Abstract
Liver fibrosis is an advanced liver disease condition, which could progress to cirrhosis and hepatocellular carcinoma. To date, there is no direct approved antifibrotic therapy, and current treatment is mainly the removal of the causative factor. Transforming growth factor (TGF)-β is a master profibrogenic cytokine and a promising target to treat fibrosis. However, TGF-β has broad biological functions and its inhibition induces non-desirable side effects, which override therapeutic benefits. Therefore, understanding the pleiotropic effects of TGF-β and its upstream and downstream regulatory mechanisms will help to design better TGF-β based therapeutics. Here, we summarize recent discoveries and milestones on the TGF-β signaling pathway related to liver fibrosis and hepatic stellate cell (HSC) activation, emphasizing research of the last five years. This comprises impact of TGF-β on liver fibrogenesis related biological processes, such as senescence, metabolism, reactive oxygen species generation, epigenetics, circadian rhythm, epithelial mesenchymal transition, and endothelial-mesenchymal transition. We also describe the influence of the microenvironment on the response of HSC to TGF-β. Finally, we discuss new approaches to target the TGF-β pathway, name current clinical trials, and explain promises and drawbacks that deserve to be adequately addressed.
Keywords: TGF-β, liver fibrosis, hepatic stellate cells, myofibroblasts
1. Introduction
1.1. Liver Fibrosis and Hepatic Stellate Cell (HSC) Activation
Liver fibrosis is the common result of chronic liver damage from any etiology, such as alcoholic steatohepatitis (ASH), non-alcoholic fatty liver disease (NAFLD), hepatitis B or C infection (HBV/HCV) or cholestatic liver injury, although the scar pattern formation may vary in dependency of the location of the primary damaged hepatocytes. Fibrosis means that an excessive amount of fibrillar extracellular matrix (ECM) proteins, e.g., collagen I and III, is deposited in the space of Disse [1,2]. The changes in ECM composition induce liver sinusoidal endothelial cells (LSEC) to loose fenestra and to form a basement membrane in a process termed LSEC capillarization [3]. These pathophysiological changes interfere with normal nutrient transport between sinusoidal blood and surrounding cells, especially hepatocytes, which leads to functional disturbance [1,3]. If the liver damage-inducing agent is not properly removed, liver fibrogenesis perpetuates (reflected by the term “chronic”) until the liver architecture is strongly distorted, and can then progress to the late stages of liver disease, liver cirrhosis and hepatocellular carcinoma (HCC), and thus could finally lead to liver failure and death [4,5,6].
Myofibroblasts (MFB) are the main producers of collagens and other ECM proteins and are therefore central in scar formation during liver fibrogenesis. The origin of MFB is intensely debated. MFB may originate from bone marrow-derived fibrocytes, portal fibroblasts, or hepatic stellate cells (HSC), depending on the type of damage and location. It has been proposed that liver epithelial cells, i.e., hepatocytes and cholangiocytes, or even endothelial cells could be additional sources for liver MFB, through epithelial mesenchymal transition (EMT) or endothelial mesenchymal transition (EndMT) processes [7,8]. There are supportive and disproving data available, however, for the latter transdifferentiation pathways, there is a broad consensus that HSC are the major contributors for the MFB pool during liver fibrosis, independent of the damaging source [4].
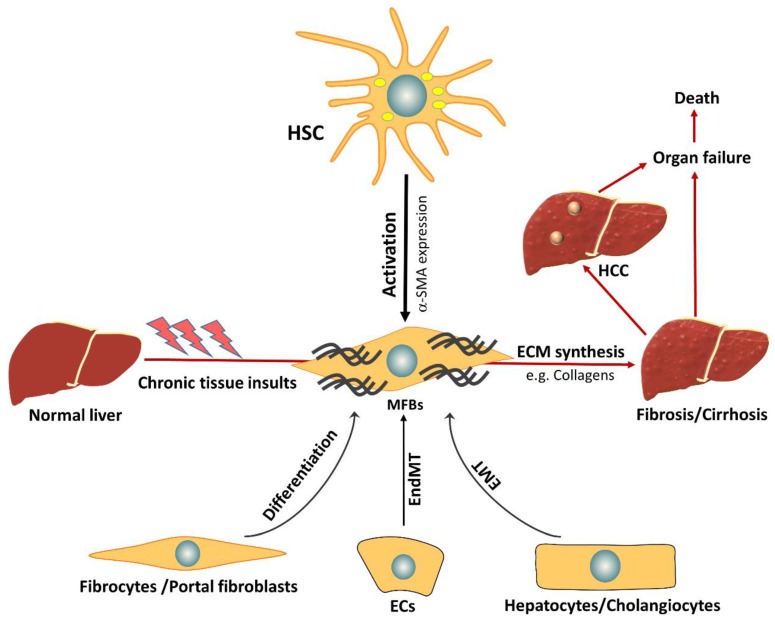
In normal liver, HSC exist in a quiescent non-proliferative state, where they have a star-like shape with intracellular lipid droplet storage containing vitamin A as retinyl palmitate [9]. During acute and chronic liver injury, transforming growth factor (TGF)-β is activated from deposits in the ECM and expressed and released from various cell types. An important target of TGF-β in this setting are HSC that are induced to activate and transdifferentiate to MFB, which comprises loss of intracellular vitamin A droplets, adaptation of a fibroblast shape, and development of a contractile, proliferative, and migratory phenotype (Figure 1) [9].
Figure 1.
Activation of hepatic stellate cells (HSCs) and origin of myofibroblasts (MFBs) in chronic liver diseases. During activation, HSCs lose intracellular lipid droplets, acquire a fibroblast-like shape, and express a large amount of alpha-smooth muscle actin (α-SMA) and extracellular matrix proteins (ECM). Beside HSCs, which represent a major source of MFBs, other cells such as pericytes, portal fibroblasts can differentiate into MFBs. Also, endothelial cells (ECs) and epithelial cells, i.e., hepatocytes and cholangiocytes, might contribute to liver MFBs pool through an endothelial-mesenchymal transition (EndMT) and epithelial-mesenchymal transition (EMT), respectively. However, unequivocal in vivo evidence of EMT during liver fibrosis is still missing.
In cooperation with other signaling pathways, triggered by e.g., reactive oxygen species (ROS), platelet-derived growth factor (PDGF), and connective tissue growth factor (CTGF), TGF-β signaling is considered the key fibrogenic pathway that drives HSC activation and induces ECM production [8,10]. In normal liver, quiescent HSC express a minute amount of TGF-β, which is upregulated shortly after liver injury. Besides HSC, there are additional cellular sources for TGF-β in the liver such as LSECs, macrophages, and hepatocytes [11]. Very recently, platelets were also described as an important source of TGF-β during liver fibrosis [12].
1.2. TGF-β Family
The TGF-β family comprises 33 members including TGF-βs, activins, and bone morphogenetic proteins (BMPs) [13]. TGF-β proteins exist in three isoforms, TGF-β1, 2, and 3, which share overlapping but non-redundant functions, as most importantly shown by knockout (KO) mice of the respective TGF-β isoforms [14,15,16]. Generally, TGF-β1 is the most widely and most intensely investigated isoform in liver fibrogenesis [10,17]. Therefore, when using the term TGF-β, the knowledge is based on experimental data from TGF-β1 throughout this review. In this regard, we previously reported a predominant role for TGF-β2 in the pathogenesis of biliary fibrosis. In liver tissue of bile-duct ligated (BDL) and Mdr2-/- mice, TGF-β2 was elevated to a higher level than TGF-β1 [18]. Moreover, a TGF-β2 antisense oligonucleotide efficiently attenuated biliary fibrosis, as shown by reduced sirius red and α-SMA staining (data under revision). Abd El-Mequid et al. describe a correlation between TGF-β2 expression in peripheral leucocytes and protein levels in serum with hepatic fibrogenesis in a cohort of 89 HCV patients and 21 healthy controls. The authors thus suggest TGF-β2 as a promising biomarker for liver fibrosis in HCV [19]. Mechanistically, the increased expression of TGF-β2 in HCV-induced liver fibrosis is mediated via a cAMP-responsive element-binding protein (CREBH) site in the promoter of the TGF-β2 gene in hepatocytes that is activated from HCV infection [20].
1.3. TGF-β Signaling
TGF-β is synthesized in the form of a latent precursor that needs to be cleaved by furin-like proteases to become activated. Subsequently, the C-terminus of the TGF-β molecule binds to the N-terminus of the latency associated protein (LAP) to form the latent TGF-β complex (LTC) [21]. The LTC is then released and deposited in the surrounding ECM through binding to latent TGF-β binding protein (LTBP), forming the large latent complex (LLC) [22]. All TGF-β isoforms undergo such process, whereas certain BMPs and activins are not released as latent complexes [13]. Entrapment of TGF-β in the form of LLC ensures focused local effects upon activation. The release of active TGF-β from LLC is actively triggered through variant physical and biochemical factors, such as high or low pH, cleavage by specific proteases, and through interaction with integrins. The integrin interactions are considered the principal activating mechanism for latent TGF-β. Through integrin binding to certain collagen molecules in the ECM, traction forces are applied, which induce a conformational change in the LLC, thereby facilitating the accessibility of proteases that mediate the liberation of the active form of TGF-β [22,23,24,25]. We recently identified extracellular matrix protein 1 (ECM1) as a stabilizer of liver ECM deposited latent TGF-β by interacting with αv integrins. In different animal models of experimental liver fibrosis and in patients with chronic liver diseases (CLD), ECM1 expression decreased along with disease severity. Ecm1-KO mice spontaneously developed severe liver fibrosis with tremendous TGF-β/Smad3 and subsequent HSC activation. The animals die between 8 and 12 weeks of age. This phenotype could be rescued by adenoassociated virus (AAV) mediated expression of ECM1 or by interfering with TGF-β signaling using AAV expressing soluble TβRII. Moreover, carbon tetrachloride (CCl4)-induced liver damage was blunted by ECM1 overexpression [25].
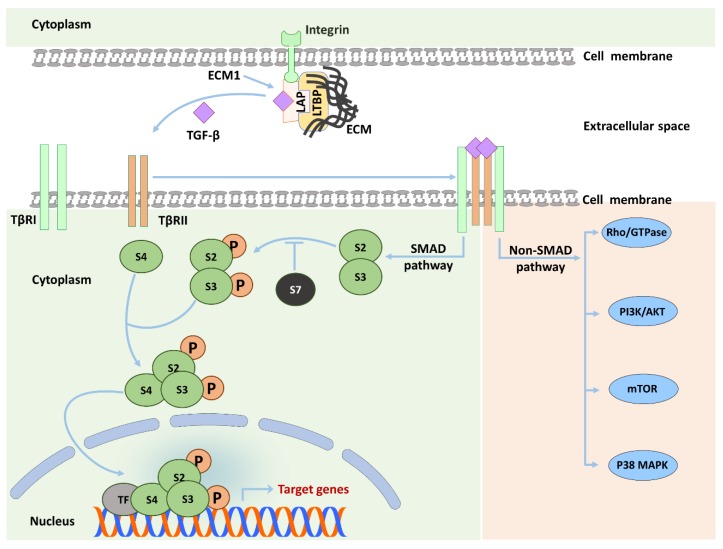
Active TGF-β starts signaling by binding to the TGF-β type II receptor (TβRII) resulting in recruitment of the TGF-β type I receptor (TβRI). Next, TβRII phosphorylates TβRI at a Gly-Ser–rich (GS) domain leading to a conformational modulation in TβRI and sensitizing it to bind and phosphorylate its substrates, i.e., SMAD2 and SMAD3 proteins (also called receptor-activated SMADs or R-SMADs). After C-terminal SMAD phosphorylation, pSMAD2 and pSMAD3 form heterocomplexes with the common SMAD4, which thereafter translocates to the nucleus to bind DNA and regulate the transcription of multiple target genes, e.g., αSMA, and CTGF (Figure 2) [13,26]. Two important facts deserve to be highlighted here. First, SMAD2 does not bind to DNA, while SMAD3 possesses a weak DNA binding affinity. Therefore, SMAD2/3/4 complexes generally recruit additional transcriptional coactivators to stabilize transactivation complexes [13,27]. Second, several TGF-β target genes can be activated by R-SMADs without the requirement of SMAD4 [28].
Figure 2.
SMAD- and Non-SMAD-dependent TGF-β signaling. Upon liver damage associated signaling, TGF-β molecules are freed from the large latent complex (LLC) through the interaction of integrins with the latent association protein (LAP). Binding of released TGF-β to TβRII results in the formation of a heterotetramer with TβRI, which then initiates the canonical signaling pathway through phosphorylation of R-SMADs, i.e., SMAD2 (S2) and SMAD3 (S3). TGF-β can also activate non-canonical SMAD-independent pathways, as exemplified here by MAPK, mTOR, PI3K/AKT, and Rho/GTPase pathways. Alongside other mechanisms, SMAD7 negatively regulates TGF-β signaling through competing with R-SMADs for TβRI binding. TF: Transcription factors, P: phosphate group, LTBP: latent TGF-β binding protein.
Canonical R-SMAD-mediated TGF-β signaling does not explain all observed effects of TGF-β. Many studies identified other signaling pathways that could be activated by TGF-β, such as mitogen-activated protein kinase (MAPK), mammalian target of rapamycin (mTOR), phosphatidylinositol-3-kinase/AKT, and Rho GTPase pathways (Figure 2). TGF-β non-canonical pathways provide a broad window for intracellular cross-talk [29,30,31] and can be classified into three major groups [29]: (I) R-SMADs interact with other pathways instead of directly transmitting the signal to the nucleus. Such interaction is illustrated by the ability of SMAD2 and SMAD3 to activate ERK and PKA [32,33]. (II) TheTβR complex can activate intracellular substrates other than SMADs, such as Daxx, a proapoptotic adaptor protein, leading to JNK activation and apoptosis [34]. (III) R-SMADs could be activated by TβR-independent mechanisms. The latter mechanism is best exemplified by phosphorylation of the linker domain of R-SMADs, e.g., by ERK, which interferes with R-SMAD nuclear translocation [35]. Non-canonical pathways provide one explanation for the versatile effects of TGF-β signaling and its dichotomal functions, as for example described in carcinogenesis [36]. In fibrosis, however, such events have not yet been thoroughly investigated, with exception of linker phosphorylation [37]. It should be emphasized here that results obtained from SMAD4 KO cells or specific kinase inhibitor treatments should be carefully attributed to non-SMAD signaling for several reasons [29,30]. Firstly, as previously mentioned, SMAD4 is not required for transcription of several specific R-SMAD dependent genes such as SNAI2, TPD52, and CDKN1A [28]. Secondly, chemical inhibitors can block several kinases dose-dependently [30]. Therefore, in our opinion, specific SMAD2 and SMAD3 KO models represent the best way to characterize non-SMAD pathways downstream to TGF-β treatment [29]. Signaling kinetics can also be utilized to shed light on SMAD and non-SMAD-dependent effects. For example, in some cells, e.g., mast cells, TGF-β mediated ERK phosphorylation occurs within 10 min in similar kinetics to EGF-induced ERK activation, which suggests a direct non-SMAD mechanism. In contrast, the same effect requires several hours in other cells, e.g., pancreatic acinar cells, highlighting the need for de novo protein synthesis, which could rely on SMAD dependent mechanisms [30,38,39,40]. Additional examples of non-canonical TGF-β signaling in the context of liver fibrogenesis are mentioned in different sections of this review.
1.3.1. SMAD2 vs. SMAD3
Although SMAD2 and SMAD3 share 92% of their amino acid sequence, they show distinct biological effects. As already mentioned above, SMAD2, in contrast to SMAD3, has no DNA binding capacity, as it has extra sequences inserted in its Mad homology (MH1) domain, which prevents DNA binding [27]. This difference in DNA binding ability provides one explanation for the variant functions of the two SMAD molecules in general, in the different liver cell types and during liver fibrosis [27]. SMAD3 is considered the crucial inducer of the fibrogenic program in HSC, whereas SMAD2 was described as having antifibrotic effects [41,42]. A mechanism mediating such antifibrotic effects of SMAD2 was recently deciphered by Xu et al. and was linked to its ability to increase ligand (TRAIL) mediated HSC apoptosis through downregulation of X-linked inhibitor of apoptosis protein (XIAP). This then leads to enhanced caspase-3 activity and cell death [42]. In addition, deletion of SMAD3, but not SMAD2, in fibroblasts efficiently reduced pressure overload-induced cardiac fibrosis [43], which again underlines the differential roles of SMAD2 and SMAD3 in mediating organ fibrosis.
1.3.2. SMAD Phosphorylation Dynamics
Noteworthy, the peak of a transient TGF-β induced SMAD phosphorylation generally occurs within 30–60 min, turning back to baseline at between 3 and 6 h. On the other hand, the full differentiation of MFB needs between 2–3 days [26,44]. An extended pSMAD kinetics investigation by Ard et al. shows that SMAD2 remains partially phosphorylated for up to 2 days after TGF-β stimulation in human lung fibroblasts. The authors claim that this sustained SMAD2 phosphorylation, besides transcription of SMAD3-dependent genes, is required for full differentiation of MFB [45]. These results suggest a co-operative rather than an antagonistic relationship between SMAD2 and SMAD3 in the context of MFB trans-differentiation, at least in the lung. Data from liver in this respect are lacking but given the cellular context-dependency of TGF-β signaling, a similar mechanism can be expected. Extended kinetic studies in HSC thus may generate valuable data.
2. Regulation of the TGF-β Pathway
Cells keep TGF-β signaling under tight control by a plethora of positive and negative feedback loops. One of these regulatory mechanisms is the transcription of inhibitory SMAD proteins (I-SMAD) i.e., SMAD6 and SMAD7 [46], which can be induced by TGF-β as a negative feedback system. Of note, SMAD6 is selective for BMP receptors, whereas SMAD7 can bind to and inhibit both TGF-β and BMP receptors [27,47]. I-SMADs negatively regulate and fine-tune TGF-β signaling through several mechanisms, including competitive inhibition of R-SMAD binding to TβRI, recruiting ubiquitin E3 ligases, e.g., SMURF2, to TβR and induce its degradation, or phosphatase enzymes, e.g., PP1C, for receptor deactivation [27,48,49]. RING finger protein 11 (RNF11) positively regulates TGF-β signaling by competing with SMAD7 for binding to SMURF2, thus decreasing degradation activity for receptor complexes [50]. On the other hand, the deubiquitination of SMAD7 by OTU domain-containing protein (OTUD) 1 stabilizes SMAD7, thus leading to enhanced negative regulation of TGF-β signaling [51]. Moreover, the sumoylation of SMURF2 by SUMO E3 ligase PIAS3 enhances its ability to degrade TGF-β receptors and thereby suppresses TGF-β effects, e.g., EMT [52].
To get a broader overview of the currently known regulatory mechanisms of TGF-β signaling, the reader is referred to the elegant review by Derynck and Budi [13]. In the next section, we will discuss more recently identified or more deeply characterized targets and effectors of the TGF-β pathway that were described in the context of HSC activation and liver fibrosis.
