line is plotted along the slope of NLC at Vh across frequency. That same red line is recast onto the two other plots of regions p13–18 and p23-28, showing alterations in the frequency response of NLC as duration of the step increases. A quantification of the frequency responses is made in Figure 3C. Power fits indicate that low frequency components are reduced over time such that the frequency cut-off effectively increases over time. Figures 3D–3G plot the fitted parameters of the power fit across holding potential duration, showing reductions in NLC0, and time-dependent changes in the power parameters that control frequency dependence, with frequency cut-offs increasing over time.
Figure 3.
NLC Frequency Response Alters Over Time
(A) Currents were averaged at three time regions, six chirp responses each, over the duration of holding potential, p3-8, p13-18, and p23-28, and NLC was extracted for each region.
(B) NLC for the three durations is shown. The red line demarks the slope of NLC at Vh for the first region (p3-8) and is recast on the others. Note the change in NLC as duration of holding voltage increases.
(C) The frequency response of NLC at Vh is fit with a power function and shows that low-frequency regions of the response decreases over time. Circles depict one-half magnitude, which increases over time.
(D–G) Plots show the parameters of the power fits ([D] NLC0, [E] a, [F] b, and [G] Fc) that alter over time.
(H and I) Vh shifts over the duration of holding voltage steps. (H) Vh across frequency (colors correspond to those in [C]). (I) Mean Vh across frequency corresponding to data and colors in (H).
It has been observed that patch size can change during the course of experiments (Sokabe et al., 1991). It is unlikely that patch size alterations across the duration of our voltage protocol account for the changes in frequency responses that we find because such changes would likely alter the size of NLC across the full frequency range (e.g., see Figure 2D, where smaller mouse patches simply shift all components of NLC downward relative to guinea pig patches). Indeed, Figures 3H and 3I illustrate that NLC across holding potential duration exhibits shifts in Vh that correspond to the nonstationary behavior of NLC that we previously found (Santos-Sacchi et al., 2009), rather than changes in patch area.
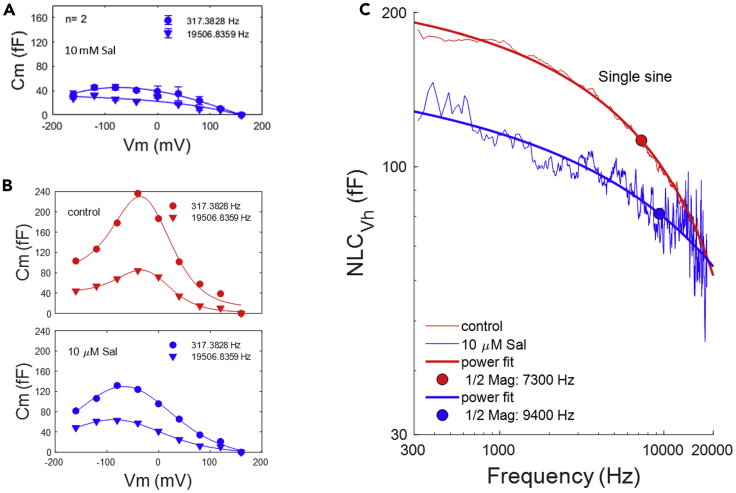
Salicylate is a known intracellular blocker of NLC (Kakehata and Santos-Sacchi, 1996, Tunstall et al., 1995), working in a competitive fashion on chloride binding (Oliver et al., 2001). Chloride anions are not extrinsic voltage sensors for prestin but instead likely work allosterically (Song and Santos-Sacchi, 2010, Rybalchenko and Santos-Sacchi, 2003) (also see our comment #1 on Walter et al. [2019]—https://elifesciences.org/articles/46986). In Figure 4, we explore the effects of salicylate on NLC frequency response. Blue symbols denote responses after salicylate treatment, and red symbols denote response before treatment. Extracellular perfusion of high concentrations of salicylate (10 mM) substantially reduces NLC but not completely (Figure 4A). The effect results from intracellular loading of the ionized form of the molecule, working in the micromolar range (Kakehata and Santos-Sacchi, 1996). In order to gauge salicylate effects on NLC frequency response, we utilized lower concentrations (10 μM), assessing the response before (Figure 4B, top panel) and after (Figure 4B, bottom panel) treatment. Figure 4C shows that low-frequency components of NLC were reduced by 10 μM salicylate, effectively increasing the frequency cut-off. None of the NLC frequency modulations we observed, due to time dependence or salicylate interference with chloride binding, sufficiently increased the response to promote very high frequency electromechanical activity.
Figure 4.
Salicylate Blocks NLC by Reducing Low Frequency Components
Blue color denotes responses after salicylate treatments. Red color denotes control prior to treatment.