New Targets and Regulators of the TGF-β Pathway in Liver Fibrosis
Caveolin is an essential component of the caveolae of the plasma membrane and is involved in cell surface receptor trafficking [53]. Receptor-mediated endocytosis is another basic mechanism for the internalization of various proteins e.g., TGF-β receptors from cell surface through formation of clathrin-coated vesicles [54]. These two endocytosis processes were described to regulate TGF-β signaling differentially. In contrast to clathrin-dependent endocytosis of TGF-β receptors, which facilitates TGF-β signaling, caveolin mediated endocytosis promotes TGF-β receptor degradation [55] and relates to non-Smad TGF-β signaling [56]. Cav1 deficiency was found to aggravate CCl4-induced liver fibrosis in mice, which was mechanistically linked to enhanced TGF-β induced oxidative stress [57]. Further, a Cav1 scaffolding domain (CSD) peptide attenuated CCl4-induced liver fibrosis as shown by significantly decreased collagen content [58]. The authors showed that Cav1 knockdown led to enhanced TGF-β signaling and SMAD2 phosphorylation. In addition, SMAD2 phosphorylation decreased after CSD treatment. Further, our group identified a crucial role for Cav1 towards hepatocyte apoptosis. Cav1 levels determine whether hepatocyte apoptosis is executed upon a TGF-β stimulus [59]. Based on the regulation of Cav1 expression in hepatocytes in vitro [60] and in vivo upon NAFLD development [61], Cav1 serves as a crucial regulator of TGF-β signaling and thus TGF-β-related phenotypes.
TGF-β-HAS2-HA: Seki and coworkers delineated another downstream route of TGF-β mediated HSC activation in liver fibrosis, which ends in hyaluronan (HA) production, a major ECM glycosaminoglycan, and biomarker of liver cirrhosis. They show that HA synthase 2 (HAS2) is transcriptionally up-regulated by TGF-β and mediates HSC activation by Wilms tumor 1, CD44, Toll-like receptor 4 (TLR4), and Notch1. HAS2 expression is elevated in human and murine liver fibrosis. Furthermore, HA production and liver fibrosis were decreased upon HAS2 depletion in HSC and enhanced upon HAS2 overexpression. Therapeutical blunting of HA synthesis by 4-methylumbelliferone decreases HSC activation and liver fibrosis [62], which may offer a new therapeutic route in future.
TGF-β-CD147 feedback loop: CD147 is a glycosylated protein that is expressed on the cellular membrane of HSC [63]. Li et al. described an interesting positive feedback loop between TGF-β1 signaling and CD147 in HSC. On one side, TGF-β1 increased expression of CD147 via a SMAD2/3/4-dependent mechanism, which can promote LX-2 cell migration and contraction. On the other side, CD147 overexpression triggered TGF-β1, α-SMA, and COL1α1 expression through upregulation of ERK1/2 and Sp1 [64]. The study raises the possibility that a combination of TβR inhibitors and an anti-CD147 antibody could be more effective than TβR inhibitors alone in treating liver fibrosis. However, more studies on this topic are warranted to determine effectiveness.
Hydrogen peroxide-inducible clone 5 (HIC-5) was previously identified as a gene induced in response to increased H2O2 and TGF-β1 and was shown to be essential for MFB differentiation [65,66]. Noteworthy, the expression of HIC-5 protein increased in liver fibrosis in mice and humans [67]. HIC5 knockdown attenuated liver fibrosis in CCl4 and BDL animal models through upregulation of SMAD7 expression [67]. Yet, the molecular mechanisms regulating HIC-5 expression during liver fibrogenesis and their link to TGF-β signaling (components, e.g., SMAD7) are still waiting further investigation.
GP73 is a type II transmembrane glycoprotein, which is highly expressed in hepatocytes during acute and CLD [68]. GP73 was recently described as a novel target and regulator of the TGF-β pathway. Its promoter contains a SMAD binding element (SBE) which is responsible for enhanced transcription upon stimulation with TGF-β [69]. Subsequently, GP73 feeds back on decision making between canonical and non-canonical arms of TGF-β signaling, thereby downregulating SMAD signaling and facilitating ERK/AKT signaling [69]. The modulatory effect of GP73 on TGF-β signaling was attributed to GP73 mediated upregulation of caveolin that subsequently modulates TGF-β signaling as discussed above [69]. However, the in vivo relevance of GP73 in HSC activation and liver fibrosis awaits further studies.
Galectin-1 (Gal-1) is a β-galactoside-binding lectin, which recognizes and binds to several glycosylated receptors leading to the regulation of diverse intracellular signaling pathways [70]. Gal-1 expression was increased in ethanol-fed HCV core-transgenic mice and participated in MFB activation in cancer [71,72]. The link to TGF-β signaling and HSC activation was shown by a recent study by Wu et al. [73]. Treatment of LX-2 cells with recombinant Gal-1 protein increased phosphorylation of SMAD2, SMAD3, and ERK1/2 leading to enhanced HSC migration. These pro-migratory properties of Gal-1 were found to be dependent on Gal-1 binding to neuropilin-1 in a glycosylation-dependent manner [73]. Based on these results, it will be interesting to test specific Gal-1 binding inhibitors in animal models of liver fibrosis.
Lipin-1 is a phosphatidic acid phosphatase enzyme that catalyzes phosphatidate conversion to diacylglycerol [74]. It also acts as a transcriptional factor by direct binding to other transcriptional factors including PPAR-γ coactivator-1 α (PGC-1α) and PPAR-α [75]. Lipin-1 was reported to have antifibrogenic effects, which are mediated by inhibition of TGF-β1 signaling [76]. Jang et al. showed that TGF-β keeps lipin-1 downregulated on protein level in activated HSC through induction of lipin-1 polyubiquitination without altering lipin-1 mRNA stability [76]. Interestingly, resveratrol, a polyphenolic compound with antioxidant properties inhibited TGF-β-mediated polyubiquitination of lipin-1 which led to a suppression of TGF-β induced expression of fibrogenic genes [76]. Whether this is a unique response to resveratrol or represents a common mechanism for other antioxidants remains an interesting open question.
Notch and Y-box binding protein (YB-1): Recent studies describe new roles for Notch signaling and YB- signaling in regulating TGF-β signaling. Wu et al. show that both TGF-β and Notch signaling were activated in concanavalin A-induced liver fibrosis in rats [77]. Inhibition of Notch signaling using γ-secretase inhibitor reduced TGF-β and SMAD3 expression at protein and mRNA levels in peripheral blood mononuclear cells of fibrotic rats [77]. In our laboratory, treatment of primary and JS-1 HSC with Delta-like (DLL)-4 notch ligand did not alter TGF-β-induced HSC activation [78]. However, we showed that DLL4 ligand could modulate CCl4- and BDL-induced liver fibrosis through inhibition of inflammatory CCL-2 chemokine secretion from macrophages [78]. These results suggest that Notch signaling alters liver fibrogenesis in TGF-β dependent and independent ways. Recently, Xiong et al. described an interesting mutual interaction between YB-1 protein and TGF-β signaling in liver fibrosis. On one hand, the TGF-β signaling pathway could induce YB-1 protein expression via a SMAD2-dependent mechanism. On the other hand, phosphorylated YB-1 acts as a stabilizer for SMAD2 protein through inhibition of ubiquitination [79].
NUAK1 and 2 are members of the AMP-activated protein kinase (AMPK) family. Both are upregulated in response to TGF-β by a SMAD2/3/4-MAPK-dependent mechanism [80]. Kolliopoulos and colleagues described a NUAK1/2 bifurcation loop in the TGF-β pathway, with NUAK1 suppressing and NUAK2 enhancing TGF-β signaling [80]. The authors show that NUAK2 is physically associated with SMAD3/TβRI, leading to stabilization of the activated receptor complex, which results in enhanced TGF-β signaling. Opposite to NUAK1 overexpression results, siRNA mediated silencing of NUAK2 attenuated TGFβ mediated α-SMA protein expression and TGF-β induced collagen gel contraction in AG1523 cells, suggesting a potential fibrogenic role [80].
STAT3/JAK1 We and others previously revealed a cross-talk between TGF-β and STAT3 in HSC [81,82,83,84]. A recent study describes a novel mechanism by which TGF-β activates STAT3 downstream signaling involving JAK1. The authors show that JAK1 associates with TβRI and mediates TGF-β induced activation of STAT3 by two distinct mechanisms. First, an early and direct SMAD independent phosphorylation of STAT3 occurs. Second, late phosphorylation of STAT3 requires SMAD activation and de novo protein synthesis. The authors further show that rapid SMAD independent activation of STAT3 is essential for expression of TGF-β targets genes, such as PDGFB, ID1, SMAD7, and SOCS3 in LX-2 cells, thus priming the cells for a second enhanced activation. Therewith, they suggest a co-operative interaction of both mechanisms in mediating TGF-β effects in liver fibrosis [82]. In contrast, Wang et al. showed that activated STAT3 physically interacts with SMAD3 leading to a disruption of SMAD2/3/4 complex formation and thus to attenuation of TGF-β signaling in cancer cells [83]. From these findings, it is concluded that the outcome of the interaction between TGF-β signaling and STAT3 is strongly cell-context dependent.
Tetratricopeptide repeat domain 3 (TTC3) is a ubiquitin E3 ligase, which is transcriptionally induced by TGF-β by a SMAD2/3 dependent mechanism. TTC3 then positively regulates TGF-β signaling through ubiquitination and by triggering proteasomal degradation of other ubiquitin E3 ligases, i.e., SMURF2, a well-known negative regulator of TGF-β signaling that mediates ubiquitination and subsequent proteasomal degradation of SMAD2/3 and TβRs [85]. In line, Kim et al. showed that TTC3 siRNA suppressed TGF-β induced MFB differentiation and EMT, whereas TTC3 overexpression, in the absence of TGFβ, induced EMT and MFB differentiation [85].
Mesothelin (MSLN) is a membrane-anchored cell surface protein and described as novel marker for activated portal fibroblasts [86,87]. The deletion of MSLN resulted in upregulation of Thy-1 expression and formation of an inhibitory complex between THY-1 and TβRI, resulting in attenuation of TGF-β induced portal fibroblast activation. Even more, blocking antibodies against MSLN were able to suppress BDL mediated cholestatic fibrosis [88].
BMPs belong to the TGF-β superfamily and signal through SMAD1/5/8 complexes [13,89]. BMPs have diverse effects on liver fibrosis development and progression. For example, we recently reported upregulation of BMP9 expression in HSC and determined its fibrogenic role in the chronic CCl4 mouse liver fibrosis model as well as in human patients with CLD, whereas BMP2 was proven to have antifibrotic effects in CCl4 and BDL models of liver fibrosis [90,91]. Further, BMP-2 suppressed expression of TGF-β, TβRI and TβRII in HSC, whereas exogenous TGF-β and TGF-β signaling decreased BMP-2 expression, suggesting the existence of mutual regulation between the pathways of these two cytokines [91]. In addition, gremlin1, a TGF-β target gene, acts as antagonist of BMP7. It was upregulated in the porcine serum-induced hepatic fibrosis model [92]. Taking into consideration the antagonistic functions of BMP7 and TGF-β in liver fibrosis, gremlin1 could act as a bridge connecting these two signaling pathways [10].
ArfGAP with GTPase domain, Ankyrin repeat and PH domain (AGAP) 2 is a GTPase activating protein, which was recently described as a target of TGF-β signaling in human HSC. AGAP2 knockdown interfered with recycling of TβRII to the cell membrane and thus diminished TGF-β signaling and downstream effects in LX-2 cells, including proliferation, migration, and profibrogenic gene expression, e.g., ACTA2, COL1A2, LOX, PDGFB, and TGFΒ2 [93].
Factor VII-activating Protease (FSAP) is synthesized and released mainly from hepatocytes and acts as a regulator of fibrinolysis and coagulation [94,95]. FSAP expression is decreased in mouse and human fibrosis [96]. In addition, single nucleotide polymorphisms in HABP2, the gene encoding FSAP, resulted in decreased FSAP enzymatic activity and is associated with the severity of liver fibrosis in patients, probably due to decreased FSAP-mediated degradation of PDGF-βa potent HSC mitogen [95,97]. Recently, a link to the TGF-β pathway was uncovered by Leiting et al. demonstrating a dose- and time-dependent TGF-β-mediated inhibition of Habp2 mRNA and protein expression via a SMAD2-based mechanism [95].
Wnt: Beljaars et al. and Spanjer et al. reported a cross-talk between Wnt and TGF-β signaling [98,99]. TGF-β, but not other cytokines like PDGF, IL-1, or TNF-α, enhanced the expression of WNT5A, a major Wnt ligand in LX-2 cells [99]. Furthermore, TGF-β induced the expression of the Wnt receptors FZD2 and FZD8 in these cells [98,99]. These results indicate that the TGF-β pathway utilizes Wnt signaling to achieve HSC activation, but also acts in a paracrine way. Supporting this notion, WNT5A RNAi decreased TGF-β induced expression of COL1A1, COL3A1, FN, and VIM in HSC [99].
3. TGF-β Activity and the Microenvironment in Liver Fibrosis
First, we want to define what we understand as microenvironment in the liver and more specifically, upon liver damage. Let us assume the hepatocytes represent the center of the liver that sends out signals that vary in stages of physiology/homeostasis or damage. These signals are then translated from the microenvironment into distinct actions (matrix, inflammatory, etc.), which then feedback to the respective hepatocytes. In this way, the microenvironment is composed of the non-parenchymal cells (NPC), which are initially HSC, KCs, and LSEC, but also later infiltrated populations of inflammatory cells, the respective composition of the ECM, the pressure of the bloodstream and the biliary system. A thus defined microenvironment is highly dynamic upon hepatocellular damage along the different progression stages of hepatitis, fibrosis, cirrhosis, and cancer. Cellular fate changes driven by secreted factors and de novo activated intracellular signaling pathways induce the respective tissue responses, like cell proliferation, cell migration, tissue inflammation, and ECM deposition respective scar formation. Hereby, TGF-β is a critical cytokine triggering canonical and non-canonical intracellular pathways leading to activated HSC, macrophages with variant polarization and LSEC capillarization. These processes can be described as major features of the microenvironment, driven by and in exchange with stressed hepatocytes (reviewed in [2,3,100,101,102,103,104,105,106]).
3.1. Composition of the ECM
During liver fibrosis, the composition of the hepatic ECM changes, among others from collagen type IV and laminin towards collagen types I and III [9,107]. This change is sensed by HSC through two types of collagen receptors, i.e., discoidin domain-containing receptors and the integrins [108,109]. Two recent studies investigated the consequences of integrin deletion from fibroblasts and hepatocytes on TGF-β signaling. In the study by Bansal et al., integrin α11 KO fibroblasts show impaired proliferation, contractility and reduced responsiveness to TGF-β [110]. In the second study, integrin αvβ8 depletion from hepatocytes protected the cells against the antiproliferative effects of TGF-β, leading to enhanced liver regeneration after partial hepatectomy [111]. The mechanisms behind these interesting observations yet need to be clarified.
Kindlin-2 is an adapter protein that mediates cell-matrix and cell-cell adhesion and has been identified as a crucial regulator of integrin activation [112,113]. It is upregulated in the livers of patients with CLD and in the mouse model of chronic CCl4-related liver damage. In LX-2 cells, TGF-β increases kindlin-2 expression via non-canonical p38 and MAPK-dependent signaling. On the other hand, Kindlin-2 overexpression enhances TGF-β induced phosphorylation of SMAD2/3, suggesting a mutual regulation between TGF-β and Kindlin-2 [113].
Another modulated ECM component in fibrogenesis, which is related to TGF-β signaling and HSC activation, is fibronectin. Fibronectin exists in an insoluble cellular form as well as in a soluble form that is released into circulation by hepatocytes [114,115]. Cellular fibronectin has two splice variants, ED-A and ED-B [116,117]. During fibrogenesis, TGF-β and ED-A-fibronectin establish a positive feed-forward cycle. That is, TGF-β modulates ED-A splicing of fibronectin and promotes its expression. Further, ED-A KO fibroblasts display a reduced response to active TGF-β [118]. It was also reported that blocking fibronectin deposition through pUR4, a fibronectin assembly inhibitor, improved liver function and decreased collagen accumulation in mouse models of liver fibrosis [119,120]. Mechanistically, fibronectin can enhance TGF-β signaling (i) by binding to integrins, thus generating a pulling force to release encaged TGF-β, and (ii) by sequestering LTBP-1 in the ECM [115,117,121]. In line with this, blocking antibodies against the ED-A domain impaired incorporation of LTBP-1 into the ECM and consequently decreased TGF-β activation [115]. On the other hand, we found, rather contradictory, increased active TGF-β concentrations and enhanced HSC activation and proliferation in hepatocyte-specific Fn-KO mice [119,120]. These conflicting results suggest a more complex interplay between TGF-β and fibronection that needs further investigation.
An additional profibrogenic micro-environmental factor is the collagen triple helix repeat containing 1 (CTHRC1), which is secreted from HSC during liver fibrosis. Although CTHRC1 can activate Wnt and TGF-β pathways in parallel, the CTHRC1-mediated activation of quiescent HSC mainly depends on TGF-β signaling. Recombinant CTHRC1 activates canonical TGF-β/SMAD2/3 signaling in rat HSC. Treating HSC with a TβRII neutralizing antibody or a TβRI inhibitor suppressed CTHRC1 mediated induction of α-SMA expression, whereas a WNT3A neutralizing antibody had no effect [122]. Antagonizing CTHRC1 function thus may be a promising avenue to interfere with HSC activity in chronic liver diseases.
3.2. Matrix Stiffness
Matrix stiffness is not considered as an endpoint of organ fibrosis anymore, but rather as a critical regulator of the process itself. In line, there are reports showing that matrix stiffness precedes fibrosis [123,124]. HSC change their phenotype in response to stiffness and softness of the surrounding ECM [125]. Transfer of activated HSC from stiff plastic dishes to collagen-coated dishes decreased the expression of TGFB1, α-SMA, and ED-A [126]. Matrix stiffness also modulates TGF-β signaling, mainly via three mechanisms: (i) activation of ECM-entrapped latent TGF-β, (ii) stimulation of TGF-β non-canonical signaling pathways, and (iii) mechano-activation of HSC. For further discussion of these mechanisms, the reader is referred to the recent review by Santos and Lagares [123].
The degree of matrix stiffness is controlled by two groups of cross-linking enzymes. One group comprises the lysyl oxidase (LOX) family that is responsible for cross-linking elastin and collagen by oxidation [127]. β-aminopropionitrile (BAPN), an irreversible LOX inhibitor, inhibited HSC activation and amplified fibrosis reversion in a CCl4 mouse model of liver fibrosis [128]. Stimulation of LX-2 cells with TGF-β increased mRNA and protein expression of LOX-like (LOXL)-1 in a SMAD2/3-dependent mechanism. Furthermore, LOXL1 siRNA suppressed TGF-β effects on proliferation and activation markers in HSC [129]. Noteworthy, LOX is discussed as a serum biomarker of liver fibrosis in patients with NAFLD [130].
Another enzyme that essentially contributes to matrix cross-linking is transglutaminase (TG)2. TG2 regulates TGF-β signaling by cross-linking latent TGF-β binding protein (LTBP) to the matrix, and thus mediates entrapment of latent TGF-β in the ECM [131,132]. Its role in CLD, however, needs to be determined.
3.3. TGF-β and Inflammatory Cells
Inflammatory cell infiltration, HSC activation, and their subsequent removal are critical features of the healing process in response to an acute liver injury. During chronic liver injury, clearance of the liver from activated HSC and inflammatory cells, in particular macrophages, is dysregulated due to the constitutive occurrence of the liver cell-damaging insult [133]. Although the reasons underlying such dysregulation are still not fully understood, several mechanisms have been delineated and are obvious. Lodyga et al. very recently reported new insights. In their work, they show that cadherin-11 (CDH11) provides intercellular junctions between activated HSC and macrophages. As a consequence, TGF-β is produced and secreted from macrophages and activated in close proximity of connected HSC, which thus show prolonged activation. The authors suggest formation of a profibrotic niche and spatial targeting for active TGF-β by this mechanism [134]. Inhibition of the CDH11 could disrupt this niche and enhance the fibrosis resolution process.
With focus on inflammatory cytokines, Cai and coworkers identified CXCL6 to be upregulated in serum and liver tissue of liver fibrosis patients. Treatment of the HSC line HSC-T6 with CXCL6 did not mediate their activation directly. Instead, CXCL6 induced TGF-β production in KC. The authors further show that HSC are activated from the supernatant of CXCL6 treated KC via a SMAD3-BRD4 complex that binds to the c-Myc promoter. Subsequent binding of c-MYC to the Ezh2 promoter then leads to profibrogenic gene expression. Parts of these findings were confirmed in a CCl4-CLD mouse model [135].
Fabre et al. reported increased IL-17 and IL-22 production in advanced liver fibrosis, whereby in human patients, neutrophils and mast cells were the main sources of IL-17. IL-22 was identified as a profibrogenic cytokine by enhancing non-SMAD TGF-β/p38 MAPK signaling in HSC. The data were then confirmed with IL-22 RA1-KO mice that display less fibrosis in both thioacetamide (TAA) and CCl4 models of CLD. Also, inhibiting IL-22 or IL-17 production by aryl hydrocarbon receptor or RAR-related orphan receptor γ antagonists improved fibrosis [136].
Natural killer (NK) cells mediate liver fibrosis reversion by inducing interferon (IFN)-γ mediated death of activated HSC. Shi et al. examined the frequency, phenotype, and function of NK subsets in HBV-driven liver disease. NK subsets were reduced in number and displayed less antifibrotic activity in cirrhotic patients as compared to earlier disease stages. Based on in vitro studies, the authors characterized TGF-β as important factor to (1) decrease antifibrotic activity of NK cells and (2) to deplete hepatic NK cells by an HSC mediated emperipolesis (a cell-in-cell structure) and subsequent apoptosis. This process was inhibited by anti-TGF-β treatment. These data were confirmed by colocalization studies of α-SMA+ cells and NK cells in patients. The authors, therefore, suggest an anti-fibrotic approach by enhancing NK cells activity [137].
3.4. TGF-β and Pathophysiological Blood Flow in Liver Fibrosis
Besides matrix stiffness, fluid pressure of blood and bile in the liver vessels represents an additional mechanical parameter that influences adjacent cells with modulation of biochemical signals that translate into cellular fate decisions. Swartz and colleagues recently showed that a slow fluid flow stimulates HSC activation through the upregulation of TGF-β [138]. Physiological fluid flow upregulated profibrotic genes, e.g., TGFB, ACTA2, Col1A1 in oral and dermal fibroblasts, in the absence of any exogenous mediators [138,139]. Interestingly, a physiological fluid flow augmented cytoplasmic internalization of caveolin and nuclear translocation of the TβRII in dermal fibroblasts [139]. The latter effects could partly explain why exogenous TGF-β combined with flow state did not lead to further augmentation of dermal fibroblast activation and even antagonized the effects of the physiological fluid flow in this study. The relevance of flow in CLD has yet to be determined, even more considering the sinusoidal structure and the impact of LSEC (defenestration).
3.5. Dynamics of TGF-β Ligand Availability
The strength of fibroblast responsiveness is determined, among others, by the amount of TGF-β, which is released from cells upon de novo synthesis in response to a distinct stimulus and the amount which is already stored in the surrounding milieu and activated. Ansorge et al. showed that fibroblasts get activated to a similar extent by a lower concentration of a “sustained release form” of TGF-β (achieved by binding of TGF-β to agarose microbeads), as compared to treatment with active recombinant cytokine in traditional cell culture experiments [140]. It is suggested that this type of TGF-β release in constitutive portions resembles more the in vivo situation than the traditional cell culture treatment approach. Using mathematical modeling based on experimental data, it was as well predicted that continuous or pulsating TGF-β treatment will induce different cellular responses [141]. It was also shown that increasing medium volume while keeping a constant number of TGF-β molecules per cell delayed TGF-β ligand depletion and reduced a switch-like response of the TGF-β pathway, as described mathematically by Hill’s coefficient [141,142].
As mentioned above, TGF-β is produced as an inactive latent complex, enveloped by its pro-peptide LAP, and then deposited in the ECM. For receptor binding and signaling, TGF-β must be released from the complex and the matrix, e.g., to initiate ECM synthesis in HSC. One factor able to induce latent TGF-β activation by cleavage is plasma kallikrein. Subsequently, the N-terminal cleavage product of LAP can be detected in vicinity of activated HSC [143]. The authors now developed an ELISA-based method to also identify the C-terminal LAP cleavage product (LAP-DP) and showed that it can be used as a blood-based biomarker for fibrogenesis. In line, LAP-DP levels were increased in both CCl4 and BDL fibrosis models in mouse, prior to an increase in hydroxyproline. Furthermore, LAP-DP levels correlated with α-SMA expression [144].
4. TGF-β Signaling, Cell Damage and Oxidative Stress in Liver Fibrosis
ROS plays crucial roles in liver fibrosis and HSC activation [9,145]. The two main enzyme families generating ROS during liver injury are nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) and the CYP450 family members [8]. ROS can act as both inducer or effector of the TGF-β signaling pathway, thereby generating a vicious cycle for fibrosis [146,147]. On the one hand, ROS induces TGF-β signaling through different mechanisms such as (i) activation of matrix metalloproteinases, (ii) induction of TGF-β expression, and (iii) augmentation of TGF-β release through activation of LAP [8,146]. On the other hand, TGF-β augments mitochondrial ROS production by activating the mTOR pathway and reducing the activity of complex III and IV [147]. Interestingly, mTOR kinase inhibitor rapamycin was recently reported to increase ROS production as well as to activate latent TGF-β which led to upregulation of CTGF in hepatic progenitor cells through SMAD2, but not SMAD3 [148]. The relationship between NOX1, 2, and 4, HSC activation, and TGF-β signaling were described previously [8,149]. NOX1 and NOX2 were increased in CCl4 and BDL mouse models of liver fibrosis. Their knockdown attenuated hepatic fibrosis and ROS generation [150]. A recent report also showed upregulation of NOX1 in non-alcoholic fatty liver disease (NAFLD), which impaired the hepatic microcirculation through formation of protein nitrotyrosine adducts and by reducing NO availability [151]. NOX4 was also upregulated in mouse and human liver fibrosis and was essential for HSC activation and maintenance of MFB fate [152]. Recently, involvement of NOX5 in HSC activation was demonstrated as well [153]. Overexpression of NOX5β and NOX5ε splice variants upregulates COL1A1 and elicits the proliferation of human HSC (LX-2), whereas NOX5 knockdown inhibits these effects [153]. Interestingly, TGF-β stimulated NOX5 expression in a ROS-dependent mechanism [153]. The role of TGF-β/NOX5 signaling in human fibrosis remains to be elucidated.
A new antagonistic player in the liver fibrosis scene is nuclear factor-erythroid 2-related factor 2 (Nrf2). Nrf2 signaling represents a cellular protection mechanism that is induced in cells exposed to oxidative stress. Nrf2 regulates the transcription of various antioxidant enzymes such as glutathione S-transferase, glutathione reductase, glutathione peroxidase, and heme oxygenase-1 [154]. Moreover, the Nrf2 pathway to some extent protects the liver against toxin-induced fibrosis [155]. An association between TGF-β, ROS, and Nrf2 was reported for the fibrogenic processes of several organs, including the liver [154]. Sulforaphane, an Nrf2 activator, inhibited TGF-β signaling and reduced hepatic fibrosis in the BDL model [156]. Nrf2-deficient HSC were much stronger activated by TGF-β treatment as compared to wild type cells [157]. Another Nrf2 activator, tBHQ, was also shown to attenuate intestinal fibrosis by inhibiting the ROS-dependent TGF-β/SMAD pathway [158].
Recently, Schisandrin B, the main bioactive ingredient of the Chinese herb “Schisandra chinensis”, was reported to improve liver fibrosis in the CCl4 mouse model by regulating Nrf2 and TGF-β/SMAD signaling pathways [159]. Of particular interest, ascorbic acid, an antioxidant with Nrf2 inhibitory activities, promoted TGF-β mediated HSC activation via a SMAD2/3-independent mechanism [160,161]. The full mechanism of the profibrogenic effect of ascorbic acid is still unclear but there are hints for a relation to epigenetics [160,162].
In alcoholic liver disease, alcoholic hepatitis (AH) represents a life-threatening disease complication with significantly reduced liver function. Identification of molecular mechanisms that lead and drive (acute) alcoholic hepatitis are lacking since there are no available animal models to phenocopy the disease setting. Aergemi et al. analyzed livers from patients with variant stages of AH by RNA sequencing. One major finding was the lack of transcription factors involved in the determination of physiological hepatocyte fate. Most interestingly, they identified TGF-β as major upstream driver of metabolic and anabolic dysfunctions. Mechanistically, there is evidence that profound changes in DNA methylation and chromatin modification are involved in severe AH [163].
5. TGF-β Signaling and Epigenetics in Liver Fibrosis
Epigenetics stands for qualitative and quantitative reversible changes in gene expression without affecting the DNA sequence itself [164]. Epigenetic regulatory mechanisms include microRNAs (miRNAs), long non-coding RNA (lncRNAs), circular RNA (circRNAs), RNA binding protein (RBP), as well as DNA and histone modifications. During recent years, much evidence has been gathered showing epigenetic regulation of TGF-β signaling. On the one hand, TGF-β induces epigenetic changes in HSC e.g., by regulating expression levels of distinct pro- and antifibrotic miRNAs [165,166]. On the other hand, epigenetic regulators modulate the activity and expression of up- and downstream components of the TGF-β signaling pathway. In the following section, we will discuss the recent findings interconnecting TGF-β signaling with epigenetics.
miRNAs are short non-coding nucleotide sequences (19–23 nucleotides), which regulate gene expression at the posttranscriptional level by binding to 3’-UTRs of target mRNAs. Upon mRNA binding, miRNAs induce either their degradation or interfere with their translation [167]. miRNA profiles of human HSC change upon culture activation [168] and many results reported the modulatory roles of miRNAs on the expression of liver fibrosis genes. Thus, several miRNAs were classified as antifibrotic and related to the TGF-β pathway, such as miR-19b, miR-34a-5p, miR-146a, miR-133, and miR-134. For example, miR-133 is downregulated in HSC during murine and human liver fibrogenesis and upon TGF-β treatment. Overexpression of miR-133 in HSC, in contrast, inhibits collagen expression [169]. miR-193 and miR-30c are TGF-β dependently downregulated in cultured HSC and in experimental liver fibrosis models, and potentially target TGF-β2 and SNAIL1 [170]. Other miRNAs have been described as profibrogenic, e.g., miR-942 and miR-125b [171,172,173,174,175,176,177]. In the past, different mechanisms were delineated how miRNAs may regulate TGF-β signaling during fibrogenesis. All these miRNAs mediate their effects by targeting various mediators of the TGF-β pathway. miRNA-30, miRNA-212-3p, and miRNA-17-5p modulate TGF-β signaling strength by targeting the SMAD7. While SMAD7 is a direct target of pro-fibrotic miRNAs 17-5p [178] and 212-3p [179], miRNA-30 inhibits expression of SMAD7 inhibitor KLF11 [180]. miRNA-9-5p targets TβRI and TβRII mRNA in LX-2 cells and is itself downregulated by TGF-β through promoter methylation [165]. TβRII expression is also downregulated by augmenter of liver regeneration (ALR) under healthy conditions in LX-2 cells, whereas TGF-β treatment induces miRNA-181a, which silences ALR expression [181]. Further, it was demonstrated that miR-19b and miR-142-3 target TβRI. As another TGF-β signaling component, SMAD4 is targeted by miR-34a-5p and miR-146a [171,172,173,182]. Microinjection of miRNA-455-39 suppressed heat shock protein factor 1 (Hsf1) in livers of CCl4, BDL and high-fat diet (HFD) fed mice, therewith improving fibrosis. Mechanistically, miRNA-455-3p binds to the 3’UTR of Hsf1. Downregulated Hsf1 reduces expression of heat shock protein 47, thus inhibiting TGF-β signaling [183]. miRNA-134 has also decreased in the CCl4 and BDL models or after HFD feeding in rats. This miRNA exerts its function in human and rat HSC by binding to the 3´ untranslated region of TGF-β activated kinase 1-binding protein 1 (TAB1), therewith interfering with its TGF-β dependent induction, which finally results in decreased HSC proliferation, α-SMA and collagen expression [175]. In portal fibroblasts, MLK1 silencing reduces TGF-β dependent expression of profibrogenic genes. Fan et al. concluded from their results that MLK1-SMAD3 interaction at the DNA levels is required for induction of TGF-β target genes. In line, MLK1 deficiency ameliorates BDL-induced fibrosis [184]. Also, SMAD3 mediated the upregulation of miRNA-31 in fibrotic samples from human and rat livers as well as in activated HSC [185]. In a very recent study, we could demonstrate that profibrogenic effects of miR-942 are mediated through targeting BAMBI, a TGF-β decoy receptor, leading to enhanced fibrogenic TGF-β signaling [176]. Further, Genz et al. showed an antifibrotic role for miR-25-3p, mediated by its ability to inhibit TGF-β induced SMAD2 phosphorylation and collagen deposition in LX-2 cells [186].
LncRNAs: In contrast to miRNAs, lncRNAs comprise longer non-coding nucleotide sequences (more than 200 nucleotides). LncRNAs regulate gene expression by acting as miRNA sponges or by competing with miRNAs for binding to mRNA, thus blocking their effects on respective mRNA targets [187,188]. Cellular availability of lncRNAs is also dysregulated through HSC activation and liver fibrosis. Long intervening noncoding RNA-p21 (lincRNA-p21) is upregulated in CCl4-induced liver fibrosis and acts as endogenous competitive inhibitor of miR-30, which inhibits TGF-β signaling through targeting KLF11 and by increasing SMAD7 expression. Therewith, lincRNA-p21 enhances TGF-β signaling [189]. LncRNA H19 is also upregulated in CCl4 and BDL induced liver fibrosis [190,191]. Mechanistically, lncRNA H19 stabilizes TβRI by abrogating the inhibitory effect of miR-148a on ubiquitin-specific protease 4 (USP4) [192].
CircRNAs are a new class of lncRNAs with a similar mechanism of action [193]. The circRNA signature is dysregulated upon radiation-mediated HSC activation [194]. Zhou et al. reported a significantly increased expression of several circRNAs, e.g., mmu_circ_33594, mmu_circ_34116, and mmu_circ_35216 in fibrotic livers and in a mouse HSC cell line (JS1) upon TGF-β stimulation [195]. The mechanistic link between TGF-β and these circRNAs still needs further investigations.
RBPs bind to specific RNA motifs, which results in modulation of their transcription, localization, and stability. RBPs, miRNAs, lncRNAs, and circRNAs together form an RNA regulon that controls the expression of numerous genes [196]. Recently, insulin-like growth factor 2 binding protein 3 (IGF2BP3) was identified as an RBP and to be an effector and target of TGF-β signaling. Igf2bp3 knockdown decreased the effects of TGF-β on HSC activation, whereas TGF-β signaling inhibition induced Igf2bp3 expression in a miRNA dependent mechanism, therewith revealing an interesting positive feedback loop between TGF-β and IGF2BP3 [197].
DNA and histone modification: DNA methylation and histone acetylation represent additional abundant epigenetic mechanisms that are currently intensely investigated [198,199] as presented in the following.
DNA methylation: Transcription of multiple genes including fibrosis-related genes can be repressed by methylation of cytosine-phosphoguanine (CpG) dinucleotides in their promoters through DNA methyltransferases (DNMT) [199]. One of these genes is angiogenic factor with G patch and FHA domains 1 (Aggf1), which is downregulated in different models of liver fibrosis and in activated HSC through increased CpG methylation of DNA surrounding the Aggf1 promoter. 5-Azacytidine, a DNMT inhibitor, restored Aggf1 expression, which led to improvement of liver fibrosis in a SMAD7-dependent mechanism [200]. In line, 3-deazaneplanocin, another DNMT inhibitor, improved CCl4 induced liver fibrosis in mice [201]. Recently, enhancer of zeste homolog 2 (EZH2), which methylates the lysine residues 9 and 27 of histone 3 was found to play a crucial role in experimental liver fibrosis through modulation of TGF-β signaling. Indeed, TGF-β specifically increased the expression of EZH2 without influencing other histone-lysine N-methyltransferase enzymes. In addition, inhibition of EZH2 diminished TGF-β-induced transcription of profibrogenic genes e.g., Fn, Col1a1, and Acta2 [202]. Noteworthy, various EZH2 inhibitors e.g., MAK683, SHR2554, and CPI-1205 are currently investigated in clinical trials for their potential in the treatment of different oncologic diseases (ClinicalTrials.gov Identifier: NCT02900651, NCT03603951, NCT03741712, and NCT03480646). Our group showed synergism between SMAD2/3 and TRIM33, which is essential in directing liver progenitor cell differentiation towards hepatocytes [203]. TRIM33 is a chromatin reader that prepares the cell genome for transcriptional activity by modulating DNA methylation, thereby relevant in determining cellular fate. Interestingly, TRIM33 owns super affinity for phosphorylated SMAD2/3 in competition with SMAD4. We found that TRIM33 formed a transcription factor complex in combination with p-SMAD2/3 in liver progenitor cells. The complexes are essential for expression of key hepatic functional genes, e.g., albumin and coagulation factors, and largely determine whether LPCs are capable of taking over hepatic function in acute-on-chronic liver failure patients suffering from massive parenchymal necrosis [203].
Histone acetylation and deacetylation: During HSC activation and liver fibrosis, histone deacetylation catalyzed by histone deacetylase (HDAC) is also crucial in regulating the expression of various profibrogenic genes [198]. Also, in this case, HDAC inhibitors display antifibrotic effects in animal models of CLD. As one example, Wang and colleagues recently reported that an HDAC inhibitor, SAHA, improved liver function and decreased hepatic fibrosis in rats [204]. SAHA mediated its antifibrotic effects by increasing SMAD7 expression, which leads to an attenuation of TGF-β signaling by enhanced negative feedback [204]. Furthermore, P300 acetyltransferase is long known to promote TGF-β signaling through acetylation of histones and SMAD2/3 [205,206,207]. Very recently, P300 was additionally shown to enhance TGF-β signaling by a non-canonical mechanism via acting as a shuttle to facilitate the nuclear transport of the SMAD2/3 complex [208]. In this study, the authors showed that p300-mediated histone acetylation enhanced the response to TGF-β in HSC [208]. In line, Jiang and his colleagues have shown that nicotinamide riboside, which increases the intracellular pool of NAD+, decreased the expression of P300, whereas it increased the activity of Sirt1, an NAD+-dependent histone deacetylate enzyme, leading to inhibition of SMAD2/3 acetylation and attenuation of TGF-β signaling [209]. Interestingly, thyroid hormone triiodothyronine (T3) inhibited TGF-β1-mediated histone 4 acetylation at the promoters of TGF-β1 target genes, i.e., Smad7, Id1, and p15 leading to suppression of TGF-β signaling [210]. The antifibrotic effects of T3 were demonstrated in liver and skin fibrosis models, which were induced by CCl4 and bleomycin, respectively [210].
6. TGF-β and Mesenchymal Transition in Liver Fibrosis
EMT is a reversible process during which epithelial cells lose polarity and change their phenotype to a mesenchymal fate [7]. Three basic types of EMT have been described so far. EMT type 1 and type 3 are connected to embryogenesis and cancer metastasis, respectively, whereas EMT type 2 is associated with wound healing, organ fibrosis and tissue regeneration [7]. Although epithelial liver cells, i.e., hepatocytes, and cholangiocytes, are able to undergo EMT in vitro, the existence of EMT in vivo is highly debated [211]. In a recent review article by Weng et al., it was hypothesized that EMT-like features in patients with advanced CLD could be a side effect of the TGF-β enriched microenvironment [211,212,213,214,215].