(A) NLC is substantially reduced by 10 mM salicylate.
(B) Top panel: control before and bottom panel: after application of 10 μM salicylate to a single patch to provide partial block of NLC.
(C) The resultant partial inhibition of fit extracted NLC illustrates a low-frequency block, which effectively increases NLC frequency response.
Discussion
The identification of AC electromotile responses of the OHC (Kachar et al., 1986) offered a clue to the OHCs' ability to promote high-frequency enhancement of hearing in mammals (Dallos, 2008). The subsequent identification of the response's voltage dependence and associated voltage-sensor activity (Santos-Sacchi, 1990, Santos-Sacchi, 1991, Santos-Sacchi and Dilger, 1988, Ashmore, 1990) suggested that OHC receptor potentials, evoked by acoustic stimulation, drive feedback of mechanical energy into the cochlear partition. It is current dogma that this feedback is cycle-by-cycle, working best at high frequencies, where tonotopic frequency tuning is best (Ashmore et al., 2010). Indeed, OHC eM had been measured in a whole-cell microchamber configuration to work without attenuation beyond 80 kHz at room temperature (Frank et al., 1999), this in spite of initial studies showing more limited charge movement (Gale and Ashmore, 1997a). More recent investigations on whole cells have challenged the concept of OHC high-frequency electromechanical activity, with both NLC and eM exhibiting significant low-pass behavior below 10 kHz (Santos-Sacchi, 2019, Santos-Sacchi et al., 2019, Bai et al., 2019). Our current data on excised and on-cell macro-patch measures of NLC, where whole-cell mechanical loads on the piezoelectric-like protein prestin are absent, directly show continuous roll-off of prestin's conformational activity and indicate that attenuation of sensor-charge movement in both mouse and guinea pig is not single Lorentzian in nature, but instead fit well by a power function of frequency. This frequency response is not very different from that of capacitance measures in the GAT1GABA transporter (Lu et al., 1995). Mechanisms that we find to enhance the frequency response of prestin, namely, time-dependence or motor block with salicylate, do not increase its high-frequency behavior, but simply work by reducing the magnitude of low-frequency components. These observations place a severe restraint on the effectiveness of voltage (i.e., receptor potentials) to drive electromechanical responses of the OHC at very high acoustic frequencies where several mammals possess hearing in the 60–160 kHz range. That is, there can be little or no OHC mechanical feedback in the absence of prestin sensor charge movement at high frequencies, assuming that prestin works as an AC motor driven by AC voltage. Interestingly, in vivo indications of OHC electromechanical activity in the gerbil obtained with OCT show low pass behavior, as well (Vavakou et al., 2019). Those authors considered the RC filter problem (Santos-Sacchi, 1989), where OHC receptor potentials are expected to be attenuated at very high frequencies by more than 20 dB relative to partition movements, and thus not be able to drive prestin. Our data show that the RC filter problem is inconsequential, in as much as the ability of prestin to follow wide-band voltage perturbations is insufficient in and of itself.
There is no doubt that normal prestin activity is involved in the process that enhances hearing capabilities (Dallos et al., 2008, Santos-Sacchi et al., 2006). Knock-in of prestin mutations that alter its voltage operating range (i.e. Vh) or manipulations of OHC chloride, known to influence prestin activity, are catastrophic to cochlear amplification in the mouse, the latter in a reversible manner. How can a slow motor protein work to enhance high-frequency hearing? Could low pass eM actually be countering low-frequency acoustic responses in order to “highlight” high-frequency input to the cochlea? Intriguing results on basilar membrane tuning have been obtained in prestin knock-out/in mice that may point to possible mechanisms of OHC action (MelladoLagarde et al., 2008). Alternatively, a rectified DC component of prestin activity (Evans et al., 1991, Santos-Sacchi, 1989) could contribute to effects on high-frequency tuning, as Vavakou et al. (Vavakou et al., 2019) have intimated. Finally, global hydromechanical influences of the OHC may be at play (He et al., 2018). In any case, the long-held cycle-by-cycle hypothesis of cochlear amplification at very high frequencies is directly countered by our present observations.
Limitations of the Study
Ion concentrations in pipette and cell over the timescale of recording are likely fixed; however, conceivably, as in any macro-patch experiment, ion depletion or accumulation on the patched membrane could influence electrical measures.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This research was supported by NIH-NIDCD R01DC000273, R01DC016318, and R01DC008130 to J.S.-S.
Author Contributions
J.S.-S. designed and performed experiments, analyzed data and wrote the paper. W.T. performed experiments, analyzed data, and edited paper.
Declaration of Interests
The authors declare no conflict of interest.
Published: December 20, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.11.036.