Noteworthy, EMT is a dynamic process characterized by transitional states. After a complete EMT, cells have totally lost their epithelial character and have acquired a mesenchymal phenotype. However, some cells only undergo partial EMT, thus presenting both epithelial and mesenchymal markers [7]. Recently, Wu et al. reported that TGF-β or activin signaling induces a partial EMT in hepatic progenitor cell (HPC) derived cell lines via the canonical SMAD signaling pathway [216]. One can conclude that cellular fate changes of HPC due to partial EMT could facilitate their contribution to liver fibro-carcinogenesis. In cancer cells, sustained TGF-β exposure induces a stable form of EMT through non-Smad mTOR signaling [217]. A sustained SMAD2 phosphorylation for more than 6 h, for example, was reported to be necessary for a complete trans-differentiation of human fibroblasts [45]. In contrast to a reversible EMT, which is crucial for wound healing processes and tumor dissemination, a more stable EMT phenotype is only partially reversible and contributes to tumor persistence and latency [217]. This study raised an interesting hypothesis on the existence of a robust EMT as a feature of liver fibro-carcinogenesis, especially pinpointing on the fibrotic tumor microenvironment that presents with high amounts of TGF-β for a prolonged time.
Sun and coworkers claim to have identified a mechanism for the occurrence of hepatocyte EMT during liver fibrosis. They treated human L02 cells and primary rat hepatocytes with advanced oxidation protein products (AOPP) and subsequently tested for EMT features. They found reduced E-cadherin and increased vimentin expression, collagen deposition and induced cell migration. AOPP also increased cellular ROS levels and activated TβRs and Smad signaling. Interfering with ROS production and TGF-β signaling blunted AOPP mediated hepatocyte EMT [218].
Mesothelial cells (MCs) form a single layer of the mesothelium and cover the liver surface. It was suggested that in biliary fibrosis upon bile duct ligation (BDL) or in CCl4-induced fibrosis in mice, MCs may migrate inside the liver and contribute to the population of activated HSC/MFB by a process termed mesothelial mesenchymal transition (MMT) [219]. Li and colleagues reported that this cell fate modulation requires TGF-β since this conversion was suppressed in TβRII deficient MCs in culture and in vivo. The authors further showed that such MCs derived HSC are predominantly located near the liver surface and contribute to capsular fibrosis. Based on FACS cell sorting, the authors claim that the MC-derived HSC population stores little vitamin A-containing lipid droplets presents with a fibrogenic fate and contributes to about 1.4 and 2.0% of activated HSC in the BDL and CCl4 models, respectively. During fibrosis reversion in the CCl4 model, 20% of MC-derived MFB survive and deactivate (senescent state) to vitamin A-poor HSC [220].
Interestingly, TGF-β could also induce a mesenchymal transition of endothelial cells (EC) in a process termed EndMT [221]. Ribera et al. recently reported that a small fraction of EC undergoes EndMT in the liver of CCl4-treated mice and they claim that this is also occurring in cirrhotic patients [222]. Inhibition of EndMT by BMP-7 improved liver fibrosis in mice [222]. In this regard, Randi and colleagues reported that endothelial transcription factor (ETS)-related gene (Erg) is essential in maintaining liver homeostasis by binding to SMAD3 and hindering its nuclear translocation [223]. Abrogation of Erg enhanced SMAD3 dependent TGF-β signaling in EC, resulting in induction of EndMT and the promotion of liver fibrogenesis [223].
7. TGF-β and Metabolic Fate Changes in Liver Fibrosis
Activated HSC are roughly characterized, among others, by α-SMA expression and to synthesize and secrete or deposit ECM. The activation process requires a metabolic reprogramming of the cells. Indeed, the HSC trans-differentiation process is energy demanding. The core metabolic changes include a switch from oxidative phosphorylation to aerobic glycolysis [224]. Although glycolysis generates less adenosine triphosphate (ATP) than oxidative phosphorylation, it requires less time, which makes this process finally more efficient to produce the required amount of ATP for HSC activation [224,225,226]. TGF-β was reported to induce glycolysis, and thus can be considered a driver of metabolic reprogramming. A very interesting mechanistic study in idiopathic pulmonary fibrosis (IPF) suggests that a TGF-β-induced glycolytic pathway leads to the accumulation of lactic acid, which acidified the pH in the microenvironment, and thus activated ECM bound latent TGF-β, therewith representing a positive feedback loop to increase TGF-β action [224,227]. Besides glycolysis, glutamine metabolism was recently shown to be essential for TGF-β induced HSC trans-differentiation [228,229]. Glutaminolysis is a two-step reaction that involves the conversion of glutamine to glutamate by glutaminase (GLS) and then to α-ketoglutarate through glutamate dehydrogenase or aminotransferase enzymes [230]. In activated HSC, TGF-β induces Gls1 through SMAD3 and p38 MAPK. On the other side, depletion of extracellular glutamine or silencing Gls1 in the presence of glutamine prevented TGF-β induced HSC activation and decreased the expression of profibrotic markers [229].
Interestingly, a recent study by Takahashi et al. assigned new metabolic functions to the TGF-β2 isoform. In this study, exercise-induced TGF-β2 stimulated cellular uptake of fatty acids and glucose, therewith improving insulin sensitivity [231,232]. The role of TGF-β2 and exercise on HSC activation and liver fibrosis remains an open question. So far, Takahashi et al. reported a decrease in liver mass and liver fat content after administration of TGF-β2 in high-fat diet (HFD) treated mice [231]. Also of relevance, knockout of Smad4 stimulated β-oxidation of fatty acids and suppressed lipid-induced fibrosis and inflammation in mouse livers [233]. The distinct metabolic roles of TGF-β1 and 2 again highlight the non-redundant functions of these TGF-β isoforms.
8. Circadian Rhythm, TGF-β Signaling, and Liver Fibrosis
The circadian rhythm regulates numerous physiological processes that require oscillation during the daily 24 h cycle e.g., heart rate, body temperature, blood pressure, and some metabolic processes such as glycolysis [224,234]. This oscillatory behavior is driven by a network of clock genes, e.g., Period 1 (Per1), Period 2 (Per2), Period 3 (Per3), brain and muscle aryl-hydrocarbon receptor nuclear translocator-like 1 (Bmal1) and Cryptochrome (Cry1 and Cry2) [224,235]. In fibrosis, several clock genes were found to play critical roles. For example, depleting Per2 in mice strongly enhanced CCl4 and BDL-induced liver fibrosis, highlighting its protective role to maintain liver homeostasis [236,237]. Moreover, there is mutual regulation between TGF-β signaling and clock genes. For instance, several regulators of TGF-β signaling, such as SMURF2 and SMAD7 contain Bmal1 binding sites in their promoter, suggesting that their transcriptional regulation has a “clock component” [238]. In HT22 neurons and NIH3T3 fibroblasts, TGF-β2 inhibits a number of clock-controlled genes including Dbp and Tef, without changing Bmal1 levels [239]. In a recent study, TGF-β increased Bmal1 expression in normal lung fibroblasts and in an animal model of lung fibrosis [240]. Bmal1 knockdown abrogated TGF-β induced EMT of lung epithelial cells and inhibited differentiation of normal lung fibroblasts [240]. These results at least suggest relevance of Bmal1 in mediating TGF-β related signals in lung fibrogenesis. The existence of a similar regulation system in liver fibrosis warrants further studies. Importantly, Bmal1 was linked to inflammation in several settings [241,242]. Inflammation, as previously described, is a vital phenomenon preceding or accompanying liver fibrosis. Also, it needs further clarification if the aforementioned connection between TGF-β and Bmal1 is indeed directly related to circadian rhythm.
9. TGF-β, Autophagy, and Senescence in Liver Fibrosis
Autophagy describes a cellular survival program during which lysosomes degrade substrates intracellularly for energy production and depletion of damaged cellular components [243]. Ligand activated nuclear receptor Rev-erb, previously shown to improve liver fibrosis in the CCl4 mouse model, is also characterized as a novel regulator of autophagy [244,245]. It is suggested that HSC need to undergo autophagy to provide energy for the activation process. Thomes et al. compared the impact of Rev-erb activation with a synthetic ligand, SR9009, and TGF-β treatment on autophagy during HSC activation in CCl4 mediated mouse fibrosis. CCl4 challenged mice display reduced AMPK signaling, upregulated P70S6K phosphorylation as well as increased P62 and decreased protein levels of autophagy-related gene (ATG), which is suggestive for a disturbed autophagosome formation. SR9009 prevented P70S6K phosphorylation only, whereas all other parameters were not changed. In vitro, both SR9009 and TGF-β inhibited AV biogenesis, whereas opposite effects were provided for fibrogenic gene expression, P70S6K phosphorylation, and HSC proliferation, TGF-β being an enhancer. Further, autophagy activator rapamycin and inhibitor wortmannin both antagonized HSC activation, proliferation, and P70S6K phosphorylation. In addition, inhibiting P70S6K blunted TGF-β induced HSC proliferation. The authors conclude that SR9009 and TGF-β both similarly affect autophagy, but differentially regulate HSC activation and fibrogenic fate. Based on these findings, the role of autophagy on HSC activation needs further investigation before a final conclusion can be drawn [246].
Cellular senescence provides another cellular survival mechanism during which the cell cycle is irreversibly arrested, resulting in the cease of a cell.
Replication [247]: Senescent cells are characterized by expression of p16, p21, p53, and β1-galactosidase [247]. Cellular senescence protects from malignant transdifferentiation and plays crucial roles in aging and wound healing [248]. In line, cellular senescence occurs in mouse and human liver fibrosis [249,250]. In a murine model of CCl4 induced liver fibrosis, Krizhanovsky et al. identified senescent cells as mainly derived from HSC. Such senescent HSC were efficiently removed by natural killer (NK) cells, suggesting that HSC senescence is a mechanism to limit liver fibrogenesis [249]. Moreover, ablation of a key senescent regulator, P53, resulted in excessive liver fibrosis, with a delayed resolution after chronic administration of CCl4 in mice [249]. In contrast, in a mouse model of IPF, the secretome of senescent fibroblasts was found to be fibrogenic, and deletion of senescent cells improved fibrogenesis [251]. TGF-β has been described as a central component of senescence-associated secretory phenotypes (SASP). In liver, TGF-β contributes to cellular senescence in acute and chronic liver injury models [252]. A number of hepatocytes undergo senescence upon acute acetaminophen (APAP) induced liver damage [253]. Furthermore, infiltrating macrophages secrete TGF-β, therewith facilitating gain of a senescent fate to neighboring viable hepatocytes. TβRI inhibition with SB525334, a small-molecule inhibitor, decreases senescence and improves survival after challenging mice with a toxic dosage of APAP [253]. In the Mdm2-KO model of biliary senescence, recruited macrophages and MFB secrete TGF-β and induce senescence in the surrounding hepatocytes and cholangiocytes [254]. Inhibition of TGF-β signaling using galunisertib, another compound inhibitor of TβRI, interferes with senescence transmission and improves liver function [254]. In a recent study from Razdan et al., they found that TGF-β via SMAD3, NOX4 and ROS may induce telomere dysfunction, which was demonstrated by immunofluorescence quantification of DNA damage foci. TGF-β-mediated telomere dysfunction subsequently promotes trans-differentiation of human fibroblasts to MFB, instead of inducing senescence [252]. These results once more highlight the cell context-dependency of TGF-β effects and the existence of overlapping pathways for cellular senescence and MFB transdifferentiation. Considering the key role of TGF-β in HSC activation and liver fibrogenesis, the results of Razdan et al. seem to contrast the findings of Krizhanovsky et al. Moreover, the findings underline again that extrapolation of senescence-associated results from mouse to human cells should be performed very cautiously. One important fact to consider is that mice, in contrast to humans, express telomerase in a wide range of cell lineages [247]. This in turn means that Krizhanovsky et al. possibly describe a senescence program that is independent of telomere erosion [247]. Taken together, a link between TGF-β, HSC senescence, and liver fibrosis needs to be carefully (re)-evaluated.
10. Targeting TGF-β in Liver Fibrosis
Reversibility of liver fibrosis is evident in patients after removal of the causative agent of damage/disease [133,255], e.g., antiviral treatment of patients with HBV infection led to regression of virus-induced liver cirrhosis [255]. Despite clear evidence for reversibility, there is still no direct antifibrotic therapy for liver fibrosis available but urgently required because removal of the fibrosis-inducing factors is often hard to achieve, or at least not completely possible, especially in the increasing CLD entities of NAFLD and ASH. Further, even upon avoidance or abrogation of the fibrosis-inducing factor, fibrosis resolution requires time. Toning down scar formation or speeding up scar resolution processes are therefore aims of a direct antifibrotic therapy [256]. Due to the major role of TGF-β in liver fibrogenesis, several studies focused on its inhibition to develop effective antifibrotic therapies. Previous attempts to target TGF-β include, for example, the use of soluble TβRII, which compete with the membrane-anchored TβRII for TGF-β binding. These soluble receptors lack the intracellular signal transduction domain and thus act as molecular sinks [257]. Wang et al. utilized the His-SUMO expression system to generate high amounts of a soluble truncated TβRII for in vivo testing. In this study, N-terminally His-SUMO-linked TβRII significantly mitigated CCl4-induced liver fibrosis [258]. Other attempts include the use of anti-receptor/anti-ligand antibodies to disrupt receptor-ligand interactions or the use of small molecule kinase inhibitors such as LY2157299 (also known as galunisertib) and LY2109761 to inhibit TβRI and interrupt intracellular downstream signaling [259]. Both LY2157299 and LY2109761 are small chemical inhibitors that block TβR activity. In comparison to LY2157299, which inhibits mainly TβRI, LY2109761 was reported as a dual inhibitor of TβRI and TβRII [260]. We and other groups have shown promising results for the use of galunisertib in preclinical animal models of liver fibrosis [261,262]. Other approaches inhibited TGF-β signaling indirectly through targeting LOXL or integrins, and therewith reducing release of active TGF-β from its ECM deposited latent form [108,129,263].
Additional efforts to develop TGF-β-based therapeutics include interference with nuclear translocation of the SMAD2/3/4 oligo-complex using an aminoacyl-tRNA synthetase interacting multifunctional protein 1 (AIMP1) peptide. Of note, the AIMP1 peptide did not influence TGF-β-induced phosphorylation of SMAD2 and SMAD3 but inhibited the nuclear translocation of SMAD3 by a still not identified mechanism [264]. Moreover, Zhang and his colleagues used magnolol to prevent the interaction between SMAD3 and SMAD4 and, thus, could attenuate TGF-β signaling and concanavalin A induced hepatic fibrosis [265]. In line with the above description on the distinct roles of SMAD3 and SMAD2 in organ fibrosis, a recent study reported the efficacy of a specific SMAD3 inhibitor (SIS3) in reducing fibrosis, apoptosis, and inflammation in a mouse model of kidney fibrosis [266], however, a therapeutic effect of SIS3 on liver fibrosis still remains poorly defined.
Many natural compounds with experimental antifibrotic activity integrate at some point with the TGF-β signaling pathway. We here will just mention two most recent findings. Ganai and Husain described Genistein, an isoflavonoid found in soy, as hepatoprotective in a rat model of liver fibrosis. Daily intragastric administration of Genistein for 12 weeks attenuated d-Galactosamine -induced fibrosis. Mechanistically, Genistein increased expression of SMAD7 in liver, which ultimately interfered with TGF-β/Smad signaling [267]. Similarly, praziquantel (PZQ), a schistosomicide used in the clinics for decades, has antifibrotic activity in schistosomiasis mice. In a recent mechanistic study, the authors showed that PZQ inhibits CCl4-induced liver fibrosis by upregulating SMAD7 in HSC. The link between PZQ, SMAD7 expression, and HSC activation was further confirmed in LX-2 and other fibroblast cell lines [268].
Unfortunately, TGF-β signaling based antifibrotic therapies have not yet been translated to human patients. The reason for this is the complexity of this pathway, and the highly dynamic, context and cell-type dependent outcome of TGF-β signaling. Further, TGF-β nearly acts ubiquitously in the whole organism thus making all organs target of TGF-β-directed therapies., Finally animal models of liver fibrosis do not reflect all aspects of human disease [257,269]. Depending on the model, mice may need 4–6 weeks of treatment to develop liver fibrosis, whereas, in humans, the process usually takes decades [270]. Another reason for translation failure is the lack of non-invasive biomarkers that could stratify patients with liver fibrosis and aid in monitoring the response of the patients during therapeutic intervention. In this regard, Kojima and his colleagues developed an antibody against LAP degradation products (LAP-DP) that represents a measure for active TGF-β availability in serum and tissue [143,271]. Such discoveries are highly encouraging, as they can provide real-time monitoring of safety and efficacy issues, thus making translation more reliable. The broad biological effects of TGF-β already indicate that a simple and general inhibition of the ligand or the receptor complex will induce unwanted effects that sometimes my override the desired beneficial outcome. Here, more specific targeting of disease stage-related regulatory parameters and cell type-directed approaches based on basic research findings need to be developed. Recently, Qiao et al. reported an elegant strategy to differentially modulate inflammatory and fibrogenic effects of TGF-β through manipulating interactions with key transcriptional factors. Administration of ICG-001 inhibited the interaction of β-catenin with T Cell factor (TCF), therewith facilitating its complex formation with FoxO. The interaction between β-catenin and TCF is considered central for several profibrogenic pathways such as TGF-β/SMAD and Wnt/β-catenin, whereas the β-catenin/FoxO interaction mediates TGF-β induced differentiation of anti-inflammatory T regulatory (Treg) cells. Through intervention with ICG-001, Qiao et al. were able to maintain the anti-inflammatory effects of TGF-β through upregulation of T regulatory (Treg) cells, while its profibrogenic effects were suppressed in a unilateral ureteral obstruction model of kidney fibrosis. Interestingly, ICG-001 also protects the liver from profibrogenic effects of systemically administered recombinant TGF-β [272]. ICG-001 reduced HSC activation, leading to attenuation of collagen deposition, as evidenced by reduced hydroxproline content and collagen I staining in the livers upon acute CCl4 injury in mice. ICG-001 also displayed anti-inflammatory effects via suppression of the chemokine CXCL12 [273]. Based on these preclinical data, Kimura and colleagues nicely demonstrated tolerability of an ICG-001 analog, PRI-724, in a small group of patients with HCV induced hepatic cirrhosis [274]. A well-controlled clinical trial including more patients is now required to adequately evaluate antifibrotic effects of these small molecules during liver fibrosis. This is a good example of the possibility of uncoupling diverse TGF-β effects in a tissue, and a similar approach can be imagined for CLD. Of course, this warrants detailed knowledge of cell type and cell fate specific components of the TGF-β pathway and their signaling from the cell surface to the nucleus that are related to the respective adverse TGF-β effects. Furthermore, they need to be druggable.
Repurposing clinically used drugs for the treatment of liver fibrosis will probably accelerate the development of new antifibrotic therapies, as these drugs have known safety profiles, and thus will facilitate the conduct of clinical trials. For example, carvedilol and metformin, approved drugs for the treatment of hypertension and type II diabetes, respectively, showed promising results in animal models of liver fibrosis [275,276]. Mechanistically, metformin suppresses TGF-β/SMAD3 signaling, thus mediating the antifibrotic effects in the CCl4 model of liver fibrosis in mice [277]. Unfortunately, a clinical pilot study launched in 2014 to evaluate the role of metformin in HCV-induced liver fibrosis was withdrawn in 2018 due to insufficient funding (ClinicalTrials.gov Identifier: NCT02306070). Nonetheless, hope rises again with new clinical trials that will evaluate the potential of AVID200 in patients with diffuse cutaneous systemic sclerosis and myelofibrosis (ClinicalTrials.gov Identifier: NCT03831438 and NCT03895112). AVID200 represents a computationally designed highly potent trap for TGF-β1 and TGF-β3 [278]. The hopefully positive results of these trials would then encourage further testing of AVID200 in fibrotic diseases of the liver.
Recruitment for another study, NCT00574613, on efficacy and safety of p144, a 14mer peptide from human TGF-β1 type III receptor (betaglycan), to treat skin fibrosis in systemic sclerosis was recently completed. P144 has been specifically designed to block the interaction between TGF-β1 and TGF-β1 type III receptor, thus blocking its biological effects. P144 has shown significant antifibrotic activity in mice receiving repeated subcutaneous injections of bleomycin, a widely accepted animal model of human scleroderma. The results of this trial are expected soon.
Finally, NCT03727802, a study with TRK-250, a nucleic acid compound that inhibits the progression of experimental pulmonary fibrosis by selectively suppressing gene expression of TGF-β1, is currently (October 2019) still recruiting. The study is a double-blind, randomized, placebo-controlled phase I study. The primary objective is to assess the safety and tolerability of single and multiple inhaled doses of TRK-250 in subjects with idiopathic pulmonary fibrosis.
11. Conclusions and Outlook
The field of TGF-β signaling has broadened with newly identified connections to cellular programs like metabolism, ROS, senescence, circadian rhythm, epigenetics, and EMT. Continuously, new downstream branches, regulatory mechanisms and transcriptional targets of the TGF-β pathway are delineated, some of these related to HSC activation and liver fibrogenesis. However, still many aspects are not fully understood, especially with regard to a successful clinical translation from animal studies that has not been achieved yet. A major reason for this is the pleiotropic effects of this cytokine. The future aim will be to suppress excessive profibrogenic TGF-β effects, while maintaining the desired wound healing and anti-inflammatory responses, therewith providing superior TGF-β based therapeutics with minimal side effects. To date, there are no FDA approved drugs for the treatment of liver fibrosis. However, there are several ongoing TGF-β directed clinical trials conducted on patients with fibrotic diseases of other organs than the liver that, if successful, could be transferred to liver diseases and tested in well-controlled clinical trials. Due to the continuously growing understanding of the cellular context-dependency of TGF-β signaling together with advancement of diagnosis and follow up procedures for patients with liver fibrosis, we believe that TGF-β based therapeutics have a chance to become reality in the near future.
Acknowledgments
We appreciate all researcher’s work on hepatic stellate cells and TGF-β in liver disease, even if not cited due to space restraints.
Author Contributions
Idea and concept, S.D., B.D., N.M.-B.; Literature search, B.D., S.D., N.M.-B.; Manuscript writing, B.D., N.M.-B., S.D., C.M.; Manuscript editing, B.D., S.D., N.M.-B., C.M.
Funding
S.D. is supported by the LiSyM program of the BMBF.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chen J.Y., Thakar D., Chang T.T. Liver Fibrosis: Current Approaches and Future Directions for Diagnosis and Treatment. In: Willis M.S., Yates C.C., Schisler J.C., editors. Fibrosis in Disease: An Organ-Based Guide to Disease Pathophysiology and Therapeutic Considerations. Springer; Cham, Germany: 2019. pp. 387–417. [Google Scholar]
- 2.Hernandez-Gea V., Friedman S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 3.Ni Y., Li J.-M., Liu M.-K., Zhang T.-T., Wang D.-P., Zhou W.-H., Hu L.-Z., Lv W.-L. Pathological process of liver sinusoidal endothelial cells in liver diseases. World J. Gastroenterol. 2017;23:7666–7677. doi: 10.3748/wjg.v23.i43.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elpek G.Ö. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J. Gastroenterol. 2014;20:7260–7276. doi: 10.3748/wjg.v20.i23.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seki E., Brenner D.A. Recent advancement of molecular mechanisms of liver fibrosis. J. Hepato-Biliary-Pancreat. Sci. 2015;22:512–518. doi: 10.1002/jhbp.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellicoro A., Ramachandran P., Iredale J.P., Fallowfield J.A. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y.-L., Zhu R.-T., Sun Y.-L. Epithelial-mesenchymal transition in liver fibrosis. Biomed. Rep. 2016;4:269–274. doi: 10.3892/br.2016.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabregat I., Caballero-Díaz D. Transforming growth factor-β-induced cell plasticity in liver fibrosis and hepatocarcinogenesis. Front. Oncol. 2018;8:357. doi: 10.3389/fonc.2018.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 10.Dewidar B., Soukupova J., Fabregat I., Dooley S. TGF-β in hepatic stellate cell activation and liver fibrogenesis: Updated. Curr. Pathobiol. Rep. 2015;3:291–305. doi: 10.1007/s40139-015-0089-8. [DOI] [Google Scholar]
- 11.Schon H.-T., Weiskirchen R. Immunomodulatory effects of transforming growth factor-β in the liver. Hepatobiliary Surg. Nutr. 2014;3:386–406. doi: 10.3978/j.issn.2304-3881.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghafoory S., Varshney R., Robison T., Kouzbari K., Woolington S., Murphy B., Xia L., Ahamed J. Platelet TGF-β1 deficiency decreases liver fibrosis in a mouse model of liver injury. Blood Adv. 2018;2:470–480. doi: 10.1182/bloodadvances.2017010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derynck R., Budi E.H. Specificity, versatility, and control of TGF-β family signaling. Sci. Signal. 2019;12:eaav5183. doi: 10.1126/scisignal.aav5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickson M.C., Martin J.S., Cousins F.M., Kulkarni A.B., Karlsson S., Akhurst R.J. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 15.Kaartinen V., Voncken J.W., Shuler C., Warburton D., Bu D., Heisterkamp N., Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 16.Sanford L.P., Ormsby I., Gittenberger-de Groot A.C., Sariola H., Friedman R., Boivin G.P., Cardell E.L., Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabregat I., Moreno-Càceres J., Sánchez A., Dooley S., Dewidar B., Giannelli G., Ten Dijke P. IT-LIVER Consortium TGF-β signalling and liver disease. FEBS J. 2016;283:2219–2232. doi: 10.1111/febs.13665. [DOI] [PubMed] [Google Scholar]
- 18.Dropmann A., Dediulia T., Breitkopf-Heinlein K., Korhonen H., Janicot M., Weber S.N., Thomas M., Piiper A., Bertran E., Fabregat I., et al. TGF-β1 and TGF-β2 abundance in liver diseases of mice and men. Oncotarget. 2016;7:19499–19518. doi: 10.18632/oncotarget.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abd El-Meguid M., Dawood R.M., Mokhles M.A., El Awady M.K. Extrahepatic upregulation of transforming growth factor beta 2 in HCV genotype 4-induced liver fibrosis. J. Int. Soc. Interferon Cytokine Res. 2018;38:341–347. doi: 10.1089/jir.2018.0045. [DOI] [PubMed] [Google Scholar]
- 20.Chida T., Ito M., Nakashima K., Kanegae Y., Aoshima T., Takabayashi S., Kawata K., Nakagawa Y., Yamamoto M., Shimano H., et al. Critical role of CREBH-mediated induction of transforming growth factor β2 by hepatitis C virus infection in fibrogenic responses in hepatic stellate cells. Hepatology. 2017;66:1430–1443. doi: 10.1002/hep.29319. [DOI] [PubMed] [Google Scholar]
- 21.Shi M., Zhu J., Wang R., Chen X., Mi L., Walz T., Springer T.A. Latent TGF-β structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson I.B., Rifkin D.B. Regulation of the Bioavailability of TGF-β and TGF-β-Related Proteins. Cold Spring Harb. Perspect. Biol. 2016;8:a021907. doi: 10.1101/cshperspect.a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan Z., Marshall J.F. The role of integrins in TGFβ activation in the tumour stroma. Cell Tissue Res. 2016;365:657–673. doi: 10.1007/s00441-016-2474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura S.L. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am. J. Pathol. 2009;175:1362–1370. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan W., Liu T., Chen W., Hammad S., Longerich T., Fu Y., Li N., He Y., Liu C., Zhang Y., et al. ECM1 Prevents activation of transforming growth factor beta, hepatic stellate cells, and fibrogenesis in mice. Gastroenterology. 2019;157:1352–1367. doi: 10.1053/j.gastro.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 26.Carthy J.M. TGFβ signaling and the control of myofibroblast differentiation: Implications for chronic inflammatory disorders. J. Cell. Physiol. 2018;233:98–106. doi: 10.1002/jcp.25879. [DOI] [PubMed] [Google Scholar]
- 27.Heldin C.-H. Transforming Growth Factor-β Signaling. In: Moustakas A., Miyazawa K., editors. TGF-β in Human Disease. Springer; Tokyo, Japan: 2013. pp. 3–32. [Google Scholar]
- 28.Levy L., Hill C.S. Smad4 dependency defines two classes of transforming growth factor β (TGF-β) target genes and distinguishes TGF-β-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol. Cell. Biol. 2005;25:8108–8125. doi: 10.1128/MCB.25.18.8108-8125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moustakas A., Heldin C.-H. Non-Smad TGF-beta signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y.E. Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2017;9:a022129. doi: 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee M.-S., Ko S.-G., Kim H.-P., Kim Y.-B., Lee S.-Y., Kim S.-G., Jong H.-S., Kim T.-Y., Lee J.W., Bang Y.-J. Smad2 mediates Erk1/2 activation by TGF-beta1 in suspended, but not in adherent, gastric carcinoma cells. Int. J. Oncol. 2004;24:1229–1234. [PubMed] [Google Scholar]
- 33.Zhang L., Duan C.J., Binkley C., Li G., Uhler M.D., Logsdon C.D., Simeone D.M. A transforming growth factor beta-induced Smad3/Smad4 complex directly activates protein kinase A. Mol. Cell. Biol. 2004;24:2169–2180. doi: 10.1128/MCB.24.5.2169-2180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perlman R., Schiemann W.P., Brooks M.W., Lodish H.F., Weinberg R.A. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat. Cell Biol. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- 35.Kretzschmar M., Doody J., Massagué J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 36.Yang L., Moses H.L. Transforming growth factor β: Tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008;68:9107–9111. doi: 10.1158/0008-5472.CAN-08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida K., Matsuzaki K., Murata M., Yamaguchi T., Suwa K., Okazaki K. Clinico-Pathological importance of TGF-β/phospho-smad signaling during human hepatic fibrocarcinogenesis. Cancers. 2018;10:183. doi: 10.3390/cancers10060183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simeone D.M., Zhang L., Graziano K., Nicke B., Pham T., Schaefer C., Logsdon C.D. Smad4 mediates activation of mitogen-activated protein kinases by TGF-beta in pancreatic acinar cells. Am. J. Physiol. Cell Physiol. 2001;281:C311–C319. doi: 10.1152/ajpcell.2001.281.1.C311. [DOI] [PubMed] [Google Scholar]
- 39.Olsson N., Piek E., Sundström M., ten Dijke P., Nilsson G. Transforming growth factor-beta-mediated mast cell migration depends on mitogen-activated protein kinase activity. Cell. Signal. 2001;13:483–490. doi: 10.1016/S0898-6568(01)00176-0. [DOI] [PubMed] [Google Scholar]
- 40.Lee M.K., Pardoux C., Hall M.C., Lee P.S., Warburton D., Qing J., Smith S.M., Derynck R. TGF-β activates Erk MAP kinase signalling through direct phosphorylation of ShcA. Embo J. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu F., Liu C., Zhou D., Zhang L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016;64:157–167. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu F., Zhou D., Meng X., Wang X., Liu C., Huang C., Li J., Zhang L. Smad2 increases the apoptosis of activated human hepatic stellate cells induced by TRAIL. Int. Immunopharmacol. 2016;32:76–86. doi: 10.1016/j.intimp.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Khalil H., Kanisicak O., Prasad V., Correll R.N., Fu X., Schips T., Vagnozzi R.J., Liu R., Huynh T., Lee S.-J., et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017;127:3770–3783. doi: 10.1172/JCI94753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carthy J.M., Sundqvist A., Heldin A., van Dam H., Kletsas D., Heldin C.-H., Moustakas A. Tamoxifen inhibits TGF-β-mediated activation of myofibroblasts by blocking non-smad signaling through ERK1/2. J. Cell. Physiol. 2015;230:3084–3092. doi: 10.1002/jcp.25049. [DOI] [PubMed] [Google Scholar]
- 45.Ard S., Reed E.B., Smolyaninova L.V., Orlov S.N., Mutlu G.M., Guzy R.D., Dulin N.O. Sustained smad2 phosphorylation is required for myofibroblast transformation in response to TGF-β. Am. J. Respir. Cell Mol. Biol. 2019;60:367–369. doi: 10.1165/rcmb.2018-0252LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park S.H. Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. J. Biochem. Mol. Biol. 2005;38:9–16. doi: 10.5483/bmbrep.2005.38.1.009. [DOI] [PubMed] [Google Scholar]
- 47.Goto K., Kamiya Y., Imamura T., Miyazono K., Miyazawa K. Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors. J. Biol. Chem. 2007;282:20603–20611. doi: 10.1074/jbc.M702100200. [DOI] [PubMed] [Google Scholar]
- 48.Shi W., Sun C., He B., Xiong W., Shi X., Yao D., Cao X. GADD34–PP1c recruited by Smad7 dephosphorylates TGFβ type I receptor. J. Cell Biol. 2004;164:291–300. doi: 10.1083/jcb.200307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kavsak P., Rasmussen R.K., Causing C.G., Bonni S., Zhu H., Thomsen G.H., Wrana J.L. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol. Cell. 2000;6:1365–1375. doi: 10.1016/S1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 50.Malonis R.J., Fu W., Jelcic M.J., Thompson M., Canter B.S., Tsikitis M., Esteva F.J., Sánchez I. RNF11 sequestration of the E3 ligase SMURF2 on membranes antagonizes SMAD7 down-regulation of transforming growth factor β signaling. J. Biol. Chem. 2017;292:7435–7451. doi: 10.1074/jbc.M117.783662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z., Fan Y., Xie F., Zhou H., Jin K., Shao L., Shi W., Fang P., Yang B., van Dam H., et al. Breast cancer metastasis suppressor OTUD1 deubiquitinates SMAD7. Nat. Commun. 2017;8:2116. doi: 10.1038/s41467-017-02029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandhoke A.S., Karve K., Dadakhujaev S., Netherton S., Deng L., Bonni S. The ubiquitin ligase Smurf2 suppresses TGFβ-induced epithelial–mesenchymal transition in a sumoylation-regulated manner. Cell Death Differ. 2016;23:876–888. doi: 10.1038/cdd.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shihata W.A., Putra M.R.A., Chin-Dusting J.P.F. Is there a potential therapeutic role for caveolin-1 in fibrosis? Front. Pharm. 2017;8:567. doi: 10.3389/fphar.2017.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Roy C., Wrana J.L. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y.-G. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19:58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 56.Meyer C., Godoy P., Bachmann A., Liu Y., Barzan D., Ilkavets I., Maier P., Herskind C., Hengstler J.G., Dooley S. Distinct role of endocytosis for Smad and non-Smad TGF-β signaling regulation in hepatocytes. J. Hepatol. 2011;55:369–378. doi: 10.1016/j.jhep.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 57.Ji D.-G., Zhang Y., Yao S.-M., Zhai X.-J., Zhang L.-R., Zhang Y.-Z., Li H. Cav-1 deficiency promotes liver fibrosis in carbon tetrachloride (CCl4)-induced mice by regulation of oxidative stress and inflammation responses. Biomed. Pharm. Biomed. Pharm. 2018;102:26–33. doi: 10.1016/j.biopha.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 58.Lu J., Zhang J., Wang Y., Sun Q. Caveolin-1 scaffolding domain peptides alleviate liver fibrosis by inhibiting tgf-β1/smad signaling in mice. Int. J. Mol. Sci. 2018;19:1729. doi: 10.3390/ijms19061729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer C., Liu Y., Kaul A., Peipe I., Dooley S. Caveolin-1 abrogates TGF-β mediated hepatocyte apoptosis. Cell Death Dis. 2013;4:e466. doi: 10.1038/cddis.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyer C., Dzieran J., Liu Y., Schindler F., Munker S., Müller A., Coulouarn C., Dooley S. Distinct dedifferentiation processes affect caveolin-1 expression in hepatocytes. Cell Commun. Signal. 2013;11:6. doi: 10.1186/1478-811X-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M., Chen D., Huang H., Wang J., Wan X., Xu C., Li C., Ma H., Yu C., Li Y. Caveolin1 protects against diet induced hepatic lipid accumulation in mice. PLoS ONE. 2017;12:e0178748. doi: 10.1371/journal.pone.0178748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y.M., Noureddin M., Liu C., Ohashi K., Kim S.Y., Ramnath D., Powell E.E., Sweet M.J., Roh Y.S., Hsin I.-F., et al. Hyaluronan synthase 2-mediated hyaluronan production mediates Notch1 activation and liver fibrosis. Sci. Transl. Med. 2019;11:eaat9284. doi: 10.1126/scitranslmed.aat9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang D.-W., Zhao Y.-X., Wei D., Li Y.-L., Zhang Y., Wu J., Xu J., Chen C., Tang H., Zhang W., et al. HAb18G/CD147 promotes activation of hepatic stellate cells and is a target for antibody therapy of liver fibrosis. J. Hepatol. 2012;57:1283–1291. doi: 10.1016/j.jhep.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 64.Li H.-Y., Ju D., Zhang D.-W., Li H., Kong L.-M., Guo Y., Li C., Wang X.-L., Chen Z.-N., Bian H. Activation of TGF-β1-CD147 positive feedback loop in hepatic stellate cells promotes liver fibrosis. Sci. Rep. 2015;5:16552. doi: 10.1038/srep16552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shibanuma M., Mashimo J., Kuroki T., Nose K. Characterization of the TGF beta 1-inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. J. Biol. Chem. 1994;269:26767–26774. [PubMed] [Google Scholar]
- 66.Varney S.D., Betts C.B., Zheng R., Wu L., Hinz B., Zhou J., Van De Water L. Hic-5 is required for myofibroblast differentiation by regulating mechanically dependent MRTF-A nuclear accumulation. J. Cell Sci. 2016;129:774–787. doi: 10.1242/jcs.170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lei X.-F., Fu W., Kim-Kaneyama J.-R., Omoto T., Miyazaki T., Li B., Miyazaki A. Hic-5 deficiency attenuates the activation of hepatic stellate cells and liver fibrosis through upregulation of Smad7 in mice. J. Hepatol. 2016;64:110–117. doi: 10.1016/j.jhep.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 68.Kladney R.D., Cui X., Bulla G.A., Brunt E.M., Fimmel C.J. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology. 2002;35:1431–1440. doi: 10.1053/jhep.2002.32525. [DOI] [PubMed] [Google Scholar]