Supplemental Information
References
- Ashmore J., Avan P., Brownell W.E., Dallos P., Dierkes K., Fettiplace R., Grosh K., Hackney C.M., Hudspeth A.J., Juelicher F. The remarkable cochlear amplifier. Hear. Res. 2010;266:1–17. doi: 10.1016/j.heares.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J.F. Forward and reverse transduction in the mammalian cochlea. Neurosci. Res. Suppl. 1990;12:S39–S50. doi: 10.1016/0921-8696(90)90007-p. [DOI] [PubMed] [Google Scholar]
- Bai J.P., Navaratnam D., Santos-Sacchi J. Prestin kinetics and corresponding frequency dependence augment during early development of the outer hair cell within the mouse organ of Corti. Sci. Rep. 2019;9:16460. doi: 10.1038/s41598-019-52965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellote M., Mooney T.A., Quakenbush L., Hobbs R., Goertz C., Gaglione E. Baseline hearing abilities and variability in wild beluga whales (Delphinapterusleucas) J. Exp. Biol. 2014;217:1682–1691. doi: 10.1242/jeb.093252. [DOI] [PubMed] [Google Scholar]
- Dallos P. Cochlear amplification, outer hair cells and prestin. Curr. Opin. Neurobiol. 2008;18:370–376. doi: 10.1016/j.conb.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P., Wu X., Cheatham M.A., Gao J., Zheng J., Anderson C.T., Jia S., Wang X., Cheng W.H., Sengupta S. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58:333–339. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H. An active process in cochlear mechanics. Hear. Res. 1983;9:79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Evans B.N., Hallworth R., Dallos P. Outer hair cell electromotility: the sensitivity and vulnerability of the DC component. Hear. Res. 1991;52:288–304. doi: 10.1016/0378-5955(91)90019-6. [DOI] [PubMed] [Google Scholar]
- Frank G., Hemmert W., Gummer A.W. Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc. Natl. Acad. Sci. U S A. 1999;96:4420–4425. doi: 10.1073/pnas.96.8.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale J.E., Ashmore J.F. Charge displacement induced by rapid stretch in the basolateral membrane of the Guinea-pig outer hair cell. Proc. R. Soc. Lond. B Biol. Sci. 1994;255:243–249. doi: 10.1098/rspb.1994.0035. [DOI] [PubMed] [Google Scholar]
- Gale J.E., Ashmore J.F. An intrinsic frequency limit to the cochlear amplifier. Nature. 1997;389:63–66. doi: 10.1038/37968. [DOI] [PubMed] [Google Scholar]
- Gale J.E., Ashmore J.F. The outer hair cell motor in membrane patches. Pflugers Arch. 1997;434:267–271. doi: 10.1007/s004240050395. [DOI] [PubMed] [Google Scholar]
- Heffner H., Masterton B. Hearing in Glires: domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J. Acoust. Soc. Am. 1980;68:1584–1599. [Google Scholar]
- He W., Kemp D., Ren T. Timing of the reticular lamina and basilar membrane vibration in living gerbil cochleae. Elife. 2018;7:e37625. doi: 10.7554/eLife.37625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner R.S., Koay G., Heffner H.E. Audiograms of five species of rodents: implications for the evolution of hearing and the perception of pitch. Hear. Res. 2001;157:138–152. doi: 10.1016/s0378-5955(01)00298-2. [DOI] [PubMed] [Google Scholar]
- Iwasa K.H. Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys. J. 1993;65:492–498. doi: 10.1016/S0006-3495(93)81053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K.H., Kachar B. Fast in vitro movement of outer hair cells in an external electric field: effect of digitonin, a membrane permeabilizing agent. Hear. Res. 1989;40:247–254. doi: 10.1016/0378-5955(89)90165-2. [DOI] [PubMed] [Google Scholar]
- Kachar B., Brownell W.E., Altschuler R., Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986;322:365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kakehata S., Santos-Sacchi J. Membrane tension directly shifts voltage dependence of outer hair cell motility and associated gating charge. Biophys. J. 1995;68:2190–2197. doi: 10.1016/S0006-3495(95)80401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakehata S., Santos-Sacchi J. Effects of salicylate and lanthanides on outer hair cell motility and associated gating charge. J.Neurosci. 1996;16:4881–4889. doi: 10.1523/JNEUROSCI.16-16-04881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.C., Kabakov A., Markin V.S., Mager S., Frazier G.A., Hilgemann D.W. Membrane transport mechanisms probed by capacitance measurements with megahertz voltage clamp. Proc. Natl. Acad. Sci. U S A. 1995;92:11220–11224. doi: 10.1073/pnas.92.24.11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MelladoLagarde M.M., Drexl M., Lukashkin A.N., Zuo J., Russell I.J. Prestin's role in cochlear frequency tuning and transmission of mechanical responses to neural excitation. Curr. Biol. 2008;18:200–202. doi: 10.1016/j.cub.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Oliver D., He D.Z., Klocker N., Ludwig J., Schulte U., Waldegger S., Ruppersberg J.P., Dallos P., Fakler B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2340–2343. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- Pollak G., Henson O.W., Jr., Novick A. Cochlear microphonic audiograms in the “pure tone” bat Chilonycterisparnelliiparnellii. Science. 1972;176:66–68. doi: 10.1126/science.176.4030.66. [DOI] [PubMed] [Google Scholar]