- 69.Yang X., Wei C., Liu N., Wu F., Chen J., Wang C., Sun Z., Wang Y., Liu L., Zhang X., et al. GP73, a novel TGF-β target gene, provides selective regulation on Smad and non-Smad signaling pathways. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:588–597. doi: 10.1016/j.bbamcr.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Earl L.A., Bi S., Baum L.G. Galectin multimerization and lattice formation are regulated by linker region structure. Glycobiology. 2011;21:6–12. doi: 10.1093/glycob/cwq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu M.-H., Hong H.-C., Hong T.-M., Chiang W.-F., Jin Y.-T., Chen Y.-L. Targeting galectin-1 in carcinoma-associated fibroblasts inhibits oral squamous cell carcinoma metastasis by downregulating MCP-1/CCL2 expression. Clin. Cancer Res. 2011;17:1306–1316. doi: 10.1158/1078-0432.CCR-10-1824. [DOI] [PubMed] [Google Scholar]
- 72.Tsutsumi T., Suzuki T., Moriya K., Shintani Y., Fujie H., Miyoshi H., Matsuura Y., Koike K., Miyamura T. Hepatitis C virus core protein activates ERK and p38 MAPK in cooperation with ethanol in transgenic mice. Hepatology. 2003;38:820–828. doi: 10.1002/hep.1840380408. [DOI] [PubMed] [Google Scholar]
- 73.Wu M.-H., Chen Y.-L., Lee K.-H., Chang C.-C., Cheng T.-M., Wu S.-Y., Tu C.-C., Tsui W.-L. Glycosylation-dependent galectin-1/neuropilin-1 interactions promote liver fibrosis through activation of TGF-β- and PDGF-like signals in hepatic stellate cells. Sci. Rep. 2017;7:11006. doi: 10.1038/s41598-017-11212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y., Rui B.-B., Tang L.-Y., Hu C.-M. Lipin family proteins—Key regulators in lipid metabolism. Ann. Nutr. Metab. 2015;66:10–18. doi: 10.1159/000368661. [DOI] [PubMed] [Google Scholar]
- 75.Finck B.N., Gropler M.C., Chen Z., Leone T.C., Croce M.A., Harris T.E., Lawrence J.C., Kelly D.P. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Jang C.H., Kim K.M., Yang J.H., Cho S.S., Kim S.J., Shin S.M., Cho I.J., Ki S.H. The Role of Lipin-1 in the Regulation of Fibrogenesis and TGF-β Signaling in Hepatic Stellate Cells. Toxicol. Sci. 2016;153:28–38. doi: 10.1093/toxsci/kfw109. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y., Shen R.-W., Han B., Li Z., Xiong L., Zhang F.-Y., Cong B.-B., Zhang B. Notch signaling mediated by TGF-β/Smad pathway in concanavalin A-induced liver fibrosis in rats. World J. Gastroenterol. 2017;23:2330–2336. doi: 10.3748/wjg.v23.i13.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen Z., Liu Y., Dewidar B., Hu J., Park O., Feng T., Xu C., Yu C., Li Q., Meyer C., et al. Delta-like ligand 4 modulates liver damage by down-regulating chemokine expression. Am. J. Pathol. 2016;186:1874–1889. doi: 10.1016/j.ajpath.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiong P., Zhang J., Xu D., Zhu J., Li W., Liu J., Liu F. Positive feedback loop of YB-1 interacting with Smad2 promotes liver fibrosis. Biochem. Biophys. Res. Commun. 2017;484:753–761. doi: 10.1016/j.bbrc.2017.01.148. [DOI] [PubMed] [Google Scholar]
- 80.Kolliopoulos C., Raja E., Razmara M., Heldin P., Heldin C.-H., Moustakas A., van der Heide L.P. Transforming growth factor β (TGFβ) induces NUAK kinase expression to fine-tune its signaling output. J. Biol. Chem. 2019;294:4119–4136. doi: 10.1074/jbc.RA118.004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y., Liu H., Meyer C., Li J., Nadalin S., Königsrainer A., Weng H., Dooley S., ten Dijke P. Transforming growth factor-β (TGF-β)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J. Biol. Chem. 2013;288:30708–30719. doi: 10.1074/jbc.M113.478685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang L.-Y., Heller M., Meng Z., Yu L.-R., Tang Y., Zhou M., Zhang Y.E. Transforming growth factor-β (TGF-β) Directly activates the JAK1-STAT3 axis to induce hepatic fibrosis in coordination with the SMAD pathway. J. Biol. Chem. 2017;292:4302–4312. doi: 10.1074/jbc.M116.773085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang G., Yu Y., Sun C., Liu T., Liang T., Zhan L., Lin X., Feng X.-H. STAT3 selectively interacts with Smad3 to antagonize TGF-β signalling. Oncogene. 2016;35:4388–4398. doi: 10.1038/onc.2015.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoon J.-H., Sudo K., Kuroda M., Kato M., Lee I.-K., Han J.S., Nakae S., Imamura T., Kim J., Ju J.H., et al. Phosphorylation status determines the opposing functions of Smad2/Smad3 as STAT3 cofactors in TH17 differentiation. Nat. Commun. 2015;6:7600. doi: 10.1038/ncomms8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim J.-H., Ham S., Lee Y., Suh G.Y., Lee Y.-S. TTC3 contributes to TGF-β1-induced epithelial-mesenchymal transition and myofibroblast differentiation, potentially through SMURF2 ubiquitylation and degradation. Cell Death Dis. 2019;10:92. doi: 10.1038/s41419-019-1308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iwaisako K., Jiang C., Zhang M., Cong M., Moore-Morris T.J., Park T.J., Liu X., Xu J., Wang P., Paik Y.-H., et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc. Natl. Acad. Sci. USA. 2014;111:E3297–E3305. doi: 10.1073/pnas.1400062111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pastan I., Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 2014;74:2907–2912. doi: 10.1158/0008-5472.CAN-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koyama Y., Wang P., Liang S., Iwaisako K., Liu X., Xu J., Zhang M., Sun M., Cong M., Karin D., et al. Mesothelin/mucin 16 signaling in activated portal fibroblasts regulates cholestatic liver fibrosis. J. Clin. Investig. 2017;127:1254–1270. doi: 10.1172/JCI88845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herrera B., Addante A., Sánchez A. BMP Signalling at the crossroad of liver fibrosis and regeneration. Int. J. Mol. Sci. 2017;19:39. doi: 10.3390/ijms19010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Breitkopf-Heinlein K., Meyer C., König C., Gaitantzi H., Addante A., Thomas M., Wiercinska E., Cai C., Li Q., Wan F., et al. BMP-9 interferes with liver regeneration and promotes liver fibrosis. Gut. 2017;66:939–954. doi: 10.1136/gutjnl-2016-313314. [DOI] [PubMed] [Google Scholar]
- 91.Chung Y.-H., Huang Y.-H., Chu T.-H., Chen C.-L., Lin P.-R., Huang S.-C., Wu D.-C., Huang C.-C., Hu T.-H., Kao Y.-H., et al. BMP-2 restoration aids in recovery from liver fibrosis by attenuating TGF-β1 signaling. Lab. Investig. J. Tech. Methods Pathol. 2018;98:999–1013. doi: 10.1038/s41374-018-0069-9. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y.-Q., Wan L.-Y., He X.-M., Ni Y.-R., Wang C., Liu C.-B., Wu J.-F. Gremlin1 Accelerates Hepatic Stellate Cell Activation Through Upregulation of TGF-Beta Expression. DNA Cell Biol. 2017;36:603–610. doi: 10.1089/dna.2017.3707. [DOI] [PubMed] [Google Scholar]
- 93.Navarro-Corcuera A., López-Zabalza M.J., Martínez-Irujo J.J., Álvarez-Sola G., Ávila M.A., Iraburu M.J., Ansorena E., Montiel-Duarte C. Role of AGAP2 in the profibrogenic effects induced by TGFβ in LX-2 hepatic stellate cells. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:673–685. doi: 10.1016/j.bbamcr.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 94.Römisch J. Factor VII activating protease (FSAP): A novel protease in hemostasis. Biol. Chem. 2002;383:1119–1124. doi: 10.1515/BC.2002.121. [DOI] [PubMed] [Google Scholar]
- 95.Leiting S., Seidl S., Martinez-Palacian A., Muhl L., Kanse S.M. Transforming growth factor-β (TGF-β) inhibits the expression of factor vii-activating protease (FSAP) in hepatocytes. J. Biol. Chem. 2016;291:21020–21028. doi: 10.1074/jbc.M116.744631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borkham-Kamphorst E., Zimmermann H.W., Gassler N., Bissels U., Bosio A., Tacke F., Weiskirchen R., Kanse S.M. Factor VII activating protease (FSAP) exerts anti-inflammatory and anti-fibrotic effects in liver fibrosis in mice and men. J. Hepatol. 2013;58:104–111. doi: 10.1016/j.jhep.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 97.Wasmuth H.E., Tag C.G., Van de Leur E., Hellerbrand C., Mueller T., Berg T., Puhl G., Neuhaus P., Samuel D., Trautwein C., et al. The Marburg I variant (G534E) of the factor VII-activating protease determines liver fibrosis in hepatitis C infection by reduced proteolysis of platelet-derived growth factor BB. Hepatology. 2009;49:775–780. doi: 10.1002/hep.22707. [DOI] [PubMed] [Google Scholar]
- 98.Spanjer A.I.R., Baarsma H.A., Oostenbrink L.M., Jansen S.R., Kuipers C.C., Lindner M., Postma D.S., Meurs H., Heijink I.H., Gosens R., et al. TGF-β-induced profibrotic signaling is regulated in part by the WNT receptor Frizzled-8. FASEB J. 2016;30:1823–1835. doi: 10.1096/fj.201500129. [DOI] [PubMed] [Google Scholar]
- 99.Beljaars L., Daliri S., Dijkhuizen C., Poelstra K., Gosens R. WNT-5A regulates TGF-β-related activities in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G219–G227. doi: 10.1152/ajpgi.00160.2016. [DOI] [PubMed] [Google Scholar]
- 100.Augustin H.G., Koh G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science. 2017;357:eaal2379. doi: 10.1126/science.aal2379. [DOI] [PubMed] [Google Scholar]
- 101.Karin D., Koyama Y., Brenner D., Kisseleva T. The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis. Differ. Res. Biol. Divers. 2016;92:84–92. doi: 10.1016/j.diff.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun M., Kisseleva T. Reversibility of liver fibrosis. Clin. Res. Hepatol. Gastroenterol. 2015;39:S60–S63. doi: 10.1016/j.clinre.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Investig. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Puche J.E., Saiman Y., Friedman S.L. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 2013;3:1473–1492. doi: 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- 105.Heymann F., Trautwein C., Tacke F. Monocytes and macrophages as cellular targets in liver fibrosis. Inflamm. Allergy Drug Targets. 2009;8:307–318. doi: 10.2174/187152809789352230. [DOI] [PubMed] [Google Scholar]
- 106.Krenkel O., Tacke F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 107.Schuppan D., Ruehl M., Somasundaram R., Hahn E.G. Matrix as a modulator of hepatic fibrogenesis. Semin. Liver Dis. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 108.Henderson N.C., Arnold T.D., Katamura Y., Giacomini M.M., Rodriguez J.D., McCarty J.H., Pellicoro A., Raschperger E., Betsholtz C., Ruminski P.G., et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olaso E., Ikeda K., Eng F.J., Xu L., Wang L.H., Lin H.C., Friedman S.L. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J. Clin. Investig. 2001;108:1369–1378. doi: 10.1172/JCI200112373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bansal R., Nakagawa S., Yazdani S., van Baarlen J., Venkatesh A., Koh A.P., Song W.-M., Goossens N., Watanabe H., Beasley M.B., et al. Integrin alpha 11 in the regulation of the myofibroblast phenotype: Implications for fibrotic diseases. Exp. Mol. Med. 2017;49:e396. doi: 10.1038/emm.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Greenhalgh S.N., Matchett K.P., Taylor R.S., Huang K., Li J.T., Saeteurn K., Donnelly M.C., Simpson E.E.M., Pollack J.L., Atakilit A., et al. Loss of integrin αvβ8 in murine hepatocytes accelerates liver regeneration. Am. J. Pathol. 2019;189:258–271. doi: 10.1016/j.ajpath.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rognoni E., Ruppert R., Fässler R. The kindlin family: Functions, signaling properties and implications for human disease. J. Cell Sci. 2016;129:17–27. doi: 10.1242/jcs.161190. [DOI] [PubMed] [Google Scholar]
- 113.Yu J., Hu Y., Gao Y., Li Q., Zeng Z., Li Y., Chen H. Kindlin-2 regulates hepatic stellate cells activation and liver fibrogenesis. Cell Death Discov. 2018;4:34. doi: 10.1038/s41420-018-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zollinger A.J., Smith M.L. Fibronectin, the extracellular glue. Matrix Biol. J. Int. Soc. Matrix Biol. 2017;60–61:27–37. doi: 10.1016/j.matbio.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 115.Klingberg F., Chau G., Walraven M., Boo S., Koehler A., Chow M.L., Olsen A.L., Im M., Lodyga M., Wells R.G., et al. The fibronectin ED-A domain enhances recruitment of latent TGF-β-binding protein-1 to the fibroblast matrix. J. Cell Sci. 2018;131:jcs201293. doi: 10.1242/jcs.201293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peters J.H., Hynes R.O. Fibronectin isoform distribution in the mouse. I. The alternatively spliced EIIIB, EIIIA, and V segments show widespread codistribution in the developing mouse embryo. Cell Adhes. Commun. 1996;4:103–125. doi: 10.3109/15419069609010766. [DOI] [PubMed] [Google Scholar]
- 117.Zent J., Guo L.-W. Signaling Mechanisms of Myofibroblastic Activation: Outside-in and Inside-Out. Cell. Physiol. Biochem. 2018;49:848–868. doi: 10.1159/000493217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muro A.F., Moretti F.A., Moore B.B., Yan M., Atrasz R.G., Wilke C.A., Flaherty K.R., Martinez F.J., Tsui J.L., Sheppard D., et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kawelke N., Vasel M., Sens C., von Au A., Dooley S., Nakchbandi I.A. Fibronectin protects from excessive liver fibrosis by modulating the availability of and responsiveness of stellate cells to active TGF-β. PLoS ONE. 2011;6:e28181. doi: 10.1371/journal.pone.0028181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Altrock E., Sens C., Wuerfel C., Vasel M., Kawelke N., Dooley S., Sottile J., Nakchbandi I.A. Inhibition of fibronectin deposition improves experimental liver fibrosis. J. Hepatol. 2015;62:625–633. doi: 10.1016/j.jhep.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 121.Phanish M.K., Heidebrecht F., Nabi M.E., Shah N., Niculescu-Duvaz I., Dockrell M.E.C. The regulation of TGFβ1 Induced fibronectin eda exon alternative splicing in human renal proximal tubule epithelial cells. J. Cell. Physiol. 2015;230:286–295. doi: 10.1002/jcp.24703. [DOI] [PubMed] [Google Scholar]
- 122.Li J., Wang Y., Ma M., Jiang S., Zhang X., Zhang Y., Yang X., Xu C., Tian G., Li Q., et al. Autocrine CTHRC1 activates hepatic stellate cells and promotes liver fibrosis by activating TGF-β signaling. EBioMedicine. 2019;40:43–55. doi: 10.1016/j.ebiom.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Santos A., Lagares D. Matrix stiffness: The conductor of organ fibrosis. Curr. Rheumatol. Rep. 2018;20:2. doi: 10.1007/s11926-018-0710-z. [DOI] [PubMed] [Google Scholar]
- 124.Lampi M.C., Reinhart-King C.A. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci. Transl. Med. 2018;10:eaao0475. doi: 10.1126/scitranslmed.aao0475. [DOI] [PubMed] [Google Scholar]
- 125.Caliari S.R., Perepelyuk M., Soulas E.M., Lee G.Y., Wells R.G., Burdick J.A. Gradually softening hydrogels for modeling hepatic stellate cell behavior during fibrosis regression. Integr. Biol. Quant. Biosci. Nano Macro. 2016;8:720–728. doi: 10.1039/C6IB00027D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peterová E., Mrkvicová A., Podmolíková L., Řezáčová M., Kanta J. The role of cytokines TGF-beta1 and FGF-1 in the expression of characteristic markers of rat liver myofibroblasts cultured in three-dimensional collagen gel. Physiol. Res. 2016;65:661–672. doi: 10.33549/physiolres.933092. [DOI] [PubMed] [Google Scholar]
- 127.Siegel R.C., Pinnell S.R., Martin G.R. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry. 1970;9:4486–4492. doi: 10.1021/bi00825a004. [DOI] [PubMed] [Google Scholar]
- 128.Liu S.B., Ikenaga N., Peng Z.-W., Sverdlov D.Y., Greenstein A., Smith V., Schuppan D., Popov Y. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2016;30:1599–1609. doi: 10.1096/fj.14-268425. [DOI] [PubMed] [Google Scholar]
- 129.Ma L., Zeng Y., Wei J., Yang D., Ding G., Liu J., Shang J., Kang Y., Ji X. Knockdown of LOXL1 inhibits TGF-β1-induced proliferation and fibrogenesis of hepatic stellate cells by inhibition of Smad2/3 phosphorylation. Biomed. Pharm. Biomed. Pharm. 2018;107:1728–1735. doi: 10.1016/j.biopha.2018.08.156. [DOI] [PubMed] [Google Scholar]
- 130.Mesarwi O.A., Shin M.-K., Drager L.F., Bevans-Fonti S., Jun J.C., Putcha N., Torbenson M.S., Pedrosa R.P., Lorenzi-Filho G., Steele K.E., et al. Lysyl Oxidase as a serum biomarker of liver fibrosis in patients with severe obesity and obstructive sleep apnea. Sleep. 2015;38:1583–1591. doi: 10.5665/sleep.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Z., Perez M., Lee E.-S., Kojima S., Griffin M. The functional relationship between transglutaminase 2 and transforming growth factor β1 in the regulation of angiogenesis and endothelial-mesenchymal transition. Cell Death Dis. 2017;8:e3032. doi: 10.1038/cddis.2017.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Verderio E., Gaudry C., Gross S., Smith C., Downes S., Griffin M. Regulation of cell surface tissue transglutaminase: Effects on matrix storage of latent transforming growth factor-beta binding protein-1. J. Histochem. Cytochem. 1999;47:1417–1432. doi: 10.1177/002215549904701108. [DOI] [PubMed] [Google Scholar]
- 133.Weiskirchen R., Tacke F. Liver fibrosis: From pathogenesis to novel therapies. Dig. Dis. 2016;34:410–422. doi: 10.1159/000444556. [DOI] [PubMed] [Google Scholar]
- 134.Lodyga M., Cambridge E., Karvonen H.M., Pakshir P., Wu B., Boo S., Kiebalo M., Kaarteenaho R., Glogauer M., Kapoor M., et al. Cadherin-11-mediated adhesion of macrophages to myofibroblasts establishes a profibrotic niche of active TGF-β. Sci. Signal. 2019;12:e3469. doi: 10.1126/scisignal.aao3469. [DOI] [PubMed] [Google Scholar]
- 135.Cai X., Li Z., Zhang Q., Qu Y., Xu M., Wan X., Lu L. CXCL6-EGFR-induced Kupffer cells secrete TGF-β1 promoting hepatic stellate cell activation via the SMAD2/BRD4/C-MYC/EZH2 pathway in liver fibrosis. J. Cell. Mol. Med. 2018;22:5050–5061. doi: 10.1111/jcmm.13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fabre T., Molina M.F., Soucy G., Goulet J.-P., Willems B., Villeneuve J.-P., Bilodeau M., Shoukry N.H. Type 3 cytokines IL-17A and IL-22 drive TGF-β-dependent liver fibrosis. Sci. Immunol. 2018;3:e7754. doi: 10.1126/sciimmunol.aar7754. [DOI] [PubMed] [Google Scholar]
- 137.Shi J., Zhao J., Zhang X., Cheng Y., Hu J., Li Y., Zhao X., Shang Q., Sun Y., Tu B., et al. Activated hepatic stellate cells impair NK cell anti-fibrosis capacity through a TGF-β-dependent emperipolesis in HBV cirrhotic patients. Sci. Rep. 2017;7:44544. doi: 10.1038/srep44544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ng C.P., Hinz B., Swartz M.A. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J. Cell Sci. 2005;118:4731–4739. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 139.Nithiananthan S., Crawford A., Knock J.C., Lambert D.W., Whawell S.A. Physiological fluid flow moderates fibroblast responses to TGF-β1. J. Cell. Biochem. 2017;118:878–890. doi: 10.1002/jcb.25767. [DOI] [PubMed] [Google Scholar]
- 140.Ansorge M., Sapudom J., Chkolnikov M., Wilde M., Anderegg U., Möller S., Schnabelrauch M., Pompe T. Mimicking paracrine TGFβ1 Signals during myofibroblast differentiation in 3D Collagen networks. Sci. Rep. 2017;7:5664. doi: 10.1038/s41598-017-05912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zi Z., Feng Z., Chapnick D.A., Dahl M., Deng D., Klipp E., Moustakas A., Liu X. Quantitative analysis of transient and sustained transforming growth factor-β signaling dynamics. Mol. Syst. Biol. 2011;7:492. doi: 10.1038/msb.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Feng Z., Zi Z., Liu X. Measuring TGF-β ligand dynamics in culture medium. Methods Mol. Biol. 2016;1344:379–389. doi: 10.1007/978-1-4939-2966-5_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hara M., Kirita A., Kondo W., Matsuura T., Nagatsuma K., Dohmae N., Ogawa S., Imajoh-Ohmi S., Friedman S.L., Rifkin D.B., et al. LAP degradation product reflects plasma kallikrein-dependent TGF-β activation in patients with hepatic fibrosis. SpringerPlus. 2014;3:221. doi: 10.1186/2193-1801-3-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hara M., Inoue I., Yamazaki Y., Kirita A., Matsuura T., Friedman S.L., Rifkin D.B., Kojima S. L59 TGF-β LAP degradation products serve as a promising blood biomarker for liver fibrogenesis in mice. Fibrogenesis Tissue Repair. 2015;8:17. doi: 10.1186/s13069-015-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Novo E., Parola M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2008;1:5. doi: 10.1186/1755-1536-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Richter K., Kietzmann T. Reactive oxygen species and fibrosis: Further evidence of a significant liaison. Cell Tissue Res. 2016;365:591–605. doi: 10.1007/s00441-016-2445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu R.-M., Desai L.P. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015;6:565–577. doi: 10.1016/j.redox.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu Y., Wang W., Peng X.-M., He Y., Xiong Y.-X., Liang H.-F., Chu L., Zhang B.-X., Ding Z.-Y., Chen X.-P. Rapamycin Upregulates connective tissue growth factor expression in hepatic progenitor cells through TGF-β-Smad2 dependent signaling. Front. Pharm. 2018;9:877. doi: 10.3389/fphar.2018.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liang S., Kisseleva T., Brenner D.A. The role of NADPH oxidases (NOXs) in liver fibrosis and the activation of myofibroblasts. Front. Physiol. 2016;7:17. doi: 10.3389/fphys.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Paik Y.-H., Iwaisako K., Seki E., Inokuchi S., Schnabl B., Osterreicher C.H., Kisseleva T., Brenner D.A. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology. 2011;53:1730–1741. doi: 10.1002/hep.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matsumoto M., Zhang J., Zhang X., Liu J., Jiang J.X., Yamaguchi K., Taruno A., Katsuyama M., Iwata K., Ibi M., et al. The NOX1 isoform of NADPH oxidase is involved in dysfunction of liver sinusoids in nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2018;115:412–420. doi: 10.1016/j.freeradbiomed.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sancho P., Mainez J., Crosas-Molist E., Roncero C., Fernández-Rodriguez C.M., Pinedo F., Huber H., Eferl R., Mikulits W., Fabregat I. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS ONE. 2012;7:e45285. doi: 10.1371/journal.pone.0045285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Andueza A., Garde N., García-Garzón A., Ansorena E., López-Zabalza M.J., Iraburu M.J., Zalba G., Martínez-Irujo J.J. NADPH oxidase 5 promotes proliferation and fibrosis in human hepatic stellate cells. Free Radic. Biol. Med. 2018;126:15–26. doi: 10.1016/j.freeradbiomed.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 154.Latella G. Redox Imbalance in intestinal fibrosis: Beware of the TGFβ-1, ROS, and Nrf2 connection. Dig. Dis. Sci. 2018;63:312–320. doi: 10.1007/s10620-017-4887-1. [DOI] [PubMed] [Google Scholar]
- 155.Xu W., Hellerbrand C., Köhler U.A., Bugnon P., Kan Y.-W., Werner S., Beyer T.A. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab. Investig. J. Tech. Methods Pathol. 2008;88:1068–1078. doi: 10.1038/labinvest.2008.75. [DOI] [PubMed] [Google Scholar]
- 156.Oh C.J., Kim J.-Y., Min A.-K., Park K.-G., Harris R.A., Kim H.-J., Lee I.-K. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. Free Radic. Biol. Med. 2012;52:671–682. doi: 10.1016/j.freeradbiomed.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 157.Prestigiacomo V., Suter-Dick L. Nrf2 protects stellate cells from Smad-dependent cell activation. PLoS ONE. 2018;13:e0201044. doi: 10.1371/journal.pone.0201044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guan Y., Tan Y., Liu W., Yang J., Wang D., Pan D., Sun Y., Zheng C. NF-E2-related factor 2 suppresses intestinal fibrosis by inhibiting reactive oxygen species-dependent TGF-β1/SMADs pathway. Dig. Dis. Sci. 2018;63:366–380. doi: 10.1007/s10620-017-4710-z. [DOI] [PubMed] [Google Scholar]
- 159.Chen Q., Zhang H., Cao Y., Li Y., Sun S., Zhang J., Zhang G. Schisandrin B attenuates CCl4-induced liver fibrosis in rats by regulation of Nrf2-ARE and TGF-β/Smad signaling pathways. Drug Des. Dev. 2017;11:2179–2191. doi: 10.2147/DDDT.S137507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Piersma B., Wouters O.Y., de Rond S., Boersema M., Gjaltema R.A.F., Bank R.A. Ascorbic acid promotes a TGFβ1-induced myofibroblast phenotype switch. Physiol. Rep. 2017;5:e13324. doi: 10.14814/phy2.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jung B.-J., Yoo H.-S., Shin S., Park Y.-J., Jeon S.-M. Dysregulation of NRF2 in cancer: From Molecular mechanisms to therapeutic opportunities. Biomology. 2018;26:57–68. doi: 10.4062/biomolther.2017.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Camarena V., Wang G. The epigenetic role of vitamin C in health and disease. Cell. Mol. Life Sci. 2016;73:1645–1658. doi: 10.1007/s00018-016-2145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Argemi J., Latasa M.U., Atkinson S.R., Blokhin I.O., Massey V., Gue J.P., Cabezas J., Lozano J.J., Van Booven D., Bell A., et al. Defective HNF4alpha-dependent gene expression as a driver of hepatocellular failure in alcoholic hepatitis. Nat. Commun. 2019;10:3126. doi: 10.1038/s41467-019-11004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Berger S.L., Kouzarides T., Shiekhattar R., Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu F., Chen B., Fan X., Li G., Dong P., Zheng J. Epigenetically-regulated MicroRNA-9-5p suppresses the activation of hepatic stellate cells via TGFBR1 and TGFBR2. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharm. 2017;43:2242–2252. doi: 10.1159/000484303. [DOI] [PubMed] [Google Scholar]
- 166.Davoodian P., Ravanshad M., Hosseini S.Y., Khanizadeh S., Almasian M., Nejati Zadeh A., Esmaiili Lashgarian H. Effect of TGF-β/smad signaling pathway blocking on expression profiles of miR-335, miR-150, miR-194, miR-27a, and miR-199a of hepatic stellate cells (HSCs) Gastroenterol. Hepatol. Bed Bench. 2017;10:112–117. [PMC free article] [PubMed] [Google Scholar]
- 167.Bowen T., Jenkins R.H., Fraser D.J. MicroRNAs, transforming growth factor beta-1, and tissue fibrosis. J. Pathol. 2013;229:274–285. doi: 10.1002/path.4119. [DOI] [PubMed] [Google Scholar]
- 168.Coll M., El Taghdouini A., Perea L., Mannaerts I., Vila-Casadesús M., Blaya D., Rodrigo-Torres D., Affò S., Morales-Ibanez O., Graupera I., et al. Integrative miRNA and Gene expression profiling analysis of human quiescent hepatic stellate cells. Sci. Rep. 2015;5:11549. doi: 10.1038/srep11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Roderburg C., Luedde M., Vargas Cardenas D., Vucur M., Mollnow T., Zimmermann H.W., Koch A., Hellerbrand C., Weiskirchen R., Frey N., et al. miR-133a mediates TGF-β-dependent derepression of collagen synthesis in hepatic stellate cells during liver fibrosis. J. Hepatol. 2013;58:736–742. doi: 10.1016/j.jhep.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 170.Roy S., Benz F., Vargas Cardenas D., Vucur M., Gautheron J., Schneider A., Hellerbrand C., Pottier N., Alder J., Tacke F., et al. miR-30c and miR-193 are a part of the TGF-β-dependent regulatory network controlling extracellular matrix genes in liver fibrosis. J. Dig. Dis. 2015;16:513–524. doi: 10.1111/1751-2980.12266. [DOI] [PubMed] [Google Scholar]
- 171.Brandon-Warner E., Benbow J.H., Swet J.H., Feilen N.A., Culberson C.R., McKillop I.H., deLemos A.S., Russo M.W., Schrum L.W. Adeno-associated virus serotype 2 vector-mediated reintroduction of microrna-19b attenuates hepatic fibrosis. Hum. Gene Ther. 2018;29:674–686. doi: 10.1089/hum.2017.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Feili X., Wu S., Ye W., Tu J., Lou L. MicroRNA-34a-5p inhibits liver fibrosis by regulating TGF-β1/Smad3 pathway in hepatic stellate cells. Cell Biol. Int. 2018;42:1370–1376. doi: 10.1002/cbin.11022. [DOI] [PubMed] [Google Scholar]
- 173.Zou Y., Li S., Li Z., Song D., Zhang S., Yao Q. MiR-146a attenuates liver fibrosis by inhibiting transforming growth factor-β1 mediated epithelial-mesenchymal transition in hepatocytes. Cell. Signal. 2019;58:1–8. doi: 10.1016/j.cellsig.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 174.Zou Y., Cai Y., Lu D., Zhou Y., Yao Q., Zhang S. MicroRNA-146a-5p attenuates liver fibrosis by suppressing profibrogenic effects of TGFβ1 and lipopolysaccharide. Cell. Signal. 2017;39:1–8. doi: 10.1016/j.cellsig.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 175.Wang P., Lei S., Wang X., Xu W., Hu P., Chen F., Zhang X., Yin C., Xie W. MicroRNA-134 deactivates hepatic stellate cells by targeting tgf-β activated kinase 1-binding protein 1. Biochem. Cell Biol. 2019;97:505–512. doi: 10.1139/bcb-2018-0211. [DOI] [PubMed] [Google Scholar]
- 176.Tao L., Xue D., Shen D., Ma W., Zhang J., Wang X., Zhang W., Wu L., Pan K., Yang Y., et al. MicroRNA-942 mediates hepatic stellate cell activation by regulating BAMBI expression in human liver fibrosis. Arch. Toxicol. 2018;92:2935–2946. doi: 10.1007/s00204-018-2278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.You K., Li S.-Y., Gong J., Fang J.-H., Zhang C., Zhang M., Yuan Y., Yang J., Zhuang S.-M. MicroRNA-125b promotes hepatic stellate cell activation and liver fibrosis by activating rhoa signaling. Mol. Nucleic Acids. 2018;12:57–66. doi: 10.1016/j.omtn.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu F., Guo Y., Chen B., Dong P., Zheng J. MicroRNA-17-5p activates hepatic stellate cells through targeting of Smad7. Lab. Investig. J. Tech. Methods Pathol. 2015;95:781–789. doi: 10.1038/labinvest.2015.58. [DOI] [PubMed] [Google Scholar]
- 179.Zhu J., Zhang Z., Zhang Y., Li W., Zheng W., Yu J., Wang B., Chen L., Zhuo Q., Chen L., et al. MicroRNA-212 activates hepatic stellate cells and promotes liver fibrosis via targeting SMAD7. Biochem. Biophys. Res. Commun. 2018;496:176–183. doi: 10.1016/j.bbrc.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 180.Tu X., Zheng X., Li H., Cao Z., Chang H., Luan S., Zhu J., Chen J., Zang Y., Zhang J. MicroRNA-30 Protects against carbon tetrachloride-induced liver fibrosis by attenuating transforming growth factor beta signaling in hepatic stellate cells. Toxicol. Sci. 2015;146:157–169. doi: 10.1093/toxsci/kfv081. [DOI] [PubMed] [Google Scholar]
- 181.Gupta P., Sata T.N., Yadav A.K., Mishra A., Vats N., Hossain M.M., Sanal M.G., Venugopal S.K. TGF-β induces liver fibrosis via miRNA-181a-mediated down regulation of augmenter of liver regeneration in hepatic stellate cells. PLoS ONE. 2019;14:e0214534. doi: 10.1371/journal.pone.0214534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang X., Dan X., Men R., Ma L., Wen M., Peng Y., Yang L. MiR-142-3p blocks TGF-β-induced activation of hepatic stellate cells through targeting TGFβRI. Life Sci. 2017;187:22–30. doi: 10.1016/j.lfs.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 183.Wei S., Wang Q., Zhou H., Qiu J., Li C., Shi C., Zhou S., Liu R., Lu L. miR-455-3p Alleviates Hepatic Stellate Cell Activation and Liver Fibrosis by Suppressing HSF1 Expression. Mol. Nucleic Acids. 2019;16:758–769. doi: 10.1016/j.omtn.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fan Z., Hao C., Li M., Dai X., Qin H., Li J., Xu H., Wu X., Zhang L., Fang M., et al. MKL1 is an epigenetic modulator of TGF-β induced fibrogenesis. Biochim. Biophys. Acta. 2015;1849:1219–1228. doi: 10.1016/j.bbagrm.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 185.Hu J., Chen C., Liu Q., Liu B., Song C., Zhu S., Wu C., Liu S., Yu H., Yao D., et al. The role of the miR-31/FIH1 pathway in TGF-β-induced liver fibrosis. Clin. Sci. 2015;129:305–317. doi: 10.1042/CS20140012. [DOI] [PubMed] [Google Scholar]
- 186.Genz B., Coleman M.A., Irvine K.M., Kutasovic J.R., Miranda M., Gratte F.D., Tirnitz-Parker J.E.E., Olynyk J.K., Calvopina D.A., Weis A., et al. Overexpression of miRNA-25-3p inhibits Notch1 signaling and TGF-β-induced collagen expression in hepatic stellate cells. Sci. Rep. 2019;9:8541. doi: 10.1038/s41598-019-44865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Peng H., Wan L.-Y., Liang J.-J., Zhang Y.-Q., Ai W.-B., Wu J.-F. The roles of lncRNA in hepatic fibrosis. Cell Biosci. 2018;8:63. doi: 10.1186/s13578-018-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tang P.M.-K., Zhang Y.-Y., Lan H.-Y. LncRNAs in TGF-β-driven tissue fibrosis. Non-Coding RNA. 2018;4:26. doi: 10.3390/ncrna4040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tu X., Zhang Y., Zheng X., Deng J., Li H., Kang Z., Cao Z., Huang Z., Ding Z., Dong L., et al. TGF-β-induced hepatocyte lincRNA-p21 contributes to liver fibrosis in mice. Sci. Rep. 2017;7:2957. doi: 10.1038/s41598-017-03175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang Y., Liu C., Barbier O., Smalling R., Tsuchiya H., Lee S., Delker D., Zou A., Hagedorn C.H., Wang L. Bcl2 is a critical regulator of bile acid homeostasis by dictating Shp and lncRNA H19 function. Sci. Rep. 2016;6:20559. doi: 10.1038/srep20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen X., Yamamoto M., Fujii K., Nagahama Y., Ooshio T., Xin B., Okada Y., Furukawa H., Nishikawa Y. Differential reactivation of fetal/neonatal genes in mouse liver tumors induced in cirrhotic and non-cirrhotic conditions. Cancer Sci. 2015;106:972–981. doi: 10.1111/cas.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhu J., Luo Z., Pan Y., Zheng W., Li W., Zhang Z., Xiong P., Xu D., Du M., Wang B., et al. H19/miR-148a/USP4 axis facilitates liver fibrosis by enhancing TGF-β signaling in both hepatic stellate cells and hepatocytes. J. Cell. Physiol. 2019;234:9698–9710. doi: 10.1002/jcp.27656. [DOI] [PubMed] [Google Scholar]
- 193.Song M., Xia L., Sun M., Yang C., Wang F. Circular RNA in Liver: Health and diseases. Adv. Exp. Med. Biol. 2018;1087:245–257. doi: 10.1007/978-981-13-1426-1_20. [DOI] [PubMed] [Google Scholar]
- 194.Chen Y., Yuan B., Wu Z., Dong Y., Zhang L., Zeng Z. Microarray profiling of circular RNAs and the potential regulatory role of hsa_circ_0071410 in the activated human hepatic stellate cell induced by irradiation. Gene. 2017;629:35–42. doi: 10.1016/j.gene.2017.07.078. [DOI] [PubMed] [Google Scholar]
- 195.Zhou Y., Lv X., Qu H., Zhao K., Fu L., Zhu L., Ye G., Guo J. Preliminary screening and functional analysis of circular RNAs associated with hepatic stellate cell activation. Gene. 2018;677:317–323. doi: 10.1016/j.gene.2018.08.052. [DOI] [PubMed] [Google Scholar]
- 196.Keene J.D. RNA regulons: Coordination of post-transcriptional events. Nat. Rev. Genet. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 197.Wang S., Jung Y., Hyun J., Friedersdorf M., Oh S.-H., Kim J., Premont R.T., Keene J.D., Diehl A.M. RNA binding proteins control transdifferentiation of hepatic stellate cells into myofibroblasts. Cell. Physiol. Biochem. 2018;48:1215–1229. doi: 10.1159/000491987. [DOI] [PubMed] [Google Scholar]
- 198.Chen P.-J., Huang C., Meng X.-M., Li J. Epigenetic modifications by histone deacetylases: Biological implications and therapeutic potential in liver fibrosis. Biochimie. 2015;116:61–69. doi: 10.1016/j.biochi.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 199.Hardy T., Mann D.A. Epigenetics in liver disease: From biology to therapeutics. Gut. 2016;65:1895–1905. doi: 10.1136/gutjnl-2015-311292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhou B., Zeng S., Li L., Fan Z., Tian W., Li M., Xu H., Wu X., Fang M., Xu Y. Angiogenic factor with G patch and FHA domains 1 (Aggf1) regulates liver fibrosis by modulating TGF-β signaling. Biochim. Biophys. Acta. 2016;1862:1203–1213. doi: 10.1016/j.bbadis.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 201.Zeybel M., Luli S., Sabater L., Hardy T., Oakley F., Leslie J., Page A., Moran Salvador E., Sharkey V., Tsukamoto H., et al. A proof-of-concept for epigenetic therapy of tissue fibrosis: Inhibition of liver fibrosis progression by 3-deazaneplanocin A. Mol. J. Am. Soc. Gene. 2017;25:218–231. doi: 10.1016/j.ymthe.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Martin-Mateos R., De Assuncao T.M., Arab J.P., Jalan-Sakrikar N., Yaqoob U., Greuter T., Verma V.K., Mathison A.J., Cao S., Lomberk G., et al. Enhancer of zeste homologue 2 inhibition attenuates TGF-β dependent hepatic stellate cell activation and liver fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2019;7:197–209. doi: 10.1016/j.jcmgh.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lin T., Wang S., Shao C., Yuan X., Wandrer F., Bantel H., Marx A., Ebert M., Ding H., Dooley S., et al. THU-375-Transcription factor TRIM33 controls liver progenitor cell towards hepatocyte differentiation through synergizing with SMAD2/3 following massive parenchymal loss. J. Hepatol. 2019;70:e318–e319. doi: 10.1016/S0618-8278(19)30623-1. [DOI] [Google Scholar]
- 204.Wang Y., Zhao L., Jiao F.-Z., Zhang W.-B., Chen Q., Gong Z.-J. Histone deacetylase inhibitor suberoylanilide hydroxamic acid alleviates liver fibrosis by suppressing the transforming growth factor-β1 signal pathway. Hepatobiliary Pancreat. Dis. Int. 2018;17:423–429. doi: 10.1016/j.hbpd.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 205.Tu A.W., Luo K. Acetylation of Smad2 by the co-activator p300 regulates activin and transforming growth factor beta response. J. Biol. Chem. 2007;282:21187–21196. doi: 10.1074/jbc.M700085200. [DOI] [PubMed] [Google Scholar]