- Rybalchenko V., Santos-Sacchi J. Allosteric modulation of the outer hair cell motor protein prestin by chloride. In: Gummer A., editor. Biophysics of the Cochlea: From Molecules to Models. World Scientific Publishing; 2003. pp. 116–126. [Google Scholar]
- Santos-Sacchi J. Asymmetry in voltage-dependent movements of isolated outer hair cells from the organ of Corti. J.Neurosci. 1989;9:2954–2962. doi: 10.1523/JNEUROSCI.09-08-02954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Fast outer hair cell motility: how fast is fast? In: Dallos P., Geisler C.D., Matthews J.W., Ruggero M.A., Steele C.R., editors. The Mechanics and Biophysics of Hearing. Springer-Verlag; 1990. pp. 69–75. [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J. Neurosci. 1991;11:3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. The speed limit of outer hair cell electromechanical activity. HNO. 2019;67:159–164. doi: 10.1007/s00106-019-0615-9. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J., Dilger J.P. Whole cell currents and mechanical responses of isolated outer hair cells. Hear. Res. 1988;35:143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J., Huang G.J., Wu M. Mapping the distribution of outer hair cell voltage-dependent conductances by electrical amputation. Biophys. J. 1997;73:1424–1429. doi: 10.1016/S0006-3495(97)78174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J., Iwasa K.H., Tan W. Outer hair cell electromotility is low-pass filtered relative to the molecular conformational changes that produce nonlinear capacitance. J. Gen. Physiol. 2019 doi: 10.1085/jgp.201812280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J., Navarrete E. Voltage-dependent changes in specific membrane capacitance caused by prestin, the outer hair cell lateral membrane motor. Pflugers Arch. 2002;444:99–106. doi: 10.1007/s00424-002-0804-2. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J., Navarrete E., Song L. Fast electromechanical amplification in the lateral membrane of the outer hair cell. Biophys. J. 2009;96:739–747. doi: 10.1016/j.bpj.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J., Song L. Chloride-driven electromechanical phase lags at acoustic frequencies are generated by SLC26a5, the outer hair cell motor protein. Biophys. J. 2014;107:126–133. doi: 10.1016/j.bpj.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J., Song L., Zheng J., Nuttall A.L. Control of mammalian cochlear amplification by chloride anions. J. Neurosci. 2006;26:3992–3998. doi: 10.1523/JNEUROSCI.4548-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J., Tan W. The frequency response of outer hair cell voltage-dependent motility is limited by kinetics of prestin. J. Neurosci. 2018;38:5495–5506. doi: 10.1523/JNEUROSCI.0425-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe M., Sachs F., Jing Z.Q. Quantitative video microscopy of patch clamped membranes stress, strain, capacitance, and stretch channel activation. Biophys. J. 1991;59:722–728. doi: 10.1016/S0006-3495(91)82285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Santos-Sacchi J. Conformational state-dependent anion binding in prestin: evidence for allosteric modulation. Biophys. J. 2010;98:371–376. doi: 10.1016/j.bpj.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski M.D., Bain D.E., Kiehl K., Pennington S., Wong S., Henry K.R. Killer whale (Orcinus orca) hearing: auditory brainstem response and behavioral audiograms. J. Acoust. Soc. Am. 1999;106:1134–1141. doi: 10.1121/1.427121. [DOI] [PubMed] [Google Scholar]
- Tunstall M.J., Gale J.E., Ashmore J.F. Action of salicylate on membrane capacitance of outer hair cells from the Guinea-pig cochlea. J. Physiol. 1995;485(Pt 3):739–752. doi: 10.1113/jphysiol.1995.sp020765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavakou A., Cooper N.P., van der Heijden M. The frequency limit of outer hair cell motility measured in vivo. Elife. 2019;8:e47667. doi: 10.7554/eLife.47667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bekesy G. McGraw-Hill; 1960. Experiments in Hearing. [Google Scholar]
- Walter J.D., Sawicka M., Dutzler R. Cryo-EM structures and functional characterization of murine Slc26a9 reveal mechanism of uncoupled chloride transport. Elife. 2019;8 doi: 10.7554/eLife.46986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Shen W., He D.Z., Long K.B., Madison L.D., Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.