- 206.Inoue Y., Itoh Y., Abe K., Okamoto T., Daitoku H., Fukamizu A., Onozaki K., Hayashi H. Smad3 is acetylated by p300/CBP to regulate its transactivation activity. Oncogene. 2007;26:500–508. doi: 10.1038/sj.onc.1209826. [DOI] [PubMed] [Google Scholar]
- 207.Janknecht R., Wells N.J., Hunter T. TGF-beta-stimulated cooperation of smad proteins with the coactivators CBP/p300. Genes Dev. 1998;12:2114–2119. doi: 10.1101/gad.12.14.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang Y., Tu K., Liu D., Guo L., Chen Y., Li Q., Maiers J.L., Liu Z., Shah V.H., Dou C., et al. p300 acetyltransferase is a cytoplasm-to-nucleus shuttle for SMAD2/3 and TAZ nuclear transport in transforming growth factor β-stimulated hepatic stellate cells. Hepatology. 2019;70:1409–1423. doi: 10.1002/hep.30668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jiang R., Zhou Y., Wang S., Pang N., Huang Y., Ye M., Wan T., Qiu Y., Pei L., Jiang X., et al. Nicotinamide riboside protects against liver fibrosis induced by CCl4 via regulating the acetylation of Smads signaling pathway. Life Sci. 2019;225:20–28. doi: 10.1016/j.lfs.2019.03.064. [DOI] [PubMed] [Google Scholar]
- 210.Alonso-Merino E., Martín Orozco R., Ruíz-Llorente L., Martínez-Iglesias O.A., Velasco-Martín J.P., Montero-Pedrazuela A., Fanjul-Rodríguez L., Contreras-Jurado C., Regadera J., Aranda A. Thyroid hormones inhibit TGF-β signaling and attenuate fibrotic responses. Proc. Natl. Acad. Sci. USA. 2016;113:E3451–E3460. doi: 10.1073/pnas.1506113113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Munker S., Wu Y.-L., Ding H.-G., Liebe R., Weng H.-L. Can a fibrotic liver afford epithelial-mesenchymal transition? World J. Gastroenterol. 2017;23:4661–4668. doi: 10.3748/wjg.v23.i26.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Taura K., Iwaisako K., Hatano E., Uemoto S. Controversies over the epithelial-to-mesenchymal transition in liver fibrosis. J. Clin. Med. 2016;5:9. doi: 10.3390/jcm5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Choi S.S., Diehl A.M. Epithelial-to-mesenchymal transitions in the liver. Hepatology. 2009;50:2007–2013. doi: 10.1002/hep.23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology. 2017;65:1039–1043. doi: 10.1002/hep.28948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zeisberg M., Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am. J. Physiol. Cell Physiol. 2013;304:C216–C225. doi: 10.1152/ajpcell.00328.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wu Y., Ding Z.-Y., Jin G.-N., Xiong Y.-X., Yu B., Sun Y.-M., Wang W., Liang H.-F., Zhang B., Chen X.-P. Autocrine transforming growth factor-β/activin A-Smad signaling induces hepatic progenitor cells undergoing partial epithelial-mesenchymal transition states. Biochimie. 2018;148:87–98. doi: 10.1016/j.biochi.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 217.Katsuno Y., Meyer D.S., Zhang Z., Shokat K.M., Akhurst R.J., Miyazono K., Derynck R. Chronic TGF-β exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci. Signal. 2019;12:eaau8544. doi: 10.1126/scisignal.aau8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sun S., Xie F., Zhang Q., Cui Z., Cheng X., Zhong F., He K., Zhou J. Advanced oxidation protein products induce hepatocyte epithelial-mesenchymal transition via a ROS-dependent, TGF-β/Smad signaling pathway. Cell Biol. Int. 2017;41:842–853. doi: 10.1002/cbin.10792. [DOI] [PubMed] [Google Scholar]
- 219.Asahina K., Zhou B., Pu W.T., Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–995. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li Y., Lua I., French S.W., Asahina K. Role of TGF-β signaling in differentiation of mesothelial cells to vitamin A-poor hepatic stellate cells in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310:G262–G272. doi: 10.1152/ajpgi.00257.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Adams D.H., Shepherd E.L., Lalor P.F. Could endothelial TGFβ signaling be a promising new target for liver disease? Expert Rev. Gastroenterol. Hepatol. 2018;12:637–639. doi: 10.1080/17474124.2018.1477587. [DOI] [PubMed] [Google Scholar]
- 222.Ribera J., Pauta M., Melgar-Lesmes P., Córdoba B., Bosch A., Calvo M., Rodrigo-Torres D., Sancho-Bru P., Mira A., Jiménez W., et al. A small population of liver endothelial cells undergoes endothelial-to-mesenchymal transition in response to chronic liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;313:G492–G504. doi: 10.1152/ajpgi.00428.2016. [DOI] [PubMed] [Google Scholar]
- 223.Dufton N.P., Peghaire C.R., Osuna-Almagro L., Raimondi C., Kalna V., Chuahan A., Webb G., Yang Y., Birdsey G.M., Lalor P., et al. Dynamic regulation of canonical TGFβ signalling by endothelial transcription factor ERG protects from liver fibrogenesis. Nat. Commun. 2017;8:895. doi: 10.1038/s41467-017-01169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vallée A., Lecarpentier Y., Vallée J.-N. Thermodynamic Aspects and Reprogramming Cellular Energy Metabolism during the Fibrosis Process. Int. J. Mol. Sci. 2017;18:2537. doi: 10.3390/ijms18122537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pfeiffer T., Schuster S., Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 227.Kottmann R.M., Kulkarni A.A., Smolnycki K.A., Lyda E., Dahanayake T., Salibi R., Honnons S., Jones C., Isern N.G., Hu J.Z., et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am. J. Respir. Crit. Care Med. 2012;186:740–751. doi: 10.1164/rccm.201201-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li J., Ghazwani M., Liu K., Huang Y., Chang N., Fan J., He F., Li L., Bu S., Xie W., et al. Regulation of hepatic stellate cell proliferation and activation by glutamine metabolism. PLoS ONE. 2017;12:e0182679. doi: 10.1371/journal.pone.0182679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bernard K., Logsdon N.J., Benavides G.A., Sanders Y., Zhang J., Darley-Usmar V.M., Thannickal V.J. Glutaminolysis is required for transforming growth factor-β1-induced myofibroblast differentiation and activation. J. Biol. Chem. 2018;293:1218–1228. doi: 10.1074/jbc.RA117.000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Altman B.J., Stine Z.E., Dang C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer. 2016;16:619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Takahashi H., Alves C.R.R., Stanford K.I., Middelbeek R.J.W., Nigro P., Ryan R.E., Xue R., Sakaguchi M., Lynes M.D., So K., et al. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 2019;1:291–303. doi: 10.1038/s42255-018-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Starling S. A new metabolic role for TGFβ2. Nat. Rev. Endocrinol. 2019;15:191. doi: 10.1038/s41574-019-0183-9. [DOI] [PubMed] [Google Scholar]
- 233.Qin G., Wang G.Z., Guo D.D., Bai R.X., Wang M., Du S.Y. Deletion of Smad4 reduces hepatic inflammation and fibrogenesis during nonalcoholic steatohepatitis progression. J. Dig. Dis. 2018;19:301–313. doi: 10.1111/1751-2980.12599. [DOI] [PubMed] [Google Scholar]
- 234.Ko C.H., Takahashi J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 235.Gekakis N., Staknis D., Nguyen H.B., Davis F.C., Wilsbacher L.D., King D.P., Takahashi J.S., Weitz C.J. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 236.Chen P., Kakan X., Wang S., Dong W., Jia A., Cai C., Zhang J. Deletion of clock gene Per2 exacerbates cholestatic liver injury and fibrosis in mice. Exp. Toxicol. Pathol. 2013;65:427–432. doi: 10.1016/j.etp.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 237.Chen P., Han Z., Yang P., Zhu L., Hua Z., Zhang J. Loss of clock gene mPer2 promotes liver fibrosis induced by carbon tetrachloride. Hepatol. Res. 2010;40:1117–1127. doi: 10.1111/j.1872-034X.2010.00695.x. [DOI] [PubMed] [Google Scholar]
- 238.Janich P., Pascual G., Merlos-Suárez A., Batlle E., Ripperger J., Albrecht U., Cheng H.-Y.M., Obrietan K., Di Croce L., Benitah S.A. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–214. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- 239.Gast H., Gordic S., Petrzilka S., Lopez M., Müller A., Gietl A., Hock C., Birchler T., Fontana A. Transforming growth factor-beta inhibits the expression of clock genes. Ann. N. Y. Acad. Sci. 2012;1261:79–87. doi: 10.1111/j.1749-6632.2012.06640.x. [DOI] [PubMed] [Google Scholar]
- 240.Dong C., Gongora R., Sosulski M.L., Luo F., Sanchez C.G. Regulation of transforming growth factor-beta1 (TGF-β1)-induced pro-fibrotic activities by circadian clock gene BMAL1. Respir. Res. 2016;17:4. doi: 10.1186/s12931-016-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gibbs J., Ince L., Matthews L., Mei J., Bell T., Yang N., Saer B., Begley N., Poolman T., Pariollaud M., et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 2014;20:919–926. doi: 10.1038/nm.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hwang J.-W., Sundar I.K., Yao H., Sellix M.T., Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 2014;28:176–194. doi: 10.1096/fj.13-232629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dikic I., Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 244.Li T., Eheim A.L., Klein S., Uschner F.E., Smith A.C., Brandon-Warner E., Ghosh S., Bonkovsky H.L., Trebicka J., Schrum L.W. Novel role of nuclear receptor Rev-erbα in hepatic stellate cell activation: Potential therapeutic target for liver injury. Hepatology. 2014;59:2383–2396. doi: 10.1002/hep.27049. [DOI] [PubMed] [Google Scholar]
- 245.Kojetin D.J., Burris T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Thomes P.G., Brandon-Warner E., Li T., Donohue T.M., Schrum L.W. Rev-erb agonist and TGF-β similarly affect autophagy but differentially regulate hepatic stellate cell fibrogenic phenotype. Int. J. Biochem. Cell Biol. 2016;81:137–147. doi: 10.1016/j.biocel.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 247.Schrader J., Fallowfield J., Iredale J.P. Senescence of activated stellate cells: Not just early retirement. Hepatology. 2009;49:1045–1047. doi: 10.1002/hep.22832. [DOI] [PubMed] [Google Scholar]
- 248.Muñoz-Espín D., Serrano M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 249.Krizhanovsky V., Yon M., Dickins R.A., Hearn S., Simon J., Miething C., Yee H., Zender L., Lowe S.W. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wiemann S.U., Satyanarayana A., Tsahuridu M., Tillmann H.L., Zender L., Klempnauer J., Flemming P., Franco S., Blasco M.A., Manns M.P., et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- 251.Schafer M.J., White T.A., Iijima K., Haak A.J., Ligresti G., Atkinson E.J., Oberg A.L., Birch J., Salmonowicz H., Zhu Y., et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Razdan N., Vasilopoulos T., Herbig U. Telomere dysfunction promotes transdifferentiation of human fibroblasts into myofibroblasts. Aging Cell. 2018;17:e12838. doi: 10.1111/acel.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bird T.G., Müller M., Boulter L., Vincent D.F., Ridgway R.A., Lopez-Guadamillas E., Lu W.-Y., Jamieson T., Govaere O., Campbell A.D., et al. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci. Transl. Med. 2018;10:eaan1230. doi: 10.1126/scitranslmed.aan1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ferreira-Gonzalez S., Lu W.-Y., Raven A., Dwyer B., Man T.Y., O’Duibhir E., Lewis P.J.S., Campana L., Kendall T.J., Bird T.G., et al. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration. Nat. Commun. 2018;9:1020. doi: 10.1038/s41467-018-03299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Marcellin P., Gane E., Buti M., Afdhal N., Sievert W., Jacobson I.M., Washington M.K., Germanidis G., Flaherty J.F., Aguilar Schall R., et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 256.Schuppan D., Ashfaq-Khan M., Yang A.T., Kim Y.O. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018;68–69:435–451. doi: 10.1016/j.matbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 257.Dooley S., ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245–256. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wang X., Li Y., Li X., Yan L., Guan H., Han R., Han Y., Gui J., Xu X., Dong Y., et al. Expression, purification, and evaluation of in vivo anti-fibrotic activity for soluble truncated TGF-β receptor II as a cleavable His-SUMO fusion protein. World J. Microbiol. Biotechnol. 2018;34:181. doi: 10.1007/s11274-018-2565-x. [DOI] [PubMed] [Google Scholar]
- 259.Dituri F., Mancarella S., Cigliano A., Chieti A., Giannelli G. TGF-β as Multifaceted Orchestrator in HCC Progression: Signaling, EMT, Immune Microenvironment, and Novel Therapeutic Perspectives. Semin. Liver Dis. 2019;39:53–69. doi: 10.1055/s-0038-1676121. [DOI] [PubMed] [Google Scholar]
- 260.Melisi D., Ishiyama S., Sclabas G.M., Fleming J.B., Xia Q., Tortora G., Abbruzzese J.L., Chiao P.J. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol. Cancer Ther. 2008;7:829–840. doi: 10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Luangmonkong T., Suriguga S., Bigaeva E., Boersema M., Oosterhuis D., de Jong K.P., Schuppan D., Mutsaers H.A.M., Olinga P. Evaluating the antifibrotic potency of galunisertib in a human ex vivo model of liver fibrosis. Br. J. Pharm. 2017;174:3107–3117. doi: 10.1111/bph.13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hammad S., Cavalcanti E., Werle J., Caruso M.L., Dropmann A., Ignazzi A., Ebert M.P., Dooley S., Giannelli G. Galunisertib modifies the liver fibrotic composition in the Abcb4Ko mouse model. Arch. Toxicol. 2018;92:2297–2309. doi: 10.1007/s00204-018-2231-y. [DOI] [PubMed] [Google Scholar]
- 263.Ikenaga N., Peng Z.-W., Vaid K.A., Liu S.B., Yoshida S., Sverdlov D.Y., Mikels-Vigdal A., Smith V., Schuppan D., Popov Y.V. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017;66:1697–1708. doi: 10.1136/gutjnl-2016-312473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ahn J., Son M.K., Jung K.H., Kim K., Kim G.J., Lee S.-H., Hong S.-S., Park S.G. Aminoacyl-tRNA synthetase interacting multi-functional protein 1 attenuates liver fibrosis by inhibiting TGFβ signaling. Int. J. Oncol. 2016;48:747–755. doi: 10.3892/ijo.2015.3303. [DOI] [PubMed] [Google Scholar]
- 265.Zhang H., Ju B., Zhang X., Zhu Y., Nie Y., Xu Y., Lei Q. Magnolol attenuates concanavalin a-induced hepatic fibrosis, inhibits cd4+ t helper 17 (Th17) cell differentiation and suppresses hepatic stellate cell activation: Blockade of smad3/smad4 signalling. Basic Clin. Pharm. Toxicol. 2017;120:560–570. doi: 10.1111/bcpt.12749. [DOI] [PubMed] [Google Scholar]
- 266.Ji X., Wang H., Wu Z., Zhong X., Zhu M., Zhang Y., Tan R., Liu Y., Li J., Wang L. Specific Inhibitor of smad3 (SIS3) attenuates fibrosis, apoptosis, and inflammation in unilateral ureteral obstruction kidneys by inhibition of transforming growth factor β (TGF-β)/Smad3 signaling. Med. Sci. Monit. Int. 2018;24:1633–1641. doi: 10.12659/MSM.909236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ganai A.A., Husain M. Genistein attenuates D-GalN induced liver fibrosis/chronic liver damage in rats by blocking the TGF-β/Smad signaling pathways. Chem. Biol. Interact. 2017;261:80–85. doi: 10.1016/j.cbi.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 268.Liu J., Kong D., Qiu J., Xie Y., Lu Z., Zhou C., Liu X., Zhang R., Wang Y. Praziquantel Ameliorates ccl4 -induced liver fibrosis in mice by inhibiting TGF-β/smad signalling via upregulating smad7 in hepatic stellate cells. Br. J. Pharm. 2019 doi: 10.1111/bph.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Delire B., Stärkel P., Leclercq I. Animal models for fibrotic liver diseases: What we have, what we need, and what is under development. J. Clin. Transl. Hepatol. 2015;3:53–66. doi: 10.14218/JCTH.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hayashi H., Sakai T. Animal models for the study of liver fibrosis: New insights from knockout mouse models. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G729–G738. doi: 10.1152/ajpgi.00013.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hara M., Matsuura T., Kojima S. TGF-β LAP Degradation Products, a Novel Biomarker and Promising Therapeutic Target for Liver Fibrogenesis. In: Nakao K., Minato N., Uemoto S., editors. Innovative Medicine: Basic Research and Development. Springer; Tokyo, Japan: 2015. [PubMed] [Google Scholar]
- 272.Qiao X., Rao P., Zhang Y., Liu L., Pang M., Wang H., Hu M., Tian X., Zhang J., Zhao Y., et al. Redirecting TGF-β signaling through the β-catenin/foxo complex prevents kidney fibrosis. J. Am. Soc. Nephrol. 2018;29:557–570. doi: 10.1681/ASN.2016121362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Akcora B.Ö., Storm G., Bansal R. Inhibition of canonical WNT signaling pathway by β-catenin/CBP inhibitor ICG-001 ameliorates liver fibrosis in vivo through suppression of stromal CXCL12. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:804–818. doi: 10.1016/j.bbadis.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 274.Kimura K., Ikoma A., Shibakawa M., Shimoda S., Harada K., Saio M., Imamura J., Osawa Y., Kimura M., Nishikawa K., et al. Safety, tolerability, and preliminary efficacy of the anti-fibrotic small molecule pri-724, a cbp/β-catenin inhibitor, in patients with hepatitis c virus-related cirrhosis: A single-center, open-label, dose escalation phase 1 trial. EBioMedicine. 2017;23:79–87. doi: 10.1016/j.ebiom.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.El-Wakeel S.A., Rahmo R.M., El-Abhar H.S. Anti-fibrotic impact of Carvedilol in a CCl-4 model of liver fibrosis via serum microRNA-200a/SMAD7 enhancement to bridle TGF-β1/EMT track. Sci. Rep. 2018;8:14327. doi: 10.1038/s41598-018-32309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Nguyen G., Park S.Y., Le C.T., Park W.S., Choi D.H., Cho E.-H. Metformin ameliorates activation of hepatic stellate cells and hepatic fibrosis by succinate and GPR91 inhibition. Biochem. Biophys. Res. Commun. 2018;495:2649–2656. doi: 10.1016/j.bbrc.2017.12.143. [DOI] [PubMed] [Google Scholar]
- 277.Fan K., Wu K., Lin L., Ge P., Dai J., He X., Hu K., Zhang L. Metformin mitigates carbon tetrachloride-induced TGF-β1/Smad3 signaling and liver fibrosis in mice. Biomed. Pharm. 2017;90:421–426. doi: 10.1016/j.biopha.2017.03.079. [DOI] [PubMed] [Google Scholar]
- 278.Batlle E., Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]